
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol. , 04 March 2024
Sec. Inflammation
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1354593
 Liang Su1,2†
Liang Su1,2† Chunyan Xu3†
Chunyan Xu3† Hong Huang1,2
Hong Huang1,2 Peilian Zhang1,2
Peilian Zhang1,2 Jinrong Wang1,2
Jinrong Wang1,2 Xiaoyong Ouyang1,2*
Xiaoyong Ouyang1,2* Xuesong Yang1,2*
Xuesong Yang1,2* Jianzhou Ye1,2*
Jianzhou Ye1,2*Background: There is no consensus on the effect of tumor necrosis factor-alpha (TNF-alpha) inhibitors on lipid profiles in patients with psoriasis. This study aimed to investigate the effects of TNF-alpha inhibitors on lipid profiles (triglycerides, total cholesterol, low-density lipoprotein, or high-density lipoprotein) in patients with psoriasis.
Methods: We searched PubMed, Embase, and Cochrane Library databases for articles published before October 17, 2023. Four TNF-alpha inhibitors (infliximab, etanercept, adalimumab, and certolizumab) were included in our study. (PROSPERO ID: CRD42023469703).
Results: A total of twenty trials were included. Overall results revealed that TNF-alpha inhibitors elevated high-density lipoprotein levels in patients with psoriasis (WMD = 2.31; 95% CI: 0.96, 3.67; P = 0.001), which was supported by the results of sensitivity analyses excluding the effect of lipid-lowering drugs. Subgroup analyses indicated that high-density lipoprotein levels were significantly increased in the less than or equal to 3 months group (WMD = 2.88; 95% CI: 1.37, 4.4; P < 0.001), the etanercept group (WMD = 3.4; 95% CI = 1.71, 5.09, P < 0.001), and the psoriasis group (WMD = 2.52; 95% CI = 0.57, 4.48, P = 0.011). Triglyceride levels were significantly increased in the 3 to 6-month group (WMD = 4.98; 95% CI = 1.97, 7.99, P = 0.001) and significantly decreased in the 6-month and older group (WMD = -19.84; 95% CI = -23.97, -15.7, P < 0.001). Additionally, Triglyceride levels were significantly increased in the psoriasis group (WMD = 5.22; 95% CI = 2.23, 8.21, P = 0.001).
Conclusion: Our results revealed that TNF-alpha inhibitors might temporarily increase high-density lipoprotein levels in patients with psoriasis. However, changes in triglycerides were not consistent among the different durations of treatment, with significant increases after 3 to 6 months of treatment. Future prospective trials with long-term follow-up contribute to confirming and extending our findings.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42023469703.
Psoriasis is a common immune-mediated disease that is mainly associated with the skin and affects approximately 125 million people worldwide (1, 2). Psoriasis is characterized by the formation of silvery-white scaly plaques, and its adverse effect on emotionally and physically relevant quality of life is comparable to other major diseases (1, 3). Of note, psoriasis is a typical inflammatory skin disease whose pathogenesis usually involves the activation of inflammatory processes that have the potential to influence systemic organ responses and functions, which in turn results in the dysfunction of various organs (4–6). Indeed, increasing clinical observations have converged to evidence the high prevalence of co-morbidities in patients with psoriasis (7, 8). Hence, comorbidities in patients with psoriasis often influence the selection of treatment, and it is necessary to consider that certain treatments may ameliorate or exacerbate psoriasis comorbidities (9).
Over the past few decades, lipid disturbances in psoriasis have attracted attention (10). Our previous published meta-analysis also revealed that psoriasis was associated with abnormal apolipoprotein A1 and B levels compared with healthy controls (11). The introduction of biologics has greatly expanded the treatment options for patients with moderate to severe psoriasis (12). Among these biologics, tumor necrosis factor-alpha (TNF-alpha) inhibitors, the first class of approved biologics, have been used for over a decade and have dramatically enhanced clinical outcomes for patients with moderate to severe psoriasis (13, 14). TNF-alpha is a multifunctional cytokine with a series of biological actions that have been reported to regulate and interfere with lipid homeostasis (15). Meanwhile, TNF-alpha inhibitors have been reported to possibly affect lipid metabolism (15). However, there is no consensus on the effect of TNF-alpha inhibitors on lipid profiles in patients with psoriasis (16–18).
Therefore, this study aimed to investigate the effects of TNF-alpha inhibitors on lipid profiles in patients with psoriasis using a systematic review a systematic review and meta-analysis.
Our results were reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (19) (PROSPERO ID: CRD42023469703).
According to a related Cochrane meta-analysis, four TNF-alpha inhibitors (infliximab, etanercept, adalimumab, and certolizumab) were searched (20). We searched PubMed, Embase, and Cochrane Library databases for articles published before October 17, 2023. English language restriction was applied (Supplementary Appendix 1). The eligibility of studies was evaluated independently by LS and C-y X, and disagreements were resolved through consultation with J-z Y.
The included studies must fulfill the following criteria: 1) patients clinically diagnosed with psoriasis (21); 2) interventions for TNF-alpha inhibitors (infliximab, etanercept, adalimumab, or certolizumab); and 3) outcomes including triglycerides, total cholesterol, low-density lipoprotein, or high-density lipoprotein. Furthermore, the exclusion criteria were as follows: 1) reporting study populations include psoriasis combined with other autoimmune diseases; 2) significant changes in medications for systemic treatment of psoriasis during the observation period compared to pre-treatment; and 2) letters, editorials, books, or studies that did not provide sufficient data.
We extracted the following data from each included study: first author, country, study design, publication year, type of psoriasis, sample size, age, psoriasis area and severity index (PASI), interventions (the type of TNF-alpha inhibitors), duration of treatment, and lipid profiles (triglycerides, total cholesterol, low-density lipoprotein, or high-density lipoprotein) data. The methodological index for non-randomized studies (MINORS) was employed to assess the quality of included studies, which consisted of eight items (22). Each item was assigned a score ranging from 0 to 2, with high scores representing adequate reporting. Two reviewers (LS and C-y X) independently extracted the data and assessed the risk of bias (RoB), with any disagreements resolved by a third reviewer (J-z Y).
Considering that random-effects model provides more conservative results, we performed a meta-analysis with a random-effects model using Stata15 software (23, 24). To evaluate the effects of TNF-alpha inhibitors on lipid profiles, the results were presented as weighted mean differences (WMDs) with their 95% confidence intervals (CIs). In the overall results, when studies reported results for different intervention durations, our analyses utilized data for the longest intervention duration. When patients with psoriasis were divided into subgroups according to the type of TNF-alpha inhibitors, we calculated the pooled mean and standard deviation (SD), as suggested by the Cochrane Handbook (25). Study heterogeneity was assessed using the Cochran’s Q and I2 statistics (26). Publication bias was assessed using the funnel plot and Egger’s test (27). We conducted sensitivity analyses by removing each study in turn. Additional sensitivity analyses were performed only included in studies that reported exclusion of lipid-lowering drugs or no significant change in lipid-lowering drugs during the observation period. Subgroup analyses were performed according to the type of TNF-alpha inhibitor and duration of intervention. Additional analyses were performed for psoriasis type (psoriasis or psoriatic arthritis) and PASI scores for psoriasis [mean or median PASI more than 10, which represents moderate to severe psoriasis (28)]. Statistical significance was defined as P < 0.05.
The literature search identified 558 publications, of which 59 were fully reviewed and 20 studies (29–48) were finally eligible for inclusion. Figure 1 and Supplementary Appendix 2 illustrate the detailed information of the literature selection procedure.
