- 1School of Clinical Medicine, Beijing University of Chinese Medicine, Beijing, China
- 2Department of Dermatology, China-Japan Friendship Hospital, Beijing, China
- 3Department of Dermatology, The First Affiliated Hospital of Hunan University of Chinese Medicine, Changsha, China
Corona virus disease 2019(COVID-19) is one of the most serious respiratory pandemic diseases threatening human health for centuries. Alopecia areata (AA) is a sudden patchy hair loss, an autoimmune disease, which seriously affects the image and mental health of patients. Evidence shows that the risk of autoimmune diseases significantly increases after COVID-19, and is positively correlated with the severity, with a significant increase in the risk of alopecia in those over 40 years old. The relationship between COVID-19 and AA has become a hot topic of current research. Strengthening the research on the correlation between COVID-19 and AA can help to identify and protect susceptible populations at an early stage. This article reviews the research progress on the epidemiological background of COVID-19 and AA, the situation and possible mechanisms of AA induced by COVID-19 or COVID-19 vaccination, and potential treatment methods.
1 Introduction
Corona virus disease 2019(COVID-19) is a systemic multi-organ injury disease mainly targeting the lungs, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It has strong transmissibility and quickly leads to a global pandemic. To resist the spread of the virus, the number of COVID-19 vaccinations has rapidly increased worldwide, followed by the occurrence of some adverse reactions, one of which is hair loss. As a systemic multi-organ injury disease, COVID-19 has been shown to be closely related to skin diseases (1), with hair loss being one of the most common sequelae of COVID-19. Alopecia areata (AA) and telogen effluvium (TE) are the most common types of hair loss in the context of COVID-19 (2).
AA is a T-cell-mediated autoimmune disease in which hair follicles lose immune privilege, related to genetic, immune, and psychological factors. Depending on the extent of hair involvement, it can be classified into localized or circumscribed alopecia areata, alopecia totalis (AT), and alopecia universalis (AU). The lifetime risk of AA is 2.1%, with a total prevalence of 0.1% to 0.2% (3). Studies estimate that the global incidence of AA ranges from 0.57% to 3.8% (4), with no significant difference in incidence between men and women. Currently, the relationship between COVID-19 and AA is not clear, but there have been many reports of new onset, recurrence, or worsening of AA after SARS-CoV-2 infection or COVID-19 vaccination. This review summarizes the relevant studies on the new onset or recurrence of AA or the exacerbation of existing AA after SARS-CoV-2 infection or COVID-19 vaccination, and deduces the possible mechanisms of inducing AA, to better clarify the association between AA and COVID-19.
2 Materials and methods
2.1 Methods
This paper explores the association between AA and COVID-19 by searching for relevant literature on new or recurrent AA after COVID-19 or COVID-19 vaccination, or exacerbation of existing AA. The specific method was as follows: CNKI, Wanfang, VIP, and Pubmed were used as data sources, and (COVID-19 OR SARS-CoV-2 OR “Coronavirus Disease-19” OR “Novel coronavirus”) AND (Alopecia Areata OR Hair loss OR Bald spot) were used as the search formula to search papers included before December 2023. Inclusion criteria for literature: Literature that is publicly published, downloadable, and with full text available for reading, reporting patients diagnosed with COVID-19 and AA, or diagnosed with AA after receiving the COVID-19 vaccine. Exclusion criteria for literature: Duplicate reports, literature on case reports not confirmed by diagnosis.
2.2 Results
A total of 51 patients reported in 35 literature articles were included.
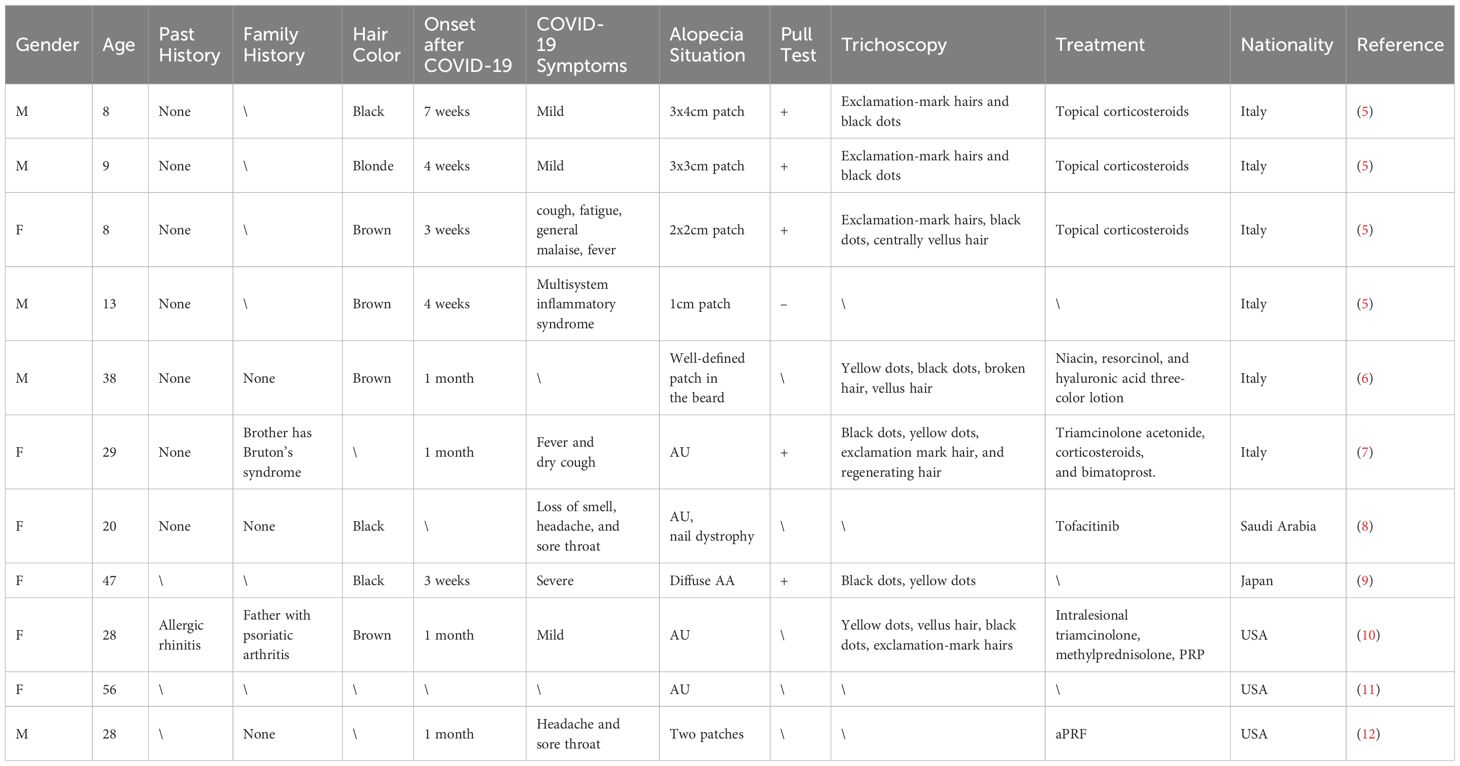
Cases of new-onset AA after COVID-19 were reported from Italy, Saudi Arabia, Japan, and the United States, accounting for 11 cases (Table 1). Most patients were adults, with 5 cases being diffuse AU and another case presenting with a distinctive pattern of AA in the beard area, characterized by well-defined circular bald patches. Only Italy reported 4 child patients, all of whom exhibited patchy hair loss.
