- 1Division of Hepatobiliary Surgery, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
- 2Guangxi Key Laboratory of Immunology and Metabolism for Liver Disease, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
- 3Department of Pathology, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Sciences, Xiangyang, China
Background: As the leading cause of chronic kidney disease, diabetic kidney disease (DKD) is an enormous burden for all healthcare systems around the world. However, its early diagnosis has no effective methods.
Methods: First, gene expression data in GEO database were extracted, and the differential genes of diabetic tubulopathy were obtained. Immune-related genesets were generated by WGCNA and immune cell infiltration analyses. Then, differentially expressed immune-related cuproptosis genes (DEICGs) were derived by the intersection of differential genes and genes related to cuproptosis and immune. To investigate the functions of DEICGs, volcano plots and GO term enrichment analysis was performed. Machine learning and protein-protein interaction (PPI) network analysis helped to finally screen out hub genes. The diagnostic efficacy of them was evaluated by GSEA analysis, receiver operating characteristic (ROC) curve, single-cell RNA sequencing and the Nephroseq website. The expression of hub genes at the animal level by STZ -induced and db/db DKD mouse models was further verified.
Results: Finally, three hub genes, including FSTL1, CX3CR1 and AGR2 that were up-regulated in both the test set GSE30122 and the validation set GSE30529, were screened. The areas under the curve (AUCs) of ROC curves of hub genes were 0.911, 0.935 and 0.922, respectively, and 0.946 when taking as a whole. Correlation analysis showed that the expression level of three hub genes demonstrated their negative relationship with GFR, while those of FSTL1 displayed a positive correlation with the level of serum creatinine. GSEA was enriched in inflammatory and immune-related pathways. Single-nucleus RNA sequencing indicated the main distribution of FSTL1 in podocyte and mesangial cells, the high expression of CX3CR1 in leukocytes and the main localization of AGR2 in the loop of Henle. In mouse models, all three hub genes were increased in both STZ-induced and db/db DKD models.
Conclusion: Machine learning was combined with WGCNA, immune cell infiltration and PPI analyses to identify three hub genes associated with cuproptosis, immunity and diabetic nephropathy, which all have great potential as diagnostic markers for DKD and even predict disease progression.
1 Introduction
Diabetic kidney disease (DKD), the main complication of diabetes, mainly features hypertension, proteinuria and progressive decreases in kidney function. It is also a leading cause of chronic and even end-stage kidney diseases worldwide (1–3). DKD is expected to experience an increase of nearly 50% in morbidity in the next two decades, which results in an estimated 783 million patients. It is estimated that the 10-year cumulative mortality rate of DKD patients is up to 31.1% (4). Detecting DKD early is important to prevent its evolution into renal failure. Unfortunately, only abnormal proteinuria can be found due to the silence at the early stages of the disease, which makes its diagnosis difficult. Various studies have identified associations of DKD with markers of renal tubule injury such as kidney injury molecule 1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL) and N-acetyl-β-D-glocosaminidase (NAG), inflammatory markers like tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), as well as markers of oxidative stress like 8-hydroxy-2’-deoxyguanosine (8-OHdG) (5). However, no available biomarkers can accurately diagnose or predict the progression of DKD.
Tubular injury in the progression of DKD has been recognized to play a significant role for the past few years and is named diabetic tubulopathy (6). Regarding mechanism, oxidative stress, hypoxia, albumin overload, inflammation, epithelial-mesenchymal transition (EMT), cellular senescence and nutrient-sensing pathways in tubule cells continue to occur during the development of diabetic nephropathy. They jointly promote the progression of tubule injury and interstitial fibrosis, which thus affects the prognosis of DKD patients (7).
Cuproptosis, a novel copper ion-dependent cell death regulatory process, has recently been recognized to be strongly correlated with mitochondrial respiration and the lipoic acid (LA) pathway (8). As an important micronutrient in the human body, copper is a key catalytic cofactor, which is vital in mitochondrial respiration, antioxidant defense, biocompound synthesis and other biological processes. Its concentration has been maintained in a low range. When accumulating abnormally, copper ions show serious cytotoxicity. However, the specific mechanisms that have evolved to do that are not fully understood.
Proximal tubules are the main site of renal reabsorption, in which a large number of mitochondria are distributed to produce energy (9). In the early phase of DKD, high glomerular filtration is accompanied by the increased reabsorption of proximal tubules. This causes a sharp increase in energy consumption and the exposure of proximal tubules to hypoxia, which leads to mitochondrial dysfunction. It was hypothesized that the process of diabetic renal tubular disease is accompanied by copper death, given the close relationship between cuproptosis and mitochondrial function. Recent studies have shown that all the stages of DKD are accompanied by immune infiltration, predominantly macrophage infiltration. In addition, the degree of immune infiltration has also been observed to have a close association with the progression of end-stage renal disease (ESRD) in DKD patients (10, 11). Therefore, the aim of this research was to investigate the associations of immune infiltration and cuproptosis with diabetic tubulopathy, and then construct and externally validate a novel DKD prediction model.
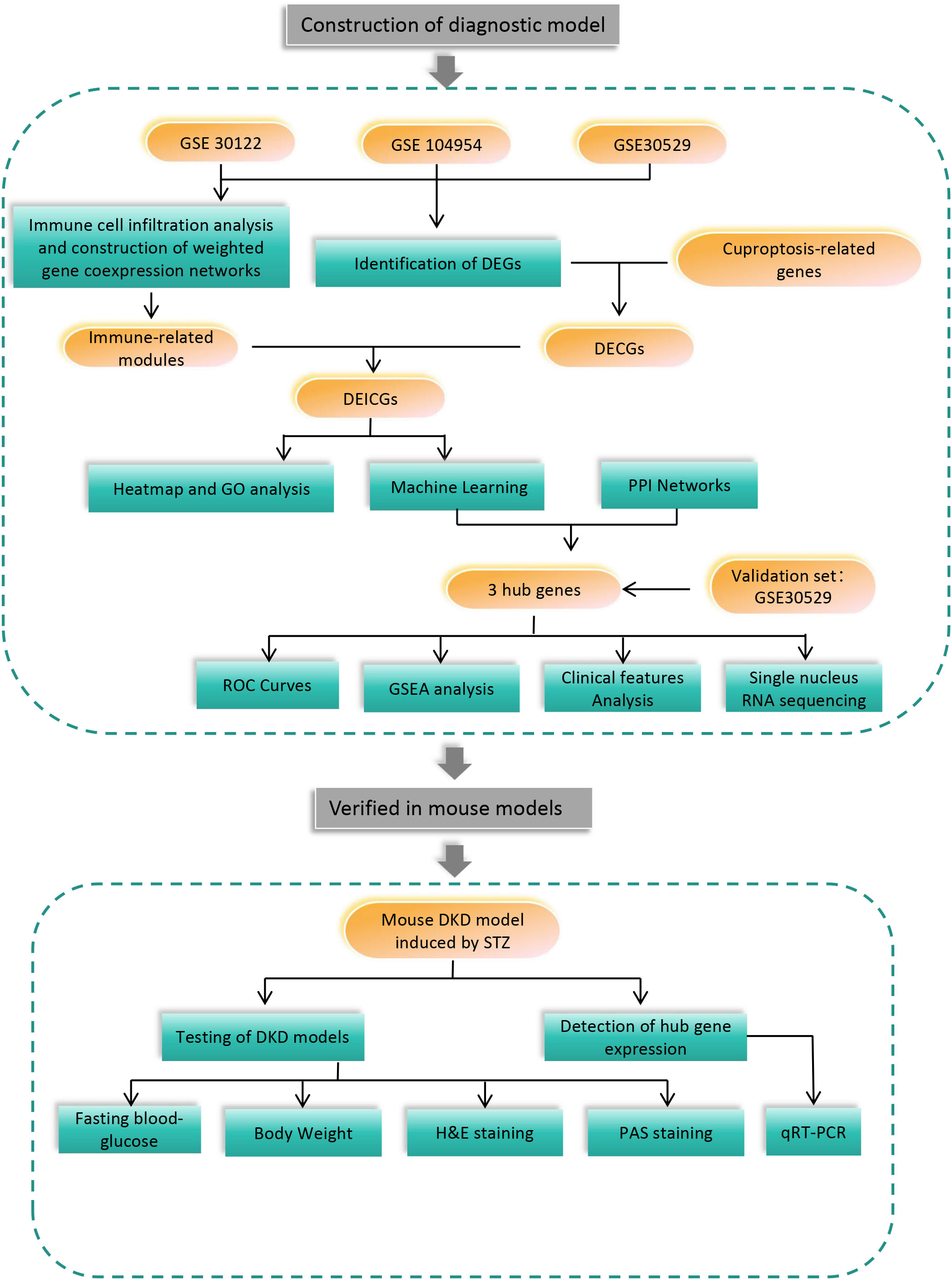
In this study, the diagnostic genes of DKD tubule injury were predicted by making a combination of the bioinformatics methods related to immune infiltration and cuproptosis. Additionally, the external data set was used as the verification set, and mouse models were also involved. The specific process of the experiment is shown in Figure 1.