The baseline characteristics of the included studies are shown in Table 1. The 20 studies included were conducted in 11 countries. The mean (median) PASI of the participants was 6-21.2, and the mean age of the participants was 34-56 years. The duration of the intervention ranged from 2 to 36 months. Sixteen studies reported data on triglycerides. Fifteen studies reported data on total cholesterol and high-density lipoprotein respectively. Twelve studies reported data on low-density lipoprotein. Fifteen studies illustrated specific types of TNF-alpha inhibitors. Nine studies reported exclusion of lipid-lowering drugs or no significant change in lipid-lowering drugs during the observation period. The RoB assessment by MINORS is shown in Table 1.
For triglycerides, the meta-analysis showed that pooled WMD was -0.85 (95% CI: -11.95, 10.26, P = 0.881; I2 = 85.6%, P < 0.001) (Figure 2). Sensitivity analysis revealed that this result did not change significantly when any individual study was removed (Supplementary Figure 1). For total cholesterol, the meta-analysis showed that pooled WMD was 0.33 (95% CI: -4.53, 5.19, P = 0.893; I2 = 36.9%, P = 0.075) (Figure 3). Sensitivity analysis revealed that this result did not change significantly when any individual study was removed (Supplementary Figure 2). For high-density lipoprotein, the meta-analysis showed that pooled WMD was 2.31 (95% CI: 0.96, 3.67, P = 0.001; I2 = 6.1%, P = 0.384) (Figure 4). Sensitivity analysis revealed that this result did not change significantly when any individual study was removed (Supplementary Figure 3). For low-density lipoprotein, the meta-analysis showed that pooled WMD was -2.23 (95% CI: -6.05, 1.59, P = 0.253; I2 = 37.9%, P = 0.089) (Figure 5). Sensitivity analysis revealed that this result did not change significantly when any individual study was removed (Supplementary Figure 4). We conducted additional sensitivity analyses, including only studies that reported exclusion of lipid-lowering drugs or no significant change in lipid-lowering drugs during the observation period. The findings were consistent with the overall results (Supplementary Figure 5-8).
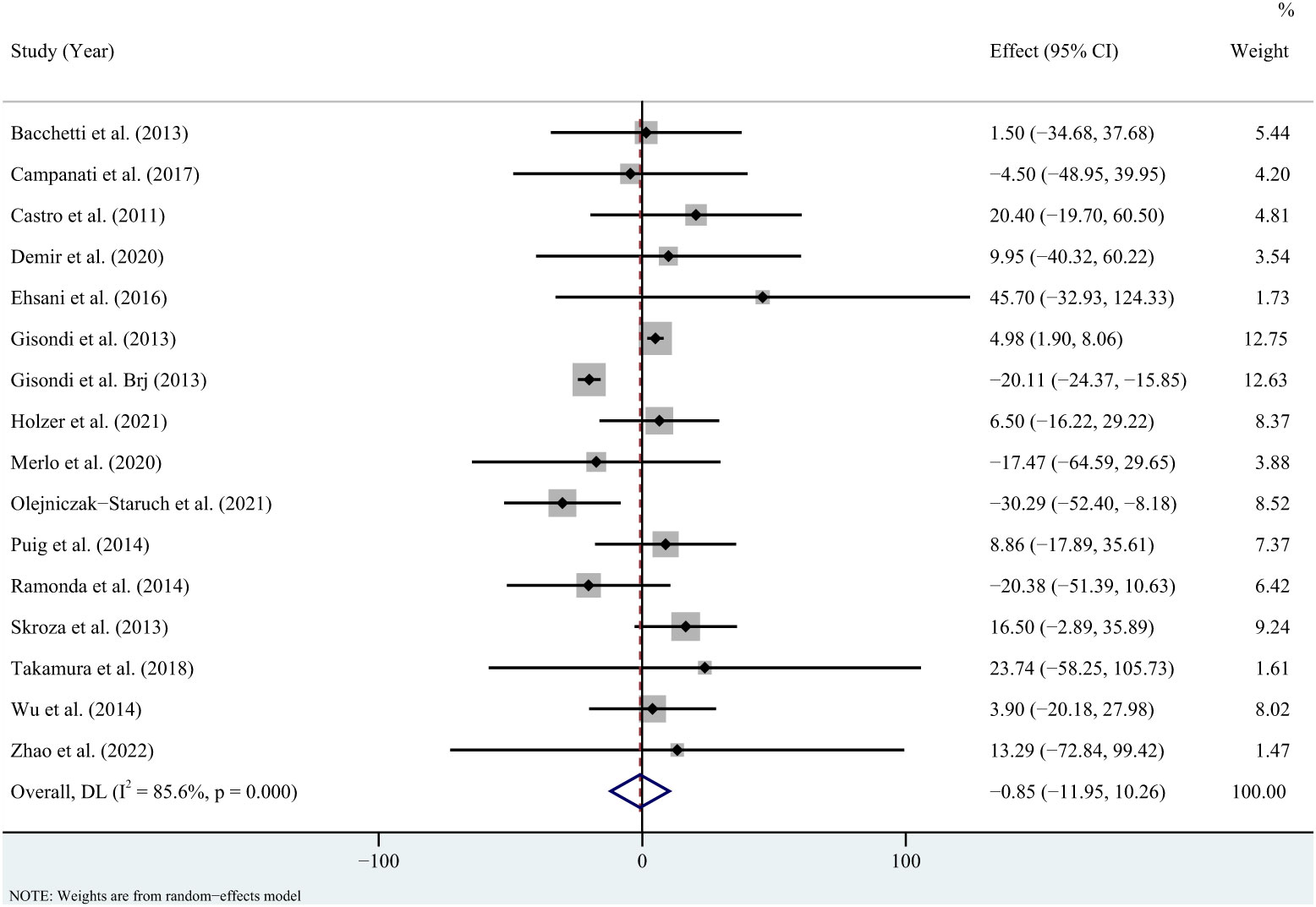
Figure 2 Forest plot for the effect of TNF-alpha inhibitors on triglycerides in patients with psoriasis (weighted mean difference).
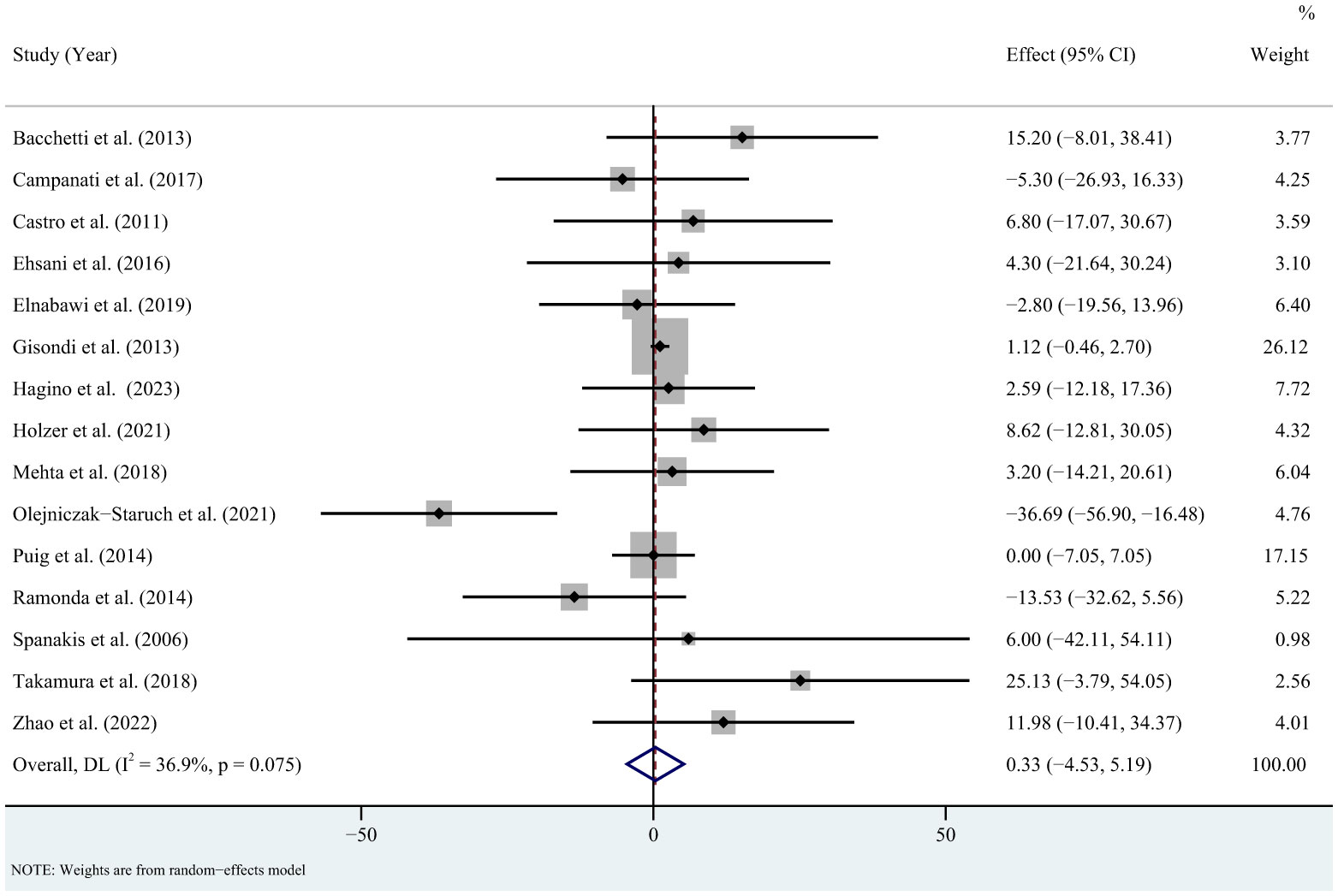
Figure 3 Forest plot for the effect of TNF-alpha inhibitors on total cholesterol in patients with psoriasis (weighted mean difference).
