
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 13 February 2024
Sec. Alloimmunity and Transplantation
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1335148
Introduction: Kidney transplant recipients (KTRs) are at a higher risk of severe coronavirus disease (COVID-19) because of their immunocompromised status. However, the effect of allograft function on the prognosis of severe COVID-19 in KTRs is unclear. In this study, we aimed to analyze the correlation between pre-infection allograft function and the prognosis of severe COVID-19 in KTRs.
Methods: This retrospective cohort study included 82 patients who underwent kidney transplantation at the Sichuan Provincial Peoples Hospital between October 1, 2014 and December 1, 2022 and were diagnosed with severe COVID-19. The patients were divided into decreased eGFR and normal eGFR groups based on the allograft function before COVID-19 diagnosis (n=32 [decreased eGFR group], mean age: 43.00 years; n=50 [normal eGFR group, mean age: 41.88 years). We performed logistic regression analysis to identify risk factors for death in patients with severe COVID-19. The nomogram was used to visualize the logistic regression model results.
Results: The mortality rate of KTRs with pre-infection allograft function insufficiency in the decreased eGFR group was significantly higher than that of KTRs in the normal eGFR group (31.25% [10/32] vs. 8.00% [4/50], P=0.006). Pre-infection allograft function insufficiency (OR=6.96, 95% CI: 1.4633.18, P=0.015) and maintenance of a mycophenolic acid dose >1500 mg/day before infection (OR=7.59, 95% CI: 1.0853.20, P=0.041) were independent risk factors, and the use of nirmatrelvir/ritonavir before severe COVID-19 (OR=0.15, 95% CI: 0.030.72, P=0.018) was a protective factor against death in severe COVID-19.
Conclusions: Pre-infection allograft function is a good predictor of death in patients with severe COVID-19. Allograft function was improved after treatment for severe COVID-19, which was not observed in patients with non-severe COVID-19.
The coronavirus disease (COVID-19) pandemic continues to pose a significant health risk to people worldwide (1), particularly kidney transplant recipients (KTRs) who are at a higher risk of severe COVID-19 because of their immunocompromised status. Allograft function (AF) plays an important role in severe COVID-19 in KTRs. A study conducted in Spain demonstrated that impaired AF increased the risk of intensive care unit admission and was a predictor of mortality (2), and it is important to determine whether this similar effect is observed on severe COVID-19. Notably, the kidney is an angiotensin-converting enzyme 2 (ACE2) receptor organ (3, 4), causing it to have a high affinity for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19 (5). The kidney-lung crosstalk theory suggests that lung and kidney damage mutually worsen each others function (6, 7). However, the effect of AF on the prognosis of severe COVID-19 in KTRs remains unclear. Therefore, investigating the prognostic role of pre-infection AF in severe COVID-19 among KTRs is crucial.
Another point of concern is the impact of severe COVID-19 on AF. On the one hand, severe COVID-19 is associated with an increased risk of acute kidney injury (AKI) (8), with a total incidence rate of up to 8% (9). The occurrence of AKI in severe COVID-19 involves various mechanisms, including a systemic inflammatory response, viral infection of renal cells, and severe hemodynamic changes in the kidneys (10), which may damage AF. The incidence of AKI is significantly higher in KTRs than in the general population (11, 12). On the other hand, severe COVID-19 often requires immunosuppressant (IS) drug discontinuation, which increases the risk of subsequent acute rejection (AR) and impairs AF. In addition, the potential effects of small-molecule antivirals, such as nirmatrelvir/ritonavir, on AF during treatment are poorly understood. The question on whether nirmatrelvir/ritonavir exacerbates AF impairment, particularly in patients with impaired AF before infection, requires urgent attention.
In this study, we aimed to utilize AF before, during, and after SARS-CoV-2 infection as indicators to explore the relationship between pre-infection AF and the outcome of severe COVID-19 and determine the factors influencing functional changes in AF during and after infection. We hope to offer valuable insights for future clinical decision-making.
In this retrospective cohort study, we included KTRs who underwent kidney transplantation at the Sichuan Provincial Peoples Hospital between October 1, 2014 and December 1, 2022 and were diagnosed with severe COVID-19. KTRs who died or had renal allograft loss before December 1, 2022 or during the follow-up were excluded. Based on the prevailing period of COVID-19 wave from December 1, 2022 to early February 2023, our follow-up started on December 1, 2022 and ended on April 1, 2023 or at the time of death, whichever came first. According to the 10th Trial Edition of the Guidelines for the Diagnosis and Treatment of COVID-19 (13), severe COVID-19 was diagnosed when adults meet any of the following conditions that cannot be explained for reasons other than COVID-19: 1) shortness of breath or respiratory rate ≥30 times/min; 2) oxygen saturation ≤93% at rest; 3) arterial partial pressure of oxygen/oxygen uptake concentration ≤300 mmHg (1 mmHg=0.133 kPa), and 4) gradual aggravation of clinical symptoms and lung imaging showing significant lesion progression (>50%) within 24-48 h. Based on the AF before COVID-19 diagnosis, AF insufficiency is defined as estimated glomerular filtration rate (eGFR) <60 mL/min, according to the KDIGO guidelines (14). We then divided the patients into decreased eGFR and normal eGFR group, with a cutoff value of estimated glomerular filtration rate <60 mL/min or ≥60 mL/min, respectively.
