- Department of General Surgery, The Second Hospital of Jilin University, Jilin University, Changchun, Jilin, China
Intestinal inflammatory imbalance and immune dysfunction may lead to a spectrum of intestinal diseases, such as inflammatory bowel disease (IBD) and gastrointestinal tumors. As the king of herbs, ginseng has exerted a wide range of pharmacological effects in various diseases. Especially, it has been shown that ginseng and ginsenosides have strong immunomodulatory and anti-inflammatory abilities in intestinal system. In this review, we summarized how ginseng and various extracts influence intestinal inflammation and immune function, including regulating the immune balance, modulating the expression of inflammatory mediators and cytokines, promoting intestinal mucosal wound healing, preventing colitis-associated colorectal cancer, recovering gut microbiota and metabolism imbalance, alleviating antibiotic-induced diarrhea, and relieving the symptoms of irritable bowel syndrome. In addition, the specific experimental methods and key control mechanisms are also briefly described.
1 Introduction
The dysregulation of the intestinal inflammations system and adaptive immune imbalance can result in a series of intestinal diseases and diseases in distant body sites, such as inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), intestinal infectious diseases, intestinal system tumor, and neurological disease (1, 2). Among them, IBD is the most prevalent disease, the incidence of which is significantly increasing and continues to rise during the twentieth century (3). Despite multiple anti-inflammation and immunomodulating agents have been used for intestinal system diseases treatment, limited efficacy and serious side effects remain major clinical challenges. With more and more immunomodulatory and anti-inflammatory pharmacological effects having been developed, traditional Chinese medicines have been used for various inflammation and immune disorders treatment. As one of the most well-known traditional Chinese medicines, ginseng has a long history as an herbal medicine for various disease (4), especially, which possessed stronger anti-inflammatory and immunoregulation effects. In the past decades, an increasing number of studies have indicated that ginseng and its major constituent, various ginsenosides, were capable of effectively relieving the severity of gastrointestinal colitis in animal models (5). In this review, we mainly focus on how ginseng and ginsenoside regulate intestinal system inflammation and the immune homeostasis.
2 Intestinal inflammations
Under normal physiological conditions, inflammation response is a self-resolving and self-protective process, through which the body attempts to counteract tissue injury damage or infection and together remove pathogens and cell debris (6). However, continuous inflammation response is harmful to the host. Therefore, once the harmful stimuli has been eliminated, the related proinflammatory response should be curtailed immediately (7). Intestinal system inflammations represent a group of relapsing and multifactorial disorder associated with physical and mental well-being, mainly including intestinal tumor and inflammatory bowel disease (IBD) such as ulcerative colitis (UC), irritable bowel disease (IBS), and Crohn’s disease (CD). According to the latest epidemiological data from the World Health Organization, IBD has become a global burden, and the incidence are rising globally, especially in newly industrialized regions of North America and Europe (8–10).
To date, multiple factors have been verified to be involved in the regulation of IBD, such as gut microbiota, gut mucosal inflammation, cell immune response, oxidative stress, and eating habits (11). The normal intestinal epithelial barrier is composed of the mucosa, the glycocalyx, and tight junctions, all of which are involved in maintaining gut barrier integrity and intestinal homeostasis (12). The intestinal tight junction proteins form a physical barrier and the commensal microbiome can further reinforce intestinal barrier homeostasis through preserving gastrointestinal physiology and intestinal immune system development (13, 14). The dysfunction of IBD is primarily associated with intestinal mucosal inflammation. Inflammation responses are a complex process involving a series of cellular and molecular changes, including altering vascular permeability, promoting aggregation of leukocyte, and regulating expression levels of inflammatory cytokines (15). Cytokines play a crucial role in the pathogenesis of IBD. In terms of UC and CD, it was found that the proinflammatory cytokines such as IL-1, TNF-α, IL-1β, and IL-6 were significantly upregulated in the inflamed intestine, thus further changing the composition of the tight junction microenvironment (16). In addition, gut microbiome composition and mucosal immune responses both play an important role in intestinal barrier homeostasis (17). A recent study by Khan and co-workers suggested that dysregulated gut microbiota was able to specifically activate T-helper cell immune response, which can result in further dysregulation of intestinal barrier function and a sustained inflammatory response (18).
3 Intestinal immune
The immune system plays an important role in the human body against foreign bacteria and virus infections. The human immune system consists of primary and secondary lymphoid systems (19). Mucosal-associated lymphoid tissue is one of the most typical secondary lymphoid systems, which works as a physical and immunological barrier (20). As the largest mucosal-associated lymphoid tissue, gut-associated lymphoid tissue is very essential for the maintenance of intestinal homeostasis, the components of which mainly include Peyer’s patches and mesenteric lymph nodes (21). Intestinal immune dysfunction also has been reckoned as a major pathogenesis of IBD (22). As the primary site of gut microbiota colonization, more than trillions of microorganisms have been discovered on the surfaces of the ileum and colon, mainly including fungi, protozoa, viruses, archaea, and predominantly bacteria (23). It has been reported that gut microbiota was capable of regulating the innate immune system and intestinal immune homeostasis (24). For example, Clostridium difficile is capable of promoting goblet cells and dendritic cells secreting cytokines (TGF-β and IL-10), thus further generating ample signals to upregulate Treg population (25). In addition, some important components such as vitamin K and SCFA are produced by intestinal microbiota, both of which play an important role in preventing intestinal disease (26). An increasing number of clinical studies have proposed that the composition and the metabolites of gut microbiota were altered in IBD patients (27–29). In addition to gut microbiota, intestinal immune cells also contribute to the intestinal immune responses, especially in IBD. The intestinal immune cells include innate immune cells and adaptive immune cells, which supplement each other and eliminate invading pathogens (30). Moreover, inflammation response is closely related with the immune system. More in detail, the initiation of inflammation is frequently accompanied by the activation of the immune response, which can further activate inflammatory cascade via activating pattern recognition receptors and damage-associated molecular patterns (31, 32). For example, the pathological mechanism of ulcerative proctitis involved multiple factors, including genetic, immune function, and inflammatory responses (33, 34). The pathogenesis of IBDs is complex and involves immune-inflammatory mechanisms. Ginseng has been seen as an important immunomodulator. Specifically, it was found that long-term treatment of ginseng soluble dietary fiber could regulate the secretion levels of immunoglobulins and affect B-cell proliferation, thus further restoring intestinal homeostasis (35).
4 Ginseng and ginsenosides
Ginseng, the root of Panax ginseng, is one of the most frequently used traditional herbal medicine (4). To date, multiple active ingredients have been isolated from ginseng, mainly including ginsenoside, ginseng polysaccharide, and ginseng polypeptide. Among them, ginsenoside is the most abundant and the most studied. The three most common ginseng types included American ginseng, Asian ginseng, and Panax notoginseng. We have described their global distribution in Figure 1. The content and composition of ginsenosides are varied in different ginseng species. American ginseng is originally grown in the mountain forests of America and Canada and recently has been cultivated in northern China. The major bioactive ginsenosides of American ginseng are F11, Rb1, Re, Rd, Rc, Rg1, and Rb3. Asian ginseng is mainly distributed in Chinese or Korean. The major ginsenosides of Asian ginseng include Rf, Rb1, Rb2, Rc, Rd, Re, and Rg1. Panax notoginseng, belonging to the Araliaceae family, is a traditional Chinese medicine, which has been widely used for various diseases, especially cardiovascular diseases. The major ginsenosides of P. notoginseng include Rg1, Rb1, Rd, and notoginsenoside R1 (36).
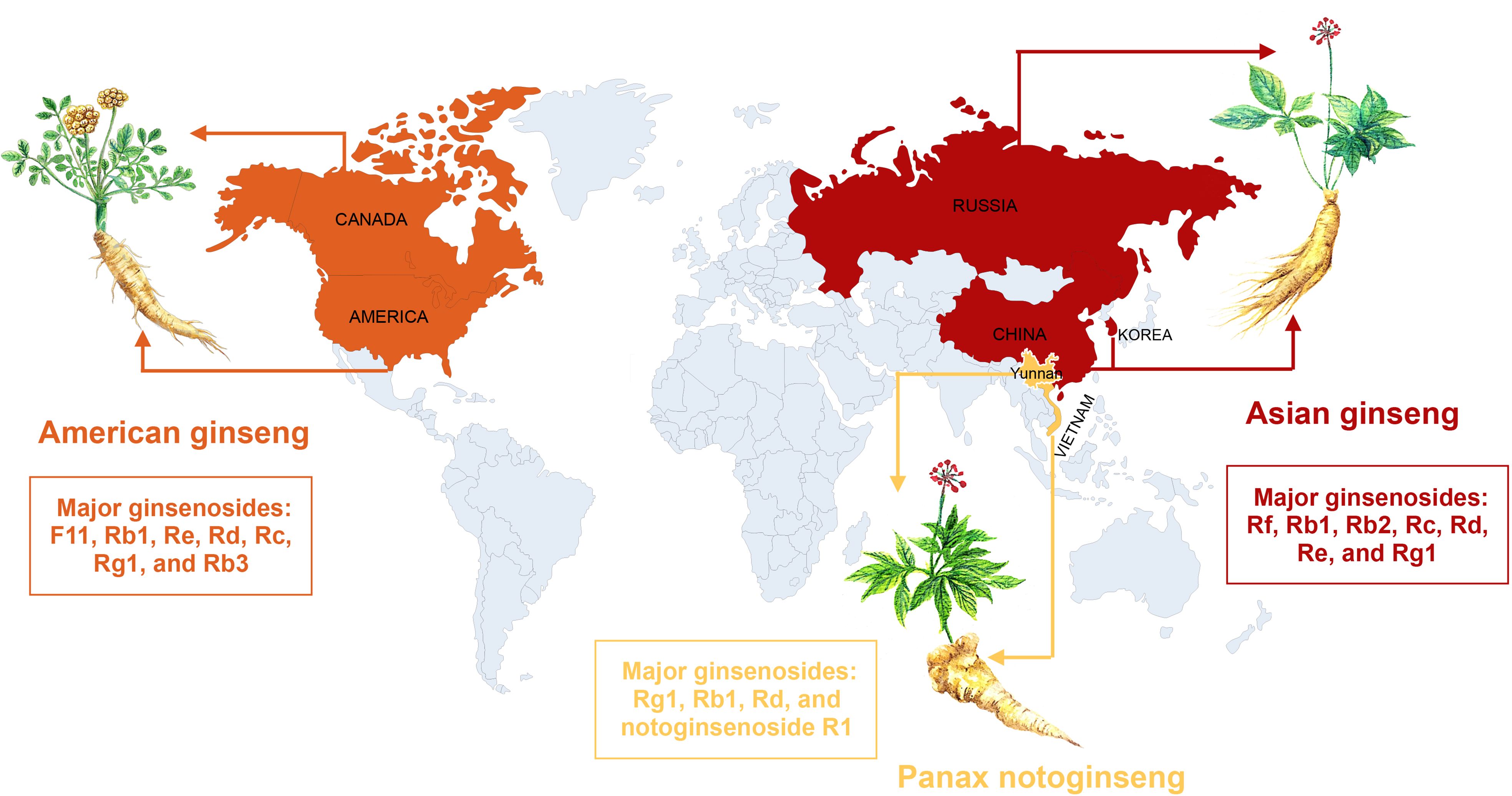
Figure 1 The global distribution of the three most common ginseng herbs, with their major ginsenosides.
