
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol. , 28 March 2024
Sec. Cancer Immunity and Immunotherapy
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1321813
This article is part of the Research Topic Advances in Immunotherapy of Hepatic-Biliary-Pancreatic Cancers - Volume II View all 5 articles
 Seung Bae Yoon1
Seung Bae Yoon1 Sang Myung Woo2*
Sang Myung Woo2* Jung Won Chun2
Jung Won Chun2 Dong Uk Kim3
Dong Uk Kim3 Jaihwan Kim4
Jaihwan Kim4 Joo Kyung Park5
Joo Kyung Park5 Hoonsub So6
Hoonsub So6 Moon Jae Chung7
Moon Jae Chung7 In Rae Cho8
In Rae Cho8 Jun Heo9
Jun Heo9Background: Recently, anti-programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) immunotherapy offers promising results for advanced biliary tract cancer (BTC). However, patients show highly heterogeneous responses to treatment, and predictive biomarkers are lacking. We performed a systematic review and meta-analysis to assess the potential of PD-L1 expression as a biomarker for treatment response and survival in patients with BTC undergoing anti-PD-1/PD-L1 therapy.
Methods: We conducted a comprehensive systematic literature search through June 2023, utilizing the PubMed, EMBASE, and Cochrane Library databases. The outcomes of interest included objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), and overall survival (OS) according to PD-L1 expression. Subgroup analyses and meta-regression were performed to identify possible sources of heterogeneity.
Results: A total of 30 studies was included in the final analysis. Pooled analysis showed no significant differences in ORR (odds ratio [OR], 1.56; 95% confidence intervals [CIs], 0.94-2.56) and DCR (OR, 1.84; 95% CIs, 0.88-3.82) between PD-L1 (+) and PD-L1 (-) patients. In contrast, survival analysis showed improved PFS (hazard ratio [HR], 0.54, 95% CIs, 0.41-0.71) and OS (HR, 0.58; 95% CI, 0.47-0.72) among PD-L1 (+) patients compared to PD-L1 (-) patients. Sensitivity analysis excluding retrospective studies showed no significant differences with the primary results. Furthermore, meta-regression demonstrated that drug target (PD-1 vs. PD-L1), presence of additional intervention (monotherapy vs. combination therapy), and PD-L1 cut-off level (1% vs. ≥5%) significantly affected the predictive value of PD-L1 expression.
Conclusion: PD-L1 expression might be a helpful biomarker for predicting PFS and OS in patients with BTC undergoing anti-PD-1/PD-L1 therapy. The predictive value of PD-L1 expression can be significantly influenced by diagnostic or treatment variables.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO, identifier CRD42023434114.
Biliary tract cancer (BTC) refers to a diverse group of malignant tumors arising from the biliary or gallbladder epithelium, including intrahepatic cholangiocarcinoma, extrahepatic cholangiocarcinoma, and gallbladder cancer. This disease has a poor prognosis, with a 5-year survival rate of <20% and increasing global mortality rates (1, 2). Surgery is the only curative treatment for BTC, although a minority are potential candidates for radial resection due to delayed diagnosis from a frequent lack of symptoms. Accordingly, palliative chemotherapy continues to be the mainstay of treatment for most patients with BTC, with gemcitabine plus cisplatin remaining the standard first-line therapy for more than a decade (3, 4). However, the limited median survival benefit of <1 year despite undergoing standard systemic chemotherapy highlights the need for more effective medical treatments.
The emergence of immunotherapy has revolutionized cancer treatment. In particular, immune checkpoint inhibitors (ICIs) that target the programmed cell death protein 1 (PD-1) or programmed death-ligand 1 (PD-L1) have shown promising outcomes for diverse solid tumors, including melanoma, non-small cell lung cancer, renal cell carcinoma, and bladder cancer (5–8). In BTC, immunotherapy using pembrolizumab was first evaluated in two cohorts of metastatic patients where cancer progressed after standard systemic chemotherapy: Keynote-158 (104 patients) and Keynote-028 (23 patients) (9). In these two cohorts, the objective response rate (ORR) with median progression-free survival (PFS) were 5.8% with 2.0 months and 13.0% with 1.8 months, respectively. Although ICIs have had considerable success in immunotherapy for some malignancies, most patients with BTC fail to achieve durable responses with ICI monotherapy. Therefore, it is crucial to develop effective combination regimens and explore potential biomarkers to identify patients who would benefit from immunotherapy. Furthermore, while recent phase 3 trials have shown promising outcomes with anti-PD-1/PD-L1 plus gemcitabine and cisplatin regimens (10, 11), a biomarker in patients with BTC undergoing immunotherapy remains to be established.