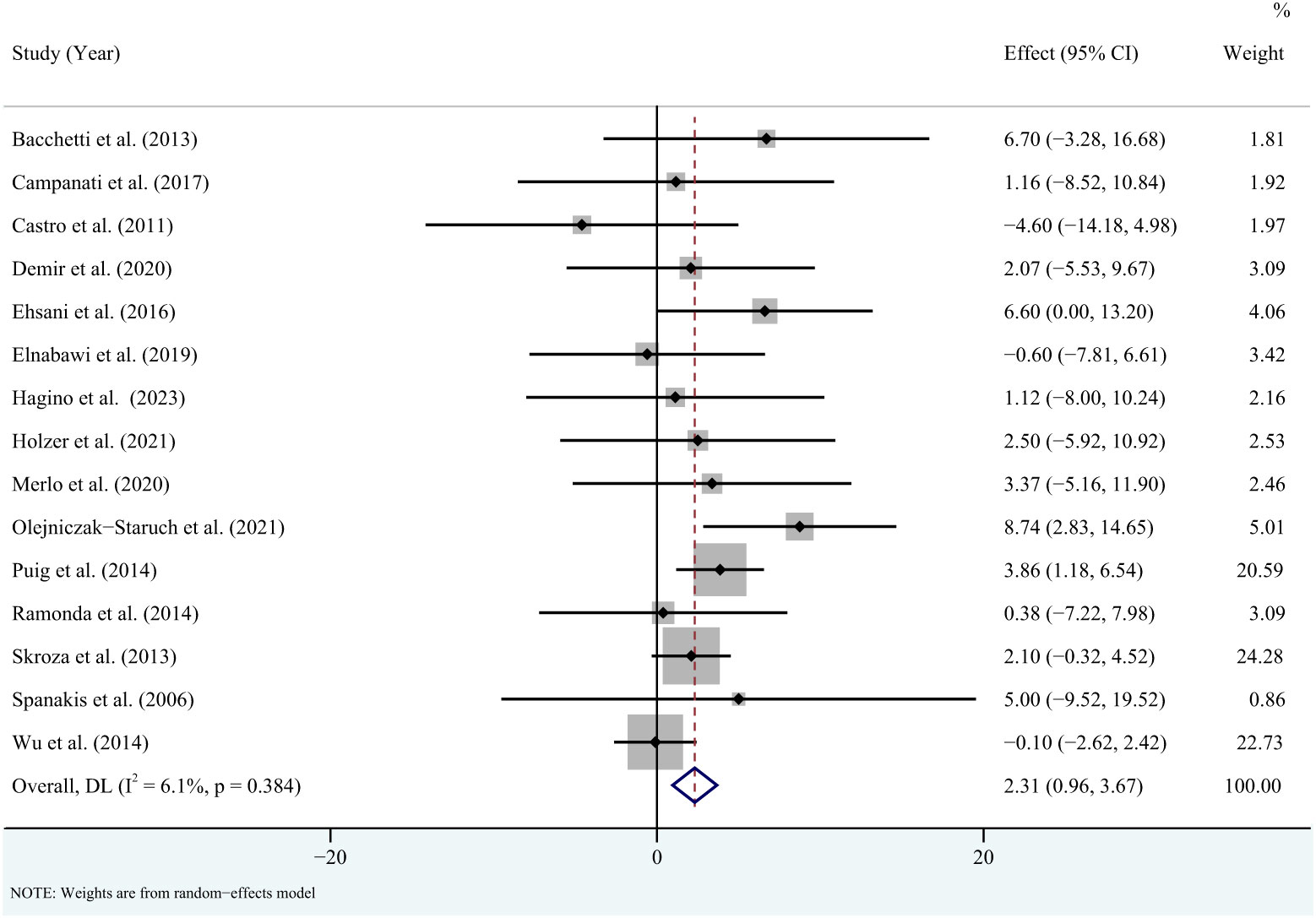
Figure 4 Forest plot for the effect of TNF-alpha inhibitors on high-density lipoprotein in patients with psoriasis (weighted mean difference).