Ginsenosides are the major bio-active component of various ginseng and could be recovered from plant roots, stems, leaves, and flowers. With the recent developments in the extraction and synthetic process, more than 300 ginsenoside monomers have been discovered, such as ginsenoside Rg1, ginsenoside Rb1, ginsenoside Rd, ginsenoside Rf, ginsenoside Re, ginsenoside Rg5, ginsenoside Ra3, and ginsenoside Rd (37). All of them have been confirmed to possess multiple pharmacological effects, including anti-inflammatory (38), anti-aging (39), antioxidant (40), anti-cancer (41), and immuno-modulatory effects (40). On the basis of their chemical structures, ginsenosides can be classed into three types: dammarane, oleanane, and oleanolic acid types. Among them, dammarane can be further divided into protopanaxadiol (PPD), protopanaxatriol (PPT), and ocotillol (OCT) types (42). PPD-type ginsenosides mainly include Rb1, Rb2, Rb3, Rc, Rd, Rg3, and Rh2. PPT-type ginsenosides mainly include Re, Rf, Rg1, Rg2, and Rh1. Previous studies have evaluated that ginsenosides were associated with inflammasome and immune responses (43, 44). For example, it has been demonstrated that ginsenoside Rh1, Rg3, Rg5, Rb1, compound K, and Rg1 were capable of inhabiting inflammatory responses by blocking NLRP3 and NLRP1 (45). Another study further proposed that ginsenosides could enhance the cellular immune function (46). For instance, ginsenoside Rg1 was capable of promoting the proliferation of lymphocytes (47). The functional funding of ginsenosides on inflammasome and immune response provides new insight into the understanding of the molecular mechanisms of ginsenoside-mediated inflammatory and immune actions. More and more pharmacological effects of ginseng and ginsenosides are characterized, especially exhibiting stronger anti-inflammatory and immunomodulatory effects, which have been reckoned as a promising drug for intestinal diseases with great clinical translational potential.
5 Effect of ginseng and ginsenosides on intestinal system inflammations
Inflammatory bowel disease is increasingly prevalent in recent years, which greatly affect the gastrointestinal tract function (48). Clinically, despite that a variety of drugs have been used for IBD administration, such as cyclosporin, mesalamine, mercaptopurine, and azathioprine, however, serious side effect and expensive medication cost have greatly limited their clinical application (49). A large number of experiments have shown that ginseng and ginsenosides were capable of effectively relieving the symptoms of IBD through multiple regulatory mechanisms, including regulating the balance of immune cells (50), mediating cytokine expression (51), restoring pathological damage (52), regulating inflammatory signaling pathway (53), and promoting the proliferation of intestinal mucosal epithelium (52). Moreover, in terms of ulcerative colitis (UC) patients, a recent randomized clinical study shows that the rectal co-administration of P. notoginseng and Colla Corii Asini suppositories could effectively alleviate their clinical symptom scores and inflammatory factors and improve colon immune function (54). Next, we will discuss all aspects of how ginseng and ginsenosides alleviate IBD in greater detail.
5.1 Regulating inflammatory mediators and cytokines
A growing number of studies have demonstrated that ginseng and its major pharmacologically active components ginsenosides possessed stronger anti-inflammatory effects (55, 56). As shown in Figure 2, ginseng and ginsenosides mainly regulate intestinal inflammatory through influencing proinflammatory cytokines expression levels and regulating inflammation-related pathways. Ullah et al. reported that Rg3-enriched Korean Red Ginseng extract could alleviate oxazolone (OXA)-induced UC through suppressing the expression level of NLRP3 and NF-κB (57). Li et al. proposed that ginseng polysaccharides can be used as a promising intervention agent for the prevention of colitis. In a (DSS)-induced rat colitis model, they found that ginseng polysaccharides could effectively alleviate symptoms of colitis and recover the intestinal barrier through downregulating colon inflammatory cytokine levels such as IL-1β, IL-2, IL-6, and IL-17, and blocking the TLR4/MyD88/NF-κB-signaling pathway (58).
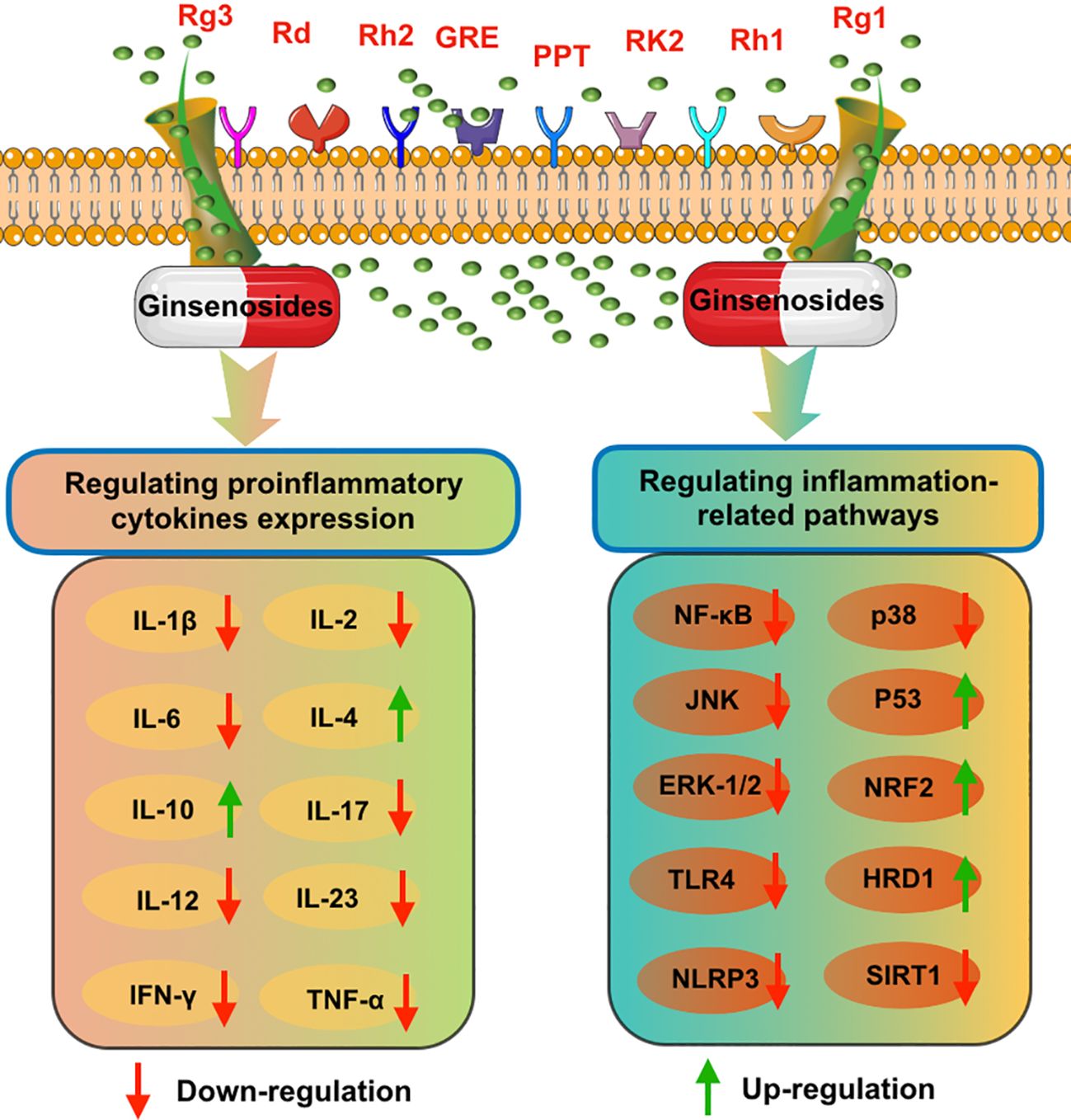
Figure 2 The possible anti-inflammatory mechanisms of various ginsenosides on intestinal system diseases, including inhibiting proinflammatory cytokines expression and blocking inflammation-related pathways.
