
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 05 February 2024
Sec. Cancer Immunity and Immunotherapy
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1332492
This article is part of the Research Topic Novel Reliable Approaches for Prediction and Clinical Decision-making in Cancer View all 12 articles
 Shu-Han Xie1,2†
Shu-Han Xie1,2† Li-Tao Yang1,2,3†
Li-Tao Yang1,2,3† Hai Zhang1,2,4†
Hai Zhang1,2,4† Zi-Lu Tang5,6†
Zi-Lu Tang5,6† Zhi-Wei Lin1,2
Zhi-Wei Lin1,2 Yi Chen1,2
Yi Chen1,2 Zhi-Nuan Hong1,7,8,9*‡
Zhi-Nuan Hong1,7,8,9*‡ Rong-Yu Xu5,6*‡
Rong-Yu Xu5,6*‡ Wan-Li Lin4*‡
Wan-Li Lin4*‡ Ming-Qiang Kang1,7,8,9*‡
Ming-Qiang Kang1,7,8,9*‡Purpose: The need for adjuvant therapy (AT) following neoadjuvant chemoimmunotherapy (nICT) and surgery in esophageal squamous cell cancer (ESCC) remains uncertain. This study aims to investigate whether AT offers additional benefits in terms of recurrence-free survival (RFS) for ESCC patients after nICT and surgery.
Methods: Retrospective analysis was conducted between January 2019 and December 2022 from three centers. Eligible patients were divided into two groups: the AT group and the non-AT group. Survival analyses comparing different modalities of AT (including adjuvant chemotherapy and adjuvant chemoimmunotherapy) with non-AT were performed. The primary endpoint was RFS. Propensity score matching(PSM) was used to mitigate inter-group patient heterogeneity. Kaplan-Meier survival curves and Cox regression analysis were employed for recurrence-free survival analysis.
Results: A total of 155 nICT patients were included, with 26 patients experiencing recurrence. According to Cox analysis, receipt of adjuvant therapy emerged as an independent risk factor(HR:2.621, 95%CI:[1.089,6.310], P=0.032), and there was statistically significant difference in the Kaplan-Meier survival curves between non-AT and receipt of AT in matched pairs (p=0.026). Stratified analysis revealed AT bring no survival benefit to patients with pathological complete response(p= 0.149) and residual tumor cell(p=0.062). Subgroup analysis showed no significant difference in recurrence-free survival between non-AT and adjuvant chemoimmunotherapy patients(P=0.108). However, patients receiving adjuvant chemotherapy exhibited poorer recurrence survival compared to non-AT patients (p= 0.016).
Conclusion: In terms of recurrence-free survival for ESCC patients after nICT and surgery, the necessity of adjuvant therapy especially the adjuvant chemotherapy, can be mitigated.
Esophageal cancer accounts for approximately 50% of cancer cases in China, with over 90% diagnosed as esophageal squamous cell carcinoma (ESCC) (1, 2). Esophagectomy plays a pivotal role in the treatment of locally advanced esophageal squamous cell carcinoma (3). However, surgery alone often results in substantial recurrence and metastasis, with rates ranging from 43.3% to 50.0% (4).
Currently, the standard treatment for locally advanced ESCC involves minimally invasive esophagectomy following neoadjuvant therapy (5). However, the standard neoadjuvant therapy for locally advanced ESCC remains uncertain. Neoadjuvant chemoradiotherapy (nCRT) is commonly used in Western countries, while neoadjuvant chemotherapy (nCT) is extensively used in China and Japan (6) (7). Despite availability of these treatments, the survival of ESCC patients following neoadjuvant therapy is poor due to high recurrence rates and limited long-term survival. The 10-year results from the CROSS trial show a 63.6% disease-free survival rate in the nCRT group, with a 24.3% distant metastasis rate (8). Therefore, there is an urgent need for more effective systemic therapies to improve long-term survival outcomes. Previous study indicates enhanced prognosis in patients receiving nCRT following the addition of adjuvant chemotherapy(aCT) (9). Additionally, neoadjuvant chemoimmunotherapy (nICT) has emerged as a promising and innovative approach for locally advanced ESCC in recent years. Our center has conducted a single-arm phase II clinical trial to evaluate the safety and efficacy of nICT in the treatment of locally advanced ESCC (LA-ESCC) (10). Furthermore, the combination of pembrolizumab (a PD-1 inhibitor) with chemotherapy has been recommended as a first-line treatment for advanced EC (11). The NICE phase-II study demonstrated a 78.1% 2-year recurrence-free survival rate and a 67.9% overall survival rate after nICT (12). The CheckMate577 study revealed that adjuvant immunotherapy following nCRT and esophagectomy significantly extended median disease-free survival to 11.0 months, highlighting the therapeutic advantage of immunotherapy as a systemic treatment option (13).
However, it is imperative to elucidate whether adjuvant therapy, including aCT and adjuvant chemoimmunotherapy(aICT), is indispensable following nICT. Considering the long-term immune memory effect of immunotherapeutic agents (14, 15), we propose that postoperative adjuvant treatment might not be necessary for improved recurrence-free survival in esophageal cancer patients undergoing nICT.
This study retrospectively enrolled patients who underwent esophagectomy at three centers(Fujian Medical University Union Hospital, Quanzhou First Hospital and Gaozhou People’s Hospital) between January 1, 2019, and December 30, 2022. The inclusion criteria of this study were as follows: 1. Patients diagnosed with cT3-4aNanyM0 or cT1-2N+M0 ESCC; 2. receiving at least one cycle of nICT without restrictions on the chemotherapy regimen and type of immunodrug; 3. undergoing radical resection(R0 resection); and 4. provided complete clinical and pathological information. The exclusion criteria were as follows: 1. Patients diagnosed with esophageal adenocarcinoma or other pathological type; 2. patients who underwent only exploratory surgery or jejunostomy; and 3. patients who received radiotherapy before or after surgery. The patient selection procedure is summarized in the flowchart (Figure S1).
Diagnostic and clinical staging procedures included gastroscopy, contrast-enhanced computed tomography of the neck, chest, and upper abdomen, as well as neck ultrasound. Positron emission computed tomography was performed when necessary.
The chemotherapy regimen primarily consisted of platinum in combination with paclitaxel or docetaxel, administered every three weeks. Common neoadjuvant chemotherapy regimens involved cisplatin(60 mg/m2) on day 1, followed by nab-paclitaxel(125 mg/m2) on days 1 and 8, or docetaxel(75 mg/m2) with cisplatin(60 mg/m2) on day 1. Following neoadjuvant chemotherapy, PD-1 monoclonal antibodies were administered, including camrelizumab, pembrolizumab, sintilimab, tislelizumab, or toripalimab, as detailed in our previous studies (16, 17). Generally, PD-1 inhibitors were administered every three weeks, including sintilimab at a dosage of 200 mg, toripalimab at a dosage of 240 mg, pembrolizumab at a dosage of 200 mg, tislelizuma at a dosage of 200 mg and camrelizumab at a dosage of 200 mg.
Suitable candidates for curative esophagectomy, without contraindications, typically underwent the procedure 4-8 weeks after the last dose of neoadjuvant therapy. Esophagectomy with standard 2-field or 3-field lymphadenectomy and gastric reconstruction was performed. Neck lymphadenectomy was conducted if preoperative imaging indicated suspected neck lymph node enlargement.