Kidney allografts from living or deceased organ donors who met the ethical guidelines for kidney donation were used. None of the KTRs received organs from executed prisoners or other institutionalized individuals. This study adhered to the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of the Sichuan Provincial Peoples Hospital (No. 20220901).
Baseline characteristics of the KTRs, including age, sex, body mass index (BMI), donor type, human leukocyte antigen (HLA) mismatch, primary kidney disease, and comorbidities, were obtained from a scientific registry of the kidney transplantation system (https://www.csrkt.org.cn/door/index). Medical history was obtained through in-patient information collection, outpatient services, online outpatient services, and telephone follow-ups. Clinical data were obtained from medical records, including creatinine (Cr) values 6 months before and 1 and 2 months after infection, vaccination, IS regimen, hospitalization, and COVID-19-related treatment.
Oral IS medications were discontinued in all patients. The general treatment for COVID-19 included ensuring adequate energy and nutrient intake and paying attention to water and electrolyte balance. The principle of supportive treatment for severe COVID-19 involved actively preventing and treating complications, treating basic diseases, preventing secondary infections, and providing timely organ function support. Respiratory support treatments were selected based on the patients oxygenation index, including nasal catheter or mask oxygen inhalation (PaO2/FiO2 <300 mmHg), nasal high-flow oxygen therapy, noninvasive ventilation (PaO2/FiO2 <200 mmHg), invasive mechanical ventilation (PaO2/FiO2 <150 mmHg), and oxygen therapy during resuscitation to target SpO2 ≥94% in KTRs with emergency signs. Once the patient was stable, SpO2 >90% was targeted. Airway management and sputum discharge were facilitated to maintain airway patency.
Nirmatrelvir/ritonavir (Pfizer, USA), azvudine (Henan Zhenzhen Biotechnology, China), and molnupiravir (Merck, USA) are recommended by the National Health Commission for COVID-19 treatment. Ritonavir, a component of nirmatrelvir/ritonavir, is a potent inhibitor of cytochrome P450 3A and P-glycoproteins. After obtaining informed consent from the patients, azvudine and nirmatrelvir/ritonavir were administered to treat KTRs infected with severe COVID-19. Therapeutic drug monitoring was continued during nirmatrelvir/ritonavir treatment, and the restart dose after nirmatrelvir/ritonavir treatment was adjusted accordingly.
The primary outcome was all-cause mortality, defined as mortality from various causes during the study period. Other outcomes were mainly related to allograft complications. AR is defined as the rapid deterioration of function caused by specific pathological changes in the allograft and can be divided into acute T cell-mediated rejection (TCMR) and acute antibody-mediated rejection (ABMR). BK polyomavirus (BKPyV) infection is mostly covert; however, its reactivation can occur in patients with impaired immune function, eventually leading to BKPyV-associated nephropathy (BKPyVAN). HLA is closely related to functioning of the human immune system and is an important antigenic substance in transplant rejection. Donor-specific antibodies (DSA) are specific antibodies the recipient produces after organ/tissue transplantation against donor tissue antigens, including HLA and non-HLA antibodies.
Continuous variables are presented as median and interquartile intervals (IQRs) and were analyzed using a t-test or MannWhitney U test. Categorical variables are reported as frequency counts and percentages and were evaluated using the chi-squared or Fisher exact test. Multivariate logistic regression analysis was used to identify the risk factors for death due to severe COVID-19. The results are reported as odds ratios (ORs), 95% confidence intervals (CIs), and P-values. Cox regression was used to construct the final nomogram prediction model. Statistical analyses were performed using GraphPad Prism 8.0 and R version 4.0.3. All tests were two-tailed, and P-values <0.05 were considered statistically significant.
In total, 926 patients underwent kidney transplantation between October 1, 2014 and December 1, 2022, and 82 KTRs were included in this study. Of these, 32 were in the decreased eGFR group, and 50 were in the normal eGFR group (Figure 1), and we compared the baseline characteristics between the groups (Table 1). The mean age of patients in the decreased eGFR and normal eGFR group was 43.00 ± 10.6 years and 41.88 ± 8.72 years, respectively (P=0.60). Compared with that in the decreased eGFR group, the proportion of complete vaccination was significantly higher in the normal eGFR group (7.32% [6/82] vs. 9.76% [8/82], P=0.047). The proportion of mortality was significantly higher in the decreased eGFR group than in the normal eGFR group (31.25% [10/32] vs. 8.00% [4/50], P=0.006). However, no significant differences were observed in the patients ages, sexes, comorbidities, primary disease, HLA mismatch, vaccine doses, induction agents, IS regimen adjustment, and donor type between the decreased eGFR and normal eGFR groups.