In addition, to investigate the protective mechanism of ginsenoside Rd on IBD, 2,4,6-trinitrobenzenesulfonic acid (TNBS) induced colitis rat models were orally administered with ginsenoside Rd for 7 days. The results demonstrated that the inflammatory response was significantly attenuated through downregulating expression levels of proinflammatory cytokines (TNF-α, IL-1β, and IL-6) and blocking the activity of p38 and JNK (59). Moreover, another study also proposed that ginsenosides Rd was able to effectively alleviate DSS colitis in mice through inhibiting proinflammatory cytokines expression (TNF-α, IFN-γ, IL-6, IL-12/23p40, and IL-17A) and inhibiting NF-κB and P38MAPK signaling pathways (60). Ginseng root extract (GRE) also exerted stronger anti-inflammatory and anti-oxidative effects in DSS-induced colitis, which could remarkably inhibit expression levels of inflammatory factors (TNF-α, IL-6, and IL-1β), blocking NF-κB and p62-Nrf2-Keap1 pathways activity, and suppressing the phosphorylation of MAPKs (JNK, ERK-1/2, and p38) (61). Panaxadiol could alleviate DSS induced acute mouse model colitis through suppressing IL-1β secretion and blocking non-canonical caspase-8 inflammasome and MAPKs (62). Huang et al. proposed that ginsenoside Rk2 may be an effective agent in the treatment of UC. They found that ginsenoside Rk2 treatment could block the secretion of proinflammatory cytokines (IL-1β, IL-6, IL-10, and TNF-α) and inactivate ERK/MEK signaling through promoting the dephosphorylation of ERK/MEK and upregulating SIRT1 pathway (63).
In an obesity-induced colonic inflammation-stimulated colitis, in was found that ginsenoside Rk3 could effectively repair the injuries of intestinal epithelial barrier through upregulating the secretion of multiple tight junction proteins and suppressing the expression levels of inflammatory cytokine (TNF-α, IL-1β, and IL-6) and oxidative stress cytokine through blocking the TLR4/NF-κB signaling pathway (64).
Ginsenoside Rb1 (GRb1), one major ginsenoside with multiple pharmacological properties, was capable of effectively alleviating colitis symptoms such as endoplasmic reticulum stress response and fas-related apoptosis through inhibiting inflammatory responses and activating Hrd1 signaling pathway (53). Rh2 has been verified to possess stronger anti-inflammatory and anticancer effects. Based on these characteristics, a recent study found that Rh2 could markedly alleviate various symptoms of (DSS)-induced colitis, mainly including body weight loss, disrupted intestinal barrier functions, colon length shortening, and disease activity index (DAI) scores (65). Luo et al. analyzed the anti-inflammatory and protective effects of P. notoginseng saponins (PNS) in IBD both in vitro and in vivo (DSS-induced colitis mouse model) (66). They found that PNS administration was capable of deregulating secretion levels of proinflammatory cytokines (TNF-α, IL-6, and MCP-1) and blocking the activity of MAPK and NF-κB signaling pathway. Similarly, another study also found that P. notoginseng saponins (PNS) could significantly alleviate (DSS)-induced intestinal inflammatory and oxidative stress reactions through upregulating apoptotic cell numbers and blocking PI3K/AKT signaling pathway (67). Moreover, Wang et al. also proposed that P. notoginseng could significantly attenuate (DSS-) or iodoacetamide (IA)-induced rat colitis through downregulating serum concentrations of VEGFA isoforms IL-6, and TNF-α, while together upregulating IL-4 and IL-10 (68). In addition, as one main bioactive constituent of P. notoginseng, Notoginsenoside R1 also been reported to possess stronger protective effect on IBD, which could effectively relieve the severity of DSS-induced colitis in mice models through suppressing the secretion levels of cytokine and related proinflammatory genes expression (69).
Saba et al. proposed that the co-treatment of red ginseng extract enriched with Rg3 (Rg3-RGE) and Persicaria tinctoria could be used for the prevention UC induced by DSS both in vitro and in vivo (70). Specifically, in an in vitro study, it could inhibit inflammation responses of RAW 264.7 cells through promoting protein kinase and NF-κB pathways. In C57BL/6 mice, this mixture could effectively relieve colitis-related symptoms through exhibiting strong anti-inflammatory effects and suppressing expression levels of NLRP3 inflammasome. In addition, they also proposed that the combination of red ginseng extracts and Epimedium koreanum Nakai could alleviate DSS-induced colitis through suppressing protein expression level of proinflammatory cytokines and blocking NF-κB and MAPK pathways (71). Lee et al. found that non-saponin fraction of Korean Red Ginseng possessed stronger intestinal protective effects in a DSS-induced colitis rat model, which could markedly ameliorate gastrointestinal inflammation through suppressing MPO activity, upregulating COX-1 protein expression level, and restoring ZO-1 and occludin secretion to normal levels (72). As one of most commonly used species of ginseng, American ginseng has been discovered to possess multiple protective functions (73). Jin et al. first reported that American ginseng could prevent and treat mouse colitis through suppressing leukocyte activation and subsequent epithelial cell DNA damage (74), promoting inflammatory cell apoptosis, and regulating the activation of P53 (75). Cui et al. found that American ginseng could treat colitis and prevent colon cancer through suppressing the expression levels of ROS and primary proinflammatory markers (76). In addition, their further study proposed that AG and its components also could activate nuclear factor erythroid-2-related factor 2 (Nrf2) pathway, which is involved in colitis progress and closely related with CRC development (77). Consistent with the above results, another study also found that American ginseng could effectively relieve AOM/DSS-induced colon inflammation and suppress tumorigenesis in mouse model through restoring intestinal microbiota function (78).
As an efficient anti-inflammatory agent, Zhu et al. first reported that ginsenoside Rg1 was capable of protecting against DSS-induced mouse colitis through markedly downregulating proinflammatory cytokines secretion (IL-1β and TNF-α) (79). A recent study further discovered that ginsenoside Rg1 could be transformed into 20(S)-protopanaxtriol via ginsenosides Rh1 and F1 through interacting with gut microbiota. Lee et al. found that ginsenosides Rg1, and its major metabolites (Rh1 and 20(S)-protopanaxtriol), all could ameliorate 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis through suppressing the secretion levels of inflammatory factors (IL-1β, IL-17, and TNF-α), restoring Th17/Treg imbalance, and blocking the binding of LPS to TLR4 on macrophages (80). Another study found that oral administration of probiotic-fermented red ginseng could significantly relieve the symptoms of colitis in (DSS)-induced mouse mode through downregulating the serum levels of inflammatory factors (IL-6 and TNF-α) (81). In addition, one study also found that fermented wild ginseng could relieve the symptoms of colitis in a DSS-induced colitis animal model through inhabiting secretion level of proinflammatory cytokines (IL-1β, IL-6, IL-12, p40, TNF-α, and IFN-γ) and blocking NF-κB signaling pathway (82).
5.2 Promoting intestinal mucosal wound healing
In a trinitrobenzenesulfonic-acid-induced rat colitis model, Toyokawa et al. proposed that Daikenchuto and its constituent, ginsenoside Rb1, could remarkably promote intestinal mucosal damage by regulating extracellular-signal-regulated kinase and Rho signaling pathway (83). In addition, another study also demonstrated that P. notoginseng administration could promote repair of colonic mucosal injury and microvessels in a (DSS-) or iodoacetamide (IA)-induced rat colitis models through blocking VEGFA isoforms and Rap1GAP/TSP1 pathway (68). Consistent with the above results, Wang et al. also proposed that P. notoginseng could repair vascular injury in DSS- and IA-induced colitis animal model through alleviating inflammation responses and oxidative stress (84).
5.3 Regulating gut microbiota and metabolism
Intestinal dysbacteriosis has been reckoned as one of the most fundamental factors leading to intestinal diseases, such as IBD, IBS, and CRC (85, 86). Changes in intestinal flora diversity and composition is capable of resulting in imbalances of immune tolerance and dysregulation of intestinal barrier function and upregulating proinflammatory cytokines expression and increasing the incidence of erosion and ulcer (87). Previous studies have found that probiotics supplementation could restore the structure of gut microbiota, thus enhancing intestinal mucosal barrier function and reducing gastrointestinal infection (88). Recent studies further proposed that restoring unbalanced gut microbiota was able to prevent the progression of IBD and intestinal cancer (89). Ginseng and ginsenosides are closely related to the role of gut microbiota, and various non-widespread initial ginsenosides have to be processed and transformed by gut microbiota before they get good biological activity (90, 91). For example, it was found that ginseng polysaccharides could alleviate (DSS)-induced colitis through restoring unbalanced gut microbiota, including increasing the relative abundance of probiotics and, at the same time, imbibing the abundance of pathogenic bacteria (92).
Li et al. also proposed that ginseng polysaccharides exerted a protective effect against (DSS)-induced rat colitis through restoring the diversity and composition of gut microbiota, such as effectively upregulating the relative abundance of Ruminococcus, which also demonstrated that Ruminococcus might be involved in the progression of colitis occurrence (58). The combination treatment of Zingiber officinale and P. ginseng could ameliorate DSS ulcerative colitis via regulating the abundance of gut microbiome, including upregulating beneficial bacteria such as Muribaculaceae_norank, Lachnospiraceae NK4A136 group, and Akkermansia, and downregulating pathogenic bacteria such as Bacteroides, Parabacteroides, and Desulfovibrio (93). Another study also found that synergistic administration of American ginseng polysaccharide and American ginseng ginsenoside could improve gut microbiota diversity and restore gut microbiota composition, including upregulating the relative abundance of probiotics (Clostridiales, Bifidobacterium, and Lachnospiraceae), while together downregulating harmful bacteria (Escherichia-Shigella and Peptococcaceae) (94).
