
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 11 March 2024
Sec. Cytokines and Soluble Mediators in Immunity
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1337070
 Daisuke Kasugai1*
Daisuke Kasugai1* Taku Tanaka1
Taku Tanaka1 Takako Suzuki2
Takako Suzuki2 Yoshinori Ito2
Yoshinori Ito2 Kazuki Nishida3
Kazuki Nishida3 Masayuki Ozaki4
Masayuki Ozaki4 Takeo Kutsuna5
Takeo Kutsuna5 Toshiki Yokoyama6
Toshiki Yokoyama6 Hitoshi Kaneko7
Hitoshi Kaneko7 Ryo Ogata8
Ryo Ogata8 Ryohei Matsui9
Ryohei Matsui9 Takahiro Goshima10
Takahiro Goshima10 Hiroshi Hamada11
Hiroshi Hamada11 Azusa Ishii12
Azusa Ishii12 Yusuke Kodama13
Yusuke Kodama13 Naruhiro Jingushi1
Naruhiro Jingushi1 Ken Ishikura14
Ken Ishikura14 Ryo Kamidani15
Ryo Kamidani15 Masashi Tada16
Masashi Tada16 Hideshi Okada15
Hideshi Okada15 Takanori Yamamoto1
Takanori Yamamoto1 Yukari Goto1,17
Yukari Goto1,17Background: Coronavirus disease 2019 (COVID-19) features a hypercoagulable state, but therapeutic anticoagulation effectiveness varies with disease severity. We aimed to evaluate the dynamics of the coagulation profile and its association with COVID-19 severity, outcomes, and biomarker trajectories.
Methods: This multicenter, prospective, observational study included patients with COVID-19 requiring respiratory support. Rotational thromboelastometry findings were evaluated for coagulation and fibrinolysis status. Hypercoagulable status was defined as supranormal range of maximum clot elasticity in an external pathway. Longitudinal laboratory parameters were collected to characterize the coagulation phenotype.
Results: Of 166 patients, 90 (54%) were severely ill at inclusion (invasive mechanical ventilation, 84; extracorporeal membrane oxygenation, 6). Higher maximum elasticity (P=0.02) and lower maximum lysis in the external pathway (P=0.03) were observed in severely ill patients compared with the corresponding values in patients on non-invasive oxygen supplementation. Hypercoagulability components correlated with platelet and fibrinogen levels. Hypercoagulable phenotype was associated with favorable outcomes in severely ill patients, while normocoagulable phenotype was not (median time to recovery, 15 days vs. 27 days, P=0.002), but no significant association was observed in moderately ill patients. In patients with severe COVID-19, lower initial C3, minimum C3, CH50, and greater changes in CH50 were associated with the normocoagulable phenotype. Changes in complement components correlated with dynamics of coagulation markers, hematocrit, and alveolar injury markers.
Conclusions: While hypercoagulable states become more evident with increasing severity of respiratory disease in patients with COVID-19, normocoagulable phenotype is associated with triggered by alternative pathway activation and poor outcomes.
Coronavirus disease 2019 (COVID-19) triggered a global pandemic by causing primarily, respiratory distress occasionally escalating into respiratory failure, thereby posing significant public health challenges worldwide (1). In this context, coagulopathy, characterized by hypercoagulation is identified as one of the key pathogenic factors in COVID-19 (2, 3). Associations of COVID-19 with macro- and microthrombosis have been established (4–6), which have spurred numerous clinical trials aiming to mitigate coagulopathy induced by this disease (7). Despite these efforts, previous studies did not consistently demonstrate the efficacy of therapeutic anticoagulation against COVID-19, irrespective of disease severity (8). While the effectiveness of therapeutic anticoagulation has been explored in moderately and critically ill patients with COVID-19 (9, 10), the contrasting results from these trials suggest that therapeutic anticoagulation might be more effective in non-critically ill patients than in their critically ill counterparts. This discrepancy could stem from the evolving nature of coagulopathy as the disease progresses. Hence, understanding how coagulopathy varies with disease severity is essential for optimizing therapeutic interventions aimed at controlling coagulation status.
This study aimed to investigate the changes in coagulation characteristics with increasing severity of COVID-19.
This prospective observational study conducted at 14 centers in Japan, from March 2021 to March 2022 included individuals aged ≥18 years, who were hospitalized for COVID-19 pneumonia and required oxygenation. COVID-19 was diagnosed based on findings from lung imaging and a positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) polymerase chain reaction or antigen test. Patients were classified into four groups based on COVID-19 severity at the time of coagulation status assessment according to the WHO ordinal scale: moderately ill patients requiring supplemental oxygen, patients on non-invasive respiratory support, patients on mechanical ventilation, and patients on extracorporeal membrane oxygenation (11). Exclusion criteria included known coagulopathy, previous oral anticoagulants administration, hematologic malignancy, and thrombocytopenia (detailed criteria listed in the Supplementary Methods in Additional File 1). Patients provided pre-enrollment informed consent. The study was approved by Nagoya University Hospital Institutional Review Board (Approval No. 2020-0548).
In addition to clinical laboratory coagulation function tests, thromboelastometry with ROTEM sigma™ was used to evaluate the coagulation profile of participants on the day of inclusion. The maximum clot elasticity (MCE) of each component was calculated from maximum clot firmness (MCF) using the previously described: MCE = (100×MCF)/(100-MCF) (12). The platelet component of MCE is evaluated using the difference between MCE EXTEM and FIBTEM. A hypercoagulable state was defined as the MCE of the external pathway exceeding the normal reference value (13). Figure 1 illustrates responentable image of EXTEM components of the thromboelastometry test for patients with normal and hypercoagulable states. A comprehensive description of the thromboelastometry parameters can be found in the previous publicaion (14).
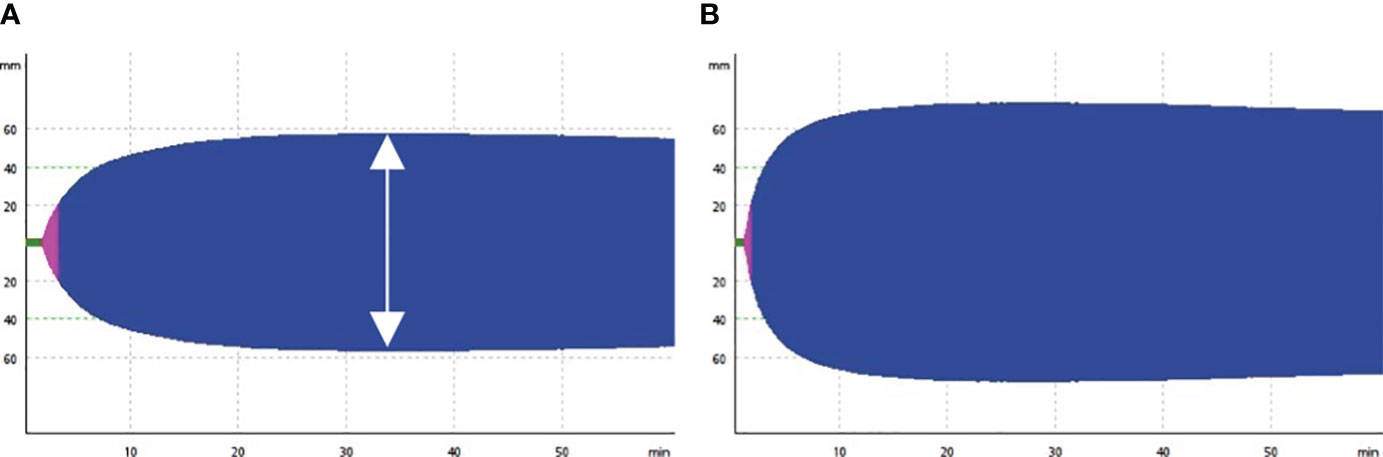
Figure 1 Representative image of thromboelastometry test. The figure shows the output of the EXTEM component of thromboelastometry, illustrating normal coagulation (A) and hypercoagulable states in patient with COVID-19 patients (B). The double arrow indicates maximum clot firmness.
