
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 02 February 2024
Sec. Inflammation
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1345625
The P2X7 receptor (P2X7R), a non-selective cation channel modulated by adenosine triphosphate (ATP), localizes to microglia, astrocytes, oligodendrocytes, and neurons in the central nervous system, with the most incredible abundance in microglia. P2X7R partake in various signaling pathways, engaging in the immune response, the release of neurotransmitters, oxidative stress, cell division, and programmed cell death. When neurodegenerative diseases result in neuronal apoptosis and necrosis, ATP activates the P2X7R. This activation induces the release of biologically active molecules such as pro-inflammatory cytokines, chemokines, proteases, reactive oxygen species, and excitotoxic glutamate/ATP. Subsequently, this leads to neuroinflammation, which exacerbates neuronal involvement. The P2X7R is essential in the development of neurodegenerative diseases. This implies that it has potential as a drug target and could be treated using P2X7R antagonists that are able to cross the blood-brain barrier. This review will comprehensively and objectively discuss recent research breakthroughs on P2X7R genes, their structural features, functional properties, signaling pathways, and their roles in neurodegenerative diseases and possible therapies.
P2X7 is a microglial protein highly expressed in the central nervous system (CNS) and functions as an ATP-gated ion channel (1). It plays a crucial role in mediating ATP-driven hazardous signaling, as its activation leads to the opening of pores and non-selective transport of Ca2+, Na+, and K+ (2). The expression of P2X7R in immunocompetent cells of the central and peripheral nervous system has been extensively described (3). It is expressed in microglia, astrocytes, and oligodendrocytes in the CNS (4). However, there is an ongoing discussion regarding its expression in neurons (5, 6). The P2X7-EFGP BAC transgenic mouse model overexpresses functional fluorescently labeled P2X7, which is located at the protein level and provides stronger signaling. However, neuronal P2X7 protein expression is not induced under pathological conditions (7). The study notes that P2X7 is predominantly found in microglia and oligodendrocytes. Recently, researchers generated humanized P2X7R (hP2RX7) by inserting human P2RX7 cDNA into the mouse P2RX7 locus. They found that P2X7R is specifically expressed in glutamatergic pyramidal neurons in the hippocampus (8). P2X7R expression was also found on neuronal progenitor cells and mature neuronal cells of human hiPSC origin. This study also indicated that P2X7R is not localized to the cell membrane of neurons and may not directly mediate neurotoxicity (9). The presence of P2X7R in neurons and glial cell populations is supported by the P2RX7-EGFP reporter mouse, which expresses enhanced green fluorescent protein (EGFP) (10).
ATP is a co-transmitter released by neurons and can be influx into the extracellular space from glial cells (astrocytes, oligodendrocytes, microglia) in the CNS to regulate neuronal activity (11). P2X7 is extensively expressed in microglial cells within the CNS (12). Activating P2X7 initiates the assembly of NLR family pyrin structural domains containing NLRP3 in microglia. This results in the activation of cysteinyl asparagine-1, which increases cellular metabolism by boosting both glycolysis and oxidative phosphorylation. In turn, this causes the secretion of IL-1β, IL-6, TNF-α, and IL-18, thereby initiating a neuroinflammatory response (13, 14). Stimulation of P2X7 also induces the discharge of different pro-inflammatory substances like TNF-α (15), IL-6 (16), CCL2 (17), excitotoxic glutamate (17), and reactive oxygen species (ROS) (18). These mediators result in neuroinflammation, proliferation of reactive glial cells, and cell death. It is important to note that these substances cause neuroinflammation and cellular damage. The main pathogenic alterations in neurodegenerative disorders, like Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), Multiple sclerosis (MS), and Amyotrophic lateral sclerosis (ALS) that are prevalent globally, consist of several neurodegenerative reactions, leading to substantial ATP release through permeable plasma membranes of neural tissues (19). This results in high ATP concentration activating the P2X7R, causing neurological damage. Therefore, extensive research explores the modulation of P2X7R-mediated pathways as a possible treatment for neurodegenerative diseases, aiming to slow or remedy their progression. Our review concentrates on the P2X7R signaling pathway, the extent of its participation in neurodegenerative disorders, and available therapeutic interventions.
The P2X7 gene, which codes for the P2X7R, is situated on the long arm of chromosome 12 at 12q24.31 with a length of 53 kb along with 13 exons (Figure 1). P2RX4 (12.q24.32) is located in proximity to a mitophagosome. (Source: www.ncbi.nlm.nih.gov/gene/5027).
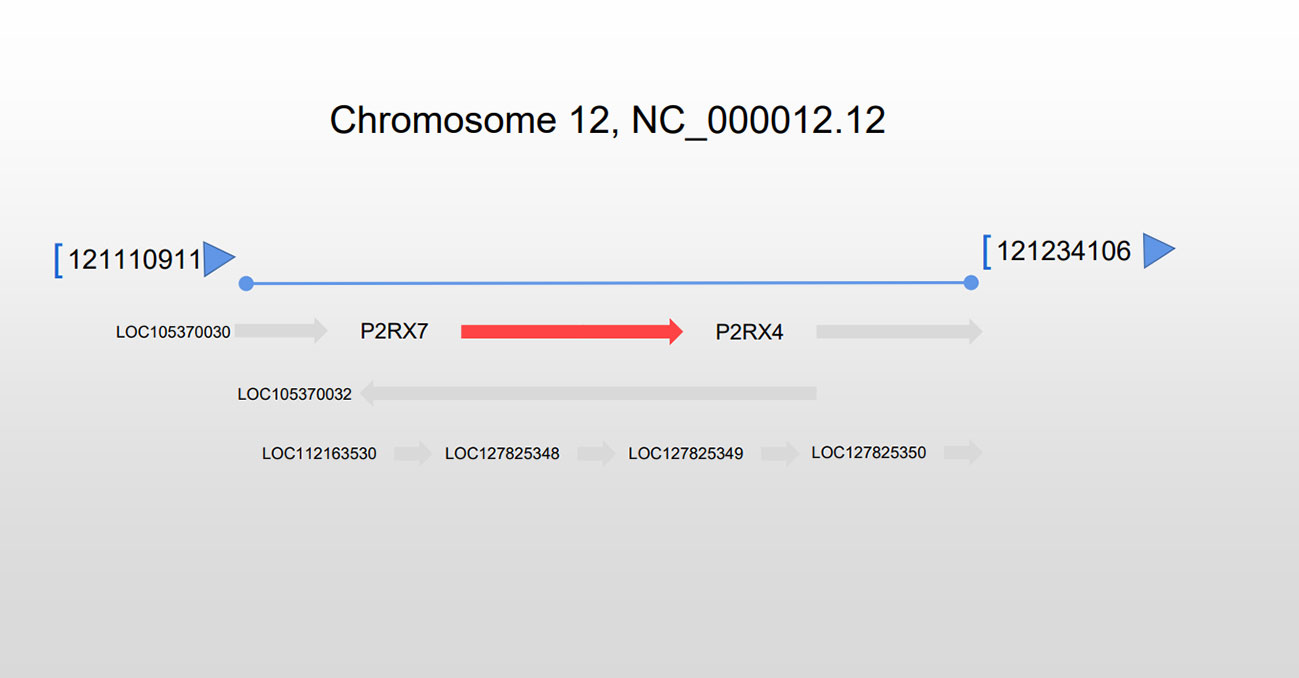
Figure 1 Location of the P2RX7 gene. The P2RX7 gene is situated on chromosome 12 at q24.31, extending 53 kb, and situated next to the P2RX4 gene.
P2XR is a trimeric ion channel composed of three subunits. Each subunit contains two structural domains: the extracellular cysteine-rich structural domain and the C- and N-termini that enhance channel function (20). P2X7R belongs to the P2XR family and is extensively expressed in body cells, particularly in macrophages and microglia (21). Furthermore, the P2X7R has ATP-gated ion channel activity (22). The P2X7R requires higher levels of ATP to activate compared to other P2XR (23). P2X7 encodes a 595-amino acid protein, which forms a trimeric ion channel assembly (23). The P2X7R protein comprises cellular extracellular, transmembrane, and cytoplasmic components (24). The ligand binding site is formed through subunit interactions among three extracellular structural domains, totaling 282 amino acids (13, 25). Six alpha subunits form two 24 amino acid transmembrane helices, consisting of three TM1 and three TM2 units (26). The cytoplasmic cap is composed of two N-terminal β strands, which converge proximally at the C-terminus with β15 and α8 (25). The N-terminus starts about 26 amino acids earlier and includes a conserved protein kinase C (PKC) phosphorylation consensus site [TX (K/R)] (13). Following this, TM1 is the first transmembrane structural domain that ranges from a26 to 46 and contains the ATP-binding pocket (27). It is then followed by the voluminous extracellular structural domain that maintains conformational stability through a highly conserved protein fold constructed with multiple disulfide bonds between cysteine residues (28). The second transmembrane structural domain following the extracellular area is TM2 (amino acid 330 to 349), which contains numerous essential pore-lining residues (28). These residues regulate channel gating: S342 is situated at the narrowest segment of the channel, and Y343 is phosphorylated to modify gating (29).
The P2X7R has the lengthiest C-terminal among all P2XR (30). Moreover, in the P2X7 subunit, the carboxyl-terminal tail (amino acid 356-595) is the most remarkable structural domain, stabilizing macropore opening, distinct to this receptor subtype. The initial region of the C-terminal tail has a cysteine-rich area and is palmitoylated on at least five residues, specifically C362, C363, C374, C377, and S360. The palmitoylated residues serve as hinges, thus enabling each C-terminal tail to form a binding site for guanosine diphosphate or triphosphate (GDP/GTP) and two zinc bins (31).
Multiple motifs that bind lipids and proteins are present in this structural domain. Specifically, the region 436-531 shares homology with a segment of tumor necrosis factor receptor 1 (TNFR1) that includes the death domain, while residues 573-590 exhibit homology with the endotoxin- binding region of the serum LPS-binding protein (LBP). Additionally, there are multiple regions that share homology (32) (Figure 2).
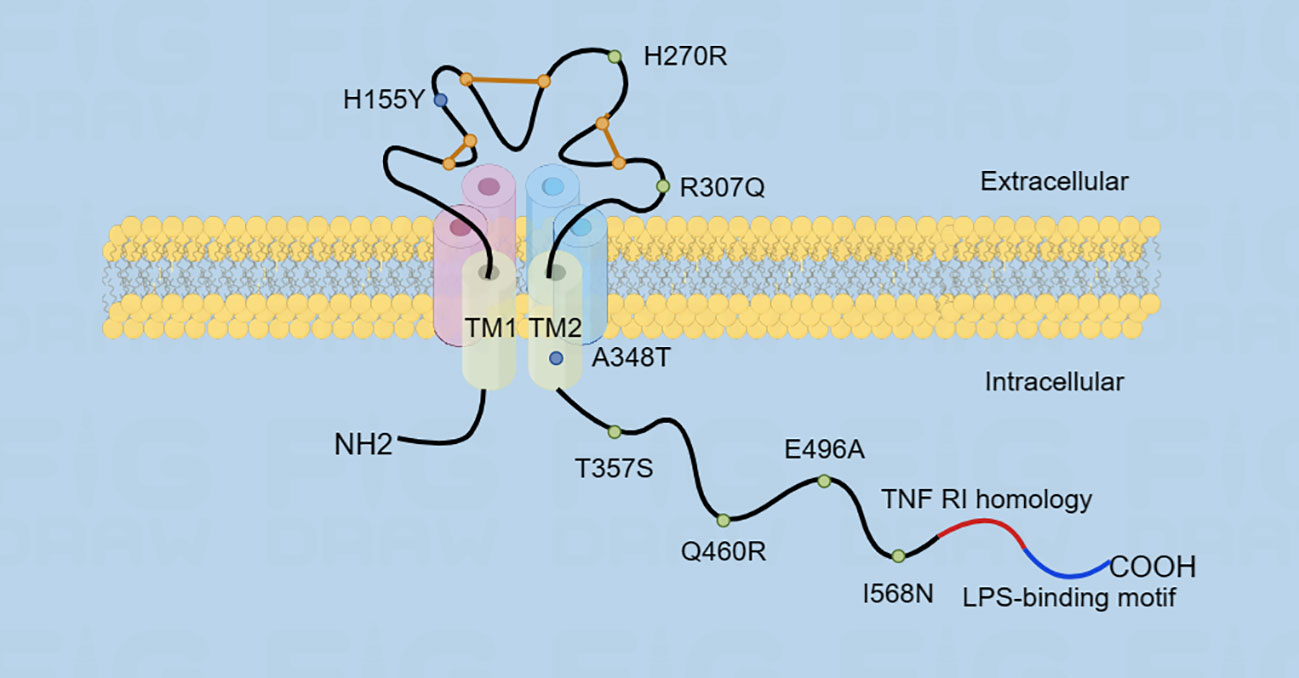
Figure 2 The figure illustrates the structure of P2X7R, which consists of transmembrane structural domains TM1 and TM2. The extracellular structural domain is represented by an orange line, which maintains the conformational stability through a disulfide bond. The carboxyl-terminal tail is very long and contains two homologous region sequences. Additionally, the figure shows the location of single nucleotide polymorphisms (SNPs) in the P2X7 receptor, with gain-of-function SNPs represented by blue dots and loss-of-function SNPs represented by green dots.
The P2X7R is notable within the P2XR family for its distinct characteristics. Structurally, it consists of 3-6 homologous subunits (33) that commonly combine to form a trimeric complex in order to create a functional P2X7R. The C-terminus of the P2X7R is lengthier compared to that of other P2X7R. It plays a role in regulating the receptor’s functions, which include signaling pathways, cellular localization, protein-protein interactions, and post-translational modifications (34, 35). As an ATP-gated non-selective cation channel, the P2X7R mediates the inward flow of Na+ and Ca2+ and the efflux of K+, resulting in inward current/depolarization (36, 37). Normal physiological conditions are maintained by extracellular divalent cations such as calcium, magnesium, zinc, and copper ions (38–40), protons (41), and anions (42), which keep receptor activity at low levels. There are two potential reasons for the expansion of P2X7R. One is its binding to the agonist-binding pocket over an extended period, which causes the channel to expand gradually (43). The other is that P2X7R is able to transport large organic cations directly (44) or form a large conductance pore (45). P2X7R has a different conductance than other P2XRs in terms of its response to activation. In comparison, other receptors show a fast and brief response that diminishes within a few seconds. On the other hand, P2X7R do not typically exhibit desensitization and allow for continued inward flow of Ca2+ (39).