Postoperative adjuvant therapy was not mandatory and was applied depending on a comprehensive assessment of pathological outcomes, treatment preferences, physical condition, and physician evaluation. Adjuvant therapy regimens in this study included chemotherapy(aCT), immunotherapy(aIT), or a combination of both(aICT).
Dosages and cycles were determined by expert oncologists and thoracic surgeons, and adjusted as needed for drug-related toxicities, patient tolerance, or tumor response to treatment.
In accordance with the NCCN and CSCO guideline, ESCC patients were subjected to regular follow-up examinations every 3 to 6 months within the initial two-year period. Subsequently, follow-ups were conducted at 6-month intervals from the third to fifth year, and annually thereafter. Commonly, the follow-up methods included outpatient visits and telephone interviews. Computed tomography (CT) scans is widely used as a routine examination method to monitor for recurrence of the disease during the follow-up period. If deemed necessary and possible, a PET-CT scan or biopsy will be conducted. Follow-up times were defined from the date of surgery to recurrence or the last date of follow-up. The cut-off date of the last follow-up was October 28, 2023. The follow-up endpoint in this study is recurrence-free survival, defined as the duration from surgical resection to the occurrence of local recurrence or distal metastasis. Moreover, we investigated recurrence patterns among patients after nICT. Locoregional recurrences were defined as cancer reappearance within the esophagus, at the surgical anastomosis site, or in adjacent regional lymph nodes. Distant recurrences were defined as cancer recurrence in distant organs or beyond the operative field.
In this study, Propensity score matching (PSM) was performed to assess the impact of adjuvant therapy and its specific regimens on survival outcomes in distinct groups of ESCC patients. For matching cohort 1, ESCC patients with AT was compared with patients without AT. Subsequent analyses, represented by matching cohorts 2 and 3, investigated the survival advantage of specific adjuvant therapy modes compared to the absence of any adjuvant therapy. Notably, patients with adjuvant chemotherapy was compared with patients without any adjuvant therapy in matching cohort 2. Similarly, patients with adjuvant chemoimmunotherapy was compared with patients without any adjuvant therapy in matching cohort 3. Statistical analysis flow is depicted in Figure 1.
Additionally, The 11-month landmark method was implemented to re-evaluate the role of AT in the nICT group by excluding patients without positive outcome events and a follow-up period of no exceeding 11 months post-surgery (18–20).
Categorical data were presented as counts and percentages, compared using Chi-square or Fisher’s exact tests. PSM reduced bias from confounders, generating scores via logistic regression and nearest neighbor matching without replacement (caliper: 0.05). Matching parameters included pCR, ypT, ypN statuses. Matching cohorts 1 and 3 had a 1:1 ratio, cohort 2 a 1:2 ratio. Survival differences were analyzed using Kaplan-Meier curves and log-rank tests. In addition, Cox regression was performed to evaluate risk factors (variables with p< 0.05 in univariate analysis were included in the multivariate analysis, using LR stepwise regression method). The reverse Kaplan-Meier method was used to calculate the median follow-up duration. In this study, the statistical test values were calculated using the chi-square test. Data were analyzed using SPSS (v25) and R (v4.3.1). Statistical significance was set at P < 0.05.
Our study included a total of 155 patients from three centers. The study consisted of 125 males (80.6%) and 30 females (19.4%). Among them, 77 patients(49.6%) received AT. All patients took a TP or DP for their neoadjuvant chemotherapy regimen. Within the AT recipients, only 20 patients receive adjuvant chemotherapy and 10 patients received adjuvant immunotherapy (aIT), while 47 patients take aICT as their adjuvant therapy regimen. The median follow-up duration of this study was 23 months (95%CI: 20.95-25.05; range:2-48 months). Detailed information about patients in nICT group is presented in Table 1.
A comparison of baseline characteristics between the AT and non-AT patient populations is detailed in Table 2. Before PSM, AT recipients had a worse RFS compared to patients without AT (p=0.027). Similarly, Kaplan-Meier curve analysis and log-rank tests indicated statistically significant differences between patients who received AT and those who did not after PSM (p=0.026), as shown in Figure 2.
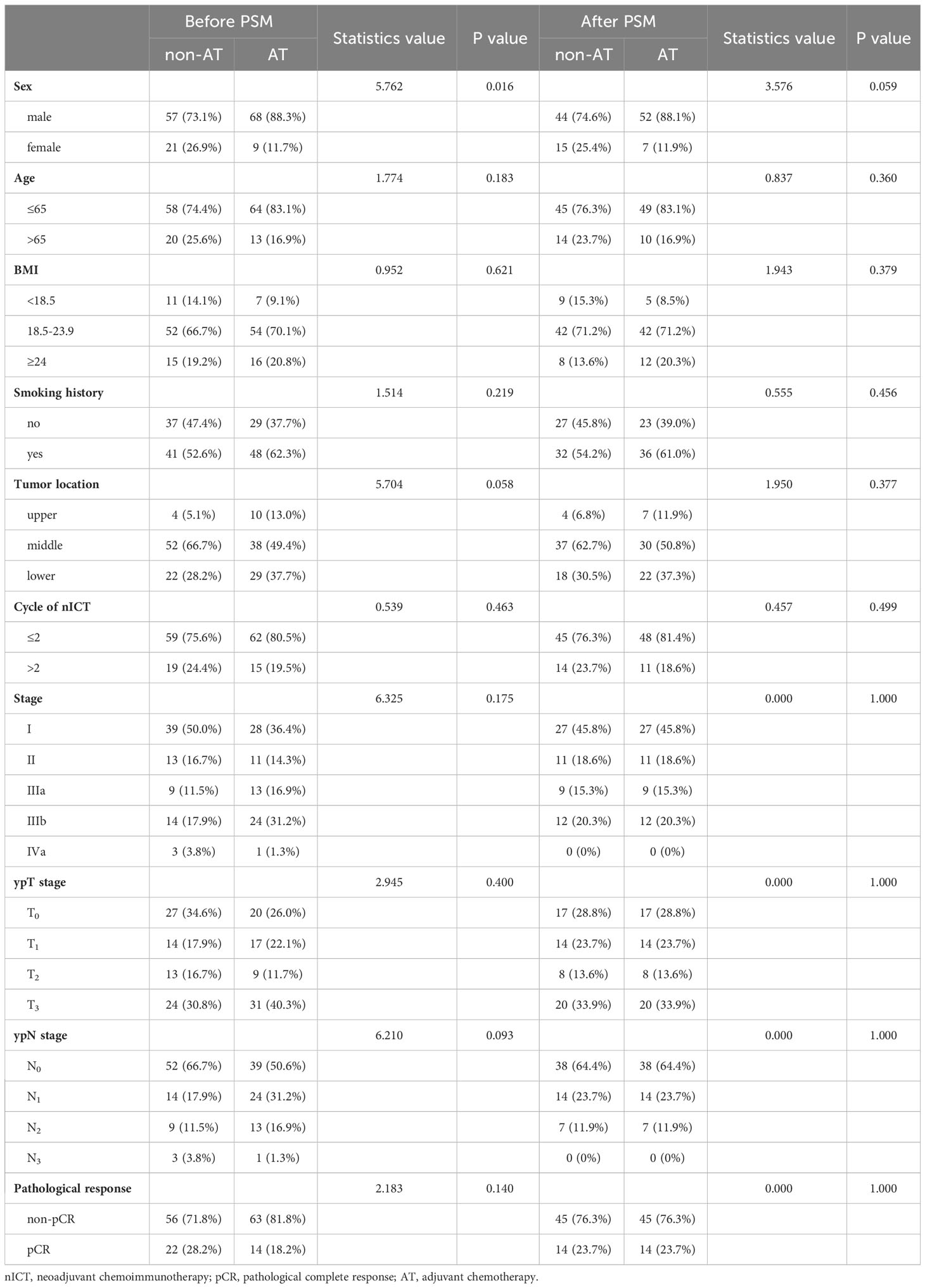
Table 2 Characteristics comparison of AT and non-AT patients in nICT group before and after matching.