2 Materials and methods
2.1 Data acquisition
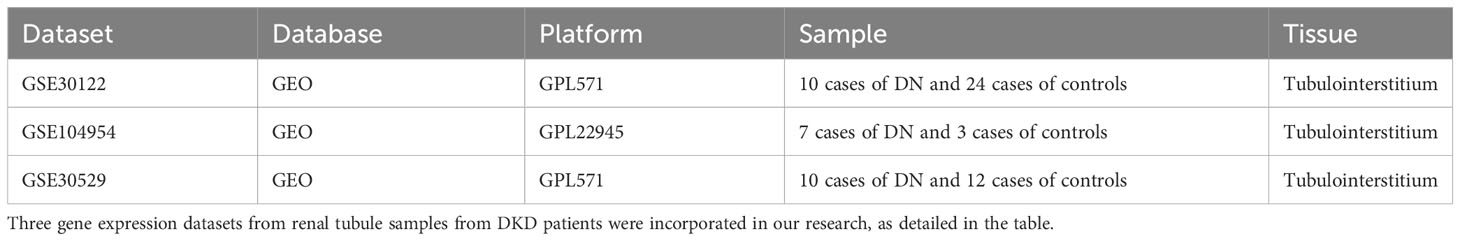
The Gene Expression Omnibus (GEO) database was searched, and three data sets were obtained from renal tubules in diabetic nephropathy patients, namely gene set enrichment 30122 (GSE30122), GSE104954 and GSE30529, which were derived from the GPL571 and GPL22945 platforms, respectively. Their detailed information is shown in Table 1. A cuproptosis-related geneset was constructed by searching for “cuproptosis” in Gene Set Enrichment Analysis (GSEA) and GeneCards databases (https://www.gsea-msigdb.org)(https://www.genecards.org), respectively. After that, they were merged with key genes for cuproptosis mentioned in an article published in 2022 (8). The specific gene list is shown in Supplementary Table 1.
2.2 Identification of differentially expressed genes
Firstly, difference analysis was conducted on the three GEO datasets respectively by the R package “limma”, and volcano maps were drawn with the help of the “ggplot” package. It was considered that P-value < 0.05 and |log2FC| >0.5 had statistical significance. A summary was made on the basis of the above three sets of differentially expressed genes(DEGs) and cuproptosis-related genes. A Venn diagram was plotted to obtain the collection of differential genes in diabetic nephropathy tubular tissues associated with copper death, which were named differentially expressed cuproptosis-related genes (DECGs).
2.3 Immune infiltration and weighted correlation network analysis
The cibersort platform (https://cibersortx.stanford.edu) helped us identify immune cell infiltration in diabetic nephropathy and control samples by matching their M22 dataset with the expression profile of this study. Weighted correlation network analysis (WGCNA) is a systems biology approach. It is used for describing patterns of gene associations between various samples by identifying high-covariance gene sets and candidate treatment targets or biomarker genes based on the interconnectivity of gene sets and the connection between gene sets and clinical features. Specifically, gene expression profiles were utilized to calculate the median absolute deviation (MAD) for each gene. The top 50% of genes with the smallest MAD were eliminated, and the “goodSamplesGenes” method of the R software package “WGCNA” was employed to remove outlier genes and samples. It was found that six modules, including dark orange, bisque4, darkgrey, orangered4, cyan and darkslateblue, exhibited the strongest correlation with immune cells. Therefore, they were selected for subsequent analysis. Afterwards, the genes in the DECGs gene set were intersected with the six key modules obtained by WGCNA. Intersection gene sets were named differentially expressed immune-related cuproptosis genes (DEICGs), and further research was carried out.
2.4 Gene ontology functional enrichment and GSEA analyses
The R software package “inSilicoMerging” was used for merging GSE104954 with GSE30122. Further, the batch effects between them were removed using the removeBatchEffect function of the R software package “limma”. The R package “ComplexHeatma” was adopted to generate heatmaps for visualization. For the functional enrichment analysis of gene sets, the gene ontology (GO) annotation of genes in the R software package “org.Hs.eg.db” was used for mapping genes into the background set. Enrichment analysis was performed using the clusterProfiler package. In the above process, the minimum and maximum gene sets were set to 5 and 5,000, respectively. It was considered that P value < 0.05 and false discovery rate (FDR) < 0.25 had statistical significance. In the follow-up study, the “clusterProfiler” package was also utilized to conduct GSEA enrichment analysis on the three finally selected hub genes.
2.5 Screening hub genes by machine learning and PPI networks
Least absolute shrinkage and selection operator (LASSO) regression is a constraint term model with L1 norm after the cost function of a linear regression model, which can be used for the variable screening and feature selection of high-dimensional data and model construction (12). The support vector machine-recursive feature elimination (SVM-RFE) algorithm is a method of machine learning that uses support vector machines as classifiers to identify the best core genes (13).
The protein interaction networks of seven proteins were retrieved from the STRING website (https://cn.string-db.org/) and imported into Cytoscape for visualization. With the aid of the cytohubba plug-in software in Cytoscape, eight kinds of algorithms such as boeeleneck, closeness, degree, density of maximum neighborhood component (DMNC), eccentricity, edge percolated component (EPC), maximal clique centrality (MCC) and newsun were obtained to forecast key genes. The top four genes in the results obtained by each algorithm were intersected. The intersection genes that overlapped in at least five algorithms were taken as a new gene set. The differential genes obtained by machine learning were further intersected. Finally, hub genes were obtained. The receiver operating characteristic (ROC) analysis of three hub genes was conducted by use of the “pROC” package and visualized by the “ggplot2” package.
2.6 Clinical analysis
The expression levels of three hub genes were compared using unpaired two-sample t-tests in the training cohort GSE30122 and the validation cohort GSE30529. Then, the correlations between the expression levels of hub genes and the serum creatinine and glomerular filtration rate (GFR) of clinical patients on Nephroseq(http://v5.nephroseq.org), a comprehensive information website related to kidney diseases, were searched.
2.7 Single nucleus RNA sequencing
The Kidney Integrative Transcriptomics (K.I.T.) database was invented in the laboratory of Ben Humphrey at Washington University (http://humphreyslab.com/SingleCell/) (14). Thanks to the database, the single-cell sequencing data of three hub genes in the renal tubular lesion samples of diabetic nephropathy were mined in this study, and the results were visualized.
2.8 DKD mouse models
The type 1 diabetes was induced by low-dose streptozocin (STZ) treatment in C57 male mice aged 8-10 weeks. All the mice received 12-week and 5-day feeding with a 60% high fat diet (HFD) (Guangdong Medical Animal Center, Guangzhou, China) and were given an intraperitoneal injection of STZ or citrate buffer after a 4-hour fast for 5 days before the start of feeding. Fasting blood glucose was measured one week after drug withdrawal. Mice with fasting blood glucose greater than 15mmol/L were considered to have successfully constructed a diabetes model. For the validation of diabetic nephropathy, the increase of urinary albumin level is an important indicator. Tubular atrophy, glomerular enlargement, thickened capsule and inflammatory cell infiltration in H&E staining and mesangial hyperplasia, basement membrane thickening and glomerular sclerosis with PAS staining can further verify the renal damage caused by hyperglycemia At the same time, we introduced 12-week-old male db/db mice from Shanghai Model Organisms Center, Inc. to model type 2 diabetes, with db/m mice as controls.
2.9 Hematoxylin-eosin and periodic acid-Schiff staining
Mouse kidneys were fixed with 4% paraformaldehyde immediately after sacrifice. After tissue processing and paraffin embedding, sections of 2 μm were cut for further staining. Hematoxylin-eosin (H&E) staining on kidney sections was performed according to standard H&E staining protocols, in which the staining time of hematoxylin and eosin was 18 minutes and 30 seconds, respectively. Periodic acid-Schiff (PAS) staining for glycogen was performed using the Solarbio kit (G1281).
2.10 RNA extraction from mouse kidney and quantitative real-time polymerase chain reaction
Ribonucleic acids (RNAs) were extracted by use of TRIzol [Invitrogen, Carlsbad, California (CA), the United States of America (USA)], followed by chloroform extraction. Subsequently, RNA precipitated in isopropanol and resuspended in nuclease-free double distilled water (ddH2O). Complementary deoxyribonucleic acids (cDNA) were synthesized using 1 μg corresponding RNAs (Takara Bio, Japan). Total cDNAs were diluted at a ratio of 1:3, and utilized for quantifying the three genome segments by SYBR Green qPCR (Vazyme,027E2220GB) with the following primer pairs: FSTL1: Forward: 5’-CTCCCACCTTCGCCTCTAAC-3’, Reverse: 3’-TTCTAGGTTCCTCCTCGCCG-5’, GAPDH: Forward:5’-GTTGGTTGGAGCAAACATCCC-3’, Reverse: 3’- TTAGGAGTGGGGGTGGCTTT-5’, AGR2: Forward: 5’-GTGGGAAGCCCAGATTTGCC-3’, Reverse: 3’-TAGTTTGGGCCGAGAGTCCT-5’, CX3CR1: Forward: 5’-CCATCTGCTCAGGACCTCAC-3’, Reverse: 3’-CACCAGACCGAACGTGAAGA-5’.The amplification process was completed on a 7500 fast real-time PCR System (Applied Biosystems, CA, USA).
2.11 Statistical analysis
Statistical Package for the Social Sciences (SPSS) software 23.0 (SPSS Inc., Chicago, USA) or GraphPad Prism 9 (GraphPad Software, CA, USA) was used to conduct statistical analysis. The expression levels of immune cells in diabetic nephropathy (DN) and control groups from the Cibersort website and those of hub genes in both groups were compared using unpaired t-tests. Statistical significance was set when p value was below 0.05.
3 Results
3.1 Identification of differential genes
Differential genes associated with renal tubulointerstitial damage in mice with diabetic nephropathy were searched for by downloading three gene sets, including GSE30122, GSE104954 and GSE30529, from the GEO database. The package “limma” was used for differential analysis to obtain 2,201, 488 and 1,649 diabetic nephropathy differential genes associated with tubulointerstitial damage in three datasets, respectively. (Figures 2A–C). The above gene sets were intersected with 1,046 cuproptosis-related gene sets collected by literature review and database mining. Then, a DECG gene set with 33 genes was obtained. (Figure 2D).
