
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol. , 01 March 2024
Sec. Inflammation
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1330386
Introduction: Chronic low-grade inflammation is an important aspect of morbidity and mortality in older adults. The level of circulating pro-inflammatory cytokines (interleukin (IL)-6, tumor necrosis factor (TNF) or IL-1β) is a risk factor in cardiovascular and neurodegenerative diseases and is also associated with sarcopenia and frailties. The objective of this study was to assess each cytokine: IL-6, TNF, and IL-1β separately in the elderly with comorbidities against controls without diseases according to the data published in the available literature.
Methods: The electronic bibliographic PubMed database was systematically searched to select all the relevant studies published up to July 2023. The total number of the subjects involved in the meta-analysis included patients with diseases (n=8154) and controls (n=33967).
Results: The overall concentration of IL-6 was found to be higher in patients with diseases compared to controls and the difference was statistically significant, with a p-value of <0.001 (SMD, 0.16; 95% CI, 0.12–0.19). The heterogeneity was considerable with Q = 109.97 (P <0.0001) and I2 = 79.2%. The potential diagnostic usefulness of IL-6 was confirmed by odds ratio (OR) analysis (OR: 1.03, 95% CI (1.01; 1.05), p=0.0029). The concentration of both TNF and IL-1β was elevated in the control group compared to patients and amounted to SMD -0.03; 95% CI, -0.09–0.02, p-value 0.533 and SMD-0.29; 95% CI, -0.47– -0.12; p = 0.001, respectively. For TNF, however, the difference was statistically insignificant.
Discussion: IL-6, unlike TNF and IL-1β, could be a useful and convenient marker of peripheral inflammation in older adults with various comorbidities.
In developed countries, a trend towards a shift in the age structure towards the elderly population can be observed, which can have health care as well as economic consequences. According to the latest estimates, the number of older people over 60 will double from 756 to 1,400 millions by 2030 (1). The aging of the immune system is a paradox of immunosenescence (deficiencies) and excessive inflammation (inflammaging), causing immune disorders. Inflammaging described as a chronic systemic inflammation associated with chronic age. The changes are mainly attributed to the somatic senescence-associated secretory phenotype (SASP) (2). It has been proposed that the basal inflammatory state and aging is a major factor in the increasing incidence of chronic diseases such as cardiovascular, neurodegenerative, and metabolic diseases (3). The coexisting multimorbidity in the elderly increases the risk of multiple organ failure and death. As the aging of the immune system progresses, the elderly become more susceptible to infectious diseases and cancer, and the risk of dying of influenza and coronavirus disease (COVID-19) also increases (4). It is therefore clear that there is an interplay between the aging of the immune system and age-related diseases. Solutions to prevent and treat age-related diseases can be sought through analyses of both the inflammation severity and immunosenescence (5).
Despite many features of acute inflammation, chronic inflammation is usually low-grade and results in tissue degeneration. There are several mechanisms which activate the development of chronic inflammation, which has been described as the following theories: 1) stress theory, which emphasizes the role of the correlation between excessive stress reaction and stronger pro-inflammatory response in the elderly (6); 2) the theory of oxidation-inflammation, according to which oxidative stress leads to inflammatory aging and affects homeostasis and prevention of health (7); 3) DNA damage theory, where damage to DNA telomeres and mitochondrial DNA leads to point mutations and chromosome rearrangements that contribute to cell aging (8); 4) theory of stem cells aging, where chronic inflammation is one of the main factors responsible for stem cells aging (9); and most widely discussed 5) theory of cytokines, where pro-inflammatory cytokines play an important role in inflammatory aging caused by chronic inflammation.
Aging facilitates a pro-inflammatory state by disrupting the peripheral immune system, which leads to excessive innate immune activity with the release of pro-inflammatory cytokines and a decrease in anti-inflammatory cytokines (10, 11). Different pro-inflammatory cytokines, such as interleukins (IL): IL-1β, IL-6, IL-12, IL-18, interferon (IFN-γ), and tumor necrosis factor (TNF), as well as anti-inflammatory ones, such as IL-4, IL-10, IL-13, and IL-19 which are secreted from immune cells, interact with body cells to mediate the immune responses and thus elicit its most optimum outcome (12–15). Elevated levels of interleukin-6 and TNF (16), as well as IL-1β (17), are associated with diseases, disability, and mortality in older adults. Interleukin-6, also known as ‘the cytokine for gerontologists’, plays a key role in the acute phase response in metabolic control and in the pathogenesis of many chronic diseases (18). IL-6 is produced mainly by the monocytes and macrophages (19). It produces a pleiotropic effect, and although in healthy and younger people its level is usually relatively low, in the elderly its elevated levels may correlate with increased mortality (20–24). Both Sánchez-Castellano et al. (25) and Mehta et al. (26) showed an association between inflammation, including IL-6 levels, and sarcopenia severity. A meta-analysis by Ng et al. (11), in which 34 studies were analyzed, demonstrated that the elderly with depression had significantly higher peripheral levels of IL-1β (p=0.026) and IL-6 (p<0.001) which was not the case in TNF (p=0.351) and C-reactive protein (CRP) levels (p=0.05). IL-1β is a key mediator of inflammatory response and participates in cell proliferation, differentiation, and apoptosis (27). This cytokine is expressed by a wide variety of cells, but macrophages and monocyes are particularly important, where IL-1β is produced in large quantities during infections and other stressful events (28, 29). Overproduction of IL-1β has also been confirmed in major depressive disorder, which may be related to the inflammasome hypothesis (27). High glucose concentration was reported to stimulate the production of IL-1β by pancreatic β cells, which implies the role of this cytokine also in type 2 diabetes (30–32). Since type 2 diabetes in the elderly increases the risk of cardiovascular disease, blocking IL-1β in these patients may reduce the incidence of myocardial infarction and stroke (31). Similar observations were recorded by Alzamil (33) in patients with obesity and with type 2 diabetes, where serum TNF levels in patients with diabetes and obesity were significantly higher than in patients without obesity (p<0.018). Significantly higher serum TNF levels were detected in patients with diabetes and obesity than in non-diabetic group with obesity (p<0.001). TNF is a pro-inflammatory mediator that can produce beneficial effects when activated locally in the tissues but it can be highly harmful when released systemically. It is one of the most important cytokines, produced by several types of cells: monocytes, T-cells, macrophages, fibroblast, adipocytes and smooth muscle cells (34). Generally, TNF is released together with interleukins usually IL-1 (35). In elderly people and centenarians, it has been shown that the level of TNF rises, which significantly increases mortality. Moreover, TNF has been shown to mediate metabolic changes and increased TNF levels have been found in patients with type 2 diabetes (24).
