
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 07 February 2024
Sec. NK and Innate Lymphoid Cell Biology
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1336666
This article is part of the Research Topic Molecular Insights of Microbiota and Innate Lymphoid Cell Interactions View all 5 articles
Background: ILCs play important roles in the brain, gut, and lungs. Researchers are attempting to establish a research framework on the brain-gut-lung axis using ILCs. However, no one has yet conducted a bibliometric analysis to summarize the findings. In this study, we utilized bibliometrics to analyze the emerging trends and focal areas of ILCs in the brain, intestine, and lung. We aim to provide references for future research on the brain-gut-lung axis.
Methods: To conduct a comprehensive bibliometric analysis on ILCs in the fields of brain, intestine, and lung, we utilized software such as HistCite, VOSviewer, and CiteSpace. Our analysis focused on various aspects, including the number of publications, countries, authors, journals, co-cited documents, and keywords. This approach allowed us to gain valuable insights into the research landscape surrounding ILCs in these specific fields.
Results: A total of 8411 articles or reviews on ILCs in the fields of brain, intestine, and lung were included. The number of published articles has shown a consistent upward trend since 2003. A total of 45279 authors from 99 countries have contributed to these articles. The United States has the highest number of publications (n=3044) and the most cited articles (TGCS=210776). The top three published authors in this field are David Artis, Marco Colonna and Andrew NJ McKenzie. The journal Immunity is the most authoritative choice for researchers. The main research focuses in this field include NK cell, ILC2, tumor immunity, multiple sclerosis, inflammatory bowel disease, airway inflammation, RORγT, and immunotherapy. In recent years, cancer and tumor microenvironment have emerged as hot keywords, particularly immunotherapy, PD-1 related directions, indicating a potential shift in research focus.
Conclusion: European and American countries have been pivotal in conducting research on ILCs, while China has produced a significant number of publications, its impact is still limited. Tumors are likely to emerge as the next focal points in this field. The connection and regulation between the brain and the lung are not yet fully understood, and further investigation is necessary to explore the role of ILCs in the brain-lung axis.
Innate lymphoid cells (ILCs) are a type of immune cells that lack adaptive antigen receptors. Similar to T and B lymphocytes, they originate from common lymphoid progenitor cells and can be rapidly activated during the early stages of disease to provide immunoprotective effects. These cells are widely distributed in mucosal barriers such as the lungs, brain, intestines, and skin. They play a crucial role in the body’s immune defense, tissue repair, inflammatory response, metabolic homeostasis, and tumor suppression (1, 2). According to the naming rules established by Spits et al., ILCs can be categorized into three major groups (3). Group 1 consists of NK cells and ILC1, which rely on T-box transcription factors to produce IFN-γ and primarily respond to viruses and tumors. Group 2 comprises ILC2 that produce IL-5 and IL-13. These cells mainly rely on the transcription factors GATA3 and RORA and respond to parasites and allergens. The final group, Group 3, includes LTi and ILC3 that produce IL-17 and IL-22. These cells primarily act on pathogenic microorganisms such as bacteria and fungi (4). Consequently, ILCs have garnered significant attention in various medical fields, becoming a prominent area of research.
The connection and regulation between the brain, intestines, and lungs are still not fully understood in detail. Recent research has emphasized the important role of ILCs. According to Quatrini and colleagues, ILC cells have a high expression of catecholamine and glucocorticoid receptors on their surface. These receptors are regulated by the hypothalamus-pituitary-adrenergic axis, sympathetic nerves, and parasympathetic nerves (4, 5). Cardoso et al. found that sympathetic nerves can regulate the activity of ILC2 in adipose tissue and activate ILC2 cells through β2AR (6). Furthermore, IL-33 can enhance the activation of ILC2, promoting macrophage activation and eosinophil aggregation to regulate insulin resistance and obesity (7, 8). Catecholamines have been found to negatively regulate the immune response of ILC2, thereby reducing type 2 inflammatory responses in lung and intestinal tissues (9).In the research conducted by von Moltke and Mjösberg (10, 11), it has been observed that helminth infection or IL-25 stimulation can lead to the activation and proliferation of a large number of ILC2s in the intestines. This activation results in the release of type 2 cytokines (IL-4, IL-5, IL-13) and the promotion of immune cell activation (such as mast cells and eosinophils). Through the mediation of sphingosine 1-phosphate (S1P), ILC2s can migrate through the blood circulation to other parts of the body (12), including the lungs. This migration contributes to both anti-worm defense and tissue repair. However, it also leads to the production of inflammatory ILC2s in the lungs, which can cause allergic diseases and support Mjösberg’s hypothesis that lung inflammation originates from the intestines (11). Another study by Cao et al. discovered that dopamine can inhibit the generation of ILC2s and type 2 inflammatory responses by regulating the mitochondrial oxidative phosphorylation process, thereby preventing the occurrence of asthma (13). Therefore, conducting in-depth research on the mechanism of action of ILCs will enhance our understanding of the correlation and regulatory mechanisms between the brain, intestines, and lungs.
Bibliometrics is a research method that focuses on analyzing the literature system and related media to identify research hotspots and development trends in various fields (14, 15). It combines mathematical and statistical methods with visual analysis to provide valuable insights for academic research and innovation. However, our search on platforms like Web of Science, PubMed, and China National Knowledge Infrastructure did not yield any bibliometric or visual analysis on ILCs related to the brain, intestines, and lungs. Therefore, This article utilizes bibliometric methods to comprehensively evaluate the research trends, hotspots, and current status of ILCs in the brain, intestine, and lung domains during the last two decades. It aims to bridge the gap in bibliometric studies in this specific area and provides valuable insights for future research on the brain-gut-lung axis.
We searched the Web of Science Core Collection (WoSCC) to find articles on ILCs in the brain, intestine, and lung fields over the past 20 years (from 2003-01-01 to 2023-12-12). The search formula used was TS=(“ILC” OR “ILCs” OR “Innate lymphoid cells” OR “natural killer cell” OR “NK cell” OR “lymphoid tissue inducer” OR “LTi”) and (“brain” OR “neurotransmitters” OR “Encephalon” OR “nerve” OR “nervous” OR “lung” OR “pulmonary” OR “gut” OR “Colon” OR “colorectal” OR “Rectum” OR “Intestine”). The search was completed on December 12, 2023, to ensure data accuracy. Articles written in English were specifically chosen, and the document types were limited to articles and reviews, excluding meeting abstracts, editorial materials, proceedings papers, and other document types. Manual literature screening was conducted to exclude irrelevant articles, such as those related to testicular interstitial cells (ILC), breast invasive lobular carcinoma (ILC), International Law Commission (ILC), etc. Additionally, content that was unrelated to the fields of brain, lung, and intestine, such as liver, uterus, pregnancy, blood system diseases, breast diseases and physical realm, was also excluded (Figure 1). The search results were then exported in Plain Text file format, and the Full Record and Cited References of the documents were collected. This included information such as authors, countries, institutions, references, keywords, journals, and other relevant details. These data were utilized for bibliometric and visual analysis purposes.
In this study, we will utilize HistCite, VOSviewer, and CiteSpace for bibliometric and visual analysis. Additionally, we will employ Microsoft Excel Office to organize relevant data including the number of publications and citation frequency.
Histcite is a document analysis tool developed by Thomson Reuters in the United States (16). It is specifically designed to analyze documents (Plain Text files) exported from the WoS database. The tool effectively identifies and counts various elements such as keywords, authors, countries, institutions, journals, publication years, languages, document types, cited documents, TLCS, TGCS, and other relevant information in each document. Additionally, it constructs the citation relationships between documents, which aids in understanding the overall context and relationships within a particular field. TLCS, also known as the total local citation score, indicates the level of attention a document has received from peers. A higher TLCS score implies greater attention and makes the document more worthy of reading. On the other hand, TGCS, or the total global citation score, takes into account the attention the document has received from other fields or disciplines.
VOSviewer is a powerful document visualization tool developed by Leiden University. It facilitates multi-dimensional bibliometric and visual analysis (17), including network visualization, coverage visualization, and density visualization. Through the construction of network relationship maps between authors, keywords, citations, etc., it aids in uncovering cooperative relationships, research hotspots, and development trends in the field. Network visualization depicts connections between documents, keywords, and authors using a network diagram. The size of nodes represents the frequency of occurrence of the corresponding unit, and clusters are indicated by the same color. Overlay visualization adds supplementary information to the original network diagram, providing further insights. Density visualization illustrates the distribution of a specific attribute in the network diagram, enabling the visualization of heat distribution.
Citespace, a document visualization software developed by Professor Chen, is capable of analyzing massive documents from various databases such as Web of Science, Pubmed, and CNKI. It provides insights on topics, keywords, author units, collaborative networks, journals, publication time, and document citations (18). By performing spatiotemporal analysis, hot spot analysis, and cluster analysis on topics, Citespace can detect research hot spots and popularity in different periods through keyword burst, thus analyzing the development trends in the field (19). In our study, we utilized cluster analysis to identify key research areas and directions of ILCs in the brain, intestines, and lungs by clustering keywords and co-cited documents. Modularity Q and mean silhouette were used as important evaluation indicators in cluster analysis. It is commonly accepted that Modularity Q > 0.3 indicates a significant clustering structure, while Mean silhouette > 0.5 suggests convincing clustering results. Furthermore, we conducted burst analysis and timeline analysis of keywords, references, and more to explore the hot spots and development trends of ILCs in the fields of brain, intestine, and lung.
