- 1Department of Endocrinology, Chengdu Eighth People’s Hospital (Geriatric Hospital of Chengdu Medical College), Chengdu, Sichuan, China
- 2Department of Endocrinology, Shengjing Hospital of China Medical University, Shenyang, Liaoning, China
Background: Immune checkpoint inhibitors (ICPis) induce autoimmune diseases, including autoimmune polyendocrine syndrome type 2 (APS-2), which is defined as a combination of at least two of the following endocrinopathies: autoimmune thyroid disease, type 1 diabetes, and Addison’s disease. Cases with the full triad are rare. We present a case of an elderly woman who developed APS-2 with the complete triad shortly after starting anti-programmed cell death 1 (anti-PD1) treatment and review the related literature.
Case: A 60-year-old woman, without any personal or family history of autoimmune and endocrine diseases, started the immunotherapy of anti-PD1 (camrelizumab) for squamous cell carcinoma of the urethral meatus. She developed primary hypothyroidism with elevated antibodies to thyroid peroxidase and thyroglobulin after 25 weeks of treatment, and developed primary adrenal insufficiency with adrenal crisis and fulminant type 1 diabetes with ketoacidosis after 45 weeks. Therefore, this patient met the diagnosis of APS-2 and was given multiple hormone replacement including glucocorticoid, levothyroxine and insulin therapy. Continuous improvement was achieved through regular monitoring and titration of the dosage.
Conclusions: Different components of APS-2 may appear at different time points after anti-PD1 administration, and can be acute and life-threatening. A good prognosis can be obtained by appropriate replacement with multiple hormones.
Insights: With the clinical application of ICPis to APS-2, the complexity of its treatment should be paid enough attention.
Introduction
Immune checkpoint inhibitors (ICPis) are novel antitumor agents. Over-activated immune cells may also lead to clinical manifestations of autoimmune abnormalities in multiple systems of the body, namely immune-related adverse events (irAEs) (1). Endocrine glands are common targets of irAEs, particularly the thyroid and pituitary. A meta-analysis has shown that patients treated with anti-programmed cell death protein 1 (c) combined with anti-cytotoxic T-lymphocyte antigen 4 (anti-CTLA-4) had the highest incidence of hypothyroidism and hyperthyroidism, patients treated with anti-PD1 alone were at even higher risk of hypothyroidism, and patients treated with anti-CTLA-4 alone were at even higher risk of developing pituitary inflammation (2). With the gradual increase in clinical use of ICPis, reports on the involvement of two or more glands are becoming more common. ICPis therapy can usually be continued with close monitoring. However, moderate to severe irAEs may cause severe organ dysfunction, a reduced quality of life, and even death, requiring early recognition and appropriate management (3).
Here, we present a case of autoimmune polyendocrine syndrome type 2 (APS-2) after anti-PD1 treatment in a patient with squamous carcinoma of the urethral meatus. APS-2 is defined as the occurrence of any type 1 diabetes mellitus (T1DM), autoimmune thyroiditis, and primary adrenal insufficiency (Addison’s disease). In this case, the patient presented with primary hypothyroidism and T1DM with ketoacidosis and primary adrenal insufficiency with adrenal crisis, i.e., all components of APS-2 were present. In this paper, we will present the clinical manifestations and treatment of this special case and also review the reported cases of anti-PD1-induced APS-2 in the literature, to help clinicians improve their awareness and understanding of this disease when anti-PD1 is widely used today.
Case description
1. The baseline situation: A 60-year-old female, without any personal or family history of autoimmune and endocrine diseases, was diagnosed with squamous cell carcinoma of the urethral meatus with inguinal lymph node metastasis and was treated with camrelizumab (anti-PD1, 200mg every 3 weeks) 14 times combined with anlotinib hydrochloride capsules (12mg/day) at regular intervals from February to December 2020. Before anti-PD1 administration, blood glucose level and thyroid function was normal except for a mild increase in thyroglobulin antibody (TgAb). However, the baseline adrenal function and islet function were not examined. The first application of anti-PD1 was on February 12, 2020. After the initial use of anti-PD1, the oncologist regularly monitored the patient’s thyroid function, adrenal function and blood glucose and found them to be within the normal range.
2. The progress of APS-2:
(1) The first occurrence of hypothyroidism: At the 25th week after anti-PD1 administration, a significant increase in thyroid autoimmune antibodies (ARCHITECT Anti-Tg/Anti-TPO Reagent Kit, Chemiluminescent Micro-Particle Immunoassay) combined with hypothyroidism was found, while adrenal function remained normal during the same period, and growth hormone (GH) and sex hormone were not tested. The patient started on levothyroxine sodium tablets (L-T4) 12.5ug/d and gradually increased to 75ug/d., When tested one month after taking L-T4, thyroid function returned to normal, so 75ug/d was continued.
(2) The simultaneous occurrence of T1DM and Addison’s disease: At the 45th week after anti-PD1 administration, the patient developed fatigue, nausea, epigastric discomfort, and vomiting, accompanied by weight loss (5 kg in 1 week). Physical examination was as follows: height 165 cm, weight 45 kg, body mass index 16.5 kg/m, body temperature 36.3°C, pulse 120 beats/min, respiration 30 breaths/min, blood pressure 127/92 mmHg, Although mentally lethargic, the patient can answer the doctor’s question correctly. No alopecia, pigmentation, vitiligo, oedema or bleeding spots on the skin and mucous membranes. No abnormalities on thyroid inspection and palpation. Heart rate 120 beats/min with normal rhythm. Mild tenderness appeared in the upper abdomen without rebound pain or muscle tension. No muscle weakness or other nervous system abnormalities were found in physical examinations.