To further explore the risk factors for death in severe COVID-19, we used the univariate and multivariate logistic regression model to analyze the risk factors (Table 2). The univariate analysis revealed that co-infection with pulmonary aspergillosis (OR=5.24, 95% CI: 1.3420.51, P=0.017), pre-infection AF insufficiency (OR=5.23, 95% CI: 1.4718.54, P=0.010), maintenance of a mycophenolic acid (MPA) dose >1500 mg/day before infection (OR=7.000, 95% CI: 1.6929.04, P=0.007), and the use of mechanical ventilation (OR=4.33, 95% CI: 1.3114.35, P=0.016) were significant risk factors death in severe COVID-19. We included covariates with P<0.1 in the univariate analysis and significant clinical variables in the multivariate analysis. The results showed that only AF insufficiency (OR=6.96, 95% CI: 1.4633.18, P=0.015) and maintenance of an MPA dose >1500 mg/day before infection (OR=7.59, 95% CI: 1.0853.20, P=0.041) were significant risk factors for death in severe COVID-19 infection, whereas the use of nirmatrelvir/ritonavir before severe COVID-19 diagnosis (OR = 0.21, 95% CI: 0.041.00, P=0.049) was a protective factor. Concomitant Aspergillus infection (OR=3.70, 95% CI: 0.7119.15, P=0.12) and mechanical ventilation (OR=2.76, 95% CI: 0.5713.49, P=0.21) were not significant risk factors (Table 2).
Regarding the prognosis of patients with severe COVID-19, we generated a nomogram based on variables included in the multivariate model (Figure 2). Each variable was assigned a score ranging from 0 to 100, and the total scores of all variables were added to estimate mortality.
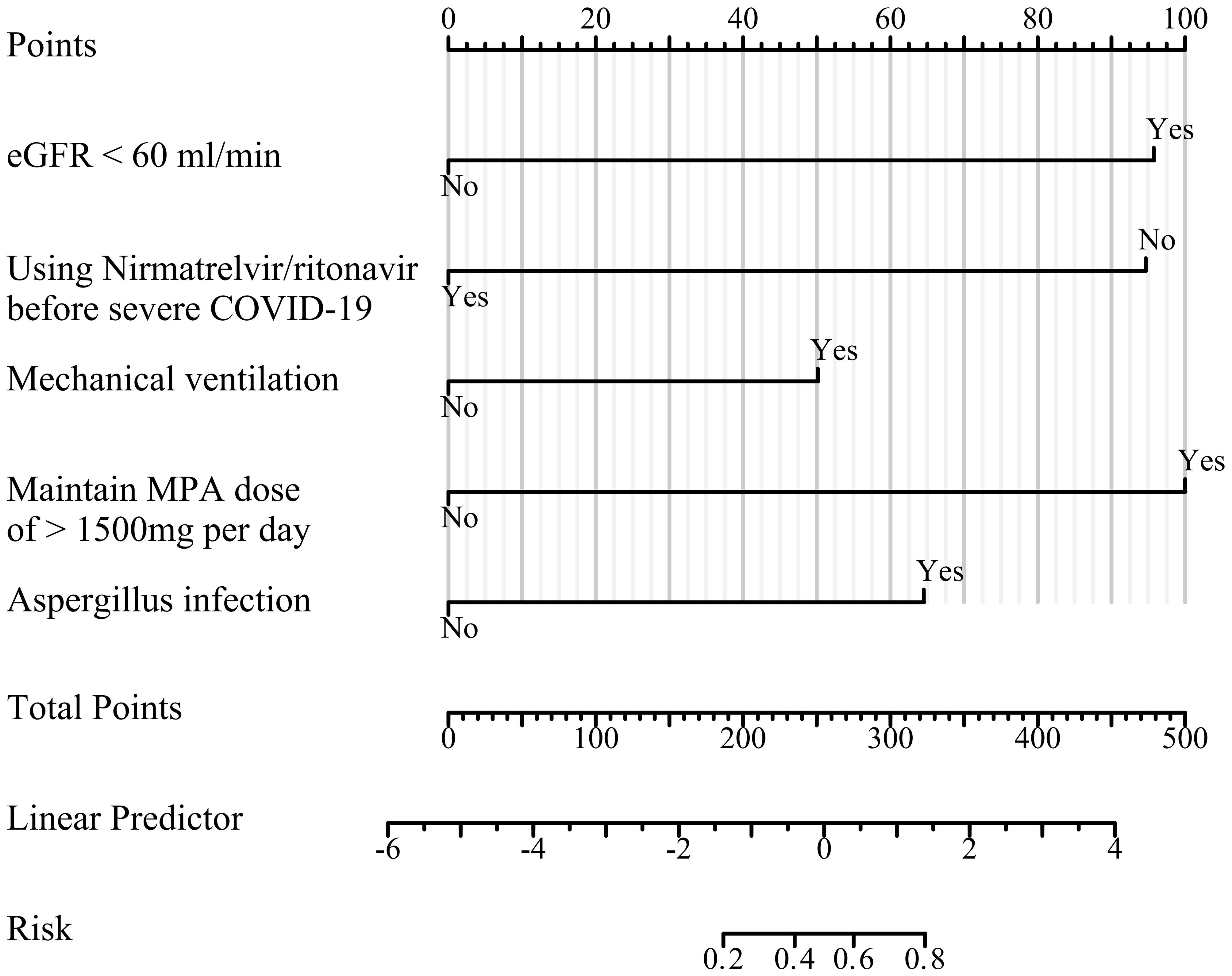
Figure 2 Prediction model nomogram. For each variable, the patients status value is plotted on the unique scale for that variable, and a vertical line is drawn from that location to the points line to determine a point value for that variable. The points for all variables are then added for a total point score.
