- 1Department of Dermatology and Cosmetology, Chongqing Hospital of Traditional Chinese Medicine, Chongqing, China
- 2College of Traditional Chinese Medicine, Chongqing Medical University, Chongqing, China
Acute generalized pustular psoriasis (GPP) is a serious illness. Despite various treatment methods, there is still lack of effective treatment plans for refractory cases with multiple comorbidities. This case report presents a 67-year-old woman with acute GPP, stage 4 chronic kidney disease (CKD), type 2 diabetes, and cardiovascular disease, in whom skin symptom disappearance and kidney function improvement were observed after the use of oral tacrolimus as the sole therapy. This is the first report on the application of tacrolimus in the treatment of acute GPP, especially refractory acute GPP. The successful treatment indicates that there are shared immune pathways between acute GPP and CKD, and the pathways can be interdicted by tacrolimus. Further studies are needed to optimize the therapy to maximize efficacy and minimize toxicity.
1 Introduction
Generalized pustular psoriasis (GPP) is a severe disease with reported mortality rates of 2%–16% (1). It characteristically presents as widespread sterile pustules over an erythematous background (2). Thus far, five subtypes have been described. Acute GPP is the most grievous subtype, and it is often accompanied by systemic symptoms, such as fever, general malaise, anorexia, nausea, and myalgias (3). Common treatments for this disease include methotrexate, cyclosporine, acitretin, corticosteroids, biologics, topical therapy, and phototherapy (4). However, some patients are unable to use these therapies due to therapeutic resistance or