- 1Department of Laboratory Medicine, Affiliated Hangzhou First People’s Hospital, Westlake University School of Medicine, Hangzhou, China
- 2Department of Laboratory Medicine, People’s Hospital of Tonglu County, Hangzhou, Zhejiang, China
- 3Marketing Department, Guilin URIT Medical Electronic Co., Ltd., Guilin, Guangxi, China
- 4Department of Neurology, Affiliated Hangzhou First People’s Hospital, Westlake University School of Medicine, Hangzhou, China
- 5Department of Nephrology, the First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Traditional Chinese Medicine), Hangzhou, Zhejiang, China
In the late stages of the COVID-19 pandemic, there’s an increasing trend in opportunistic infections, including bacterial and fungal infections. This study discusses the treatment process of two cases of cryptococcal meningitis during the COVID-19 pandemic. It highlights the importance of laboratory testing for these co-infections and stresses the need for vigilance, early diagnosis, and proactive treatment to improve patient outcomes in the post-pandemic era.
Introduction
Since the outbreak of the novel coronavirus in 2019, COVID-19 continues to be a focal issue in the global public health arena (1).This virus is a ribonucleic acid (RNA) virus capable of causing severe infections accompanied by various complications. Under the influence of COVID-19, older individuals and those with compromised immune systems are more susceptible to opportunistic fungal infections. The immunosuppressive effects of COVID-19, coupled with the use of immunosuppressive medications in critically ill patients, have continually elevated the risk of opportunistic infections. These pathogens exacerbate the severity of the disease and increase the infection and mortality rates among global COVID-19 patients (2). There have been reported cases indicating the occurrence of cryptococcosis in patients infected with SARS-CoV-2, manifesting as cryptococcal meningitis(CM)or pneumonia. Currently reported cases of cryptococcosis in COVID-19 patients have been observed not only in individuals with compromised immune function but also in those with normal immune function (3, 4).
Cryptococcal infection is an opportunistic infection caused by Cryptococcus neoformans or Cryptococcus gattii (5). Cryptococcus reproduces asexually as sterile filamentous single-budded yeasts, lacking capsules or having only small ones in the environment. However, upon entering the human body, they rapidly develop thick capsules. The presence of capsules significantly increases the diameter of Cryptococcus spores and enhances their pathogenicity. Initial infection of Cryptococcus neoformans occurs via inhalation of infectious propagules and subsequent colonization of the respiratory tract (6).When the immune system is compromised, the primary infection occurs in the lungs, then it disseminates and invades the central nervous system, resulting in CM as the most significant and commonly affected location (7, 8). Furthermore, there are pulmonary cryptococcosis. they have the capability to invade bones, muscles, and mucous membranes of the skin, giving rise to chronic inflammation and abscess formation. Cryptococcus is widely distributed in the natural environment, and susceptible individuals include those with chronic illnesses or patients on long-term immunosuppressive, cytotoxic, or glucocorticoid therapy.
In this case report, we present the treatment of two patients with SARS-CoV-2 infection complicated by Cryptococcus neoformans during the post-pandemic period. This underscores the critical role of laboratory testing in accurately diagnosing whether an individual has COVID-19, Cryptococcus infection, or a co-infection of both. In COVID-19 patients, it is crucial to enhance pathogen detection to prevent concurrent infections with rare pathogens. Timely diagnosis and treatment of CM patients, along with preventive measures against COVID-19, contribute to improving patient outcomes.
This study focuses on determining cases of Cryptococcus infection in the post-pandemic period and explores the observation and treatment experiences of CM cases occurring in the later stages of the COVID-19 pandemic. Case 1 has no history of COVID-19 infection or vaccination, which is crucial for understanding the susceptibility of Cryptococcus in the absence of exposure to the coronavirus. On the other hand, Case 2 not only has a history of COVID-19 infection but also underlying conditions like hypertension and diabetes, emphasizing the importance of these factors in understanding the dynamics of Cryptococcus infection post-pandemic. We emphasize that studying these two cases aligns with the background and theme of the research, as they provide unique experiences and treatment insights into Cryptococcus infection during the later stages of the COVID-19 pandemic, offering valuable insights into the potential relationship between COVID-19 and subsequent Cryptococcus susceptibility.
Case presentation
Case 1
A 47-year-old male was admitted with a history of palpitations and fatigue persisting for 10 years and a recent headache lasting 10 days. He has a medical history of hypertension, diabetes, hyperthyroidism, and heart disease. He has no family history of hereditary diseases, smoking, or alcohol consumption. He is allergic to strawberries. The patient has no history of previous COVID-19 infection, denies having visited any COVID-19 outbreak areas or countries in the past 14 days, and has had no direct or indirect contact with individuals from those regions. There has been no recent travel abroad, no involvement in any clustered outbreak events, and no contact with individuals exhibiting fever symptoms. Additionally, the patient has not received the COVID-19 vaccine. Physical examination revealed thyroid enlargement, irregular heart rhythm, and bilateral lower limb edema. Laboratory tests indicated a high white blood cell(WBC)count, abnormal neutrophil percentage, elevated high-sensitivity C-reactive protein, and positive results for syphilis and cryptococcal infections. The head magnetic resonance imaging (MRI) revealed a potential brain-related condition as shown in Figure 1. Analysis of the cerebrospinal fluid (CSF) indicated abnormalities, including a positive finding for cryptococcal infection. Utilizing CSF microscopy examination, morphological analysis can be conducted to diagnose Cryptococcus infection using India ink staining and Wright-Giemsa stain. Confirmation of the Cryptococcus species could be obtained through microbial culture and mass spectrometry identification. The final results showed a medium quantity of Cryptococcus neoformans in the CSF. Based on the comprehensive examination results, the diagnosis of cryptococcal intracranial infection has been confirmed. Initially, treatment was initiated with amphotericin B cholesterol sulfate complex and fluconazole. On the third day, the patient developed symptoms consistent withCOVID-19 and tested positive for SARS-CoV-2 using nucleic acid testing (PCR). The condition rapidly deteriorated, leading to multiple systemic complications. The medical team discontinued amphotericin B cholesterol sulfate complex and fluconazole, switching to voriconazole, and initiated antiviral therapy with remdesivir. Despite aggressive antiviral and resuscitative treatments, the patient unfortunately passed away on the 13th day of admission due to respiratory failure.