In a high-fat diet-induced colitis mice model, it was found that Rk3 could reduce chronic-obesity-induced colitis through alleviating metabolic dysbiosis of gut microbiota and significantly suppressing the ratios of Firmicute/Bacteroidete (64). Prior research has shown that red ginseng could improve the functions of gastrointestinal tract (95). A recent study further proposed that red ginseng could be reckoned as a promising agent for the ulcerative colitis treatment. They found that red ginseng administration significantly relieved the symptoms of trinitro-benzene-sulfonic acid induced ulcerative colitis in a rat model through improving the structure of gut microbiota, including increasing the abundance of probiotics (Bifidobacterium and Lactobacillus) while inhibiting the growth of some pathogen strains (96).
5.4 Preventing colitis-associated colorectal cancer
In addition to ameliorating various symptoms of IBD, some researchers also found that ginseng and ginsenosides also can be used for the prevention of the progression of colitis-associated CRC progression. For example, Wang et al. proposed that oral administration of American ginseng could attenuate AOM/DSS-induced colitis and associated CRC carcinogenesis through downregulating inflammatory factors secretion and restoring the metabolomics and intestinal homeostasis (97). Similarly, Yu et al. also proposed that American ginseng administration was capable of preventing azoxymethane/DSS-induced CRC carcinogenesis through downregulating inflammatory cytokine gene expression (IL-1α, IL-1β, IL-6, IFN-γ, G-CSF, and GM-CSF) (98). Consistent with above results, Poudyal et al. reported that American ginseng possessed stronger anti-inflammatory properties and could prevent azoxymethane/DSS-induced CRC carcinogenesis (99).
In addition, Chen et al. proposed that P. notoginseng saponins (PNS) could effectively suppress the progression and development of AOM/DSS-induced colon tumor through regulating the abundance and diversity of gut microbiota, especially obviously increasing the abundance of Akkermansia spp., which was negatively associated with the development of CRC (100). It should be noted that P. notoginseng saponins (PNS) can be bio-transformed to ginsenoside compound K (GCK) by gut microbiota. Another study found that ginsenoside compound K (GCK) also could inhibit the progression of AOM/DSS-induced colitis-associated CRC by upregulating the relative abundance of A. muciniphila (101).
5.5 Alleviate antibiotic-induced diarrhea
Qu et al. proposed that fermented ginseng could alleviate antibiotic-induced diarrhea and colon inflammation through regulating inflammation-related factors such as TNF-α, IL-1β, IL-6, and IL-10 (102). In addition, this study also found that the intestinal flora changes were associated with immune-related factors expression, and the fermented ginseng treatment could recover the alterations of gut microbiota. Similar results were reported by Qu and co-workers. They found that fermented ginseng was able to relieve the symptoms of antibiotic-associated diarrhea through downregulating colon inflammation factors and immune factors (TLR4 and NF-κB) and restoring the gut flora to original intestinal homeostasis (103).
5.6 Improving symptoms of irritable bowel syndrome
Irritable bowel syndrome (IBS) is one of the most common gastrointestinal diseases, which affects approximately 10%–20% of the population worldwide, especially in developed countries (104). The symptoms of IBS are experienced as recurrent abdominal pain or discomfort and psychological and physical stressors such as depression or anxiety disorder (105). To date, the pathophysiology of IBS is still unclear (106), mainly including gut microbial dysbiosis, gut–brain axis homeostasis, gut inflammation, and immune dysfunction (107, 108). Ginseng has been confirmed to ameliorate various inflammation responses and help combat depression through suppressing stress (109). An increasing number of studies also demonstrated that ginseng and its major constituent can be reckoned as a promising candidate to treat IBS. For example, Yu et al. proposed that red ginseng (RG) extract significantly improved various symptoms of IBS through downregulating expression level of IL-1β and c-fos, regulating the plasma levels of corticosterone, and restoring the abundance of gut microbiota, including increasing the growth of probiotics microbes (Lactobacillus johnsonii, Lactobacillus reuteri, and Parabacteroides goldsteinii) (110).
6 Effect of ginseng and ginsenosides on intestinal immune disorders
Intestine immune balance is very essential to human body health. Once the balance is broken, it can result in a series of intestinal diseases, such as most common IBD and intestinal tumor (111). Immune dysregulation has been implicated in the pathogenesis of a group of autoimmune diseases. As one of the most common autoimmune diseases, IBD is believed to exhibit a complex and dysregulated response intestinal immune homeostasis, in which the intestinal immune system becomes hyperactive and causes unnecessary impaired integrity of the epithelial barrier (112). As an important immunomodulator, various ginsengs and ginsenosides have been reported to possess a wide range of immuno-modulatory effects, including enhancing host immunity, protecting against various infections and treating immunity-related disorders (113–115).
Specifically, one latest research suggests that, in an oxazolone (OXA)-induced mice UC model, Rg3-enriched Korean Red Ginseng extract was capable of upregulating the number of immune cell subtypes of CD4+ T-helper cells, CD19+ B-cells, and CD4+ and CD25+ regulatory T-cells (Tregs), thus significantly improving the colon length and body weight and decreasing disease activity index and histological injury (57). American ginseng has always been recommended as an edible and medicinal functional food use for immunological disorder. One study found that American ginseng and its primary extract (such as polysaccharide and ginsenoside) could significantly reverse the lymphocyte subsets ratio in spleen and peripheral blood and at the same time stimulate CD4+T cells and IgA-secreting cells in the small intestine (116). Lu et al. found that P. notoginseng saponin (PNS) could significantly relieve (DSS)-induced intestinal colitis through increasing M1 macrophages while decreasing M2 macrophages both in the spleen and colon tissues (67). Kim et al. first reported that Fermented Red Ginseng could obviously relieve 2,4,6-trinitrobenzenesulfonic acid-induced colitis through suppressing macrophage activity and modulating Th1 and Treg cell differentiation (117). In addition to ginseng roots and its various extract, Zhang et al. first reported that ginseng berry extract also could relieve (DSS)-induced colitis through improving the macroscopic appearance of the colon wall, suppressing the activation and number of immune cell (T cells, neutrophils, and CD103+CD11c+ cDCs), and promoting the migration of CD103+CD11c+ cDCs (118).
During the most recent years under study, it was found that gut microbiota was involved in regulating mucosal immune balance and host immune response (111, 119). Ginseng and ginsenosides were capable of closely interacting with gut microbiota in the human digestive tract. In addition to directly modulating intestine immune responses, a growing number of studies also reported that ginseng and its various extracts could influence intestinal immune functions via controlling intestinal homeostasis. For example, two previous studies proposed that Korean Red Ginseng-derived polysaccharides could enhancing gut-associated immune functions through increasing the activity of macrophage and promoting Peyer’s patches secretion levels both in vitro and in an animal model (120, 121). In addition, Wang et al. reported that oral administration with ginseng polysaccharide was able to relieve lipopolysaccharide induced immunological stress and significantly improve intestinal barrier function (122). Another study found that ginseng-derived small molecule oligopeptides could alleviate irradiation-induced intestinal injury and immune dysfunction through upregulating concentrations of lymphocytes (CD3+, CD4+, and CD8+) and restoring normal baseline intestinal permeability (123). In addition, in another study, Zhu and co-workers found that the intestinal metabolomic effects were significantly different between normal and immunosuppressed rats after ginseng administration (124).
7 Conclusions
In this article, we systematically summarized the anti-inflammation and immune modulatory effects of various ginseng and ginsenosides in intestinal system (Table 1). Intestinal inflammatory imbalance and immune dysfunction may cause a series of intestinal system diseases (125). Ginseng and ginsenosides exert a strong anti-inflammatory and immunomodulatory effect in the intestinal system, and the specific molecular mechanisms were also summarized in this review (Figure 3). We can conclude that ginseng and ginsenosides are becoming promising therapeutic options for various gastrointestinal disorders through regulating the immune balance, regulating inflammatory mediators and cytokines, preventing colitis-associated colorectal cancer, regulating gut microbiota and metabolism, alleviating antibiotic-induced diarrhea, and relieving the symptoms of antibiotic-associated diarrhea.