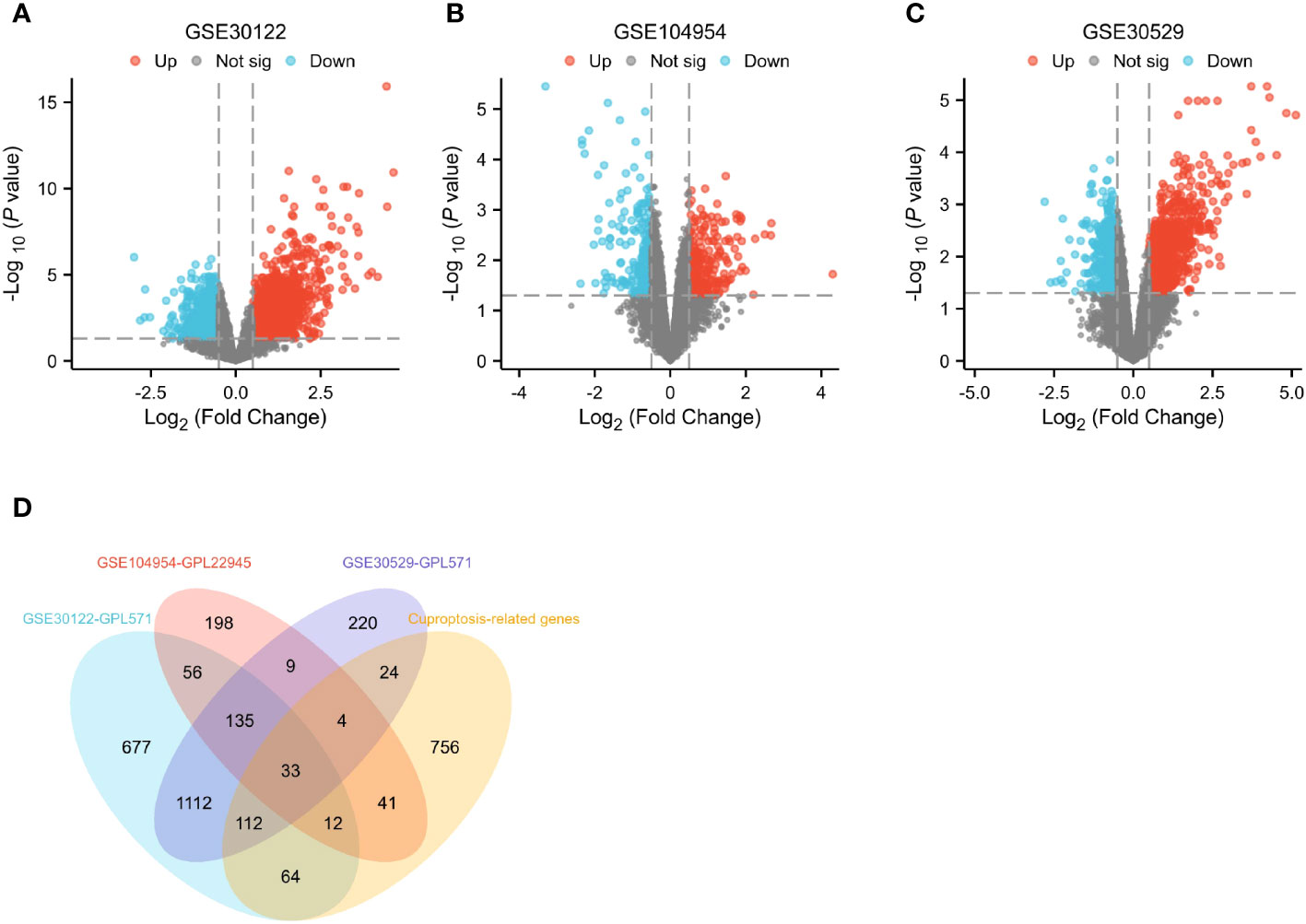
Figure 2 Identification of differential genes. (A–C) Differential gene expression on training cohort (GSE30122, GSE104954 and GSE30529). (D) Venn diagrams of differentially expressed genes.
3.2 Immune infiltration analysis and construction of weighted gene co-expression networks
Cibersort analysis suggested that the infiltration levels of nine kinds of immune cells in the GSE30529 database showed statistical differences in the tubulointerstitial damage of the diabetic nephropathy group. These immune cells included plasma cells, T cells cluster of differentiation 8 (CD8), T cells CD4 memory resting, T cells regulatory (Tregs), T cells gamma delta, natural killer (NK) cells resting, macrophages M1, and mast cells resting and activated. (Figure 3A). Meanwhile, 10 immune cell types were also shown to differ in infiltration level between DKD and control groups in the GSE 30122 dataset. These immune cell types contained T cells CD8, CD4 memory resting, Tregs, T cells gamma delta, NK cells resting, macrophages M0 and M1, dendritic cells resting, and mast cells resting and activated. (Figure 3B) In addition, two differentially distributed immune infiltrating cells were in the GSE104945 dataset, namely dendritic cells and mast resting. (Supplementary Figure 1).
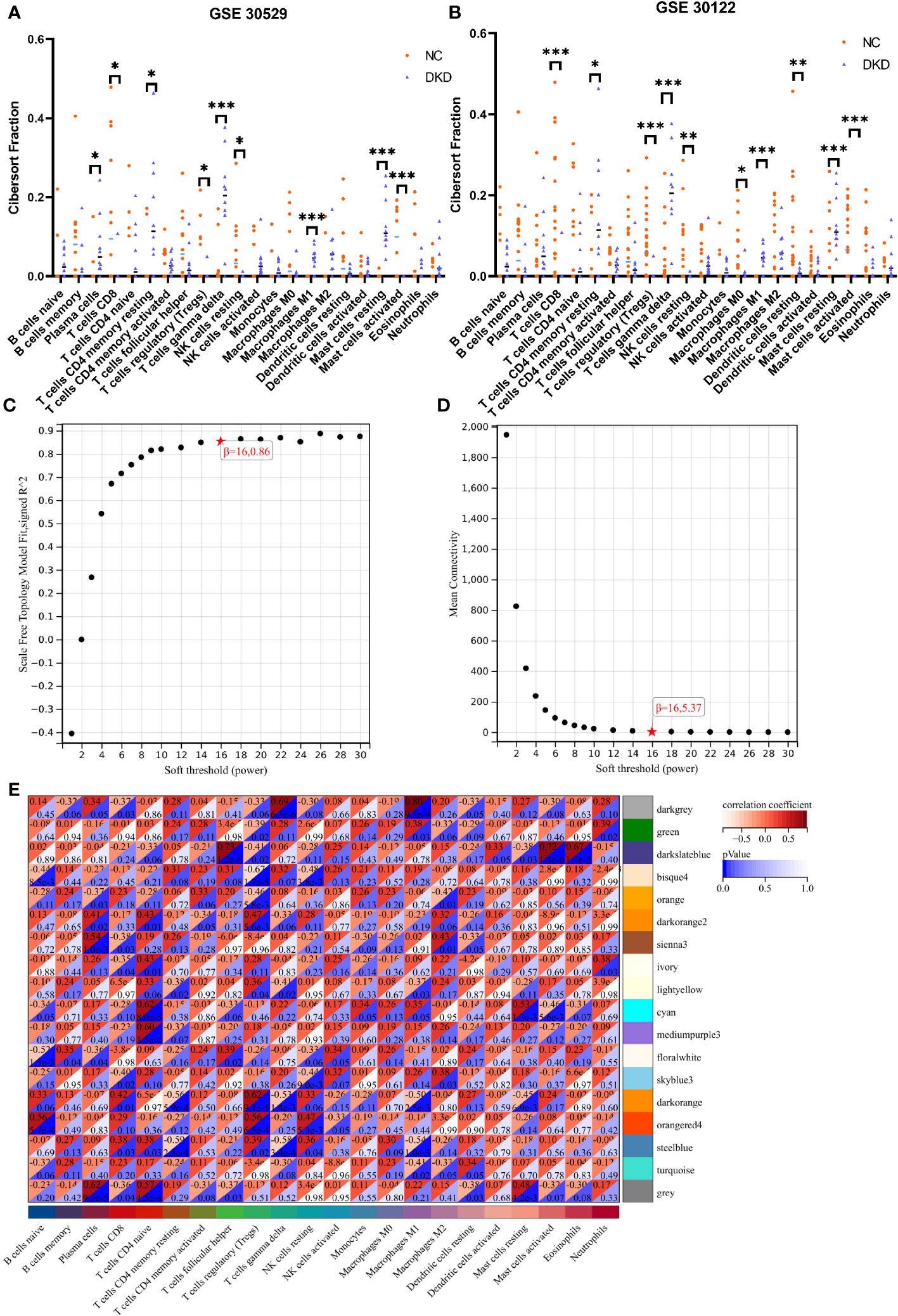
Figure 3 Immune infiltration analysis and construction of weighted gene co-expression networks. (A, B) Comparison of infiltration levels of immune cells in DKD group and control group inGSE30529 and GSE30122 database. (C, D). The mean connectivity plot and scale-free topology plots, 16 was an appropriate soft-power. (E) 18 modules revealed by the WGCNA analysis. DKD, diabetic kidney disease; WGCNA, weighted gene co-expression network analysis. p values were showed as: *,p < 0.05; **,p < 0.01, ***,p <0.001.
The soft-threshold power, which was more than a scale-free topology fit index of 0.86 for each network, was 16. Thus, 16 was selected as the soft power threshold for the construction of WGCNA. (Figure 3C). In the meantime, the network connectivity under different soft thresholds is shown in Figure 3D. Subsequently, 18 modules were presented in the analysis process. Among them, six modules, including dark orange, bisque4, darkgrey, orangered4, cyan and darkslateblue, demonstrated the strongest positive correlation with the infiltration level of immune cells and were taken into consideration for further investigation (Figure 3E).
3.3 Identification and GO enrichment of DEICGs
DEICGs were further harvested by intersecting the six correlation modules obtained from WGCNA with the DECGs obtained from the previous analysis. (Figure 4A). To increase the representativeness of the subsequent results, the GSE104954 dataset was combined with the GSE30122, and the batch effect was removed. (Figures 4B, C). Increased messenger RNA (mRNA) levels of DEICGs were observed in the heatmaps of two datasets. (Figure 4D). The GO enrichment analysis results demonstrated that DEICGs mainly got involved in biological processes like response to stress and anatomical structure morphogenesis, were distributed in cellular components like extracellular space, and played a role in cytokine receptor activity and other molecular functions (Figures 4E–G).