The primary endpoint was the time to recovery, with the recovery day defined as the first day during the 28 days after enrollment, on which patients did not require any respiratory support (11). Secondary outcomes included ventilator-free days within the 28 days and in-hospital mortality. Other recorded clinical data included demographics, comorbidity, activities of daily living (15), history of COVID-19 vaccinations, respiratory mechanics, laboratory values at the time of thromboelastometry evaluation, and treatments. In patients requiring mechanical ventilation at the Nagoya University Hospital, daily laboratory results within the initial 7 days from intubation to ICU discharge were recorded to assess the association between biomarker dynamics. The collected longitudinal data were analyzed to identify the initial, the highest, and lowest value, and calculate the maximum changes(ΔC3 = lowest C3/initial C3 ×100%). These routine lab tests were conducted by the central laboratory, with the specific testing methods and reagents detailed in Table E1 in the Supplementary Material.
To explore the relationship with coagulation profiles, plasma viral loads were analyzed using quantitative reverse-transcription polymerase chain reaction. To investigate the relationship between coagulation profiles and viral genotypes in SARS-CoV-2, we performed whole-genome sequencing using stored specimens from the lower respiratory tract.
Spearman’s rank correlation test was used for statistical analysis, focusing on assessing the relationships between thromboelastometry parameters and clinical laboratory tests, such as coagulation function tests, using Spearman’s rank correlation test. The results were visualized using a heatmap. This method was also employed to examine longitudinal associations between complements and laboratory biomarkers. To assess differences in thromboelastometry results by severity, the Kruskal–Wallis test was used. P values for pairwise comparisons were adjusted using the Bonferroni method. The association between hypercoagulable state and baseline respiratory and laboratory parameters was evaluated using the Mann–Whitney U test. To decrease type-I error of multiple comparisons for 29 variables, the Benjamini–Hochberg procedure with a false discovery rate of 0.05 was applied to adjust the P value. Time to recovery was evaluated using the log-rank test, with a competing event of death regarded as “not recovered” on the last observation day, following an approach similar to the Fine–Grey method (11, 16). A P value less than 0.05 was considered statistically significant. All statistical analyses were performed using R software (version 4.2.2) and RStudio.
Of the 192 cases identified during the observation period, 166 were finally included in the study (Figure E1 in the Additional File 1). Among the enrolled patients, 76 had moderate respiratory failure at the time of inclusion (conventional oxygen supplementation, 62; non-invasive respiratory support, 14), and 90 had severe respiratory failure (mechanical ventilation, 84; extracorporeal membrane oxygenation, 6) (Table 1). The most common comorbidities were hypertension (40%) and diabetes mellitus (28%). Prior to disease onset, 159 (96%) patients could independently perform activities of daily living. Of the enrolled patients, 26 (16%) had received two doses of SARS-CoV-2 vaccine before disease onset. Unfractionated heparin was more commonly administered in severe cases, 88 (98%), than in moderate ones, 34 (45%), and was mainly used for therapeutic dose titration. Among patients with moderate respiratory failure at the time of inclusion, 10 (13%) experienced disease progression. Of the patients who received mechanical ventilation, 59 (66%) recovered after 28 days, 11 (12%) died, and 20 (22%) became ventilator dependent.
Figure 2 demonstrates a correlation between thromboelastometry parameters and laboratory biomarkers, where MCF and MCE were positively correlated with platelet counts (rho for MCEEXTEM = 0.67, P < 0.001) in all components except for in FIBTEM. Maximum lysis parameters were weak and negatively associated with white blood cell count, D-dimer levels, and platelet count (rho = -0.3, -0.32, and -0.32, respectively, for maximum lysis (EXTEM), P < 0.001). Figures 3, 4 illustrate the relationship between elasticity and fibrinolytic profile among different severities of respiratory failure. Patients receiving non-invasive respiratory support exhibited the highest MCEEXTEM values among other severity levels (Figure 3A, median [interquartile range (IQR)], 223 [186-245] for supplemental oxygen, 257 [213-281] for on-invasive respiratory support, 244 [203-270] for mechanical ventilation, and 178 [170-213] for extracorporeal membrane oxygenation, respectively).MCE was higher in patients on mechanical ventilation than in those receiving supplemental oxygen, which mainly reflects the platelet component (Figure 3B, median [IQR] MCEplatelet of supplemental oxygen vs mechanical ventilation, 184 [154–213] vs. 206 [170–230], P = 0.02). Meanwhile, maximum lysis declined as respiratory failure progressed (Figures 4A, B, median [IQR] MLEXTEM of supplemental oxygen vs mechanical ventilation, 11 [6–13] vs. 8 [5–11], P = 0.03). No significant differences between the groups were observed in terms of the fibrinogen component (Figures 3D, 4C).