Considering the underlying mechanism of PD-1/PD-L1 inhibitor therapy, PD-L1 expression on tumor or immune cells serves as a promising biomarker for predicting ICI response. Immunohistochemistry (IHC) of PD-L1 is the most widely validated method for selecting patients for ICI therapy, demonstrating robust predictive values in various cancers (12–14). Recent meta-analyses have demonstrated the utility of PD-L1 expression as a valuable predictive biomarker of anti-PD-1/PD-L1 therapy in digestive malignancies, including gastroesophageal and hepatocellular carcinoma (15–17). However, the value of PD-L1 expression in patients with BTC receiving anti-PD-1/PD-L1 therapy remains controversial. Results from the initial Keynote-158 cohort reported no differences in ORR, PFS, or OS between PD-L1 (+) and PD-L1 (-) patients treated with pembrolizumab (9). Conversely, in a phase 2 multi-institutional study of nivolumab for patients with advanced BTC, positive PD-L1 expression was associated with prolonged PFS (18). In addition to these conflicting results, the definition of PD-L1 positivity remains unclear, resulting in the use of several PD-L1 scoring methods and cut-offs in clinical trials. Moreover, no optimal anti-PD-1/PD-L1 drug or combination immunotherapy regimen has been identified for BTC.
Given these circumstances, the aim of this meta-analysis and systematic review was to assess the value of PD-L1 expression for predicting tumor response and survival outcomes with PD-1/PD-L1 inhibitors in patients with BTC. We also aimed to identify variables associated with the predictive performance of PD-L1 expression in this cohort.
This systematic review and meta-analysis has been registered in PROSPERO (CRD42023434114) and was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (Supplementary Table 1) (19).
A comprehensive systematic literature search through June 20, 2023, utilizing the PubMed, EMBASE, and Cochrane Library databases. The key search terms included “cholangiocarcinoma,” “biliary tract neoplasms,” “gallbladder neoplasms,” “Immune checkpoint inhibitors,” “PD-1,” and “PD-L1.” The full search details are presented in Supplementary Table 2. Included studies were restricted to English publications. Additional relevant studies by manually cross-checking the reference lists in the retrieved articles.
All randomized trials and prospective/retrospective studies fulfilling the following criteria were included: [1] patients with BTC treated with PD-1 or PD-L1 inhibitors; [2] PD-L1 status based on IHC staining methods; and [3] presentation of at least one of the clinical outcomes of ORR, disease control rate (DCR), PFS, or OS. The exclusion criteria were as follows: [1] the inclusion of only PD-L1 (+) patients; [2] lacking or no data on ORR, DCR, PFS, or OS; and [3] non-English publications, case series (n<10), letters, commentaries, or review papers. In cases where multiple studies reported data from the same cohort, the higher quality study was included. Two investigators (SBY and SMW) independently reviewed and evaluated all titles, abstracts, and full texts. Discrepancies between the two investigators were resolved through discussion with a third reviewer (JWC).
Data were extracted independently by two investigators (SBY and SMW) using a standardized table. The following items were documented for each study: [1] study characteristics, including the name of the first author, publication year, study design, region, and median follow-up period; [2] participant characteristics, including number of patients, median age, and sex; [3] intervention details, including name of drug, line of therapy, and combined treatment; [4] PD-L1 expression assessment, including type of PD-L1 antibody clone, PD-L1 IHC scoring method, and respective cut-off values; and [5] clinical outcomes (ORR, DCR, median PFS, and median OS) and safety (grade 3-5 adverse event rates) profiles. For studies with multiple combination drug modalities including anti-PD-1/PD-L1 therapy, data were collected separately and analyzed as individual datasets. If the hazard ratio (HR) of survival data was not provided in the original paper, the values were extracted by replicating Kaplan-Meier survival curves using WebPlotDigitizer software Version 4.5 (PLOTCON; Oakland, CA, USA). As a meta-analysis of biomarker assessment, the Quality in Prognosis Studies (QUIPS) tool was used to appraise the quality of the included studies based on six domains: [1] study participants, [2] study attrition, [3] prognostic factor measurement, [4] outcome measurement, [5] adjustment for other prognostic factors, and [6] statistical analysis and reporting (20, 21).
Pooled ORR and DCR result were derived using the random-effects model, as suggested by DerSimonian and Laird (22). Time-to-event (PFS and OS) data were incorporated into the meta-analysis, according to the methodology employed by Tierney et al. (23). Forest plots were constructed for the visual representation of individual study results and pooled data. Sensitivity analyses were performed for clinical trials and prospective studies, excluding retrospective studies. Meanwhile, subgroup analyses and meta-regression were performed according to region (Eastern vs. Western), target of drug (PD-1 vs. PD-L1), line of therapy (1st vs. 2nd or later), presence of additional interventions (monotherapy vs. combination therapy), PD-L1 scoring method (tumor proportion score [TPS] vs. combined positive score [CPS]), and PD-L1 cut-off level (1% vs. ≥5%). Furthermore, publication bias was qualitatively evaluated by visual inspection of the funnel plot and statistically confirmed using Egger’s test. This meta-analysis was conducted using the Comprehensive Meta-Analysis Software Version 4.0 (Biostat, Englewood, NJ, USA).
The initial literature search retrieved 2005 articles. After removing duplicates, 1619 remained for further title and abstract review (Figure 1). After excluding case reports, reviews, commentaries, and irrelevant articles, 85 studies underwent full analysis. Of these 85 studies, 55 were excluded due to the following reasons: [1] lack of biomarker analysis for PD-L1 (n=38), [2] insufficient data on clinical responses (n=5), [3] non-use of anti-PD-1/PD-1 therapy (n=4), [4] analysis only in PD-L1 (+) patients (n=3), [5] duplicated data (n=2), [6] small number (N<10) of patients (n=2), and (7) discussion of study protocol only (n=1). Ultimately, a total of 30 studies was included in final analysis (9, 18, 24–51).