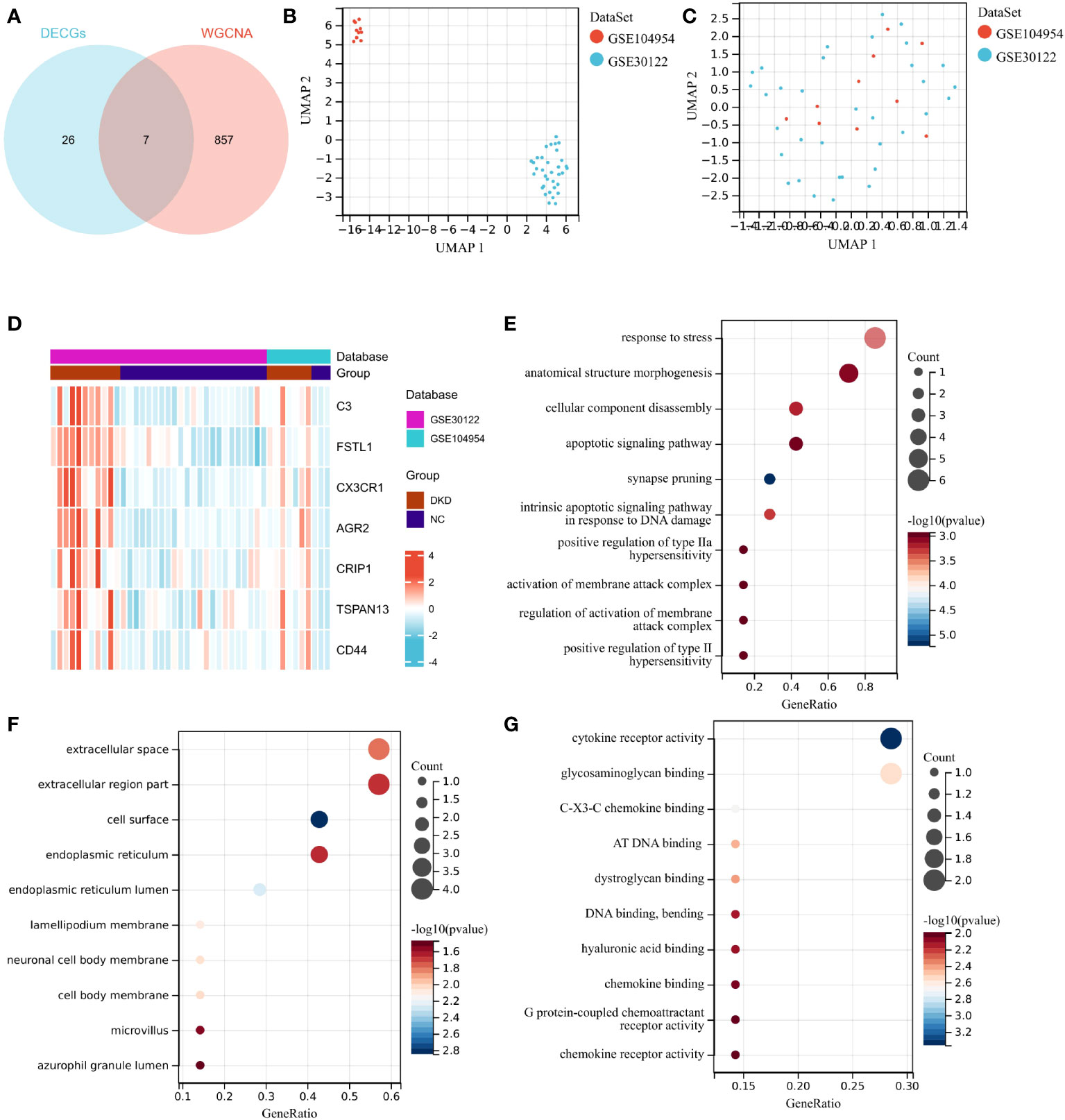
Figure 4 Identification of DEICGs and GO enrichment of them. (A) The immune-related genes screened by WGCNA analysis were combined with DECGs to draw the Venn diagrams, and thus DEICGs was obtained. (B, C) Sample distribution of GSE104954 and GSE30122 data sets before and after batch effect correction. (D) Distribution of DEICGs in GSE104954 and GSE30122 datasets. (E–G) Gene functions were categorized based on BP, MF, and CC by GO enrichment analysis. DECGs, differentially expressed cuproptosis-related genes; DEICGs, differentially expressed immune-related cuproptosis genes; BP, Biological Process; CC, Cell Component; MF, Molecular Function).
3.4 Screening of hub genes by machine learning and PPI networks
A machine learning algorithm model was further built to extract key genes from DEICGs that have more diagnostic significance for diabetic nephropathy. We first tested the machine learning algorithm on the dataset merged with GSE30122 and GSE104954. Through the LASSO regression algorithm, a signature of five genes was obtained. (Figures 5A, B). As for SVM-RFE, seven genes experienced the lowest classifier error and highest classifier accuracy (minimal error = 0.0837, maximal accuracy = 0.916) (Figures 5C, D). We then tested the above analysis with GSE30122, and five and seven genes were validated in LASSO regression algorithm and SVM-RFE analyses, respectively. The overlapping part of the test and validation results were selected to represent the final results of LASSO regression algorithm and SVM-RFE analyze to participate in the subsequent produce the final hub genes. Meanwhile, five genes were predicted from the PPI network by using the CytoHubba plug-in software in Cytoscape (Figure 5E). By intersecting the above three gene sets through the Venn diagram, three hub genes were obtained at last (Figure 5F). Among them, FSTL1 has been proved to promote the progression of focal segmental glomerular sclerosis(FSGS), membranous nephropathy (MN), immunoglobulin A (IgA) nephropathy (IgAN), and other chronic kidney diseases. CX3CR1 has been reported to regulate immune responses such as inflammation, cell adhesion, and chemotaxis, and therefore plays a crucial role in the progression of kidney diseases such as IgA nephritis, nephrotoxic nephritis, and renal candidiasis. The role of AGR2 in DKD disease progression has been predicted but not confirmed by experimental data. Combined with the above information, we believe that the three hub genes have great potential as markers of progression of diabetic tubulopathy. In further evaluation, the ROC curve showed high AUC levels for FSTL1, CX3CR1 and AGR2, which were 0.911, 0.935 and 0.922, respectively. The cut-off values of FSTL1,CX3CR1 and AGR2 genes were 4.636, 3.425 and 3.101, respectively. The sensitivity and specificity were 0.926 and 0.765 for FSTL1, 1 and 0.882 for CX3CR1 and those of AGR2 is 0.926 and 0.765 (Figure 5G). The above data indicated the three genes’ high diagnostic accuracy. When taken as a whole, the AUC of the ROC curve of the disease prediction model composed of the three hub genes was 0.946, the cut-off value was 0.633 and the sensitivity and specificity were 0.963 and 0.941 respectively (Figure 5H).
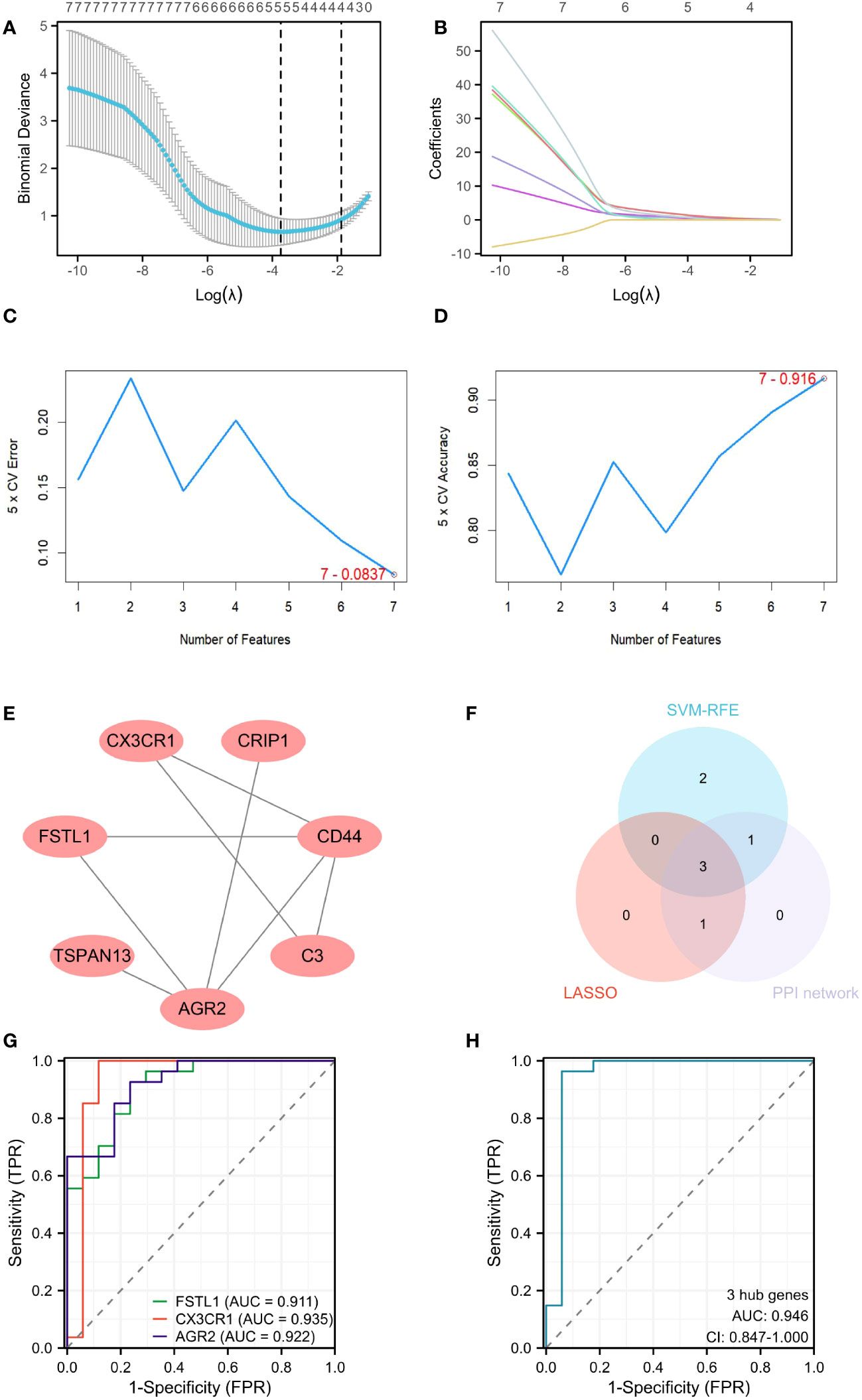
Figure 5 Screening hub genes by machine learning. (A, B) Cross-validation curves and regression coefficient path diagram in LASSO logistic regression algorithm. (C, D) The curve of change in the predicted true and error value of each gene in SVM-RFE algorithm. (E) Plot the PPI interaction network for DEICGs. (F) Venn diagram demonstrates the intersection of the above three analyses. (G) ROC curve analysis for 3 hub genes. (H) ROC analysis of prediction model composed of 3 hub genes. LASSO, least absolute shrinkage and selection operator; SVM-RFE, support vector machine-recursive feature elimination; PPI, protein-protein interaction; ROC, receiver operating characteristic.