The assessment of chronic inflammation, including the level of pro-inflammatory cytokines in elderly people with comorbidities, may be the key to more effective treatment. Our hypothesis was that independent measurements of cytokines such as IL-6, TNF and IL-1β were significantly associated with the development of age-related diseases. Therefore, the aim of this study was to independently evaluate three cytokines: IL-6, TNF and IL-1β in elderly people with comorbidities compared to disease-free controls.
This systematic literature review and meta-analysis followed the requirements of the PRISMA statement (36). We comprehensively searched the Internet literature in the PubMed/Medline database. We focused on scientific papers that contained the word “cytokines” and at least one of the cytokines: IL6 or/and TNF alpha or/and IL-1 beta, and one of the following terms: “cut off” or/and “odds ratio”. Next, we excluded all the papers that involved training or therapy, as such interventions could affect the cytokine levels. Finally, the following filters were also used to obtain our working database: papers in English, free full text available, and study patients’ age above 65. The queries were last updated on 14 August 2023, and Table 1 presents the search strategy using MeSH terms. Detailed search code attached in the Supplementary Material File.
The following eligibility requirements were applied: (1) the participants of the study were elderly, additionally filtered in PubMed by the search terms: ‘aged: 65+ years’ (2) the older adults in the study design were clearly divided into two groups: the group with a disease entity and without diseases as the control group; (3) cytokine levels were measured quantitatively; (4) sufficient information and data were provided to estimate the mean value and standard deviation (SD). Reviewers independently evaluated the eligibility of the research papers found, and any disagreement was resolved through discussion. The exclusion criteria were as follows: (1) duplicate publications; (2) reviews, meta-analyses, protocols, editorials, letters, preprints, and unavailable full texts; (3) studies in which a healthy control group was missing or another disease entity was selected as the control group; (4) studies in which interventions were described, i.e. the impact of treatment (therapy), diet, physical activity; (5) studies on animals, (6) non-English research papers.
Each paper was annotated using the following data: the name of first author and the year of publication, the number of included individuals with respect to sex in both study and control groups, the age of study and control groups, IL-6, TNF and IL-1β level, and ratio (OR) values (if applicable in the single model).
Statistical analyses were performed using R 4.2.1 software (37), and a “meta” package (38) using a random effects model. For statistical homogeneity, medians and IQRs were converted to means with SDs to maximize the number of studies eligible for meta-analysis (39). Chi-squared and Higgin’s I2 tests were used to measure heterogeneity between studies. Cut-off values of 25%, 50%, and 75% were applied to label heterogeneity as low, moderate, or high respectively (40). Heterogeneity between studies was tested using Standardized Mean Difference (SMD) and “metacont” function, whereas studies with OR were analyzed using “metagen” function. Funnel plots were used to assess publication bias. Asymmetry was present in the funnel plots, Egger’s and Begg’s tests were applied to quantitatively assess whether there was any publication bias. The significance threshold for all statistical tests was set at p < 0.05.
A detailed selection of studies which were included in our analysis is shown in Figure 1. The initial search yielded 371 records. After removing duplicates (n=3), 368 studies were screened. After reviewing the abstracts and titles of the manuscripts, we excluded a total of 330 records from further analysis. Thirty eight publications (records) were qualified for the next stage, of which n = 21 records were removed. The reasons for deleting the publication was: n=11 lack of control group and n=10 irrelevant data - data presented in the form of unadjusted and adjusted models. Finally, n=17 publications were assessed. Due to the fact that some of the publications included more than one tested group, the total number of studies was n=38. For OR analyses, n=6 publications were included, and the total number of studies was n=10.