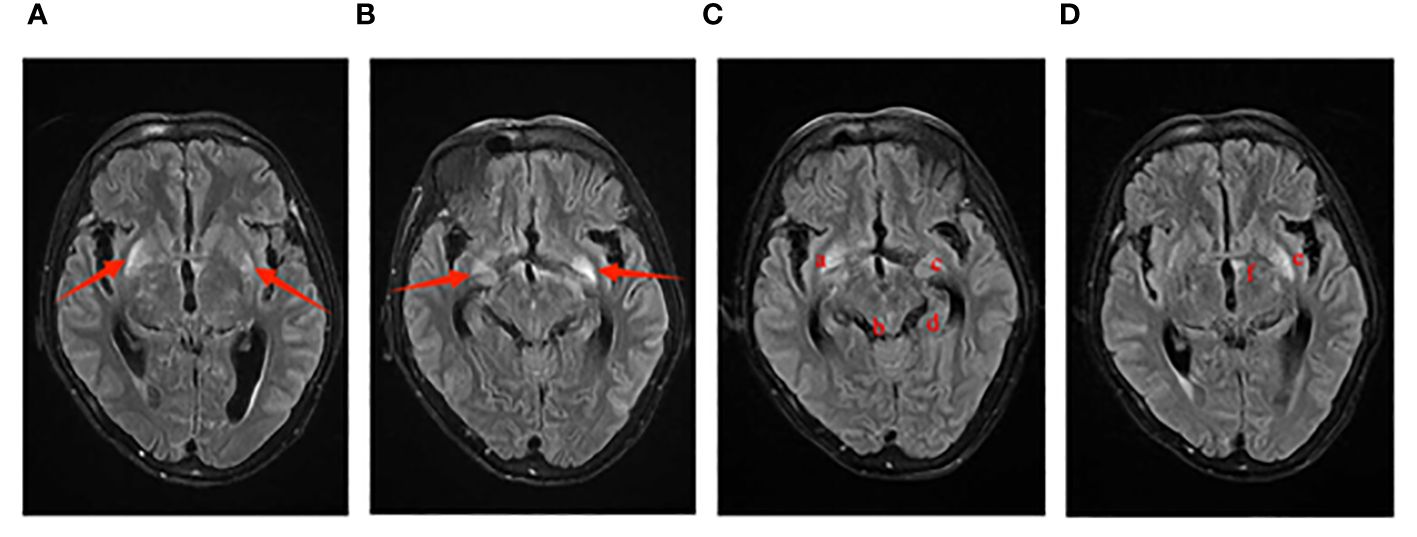
Figure 1 Imaging manifestations of Case 1 on cranial MRI. The T2 Flair sequence is as follows: Images (A, B) display small patchy abnormal signal foci symmetrically located at the base of the bilateral basal ganglia on January 4th. Images (C, D) reveal multiple abnormal signal shadows on the cranial MRI on January 10th, including the bottom of the bilateral basal ganglia (a, c), the globus pallidus (e), the cingulate gyrus of the temporal lobe (d), the left edge of the third ventricle (f), and the cerebral aqueduct (b).
Case 2
A 52-year-old male, previously infected with COVID-19, has a history of hypertension and pancreatitis. He has a smoking and alcohol consumption habit, with no family history of hereditary diseases, and no history of drug or food allergies. He has received the COVID-19 vaccine before. He was admitted due to a four-day history of headache and two days of dizziness. Prior to admission, he had received ceftriaxone for infection control, amlodipine for blood pressure management, and mannitol for intracranial pressure reduction, but his condition did not improve. Physical examination revealed increased neck stiffness and a positive Kernig sign on both sides. Laboratory tests indicated elevated WBC count, increased neutrophil percentage, accelerated erythrocyte sedimentation rate, with all other blood parameters falling within the normal range. Chest CT revealed bronchitis, diffuse fibrous proliferation in both lungs, and mild thickening of the bilateral pleura. The head MRI revealed an acute cerebral infarction adjacent to the left lateral ventricle, as shown in Figure 2. accompanied by increased intracranial pressure of 400+ mmHg (normal range: 80-180 mmHg). The cerebrospinal fluid (CSF) routine test showed that the appearance was colorless and transparent, with a nucleated cell count of 31/μL. The Pandy test was weakly positive, and the CSF India ink staining indicated the presence of Cryptococcus. Further culture and identification of bacteria and fungi in the CSF revealed the detection of Cryptococcus neoformans. Cryptococcus neoformans was detected in blood culture, as depicted in Figure 3. Peripheral blood cultures from both hands were cultured for aerobic and anaerobic bacteria. After incubation, no bacterial growth was observed in the anaerobic bottles and one of the aerobic bottles; however, positive growth triggering an alarm was noted in the other aerobic bottle. Gram staining revealed fungal spores, as shown in Figure 3C. Subsequent subculturing on specific agar mediums confirmed fungal growth and identified a Cryptococcus neoformans strain through mass spectrometry. The patient had a Cryptococcus neoformans infection, ultimately diagnosed as CM. A four-week course of antifungal therapy is being conducted, which includes amphotericin B with flucytosine. In addition, adjunctive therapies such as mannitol, glycerol fructose, and albumin dehydration are being used to reduce intracranial pressure, alleviate symptoms, and prevent certain complications like brain herniation.