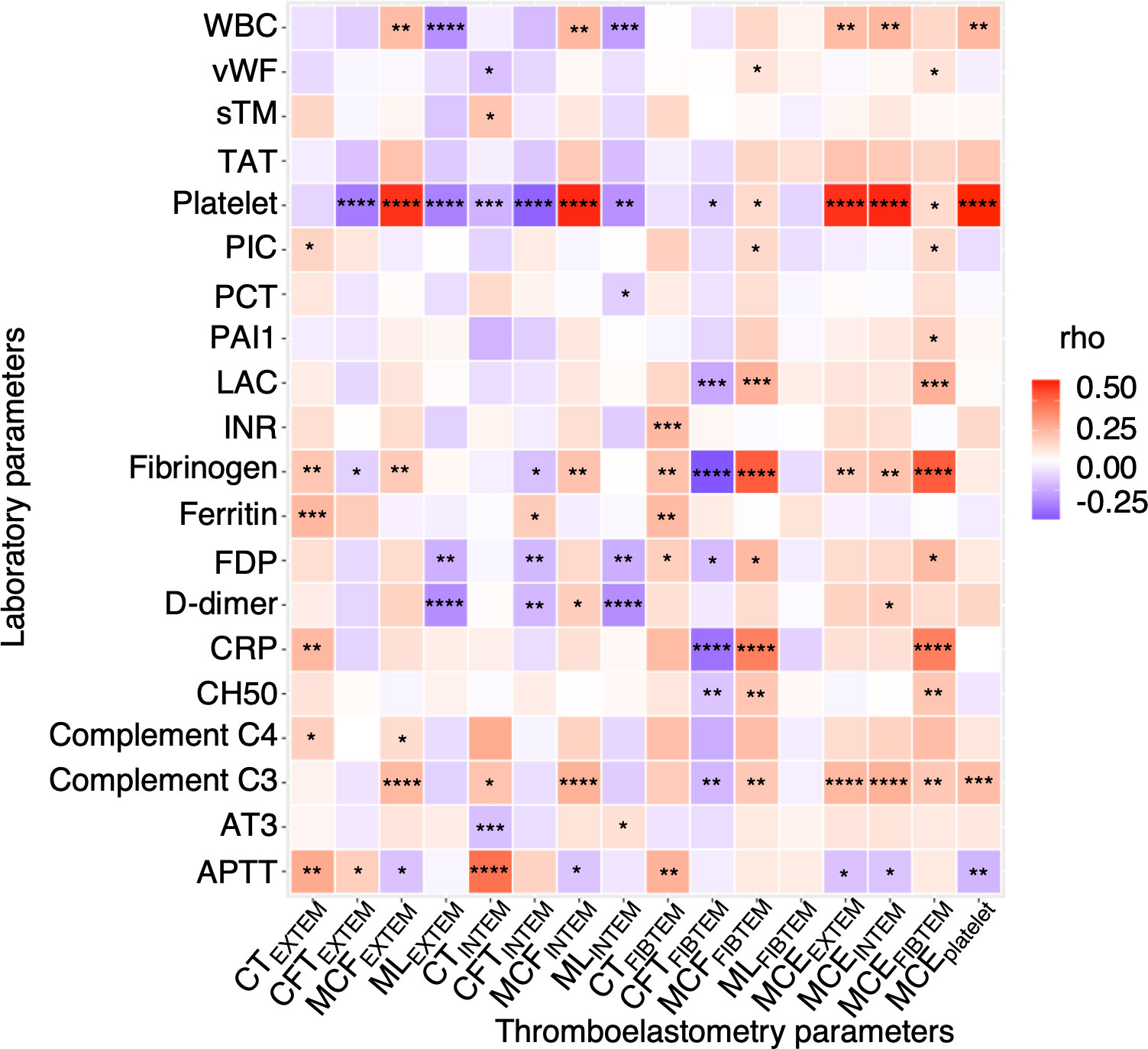
Figure 2 Correlation of thromboelastometry results with clinical laboratory test results. A heatmap showing correlation of viscoelasticity test parameters with clinical laboratory test results. Each rectangle represents Spearman’s rank correlation coefficient. P values are displayed in asterisks. * P value < 0.05, ** P value < 0.01, *** P value < 0.001, ****P value < 0.0001. WBC, white blood cell; vWF, von Willebrand factor; sTM, soluble thrombomodulin; TAT, thrombin-antithrombin complex; PIC, plasmin-alpha 2-plasmin inhibitor complex; PCT, procalcitonin; PAI1, plasminogen activator inhibitor-1; LAC, lupus anticoagulant; INR, international normalized ratio; FDP, fibrin degradation product; CRP, c reactive protein; CH50, total hemolytic complement; AT3, antithrombin 3; APTT, activated partial thrombin time; CT, clotting time; CFT, clot formation time; MCF, maximum clot firmness; ML, maximum lysis; MCE, maximum clot elasticity.
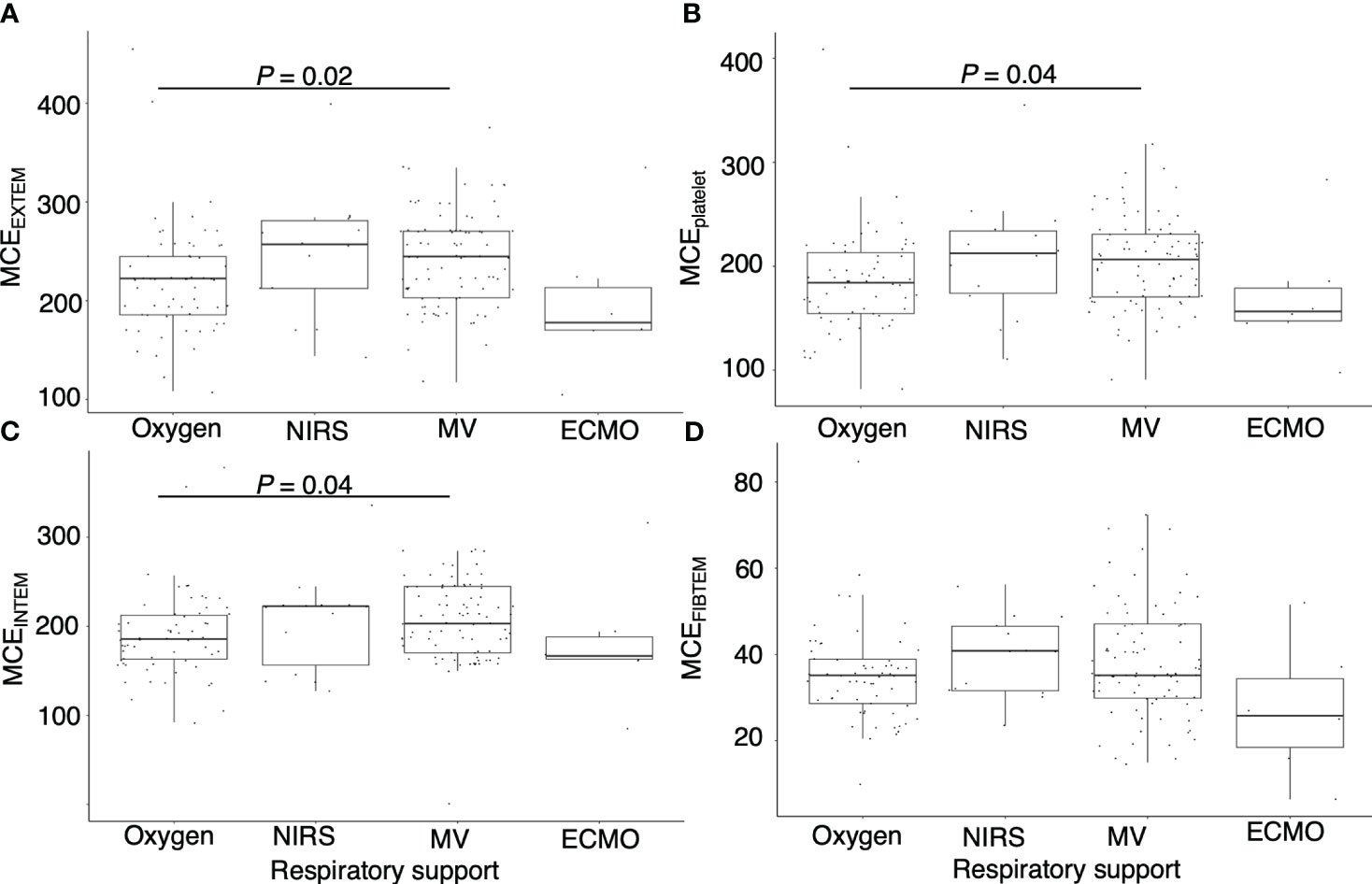
Figure 3 Association between maximum clot elasticity and severity of respiratory distress. Box plots showing (A) maximum clot elasticity of EXTEM for each severity level, (B) maximum clot elasticity of platelet components for each severity level, (C) maximum clot elasticity of INTEM for each severity level, and (D) maximum clot elasticity of FIBTEM for each severity level. MCE, maximum clot elasticity; NIRS= non-invasive respiratory support; MV, mechanical ventilation; ECMO, extracorporeal membrane oxygenation. P values calculated from the Mann–Whitney U test for pairwise comparison and adjusted using the Bonferroni method. Adjusted P values are displayed only if <0.05.