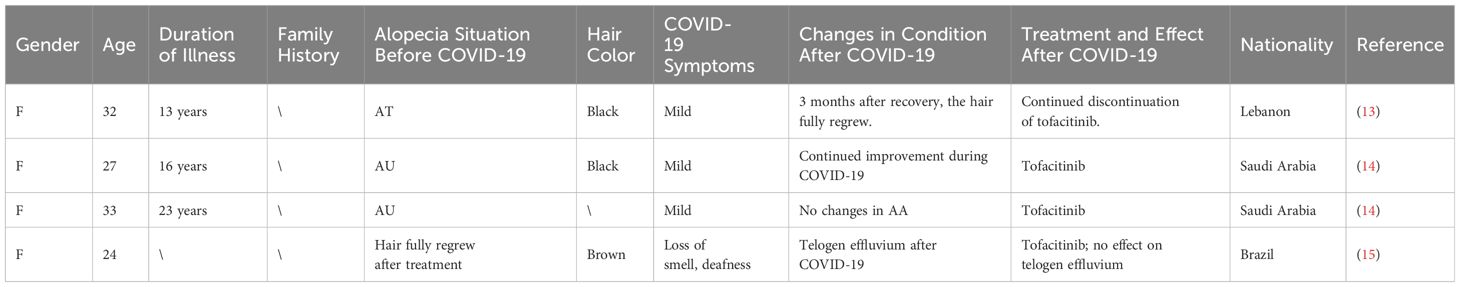
Lebanon, Saudi Arabia, and Brazil collectively reported on the post-COVID-19 changes in condition for 4 AA patients (Table 2). Among these, the condition did not deteriorate for 3 patients after COVID-19; rather, it continued to improve. The remaining patient did not experience a recurrence of AA following COVID-19 but instead developed TE.
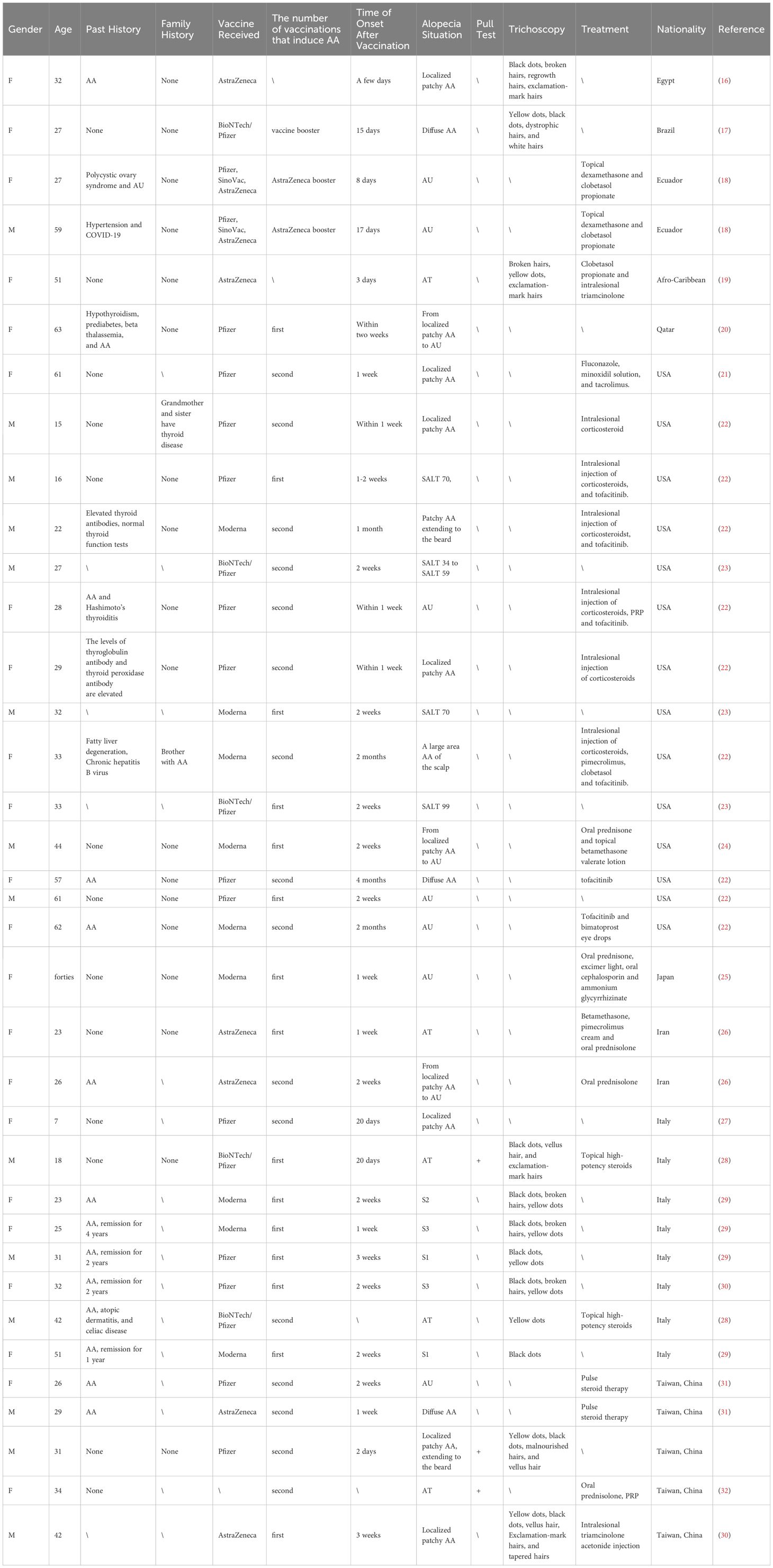
Cases of AA after COVID-19 vaccination have been reported more frequently in the United States and Italy, and also in Egypt, Brazil, Uruguay, Qatar, Japan, Iran, and Taiwan, China (Table 3). Most patients are adults, with only one pediatric patient in Italy, and the majority of patients have received the Pfizer/BioNTech vaccine, followed by AstraZeneca and Moderna.
3 Discussion
3.1 The interplay between COVID-19 and AA
3.1.1 COVID-19 patients seem to be prone to AA
Since the start of the COVID-19 pandemic, the incidence of AA has significantly increased, suggesting that new onset AA may be a dermatological manifestation of SARS-CoV-2 infection (33). A study in Turkey found that the number of AA cases in dermatology outpatient clinics increased after the pandemic, affecting a wide age range of patients. Still, most were adults with no personal or family history of AA (34). A large retrospective cohort study in Korea showed that the risk of autoimmune diseases significantly increased after COVID-19 and was positively correlated with severity, with patients over 40 years old having a significantly increased risk of AA and total alopecia (35). In addition, Rinaldi et al. found that the recurrence rate of AA increased after COVID-19 through a large-scale questionnaire survey (36). This article includes 11 cases of patients who developed new-onset AA after COVID-19, with an average time to onset of about 4 weeks post-infection.
However, there is some disagreement in the research on the relationship between COVID-19 and AA. Rudnicka et al. assessed the relationship between COVID-19 and the severity of existing AA and found that AA symptoms did not significantly worsen after COVID-19, especially in patients who were receiving treatment for AA, thus proposing that mild to moderate COVID-19 is not associated with worsening AA (37). In line with the above findings, among 4 AA patients who contracted COVID-19, there was no significant exacerbation of AA symptoms post-infection.
3.1.2 AA patients may be less susceptible to COVID-19