The stimulation of P2X7R leads to neurodegeneration through the release of several bioactive substances, including pro-inflammatory cytokines (IL16, IL1β, IL18, TNF-α) (46–48), chemokines (CCL3, CXCL2) (49, 50), proteases (Rac1, NADPH oxidase 2) (48), reactive oxygen species (ROS) (51, 52), and NO (53, 54), as well as excitotoxic glutamate (55, 56) or ATP (19). P2X7R acts as a crucial initiator of inflammation since microglia recognize pathogen-associated molecules (PAMP), such as lipopolysaccharide (LPS), or danger-associated molecular patterns (DAMP), like ATP (57). Upon exposure to these substances, P2X7R stimulates the release of the cytokine interleukin-1β (IL-1β) that facilitates the inflammatory response. Conversely, the inability of astrocytes to release IL-1β is attributed to NLRP3 deficiency (58).
MAPKs, or mitogen-activated protein kinases, are serine/threonine kinases that regulate gene expression. This includes p38MAPK, ERK (extracellular signal-regulated kinase), and JNK (c-jun N-terminal kinase). They respond to extracellular stimuli and regulate various physiological processes, such as gene expression, mitosis, metabolism, cellular differentiation and motility, stress response, and cell survival or death (59, 60).
LPS can cause inflammation in microglia by activating the phosphorylation of three vital MAPK pathways - p38, JNK, and ERK - resulting in the secretion of pro-inflammatory cytokines (61). Researchers observed that inhibiting P2X7R antagonist brilliant blue G (BBG)and treating BV2 cells with LPS can halt MAPK activation by preventing the phosphorylation of p38MAPK, JNK, and ERK. This treatment decreased the secretion and expression of pro-inflammatory cytokines, such as IL-16, IL-1β, and TNF-α mRNA. Remarkably, using a MAPK inhibitor further intensified the inhibitory effect on MAPK. The findings indicate that BBG can effectively alleviate the neuroinflammatory response triggered by LPS in BV2 cells by inhibiting the MAPK signaling pathway (46). Inhibiting MAPKs led to significant neuroprotection in models of subarachnoid hemorrhage, cerebral hemorrhage, and PD (62, 63). ATP activates JNK, p38, and ERK. While JNK and ERK contribute to the production of TNF mRNA, p38 does not affect elevated TNF mRNA levels. Instead, it inhibits TNF mRNA transport from the nucleus to the cytoplasm and stimulates TNF release from microglial cells. The release is dependent on P2X7R, which may play a role in activating JNK and p38. Downstream from P2X7R, members of the tyrosine-protein kinase SRC (SRC) family (possibly PTK) are involved in activating JNK and p38 (15, 64). TNF release from microglia treated with 2’(3)-omicron-(4-Benzoylbenzoyl) adenosine-5’-triphosphate (BzATP) in neuron-microglia co-cultures reduced glutamate-induced neuronal cell death. P38 and JNK activation appears to be independent of inward Ca2+ flow (64), while a different study suggests a potential relationship between the inward flow of Ca2+ through P2X7R and the activation of p38 and JNK (65).
In a rat model of PD, the administration of LPS triggers an inflammatory response that results in the degeneration of dopaminergic neurons in the nigrostriatal pathway (66, 67). Microglial activation and the loss of dopaminergic neurons in the nigrostriatal system have been linked to enhanced expression of P2X7R in microglia and elevated levels of p38MAPK phosphorylation. The inhibition of P2X7R with BBG reduces microglial activation and prevents p38MAPK-induced degeneration of dopaminergic neurons (68). P2X7R antagonists effectively prevented the depletion of striatal dopamine stores induced by 6-hydroxydopamine (69, 70). Nonetheless, other studies found that P2X7R deficiency or inhibition did not impact dopaminergic neuron loss induced by 1-methyl-4-phenylpyridine or rotenone in chemical PD models (71). This lack of consistency might be ascribed to the extent of nigral damage induced by different models or the duration of P2X7R antagonist treatment.
Activation of P2X7 is associated with the AKT and ERK pathways, leading to cell death. However, it is not linked to the release of IL-1 family cytokines (IL-18, IL-1β, IL-1α) (47). In cortical astrocytes of rats, P2X7 activation induces AKT phosphorylation (72). However, the stimulation of P2X7 with BzATP in microglial cells results in ERK and AKT dephosphorylation (47), which might be attributed to their different cell types (73).
Stimulating P2X7R activates MAP kinases, leading to increased a disintegrin and metalloproteases (ADAM) phosphorylation. These ADAMs, specifically ADAM9, -10, and -17, facilitate the non-amyloidogenic deformation α-processing of the amyloid precursor protein (APP) (74–76). Furthermore, a separate ADAM-independent process for APP α processing has been observed in mouse and human neuroblastoma cells, primary mouse astrocytes, and neural precursor cells. This process differs from the α-secretase activity of ADAM9, -10, and -17 in response to APP, primarily leading to α-cleavage. Moreover, it promotes the release of the soluble ectodomain of APP (sAPPα) while inhibiting the production of sAPPβ and amyloid β (A-β) peptides. This process involves the Erk1/2 and JNK pathways and is dependent on P2X7R (77). Another study on glioma U251 cells also reported the joint involvement of Erk1/2 and JNK in APP α-cleavage (78). Furthermore, stimulation of P2X7R leads to ERM (Ezrin/Radixin/Moesin) phosphorylation, which relocates to the plasma membrane and interacts with P2X7R. This interaction causes APP processing and subsequent protein hydrolysis, resulting in sAPPα shedding dependent on P2X7R. Additionally, P2X7R signaling triggers ERM phosphorylation in Neuro2a cells via upstream Rho kinase and MAPK activation, while downstream PI3K activity is stimulated (79) (Figure 3).

Figure 3 Overview of the P2X7-mediated signaling pathway. P2X7R triggers the activation of P38/ERK/JNK MAPK, leading to the build-up of TNF mRNA in the cytosol, which contributes to neuroinflammation. Moreover, sAPPα, released due to non-amyloid α cleavage of APP, is also impacted by P2X7R, following two distinct pathways, one ADAM-dependent and the other ADAM-independent. ROS can be toxic to neurons both inside and outside the cell, leading to neuronal death and promoting neurodegenerative disease progression. P2X7R mediates NOX-2 activation, resulting in the accumulation of ROS. P2X7R also stimulates the production of NLRP3 inflammatory vesicles and IL-1β secretion, resulting in pro-inflammatory effects outside of the cell.
Mitochondrial damage from inflammation and metabolic stress not only impairs energy production but also triggers the accumulation of ROS. This ultimately causes neuronal cell death and exacerbates the progression of neurodegenerative conditions (80, 81). The primary cause of progressive neuronal death is thought to be the inflammatory response that results from the activation of microglia, which causes ROS to accumulate (82, 83). ATP-induced neurodegeneration and oxidative stress are significant contributors to neurodegenerative diseases due to P2X7R-mediated mitochondrial dysfunction and inward Ca2+ flow into neurons (84–86).
Mitochondrial dysfunction results in decreased ATP production, Ca2+ dysregulation, and the generation of ROS. Mitochondria produce superoxide, a significant source of reactive oxygen species during ischemic and hypoxic conditions at the respiratory chain’s origin (85). Reactive oxygen species can damage macromolecules in the plasma membrane of neurons through oxidative modifications and harm (87). Activation of P2X7R by α-synuclein causes a reduction in mitochondrial membrane potential and an increase in the production of mitochondrial ROS (84). Subsequently, ROS stimulate the mitochondria-dependent intrinsic apoptotic pathway and activate pro-apoptotic proteins (88), which cause mitochondrial dysfunction, decreased cellular energy production, and cell death (89).
Oxidative stress is mainly characterized by increased levels of ROS and reduced antioxidant system capacity to combat free radicals (90–92). Oxidative stress can modify the inflammatory response in multiple ways, activating transient receptor potential (TRP) channels, specifically TRPV1, and signaling pathways (93). Additionally, stress can activate other mechanisms that increase the secretion of pro-inflammatory mediators (94), resulting in neuronal damage (95). Both oxidative stress and mitochondria significantly impact triggering apoptosis, where mitochondria serve as a source and target of ROS (96).
NADPH oxidase 2 (NOX2 or phagocytic oxidase PHOX) is a significant contributor of extracellular and intracellular ROS in microglia (97). The generation of ROS is a natural byproduct of cellular metabolism and plays a role in intracellular and extracellular signaling (98). Extracellular ROS are harmful to neurons, while intracellular ROS function as signaling mechanisms in microglia, activating p38 and ERK1/2 to prompt the production of various pro-inflammatory and neurotoxic cytokines, such as tumor necrosis factor-α, prostaglandin E2, and IL-1β (99). In SOD1-G93A mice, the absence of NOX2 enhances disease progression and survival (48).
In SOD1-G93A microglia, BzATP stimulates P2X7R, activating Rac1 (48, 98). Rac1 is a crucial activator of NOX1 and NOX2 from the Rho GTPase family (100). The activation of Rac1 enhances NOX2 activity, leading to an increase in ROS generation (48). P2X7R-mediated activation of NOX2 and consecutive ROS generation in microglia relies entirely on Rac1 (48). Additionally, phosphorylation of ERK1/2 increased in ALS microglia triggered by ATP stimulation of P2X7R. There exists an interdependence between the NOX2 and ERK1/2 pathways that combine to produce ROS. NOX2 activation results in further ERK1/2 phosphorylation, causing excessive ROS production (48, 101). Intrathecal injection of BzATP results in spinal ROS production and oxidative DNA damage in dorsal horn neurons (98).
Activation of P2X7R induced ROS production and IL-6 release in spinal cord astrocytes. Both releases partially passed through NADPH oxidase. The P2X7R antagonist A438079 inhibited ROS increase, whereas the P2X7R scavenger N-acetylcysteine (NAC) partially inhibited BzATP-mediated IL-6 release (52). Meanwhile, P2X7 activation induced ROS formation in EOC13 cells, leading to subsequent cell death (102, 103). ROS formation occurs through a mechanism independent of Ca2+ inward flow and K+ efflux (104). In contrast, P2X7-induced ROS formation in primary rat microglia depends on Ca2+ inward flow (105).
ROS-induced oxidative stress is a significant factor in the development of AD (106). ATP released from microglia, stimulated by A-β, activates NADPH oxidase-mediated ROS production through P2X7R (105). The upregulation of P2X7R activation and ROS production coincides with A-β growth, and increased levels of oxidative stress are strongly linked with synaptic loss in the AD mouse model mediated by A-β (51). This potentially explains the microglia-induced neuronal damage in the brain affected by AD.
The production of pro-inflammatory cytokines has been associated with several neurodegenerative diseases, such as AD, PD, and MS (107). In animal models, the evidence suggests that inflammation may contribute to disease progression but not necessarily be the primary initiator of neurodegenerative diseases (108). The NLRP3 inflammasome comprises the sensory protein NLRP3, the junction protein (ASC), and effector proteins (caspase-1) (109). The activation of the NLRP3 inflammasome occurs through a two-step process consisting of “initiation” and “activation” (110). The process that ignites the activation of inflammatory vesicles is called “initiation.” Initiation is instigated by Toll-like receptors (TLRs) that recognize PAMPs, DAMPs, environmental stress, or by NF-κB which is activated by TNF-α (111). Moreover, ROS are indispensable for NF-κB activation (112). Following this, NF-κB induces the upregulation of expression levels of NLRP3, pro-IL-1β, and pro-IL-18 (113). However, NLRP3 remains inactive (114). The second signal is called “triggering” or “activation”. NLRP3 and ASC form a complex with Pro-caspase-1 under certain conditions, leading to the conversion pro-caspase-1 to caspase-1. The activated caspase-1 converts pro-IL-1β and pro-IL-18 to their active forms, which are then released extracellularly to promote pro-inflammatory effects (115). IL-1β is produced in response to stimulation from various inflammatory vesicle activators, such as ATP, Nigericin, and alum. Furthermore, microglia release IL-18 and IL-1α. However, functional NLRP3 inflammatory vesicle formation and IL-1β secretion are unique to microglia in the mouse brain and do not occur in astrocytes (58).
With an aging population, the worldwide prevalence and rates of disability associated with neurodegenerative diseases are increasing, significantly impacting societal development and progress. This section provides a detailed analysis of the major neurodegenerative diseases, including AD, PD, HD, MS, and ALS. Neuronal damage is the main pathological feature of the above-mentioned neurodegenerative diseases (116). While the five disorders have diverse origins (Table 1), they share a common characteristic: chronic inflammatory damage in the CNS, which results in the persistent activation of innate immune cells. This includes the infiltration of peripheral immune cells across the blood-brain barrier (BBB), which is observed in MS (116). Moreover, there is no doubt that P2X7R, which is present in high densities in microglia (12), astrocytes (134), and oligodendrocytes (135), largely determines changes in neuronal function and activity in the CNS. Furthermore, in addition to amplifying inflammatory damage through glial cells (19), P2X7R receptors on the surface of neurons themselves can actively induce autophagy in neurons (136).
AD is a neurodegenerative disorder characterized by neuronal fibrillary tangles and senile plaques. Neuronal fibrillary tangles result from accumulations of hyperphosphorylated tau protein inside neurons, while extracellular A-β peptides lead to the formation of senile plaques (137). Nevertheless, the degree of cognitive impairment in AD does not correlate with the presence of amyloid plaques or neuroprotective fibril tangles (138). Most animal disease models are transgenic mice resulting from random mutations in genes that encode proteins associated with AD pathology or rodents injected with A-β into their brains (139).
P2X7R is involved in various processes, such as APP processing to produce A-β (140), synaptic dysfunction, oxidative stress (51), and neural inflammation (105). Specifically, in the production of A-β, P2X7R may be involved in the cleavage of APP, which has three different secretases - α-, β-, and γ-, cleaving at different sites on APP. The β segment produces A-β and γ-secretases present in amyloid plaques in AD patients’ brains. When α-secretase processes APP in a non-amyloidogenic manner, it leads to hydrolysis of A-β peptide sequences and shedding of sAPPα, which has neuroprotective and neurotrophic effects (141).
Stimulation of P2X7R activates ADAM9, -10, and -17, which have α-secretase activity (74, 75). They mediate the non-amyloidogenic processing of APP (76). Additionally, P2X7R triggers a new non-amyloidogenic pathway independent of ADAM9, -10, and -17 (77), promoting a significant shift in APP processing towards α-cleavage. This process increases the release of sAPPα while inhibiting the production of sAPPβ and A-β peptides (77). Stimulation of P2X7R-induced release of sAPPα was observed in human APP-expressing mouse and human neuroblastoma cells, mouse primary astrocytes, and neural progenitor cells. The knockdown of P2X7R could inhibit this release through a P2X7R antagonist or specific small interfering RNAs (siRNAs). It was not observed in neuronal cells from P2X7R-deficient mice (77). Inhibitors of β- and γ-secretase have been extensively researched, but therapy using these inhibitors is limited by associated side effects resulting from reduced β-secretase 1 and γ-secretase activity. Therefore, alternative approaches to treating AD, such as modulation of α-secretase activity, are being explored (142).