Of the 30 studies, 18 were phase I/II clinical trials, two were prospective studies, and the remaining 10 were retrospective studies. Most studies (83.3%, 25/30) were conducted in Asian countries. PD-1 inhibitors, including pembrolizumab, nivolumab, camrelizumab, sintilimab, toripalimab, and tislelizumab, were used in 24 studies, whereas PD-L1 inhibitors, including durvalumab, avelumab, and bintrafusp alfa, were used in six studies. Since three studies included two or more intervention modalities (26, 37, 41), a total of 34 datasets with 1310 patients were included in the meta-analysis. Among these patients, 631 (48.2%) were PD-L1 (+). Main characteristics of the included studies are summarized in Table 1.
The QUIPS tools, which assessed the risk of bias in the included studies, showed that 63% (19/30) of the studies had at least one domain at high risk of bias. Reasons for high risk of bias included the following: [1] retrospective design (n=10), [2] varied ICIs and treatment modalities (n=7), [3] insufficient reporting data on clinical outcomes (n=7). and [4] inconsistency of PD-L1 IHC methods or clones (n=4). Further details of the quality assessment are presented in Supplementary Table 3.
Pooled analysis revealed an ORR of 28.8% (95% confidence intervals [CIs], 23.8–34.3%; I2 = 87.4%) and a DCR of 68.6% (95% CIs, 62.6–74.0%; I2 = 90.4%). The pooled rate of grade 3-5 adverse events was 36.7% (95% CIs, 30.1–43.8%; I2 = 84.8%). Compared to the ICI-monotherapy group, pooled ORR (9.0%; 95% CIs, 5.6–14.2%; I2 = 5.3% vs. 40.2%; 95% CIs, 33.2–47.6%; I2 = 80.0%; P<0.001), DCR (36.4%; 95% CIs, 26.9–47.1%; I2 = 77.7% vs. 83.0%; 95% CIs, 77.7–87.3%; I2 = 68.8%; P<0.001), and grade 3-5 adverse event rates (20.7%; 95% CIs, 13.2–30.9%; I2 = 73.9% vs. 47.4%; 95% CIs, 38.5–56.4%; I2 = 78.0%; P<0.001) were significantly higher in the ICI-based combination therapy group. The detailed clinical outcomes of all patients from the included studies are summarized in Supplementary Table 4. The forest plots of these clinical outcomes are presented in Supplementary Figure 1.
Primary clinical outcomes according to PD-L1 expression are presented in Table 2. The ORR, DCR, PFS, and OS according to PD-L1 expression were compared between PD-L1 (+) and PD-L1 (-) groups. Overall, 25 (n=1055) and 8 (n=310) studies reported ORR and DCR according to PD-L1 expression, respectively. A direct comparison between PD-L1 (+) and PD-L1 (-) groups showed no significant differences in ORR (odds ratio [OR], 1.56; 95% CIs, 0.94-2.56; I2 = 37.7%; P=0.085) and DCR (OR, 1.84; 95% CIs, 0.88-3.82; I2 = 39.7%; P=0.104) (Figures 2A, B). On the other hand, 24 (n=802) and 21 (n=704) studies reported PFS and OS according to PD-L1 expression, respectively. Pooled analysis showed significantly improved PFS (HR, 0.54; 95% CIs, 0.41-0.71; I2 = 54.2%; P<0.001) and OS (HR, 0.58; 95% CIs, 0.47-0.72; I2 = 0.0%; P<0.001) in PD-L1 (+) compared to PD-L1 (-) patients (Figures 2C, D).
