
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 30 January 2024
Sec. Inflammation
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1337103
 Cheng Yang1,2,3†
Cheng Yang1,2,3† Yinghan Tian1,2,3†
Yinghan Tian1,2,3† Xiaoxue Yang1,2,3†
Xiaoxue Yang1,2,3† Lewei Liu1,2,3
Lewei Liu1,2,3 Chen Ling1,2,3
Chen Ling1,2,3 Lei Xia2,3*
Lei Xia2,3* Huanzhong Liu2,3*
Huanzhong Liu2,3*Background: There is a growing amount of evidence suggesting that immunity and inflammation play an important role in the pathophysiology of schizophrenia. In this study, we aimed to examine the relationship between hematological and inflammatory markers with symptom severity in Han Chinese patients with drug-free schizophrenia.
Methods: This retrospective study was conducted at Chaohu Hospital of Anhui Medical University and data were extracted from the electronic medical record system over a 5-year period (May 2017 to April 2022), including participants’ general and clinical information as well as Brief Psychiatric Rating Scale (BPRS) scores and hematological parameters.
Results: A total of 2,899 patients with schizophrenia were identified through the initial search. After screening, 91 patients and 141 healthy controls (HCs) were included. The patients had a higher value of neutrophils/lymphocytes ratio (NLR), monocyte/lymphocyte ratio (MLR), and platelet/lymphocyte ratio (PLR) than HCs (all P < 0.001). MLR was positively correlated with BPRS total score (r = 0.337, P = 0.001) and resistance subscale score (r = 0.350, P = 0.001). Binary logistic regression analyses revealed that severely ill was significantly associated with being male and a higher value of MLR (Natural Logaruthm, Ln) (all P < 0.05), and the receiver operating characteristic (ROC) analysis showed good performance of a regression model with an area under the curve (AUC) value of 0.787.
Conclusion: Patients with drug-free schizophrenia have an unbalanced distribution of peripheral blood granulocytes, and elevated NLR, MLR and PLR. Patients with higher value of MLR tend to have more psychotic symptoms, especially those symptoms of hostility, uncooperativeness, and suspiciousness. Our study gives a preliminary indication that MLR is a potential predictor of disease severity in patients with drug-free schizophrenia.
The inflammatory response may play an important role in the pathology of schizophrenia (1). Elevated levels of inflammation are frequently found in patients with schizophrenia, which might predict the disease prognosis and relapse (2, 3). The positive association was confirmed by the fact that psychiatric symptoms improve with the decrease in inflammation levels during treatment with antipsychotics (4).
Cytokines are key molecules that regulate inflammation, including pro-inflammatory cytokines and anti-inflammatory cytokines (5). Numerous studies have found that some pro-inflammatory and anti-inflammatory cytokines are elevated in the blood of patients with schizophrenia (6, 7). For example, a meta-analysis of cytokines in schizophrenia found higher levels of pro-inflammatory cytokines such as IL-6, TNF-α and sIL-2R in acute patients, and IL-6, IL-1β and sIL-2R in chronic patients than in healthy controls (8). It also found higher levels of anti-inflammatory cytokines IL-1RA and TGF-β in the unspecified (not acute or chronic) patients (8). Also, another meta-analysis with a large scale of 59 studies found first-episode patients (FEP) with schizophrenia have higher levels of IL-6 and TNF-α than the general population (9). As the inflammatory response occurs, activated cytokines can not only enter the brain through peripheral organs lacking the blood-brain barrier (BBB), but also induce the production of cytokines by cells that compose the BBB. Activated cytokines modulate dopaminergic neurotransmission directly or glutamatergic neurotransmitters indirectly through tryptophan metabolism (10–14). This action leads to brain dysfunction and ultimately to schizophrenia-like symptoms (15).
Since the physiological response of leukocytes under stressful conditions such as inflammation leads to an increase in the neutrophil count and a decrease in the lymphocyte count, the ratios between neutrophil and lymphocyte (NLR), monocyte and lymphocyte (MLR), and platelet and lymphocyte (PLR) are often used as inflammatory markers (16). NLR, MLR and PLR are more accessible than inflammatory cytokines in clinical practice and scientific research, which have been used to predict progression and prognosis of many non-psychiatric diseases, such as hepatocellular carcinoma, gastric cancer, autoimmune encephalitis etc. (17–19). In recent years, there has been an increase in studies of psychiatric disorders involving these inflammatory markers. A meta-analysis of eight observational studies showed that patients with psychotic disorders had significantly higher NLR, MLR and PLR compared to healthy controls (20). In previous studies, NLR was found to be positively associated with the severity of psychotic symptoms as measured by Positive and Negative Syndrome Scale (PANSS) and Clinical Global Impressions Severity (CGI-S) scale in patients with schizophrenia (21–23). Furthermore, Dawidowski et al. demonstrated that NLR decreased after treatment and could be an indicator of response to antipsychotic treatment (24).
However, inconsistent results have been reported in some other studies. For example, a large retrospective study in China found a significant decrease of PLR in patients with acute-onset schizophrenia compared with healthy controls (25). Two cross-sectional studies conducted in Turkey and a cohort study conducted in Europe showed that there was no correlation between NLR and psychotic symptom scores as measured by the BPRS or PANSS in patients with schizophrenia (26–28). Such differences may be attributed to the heterogeneity of subjects across studies (e.g., different ethnicities, antipsychotic intake, and periods of illness). Therefore, this current study aimed to evaluate (I) hematological and inflammatory markers (NLR, MLR and PLR) in Han Chinese patients with acute episode and drug-free schizophrenia; to explore (II) their correlations with total and subscale scores of BPRS; and (III) their potential associations with symptom severity in a predictive model.
This retrospective study extracted 5-year data (May 2017 - April 2022) from the electronic medical record system of Chaohu Hospital of Anhui Medical University, which have a total of 300 psychiatric beds, and serve more than 1 million people in Anhui Province, China. Participants were included if they fulfilled the following criteria: (I) Han Chinese, aged between 18 and 70 years; (II) with a diagnosis of schizophrenia according to the International Classification of Diseases (ICD-10) made by two independent senior psychiatrists; (III) drug-free defined as not taking any psychiatric medication before or off psychiatric medication for one month prior to blood sampling (23). During the same period, age- and sex-matched healthy controls (HCs) without personal or family history of mental illness were recruited from the medical screening center of Chaohu Hospital, which is the largest hospital in Chaohu, serving a population of about 700,000 people. All participants (patients and HCs) were excluded if they had somatic conditions that could alter the inflammatory state, such as acute or chronic infections (High-sensitivity C-reactive protein > 10mg/L), and immune-inflammatory diseases based on recorded diagnosis from the electronic medical record system; or they had blood diseases or were taking medications that would alter blood cell counts.