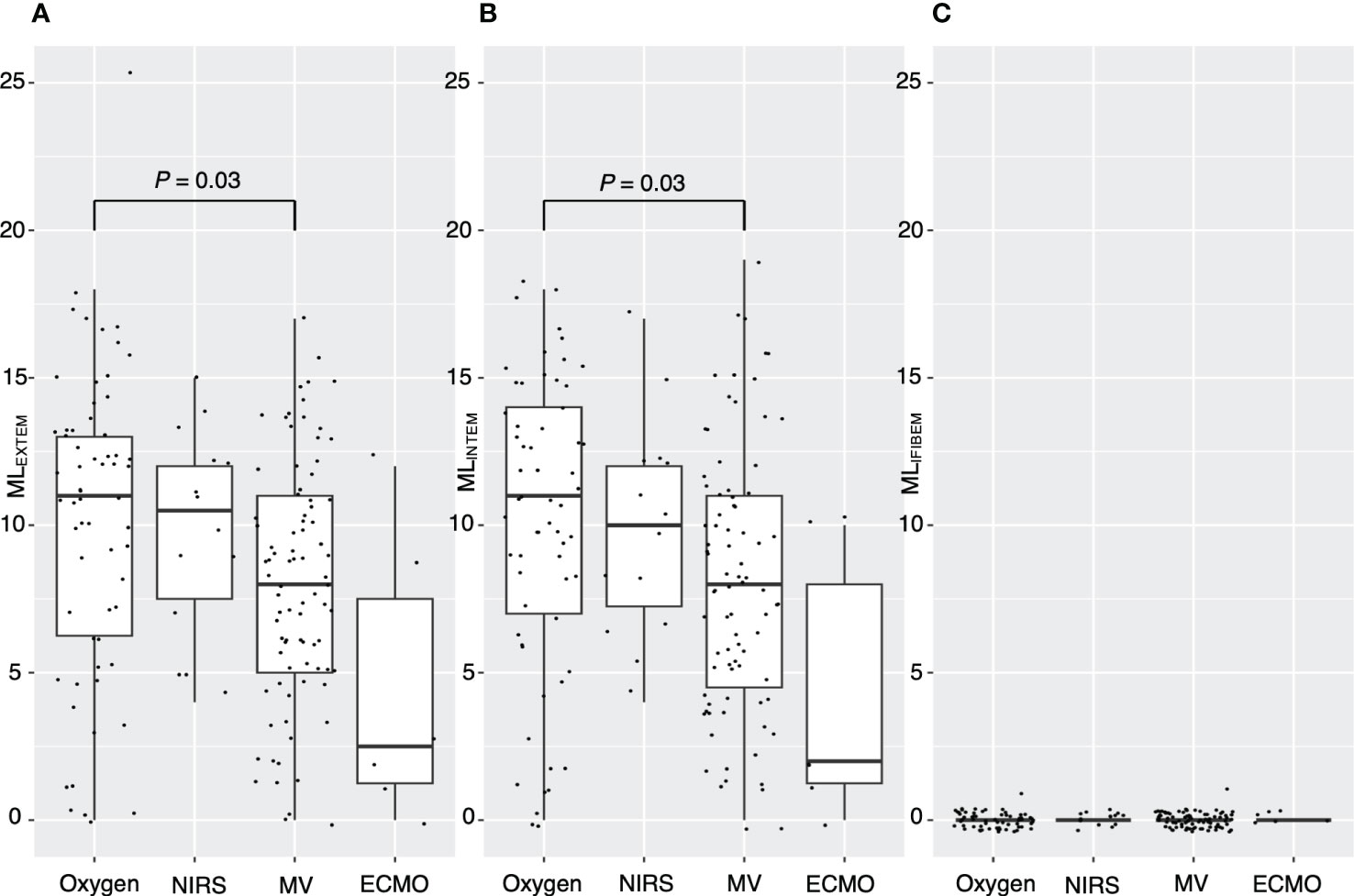
Figure 4 Association between maximum lysis and severity of respiratory distress. Box plots showing (A) maximum lysis of EXTEM for each severity level, (B) maximum lysis of INTEM for each severity level, and (C) maximum lysis of FIBTEM for each severity level. ML, maximum lysis; NIRS, non-invasive respiratory support; MV, mechanical ventilation; ECMO, extracorporeal membrane oxygenation. P values calculated from the Mann–Whitney U test for pairwise comparison and adjusted using the Bonferroni method. P values are displayed if P <0.05.
Figure 5 illustrates the association between hypercoagulable status and clinical outcomes. No correlation was observed between hypercoagulable state and the disease course in moderate COVID-19 cases (Figure 5A, median [95% confidence interval (CI)] time to recovery of hypercoagulable group vs. normocoagulable group, 6 [5–11] days vs. 6 [5–9] days, P = 0.66). However, in severe COVID-19 cases, hypercoagulable state was associated with a favorable prognosis (Figures 5B, C, median [95% CI] time to recovery, 15 [13–18] days vs. 27 [19–censored] days, P = 0.002; median [IQR] ventilator-free days, 18 [2–21] vs. 21 [16–23], P = 0.01). There was no significant difference in mortality associated with the two coagulation states (15% vs. 13%). The normocoagulable state in severe COVID-19 cases was associated with lower fibrinogen (median [IQR], 551 [481–579] mg/dL vs. 428 [359–534] mg/dL, adjusted P value = 0.03), lower platelet count (median [IQR], 246 [194–308] × 103/μL vs. 154 [126–204] × 103/μL, adjusted P value <0.001), and lower complement C3 value (median [IQR], 116 [100–128] mg/dL vs. 96 [73–118] mg/dL, adjusted P value = 0.05) (Table E3 in Additional File 1). No significant differences in respiratory parameters were observed between the two coagulation states. Further analysis in severe COVID-19 cases demonstrated no correlation between viral load and hypercoagulable state; there was no clear association between the viral genotype and hypercoagulable state (Figure 6).