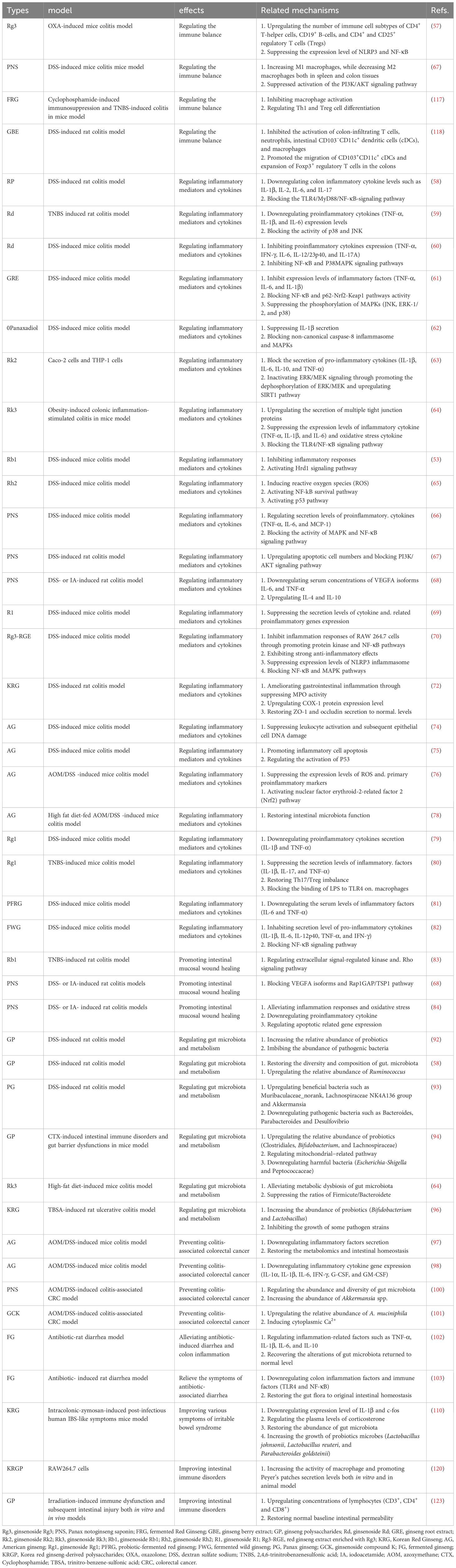
Table 1 Anti-inflammation and immune modulatory effects of various ginseng and ginsenosides in intestinal system.
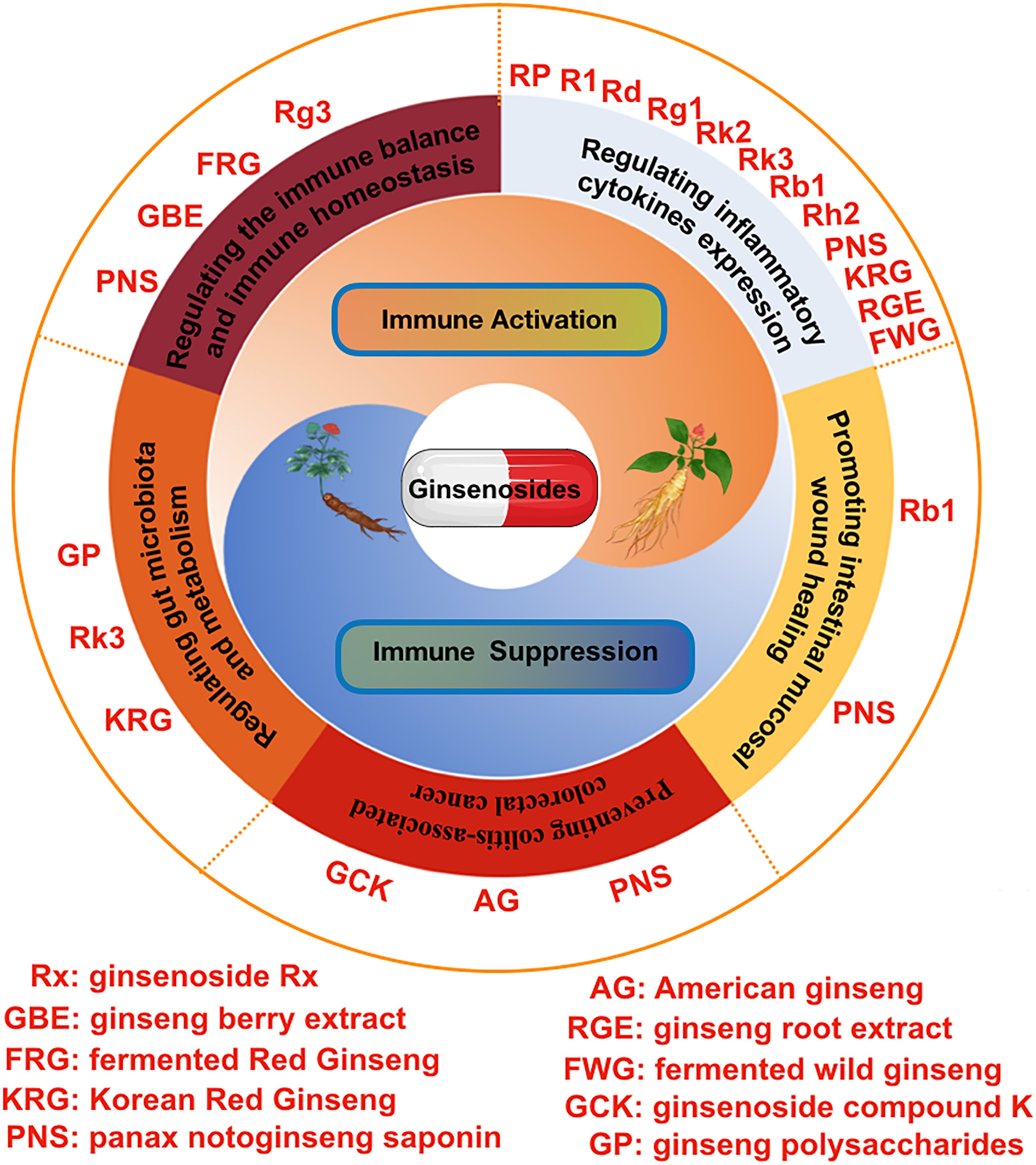
Figure 3 The pharmacological effects of various ginsenosides on intestinal inflammation and the immune system, including regulating inflammatory cytokines expression, regulating the immune balance and immune homeostasis, regulating gut microbiota and metabolism, promoting intestinal mucosal wound healing, and preventing colitis-associated colorectal cancer.
Author contributions
LZ: Writing – original draft, Supervision, Conceptualization. TZ: Writing – original draft, Investigation, Conceptualization. KZ: Writing – original draft, Supervision, Project administration, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
IBD, inflammatory bowel disease; UC, ulcerative colitis; IBS, irritable bowel disease; CD, Crohn’s disease; PPD, protopanaxadiol; PPT, protopanaxatriol; OCT, ocotillol; OXA, oxazolone; PNS, Panax notoginseng saponin; TNBS, 2,4,6-trinitrobenzenesulfonic acid; GRE, ginseng root extract; GRb1, ginsenoside Rb1; DIA, disease activity index; RG, red ginseng.
References
1. Kühl AA, Erben U, Kredel LI, Siegmund B. Diversity of intestinal macrophages in inflammatory bowel diseases. Front Immunol. (2015) 6:613. doi: 10.3389/fimmu.2015.00613
2. Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. (2017) 389:1741–55. doi: 10.1016/S0140-6736(16)31711-1
3. Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. (2012) 142:46–54.e42; quiz e30.
4. Yun TK. Brief introduction of Panax ginseng C.A. Meyer. J Korean Med Sci. (2001) 16 Suppl:S3–5. doi: 10.3346/jkms.2001.16.S.S3
5. Kang Z, Zhonga Y, Wu T, Huang J, Zhao H, Liu D. Ginsenoside from ginseng: a promising treatment for inflammatory bowel disease. Pharmacol Rep. (2021) 73:700–11. doi: 10.1007/s43440-020-00213-z
6. Zhou X, Liao WJ, Liao JM, Liao P, Lu H. Ribosomal proteins: functions beyond the ribosome. J Mol Cell Biol. (2015) 7:92–104. doi: 10.1093/jmcb/mjv014
8. Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. (2017) 390:2769–78. doi: 10.1016/S0140-6736(17)32448-0
9. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. (2020) 5:17–30.