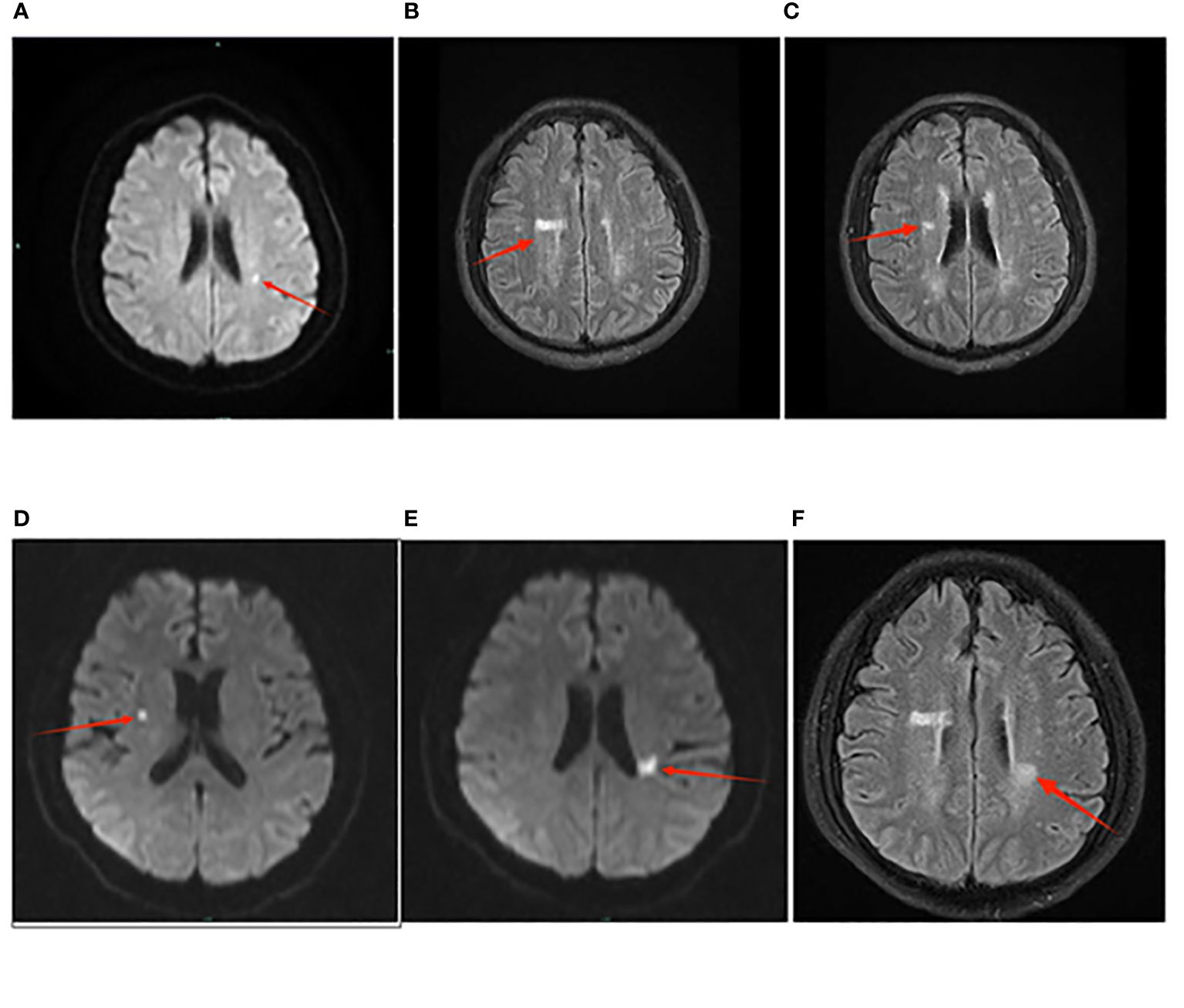
Figure 2 The presentation of cerebral infarction in Case 2 on the MRI images. Panel (A–C) represents the examination results from April 30th. In Panel (A), the diffusion-weighted imaging (DWI) sequence shows punctate high signal foci adjacent to the left lateral ventricle, indicating acute cerebral infarction. In Panel (B), T2 Flair sequence reveals patchy high signals adjacent to the right lateral ventricle. In Panel (C), T2 Flair sequence shows patchy high signals adjacent to the right lateral ventricle. Panel (D–F) represents the examination results from May 23rd. In Panel (D), T2 Flair sequence reveals punctate high signals adjacent to the right lateral ventricle. In Panel (E), the T2 Flair sequence demonstrates patchy high signal intensities adjacent to the left lateral ventricle, particularly in the posterior horn region. In Panel (F), there are also patchy high signal areas observed near the left lateral ventricle on the T2 Flair sequence.