drug-related contraindications, so another effective and relatively safe treatment option is needed. Here, we present the case of a female patient who had chronic renal failure, type 2 diabetes, and cardiovascular disease, and then developed acute GPP. The patient’s skin symptoms disappeared and kidney function improved without adverse reactions after only oral tacrolimus was administered. Tacrolimus is a commonly used treatment for CKD, but not a standard treatment for acute GPP. To the best of our knowledge, this study is the first attempt to present tacrolimus in acute GPP. We discuss the same immunological pathogenesis between acute GPP and other chronic diseases as well as the role of tacrolimus in treating these conditions based on treatment experience and relevant literature.
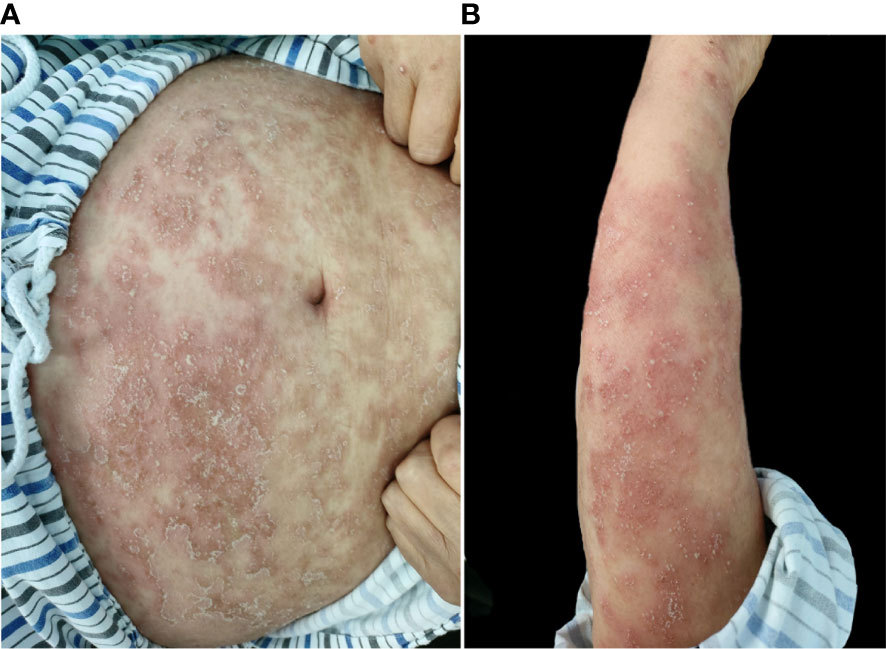
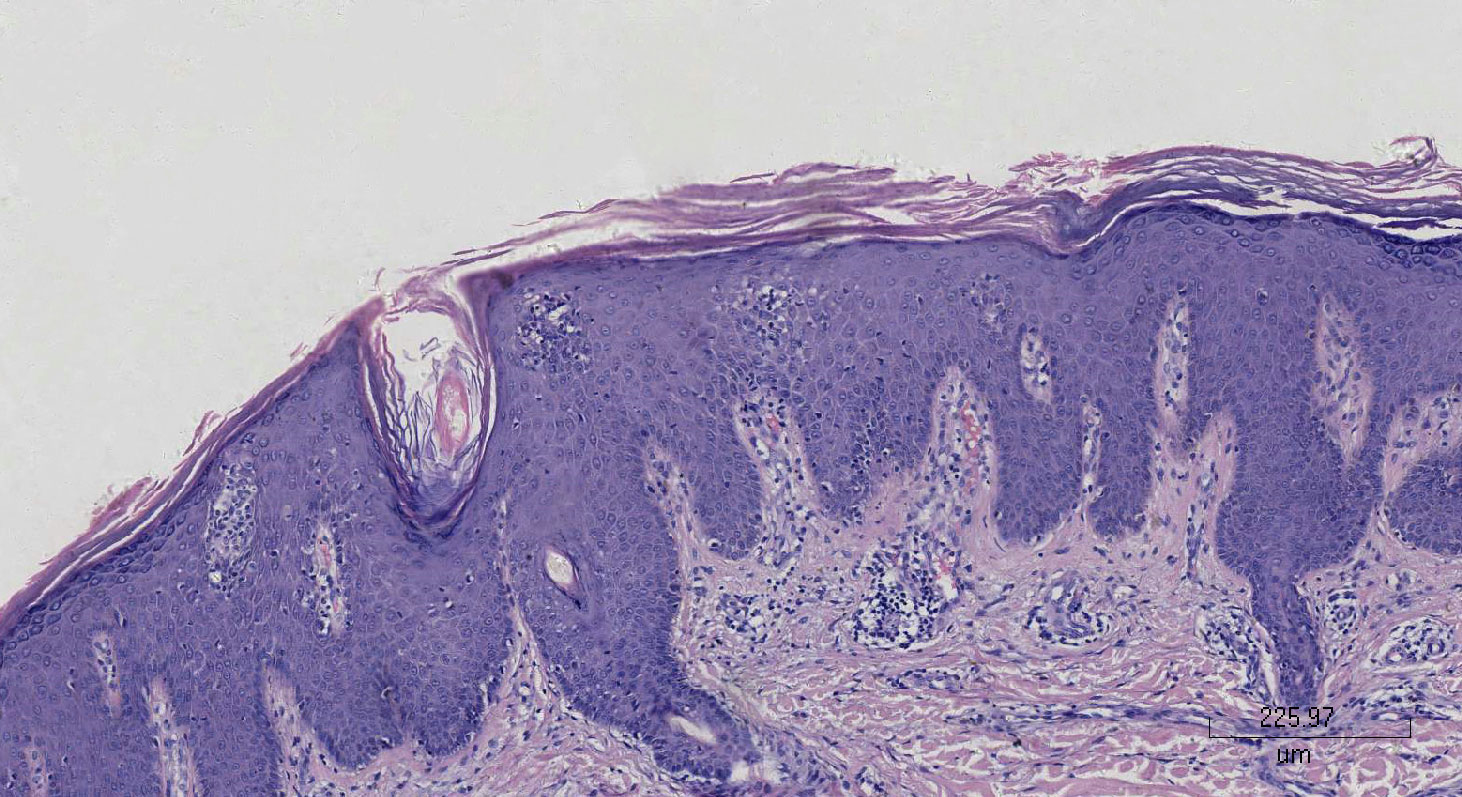
A 67-year-old woman had psoriasis vulgaris for 1 year. She was treated with topical hormone and narrow-band ultraviolet B phototherapy for a prolonged period of time. Neither of the therapies led to a consistent improvement of cutaneous manifestations, and she developed fever and extensive erythema along with pinpoint pustules for 2 weeks prior to our treatment (Figures 1A, B). Her medical history included type 2 diabetes, hypertension, cerebral infarction, and stage 4 CKD. Histopathological examination revealed psoriatic hyperplasia of the epidermis with keratosis, along with Kogoj abscesses in the horny layer. Lymphocytic infiltrate was present in the superficial dermal layer along with dilatation of capillary vessels (Figure 2). The patient was diagnosed with acute GPP based on pathological and clinical manifestations.