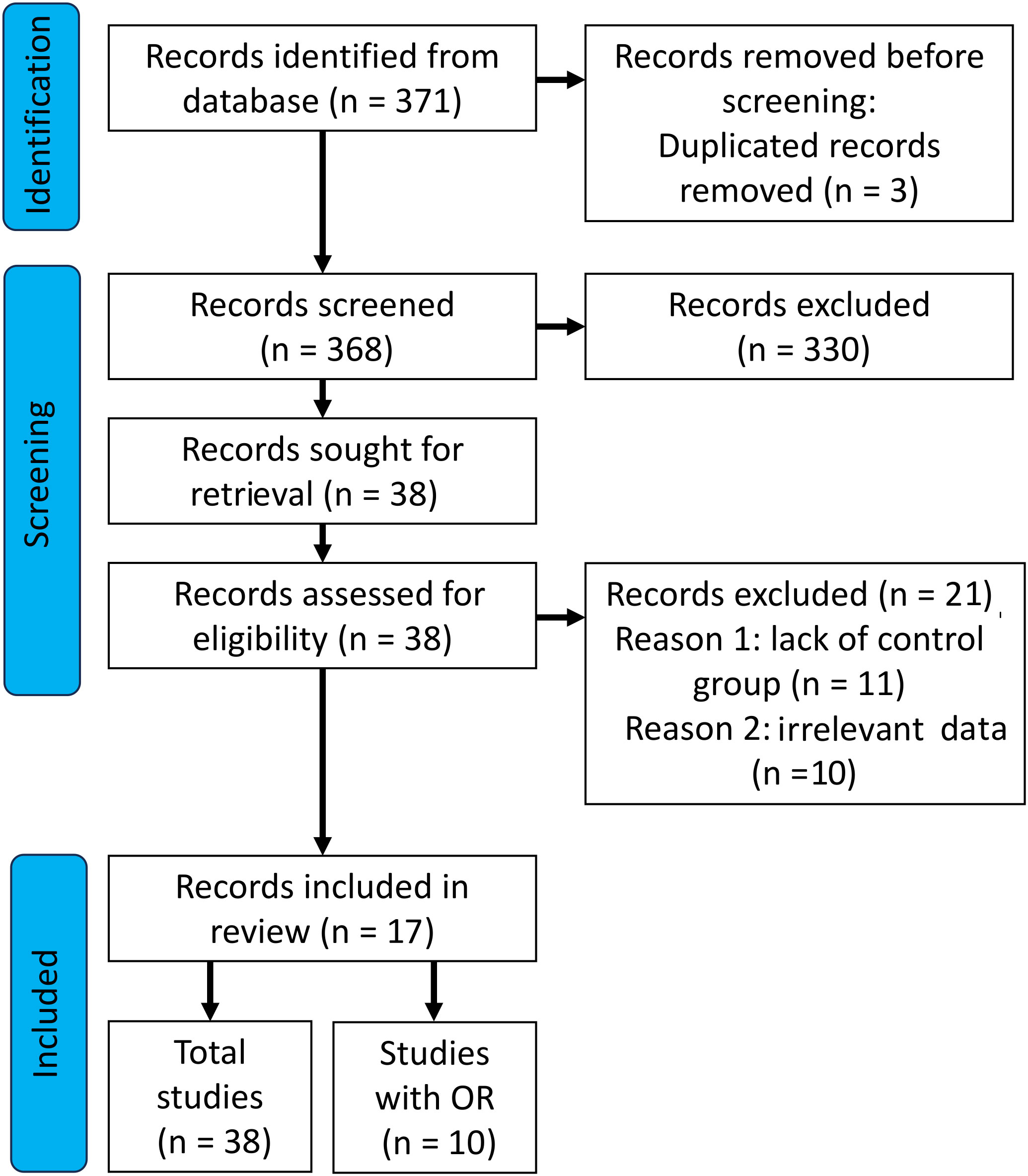
Figure 1 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram showing the paper selection process for inclusion.
Publication dates of the included studies ranged from 2008 to 2023. All the included studies reported the measurement of cytokine levels quantified in patients/controls blood and serum or plasma. The total number of the included subjects in the meta-analysis equaled n=8154 patients with diseases and n=33967 controls. With regard to IL-6 concentration investigation,15 publications were included, five of which were used twice in the analysis (41–45) because they involved two groups of patients with a disease and one control group. In turn, the research conducted by Sánchez-Castellano et al. (25) was used three times in IL-6 level assessment (as there were three groups of patients with sarcopenia described by different criteria). Nine publications were included in the evaluation of TNF level, one of which i.e., the research by Sánchez-Castellano et al. (25), was used three times and the research conducted by Jefferis et al. (41) was used twice. Two publications were investigated for IL-1β evaluation and the research carried out by Sánchez-Castellano et al. (25) was used three times (Table 2). For the analysis of the OR for IL-6, five manuscripts were selected where the OR values were publicly available and were reported as 95% Cl. The publications by Tattersall et al. (44) and Wennberg et al. (52) were used twice because two independent models were applied in the study design. The OR analysis for TNF-α included two manuscripts: Wennberg et al. (52) and Anaszewicz et al. (54). OR analysis was not performed for IL-1β due to a lack of data (Table 3).
Twenty-two studies with the total of 5213 patients with diseases and 28326 controls reported the data on IL-6 concentration. The overall concentration of IL-6 was found to be higher in patients with diseases compared to controls and the difference was significant, with a p-value of <0.001 (SMD, 0.16; 95% CI, 0.12–0.19). The heterogeneity was considerable with Q = 109.97 (P <0.0001) and I2 = 79.2% (Figure 2A). Egger’s test for the publication bias was also significant (p=0.02) (Figure 2B) and the asymmetry of the funnel plot was observed (Figure 2C).
The meta-analysis demonstrated that TNF concentration was higher in the controls when compared to patients with diseases (n patients = 2591; n controls = 5262; SMD, -0.03; 95% CI, -0.09–0.02), however, the value was not significant with p – value of 0.533. The observed heterogeneity of the reported studies was high (Q =209.53; P < 0.0001; I2 = 94.8%) (Figure 3A). Egger’s test for the publication bias was insignificant (p = 0.947) (Figure 3B), and the asymmetry of the funnel plot was observed (Figure 3C). The outliers analysis revealed that two studies had an exceptional impact on the overall effect.