Research has shown that IFN-γ can lead to the downregulation of ACE2, the receptor for SARS-CoV. Based on this, some scholars have proposed that the elevated levels of IFN-γ in the serum of AA patients may have some role in combating SARS-CoV-2 infection, and this has been confirmed by large-scale cohort studies showing that the risk of COVID-19 is lower in AA patients than in non-AA individuals (38).
3.2 The association between COVID-19 vaccination and AA is controversial
Throughout human history, the development of vaccines has greatly changed the course of disease and human societal development. Usually, the development of a new vaccine takes several decades, but due to the urgency of the SARS-CoV-2 pandemic, the process of developing and administering the COVID-19 vaccine has been greatly accelerated. As a large number of people around the world are being vaccinated, reports of adverse events are also very common (18). This article compiles 36 cases of AA following COVID-19 vaccination that have been reported both domestically and internationally.
However, a nationwide population-based study in Korea found that the incidence of autoimmune connective tissue diseases such as AA, psoriasis, and vitiligo did not significantly increase after COVID-19 vaccination. The study pointed out that COVID-19 vaccination does not increase the risk of most autoimmune diseases, including AA (39). Subsequently, another retrospective cohort study in the country came to a consistent conclusion, that the risk of autoimmune diseases did not increase after vaccination, and that vaccination has potential protective effects against the development of COVID-19-related diseases (35).
3.3 Pathogenesis
3.3.1 Pathogenesis of AA
The current mechanism of AA is not clear. Still, it is generally believed to be related to the imbalance of various immune genes, immune cells, and immune molecules, leading to the destruction of hair follicle immune privilege. Abnormal immune genes are the basis for the occurrence of AA. Genome-wide association studies have found 139 single nucleotide polymorphism sites significantly related to AA patients, including genomic regions that control immune cell phenotypes, such as Treg cells, human leukocyte antigen (HLA), UL16 binding protein (ULBP), etc (40).
The destruction of hair follicle immune privilege is an important link in the pathogenesis of AA. Local micro-trauma, neurological and psychological factors, and infections can all cause the release of cytokines such as interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), thereby destroying the immune privilege state of the hair follicles. This process involves the mediation of various immune cells, including activated natural killer group 2D (NKG 2D+) and cytotoxic T cells (CD 8+) (40, 41).