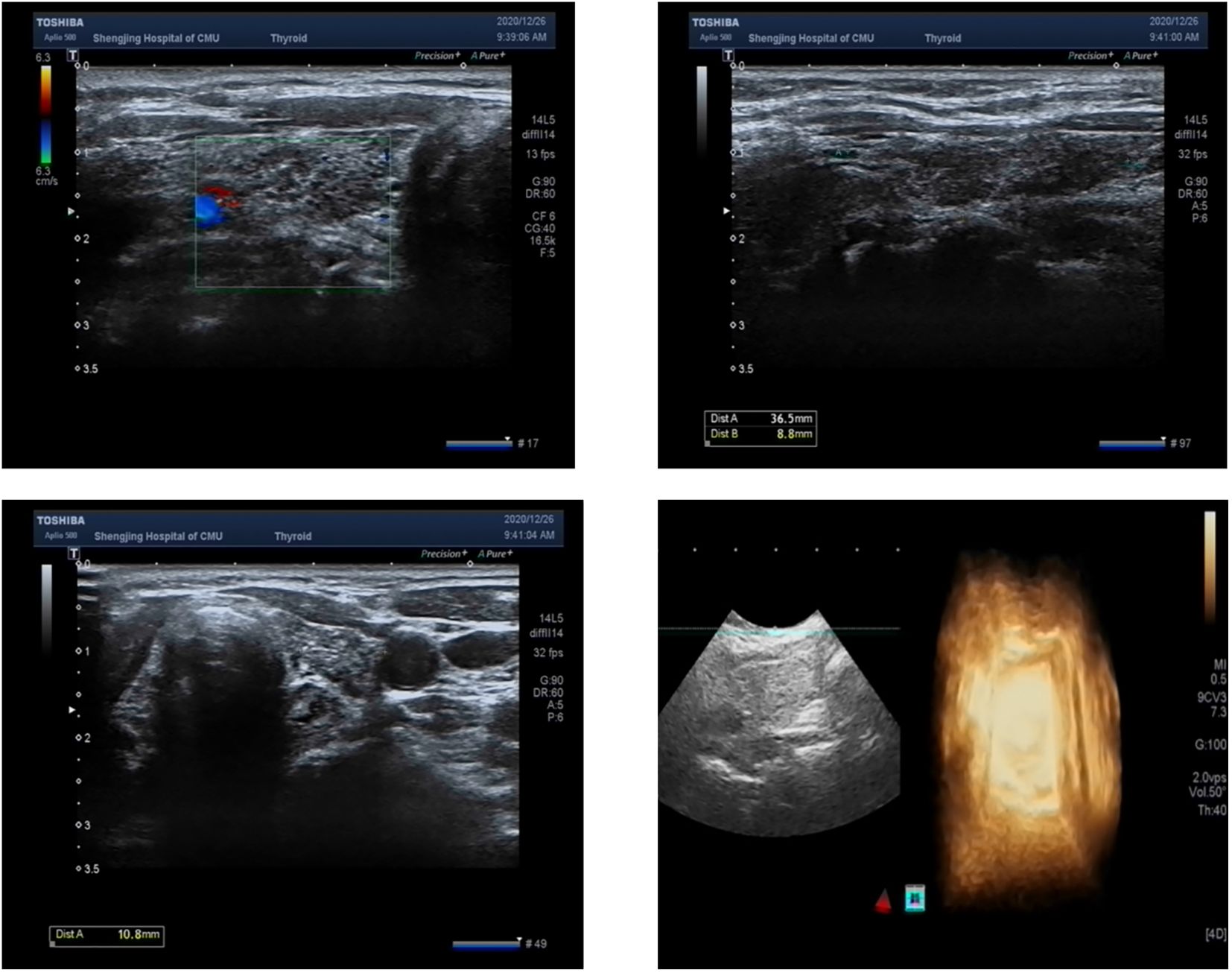
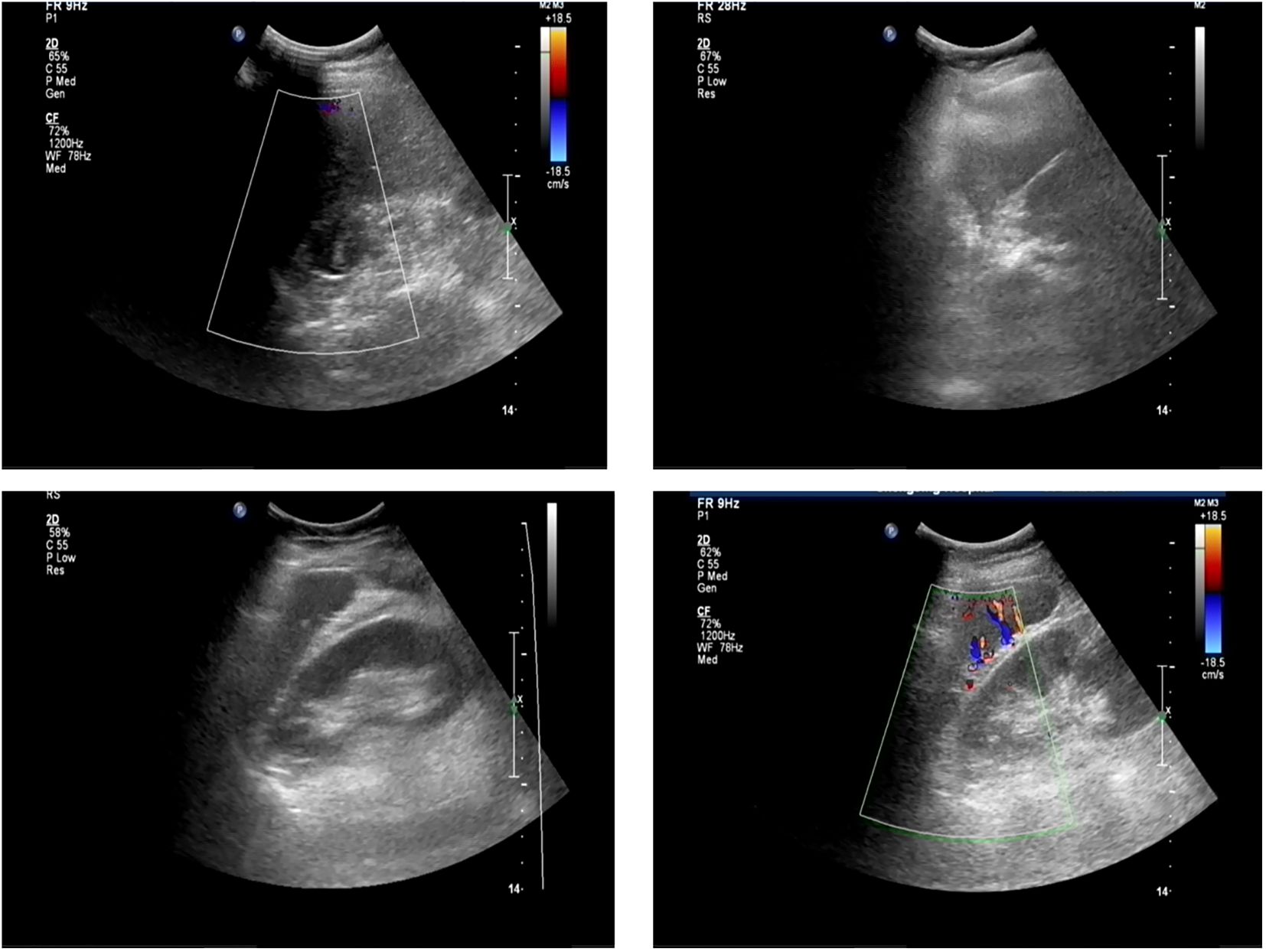
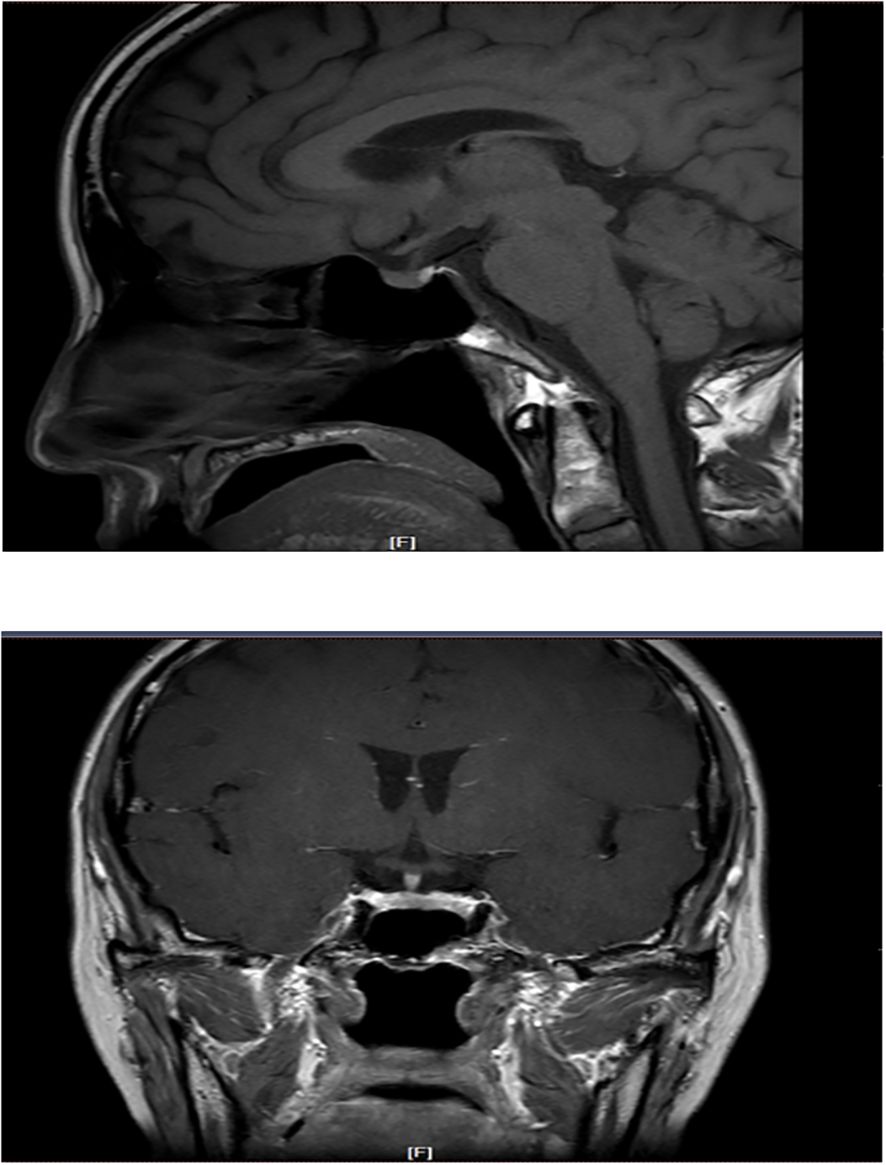
The patient had significantly elevated blood glucose, with fasting glucose of 21.06 mmol/L, reduced fasting C-peptide and insulin, urinary ketone bodies 4+, and significantly lower PH indicating diabetic ketoacidosis (DKA), and then admitted to the hospital. Other laboratory and imaging findings included negative autoimmune antibodies for type 1 diabetes (Line Immuno Assay for the Detection of Antibodies in Autoimmune Diabetes, Western blotting), normal levels of sex hormone, human growth hormone (GH), insulin growth factor-1 (IGF-1), parathyroid hormone (PTH), serum transaminase and creatinine (Table 1). The ultrasound indicated that the echo of thyroid tissue was rough without other obvious abnormality in the thyroid or parathyroid area (Figure 1). Also, the ultrasound indicated no obvious lesions in bilateral adrenals (Figure 2). Contrast-enhanced magnetic resonance imaging (MRI) of pituitary indicated no definite abnormalities (Figure 3).