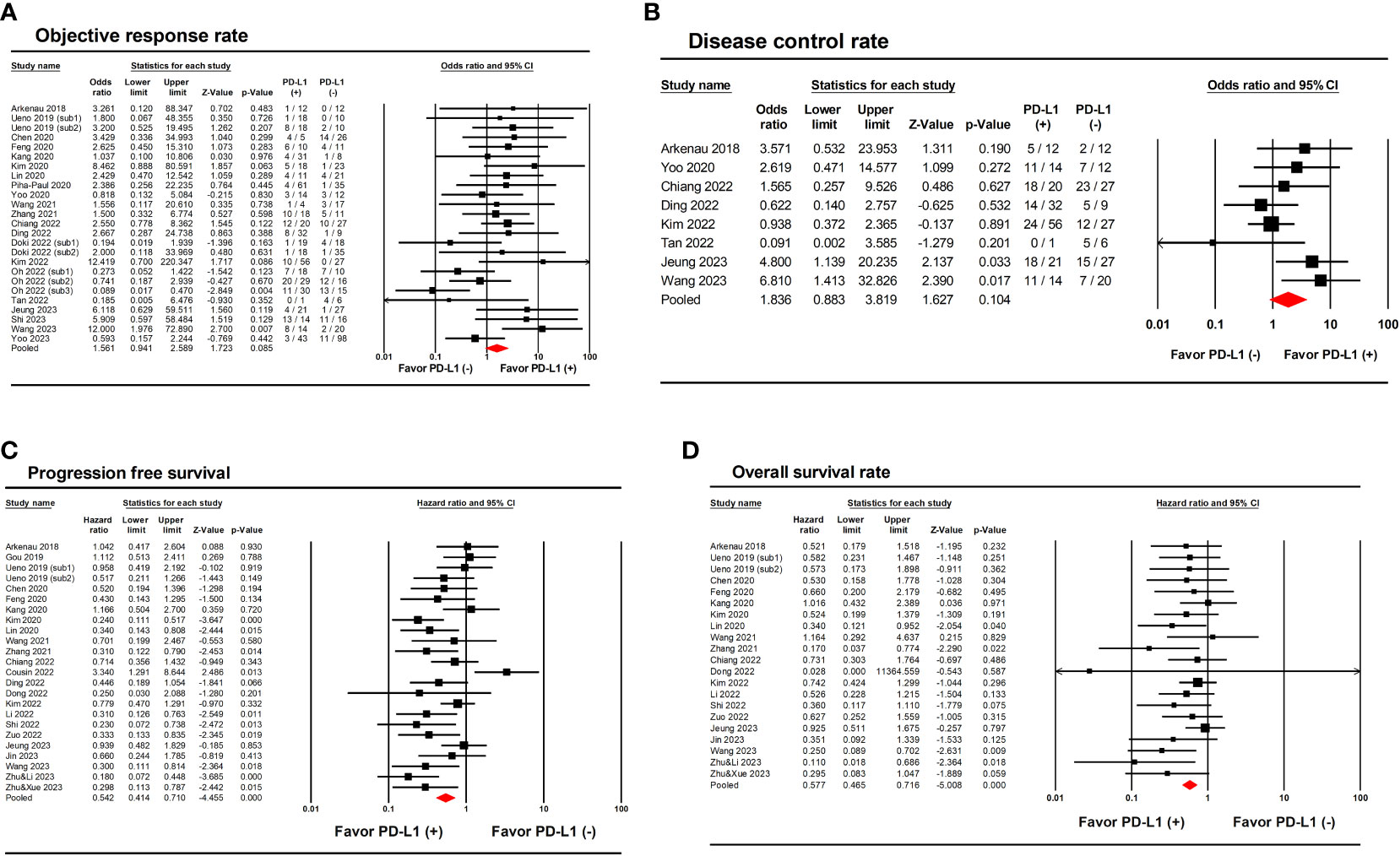
Figure 2 Forest plots showing the results of primary outcomes in patients with biliary tract cancer treated with anti PD-1/PD-L1 therapy according to PD-L1 expression. (A) Objective response rate. (B) Disease control rate. (C) Progression-free survival. (D) Overall survival.
Sensitivity analysis was performed only for clinical trials and prospective studies. This analysis revealed that PD-L1 (+) expression did not significantly improve ORR (OR, 1.25; 95% CIs, 0.75-2.09; I2 = 30.4%; P=0.387) and DCR (OR, 2.42; 95% CIs, 0.86-6.86; I2 = 0.0%; P=0.096) between groups (Supplementary Figures 2A, B). In contrast, sensitivity analysis showed that PD-L1 (+) expression was associated with improved PFS (HR, 0.60; 95% CIs, 0.42-0.86; I2 = 53.6%; P=0.005) and OS (HR, 0.59; 95% CIs, 0.43-0.76; I2 = 0.0%; P<0.001) (Supplementary Figures 2C, D). This confirms the robustness of the findings since sensitivity analysis did not significantly change the primary results.
Subgroup analyses and meta-regression were performed for ORR, PFS, and OS using six variables (Table 3). The predictive value of PD-L1 expression was significantly affected by drug target (PD-1 vs. PD-L1), presence of additional interventions (monotherapy vs. combination therapy), and PD-L1 cut-off level (1% vs. ≥5%). Specifically, use of anti-PD-1 inhibitors was associated with higher OR of ORR (2.97; 95% CIs, 1.84–4.79 vs. 0.42; 95% CIs, 0.22-0.81; P<0.001) and lower HR of PFS (0.53; 95% CIs, 0.41-0.67 vs. 3.34; 95% CIs, 1.01-11.01; P=0.003) compared to use of anti-PD-L1 inhibitors. Studies with ICI-based combination therapy showed lower HR of OS (0.45; 95% CIs, 0.34-0.61 vs. 0.75; 95% CIs, 0.55-1.02; P=0.019) than studies with ICI-monotherapy. Furthermore, higher PD-L1 cut-off values (≥5%) were associated with lower HR of PFS (0.31; 95% CIs, 0.18-0.54 vs. 0.59; 95% CIs, 0.44-0.79; P=0.017) and lower HR of OS (0.30; 95% CIs, 0.17-0.53 vs. 0.65; 95% CIs, 0.51-0.82; P=0.015) compared to lower PD-L1 cut-off values (1%).