During the study period, 16 KTRs received azvudine, whereas 24 received nirmatrelvir/ritonavir. Among the surviving KTRs, 29.03% (9/31) had pre-infection AF insufficiency, with 9.68% (3/31) and 19.35% (6/31) of them receiving azvudine and nirmatrelvir/ritonavir, respectively (Figures 3A, C). However, the results indicated that AF did not undergo irreversible damage following the administration of small-molecule antivirals (Figure 3).
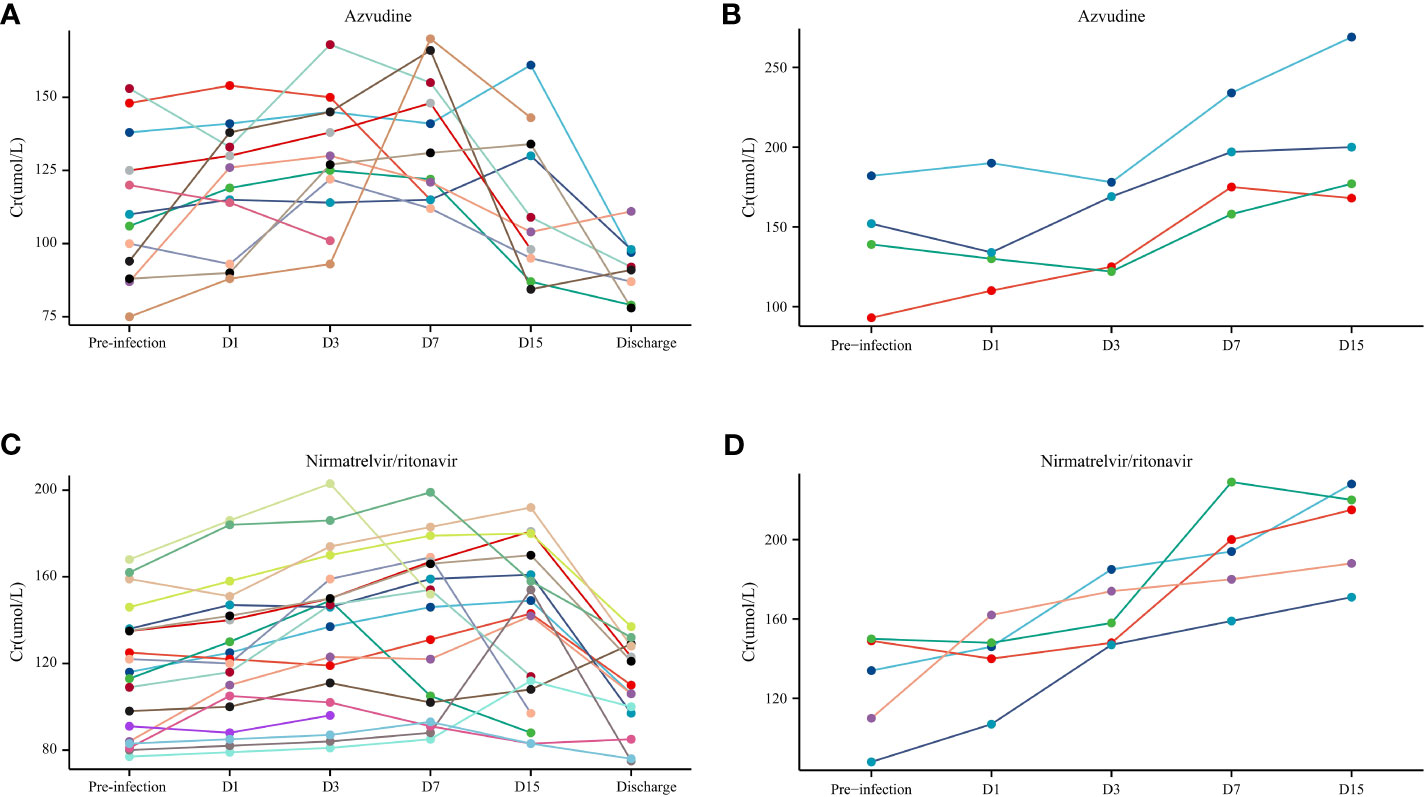
Figure 3 The allograft function (Cr) in patients with coronavirus disease (COVID-19) who used small-molecule antivirals. (A) Azvudine non-death group. (B) Azvudine death group. (C) Nirmatrelvir/ritonavir non-death group. (D) Nirmatrelvir/ritonavir death group.