(3) Diagnosis: Considering the patient had no previous history of diabetes, the sudden onset of T1DM and DKA were speculated to be drug-induced. The patient had an acute onset of DKA, fasting blood glucose > 16mmol/L, HbA1c < 8.5% and serum fasting C-peptide < 0.3ng/mL, which met the diagnostic criteria of fulminant type 1 diabetes. Decreased blood cortisol and significantly increased ACTH was found accompanied by low sodium, which supported the appearance of primary adrenal insufficiency (Addison’s disease). Considering the patient’s concurrent severe symptoms of nausea, vomiting, and lethargy, we diagnosed adrenal crisis. The patient had T1DM, primary hypothyroidism together with Addison’s disease, therefore, she can be diagnosed as APS-2 secondary to anti PD-1 administration.
(4) Treatment: The treatment of DKA included sufficient liquid supplementation, insulin therapy, and immediate correction of ionic disturbances. Finally, due to economic reasons, the patient did not choose insulin pump, but chose subcutaneous injection of short-acting insulin three times a day and long-acting insulin once a day for insulin replacement. The treatment of adrenal crisis involved sufficient liquid supplementation, glucocorticoid replacement including hydrocortisone intravenous infusion 200 mg/day and gradual reduction to oral dosage of 15mg/day. After pituitary crisis and ketoacidosis were corrected, the patient’s condition was greatly improved and allowed to leave the hospital.
3. Follow-up: This patient was given multiple hormone replacement including: (1) glucocorticoid replacement: 10 mg hydrocortisone p.o. in the morning and 5 mg p.o. in the afternoon, and was suggested to increase the glucocorticoid dose by 3–5 times when under stress; (2) thyroid hormone supplementation: levothyroxine 75 µg p.o. in the morning; (3) insulin therapy of T1DM: insulin glargine 7 U subcutaneously before bedtime, insulin aspart 4 U before three meals, and the patient was taught and trained to titrate insulin dosage according to blood sugar levels. The patient’s blood glucose fluctuated between 4 and 14 mmol/L (72-252mg/dL), with occasional appearance of hypoglycaemia. About six 6 months after discharge from the hospital, the patient measured HbA1c 7.6%, and the thyroid function and serum ion levels were in the normal range. Up to the last visit in our hospital, the hormone supplements remained the same dose.
As to the treatment of squamous cell carcinoma of the urethral meatus, the patient refused to continue using anti-PD1 because of the occurrence of APS-2. After full discussion with the doctor, the patient still chose to start chemotherapy (cisplatin combined with gemcitabine hydrochloride, which was adjusted to gemcitabine monotherapy due to poor tolerance). Six months later, local boost radiotherapy was performed, and the tumor was reduced after radiotherapy. Eleven months after discharge, the tumor progressed and the physical condition was not good. The patient was given Paclitaxel liposome for injection chemotherapy until January 27, 2022, when the last chemotherapy was performed. Since the last visit, the patient could not be contacted and was lost to follow-up.
Discussion and conclusions
This is a case of APS-2 with the complete triad (type 1 diabetes, hypothyroidism, and adrenal insufficiency) following treatment with anti-PD1. In the first reported case (4), a patient in their 70s developed the full triad of APS-2 shortly after commencing anti-PD1 for unresectable melanoma in 2019. The primary manifestation of APS is the impaired function of multiple endocrine glands caused by autoimmune reactions. APS-2 is more frequent in women and usually develops in the 3rd to 4th decade of life. It is thought to be polygenic and is associated with mutations in cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and human leukocyte antigen (HLA) DR3 and DR4 serotypes (5).
The initial endocrine gland injury in this patient involved the thyroid. The patient developed hypothyroidism 25 weeks after the first use of camrelizumab, presenting as significantly elevated TSH, TPOAb, and TgAb titres. The patient was diagnosed with primary hypothyroidism secondary to autoimmune destruction of the thyroid due to pharmacological factors rather than damage to the anterior pituitary. Six months after the onset of primary hypothyroidism, the patient developed fulminant type 1 diabetes with diabetic ketoacidosis as the first symptom. The patient met all three diagnostic criteria established in a 2007 Japanese study of fulminant type 1 diabetes (6): (1) Occurrence of diabetic ketosis or ketoacidosis soon (around 7 days) after the onset of hyperglycaemic symptoms (elevation of urinary and/or serum ketone bodies at first visit); (2)Plasma glucose level ≥16.0 mmol/l (>288 mg/dl) and glycated haemoglobin (HbA1c) level <8.5% at first visit; (3) Urinary C-peptide excretion <10 μg/day or fasting serum C-peptide level <0.3 ng/ml (<0.10 nmol/l) and <0.5 ng/ml (<0.17 nmol/l) after intravenous glucagon (or after meal) load at onset. Under the stress of diabetic ketoacidosis, the patient exhibited a potential latent adrenocortical insufficiency with an initial presentation of “adrenal crisis.” We need to distinguish whether this adrenal insufficiency is primary or secondary. Common laboratory findings of primary adrenal insufficiency are a random or early-morning decrease in cortisol and an increase in ACTH, in some cases with the detection of 21-hydroxylase antibody (21-OH Ab) and adrenal cortex antibody (ACA) (7, 8). Imaging often indicates bilateral enlargement of the adrenal glands with clear margins. Secondary adrenal insufficiency presents with a random or early-morning decrease in cortisol without a substantial increase or even a decrease in ACTH and may be combined with a decrease in other endocrine hormones of the pituitary gland (e.g. TSH, LH, or FSH) (9). The reduced blood cortisol level and significantly elevated ACTH were consistent with primary adrenal insufficiency (Addison’s disease). Moreover, although adrenal crisis was diagnosed at the same time as diabetes mellitus, it may have appeared earlier and presented only as mild hyponatremia or it may have appeared after the major stress of diabetic ketoacidosis.