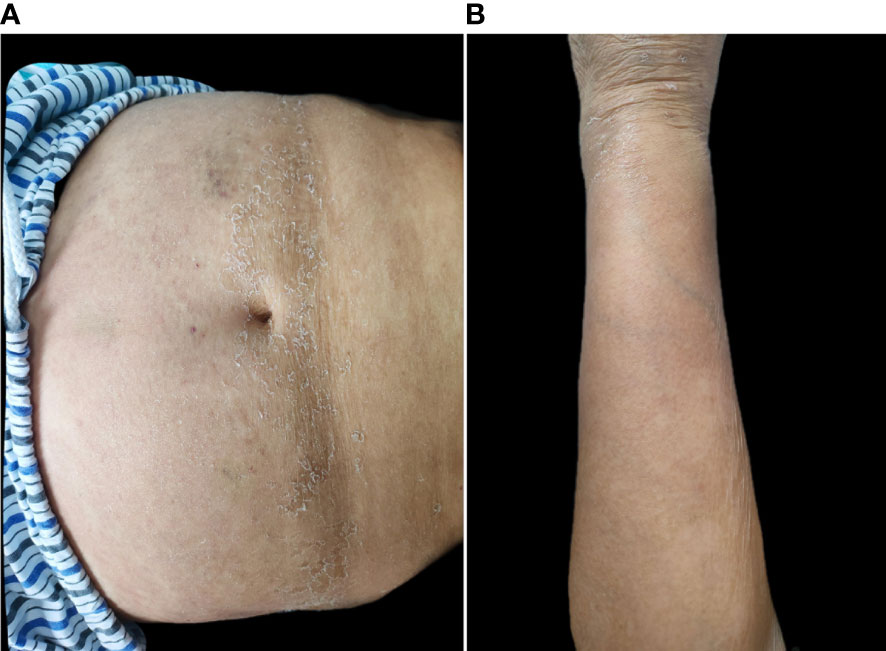
Prior to starting systemic treatment, blood tests (blood count, biochemical tests, and inflammatory markers) were performed. We also decided to perform a computer tomography (CT) scan of her chest to exclude an underlying infection, because she had a self-limiting fever episode 2 weeks earlier. The results were unremarkable except for creatinine (247 μmol/L), glomerular filtration (18.3 mL/min/1.73 m2), and D-dimers (13.04 mg/L), and the CT scan revealed pulmonary interstitial fibrosis. Acitretin, methotrexate, and cyclosporine were avoided due to serious renal insufficiency. The patient declined systemic corticosteroids due to type 2 diabetes and cardiovascular disease, and the use of topical emollients and steroids was not efficient. With informed consent, the patient received oral tacrolimus at an initial dose of 3 mg/day (0.05 mg/kg/day). The patient was afebrile, and her lesions were almost completely resolved after 2 weeks (Figures 3A, B). Creatinine dropped to 213 μmol/L, the glomerular filtration rate was 22 mL/min/1.73 m2, and the D-dimers dramatically improved (2.99 mg/L). The patient’s blood pressure and glycemia were well controlled, and no adverse events such as diarrhea, infection, or paresthesia, were observed. The drug dose was reduced by 1 mg every month, and the entire treatment duration was 3 months. No recurrence was observed after 6 months of follow-up.
2 Discussion
Acute GPP is an immune-mediated disease that can significantly affect social function and interpersonal relationships (5). GPP is a consequence of genetic susceptibility, and inducements include infections, medications, pregnancy, and environmental factors (6). These inducements lead to the acute activation and infiltration of neutrophils, and the dysregulated inflammatory process has a pivotal part in the development of acute GPP (7). The key cytokines of this pathogenesis include tumor necrosis factor (TNF), interleukin (IL)-1, IL-6, IL-12/23, IL-17A, and IL-36 (8), and they locate on a number of skin cells, such as dendritic cells, monocytes, and keratinocytes. Mitogen-activated protein kinase signaling pathways and their downstream inflammatory pathways are activated when the cytokines express abundantly in skin (9). In addition, CD4+ T cells in blood and skin lesions are activated and intense hyperproliferation leads to the T-cell-mediated inflammation (10). These processes ultimately develop into acute GPP inflammatory skin conditions.