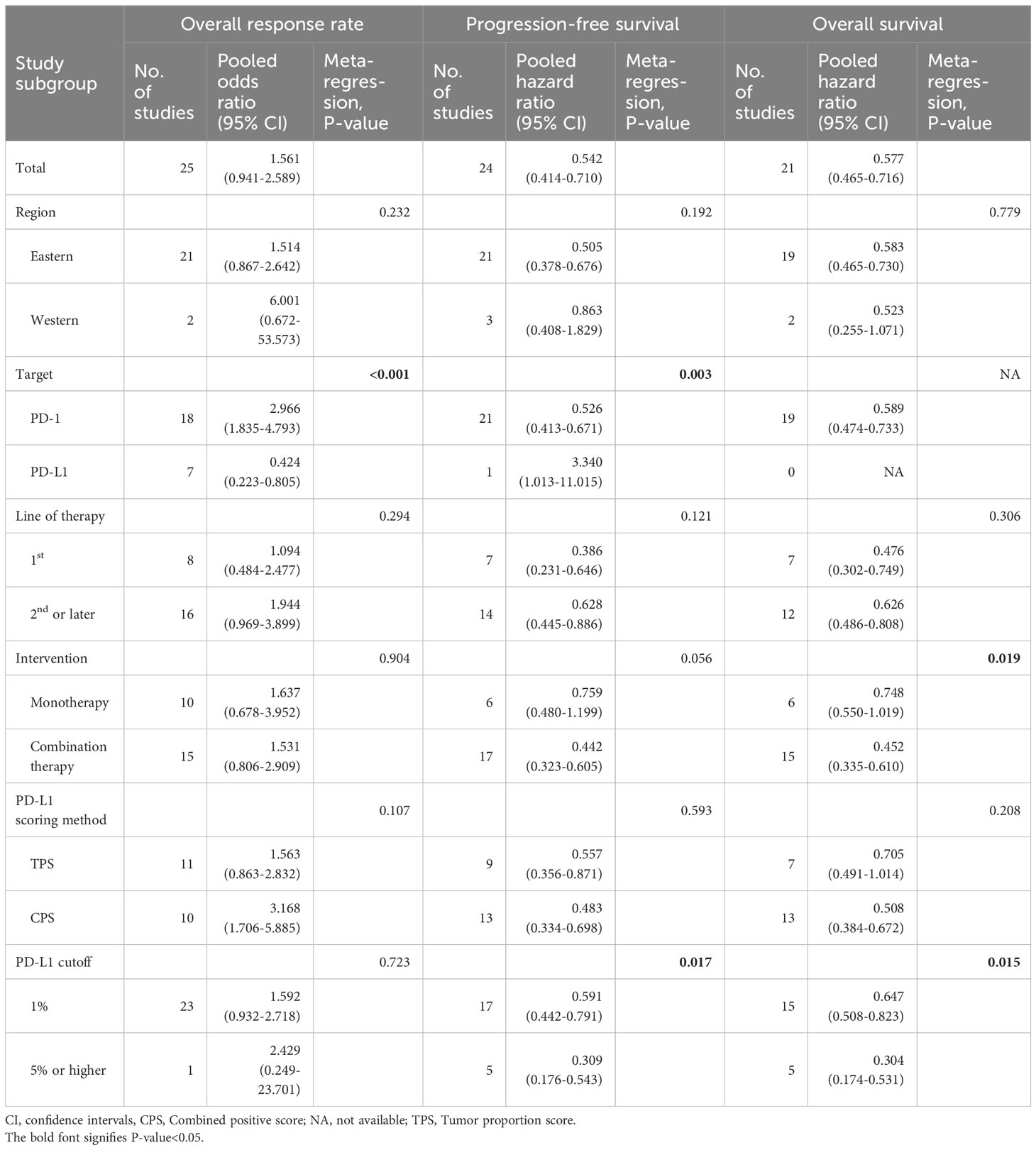
Table 3 Subgroup analyses and meta-regression of studies reporting associations of PD-L1 expression and clinical outcomes.
Visual inspection of the funnel plots and clinical outcomes from included studies did not suggest asymmetry. Statistical analysis using Egger’s test showed similar results, confirming the absence of publication biases in these analyses (P=0.332, P=0.939, and P=0.133, respectively). Meanwhile, the funnel plot of OS revealed asymmetry, which was supported by a P-value of 0.002 on Egger’s test, indicating publication bias (Supplementary Figure 3).
This meta-analysis revealed no significant differences in ORR and DCR between PD-L1 (+) and PD-L1 (-) patients with BTC undergoing anti-PD-1/PD-L1 therapy. However, PD-L1 (+) patients showed longer PFS and OS compared to that in PD-L1 (-) patients. These findings suggest the potential of PD-L1 expression as a helpful biomarker for predicting survival but not treatment responsiveness in patients with advanced BTC. Notably, clinical values of PD-L1 expression were significantly affected by the target of immunotherapy, presence of combined treatment, and PD-L1 cut-off values.