Three hundred and fifty patients with diseases and 379 controls were included in IL-1β serum concentration meta-analysis which showed a tendency toward increased IL-1β concentration in the control group (SMD, -0.29; 95% CI, -0.47– -0.12; p = 0.001). Both overall heterogeneity and Egger’s test for the publication bias were found to be insignificant (Q = 4.48; p=0.214; I2 = 33.0% and p =0.213, respectively) (Figures 4A, B), but the asymmetry of the funnel plot was observed (Figure 4C).
The outcomes of seven studies comprising patients and a control group showed OR higher than 1 (OR: 1.03, 95% CI (1.01; 1.05), p=0.0029) (Figure 5A), which indicates a diagnostic usefulness of the analyzed cytokine. The heterogeneity was Q=25.18, p=0.142, I2 = 76.2%. The Egger’s test for the publication bias was found significant (p=0.0013, Figure 5B), and the asymmetry of the funnel plot was observed (Figure 5C).
The outcomes of three studies including patients showed OR ratio lower than 1 (OR: 0.99, 95% CI (0.98; 1.00), p = 0.07) (Figure 6A). The heterogeneity was Q=3.90, p=0.0003, I2 = 48.7%. The Egger’s test for the publication bias was not significant (p=0.08, Figure 6B) and the asymmetry of the funnel plot was not observed (Figure 6C).
The adaptive immune response weakens with age whereas the innate immune system becomes chronically activated, which is reflected by an increase in the level of pro-inflammatory cytokines. Persistent, low-grade inflammation contributes significantly to the pathophysiology of a variety of age-related conditions and diseases (56). Sex differences in immune response include the production of cytokines that mediate the immune response between cells. Women show greater production of type 2 cytokines, while men show greater production of type 1 cytokines, which include, among others, IL-1β and TNF (57). This analysis and literature review was conducted to analyze the level/concentration of selected cytokines in the elderly. PubMed database papers were selected to assess the level of IL-6, TNF, and IL-1β in a group of elderly people with diverse comorbidities in comparison to a control group of elderly people without coexisting diseases. Significantly increased levels of IL-6 were recorded in patients compared to disease-free group and the outcomes of seven studies showed OR at a slightly higher level than 1. In turn, no statistically significant difference in TNF level was observed between the controls and the patients, whereas a higher concentration of IL-1β was observed in the control group
Most researchers investigating inflammation and aging focus on IL-6 level assessment mainly in the context of the acute phase reaction while there is a growing body of evidence emphasizing the role of this cytokine in the pathogenesis of chronic diseases (58, 59). Research conducted by Xing et al. (60) also suggested that one of the main functions of IL-6 was to self-limit the inflammatory response by inhibiting the production of TNF and IL-1β. IL-6 confines neutrophil recruitment, favoring their replacement by mononuclear cells (61). Consequently, IL-6 may simultaneously regulate both pro-inflammatory and anti-inflammatory activity contributing to both the development/intensification and the suppression of the acute phase reaction. The switch from the inflammatory burst that follows an inflammatory stimulus to the chronic elevation of IL-6 typical of immune-mediated diseases and encountered in many older adults is still much less understood (59). In our meta-analysis, 15 manuscripts met the inclusion criteria. Five publications were evaluated twice, taking into account two groups of patients with diseases vs. one control group, and one manuscript was analyzed three times. A total of 22 records were assessed. Since IL-6 is produced in skeletal muscles and physical activity can cause up to a 10-fold increase in IL-6 levels (62), our meta-analysis excluded the studies which involved any type of intervention (physical activity). Similarly, studies which assessed the effect of diet were also excluded because a high-fat meal increases plasma IL-6 levels (63), which could bias our analyses. In 17 out of 22 papers analyzed, IL-6 levels were higher in the disease group than in the control group with the highest IL-6 levels reported by Tylutka et al. (53), where the patients with the disease (metabolic syndrome) showed an IL-6 value of 45.7 ± 41.4 pg/ml, which was the highest mean value found in all the manuscripts included in the study. This can be explained by the fact that IL-6 produced by adipose tissue accounts for on average 10 to 35% of its basal circulating level in the organism (64). Taking into account the relationship between IL-6 and obesity, it was hypothesized that the main cause of insulin resistance is the increasing level of IL-6 and other pro-inflammatory cytokines (65). Higher mean IL-6 levels in the control group were reported in the studies by Sánchez-Castellano et al. (25), where sarcopenia was analyzed according to the criterion (Masanés), as well as in studies on liver diseases by Mehta et al. (26). In the recorded assessment of Parkinson’s disease and IL-6 levels, neither incident nor prevalent Parkinson’s disease differed significantly compared to the control group (45). No difference in IL-6 levels between diabetic retinopathy (DR) group and control group was shown in the studies by Ngyuen et al. (50). However, the meta-analysis conducted by Yao et al. (66) with 31 case-control studies (groups with and without diabetic retinopathy) demonstrated a relationship between DR and IL-6 level (SMD: 2.12, 95% CI: 1.53-2.70, P<0.00001). This shows that an increased or decreased level of IL-6 depends on the analyzed disease entity, the group of people included in the study and the age or gender of the study subjects. Our meta-analysis showed that IL-6 level in the group of elderly people with various comorbidities was statistically significantly higher compared to the control group with a p-value of <0.001 (SMD, 0.16; 95% CI, 0.12–0.19), and obvious heterogeneity existed between the studies p <0.0001 and I2 = 79.2%. The OR analysis also demonstrated higher values than 1 (OR: 1.03, 95% CI (1.01; 1.05), p=0.0029), which indicates the potential diagnostic usefulness of IL-6 (Figure 5A).