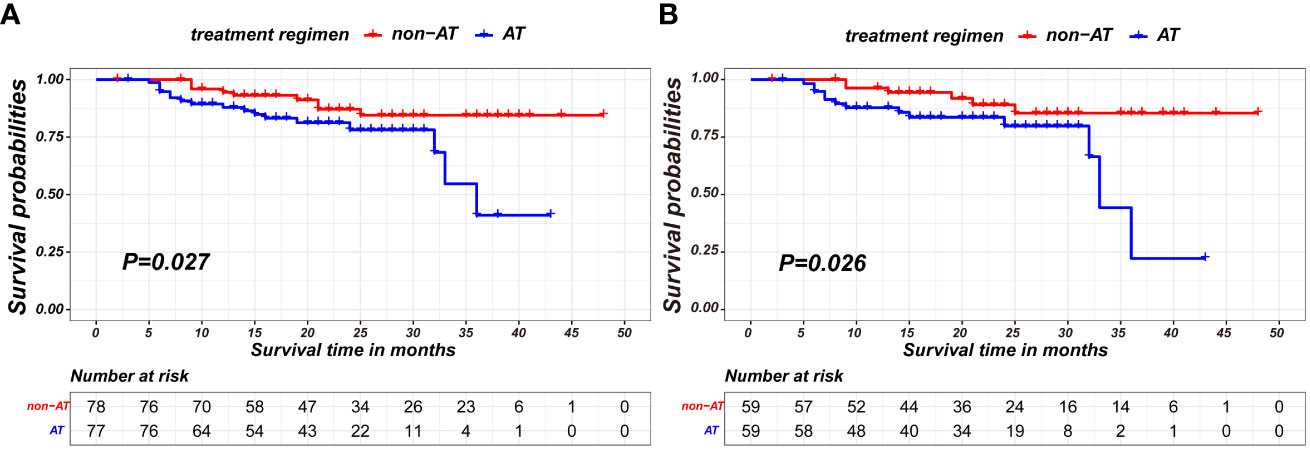
Figure 2 Kaplan-Meier survival curves of recurrence-free survival between non-AT and AT recipients in the nICT group before PSM (A) and after PSM (B).
Subsequently, Cox regression analysis was conducted in unmatched pairs to analyze the risk factors affecting the RFS of nICT patients. In univariate Cox analysis, ypN status, ypT status, smoking history and AT were identified as the significant influencing factor for RFS in nICT patients. While, AT(HR:2.621, 95%CI: [1.089,6.310], P=0.032) and ypN status were significant independent risk factor for RFS in multivariate Cox analysis, as shown in Table 3.
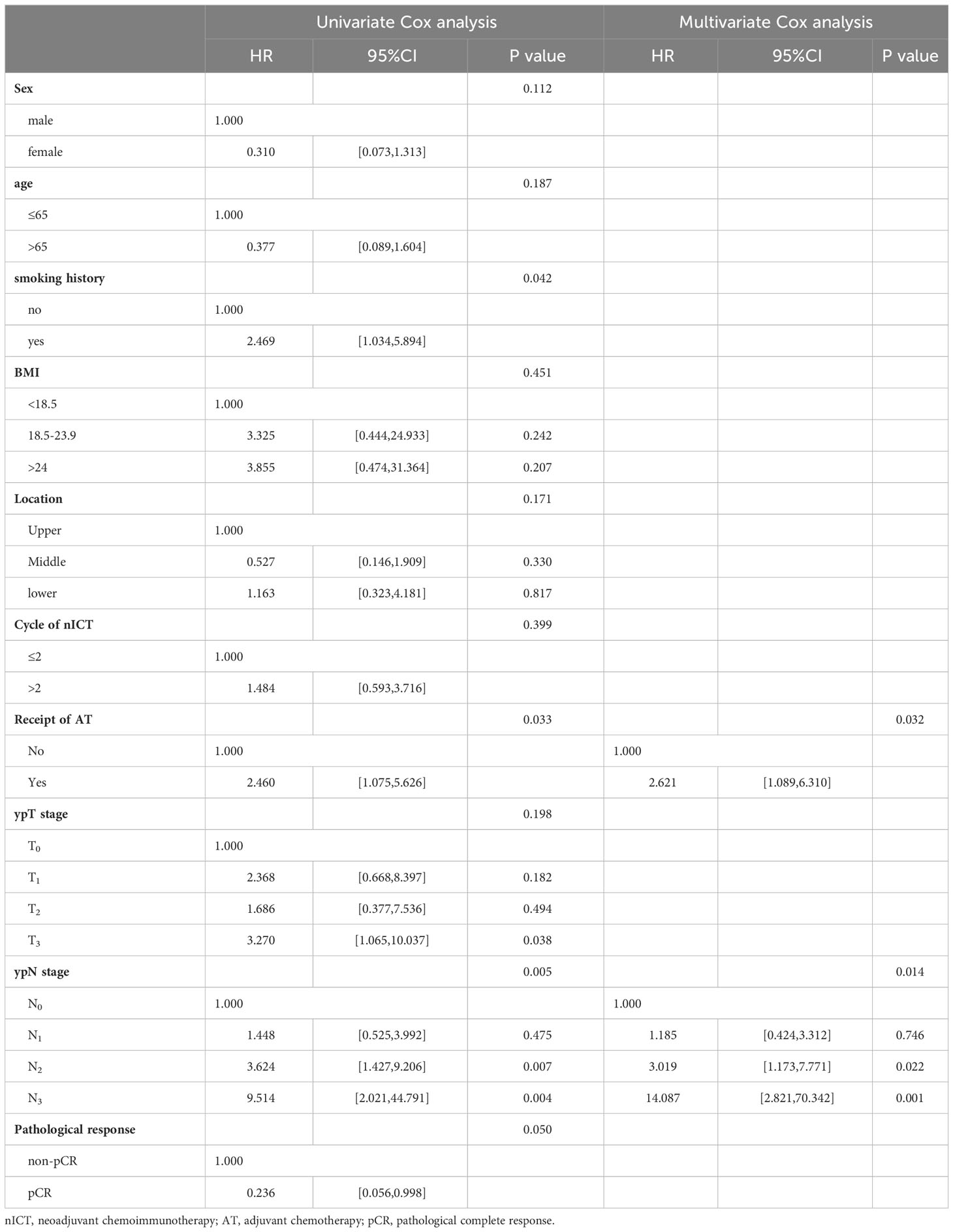
Table 3 Univariate and multivariate Cox analysis for recurrence-free survival in a unmatched population.