3.5 Expression of hub genes and validation of external datasets
Both the training set GSE30122 and the validation set GSE30529 from the GEO database showed a consistent increase in the mRNA levels of three hub genes, including FSTL1, CX3CR1 and AGR2, in the DKD group. (Figures 6A–F). When it comes to clinical patients, correlation analysis showed that the expression level of FSTL1 had a positive correlation with the level of serum creatinine (p=0.02, R2 = 0.311) and a negative correlation with GFR(p<0.001, R2 = 0.597) (Figures 6G, H). The expression levels of CX3CR1 and AGR2 also displayed a negative correlation with GFR (p1<0.001,p2<0.001,R12 = 0.614,R22 = 0.742) (Figures 6I, J).
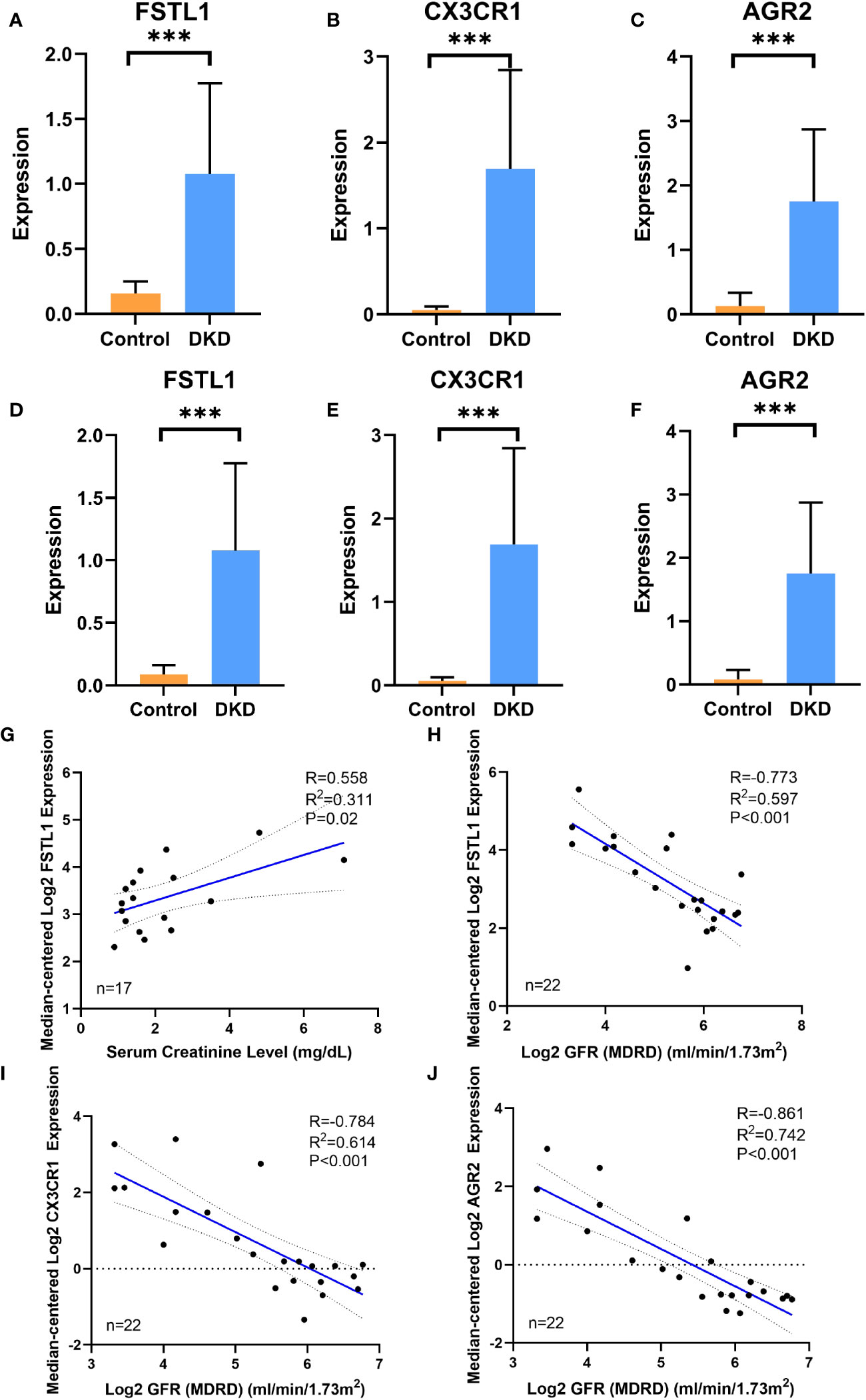
Figure 6 The expression of hub genes in disease database and its correlation with clinical features (A–C). The expression level of FSTL1, CX3CR1 and AGR2 in DN group and control group in training cohort GSE30122. (D–F) The expression level of FSTL1, CX3CR1 and AGR2 in DKD group and control group in validation cohort GSE30529. (G, H) Correlation analysis of FSTL1 expression with serum creatinine and GFR level. (I) Correlation analysis of CX3CR1 expression with GFR level. (J) Correlation analysis of AGR2 expression with GFR level. GFR, glomerular filtration rate. p values were showed as: ***,p <0.001.
3.6 GSEA analysis
GSEA analysis revealed that the FSTL1 high-expression group was mostly concentrated in pathways like primary focal segmental glomerulosclerosis (FSGS), nephrotic syndrome, and IL-4 and IL-13 signaling and neutrophil degranulation (Figure 7A). The CX3CR1 high-expression group was highly enriched for the innate immune system, signaling by ILs and the complement cascade (Figure 7B). Reactomes like the innate immune system, cytokine signaling in the immune system and signaling by ILs were all associated with elevated AGR2 levels. (Figure 7C).
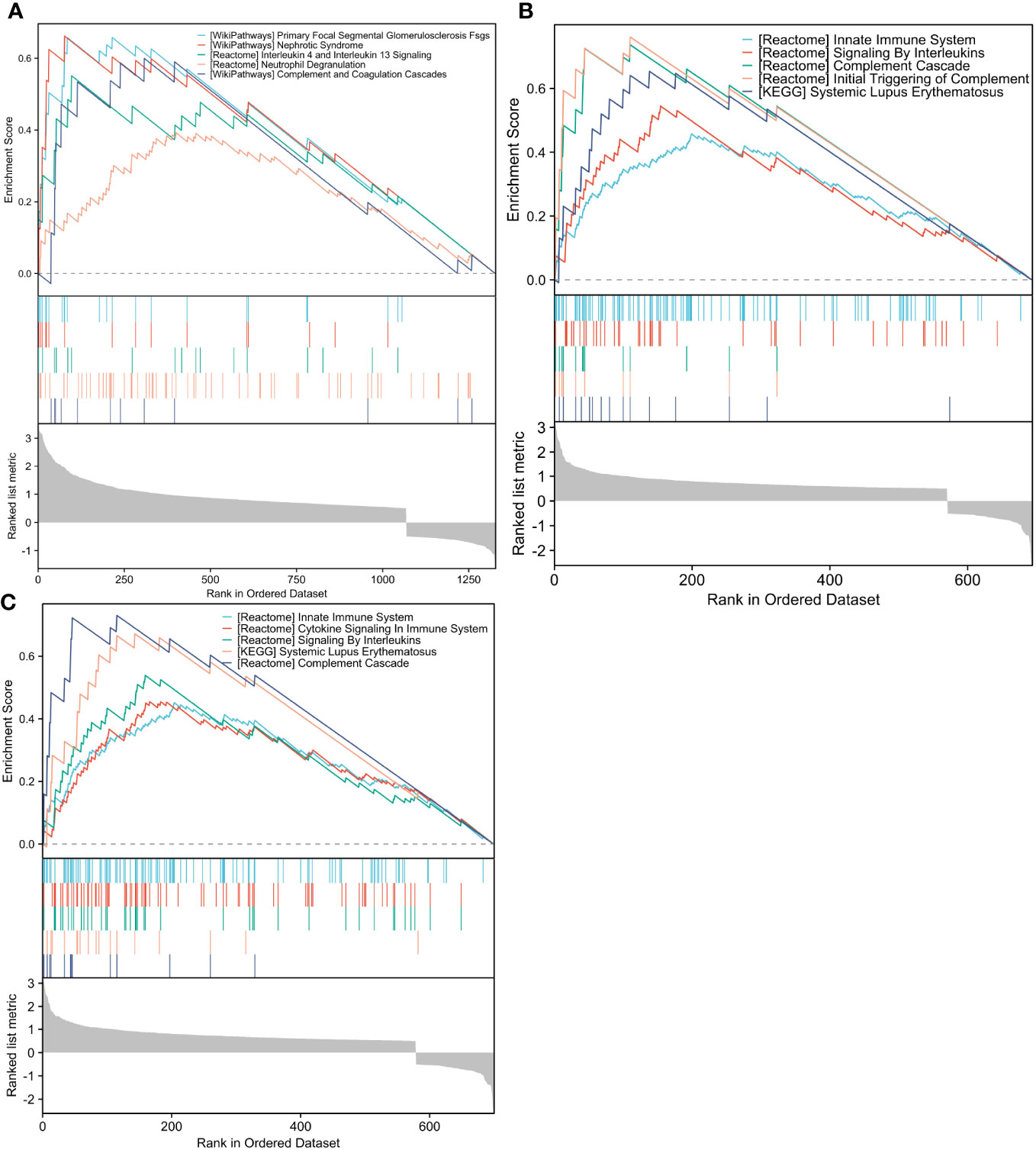
Figure 7 GSEA analysis of hub genes. (A–C). The differential genes with high and low FSTL1, CX3CR1 and AGR2 expression in GSE30122 database were enriched by ESEA analysis. GSEA, Gene Set Enrichment Analysis.