Figure 3 (A) displays CSF stained with India ink, magnified 1000x. Due to the lack of staining on the capsule, Cryptococcus appears as a “starry sky” against the ink background, with transparent spore bodies and prominent, thick capsules. (B) displays the CSF stained with Wright-Giemsa staining at a magnification of 1000x. Cryptococcus spores display an irregular size and may present as either circular or “8”-shaped structures, showing staining in various hues of purple-black or mauve. Spore bodies show purple-black or mauve pigmentation, while their enclosing capsules are either lightly pigmented or colorless. Prior to treatment, the spores exhibit vigorous growth, with radial fluff covering their surface, as indicated by the black arrows. The dense fluff is prone to contamination by residual dyes, which are difficult to remove, as indicated by the red arrow. (C) displays a Gram-stained smear of a positive blood culture from one side, magnified 400x. In the image, fungal spores are clearly visible, as indicated by the black arrows. These spores have a smooth surface and lack capsules. India ink staining yielded a negative result, but identification following inoculation on blood agar confirmed the presence of Cryptococcus neoformans.
On the 16th day of antifungal treatment for Cryptococcal infection, the patient developed fever and symptoms of a pulmonary infection. Upon examination, signs of a lung infection were detected. The patient was initially infected with the novel coronavirus approximately 5 months ago, and they are currently experiencing a surge in secondary COVID-19 infections. Due to the patient’s compromised immune system and the potential for secondary infections, the doctor has opted to incorporate a 5-day course of Molnupiravir as part of the antiviral treatment plan. On day 21 of the treatment, the patient developed acute pancreatitis, confirmed by abdominal CT scans. Treatment includes fasting, omeprazole, and somatostatin to control pancreatic enzyme secretion. After completing a 4-week course of treatment for CM, the patient’s symptoms showed significant improvement. He has been discharged and will continue treatment at a local hospital. The treatment timelines for Case 1 and Case 2 are shown in Figure 4.
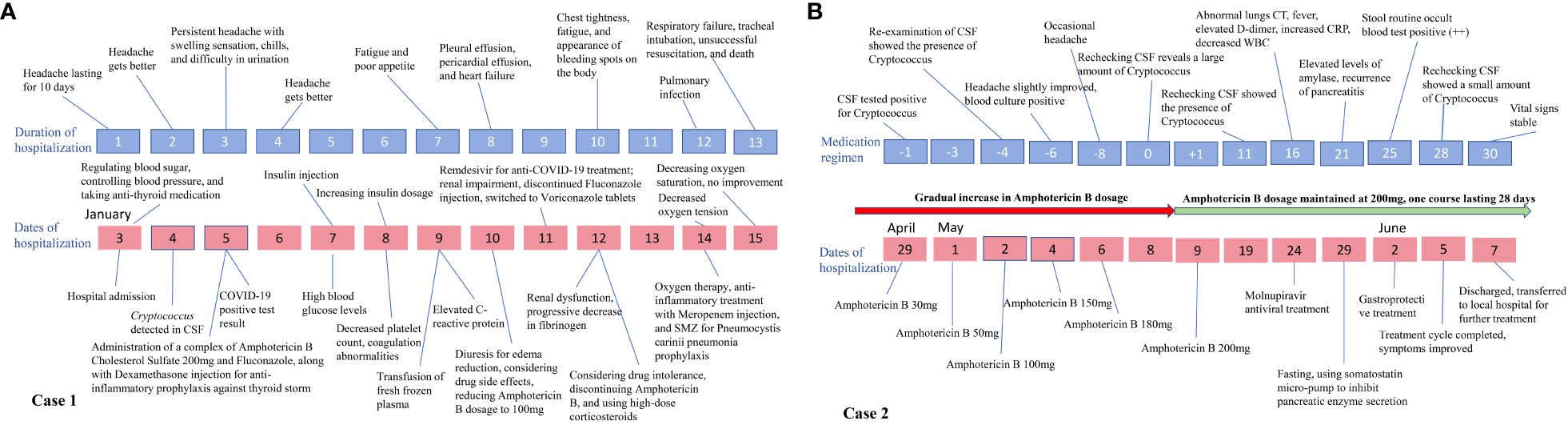
Figure 4 Timeline of changes in the condition of the two cases. (A) provides an illustration of how the symptoms and overall disease condition of case 1 evolved over time with increasing duration of hospital stay, along with depicting the implemented therapeutic interventions during this period; (B) shows the application of treatment plans and changes in disease condition over time for case 2 during hospitalization.
Discussion
Patient 1 had various risk factors for Cryptococcus infection, such as prior illnesses, poor health, and weakened immunity. Their 10-day delay in seeking medical care worsened the situation. After admission, the patient was diagnosed with CM through various tests and received treatment. However, three days after admission, the patient developed chills and a fever, initially thought to be medication side effects. Later, a positive COVID-19 test was confirmed, and the patient experienced urinary issues, ongoing headaches, and altered consciousness. Additionally, there was a continuous drop in platelet count and the development of a hypercoagulable state, complicating diagnosis and treatment. The patient’s condition worsened rapidly, leading to multi-organ failure and eventual death. This complex case involved medication side effects resembling COVID-19 symptoms, and the interaction between COVID-19 and the cryptococcal infection led to severe complications.