The Medical Ethics Committee of Chaohu Hospital of Anhui Medical University reviewed and approved the protocol of this retrospective study (No. 202210-kyxm-015).
We collected participants’ demographic and clinical information including age (years), sex (male/female), BMI (kg/m2) and duration of illness (months). BMI was calculated by the formula: weight (kg)/height (meters2).
Blood samples were collected between 06:00 and 08:00 AM after an overnight fast. Blood counts were measured by the Sysmex XN-9000 automated counter in the clinical laboratory department of Chaohu Hospital, including white blood cell (WBC), neutrophil, lymphocyte, monocyte, and platelet counts. NLR (neutrophils/lymphocytes ratio), MLR (monocyte/lymphocyte ratio), and PLR (platelet/lymphocyte) were calculated accordingly.
Psychotic symptoms were assessed by Chinese version of the 18-item Brief Psychiatric Rating Scale (BPRS). All items were rated on a 7-point Likert scale ranging from 1 (not present) to 7 (extremely severe), with higher total scores indicating more severe symptoms (29). According to previous studies, the 18 items of the BPRS can be divided into the five subscales of affect, positive symptoms, negative symptoms, resistance, and activation (30). In this study, a cut-off value of 53 for the BPRS total score was used (mildly or moderately ill: 18-53; and severely ill: >53) (31).
Statistical analyses were performed using SPSS version 23.0 (SPSS IBM, Chicago, IL, USA). Demographic and clinical data are presented as mean (standard deviation, SD), and frequency distribution (%). For comparison between groups, independent samples t-test, Mann-Whitney U test, and Chi-square test were used as appropriate. When we compared the hematological and inflammatory markers between patients and HCs, analysis of covariance (ANCOVA) was used to control for potential confounding factors. Pearson (for normally distributed variables) or Spearman (for non-normally distributed variables) correlation analyses were used to examine the correlation between BPRS scores and hematologic parameters as well as inflammatory markers. Bonferroni corrections were applied to these analyses to adjust for multiple tests. Logarithmic conversions were performed for NLR and MLR in the logistic regression model. A logistic regression analysis (Forward Stepwise method) was used to examine the independent correlates associated with “severe illness” (yes or no), with variables that were significant (P < 0.05) in the univariate analyses as independent variables. We also conducted logistic regression analyses (Enter method) to examine the association between each hematological and inflammatory marker and “severe illness” after adjusting for age, gender, BMI, and duration of illness (Supplementary Table 1). Then, a receiver operating characteristic (ROC) curve was plotted and area under the curve (AUC) values were also calculated to assess the discriminatory power of the fitted model. The statistical significance was set at P < 0.05 (two-tailed).
We obtained 2,899 potentially relevant case records from the initial search (Figure 1). After duplicates excluded (n = 1,431) and screening according to the specific criteria (n = 1,377), 91 patients with drug-free schizophrenia were finally included. Meanwhile, 141 age- and sex-matched HCs from the local medical screening center were included. In this study, the mean age of patients and HCs was 39.27 ± 14.04 years and 41.52 ± 10.82 years, respectively, and 40.7% of patients and 46.8% of HCs were male. There were no significant differences in age, gender and BMI between groups (P > 0.05) (Table 1).
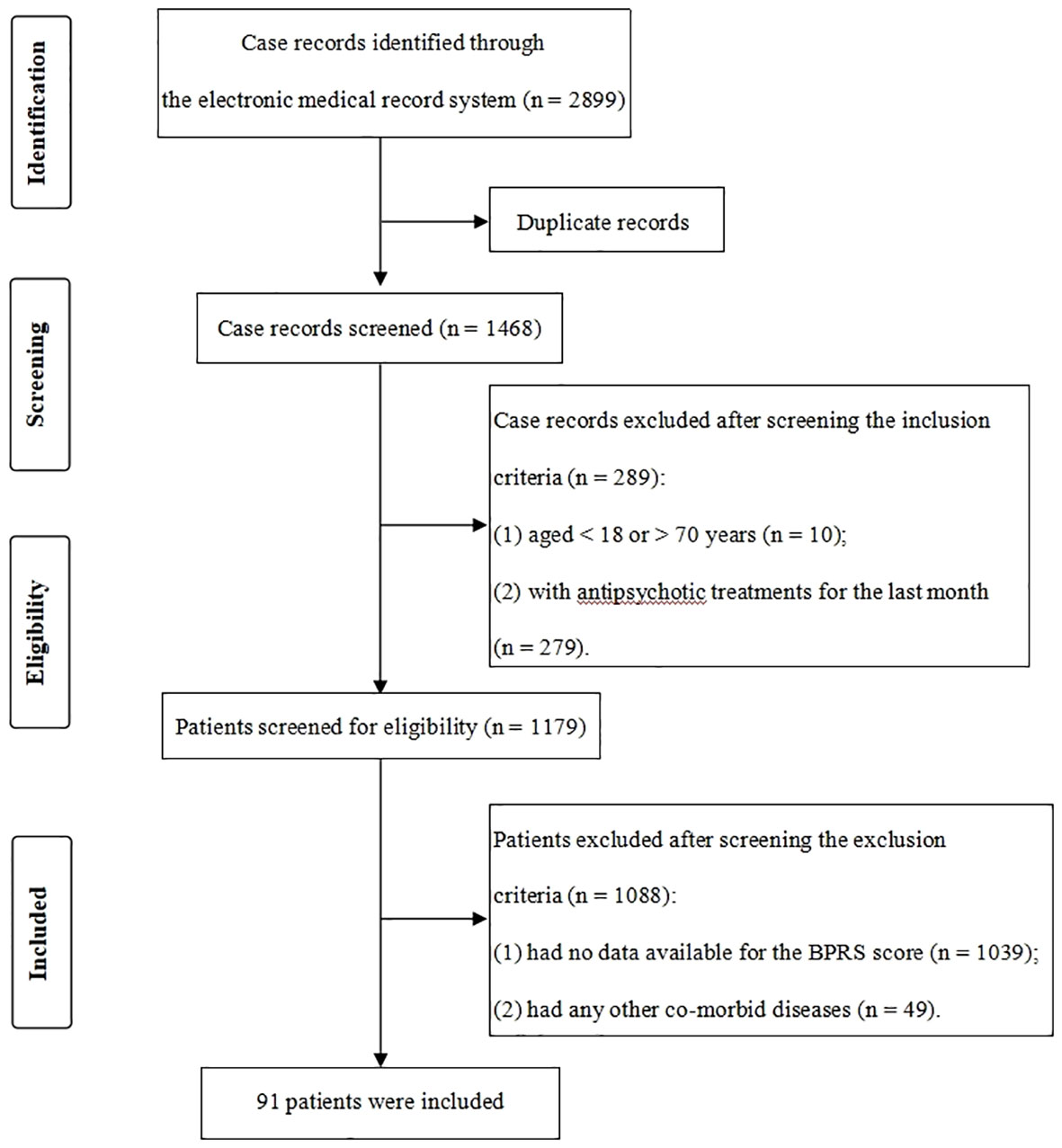
Figure 1 Flowchart of the case records selection. For patients with multiple admissions, only their first admission data were extracted for analyses.