When extracellular particles, specifically A-β peptides (143), are present, ATP is released from damaged neurons, microglia, and astrocytes (144). High levels of extracellular ATP activate P2X7R, which is expressed at significantly higher levels during microgliocytosis and significant cognitive and motor impairment (145). A similar phenomenon was observed in microglia surrounding A-β in patients with Alzheimer’s disease (AD), and AD mouse models (146), and the increase in P2X7R levels mirrored the progression of AD (147). Additionally, activating P2X7R boosted microglia migration towards senile plaques while simultaneously inhibiting phagocytosis (147). In familial Alzheimer’s disease (FAD), the aggregation of A-β peptide occurred prior to P2X7R expression in microglia. This was found by investigating a new transgenic mouse model - P2X7R-EGFP/J20 mice. Furthermore, microglia expressing P2X7R were closer to emerging plaques than those without P2X7R expression133.
P2X7R produces chemokines induced by A-β peptides and recruits CD8T cells in brain parenchyma. Chemokines are expressed excessively in vitro and AD mouse models in response to A-β peptides, leading to the inflammatory process and recruitment of immune cells (148). Furthermore, this overexpression of chemokines contributes to the subsequent neurodegenerative process (149).
In the Neuro-2a cell line expressing APP, activation of the P2X7R receptor results in decreased α-secretase activity, whereas activation of the P2Y2 receptor leads to the opposite effect. In cultured cerebellar granule neurons, activating P2X7R inhibits and neuroprotects glycogen synthase kinase 3 (GSK-3), the less active form of α-secretase (150). In J20 mice, a transgenic model of FAD that expresses the human mutant APP protein, inhibiting P2X7R in vivo, reduced the number and size of hippocampal A-β significantly. This reduction was facilitated by an increase in the phosphorylated form of GSK-3, which, in turn, enhanced the α-secretase-induced non-amyloidogenic degradation of APP (150). Multiple lines of evidence suggest that the inhibition of α-secretase by BzATP is dependent solely on P2X7R. To prevent the inhibitory effects of BzATP on α-secretase activity, both the pharmacological blockade and the synthesis inhibition of P2X7R with RNA interference be effective (142). Several studies demonstrate that a lack of P2X7R restores hippocampal synaptic integrity and plasticity, rescues memory deficits in APPPS1 mice, and reduces A-β pathology. However, the effects are not modulated by the sAPPα pathway, IL-1β treatment, microglial activation, or phagocytosis (12).
PD is the second most common neurodegenerative disorder globally, affecting over 6 million individuals (151). It is also a leading contributor to neurological disabilities. Typical symptoms of PD consist of bradykinesia, resting tremor, tonus, and changes in posture and gait, significantly impacting patients’ quality of life (95). The pathological characteristics of PD involve the creation of neural inclusion bodies that comprise eosinophilic material, Lewy vesicles, and the demise of dopaminergic neurons with injury to the central region of nigrostriatum (122). Misfolded α-synuclein aggregates predominantly constitute the Lewy bodies among these characteristics (122).
After an extensive investigation, it was found that postmortem PD patients have an elevated number of reactive microglia with phagocytic activity in their brains (152). Furthermore, similar results as well as high expression of P2X7R were observed in a mouse model of PD (153), emphasizing the strong correlation between microglia-induced neuroinflammation and PD in this disease. P2X7R plays a significant role in the pathogenesis of PD, as it produces a profoundly pronounced facilitatory effect. When neuroinflammation occurs, dying neurons release a significant amount of ATP, which then activates the P2X7R located on the surface of glial cells. The P2X7R induces a positive feedback loop of Ca2+ influx, promoting the opening of P2X7R and pannexin-1 channels on the membrane surface, which then releases more ATP (154). This increased ATP release also boosts the exocytosis of K+ ions and triggers the assembly of NLRP3 inflammasomes. Subsequently, the activation of caspase-1 cleaves pro-IL-1β to IL-1β, thus facilitating its release (155). As a result, neuroinflammation worsens, and this ultimately accelerates neuronal death.
Several studies have identified the interaction between the pathological protein α-Syn and microglia as a crucial factor in the neuroinflammatory process of PD. Alpha-synuclein not only binds to microglial NOX2, thereby activating the NOX complex and triggering oxidative stress in vivo (156) but also directly activates P2X7R on its surface to exert its effects; this activation persists in the presence of exogenous ATP withdrawal (157). During this process, activated microglia release excitotoxic glutamate, which damages dopaminergic neurons through ATP/glutamate secretion on the one hand (158), and destroys dopaminergic neurons through the release of ROS on the other hand, leading to the extensive destruction of dopaminergic neurons and degeneration, thus triggering the development of PD (159). In addition, the pathological changes in PD are closely related to excessive Ca2+ inward flow due to P2X7R activation, and the high intracytoplasmic calcium environment also directly induces apoptotic necrotic loss of dopaminergic neurons (160). Moreover, other studies have demonstrated that Ca2+ binds to the C-terminus of α-Syn to increase its localization in presynaptic terminals and synaptic vesicles (161).
A study demonstrated that BBG, a P2X7R antagonist, successfully reduced neuronal apoptosis in rat subarachnoid hemorrhage by inhibiting p38MAPK (162). Peroxisome proliferator-activated receptor-γ (PPARγ) coactivator 1α (PGC-1α) is a protein that interacts with nuclear receptors like PPARγ, which negatively regulates the transcription of the NF-KB pathway, a pro-inflammatory pathway, thereby inhibiting inflammatory responses. In contrast, PGC-1α regulation is accomplished through phosphorylation at multiple sites by several phosphokinases, such as MAPK and AKT (163). Another experiment simulating PD treatment observed that attenuation of DA neuronal damage in an LPS rat model of PD was achieved by inhibiting the P38MAPK pathway with a P2X7R antagonist. The criterion for efficacy was counted using tyrosine hydroxylase-immunoreactive (TH-ir) neurons in the substantia nigra. The study revealed a significant reduction in TH-ir levels in the LPS-treated group. After 15 days of treatment with BBG, the LPS group witnessed an effective reversal of the reduction in TH-ir levels (68). The studies above highlight the critical role played by P2X7R-mediated stimulation of oxidative stress via the MAPK pathway in promoting neuroinflammation during the pathogenesis of PD. In PD animal model experiments, the nigrostriatal region of rats was damaged with 6-OHDA to induce PD. Apomorphine caused the rats to exhibit rotating behavior, confirming the establishment of the animal model of PD damage. In batches, the rats were treated with BBG. The number of rotations per minute of the saline-treated rats remained unchanged from seven days prior. However, the BBG-treated rats significantly decreased the number of rotations, providing further evidence that BBG treatment alleviates motor deficits in lateralized parkinsonism (163). Additional studies suggest that the P2X7R-mediated neuronal cell swelling and necrosis may be linked to abnormal functioning of the substantia nigra striatal region in PD. When the SN4741 neuronal cells derived from transgenic mouse embryos were exposed to high concentrations of ATP, their cell volume increased dramatically and in a concentration-dependent manner within twenty minutes. Subsequently, the cells exhibited necrotic manifestations typical of cellular necrosis, such as nuclear swelling, DNA leakage, ER integrity loss, and cytoplasmic vacuole formation (164). Furthermore, prior research has indicated that the sensitivity of P2X7R to ATP activation amplifies with decreasing levels of extracellular divalent cations. This implies that a positive feedback loop occurs when there is neuroinflammation in the substantia nigra striatal region, as lower concentrations of ATP activate P2X7R, worsening the lesion. In summary, ATP binding to the P2X7R stimulates microglia and recruits peripheral immune cells through a variety of complex pathways. These pathways include induction of calcium influx, activation of NLRP3 inflammatory vesicles that induce cell death and tissue damage, and release of more ATP (19). The ATP release/P2X7R activation/apoptosis axis then acts in a positive feedback loop, which contributes significantly and continuously to the neurodegenerative process of PD (19).
HD is an autosomal dominant neurodegenerative disorder characterized by motor, cognitive, and psychiatric deficits (165). Commonly observed motor symptoms include chorea and coordination difficulties (166). The primary cause of HD is believed to be the amplification of a CAG repeat in the first exon of the huntingtin gene (HTT), leading to the production of the mutant Huntington’s protein (mHTT) (167). The expansion of a bundle of polyQ in the N-terminal segment of the encoded protein, due to CAG repetition, causes abnormal folding of mHTT and its accumulation in brain cells (168). Subsequently, earlier transcriptional dysregulation occurs, along with abnormalities in synaptic and axonal transport, disruption of the protein homeostasis network, aggregation pathology, compromised function of the nuclear pore complex, oxidative damage, mitochondrial malfunction, and extrasynaptic excitotoxicity (169, 170).
There is now extensive direct evidence suggesting that pathways mediated by the P2X7R contribute to the development of Huntington’s chorea. This makes it a potentially interesting target for HD patient treatment. It is worth noting that the increase in the number of CAG triplet repeats is not related to either the transcriptional process of P2X7 gene expression or the role of the P2X7R (171). However, research has suggested that the expression of HTT mutant genes may render neurons more vulnerable to P2X7R-mediated apoptosis, effectively increasing susceptibility (171). In this experiment, it was observed that Tet/HD94 mutant mice showed a significant decrease in the survival of their cortical and striatal neurons when exposed to 10 Mm BzATP, whereas this ATP analogue had no discernible effect on wild-type mice (171). Administration of BBG, a P2X7R receptor antagonist, was discovered to slow or prevent the negative impacts of ATP analogues on neuronal viability in mice with the HTT mutant phenotype (171). The administration of BBG to R6/1 HD gene mouse models also had a similar effect, improving motor parameters and alleviating weight loss after treatment (171).
In a certain centralized investigative study of HD patients, it was shown that there was a significant fold increase in P2X7R protein levels (including protein bands of all four isoforms of P2X7R-A, B, H, and J) in affected individuals (172). In addition, immunohistochemistry revealed that more diffuse and intense reactivity and a greater number of immunoreactive cells can be observed in striatal sections of HD patients (172).
A reported study has demonstrated that after using BzATP in extracellular electrophysiology experiments in cortical striatal slices from both wild-type mice (WT) and R6/2 (HD-type mice), a decrease in FP amplitude was induced (173). Nonetheless, BzATP’s reduction in FP proved much more statistically substantial in transgenic mice than in WT mice (173). This indicates that the ATP-activated P2X7R pathway hinders synaptic transmission to a greater extent in HD genotypes. This could contribute to the gradual impairment of neuronal viability in HD patients. Moreover, there is evidence of mHTT mutant protein accumulation in astrocytes of both HD patients and animal models. This accumulation is linked to reduced astroglial potassium channel (Kir4.1) (174). In the R6/2 mouse model of HD, it has been demonstrated that astrocytes exhibit anomalous electrophysiology and significantly elevated extracellular levels of potassium ions (174). These manifestations may stem from irregular ion exchange caused by the opening of large pores influenced by the activation of P2X7R (174).
Regardless, the activation of P2X7R is highly significant in initiating an expedited process of neuronal degeneration within the striatal region during the onset and advancement of the disease in Huntington’s patients. This could be a pivotal breakthrough in treating individuals with HD.
MS is a chronic inflammatory disease of the CNS that is mediated by autoreactive helper T cells (Th1 and Th17 cells). Patients with MS are typically between the ages of 20 and 40, and it is the leading cause of disability among young people in the United States and Europe (175). MS is characterized by demyelination and axonal degeneration (176). Neurological symptoms may happen during seizures, including weakness, altered sensation, balance disturbances, visual impairment, and color vision or diplopia (127). The critical pathological characteristic of MS is the emergence of inflammatory plaques, causing damage to the myelin sheaths and specialized cells (e.g., oligodendrocytes) in both the white and gray matter of the brain and spinal cord, leading to neuronal loss. Upon initial exposure to unfamiliar antigens, Th1 cells produce pro-inflammatory cytokines IL-1 and IFN-γ, while Th17 cells produce IL-17 (127).
The pathology of MS is primarily driven by the interaction of neuroglial cells and autoreactive immune cells. Activation of P2X7R on astrocytes and microglia, which mediates purinergic signaling, has been suggested as a significant causative factor in these pathologic processes (4). Some experimental studies have indicated a significant presence of P2X7-immunoreactive microglia/macrophages in the spinal cord of patients with MS, particularly in the dorsolateral white matter region of the degenerating corticospinal tracts (177). Additionally, inflammation-related substances such as IL-β and COX-2 are also heightened in the affected areas (177).
Cell death is thought to raise extracellular ATP levels (178), boosting P2X7R expression in microglia, which sequentially releases IL-1β, COX-2, and PGE2, ultimately leading to additional cell death and ATP release. This cascading cyclic mechanism could play a significant role in the demyelination process observed in MS patients (177). In a study conducted on SP(Secondary progressive)-type MS patients, researchers found that astrocytes in the frontal cortical region had an increased expression of P2X7R and promoted neuroinflammation in a manner similar to microglial cells. Additionally, they discovered that direct activation of P2X7R by BzATP increased MCP-1 (Monocyte chemoattractant protein-1) levels in astrocytes, a protein that is responsible for leukocyte recruitment during MS progression (179). In astrocytes, P2X7 activation induces phosphorylation of ERK1/2 and activation of the PI3/AKT signaling pathway, both of which contribute to promoting neuroinflammatory responses (180). Moreover, exposure to high concentrations of ATP or the selective agonist BzATP acting on P2X7R stimulates the shedding of MPs (microparticles) from particulate vesicles on the surface of microglia or astrocytes. Exocytosis of MP results in the release of significant quantities of pro-inflammatory factors, including IL-1β, which expedites the neuroinflammatory process in MS development (181). As previously stated, MS is a demyelinating disease that centers on the death of oligodendrocytes. According to a study, ATP accumulation triggers Ca2+ signaling in oligodendrocyte progenitor cells (OPCs), leading to the activation of the P2X7R and P2Y1R pathways. This impacts the cells’ growth, development, differentiation, and other processes, potentially acting as a mechanism for the onset of demyelination (182).
There are numerous immune cell types that participate in the development of pathogenic neuroinflammation in MS. Among them, the monocyte macrophage lineage expresses the P2X7R at the highest level. Activated monocytes are often one of the first phenotypes to arrive at the site of CNS neuropathy (183). In a particular study on patients with MS, it was discovered that despite a significant increase in P2X7R mRNA in total cell extracts from the frontal cortex in SPMS (Secondary progressive multiple sclerosis) patients, there was an unexpected decrease in P2X7R expression on the surface of monocytes (184). It is hypothesized that monocytes decrease P2X7R protein expression when the efficiency of excess toxic ATP removal reduces, efficiently preventing diminished viability caused by calcium overload. This sustains their activity for better participation in neuroinflammation (184).