In stratified analysis, it was observed that patients with pathological complete response showed no statistically significant differences in prognosis based on the receipt of AT(p=0.072 in unmatched pairs; p= 0.149 in matched pairs), as shown in Figures 3A, B. Similarly, among patients with residual tumor cell(non-pCR), the receipt of AT did not result in statistically significant differences in prognosis(p=0.142 in unmatched pairs; p= 0.062 in matched pairs), as shown in Figures 3C, D.
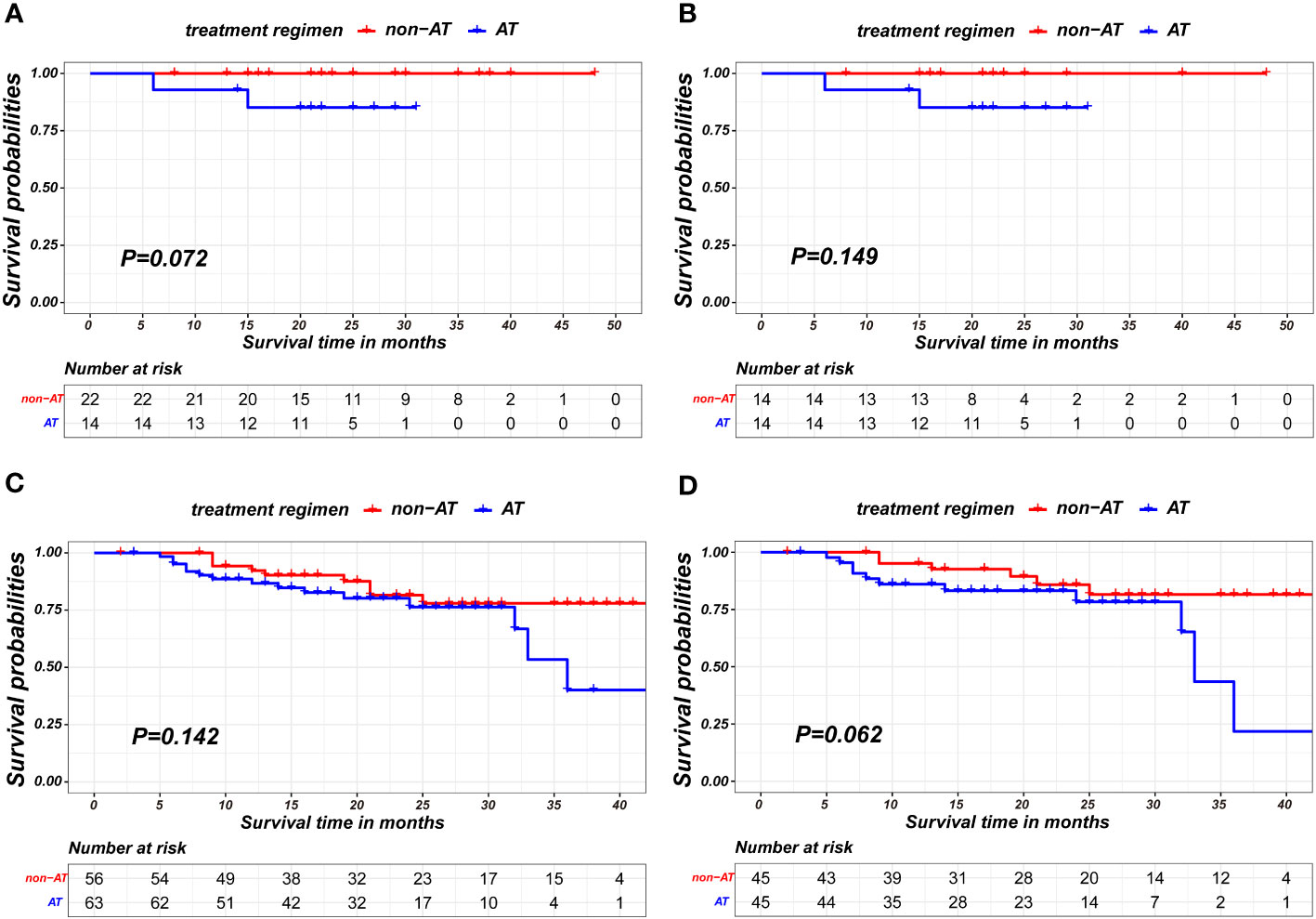
Figure 3 Kaplan-Meier survival curves of recurrence-free survival between non-AT and AT recipient in subgroup of pCR patients in unmatched pairs (A), pCR patients in matched pairs (B), non-pCR patients in unmatched pairs (C) and non-pCR patients in matched pairs (D).
In landmark analysis, patients without positive outcome events and a follow-up period of no more than 11 months post-surgery was excluded. The AT recipients have worse recurrence-free survival compared to patients without AT in both pre-PSM and after-PSM cohorts(P=0.024; p= 0.011, respectively), as is shown in Figure 4. The matching parameters in this landmark analysis including Sex, pCR, ypT and ypN status. Detailed baseline information about patients on landmark method basis is presented in Table 4.
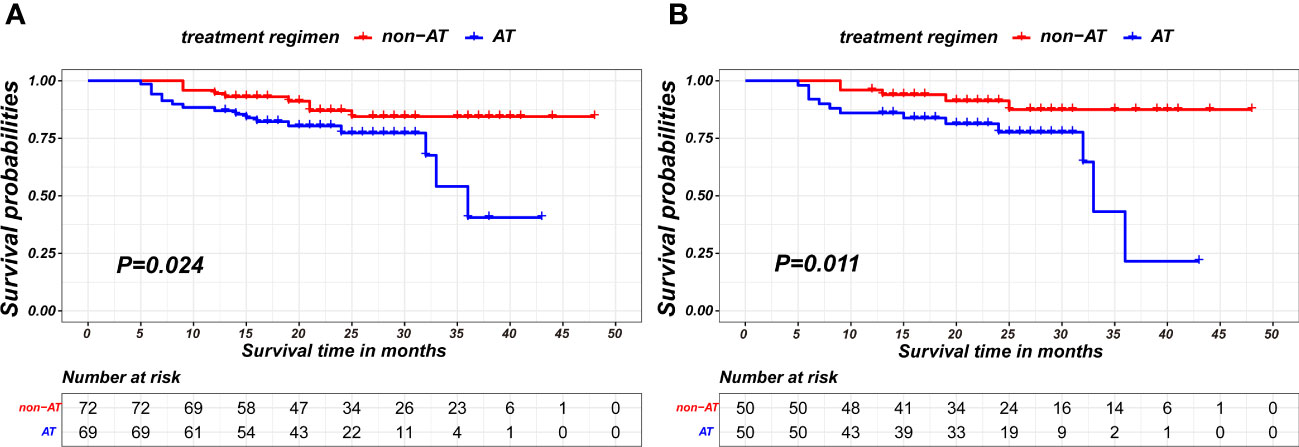
Figure 4 Kaplan-Meier survival curves of recurrence-free survival between non-AT and aCT patients on the 11-month landmark basis before PSM (A) and after PSM (B).