3.7 Single Nucleus RNA sequencing of hub genes
Single-nucleus RNA sequencing was performed to identify how the three hub genes were distributed in 12 cell populations (Figure 8A). Among them, FSTL1 was mainly distributed in podocyte and mesangial cells (Figure 8B), CX3CR1 was highly expressed in leukocytes (Figure 8C) and AGR2 was mostly localized in the loop of Henle (Figure 8D).
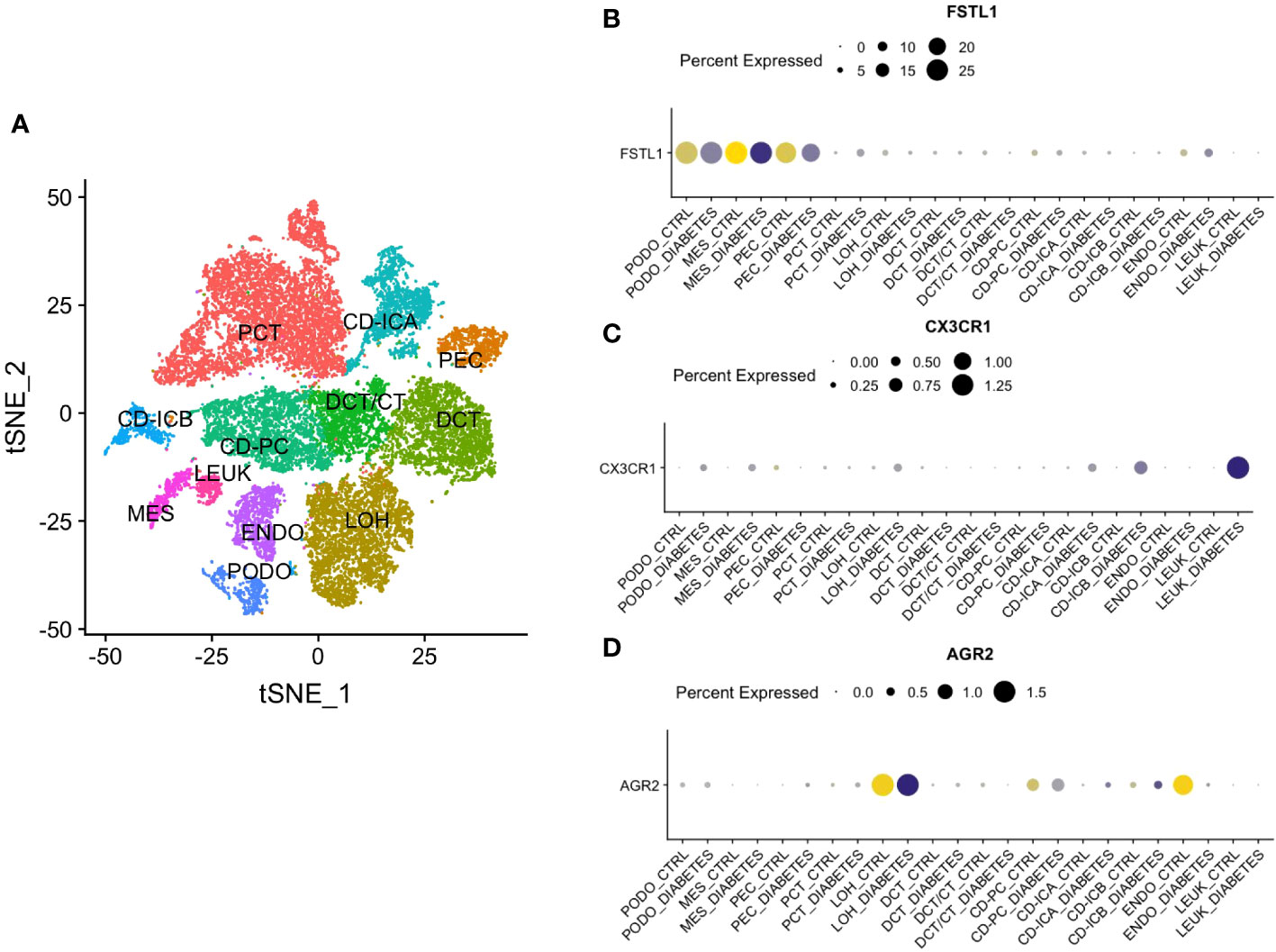
Figure 8 Single Nucleus RNA Sequencing of hub genes. (A) The division of 12 cell types. (B) The distribution of FSTL1. (C) The distribution of CX3CR1. (D) The distribution of AGR2. CD, collecting duct. ICA, Type A intercalated cells. ICB, Type B intercalated cells. PEC, parietal epithelial cells. PC, principal cell. DCT, distal convoluted tubule. CT, connecting tubule. LOH, loop of Henle. PODO, podocyte. ENDO, endothelium. MES, mesangial cell. LEUK, leukocyte.
3.8 Expression level of hub genes in the kidneys of DKD mice
To assess the diagnostic value of hub genes at the mouse level, a mouse model of type 1 diabetes with STZ was constructed, and the specific methods are described in Figure 9A. One week after the injection, the fasting blood glucose of the DKD group was all greater than 15mmol/L, which indicated the successful construction of the diabetes model. (Figure 9B). After the mice were weighed weekly for 12 weeks, it was found that the DKD group was lighter than the control one, which is consistent with the clinical manifestations of diabetes (Figure 9C). H&E staining indicated that the DKD group was characterized by the hypertrophy of glomerular capillary loops, glomerular enlargement and the vacuolar degeneration of renal tubular epithelial cells (Figure 9D). PAS staining demonstrated mesangial hyperplasia, basement membrane thickening, glomerular sclerosis and other additional abnormalities in the DKD group (Figure 9D). The quantitative real-time polymerase chain reaction (RT-qPCR) results of the mouse renal cortex sample suggested that the expression levels of all hub genes showed an increase in both STZ treatment group and db/db group. (Figures 9E–G).
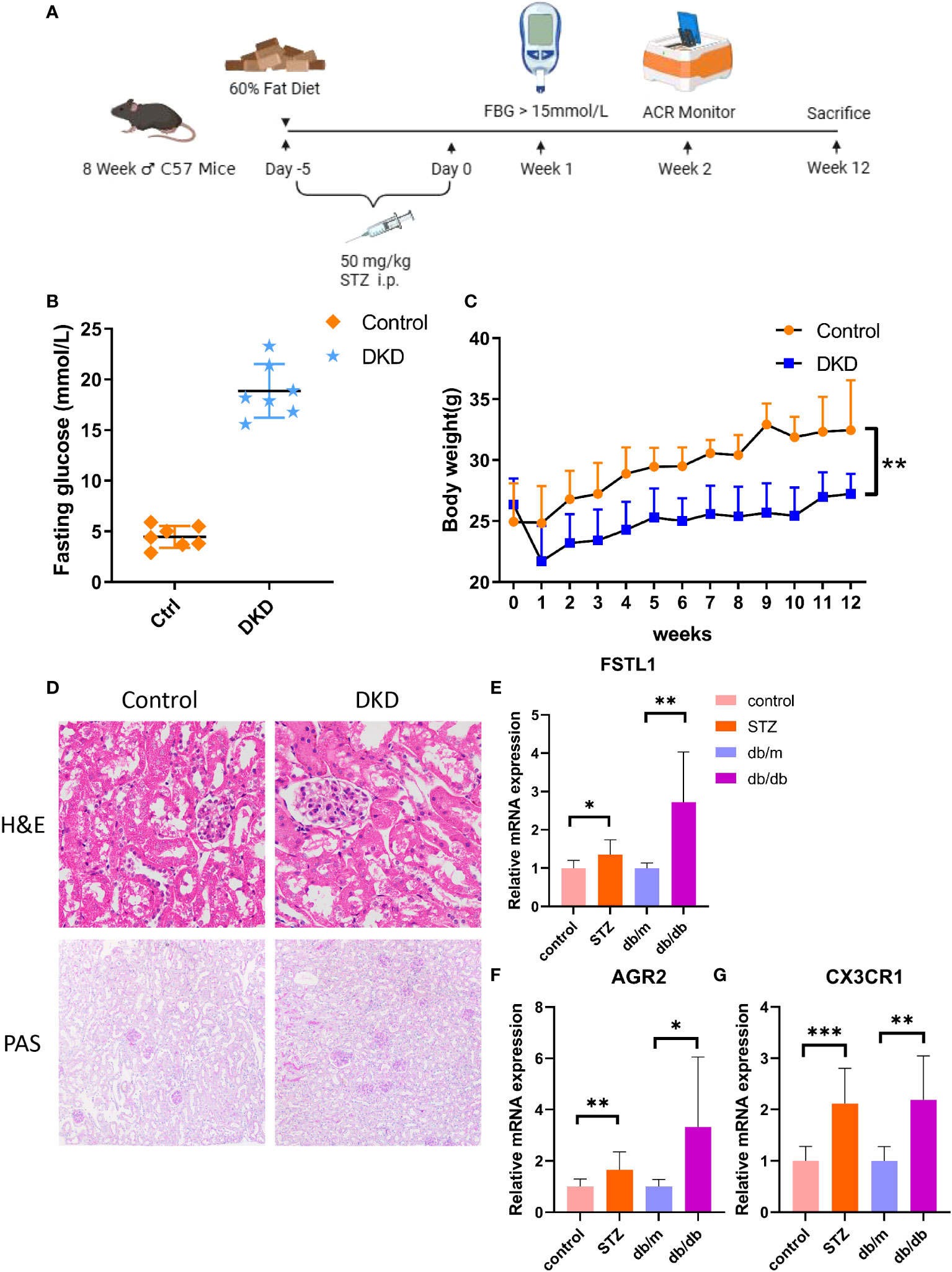
Figure 9 The expression level of hub genes in kidney of DKD mice. (A) Schematic diagram of STZ-induced mouse DKD models. (B) Fasting glucose in both control and STZ-induced DKD mice. (C) Body weight in both control and STZ-induced DKD mice. (D) The H&E and PAS staining in control and STZ-induced DKD mice. (E–G). mRNA expression of FSTL1, AGR2 and CX3CR1 by RT-qPCR. p values were showed as: *,p < 0.05; **,p < 0.01, ***,p < 0.001. STZ, streptozocin; H&E, hematoxylin-eosin; PAS, schiff periodic acid shiff.