Although definitive studies have not confirmed the inevitability of MS occurrence with the presence of P2X7R, many experiments have shown that deleting P2X7R can significantly reduce the incidence of EAE disease in mice. One study discovered that P2X7-deficient mice had significantly lower mean scores of clinical symptoms in comparison to WT mice, notwithstanding the insignificant alteration of the mean number of days of disease onset. This finding effectively implies that the deletion of P2X7R reduces the incidence of EAE (encephalomyelitis) (184).High levels of astrocyte activation were detected in various areas of white matter in WT EAE mice as well as in Bergman’s radial glial fibers. However, the level of activation was not significant in the P2X7null group (184).
Furthermore, related studies have identified a twofold function of P2X7R in the development of MS. The studies have demonstrated that the activation of P2X7R on erythrocytes can disrupt the regulation of cation fluxes, which are responsible for maintaining extracellular K-ion homeostasis and removing excessive toxic glutamate. These findings contribute to the intricate nature of the relationship between P2X7R and MS (185).
ALS is a long-term neurological disorder defined by the invasion of lesions, mainly in the anterior horn cells of the spinal cord, brainstem motor nerve nuclei, and the pyramidal tract. This can lead to the loss of upper and lower motor neurons in the motor cortex, brainstem nuclei, and anterior horn of the spinal cord (186). This condition primarily affects the motor system and presents accompanying symptoms, such as skeletal muscle atrophy, progressive paralysis, respiratory failure, and death within 2-5 years (187). The pathophysiology of ALS is characterized by neuromuscular junction loss in both the upper and lower motor neurons, axonal retraction, subsequent cell death, astrocytic hyperplasia, and an increase in microglia around the lesion (188).
Neuroinflammation is a key pathological mechanism in ALS, resulting from the activation of P2X7R and leading to chronic microglial activation (189). This is considered a mechanism that contributes to the death of motor neurons (190). In addition, impaired autophagy can also lead to the damage and death of motor neurons (191). Activation of P2X7 in vitro exacerbates pro-inflammatory responses, including NOX2 activation in microglia, elevated levels of TNF-α, COX-2, MAPK, and neuronal toxicity (48). Stimulating P2X7R using the P2X7R-specific agonist BzATP before the onset of pathological neuromuscular symptoms in SOD1-G93A mice resulted in enhanced muscle fiber innervation and metabolism, preserved neuromuscular junction morphology, and stimulated satellite cell proliferation and differentiation. This intervention effectively prevented skeletal muscle denervation in SOD1-G93A mice (192). During the presymptomatic stage of ALS disease, administering BzATP via intramuscular injection improved locomotor activity in mice by revitalizing muscle cells and infiltrating macrophages. The treatment not only protected the retrograde propagation of skeletal muscle to the CNS but also enabled direct and immune-mediated protection. Additionally, it reduced neuroinflammation and promoted spinal motor neuron survival (193).
Repeated stimulation of spinal astrocyte P2X7R with ATP or BzATP activates it, leading to a neurotoxic phenotype that causes motor neuron death. Conversely, inhibiting P2X7R or apyrase, an enzyme that degrades ATP, by using BBG eliminates their toxicity to motor neurons (194). Brief stimulation of the P2X7R initiated autophagy activation and enhanced the expression of anti-inflammatory biomarkers in microglia of SOD1-G93A mice (M2 microglia). In contrast, prolonged activation of P2X7R caused disruption of autophagic fluxes, which could lead to a shift towards a pro-inflammatory phenotype (M1 microglia). These results indicate a dual function of the receptor in the pathway (195).
Previous research suggests that P2X7R ablation accelerates clinical onset and worsens disease progression in mSOD1 mice (196). In contrast, BBG-induced P2X7R antagonism suppresses microglial proliferation, alters microglial-associated inflammatory genes, enhances motoneuron survival, mildly delays onset, and improves motor function but does not impact survival (197).
In the SOD1-G93A mouse model, BBG was used to antagonize P2X7R, resulting in reduced neuroinflammation and promotion of the survival of lumbar medullary motor neurons. This may ultimately delay the onset of ALS in a mouse model of ALS (198). Similarly, AXX71 and AXX13 were found to reduce proinflammatory markers, delay the onset of neuromuscular injury, and transiently preserve motor function and muscle strength by antagonizing P2X7R (199). The results indicate that P2X7R may play a role in ALS, a neurodegenerative disease. P2X7R antagonists have the potential to delay the onset and reduce the clinical symptoms of ALS.
In addition to the five neurodegenerative diseases mentioned above, P2X7R may also play a role in neuropsychiatric disorders, including depression, anxiety disorders, and post-traumatic stress disorder (PTSD). These disorders are a frequent cause of death in the elderly and are characterized by behavioral changes (200).
Depression is a prevalent psychiatric disorder characterized by persistent sadness, anhedonia, and diminished interest, which can lead to impairment of daily functioning (201). The onset or development of depression may be influenced by neuroinflammation (202). The involvement of the pro-inflammatory cytokine IL-1β, released by microglia, induces the secretion of corticotropin-releasing hormone (CRH), which in turn secretes adrenocorticotropic hormone (ACTH) and cortisol, along with a large number of other cytokines/chemokines, leading to mood dysregulation. Therefore, microglia may be a potential target for the treatment of depressive symptoms. Hyperactivation of P2X7 leads to increased release of inflammatory cytokines, such as IL-1β, which are involved in depression. Inhibiting P2X7R expression in the hippocampus, spinal cord, and dorsal root ganglia may alleviate visceral pain and depression. In a rat model of bone cancer, cancer-complicated pain and depression-like behavior were reduced by intrahippocampal injection of the P2X7R antagonist A438079. Microinjection of the P2X7R antagonist A-438079 into the amygdala significantly attenuated depressive and anxiety-like behaviors in neuropathic pain. This effect may be attributed to the antagonist’s inhibitory effects on microglia and astrocytes. These findings suggest that P2X7R may play a role in depression complicated by various kinds of pain. Additionally, salidroside, a bioactive extract from Rhodiola rosea L, may mediate depression by inhibiting P2X7/NF-KB/NLRP3-mediated focal death.
Anxiety is defined as excessive fear, anxiety, or avoidance of perceived threats. Studies have shown that ATP/P2X7R-initiated microglia in the ipsilateral hippocampus can drive anxiety-depressive-like behaviors associated with trigeminal neuralgia via IL-1β. P2X7 is also involved in brain monocyte aggregation associated with repeated social failure, IL-1β mRNA expression in enriched myeloid cells, plasma IL-6, and anxiety-like behavior. Additionally, IL-1β accumulation plays a role in the pathophysiology of PTSD. However, the relationship between P2X7R and PTSD has not yet been definitively investigated.
P2X7R are a major therapeutic target in the treatment of neurodegenerative diseases. To categorize common P2X7 antagonists, we have identified five groups (Table 2). The first group comprises divalent cations like Ca2+, Mg2+, Zn2+, and Cu2+. These cations hinder the activation of ATP-induced P2X7R. Experiments have demonstrated that their in vitro values of IC50 (μM) were 2900, 500, 11, and 0.5 at pH 6.1. A decrease in pH modifies the charge on histidine residues and prevents ATP-gated currents. In AD and PD, Mg2+ administration may be a potential strategy to reduce the deleterious effects of Ca2+ induced neuroinflammation.The second group, consisting of nonselective P2XR antagonists such as sulforaphane, RB-2(Reactive Blue 2), PPADS (pyridoxal phosphate-6-azobenzene-2′,4′-disulfonic acid), and iso-PPADS, exhibit lower potency and selectivity for the P2X7R as compared to other P2Rs. Furthermore, some other P2R antagonists display moderate selectivity for P2X7R while also having either low potency, as seen in oxATP(oxidized ATP), or high potency, as seen in BBG. OxATP inhibits P2X7 activation in microglia from both AD and non-demented brains, indicating therapeutic potential for oxATP in AD (146). BBG blocks rP2X7 at 10 nM concentration, and human P2X7R (hP7X2R) at 200 nM concentration (203, 204). Additionally, BBG blocks voltage-gated sodium channels at low micromolar concentrations (205). In the J20 hAPP transgenic mouse model of AD, BBG inhibits glycogen synthase kinase 3-β (GSK-3β) via P2X7R. This increases α-secretase activity in hippocampal neurons, thereby reducing amyloid-beta (Aβ) and subsequent plaque production (206). In an animal model of AD, BBG reduced levels of purinergic receptors, decreased gliosis, and mitigated blood-brain barrier leakage. Additionally, it exhibited neuroprotective properties and acted as an antagonist to the inflammatory response triggered by the P2X7R agonist 2’,3’-(benzoyl-4-benzoyl)-ATP (207). In vivo data obtained from the administration of BBG in HD mice strongly suggests that BBG prevents neuronal apoptosis and attenuates weight loss and motor coordination deficits. Alterations in P2X7 receptor levels and function are believed to contribute to the pathogenesis of HD.A third category of organic cations, including calmidazolium and 1-[N,O-Bis(5-isoquinolinesulfonyl)-N-methyl-L-tyrosyl]-4-phenylpipera zine(KN-62) (at 10 nM), inhibits rP2X7R activation by blocking BzATP-induced currents. However, they demonstrate less efficacy in inhibiting hP2X7R activation (40). The piperazine antagonist KN-62 blocks CaM kinase II, and experiments show inhibited ionic currents in KN-62-treated cells expressing hP2X7R or mP2X7R (206). The fourth group mainly consists of naturally synthesized compounds. Chelerythrine, among the benzophenanthridine alkaloids tested, is the only one that can effectively inhibit P2X7R function noncompetitively at ATP concentrations ranging from 0 to 1,000 μM (207). Mineral oil can also inhibit P2X7R function by reducing P2X7-dependent multinucleated giant cell formation, thus downregulating P2X7R expression (208). The fifth group comprises novel synthetic compounds with varied chemical structures and conformation types, including GSK-1482160, GSK-314181A, AZ1060612, AXX71, JNJ-54175446, and JNJ-55308942. An example of one such compound is 11C-GSK1482160, which exhibited high affinity and good binding-dissociation kinetics for P2X7R, as demonstrated by 11C-GSK1482160 in vivo PET/CT tracer kinetics in the experimenter (209). V-T exhibited the expected trend in lipopolysaccharide-treated mice (210). The results confirm the potential use of 11C-GSK1482160 as a novel radioligand for targeting P2X7R and as a biomarker of neuroinflammation, as evidenced by the activation of microglial cells via peripheral lipopolysaccharide treatment and the receptor-dependent regional binding (211). Interestingly, the specific P2X7 antagonist AZ10606120 completely blocked the ATP-induced release of SOD1 from NSC-34 cells (212). In contrast, the SOD1-G93A protein has recently been shown to be rapidly released into the extracellular space upon P2X7 activation and is then re-uptaken by naïve NSC-34 cells or microglia cell lines to induce endoplasmic reticulum stress and TNF-α release, which mediate neurodegenerative disease and neuroinflammation-related ALS events respectively (213). In the SOD1-G93A mouse model of ALS, AXX71 treatment resulted in a significant down-regulation of pro-inflammatory markers such as IL-1β, NOX2, and NF-κB in the spinal cord at the end of the disease. Additionally, AXX71 treatment regulated the levels of autophagy-related proteins LC3B-II and SQSTM1/p62 (199).
The P2X7R is a non-selective ATP-gated cation channel that is widely expressed on the surface of various types of human cells. It plays an important role in the physiology and disease mechanisms of many human systems. For instance, the activation of P2X7R on skeletal muscle cells is involved in the pathogenesis of osteoarthritis (OA). Similarly, the activation of P2X7R in the cardiovascular system can induce small-vessel vasculitis, which may stimulate the development of hypertension and atherosclerosis. Additionally, ocular P2X7R may be associated with diseases of the retina. Finally, it has been demonstrated that P2X7R is inextricably involved in hematologic malignancies.
Due to its high-density localization in the nervous and immune systems, P2X7R plays a unique role in neuroinflammatory processes and is a significant factor in inducing various types of neurodegenerative diseases. When activated, P2X7R triggers the NLRP3 inflammasome, disrupting mitochondrial function and inducing Ca2+ influx into neurons. Elevated extracellular ATP levels resulting from neuronal apoptosis subsequently lead to ATP-induced oxidative stress and neurodegeneration, resulting in neuronal apoptosis and a significant degree of microglia activation and aggregation. Elevated extracellular ATP levels resulting from neuronal apoptosis subsequently lead to ATP-induced oxidative stress and neurodegeneration, resulting in neuronal apoptosis and a significant degree of microglia activation and aggregation. This, in turn, triggers a cascade of reactions by further activating P2X7R. Although microglia activation initially plays a neuroprotective role, as the disease progresses, it transitions from phagocytosis to the production of pro-inflammatory cytokines that worsen the disease. Activated microglia release excitotoxic glutamate, which can injure dopaminergic neurons.
Therefore, blocking the P2X7R pathway using a variety of approaches may be a practical and effective way to treat neurodegenerative disease and slow down its progression. This treatment has been shown to be effective in a number of neurodegenerative diseases. For instance, in AD, the P2X7R antagonist oxATP, as well as BBG and the Ca2+ efflux blocker Mg2+, have been shown to reduce A-β protein aggregation and subsequent plaque production. In HD, BBG has been found to prevent neuronal apoptosis and alleviate symptoms such as weight loss and motor coordination deficits. In ALS, the P2X7 antagonist AZ10606120 can reduce endoplasmic reticulum stress and the release of TNF-α, thereby counteracting the neuroinflammatory events associated with the disease. However, while antagonizing the P2X7R-mediated pathway shows promise for treating neurodegenerative diseases, it has not yet gained popularity in clinical settings. Therefore, the focus of major pharmaceutical laboratories studying P2X7 and neurodegenerative diseases is on refining and developing multiple forms of P2X7R antagonists and conducting clinical trials.
The relationship between P2X7R and microglia-specific phenotypic transformation is a controversial topic. Microglia are distinct from macrophages, as they have two cell types: pro-inflammatory phenotype (M1) and anti-inflammatory phenotype (M2), which express different markers on the cell membrane surface and play different roles. The former is involved in neurotoxicity, while the latter is associated with inflammation abatement and tissue repair. Environmental factors, such as LPS and IFN-γ, facilitate the conversion of microglia to the M1 phenotype. Conversely, IL-4 enables the M2 phenotype. Previous studies have shown that the activation of P2X7R by BzATP induces the polarization of the M1 phenotype, which promotes neuroinflammation. Other studies have found that astragalus polysaccharides, which are ATP-degrading agents, can enhance M2 polarization by reducing P2X7R activation. This exerts a protective effect on the nervous system. However, a related experiment found that P2X7R activation not only stimulates the formation of M1 markers but also promotes the production of M2 markers such as Arg-1(arginase-1). Controlling the activation of P2X7R by a certain amount of ATP may have neuroprotective effects. This finding is important for further research in this area.