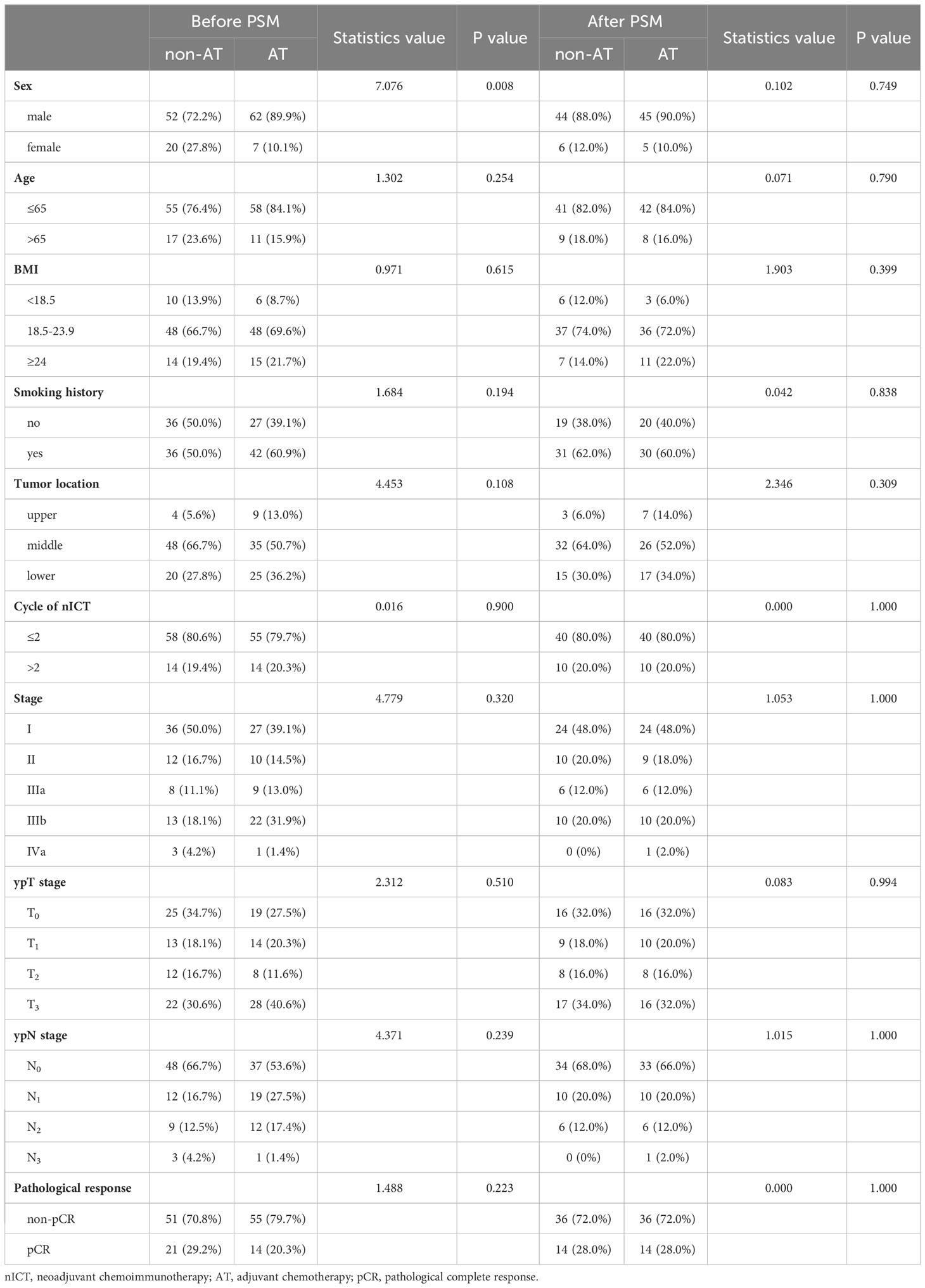
Table 4 Characteristics comparison of AT patients and non-AT patients in the nICT group on the landmark basis before and after matching.
In the nICT group, both before and after PSM, patients receiving aCT exhibited poorer prognosis in terms of recurrence-free survival compared to non-AT patients (p=0.008;p= 0.016, respectively), as shown in Figures 5A, B. Detailed baseline information for these two matched pairs before and after matching is presented in Table 5.
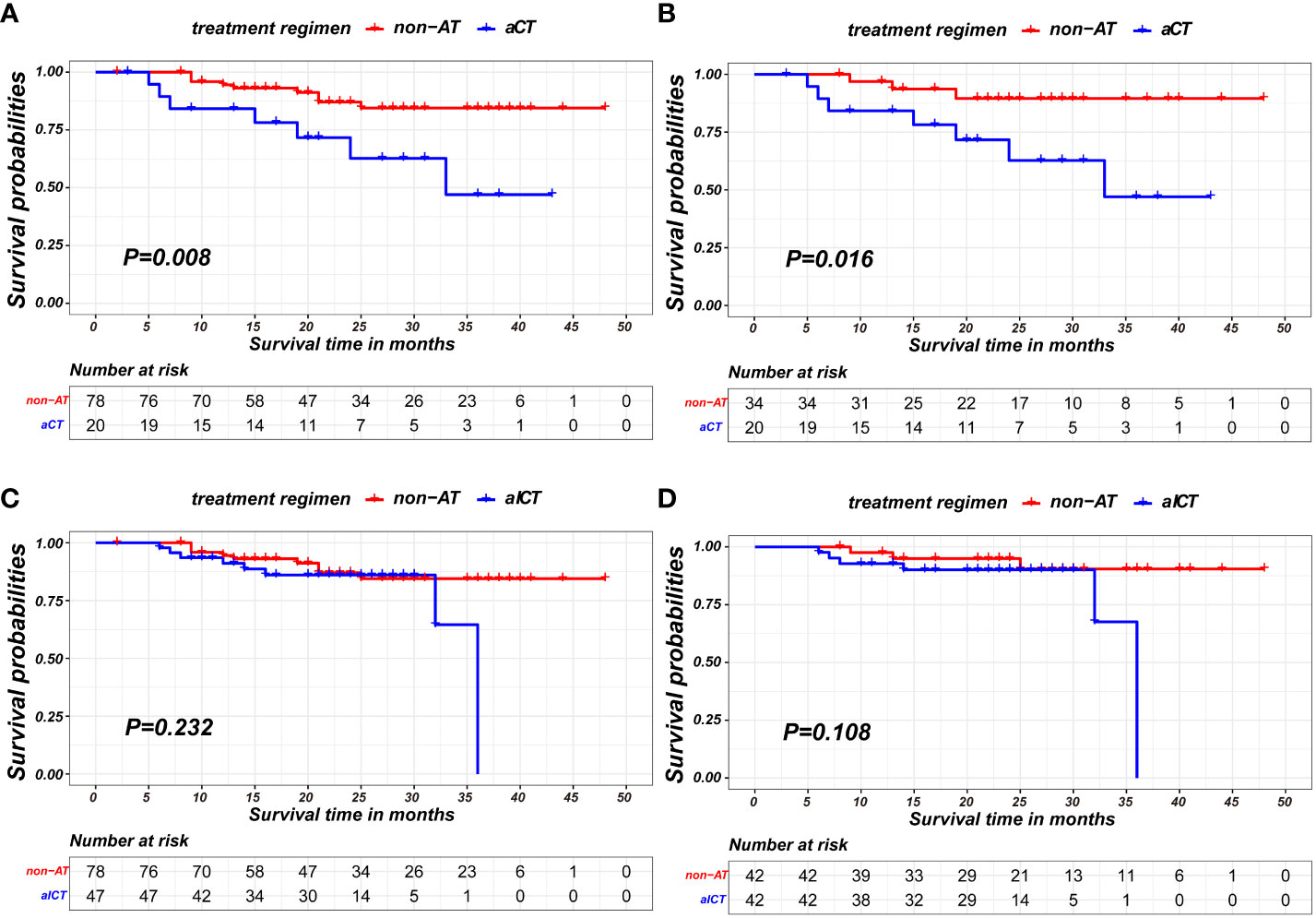
Figure 5 Kaplan-Meier survival curves of recurrence-free survival between patients of non-AT and two modalities of AT: (A) comparison of patients of non-AT with aCT before PSM; (B) comparison of patients of non-AT with aCT after PSM; (C) comparison of patients of non-AT with aICT before PSM; (D) comparison of patients of non-AT with aICT after PSM).