Genetics plays an important role in AA, with 10% to 20% of AA patients having a family history of the disease. The affected genes are related to immune and inflammatory response genes, such as MHC, CTLA 4, and PRDX 5 (18). In addition, there is a high correlation between AA and other immune-mediated diseases (such as thyroiditis, diabetes, and vitiligo), which further supports the theory of immune pathogenesis in AA (42).
Scientists speculate that the autoimmune attack on hair follicles may also be related to viral infections. Viral infections (such as COVID-19) may induce oxidative stress, leading to upregulation of major histocompatibility complex class I (MHC-I) ligands on hair follicles, further activating T cells, destroying hair follicle cells, and releasing IFN-γ and TNF-α around hair follicles, thereby causing a vicious cycle of inflammation (42).
Psychological stress has also gradually become an important cause of AA. Studies have found that the incidence of life stress events before the onset of AA is significantly higher than in normal people, and people with tendencies towards anxiety, depression, and mental stress are also more likely to develop AA (43).
In summary, the development of AA is the result of multiple factors. Genetic factors, hypersensitivity, autoimmunity, stress, trace element deficiency, hormonal changes, vaccination, and infections are all related to the evolution of AA.
3.3.2 Potential association mechanism between COVID-19 and AA
COVID-19 is a disease associated with systemic immune activation caused by SARS-CoV-2. SARS-CoV-2 has an envelope structure, covered with spike proteins, which infect host cells by binding to the angiotensin-converting enzyme 2 (ACE2) receptor on the surface of host cells. The immune response to COVID-19 is multi-layered, and there is a complex interaction between SARS-CoV-2 infection and the host immune system.
At present, the exact mechanism by which COVID-19 induces alopecia is not clear, but based on the pathophysiology of SARS-CoV-2 and alopecia, some possible mechanisms can be speculated. Research suggests that SARS-CoV-2 infection may be related to autoimmunity, and widely distributed tissue antigens may be targets for cross-reactive antibodies directed against SARS-CoV-2 epitopes (44). COVID-19 may trigger an overactivation of the immune system leading to a cytokine storm and inflammatory response. It has been reported that patients with COVID-19 have lower complement levels and positive autoantibodies (45, 46), while plasma concentrations of pro-inflammatory cytokines such as IFN-γ, TNF-α, IL-6, IL-1, IL-2, and IL-17 are significantly elevated, among which IL-6 is believed to be associated with the inhibition of hair shaft elongation and matrix cell proliferation (47, 48). The increase in these pro-inflammatory cytokines can lead to exacerbated inflammation, impairing the immunotolerance of hair follicles and resulting in autoimmune attacks where immune cells mistakenly target hair follicles or hair papilla cells, thereby inducing alopecia.
In addition, dysfunctional ACE2 and its variants may be one of the reasons for imbalance in the inflammatory microenvironment (49, 50). ACE2 is not only the functional receptor for COVID-19 but also an important endogenous antagonist of the renin-angiotensin system (RAS), which plays a key role in maintaining blood pressure and cardiovascular function. Some studies suggest that RAS also plays a significant role in inflammation and autoimmune processes, and an imbalance in RAS may lead to excessive inflammatory responses (51). Research has found that serum ACE levels are higher in more severe cases of AA, while ACE levels in alopecia tissue are significantly lower than in controls. Angiotensin I may play a role in the inflammation of AA, leading to ACE consumption and reduced tissue levels of this enzyme (52).
Simultaneously, activation of the coagulation cascade can lead to microthrombosis formation and blood vessel obstruction in hair follicles, which may also contribute to the development of alopecia. Furthermore, psychological burdens caused by COVID-19, such as mental stress, strict control measures, social restrictions, and unemployment, may also have significant impacts on the development of alopecia (53).
Given the larger proportion of female patients in hair loss caused by COVID-19, female hormones such as estrogen and progesterone may play important roles. Estrogen and progesterone have immunoregulatory and anti-inflammatory effects and can inhibit the release of pro-inflammatory cytokines. Moreover, estrogen and progesterone have protective effects on hair follicles. Estradiol can regulate hair follicle growth and hair cycle through its receptor, while progesterone can reduce the conversion of testosterone to dihydrotestosterone. Dihydrotestosterone is an active form of testosterone, and its increase may lead to hair loss. Therefore, it can be speculated that the acute damage caused by viral infection leads to a significant reduction in systemic estrogen and progesterone levels in female patients, making female COVID-19 patients more susceptible to AA (54).
In summary, the induction of AA by COVID-19 may be a multifactorial process, including abnormal immune responses, the influence of cytokines, vascular dysfunction, and genetic, psychological, and endocrine factors, etc. Further research and clinical observation are needed to clarify the exact roles of these mechanisms in COVID-19-induced AA.
3.3.3 Potential mechanisms by which COVID-19 vaccination could trigger AA
The possible mechanism for the induction of AA by COVID-19 vaccination is the molecular mimicry phenomenon caused by the induction of spike proteins. The COVID-19 mRNA vaccine delivers an mRNA that encodes a protein identical to the spike protein of SARS-CoV-2, thereby inducing the production of immunogenic spike proteins and triggering T-cell and B-cell adaptive immune responses (55). The antibody-mediated response caused by vaccination may cross-react with self-antigens, leading to autoimmunity. These mRNA vaccines continuously produce specific antibodies, including complement products, anti-platelet factor 4, and polyethylene glycol, thereby stimulating the immune system to switch to a chronic inflammatory state.
Research has found that, compared to a control group, serum levels of PARC/CCL18 and GNLY (granulysin), as well as IgG levels against MX1 and METTL3, are elevated in patients with AA related to the COVID-19 vaccine. Among these, PARC/CCL18 promotes the recruitment of CD4+Th2 cells to the site of lesions (56), while GNLY is a key mediator released by CD8+CTLs, responsible for severe drug-induced delayed hypersensitivity reactions (57). MX1 belongs to interferon-induced GTP-binding proteins (58), and METTL3 plays a role in NK cell activation (59).