To the best of our knowledge, this study is the first meta-analysis evaluating the predictive value of PD-L1 expression in patients with BTC undergoing anti-PD-1/PD-L1 therapy. In immunotherapy-naïve patients with BTC, PD-L1 expression has been associated with tumor aggressiveness and poor prognosis (52–54). However, with the emergence of cancer immunotherapy, PD-L1 expression has been reported to be a potential predictive biomarker in various types of malignancies (16, 55, 56). Our findings similarly suggest that PD-L1 (+) expression was associated with prolonged PFS and OS, indicating its promise as a predictive biomarker in BTC. While the TOPAZ-1 trial, a representative phase III trial of immunotherapy for BTC, was excluded from this meta-analysis due to lacking data on direct comparisons between PD-L1 (+) and PD-L1 (-) patients within the experimental (durvalumab) group, the OS improvement of durvalumab compared to placebo has been reported to be more pronounced in the PD-L1(+) subset rather than the PD-L1(-) subset (HR, 0.79 vs. 0.86) (10). Considering that positive PD-L1 expression has been regarded as a poor prognostic factor in the absence of immunotherapy, our findings solidify the need for immunotherapy in PD-L1 (+) patients, allowing the prognostication of favorable clinical outcomes in this cohort. In contrast, PD-L1 expression did not show a significant association with ORR and DCR in this study. The reasons for the observed lack of correlation between PD-L1 expression and radiologic assessment, such as ORR and DCR, are unclear. Despite this, some underlying mechanisms have been proposed. Primarily, immunotherapies often induce robust inflammatory and immune responses within the tumor microenvironment. These dynamic reactions can result in intricate radiological patterns, posing challenges in the accurate assessment of tumor changes (e.g., morphology, size, metabolic activity). Additionally, BTC exhibits significant anatomical heterogeneity and inherent desmoplastic stroma, making it difficult to accurately delineate the tumor boundaries radiologically. This difficulty may result in the discrepancies between imaging assessments and survival prognosis.
Our findings indicate that PD-L1 may not be an absolute biomarker for immunotherapy response in patients with BTC. The difference in pooled ORR values for anti-PD-1/PD-L1 therapy between PD-L1 (+) and PD-L1 (-) patients was <5% (28.8% vs. 24.0%). Moreover, durable clinical benefits were reported even in PD-L1 (-) individuals. Therefore, PD-L1 expression in patients with BTC is more appropriately used as a biomarker to predict prognosis, rather than as an independent marker in determining the choice of anti-PD-1/PD-L1 therapy. Interestingly, the predictive value of PD-L1 expression significantly decreased in patients receiving anti-PD-L1 agents compared to those receiving anti-PD-1 agents. This trend has also been observed in carcinomas other than BTC (17, 56, 57). Further studies are warranted to verify whether the predictive value of PD-L1 expression differs depending on the target of ICIs (PD-1 vs. PD-L1) in cancer immunotherapy.
Various drugs, including conventional chemotherapeutic agents, anti-angiogenic agents, and inhibitors targeting other immune checkpoints (e.g., cytotoxic T lymphocyte-4), have been introduced in combination regimens with anti-PD-1/PD-L1 agents for the treatment of BTC (27, 28, 30, 37). A recent meta-analysis involving patients with BTC showed superior treatment response and clinical outcomes using combination regimens with anti-PD-1/PD-L1 therapy compared to anti-PD-1/PD-L1 monotherapy (58). This was consistent with our results, which demonstrated a higher response rate in combination therapy. Our study also showed that the role of PD-L1 expression as a predictive biomarker in OS was more prominent in anti-PD-1/PD-L1-based combination therapy than in monotherapy. Promising results of recent phase 3 studies strongly indicate that ICI-based combination therapy will likely become the first-line standard treatment for advanced BTC (10, 11). As such, future studies should further explore PD-L1 expression as a biomarker in combination therapy.
Although various methods in IHC have been reported for evaluating PD-L1 expression, the optimal method in patients with BTC remain unclear. Different PD-L1 antibodies, staining platforms, scoring methods, and cut-offs have been used in clinical trials and practices. Dako’s 22C3 and Ventana’s SP263 are two major antibodies commonly used in PD-L1 IHC, with more than half of the included studies reporting the use of these assays. As reported in other cancer types, both assays showed high concordance and may be utilized interchangeably (59). For PD-L1 scoring methods, TPS and CPS were the most frequently used for BTC studies. According to subgroup analyses and meta-regression in our study, scoring method did not significantly affect the predictive or prognostic value of PD-L1 expression in BTC. Conversely, when PD-L1 expression was determined at a high cut-off level (≥5%), the predictive value of survival was greatly improved. In a recent study including only PD-L1 (+) patients with BTC, higher PD-L1 values (≥50%) were associated with better therapeutic response to pembrolizumab compared to the lower PD-L1 values (1% to <50%) (60). Thus, similar to the findings in non-small cell lung cancer, further research is required to assess the prognosis of patients with BTC using subgroup analyses based on PD-L1 expression levels (61).