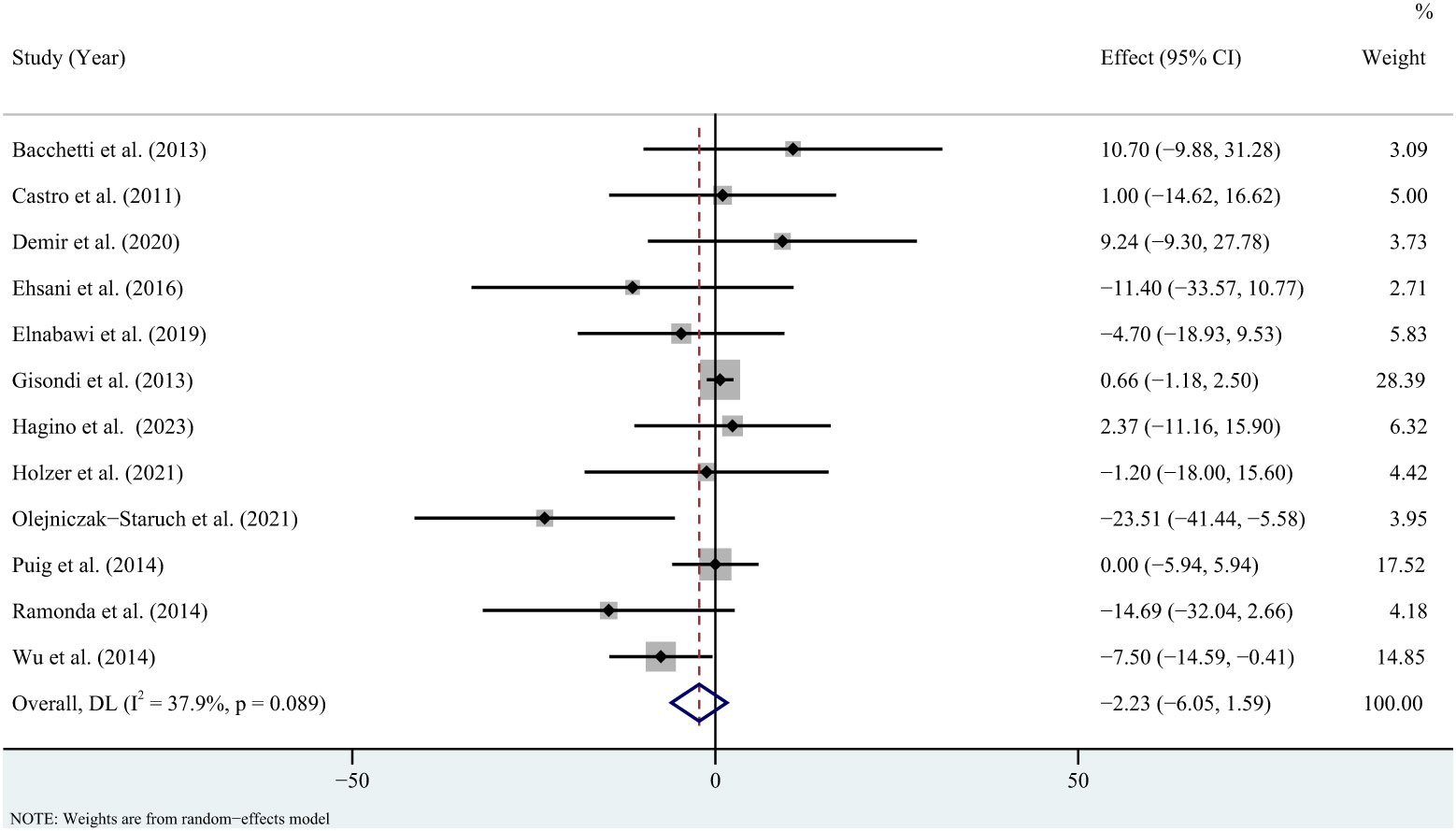
Figure 5 Forest plot for the effect of TNF-alpha inhibitors on low-density lipoprotein in patients with psoriasis (weighted mean difference).
The subgroup analysis revealed that triglycerides levels were not significantly increased in the less than or equal to 3 months group (WMD = 11.82; 95% CI = -2.8, 26.44, P = 0.113; I2 = 90.9%), while were significantly increased in the 3 to 6 months group (WMD = 4.98; 95% CI = 1.97, 7.99, P = 0.001; I2 = 0%) and decreased in the greater than 6 months group (WMD = -19.84; 95% CI = -23.97, -15.7, P < 0.001; I2 = 0%) (Supplementary Figure 9). Total cholesterol levels did not change significantly in the less than or equal to 3 months group (WMD = 1.67; 95% CI = -3.1, 6.45, P = 0.492; I2 = 30.3%), the 3 to 6 months group (WMD = 1.21; 95% CI = -0.36, 2.77, P = 0.13; I2 = 0%) and the greater than 6 months group (WMD = -1.77; 95% CI = -11.08, 7.55, P = 0.71; I2 = 31.9%) (Supplementary Figure 10). High-density lipoprotein levels were significantly increased in the less than or equal to 3 months group (WMD = 2.88; 95% CI = 1.37, 4.4, P < 0.001; I2 = 0%), while were not significantly increased in the 3 to 6 months group (WMD = 1.39; 95% CI = -0.87, 3.65, P = 0.229; I2 = 2.2%) and the greater than 6 months group (WMD = 2.16; 95% CI = -1.01, 5.33, P = 0.182; I2 = 0%) (Supplementary Figure 11). Low-density lipoprotein levels did not change significantly in the less than or equal to 3 months group (WMD = 1.12; 95% CI = -3.25, 5.49, P = 0.617; I2 = 36.2%), the 3 to 6 months group (WMD = -1.85; 95% CI = -7.3, 3.61, P = 0.507; I2 = 41.6%) and the greater than 6 months group (WMD = -5.19; 95% CI = -13.04, 2.66, P = 0.195; I2 = 0%) (Supplementary Figure 12).
The subgroup analysis revealed that triglycerides levels did not change significantly in the adalimumab group (WMD = -5.31; 95% CI = -15.47, 4.85, P = 0.305; I2 = 0%), the etanercept group (WMD = 1.67; 95% CI = -12.51, 15.85, P = 0.817; I2 = 55.6%) and the infliximab group (WMD = -7.45; 95% CI = -21.64, 6.74, P = 0.303; I2 = 79.4%) (Supplementary Figure 13). Total cholesterol levels did not change significantly in the adalimumab group (WMD = 7.87; 95% CI = -0.91, 16.65, P = 0.079; I2 = 28.6%), the etanercept group (WMD = -0.46; 95% CI = -9.71, 8.79, P = 0.923; I2 = 72.1%) and the infliximab group (WMD = -1.57; 95% CI = -11.91, 8.76, P = 0.765; I2 = 38.6%) (Supplementary Figure 14). High-density lipoprotein levels were significantly increased in the etanercept group (WMD = 3.4; 95% CI = 1.71, 5.09, P < 0.001; I2 = 0%), while were not significantly increased in the adalimumab group (WMD = 2.85; 95% CI = -2.8, 8.5, P = 0.323; I2 = 0%) and the infliximab group (WMD = 6.2; 95% CI = -3.27, 15.67, P = 0.199; I2 = 84.4%) (Supplementary Figure 15). Low-density lipoprotein levels did not change significantly adalimumab group (WMD = 2.67; 95% CI = -7.63, 12.98, P = 0.611; I2 = 27.1%), the etanercept group (WMD = -0.01; 95% CI = -5.67, 5.65, P = 0.998; I2 = 50.8%) and the infliximab group (WMD = -9.16; 95% CI = -21.18, 2.86, P = 0.135; I2 = 75.6%) (Supplementary Figure 16).
According to the PASI scores for psoriasis (mean or median PASI more than 10), the findings were consistent with the overall results (Supplementary Figures 17-20). According to the psoriasis type (psoriasis or psoriatic arthritis), the results of total cholesterol and low-density lipoprotein were consistent with the overall results (Supplementary Figures 21, 22). Triglycerides levels were significantly increased in the psoriasis group (WMD = 5.22; 95% CI = 2.23, 8.21, P = 0.001; I2 = 0%), while were not change significantly in the psoriatic arthritis group (WMD = -2.05; 95% CI = -41.81, 37.7, P = 0.919; I2 = 59.8%) (Supplementary Figure 23). High-density lipoprotein levels were significantly increased in the psoriasis group (WMD = 2.52; 95% CI = 0.57, 4.48, P = 0.011; I2 = 0%), while were not change significantly in the psoriatic arthritis group (WMD = -0.6; 95% CI = -6.11, 4.9, P = 0.83; I2 = 0%) (Supplementary Figure 24).
For the overall results, a funnel plot showed that a possible publication bias may exist in triglycerides (Supplementary Figure 25) and total cholesterol (Supplementary Figure 26), although Egger’s test was not statistically significant in triglycerides (P = 0.835; Supplementary Figure 27) and total cholesterol (P = 0.931; Supplementary Figure 28). There was no obvious funnel plot asymmetry for the high-density lipoprotein (Supplementary Figure 29), and low-density lipoprotein (Supplementary Figure 30). Furthermore, Egger’s test revealed no statistical evidence of publication bias in high-density lipoprotein (P = 0.641; Supplementary Figure 31) and low-density lipoprotein (P = 0.188; Supplementary Figure 32).