TNF is a pro-inflammatory cytokine that affects macrophage functioning and its numerous functions include a key role in the activation of the pro-inflammatory cytokine cascade, which is why it is called the ‘master regulator’ of pro-inflammatory cytokines (67). TNF is an important contributor to tumorigenesis, progression, invasion, and metastasis and it also plays a critical role in autoimmune diseases (68). In our meta-analysis, nine manuscripts were selected for further analyses. One of them was included twice (41) and one underwent our investigation three times (25), which gave us a total of 12 records. Higher values of TNF compared to the control group were noted in the studies conducted on patients with type 2 diabetes and coronary artery disease by Pu et al. (55), as well as in the studies by Tylutka et al. (53), where patients with metabolic syndrome were analyzed. So far, research focused on the relationship between TNF and insulin resistance has provided ambiguous outcomes. Studies on rats conducted by Hotamisligil et al. (69) proved catabolic effect of TNF on adipose tissue and increased peripheral glucose uptake after neutralizing TNF in obese rats. Research on elderly people conducted by Alzamir (33) demonstrated that serum TNF levels in patients with diabetes and obesity were significantly higher than in non-obese counterparts, however, reports by Miyazaki et al. (70) did not confirm such an association. Interestingly, Jefferis et al. (41) showed that TNF was statistically significantly different in the patients with myocardial infarction than in the control group (p<0.001), but such a difference was not recorded in the group of patients with stroke vs control group (p=0.079), which implied associations between circulating acute phase inflammatory markers and coronary heart disease. Nevertheless, most research papers in our meta-analyses indicated lower TNF values in the group of people with diseases compared to the control, where SMD reached: -0.03; 95% CI, -0.09–0.02, and the value was not significant with p -value of 0.533. Additionally, low OR results: 0.99, 95% CI (0.98; 1.00), p = 0.07 confirmed our assumption that TNF was a poor predictor in diagnostics.
We also evaluated IL-1β, but only two studies that met the selection criteria were reviewed; therefore, the results should be applied with caution. IL-1β is the best characterized and studied pro-inflammatory cytokine of the 11 members of the IL-1 family. Although most research focuses on its production in monocytes or macrophages it is actually produced by various types of immune cells (71). IL-1β is known to contribute to disease pathogenesis in rheumatoid arthritis, gout, inflammatory bowel disease, and type 2 diabetes (72). More recent evidence highlighted a paradoxical role of IL-1β in diabetic retinopathy, multiple sclerosis, and Alzheimer’s disease (73). Research conducted by Sánchez-Castellano et al. (25) in the group of patients with sarcopenia did not reveal any differences between the groups (regardless of the adopted classification scheme for the study). In turn, the research conducted by Morawin et al. (74) showed statistically significantly higher IL-1β values (p=0.02) in the group of patients with sarcopenia n=39, 1406.74 ± 1120.23 pg/mL when compared to patients without diagnosed sarcopenia (n=134, 755.11 ± 353.90 pg/mL). Our meta-analysis showed higher cytokine values in the control group (SMD, -0.29; 95% CI, -0.47– -0.12; p = 0.001), and the overall heterogeneity was not significant (Q = 4.48; p = 0.214; I2 = 33.0%). Since the data on cut-offs and ORs was missing, the diagnostic utility of this cytokine could not be assessed and further research needs to be conducted.
Several shortcomings of this meta-analysis should be acknowledged. Firstly, various disease entities were included in the analyses, which may affect the overall results. Moreover, the individuals included in this meta-analysis have a different geographical nationality. The gender of the examined persons is another point that may affect the analyzed cytokines. Another limitation is related to a disproportion between the size of the control group vs. the group with diseases. Some of the included cytokines lacked enough original studies for the analyses of OR (IL-1β) to be performed. Finally, the analyzed cytokines were determined only in peripheral blood: plasma or serum.
Our meta-analysis assessed the levels of IL-6, TNF and IL-1β in older adults in relation to disease entities. An increase in IL-6 levels was found in the group with diseases compared to the control group, regardless of the analyzed disease entity. What may suggest a potential role of IL-6 in the progression/development of age-related diseases is the OR result (OR: 1.03, 95% CI (1.01; 1.05), p=0.0029). The evaluation of IL-6 levels may therefore be helpful in the assessment of a disease progression or treatment optimization. Higher values of TNF and IL-1β were found in the control group. Unfortunately, a limited volume of the available scientific literature on IL-1β means that further research is necessary.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
AT: Conceptualization, Writing – original draft, Writing – review and editing, Methodology. ŁW: Data curation, Formal analysis, Writing – review and editing. AZ-L: Conceptualization, Supervision, Writing – review and editing, Methodology.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1330386/full#supplementary-material
1. De Luca d'Alessandro E, Bonacci S, Giraldi G. Aging populations: the health and quality of life of the elderly. La Clinica Terapeutica. (2011) 162:e13–8.