HZ: Writing – original draft. QL: Writing – original draft. SZ: Writing – original draft. HL: Writing – review & editing. WZ: Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bhattacharya A. Recent advances in CNS P2X7 physiology and pharmacology: focus on neuropsychiatric disorders. Front Pharmacol (2018) 9:30. doi: 10.3389/fphar.2018.00030
2. Bartlett R, Stokes L, Sluyter R. The P2X7 receptor channel: recent developments and the use of P2X7 antagonists in models of disease. Pharmacol Rev (2014) 66(3):638–75. doi: 10.1124/pr.113.008003
3. Kim M, Spelta V, Sim J, North RA, Surprenant A. Differential assembly of rat purinergic P2X7 receptor in immune cells of the brain and periphery. J Biol Chem (2001) 276(26):23262–7. doi: 10.1074/jbc.M102253200
4. Sidoryk-Węgrzynowicz M, Strużyńska L. Astroglial and microglial purinergic P2X7 receptor as a major contributor to neuroinflammation during the course of multiple sclerosis. Int J Mol Sci (2021) 22(16):8404. doi: 10.3390/ijms22168404
5. Illes P, Khan TM, Rubini P. Neuronal P2X7 receptors revisited: do they really exist? J Neurosci (2017) 37(30):7049–62. doi: 10.1523/JNEUROSCI.3103-16.2017
6. Miras-Portugal MT, Sebastián-Serrano Á, de Diego García L, Díaz-Hernández M. Neuronal P2X7 receptor: involvement in neuronal physiology and pathology. J Neurosci (2017) 37(30):7063–72. doi: 10.1523/JNEUROSCI.3104-16.2017
7. Kaczmarek-Hajek K, Zhang J, Kopp R, Grosche A, Rissiek B, Saul A, et al. Re-evaluation of neuronal P2X7 expression using novel mouse models and a P2X7-specific nanobody. Elife (2018) 7:e36217. doi: 10.7554/eLife.36217.030
8. Metzger MW, Walser SM, Aprile-Garcia F, Dedic N, Chen A, Holsboer F, et al. Genetically dissecting P2rx7 expression within the central nervous system using conditional humanized mice. Purinergic Signal (2017) 13(2):153–70. doi: 10.1007/s11302-016-9546-z
9. Francistiová L, Vörös K, Lovász Z, Dinnyés A, Kobolák J. Detection and functional evaluation of the P2X7 receptor in hiPSC derived neurons and microglia-like cells. Front Mol Neurosci (2022) 14:793769. doi: 10.3389/fnmol.2021.793769
10. Ortega F, Gomez-Villafuertes R, Benito-León M, Martínez de la Torre M, Olivos-Oré LA, Arribas-Blazquez M, et al. Salient brain entities labelled in P2rx7-EGFP reporter mouse embryos include the septum, roof plate glial specializations and circumventricular ependymal organs. Brain Struct Funct (2021) 226(3):715–41. doi: 10.1007/s00429-020-02204-5
11. Illes P, Ulrich H, Chen JF, Tang Y. Purinergic receptors in cognitive disturbances. Neurobiol Dis (2023) 185:106229. doi: 10.1016/j.nbd.2023.106229
12. Bhattacharya A, Biber K. The microglial ATP-gated ion channel P2X7 as a CNS drug target. Glia (2016) 64(10):1772–87. doi: 10.1002/glia.23001
13. Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. The P2X7 receptor in infection and inflammation. Immunity (2017) 47(1):15–31. doi: 10.1016/j.immuni.2017.06.020
14. von Muecke-Heim IA, Ries C, Urbina L, Deussing JM. P2X7R antagonists in chronic stress-based depression models: a review. Eur Arch Psychiatry Clin Neurosci (2021) 271(7):1343–58. doi: 10.1007/s00406-021-01306-3
15. Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci (2004) 24(1):1–7. doi: 10.1523/JNEUROSCI.3792-03.2004
16. Solini A, Chiozzi P, Morelli A, Fellin R, Di Virgilio F. Human primary fibroblasts in vitro express a purinergic P2X7 receptor coupled to ion fluxes, microvesicle formation and IL-6 release. Pt 3J. Cell Sci (1999) 112:297–305. doi: 10.1242/jcs.112.3.297
17. Shieh C-H, Heinrich A, Serchov T, van Calker D, Biber K. P2X7-dependent, but differentially regulated release of IL-6, CCL2, and TNF-α in cultured mouse microglia. Glia (2014) 62:592–607. doi: 10.1002/glia.22628
18. Lee HG, Won SM, Gwag BJ, Lee YB. Microglial P2X7 receptor expression is accompanied by neuronal damage in thexxx cerebral xxxcortex xxxof the APPswe/PS1dE9 mouse model of alzheimer’s disease. Exp Mol Med (2011) 43:7–14. doi: 10.3858/emm.2011.43.1.001
19. Illes P. P2X7 receptors amplify CNS damage in neurodegenerative diseases. Int J Mol Sci (2020) 21(17):5996. doi: 10.3390/ijms21175996
20. Oken AC, Krishnamurthy I, Savage JC, Lisi NE, Godsey MH, Mansoor SE. Molecular pharmacology of P2X receptors: exploring druggable domains revealed by structural biology. Front Pharmacol (2022) 13:925880. doi: 10.3389/fphar.2022.925880
21. Collo G, Neidhart S, Kawashima E, Kosco-Vilbois M, North RA, Buell G. Tissue distribution of the P2X7 receptor. Neuropharmacology (1997) 36(9):1277–83. doi: 10.1016/S0028-3908(97)00140-8
22. Martínez-Cuesta MÁ, Blanch-Ruiz MA, Ortega-Luna R, Sánchez-López A, Álvarez Á. Structural and functional basis for understanding the biological significance of P2X7 receptor. Int J Mol Sci (2020) 21(22):8454. doi: 10.3390/ijms21228454
23. Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science (1996) 272(5262):735–8. doi: 10.1126/science.272.5262.735
24. Tassetto M, Scialdone A, Solini A, Di Virgilio F. The P2X7 receptor: A promising pharmacological target in diabetic retinopathy. Int J Mol Sci (2021) 22(13):7110. doi: 10.3390/ijms22137110
25. Jiang LH, Caseley EA, Muench SP, Roger S. Structural basis for the functional properties of the P2X7 receptor for extracellular ATP. Purinergic Signal (2021) 17(3):331–44. doi: 10.1007/s11302-021-09790-x
26. McCarthy AE, Yoshioka C, Mansoor SE. Full-length P2X7 structures reveal how palmitoylation prevents channel desensitization. Cell (2019) 179(3):659–70. doi: 10.1016/j.cell.2019.09.017
27. Lara R, Adinolfi E, Harwood CA, Philpott M, Barden JA, Di Virgilio F, et al. P2X7 in cancer: from molecular mechanisms to therapeutics. Front Pharmacol (2020) 11:793. doi: 10.3389/fphar.2020.00793
28. Hansen MA, Barden JA, Balcar VJ, Keay KA, Bennett MR. Structural motif and characteristics of the extracellular domain of P2X receptors. Biochem Biophys Res Commun (1997) 236(3):670–5. doi: 10.1006/bbrc.1997.6815
29. Pippel A, Stolz M, Woltersdorf R, Kless A, Schmalzing G, Markwardt F. Localization of the gate and selectivity filter of the full-length P2X7 receptor. Proc Natl Acad Sci U S A. (2017) 114(11):E2156–65. doi: 10.1073/pnas.1610414114
30. Kopp R, Krautloher A, Ramírez-Fernández A, Nicke A. P2X7 interactions and signaling - making head or tail of it. Front Mol Neurosci (2019) 12:183. doi: 10.3389/fnmol.2019.00183
31. Durner A, Durner E, Nicke A. Improved ANAP incorporation and VCF analysis reveal details of P2X7 current facilitation and a limited conformational interplay between ATP binding and the intracellular ballast domain. Elife (2023) 12:e82479. doi: 10.7554/eLife.82479
32. Denlinger LC, Fisette PL, Sommer JA, Watters JJ, Prabhu U, Dubyak GR, et al. Cutting edge: the nucleotide receptor P2X7 contains multiple protein- and lipid-interaction motifs including a potential binding site for bacterial lipopolysaccharide. J Immunol (2001) 167(4):1871–6. doi: 10.4049/jimmunol.167.4.1871
33. Tewari M, Seth P. Emerging role of P2X7 receptors in CNS health and disease. Ageing Res Rev (2015) 24(Pt B):328–42. doi: 10.1016/j.arr.2015.10.001
35. Peverini L, Beudez J, Dunning K, Chataigneau T, Grutter T. New insights into permeation of large cations through ATP-gated P2X receptors. Front Mol Neurosci (2018) 11:265. doi: 10.3389/fnmol.2018.00265
36. Vasileiou E, Montero RM, Turner CM, Vergoulas G. P2X(7) receptor at the heart of disease. Hippokratia (2010) 14(3):155–63.
37. Li Z, Huang Z, Zhang H, Lu J, Tian Y, Wei Y, et al. P2X7 receptor induces pyroptotic inflammation and cartilage degradation in osteoarthritis via NF-κB/NLRP3 crosstalk. Oxid Med Cell Longev (2021) 2021:8868361. doi: 10.1155/2021/8868361
38. Acuña-Castillo C, Coddou C, Bull P, Brito J, Huidobro-Toro JP. Differential role of extracellular histidines in copper, zinc, magnesium and proton modulation of the P2X7 purinergic receptor. J Neurochem (2007) 101(1):17–26. doi: 10.1111/j.1471-4159.2006.04343.x
39. Dutot M, Liang H, Pauloin T, Brignole-Baudouin F, Baudouin C, Warnet JM, et al. Effects of toxic cellular stresses and divalent cations on the human P2X7 cell death receptor. Mol Vis (2008) 14:889–97.
40. Michel AD, Chessell IP, Humphrey PP. Ionic effects on human recombinant P2X7 receptor function. Naunyn Schmiedebergs Arch Pharmacol (1999) 359(2):102–9. doi: 10.1007/PL00005328
41. Flittiger B, Klapperstück M, Schmalzing G, Markwardt F. Effects of protons on macroscopic and single-channel currents mediated by the human P2X7 receptor. Biochim Biophys Acta (2010) 1798(5):947–57. doi: 10.1016/j.bbamem.2010.01.023
42. Kubick C, Schmalzing G, Markwardt F. The effect of anions on the human P2X7 receptor. Biochim Biophys Acta (2011) 1808(12):2913–22. doi: 10.1016/j.bbamem.2011.08.017
43. Virginio C, MacKenzie A, Rassendren FA, North RA, Surprenant A. Pore dilation of neuronal P2X receptor channels. Nat Neurosci (1999) 2(4):315–21. doi: 10.1038/7225
44. Harkat M, Peverini L, Cerdan AH, Dunning K, Beudez J, Martz A, et al. On the permeation of large organic cations through the pore of ATP-gated P2X receptors. Proc Natl Acad Sci U S A. (2017) 114(19):E3786–95. doi: 10.1073/pnas.1701379114
45. Di Virgilio F, Schmalzing G, Markwardt F. The elusive P2X7 macropore. Trends Cell Biol (2018) 28(5):392–404. doi: 10.1016/j.tcb.2018.01.005
46. Wang W, Huang F, Jiang W, Wang W, Xiang J. Brilliant blue G attenuates neuro-inflammation via regulating MAPKs and NF-κB signaling pathways in lipopolysaccharide-induced BV2 microglia cells. Exp Ther Med (2020) 20(5):116. doi: 10.3892/etm.2020.9244
47. He Y, Taylor N, Fourgeaud L, Bhattacharya A. The role of microglial P2X7: modulation of cell death and cytokine release. J Neuroinflammation. (2017) 14(1):135. doi: 10.1186/s12974-017-0904-8
48. Apolloni S, Parisi C, Pesaresi MG, Rossi S, Carrì MT, Cozzolino M, et al. The NADPH oxidase pathway is dysregulated by the P2X7 receptor in the SOD1-G93A microglia model of amyotrophic lateral sclerosis. J Immunol (2013) 190(10):5187–95. doi: 10.4049/jimmunol.1203262
49. Ochi-ishi R, Nagata K, Inoue T, Tozaki-Saitoh H, Tsuda M, Inoue K. Involvement of the chemokine CCL3 and the purinoceptor P2X7 in the spinal cord in paclitaxel-induced mechanical allodynia. Mol Pain. (2014) 10:53. doi: 10.1186/1744-8069-10-53
50. Shiratori M, Tozaki-Saitoh H, Yoshitake M, Tsuda M, Inoue K. P2X7 receptor activation induces CXCL2 production in microglia through NFAT and PKC/MAPK pathways. J Neurochem (2010) 114(3):810–9. doi: 10.1111/j.1471-4159.2010.06809.x
51. Lee HG, Won SM, Gwag BJ, Lee YB. Microglial P2X7 receptor expression is accompanied by neuronal damage in the cerebral cortex of the APPswe/PS1dE9 mouse model of Alzheimer’s disease. Exp Mol Med (2011) 43(1):7–14. doi: 10.3858/emm.2011.43.1.001
52. Munoz FM, Patel PA, Gao X, Mei Y, Xia J, Gilels S, et al. Reactive oxygen species play a role in P2X7 receptor-mediated IL-6 production in spinal astrocytes. Purinergic Signal (2020) 16(1):97–107. doi: 10.1007/s11302-020-09691-5
53. Gandelman M, Levy M, Cassina P, Barbeito L, Beckman JS. P2X7 receptor-induced death of motor neurons by a peroxynitrite/FAS-dependent pathway. J Neurochem (2013) 126(3):382–8. doi: 10.1111/jnc.12286
54. Chisari M, Barraco M, Bucolo C, Ciranna L, Sortino MA. Purinergic ionotropic P2X7 and metabotropic glutamate mGlu5 receptors crosstalk influences pro-inflammatory conditions in microglia. Eur J Pharmacol (2023) 938:175389. doi: 10.1016/j.ejphar.2022.175389
55. Song P, Hu J, Liu X, Deng X. Increased expression of the P2X7 receptor in temporal lobe epilepsy: Animal models and clinical evidence. Mol Med Rep (2019) 19(6):5433–9. doi: 10.3892/mmr.2019.10202
56. Li T, Gao Y, He M, Gui Z, Zhao B, Cao Y, et al. P2X7 receptor-activated microglia in cortex is critical for sleep disorder under neuropathic pain. Front Neurosci (2023) 17:1095718. doi: 10.3389/fnins.2023.1095718
57. Butt AM. ATP: a ubiquitous gliotransmitter integrating neuron-glial networks. Semin Cell Dev Biol (2011) 22(2):205–13. doi: 10.1016/j.semcdb.2011.02.023
58. Gustin A, Kirchmeyer M, Koncina E, Felten P, Losciuto S, Heurtaux T, et al. NLRP3 inflammasome is expressed and functional in mouse brain microglia but not in astrocytes. PloS One (2015) 10(6):e0130624. doi: 10.1371/journal.pone.0130624
59. Plotnikov A, Zehorai E, Procaccia S, Seger R. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim Biophys Acta (2011) 1813(9):1619–33. doi: 10.1016/j.bbamcr.2010.12.012
60. Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta (2010) 1802(4):396–405. doi: 10.1016/j.bbadis.2009.12.009
61. Chew J, Biswas S, Shreeram S, Humaidi M, Wong ET, Dhillion MK, et al. WIP1 phosphatase is a negative regulator of NF-kappaB signalling. Nat Cell Biol (2009) 11(5):659–66. doi: 10.1038/ncb1873
62. Wen Z, Mei B, Li H, Dou Y, Tian X, Shen M, et al. P2X7 participates in intracerebral hemorrhage-induced secondary brain injury in rats via MAPKs signaling pathways. Neurochem Res (2017) 42(8):2372–83. doi: 10.1007/s11064-017-2257-1
63. Kumar S, Mishra A, Krishnamurthy S. Purinergic antagonism prevents mitochondrial dysfunction and behavioral deficits associated with dopaminergic toxicity induced by 6-OHDA in rats. Neurochem Res (2017) 42(12):3414–30. doi: 10.1007/s11064-017-2383-9
64. Hide I, Tanaka M, Inoue A, Nakajima K, Kohsaka S, Inoue K, et al. Extracellular ATP triggers tumor necrosis factor-alpha release from rat microglia. J Neurochem (2000) 75(3):965–72. doi: 10.1046/j.1471-4159.2000.0750965.x