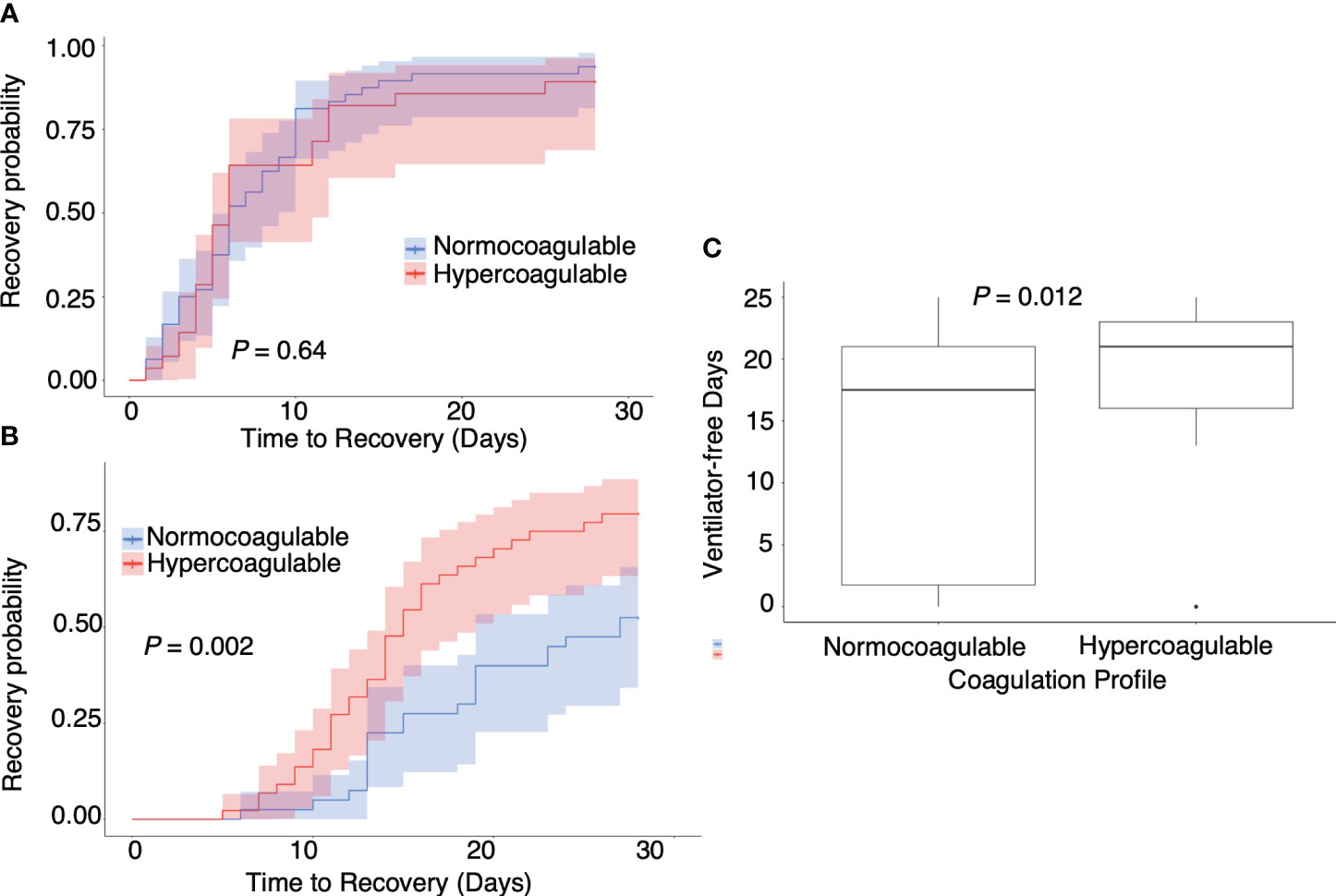
Figure 5 Association between coagulation profile and outcomes. Association between (A) coagulation profile and time to recovery in moderate COVID-19 cases, (B) coagulation profile and time to recovery in severe COVID-19 cases, and (C) coagulation profile and ventilator-free days in severe COVID-19 cases. P values calculated from the Mann–Whitney U test for pairwise comparison and adjusted using the Bonferroni method. P values are displayed if P <0.05.
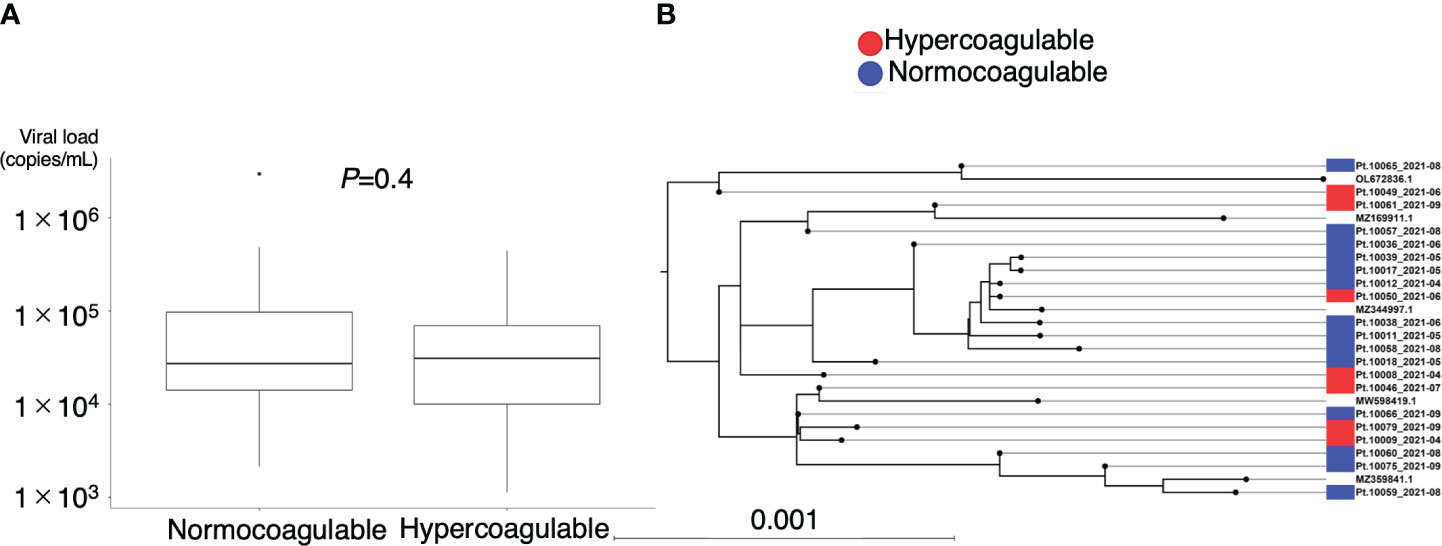
Figure 6 Association between coagulation profile and viral load and genotype. (A) Plasma viral load of SARS-CoV-2 at the time of coagulation profile evaluation. (B) Phylogenetic analysis of SARS-CoV-2 sequences obtained from the patients (n = 21) and their coagulation phenotypes. Scale bar indicates the measure of evolutionary distance calculated from the proportion of differing bases in the total sequence. Representative strains are indicated by GenBank accession number as references. OL672836.1(Omicron BA.1); MZ169911.1(Gamma P.1); MZ344997.1(Alpha B.1.1.7); MW598419.1(Beta B.1.351); MZ359841.1(Delta B.1.617.2).
Figure 7 illustrates the relationship between coagulation profiles and the dynamics of complement components. Complement C3, in addition to the initial assessment, had a significantly lower minimum value in the normocoagulable group (median [IQR], 85.9 [74.8–94.1] mg/dL vs. 77.3 [51.6–86.6] mg/dL, P = 0.016) (Figure 7A). Meanwhile, any significant difference in the dynamics of C4 was not found between the two coagulation profile groups. Total complement activity was significantly reduced in patients in the normocoagulable group compared to those in the hypercoagulable group (median [IQR] reductions of 58 [42–69]% versus 43 [24–52]%, respectively, P = 0.001) (Figure 7B). Decreases in C3 minimum correlated with the decrease in platelet count(rho = 0.56, P < 0.001), and hematocrit minimums (rho = 0.61, P < 0.001) and were associated with increases in D-dimer levels(rho = -0.56, P < 0.001), fibrin degradation product(rho = -0.51, P < 0.001), KL-6(rho = -0.45, P < 0.001), and soluble thrombomodulin maxima (rho = -0.33, P = 0.01) (Figure 7C). Notably, changes in each component of complement system (ΔC3, ΔC4, and ΔCH50) were positively correlated with changes in hematocrit (rho = 0.53, 0.46, and 0.53, respectively, P < 0.001 for each) and ventilator-free days (rho = 0.45, 0.5, and 0.53, respectively, P < 0.001 for each), and negatively correlated with changes in KL-6 (rho = -0.34, -0.48, and -0.45, respectively, P = 0.007, <0.001, and <0.001, respectively) (Figure 7D).