When conducting visual analysis using Citespace, we typically analyze the data with the system’s default parameters: time slice=1, link strength measured by cosine, g-index K=25, and top N=50. For clustering, we employ the LLR algorithm. The algorithm used for burst analysis is based on a conference paper titled ‘Bursty and Hierarchical Structure in Streams’ by Kleinberg J in 2002. The number of nodes within a cluster indicates the level of activity in a field, representing an emerging trend in research. During burst analysis, we also follow the system’s default parameters, which involve configuring the detection model with F(x)=ae-ax, a1/a0 = 2.0, ai/ai-1 = 2.0, The Number of States=2, γ [0,1]=1.0, Minimum Duration=2.
We conducted a comprehensive search of academic articles and reviews on ILCs in the fields of brain, intestine, and lung using Web of Science. A total of 8411 articles were screened, including 6070 articles and 2341 reviews. During the search period, Lin et al. published the first article in this field, which revealed that the expression levels of ICAM-1 and L-selectin on peripheral blood NK cells decreased during acute attacks of childhood asthma. This reduction promoted the migration of NK cells to the lung inflammation site (20). NK cells, the first ILCs discovered in the 1970s, have been the subject of extensive research due to their crucial role in both innate and adaptive immunity. Figure 2A illustrates a fitting curve that demonstrates an overall upward trend in the number of publications on ILCs in the brain, intestine, and lung fields since 2003. The average number of publications is approximately 400, with the highest number recorded in 2021 (n=953). In Figure 2B, it is evident that the growth rate of published articles in 2020-2021 was the highest at 36%, while the number of published articles declined in the past two years. Over the past 20 years, the total number of local citations for these articles reached 36672, with a global citation count of 417918. The peak values for total local citations (TLCS) and total global citations (TGCS) were observed in 2014 (TLCS=4110, TGCS=36160). However, starting from 2021, there has been a significant decrease in TLCS and TGCS compared to the previous year, reaching the lowest point in 2023 (TGCS=631).
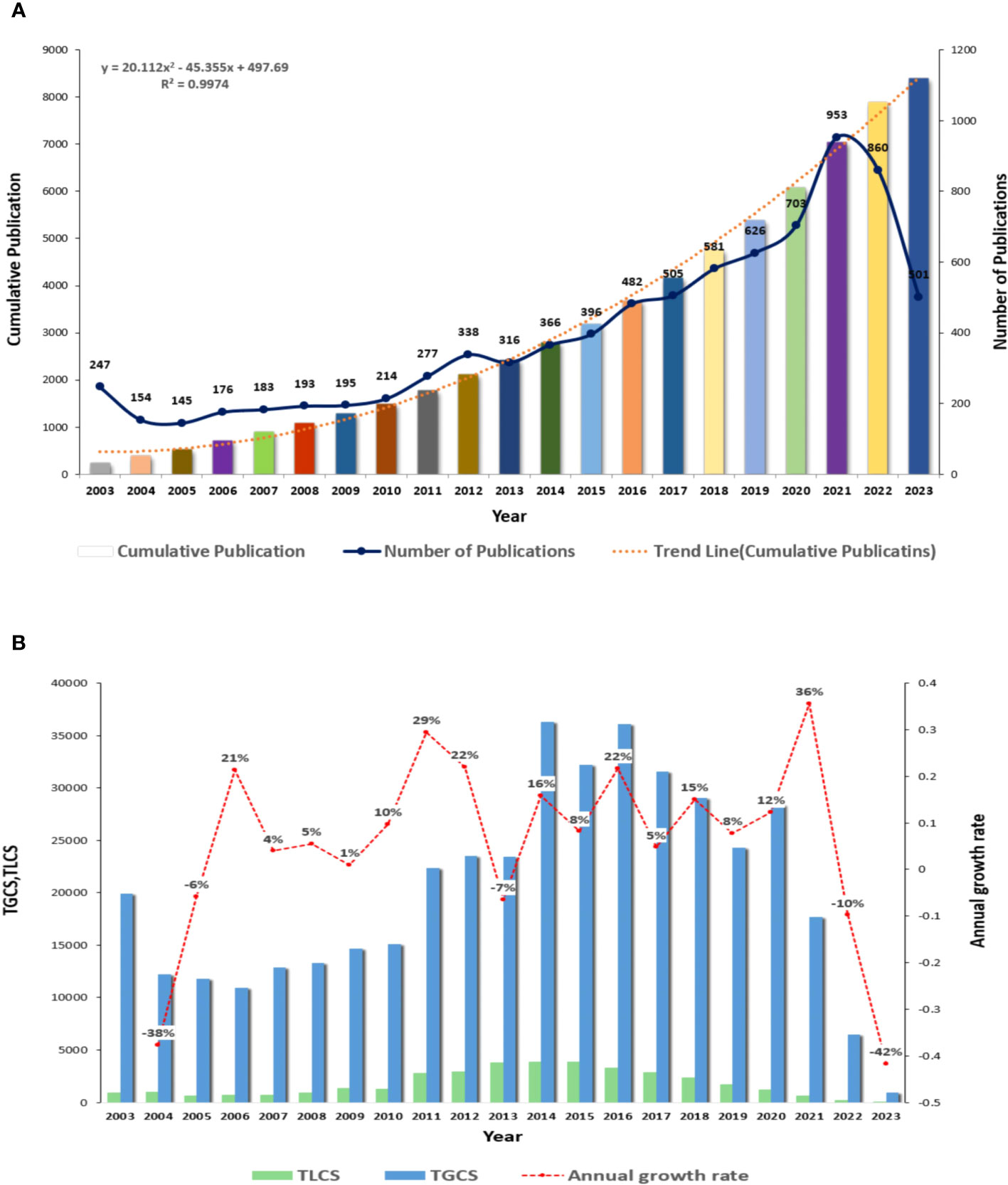
Figure 2 Overall distribution of publications and citations in this field (A) Global annual output trends. (B) TGCS, TLCS and growth rate in annual publications.
From 2003 to 2023, a total of 99 countries have published articles on ILCs in the fields of brain, intestine, and lung. The geographical distribution of these countries is shown in Figure 3A. Table 1 displays the top ten countries with the most publications. According to Figure 3B, the United States has the highest number of publications, with a total of 3044 articles. China (including Taiwan) follows with 1559 articles, and Germany ranks third with 788 articles. In terms of citations, the United States leads with a total of 210776 citations. Germany comes second with 55098 citations, followed by the United Kingdom with 50887 citations. China ranks fourth, as shown in Figure 3D. Israel has the highest average number of citations, with 115 publications, 11390 citations, and an average of 99.00 citations per article. France follows with 443 publications, 39235 citations, and an average of 88.57 citations per article. China’s average number of citations is 26.86. Figure 3E visually displays the national cooperation relationships in the research on ILCs in the fields of brain, intestine, and lung. Notably, there is strong international research cooperation between China and the United States, followed by the United States and Germany, the United Kingdom, Japan, Canada, and other countries. The annual publication trend chart by country (Figure 3C) reveals that research in the United States, the United Kingdom, Australia, and other countries began earlier compared to China. However, the number of publications in China has been steadily increasing since 2010, and in recent years, China has become the leading country in terms of publication output in this field.
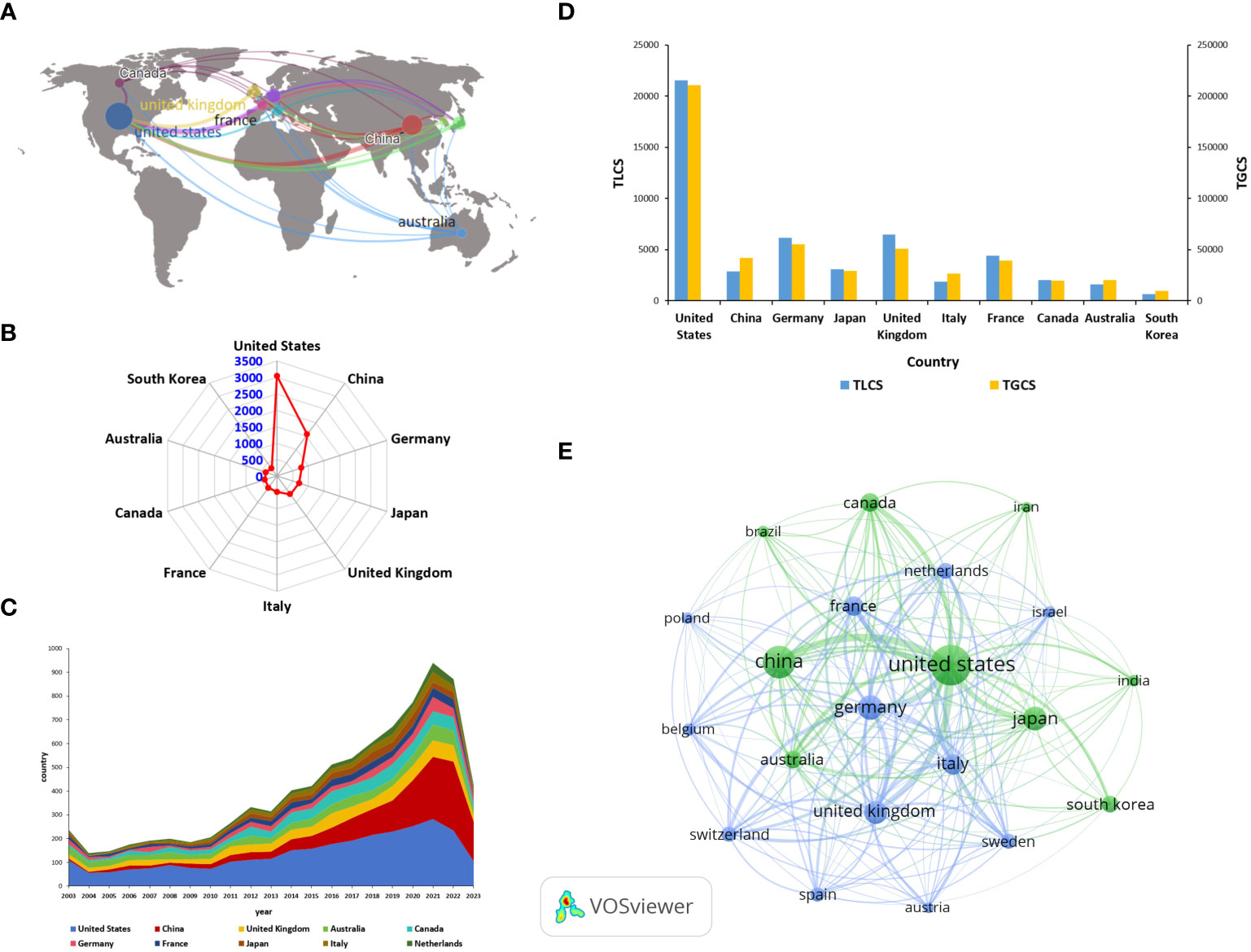
Figure 3 Major countries contributing in this field (A) Geographical distribution of global output. (B) Radar map of the top 10 productive countries. (C) Annual output trend of the top 10 productive countries (The areas of different colors represent the output in different countries). (D) Bar chart of TGCS, TLCS of the top 10 productive countries. (E) Visual cluster analysis of cooperation among countries (The size of the circle is proportional to the number of articles, and the thickness of the line indicates the strength of the link).