Various difficulties were encountered during the treatment of this patient. (1) The lack of glucocorticoids reduced the ability to regulate blood glucose; accordingly, the patient was more prone to large fluctuations in blood glucose and was at higher risk of hypoglycaemia than that of other patients with type 1 diabetes. (2) Insufficient hydrocortisone supplementation induces adrenal crisis, whereas excessive supplementation is detrimental to glycaemic control. Therefore, every effort needed be made to maintain a balance between the doses of supplemental glucocorticoids and insulin.
Immune checkpoints are small molecules present on the cell surface of T-lymphocytes. They play critical roles in maintaining immune homeostasis and self-tolerance and modulating the duration and amplitude of physiological immune responses. Some immune checkpoints, such asCTLA-4 and PD-1, mediate inhibitory signals to blunt T-cell activity. ICPis serve to induce an anti-tumour immune response by blocking immune checkpoints. Normally, immune checkpoints downregulate T cell responses and act to protect the body from possibly damaging immune responses, such as autoimmune disease. However, tumours can evade the immune system through the activation of immune checkpoints and inhibition of the T cell response. Although the exact mechanism by which ICPis induce apoptosis remains unclear, We briefly summarise the mechanism of action of immune checkpoints and immune checkpoint inhibitors (Figure 4) (10, 11). The mechanisms underlying immune-related adverse events owing to ICPis depend on the type of ICPis therapy used (anti-PD1 or anti-PD-L1 versus anti-CTLA-4). anti-CTLA-4 can induce several cellular alterations, such as T cell activation and proliferation, impaired CD4+CD25+ regulatory T cell (Treg cell) survival and increased counts of type 17 T helper cells, in addition to the induction of cross-reactivity between anti-tumour T cells and antigens on healthy cells and autoantibody production. PD-1 and PD- L1 inhibitors reduce Treg cell survival and increase cytokine production (12).
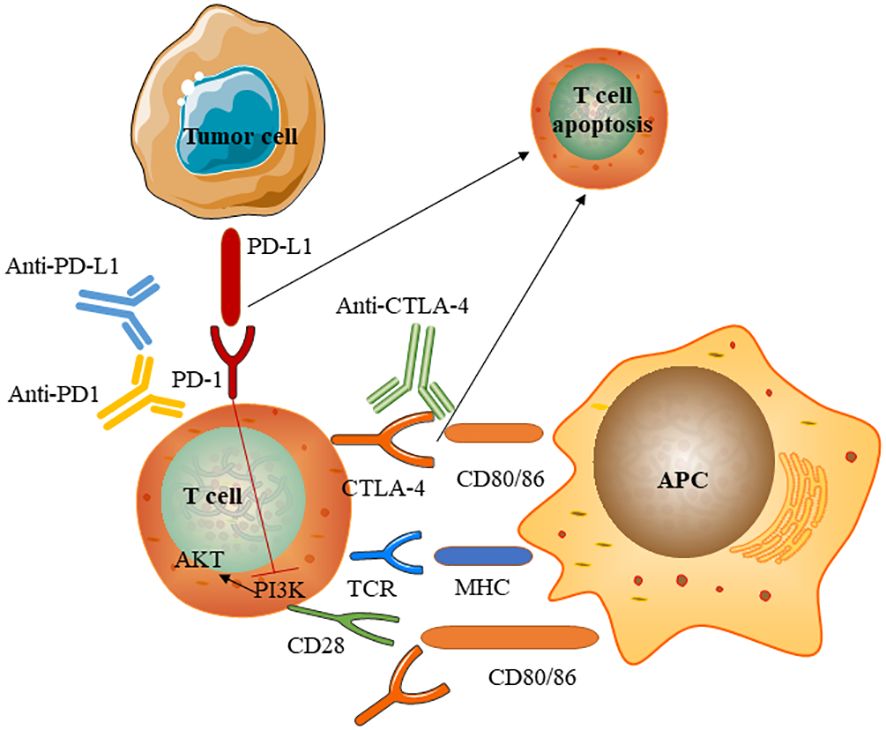
Figure 4 Mechanism of immune checkpoints and immune checkpoint inhibitors. Immune checkpoint inhibitors are monoclonal antibodies that target the CTLA-4 and PD-1 receptors and the PD-1 ligand PD- L1.T cell activation requires two signals: first, antigen recognition by the T cell receptor (TCR) following antigen presentation by major histocompatibility complex (MHC) class II molecules on the surface of antigen- presenting cells(APC); and, second, signal modulation by CD80 or CD86 binding to the CD28 receptor. CTLA-4 expressed on conventional T-cells interacts with its ligands CD80 and CD86 to initiate inhibitory signalling, enhance adhesion, and compete with CD28 to block interaction between CD28 and CD80/CD86.CTLA-4 inhibitors block CTLA-4 binding to CD80 or CD86 to promote T-cell activation PD-1 is a surface receptor that is expressed by T cells and promotes apoptosis of antigen- specific T cells and reduces apoptosis of regulatory T cells through its interaction with its ligand, PD- L1, which is expressed by tumour cells. Engagement of PD-1 with PD-L1 induces an intracellular inhibitory pathway to inhibit costimulatory CD28-activated PI3K pathways, leading to T-cell exhaustion. PD-1 and PD- L1 inhibitors block the PD-1–PD- L1 interaction, facilitating T cell activation.
A meta-analysis has revealed that endocrine disorders are among the most common irAEs in ICPis therapy (13). In particular, anti-CTLA-4 therapy was associated with pituitary inflammation, anti-PD1 therapy was associated with thyroid dysfunction, and the combination of anti-CTLA-4 and anti-PD1 therapy was associated with the highest incidence of ICPis-associated endocrine disorders. ICPis-associated endocrine disorders typically occur within 12 weeks after the start of ICPis treatment (13); however, they can occur months to years after ICPis are started. Some ICPis-related endocrine disorders may resolve spontaneously, but adrenal insufficiency and primary hypothyroidism are typically persistent (13). Similarly, in another meta-analysis, the most common endocrine disorders were hypothyroidism and hyperthyroidism, followed by hyperglycaemia, thyroiditis, and adrenal insufficiency. The risk ratios for adverse events were RR = 26.09% for adrenocortical insufficiency, RR = 26.67% for pituitary inflammation, and RR = 26.92% for hypopituitarism (14).