Conversely, no statistically significant differences were observed between non-AT patients and those receiving aICT before and after PSM (p=0.232; p= 0.108, respectively), as illustrated in Figures 5C, D. The baseline information for non-AT and aICT patients before and after matching is presented in Table 6.
Within the nICT group, 26 patients experienced recurrence. The median time to recurrence was 12.5 months. Specifically, 14 patients had locoregional recurrences, 11 patients had distant metastasis, and 1 patients experienced both locoregional recurrence and distant metastasis. Additionally, 2 patients developed supraclavicular lymph node metastasis, classified as a locoregional recurrence in our study, as shown in Figure 6.
In recent years, immunotherapy has increasingly been used for esophageal cancer patients, especially those with locally advanced stages. However, the necessity and benefits of adjuvant therapy for ESCC patients after nICT and surgery remain contentious in international medical consensus. Given the reported finding that nICT does not increase postoperative complications (21), our study is keen to investigate the prognostic factors influencing the survival of esophageal cancer patients undergoing nICT and evaluate the necessity of adjuvant therapy and different AT modalities (including aCT and aICT) in order to better guide the selection of treatment and surveillance model after nICT and surgery. In this study, data from three centers of 155 nICT cases were analyzed. After propensity score matching, there were no statistically significant differences observed in baseline characteristics between patients who received AT and those who did not. According to the Cox analysis and Kaplan-Meier curve analysis, the addition of adjuvant therapy significantly compromised the recurrence-free survival rate of ESCC following nICT in our study. These findings suggest that the administration of adjuvant therapy had a detrimental impact on recurrence-free survival following nICT and the unnecessity of AT in this patient population. This aligns with prior research indicating patients receiving adjuvant therapy exhibited a significantly diminished disease-free survival following resection and neoadjuvant chemoradiation, in comparison to those not undergoing adjuvant therapy (22).
Previous studies have reported that ESCC patients with a pathological complete response have higher 5-year overall survival than incomplete responders (23). The correlation between pathological response and prognosis influences the choice of postoperative treatment. However, the recommendation of adjuvant therapy for ESCC patients exhibiting diverse pathological responses remains a subject of ongoing controversy (9, 24). Therefore, stratified analysis was conducted in our study to evaluate survival outcomes of AT among patients achieving pCR and those with residual pathological tumor cells (ypT+ status or/and ypN+ status) after nICT. Interestingly, AT did not significantly enhance recurrence-free survival benefits for pCR cases, indicating that these individuals can adopt a “watch and see” follow-up strategy, consistent with the current postoperative follow-up strategy (25, 26). However, in cases with incomplete response, the survival advantages of AT were still not found to be statistically significant. Previous studies have demonstrated that the administration of postoperative chemotherapy does not confer any additional survival benefit to patients with lymph node metastasis following neoadjuvant chemotherapy, which aligns with our study (27). The clinical trial CheckMate-577 showed that nivolumab, as an adjuvant agent, can lead to longer disease-free survival for patients with residual pathological tumors after nCRT and surgery. However, there is insufficient evidence and research to substantiate the necessity of AT in ESCC patients who had received nICT and exhibit positive postoperative pathological findings. Previous research has demonstrated that, in comparison to as adjuvants, immune checkpoint inhibitors (ICIs) offer distinct advantages as neoadjuvant therapy for eradicating distant metastases (28). This potentially explains the improved recurrence-free survival observed in nICT cases without AT of our study, even in cases of lymph node metastasis or residual tumor cells.
Administration of different adjuvant therapy regimens may exert varying benefits in terms of recurrence-free survival of ESCC patients. Therefore, whether different adjuvant therapy regimens (aCT and aICT) could confer survival benefits to nICT cases was analyzed. In this study, the patients receiving aICT did not show any statistically significant differences compared to those without any AT. However, it was noted that recipients of aCT exhibited inferior survival rates compared to non-AT individuals following nICT and surgery in both pre-match and post-match cohorts, suggesting the absence of requirement for aCT. Previous research conducted by Yan demonstrated that postoperative adjuvant chemotherapy is not necessary for reducing recurrence in patients who have undergone neoadjuvant chemotherapy and a trend towards inferior disease-free survival was observed in patients who underwent adjuvant therapy (29), aligning with our study’s perspective on the need for adjuvant chemotherapy following neoadjuvant therapy. In addition, it is widely acknowledged that not all patients derive benefits from chemotherapy. Considering the potential impact of esophagectomy on patients, factors such as impaired postoperative food intake and swallowing ability, physiological and psychological stress, as well as postoperative complications, compromise the immune system of individuals with esophageal cancer after surgery (30–32). Consequently, the adverse effects of chemotherapy may further impede an already compromised immune system’s capacity to effectively recognize and target cancer cells, thereby diminishing the efficacy of chemotherapy or immunotherapy and leading to cancer recurrence. In addition, administration of neoadjuvant therapy may potentially suppress the responsiveness of patients towards subsequent systemic therapy post-surgery (33, 34). In this study, we identified adjuvant therapy and higher ypN status as independent risk factors of recurrence in ESCC patients following nICT and surgery. Furthermore, subgroup analysis revealed that patients receiving adjuvant chemotherapy exhibited a poorer prognosis compared to those who did not receive any adjuvant therapy. This may be due to the outweighing side effects of chemotherapy compared to its survival benefit. These findings suggest that in our clinical practice, a close follow-up strategy is preferable over continued administration of adjuvant therapy, particularly chemotherapy, for patients undergoing nICT and surgery.
For the recurrence pattern in nICT patients, the proportion of local recurrence and distant metastasis was 54% and 46%, respectively. However, the majority of patients with distant recurrence had bone metastases and respiratory system metastases, suggesting that in addition to routine CT examination, PET/CT or bone scintigraphy may help to facilitate early detection of distant metastasis.
To the best of our knowledge, this study represents the first analysis of the survival benefit associated with adjuvant therapy in ESCC patients with nICT following surgery. According to previous research, the median time to recurrence for ESCC after nCRT is approximately 11 months (35–37), which is similar with our study. Therefore, the follow-up period in this study is adequate to reflect the effectiveness of adjuvant treatment for ESCC for recurrence. Additionally, to further mitigate the potential bias caused by shorter follow-up durations compared to the median recurrence time, a sensitivity analysis(landmark method) was conducted on patients with follow-up durations exceeding 11 months. The results of this landmark analysis mirrored those of the primary analysis, suggesting that the observed lack of benefit from adjuvant therapy is robust and reliable.