A total of 45279 authors from 6585 institutions participated in the research on ILCs in the fields of brain, intestine, and lung. The top 10 institutions with the most research results are presented in Figure 4A. Harvard Medical School in the United States leads with the highest number of publications (n=140, TGCS =6997, TLCS=800), followed by Karolinska Institutet in Sweden (n=134, TGCS=6709, TLCS=860), and University of California in the United States (n=124, TGCS=13404, TLCS=1914). In terms of total global citations (TGCS), the University of Pennsylvania in the United States holds the top position with 14,778 citations, followed by the University of California in the United States (TGCS=13404) and Inserm in France (TGCS=13200) (Figure 4B). The social network diagram of research institutions illustrates the division of institutions into 7 clusters based on an occurrence frequency of ≥10 and cooperative relationships (Figure 4C). Notably, there is significant collaboration between institutions, both domestically and internationally, with the strongest partnership observed between France’s Inst Pasteur and Inserm. According to Figure 4D, articles published by Harvard University and NIAID in the United States were published earlier. In contrast, Chinese institutions such as Shanghai Jiao Tong University and Huazhong University of Science and Technology have published a higher number of articles around 2020.
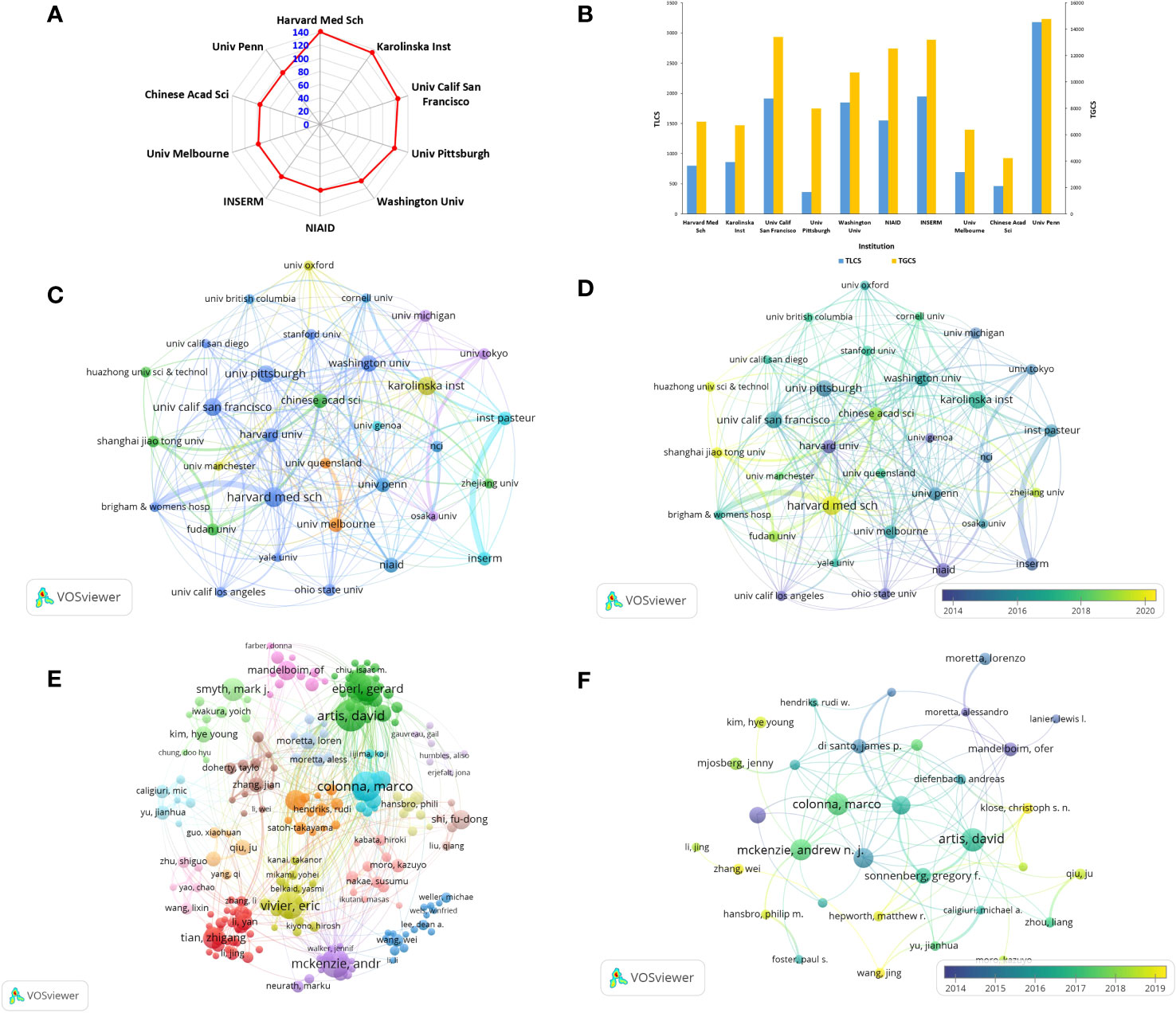
Figure 4 Contributions and relationships with major research institutions or authors (A) The top10 institutions with the number of publications. (B) The number of citations corresponding to the top 10 institutions. (C) Visual cluster analysis of cooperation among institutions. (D) Timeline visualization of cooperation among institutions (different colors represent different times). (E) Visual cluster analysis of cooperation among authors (different colors represent different clusters). (F) Timeline visualization of cooperation among authors.
The top three authors with the most published articles on innate lymphoid cells (ILCs) in the fields of brain, intestines, and lungs are David Artis from the University of Pennsylvania (n=40, TGCS=10327, TLCS=2857), Marco Colonna from Washington University (n=37, TGCS=6803, TLCS=1510), and Andrew NJ McKenzie from the MRC Laboratory of Molecular Biology (n=36, TGCS=6866, TLCS=2158) (Table 2). David Artis is highly regarded as an authoritative expert in the fields of brain, intestines, and lungs, as evidenced by his extensive research publications on ILCs. His articles have received the highest number of citations in this field. Notably, he published the first review on this topic in 2010 and has since continued his uninterrupted research on ILCs. In October 2023, David Artis published a comprehensive review in the journal Immunology, providing an in-depth analysis of ILC2s and TH2 cells and their synergistic effect in the expulsion of intestinal parasites (21). After conducting visualization (Figure 4E), we observed a relatively close cooperation among the authors. We formed 18 clusters consisting of authors who had an occurrence frequency of ≥10 and a cooperative relationship. The strongest collaboration was found between Colonna and Gilfillan. In terms of publication timing, most authors published their papers after 2015, as depicted in Figure 4F.
All articles were published in 1350 magazines. The top 10 magazines with the most articles are shown in Table 3. According to Figure 5A, The Journal of Immunology leads with 330 articles (n=330, TGCS=20331, TLCS=2247), followed by Frontiers in Immunology with 289 articles (n=289, TGCS=9135, TLCS=2), and Plos One with 199 articles (n=199, TGCS=6294, TLCS=0). However, these journals have relatively low citation rates in the field. The top three journals with the highest citation counts are Immunity (TGCS=22719), Journal of Immunology (TGCS=20331), and Nature Immunology (TGCS=17537). Figures 5B, C depict the journal time series diagram and heat map, respectively. Notably, Frontiers in Immunology has gained popularity for submissions in recent years. According to the double overlay diagram, there are three main citation paths. The published articles were predominantly found in journals related to molecular biology, immunology, and clinical medicine. On the other hand, the cited articles were mostly published in journals covering molecular biology, genetics, health, nursing, and medicine (Figure 5D).