Comparison of AF before and after COVID-19 diagnosis revealed that AF was significantly better 1 month after infection than before infection in the decreased eGFR (P<0.05) and normal eGFR (P<0.01) groups; however, there was no difference before and 2 months after infection (Figures 4A, B). We found no difference in AF before infection and one or two months after infection in the non-severe COVID-19 group (Figure 4C).
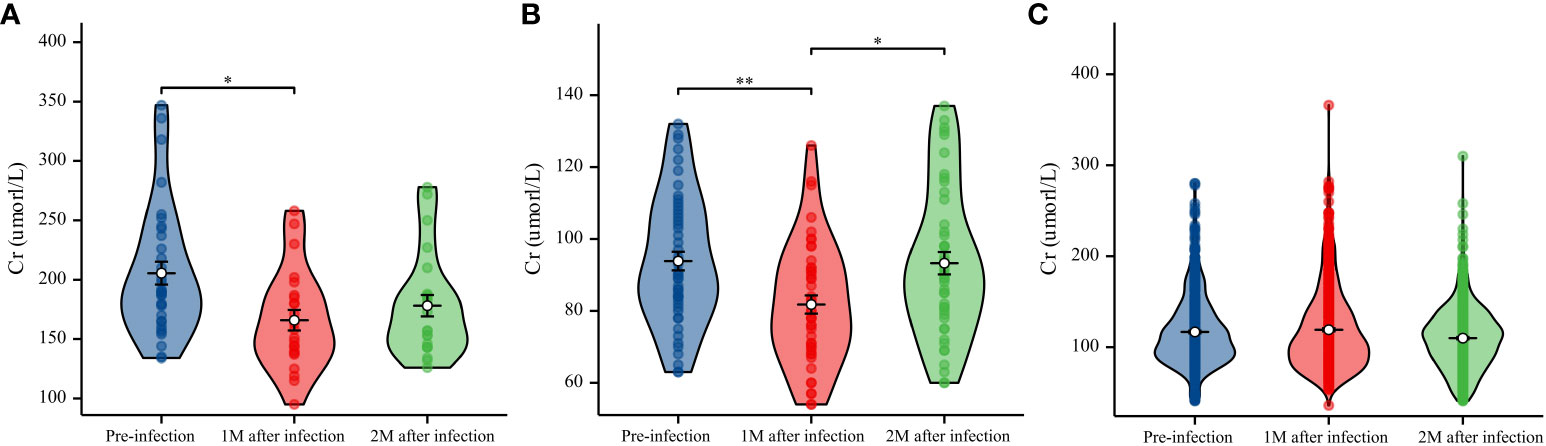
Figure 4 Data on creatinine (Cr) (μmol/L) levels were collected before infection and 1 and 2 months after infection. (A) Boxplot with bold line represents the median Cr (μmol/L) level in the decreased eGFR group. (B) Boxplot with bold line represents the median Cr (μmol/L) level in the normal eGFR group. (C) Boxplot with bold line represents the median Cr (μmol/L) level in the non-severe COVID-19 group. *P<0.05; **P<0.01.
In the entire study population, no statistical difference was observed in complications related to allograft, including TCMR, urine BKPyV DNA load >8 log10, neo-HLA, neo-DSA before and after severe COVID-19 (2.44% [2/82] vs. 6.10% [5/82], P=0.44; 3.66% [3/82] vs. 9.76% [8/82], P=0.119; 3.66% [3/82] vs. 7.32% [6/82], P=0.49; 1.22% [1/82] vs. 2.44% [2/82], P=1.000; respectively). Similarly, there were no differences in complications unrelated to allograft, including lung superinfection, thrombus, and abnormal glucose levels (1.22% [1/82] vs. 1.22% [1/82], P=1.000; 2.44% [2/82] vs. 4.88% [4/82], P=0.677; 4.88% [4/82] vs. 7.32% [6/82], P=0.514; respectively). Notably, weight (61.79 [50.0965.84] vs. 56.71 [49.9660.93], P<0.001), hip circumference (92.90 [87.9197.61] vs. 91.13 [87.4494.97], P=0.03), and BMI (21.85 [20.6023.79] vs. 20.25 [18.5922.24], P<0.001) after severe COVID-19 were significantly lower than those before infection (Table 3).

Table 3 Other complications of kidney transplant recipients with severe COVID-19 before and after COVID-19 diagnosis.