2. Liberale L, Badimon L, Montecucco F, Lüscher TF, Libby P, Camici GG. Inflammation, aging, and cardiovascular disease: JACC review topic of the week. J Am Coll Cardiol. (2022) 79:837–47. doi: 10.1016/j.jacc.2021.12.017
3. Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. (2019) 25:1822–32. doi: 10.1038/s41591-019-0675-0
4. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
5. Barbé-Tuana F, Funchal G, Schmitz CRR, Maurmann RM, Bauer ME. The interplay between immunosenescence and age-related diseases. Semin immunopathol. (2020) 42:545–557). doi: 10.1007/s00281-020-00806-z
6. Ottaviani E, Franceschi C. The neuroimmunology of stress from invertebrates to man. Prog Neurobiol. (1996) 48:421–40. doi: 10.1016/0301-0082(95)00049-6
7. De la Fuente M, Miquel J. An update of the oxidation-inflammation theory of aging: the involvement of the immune system in oxi-inflamm-aging. Curr Pharm design. (2009) 15:3003–26. doi: 10.2174/138161209789058110
8. Meier A, Fiegler H, Muñoz P, Ellis P, Rigler D, Langford C, et al. Spreading of mammalian DNA-damage response factors studied by ChIP-chip at damaged telomeres. EMBO J. (2007) 26:2707–18. doi: 10.1038/sj.emboj.7601719
9. Jones DL, Rando TA. Emerging models and paradigms for stem cell ageing. Nat Cell Biol. (2011) 13:506–12. doi: 10.1038/ncb0511-506
10. Gruver AL, Hudson LL, Sempowski G. Immunosenescence of ageing. J Pathol: A J Pathological Soc Great Britain Ireland. (2007) 211:144–56. doi: 10.1002/path.2104
11. Ng A, Tam WW, Zhang MW, Ho CS, Husain SF, McIntyre RS, et al. IL-1β, IL-6, TNF-α and CRP in elderly patients with depression or Alzheimer’s disease: systematic review and meta-analysis. Sci Rep. (2018) 8:12050. doi: 10.1038/s41598-018-30487-6
12. Dinarello CA. IL-18: A TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J Allergy Clin Immunol. (1999) 103:11–24. doi: 10.1016/S0091-6749(99)70518-X
13. Luttmann W, Matthiesen T, Matthys H, Virchow JC Jr. Synergistic effects of interleukin-4 or interleukin-13 and tumor necrosis factor-α on eosinophil activation in vitro. Am J Respir Cell Mol Biol. (1999) 20:474–80. doi: 10.1165/ajrcmb.20.3.3326
14. Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. (2000) 117:1162–72. doi: 10.1378/chest.117.4.1162
15. Wong CK, Ho CY, Ko FWS, Chan CHS, Ho ASS, Hui DSC, et al. Proinflammatory cytokines (IL-17, IL-6, IL-18 and IL-12) and Th cytokines (IFN-γ, IL-4, IL-10 and IL-13) in patients with allergic asthma. Clin Exp Immunol. (2001) 125:pp.177–183. doi: 10.1046/j.1365-2249.2001.01602.x
16. De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett. (2005) 579:2035–9. doi: 10.1016/j.febslet.2005.02.055
17. Adams AA, Breathnach CC, Katepalli MP, Kohler K, Horohov DW. Advanced age in horses affects divisional history of T cells and inflammatory cytokine production. Mech Ageing Dev. (2008) 129:656–64. doi: 10.1016/j.mad.2008.09.004
18. Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. (2000) 51:245–70. doi: 10.1146/annurev.med.51.1.245
19. Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. (2006) 8:1–6. doi: 10.1186/ar1917
20. Hubbard RE, O’Mahony MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med. (2009) 13:3103–9. doi: 10.1111/j.1582-4934.2009.00733.x
21. Varadhan R, Yao W, Matteini A, Beamer BA, Xue QL, Yang H, et al. Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. Journals Gerontol Ser A: Biomed Sci Med Sci. (2014) 69:165–73. doi: 10.1093/gerona/glt023
22. Puzianowska-Kuźnicka M, Owczarz M, Wieczorowska-Tobis K, Nadrowski P, Chudek J, Slusarczyk P, et al. Interleukin-6 and C-reactive protein, successful aging, and mortality: the PolSenior study. Immun Ageing. (2016) 13:1–12. doi: 10.1186/s12979-016-0076-x
23. Van Epps P, Oswald D, Higgins PA, Hornick TR, Aung H, Banks RE, et al. Frailty has a stronger association with inflammation than age in older veterans. Immun Ageing. (2016) 13:1–9. doi: 10.1186/s12979-016-0082-z
24. Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, Ross OA. Age and age-related diseases: role of inflammation triggers and cytokines. Front Immunol. (2018) 586. doi: 10.3389/fimmu.2018.00586
25. Sánchez-Castellano C, Martín-Aragón S, Bermejo-Bescós P, Vaquero-Pinto N, Miret-CorChado C, Merello de Miguel A, et al. Biomarkers of sarcopenia in very old patients with hip fracture. J Cachexia Sarcopenia Muscle. (2020) 11:478–86. doi: 10.1002/jcsm.12508
26. Mehta M, Louissaint J, Parikh NS, Long MT, Tapper EB. Cognitive function, sarcopenia, and inflammation are strongly associated with frailty: a Framingham cohort study. Am J Med. (2021) 134:1530–8. doi: 10.1016/j.amjmed.2021.07.012
27. Ferentinos P, Maratou E, Antoniou A, Serretti A, Smyrnis N, Moutsatsou P. Interleukin-1 Beta in peripheral blood mononuclear cell lysates as a longitudinal biomarker of response to antidepressants: a pilot study. . Front Psychiatry. (2021) 12:801738. doi: 10.3389/fpsyt.2021.801738
28. Kaneko N, Kurata M, Yamamoto T, Morikawa S, Masumoto J. The role of interleukin-1 in general pathology. Inflammation regeneration. (2019) 39:1–16. doi: 10.1186/s41232-019-0101-5
29. Boraschi D. What is IL-1 for? The functions of interleukin-1 across evolution. Front Immunol. (2022) 13:872155. doi: 10.3389/fimmu.2022.872155
30. Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, et al. Glucose-induced β cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J Clin Invest. (2002) 110:851–60. doi: 10.1172/JCI200215318
31. Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1β in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. (2010) 17:314–21. doi: 10.1097/MED.0b013e32833bf6dc
32. Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood J Am Soc Hematol. (2011) 117:3720–32. doi: 10.1182/blood-2010-07-273417
33. Alzamil H. Elevated serum TNF-α is related to obesity in type 2 diabetes mellitus and is associated with glycemic control and insulin resistance. J Obes. (2020) 2020 5076858. doi: 10.1155/2020/5076858
34. Popa C, Netea MG, Van Riel PL, van der Meer JW, Stalenhoef AF. The role of TNF-α in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res. (2007) 48:751–62. doi: 10.1194/jlr.R600021-JLR200
35. Harvanová G, Duranková S, Bernasovská J. The role of cytokines and chemokines in the inflammatory response. Alergologia Polska-Polish J Allergol. (2023) 10:210–9. doi: 10.5114/pja.2023.131708
36. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
37. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing (2023). Available at: https://www.R-project.org/.
38. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evidence-Based Ment Health. (2019) 22:153–60. doi: 10.1136/ebmental-2019-300117
39. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Method. (2014) 14:1–13. doi: 10.1186/1471-2288-14-135
40. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
41. Jefferis BJ, Whincup PH, Welsh P, Wannamethee SG, Rumley A, Lennon LT, et al. Circulating TNFα levels in older men and women do not show independent prospective relations with MI or stroke. Atherosclerosis. (2009) 205:302–8. doi: 10.1016/j.atherosclerosis.2008.12.001
42. Pine SR, Mechanic LE, Enewold L, Chaturvedi AK, Katki HA, Zheng YL, et al. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. J Natl Cancer Institute. (2011) 103:1112–22. doi: 10.1093/jnci/djr216
43. Porta C, De Amici M, Quaglini S, Paglino C, Tagliani F, Boncimino A, et al. Circulating interleukin-6 as a tumor marker for hepatocellular carcinoma. Ann Oncol. (2008) 19:353–8. doi: 10.1093/annonc/mdm448
44. Tattersall MC, Dasiewicz AS, McClelland RL, Jarjour NN, Korcarz CE, Mitchell CC, et al. Persistent asthma is associated with carotid plaque in MESA. J Am Heart Assoc. (2022) 11:e026644. doi: 10.1161/JAHA.122.026644
45. Ton TG, Jain S, Biggs ML, Thacker EL, Strotmeyer ES, Boudreau R, et al. Markers of inflammation in prevalent and incident Parkinson’s disease in the Cardiovascular Health Study. Parkinsonism related Disord. (2012) 18:274–8. doi: 10.1016/j.parkreldis.2011.11.003
46. Garcia JM, Splenser AE, Kramer J, Alsarraj A, Fitzgerald S, Ramsey D, et al. Circulating inflammatory cytokines and adipokines are associated with increased risk of Barrett's esophagus: a case–control study. Clin Gastroenterol Hepatol. (2014) 12:229–38. doi: 10.1016/j.cgh.2013.07.038
47. Marcus GM, Whooley MA, Glidden DV, Pawlikowska L, Zaroff JG, Olgin JE. Interleukin-6 and atrial fibrillation in patients with coronary artery disease: data from the Heart and Soul Study. Am Heart J. (2008) 155:303–9. doi: 10.1016/j.ahj.2007.09.006
48. Kaplan RC, McGinn AP, Baird AE, Hendrix SL, Kooperberg C, Lynch J, et al. Inflammation and hemostasis biomarkers for predicting stroke in postmenopausal women: the Women's Health Initiative Observational Study. J Stroke Cerebrovascular Dis. (2008) 17:344–55. doi: 10.1016/j.jstrokecerebrovasdis.2008.04.006
49. Lee JH, Jang JH, Park JH, Jang HJ, Park CS, Lee S, et al. The role of interleukin-6 as a prognostic biomarker for predicting acute exacerbation in interstitial lung diseases. PLoS One. (2021) 16:e0255365. doi: 10.1371/journal.pone.0255365
50. Nguyen TT, Alibrahim E, Islam FA, Klein R, Klein BE, Cotch MF, et al. Inflammatory, hemostatic, and other novel biomarkers for diabetic retinopathy: the multi-ethnic study of atherosclerosis. Diabetes Care. (2009) 32:1704–9. doi: 10.2337/dc09-0102
51. Jenny NS, French B, Arnold AM, Strotmeyer ES, Cushman M, Chaves PH, et al. Long-term assessment of inflammation and healthy aging in late life: the Cardiovascular Health Study All Stars. Journals Gerontol Ser A: Biomed Sci Med Sci. (2012) 67:970–6. doi: 10.1093/gerona/glr261
52. Wennberg AM, Hagen CE, Machulda MM, Knopman DS, Petersen RC, Mielke MM. The cross-sectional and longitudinal associations between IL-6, IL-10, and TNFα and cognitive outcomes in the Mayo Clinic Study of Aging. Journals Gerontol: Ser A. (2019) 74:1289–95. doi: 10.1093/gerona/gly217
53. Tylutka A, Morawin B, Walas Ł., Michałek M, Gwara A, Zembron-Lacny A. Assessment of metabolic syndrome predictors in relation to inflammation and visceral fat tissue in older adults. Sci Rep. (2023) 13:89. doi: 10.1038/s41598-022-27269-6
54. Anaszewicz M, Wawrzeńczyk A, Czerniak B, Banaś W, Socha E, Lis K, et al. Leptin, adiponectin, tumor necrosis factor α, and irisin concentrations as factors linking obesity with the risk of atrial fibrillation among inpatients with cardiovascular diseases. Kardiologia Polska (Polish Heart Journal). (2019) 77:1055–61. doi: 10.33963/KP.14989