Despite the implementation of rigorous inclusion and exclusion criteria, as well as propensity score matching to ensure baseline comparability, the inherent limitations of a retrospective study design may introduce some degree of bias. Additionally, the majority of patients in our study were treated with the TP/DP regimen-based protocol for neoadjuvant and adjuvants and received no more than two cycles of treatment. Consequently, conducting subgroup analysis regarding chemotherapy regimens and cycles was not feasible in this study. We are currently making efforts to collaborate with more institutions to expand our database and plan to conduct subgroup analyses in future studies. Since the incidence of ESCC is more than 90% in Asian populations, it is important to note that this study specifically focused on patients diagnosed with esophageal squamous cell carcinoma; thus, the applicability of our research findings to patients with adenocarcinoma remains uncertain.
In terms of recurrence-free survival, the need for postoperative adjuvant therapy can be reduced for patients who have undergone nICT and surgery. Meanwhile, the adverse effects of postoperative adjuvant chemotherapy for patients already receiving nICT appear to outweigh its therapeutic benefits in preventing recurrence. A well-designed prospective study on a large scale is necessary to validate these findings.
The raw data supporting the conclusions of this article will be made available by the authors at reasonable request.
The studies involving humans were approved by Fujian Medical University Union Hospital (Project identification code: 2023KY241). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this is a retrospective study.
SX: Conceptualization, Data curation, Formal analysis, Methodology, Software, Supervision, Writing – original draft, Writing – review & editing. LY: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. HZ: Data curation, Formal analysis, Investigation, Writing – original draft. ZT: Data curation, Investigation, Writing – original draft. ZL: Data curation, Investigation, Software, Writing – original draft. YC: Data curation, Investigation, Software, Writing – original draft. ZH: Conceptualization, Data curation, Supervision, Writing – review & editing. RX: Conceptualization, Data curation, Supervision, Writing – review & editing. WL: Conceptualization, Data curation, Supervision, Writing – review & editing. MK: Conceptualization, Data curation, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We express our sincere appreciation for the technical support provided by Figdraw. The authors express their gratitude to Figdraw for providing an online drawing platform. Finally, we express huge gratitude towards Frontier in Immunology for providing us with this opportunity to check our manuscript again.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1332492/full#supplementary-material
nCT, neoadjuvant chemotherapy; nCRT, neoadjuvant chemoradiotherapy; nICT, neoadjuvant chemoimmunotherapy; aIT, adjuvant immunotherapy; aICT, adjuvant chemoimmunotherapy; aCT, adjuvant chemotherapy; AT, adjuvant therapy; LA-ESCC, locally advanced ESCC; PSM, propensity score matching; MIE, minimally invasive esophagectomy; RFS, recurrence-free survival; ESCC, esophageal squamous cell cancer.
1. Li J, Xu J, Zheng Y, Gao Y, He S, Li H, et al. Esophageal cancer: idemiology, risk factors and screening. Chin J Cancer Res (2021) 33(5):535–47. doi: 10.21147/j.issn.1000-9604.2021.05.01
2. He F, Wang J, Liu L, Qin X, Wan Z, Li W, et al. Esophageal cancer: trends in incidence and mortality in China from 2005 to 2015. Cancer Med (2021) 10(5):1839–47. doi: 10.1002/cam4.3647
3. Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. (2017) 390(10110):2383–96. doi: 10.1016/S0140-6736(17)31462-9
4. Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2019) 17(7):855–83. doi: 10.6004/jnccn.2019.0033
5. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med (2012) 366(22):2074–84. doi: 10.1056/NEJMoa1112088
6. Kadono T, Yamamoto S, Hirose T, Ikeda G, Ohara A, Itoyama M, et al. Safety and short-term efficacy of preoperative FOLFOX therapy in patients with resectable esophageal squamous cell carcinoma who are ineligible for cisplatin. Esophagus. (2023) 20(1):109–15. doi: 10.1007/s10388-022-00951-4
7. Nishiwaki N, Noma K, Kunitomo T, Hashimoto M, Maeda N, Tanabe S, et al. Neoadjuvant chemotherapy for locally advanced esophageal cancer comparing cisplatin and 5-fluorouracil versus docetaxel plus cisplatin and 5-fluorouracil: a propensity score matching analysis. Esophagus. (2022) 19(4):626–38. doi: 10.1007/s10388-022-00934-5
8. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Long-term efficacy of neoadjuvant chemoradiotherapy plus surgery for the treatment of locally advanced esophageal squamous cell carcinoma: the NEOCRTEC5010 randomized clinical trial. JAMA Surg (2021) 156(8):721–9. doi: 10.1001/jamasurg.2021.2373
9. Park SY, Kim HK, Jeon YJ, Lee J, Cho JH, Choi YS, et al. The role of adjuvant chemotherapy after neoadjuvant chemoradiotherapy followed by surgery in patients with esophageal squamous cell carcinoma. Cancer Res Treat (2023) 55(4):1231–9. doi: 10.4143/crt.2022.1417
10. Zhang Z, Hong ZN, Xie S, Lin W, Lin Y, Zhu J, et al. Neoadjuvant sintilimab plus chemotherapy for locally advanced esophageal squamous cell carcinoma: a single-arm, single-center, phase 2 trial (ESONICT-1). Ann Transl Med (2021) 9(21):1623. doi: 10.21037/atm-21-5381
11. Smyth EC, Gambardella V, Cervantes A, Fleitas T. Checkpoint inhibitors for gastroesophageal cancers: dissecting heterogeneity to better understand their role in first-line and adjuvant therapy. Ann Oncol (2021) 32(5):590–9. doi: 10.1016/j.annonc.2021.02.004
12. Yang Y, Liu J, Liu Z, Zhu L, Chen H, Yu B, et al. Two-year outcomes of clinical N2-3 esophageal squamous cell carcinoma after neoadjuvant chemotherapy and immunotherapy from the phase 2 NICE study. J Thorac Cardiovasc Surg (2023) S0022-5223(23):00782–1. doi: 10.1016/j.jtcvs.2023.08.056
13. Yarchoan M, Johnson BA 3rd, Lutz ER, Laheru DA, Jaffee EM. Targeting neoantigens to augment antitumour immunity. Nat Rev Canc (2017) 17(9):569. doi: 10.1038/nrc.2017.74
14. Pauken KE, Torchia JA, Chaudhri A, Sharpe AH, Freeman GJ. Emerging concts in PD-1 checkpoint biology. Semin Immunol (2021) 52:101480. doi: 10.1016/j.smim.2021.101480
15. He J, Hu Y, Hu M, Li B. Development of PD-1/PD-L1 pathway in tumor immune microenvironment and treatment for non-small cell lung cancer. Sci R (2015) 5:13110. doi: 10.1038/sr13110