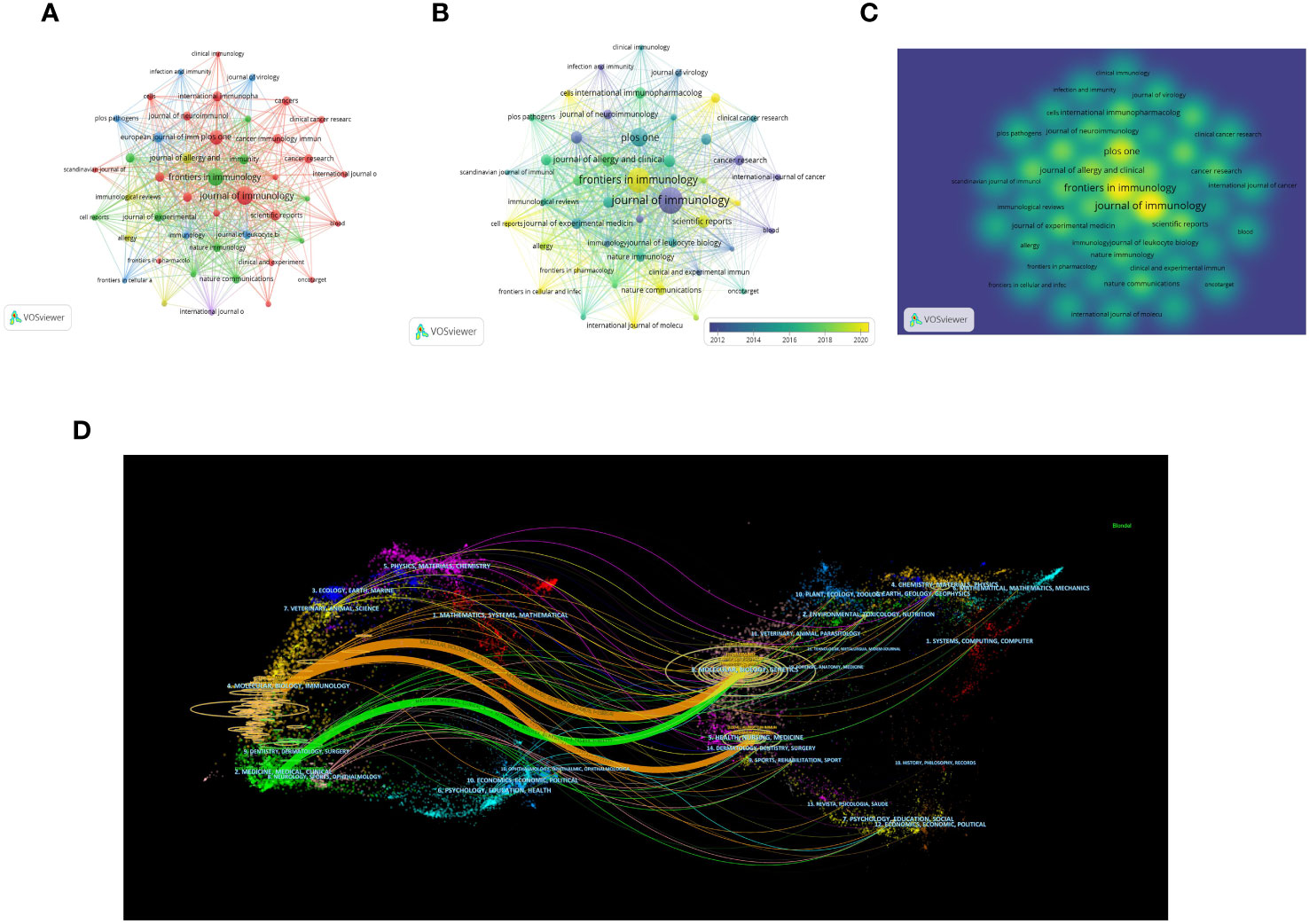
Figure 5 Distribution of the main journals publishing research in the field (A) Visual cluster analysis among Journals. (B) Timeline visualization among Journals. (C) Density visualization of journals (The color brightness is directly proportional to the density of the publication). (D) The dual-map overlap of journals (The left side represents the citing journal, the right side represents the cited journal, and the line path represents the citation relationship).
Table 4 presents the top 10 most frequently cited articles. These articles provide comprehensive descriptions of the roles and mechanisms of various types of ILCs in inflammation, allergy, and infection. By conducting a co-citation analysis of the 278,967 references, it was discovered that there were 139 articles with more than 100 citations. The article with the highest number of citations, authored by Hergen Spits et al. and published in the journal Nature reviews Immunology in 2013. In their review, the authors proposed a classification of ILCs into three categories based on the cytokines produced by ILCs and the transcription factors that regulate their development and function. This classification has been widely accepted and is now considered the standard for categorizing ILCs. It has significantly advanced research on ILCs in the field of brain-gut-lung axis (3). We constructed a network visualization of co-cited documents (Figure 6A) and conducted cluster analysis, which led to the identification of 19 cluster modules (Figure 6B). The modularity Q value was determined to be 0.8900, and the mean silhouette value was 0.9055. These cluster modules encompassed various topics such as Innate lymphoid cells, Tumor immunity, Airway inflammation, Inflammatory bowel disease, Cytokine storm, and ILC2 activation. Additionally, we visualized the clustering timeline (Figure 6C) and observed frequent mentions of Tumor immunity, NK cells, Multiple sclerosis, Inflammatory bowel disease, Ligands, Airway hyperreactivity, etc. in the early research on ILCs in the brain, intestine, and lung fields. The inclusion of these references suggests that they are considered research hotspots in this particular domain. The current focus in this research field is on Innate lymphoid cells, Immunity, Airway inflammation, Cytokine storm, and Tissue migration. Upon closer examination of the clustering patterns, it was observed that ‘#0 Innate lymphoid cells’ mainly refers to ILC1, ILC2, and ILC3. These three types of cells have been progressively recognized since 2010. Notably, in 2010, Sawa et al. proposed in Science that RORγt(+) innate lymphocytes (ILC) play a beneficial role in maintaining intestinal bacteria, stabilizing the population, and promoting the production of intestinal defense cells (22). This groundbreaking study paved the way for further research on these three types of ILCs in various organs such as the brain, intestine, and lungs. An burst analysis was conducted on the co-cited documents (Figure 6D). The review by Vivier et al. in 2018 (4) had the highest burst strength. This review provided detailed information on the research conducted in the past ten years, emphasizing the importance of ILCs in environmental homeostasis, tissue metabolism, and cell repair. Currently, the focus of the cited literature is on an article published by Huang et al. in Nature in 2018. They discovered that interleukin-25 or helminth-induced inflammation ILC2s can facilitate the chemotaxis of sphingosine 1-phosphate (S1P), enabling migration from the intestinal lamina propria to various tissues through blood and lymph circulation (12). In 2017, Cardoso et al. found that neuromodulation of type 2 innate lymphocytes can be achieved through neuromedin U (23). Additionally, Klose et al (24). published an article in Nature in the same year, suggesting that the nervous system regulates ILCs and type 2 inflammatory cells through the neuropeptide neuromedin U to mediate intestinal immune protection. This research area has gained considerable attention, highlighting the significance of neural regulation in intestinal immunity in recent years.