As shown in Table 1, the patients had a higher count of WBC, neutrophil, monocyte, and platelet, a lower count of lymphocyte, and higher values of NLR, MLR, and PLR than the HCs (all P < 0.05). ANCOVA showed that differences in the WBC, neutrophil, monocyte, platelet, lymphocyte, NLR, MLR, and PLR between the two groups remained significant after controlling for age, sex and BMI. The comparisons of NLR, MLR and PLR between different groups were shown in Figure 2.

Figure 2 NLR, MLR and PLR between different groups. NLR, neutrophil/lymphocyte ratio; MLR, monocyte/lymphocyte ratio; PLR, platelet/lymphocyte ratio; *P < 0.05.
The total score of BPRS was positively correlated with the counts of WBC (r = 0.250, p = 0.017), neutrophil (r = 0.337, P = 0.001) and monocyte (r = 0.331, P = 0.001), and the value of NLR (r = 0.285, P = 0.006) and MLR (r = 0.337, P = 0.001). After Bonferroni correction (α = 0.05/48 = 0.001), the correlations between the total score of BPRS with neutrophils, monocytes and MLR remained significant. The correlations between the subscale scores of BPRS with hematological parameters were shown in Table 2. The scatterplots of the correlations between total and subscale scores of BPRS and MLR (Ln) were shown in Supplementary Figure S1.
The patients in severely ill group had a higher count of WBC, neutrophil and monocyte, and higher values of NLR and MLR than those in mildly/moderately ill group (all P < 0.05) (Table 3). Further binary logistic regression analysis (forward stepwise method) found that severely ill was significantly associated with being male (OR = 6.427, 95% CI = 2.292-18.024) and a higher value of MLR (Ln) (OR = 4.236, 95% CI = 1.297-13.837) (all P < 0.05) (Table 4).
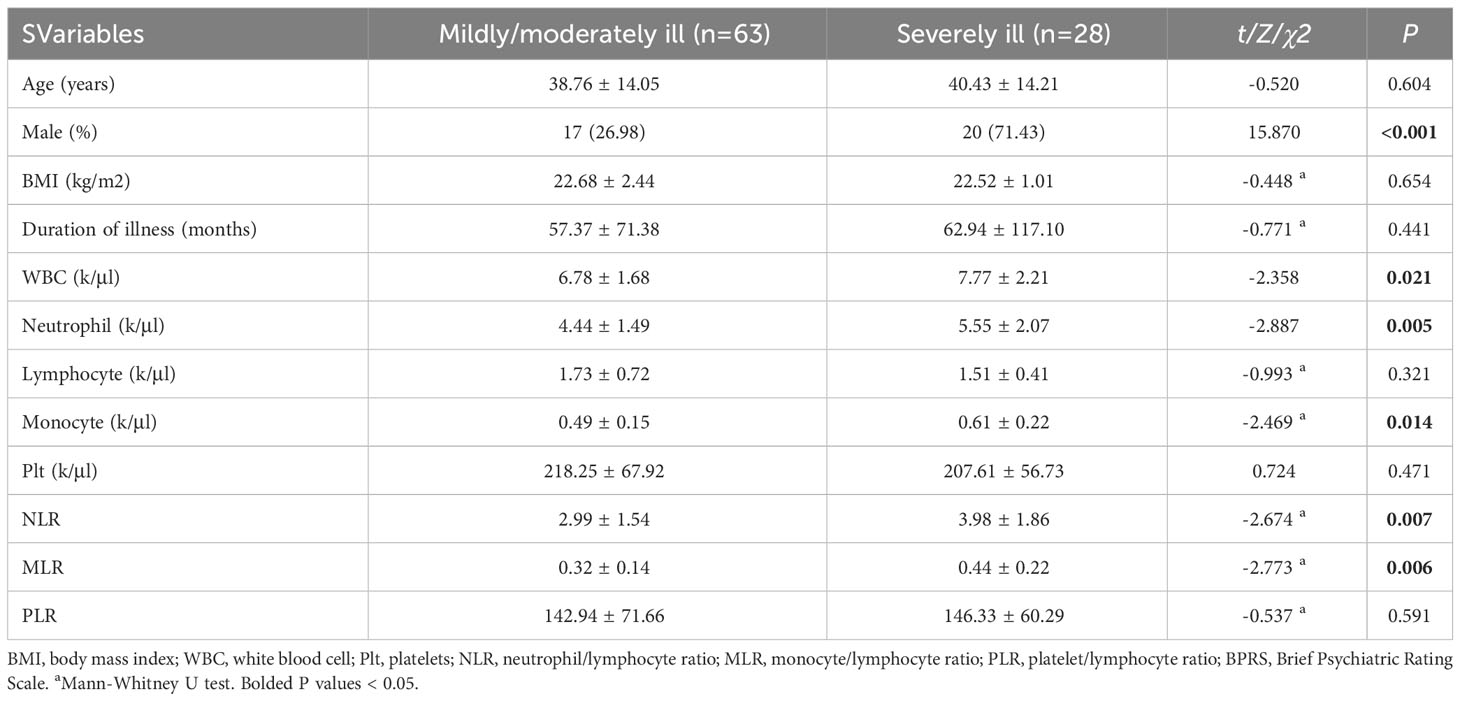
Table 3 Socio-demographic and clinical characteristics of patients in mildly/moderately ill and severely ill group.