In this study, we evaluated the effect of TNF-alpha inhibitors on lipid profiles (triglycerides, total cholesterol, low-density lipoprotein, and high-density lipoprotein) in patients with psoriasis. The overall findings revealed that TNF-alpha inhibitors elevated high-density lipoprotein levels in patients with psoriasis, which was supported by the results of sensitivity analyses excluding the effect of lipid-lowering drugs. We also performed subgroup analyses according to the type of TNF-alpha inhibitor, treatment duration, PASI scores, and psoriasis type. High-density lipoprotein levels were significantly increased in the less than or equal to 3 months group, the etanercept group, and the psoriasis group. Changes in triglyceride levels were not consistent among the different durations of treatment. Specifically, triglycerides were significantly increased in the 3 to 6-month group and significantly decreased in the 6-month and older group. In addition, triglycerides significantly increased the psoriasis group.
The negative relationship between high-density lipoprotein levels and the risk of coronary heart disease dates back to the 1950s, and it remains an important and powerful risk marker for the developing risk of atherosclerotic cardiovascular disease (49). The increase in high-density lipoprotein levels may represent a cardioprotective effect (50). However, a previous meta-analysis found no significant difference in the rate of major adverse cardiovascular events in psoriasis patients treated with TNF-alpha inhibitors compared with placebo (51). Thus, the increase of high-density lipoprotein levels observed in this meta-analysis may limited. Actually, our subgroups also revealed that high-density lipoprotein levels were significantly elevated only in the less-than-or-equal-to-3-months group. On the other hand, high-density lipoprotein levels were significantly increased in the etanercept group, while were not significantly increased in the adalimumab group and the infliximab group. Head-to-head trials comparing the effectiveness of etanercept, adalimumab, and infliximab in the treatment of psoriasis are limited, and there is a lack of consensus on the difference in effectiveness between them (52–54). Thus, the elevated high-density lipoprotein levels in the etanercept group may be explained by the fact that this result was primarily driven by the studies from Puig, L (42). and Skroza, N (44)., both of which were treated for three months. Conversely, low-density lipoprotein is the major atherogenic lipoprotein and has been reported not to modify in patients with rheumatoid arthritis after treatment with TNF-alpha inhibitors (50). Similarly, the decrease of low-density lipoprotein levels observed in this meta-analysis lacked statistical significance. Total cholesterol consists mainly of low-density lipoprotein and high-density lipoprotein cholesterol (55, 56). Hence, a potential explanation for the total cholesterol results observed in this meta-analysis is the limited increase in high-density lipoprotein levels and the lack of statistical significance of the decrease in low-density lipoprotein levels. TNF-alpha inhibitors have been reported to exhibit a tendency to increase triglycerides in the treatment of patients with rheumatoid arthritis (57). A recent meta-analysis investigating the effect of TNF-alpha inhibitors on the lipid profile of patients with rheumatic diseases showed that changes in triglycerides were not consistent among the different time point assessments (58). Specifically, triglycerides were marginally significantly increased at short-term and middle-term assessments and significantly increased at the long-term assessment (58). Our subgroup analysis revealed that triglyceride levels were not significantly increased in the less than or equal to 3 months group, while were significantly increased in the 3 to 6 months group and decreased in the greater than 6 months group. Considering the large change in effect size in the greater than 6 months group, a potential explanation for the triglycerides results may be related to the use of lipid-lowering drugs. Actually, more than half of the studies we included did not report data on the use of lipid-lowering drugs. The bias in the efficacy of lipid-lowering drugs may provide a plausible explanation for the significant increase in the 3 to 6-month group and the significant decrease in the greater than 6-month group. As for the differences in results between psoriasis and psoriatic arthritis, one potential reason may be that their pathophysiological mechanisms are not entirely identical (59, 60). In addition, psoriatic arthritis may be comorbid with more severe complications and respond relatively inadequately to biologics, which may be another potential explanation for our results (61). Notably, this result needs further investigation, especially given the small number of trials included in the psoriatic arthritis group.
Psoriasis is a typical inflammatory skin disease (62). An underlying mechanism for our results may be related to inflammation and lipid metabolism, which has been reported in other inflammatory skin diseases (63, 64). Hidradenitis suppurativa is a chronic inflammatory skin disease (63). Excessive obesity, especially in visceral depots, is connected with adipose tissue dysfunction, which manifests as a potentially pro-inflammatory state (63). The development of hidradenitis suppurativa may be partly driven by excess visceral adiposity and chronic inflammation (63). Therefore, the assessment of metabolic risk may be an important component in the clinical management of inflammatory skin diseases (64). High-density lipoprotein possesses anti-inflammatory effects, while inflammation also reduces high-density lipoprotein levels (65). Correspondingly, psoriasis patients were noted to have reduced high-density lipoprotein levels than healthy controls (65). It has been reported that cytokine expression can reduce high-density lipoprotein levels during inflammation, and the specific mechanism may be related to mediating the downregulation of peroxisome proliferator-activated receptor gamma expression (57, 66). Actually, TNF-alpha is a key inflammatory mediator, and it has been reported to interfere with cholesterol metabolism possibly (67, 68). TNF-alpha inhibitors are known to possess anti-inflammatory effects. Thus, the increase of high-density lipoprotein levels observed in this meta-analysis may be associated with TNF-alpha inhibitors alleviating inflammation in patients with psoriasis. In addition, TNF-alpha is involved in body weight homeostasis by enhancing lipolysis and depressing adipogenesis, and TNF-alpha inhibitor therapy appears to be linked to increased body weight in psoriasis patients (67). Further studies have shown that TNF-alpha increases lipolysis and promotes muscle cell catabolism by mediating the activation of the nuclear transcription factor NF-kB (30). In contrast, TNF-alpha inhibitors possess the ability to induce muscle and adipocytes to take up glucose and convert it to triglycerides and glycogen (58). In rheumatoid arthritis or ankylosing spondylitis patients, long-term TNF-alpha inhibitors have also been reported to be associated with a significant increase in fat mass, with a shift to the visceral region (69). Notably, classical methotrexate therapy for psoriasis did not appear to significantly increase body mass index (47). Obesity, in particular abdominal obesity, seems to determine triglyceride levels (70, 71). Therefore, the total cholesterol results observed in this meta-analysis may be related to the effect of TNF-alpha inhibitors on the metabolism of adipose and muscle tissue.
To the best of our knowledge, this study is the first meta-analysis to evaluate the effect of TNF-alpha inhibitors on lipid profiles (triglycerides, total cholesterol, low-density lipoprotein, and high-density lipoprotein) in patients with psoriasis. Meanwhile, the heterogeneity of most results is acceptable. Certainly, this study has limitations that cannot be denied. Firstly, we must acknowledge that this study lacked a placebo control group. Therefore, the influence of study design cannot be excluded. However, we cannot deny the fact that the ethics of using placebo control in patients with moderate to severe psoriasis are widely debated (72). Thus, placebo-controlled data are limited. A patient registry is a structured set of observational data on a population defined by a particular disease or condition, which contains relatively broader inclusion and exclusion criteria than randomized clinical trials (73). Hence, patient registries have larger sample sizes, which may increase the generalizability of results to clinical practice (73). Actually, patient registries have gained attention in psoriasis research (74, 75). Establishing a specific patient registry of biologics for the treatment of psoriasis may contribute to extending our results. Second, owing to limited data, our study failed to investigate the effect of certolizumab in subgroup analyses. Third, although we performed subgroup analyses according to the type of TNF-alpha inhibitors, we did not compare the effects of different TNF-alpha inhibitors. Thus, network meta-analysis contributes to extending our findings. Finally, the prevalence of psoriasis varies significantly in the global population, suggesting that there may be population differences in the pathogenesis of psoriasis (76). Hence, whether our findings vary across regions and countries is a question worth exploring. However, limited data restricted our ability to perform stratified analyses according to region and nation. Further well-designed controlled studies contribute to confirming and extending our findings.