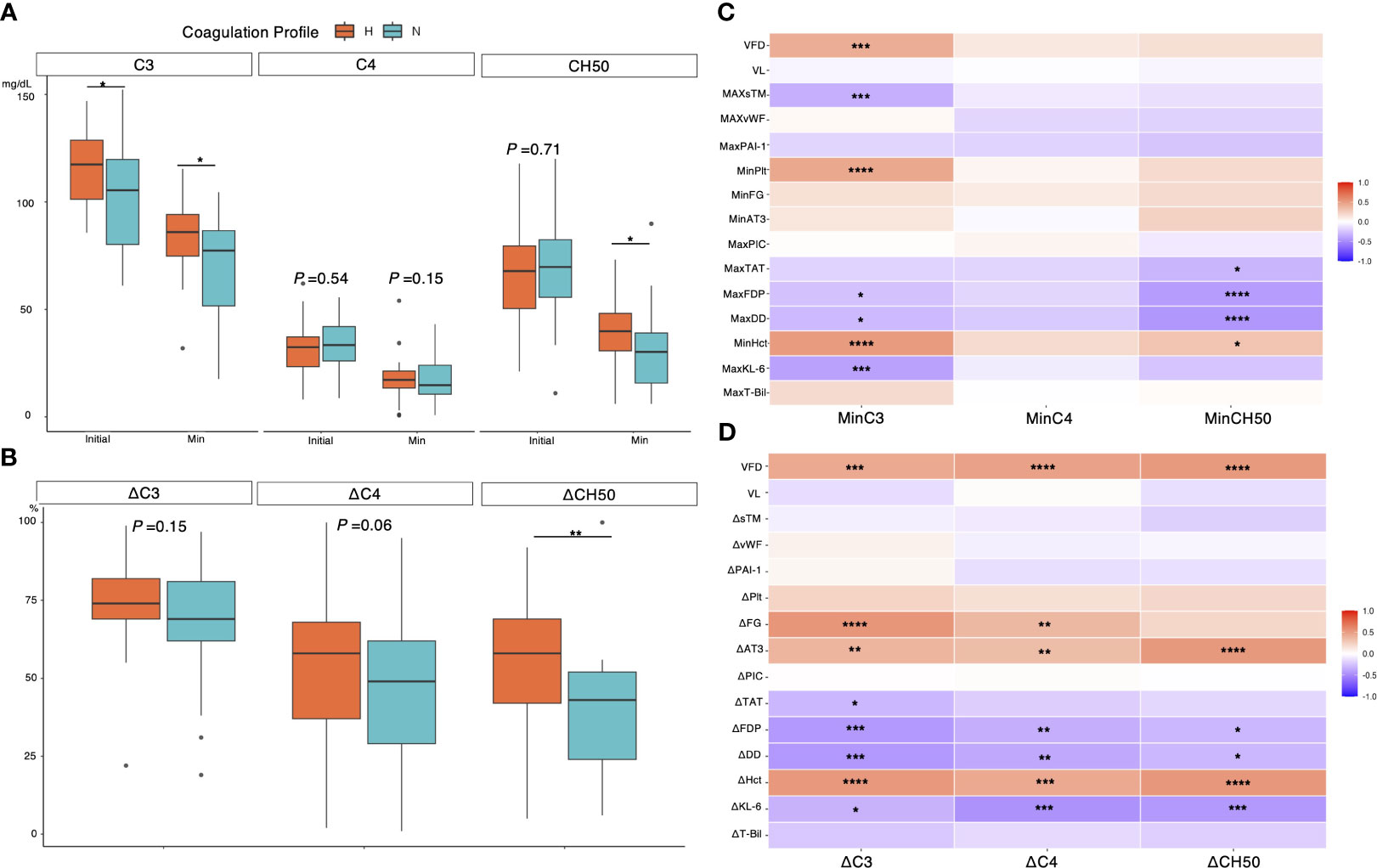
Figure 7 Association between complement dynamic and biomarkers in severe COVID-19 cases. (A) Association between coagulation profiles and initial minimum value for each complement component. (B) Association between coagulation profiles and changes in each complement component. (C) A heatmap showing association between minimum complement value and biomarkers, clinical outcome, and viral load evaluated with Spearman’s rank correlation coefficient. (D) A heatmap showing association between changes for each complement component and variables. Each laboratory variable was evaluated daily for 7 days from the day of intubation until ICU discharge. Minimum/Maximum values of each variable within 7 days were recorded and used to calculate the changes (e.g., ΔC3 = minimum C3/initial C3 ×100%). P values are displayed in asterisks. * P value < 0.05, ** P value < 0.01, *** P value < 0.001, ****P value < 0.0001. H= hypercoagulable status; N, normocoagulable status; CH50= total complement activity; VFD, ventilator-free days; VL, plasma viral load; sTM, soluble Thrombomodulin; vWF= von Willebrand factor; PAI-1= plasminogen activator inhibitor-1; Plt, platelet count; FG, fibrinogen; AT3, antithrombin 3; PIC, plasmin-alpha 2-plasmin inhibitor complex; TAT, thrombin-antithrombin complex; FDP, fibrin degradation product; DD, D-dimer; Hct, hematocrit; KL-6, Krebs von den lungen-6; T-bil, total bilirubin.
In this study, we evaluated the coagulation profiles of patients with different COVID-19 severities and found that while hypercoagulation was common with worsening respiratory failure, absence of hypercoagulation was associated with poor prognosis in severely ill patients. Platelet components were mainly involved in changes in blood viscoelasticity. Furthermore, the absence of hypercoagulation was also associated with coagulopathy, with complement consumption initiated by the alternative pathway activation.
Hypercoagulable state is considered a risk for COVID-19 macro-micro thrombosis, and treatment aimed at controlling hypercoagulation has been well studied so far (17). However, the efficacy of therapeutic anticoagulation and antiplatelet therapy has not been illustrated in critically ill patients (18). Our study depicted that hypercoagulable status was associated with rather favorable outcomes, which might be one of the reasons that previous randomized control trials failed to show the efficacy of anticoagulation in critically ill patients with COVID-19 (7–10). The hypercoagulable state is known to be influenced primarily by changes in platelet component (12), and individualized anticoagulation therapies to control the component of hypercoagulability in critically ill patients may be promising (19, 20). We demonstrated that thromboelastometry may be a simple and effective way to stratify severity and to identify different phenotypes of coagulation status. In this study, therapeutic anticoagulation and anti-inflammatory therapy were the standard of care in most critically ill patients. This may have partially led to a favorable outcome in patients with coagulable phenotype compared to the outcome in patients with normocoagulable phenotype. Whether phenotypic differences in coagulation profiles can help in individual treatment selection, such as anticoagulation therapy, needs further evaluation.