In this study, by constructing a multivariate logistic regression analysis, we found that pre-infection AF insufficiency was an independent risk factor for death in patients with severe COVID-19. AF insufficiency alters the homeostasis of fluid balance, electrolyte balance, and vascular tension, thereby exacerbating pulmonary infection (15). In addition, AF insufficiency can cause renal anemia and hypoproteinemia, leading to decreased immunity, which can increase the risk of death in an individual with COVID-19 (16). AF insufficiency can also lead to systemic damage, including dysfunction of the brain, heart, liver, and intestines (17, 18) and increased susceptibility to sepsis. Patients with chronic kidney disease and renal insufficiency have a significantly increased risk of death from severe COVID-19 (19), which is 10 times higher than that of patients with healthy renal function (20). This phenomenon was consistent in the KTRs. Nevertheless, our findings are not entirely consistent with the conclusions of a multicenter retrospective study (21). In contrast to previous studies, our analysis was based on pre-infection AF insufficiency, which was not affected by the SARS-CoV-2 infection. Therefore, for KTRs with pre-infection AF insufficiency, close attention should be paid to the changes following COVID-19. Interestingly, using nirmatrelvir/ritonavir before severe COVID-19 diagnosis reduced the risk of death in KTRs because nirmatrelvir/ritonavir can effectively inhibit SARS-CoV-2 (22). In addition, maintaining an MPA dose >1500 mg/day before severe COVID-19 and comorbidity with Aspergillus infection were risk factors for death in KTRs with severe COVID-19, similar to the findings of some studies (22, 23).
The kidney is one of the most common target organs of SARS-CoV-2 infection, and the incidence of AKI is considerably higher in KTRs than in the general population (11, 12). This raises one of the most concerning topics: Will small-molecule antiviral drugs exacerbate COVID-19-induced renal injury? Before answering this question, we should consider arguments regarding whether coronaviruses directly attack allografts. Su et al. (3) reported that autopsies of patients with COVID-19 revealed viral particles in the tubular epithelium and podocytes of the kidneys, which are ACE2-expressing cells, suggesting that severe COVID-19 significantly impacts the kidneys. Conversely. Golmai et al. (24) performed kidney autopsies on patients with COVID-19 diagnosed with stage 2 or 3 AKI; however, SARS-CoV-2 was not detected by immunohistochemistry. If the virus does not attack the kidney directly via an inflammatory response, drug-induced renal injury resulting from antiviral medications can exacerbate its impact on the allograft. Nirmatrelvir/ritonavir and azvudine are recommended antiviral drugs for COVID-19 treatment. As a reverse transcriptase inhibitor, azvudine shortens the nucleic acid negative conversion time (25), and nirmatrelvir/ritonavir can effectively inhibit SARS-CoV-2, significantly reducing the viral load in patients and thus decreasing the risk of death (22). However, in severe COVID-19, the effects of small-molecule antiviral drugs are still unclear, with no data on whether they exacerbate AF impairment. In our study, we found that the use of two small-molecule antiviral drugs in KTRs with severe COVID-19 did not further worsen AF impairment. Similarly, Toussi et al. (26) demonstrated that the safety of nirmatrelvir/ritonavir in patients with renal impairment was similar to that of azvudine (27). Adverse reactions during COVID-19 treatment require further exploration in future studies.
Another question we sought to clarify is whether AF will recover after severe COVID-19. Interestingly, we observed an improvement in AF 1 month after treatment for severe COVID-19 compared with that before infection. This phenomenon was also reported in a study from Italy, in which hospitalized KTRs had better AF after discharge than before infection, with no difference in non-hospitalized KTRs (28). However, this study did not specify whether the phenomenon occurred in patients with non-severe or severe COVID-19. Our data show that this paradox typically occurs in KTRs with severe COVID-19. Calcineurin inhibitors (tacrolimus and cyclosporine) are associated with AF impairment in KTRs (29). Thus, discontinuing calcineurin inhibitors in KTRs with severe COVID-19 may improve AF (30). Additionally, Cr is related to systemic nutritional status, and severe COVID-19 is a systemic-wasting disease that often causes malnutrition, which explains the temporary improvement in AF (31). For example, in our study, BMI, hip circumference, and weight were significantly lower after severe COVID-19 treatment conclusion than before the infection. A slightly worse AF at 2 months than at 1 month after treatment for severe COVID-19 confirms our conjecture. In addition, the occurrence of allograft-related complications after infection, such as TCMR, BKPyV, neo-HLA, and neo-DSA, indicates that the improvement in AF was temporary.
Our study had some limitations. First, this was a retrospective study, which inevitably involved information bias and potential confounding factors. Second, this was a single-center study with a limited sample size, and the results require further verification using large-sample multicenter research. Third, we did not confirm the variant of SARS-CoV-2 infection by conducting a specific PCR but instead used an antigen test paper or SARS-CoV-2 PCR.
In conclusion, this is the first report of a correlation between pre-infection AF insufficiency and mortality in patients with severe COVID-19. Pre-infection AF was a good predictor of death in KTRs with severe COVID-19. Additionally, an MPA dose >1500 mg/day before infection, non-use of nirmatrelvir/ritonavir before severe COVID-19, use of mechanical ventilation, and co-infection with pulmonary aspergillosis were associated with death in KTRs with severe COVID-19. AF was improved after the treatment of severe COVID-19, whereas this effect was not detected in non-severe COVID-19.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Committee of the Sichuan Provincial Peoples Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
HL: Writing – original draft, Conceptualization, Data curation. JW: Data curation, Methodology, Writing – original draft, Funding acquisition. HY: Data curation, Writing – review & editing. QR: Data curation, Supervision, Writing – review & editing. YH: Data curation, Project administration, Writing – original draft, Funding acquisition.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study has received funding from the Sichuan Province Science and Technology Support Program (no. 2022YFS0093), Cadre Health Care in Sichuan Province (no. 2023-211) and Youth Fund of Sichuan Provincial Peoples Hospital (no. 2022QN26).