In Case 2, the patient had a history of hypertension, pancreatitis, and a prior COVID-19 infection five months ago. They sought prompt medical care when symptoms appeared and were admitted after receiving treatment elsewhere. Tests confirmed a Cryptococcus neoformans infection affecting the blood and central nervous system. Considering the positive result of blood culture on one side, lack of fever and symptoms of multi-organ involvement, a transient bloodstream infection is initially suspected. However, on the 16th day of treatment, the patient developed fever, decreased white blood cell count, and lung inflammation. Considering the patient’s medical history and environmental factors, we have now taken into account the potential impact of COVID-19 and opted to commence preventive treatment for the virus. Following the administration of preventive anti-COVID-19 treatment, there has been improvement in the patient’s lung infection, confirming our initial suspicion. On the 21st day, pancreatitis recurred but was promptly managed. The treatment lasted 28 days, with significant symptom improvement, and the patient was successfully discharged.
Both Case 1 and Case 2 had CM, but Case 1’s treatment failed while Case 2 succeeded due to the following reasons: ①Health Differences: Case 1 had multiple health issues, poor health, and a weakened immune system, making them more vulnerable. In contrast, Case 2 was in better health. ②Timing: Case 1 delayed seeking help by 10 days, contributing to treatment failure. Case 2 sought timely treatment, effectively managing the condition. ③Immunity and the Role of Vaccines: Patient 1 was a first-time infection of the COVID-19, with no prior vaccination. After contracting COVID-19 in addition to CM, severe complications arose, and the condition rapidly deteriorated. On the other hand, Patient 2 experienced a secondary infection but had previously been vaccinated following the prevention and control policies. This vaccination helped alleviate the symptoms of the COVID-19 infection to some extent. Moreover, proactive treatment measures were taken, successfully preventing the occurrence of complications. ④Treatment Challenges: Patient 1 contracted COVID-19 during hospitalization, and the side effects of the medications were similar to the symptoms of the infection, complicating the symptoms and increasing the difficulty of treatment. On the other hand, Patient 2 received preventive treatment for COVID-19, effectively controlling the condition in a timely manner.
CM is an opportunistic infection with a high mortality rate (9). In the post-COVID-19 era, heightened attention to this disease is imperative. Currently, there is limited research on the co-infection of SARS-CoV-2 and CM (10). There have been numerous case reports and small reviews describing cryptococcal infection following COVID-19 (11, 12). The virus-induced excessive inflammatory immune response typically plays a central role in the pathogenesis of CM. Following SARS-CoV-2 infection, patients with cryptococcosis exhibited a mortality rate of 59%, which surpasses that of contemporary Cryptococcus cohorts. An evident correlation was observed between compromised immune status and the presentation of cryptococcal disease, along with mortality (13). Furthermore, the study emphasizes the necessity of conducting clinical and laboratory assessments for opportunistic infections for more than 30 days when relevant symptoms develop. Cryptococcus infection was predominantly observed in individuals with severe cases of COVID-19 who were treated with corticosteroids and hospitalized in the intensive care unit (12). There have been reports suggesting a potential association between the administration of steroids and tocilizumab in patients with COVID-19 in 2019, which may potentially contribute to the onset of CM (14–16).Research indicates that reduced T-cell response may drive CM. Given potential effects of SARS-CoV-2 on T-cells, more study is needed (17). On the contrary, studies have shown that there is no observed difference in mortality rates between patients with CM infected with COVID-19 and those without COVID-19 infection (18). Considering the potential overlap in symptoms between cryptococcal disease and COVID-19, rapid diagnosis of Cryptococcus through blood culture testing is crucial for prompt patient identification. Furthermore, ferroptosis has also been identified as playing a role in the pathogenesis of CM (19). In the field of CM, several studies have observed rapid iron accumulation and lipid peroxidation within the brain, both of which are hallmarks of ferroptosis, a type of programmed cell death characterized by iron dependence and lipid peroxidation (20). In recent years, numerous studies have confirmed the involvement of ferroptosis in various diseases, including infectious diseases like Mycobacterium tuberculosis infection and coronavirus disease-2019 (COVID-19) (6, 21). Furthermore, ferroptosis is considered immunogenic and pro-inflammatory as ferroptosis cells release damage-associated molecular pattern molecules (DAMPs) and alarmins, which both regulate immunity and pro-inflammatory activity (22). Therefore, we hypothesize that there may be a relationship between this unique cell death modality and CM. This suggests that alterations in the body’s immune function may increase the risk of infection.