65. Jandy M, Noor A, Nelson P, Dennys CN, Karabinas IM, Pestoni JC, et al. Peroxynitrite nitration of Tyr 56 in Hsp90 induces PC12 cell death through P2X7R-dependent PTEN activation. Redox Biol (2022) 50:102247. doi: 10.1016/j.redox.2022.102247
66. Tufekci KU, Genc S, Genc K. The endotoxin-induced neuroinflammation model of Parkinson’s disease. Parkinsons Dis (2011) 2011:487450. doi: 10.4061/2011/487450
67. Herrera AJ, Castaño A, Venero JL, Cano J, MaChado A. The single intranigral injection of LPS as a new model for studying the selective effects of inflammatory reactions on dopaminergic system. Neurobiol Dis (2000) 7(4):429–47. doi: 10.1006/nbdi.2000.0289
68. Wang XH, Xie X, Luo XG, Shang H, He ZY. Inhibiting purinergic P2X7 receptors with the antagonist brilliant blue G is neuroprotective in an intranigral lipopolysaccharide animal model of Parkinson’s disease. Mol Med Rep (2017) 15(2):768–76. doi: 10.3892/mmr.2016.6070
69. Carmo MR, Menezes AP, Nunes AC, Pliássova A, Rolo AP, Palmeira CM, et al. The P2X7 receptor antagonist Brilliant Blue G attenuates contralateral rotations in a rat model of Parkinsonism through a combined control of synaptotoxicity, neurotoxicity and gliosis. Neuropharmacology (2014) 81:142–52. doi: 10.1016/j.neuropharm.2014.01.045
70. Marcellino D, Suárez-Boomgaard D, Sánchez-Reina MD, Aguirre JA, Yoshitake T, Yoshitake S, et al. On the role of P2X(7) receptors in dopamine nerve cell degeneration in a rat model of Parkinson’s disease: studies with the P2X(7) receptor antagonist A-438079. J Neural Transm (Vienna). (2010) 117(6):681–7. doi: 10.1007/s00702-010-0400-0
71. Hracskó Z, Baranyi M, Csölle C, Gölöncsér F, Madarász E, Kittel A, et al. Lack of neuroprotection in the absence of P2X7 receptors in toxin-induced animal models of Parkinson’s disease. Mol Neurodegener. (2011) 6:28. doi: 10.1186/1750-1326-6-28
72. Jacques-Silva MC, Rodnight R, Lenz G, Liao Z, Kong Q, Tran M, et al. P2X7 receptors stimulate AKT phosphorylation in astrocytes. Br J Pharmacol (2004) 141(7):1106–17. doi: 10.1038/sj.bjp.0705685
73. Bian S, Sun X, Bai A, Zhang C, Li L, Enjyoji K, et al. P2X7 integrates PI3K/AKT and AMPK-PRAS40-mTOR signaling pathways to mediate tumor cell death. PloS One (2013) 8(4):e60184. doi: 10.1371/journal.pone.0060184
74. Camden JM, Schrader AM, Camden RE, González FA, Erb L, Seye CI, et al. P2Y2 nucleotide receptors enhance alpha-secretase-dependent amyloid precursor protein processing. J Biol Chem (2005) 280(19):18696–702. doi: 10.1074/jbc.M500219200
75. Surprenant A, North RA. Signaling at purinergic P2X receptors. Annu Rev Physiol (2009) 71:333–59. doi: 10.1146/annurev.physiol.70.113006.100630
76. Allinson TM, Parkin ET, Turner AJ, Hooper NM. ADAMs family members as amyloid precursor protein alpha-secretases. J Neurosci Res (2003) 74(3):342–52. doi: 10.1002/jnr.10737
77. Delarasse C, Auger R, Gonnord P, Fontaine B, Kanellopoulos JM. The purinergic receptor P2X7 triggers alpha-secretase-dependent processing of the amyloid precursor protein. J Biol Chem (2011) 286(4):2596–606. doi: 10.1074/jbc.M110.200618
78. Ma G, Chen S, Wang X, Ba M, Yang H, Lu G. Short-term interleukin-1(beta) increases the release of secreted APP(alpha) via MEK1/2-dependent and JNK-dependent alpha-secretase cleavage in neuroglioma U251 cells. J Neurosci Res (2005) 80(5):683–92. doi: 10.1002/jnr.20515
79. Darmellah A, Rayah A, Auger R, Cuif MH, Prigent M, Arpin M, et al. Ezrin/radixin/moesin are required for the purinergic P2X7 receptor (P2X7R)-dependent processing of the amyloid precursor protein. J Biol Chem (2012) 287(41):34583–95. doi: 10.1074/jbc.M112.400010
80. Exner N, Lutz AK, Haass C, Winklhofer KF. Mitochondrial dysfunction in Parkinson’s disease: molecular mechanisms and pathophysiological consequences. EMBO J (2012) 31:3038–62. doi: 10.1038/emboj.2012.170
81. Kubli DA, Gustafsson AB. Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res (2012) 111:1208–21. doi: 10.1161/CIRCRESAHA.112.265819
82. Lull ME, Block ML. Microglial activation and chronic neurodegeneration. Neurotherapeutics (2010) 7(4):354–65. doi: 10.1016/j.nurt.2010.05.014
83. Cunningham C. Microglia and neurodegeneration: the role of systemic inflammation. Glia (2013) 61(1):71–90. doi: 10.1002/glia.22350
84. Jiang T, Hoekstra J, Heng X, Kang W, Ding J, Liu J, et al. P2X7 receptor is critical in α-synuclein–mediated microglial NADPH oxidase activation. Neurobiol Aging. (2015) 36(7):2304–18. doi: 10.1016/j.neurobiolaging.2015.03.015
85. Zelentsova AS, Deykin AV, Soldatov VO, Ulezko AA, Borisova AY, Belyaeva VS, et al. P2X7 receptor and purinergic signaling: orchestrating mitochondrial dysfunction in neurodegenerative diseases. eNeuro (2022) 9(6):ENEURO.0092-22.2022. doi: 10.1523/ENEURO.0092-22.2022
86. Nishida K, Nakatani T, Ohishi A, Okuda H, Higashi Y, Matsuo T, et al. Mitochondrial dysfunction is involved in P2X7 receptor-mediated neuronal cell death. J Neurochem (2012) 122(6):1118–28. doi: 10.1111/j.1471-4159.2012.07868.x
87. Subramaniam SR, Chesselet MF. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog Neurobiol (2013) 106–107:17–32. doi: 10.1016/j.pneurobio.2013.04.004
88. Wu CC, Bratton SB. Regulation of the intrinsic apoptosis pathway by reactive oxygen species. Antioxid Redox Signal (2013) 19(6):546–58. doi: 10.1089/ars.2012.4905
89. Wilkaniec A, Cieślik M, Murawska E, Babiec L, Gąssowska-Dobrowolska M, Pałasz E, et al. P2X7 receptor is involved in mitochondrial dysfunction induced by extracellular alpha synuclein in neuroblastoma SH-SY5Y cells. Int J Mol Sci (2020) 21(11):3959. doi: 10.3390/ijms21113959
90. Rivas-Arancibia S, Guevara-Guzmán R, López-Vidal Y, Rodríguez-Martínez E, Zanardo-Gomes M, Angoa-Pérez M, et al. Oxidative stress caused by ozone exposure induces loss of brain repair in the hippocampus of adult rats. Toxicol Sci (2010) 113:187–97. doi: 10.1093/toxsci/kfp252
91. Halliwell B. Free radicals and antioxidants: Updating a personal view. Nutr Rev (2012) 70:257–65. doi: 10.1111/j.1753-4887.2012.00476.x
92. Solleiro-Villavicencio H, Rivas-Arancibia S. Effect of chronic oxidative stress on neuroinflammatory response mediated by CD4+T cells in neurodegenerative diseases. Front Cell Neurosci (2018) 12:114. doi: 10.3389/fncel.2018.00114
93. Adhya P, Sharma SS. Redox TRPs in diabetes and diabetic complications: Mechanisms and pharmacological modulation. Pharmacol Res (2019) 146:104271. doi: 10.1016/j.phrs.2019.104271
94. Hsieh HL, Yang CM. Role of redox signaling in neuroinflammation and neurodegenerative diseases. BioMed Res Int (2013) 2013:484613. doi: 10.1155/2013/484613
95. Pajares M, Rojo A, Manda G, Boscá L, Cuadrado A. Inflammation in Parkinson’s disease: Mechanisms and therapeutic implications. Cells (2020) 9(7):1687. doi: 10.3390/cells9071687
96. Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta (2016) 1863(12):2977–29922. doi: 10.1016/j.bbamcr.2016.09.012
97. Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev (2007) 87(1):245–313. doi: 10.1152/physrev.00044.2005
98. Munoz FM, Gao R, Tian Y, Henstenburg BA, Barrett JE, Hu H. Neuronal P2X7 receptor-induced reactive oxygen species production contributes to nociceptive behavior in mice. Sci Rep (2017) 7(1):3539. doi: 10.1038/s41598-017-03813-7
99. Block ML. NADPH oxidase as a therapeutic target in Alzheimer’s disease. BMC Neurosci (2008) 9 Suppl 2(Suppl 2):S8. doi: 10.1186/1471-2202-9-S2-S8
100. Hernandes MS, Britto LR. NADPH oxidase and neurodegeneration. Curr Neuropharmacol. (2012) 10(4):321–7. doi: 10.2174/157015912804499483
101. Lenertz LY, Gavala ML, Hill LM, Bertics PJ. Cell signaling via the P2X(7) nucleotide receptor: linkage to ROS production, gene transcription, and receptor trafficking. Purinergic Signal (2009) 5(2):175–87. doi: 10.1007/s11302-009-9133-7
102. Hewinson J, Mackenzie AB. P2X(7) receptor-mediated reactive oxygen and nitrogen species formation: from receptor to generators. Biochem Soc Trans (2007) 35(Pt 5):1168–70. doi: 10.1042/BST0351168
103. Di Virgilio F, Ferrari D, Adinolfi E. P2X(7): a growth-promoting receptor-implications for cancer. Purinergic Signal (2009) 5(2):251–6. doi: 10.1007/s11302-009-9145-3
104. Bartlett R, Yerbury JJ, Sluyter R. P2X7 receptor activation induces reactive oxygen species formation and cell death in murine EOC13 microglia. Mediators Inflamm (2013) 2013:271813. doi: 10.1155/2013/271813
105. Kim SY, Moon JH, Lee HG, Kim SU, Lee YB. ATP released from beta-amyloid-stimulated microglia induces reactive oxygen species production in an autocrine fashion. Exp Mol Med (2007) 39(6):820–7. doi: 10.1038/emm.2007.89
106. Cenini G, Voos W. Mitochondria as potential targets in alzheimer disease therapy: an update. Front Pharmacol (2019) 10:902. doi: 10.3389/fphar.2019.00902
107. Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci (2001) 2(10):734–44. doi: 10.1038/35094583
108. Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell (2010) 140(6):918–34. doi: 10.1016/j.cell.2010.02.016
109. Xu Z, Chen ZM, Wu X, Zhang L, Cao Y, Zhou P. Distinct molecular mechanisms underlying potassium efflux for NLRP3 inflammasome activation. Front Immunol (2020) 11:609441. doi: 10.3389/fimmu.2020.609441
110. Bai R, Lang Y, Shao J, Deng Y, Refuhati R, Cui L. The role of NLRP3 inflammasome in cerebrovascular diseases pathology and possible therapeutic targets. ASN Neuro (2021) 13:17590914211018100. doi: 10.1177/17590914211018100
111. Toma C, Higa N, Koizumi Y, Nakasone N, Ogura Y, McCoy AJ, et al. Pathogenic Vibrio activate NLRP3 inflammasome via cytotoxins and TLR/nucleotide-binding oligomerization domain-mediated NF-kappa B signaling. J Immunol (2010) 184(9):5287–97. doi: 10.4049/jimmunol.0903536
112. Zhang X, Fu Y, Li H, Shen L, Chang Q, Pan L, et al. H3 relaxin inhibits the collagen synthesis via ROS- and P2X7R-mediated NLRP3 inflammasome activation in cardiac fibroblasts under high glucose. J Cell Mol Med (2018) 22(3):1816–25. doi: 10.1111/jcmm.13464
113. Saha S, Buttari B, Panieri E, Profumo E, Saso L. An overview of nrf2 signaling pathway and its role in inflammation. Molecules (2020) 25(22):5474. doi: 10.3390/molecules25225474
114. Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol (2013) 13(6):397–411. doi: 10.1038/nri3452
115. Song N, Li T. Regulation of NLRP3 inflammasome by phosphorylation. Front Immunol (2018) 9:2305. doi: 10.3389/fimmu.2018.02305
116. Stephenson J, Nutma E, van der Valk P, Amor S. Inflammation in CNS neurodegenerative diseases. Immunology (2018) 154(2):204–19. doi: 10.1111/imm.12922
117. Wimo A, Guerchet M, Ali GC, Wu YT, Prina AM, Winblad B, et al. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement. (2017) 13(1):1–7. doi: 10.1016/j.jalz.2016.07.150
118. Cova I, Markova A, Campini I, Grande G, Mariani C, Pomati S. Worldwide trends in the prevalence of dementia. J Neurol Sci (2017) 379:259. doi: 10.1016/j.jns.2017.06.030
119. Amor S, Peferoen LA, Vogel DY, Breur M, van der Valk P, Baker D, et al. Inflammation in neurodegenerative diseases–an update. Immunology (2014) 142(2):151–66. doi: 10.1111/imm.12233
120. Cummings JL, Tong G, Ballard C. Treatment combinations for alzheimer’s disease: current and future pharmacotherapy options. J Alzheimers Dis (2019) 67(3):779–94. doi: 10.3233/JAD-180766
121. Atri A. The alzheimer’s disease clinical spectrum: diagnosis and management. Med Clin North Am (2019) 103(2):263–93. doi: 10.1016/j.mcna.2018.10.009
122. Tolosa E, Garrido A, Scholz SW, Poewe W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol (2021) 20(5):385–97. doi: 10.1016/S1474-4422(21)00030-2
123. Myall DJ, Pitcher TL, Pearson JF, Dalrymple-Alford JC, Anderson TJ, MacAskill MR. Parkinson’s in the oldest old: impact on estimates of future disease burden. Parkinsonism Relat Disord (2017) 42:78–84. doi: 10.1016/j.parkreldis.2017.06.018
124. Tysnes OB, Storstein A. Epidemiology of parkinson’s disease. J Neural Transm (2017) 124:901–5. doi: 10.1007/s00702-017-1686-y
125. Rawlins MD, Wexler NS, Wexler AR, Tabrizi SJ, Douglas I, Evans SJ, et al. The prevalence of huntington’s disease. Neuroepidemiology (2016) 46(2):144–53. doi: 10.1159/000443738
126. Tabrizi SJ, Estevez-Fraga C, van Roon-Mom WMC, Flower MD, Scahill RI, Wild EJ, et al. Potential disease-modifying therapies for Huntington’s disease: lessons learned and future opportunities. Lancet Neurol (2022) 21(7):645–58. doi: 10.1016/S1474-4422(22)00121-1
127. Klineova S, Lublin FD. Clinical course of multiple sclerosis. Cold Spring Harb Perspect Med (2018) 8(9):a028928. doi: 10.1101/cshperspect.a028928
128. Mackenzie IS, Morant SV, Bloomfield GA, MacDonald TM, O’riordan J. Incidence and prevalence of multiple sclerosis in the UK 1990–2010: a descriptive study in the General Practice Research Database. J Neurol Neurosurg Psychiatry (2014) 85:76–84. doi: 10.1136/jnnp-2013-305450
129. Doshi A, Chataway J. Multiple sclerosis, a treatable disease. Clin Med (Lond). (2016) 16(Suppl 6):s53–9. doi: 10.7861/clinmedicine.16-6-s53
130. Chiò A, Logroscino G, Traynor BJ, Collins J, Simeone JC, Goldstein LA, et al. Global epidemiology of amyotrophic lateral sclerosis: a systematic review of the published literature. Neuroepidemiology (2013) 41(2):118–30. doi: 10.1159/000351153
131. Arthur KC, Calvo A, Price TR, Geiger JT, Chio A, Traynor BJ. Projected increase in amyotrophic lateral sclerosis from 2015 to 2040. Nat Commun (2016) 7:12 408. doi: 10.1038/ncomms12408