Our results revealed that TNF-alpha inhibitors might temporarily increase high-density lipoprotein levels in patients with psoriasis. However, changes in triglycerides were not consistent among the different durations of treatment, with significant increases after 3 to 6 months of treatment. Our findings emphasized the importance of screening lipids in the treatment of psoriasis with TNF-alpha inhibitors. Considering the limitations of our study, future prospective trials with long-term follow-up contribute to confirming and extending our findings.
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
LS: Data curation, Formal analysis, Writing – original draft. CX: Data curation, Formal analysis, Writing – review and editing. HH: Writing – review and editing. PZ: Writing – review and editing. JW: Writing – review and editing. XO: Conceptualization, Methodology, Project administration, Writing – review and editing. XY: Conceptualization, Methodology, Project administration, Writing – review and editing. JY: Conceptualization, Methodology, Project administration, Writing – review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by The Yunnan Provincial Science and Technology Department Science and Technology Programme Projects (202101AZ070001-168 and 202101AZ070001-289), Health Commission of Yunnan Province-Scientific and Technological Talents and Platform Program Academician (Expert) Workstation Project (202005AF150075), Yunnan Provincial Clinical Medical Centre for Traditional Chinese Medicine Project (Dermatology), and high-level key discipline construction project of the National Administration of Traditional Chinese Medicine.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1354593/full#supplementary-material
1. Takeshita J, Grewal S, Langan SM, Mehta NN, Ogdie A, Van Voorhees AS, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. (2017) 76:377–90. doi: 10.1016/j.jaad.2016.07.064
2. Li L, Lu J, Liu J, Wu J, Zhang X, Meng Y, et al. Immune cells in the epithelial immune microenvironment of psoriasis: emerging therapeutic targets. Front Immunol. (2023) 14:1340677. doi: 10.3389/fimmu.2023.1340677
3. Korman NJ. Management of psoriasis as a systemic disease: what is the evidence? Br J Dermatol. (2020) 182:840–8. doi: 10.1111/bjd.18245
4. Maurelli M, Gisondi P, Girolomoni G. Advanced glycation end products and psoriasis. Vaccines (Basel). (2023) 11:617. doi: 10.3390/vaccines11030617
5. Purzycka-Bohdan D, Kisielnicka A, Bohdan M, Szczerkowska-Dobosz A, Sobalska-Kwapis M, Nedoszytko B, et al. Analysis of the potential genetic links between psoriasis and cardiovascular risk factors. Int J Mol Sci. (2021) 22:9063. doi: 10.3390/ijms22169063
6. Huang D, Ma R, Zhong X, Jiang Y, Lu J, Li Y, et al. Positive association between different triglyceride glucose index-related indicators and psoriasis: evidence from nhanes. Front Immunol. (2023) 14:1325557. doi: 10.3389/fimmu.2023.1325557
7. De Brandt E, Hillary T. Comorbid psoriasis and metabolic syndrome: clinical implications and optimal management. Psoriasis (Auckland NZ). (2022) 12:113–26. doi: 10.2147/PTT.S293107
8. de Carvalho AVE, Romiti R, Souza CS, Paschoal RS, Milman LM, Meneghello LP. Psoriasis comorbidities: complications and benefits of immunobiological treatment. Anais Brasileiros Dermatologia. (2016) 91:781–9. doi: 10.1590/abd1806-4841.20165080
9. Jiang Y, Chen Y, Yu Q, Shi Y. Biologic and small-molecule therapies for moderate-to-severe psoriasis: focus on psoriasis comorbidities. BioDrugs. (2023) 37:35–55. doi: 10.1007/s40259-022-00569-z
10. Pietrzak A, Michalak-Stoma A, Chodorowska G, Szepietowski JC. Lipid disturbances in psoriasis: an update. Mediators Inflammation. (2010) 2010:535612. doi: 10.1155/2010/535612
11. Wang F, Wang Y, Kong X, Mu J, Wang Z, Yang X, et al. Association between psoriasis and serum apolipoprotein A1 and B: A systematic review and meta-analysis. Heliyon. (2023) 9:e21168. doi: 10.1016/j.heliyon.2023.e21168
12. Madani AN, Al-Saif FM, Alzamil LR, Almazroua AM, Alfurayh NA, Aldokhayel SD, et al. Monitoring the effect of tnf-alpha inhibitors on laboratory parameters and adverse effects in different diseases: A retrospective, single-center study. Ann Saudi Med. (2022) 42:309–18. doi: 10.5144/0256-4947.2022.309
13. Rusinol L, Carmona-Rocha E, Puig L. Durability and long-term outcomes of biologic therapies in psoriasis. Expert Rev Clin Immunol. (2024) 20:71–82. doi: 10.1080/1744666X.2023.2250918
14. Yamauchi PS, Bissonnette R, Teixeira HD, Valdecantos WC. Systematic review of efficacy of anti-tumor necrosis factor (Tnf) therapy in patients with psoriasis previously treated with a different anti-tnf agent. J Am Acad Dermatol. (2016) 75:612–8 e6. doi: 10.1016/j.jaad.2016.02.1221
15. Chen X, Xun K, Chen L, Wang Y. Tnf-alpha, a potent lipid metabolism regulator. Cell Biochem Funct. (2009) 27:407–16. doi: 10.1002/cbf.1596
16. Lestre S, Diamantino F, Veloso L, Fidalgo A, Ferreira A. Effects of etanercept treatment on lipid profile in patients with moderate-to-severe chronic plaque psoriasis: A retrospective cohort study. Eur J Dermatol EJD. (2011) 21:916–20. doi: 10.1684/ejd.2011.1548
17. Botelho KP, Pontes MAA, Rodrigues CEM, Freitas MVC. Prevalence of metabolic syndrome among patients with psoriasis treated with tnf inhibitors and the effects of anti-tnf therapy on their lipid profile: A prospective cohort study. Metab syndrome related Disord. (2020) 18:154–60. doi: 10.1089/met.2019.0092
18. Marsche G, Holzer M, Wolf P. Antipsoriatic treatment extends beyond the skin: recovering of high-density lipoprotein function. Exp Dermatol. (2014) 23:701–4. doi: 10.1111/exd.12483
19. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. Ann Intern Med. (2009) 151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135
20. Sbidian E, Chaimani A, Guelimi R, Garcia-Doval I, Hua C, Hughes C, et al. Systemic pharmacological treatments for chronic plaque psoriasis: A network meta-analysis. Cochrane Database systematic Rev. (2023) 7:CD011535. doi: 10.1002/14651858.CD011535.pub6
21. Samarasekera EJ, Smith CH, National Institute of H, Care E, Royal College of P. Psoriasis: guidance on assessment and referral. Clin Med (Lond). (2014) 14:178–82. doi: 10.7861/clinmedicine.14-2-178
22. Shang Z, Wang M, Zhang B, Wang X, Wanyan P. Clinical translation of stem cell therapy for spinal cord injury still premature: results from a single-arm meta-analysis based on 62 clinical trials. BMC Med. (2022) 20:284. doi: 10.1186/s12916-022-02482-2
23. Laird NM, Mosteller F. Some statistical methods for combining experimental results. Int J Technol Assess Health Care. (1990) 6:5–30. doi: 10.1017/s0266462300008916
24. Su L, Yang ZT, Qu H, Luo CL, Yuan GX, Wu J, et al. Effect of antioxidants supplementation on erectile dysfunction: A systematic review and meta-analysis of randomized controlled trials. Sex Med Rev. (2022) 10:754–63. doi: 10.1016/j.sxmr.2022.01.002
25. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0). UK: The Cochrane Collaboration. (2011).
26. Hardy RJ, Thompson SG. Detecting and describing heterogeneity in meta-analysis. Stat Med. (1998) 17:841–56. doi: 10.1002/(sici)1097-0258(19980430)17:8<841::aid-sim781>3.0.co;2-d
27. Langhorne P. Bias in meta-analysis detected by a simple, graphical test. Prospectively identified trials could be used for comparison with meta-analyses. BMJ (Clinical Res ed). (1998) 316:471.