To review all published cases of APS-2 triggered by anti-PD1, PubMed was searched using the terms “autoimmune polyendocrine syndrome type 2” and “immune checkpoint inhibitors,” revealing 34 relevant case reports since 2000 (Table 2). All 34 patients were treated with anti-PD1, of which five were treated with anti-PD1 in conjunction with anti-CTLA-4. The largest proportion of patients were in the United States (9/34, 26.4%), with other cases reported in various countries, including France, Australia, the United Kingdom, and Ireland. The median age at diagnosis of APS-2 was 61 years. Of the 34 patients, 15.7% (5/34) had a history of autoimmune thyroid disease. The tumour types were primarily melanoma (20/34, 58.8%) and lung cancer (7/34, 20.6%), with cases of renal cell carcinoma and cervical cancer. APS-2 includes type 1 diabetes, autoimmune thyroid disease, and Addison’s disease. Among them, 88.2% (30/34) developed autoimmune thyroid disease, 85.3% (29/34) developed type 1 diabetes, 35.3% (12/34) developed Addison’s disease, and related autoantibodies were detected in 58.8% (20/34) of cases.
There is no specific differential diagnosis for patients with APS-2. Autoantibodies such as thyroid peroxidase antibodies in AITD, GADA in type 1 diabetes mellitus(T1DM), and 21-OH Ab in Addison’s disease contribute to the diagnosis and follow-up (43). The relationship between ICPis-related T1DM and islet autoantibodies remains unclear, and islet autoantibodies are present in about half of cases according to a review (44). Compared to islet autoantibody-negative individuals, islet autoantibody-positive individuals have a shorter onset and a higher rate of diabetic ketoacidosis (45). However, some patients treated with ICPis are antibody-positive but do not develop diabetes (46). A Japanese study also reported no correlation between thyroid autoantibodies and ICPis-related thyroid dysfunction (47). Therefore, the relationship between endocrine gland damage caused by ICPis and autoantibodies remains unclear, and threshold values for treatment and outcome prediction warrant further study.
The present case emphasises that immunosuppression-induced endocrine adverse reactions may involve multiple glands, especially the coexistence of life-threatening diabetic ketoacidosis and adrenal crisis, making early recognition, rapid diagnosis, and timely treatment especially important. In particular, the following points are noted. (1) When ICPis damage one gland, it is important to consider whether there is concomitant autoimmune damage to other glands. (2) It is recommended that all patients with hypothyroidism be evaluated for adrenal function prior to treatment. Special attention should be paid to clinical manifestations after treatment with levothyroxine, and the combination of adrenal insufficiency should be considered if fatigue worsens or if nausea, vomiting, or other discomfort develops. If both hypothyroidism and hypoadrenalism are present, levothyroxine alone may induce adrenal crisis. (3) Physicians should be aware of concomitant adrenal insufficiency in patients with T1DM when the dose of insulin required is lower than before, and blood glucose monitoring should be enhanced when glucocorticoids are given to patients with hypoadrenalism.
With the increased use of ICPis in clinical practice, several guidelines/consensus statements recommend the management of immune-related adverse events caused by ICPis (3, 48). Detailed medical history (especially the history of endocrine and autoimmune diseases), rational baseline screening (including adrenal function, thyroid function, and blood glucose tests), and regular follow-up (monitoring changes in endocrine indexes and the development of symptoms and signs) are needed to detect endocrine gland damage in a timely manner and avoid life-threatening diabetic ketoacidosis, adrenal crisis, thyroid crisis, and other serious events.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving humans because the study did not identify data. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by-product of routine care or industry. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
QP: Writing – original draft. PL: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Liaoning Provincial Public Welfare Research Fund for Science (Soft Science Research Program), 2023JH4/10600030.
Acknowledgments
The authors are grateful to the patient and their family for allowing us to share her story.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dougan M, Pietropaolo M. Time to dissect the autoimmune etiology of cancer antibody immunotherapy. J Clin Invest. (2020) 130:51–61. doi: 10.1172/JCI131194
2. Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: A systematic review and meta-analysis. JAMA Oncol. (2018) 4:173–82. doi: 10.1001/jamaoncol.2017.3064
3. Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. (2021) 39:4073–126. doi: 10.1200/JCO.21.01440
4. Gunjur A, Klein O, Kee D, Cebon J. Anti-programmed cell death protein 1 (anti-PD1) immunotherapy induced autoimmune polyendocrine syndrome type II (APS-2): a case report and review of the literature. J Immunother Cancer. (2019) 7:241. doi: 10.1186/s40425-019-0713-y