Patients with psoriasis are at high risk of developing other chronic health diseases, such as CKD, cardiovascular disorders, and metabolic syndrome (11, 12). Furthermore, there may be a bidirectional association between psoriasis and these chronic diseases. A GPP study has indicated that hypertension is the most commonly identified comorbidity, followed by diabetes (13). In addition, researchers (14–16) have reported the association of GPP with renal disease, including immunoglobulin A (IgA) nephropathy, and renal amyloidosis. Damasiewicz-Bodzek et al. demonstrated that all peptides, including IgA, increased in patients with psoriasis due to oxidative stress stimulating the immune system (17). There may be a link between psoriasis and IgA nephropathy. Acute phase proteins, including serum amyloid A, accumulated in high amounts in the tissues of patients with GPP, which could increase the risk of developing renal amyloidosis (18). There is a significantly increased risk of incident CKD and end-stage renal disease (ESRD) among patients with psoriasis as compared with the general population (19). Furthermore, patients with GPP may develop acute respiratory distress syndrome, which manifests as noninfectious pneumonitis (20). Renal disease, myocardial infarction history, liver disease, and diabetes mellitus are established as predictors of severe GPP (21). The development of these chronic health diseases may be related to an immune-mediated process. Cytokines, such as IL-36 and TNF-α, activate a broad spectrum of immune and non-immune cells that control various inflammatory processes in the skin, angiocarpy, lung, liver, and kidney (22). T cells are critical drivers of related organ damage by directly promoting inflammation and cytotoxicity or via supporting B-cell differentiation and antibody production (23–25).
Since acute GPP is a potentially life-threatening variant of psoriasis, therapy should be initiated without delay. However, GPP is a multisystem disease that is difficult to treat, so personalized treatment should be selected to improve symptoms and minimize psychological harm. Multiple comorbidities were a limitation for the use of common systemic therapies in our patient. Phototherapy and topical corticosteroids are applied only as adjuvant therapies for psoriatic skin lesions on account of the limited remission rates. Topical vitamin D3 analog is not strongly recommended in the acute-phase treatment of GPP because of skin irritation. Although the literatures suggest that topical tacrolimus is effective in treating GPP (26, 27), application of the therapeutic method is limited because of inadequate skin penetrance. In recent years, there have been increasing biologic agents, including TNF, IL-1, IL-12/23, IL-36, and IL-17 inhibitors, that have been reported in the treatment of acute GPP (28), and patients treated with biologics have demonstrated positive responses. Our patient declined biologics due to the medical expense and relatively weak literature support for patients with stage 4 CKD (29). Granulocyte and monocyte adsorption (GMA) apheresis is also an effective treatment for acute GPP, and it can remove activated granulocytes and monocytes from the patient’s circulation (30). Nevertheless, this therapy relies on an arteriovenous fistula and may lead to a poor dialysis outcome.
Effective and safe therapy was needed in this case. Tacrolimus is a calcineurin inhibitor that prevents calcineurin from dephosphorylating the nuclear factor of activated T cells (31). It results in the blockage of signal transduction pathways in T cells and the inhibition of the synthesis of inflammatory cytokines, such as INF, IL-2, and IL-3 (32). This medicine has been shown to produce successful outcomes in patients with plaque psoriasis with no severe adverse events (33). The average psoriasis area and severity index (PASI) of participants with oral tacrolimus was reduced by 70% (p < 0.05) at the end of the 9-week treatment period in a placebo-controlled trial (34). The average PASI had improved by 80.37% (p < 0.001) in an open-label prospective study investigating the efficacy and safety of oral tacrolimus treatment for 12 weeks in adults with severe recalcitrant psoriasis (35). However, acute GPP differs from plaque psoriasis in phenotype, genetics, and immunity (36). Fortunately, oral tacrolimus proved to be effective for this patient, and adverse events were not observed. The most frequently reported adverse reactions of tacrolimus are insomnia, tremors, headache, paraesthesia, myalgia, pruritus, and gastrointestinal effects, while significant adverse effects include infection, hypertension, hyperglycemia, hyperkalemia, nephrotoxicity, and neurotoxicity (33). The toxic effects of tacrolimus are not significant due to the low doses and short treatment duration. Furthermore, studies have shown that tacrolimus is a better option than cyclosporine for patients who have comorbidities that include cardiovascular diseases, renal disease, and metabolic syndrome (37, 38). The psoriasis lesions, GFR, creatinine, and D-dimers of this patient improved over time as those diseases shared immune pathways and cytokines. This study supports the results of prior studies on the treatment of renal diseases with tacrolimus, which can induce remissions of creatinine, uric acid, glomerular filtration rate, and proteinuria (39, 40).