The authors thank Sichuan Provincial Peoples Hospital, University of Electronic Science and Technology of China, for their support of this work and the reviewers for allowing us to make improvements to the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ABMR, acute antibody-mediated rejection; ACE2, angiotensin-converting enzyme 2; AKI, incidence of acute kidney injury; BKPyV, BK polyomavirus; BKPyVAN, BK polyomavirus-associated nephropathy; CI, confidence interval; COVID-19, coronavirus 2019; Cr, creatinine; DSA, donor-specific antibody; HLA, human leukocyte antigen; IQR, interquartile range; IS, immunosuppressant; KTRs, kidney transplant recipients; MPA, mycophenolic acid; OR, odds ratio; ROC, receiver operating characteristic; TCMR, T cell-mediated rejection.
1. Vinson AJ, Chong AS, Clegg D, Falk C, Foster BJ, Halpin A, et al. Sex matters: COVID-19 in kidney transplantation. Kidney Int. (2021) 99(3):555–58. doi: 10.1016/j.kint.2020.12.020
2. Sacristan PG, Garcia EC, Perez EBP, Marfil AP, Sanchez MJT, Moratalla JMO, et al. Risk of severe coronavirus disease 2019 infection in kidney transplant recipients. Transplant Proc. (2022) 54(1):18–21. doi: 10.1016/j.transproceed.2021.08.060
3. Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. (2020) 98(1):219–27. doi: 10.1016/j.kint.2020.04.003
4. Puelles VG, Lutgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. (2020) 383(6):590–92. doi: 10.1056/NEJMc2011400
5. Jayant K, Reccia I, Bachul PJ, Al-Salmay Y, Pyda JS, Podda M, et al. The impact of COVID-19 on kidney transplant recipients in pre-vaccination and delta strain era: A systematic review and meta-analysis. J Clin Med. (2021) 10(19). doi: 10.3390/jcm10194533
6. Bao PL, Deng KL, Yuan AL, Yan YM, Feng AQ, Li T, et al. Early renal impairment is associated with in-hospital death of patients with COVID-19. Clin Respir J. (2022) 16(6):441–49. doi: 10.1111/crj.13496
7. Teixeira JP, Ambruso S, Griffin BR, Faubel S. Pulmonary consequences of acute kidney injury. Semin Nephrol. (2019) 39(1):3–16. doi: 10.1016/j.semnephrol.2018.10.001
8. Braun F, Lutgehetmann M, Pfefferle S, Wong MN, Carsten A, Lindenmeyer MT, et al. SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet. (2020) 396(10251):597–98. doi: 10.1016/S0140-6736(20)31759-1
9. Jafari-Oori M, Fiorentino M, Castellano G, Ebadi A, Rahimi-Bashar F, Guest PC, et al. Acute kidney injury and Covid-19: A scoping review and meta-analysis. In: Clinical, Biological and Molecular Aspects of COVID-19. Adv Exp Med Biol (2021) 1321:309–24. doi: 10.1007/978-3-030-59261-5_28
10. Fiorentino M, Bagagli F, Deleonardis A, Stasi A, Franzin R, Conserva F, et al. Acute kidney injury in kidney transplant patients in intensive care unit: from pathogenesis to clinical management. Biomedicines. (2023) 11(5). doi: 10.3390/biomedicines11051474
11. Shrivastava P, Prashar R, Khoury N, Patel A, Yeddula S, Kitajima T, et al. Acute kidney injury in a predominantly African American cohort of kidney transplant recipients with COVID-19 infection. Transplantation. (2021) 105(1):201–05. doi: 10.1097/TP.0000000000003498
12. Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. (2020) 31(16):1157–65. doi: 10.1681/ASN.2020030276