The ROC analysis indicates a good discriminatory ability with a AUC value of 0.787 (95% CI: 0.674-0.899). The cut-off point was 0.443, with a sensitivity of 64.3% and a specificity of 87.3% (Figure 3).
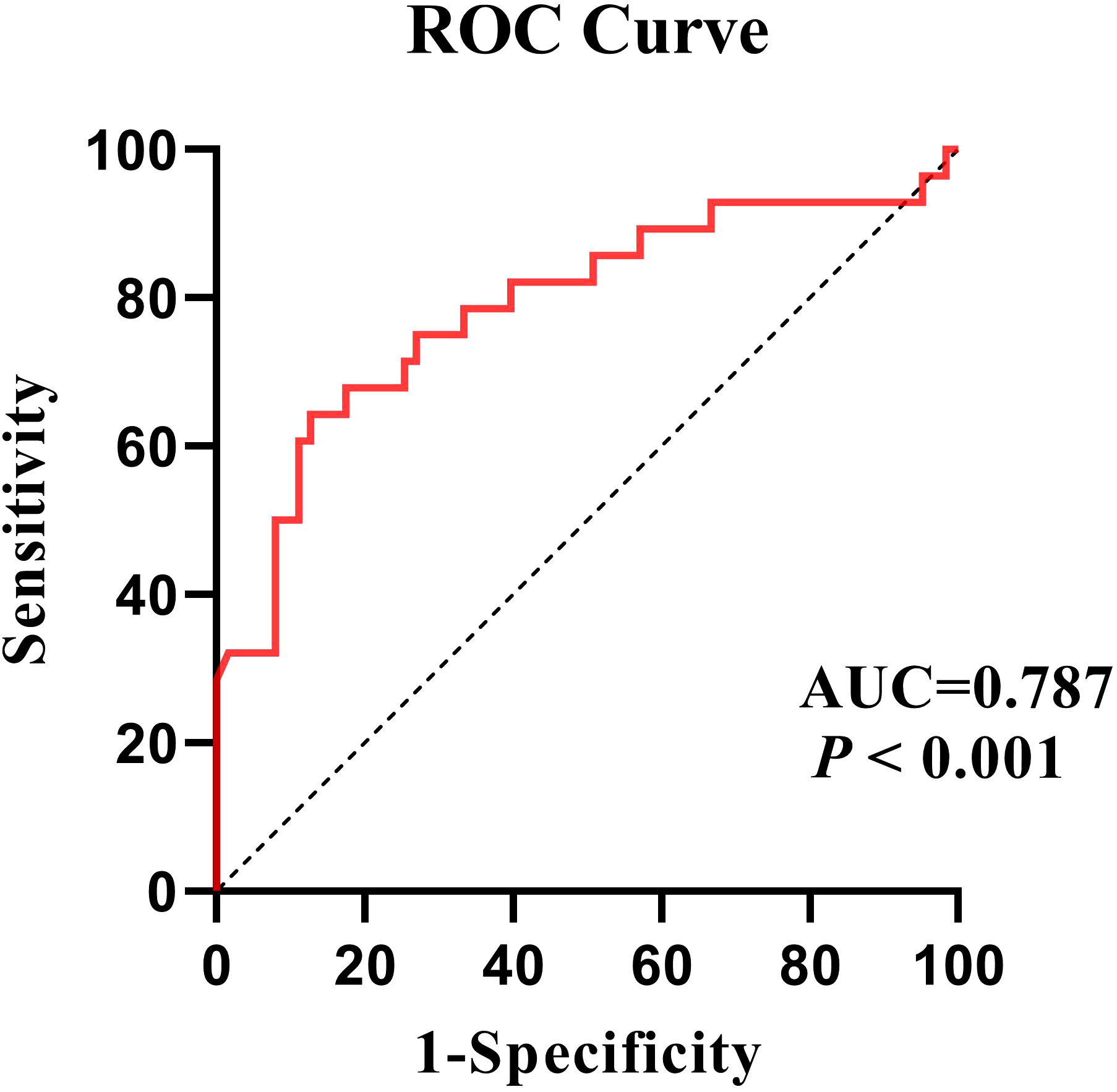
Figure 3 Using the severity of illness as a classification variable, ROC curves for the efficacy of the regression model.
In this study, we explored the changes of hematological parameters and inflammatory markers (NLR, MLR and PLR), and their associations with symptom severity in Han Chinese patients with drug-free schizophrenia. First, we found elevated and unbalanced distribution of peripheral blood leukocytes in these patients, characterized by elevated neutrophil and monocyte counts and decreased lymphocyte counts. Similarly, in a cohort study, elevated neutrophil and monocyte counts were found in drug-naïve FEP and unmedicated patients with schizophrenia at baseline (32). The mechanism of such changes may be related to the deficiency in immunoregulation mediated by the endocannabinoid system in patients with schizophrenia. A study conducted a network analysis to assess the association between cannabinoid receptor expression on immune response-associated cells and cytokine levels in patients with schizophrenia and found that patients with schizophrenia had higher expression of cannabinoid receptors and a simpler network of correlations with cytokines compared to controls (33). Recent genome-wide association studies found genetically determined Immune abnormalities in patients with schizophrenia and identified shared genetic variants between schizophrenia and WBC counts (34). While, a meta-analysis of hematological parameters in patients with schizophrenia found that there were no significant differences in lymphocyte counts between patients (not limited to unmedicated patients) and the general population (35), which may be due to the heterogeneity of the participants across studies. Second, we also found that platelet levels were higher in patients who had not taken medication during the prior month than in healthy controls. A retrospective investigation in India found a decrease in platelet levels in patients with schizophrenia following clozapine administration (36). These findings suggest that platelet counts in the circulation are susceptible to the antipsychotics taken. As calculated by the counts of these blood cells, the levels of inflammatory markers (NLR, MLR, and PLR) were higher in patients with drug-free schizophrenia than in HCs in this study, which is consistent with the results of most previous studies. For example, a cross-sectional study conducted in China found elevated NLR, MLR and PLR in first-episode patients with schizophrenia (37). Furthermore, Bustan et al. found that NLR was elevated in acute psychotic states and relatively declined during periods of clinical remission in adolescents with non-affective psychosis (38).