Given the limitations of PD-L1 expression as the only biomarker for immunotherapy in BTC, efforts have been made to identify other promising biomarkers. The overall number of somatic mutations in a tumor cell, referred to as “tumor mutation burden (TMB),” is an alternative candidate, since tumors with high TMB are expected to be more immunogenic. According to a study by Chiang et al., higher TMB (top 20%; ≥7.1 mut/Mb) predicted prolonged PFS but not OS in patients receiving chemotherapy plus nivolumab (34). However, data regarding TMB in BTC are limited, and previous studies have employed arbitrary and different cut-off thresholds (27, 36, 38). Another potential predictive biomarker for ICI responsiveness is microsatellite instability (MSI). Despite its modest predictive value for ICI response in most solid malignancies, MSI-high BTC seems to be quite rare, with a prevalence of 1-2% (34, 60, 62). Recently, a retrospective study reported that hematologic parameters, such as neutrophil-to-lymphocyte ratio or inflammatory cytokines, can be used for predicting therapeutic response to anti-PD-1 therapy in BTC (63). Since existing biomarkers, including PD-L1, have not yet shown satisfactory results, the identification of other validated predictors of immunotherapy response is equally important.
Despite the insights offered by this study, certain limitations should be acknowledged. First, significant heterogeneity was noted in pooled analyses, which was likely due to variations in treatment modalities and the lack of standardized analytical methods for PD-L1 IHC. Subgroup analyses and meta-regression were undertaken to address this matter; however, the persisting heterogeneity may still influence the results. Prospective biomarker studies conducted with uniform criteria for PD-L1 IHC are recommended to address this issue. Second, relevant studies might have been excluded due to lacking data on biomarker analysis or missing reports due to non-significant results. Third, the inclusion of several retrospective studies may have introduced bias. To address this, sensitivity analysis of prospective studies was performed for the primary outcomes. Lastly, while BTC exhibits diverse prognoses depending on its location, this meta-analysis was unable to provide the predictive value of PD-L1 according to location due to insufficient information from individual studies. Ultimately, a prospective combination of deep sequencing and experimental study is indispensable for biomarker research in immunotherapy for BTC.
In conclusion, PD-L1 expression may be a helpful predictive biomarker for survival in patients with BTC undergoing anti-PD-1/PD-L1 therapy. However, its utility as a biomarker for radiologic tumor response is less reliable. Various regimens of ICIs and the lack of standardized analytic methods can significantly affect the predictive value of PD-L1 expression. Therefore, we expect that further biomarker studies, including PD-L1, must be reported using highly efficacious regimens and established IHC methods to fully support the use of anti-PD-1/PD-L1 therapy in BTC.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
SY: Writing – original draft, Validation, Software, Methodology, Investigation, Formal analysis, Conceptualization. SW: Writing – original draft, Validation, Supervision, Formal analysis, Conceptualization. JC: Writing – original draft, Formal analysis, Data curation. DK: Writing – review & editing, Investigation, Data curation. JK: Writing – review & editing, Supervision, Investigation. JP: Writing – review & editing, Investigation, Data curation. HS: Writing – review & editing, Methodology, Data curation. MC: Writing – review & editing, Supervision, Methodology. IC: Writing – review & editing, Investigation, Data curation. JH: Writing – review & editing, Methodology, Formal analysis.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the National Cancer Center, South Korea (grant numbers: 2210610, 2310280).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1321813/full#supplementary-material
Supplementary Figure 1 | Forest plots showing the primary outcomes in total patients with biliary tract cancer treated with anti PD-1/PD-L1 therapy. (A) Objective response rate. (B) Disease control rate. (C) Grade 3-5 adverse event rate.
Supplementary Figure 2 | Sensitivity analysis excluding retrospective studies showing the results of primary outcomes in patients with biliary tract cancer treated with anti PD-1/PD-L1 therapy according to PD-L1 expression. (A) Objective response rate. (B) Disease control rate. (C) Progression-free survival. (D) Overall survival.
Supplementary Figure 3 | Funnel plot evaluation for publication bias. (A) Objective response rate. (B) Disease control rate. (C) Progression-free survival. (D) Overall survival.