10. Cao Q, Huang YH, Jiang M, Dai C. The prevalence and risk factors of psychological disorders, malnutrition and quality of life in IBD patients. Scand J Gastroenterol. (2019) 54:1458–66. doi: 10.1080/00365521.2019.1697897
11. Sanmarco LM, Chao CC, Wang YC, Kenison JE, Li Z, Rone JM, et al. Identification of environmental factors that promote intestinal inflammation. Nature. (2022) 611:801–9. doi: 10.1038/s41586-022-05308-6
12. Merga Y, Campbell BJ, Rhodes JM. Mucosal barrier, bacteria and inflammatory bowel disease: possibilities for therapy. Dig Dis. (2014) 32:475–83. doi: 10.1159/000358156
13. Lee B, Moon KM, Kim CY. Tight junction in the intestinal epithelium: its association with diseases and regulation by phytochemicals. J Immunol Res. (2018) 2018:2645465. doi: 10.1155/2018/2645465
14. Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. (2014) 146:1489–99. doi: 10.1053/j.gastro.2014.02.009
15. Freire MO, Van Dyke TE. Natural resolution of inflammation. Periodontol 2000. (2013) 63:149–64. doi: 10.1111/prd.12034
16. Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. (2014) 14:329–42. doi: 10.1038/nri3661
17. Ahluwalia B, Moraes L, Magnusson MK, Öhman L. Immunopathogenesis of inflammatory bowel disease and mechanisms of biological therapies. Scand J Gastroenterol. (2018) 53:379–89. doi: 10.1080/00365521.2018.1447597
18. Khan I, Ullah N, Zha L, Bai Y, Khan A, Zhao T, et al. Alteration of gut microbiota in inflammatory bowel disease (IBD): cause or consequence? IBD treatment targeting the gut microbiome. Pathogens. (2019) 8. doi: 10.3390/pathogens8030126
19. Sminia T, Wilders MM, Janse EM, Hoefsmit EC. Characterization of non-lymphoid cells in Peyer's patches of the rat. Immunobiology. (1983) 164:136–43. doi: 10.1016/S0171-2985(83)80005-9
20. Kagnoff MF. Mucosal immunology: new frontiers. Immunol Today. (1996) 17:57–9. doi: 10.1016/0167-5699(96)80579-2
21. Mowat AM, Viney JL. The anatomical basis of intestinal immunity. Immunol Rev. (1997) 156:145–66. doi: 10.1111/j.1600-065X.1997.tb00966.x
22. Lu Q, Yang MF, Liang YJ, Xu J, Xu HM, Nie YQ, et al. Immunology of inflammatory bowel disease: molecular mechanisms and therapeutics. J Inflammation Res. (2022) 15:1825–44. doi: 10.2147/JIR.S353038
23. Shastry RP, Rekha PD. Bacterial cross talk with gut microbiome and its implications: a short review. Folia Microbiol (Praha). (2021) 66:15–24. doi: 10.1007/s12223-020-00821-5
24. Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. (2020) 17:223–37. doi: 10.1038/s41575-019-0258-z
25. Khanna S. Management of Clostridioides difficile infection in patients with inflammatory bowel disease. Intest Res. (2021) 19:265–74. doi: 10.5217/ir.2020.00045
26. Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. (2012) 489:220–30. doi: 10.1038/nature11550
27. Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. (2007) 104:13780–5. doi: 10.1073/pnas.0706625104
28. Walker AW, Sanderson JD, Churcher C, Parkes GC, Hudspith BN, Rayment N, et al. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. (2011) 11:7. doi: 10.1186/1471-2180-11-7
29. Liu S, Zhao W, Lan P, Mou X. The microbiome in inflammatory bowel diseases: from pathogenesis to therapy. Protein Cell. (2021) 12:331–45. doi: 10.1007/s13238-020-00745-3
30. Knutson CG, Mangerich A, Zeng Y, Raczynski AR, Liberman RG, Kang P, et al. Chemical and cytokine features of innate immunity characterize serum and tissue profiles in inflammatory bowel disease. Proc Natl Acad Sci U S A. (2013) 110:E2332–41. doi: 10.1073/pnas.1222669110
31. Roh JS, Sohn DH. Damage-associated molecular patterns in inflammatory diseases. Immune Netw. (2018) 18:e27. doi: 10.4110/in.2018.18.e27
32. Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. (2009) 22:240–73. doi: 10.1128/CMR.00046-08
33. Wu XR, Liu XL, Katz S, Shen B. Pathogenesis, diagnosis, and management of ulcerative proctitis, chronic radiation proctopathy, and diversion proctitis. Inflammation Bowel Dis. (2015) 21:703–15. doi: 10.1097/MIB.0000000000000227
34. Chojnacki C, Wiśniewska-Jarosińska M, Kulig G, Majsterek I, Reiter RJ, Chojnacki J. Evaluation of enterochromaffin cells and melatonin secretion exponents in ulcerative colitis. World J Gastroenterol. (2013) 19:3602–7. doi: 10.3748/wjg.v19.i23.3602
35. Hua M, Liu Z, Sha J, Li S, Dong L, Sun Y. Effects of ginseng soluble dietary fiber on serum antioxidant status, immune factor levels and cecal health in healthy rats. Food Chem. (2021) 365:130641. doi: 10.1016/j.foodchem.2021.130641
36. Yang X, Xiong X, Wang H, Wang J. Protective effects of panax notoginseng saponins on cardiovascular diseases: a comprehensive overview of experimental studies. Evid Based Complement Alternat Med. (2014) 2014:204840. doi: 10.1155/2014/204840
37. Yang WZ, Hu Y, Wu WY, Ye M, Guo DA. Saponins in the genus Panax L. (Araliaceae): a systematic review of their chemical diversity. Phytochemistry. (2014) 106:7–24. doi: 10.1016/j.phytochem.2014.07.012
38. Hu S, Concha C, Lin F, Persson Waller K. Adjuvant effect of ginseng extracts on the immune responses to immunisation against Staphylococcus aureus in dairy cattle. Vet Immunol Immunopathol. (2003) 91:29–37. doi: 10.1016/S0165-2427(02)00264-7
39. Lee J, Jung E, Lee J, Huh S, Kim J, Park M, et al. Panax ginseng induces human Type I collagen synthesis through activation of Smad signaling. J Ethnopharmacol. (2007) 109:29–34. doi: 10.1016/j.jep.2006.06.008
40. Hong CE, Lyu SY. Anti-inflammatory and anti-oxidative effects of Korean red ginseng extract in human keratinocytes. Immune Netw. (2011) 11:42–9. doi: 10.4110/in.2011.11.1.42
41. Yun TK, Choi SY. Preventive effect of ginseng intake against various human cancers: a case-control study on 1987 pairs. Cancer Epidemiol Biomarkers Prev. (1995) 4:401–8.
42. Zhao L, Zhang Y, Li Y, Li C, Shi K, Zhang K, et al. Therapeutic effects of ginseng and ginsenosides on colorectal cancer. Food Funct. (2022) 13:6450–66. doi: 10.1039/D2FO00899H
43. Kim MY, Cho JY. 20S-dihydroprotopanaxadiol, a ginsenoside derivative, boosts innate immune responses of monocytes and macrophages. J Ginseng Res. (2013) 37:293–9. doi: 10.5142/jgr.2013.37.293
44. Yu T, Rhee MH, Lee J, Kim SH, Yang Y, Kim HG, et al. Ginsenoside Rc from Korean red ginseng (Panax ginseng C.A. Meyer) attenuates inflammatory symptoms of gastritis, hepatitis and arthritis. Am J Chin Med. (2016) 44:595–615. doi: 10.1142/S0192415X16500336
45. Yi YS. Roles of ginsenosides in inflammasome activation. J Ginseng Res. (2019) 43:172–8. doi: 10.1016/j.jgr.2017.11.005
46. Block KI, Mead MN. Immune system effects of eChinacea, ginseng, and astragalus: a review. Integr Cancer Ther. (2003) 2:247–67. doi: 10.1177/1534735403256419
47. Ogawa-Ochiai K, Kawasaki K. Panax ginseng for frailty-related disorders: A review. Front Nutr. (2018) 5:140. doi: 10.3389/fnut.2018.00140
48. Fodil N, Moradin N, Leung V, Olivier JF, Radovanovic I, Jeyakumar T, et al. CCDC88B is required for pathogenesis of inflammatory bowel disease. Nat Commun. (2017) 8:932. doi: 10.1038/s41467-017-01381-y
49. Lubbad A, Oriowo MA, Khan I. Curcumin attenuates inflammation through inhibition of TLR-4 receptor in experimental colitis. Mol Cell Biochem. (2009) 322:127–35. doi: 10.1007/s11010-008-9949-4
50. Li XM, Yuan DY, Liu YH, Zhu L, Qin HK, Yang YB, et al. Panax notoginseng saponins prevent colitis-associated colorectal cancer via inhibition IDO1 mediated immune regulation. Chin J Nat Med. (2022) 20:258–69. doi: 10.1016/S1875-5364(22)60179-1
51. Wang CZ, Yao H, Zhang CF, Chen L, Wan JY, Huang WH, et al. American ginseng microbial metabolites attenuate DSS-induced colitis and abdominal pain. Int Immunopharmacol. (2018) 64:246–51. doi: 10.1016/j.intimp.2018.09.005
52. Yang N, Liang G, Lin J, Zhang S, Lin Q, Ji X, et al. Ginsenoside Rd therapy improves histological and functional recovery in a rat model of inflammatory bowel disease. Phytother Res. (2020) 34:3019–28. doi: 10.1002/ptr.6734
53. Dong JY, Xia KJ, Liang W, Liu LL, Yang F, Fang XS, et al. Ginsenoside Rb1 alleviates colitis in mice via activation of endoplasmic reticulum-resident E3 ubiquitin ligase Hrd1 signaling pathway. Acta Pharmacol Sin. (2021) 42:1461–71. doi: 10.1038/s41401-020-00561-9
54. Zeng L, Li X, Bai G, Liu Y, Lu Q. Rectal administration of Panax notoginseng and Colla Corii Asini suppositories in ulcerative colitis: clinical effect and influence on immune function. Am J Transl Res. (2022) 14:603–11.