13. Available online at: http://www.nhc.gov.cn/ylyjs/pqt/202301/32de5b2ff9bf4eaa88e75bdf7223a65a.shtml.
14. Levey AS, Eckardt K-U, Dorman NM, Christiansen SL, Hoorn EJ, Ingelfinger JR, et al. Nomenclature for kidney function and disease: report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. (2020) 97(6):1117–29. doi: 10.1016/j.kint.2020.02.010
15. Basu RK, Wheeler DS. Kidney-lung cross-talk and acute kidney injury. Pediatr Nephrol. (2013) 28(12):2239–48. doi: 10.1007/s00467-012-2386-3
16. Bobeck EA. NUTRITION AND HEALTH: COMPANION ANIMAL APPLICATIONS: Functional nutrition in livestock and companion animals to modulate the immune response. J Anim Sci. (2020) 98(3). doi: 10.1093/jas/skaa035
17. Kelly KJ. Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol. (2003) 14(6):1549–58. doi: 10.1097/01.asn.0000064946.94590.46
18. Park SW, Kim M, Kim JY, Ham A, Brown KM, Mori-Akiyama Y, et al. Paneth cell-mediated multiorgan dysfunction after acute kidney injury. J Immunol. (2012) 189(11):5421–33. doi: 10.4049/jimmunol.1200581
19. Carlson N, Nelveg-Kristensen KE, Freese Ballegaard E, Feldt-Rasmussen B, Hornum M, Kamper AL, et al. Increased vulnerability to COVID-19 in chronic kidney disease. J Intern Med. (2021) 290(1):166–78. doi: 10.1111/joim.13239
20. Vallejos A, Baldani AEM, Gauto MA, Rueda DV, Santoro FM, Abriata G. COVID-19 among Chronic Dialysis Patients after First Year of Pandemic, Argentina. Emerg Infect Dis. (2022) 28(11):2294–97. doi: 10.3201/eid2811.212597
21. Oto OA, Ozturk S, Turgutalp K, Arici M, Alpay N, Merhametsiz O, et al. Predicting the outcome of COVID-19 infection in kidney transplant recipients. BMC Nephrol. (2021) 22(1):100. doi: 10.1186/s12882-021-02299-w
22. Singh RSP, Toussi SS, Hackman F, Chan PL, Rao R, Allen R, et al. Innovative randomized phase I study and dosing regimen selection to accelerate and inform pivotal COVID-19 trial of nirmatrelvir. Clin Pharmacol Ther. (2022) 112(1):101–111. doi: 10.1101/2022.02.08.22270649
23. Salmanton-Garcia J, Sprute R, Stemler J, Bartoletti M, Dupont D, Valerio M, et al. COVID-19-associated pulmonary aspergillosis, March-August 2020. Emerg Infect Dis. (2021) 27(4):1077–86. doi: 10.3201/eid2704.204895
24. Golmai P, Larsen CP, DeVita MV, Wahl SJ, Weins A, Rennke HG, et al. Histopathologic and ultrastructural findings in postmortem kidney biopsy material in 12 patients with AKI and COVID-19. J Am Soc Nephrol. (2020) 31(9):1944–47. doi: 10.1681/ASN.2020050683
25. Ren Z, Luo H, Yu Z, Song J, Liang L, Wang L, et al. A randomized, open-label, controlled clinical trial of Azvudine tablets in the treatment of mild and common COVID-19, a pilot study. Adv Sci (Weinh). (2020) 7(19):e2001435. doi: 10.1002/advs.202001435
26. Toussi SS, Neutel JM, Navarro J, Preston RA, Shi H, Kavetska O, et al. Pharmacokinetics of oral nirmatrelvir/ritonavir, a protease inhibitor for treatment of COVID-19, in subjects with renal impairment. Clin Pharmacol Ther. (2022) 112(4):892–900. doi: 10.1002/cpt.2688
27. Shang S, Fu B, Geng Y, Zhang J, Zhang D, Xiao F, et al. Azvudine therapy of common COVID-19 in hemodialysis patients. J Med Virol. (2023) 95(8):e29007. doi: 10.1002/jmv.29007
28. Alfano G, Damiano F, Fontana F, Ferri C, Melluso A, Montani M, et al. Immunosuppressive therapy reduction and early post-infection graft function in kidney transplant recipients with COVID-19. G Ital Nefrol. (2021) 38(6). doi: 10.1101/2021.06.06.21258414
29. Flechner SM, Kobashigawa J, Klintmalm G. Calcineurin inhibitor-sparing regimens in solid organ transplantation: focus on improving renal function and nephrotoxicity. Clin Transplant. (2008) 22(1):1–15. doi: 10.1111/j.1399-0012.2007.00739.x
30. Cantarovich M, Giannetti N, Routy JP, Cecere R, Barkun J. Long-term immunosuppression with anti-CD25 monoclonal antibodies in heart transplant patients with chronic kidney disease. J Heart Lung Transplant. (2009) 28(9):912–8. doi: 10.1016/j.healun.2009.05.021
Keywords: kidney transplant recipient, allograft function, estimated glomerular filtration rate, creatinine, severe COVID-19
Citation: Luo H, Wen J, Yang H, Ran Q and Hou Y (2024) Allograft function predicts mortality in kidney transplant recipients with severe COVID-19: a paradoxical risk factor. Front. Immunol. 15:1335148. doi: 10.3389/fimmu.2024.1335148
Received: 08 November 2023; Accepted: 29 January 2024;
Published: 13 February 2024.
Edited by:
Zhenhua Dai, Guangdong Provincial Academy of Chinese Medical Sciences, ChinaReviewed by:
Marco Fiorentino, University of Bari Aldo Moro, ItalyCopyright © 2024 Luo, Wen, Yang, Ran and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yifu Hou, aG91eWlmdTA3MjZAZm94bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.