4 Discussion
Proximal tubular injury associated with DKD, a microvascular complication with the highest mortality rate in diabetes, has always been a vital focus of investigation on the pathogenesis of the disease (15). It has been shown that oxidative stress, hypoxia, albumin overload, inflammation, EMT, cellular senescence and dysfunctional nutrient-sensing pathways all play a vital role in the progression of diabetic tubulopathy (7). However, it remains difficult to diagnose DKD early due to the complexity of the disease, and effective biomarkers are insufficient to diagnose the disease and predict its prognosis. It is known that the progression of diabetic tubulopathy is closely related to immune infiltration and mitochondrial dysfunction (16).
In this study, a series of differentially expressed genes were obtained in DKD renal tubules by mining the GEO database. They were intersected with genes related to cuproptosis and immunity to get the DEICGs gene set. Machine learning algorithms, which process large amounts of biological data to group them and build models, are widely used for predicting disease markers and treatment targets (17). During the analysis process, genes were further screened with the help of machine learning, the WGCNA algorithm and PPI protein interaction analysis. Eventually, three hub genes were found, namely FSTL1, CX3CR1 and AGR2. The ROC curve mapping of hub genes and the correlation analysis of the gene expression levels, serum creatinine and GFR of clinical patients further suggested their significant diagnostic and prognostic efficacy. To explore the possible mechanism of hub genes, GO and GSEA pathway enrichment analyses were also conducted on them. It was found that inflammatory and immune-related pathways like IL-4 and IL-13 signaling and the innate immune system occurred frequently, which may be crucial for the progression of DKD. After the validation of the aforementioned hug genes in the external dataset GSE30529, the higher levels of hub genes in STZ-induced type 1 and db/db type 2 diabetic mouse models were further demonstrated, respectively.
FSTL1 is a secreted glycoprotein that is important in various physiological processes, such as angiogenesis, immune response regulation, and cell proliferation and differentiation. In human kidneys, single-cell transcriptomic data suggest that FSTL1 is only distributed in interstitial fibroblasts and myofibroblasts (18). By activating nuclear factor-kappa B (NF-κB) and wingless (Wnt)/β-catenin pathways, FSTL1 has been found to promote the progression of different forms of CKD, including focal segmental glomerular sclerosis(FSGS), membranous nephropathy (MN), immunoglobulin A (IgA) nephropathy (IgAN), etc. (19, 20). In the present study, the expression level of FSTL1 in the renal tubules of DKD individuals was increased in both test and validation sets and displayed a positive correlation with the serum creatinine level of patients and a negative correlation with GFR levels. The above results were further validated in both type 2 and 1 diabetic mouse models. ROC analysis indicated that the AUC area of FSTL1 in diabetic tubulopathy was 0.911, which indicated its strong diagnostic efficacy. The association between FSTL1 and DKD has not been reported previously. However, it has been reported that FSTL1 influences the progression of fibrosis in diabetic retinopathy, another type of diabetic microangiopathy, through the extracellular matrix (ECM) receptor pathway (21). GSEA strongly implicated that immune-related pathways such as IL-4 and IL-13 signaling, neutrophil degranulation and complement and coagulation cascades pathways are significantly affected by FSTL1 in DKD.
CX3CR1 belongs to the G-protein coupling receptor (GPCR) superfamily and is a cell surface receptor mostly expressed in T lymphocytes, monocytes and NK and mast cells (22–24). Studies suggest that CX3CR1 regulates many aspects of the immune response, such as inflammation, cell adhesion and chemotaxis. CX3CR1 is crucial in the progression of IgA nephritis, nephrotoxic nephritis, renal candidiasis and other renal diseases (25). Few relevant studies have focused on CX3CR1 in DKD. It was only noted that CX3CR1 was up-regulated in DKD many years ago. In the current study, CX3CR1 was observed to be up-regulated in the renal tissues of DKD patients and have a diagnostic accuracy value (AUC = 0.935). The expression levels of CX3CR1 were negatively correlated with GFR levels in DKD patients. The related mechanism mainly involved the innate immune system, signaling by interleukins and complement cascade pathways.
AGR2 was screened from MCF7, an estrogen receptor positive breast cancer cell line, and its transcription is responsive to estrogen at the molecular level (26). AGR2 has been implicated in inflammatory bowel disease and cancer progression (27, 28), and mechanically, promotes angiogenesis and increases the invasiveness of tumor cells (29). Previous bioinformatics studies predicted the efficacy of the AGR2 gene in the detection of diabetic renal tubular disease but failed to verify their conclusions in disease samples (30). In this study, it was noticed that AGR2 was up-regulated in the tubule tissues of DKD individuals and higher expression levels of AGR2 were often associated with lower GER levels, which signified a close association between AGR2 and diabetic tubulopathy progression. The ROC curve showed that AGR2 had a high AUC level of 0.992, which indicated its high diagnostic accuracy. In addition, the mechanism of AGR2 getting involved in DKD is mostly ascribed to the innate immune system, cytokine signaling in the immune system and signaling by interleukins.
Common mechanisms discovered in DKD include metabolic, hemodynamic, inflammatory and fibrotic mechanisms, each of which provides potential therapeutic targets (31–33). A plethora of studies have demonstrated the functional role of inflammation in DKD, which can be triggered by the interaction of metabolic defects, secondary injury mediators (like angiotensin II and aldosterone) and pathological consequences of kidney cell injury or dysfunction (such as albuminuria, loss of Klotho production, lipid accumulation and lipotoxicity) (34, 35). At this point, the infiltration of massive immune cells occurs. The enrichment analysis of hub genes found that they affect the progression of DKD mainly through immune and inflammatory pathways, which corresponds to the pathophysiological process of DKD and indicates their diagnostic efficacy.
In this study, three hub genes in diabetic tubulopathy, which are closely related to cuproptosis and immunity and have high diagnostic efficacy, were innovatively identified. Some limitations are worth noting. First, the specific mechanism of hub genes in DKD needs to be further verified, which is also the focus of our follow-up work. Moreover, the expression level and predictive performance of hub gene in other renal diseases that are often differentiated from diabetic nephropathy, such as hypertensive nephropathy, IgA nephropathy and membranous nephropathy, should also be considered, which is crucial for the subsequent application of the prediction model composed of these three hub genes, although their diagnostic efficacy of this model for diabetic kidney disease has been fully verified. As a result, it is necessary to test the expression of hub genes in other chronic kidney diseases in future work.
5 Conclusion
In this study, immune infiltration analysis was combined with WGCNA analysis, machine learning and PPI network analysis to obtain three valuable hub genes: FSTL1, CX3CR1 and AGR2. Then, the valuable clinical efficacy of these hub genes was further demonstrated, and their possible mechanisms were predicted with the help of GSEA analysis, a ROC curve, single-cell RNA sequencing and some online clinical databases. Finally, a DKD model of C57 mice was constructed, and the expression of animal-level hub genes was verified. FSTL1, CX3CR1 and AGR2 all have great potential as diagnostic markers for DKD and even predict disease progression.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was approved by Animal Care and Use Committee of Guangxi Medical University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YC: Conceptualization, Formal Analysis, Investigation, Visualization, Writing – original draft. LL: Writing – original draft, Investigation, Validation. BW: Writing – review & editing, Data curation, Methodology, Software, Supervision. ZW: Funding acquisition, Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Innovation Project of Guangxi Graduate Education (YCBZ2023112).
Acknowledgments
Thanks to GEO(https://www.ncbi.nlm.nih.gov/geo/), GSEA(https://www.ncbi.nlm.nih.gov/geo/), Geencards (https://www.genecards.org), Cibersort (https://cibersortx.stanford.edu), STRING (https://cn.string-db.org/), Nephroseq (http://v5.nephroseq.org) and K.I.T (http://humphreyslab.com/SingleCell/) database contributors for sharing the data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1332279/full#supplementary-material
Supplementary Figure 1 | Comparison of infiltration levels of immune cells in DKD group and control group in GSE104954.