55. Choi KT. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin. (2008) 29:1109–18. doi: 10.1111/aphs.2008.29.issue-9
56. Im DS. Pro-resolving effect of ginsenosides as an anti-inflammatory mechanism of panax ginseng. Biomolecules. (2020) 10. doi: 10.3390/biom10030444
57. Ullah HMA, Saba E, Lee YY, Hong SB, Hyun SH, Kwak YS, et al. Restorative effects of Rg3-enriched Korean Red Ginseng and Persicaria tinctoria extract on oxazolone-induced ulcerative colitis in mice. J Ginseng Res. (2022) 46:628–35. doi: 10.1016/j.jgr.2021.07.001
58. Li S, Huo X, Qi Y, Ren D, Li Z, Qu D, et al. The protective effects of ginseng polysaccharides and their effective subfraction against dextran sodium sulfate-induced colitis. Foods. (2022) 11. doi: 10.3390/foods11060890
59. Yang XL, Guo TK, Wang YH, Huang YH, Liu X, Wang XX, et al. Ginsenoside Rd attenuates the inflammatory response via modulating p38 and JNK signaling pathways in rats with TNBS-induced relapsing colitis. Int Immunopharmacol. (2012) 12:408–14. doi: 10.1016/j.intimp.2011.12.014
60. Qu B, Cao T, Wang M, Wang S, Li W, Li H. Ginsenosides Rd monomer inhibits proinflammatory cytokines production and alleviates DSS-colitis by NF-κB and P38MAPK pathways in mice. Immunopharmacol Immunotoxicol. (2022) 44:110–8. doi: 10.1080/08923973.2021.2012482
61. Yang S, Li F, Lu S, Ren L, Bian S, Liu M, et al. Ginseng root extract attenuates inflammation by inhibiting the MAPK/NF-κB signaling pathway and activating autophagy and p62-Nrf2-Keap1 signaling in vitro and in vivo. J Ethnopharmacol. (2022) 283:114739. doi: 10.1016/j.jep.2021.114739
62. Wang JY, Xing Y, Li MY, Zhang ZH, Jin HL, Ma J, et al. Panaxadiol inhibits IL-1β secretion by suppressing zinc finger protein 91-regulated activation of non-canonical caspase-8 inflammasome and MAPKs in macrophages. J Ethnopharmacol. (2022) 283:114715. doi: 10.1016/j.jep.2021.114715
63. Huang X, Xiao J, Wen M, Liang J. Ginsenoside Rk2 Protects against Ulcerative Colitis via Inactivating ERK/MEK Pathway by SIRT1. J Environ Pathol Toxicol Oncol. (2022) 41:89–98. doi: 10.1615/JEnvironPatholToxicolOncol.v41.i2
64. Chen H, Yang H, Deng J, Fan D. Ginsenoside Rk3 ameliorates obesity-induced colitis by regulating of intestinal flora and the TLR4/NF-κB signaling pathway in C57BL/6 mice. J Agric Food Chem. (2021) 69:3082–93. doi: 10.1021/acs.jafc.0c07805
65. Chen X, Xu T, Lv X, Zhang J, Liu S. Ginsenoside Rh2 alleviates ulcerative colitis by regulating the STAT3/miR-214 signaling pathway. J Ethnopharmacol. (2021) 274:113997. doi: 10.1016/j.jep.2021.113997
66. Luo H, Vong CT, Tan D, Zhang J, Yu H, Yang L, et al. Panax notoginseng Saponins Modulate the Inflammatory Response and Improve IBD-Like Symptoms via TLR/NF-[Formula: see text]B and MAPK Signaling Pathways. Am J Chin Med. (2021) 49:925–39. doi: 10.1142/S0192415X21500440
67. Lu QG, Zeng L, Li XH, Liu Y, Du XF, Bai GM, et al. Protective effects of panax notoginseng saponin on dextran sulfate sodium-induced colitis in rats through phosphoinositide-3-kinase protein kinase B signaling pathway inhibition. World J Gastroenterol. (2020) 26:1156–71. doi: 10.3748/wjg.v26.i11.1156
68. Wang S, Tao P, Zhao L, Zhang W, Hu H, Lin J. Panax notoginseng promotes repair of colonic microvascular injury in sprague-dawley rats with experimental colitis. Evid Based Complement Alternat Med. (2018) 2018:4386571. doi: 10.1155/2018/4386571
69. Zhang J, Ding L, Wang B, Ren G, Sun A, Deng C, et al. Notoginsenoside R1 attenuates experimental inflammatory bowel disease via pregnane X receptor activation. J Pharmacol Exp Ther. (2015) 352:315–24. doi: 10.1124/jpet.114.218750
70. Saba E, Lee YY, Rhee MH, Kim SD. Alleviation of Ulcerative Colitis Potentially through th1/th2 Cytokine Balance by a Mixture of Rg3-enriched Korean Red Ginseng Extract and Persicaria tinctoria. Molecules. (2020) 25. doi: 10.3390/molecules25225230
71. Saba E, Lee YY, Kim M, Hyun SH, Park CK, Son E, et al. A novel herbal formulation consisting of red ginseng extract and Epimedium koreanum Nakai-attenuated dextran sulfate sodium-induced colitis in mice. J Ginseng Res. (2020) 44:833–42. doi: 10.1016/j.jgr.2020.02.003
72. Lee JO, Kim JH, Kim S, Kim MY, Hong YH, Kim HG, et al. Gastroprotective effects of the nonsaponin fraction of Korean Red Ginseng through cyclooxygenase-1 upregulation. J Ginseng Res. (2020) 44:655–63. doi: 10.1016/j.jgr.2019.11.001
73. Wang L, Huang Y, Yin G, Wang J, Wang P, Chen ZY, et al. Antimicrobial activities of Asian ginseng, American ginseng, and notoginseng. Phytother Res. (2020) 34:1226–36. doi: 10.1002/ptr.6605
74. Jin Y, Kotakadi VS, Ying L, Hofseth AB, Cui X, Wood PA, et al. American ginseng suppresses inflammation and DNA damage associated with mouse colitis. Carcinogenesis. (2008) 29:2351–9. doi: 10.1093/carcin/bgn211
75. Jin Y, Hofseth AB, Cui X, Windust AJ, Poudyal D, Chumanevich AA, et al. American ginseng suppresses colitis through p53-mediated apoptosis of inflammatory cells. Cancer Prev Res (Phila). (2010) 3:339–47. doi: 10.1158/1940-6207.CAPR-09-0116
76. Cui X, Jin Y, Poudyal D, Chumanevich AA, Davis T, Windust A, et al. Mechanistic insight into the ability of American ginseng to suppress colon cancer associated with colitis. Carcinogenesis. (2010) 31:1734–41. doi: 10.1093/carcin/bgq163
77. Chaparala A, Tashkandi H, Chumanevich AA, Witalison EE, Windust A, Cui T, et al. Molecules from American ginseng suppress colitis through nuclear factor erythroid-2-related factor 2. Nutrients. (2020) 12. doi: 10.3390/nu12061850
78. Wang CZ, Huang WH, Zhang CF, Wan JY, Wang Y, Yu C, et al. Role of intestinal microbiome in American ginseng-mediated colon cancer prevention in high fat diet-fed AOM/DSS mice [corrected]. Clin Transl Oncol. (2018) 20:302–12. doi: 10.1007/s12094-017-1717-z
79. Zhu G, Wang H, Wang T, Shi F. Ginsenoside Rg1 attenuates the inflammatory response in DSS-induced mice colitis. Int Immunopharmacol. (2017) 50:1–5. doi: 10.1016/j.intimp.2017.06.002
80. Lee SY, Jeong JJ, Eun SH, Kim DH. Anti-inflammatory effects of ginsenoside Rg1 and its metabolites ginsenoside Rh1 and 20(S)-protopanaxatriol in mice with TNBS-induced colitis. Eur J Pharmacol. (2015) 762:333–43. doi: 10.1016/j.ejphar.2015.06.011
81. Jang SH, Park J, Kim SH, Choi KM, Ko ES, Cha JD, et al. Oral administration of red ginseng powder fermented with probiotic alleviates the severity of dextran-sulfate sodium-induced colitis in a mouse model. Chin J Nat Med. (2017) 15:192–201. doi: 10.1016/S1875-5364(17)30035-3
82. Seong MA, Woo JK, Kang JH, Jang YS, Choi S, Jang YS, et al. Oral administration of fermented wild ginseng ameliorates DSS-induced acute colitis by inhibiting NF-κB signaling and protects intestinal epithelial barrier. BMB Rep. (2015) 48:419–25. doi: 10.5483/BMBRep.2015.48.7.039
83. Toyokawa Y, Takagi T, Uchiyama K, Mizushima K, Inoue K, Ushiroda C, et al. Ginsenoside Rb1 promotes intestinal epithelial wound healing through extracellular signal-regulated kinase and Rho signaling. J Gastroenterol Hepatol. (2019) 34:1193–200. doi: 10.1111/jgh.14532
84. Wang SY, Tao P, Hu HY, Yuan JY, Zhao L, Sun BY, et al. Effects of initiating time and dosage of Panax notoginseng on mucosal microvascular injury in experimental colitis. World J Gastroenterol. (2017) 23:8308–20. doi: 10.3748/wjg.v23.i47.8308
85. Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan TJ, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. (2012) 338:120–3. doi: 10.1126/science.1224820
86. Arthur JC, Jobin C. The struggle within: microbial influences on colorectal cancer. Inflammation Bowel Dis. (2011) 17:396–409. doi: 10.1002/ibd.21354
87. Hering NA, Fromm M, Schulzke JD. Determinants of colonic barrier function in inflammatory bowel disease and potential therapeutics. J Physiol. (2012) 590:1035–44. doi: 10.1113/jphysiol.2011.224568
88. Shen ZH, Zhu CX, Quan YS, Yang ZY, Wu S, Luo WW, et al. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J Gastroenterol. (2018) 24:5–14. doi: 10.3748/wjg.v24.i1.5
89. Shin HJ, Kim DH, Zhong X, Yum HW, Kim SJ, Chun KS, et al. Preventive effects of Korean red ginseng on experimentally induced colitis and colon carcinogenesis. J Tradit Complement Med. (2020) 10:198–206. doi: 10.1016/j.jtcme.2020.04.004
90. Li X, Liu J, Zuo TT, Hu Y, Li Z, Wang HD, et al. Advances and challenges in ginseng research from 2011 to 2020: the phytochemistry, quality control, metabolism, and biosynthesis. Nat Prod Rep. (2022) 39:875–909. doi: 10.1039/D1NP00071C