Additionally, polyethylene glycol 2000 (PEG 2000) and polysorbate 80, present in mRNA COVID-19 vaccines and the AZD1222/MVCCOV1901 vaccine, are major allergens. T cells from patients with AA associated with mRNA COVID-19 vaccines are significantly activated by PEG 2000 or spike protein, whereas those associated with the AstraZeneca COVID-19 vaccine are activated by polysorbate 80 or spike protein (60).
In summary, post-vaccination with the COVID-19 vaccine, patients may develop AA through an immune response mediated by CD4+Th2 and CD8+Tc2 cells, triggered by vaccine components or spike protein. CD8+CTLs may migrate to hair follicle sites and produce cytotoxic proteins like GNLY, leading to apoptosis of hair follicle cells and ultimately causing AA. The production of autoreactive antibodies and eosinophils by CD4+Th2 cells may also be involved in the immunopathological mechanisms of AA related to the COVID-19 vaccine.
Furthermore, studies have found that autoantibodies such as ANA (antinuclear antibodies), IgG-anti-MX1, and IgG-anti-METTL3 are elevated in patients with autoimmune diseases related to the COVID-19 vaccine (60). However, a direct link between AA induced by COVID-19 or vaccination and autoantibodies like anti-spike protein has not yet been established. More research is needed to elucidate this potential association and the underlying mechanisms.
3.4 Treatment
3.4.1 Treatment for AA caused by COVID-19
Although hair loss caused by COVID-19 is mostly reversible, patients may need a considerable amount of time to recover from it (14). Therefore, it is particularly important to propose specific treatment and care plans for patients with COVID-19 combined with AA.
Western medicine treatments for AA mainly include oral medications such as corticosteroids and immunosuppressants, and topical medications include biological response modifiers and immunostimulants. Some doctors have suggested that immunosuppressive drugs, including JAK inhibitors like tofacitinib, may alter the immune response leading to a worsening of the infection process, and therefore recommend discontinuing these drugs during a COVID-19 (61). However, studies investigating the impact of JAK inhibitors like tofacitinib on the outcome of COVID-19 have found no statistically significant differences in hospitalization rates, ICU admission rates, or severe infections between patients treated with JAK inhibitors like tofacitinib and other patients (61, 62). A recently published systematic review clarified the potential therapeutic effects of JAK inhibitors and type I IFN on treating COVID-19 and confirmed that they can increase the discharge rate of COVID-19, and reduce ICU admission rates and mortality (63). Therefore, JAK inhibitors may be a potential method for treating COVID-19, by disrupting AP2-associated protein kinase 1 (AAK1) signaling to interrupt the integration of the virus with host cells (64).
In addition, as female hormones such as estrogen and progesterone may play important roles in COVID-19, current research is exploring the possibility of using estrogen and progesterone to treat COVID-19 (65). Some researchers suggest that ACE2 inhibitors could benefit autoimmune patients who are more susceptible to infection by preventing organ damage (66). However the use of ACE2 inhibitors is still challenging, and corticosteroid administration in autoimmune diseases can make it more difficult to diagnose and treat COVID-19 because ACE2 inhibitors prevent fever during the disease.
3.4.2 Treatment for alopecia areata caused by COVID-19 vaccination
Since 1984, there have been reports of hair loss following routine immunizations, with vaccine antigens and adjuvants being considered potential triggers for T-cell-mediated immune responses. Topical corticosteroids, intralesional corticosteroid injections, and topical immunotherapy are the main treatments for AA triggered by COVID-19. A study in South Korea reported a recurrence rate of 27.6% in patients with severe AA who received pulse steroid therapy, with all recurrent patients achieving complete remission after repeated pulse steroid therapy (67). However, patients who relapsed after the COVID-19 vaccine continued to show disease progression despite repeated pulse steroid therapy. It is speculated that elevated levels of vaccine-induced INF-γ may promote the outbreak of T-cell-mediated immune responses, leading to immune dysregulation in susceptible individuals and resulting in treatment-resistant AA (31).
There are reports of patients completely recovering from severe AA using PRP (platelet-rich plasma) therapy, which is more convenient and less toxic than other treatments (32). In addition, studies have shown that tofacitinib and baricitinib can successfully treat AA related to the COVID-19 vaccine (60). However, larger sample size studies are still needed to assess the efficacy of JAK inhibitors in treating AA related to the COVID-19 vaccine.
4 Conclusion
Currently, there is not enough evidence to prove a direct relationship between COVID-19 and an increase in the incidence of AA, but studies have found that COVID-19 patients seem to be more prone to trigger AA, while AA patients may be less likely to contract COVID-19. The induction of AA by SARS-CoV-2 infection or COVID-19 vaccination may be a multi-factorial process, including abnormal immune responses, the influence of cytokines, vascular dysfunction, genetic, psychological, and endocrine factors, etc. JAK inhibitors, estrogen, and progesterone may be potential treatments for COVID-19. Further research into the possible pathogenic mechanisms of COVID-19 and AA is of great significance for the prevention, diagnosis, treatment, and prognosis of susceptible populations.