132. Hulisz D. Amyotrophic lateral sclerosis: disease state overview. Am J Manag Care (2018) 24(15 Suppl):S320–6.
133. Grad LI, Rouleau GA, Ravits J, Cashman NR. Clinical spectrum of amyotrophic lateral sclerosis (ALS). Cold Spring Harb Perspect Med (2017) 7(8):a024117. doi: 10.1101/cshperspect.a024117
134. Illes P, Verkhratsky A, Burnstock G, Franke H. P2X receptors and their roles in astroglia in the central and peripheral nervous system. Neuroscientist (2012) 18(5):422–38. doi: 10.1177/1073858411418524
135. Illes P, Burnstock G, Tang Y. Astroglia-derived ATP modulates CNS neuronal circuits. Trends Neurosci (2019) 42(12):885–98. doi: 10.1016/j.tins.2019.09.006
136. Di Virgilio F, Chiozzi P, Falzoni S, Ferrari D, Sanz JM, Venketaraman V, et al. Cytolytic P2X purinoceptors. Cell Death Differ (1998) 5(3):191–9. doi: 10.1038/sj.cdd.4400341
137. Martin E, Amar M, Dalle C, Youssef I, Boucher C, Le Duigou C, et al. New role of P2X7 receptor in an Alzheimer’s disease mouse model. Mol Psychiatry (2019) 24(1):108–25. doi: 10.1038/s41380-018-0108-3
138. Reitz C, Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol (2014) 88(4):640–51. doi: 10.1016/j.bcp.2013.12.024
139. Götz J, Bodea LG, Goedert M. Rodent models for Alzheimer disease. Nat Rev Neurosci (2018) 19(10):583–98. doi: 10.1038/s41583-018-0054-8
140. Diaz-Hernandez JI, Gomez-Villafuertes R, León-Otegui M, Hontecillas-Prieto L, Del Puerto A, Trejo JL, et al. In vivo P2X7 inhibition reduces amyloid plaques in Alzheimer’s disease through GSK3β and secretases. Neurobiol Aging. (2012) 33(8):1816–28. doi: 10.1016/j.neurobiolaging.2011.09.040
141. Mattson MP. Cellular actions of beta-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol Rev (1997) 77(4):1081–132. doi: 10.1152/physrev.1997.77.4.1081
142. León-Otegui M, Gómez-Villafuertes R, Díaz-Hernández JI, Díaz-Hernández M, Miras-Portugal MT, Gualix J. Opposite effects of P2X7 and P2Y2 nucleotide receptors on α-secretase-dependent APP processing in Neuro-2a cells. FEBS Lett (2011) 585(14):2255–62. doi: 10.1016/j.febslet.2011.05.048
143. Sanz JM, Chiozzi P, Ferrari D, Colaianna M, Idzko M, Falzoni S, et al. Activation of microglia by amyloid {beta} requires P2X7 receptor expression. J Immunol (2009) 182(7):4378–85. doi: 10.4049/jimmunol.0803612
144. Fiebich BL, Akter S, Akundi RS. The two-hit hypothesis for neuroinflammation: role of exogenous ATP in modulating inflammation in the brain. Front Cell Neurosci (2014), 8:260. doi: 10.3389/fncel.2014.00260
145. Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, et al. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci (2000) 20(11):4050–8. doi: 10.1523/JNEUROSCI.20-11-04050.2000
146. McLarnon JG, Ryu JK, Walker DG, Choi HB. Upregulated expression of purinergic P2X(7) receptor in Alzheimer disease and amyloid-beta peptide-treated microglia and in peptide-injected rat hippocampus. J Neuropathol Exp Neurol (2006) 65(11):1090–7. doi: 10.1097/01.jnen.0000240470.97295.d3
147. Martínez-Frailes C, Di Lauro C, Bianchi C, de Diego-García L, Sebastián-Serrano Á, Boscá L, et al. Amyloid peptide induced neuroinflammation increases the P2X7 receptor expression in microglial cells, impacting on its functionality. Front Cell Neurosci (2019) 13:143. doi: 10.3389/fncel.2019.00143
148. Cartier L, Hartley O, Dubois-Dauphin M, Krause KH. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev (2005) 48(1):16–42. doi: 10.1016/j.brainresrev.2004.07.021
149. Jaerve A, Müller HW. Chemokines in CNS injury and repair. Cell Tissue Res (2012) 349(1):229–48. doi: 10.1007/s00441-012-1427-3
150. Miras-Portugal MT, Diaz-Hernandez JI, Gomez-Villafuertes R, Diaz-Hernandez M, Artalejo AR, Gualix J. Role of P2X7 and P2Y2 receptors on α-secretase-dependent APP processing: Control of amyloid plaques formation “in vivo” by P2X7 receptor. Comput Struct Biotechnol J (2015) 13:176–81. doi: 10.1016/j.csbj.2015.02.005
151. GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol (2019) 18(5):459–80. doi: 10.1016/S1474-4422(18)30499-X
152. Pasqualetti G, Brooks DJ, Edison P. The role of neuroinflammation in dementias. Curr Neurol Neurosci Rep (2015) 15(4):17. doi: 10.1007/s11910-015-0531-7
153. Crabbé M, van der Perren A, Bollaerts I, Kounelis S, Baekelandt V, Bormans G, et al. Increased P2X7 receptor binding is associated with neuroinflammation in acute but not chronic rodent models for parkinson’s disease. Front Neurosci (2019) 13:799. doi: 10.3389/fnins.2019.00799
154. Oliveira-Giacomelli Á, Petiz LL, Andrejew R, Turrini N, Silva JB, Sack U, et al. Role of P2X7 receptors in immune responses during neurodegeneration. Front Cell Neurosci (2021) 15:662935. doi: 10.3389/fncel.2021.662935
155. He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res (2015) 25(12):1285–98. doi: 10.1038/cr.2015.139
156. Wang S, Chu CH, Guo M, Jiang L, Nie H, Zhang W, et al. Identification of a specific α-synuclein peptide (α-Syn 29-40) capable of eliciting microglial superoxide production to damage dopaminergic neurons. J Neuroinflammation. (2016) 13(1):158. doi: 10.1186/s12974-016-0606-7
157. Wilkaniec A, Gąssowska M, Czapski GA, Cieślik M, Sulkowski G, Adamczyk A. P2X7 receptor-pannexin 1 interaction mediates extracellular alpha-synuclein-induced ATP release in neuroblastoma SH-SY5Y cells. Purinergic Signal (2017) 13(3):347–61. doi: 10.1007/s11302-017-9567-2
158. Sperlágh B, Köfalvi A, Deuchars J, Atkinson L, Milligan CJ, Buckley NJ, et al. Involvement of P2X7 receptors in the regulation of neurotransmitter release in the rat hippocampus. J Neurochem (2002) 81(6):1196–211. doi: 10.1046/j.1471-4159.2002.00920.x
159. Simon DK, Tanner CM, Brundin P. Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clin Geriatr Med (2020) 36(1):1–12. doi: 10.1016/j.cger.2019.08.002
160. Glaser T, Andrejew R, Oliveira-Giacomelli Á, Ribeiro DE, Bonfim Marques L, Ye Q, et al. Purinergic receptors in basal ganglia diseases: shared molecular mechanisms between huntington’s and parkinson’s disease. Neurosci Bull (2020) 36(11):1299–314. doi: 10.1007/s12264-020-00582-8
161. Lautenschläger J, Stephens AD, Fusco G, Ströhl F, Curry N, Zacharopoulou M, et al. C-terminal calcium binding of α-synuclein modulates synaptic vesicle interaction. Nat Commun (2018) 9(1):712. doi: 10.1038/s41467-018-03111-4
162. Chen S, Ma Q, Krafft PR, Chen Y, Tang J, Zhang J, et al. P2X7 receptor antagonism inhibits p38 mitogen-activated protein kinase activation and ameliorates neuronal apoptosis after subarachnoid hemorrhage in rats. Crit Care Med (2013) 41(12):e466–74. doi: 10.1097/CCM.0b013e31829a8246
163. Ferrazoli EG, de Souza HD, Nascimento IC, Oliveira-Giacomelli Á, Schwindt TT, Britto LR, et al. But not fenofibrate, treatment reverts hemiparkinsonian behavior and restores dopamine levels in an animal model of parkinson’s disease. Cell Transplant. (2017) 26(4):669–77. doi: 10.3727/096368917X695227
164. Jun DJ, Kim J, Jung SY, Song R, Noh JH, Park YS, et al. Extracellular ATP mediates necrotic cell swelling in SN4741 dopaminergic neurons through P2X7 receptors. J Biol Chem (2007) 282(52):37350–8. doi: 10.1074/jbc.M707915200
165. Stahl CM, Feigin A. Medical, surgical, and genetic treatment of huntington disease. Neurol Clin (2020) 38(2):367–78. doi: 10.1016/j.ncl.2020.01.010
166. Jimenez-Sanchez M, Licitra F, Underwood BR, Rubinsztein DC. Huntington’s disease: mechanisms of pathogenesis and therapeutic strategies. Cold Spring Harb Perspect Med (2017) 7(7):a024240. doi: 10.1101/cshperspect.a024240
167. Leavitt BR, Kordasiewicz HB, Schobel SA. Huntingtin-lowering therapies for huntington disease. JAMA Neurol (2020) 77:764. doi: 10.1001/jamaneurol.2020.0299
168. Tabrizi SJ, Ghosh R, Leavitt BR. Huntingtin lowering strategies for disease modification in huntington’s disease. Neuron (2019) 101:801–19. doi: 10.1016/j.neuron.2019.01.039
169. Grima JC, Daigle JG, Arbez N, Cunningham KC, Zhang K, Ochaba J, et al. Mutant huntingtin disrupts the nuclear pore complex. Neuron (2017) 94(1):93–107.e6. doi: 10.1016/j.neuron.2017.03.023
170. Jones L, Hughes A. Pathogenic mechanisms in Huntington’s disease. Int Rev Neurobiol (2011) 98:373–418. doi: 10.1016/B978-0-12-381328-2.00015-8
171. Díaz-Hernández M, Díez-Zaera M, Sánchez-Nogueiro J, Gómez-Villafuertes R, Canals JM, Alberch J, et al. Altered P2X7-receptor level and function in mouse models of Huntington’s disease and therapeutic efficacy of antagonist administration. FASEB J (2009) 23(6):1893–906. doi: 10.1096/fj.08-122275
172. Ollà I, Santos-Galindo M, Elorza A, Lucas JJ. P2X7 receptor upregulation in huntington’s disease brains. Front Mol Neurosci (2020) 13:567430. doi: 10.3389/fnmol.2020.567430
173. Martire A, Pepponi R, Liguori F, Volonté C, Popoli P. P2X7 receptor agonist 2’(3’)-O-(4-benzoylbenzoyl)ATP differently modulates cell viability and corticostriatal synaptic transmission in experimental models of huntington’s disease. Front Pharmacol (2021) 11:633861. doi: 10.3389/fphar.2020.633861
174. Wang S, Wang B, Shang D, Zhang K, Yan X, Zhang X. Ion channel dysfunction in astrocytes in neurodegenerative diseases. Front Physiol (2022) 13:814285. doi: 10.3389/fphys.2022.814285
175. Tullman MJ. Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am J Manag Care (2013) 19(2 Suppl):S15–20.
176. Cotsapas C, Mitrovic M, Hafler D. Multiple sclerosis. Handb Clin Neurol (2018) 148:723–30. doi: 10.1016/B978-0-444-64076-5.00046-6
177. Yiangou Y, Facer P, Durrenberger P, Chessell IP, Naylor A, Bountra C, et al. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol (2006) 6:12. doi: 10.1186/1471-2377-6-12
178. Ahmed SM, Rzigalinski BA, Willoughby KA, Sitterding HA, Ellis EF. Stretch-induced injury alters mitochondrial membrane potential and cellular ATP in cultured astrocytes and neurons. J Neurochem (2000) 74(5):1951–60.