91. Chen Z, Zhang Z, Liu J, Qi H, Li J, Chen J, et al. Gut microbiota: therapeutic targets of ginseng against multiple disorders and ginsenoside transformation. Front Cell Infect Microbiol. (2022) 12:853981. doi: 10.3389/fcimb.2022.853981
92. Shen H, Gao XJ, Li T, Jing WH, Han BL, Jia YM, et al. Ginseng polysaccharides enhanced ginsenoside Rb1 and microbial metabolites exposure through enhancing intestinal absorption and affecting gut microbial metabolism. J Ethnopharmacol. (2018) 216:47–56. doi: 10.1016/j.jep.2018.01.021
93. Wan Y, Yang L, Li H, Ren H, Zhu K, Dong Z, et al. Zingiber officinale and Panax ginseng ameliorate ulcerative colitis in mice via modulating gut microbiota and its metabolites. J Chromatogr B Analyt Technol BioMed Life Sci. (2022) 1203:123313. doi: 10.1016/j.jchromb.2022.123313
94. Zhou R, He D, Xie J, Zhou Q, Zeng H, Li H, et al. The synergistic effects of polysaccharides and ginsenosides from American ginseng (Panax quinquefolius L.) ameliorating cyclophosphamide-induced intestinal immune disorders and gut barrier dysfunctions based on microbiome-metabolomics analysis. Front Immunol. (2021) 12:665901. doi: 10.3389/fimmu.2021.665901
95. Wang HY, Hua HY, Liu XY, Liu JH, Yu BY. In vitro biotransformation of red ginseng extract by human intestinal microflora: metabolites identification and metabolic profile elucidation using LC-Q-TOF/MS. J Pharm BioMed Anal. (2014) 98:296–306. doi: 10.1016/j.jpba.2014.06.006
96. Guo M, Ding S, Zhao C, Gu X, He X, Huang K, et al. Red Ginseng and Semen Coicis can improve the structure of gut microbiota and relieve the symptoms of ulcerative colitis. J Ethnopharmacol. (2015) 162:7–13. doi: 10.1016/j.jep.2014.12.029
97. Wang CZ, Yu C, Wen XD, Chen L, Zhang CF, Calway T, et al. American ginseng attenuates colitis-associated colon carcinogenesis in mice: impact on gut microbiota and metabolomics. Cancer Prev Res (Phila). (2016) 9:803–11. doi: 10.1158/1940-6207.CAPR-15-0372
98. Yu C, Wen XD, Zhang Z, Zhang CF, Wu XH, Martin A, et al. American ginseng attenuates azoxymethane/dextran sodium sulfate-induced colon carcinogenesis in mice. J Ginseng Res. (2015) 39:14–21. doi: 10.1016/j.jgr.2014.07.001
99. Poudyal D, Le PM, Davis T, Hofseth AB, Chumanevich A, Chumanevich AA, et al. A hexane fraction of American ginseng suppresses mouse colitis and associated colon cancer: anti-inflammatory and proapoptotic mechanisms. Cancer Prev Res (Phila). (2012) 5:685–96. doi: 10.1158/1940-6207.CAPR-11-0421
100. Chen L, Chen MY, Shao L, Zhang W, Rao T, Zhou HH, et al. Panax notoginseng saponins prevent colitis-associated colorectal cancer development: the role of gut microbiota. Chin J Nat Med. (2020) 18:500–7. doi: 10.1016/S1875-5364(20)30060-1
101. Shao L, Guo YP, Wang L, Chen MY, Zhang W, Deng S, et al. Effects of ginsenoside compound K on colitis-associated colorectal cancer and gut microbiota profiles in mice. Ann Transl Med. (2022) 10:408. doi: 10.21037/atm
102. Qu Q, Zhao C, Yang C, Zhou Q, Liu X, Yang P, et al. Limosilactobacillus fermentum-fermented ginseng improved antibiotic-induced diarrhoea and the gut microbiota profiles of rats. J Appl Microbiol. (2022). doi: 10.1111/jam.15780
103. Qu Q, Yang F, Zhao C, Liu X, Yang P, Li Z, et al. Effects of fermented ginseng on the gut microbiota and immunity of rats with antibiotic-associated diarrhea. J Ethnopharmacol. (2021) 267:113594. doi: 10.1016/j.jep.2020.113594
104. Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med. (2012) 367:1626–35. doi: 10.1056/NEJMra1207068
105. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. (2006) 130:1480–91. doi: 10.1053/j.gastro.2005.11.061
106. Halkjær SI, Boolsen AW, Günther S, Christensen AH, Petersen AM. Can fecal microbiota transplantation cure irritable bowel syndrome? World J Gastroenterol. (2017) 23:4112–20. doi: 10.3748/wjg.v23.i22.4112
107. Labus JS, Hollister EB, Jacobs J, Kirbach K, Oezguen N, Gupta A, et al. Differences in gut microbial composition correlate with regional brain volumes in irritable bowel syndrome. Microbiome. (2017) 5:49. doi: 10.1186/s40168-017-0260-z
108. Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. (2015) 28:203–9.
109. Lee S, Rhee DK. Effects of ginseng on stress-related depression, anxiety, and the hypothalamic-pituitary-adrenal axis. J Ginseng Res. (2017) 41:589–94. doi: 10.1016/j.jgr.2017.01.010
110. Yu S, Chun E, Ji Y, Lee YJ, Jin M. Effects of red ginseng on gut, microbiota, and brain in a mouse model of post-infectious irritable bowel syndrome. J Ginseng Res. (2021) 45:706–16. doi: 10.1016/j.jgr.2021.03.008
111. Shi N, Li N, Duan X, Niu H. Interaction between the gut microbiome and mucosal immune system. Mil Med Res. (2017) 4:14. doi: 10.1186/s40779-017-0122-9
112. Matricon J. [Immunopathogenesis of inflammatory bowel disease]. Med Sci (Paris). (2010) 26:405–10. doi: 10.1051/medsci/2010264405
113. Culley FJ, Pennycook AM, Tregoning JS, Hussell T, Openshaw PJ. Differential chemokine expression following respiratory virus infection reflects Th1- or Th2-biased immunopathology. J Virol. (2006) 80:4521–7. doi: 10.1128/JVI.80.9.4521-4527.2006
114. Ratan ZA, Youn SH, Kwak YS, Han CK, Haidere MF, Kim JK, et al. Adaptogenic effects of Panax ginseng on modulation of immune functions. J Ginseng Res. (2021) 45:32–40. doi: 10.1016/j.jgr.2020.09.004
115. Lee JI, Park KS, Cho IH. Panax ginseng: a candidate herbal medicine for autoimmune disease. J Ginseng Res. (2019) 43:342–8. doi: 10.1016/j.jgr.2018.10.002
116. Lee HC, Jenner AM, Low CS, Lee YK. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res Microbiol. (2006) 157:876–84. doi: 10.1016/j.resmic.2006.07.004
117. Kim JK, Kim JY, Jang SE, Choi MS, Jang HM, Yoo HH, et al. Fermented red ginseng alleviates cyclophosphamide-induced immunosuppression and 2,4,6-trinitrobenzenesulfonic acid-induced colitis in mice by regulating macrophage activation and T cell differentiation. Am J Chin Med. (2018) 46:1879–97. doi: 10.1142/S0192415X18500945
118. Zhang W, Xu L, Cho SY, Min KJ, Oda T, Zhang L, et al. Ginseng berry extract attenuates dextran sodium sulfate-induced acute and chronic colitis. Nutrients. (2016) 8:199. doi: 10.3390/nu8040199
119. Takiishi T, Fenero CIM, Câmara NOS. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers. (2017) 5:e1373208. doi: 10.1080/21688370.2017.1373208
120. Byeon SE, Lee J, Kim JH, Yang WS, Kwak YS, Kim SY, et al. Molecular mechanism of macrophage activation by red ginseng acidic polysaccharide from Korean red ginseng. Mediators Inflamm. (2012) 2012:732860. doi: 10.1155/2012/732860
121. Li B, Zhang N, Feng Q, Li H, Wang D, Ma L, et al. The core structure characterization and of ginseng neutral polysaccharide with the immune-enhancing activity. Int J Biol Macromol. (2019) 123:713–22. doi: 10.1016/j.ijbiomac.2018.11.140
122. Wang K, Zhang H, Han Q, Lan J, Chen G, Cao G, et al. Effects of astragalus and ginseng polysaccharides on growth performance, immune function and intestinal barrier in weaned piglets challenged with lipopolysaccharide. J Anim Physiol Anim Nutr (Berl). (2020) 104:1096–105. doi: 10.1111/jpn.13244
123. He LX, Zhang ZF, Zhao J, Li L, Xu T, Bin S, et al. Ginseng oligopeptides protect against irradiation-induced immune dysfunction and intestinal injury. Sci Rep. (2018) 8:13916. doi: 10.1038/s41598-018-32188-6
124. Zhu JH, Xu JD, Zhou SS, Zhang XY, Zhou J, Kong M, et al. Differences in intestinal metabolism of ginseng between normal and immunosuppressed rats. Eur J Drug Metab Pharmacokinet. (2021) 46:93–104. doi: 10.1007/s13318-020-00645-1
Keywords: ginseng, ginsenosides, intestinal system diseases, intestinal immune, intestinal inflammation
Citation: Zhao L, Zhang T and Zhang K (2024) Pharmacological effects of ginseng and ginsenosides on intestinal inflammation and the immune system. Front. Immunol. 15:1353614. doi: 10.3389/fimmu.2024.1353614
Received: 11 December 2023; Accepted: 03 April 2024;
Published: 18 April 2024.
Edited by:
Zhenhua Li, Jinan University, ChinaReviewed by:
Jin-Yi Wan, Beijing University of Chinese Medicine, ChinaJian-lin Wu, Macau University of Science and Technology, Macao SAR, China
Copyright © 2024 Zhao, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kai Zhang, emhhbmdfa2FpQGpsdS5lZHUuY24=
†These authors have contributed equally to this work
 Linxian Zhao†
Linxian Zhao† Kai Zhang
Kai Zhang