3 Conclusions
Oral tacrolimus is an effective and relatively safe treatment option for acute GPP patients, especially refractory cases with multiple comorbidities. It can improve systemic inflammation and does not increase cardiovascular burden. This treatment could be a well-tolerated alternative for refractory psoriasis. However, the long-term efficacy and adverse reactions of this therapy are currently unclear. Further studies are necessary to maximize the efficacy and minimize the toxicity of oral tacrolimus.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the ethics committee of Chongqing Hospital of Traditional Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MDZ: Resources, Writing – original draft. LT: Writing – original draft, Investigation. XZ: Investigation, Writing – original draft. FH: Methodology, Writing – review & editing. MZ: Validation, Writing – original draft. ML: Writing – original draft, Supervision. LL: Data curation, Writing – review & editing. MH: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by grants from the Chongqing Talent Plan Project of Chongqing Municipal Health Commission (Grant NO.CQYC20200303138) and the Top Young Talent of Chongqing Hospital of Traditional Chinese Medicine.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Choon SE, Navarini AA, Pinter A. Clinical course and characteristics of generalized pustular psoriasis. Am J Clin Dermatol. (2022) 23:21–9. doi: 10.1007/s40257-021-00654-z
2. Romiti R, Hirayama ALDS, Arnone M, Magalhães RF. Generalized pustular psoriasis (von Zumbusch). Bras Dermatol. (2022) 97:63–74. doi: 10.1016/j.abd.2021.05.011
3. Xue G, Lili M, Yimiao F, Miao W, Xiaohong Y, Dongmei W. Case report: Successful treatment of acute generalized pustular psoriasis of puerperium with secukinumab. Front Med (Lausanne). (2022) 9:1072039. doi: 10.3389/fmed.2022.1072039
4. Hoegler KM, John AM, Handler MZ, Schwartz RA. Generalized pustular psoriasis: a review and update on treatment. J Eur Acad Dermatol Venereo. (2018) 32:1645–51. doi: 10.1111/jdv.14949
6. Bachelez H, Choon SE, Marrakchi S, Burden AD, Tsai TF, Morita A, et al. Effisayil 1 trial investigators. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. (2021) 385:2431–40. doi: 10.1056/NEJMoa2111563
7. Iizuka H, Takahashi H, Ishida-Yamamoto A. Pathophysiology of generalized pustular psoriasis. Arch Dermatol Res. (2003) 295 Suppl 1:S55–9. doi: 10.1007/s00403-002-0372-5
8. Javor J, Buc M, Bucová M. Autoinflammatory process in the pathogenesis of generalized pustular psoriasis and perspectives of its targeted therapy. Epidemiol Mikrobiol Imunol. (2021) 70:199–207.