The current treatment for CM often involves the combined use of antifungal medications, such as amphotericin B, flucytosine and fluconazole (23). The two patients respectively received treatment with amphotericin B and fluconazole, as well as amphotericin B and flucytosine, while concurrently using mannitol to reduce intracranial pressure, aiming to prevent complications, which is in line with the standard of care. The treatment had better efficacy for Case 2, whereas Case 1, due to multiple underlying conditions and delayed medical intervention, missed the treatment window and ultimately resulted in fatality. Past studies have involved successful cases of treating CM combined with COVID-19 infection using amphotericin B and flucytosine (12). but there have also been cases where treatment was not successful in saving the patients (24). We discuss these cases as presented in Table 1. These cases indicate that in immunocompromised patients, such as those with COVID-19, HIV infection, or organ transplants, caution should be exercised to avoid the use of corticosteroids or immunosuppressants, as this may increase the risk of developing fungal meningitis. Considering the widespread use of corticosteroids in severe COVID-19 infections, it is anticipated that there may be an increase in COVID-19 cases combined with fungal meningitis during the pandemic. When differentiating and diagnosing COVID-19-related brain disorders, CM should be considered as a primary concern, and prompt testing should be conducted accordingly.
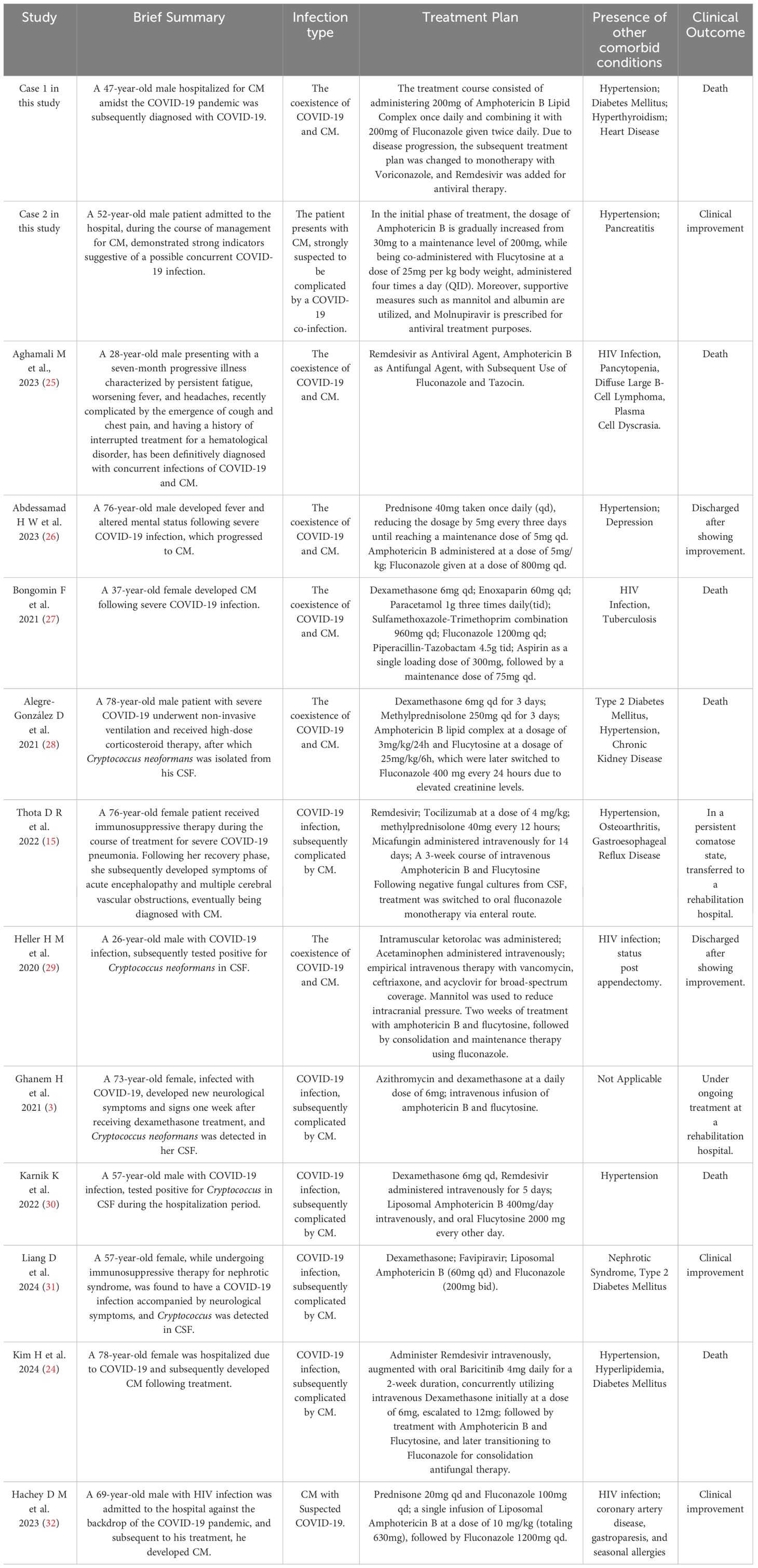
Table 1 Clinical characteristics and treatment strategies for patients with concurrent COVID-19 and CM.
The two confirmed cases of CM underscore an elevated risk and severity of opportunistic infections in the context of the novel coronavirus. Even in subclinical conditions, the immune response remains active and may potentially trigger underlying or prior medical histories. This emphasizes the need for healthcare professionals to gain a deeper understanding of this condition and to offer comprehensive diagnosis and treatment. Regarding diagnosis, proficiency in laboratory testing methods is crucial, along with considering pathogenic infections, including less common opportunistic ones. Timely diagnosis and treatment play a crucial role in enhancing patient prognosis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Hangzhou First People’s Hospital, Westlake University School of Medicine. The patients/participants provided written informed consent to participate in this study. Written informed consent for publication of this case report has been obtained from the participant/patient.