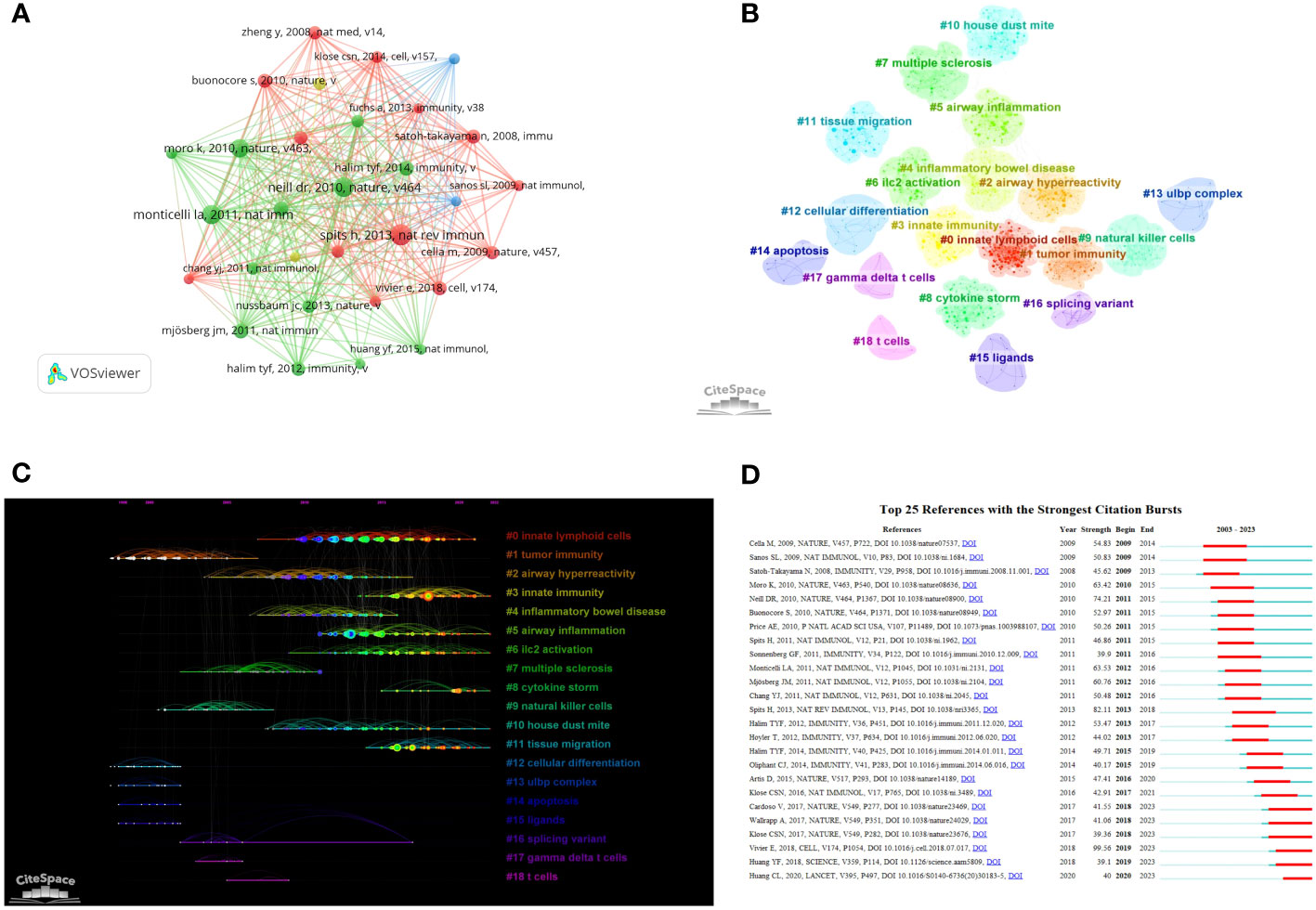
Figure 6 Clustering and bursting of co-cited references (A) Visual analysis of Co-cited References. (B) Cluster Analysis of Co-cited References. (C) Timeline distribution of the 19 clusters (The length of the lit line indicates the start and end time of the hotspot. The further back the line is, the closer it is to the present moment). (D) The top 25 references with the strongest citation bursts (The length of the red line indicates the duration of the hotspot).
A visual analysis was conducted on 21,439 keywords in this field (Figure 7A). Keywords such as ILCs, NK cells, inflammation, asthma, cancer, dendritic cell, and immunotherapy appeared frequently in this field, with a frequency of more than 500 times. The cluster analysis resulted in 20 clusters (Figure 7B) with a modularity Q of 0.816 and a mean silhouette value of 0.9363. Combining the clustering timeline results, Figure 7C demonstrates that cancer, immunity, airway inflammation, asthma, RORγT, immunotherapy, inflammatory bowel disease, allergic inflammation, and other related topics have been the primary areas of focus in the research on ILCs in the brain, intestine, and lung since 2003. These findings highlight the key research topics in this field. We conducted an burst analysis on keywords and identified the top 25 burst keywords. Figure 7D displays the keyword with the highest burst strength, which is ‘in vivo’, and it remains burst for a duration of 10 years. Additionally, our analysis revealed that lung adenocarcinoma and tumor microenvironment are prominent keywords in recent research. This suggests that ILCs may significantly impact the diagnosis and treatment of brain, intestinal, and lung tumors.
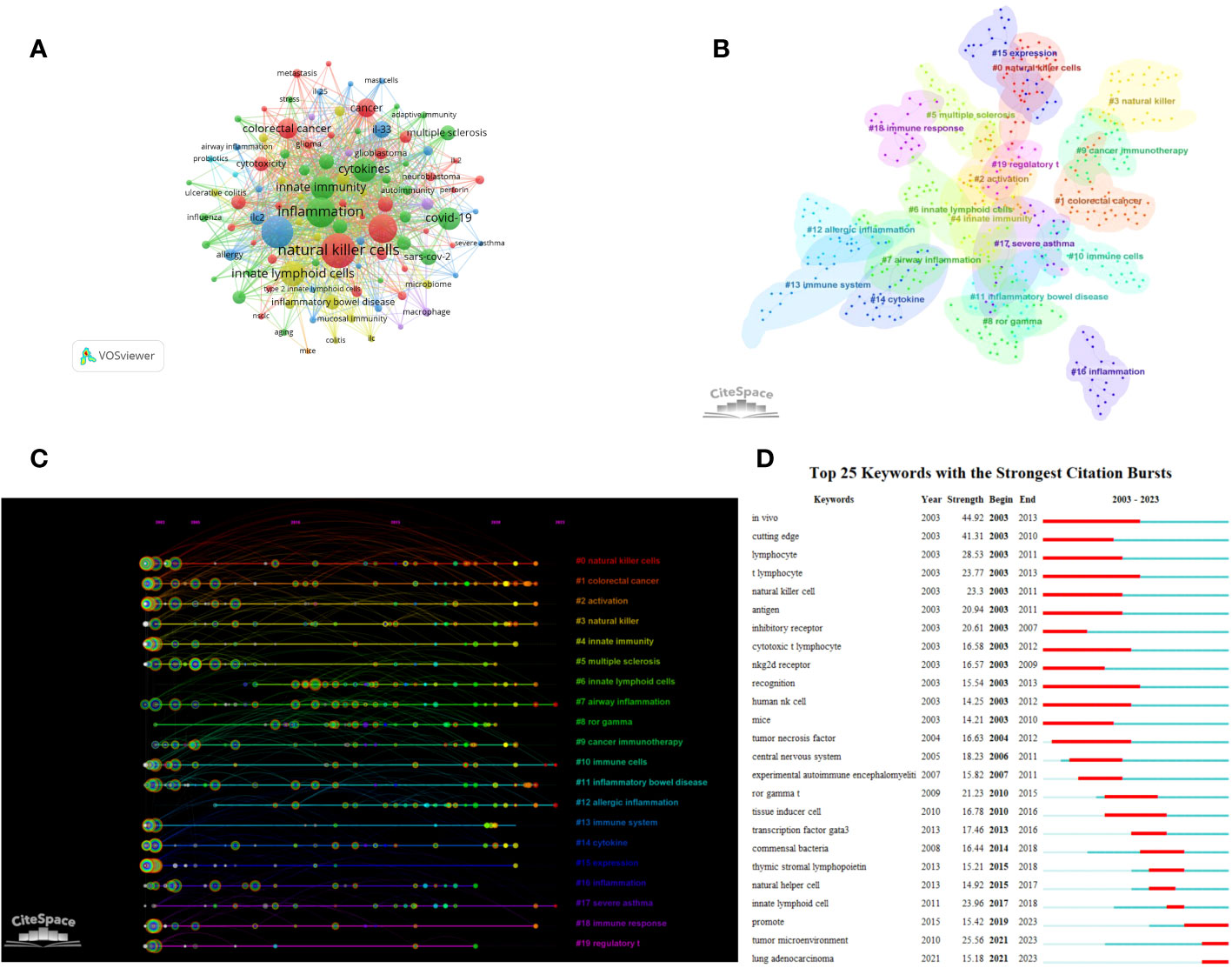
Figure 7 Clustering and bursting of keywords (A) The network map of keywords. (B) Cluster Analysis of keywords. (C) Timeline distribution of cluster analysis of keywords. (D) The top 25 keywords with the strongest citation bursts.
After analyzing the keywords and co-cited documents, recent studies have focused on investigating ILCs in the brain, intestine, and lung. These studies primarily explore various aspects including allergic diseases, intestinal inflammation, infection, tumors, and neuroendocrine regulation. Notably, ILCs have been found to play a significant role in the brain-gut axis and the lung-gut axis. However, the specific role of ILCs in the brain-lung axis remains unclear, and there is a lack of research in this area. Understanding the contribution of ILCs in the brain-lung axis is crucial for establishing a comprehensive research framework on the brain-gut-lung axis. Therefore, further research is needed to fill the existing gaps and advance our knowledge in this domain.
Based on the above search terms, we added the conditions “Tumor” OR “Neoplasm” OR “Neoplasia” OR”Cancer” OR “Malignan”, A total of 5597 articles were retrieved, and after screening, 2709 articles related to ILCs in brain, intestinal, and lung tumors were included. In terms of publications, the United States leads with the highest number (n=821), followed by China (n=740) and Japan (n=252) (Figure 8A). The United States is also the most cited country (TGCS=53717), followed by China (TGCS=18282). The most cited article is a 2009 article by Castriconi et al., they discovered that NK cells have the ability to identify and eliminate human glioblastoma cells that exhibit stem cell-like characteristics (25). Moving on to authors, Smyth has the highest number of publications in this field (n=23) (Figure 8B). Keyword clustering of the literature resulted in 17 clusters (Q=0.7532, S=0.8871) as shown in Figure 8C, Among the various types of ILCs, NK cells have received the most attention in research. Time visualization and burst analysis of the clusters reveal that recent research has focused on hot topics like IFN-gamma, mechanism, immunotherapy, tumor microenvironment, immune checkpoint, lung cancer, colorectal cancer, and PD-1 (Figures 8D, E). PD-1 is a crucial immunosuppressive molecule (26, 27). The combination of PD-1 and PD-L1 reduces T cell activity and suppresses the immune system’s attack on tumor cells. Blocking the PD-1 and PD-L1 combination restores T cell activity, leading to effective tumor cell killing and remarkable efficacy in various cancer treatments. PD-1/PD-L1 monoclonal antibodies are currently the most extensively studied immune checkpoint inhibitors in clinical research and application, making them a prominent topic in tumor immunotherapy in recent years.