9. Benjegerdes KE, Hyde K, Kivelevitch D, Mansouri B. Pustular psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckl). (2016) 6:131–44. doi: 10.2147/PTT.S98954
10. Arakawa A, Vollmer S, Besgen P, Galinski A, Summer B, Kawakami Y, et al. Unopposed IL-36 activity promotes clonal CD4+ T-cell responses with IL-17A production in generalized pustular psoriasis. J Invest Dermatol. (2018) 138:1338–47. doi: 10.1016/j.jid.2017.12.024
11. Masson W, Lobo M, Molinero G. Psoriasis and cardiovascular risk: A comprehensive review. Adv Ther. (2020) 37:2017–33. doi: 10.1007/s12325-020-01346-6
12. Bu J, Ding R, Zhou L, Chen X, Shen E. Epidemiology of psoriasis and comorbid diseases: A narrative review. Front Immunol. (2022) 13:880201. doi: 10.3389/fimmu.2022.880201
13. Ohata C, Tsuruta N, Yonekura K, Higashi Y, Saito K, Katayama E, et al. Clinical characteristics of Japanese pustular psoriasis: A multicenter observational study. J Dermatol. (2022) 49:142–50. doi: 10.1111/1346-8138.16217
14. David M, Abraham D, Weinberger A, Feuerman EJ. Generalised pustular psoriasis, psoriatic arthritis and nephrotic syndrome associated with systemic amyloidosis. Dermatologica. (1982) 165:168–71. doi: 10.1159/000249936
15. Klimko A, Toma GA, Ion L, Mehedinti AM, Andreiana I. A case report of generalized pustular psoriasis associated with igA nephropathy. Cureus. (2020) 12:e10090. doi: 10.7759/cureus.10090
16. Li SP, Tang WY, Lam WY, Wong SN. Renal failure and cholestatic jaundice as unusual complications of childhood pustular psoriasis. Br J Dermatol. (2000) 143:1292–6. doi: 10.1046/j.1365-2133.2000.03904.x
17. Damasiewicz-Bodzek A, Wielkoszyński T. Advanced protein glycation in psoriasis. J Eur Acad Dermatol Venereol. (2012) 26:172–9. doi: 10.1111/j.1468-3083.2011.04024.x
18. Berger PA. Amyloidosis–a complication of pustular psoriasis. Br Med J. (1969) 2:351–3. doi: 10.1136/bmj.2.5653.351
19. Ungprasert P, Raksasuk S. Psoriasis and risk of incident chronic kidney disease and end-stage renal disease: a systematic review and meta-analysis. Int Urol Nephrol. (2018) 50:1277–83. doi: 10.1007/s11255-018-1868-z
20. Kluger N, Bessis D, Guillot B, Girard C. Acute respiratory distress syndrome complicating generalized pustular psoriasis (psoriasis-associated aseptic pneumonitis). J Am Acad Dermatol. (2011) 64:1154–8. doi: 10.1016/j.jaad.2009.11.022
21. Ohn J, Choi YG, Yun J, Jo SJ. Identifying patients with deteriorating generalized pustular psoriasis: Development of a prediction model. J Dermato. (2022) 49:675–81. doi: 10.1111/1346-8138.16383
22. Neurath MF. IL-36 in chronic inflammation and cancer. Cytokine Growth Factor Rev. (2020) 55:70–9. doi: 10.1016/j.cytogfr.2020.06.006
23. Suárez-Fueyo A, Bradley SJ, Klatzmann D, Tsokos GC. T cells and autoimmune kidney disease. Nat Rev Nephrol. (2017) 13:329–43. doi: 10.1038/nrneph.2017.34
24. Gisterå A, Hansson GK. The immunology of atherosclerosis. Nat Rev Nephrol. (2017) 13:368–80. doi: 10.1038/nrneph.2017.51
25. Mi LL, Guo WW. Crosstalk between ILC2s and th2 CD4+ T cells in lung disease. J Immunol Res. (2022) 10:2022. doi: 10.1155/2022/8871037
26. Rodríguez García F, Fagundo González E, Cabrera-Paz R, Rodriguez Martín M, Sáez Rodríguez M, Martín-Neda F, et al. Generalized pustular psoriasis successfully treated with topical tacrolimus. Br J Dermatol. (2005) 152:587–8. doi: 10.1111/j.1365-2133.2005.06468.x
27. Tanikawa A. Amagai M. A Case Generalized Pustular Psoriasis Treated Topical Tacrolimus Arch Dermatol. (2003) 139:1219. doi: 10.1001/archderm.139.9.1219-a
28. Wang WM, Jin HZ. Biologics in the treatment of pustular psoriasis. Expert Opin Drug Saf. (2020) 19:969–80. doi: 10.1080/14740338.2020.1785427