Author contributions
YX: Writing – original draft. SL: Methodology, Supervision, Writing – original draft. SL: Data curation, Formal analysis, Writing – original draft. YC: Data curation, Formal analysis, Writing – original draft. MD: Data curation, Formal analysis, Writing – original draft. YX: Data curation, Formal analysis, Writing – original draft. DY: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Henry BM, Vikse J. Clinical characteristics of covid-19 in China. N Engl J Med. (2020) 382:1860–1. doi: 10.1056/NEJMc2005203
2. Wambier CG, Vaño-Galván S, McCoy J, Gomez-Zubiaur A, Herrera S, Hermosa-Gelbard Á, et al. Androgenetic alopecia present in the majority of patients hospitalized with COVID-19: The ‘Gabrin sign’. J Am Acad Dermatol. (2020) 83:680–2. doi: 10.1016/j.jaad.2020.05.079
3. Harries M, Macbeth AE, Holmes S, Thompson AR, Chiu WS, Gallardo WR, et al. Epidemiology, management and the associated burden of mental health illness, atopic and autoimmune conditions, and common infections in alopecia areata: protocol for an observational study series. BMJ Open. (2021) 11:e045718. doi: 10.1136/bmjopen-2020-045718
4. Simakou T, Butcher JP, Reid S, Henriquez FL. Alopecia areata: A multifactorial autoimmune condition. J Autoimmun. (2019) 98:74–85. doi: 10.1016/j.jaut.2018.12.001
5. Hayran Y, Yorulmaz A, Gür G, Aktaş A. Different hair loss patterns in two pediatric patients with COVID-19-associated multisystem inflammatory syndrome in children. Dermatol Ther. (2021) 34:e14820. doi: 10.1111/dth.14820
6. Capalbo A, Giordano D, Gagliostro N, Balampanos CG, Persechino F, Orrù F, et al. Alopecia areata in a COVID-19 patient: A case report. Dermatol Ther. (2021) 34:e14685. doi: 10.1111/dth.14685
7. New onset of alopecia areata in a patient with SARS-CoV-2 infection: Possible pathogenetic correlations? (2023). Available online at: https://pubmed.ncbi.nlm.nih.gov/33738910/
8. Alotaibi MA, Altaymani A, Al-Omair A, Alghamdi W. Viral-induced rapidly progressive alopecia universalis: A case report and literature review. Cureus. (2023) 15:e37406. doi: 10.7759/cureus.37406
9. Kageyama R, Ito T, Nakazawa S, Shimauchi T, Fujiyama T, Honda T. A case of telogen effluvium followed by alopecia areata after SARS-CoV-2 infection. J Dermatol. (2023) 50:e32–4. doi: 10.1111/1346-8138.16590
10. Phong CH, Babadjouni A, Nguyen C, Kraus CN, Mesinkovska NA. Not just thinning: A case of alopecia universalis after mild COVID-19. JAAD Case Rep. (2022) 25:1–3. doi: 10.1016/j.jdcr.2022.04.024
11. FIvenson D. COVID-19: association with rapidly progressive forms of alopecia areata. Int J Dermatol. (2021) 60:127. doi: 10.1111/ijd.15317
12. Vazquez OA, Safeek RH, Komberg J, Becker H. Alopecia areata treated with advanced platelet-rich fibrin using micronization. Plast Reconstr Surg Glob Open. (2022) 10:e4032. doi: 10.1097/GOX.0000000000004032
13. Chidiac G, Chrabieh R, Maamari M, El Khoury J, Ayoub N. Spontaneous complete resolution of alopecia totalis post SARS-CoV-2 infection. JAAD Case Rep. (2022) 27:106–9. doi: 10.1016/j.jdcr.2022.07.027
14. Alajlan AM, AlZamil LR, Aseri AM. Tofacitinib treatment in patients with active COVID-19 infection. Cureus. (2021) 13:e17957. doi: 10.7759/cureus.17957
15. Berbert Ferreira S, Gavazzoni Dias MFR, Berbert Ferreira R, Neves Neto AC, Trüeb RM, Lupi O. Rapidly progressive alopecia areata totalis in a COVID-19 patient, unresponsive to tofacitinib. J Eur Acad Dermatol Venereol. (2021) 35:e411–2. doi: 10.1111/jdv.17170
16. Essam R, Ehab R, Al-Razzaz R, Khater MW, Moustafa EA. Alopecia areata after ChAdOx1 nCoV-19 vaccine (Oxford/AstraZeneca): a potential triggering factor? J Cosmet Dermatol. (2021) 20:3727–9. doi: 10.1111/jocd.14459
17. Gamonal SBL, Marques NCV, Pereira HMB, Gamonal ACC. New-onset systemic lupus erythematosus after ChAdOX1 nCoV-19 and alopecia areata after BNT162b2 vaccination against SARS-CoV-2. Dermatol Ther. (2022) 35:e15677. doi: 10.1111/dth.15677
18. Hernández Arroyo J, Izquierdo-Condoy JS, Ortiz-Prado E. A case series and literature review of telogen effluvium and alopecia universalis after the administration of a heterologous COVID-19 vaccine scheme. Vaccines (Basel). (2023) 11:444. doi: 10.3390/vaccines11020444
19. Ho JD, McNish A, McDonald L, Burrell C, Smith-Matthews S. Alopecia universalis with unusual histopathologic features after vaccination with ChAdOx1 nCoV-19 (AZD1222). JAAD Case Rep. (2022) 25:4–8. doi: 10.1016/j.jdcr.2022.05.002
20. Abdalla H, Ebrahim E. Alopecia areata universalis precipitated by SARS-coV-2 vaccine: A case report and narrative review. Cureus. (2022) 14:e27953. doi: 10.7759/cureus.27953
21. Lo A, Mir A, Sami N. Letter in Reply: Alopecia areata after SARS-CoV-2 vaccination. JAAD Case Rep. (2022) 25:25–6. doi: 10.1016/j.jdcr.2022.04.022