55. Pu LJ, Lu L, Shen WF, Zhang Q, Zhang RY, Zhang JS, et al. Increased serum glycated albumin level is associated with the presence and severity of coronary artery disease in type 2 diabetic patients. Circ J. (2007) 71:1067–73. doi: 10.1253/circj.71.1067
56. Liberale L, Bonetti NR, Puspitasari YM, Vukolic A, Akhmedov A, Diaz-Cañestro C, et al. TNF-α antagonism rescues the effect of ageing on stroke: Perspectives for targeting inflamm-ageing. Eur J Clin Invest. (2021) 51:e13600. doi: 10.1111/eci.13600
57. Bernardi S, Toffoli B, Tonon F, Francica M, Campagnolo E, Ferretti T, et al. Sex differences in proatherogenic cytokine levels. Int J Mol Sci. (2020) 21:3861. doi: 10.3390/ijms21113861
58. Bauer J, Bauer TM, Kalb T, Taga T, Lengyel G, Hirano T, et al. Regulation of interleukin 6 receptor expression in human monocytes and monocyte-derived macrophages. Comparison with the expression in human hepatocytes. J Exp Med. (1989) 170:1537–49. doi: 10.1084/jem.170.5.1537
59. Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. Journals Gerontol Ser A: Biol Sci Med Sci. (2006) 61:575–84. doi: 10.1093/gerona/61.6.575
60. Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, et al. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. (1998) 101:311–20. doi: 10.1172/JCI1368
61. Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol. (2005) 175:3463–8. doi: 10.4049/jimmunol.175.6.3463
62. Weigert C, Hennige AM, Brodbeck K, Haring HU, Schleicher ED. Interleukin-6 acts as insulin sensitizer on glycogen synthesis in human skeletal muscle cells by phosphorylation of Ser473 of Akt. Am J physiology-Endocrinol Metab. (2005) 289:E251–7. doi: 10.1152/ajpendo.00448.2004
63. Nappo F, Esposito K, Cioffi M, Giugliano G, Molinari AM, Paolisso G, et al. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: role of fat and carbohydrate meals. J Am Coll Cardiol. (2002) 39:1145–50. doi: 10.1016/S0735-1097(02)01741-2
64. Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-α, in vivo. J Clin Endocrinol Metab. (1997) 82:4196–200. doi: 10.1210/jc.82.12.4196
65. Abbatecola AM, Ferrucci L, Grella R, Bandinelli S, Bonafè M, Barbieri M, et al. Diverse effect of inflammatory markers on insulin resistance and insulin-resistance syndrome in the elderly. J Am Geriatrics Soc. (2004) 52:399–404. doi: 10.1111/j.1532-5415.2004.52112.x
66. Yao Y, Li R, Du J, Long L, Li X, Luo N. Interleukin-6 and diabetic retinopathy: a systematic review and meta-analysis. Curr eye Res. (2019) 44:564–74. doi: 10.1080/02713683.2019.1570274
67. Parameswaran N, Patial S. Tumor necrosis factor-α signaling in macrophages. Crit Reviews™ Eukaryotic Gene Expression. (2010) 20:87–103. doi: 10.1615/CritRevEukarGeneExpr.v20.i2
69. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. (1993) 259:87–91. doi: 10.1126/science.7678183
70. Miyazaki Y, Pipek R, Mandarino LJ, DeFronzo RA. Tumor necrosis factor α and insulin resistance in obese type 2 diabetic patients. Int J Obes. (2003) 27:88–94. doi: 10.1038/sj.ijo.0802187
71. Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1β secretion. Cytokine Growth factor Rev. (2011) 22:189–95. doi: 10.1016/j.cytogfr.2011.10.001
72. Dinarello CA, Simon A, Van Der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discovery. (2012) 11:633–52. doi: 10.1038/nrd3800
73. Mendiola AS, Cardona AE. The IL-1β phenomena in neuroinflammatory diseases. J Neural Transm. (2018) 125:781–95. doi: 10.1007/s00702-017-1732-9
Keywords: comorbidities, immunosenescence, interleukin 1β, interleukin 6, tumor necrosis factor
Citation: Tylutka A, Walas Ł and Zembron-Lacny A (2024) Level of IL-6, TNF, and IL-1β and age-related diseases: a systematic review and meta-analysis. Front. Immunol. 15:1330386. doi: 10.3389/fimmu.2024.1330386
Received: 30 October 2023; Accepted: 19 February 2024;
Published: 01 March 2024.
Edited by:
Juan Pablo de Rivero Vaccari, University of Miami, United StatesReviewed by:
Rosaura Leis, University of Santiago de Compostela, SpainCopyright © 2024 Tylutka, Walas and Zembron-Lacny. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Agnieszka Zembron-Lacny, YS56ZW1icm9uLWxhY255QGNtLnV6Lnpnb3JhLnBs
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.