28. Nast A, Smith C, Spuls PI, Avila Valle G, Bata-Csorgo Z, Boonen H, et al. Euroguiderm guideline on the systemic treatment of psoriasis vulgaris - part 1: treatment and monitoring recommendations. J Eur Acad Dermatol Venereology JEADV. (2020) 34:2461–98. doi: 10.1111/jdv.16915
29. Bacchetti T, Campanati A, Ferretti G, Simonetti O, Liberati G, Offidani AM. Oxidative stress and psoriasis: the effect of antitumour necrosis factor-A Inhibitor treatment. Br J Dermatol. (2013) 168:984–9. doi: 10.1111/bjd.12144
30. Campanati A, Molinelli E, Ganzetti G, Giuliodori K, Minetti I, Taus M, et al. The effect of low-carbohydrates calorie-restricted diet on visceral adipose tissue and metabolic status in psoriasis patients receiving tnf-alpha inhibitors: results of an open label controlled, prospective, clinical study. J Dermatol Treat. (2017) 28:206–12. doi: 10.1080/09546634.2016.1214666
31. Castro KR, Aikawa NE, Saad CG, Moraes JC, Medeiros AC, Mota LM, et al. Infliximab induces increase in triglyceride levels in psoriatic arthritis patients. Clin Dev Immunol. (2011) 2011:352686. doi: 10.1155/2011/352686
32. Demir D, Aktaş Karabay E, Kivanç Altunay I, Yener Öztürk F. Impact of treatment with methotrexate and tnf alpha inhibitors on insulin resistance in patients with psoriasis. Turkiye Klinikleri Dermatoloji. (2020) 30:35–43. doi: 10.5336/DERMATO.2020-74503
33. Ehsani AH, Mortazavi H, Balighi K, Hosseini MS, Azizpour A, Hejazi SP, et al. Changes in body mass index and lipid profile in psoriatic patients after treatment with standard protocol of infliximab. Acta Med Iranica. (2016) 54:570–5.
34. Elnabawi YA, Dey AK, Goyal A, Groenendyk JW, Chung JH, Belur AD, et al. Coronary artery plaque characteristics and treatment with biologic therapy in severe psoriasis: results from a prospective observational study. Cardiovasc Res. (2019) 115:721–8. doi: 10.1093/cvr/cvz009
35. Gisondi P, Cazzaniga S, Chimenti S, Giannetti A, Maccarone M, Picardo M, et al. Metabolic abnormalities associated with initiation of systemic treatment for psoriasis: evidence from the italian psocare registry. J Eur Acad Dermatol Venereology JEADV. (2013) 27:e30–41. doi: 10.1111/j.1468-3083.2012.04450.x
36. Gisondi P, Lora V, Bonauguri C, Russo A, Lippi G, Girolomoni G. Serum chemerin is increased in patients with chronic plaque psoriasis and normalizes following treatment with infliximab. Br J Dermatol. (2013) 168:749–55. doi: 10.1111/bjd.12118
37. Hagino T, Saeki H, Fujimoto E, Kanda N. Effects of biologic therapy on laboratory indicators of cardiometabolic diseases in patients with psoriasis. J Clin Med. (2023) 12:1934. doi: 10.3390/jcm12051934
38. Holzer G, Hoke M, Sabeti-Sandor S, Perkmann T, Rauscher A, Strassegger B, et al. Disparate effects of adalimumab and fumaric acid esters on cardiovascular risk factors in psoriasis patients: results from a prospective, randomized, observer-blinded head-to-head trial. J Eur Acad Dermatol Venereology JEADV. (2021) 35:441–9. doi: 10.1111/jdv.16635
39. Mehta NN, Shin DB, Joshi AA, Dey AK, Armstrong AW, Duffin KC, et al. Effect of 2 psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers: A randomized placebo-controlled trial. Circ Cardiovasc Imaging. (2018) 11:e007394. doi: 10.1161/circimaging.117.007394
40. Merlo G, Cozzani E, Burlando M, Calvieri S, Potenza C, Stingeni L, et al. Effects of tnfα Inhibitors in patients with psoriasis and metabolic syndrome: A preliminary study. Giornale italiano di dermatologia e venereologia organo ufficiale Societa italiana di dermatologia e sifilografia. (2020) 155:14–8. doi: 10.23736/s0392-0488.17.05621-8
41. Olejniczak-Staruch I, Narbutt J, Ceryn J, Skibińska M, Bednarski I, Woźniacka A, et al. Antitnf-alpha therapy normalizes levels of lipids and adipokines in psoriatic patients in the real-life settings. Sci Rep. (2021) 11:9289. doi: 10.1038/s41598-021-88552-6
42. Puig L, Strohal R, Fuiman J, Pedersen R, Szumski A, Koenig AS, et al. Cardiometabolic Biomarkers in Chronic Plaque Psoriasis before and after Etanercept Treatment. J Dermatol Treat. (2014) 25:470–81. doi: 10.3109/09546634.2013.848260
43. Ramonda R, Puato M, Punzi L, Rattazzi M, Zanon M, Balbi G, et al. Atherosclerosis progression in psoriatic arthritis patients despite the treatment with tumor necrosis factor-alpha blockers: A two-year prospective observational study. Joint Bone Spine. (2014) 81:421–5. doi: 10.1016/j.jbspin.2014.02.005
44. Skroza N, Proietti I, Bernardini N, La Viola G, Nicolucci F, Pampena R, et al. Efficacy of food supplement to improve metabolic syndrome parameters in patients affected by moderate to severe psoriasis during anti-tnfα Treatment. Giornale italiano di dermatologia e venereologia organo ufficiale Societa italiana di dermatologia e sifilografia. (2013) 148:661–5.
45. Spanakis E, Sidiropoulos P, Papadakis J, Ganotakis E, Katsikas G, Karvounaris S, et al. Modest but sustained increase of serum high density lipoprotein cholesterol levels in patients with inflammatory arthritides treated with infliximab. J Rheumatol. (2006) 33:2440–6.