Author contributions
YZ: Software, Resources, Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. BQ: Writing – original draft, Methodology, Conceptualization. JJiang: Software, Methodology, Writing – original draft. TC: Writing – original draft, Methodology, Formal analysis. LW: Supervision, Writing – review & editing. YY: Visualization, Writing – review & editing, Conceptualization. SR: Writing – original draft, Software, Formal analysis. YC: Writing – original draft, Project administration, Software. HF: Writing – original draft, Visualization, Resources, Conceptualization. JJ: Writing – review & editing, Methodology, Supervision, Conceptualization, Validation, Visualization. NY: Visualization, Supervision, Writing – review & editing, Writing – original draft, Methodology, Investigation, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. 2022 Ministry of Education Collaborative Education Program for Industry-Academia Cooperation (Project No. 220906053235359); Hangzhou Biomedical and Health Industry Development Special Support Science and Technology Project (Third Phase, 2021) (2021WJCY090); Hangzhou Biomedical and Health Industry Development Special Support Science and Technology Project (Fifth Phase, 2021) (2021WJCY272).
Acknowledgments
We would like to thank the patient for providing written informed consent for publication, as well as thanking the research staff involved in the study.
Conflict of interest
Author JJiang was employed by Guilin URIT Medical Electronic Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
RNA, ribonucleic acid; CM, cryptococcal meningitis; WBC, white blood cell; CSF, cerebrospinal fluid.
References
1. Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. (2020) 55:105924. doi: 10.1016/j.ijantimicag.2020.105924
2. Chan KS, Lai CC, Yu WL, Chao CM. COVID-19 associated with cryptococcosis: A new challenge during the pandemic. J Fungi (Basel). (2022) 8(10):1111. doi: 10.3390/jof8101111
3. Ghanem H, Sivasubramanian G. Cryptococcus neoformans Meningoencephalitis in an Immunocompetent Patient after COVID-19 Infection. Case Rep Infect Dis. (2021) 2021:5597473. doi: 10.1155/2021/5597473
4. Shokouhi S, Hakamifard A. Meningitis caused by cryptococcus neoformans in an apparently immunocompetent patient. J Investig Med High Impact Case Rep. (2022) 10:23247096221111779. doi: 10.1177/23247096221111779
5. May RC, Stone NR, Wiesner DL, Bicanic T, Nielsen K. Cryptococcus: from environmental saprophyte to global pathogen. Nat Rev Microbiol. (2016) 14:106–17. doi: 10.1038/nrmicro.2015.6
6. Decote-Ricardo D, LaRocque-de-Freitas IF, Rocha JDB, Nascimento DO, Nunes MP, Morrot A, et al. Immunomodulatory role of capsular polysaccharides constituents of cryptococcus neoformans. Front Med (Lausanne). (2019) 6:129. doi: 10.3389/fmed.2019.00129
7. Lan J, Lv L, Ye L, Wang T, Wu Z, Wu S, et al. Post-infectious inflammatory response syndrome in an HIV-negative patient after Cryptococcus gattii meningoencephalitis: a case report and review of the literature. J Med Case Rep. (2023) 17:332. doi: 10.1186/s13256-023-04066-x
8. Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis. (2010) 50:291–322. doi: 10.1086/649858
9. Stott KE, Loyse A, Jarvis JN, Alufandika M, Harrison TS, Mwandumba HC, et al. Cryptococcal meningoencephalitis: time for action. Lancet Infect Dis. (2021) 21:e259–71. doi: 10.1016/S1473-3099(20)30771-4
10. Cafardi J, Haas D, Lamarre T, Feinberg J. Opportunistic fungal infection associated with COVID-19. Open Forum Infect Dis. (2021) 8:ofab016. doi: 10.1093/ofid/ofab016
11. Khatib MY, Ahmed AA, Shaat SB, Mohamed AS, Nashwan AJ. Cryptococcemia in a patient with COVID-19: A case report. Clin Case Rep. (2021) 9:853–5. doi: 10.1002/ccr3.3668
12. Regalla D, VanNatta M, Alam M, Malek AE. COVID-19-associated Cryptococcus infection (CACI): a review of literature and clinical pearls. Infection. (2022) 50:1007–12. doi: 10.1007/s15010-022-01805-y
13. Walker J, McCarty T, McGwin G, Ordaya EE, Vergidis P, Ostrosky-Zeichner L, et al. Description of cryptococcosis following SARS-CoV-2 infection: A disease survey through the mycosis study group education and research consortium (MSG-19). Clin Infect Dis. (2024) 78:371–7. doi: 10.1093/cid/ciad551
14. Erturk Sengel B, Tukenmez Tigen E, Can Sarinoglu R, Midi I, Cerikcioglu N, Odabasi Z. Cryptococcus meningitis presented with multiple cerebral infarcts in an immunocompetent patient. IDCases. (2021) 24:e01154. doi: 10.1016/j.idcr.2021.e01154