5. Kraus AU, Penna-Martinez M, Shoghi F, Meyer G, Badenhoop K. Monocytic cytokines in autoimmune polyglandular syndrome type 2 are modulated by vitamin D and HLA-DQ. Front Immunol. (2020) 11:583709. doi: 10.3389/fimmu.2020.583709
6. Hanafusa T, Imagawa A. Fulminant type 1 diabetes: a novel clinical entity requiring special attention by all medical practitioners. Nat Clin Pract Endocrinol Metab. (2007) 3:36–45. doi: 10.1038/ncpendmet0351
7. Druce I, Tawagi K, Shaw J, Ibrahim A, Lochnan H, Ong M. Routine screening for central and primary adrenal insufficiency during immune-checkpoint inhibitor therapy: an endocrinology perspective for oncologists. Curr Oncol. (2022) 29:4665–77. doi: 10.3390/curroncol29070370
8. Paepegaey AC, Lheure C, Ratour C, Lethielleux G, Clerc J, Bertherat J, et al. Polyendocrinopathy resulting from pembrolizumab in a patient with a Malignant melanoma. J Endocr Soc. (2017) 1:646–9. doi: 10.1210/js.2017-00170
9. Husebye ES, Pearce SH, Krone NP, Kämpe O. Adrenal insufficiency. Lancet. (2021) 397:613–29. doi: 10.1016/S0140-6736(21)00136-7
10. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. (2021) 16:223–49. doi: 10.1146/annurev-pathol-042020-042741
11. Poto R, Troiani T, Criscuolo G, Marone G, Ciardiello F, Tocchetti CG, et al. Holistic approach to immune checkpoint inhibitor-related adverse events. Front Immunol. (2022) 13:804597. doi: 10.3389/fimmu.2022.804597
12. Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. (2020) 6:38. doi: 10.1038/s41572-020-0160-6
13. Chang LS, Barroso-Sousa R, Tolaney SM, Hodi FS, Kaiser UB, Min L. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr Rev. (2019) 40:17–65. doi: 10.1210/er.2018-00006
14. Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: A systematic review and meta-analysis. JAMA Oncol. (2019) 5:1008–19. doi: 10.1001/jamaoncol.2019.0393
15. Kim HI, Kim M, Lee SH, Park SY, Kim YN, Kim H, et al. Development of thyroid dysfunction is associated with clinical response to PD-1 blockade treatment in patients with advanced non-small cell lung cancer. Oncoimmunology. (2017) 7:e1375642. doi: 10.1080/2162402X.2017.1375642
16. Sakurai K, Niitsuma S, Sato R, Takahashi K, Arihara Z. Painless thyroiditis and fulminant type 1 diabetes mellitus in a patient treated with an immune checkpoint inhibitor, nivolumab. Tohoku J Exp Med. (2018) 244:33–40. doi: 10.1620/tjem.244.33
17. Gauci ML, Laly P, Vidal-Trecan T, Baroudjian B, Gottlieb J, Madjlessi-Ezra N, et al. Autoimmune diabetes induced by PD-1 inhibitor-retrospective analysis and pathogenesis: a case report and literature review. Cancer Immunol Immunother. (2017) 66:1399–410. doi: 10.1007/s00262-017-2033-8
18. Li L, Masood A, Bari S, Yavuz S, Grosbach AB. Autoimmune diabetes and thyroiditis complicating treatment with nivolumab. Case Rep Oncol. (2017) 10:230–4. doi: 10.1159/000456540
19. Alhusseini M, Samantray J. Autoimmune diabetes superimposed on type 2 diabetes in a patient initiated on immunotherapy for lung cancer. Diabetes Metab. (2017) 43:86–8. doi: 10.1016/j.diabet.2016.05.007
20. Lowe JR, Perry DJ, Salama AK, Mathews CE, Moss LG, Hanks BA. Genetic risk analysis of a patient with fulminant autoimmune type 1 diabetes mellitus secondary to combination ipilimumab and nivolumab immunotherapy. J Immunother Cancer. (2016) 4:89. doi: 10.1186/s40425-016-0196-z
21. Kong SH, Lee SY, Yang YS, Kim TM, Kwak SH. Anti-programmed cell death 1 therapy triggering diabetic ketoacidosis and fulminant type 1 diabetes. Acta Diabetol. (2016) 53:853–6. doi: 10.1007/s00592-016-0872-y
22. Hansen E, Sahasrabudhe D, Sievert L. A case report of insulin-dependent diabetes as immune-related toxicity of pembrolizumab: presentation, management and outcome. Cancer Immunol Immunother. (2016) 65:765–7. doi: 10.1007/s00262-016-1835-4
23. Mellati M, Eaton KD, Brooks-Worrell BM, Hagopian WA, Martins R, Palmer JP, et al. Anti-PD-1 and anti-PDL-1 monoclonal antibodies causing type 1 diabetes. Diabetes Care. (2015) 38:e137–8. doi: 10.2337/dc15-0889
24. Hughes J, Vudattu N, Sznol M, Gettinger S, Kluger H, Lupsa B, et al. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care. (2015) 38:e55–7. doi: 10.2337/dc14-2349
25. Hofmann L, Forschner A, Loquai C, Goldinger SM, Zimmer L, Ugurel S, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer. (2016) 60:190–209. doi: 10.1016/j.ejca.2016.02.025
26. Gaudy C, Clévy C, Monestier S, Dubois N, Préau Y, Mallet S, et al. Anti-PD1 pembrolizumab can induce exceptional fulminant type 1 diabetes. Diabetes Care. (2015) 38:e182–3. doi: 10.2337/dc15-1331
27. Scott ES, Long GV, Guminski A, Clifton-Bligh RJ, Menzies AM, Tsang VH. The spectrum, incidence, kinetics and management of endocrinopathies with immune checkpoint inhibitors for metastatic melanoma. Eur J Endocrinol. (2018) 178:173–80. doi: 10.1530/EJE-17-0810
28. Kurihara S, Oikawa Y, Nakajima R, Satomura A, Tanaka R, Kagamu H, et al. Simultaneous development of Graves' disease and type 1 diabetes during anti-programmed cell death-1 therapy: A case report. J Diabetes Investig. (2020) 11:1006–9. doi: 10.1111/jdi.13212
29. de Filette J, Pen JJ, Decoster L, Vissers T, Bravenboer B, van der Auwera BJ, et al. Immune checkpoint inhibitors and type 1 diabetes mellitus: a case report and systematic review. Eur J Endocrinol. (2019) 181:363–74. doi: 10.1530/EJE-19-0291