22. Scollan ME, Breneman A, Kinariwalla N, Soliman Y, Youssef S, Bordone LA, et al. Alopecia areata after SARS-CoV-2 vaccination. JAAD Case Rep. (2022) 20:1–5. doi: 10.1016/j.jdcr.2021.11.023
23. Babadjouni A, Phong CH, Nguyen C, Mesinkovska NA. COVID-19 vaccination related exacerbations of hair loss in patients with moderate-to-severe alopecia areata on systemic therapy. JAAD Case Rep. (2022) 29:181–5. doi: 10.1016/j.jdcr.2022.08.016
24. AlZahrani F, Perlau MJ, Fiorillo L. Alopecia areata and subsequent Marie Antoinette syndrome following COVID-19 infection and vaccination: A case report. SAGE Open Med Case Rep. (2023) 11:2050313X231152065. doi: 10.1177/2050313X231152065
25. Iwata K, Kunisada M. Alopecia universalis after injection of messenger RNA COVID-19 vaccine. A Case Rep IDCases. (2023) 33:e01830. doi: 10.1016/j.idcr.2023.e01830
26. Ganjei Z, Yazdan Panah M, Rahmati R, Zari Meidani F, Mosavi A. COVID-19 vaccination and alopecia areata: a case report and literature review. Clin Case Rep. (2022) 10:e6039. doi: 10.1002/ccr3.6039
27. Martora F, Fornaro L, Picone V, Marasca D, Gargiulo M, Annunziata MC, et al. Herpes zoster and alopecia areata following mRNA BNT162b2 COVID-19 vaccine: Controversial immune effects. J Cosmet Dermatol. (2023) 22:36–8. doi: 10.1111/jocd.15465
28. Fusano M, Zerbinati N, Bencini PL. Alopecia areata after COVID- 19 vaccination: Two cases and review of the literature. Dermatol Rep. (2022) 14:9495. doi: 10.4081/dr.2022.9495
29. Genco L, Cantelli M, Noto M, Battista T, Patrì A, Fabbrocini G, et al. Alopecia areata after COVID-19 vaccines. Skin Appendage Disord. (2023) 9:141–3. doi: 10.1159/000528719
30. Su HA, Juan CK, Chen YC. Alopecia areata following ChAdOx1 nCoV-19 vaccination (Oxford/AstraZeneca). J Formos Med Assoc. (2022) 121:2138–40. doi: 10.1016/j.jfma.2022.03.006
31. Chen CH, Chen YY, Lan CCE. Intractable alopecia areata following the second dose of COVID-19 vaccination: Report of two cases. Dermatol Ther. (2022) 35:e15689. doi: 10.1111/dth.15689
32. Teng HC, Chen HH. Platelet-rich plasma in the treatment of alopecia areata after COVID-19 vaccination. Clin Case Rep. (2023) 11:e7342. doi: 10.1002/ccr3.7342
33. Christensen RE, Jafferany M. Association between alopecia areata and COVID-19: A systematic review. JAAD Int. (2022) 7:57–61. doi: 10.1016/j.jdin.2022.02.002
34. Kutlu Ö, Aktaş H, İmren IG, Metin A. Short-term stress-related increasing cases of alopecia areata during the COVID-19 pandemic. J Dermatolog Treat. (2022) 33:1177. doi: 10.1080/09546634.2020.1782820
35. Lim SH, Ju HJ, Han JH, Lee JH, Lee WS, Bae JM, et al. Autoimmune and autoinflammatory connective tissue disorders following COVID-19. JAMA Netw Open. (2023) 6:e2336120. doi: 10.1001/jamanetworkopen.2023.36120
36. Rinaldi F, Trink A, Giuliani G, Pinto D. Italian survey for the evaluation of the effects of coronavirus disease 2019 (COVID-19) pandemic on alopecia areata recurrence. Dermatol Ther (Heidelb). (2021) 11:339–45. doi: 10.1007/s13555-021-00498-9
37. Rudnicka L, Rakowska A, Waskiel-Burnat A, Kurzeja M, Olszewska M. Mild-to-moderate COVID-19 is not associated with worsening of alopecia areata: A retrospective analysis of 32 patients. J Am Acad Dermatol. (2021) 85:723–5. doi: 10.1016/j.jaad.2021.05.020
38. Ahn GS, Oulee A, Shahsavari S, Martin A, Wu JJ. The risk of COVID-19 infection in individuals with alopecia areata. J Clin Aesthet Dermatol. (2022) 15:19–21.
39. Ju HJ, Lee JY, Han JH, Lee JH, Bae JM, Lee S. Risk of autoimmune skin and connective tissue disorders after mRNA-based COVID-19 vaccination. J Am Acad Dermatol. (2023) 89:685–93. doi: 10.1016/j.jaad.2023.05.017
40. Petukhova L, Duvic M, Hordinsky M, Norris D, Price V, Shimomura Y, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. (2010) 466:113–7. doi: 10.1038/nature09114
41. Zhang X, Liu Y. Pathogenesis and treatment progress of alopecia areata: an immunological perspective. J Clin Dermatol. (2021) 50:125–8. doi: 10.16761/j.cnki.1000-4963.2021.02.018
42. Herzum A, Viglizzo G, Gariazzo L, Garibeh E, Occella C. Pediatric alopecia areata following COVID-19 infection. J Cosmet Dermatol. (2023) 22:734–6. doi: 10.1111/jocd.15618
43. Lu S, Zhong J, Wei D, Su T, Sun X. Overview of research on the influence of emotional factors on alopecia areata. Hunan J Traditional Chin Med. (2017) 33:165–6. doi: 10.16808/j.cnki.issn1003-7705.2017.12.076
44. Mohkhedkar M, Venigalla SSK, Janakiraman V. Untangling COVID-19 and autoimmunity: Identification of plausible targets suggests multi organ involvement. Mol Immunol. (2021) 137:105–13. doi: 10.1016/j.molimm.2021.06.021
45. High prevalence of antinuclear antibodies and lupus anticoagulant in patients hospitalized for SARS-CoV2 pneumonia (2023). Available online at: https://pubmed.ncbi.nlm.nih.gov/32462425/
46. Autoantibodies related to systemic autoimmune rheumatic diseases in severely ill patients with COVID-19 (2023). Available online at: https://pubmed.ncbi.nlm.nih.gov/32581086/