In addition, the present study found a positive association between these peripheral blood inflammatory markers and psychopathology in patients with drug-free schizophrenia. The total score of BPRS was positively associated with the counts of WBC, neutrophil and monocyte, and the value of NLR and MLR. In fact, there is a possible vicious circle of interactions between schizophrenia and immune-inflammatory dysregulation. Increased levels of inflammation may be closely intertwined with the onset of schizophrenia and exacerbation of psychotic symptoms. A cross-sectional study conducted in China found that NLR was positively associated with the CGI-S score and the BPRS total score in drug-free patients (23). In addition, a retrospective study found that there were significant differences in MLR between three groups (relapse schizophrenia > remission schizophrenia = healthy control), suggesting that MLR may be a state marker for schizophrenia (39). Although the mechanisms underlying these associations are complex, the immune-inflammatory hypothesis of schizophrenia may provide a partial explanation. Since NLR and MLR are calculated as neutrophils/lymphocytes and monocytes/lymphocytes, they reflect discuss neutrophil- and monocyte-mediated immune responses (innate immune response) as well as lymphocyte-mediated immune responses (adaptive immune response), which are possibly related to the manifestation of schizophrenia symptoms.
First, the BBB appears to be disrupted in schizophrenia (40). Neutrophils directed by specific chemokines can infiltrate the brain through the damaged BBB (41), which may lead to brain tissue damage and more psychotic symptoms in schizophrenia. Neuroimaging studies found that higher blood neutrophil levels are associated with gray matter reduction and ventricular enlargement in patients with first-episode schizophrenia (42). A clinical study also found peripheral blood neutrophil counts were significantly and positively correlated with PANSS total and positive subscale scores in patients with schizophrenia (22). Second, the monocyte macrophage system is also a critical component of innate immunity and plays an important role in the immune-inflammatory hypothesis of schizophrenia. Increased peripheral monocytes may suggest activation of microglia in central nervous system (43). As a type of macrophage, activated microglia interferes with neuronal development and disrupts the function of neuronal circuits in the brain (44).For example, Cui et al. (45) found that high transcript levels of monocyte genes across cortical regions were associated with cortical thinning in patients with psychosis by a high-resolution T1-weighted structural image, while cortical thickness could reflect neurodevelopmental status. Third, our study found that lymphocytes significantly decreased in patients but did not correlate with the total score of BPRS. Our interpretation was that adaptive immunity may be involved in the pathophysiological mechanisms of schizophrenia, but its action may be related to the activation phenotype of lymphocytes and the subtype of schizophrenia. For example, a meta-analysis reported that an increased number of CD56-positive cells are important markers of disease exacerbation in schizophrenia (46). Autopsy studies from post-mortem brain samples found that T and B cell numbers were increased in the hippocampus of patients with schizophrenia, especially those with predominantly negative symptoms (47). Therefore, we think it is necessary to stratify lymphocyte activation phenotypes and schizophrenia subtype for investigating the pathophysiological role of lymphocytes in schizophrenia.
Regarding the BPRS subscales, we found that NLR was positively correlated with the resistance subscale score, and MLR was positively correlated with the positive symptoms, activitate, and resistance subscale scores. However, only the correlation between MLR and resistance subscale score remained significant after Bonferroni correction. To the best of our knowledge, this is the first study revealing a correlation between MLR and resistance subscale scores in patients with drug-free schizophrenia. Resistance is defined as hostility, uncooperativeness and suspiciousness in patients with schizophrenia. A cross-sectional study found that higher level of IL-1β was associated with higher resistance subscale scores in patients with first-episode schizophrenia (48). Since IL-1β is produced by monocytes, and the higher level of IL-1β often reflects activation of the monocyte-macrophage system, which is equivalent to elevated value of MLR (monocyte count as numerator) to some extent (49). Therefore, in clinical practice, it is necessary to monitor the MLR, which could contribute to a comprehensive assessment of psychotic symptoms in patients with schizophrenia, especially those with hostility, uncooperativeness, and suspiciousness.
We further divided the patients into two groups of mildly/moderately or severely ill, and found that severely ill was significantly associated with being male and a higher value of MLR (Ln). Clinical studies have shown that male patients with schizophrenia tend to have a higher score of BPRS or PANSS, so they are more inclined to be defined as being severely ill (50, 51). This may be attributed to the differences in brain structure and function, neurodevelopment, and psychosocial factors between males and females (52). For example, a clinical study found differences in brain functional alterations between male and female patients with schizophrenia and were correlated with a series of psychotic symptoms (53). An animal study found that there were sex differences in dopaminergic projections from the midbrain to the basolateral amygdala in mice that were associated with schizophrenia-like behaviors (54). In addition, there is an “estrogen-protective” effect in female patients with schizophrenia (55). A randomized placebo-controlled trials found that estradiol are effective and safe adjunctive treatments to improve schizophrenia symptoms for female patients (56). Sex differences in immune cells and their signaling in the adult brain have been found to be associated with many diseases with altered brain development or plasticity, such as schizophrenia (57). However, there were no differences in inflammatory markers (NLR, MLR and PLR) between male and female patients in this study.
Although NLR, MLR, PLR, and other inflammatory markers may have potential diagnostic value in distinguishing schizophrenia from the general population, their specificity for schizophrenia is low because they are also elevated in many other psychiatric disorders (58, 59). In this cross-sectional study, we have a preliminary finding that the MLR may has a good predictive power on whether or not being severely ill in patients with schizophrenia. Specifically, the ROC analysis showed good performance of a regression model with an AUC value of 0.787. Thus, MLR is a potential biomarker for predicting the symptom severity in drug-free patients with schizophrenia. This finding provides a new insight that monitoring the MLR may allow for an objective assessment of the efficacy of antipsychotic treatment. Moreover, a meta-analysis revealed that some agents with anti-inflammatory properties such as aspirin, minocycline, and N-acetylcysteine have an adjunctive efficacy for schizophrenia, especially for first-episode psychosis and early-phase schizophrenia (60). These findings illustrate the importance of monitoring and intervening against immune-inflammatory abnormalities, which probably play a critical role in the pathogenesis, diagnosis, and treatment in early-phase or untreated schizophrenia.