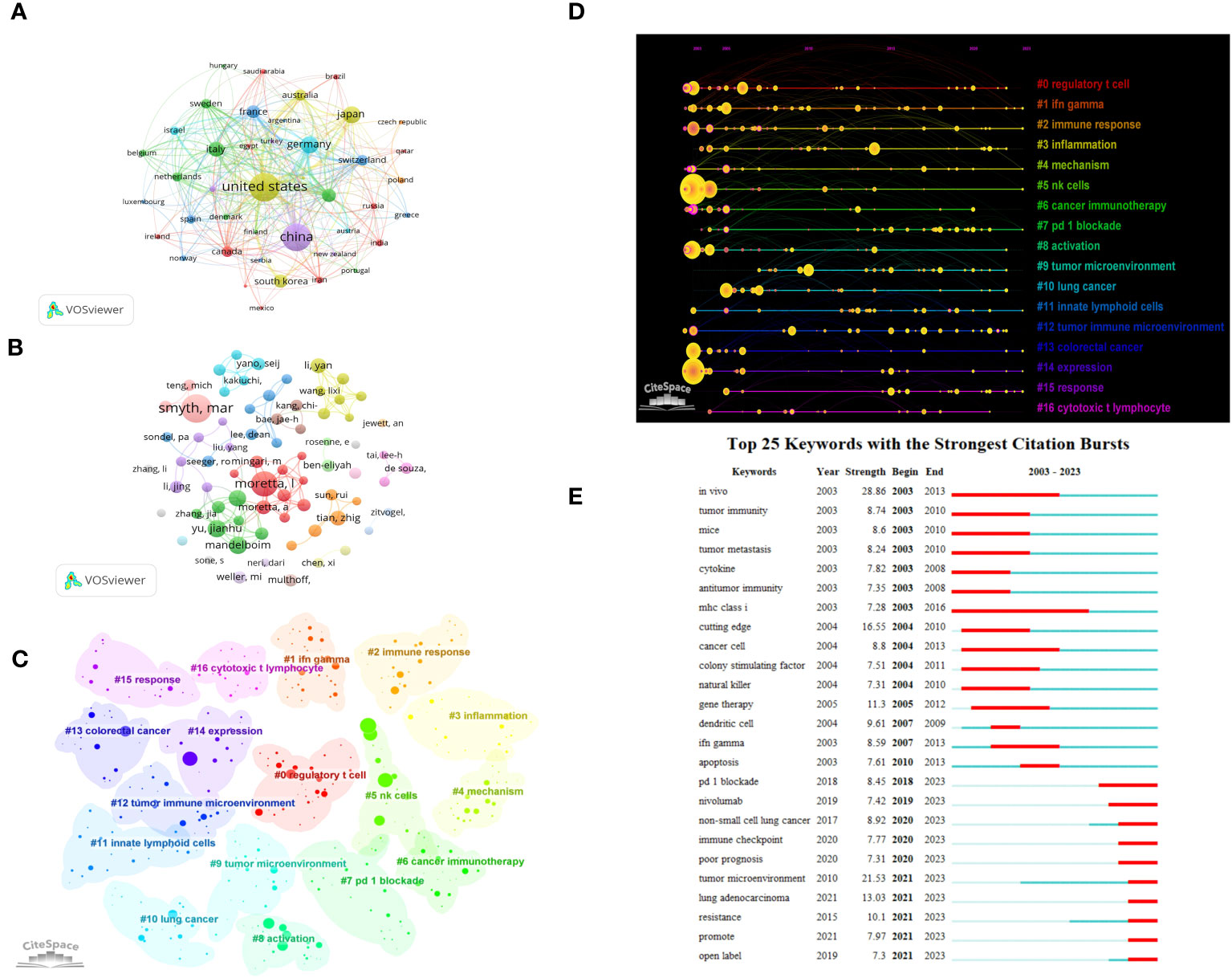
Figure 8 Visualization of oncology-related articles in the field (A) Visual cluster analysis of cooperation among countries. (B) Visual cluster analysis of cooperation among authors. (C) Cluster Analysis of keywords. (D) Timeline distribution of cluster analysis of keywords. (E) The top 25 keywords with the strongest citation bursts.
We conducted an analysis of 8411 documents that were published by 45279 authors affiliated with 6585 institutions across 99 countries. Over time, the annual publication volume has been consistently increasing. While there has been a slight decrease in the number of citations for articles in the past two years, there is still significant attention and understanding in this field. The United States stands out as the country with the highest number of publications and the highest TGCS, indicating its significant influence in this field. One noteworthy contributor is David Artis from the University of Pennsylvania, who has made substantial contributions to the study of the role of ILCs in immune regulation and tissue homeostasis. His research focuses on the relationship between ILCs and various aspects such as the intestines, lungs, skin, mucosal barriers, and obesity (1, 28). Recently, he has shifted his focus towards understanding the non-redundant functions of ILC2 in the lungs and intestines. He has proposed that neuropeptides, specifically a cholinergic neuropeptide, regulate the anti-parasitic and protective effects of ILC2 through neurons, thereby establishing a connection between the lung-gut axis and neurotransmitters. This advancement in research sheds light on the brain-gut-lung axis and provides valuable insights into the interplay between ILCs and this axis (29, 30). According to Figure 3C, it is evident that developed countries such as the United States, Britain, and Canada have been researching in this field for a longer period, while China started its research later. However, China has maintained close collaboration with European and American countries, resulting in a significant increase in the number of publications in recent years.
Among all the journals to which articles were submitted, Immunity, Journal of Immunology, and Nature Immunology are the top three journals with the highest number of citations. They have a larger number of publications compared to traditional top journals such as Nature, Science, Cell, and Lancet. This suggests that they are esteemed journals in the field and are preferred by many scholars studying the relationship between ILCs and the brain, intestines, and lungs. This preference is due to the fact that the research scope of ILCs is related to immunity, and Nature and Science are comprehensive journals. In Immunity magazine, the highest TLCS was published by Halim et al. in 2014: ‘Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation’ (31). This publication emphasizes the role of ILC2 in asthma and other allergic diseases, attracting significant attention from colleagues. Another immunology journal, Frontiers in Immunology, has witnessed a significant increase in the number of articles published in recent years, reaching 7792 articles in 2022-2023. However, its TLCS remains relatively low.
Cluster analysis and timeline chart of co-cited documents and keywords indicate that research on ILCs in the brain, intestine, and lung fields may involve asthma, tumor, Cytokine storm, Multiple sclerosis, Inflammatory bowel disease, immunotherapy, RORγT. Asthma, characterized by airway hyperresponsiveness, is closely linked to ILCs, particularly ILC2, which plays a significant role. Activation of ILC2 by external allergens or cytokines (such as IL-25 and IL-33) leads to the production of type 2 cytokines, resulting in an increasing in eosinophils, mast cells, mucus secretion, and mucosal tissue remodeling. These processes contribute to the development of allergic diseases like asthma (32–34). As a result, researchers have been focused on studying the excessive activation of ILC2 and the associated type 2 inflammatory responses, aiming to identify new targets for asthma treatment. Multiple sclerosis (MS) is a well-known autoimmune disease that affects the central nervous system. Numerous studies have demonstrated the significant involvement of ILCs in both the onset and progression of this disease (35, 36). The interaction between the nervous system and immune cells, such as ILCs, is complex and not fully understood (37). The nervous system, including the central nervous system, plays a crucial role in regulating the immune response of tissues such as the intestines and lungs, maintaining the body’s physiological homeostasis (5). In a study by Ibiza et al. (38), they discovered that glial cells promote the activation of RET tyrosine kinase by regulating the expression of neurotrophic factors. This activation induces intestinal ILC3 to produce a significant amount of IL-22, which helps maintain intestinal stability, inhibits inflammation, and prevents infection. Neuromedin U (NMU) is a highly conserved sequence that plays a crucial role in activating ILC2s in intestinal and lung tissues. Cholinergic neurons produce NMU, which promotes the production of types 2 inflammation (24). NMU activation leads to the activation of eosinophils and mast cells, resulting in smooth muscle contraction and contributing to the development of allergic diseases (39, 40). Therefore, NMU is vital for maintaining the homeostasis of ILC2s and overall body balance. These findings provide evidence of the crucial role of the nervous system in regulating ILCs and maintaining the stability of the intestine and lungs. This lays the foundation for further exploration of the brain-gut-lung axis and the role of ILCs. The transcription factor RORγT is responsible for regulating the expression and development of ILC3 (41, 42). It promotes the production of inflammatory factors IL-22 and IL-17, leading to increased goblet cell mucin secretion and inducing intestinal epithelial fucosylation. These effects enhance the integrity of the intestinal barrier and help maintain the homeostasis of the intestinal flora. However, some studies have shown (43, 44) that ILCs can respond to IL-23-mediated inflammatory bowel disease (IBD) by producing IL-22 and IL-17. This has led to an increasing focus on the role of ILCs in IBD, with researchers striving to discover new targets for its treatment (45, 46).