30. Hong AR, Yoon JH, Kim HK, Kang HC. Immune checkpoint inhibitor-induced diabetic ketoacidosis: A report of four cases and literature review. Front Endocrinol (Lausanne). (2020) 11:14. doi: 10.3389/fendo.2020.00014
31. Galligan A, Xu W, Fourlanos S, Nankervis A, Chiang C, Mant AM, et al. Diabetes associated with immune checkpoint inhibition: presentation and management challenges. Diabetes Med. (2018) 35(9):1283–90. doi: 10.1111/dme.13762
32. Marchand L, Thivolet A, Dalle S, Chikh K, Reffet S, Vouillarmet J, et al. Diabetes mellitus induced by PD-1 and PD-L1 inhibitors: description of pancreatic endocrine and exocrine phenotype. Acta Diabetol. (2019) 56:441–8. doi: 10.1007/s00592-018-1234-8
33. Hescot S, Haissaguerre M, Pautier P, Kuhn E, Schlumberger M, Berdelou A. Immunotherapy-induced Addison's disease: A rare, persistent and potentially lethal side-effect. Eur J Cancer. (2018) 97:57–8. doi: 10.1016/j.ejca.2018.04.001
34. Humayun MA, Poole R. A case of multiple immune toxicities from Ipilimumab and pembrolizumab treatment. Hormones (Athens). (2016) 15:303–6. doi: 10.14310/horm.2002.1656
35. Kuru S, Khan N, Shaaban H. Acute hypophysitis secondary to nivolumab immunotherapy in a patient with metastatic melanoma. Int J Crit Illn Inj Sci. (2017) 7:177–80. doi: 10.4103/IJCIIS.IJCIIS_15_17
36. Marchand L, Paulus V, Fabien N, Pérol M, Thivolet C, Vouillarmet J, et al. Nivolumab-induced acute diabetes mellitus and hypophysitis in a patient with advanced pulmonary pleomorphic carcinoma with a prolonged tumor response. J Thorac Oncol. (2017) 12:e182–182e184. doi: 10.1016/j.jtho.2017.07.021
37. Sum M, Garcia FV. Immunotherapy-induced autoimmune diabetes and concomitant hypophysitis. Pituitary. (2018) 21:556–7. doi: 10.1007/s11102-018-0880-8
38. Tzoulis P, Corbett RW, Ponnampalam S, Baker E, Heaton D, Doulgeraki T, et al. Nivolumab-induced fulminant diabetic ketoacidosis followed by thyroiditis. Endocrinol Diabetes Metab Case Rep. (2018) 2018(1). doi: 10.1530/EDM-18-0111
39. Okahata S, Sakamoto K, Mitsumatsu T, Kondo Y, Noso S, Ikegami H, et al. Fulminant type 1 diabetes associated with Isolated ACTH deficiency induced by anti-programmed cell death 1 antibody-insight into the pathogenesis of autoimmune endocrinopathy. Endocr J. (2019) 66:295–300. doi: 10.1507/endocrj.EJ18-0328
40. Lupi I, Brancatella A, Cosottini M, Viola N, Lanzolla G, Sgrò D, et al. Clinical heterogeneity of hypophysitis secondary to PD-1/PD-L1 blockade: insights from four cases. Endocrinol Diabetes Metab Case Rep. (2019) 2019(1). doi: 10.1530/EDM-19-0102
41. Martins MaChado C, Almeida Santos L, Barroso A, Oliveira MJ. Nivolumab-induced hypothyroidism followed by isolated ACTH deficiency. BMJ Case Rep. (2019) 12(8). doi: 10.1136/bcr-2019-231
42. Takata M, Nomura M, Yamamura K, Muto M, Komori T, Otsuka A, et al. Autoimmune polyendocrine syndrome type 3, characterized by autoimmune thyroid disease, type 1 diabetes mellitus, and isolated ACTH deficiency, developed during adjuvant nivolumab treatment. Asia Pac J Clin Oncol. (2022) 18:481–2. doi: 10.1111/ajco.13573
43. Savvateeva EN, Yukina MY, Nuralieva NF, Filippova MA, Gryadunov DA, Troshina EA. Multiplex autoantibody detection in patients with autoimmune polyglandular syndromes. Int J Mol Sci. (2021) 22(11). doi: 10.3390/ijms22115502
44. Marchand L, Disse E, Dalle S, Reffet S, Vouillarmet J, Fabien N, et al. The multifaceted nature of diabetes mellitus induced by checkpoint inhibitors. Acta Diabetol. (2019) 56:1239–45. doi: 10.1007/s00592-019-01402-w
45. Akturk HK, Kahramangil D, Sarwal A, Hoffecker L, Murad MH, Michels AW. Immune checkpoint inhibitor-induced Type 1 diabetes: a systematic review and meta-analysis. Diabetes Med. (2019) 36:1075–81. doi: 10.1111/dme.14050
46. Stamatouli AM, Quandt Z, Perdigoto AL, Clark PL, Kluger H, Weiss SA, et al. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes. (2018) 67:1471–80. doi: 10.2337/dbi18-0002
47. Yano S, Ashida K, Nagata H, Ohe K, Wada N, Takeichi Y, et al. Nivolumab-induced thyroid dysfunction lacking antithyroid antibody is frequently evoked in Japanese patients with Malignant melanoma. BMC Endocr Disord. (2018) 18:36. doi: 10.1186/s12902-018-0267-x
48. Castinetti F, Albarel F, Archambeaud F, Bertherat J, Bouillet B, Buffier P, et al. French Endocrine Society Guidance on endocrine side effects of immunotherapy. Endocr Relat Cancer. (2019) 26:G1–1G18. doi: 10.1530/ERC-18-0320
Glossary
Keywords: anti-programmed cell death protein 1, autoimmune polyendocrine syndrome type 2, adrenal insufficiency, adrenal crisis, hypothyroidism, type 1 diabetes mellitus
Citation: Pan Q and Li P (2024) Challenges in autoimmune polyendocrine syndrome type 2 with the full triad induced by anti-programmed cell death 1: a case report and review of the literature. Front. Immunol. 15:1366335. doi: 10.3389/fimmu.2024.1366335
Received: 06 January 2024; Accepted: 02 April 2024;
Published: 18 April 2024.
Edited by:
Mattia Bellan, University of Eastern Piedmont, ItalyReviewed by:
Giulia Lanzolla, University of Pennsylvania, United StatesFrançois-Xavier Mauvais, Université Paris Cité, France
Copyright © 2024 Pan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Li, czY4MDBAMTYzLmNvbQ==
 Qin Pan
Qin Pan Ping Li
Ping Li