46. Takamura S, Takahashi A, Inoue Y, Teraki Y. Effects of tumor necrosis factor-A, interleukin-23 and interleukin-17a inhibitors on bodyweight and body mass index in patients with psoriasis. J Dermatol. (2018) 45:1130–4. doi: 10.1111/1346-8138.14526
47. Wu JJ, Liu L, Asgari MM, Curtis JR, Harrold L, Salman C, et al. Initiation of tnf inhibitor therapy and change in physiologic measures in psoriasis. J Eur Acad Dermatol Venereology JEADV. (2014) 28:1380–7. doi: 10.1111/jdv.12296
48. Zhao L, Zhang X, Zhu L, Geng S, Guo K. Effectiveness and safety of adalimumab in psoriasis and its influence on gut microbiome. Microbial Pathogenesis. (2022) 162:105308. doi: 10.1016/j.micpath.2021.105308
49. von Eckardstein A, Nordestgaard BG, Remaley AT, Catapano AL. High-density lipoprotein revisited: biological functions and clinical relevance. Eur Heart J. (2023) 44:1394–407. doi: 10.1093/eurheartj/ehac605
50. Daien CI, Duny Y, Barnetche T, Daures JP, Combe B, Morel J. Effect of tnf inhibitors on lipid profile in rheumatoid arthritis: A systematic review with meta-analysis. Ann rheumatic Dis. (2012) 71:862–8. doi: 10.1136/annrheumdis-2011-201148
51. Ryan C, Leonardi CL, Krueger JG, Kimball AB, Strober BE, Gordon KB, et al. Association between biologic therapies for chronic plaque psoriasis and cardiovascular events: A meta-analysis of randomized controlled trials. Jama. (2011) 306:864–71. doi: 10.1001/jama.2011.1211
52. Reich K, Burden AD, Eaton JN, Hawkins NS. Efficacy of biologics in the treatment of moderate to severe psoriasis: A network meta-analysis of randomized controlled trials. Br J Dermatol. (2012) 166:179–88. doi: 10.1111/j.1365-2133.2011.10583.x
53. Fenix-Caballero S, Alegre-del Rey EJ, Castano-Lara R, Puigventos-Latorre F, Borrero-Rubio JM, Lopez-Vallejo JF. Direct and indirect comparison of the efficacy and safety of adalimumab, etanercept, infliximab and golimumab in psoriatic arthritis. J Clin Pharm Ther. (2013) 38:286–93. doi: 10.1111/jcpt.12045
54. Mossner R, Reich K. Management of severe psoriasis with tnf antagonists. Adalimumab, etanercept and infliximab. Curr Probl Dermatol. (2009) 38:107–36. doi: 10.1159/000232307
55. Zhang F, Tapera TM, Gou J. Application of a new dietary pattern analysis method in nutritional epidemiology. BMC Med Res Method. (2018) 18:119. doi: 10.1186/s12874-018-0585-8
56. da Silva TPR, Mendes LL, Barreto VMJ, Matozinhos FP, Duarte CK. Total cholesterol and low-density lipoprotein alterations in children and adolescents from Brazil: A prevalence meta-analysis. Arch Endocrinol Metab. (2023) 67:19–44. doi: 10.20945/2359-3997000000508
57. Pollono EN, Lopez-Olivo MA, Lopez JA, Suarez-Almazor ME. A systematic review of the effect of tnf-alpha antagonists on lipid profiles in patients with rheumatoid arthritis. Clin Rheumatol. (2010) 29:947–55. doi: 10.1007/s10067-010-1405-7
58. Di Minno MN, Ambrosino P, Peluso R, Di Minno A, Lupoli R, Dentali F, et al. Lipid profile changes in patients with rheumatic diseases receiving a treatment with tnf-alpha blockers: A meta-analysis of prospective studies. Ann Med. (2014) 46:73–83. doi: 10.3109/07853890.2013.874661
59. Sakkas LI, Bogdanos DP. Are psoriasis and psoriatic arthritis the same disease? The il-23/il-17 axis data. Autoimmun Rev. (2017) 16:10–5. doi: 10.1016/j.autrev.2016.09.015
60. Natoli V, Charras A, Hofmann SR, Northey S, Russ S, Schulze F, et al. DNA methylation patterns in cd4(+) T-cells separate psoriasis patients from healthy controls, and skin psoriasis from psoriatic arthritis. Front Immunol. (2023) 14:1245876. doi: 10.3389/fimmu.2023.1245876
61. Boutet MA, Nerviani A, Gallo Afflitto G, Pitzalis C. Role of the il-23/il-17 axis in psoriasis and psoriatic arthritis: the clinical importance of its divergence in skin and joints. Int J Mol Sci. (2018) 19:530. doi: 10.3390/ijms19020530
62. Jiang Z, Jiang X, Chen A, He W. Platelet activation: A promoter for psoriasis and its comorbidity, cardiovascular disease. Front Immunol. (2023) 14:1238647. doi: 10.3389/fimmu.2023.1238647
63. Mintoff D, Agius R, Benhadou F, Das A, Frew JW, Pace NP. Obesity and hidradenitis suppurativa: targeting meta-inflammation for therapeutic gain. Clin Exp Dermatol. (2023) 48:984–90. doi: 10.1093/ced/llad182
64. Mintoff D, Agius R, Fava S, Pace NP. Investigating adiposity-related metabolic health phenotypes in patients with hidradenitis suppurativa: A cross-sectional study. J Clin Med. (2023) 12:4847. doi: 10.3390/jcm12144847
65. Luo L, Guo Y, Chen L, Zhu J, Li C. Crosstalk between cholesterol metabolism and psoriatic inflammation. Front Immunol. (2023) 14:1124786. doi: 10.3389/fimmu.2023.1124786
66. Zhao SP, Yang J, Li J, Dong SZ, Wu ZH. Effect of niacin on lxralpha and ppargamma expression and hdl-induced cholesterol efflux in adipocytes of hypercholesterolemic rabbits. Int J Cardiol. (2008) 124:172–8. doi: 10.1016/j.ijcard.2006.12.032
67. Wu MY, Yu CL, Yang SJ, Chi CC. Change in body weight and body mass index in psoriasis patients receiving biologics: A systematic review and network meta-analysis. J Am Acad Dermatol. (2020) 82:101–9. doi: 10.1016/j.jaad.2019.07.103
68. Shih CM, Chen CC, Chu CK, Wang KH, Huang CY, Lee AW. The roles of lipoprotein in psoriasis. Int J Mol Sci. (2020) 21:859. doi: 10.3390/ijms21030859
69. Toussirot E, Mourot L, Dehecq B, Wendling D, Grandclement E, Dumoulin G, et al. Tnfalpha blockade for inflammatory rheumatic diseases is associated with a significant gain in android fat mass and has varying effects on adipokines: A 2-year prospective study. Eur J Nutr. (2014) 53:951–61. doi: 10.1007/s00394-013-0599-2
70. Zoratti R. A review on ethnic differences in plasma triglycerides and high-density-lipoprotein cholesterol: is the lipid pattern the key factor for the low coronary heart disease rate in people of african origin? Eur J Epidemiol. (1998) 14:9–21. doi: 10.1023/a:1007492202045
71. Bjornson E, Adiels M, Taskinen MR, Boren J. Kinetics of plasma triglycerides in abdominal obesity. Curr Opin lipidology. (2017) 28:11–8. doi: 10.1097/MOL.0000000000000375
72. Katz KA, Karlawish JH, Chiang DS, Bognet RA, Propert KJ, Margolis DJ. Prevalence and factors associated with use of placebo control groups in randomized controlled trials in psoriasis: A cross-sectional study. J Am Acad Dermatol. (2006) 55:814–22. doi: 10.1016/j.jaad.2006.07.005
73. Amin M, Lee EB, Bhutani T, Wu JJ. Review of european registries for psoriasis. J Dermatol Treat. (2019) 30:227–36. doi: 10.1080/09546634.2018.1506084
74. Teoh XY, Suganthy R, Voo SYM, Tang MM, Malaysian Psoriasis Registry Working G. Pustular psoriasis in Malaysia: A review of the Malaysian psoriasis registry 2007-2018. Exp Dermatol. (2023) 32:1253–62. doi: 10.1111/exd.14770
75. Lu C, Yang F, Liu H, Dou L, Wang Y, Li H, et al. Chinese registry of psoriatic arthritis (Crepar): I. Clinical characteristics of chinese patients with psoriatic arthritis. Int J rheumatic Dis. (2023) 26:1737–44. doi: 10.1111/1756-185X.14805
Keywords: tumor necrosis factor alpha (TNF-alpha), tumor necrosis factor alpha inhibitors, psoriasis, lipid profiles, systematic review, meta-analysis
Citation: Su L, Xu C, Huang H, Zhang P, Wang J, Ouyang X, Yang X and Ye J (2024) Effects of tumor necrosis factor-alpha inhibitors on lipid profiles in patients with psoriasis: a systematic review and meta-analysis. Front. Immunol. 15:1354593. doi: 10.3389/fimmu.2024.1354593
Received: 12 December 2023; Accepted: 21 February 2024;
Published: 04 March 2024.
Edited by:
James Cheng-Chung Wei, Chung Shan Medical University Hospital, TaiwanReviewed by:
Dillon Mintoff, University of Malta, MaltaCopyright © 2024 Su, Xu, Huang, Zhang, Wang, Ouyang, Yang and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyong Ouyang, b3l4eTY4QDEyNi5jb20=; Xuesong Yang, ODcxODg5Njk3QHFxLmNvbQ==; Jianzhou Ye, a215ano2M0BzaW5hLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.