47. Lee JS, Shin EC. The type I interferon response in COVID-19: implications for treatment. Nat Rev Immunol. (2020) 20:585–6. doi: 10.1038/s41577-020-00429-3
48. Gadotti AC, de Castro Deus M, Telles JP, Wind R, Goes M, Garcia Charello Ossoski R, et al. IFN-γ is an independent risk factor associated with mortality in patients with moderate and severe COVID-19 infection. Virus Res. (2020) 289:198171. doi: 10.1016/j.virusres.2020.198171
49. Najafi S, Rajaei E, Moallemian R, Nokhostin F. The potential similarities of COVID-19 and autoimmune disease pathogenesis and therapeutic options: new insights approach. Clin Rheumatol. (2020) 39:3223–35. doi: 10.1007/s10067-020-05376-x
50. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. (2020) 8:e21. doi: 10.1016/S2213-2600(20)30116-8
51. Zhu H, Zhang L, Ma Y, Zhai M, Xia L, Liu J, et al. The role of SARS-CoV-2 target ACE2 in cardiovascular diseases. J Cell Mol Med. (2021) 25:1342–9. doi: 10.1111/jcmm.16239
52. Fahim S, Montazer F, Tohidinik HR, Naraghi ZS, Abedini R, Nasimi M, et al. Serum and tissue angiotensin-converting enzyme in patients with alopecia areata. Indian J Dermatol Venereol Leprol. (2019) 85:295–9. doi: 10.4103/ijdvl.IJDVL_158_17
53. Aryanian Z, Balighi K, Hatami P, Afshar ZM, Mohandesi NA. The role of SARS-CoV-2 infection and its vaccines in various types of hair loss. Dermatol Ther. (2022) 35:e15433. doi: 10.1111/dth.15433
54. Czech T, Sugihara S, Nishimura Y. Characteristics of hair loss after COVID-19: A systematic scoping review. J Cosmet Dermatol. (2022) 21:3655–62. doi: 10.1111/jocd.15218
55. Magro C, Nuovo G, Mulvey JJ, Laurence J, Harp J, Crowson AN. The skin as a critical window in unveiling the pathophysiologic principles of COVID-19. Clin Dermatol. (2021) 39:934–65. doi: 10.1016/j.clindermatol.2021.07.001
56. Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. (2014) 32:659–702. doi: 10.1146/annurev-immunol-032713-120145
57. Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. (2008) 14:1343–50. doi: 10.1038/nm.1884
58. Braun BA, Marcovitz A, Camp JG, Jia R, Bejerano G. Mx1 and Mx2 key antiviral proteins are surprisingly lost in toothed whales. Proc Natl Acad Sci U.S.A. (2015) 112:8036–40. doi: 10.1073/pnas.1501844112
59. Song H, Song J, Cheng M, Zheng M, Wang T, Tian S, et al. METTL3-mediated m6A RNA methylation promotes the anti-tumour immunity of natural killer cells. Nat Commun. (2021) 12:5522. doi: 10.1038/s41467-021-25803-0
60. Wang CW, Wu MY, Chen CB, Lin WC, Wu J, Lu CW, et al. Clinical characteristics and immune profiles of patients with immune-mediated alopecia associated with COVID-19 vaccinations. Clin Immunol. (2023) 255:109737. doi: 10.1016/j.clim.2023.109737
61. Aşkın Ö, Özkoca D, Uzunçakmak TK, Serdaroğlu S. Evaluation of the alopecia areata patients on tofacitinib treatment during the COVID-19 pandemic. Dermatol Ther. (2021) 34:e14746. doi: 10.1111/dth.14746
62. Agrawal M, Brenner EJ, Zhang X, Modesto I, Woolcott J, Ungaro RC, et al. Characteristics and outcomes of IBD patients with COVID-19 on tofacitinib therapy in the SECURE-IBD registry. Inflammation Bowel Dis. (2021) 27:585–9. doi: 10.1093/ibd/izaa303
63. Walz L, Cohen AJ, Rebaza AP, Vanchieri J, Slade MD, Dela Cruz CS, et al. JAK-inhibitor and type I interferon ability to produce favorable clinical outcomes in COVID-19 patients: a systematic review and meta-analysis. BMC Infect Dis. (2021) 21:47. doi: 10.1186/s12879-020-05730-z
64. Seif F, Aazami H, Khoshmirsafa M, Kamali M, Mohsenzadegan M, Pornour M, et al. JAK inhibition as a new treatment strategy for patients with COVID-19. Int Arch Allergy Immunol. (2020) 181:467–75. doi: 10.1159/000508247
65. Lovre D, Bateman K, Sherman M, Fonseca VA, Lefante J, Mauvais-Jarvis F. Acute estradiol and progesterone therapy in hospitalised adults to reduce COVID-19 severity: a randomised control trial. BMJ Open. (2021) 11:e053684. doi: 10.1136/bmjopen-2021-053684
66. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. (2020) 395:1417–8. doi: 10.1016/S0140-6736(20)30937-5
Keywords: COVID-19, alopecia areata, vaccination, correlation, pathogenesis, treatment
Citation: Xie Y, Lv S, Luo S, Chen Y, Du M, Xu Y and Yang D (2024) The correlation between corona virus disease 2019 and alopecia areata: a literature review. Front. Immunol. 15:1347311. doi: 10.3389/fimmu.2024.1347311
Received: 30 November 2023; Accepted: 01 May 2024;
Published: 03 July 2024.
Edited by:
Pier Paolo Sainaghi, University of Eastern Piedmont, ItalyReviewed by:
Zaiga Nora-Krukle, RSU, LatviaChuang-Wei Wang, Linkou Chang Gung Memorial Hospital, Taiwan
Copyright © 2024 Xie, Lv, Luo, Chen, Du, Xu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dingquan Yang, eWRxbHhAMTYzLmNvbQ==
 Ying Xie
Ying Xie Shuying Lv1,2
Shuying Lv1,2 Dingquan Yang
Dingquan Yang