29. Maghfour J, Elliott E, Gill F, Stumpf B, Murina A. Effect of biologic drugs on renal function in psoriasis patients with chronic kidney disease. J Am Acad Dermatol. (2020) 82:1249–51. doi: 10.1016/j.jaad.2019.12.043
30. Mabuchi T, Manabe Y, Yamaoka H, Ota T, Kato M, Ikoma N, et al. Case of generalized pustular psoriasis with end-stage renal disease successfully treated with granulocyte monocyte apheresis in combination with hemodialysis. J Dermatol. (2014) 41:521–4. doi: 10.1111/1346-8138.12501
31. Reynolds NJ, Al-Daraji WI. Calcineurin inhibitors and sirolimus: mechanisms of action and applications in dermatology. Clin Exp Dermatol. (2002) 27:555–61. doi: 10.1046/j.1365-2230.2002.01148.x
32. Taylor DO. Cardiac transplantation: drug regimens for the 21st century. Ann Thorac Surg. (2003) 75:S72–8. doi: 10.1016/S0003-4975(03)00482-X
33. Malecic N, Young H. Tacrolimus for the management of psoriasis: clinical utility and place in therapy. Psoriasis (Auckl). (2016) 7:153–63. doi: 10.2147/PTT.S101233
34. The European FK 506 Multicentre Psoriasis Study Group. Systemic tacrolimus (FK 506) is effective for the treatment of psoriasis in a double-blind, placebo-controlled study. Arch Dermatol. (1996) 132:419–23. doi: 10.1001/archderm.132.4.419
35. Mittal A, Dogra S, Narang T, Sharma A. Pilot study to evaluate the efficacy and safety of oral tacrolimus in adult patients with refractory severe plaque psoriasis. J Cutan Med Surg. (2016) 20:228–32. doi: 10.1177/1203475415616964
36. Gooderham MJ, Van Voorhees AS, Lebwohl MG. An update on generalized pustular psoriasis. Expert Rev Clin Immunol. (2019) 15:907–19. doi: 10.1080/1744666X.2019.1648209
37. Artz MA, Boots JM, Ligtenberg G, Roodnat JI, Christiaans MH, Vos PF, et al. Conversion from cyclosporine to tacrolimus improves quality-of-life indices, renal graft function and cardiovascular risk profile. Am J Transplant. (2004) 4:937–45. doi: 10.1111/j.1600-6143.2004.00427.x
38. Xue W, Zhang Q, Xu Y, Wang W, Zhang X, Hu X. Effects of tacrolimus and cyclosporine treatment on metabolic syndrome and cardiovascular risk factors after renal transplantation: a meta-analysis. Chin Med J (Engl). (2014) 127:2376–81. doi: 10.3760/cma.j.issn.0366-6999.20140518
39. Zhao L, Yang Y, Xu H, Leng W, Xu G. Efficacy and safety of tacrolimus-based treatment for non-rapidly progressive IgA nephropathy. Front Pharmacol. (2023) 14:1189608. doi: 10.3389/fphar.2023.1189608
40. Kostopoulou M, Fanouriakis A, Cheema K, Boletis J, Bertsias G, Jayne D, et al. Management of lupus nephritis: a systematic literature review informing the 2019 update of the joint EULAR and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations. RMD Open. (2020) 6:e001263. doi: 10.1136/rmdopen-2020-001263
Keywords: case report, treatment, acute generalized pustular psoriasis, oral, tacrolimus
Citation: Zhao M, Huang F, Tang L, Zhou X, Zhang M, Liao M, Liu L and Huang M (2024) Case report: Successful treatment of acute generalized pustular psoriasis with multiple comorbidities with oral tacrolimus. Front. Immunol. 15:1354578. doi: 10.3389/fimmu.2024.1354578
Received: 12 December 2023; Accepted: 28 February 2024;
Published: 19 March 2024.
Edited by:
Ronald Vender, McMaster University, CanadaReviewed by:
Nahide Onsun, Bezmialem Vakıf University, TürkiyeKaterina Laskari, National and Kapodistrian University of Athens, Greece
Copyright © 2024 Zhao, Huang, Tang, Zhou, Zhang, Liao, Liu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mengya Huang, aHVtZXlhQHNpbmEuY29t
 Mingdan Zhao1
Mingdan Zhao1 Fujun Huang
Fujun Huang Xun Zhou
Xun Zhou Mengya Huang
Mengya Huang