Several limitations in this study should be noted. First, because this was a retrospective cross-sectional study, we were not able to address the direction of causality between inflammatory markers with symptom severity. Second, the study initially screened more than 2,000 cases over a five-year period, yet all of the cases were from a single center, with a weak representativeness. Third, it were not taken into account the fact that “drug-free” patients may receive psychotherapy and counseling intervention, which people often use to prevent or alleviate symptoms at the onset. Finally, relevant factors which may be associated with inflammation in patients with schizophrenia, such as lifestyle habits (smoking, drinking and exercise condition) and other clinical symptoms (sleep condition), were not examined.
In conclusion, patients with drug-free schizophrenia have an unbalanced distribution of peripheral blood granulocytes, and elevated NLR, MLR and PLR compared to healthy controls. Patients with higher value of MLR tend to have more psychotic symptoms, especially those symptoms of hostility, uncooperativeness, and suspiciousness. Our results also revealed that MLR is a potential marker for predicting the severity of illness in drug-free patients with drug-free schizophrenia. Given the accessibility in clinical practice, monitoring the MLR allows for a comprehensive assessment of psychotic symptoms in schizophrenic patients. However, as the findings of this study have not been generalized to all subtypes of schizophrenic patients, the relationship between MLR and psychopathology in schizophrenia needs to be treated with caution due to the heterogeneity of subjects across studies.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by Chaohu hospital of Anhui medical university. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
CY: Writing – original draft, Writing – review & editing. YT: Writing – review & editing. XY: Writing – review & editing. LL: Writing – review & editing. CL: Writing – review & editing. LX: Writing – review & editing. HL: Funding acquisition, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was supported by the Anhui Provincial Natural Science Foundation(2108085MH275) and the National Clinical Key Specialty Project Foundation (CN).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1337103/full#supplementary-material
1. Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry (2015) 2(3):258–70. doi: 10.1016/S2215-0366(14)00122-9
2. Enache D, Nikkheslat N, Fathalla D, Morgan BP, Lewis S, Drake R, et al. Peripheral immune markers and antipsychotic non-response in psychosis. Schizophr Res (2021) 230:1–8. doi: 10.1016/j.schres.2020.12.020
3. Miller BJ, Lemos H, Schooler NR, Goff DC, Kopelowicz A, Lauriello J, et al. Longitudinal study of inflammation and relapse in schizophrenia. Schizophr Res (2023) 252:88–95. doi: 10.1016/j.schres.2022.12.028
4. Marcinowicz P, Więdłocha M, Zborowska N, Dębowska W, Podwalski P, Misiak B, et al. A meta-analysis of the influence of antipsychotics on cytokines levels in first episode psychosis. J Clin Med (2021) 10(11):2488. doi: 10.3390/jcm10112488
5. Shnayder NA, Khasanova AK, Strelnik AI, Al-Zamil M, Otmakhov AP, Neznanov NG, et al. Cytokine imbalance as a biomarker of treatment-resistant schizophrenia. Int J Mol Sci (2022) 23(19):11324. doi: 10.3390/ijms231911324
6. Upthegrove R, Manzanares-Teson N, Barnes NM. Cytokine function in medication-naive first episode psychosis: A systematic review and meta-analysis. Schizophr Res (2014) 155(1-3):101–8. doi: 10.1016/j.schres.2014.03.005
7. Corsi-Zuelli F, Loureiro CM, Shuhama R, Fachim HA, Menezes PR, Louzada-Junior P, et al. Cytokine profile in first-episode psychosis, unaffected siblings and community-based controls: the effects of familial liability and childhood maltreatment. Psychol Med (2020) 50(7):1139–47. doi: 10.1017/S0033291719001016
8. Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry (2016) 21(12):1696–709. doi: 10.1038/mp.2016.3
9. Fraguas D, Díaz-Caneja CM, Ayora M, Hernández-Álvarez F, Rodríguez-Quiroga A, Recio S, et al. Oxidative stress and inflammation in first-episode psychosis: A systematic review and meta-analysis. Schizophr Bull (2019) 45(4):742–51. doi: 10.1093/schbul/sby125
10. Song C, Merali Z, Anisman H. Variations of nucleus accumbens dopamine and serotonin following systemic interleukin-1, interleukin-2 or interleukin-6 treatment. Neuroscience (1999) 88(3):823–36. doi: 10.1016/s0306-4522(98)00271-1
11. Zalcman S, Green-Johnson JM, Murray L, Nance DM, Dyck D, Anisman H, et al. Cytokine-specific central monoamine alterations induced by interleukin-1, -2 and -6. Brain Res (1994) 643(1-2):40–9. doi: 10.1016/0006-8993(94)90006-x
12. Barry S, Clarke G, Scully P, Dinan TG. Kynurenine pathway in psychosis: evidence of increased tryptophan degradation. J Psychopharmacol (2009) 23(3):287–94. doi: 10.1177/0269881108089583
13. Müller N, Myint A-M, Schwarz MJ. Kynurenine pathway in schizophrenia: pathophysiological and therapeutic aspects. Curr Pharm Des (2011) 17(2):130–6. doi: 10.2174/138161211795049552
14. Sathyasaikumar KV, Stachowski EK, Wonodi I, Roberts RC, Rassoulpour A, McMahon RP, et al. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr Bull (2011) 37(6):1147–56. doi: 10.1093/schbul/sbq112
15. Müller N. Inflammation in schizophrenia: pathogenetic aspects and therapeutic considerations. Schizophr Bull (2018) 44(5):973–82. doi: 10.1093/schbul/sby024