Supplementary Figure 2 | Screening hub genes by machine learning in GSE30122. (A, B) Cross-validation curves and regression coefficient path diagram in LASSO logistic regression algorithm; (C, D) The curve of change in the predicted true and error value of each gene in SVM-RFE algorithm.
Supplementary Table 1 | List of genes related to cuproptosis.
References
1. Bikbov B, Purcell CA, Levey AS, Smith M, Abdoli A, Abebe M, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (2020) 395(10225):709–33. doi: 10.1016/S0140-6736(20)30045-3
2. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease. Clin J Am Soc Nephrology. (2017) 12(12):2032–45. doi: 10.2215/CJN.11491116
3. Duru OK, Middleton T, Tewari MK, Norris K. The landscape of diabetic kidney disease in the United States. Curr Diabetes Rep (2018) 18(3):14. doi: 10.1007/s11892-018-0980-x
4. Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrology. (2013) 24(2):302–8. doi: 10.1681/ASN.2012070718
5. Jung C-Y, Yoo T-H. Pathophysiologic mechanisms and potential biomarkers in diabetic kidney disease. Diabetes Metab J (2022) 46(2):181–97. doi: 10.4093/dmj.2021.0329
6. Tang S, Leung J, Lai K. Diabetic tubulopathy: an emerging entity. Contributions to nephrology. (2011) 170:124–34. doi: 10.1159/000325647
7. Faria J, Gerritsen KGF, Nguyen TQ, Mihaila SM, Masereeuw R. Diabetic proximal tubulopathy: Can we mimic the disease for in vitro screening of SGLT inhibitors? Eur J Pharmacol (2021) 908:174378. doi: 10.1016/j.ejphar.2021.174378
8. Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Sci (New York NY). (2022) 375(6586):1254–61. doi: 10.1126/science.abf0529
9. Bhargava P, Schnellmann RG. Mitochondrial energetics in the kidney. Nat Rev Nephrology. (2017) 13(10):629–46. doi: 10.1038/nrneph.2017.107
10. Klessens CQF, Zandbergen M, Wolterbeek R, Bruijn JA, Rabelink TJ, Bajema IM, et al. Macrophages in diabetic nephropathy in patients with type 2 diabetes. Nephrol Dialysis Transplantation. (2016) 32(8):1322–1329. doi: 10.1093/ndt/gfw260
11. Nguyen DUY, Ping FU, Mu WEI, Hill P, Atkins RC, Chadban SJ. Macrophage accumulation in human progressive diabetic nephropathy. Nephrology (2006) 11(3):226–31. doi: 10.1111/j.1440-1797.2006.00576.x
12. Tibshirani R. The lasso method for variable selection in the Cox model. Stat Med (1997) 16(4):385–95. doi: 10.1002/(SICI)1097-0258(19970228)16:4<385::AID-SIM380>3.0.CO;2-3
13. Lin X, Li C, Zhang Y, Su B, Fan M, Wei H. Selecting feature subsets based on SVM-RFE and the overlapping ratio with applications in bioinformatics. Molecules (Basel Switzerland). (2017) 23(1):52. doi: 10.3390/molecules23010052
14. Wilson P, Wu H, Kirita Y, Uchimura K, Ledru N, Rennke H, et al. The single-cell transcriptomic landscape of early human diabetic nephropathy. Proc Natl Acad Sci United States America. (2019) 116(39):19619–25. doi: 10.1073/pnas.1908706116
15. Barkoudah E, Skali H, Uno H, Solomon SD, Pfeffer MA. Mortality rates in trials of subjects with type 2 diabetes. J Am Heart Assoc (2012) 1(1):8–15. doi: 10.1161/JAHA.111.000059
16. Forbes JM, Thorburn DR. Mitochondrial dysfunction in diabetic kidney disease. Nat Rev Nephrology. (2018) 14(5):291–312. doi: 10.1038/nrneph.2018.9
17. Greener JG, Kandathil SM, Moffat L, Jones DT. A guide to machine learning for biologists. Nat Rev Mol Cell Biol (2021) 23(1):40–55. doi: 10.1038/s41580-021-00407-0
18. Maksimowski NA, Song X, Bae EH, Reich H, John R, Pei Y, et al. Follistatin-like-1 (FSTL1) is a fibroblast-derived growth factor that contributes to progression of chronic kidney disease. Int J Mol Sci (2021) 22(17):9513. doi: 10.3390/ijms22179513
19. Zhang Y, Wang Y, Zheng G, Liu Y, Li J, Huang H, et al. Follistatin-like 1 (FSTL1) interacts with Wnt ligands and Frizzled receptors to enhance Wnt/β-catenin signaling in obstructed kidneys in vivo. J Biol Chem (2022) 298(7):102010. doi: 10.1016/j.jbc.2022.102010
20. Hayakawa S, Ohashi K, Shibata R, Kataoka Y, Miyabe M, Enomoto T, et al. Cardiac myocyte-derived follistatin-like 1 prevents renal injury in a subtotal nephrectomy model. J Am Soc Nephrology. (2015) 26(3):636–46. doi: 10.1681/ASN.2014020210
21. Niu R, Nie Z, Liu L, Chang Y, Shen J, Chen Q, et al. Follistatin-like protein 1 functions as a potential target of gene therapy in proliferative diabetic retinopathy. Aging (2021) 13(6):8643–64. doi: 10.18632/aging.202678
22. Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell (1997) 91(4):521–30. doi: 10.1016/S0092-8674(00)80438-9
23. Landsman L, Bar-On L, Zernecke A, Kim K, Krauthgamer R, Shagdarsuren E, et al. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood (2009) 113(4):963–72. doi: 10.1182/blood-2008-07-170787
24. Park M, Lee J, Yoon J. High expression of CX3CL1 by tumor cells correlates with a good prognosis and increased tumor-infiltrating CD8+ T cells, natural killer cells, and dendritic cells in breast carcinoma. J Surg Oncol (2012) 106(4):386–92. doi: 10.1002/jso.23095
25. von Vietinghoff S, Kurts C. Regulation and function of CX3CR1 and its ligand CX3CL1 in kidney disease. Cell Tissue Res (2021) 385(2):335–44. doi: 10.1007/s00441-021-03473-0
26. Zhang K, Li Y, Kong X, Lei C, Yang H, Wang N, et al. AGR2: a secreted protein worthy of attention in diagnosis and treatment of breast cancer. Front Oncol (2023) 13. doi: 10.3389/fonc.2023.1195885
27. Tian S, Chu Y, Hu J, Ding X, Liu Z, Fu D, et al. Tumour-associated neutrophils secrete AGR2 to promote colorectal cancer metastasis via its receptor CD98hc-xCT. Gut (2022) 71(12):2489–2501. doi: 10.1136/gutjnl-2021-325137
28. Al-Shaibi AA, Abdel-Motal UM, Hubrack SZ, Bullock AN, Al-Marri AA, Agrebi N, et al. Human AGR2 deficiency causes mucus barrier dysfunction and infantile inflammatory bowel disease. Cell Mol Gastroenterol Hepatology. (2021) 12(5):1809–30. doi: 10.1016/j.jcmgh.2021.07.001
29. Zhang H, Chi J, Hu J, Ji T, Luo Z, Zhou C, et al. Intracellular AGR2 transduces PGE2 stimuli to promote epithelial–mesenchymal transition and metastasis of colorectal cancer. Cancer letters. (2021) 518:180–95. doi: 10.1016/j.canlet.2021.06.025
30. Zhou L-T, Zhang Z-J, Cao J-Y, Chen H, Zhu Y-S, Wu X, et al. The unique molecular mechanism of diabetic nephropathy: a bioinformatics analysis of over 250 microarray datasets. Clin Kidney J (2021) 14(6):1626–38. doi: 10.1093/ckj/sfaa190
31. Alicic R, Rooney M, Tuttle K. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol CJASN. (2017) 12(12):2032–45. doi: 10.2215/CJN.11491116
32. Pérez-Morales R, Del Pino M, Valdivielso J, Ortiz A, Mora-Fernández C, Navarro-González J. Inflammation in diabetic kidney disease. Nephron (2019) 143(1):12–6. doi: 10.1159/000493278
33. Mora-Fernández C, Domínguez-Pimentel V, de Fuentes M, Górriz J, Martínez-Castelao A, Navarro-González J. Diabetic kidney disease: from physiology to therapeutics. J Physiol (2014) 592(18):3997–4012. doi: 10.1113/jphysiol.2014.272328
34. Ruiz-Ortega M, Rayego-Mateos S, Lamas S, Ortiz A, Rodrigues-Diez R. Targeting the progression of chronic kidney disease. Nat Rev Nephrology. (2020) 16(5):269–88. doi: 10.1038/s41581-019-0248-y
Keywords: diabetic nephropathy, bioinformatic analysis, machine learning, WGCNA, biomarker
Citation: Chen Y, Liao L, Wang B and Wu Z (2024) Identification and validation of immune and cuproptosis - related genes for diabetic nephropathy by WGCNA and machine learning. Front. Immunol. 15:1332279. doi: 10.3389/fimmu.2024.1332279
Received: 02 November 2023; Accepted: 26 January 2024;
Published: 08 February 2024.
Edited by:
Ramcés Falfán-Valencia, National Institute of Respiratory Diseases-Mexico (INER), MexicoReviewed by:
Yu Zhou, Yangzhou University, ChinaHaofuzi Zhang, Fourth Military Medical University, China
Copyright © 2024 Chen, Liao, Wang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhan Wu, d3V6aGFuY3JpY2tAMTYzLmNvbQ==; Baoju Wang, NTg0OTYzODg2QHFxLmNvbQ==
 Yubing Chen
Yubing Chen Lijuan Liao
Lijuan Liao Baoju Wang
Baoju Wang Zhan Wu
Zhan Wu