179. Amadio S, Parisi C, Piras E, Fabbrizio P, Apolloni S, Montilli C, et al. Modulation of P2X7 receptor during inflammation in multiple sclerosis. Front Immunol (2017) 8:1529. doi: 10.3389/fimmu.2017.01529
180. Gendron FP, Neary JT, Theiss PM, Sun GY, Gonzalez FA, Weisman GA. Mechanisms of P2X7 receptor-mediated ERK1/2 phosphorylation in human astrocytoma cells. Am J Physiol Cell Physiol (2003) 284(2):C571–81. doi: 10.1152/ajpcell.00286.2002
181. Bianco F, Perrotta C, Novellino L, Francolini M, Riganti L, Menna E, et al. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J (2009) 28(8):1043–54. doi: 10.1038/emboj.2009.45
182. Hamilton N, Vayro S, Wigley R, Butt AM. Axons and astrocytes release ATP and glutamate to evoke calcium signals in NG2-glia. Glia (2010) 58(1):66–79. doi: 10.1002/glia.20902
183. Wiley JS, Sluyter R, Gu BJ, Stokes L, Fuller SJ. The human P2X7 receptor and its role in innate immunity. Tissue Antigens (2011) 78(5):321–32. doi: 10.1111/j.1399-0039.2011.01780.x
184. Sharp AJ, Polak PE, Simonini V, Lin SX, Richardson JC, Bongarzone ER, et al. P2x7 deficiency suppresses development of experimental autoimmune encephalomyelitis. J Neuroinflammation. (2008) 5:33. doi: 10.1186/1742-2094-5-33
185. Sluyter R, Shemon AN, Barden JA, Wiley JS. Extracellular ATP increases cation fluxes in human erythrocytes by activation of the P2X7 receptor. J Biol Chem (2004) 279(43):44749–55. doi: 10.1074/jbc.M405631200
186. Feldman EL, Goutman SA, Petri S, Mazzini L, Savelieff MG, Shaw PJ. Sobue G.Amyotrophic lateral sclerosis. Lancet (2022) 400(10360):1363–80. doi: 10.1016/S0140-6736(22)01272-7
187. Amado DA, Davidson BL. Gene therapy for ALS: A review. Mol Ther (2021) 29(12):3345–58. doi: 10.1016/j.ymthe.2021.04.008
188. Masrori P, Van Damme P. Amyotrophic lateral sclerosis: a clinical review. Eur J Neurol (2020) 27(10):1918–29. doi: 10.1111/ene.14393
189. Ruiz-Ruiz C, García-Magro N, Negredo P, Avendaño C, Bhattacharya A, Ceusters M, et al. Chronic administration of P2X7 receptor antagonist JNJ-47965567 delays disease onset and progression, and improves motor performance in ALS SOD1G93A female mice. Dis Model Mech (2020) 13(10):dmm045732. doi: 10.1242/dmm.045732
190. McCauley ME, Baloh RH. Inflammation in ALS/FTD pathogenesis. Acta Neuropathol. (2019) 137(5):715–30. doi: 10.1007/s00401-018-1933-9
191. Gonzalez Porras MA, Sieck GC, Mantilla CB. Impaired autophagy in motor neurons: A final common mechanism of injury and death. Physiol (Bethesda). (2018) 33(3):211–24. doi: 10.1152/physiol.00008.2018
192. Fabbrizio P, Apolloni S, Bianchi A, Salvatori I, Valle C, Lanzuolo C, et al. P2X7 activation enhances skeletal muscle metabolism and regeneration in SOD1G93A mouse model of amyotrophic lateral sclerosis. Brain Pathol (2020) 30(2):272–82. doi: 10.1111/bpa.12774
193. Fabbrizio P, D’Agostino J, Margotta C, Mella G, Panini N, Pasetto L, et al. Contingent intramuscular boosting of P2XR7 axis improves motor function in transgenic ALS mice. Cell Mol Life Sci (2021) 79(1):7. doi: 10.1007/s00018-021-04070-8
194. Gandelman M, Peluffo H, Beckman JS, Cassina P, Barbeito L. Extracellular ATP and the P2X7 receptor in astrocyte-mediated motor neuron death: implications for amyotrophic lateral sclerosis. J Neuroinflammation. (2010) 7:33. doi: 10.1186/1742-2094-7-33
195. Fabbrizio P, Amadio S, Apolloni S, Volonté C. P2X7 receptor activation modulates autophagy in SOD1-G93A mouse microglia. Front Cell Neurosci (2017) 11:249. doi: 10.3389/fncel.2017.00249
196. Apolloni S, Amadio S, Montilli C, Volonté C, D’Ambrosi N. Ablation of P2X7 receptor exacerbates gliosis and motoneuron death in the SOD1-G93A mouse model of amyotrophic lateral sclerosis. Hum Mol Genet (2013) 22(20):4102–16. doi: 10.1093/hmg/ddt259
197. Apolloni S, Amadio S, Parisi C, Matteucci A, Potenza RL, Armida M, et al. Spinal cord pathology is ameliorated by P2X7 antagonism in a SOD1-mutant mouse model of amyotrophic lateral sclerosis. Dis Model Mech (2014) 7(9):1101–9. doi: 10.1242/dmm.017038
198. Bartlett R, Sluyter V, Watson D, Sluyter R, Yerbury JJ. P2X7 antagonism using Brilliant Blue G reduces body weight loss and prolongs survival in female SOD1G93A amyotrophic lateral sclerosis mice. PeerJ (2017) 5:e3064. doi: 10.7717/peerj.3064
199. Apolloni S, Fabbrizio P, Amadio S, Napoli G, Freschi M, Sironi F, et al. Novel P2X7 antagonist ameliorates the early phase of ALS disease and decreases inflammation and autophagy in SOD1-G93A mouse model. Int J Mol Sci (2021) 22(19):10649. doi: 10.3390/ijms221910649
200. Gupta R, Advani D, Yadav D, Ambasta RK, Kumar P. Dissecting the relationship between neuropsychiatric and neurodegenerative disorders. Mol Neurobiol (2023) 60(11):6476–529. doi: 10.1007/s12035-023-03502-9
201. Malhi GS, Mann JJ. Depression. Lancet (2018) 392(10161):2299–312. doi: 10.1016/S0140-6736(18)31948-2
202. Troubat R, Leman S, Pinchaud K, Surget A, Barone P, Roger S, et al. Brain immune cells characterization in UCMS exposed P2X7 knock-out mouse. Brain Behav Immun (2021) 94:159–74. doi: 10.1016/j.bbi.2021.02.012
203. Jacobson KA, Jarvis MF, Williams M. Purine and pyrimidine (P2) receptors as drug targets. J Med Chem (2002) 45(19):4057–93. doi: 10.1021/jm020046y
204. Jiang LH, Mackenzie AB, North RA, Surprenant A. Brilliant blue G selectively blocks ATP-gated rat P2X(7) receptors. Mol Pharmacol (2000) 58(1):82–8. doi: 10.1124/mol.58.1.82
205. Jo YH, Donier E, Martinez A, Garret M, Toulmé E, Boué-Grabot E. Cross-talk between P2X4 and gamma-aminobutyric acid, type A receptors determines synaptic efficacy at a central synapse. J Biol Chem (2011) 286(22):19993–20004. doi: 10.1074/jbc.M111.231324
206. Humphreys BD, Virginio C, Surprenant A, Rice J, Dubyak GR. Isoquinolines as antagonists of the P2X7 nucleotide receptor: high selectivity for the human versus rat receptor homologues. Mol Pharmacol (1998) 54(1):22–32. doi: 10.1124/mol.54.1.22
207. Shemon AN, Sluyter R, Conigrave AD, Wiley JS. Chelerythrine and other benzophenanthridine alkaloids block the human P2X7 receptor. Br J Pharmacol (2004) 142(6):1015–9. doi: 10.1038/sj.bjp.0705868
208. Marques da Silva C, Miranda Rodrigues L, Passos da Silva Gomes A, Mantuano Barradas M, Sarmento Vieira F, Persechini PM, et al. Modulation of P2X7 receptor expression in macrophages from mineral oil-injected mice. Immunobiology (2008) 213(6):481–92. doi: 10.1016/j.imbio.2007.11.006
209. Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, et al. PubChem 2019 update: improved access to chemical data. Nucleic Acids Res (2019) 47(D1):D1102–9. doi: 10.1093/nar/gky1033
210. Chrovian CC, Soyode-Johnson A, Peterson AA, Gelin CF, Deng X, Dvorak CA, et al. A dipolar cycloaddition reaction to access 6-methyl-4,5,6,7-tetrahydro-1H-[1,2,3]triazolo[4,5-c]pyridines enables the discovery synthesis and preclinical profiling of a P2X7 antagonist clinical candidate. J Med Chem (2018) 61(1):207–23. doi: 10.1021/acs.jmedchem.7b01279
211. Territo PR, Meyer JA, Peters JS, Riley AA, McCarthy BP, Gao M, et al. Characterization of 11C-GSK1482160 for targeting the P2X7 receptor as a biomarker for neuroinflammation. J Nucl Med (2017) 58(3):458–65. doi: 10.2967/jnumed.116.181354
212. Volonté C, Amadio S. Rethinking purinergic concepts and updating the emerging role of P2X7 and P2X4 in amyotrophic lateral sclerosis. Neuropharmacology (2022) 221:109278. doi: 10.1016/j.neuropharm.2022.109278
213. Bartlett R, Ly D, Cashman NR, Sluyter R, Yerbury JJ. P2X7 receptor activation mediates superoxide dismutase 1 (SOD1) release from murine NSC-34 motor neurons. Purinergic Signal (2022) 18(4):451–67. doi: 10.1007/s11302-022-09863-5
214. Sun S, Gong D, Liu R, Wang R, Chen D, Yuan T, et al. Puerarin inhibits NLRP3-caspase-1-GSDMD-mediated pyroptosis via P2X7 receptor in cardiomyocytes and macrophages. Int J Mol Sci (2023) 24(17):13169. doi: 10.3390/ijms241713169
215. Liu X, Huang R, Wan J. Puerarin: a potential natural neuroprotective agent for neurological disorders. BioMed Pharmacother. (2023) 162:114581. doi: 10.1016/j.biopha.2023.114581
216. Skaper SD, Facci L, Culbert AA, Evans NA, Chessell I, Davis JB, et al. P2X(7) receptors on microglial cells mediate injury to cortical neurons in vitro. Glia (2006) 54(3):234–42. doi: 10.1002/glia.20379
217. Matute C, Torre I, Pérez-Cerdá F, Pérez-Samartín A, Alberdi E, Etxebarria E, et al. P2X(7) receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. J Neurosci (2007) 27(35):9525–33. doi: 10.1523/JNEUROSCI.0579-07.2007
218. Ryu JK, McLarnon JG. Block of purinergic P2X(7) receptor is neuroprotective in an animal model of Alzheimer’s disease. Neuroreport (2008) 19(17):1715–9. doi: 10.1097/WNR.0b013e3283179333
219. Jimenez-Pacheco A, Mesuret G, Sanz-Rodriguez A, Tanaka K, Mooney C, Conroy R, et al. Increased neocortical expression of the P2X7 receptor after status epilepticus and anticonvulsant effect of P2X7 receptor antagonist A-438079. Epilepsia (2013) 54(9):1551–61. doi: 10.1111/epi.12257
220. Gao C, Zhang GY. KN-62 provides neuroprotection against glutamate-induced excitotoxicity in neurons. Zhongguo Yao Li Xue Bao. (1999) 20(11):991–4.
221. Broom DC, Matson DJ, Bradshaw E, Buck ME, Meade R, Coombs S, et al. Characterization of N-(adamantan-1-ylmethyl)-5-[(3R-amino-pyrrolidin-1-yl)methyl]-2-chloro-benzamide, a P2X7 antagonist in animal models of pain and inflammation. J Pharmacol Exp Ther (2008) 327(3):620–33. doi: 10.1124/jpet.108.141853
222. Recourt K, de Boer P, van der Ark P, Benes H, van Gerven JMA, Ceusters M, et al. Characterization of the central nervous system penetrant and selective purine P2X7 receptor antagonist JNJ-54175446 in patients with major depressive disorder. Transl Psychiatry (2023) 13(1):266. doi: 10.1038/s41398-023-02557-5
223. Bhattacharya A, Lord B, Grigoleit JS, He Y, Fraser I, Campbell SN, et al. Neuropsychopharmacology of JNJ-55308942: evaluation of a clinical candidate targeting P2X7 ion channels in animal models of neuroinflammation and anhedonia. Neuropsychopharmacology (2018) 43(13):2586–96. doi: 10.1038/s41386-018-0141-6
224. Yang M, Qiu R, Wang W, Liu J, Jin X, Li Y, et al. P2X7 receptor antagonist attenuates retinal inflammation and neovascularization induced by oxidized low-density lipoprotein. Oxid Med Cell Longev (2021) 2021:5520644. doi: 10.1155/2021/5520644
225. Duplantier AJ, Dombroski MA, Subramanyam C, Beaulieu AM, Chang SP, Gabel CA, et al. Optimization of the physicochemical and pharmacokinetic attributes in a 6-azauracil series of P2X7 receptor antagonists leading to the discovery of the clinical candidate CE-224,535. Bioorg Med Chem Lett (2011) 21(12):3708–11. doi: 10.1016/j.bmcl.2011.04.077
226. Staal RGW, Gandhi A, Zhou H, Cajina M, Jacobsen AM, Hestehave S, et al. Inhibition of P2X7 receptors by Lu AF27139 diminishes colonic hypersensitivity and CNS prostanoid levels in a rat model of visceral pain. Purinergic Signal (2022) 18(4):499–514. doi: 10.1007/s11302-022-09892-0
227. Hansen T, Karimi Galougahi K, Besnier M, Genetzakis E, Tsang M, Finemore M, et al. The novel P2X7 receptor antagonist PKT100 improves cardiac function and survival in pulmonary hypertension by direct targeting of the right ventricle. Am J Physiol Heart Circ Physiol (2020) 319(1):H183–91. doi: 10.1152/ajpheart.00580.2019
Keywords: P2X7 receptor, neurodegenerative disease, neuroinflammation, antagonist, microglia
Citation: Zheng H, Liu Q, Zhou S, Luo H and Zhang W (2024) Role and therapeutic targets of P2X7 receptors in neurodegenerative diseases. Front. Immunol. 15:1345625. doi: 10.3389/fimmu.2024.1345625
Received: 28 November 2023; Accepted: 16 January 2024;
Published: 02 February 2024.
Edited by:
Juan Pablo de Rivero Vaccari, University of Miami, United StatesReviewed by:
Orlando Torres-Rodríguez, University of Puerto Rico, Puerto RicoCopyright © 2024 Zheng, Liu, Zhou, Luo and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongliang Luo, bmRlZnkxMzAyOEBuY3UuZWR1LmNu; Wenjun Zhang, bmRlZnkyMjA1N0BuY3UuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.