16. Gao L, Hong ZN, Wu L, Yang Y, Kang M. Residual tumor model in esophageal squamous cell carcinoma after neoadjuvant immunochemotherapy: Frequently involves the mucosa and/or submucosa. Front Immunol (2022) 13:1008681. doi: 10.3389/fimmu.2022.1008681
17. Hong ZN, Gao L, Weng K, Huang Z, Han W, Kang M. Safety and feasibility of esophagectomy following combined immunotherapy and chemotherapy for locally advanced esophageal squamous cell carcinoma: A propensity score matching analysis. Front Immunol (2022) 13:836338. doi: 10.3389/fimmu.2022.836338
18. Brandão M, Martins-Branco D, De Angelis C, Vuylsteke P, Gelber RD, Van Damme N, et al. Surgery of the primary tumor in patients with de novo metastatic breast cancer: a nationwide population-based retrospective cohort study in Belgium. Breast Cancer Res Treat (2023) 203:351–63. doi: 10.1007/s10549-023-07116-6
19. Mazzarella L, Giugliano F, Nicolo E, Esposito A, Crimini E, Tini G, et al. Immune-related adverse event likelihood score identifies "Pure" IRAEs strongly associated with outcome in a phase I-II trial population. Oncologist (2023), oyad239. doi: 10.1093/oncolo/oyad239
20. Heymach JV, Harpole D, Mitsudomi T, Taube JM, Galffy G, Hochmair M, et al. Perioperative durvalumab for resectable non-small-cell lung cancer. N Engl J Med (2023) 389:18. doi: 10.1056/NEJMoa2304875
21. Hong Z, Xu J, Chen Z, Xu H, Huang Z, Weng K, et al. Additional neoadjuvant immunotherapy does not increase the risk of anastomotic leakage after esophagectomy for esophageal squamous cell carcinoma: a multicenter retrospective cohort study. Int J Surg (2023) 109(8):2168–78. doi: 10.1097/JS9%0000000000487
22. Li X, Luan S, Yang Y, Zhou J, Shang Q, Fang P, et al. Trimodal therapy in esophageal squamous cell carcinoma: role of adjuvant therapy following neoadjuvant chemoradiation and surgery. Cancers (Basel) (2022) 14(15):3721. doi: 10.3390/cancers14153721
23. Al-Kaabi A, van der Post RS, van der Werf LR, Wijnhoven BPL, Rosman C, Hulshof MCCM, et al. Impact of pathological tumor response after CROSS neoadjuvant chemoradiotherapy followed by surgery on long-term outcome of esophageal cancer: a population-based study. Acta Oncol (2021) 60(4):497–504. doi: 10.1080/0284186X.2020.1870246
24. Gao H, Wang Y, Jiang Z, Shi G, Hu S, Ai J, et al. Association of survival with adjuvant radiotherapy for pN0 esophageal cancer. Aging (Albany NY) (2023) 15(8):3158–70. doi: 10.18632/aging.204677
25. Network NCC. NCCN clinical practice guidelines in oncology (NCCN guidelines): esophageal and esophagogastric junction cancers. Version 4.2020. The National Comprehensive Cancer Network: Plymouth Meeting, PA (2020).
26. Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D, ESMO Guidelines Working Group. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2013) 24 Suppl 6:vi51–6. doi: 10.1093/annonc/mdt342
27. Stiles BM, Christos P, Port JL, Lee PC, Paul S, Saunders J, et al. Predictors of survival in patients with persistent nodal metastases after preoperative chemotherapy for esophageal cancer. J Thorac Cardiovasc Surg (2010) 139(2):387–94. doi: 10.1016/j.jtcvs.2009.10.003
28. Liu J, Blake SJ, Yong MC, Harjunpää H, Ngiow SF, Takeda K, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discovery (2016) 6(12):1382–99. doi: 10.1158/2159-8290.CD-16-0577
29. Yan W, Zhao P, Fu H, Lin Y, Li Z, Dai L, et al. Survival after induction chemotherapy and esophagectomy is not improved by adjuvant chemotherapy. Ann Thorac Surg (2019) 108(5):1505–13. doi: 10.1016/j.athoracsur.2019.04.106
30. Shiozaki A, Fujiwara H, Okamura H, Murayama Y, Komatsu S, Kuriu Y, et al. Risk factors for postoperative respiratory complications following esophageal cancer resection. Oncol Lett (2012) 3(4):907–12. doi: 10.3892/ol.2012.589
31. van Sandick JW, Gisbertz SS, ten Berge IJ, Boermeester MA, van der Pouw Kraan TC, Out TA, et al. Immune responses and prediction of major infection in patients undergoing transhiatal or transthoracic esophagectomy for cancer. Ann Surg (2003) 237(1):35–43. doi: 10.1097/00000658-200301000-00006
32. Kubo Y, Miyata H, Sugimura K, Shinno N, Asukai K, Hasegawa S, et al. Prognostic implication of postoperative weight loss after esophagectomy for esophageal squamous cell cancer. Ann Surg Oncol (2021) 28(1):184–93. doi: 10.1245/s10434-020-08762-6
33. Ji Y, Du X, Zhu W, Yang Y, Ma J, Zhang L, et al. Efficacy of concurrent chemoradiotherapy with S-1 vs radiotherapy alone for older patients with esophageal cancer: A multicenter randomized phase 3 clinical trial. JAMA Oncol (2021) 7(10):1459–66. doi: 10.1001/jamaoncol.2021.2705
34. Cheraghi A, Barahman M, Hariri R, Nikoofar A, Fadavi P. Comparison of the pathological response and adverse effects of oxaliplatin and capecitabine versus paclitaxel and carboplatin in the neoadjuvant chemoradiotherapy treatment approach for esophageal and gastroesophageal junction cancer: A randomized control trial study. Med J Islam Repub Iran (2021) 35:140. doi: 10.47176/mjiri.35.140
35. Sugiyama M, Morita M, Yoshida R, Ando K, Egashira A, Takefumi O, et al. Patterns and time of recurrence after complete resection of esophageal cancer. Surg Today (2012) 42(8):752–8. doi: 10.1007/s00595-012-0133-9
36. Dresner SM, Griffin SM. Pattern of recurrence following radical oesophagectomy with two-field lymphadenectomy. Br J Surg (2000) 87(10):1426–33. doi: 10.1046/j.1365-2168.2000.01541.x
Keywords: adjuvant therapy, neoadjuvant chemoimmunotherapy, esophageal cancer, propensity score matching, recurrence-free survival
Citation: Xie S-H, Yang L-T, Zhang H, Tang Z-L, Lin Z-W, Chen Y, Hong Z-N, Xu R-Y, Lin W-L and Kang M-Q (2024) Adjuvant therapy provides no additional recurrence-free benefit for esophageal squamous cell carcinoma patients after neoadjuvant chemoimmunotherapy and surgery: a multi-center propensity score match study. Front. Immunol. 15:1332492. doi: 10.3389/fimmu.2024.1332492
Received: 03 November 2023; Accepted: 17 January 2024;
Published: 05 February 2024.
Edited by:
Vera Rebmann, University of Duisburg-Essen, GermanyReviewed by:
Cui Youbin, First Affiliated Hospital of Jilin University, ChinaCopyright © 2024 Xie, Yang, Zhang, Tang, Lin, Chen, Hong, Xu, Lin and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming-Qiang Kang, OTE5OTExNTA0NUBmam11LmVkdS5jbg==; Wan-Li Lin, d2FubGlsaW4yMDIwQDE2My5jb20=; Rong-Yu Xu, eHJ5NjQxMTI3QHNpbmEuY29t; Zhi-Nuan Hong, aG9uZ3poaW51YW5AMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.