Our study showed that complement consumption was associated with normocoagulable phenotype and with poor prognosis in mechanically ventilated patients with COVID-19. Lower C3 but not C4 observed in the normocoagulable group at the initial assessment is consistent with similar results of previous in vivo studies that reported that SARS-CoV-2 triggers the alternative pathway overactivation (21). Previous studies reported indirect activation of the alternative pathway in addition to direct activation by viral components (22): viral infection of the alveolar epithelium activates the alternative pathway via the Janus kinase/signal transducer and activator of transcription pathway (23). In our study, elevated KL-6, a biomarker of type II alveolar cells, was associated with decreased C3, and there was no significant association between the viral load and decreased C3. Based on these findings, we speculate that the host response, via type 2 alveolar epithelial cell injury rather than pathogen factors, leads to overactivation of the alternative pathway, which results in phenotypic changes in coagulation phenotype and poor prognosis. The phenotypic difference may be triggered by host heterogeneity such as genetic variants in C3 genes reported in a previous study (24). We also showed that complement consumptions were correlated with reduction in coagulation components. These findings are also consistent with those of a recent randomized trial, which reported that target C3 blockage suppressed thrombin production (25). In contrast to the findings of a previous study (26), we observed that the alternative pathway consumption was associated with consumptive coagulopathy but not with hypercoagulable profile. Higher levels of D-dimer and fibrin degradation product in relation to complement dynamics, together with the findings that maximum lysis decreases as the severity increases, may imply the activity of microthrombosis. Our findings are further supported by ex vivo experiments on virus-induced senescence, which demonstrate that the secretome enhances coagulation, promotes the formation of the lytic complement complex C5b-C9, and stimulates the formation of Neutrophil Extracellular Traps (27, 28). Additionally, a rise in C3a enhances the differentiation of CD16 positive cytotoxic T cells in COVID-19 (29). These underlying processes are implicated in causing lung damage from COVID-19 and result in delayed recovery. The higher maximum soluble thrombomodulin and lower minimum hematocrit may suggest the feature of thrombotic microangiopathy (30, 31). Further studies are warranted to understand the control overactivation of the alternative pathway, especially in high-risk phenotypes.
The strength of this study was the standardized introduction of evidence-based treatments in many patients from multiple hospitals, which was useful in visualizing uncontrolled pathologies in a real-world setting. However, this study had some limitations. First, as the current cohort was a predominantly unvaccinated population, extrapolation to a post-vaccinated population may not be possible. Second, considering that the SARS-CoV-2 outbreak in the region was sporadic and the local health office conducted triage of hospitalized patients during the outbreak, selection bias may have occurred in hospitalized patients. Lastly, the present study is a hypothesis-generating analysis, and further investigation is needed to validate the stratification of severity by coagulation profile and the possibility of individualizing treatment for each phenotype.
In summary, the coagulation profile changes according to the severity of respiratory failure in COVID-19. As per our findings, normocoagulable status characterized by C3 consumption is associated with delayed recovery in patients with severe COVID-19. These findings support the heterogeneous treatment effect of anticoagulation depending on COVID-19 severity. Further studies are warranted to develop phenotype-specific treatments based on the coagulation profiles.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s. Nucleotide sequence data reported are available in the DDBJ Sequenced Read Archive under the accession numbers DRX512987-DRX513007.
The studies involving humans were approved by Nagoya University Hospital Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
DK: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. TT: Data curation, Investigation, Supervision, Visualization, Writing – review & editing. TS: Data curation, Investigation, Methodology, Supervision, Writing – review & editing. YI: Data curation, Methodology, Supervision, Writing – review & editing. KN: Formal Analysis, Funding acquisition, Methodology, Supervision, Writing – review & editing. MO: Investigation, Supervision, Writing – review & editing. TK: Data curation, Investigation, Supervision, Writing – review & editing. TYo: Data curation, Investigation, Supervision, Writing – review & editing. HK: Data curation, Investigation, Supervision, Writing – review & editing. RO: Data curation, Investigation, Supervision, Writing – review & editing. RM: Data curation, Investigation, Supervision, Writing – review & editing. TG: Data curation, Investigation, Supervision, Writing – review & editing. HH: Data curation, Investigation, Supervision, Writing – review & editing. AI: Investigation, Supervision, Writing – review & editing, Data curation. YK: Data curation, Investigation, Supervision, Writing – review & editing. NJ: Data curation, Investigation, Supervision, Writing – review & editing. KI: Data curation, Investigation, Supervision, Writing – review & editing. RK: Data curation, Investigation, Supervision, Writing – review & editing. MT: Data curation, Investigation, Supervision, Writing – review & editing. HO: Data curation, Investigation, Supervision, Writing – review & editing. TYa: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. YG: Conceptualization, Data curation, Funding acquisition, Investigation, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the Japan Agency for Medical Research and Development (AMED) to DK, TYa, and YG (grant no., 20fk0108532h0001) and Japan Society for the Promotion of Science (JSPS) to DK and KN (grant no., JP 22K09180).
The authors thank Yasushi Ogawa for the invaluable help in conducting this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1337070/full#supplementary-material
1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from Patients with pneumonia in China, 2019. N Engl J Med. (2020) 382:727–33. doi: 10.1056/NEJMoa2001017
2. Conway EM, Mackman N, Warren RQ, Wolberg AS, Mosnier LO, Campbell RA, et al. Understanding COVID-19-associated coagulopathy. Nat Rev Immunol. (2022) 22:639–49. doi: 10.1038/s41577-022-00762-9
3. Moores LK, Tritschler T, Brosnahan S, Carrier M, Collen JF, Doerschug K, et al. Thromboprophylaxis in patients with COVID-19: A brief update to the CHEST guideline and expert panel report. Chest. (2022) 162:213–25. doi: 10.1016/j.chest.2022.02.006