With the advancement of research on ILCs in the brain, intestine, and lung, new research directions in this field have gained prominence. The investigation of ILCs in brain tumors, intestinal tumors, and lung tumors has become a major area of interest in recent years, attracting significant attention from researchers. Particularly, keywords such as monoclonal antibodies and PD-1 have garnered significant interest. However, the precise mechanism of action of ILCs in tumors remains unclear. ILCs exhibit dual characteristics, resembling a politician with affiliations to both parties (47–49). This is because the function of ILCs is influenced by various factors, including phenotypic subgroups, signaling pathways, tissue sites, and the tumor microenvironment. These factors contribute to the divergent effects of ILCs in tumor development. NK cells, the first type of ILCs to be discovered, are known for their high plasticity (50). In the context of tumors, NK cells can exhibit both pro-tumor and anti-tumor phenotypes. They possess the ability to inhibit tumor growth and metastasis (51), thereby extending the lifespan of patients. While studies have highlighted the improved prognosis and overall survival rate associated with NK cells (52), there are also concerns about their potential carcinogenic risk (53). Apart from NK cells, researchers have shown significant interest in the other three types of ILCs - ILC1, ILC2, and ILC3. In a study by Saranchova et al. (54, 55), it was reported that IL-33 can alter the tumor microenvironment, leading to the activation of anti-tumor CD8+ T cells and eosinophils by ILC2. This activation inhibits tumor formation and suppresses lung tumor metastasis, with the ILC2-IL-5-GM-CSF axis playing a crucial role in this process (47). According to research conducted by Jacquelot and others, IL-33 has the ability to enhance tumor sensitivity to PD-1 by activating ILC2. This activation results in an improved anti-tumor effect of PD-1, leading to positive outcomes for the majority of patients (56, 57). However, Schuijs et al. discovered in their study that ILC2s activated by IL-33 can inhibit the tumor-killing effect of NK cells, leading to tumor metastasis (58). Jou et al. also found in their research (59) that IL-25 can activate ILC2 and recruit myeloid-derived suppressor cells (MDSC) in colorectal tumors. This activation inhibits the function of anti-tumor CD8+ T cells and promotes tumor development. Both NK cells and ILC1s can eliminate tumor cells by secreting granzyme, perforin, and IFN-γ. Under certain conditions, NK cells can differentiate into ILC1s, with NK cells primarily inhibiting tumor growth and ILC1s more effectively inhibiting tumor metastasis (60, 61). Additionally, ILC3s accumulate in the meninges, where they promote anti-tumor immunity in brain cancer by facilitating T lymphocyte infiltration into the brain and supporting their survival (62).
This study has a few limitations that need to be acknowledged. Firstly, our study is primarily focused on examining the function and role of ILCs in the brain, intestine, and lung. Although we made efforts to enhance our search terms and strategies, it is still possible that we may have missed some relevant studies. Secondly, our data is solely obtained from the WOSCC database, which means our analysis is restricted to English articles and reviews. Lastly, it is important to mention that a substantial portion of our results depend on machine algorithms, with limited manual induction and organization.
Currently, there is a growing research interest in the field of innate lymphoid cells (ILCs) in the brain, intestine, and lung. In this study, we conducted a bibliometric analysis to identify research trends and hot spots in this field. Our findings reveal that asthma, tumor, Cytokine storm, Multiple sclerosis, Inflammatory bowel disease, immunotherapy, RORγT, neuroimmunology, and PD-1 are the primary focus and hot spots of ILC research in the brain, intestine, and lung. Among the various types of ILCs, NK cells have received the most attention in research. Researchers have dedicated significant efforts to these areas. However, most studies tend to concentrate on a single system or organ, or the brain-gut and lung-gut axes. The relationship and regulation between ILCs in the brain-lung axis remain unclear. It is essential for researchers to investigate the role of ILCs in the brain-lung axis, the specific mechanisms underlying related diseases, and the reasons behind the high metastasis of lung cancer to the brain. These questions warrant further exploration.
All datasets presented in this study can be found in the article and WoSCC database.
JH: Writing – original draft, Formal Analysis, Visualization. KD: Formal Analysis, Visualization, Writing – original draft. YL: Formal Analysis, Visualization, Writing – original draft. MX: Formal Analysis, Visualization, Writing – original draft. ML: Formal Analysis, Visualization, Writing – original draft, Writing – review & editing. MW: Formal Analysis, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study received financial support from the Hunan Provincial Natural Science Foundation of China (No.2022JJ40578), the Scientific Research Program of FuRong Laboratory (No. 2023SK2085), and the Fundamental Research Funds for the Central Universities of Central South University (CX20230120).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Artis D, Spits H. The biology of innate lymphoid cells. Nature (2015) 517(7534):293–301. doi: 10.1038/nature14189
2. Yin Z, Zhou Y, Turnquist HR, Liu Q. Neuro-epithelial-ILC2 crosstalk in barrier tissues. Trends Immunol (2022) 43(11):901–16. doi: 10.1016/j.it.2022.09.006
3. Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells–a proposal for uniform nomenclature. Nat Rev Immunol (2013) 13(2):145–9. doi: 10.1038/nri3365
4. Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells: 10 years on. Cell (2018) 174(5):1054–66. doi: 10.1016/j.cell.2018.07.017
5. Quatrini L, Vivier E, Ugolini S. Neuroendocrine regulation of innate lymphoid cells. Immunol Rev (2018) 286(1):120–36. doi: 10.1111/imr.12707
6. Cardoso F, Klein Wolterink RGJ, Godinho-Silva C, Domingues RG, Ribeiro H, da Silva JA, et al. Neuro-mesenchymal units control ILC2 and obesity via a brain-adipose circuit. Nature (2021) 597(7876):410–4. doi: 10.1038/s41586-021-03830-7
7. Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med (2013) 210(3):535–49. doi: 10.1084/jem.20121964
8. Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature (2015) 519(7542):242–6. doi: 10.1038/nature14115
9. Moriyama S, Brestoff JR, Flamar AL, Moeller JB, Klose CSN, Rankin LC, et al. β2-adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science (2018) 359(6379):1056–61. doi: 10.1126/science.aan4829
10. von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature (2016) 529(7585):221–5. doi: 10.1038/nature16161
11. Mjösberg J, Rao A. Lung inflammation originating in the gut. Science (2018) 359(6371):36–7. doi: 10.1126/science.aar4301
12. Huang Y, Mao K, Chen X, Sun MA, Kawabe T, Li W, et al. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science (2018) 359(6371):114–9. doi: 10.1126/science.aam5809
13. Cao Y, Li Y, Wang X, Liu S, Zhang Y, Liu G, et al. Dopamine inhibits group 2 innate lymphoid cell-driven allergic lung inflammation by dampening mitochondrial activity. Immunity (2023) 56(2):320–335.e9. doi: 10.1016/j.immuni.2022.12.017
14. Liu T, Yang L, Mao H, Ma F, Wang Y, Zhan Y. Knowledge domain and emerging trends in podocyte injury research from 1994 to 2021: A bibliometric and visualized analysis. Front Pharmacol (2021) 12:772386. doi: 10.3389/fphar.2021.772386
15. Wu YY, Li CC, Lin X, Xu F, Shan SK, Guo B, et al. Global publication trends and research trends of necroptosis application in tumor: A bibliometric analysis. Front Pharmacol (2023) 14:1112484. doi: 10.3389/fphar.2023.1112484
16. Garfield E, Paris SW, Stock WG. HistCite: A software tool for informetric analysis of citation linkage. NFD Information-Wissenschaft und Praxis (2006) 57(8):391–400.