15. Thota DR, Ray B, Hasan M, Sharma K. Cryptococcal meningoencephalitis during convalescence from severe COVID-19 pneumonia. Neurohospitalist. (2022) 12:96–9. doi: 10.1177/19418744211009766
16. Dos Santos WM, Rogerio AP. Glucocorticoids, COVID-19 and cryptococcosis. Antiinflamm Antiallergy Agents Med Chem. (2023) 22(2):67–70. doi: 10.2174/1871523022666230912142540
17. Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis Jul 28. (2020) 71:762–8. doi: 10.1093/cid/ciaa248
18. Messina FA, Benchetrit A, Bocassi A, de Las Mercedes Romero M, Bauer S, Marín E, et al. Meningeal cryptococcosis and SARS-CoV-2 infection in people living with HIV/AIDS. Biomedica. Aug 31. (2023) 43:206–16. doi: 10.7705/biomedica.6872
19. Xu X, Lin D, Tu S, Gao S, Shao A, Sheng J. Is ferroptosis a future direction in exploring cryptococcal meningitis? Front Immunol. (2021) 12:598601. doi: 10.3389/fimmu.2021.598601
20. Rochette L, Dogon G, Rigal E, Zeller M, Cottin Y, Vergely C. Lipid peroxidation and iron metabolism: Two corner stones in the homeostasis control of ferroptosis. Int J Mol Sci. (2022) 24(1):499. doi: 10.3390/ijms24010449
21. Nuszkiewicz J, Sutkowy P, Wróblewski M, Pawłowska M, Wesołowski R, Wróblewska J, et al. Ferroptosis and SARS-CoV-2 infection. Antioxidants (Basel). (2023) 12(3):733. doi: 10.3390/antiox12030733
22. Yamaga S, Aziz M, Murao A, Brenner M, Wang P. DAMPs and radiation injury. Front Immunol. (2024) 15:1353990. doi: 10.3389/fimmu.2024.1353990
23. Tugume L, Ssebambulidde K, Kasibante J, Ellis J, Wake RM, Gakuru J, et al. Cryptococcal meningitis. Nat Rev Dis Primers. (2023) 9:62. doi: 10.1038/s41572-023-00472-z
24. Kim H, Kim S, Ahn MY, Oh DH, Choi JP, Yang E. Case report: Cryptococcal meningitis in a previously immunocompetent patient with coronavirus disease 2019. Am J Trop Med Hyg. (2024) 110:270–3. doi: 10.4269/ajtmh.23-0457
25. Aghamali M, Kazemi A, Asgharzadeh M, Kafil HS. COVID-19, HIV, and cryptococcal meningitis coinfections with abnormal laboratory findings. Case Rep Pulmonol. (2023) 2023:2868290. doi: 10.1155/2023/2868290
26. Abdessamad HW, Achkar M, Al Zoghbi A, Fleifel M, Ousta AR, Kassar A, et al. Cryptococcal meningitis post-covid-19 infection: Immunomodulation, a double-edged sword. J Infect Dev Ctries. (2023) 17:623–30. doi: 10.3855/jidc.17170
27. Bongomin F, Sereke SG, Okot J, Katsigazi R, Kandole TK, Oriekot A, et al. COVID-19, HIV-associated cryptococcal meningitis, disseminated tuberculosis and acute ischaemic stroke: A fatal foursome. Infect Drug Resist. (2021) 14:4167–71. doi: 10.2147/IDR.S335711
28. Alegre-González D, Herrera S, Bernal J, Soriano A, Bodro M. Disseminated Cryptococcus neoformans infection associated to COVID-19. Med Mycol Case Rep. (2021) 34:35–7. doi: 10.1016/j.mmcr.2021.10.001
29. Heller HM, Gonzalez RG, Edlow BL, Ard KL, Gogakos T. Case 40-2020: A 24-year-old man with headache and Covid-19. N Engl J Med. (2020) 383:2572–80. doi: 10.1056/NEJMcpc2027083
30. Karnik K, Wu Y, Ruddy S, Quijano-Rondan B, Urban C, Turett G, et al. Fatal case of disseminated cryptococcal infection and meningoencephalitis in the setting of prolonged glucocorticoid use in a Covid-19 positive patient. IDCases. (2022) 27:e01380. doi: 10.1016/j.idcr.2022.e01380
31. Liang D, Li X. Concomitant of cryptococcal meningitis and COVID-19 in a female patient with primary nephrotic syndrome and type 2 diabetes. Risk Manag Healthc Policy. (2024) 17:279–85. doi: 10.2147/RMHP.S451991
Keywords: novel coronavirus, cryptococcal meningitis, opportunistic infections, cerebrospinal fluid morphology, laboratory examinations
Citation: Zhou Y, Qiu B, Jiang J, Chen T, Wang L, Yang Y, Ruan S, Chen Y, Fang H, Jin J and Yang N (2024) Case report: Experience and insights on the treatment of two cases of cryptococcal meningitis during the later stages of the COVID-19 pandemic. Front. Immunol. 15:1361277. doi: 10.3389/fimmu.2024.1361277
Received: 11 January 2024; Accepted: 03 April 2024;
Published: 22 April 2024.
Edited by:
Helen Carolyn Steel, University of Pretoria, South AfricaReviewed by:
Namarta Kalia, University of California, San Diego, United StatesAlicia Arechavala, Hospital de Enfermedades Infecciosas F. J. Muñiz, Argentina
Copyright © 2024 Zhou, Qiu, Jiang, Chen, Wang, Yang, Ruan, Chen, Fang, Jin and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nan Yang, eTE1Njk4MTA1ODEyQDE2My5jb20=; Juan Jin, bGFuZ18wMThAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yuli Zhou1†
Yuli Zhou1† Yunxing Yang
Yunxing Yang Nan Yang
Nan Yang