16. Zahorec R. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy (2001) 102(1):5–14.
17. Mouchli M, Reddy S, Gerrard M, Boardman L, Rubio M. Usefulness of neutrophil-to-lymphocyte ratio (Nlr) as a prognostic predictor after treatment of hepatocellular carcinoma." Review article. Ann Hepatol (2021) 22:100249. doi: 10.1016/j.aohep.2020.08.067
18. Fang T, Wang Y, Yin X, Zhai Z, Zhang Y, Yang Y, et al. Diagnostic sensitivity of nlr and plr in early diagnosis of gastric cancer. J Immunol Res (2020) 2020:9146042. doi: 10.1155/2020/9146042
19. Liu Z, Li Y, Wang Y, Zhang H, Lian Y, Cheng X. The neutrophil-to-lymphocyte and monocyte-to-lymphocyte ratios are independently associated with the severity of autoimmune encephalitis. Front In Immunol (2022) 13:911779. doi: 10.3389/fimmu.2022.911779
20. Mazza MG, Lucchi S, Rossetti A, Clerici M. Neutrophil-lymphocyte ratio, monocyte-lymphocyte ratio and platelet-lymphocyte ratio in non-affective psychosis: A meta-analysis and systematic review. World J Biol Psychiatry Off J World Fed Societies Biol Psychiatry (2020) 21(5):326–38. doi: 10.1080/15622975.2019.1583371
21. Kovács MÁ, Tényi T, Kugyelka R, Prenek L, Hau L, Magyar ÉE, et al. Elevated osteopontin and interferon gamma serum levels and increased neutrophil-to-lymphocyte ratio are associated with the severity of symptoms in schizophrenia. Front In Psychiatry (2019) 10:996. doi: 10.3389/fpsyt.2019.00996
22. Kulaksizoglu B, Kulaksizoglu S. Relationship between neutrophil/lymphocyte ratio with oxidative stress and psychopathology in patients with schizophrenia. Neuropsychiatr Dis Treat (2016) 12:1999–2005. doi: 10.2147/NDT.S110484
23. Zhou X, Wang X, Li R, Yan J, Xiao Y, Li W, et al. Neutrophil-to-lymphocyte ratio is independently associated with severe psychopathology in schizophrenia and is changed by antipsychotic administration: A large-scale cross-sectional retrospective study. Front In Psychiatry (2020) 11:581061. doi: 10.3389/fpsyt.2020.581061
24. Dawidowski B, Grelecki G, Biłgorajski A, Podwalski P, Misiak B, Samochowiec J. Effect of antipsychotic treatment on neutrophil-to-lymphocyte ratio during hospitalization for acute psychosis in the course of schizophrenia-a cross-sectional retrospective study. J Clin Med (2021) 11(1). doi: 10.3390/jcm11010232
25. Xu H, Wei Y, Zheng L, Zhang H, Luo T, Li H, et al. Relation between unconjugated bilirubin and peripheral biomarkers of inflammation derived from complete blood counts in patients with acute stage of schizophrenia. Front In Psychiatry (2022) 13:843985. doi: 10.3389/fpsyt.2022.843985
26. Semiz M, Yildirim O, Canan F, Demir S, Hasbek E, Tuman TC, et al. Elevated neutrophil/lymphocyte ratio in patients with schizophrenia. Psychiatria Danubina (2014) 26(3):220–5.
27. Yüksel RN, Ertek IE, Dikmen AU, Göka E. High neutrophil-lymphocyte ratio in schizophrenia independent of infectious and metabolic parameters. Nordic J Psychiatry (2018) 72(5):336–40. doi: 10.1080/08039488.2018.1458899
28. Bioque M, Catarina Matias-Martins A, Llorca-Bofí V, Mezquida G, Cuesta MJ, Vieta E, et al. Neutrophil to lymphocyte ratio in patients with a first episode of psychosis: A two-year longitudinal follow-up study. Schizophr Bull (2022) 48(6):1327–35. doi: 10.1093/schbul/sbac089
29. Overall JE, Hollister LE, Pichot P. Major psychiatric disorders. A four-dimensional model. Arch Gen Psychiatry (1967) 16(2):146–51. doi: 10.1001/archpsyc.1967.01730200014003
30. Shafer A. Meta-analysis of the brief psychiatric rating scale factor structure. Psychol Assess (2005) 17(3):324–35. doi: 10.1037/1040-3590.17.3.324
31. Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of brief psychiatric rating scale scores. Br J Psychiatry J Ment Sci (2005) 187:366–71. doi: 10.1192/bjp.187.4.366
32. Steiner J, Frodl T, Schiltz K, Dobrowolny H, Jacobs R, Fernandes BS, et al. Innate immune cells and C-reactive protein in acute first-episode psychosis and schizophrenia: relationship to psychopathology and treatment. Schizophr Bull (2020) 46(2):363–73. doi: 10.1093/schbul/sbz068
33. de Campos-Carli SM, Araújo MS, de Oliveira Silveira AC, de Rezende VB, Rocha NP, Ferretjans R, et al. Cannabinoid receptors on peripheral leukocytes from patients with schizophrenia: evidence for defective immunomodulatory mechanisms. J Psychiatr Res (2017) 87:44–52. doi: 10.1016/j.jpsychires.2016.12.001
34. Steen NE, Rahman Z, Szabo A, Hindley GFL, Parker N, Cheng W, et al. Shared genetic loci between schizophrenia and white blood cell counts suggest genetically determined systemic immune abnormalities. Schizophr Bull (2023) 49(5):1345–54. doi: 10.1093/schbul/sbad082
35. Jackson AJ, Miller BJ. Meta-analysis of total and differential white blood cell counts in schizophrenia. Acta Psychiatr Scand (2020) 142(1):18–26. doi: 10.1111/acps.13140
36. Grover S, Shouan A, Chakrabarti S, Avasthi A. Haematological side effects associated with clozapine: A retrospective study from India. Asian J Psychiatr (2020) 48:101906. doi: 10.1016/j.ajp.2019.101906
37. Yu Q, Weng W, Zhou H, Tang Y, Ding S, Huang K, et al. Elevated platelet parameter in first-episode schizophrenia patients: A cross-sectional study. J Interferon Cytokine Res (2020) 40(11):524–9. doi: 10.1089/jir.2020.0117
38. Bustan Y, Drapisz A, Ben Dor DH, Avrahami M, Schwartz-Lifshitz M, Weizman A, et al. Elevated neutrophil to lymphocyte ratio in non-affective psychotic adolescent inpatients: evidence for early association between inflammation and psychosis. Psychiatry Res (2018) 262:149–53. doi: 10.1016/j.psychres.2018.02.002