17. van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics (2010) 84(2):523–38. doi: 10.1007/s11192-009-0146-3
18. Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci United States America (2004) 101 Suppl 1(Suppl 1):5303–10. doi: 10.1073/pnas.0307513100
19. Chen C, Song M. Visualizing a field of research: A methodology of systematic scientometric reviews. PloS One (2019) 14(10):e0223994. doi: 10.1371/journal.pone.0223994
20. Lin SJ, Chang LY, Yan DC, Huang YJ, Lin TJ, Lin TY. Decreased intercellular adhesion molecule-1 (CD54) and L-selectin (CD62L) expression on peripheral blood natural killer cells in asthmatic children with acute exacerbation. Allergy (2003) 58(1):67–71. doi: 10.1034/j.1398-9995.2003.t01-1-23697.x
21. Zaiss DMW, Pearce EJ, Artis D, McKenzie ANJ, Klose CSN. Cooperation of ILC2s and TH2 cells in the expulsion of intestinal helminth parasites. Nature reviews. Immunology (2023). doi: 10.1038/s41577-023-00942-1
22. Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, et al. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science (2010) 330(6004):665–9. doi: 10.1126/science.1194597
23. Cardoso V, Chesné J, Ribeiro H, García-Cassani B, Carvalho T, Bouchery T, et al. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature (2017) 549(7671):277–81. doi: 10.1038/nature23469
24. Klose CSN, Mahlakõiv T, Moeller JB, Rankin LC, Flamar AL, Kabata H, et al. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature (2017) 549(7671):282–6. doi: 10.1038/nature23676
25. Castriconi R, Daga A, Dondero A, Zona G, Poliani PL, Melotti A, et al. NK cells recognize and kill human glioblastoma cells with stem cell-like properties. J Immunol (Baltimore Md. 1950) (2009) 182(6):3530–9. doi: 10.4049/jimmunol.0802845
26. Li Z, Sun G, Sun G, Cheng Y, Wu L, Wang Q, et al. Various uses of PD1/PD-L1 inhibitor in oncology: opportunities and challenges. Front Oncol (2021) 11:771335. doi: 10.3389/fonc.2021.771335
27. Chamoto K, Yaguchi T, Tajima M, Honjo T. Insights from a 30-year journey: function, regulation and therapeutic modulation of PD1. Nat Rev Immunol (2023) 23(10):682–95. doi: 10.1038/s41577-023-00867-9
28. Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol (2016) 17(7):765–74. doi: 10.1038/ni.3489
29. Tsou AM, Yano H, Parkhurst CN, Mahlakõiv T, Chu C, Zhang W, et al. Neuropeptide regulation of non-redundant ILC2 responses at barrier surfaces. Nature (2022) 611(7937):787–93. doi: 10.1038/s41586-022-05297-6
30. Jarick KJ, Topczewska PM, Jakob MO, Yano H, Arifuzzaman M, Gao X, et al. Non-redundant functions of group 2 innate lymphoid cells. Nature (2022) 611(7937):794–800. doi: 10.1038/s41586-022-05395-5
31. Halim TY, Steer CA, Mathä L, Gold MJ, Martinez-Gonzalez I, McNagny KM, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity (2014) 40(3):425–35. doi: 10.1016/j.immuni.2014.01.011
32. Hsu AT, Gottschalk TA, Tsantikos E, Hibbs ML. The role of innate lymphoid cells in chronic respiratory diseases. Front Immunol (2021) 12:733324. doi: 10.3389/fimmu.2021.733324
33. Ham J, Lim M, Kim D, Kim HY. Memory-like innate lymphoid cells in the pathogenesis of asthma. Front Immunol (2022) 13:1005517. doi: 10.3389/fimmu.2022.1005517
34. Bartemes KR, Kita H. Roles of innate lymphoid cells (ILCs) in allergic diseases: The 10-year anniversary for ILC2s. J Allergy Clin Immunol (2021) 147(5):1531–47. doi: 10.1016/j.jaci.2021.03.015
35. Sadeghi Hassanabadi N, Broux B, Marinović S, Gotthardt D. Innate lymphoid cells - neglected players in multiple sclerosis. Front Immunol (2022) 13:909275. doi: 10.3389/fimmu.2022.909275
36. Beliën J, Goris A, Matthys P. Natural killer cells in multiple sclerosis: entering the stage. Front Immunol (2022) 13:869447. doi: 10.3389/fimmu.2022.869447
37. Yano H, Artis D. Neuronal regulation of innate lymphoid cell responses. Curr Opin Immunol (2022) 76:102205. doi: 10.1016/j.coi.2022.102205
38. Ibiza S, García-Cassani B, Ribeiro H, Carvalho T, Almeida L, Marques R. Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature (2016) 535(7612):440–3. doi: 10.1038/nature18644
39. Moriyama M, Fukuyama S, Inoue H, Matsumoto T, Sato T, Tanaka K. The neuropeptide neuromedin U activates eosinophils and is involved in allergen-induced eosinophilia. Am J Physiol Lung Cell Mol Physiol (2016) 290(5):L971–7. doi: 10.1152/ajplung.00345.2005
40. Moriyama M, Sato T, Inoue H, Fukuyama S, Teranishi H, Kangawa K, et al. The neuropeptide neuromedin U promotes inflammation by direct activation of mast cells. J Exp Med (2015) 202(2):217–24. doi: 10.1084/jem.20050248
41. Domingues RG, Hepworth MR. Immunoregulatory sensory circuits in group 3 innate lymphoid cell (ILC3) function and tissue homeostasis. Front Immunol (2020) 11:116. doi: 10.3389/fimmu.2020.00116
42. Melo-Gonzalez F, Hepworth MR. Functional and phenotypic heterogeneity of group 3 innate lymphoid cells. Immunology (2017) 150(3):265–75. doi: 10.1111/imm.12697
43. Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity (2008) 29(6):958–70. doi: 10.1016/j.immuni.2008.11.001
44. Geremia A, Arancibia-Cárcamo CV, Fleming MP, Rust N, Singh B, Mortensen NJ, et al. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med (2011) 208(6):1127–33. doi: 10.1084/jem.20101712
45. Coman D, Coales I, Roberts LB, Neves JF. Helper-like type-1 innate lymphoid cells in inflammatory bowel disease. Front Immunol (2022) 13:903688. doi: 10.3389/fimmu.2022.903688
46. Saez A, Gomez-Bris R, Herrero-Fernandez B, Mingorance C, Rius C, Gonzalez-Granado JM. Innate lymphoid cells in intestinal homeostasis and inflammatory bowel disease. Int J Mol Sci (2021) 22(14):7618. doi: 10.3390/ijms22147618
47. Zebboudj A, Amisaki M, Balachandran VP. ILC2s-Bipartisan politicians in cancer. Sci Immunol (2022) 7(72):eabq2791. doi: 10.1126/sciimmunol.abq2791
48. Huang Q, Cao W, Mielke LA, Seillet C, Belz GT, Jacquelot N. Innate lymphoid cells in colorectal cancers: A double-edged sword. Front Immunol (2020) 10:3080. doi: 10.3389/fimmu.2019.03080
49. Jacquelot N, Seillet C, Vivier E, Belz GT. Innate lymphoid cells and cancer. Nat Immunol (2022) 23(3):371–9. doi: 10.1038/s41590-022-01127-z
50. Corvino D, Kumar A, Bald T. Plasticity of NK cells in cancer. Front Immunol (2022) 13:888313. doi: 10.3389/fimmu.2022.888313
51. López-Soto A, Gonzalez S, Smyth MJ, Galluzzi L. Control of metastasis by NK cells. Cancer Cell (2017) 32(2):135–54. doi: 10.1016/j.ccell.2017.06.009
52. Coca S, Perez-Piqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C, et al. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer (1997) 79(12):2320–8. doi: 10.1002/(SICI)1097-0142(19970615)79:12<2320::AID-CNCR5>3.0.CO;2-P
53. Liu S, Dhar P, Wu JD. NK cell plasticity in cancer. J Clin Med (2019) 8(9):1492. doi: 10.3390/jcm8091492
54. Saranchova I, Han J, Zaman R, Arora H, Huang H, Fenninger F, et al. Type 2 innate lymphocytes actuate immunity against tumours and limit cancer metastasis. Sci Rep (2018) 8(1):2924. doi: 10.1038/s41598-018-20608-6
55. Ikutani M, Yanagibashi T, Ogasawara M, Tsuneyama K, Yamamoto S, Hattori Y. Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. J Immunol (2012) 188(2):703–13. doi: 10.4049/jimmunol.1101270
56. Jacquelot N, Seillet C, Wang M, Pizzolla A, Liao Y, Hediyeh-Zadeh S, et al. Blockade of the co-inhibitory molecule PD-1 unleashes ILC2-dependent antitumor immunity in melanoma. Nat Immunol (2021) 22(7):851–64. doi: 10.1038/s41590-021-00943-z
57. Moral JA, Leung J, Rojas LA, Ruan J, Zhao J, Sethna Z, et al. ILC2s amplify PD-1 blockade by activating tissue-specific cancer immunity. Nature (2020) 579(7797):130–5. doi: 10.1038/s41586-020-2015-4
58. Schuijs MJ, Png S, Richard AC, Tsyben A, Hamm G, Stockis J, et al. ILC2-driven innate immune checkpoint mechanism antagonizes NK cell antimetastatic function in the lung. Nat Immunol (2020) 21(9):998–1009. doi: 10.1038/s41590-020-0745-y
59. Jou E, Rodriguez-Rodriguez N, Ferreira AF, Jolin HE, Clark PA, Sawmynaden K, et al. An innate IL-25-ILC2-MDSC axis creates a cancer-permissive microenvironment for Apc mutation-driven intestinal tumorigenesis. Sci Immunol (2022) 7(72):eabn0175. doi: 10.1126/sciimmunol.abn0175
60. Lopes N, Vivier E, Narni-Mancinelli E. Natural killer cells and type 1 innate lymphoid cells in cancer. Semin Immunol (2023) 66:101709. doi: 10.1016/j.smim.2022.101709
61. Ducimetière L, Lucchiari G, Litscher G, Nater M, Heeb L, Nuñez NG, et al. Conventional NK cells and tissue-resident ILC1s join forces to control liver metastasis. Proc Natl Acad Sci United States America (2021) 118(27):e2026271118. doi: 10.1073/pnas.2026271118
Keywords: bibliometric analysis, ILCs, brain-gut-lung axis, CiteSpace, VOSviewer, HistCite
Citation: Huang J, Deng K, Liu Y, Xia M, Lei M and Wu M (2024) Global research trends on innate lymphoid cells in the brain, gut and lung field: a bibliometric and visualized analysis. Front. Immunol. 15:1336666. doi: 10.3389/fimmu.2024.1336666
Received: 11 November 2023; Accepted: 17 January 2024;
Published: 07 February 2024.
Edited by:
Cyril Seillet, The University of Melbourne, AustraliaReviewed by:
Kapil Sirohi, National Jewish Health, United StatesCopyright © 2024 Huang, Deng, Liu, Xia, Lei and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minghua Wu, d3VtaW5naHVhNTU0QGFsaXl1bi5jb20=; Mingsheng Lei, bWluZ3NoZW5nbGVpQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.