4. Gómez CA, Sun CK, Tsai IT, Chang YP, Lin MC, Hung IY, et al. Mortality and risk factors associated with pulmonary embolism in coronavirus disease 2019 patients: a systematic review and meta-analysis. Sci Rep. (2021) 11:16025. doi: 10.1038/s41598-021-95512-7
5. Pellegrini D, Kawakami R, Guagliumi G, Sakamoto A, Kawai K, Gianatti A, et al. Microthrombi as a major cause of cardiac injury in COVID-19: A pathologic study. Circulation. (2021) 143:1031–42. doi: 10.1161/CIRCULATIONAHA.120.051828
6. Fahmy OH, Daas FM, Salunkhe V, Petrey JL, Cosar EF, Ramirez J, et al. Is microthrombosis the main pathology in coronavirus disease 2019 severity?-A systematic review of the postmortem pathologic findings. Crit Care Explor. (2021) 3:e0427. doi: 10.1097/CCE.0000000000000427
7. Reis S, Popp M, Schießer S, Metzendorf MI, Kranke P, Meybohm P, et al. Anticoagulation in COVID-19 patients - An updated systematic review and meta-analysis. Thromb Res. (2022) 219:40–8. doi: 10.1016/j.thromres.2022.09.001
8. Pilia E, Belletti A, Fresilli S, Lee TC, Zangrillo A, Finco G, et al. The effect of heparin full-dose anticoagulation on survival of hospitalized, non-critically ill COVID-19 patients: A meta-analysis of high quality studies. Lung. (2023) 201:135–47. doi: 10.1007/s00408-023-00599-6
9. REMAP-CAP Investigators, ACTIV-4a Investigators, ATTACC Investigators, Goligher EC, Bradbury CA, McVerry BJ, et al. Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med. (2021) 385:777–89. doi: 10.1056/NEJMoa2103417
10. ATTACC Investigators, ACTIV-4a Investigators, REMAP-CAP Investigators, Lawler PR, Goligher EC, Berger JS, et al. Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med. (2021) 385:790–802. doi: 10.1056/NEJMoa2105911
11. Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. (2021) 384:795–807. doi: 10.1056/NEJMoa2031994
12. Solomon C, Ranucci M, Hochleitner G, Schöchl H, Schlimp CJ. Assessing the methodology for calculating platelet contribution to clot Strength (platelet component) in thromboelastometry and thrombelastography. Anesth Analg. (2015) 121:868–78. doi: 10.1213/ANE.0000000000000859
13. Lang T, Bauters A, Braun SL, Pötzsch B, von Pape KW, Kolde HJ, et al. Multi-centre investigation on reference ranges for ROTEM thromboelastometry. Blood Coagul Fibrinolysis. (2005) 16:301–10. doi: 10.1097/01.mbc.0000169225.31173.19
14. Whiting D, DiNardo JA. TEG and ROTEM: technology and clinical applications. Am J Hematol. (2014) 89:228–32. doi: 10.1002/ajh.23599
15. Tsutsui T, Muramatsu N. Care-needs certification in the long-term care insurance system of Japan. J Am Geriatr Soc. (2005) 53:522–7. doi: 10.1111/j.1532-5415.2005.53175.x
16. Dodd LE, Follmann D, Wang J, Koenig F, Korn LL, Schoergenhofer C, et al. Endpoints for randomized controlled clinical trials for COVID-19 treatments. Clin Trials. (2020) 17:472–82. doi: 10.1177/1740774520939938
17. Poor HD. Pulmonary thrombosis and thromboembolism in COVID-19. Chest. (2021) 160:1471–80. doi: 10.1016/j.chest.2021.06.016
18. REMAP-CAP Writing Committee for the REMAP-CAP Investigators;, Bradbury CA, Lawler PR, Stanworth SJ, Wolberg AS, Mosnier LO, Campbell RA, et al. Effect of antiplatelet therapy on survival and organ support-free days in critically ill patients with COVID-19: A randomized clinical trial. JAMA. (2022) 327:1247–59. doi: 10.1001/jama.2022.2910
19. Kruse JM, Magomedov A, Kurreck A, Münch FH, Koerner R, Kamhieh-Milz J, et al. Thromboembolic complications in critically ill COVID-19 patients are associated with impaired fibrinolysis. Crit Care. (2020) 24:676. doi: 10.1186/s13054-020-03401-8
20. Roh DJ, Eiseman K, Kirsch H, Yoh N, Boehme A, Agarwal S, et al. Hypercoagulable viscoelastic blood clot characteristics in critically ill coronavirus disease 2019 patients and associations with thrombotic complications. J Trauma Acute Care Surg. (2021) 90:e7–12. doi: 10.1097/TA.0000000000002963
21. Lo MW, Amarilla AA, Lee JD, Albornoz EA, Modhiran N, Clark RJ, et al. SARS-CoV-2 triggers complement activation through interactions with heparan sulfate. Clin Transl Immunol. (2022) 11:e1413. doi: 10.1002/cti2.1413
22. Yu J, Yuan X, Chen H, Chaturvedi S, Braunstein EM, Brodsky RA. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood. (2020) 136:2080–9. doi: 10.1182/blood.2020008248
23. Yan B, Freiwald T, Chauss D, Wang L, West E, Mirabelli C, et al. SARS-CoV-2 drives JAK1/2-dependent local complement hyperactivation. Sci Immunol. (2021) 6:eabg0833. doi: 10.1126/sciimmunol.abg0833
24. Asteris PG, Gavriilaki E, Touloumenidou T, Koravou EE, Koutra M, Papayanni PG, et al. Genetic prediction of ICU hospitalization and mortality in COVID-19 patients using artificial neural networks. J Cell Mol Med. (2022) 26:1445–55. doi: 10.1111/jcmm.17098
25. Skendros P, Germanidis G, Mastellos DC, Antoniadou C, Gavriilidis E, Kalopitas G, et al. Complement C3 inhibition in severe COVID-19 using compstatin AMY-101. Sci Adv. (2022) 8:eabo2341. doi: 10.1126/sciadv.abo2341
26. Ma L, Sahu SK, Cano M, Kuppuswamy V, Bajwa J, McPhatter J, et al. Increased complement activation is a distinctive feature of severe SARS-CoV-2 infection. Sci Immunol. (2021) 6:eabh2259. doi: 10.1126/sciimmunol.abh2259
27. Lee S, Yu Y, Trimpert J, Benthani F, Mairhofer M, Richter-Pechanska P, et al. Virus-induced senescence is a driver and therapeutic target in COVID-19. Nature. (2021) 599:283–9. doi: 10.1038/s41586-021-03995-1
28. Schmitt CA, Tchkonia T, Niedernhofer LJ, Robbins PD, Kirkland JL, Lee S. COVID-19 and cellular senescence. Nat Rev Immunol. (2023) 23:251–63. doi: 10.1038/s41577-022-00785-2
29. Georg P, Astaburuaga-García R, Bonaguro L, Brumhard S, Michalick L, Lippert LJ, et al. Complement activation induces excessive T cell cytotoxicity in severe COVID-19. Cell. (2022) 185:493–512.e25. doi: 10.1016/j.cell.2021.12.040
30. Lam LKM, Reilly JP, Rux AH, Murphy SJ, Kuri-Cervantes L, Weisman AR, et al. Erythrocytes identify complement activation in patients with COVID-19. Am J Physiol Lung Cell Mol Physiol. (2021) 321:L485–9. doi: 10.1152/ajplung.00231.2021
Keywords: COVID-19, blood coagulation disorders, rotational thromboelastometry, alternative complement pathway, microthrombosis
Citation: Kasugai D, Tanaka T, Suzuki T, Ito Y, Nishida K, Ozaki M, Kutsuna T, Yokoyama T, Kaneko H, Ogata R, Matsui R, Goshima T, Hamada H, Ishii A, Kodama Y, Jingushi N, Ishikura K, Kamidani R, Tada M, Okada H, Yamamoto T and Goto Y (2024) Association between loss of hypercoagulable phenotype, clinical features and complement pathway consumption in COVID-19. Front. Immunol. 15:1337070. doi: 10.3389/fimmu.2024.1337070
Received: 12 November 2023; Accepted: 27 February 2024;
Published: 11 March 2024.
Edited by:
Nicolas Stephane Merle, INSERM Public Health, FranceReviewed by:
Dhaneshwar Kumar, National Institute of Diabetes and Digestive and Kidney Diseases (NIH), United StatesCopyright © 2024 Kasugai, Tanaka, Suzuki, Ito, Nishida, Ozaki, Kutsuna, Yokoyama, Kaneko, Ogata, Matsui, Goshima, Hamada, Ishii, Kodama, Jingushi, Ishikura, Kamidani, Tada, Okada, Yamamoto and Goto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daisuke Kasugai, ZGthc3VnYWlAbWVkLm5hZ295YS11LmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.