39. Özdin S, Böke Ö. Neutrophil/lymphocyte, platelet/lymphocyte and monocyte/lymphocyte ratios in different stages of schizophrenia. Psychiatry Res (2019) 271:131–5. doi: 10.1016/j.psychres.2018.11.043
40. Kirkpatrick B, Miller BJ. Inflammation and schizophrenia. Schizophr Bull (2013) 39(6):1174–9. doi: 10.1093/schbul/sbt141
41. Balog BM, Sonti A, Zigmond RE. Neutrophil biology in injuries and diseases of the central and peripheral nervous systems. Prog Neurobiol (2023) 228:102488. doi: 10.1016/j.pneurobio.2023.102488
42. Núñez C, Stephan-Otto C, Usall J, Bioque M, Lobo A, González-Pinto A, et al. Neutrophil count is associated with reduced gray matter and enlarged ventricles in first-episode psychosis. Schizophr Bull (2019) 45(4):846–58. doi: 10.1093/schbul/sby113
43. Bergink V, Gibney SM, Drexhage HA. Autoimmunity, inflammation, and psychosis: A search for peripheral markers. Biol Psychiatry (2014) 75(4):324–31. doi: 10.1016/j.biopsych.2013.09.037
44. Marques TR, Ashok AH, Pillinger T, Veronese M, Turkheimer FE, Dazzan P, et al. Neuroinflammation in schizophrenia: meta-analysis of in vivo microglial imaging studies. Psychol Med (2019) 49(13):2186–96. doi: 10.1017/S0033291718003057
45. Cui L-B, Wang X-Y, Fu Y-F, Liu X-F, Wei Y, Zhao S-W, et al. Transcriptional level of inflammation markers associates with short-term brain structural changes in first-episode schizophrenia. BMC Med (2023) 21(1):250. doi: 10.1186/s12916-023-02963-y
46. Miller BJ, Gassama B, Sebastian D, Buckley P, Mellor A. Meta-analysis of lymphocytes in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry (2013) 73(10):993–9. doi: 10.1016/j.biopsych.2012.09.007
47. Busse S, Busse M, Schiltz K, Bielau H, Gos T, Brisch R, et al. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behavior Immun (2012) 26(8):1273–9. doi: 10.1016/j.bbi.2012.08.005
48. Yan F, Meng X, Cheng X, Pei W, Chen Y, Chen L, et al. Potential role between inflammatory cytokines and tie-2 receptor levels and clinical symptoms in patients with first-episode schizophrenia. BMC Psychiatry (2023) 23(1):538. doi: 10.1186/s12888-023-04913-7
49. Piccioli P, Rubartelli A. The secretion of il-1β and options for release. Semin Immunol (2013) 25(6):425–9. doi: 10.1016/j.smim.2013.10.007
50. Sulejmanpasic G, Memic-Serdarevic A, Sabanagic-Hajric S, Bajramagic N. The correlation of positive and negative symptoms (Panss scores) in patients with schizophrenia according to gender. Med Arch (2023) 77(2):123–6. doi: 10.5455/medarh.2023.77.123-126
51. Li Z, Liu X, Xu H, Zhao L, Zhou Y, Wu X, et al. Sex difference in comorbid depression in first-episode and drug-naive patients with schizophrenia: baseline results from the depression in schizophrenia in China study. Psychosom Med (2021) 83(9):1082–8. doi: 10.1097/PSY.0000000000000998
52. Leung A, Chue P. Sex differences in schizophrenia, a review of the literature. Acta Psychiatr Scand Suppl (2000) 401:3–38. doi: 10.1111/j.0065-1591.2000.0ap25.x
53. Lei W, Li M, Deng W, Zhou Y, Ma X, Wang Q, et al. Sex-specific patterns of aberrant brain function in first-episode treatment-naive patients with schizophrenia. Int J Mol Sci (2015) 16(7):16125–43. doi: 10.3390/ijms160716125
54. Manion MTC, Glasper ER, Wang KH. A sex difference in mouse dopaminergic projections from the midbrain to basolateral amygdala. Biol Sex Differ (2022) 13(1):75. doi: 10.1186/s13293-022-00486-4
55. Kulkarni J, Hayes E, Gavrilidis E. Hormones and schizophrenia. Curr Opin Psychiatry (2012) 25(2):89–95. doi: 10.1097/YCO.0b013e328350360e
56. Li Z, Wang Y, Wang Z, Kong L, Liu L, Li L, et al. Estradiol and raloxifene as adjunctive treatment for women with schizophrenia: A meta-analysis of randomized, double-blind, placebo-controlled trials. Acta Psychiatr Scand (2023) 147(4):360–72. doi: 10.1111/acps.13530
57. Nelson LH, Saulsbery AI, Lenz KM. Small cells with big implications: microglia and sex differences in brain development, plasticity and behavioral health. Prog Neurobiol (2019) 176:103–19. doi: 10.1016/j.pneurobio.2018.09.002
58. Bulut NS, Yorguner N, Çarkaxhiu Bulut G. The severity of inflammation in major neuropsychiatric disorders: comparison of neutrophil-lymphocyte and platelet-lymphocyte ratios between schizophrenia, bipolar mania, bipolar depression, major depressive disorder, and obsessive compulsive disorder. Nordic J Psychiatry (2021) 75(8):624–32. doi: 10.1080/08039488.2021.1919201
59. Mazza MG, Lucchi S, Tringali AGM, Rossetti A, Botti ER, Clerici M. Neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in mood disorders: A meta-analysis. Prog In Neuropsychopharmacol Biol Psychiatry (2018) 84(Pt A):229–36. doi: 10.1016/j.pnpbp.2018.03.012
Keywords: neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, schizophrenia, symptom severity, drug-free
Citation: Yang C, Tian Y, Yang X, Liu L, Ling C, Xia L and Liu H (2024) Hematological and inflammatory markers in Han Chinese patients with drug-free schizophrenia: relationship with symptom severity. Front. Immunol. 15:1337103. doi: 10.3389/fimmu.2024.1337103
Received: 12 November 2023; Accepted: 15 January 2024;
Published: 30 January 2024.
Edited by:
Pål Aukrust, Oslo University Hospital, NorwayReviewed by:
Nils Eiel Steen, University of Oslo, NorwayCopyright © 2024 Yang, Tian, Yang, Liu, Ling, Xia and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huanzhong Liu, aHVhbnpob25nbGl1QGFobXUuZWR1LmNu; Lei Xia, eGlhbGVpQGFobXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.