1. Bertuccio P, Malvezzi M, Carioli G, Hashim D, Boffetta P, El-Serag HB, et al. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. (2019) 71:104–14. doi: 10.1016/j.jhep.2019.03.013
2. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
3. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. (2010) 362:1273–81. doi: 10.1056/NEJMoa0908721
4. Valle JW, Furuse J, Jitlal M, Beare S, Mizuno N, Wasan H, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol. (2014) 25:391–8. doi: 10.1093/annonc/mdt540
5. Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. (2013) 369:134–44. doi: 10.1056/NEJMoa1305133
6. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. (2018) 379:2040–51. doi: 10.1056/NEJMoa1810865
7. Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. (2015) 373:1803–13. doi: 10.1056/NEJMoa1510665
8. Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. (2014) 515:558–62. doi: 10.1038/nature13904
9. Piha-Paul SA, Oh DY, Ueno M, Malka D, Chung HC, Nagrial A, et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int J Cancer. (2020) 147:2190–8. doi: 10.1002/ijc.33013
10. Oh D-Y, Ruth He A, Qin S, Chen L-T, Okusaka T, Vogel A, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evidence. (2022) 1. doi: 10.1056/EVIDoa2200015
11. Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2023) 401:1853–65. doi: 10.1016/s0140-6736(23)00727-4
12. Larkin J, Minor D, D'Angelo S, Neyns B, Smylie M, Miller WH Jr., et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator's choice chemotherapy in checkMate 037: A randomized, controlled, open-label phase III trial. J Clin Oncol. (2018) 36:383–90. doi: 10.1200/JCO.2016.71.8023
13. Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. (2017) 376:2415–26. doi: 10.1056/NEJMoa1613493
14. Mori K, Abufaraj M, Mostafaei H, Quhal F, Fajkovic H, Remzi M, et al. The predictive value of programmed death ligand 1 in patients with metastatic renal cell carcinoma treated with immune-checkpoint inhibitors: A systematic review and meta-analysis. Eur Urol. (2021) 79:783–92. doi: 10.1016/j.eururo.2020.10.006
15. Noori M, Yousefi AM, Zali MR, Bashash D. Predictive value of PD-L1 expression in response to immune checkpoint inhibitors for esophageal cancer treatment: A systematic review and meta-analysis. Front Oncol. (2022) 12:1021859. doi: 10.3389/fonc.2022.1021859
16. Yoon HH, Jin Z, Kour O, Kankeu Fonkoua LA, Shitara K, Gibson MK, et al. Association of PD-L1 expression and other variables with benefit from immune checkpoint inhibition in advanced gastroesophageal cancer: systematic review and meta-analysis of 17 phase 3 randomized clinical trials. JAMA Oncol. (2022) 8:1456–65. doi: 10.1001/jamaoncol.2022.3707
17. Yang Y, Chen D, Zhao B, Ren L, Huang R, Feng B, et al. The predictive value of PD-L1 expression in patients with advanced hepatocellular carcinoma treated with PD-1/PD-L1 inhibitors: A systematic review and meta-analysis. Cancer Med. (2023) 12:9282–92. doi: 10.1002/cam4.5676
18. Kim RD, Chung V, Alese OB, El-Rayes BF, Li D, Al-Toubah TE, et al. A phase 2 multi-institutional study of nivolumab for patients with advanced refractory biliary tract cancer. JAMA Oncol. (2020) 6:888–94. doi: 10.1001/jamaoncol.2020.0930
19. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
20. Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. (2013) 158:280–6. doi: 10.7326/0003-4819-158-4-201302190-00009
21. Riley RD, Moons KGM, Snell KIE, Ensor J, Hooft L, Altman DG, et al. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ. (2019) 364:k4597. doi: 10.1136/bmj.k4597
22. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
23. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
24. Arkenau HT, Martin-Liberal J, Calvo E, Penel N, Krebs MG, Herbst RS, et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced or metastatic biliary tract cancer: nonrandomized, open-label, phase I trial (JVDF). Oncologist. (2018) 23:1407–e136. doi: 10.1634/theoncologist.2018-0044
25. Gou M, Zhang Y, Si H, Dai G. Efficacy and safety of nivolumab for metastatic biliary tract cancer. Onco Targets Ther. (2019) 12:861–7. doi: 10.2147/OTT.S195537
26. Ueno M, Ikeda M, Morizane C, Kobayashi S, Ohno I, Kondo S, et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: a non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol Hepatol. (2019) 4:611–21. doi: 10.1016/S2468-1253(19)30086-X
27. Chen X, Wu X, Wu H, Gu Y, Shao Y, Shao Q, et al. Camrelizumab plus gemcitabine and oxaliplatin (GEMOX) in patients with advanced biliary tract cancer: a single-arm, open-label, phase II trial. J Immunother Cancer. (2020) 8:e001240. doi: 10.1136/jitc-2020-001240
28. Feng K, Liu Y, Zhao Y, Yang Q, Dong L, Liu J, et al. Efficacy and biomarker analysis of nivolumab plus gemcitabine and cisplatin in patients with unresectable or metastatic biliary tract cancers: results from a phase II study. J Immunother Cancer. (2020) 8:e000367. doi: 10.1136/jitc-2019-000367
29. Kang J, Jeong JH, Hwang HS, Lee SS, Park DH, Oh DW, et al. Efficacy and safety of pembrolizumab in patients with refractory advanced biliary tract cancer: tumor proportion score as a potential biomarker for response. Cancer Res Treat. (2020) 52:594–603. doi: 10.4143/crt.2019.493
30. Lin J, Yang X, Long J, Zhao S, Mao J, Wang D, et al. Pembrolizumab combined with lenvatinib as non-first-line therapy in patients with refractory biliary tract carcinoma. Hepatobiliary Surg Nutr. (2020) 9:414–24. doi: 10.21037/hbsn-20-338
31. Yoo C, Oh DY, Choi HJ, Kudo M, Ueno M, Kondo S, et al. Phase I study of bintrafusp alfa, a bifunctional fusion protein targeting TGF-beta and PD-L1, in patients with pretreated biliary tract cancer. J Immunother Cancer. (2020) 8:e000564. doi: 10.1136/jitc-2020-000564
32. Wang D, Yang X, Long J, Lin J, Mao J, Xie F, et al. The efficacy and safety of apatinib plus camrelizumab in patients with previously treated advanced biliary tract cancer: A prospective clinical study. Front Oncol. (2021) 11:646979. doi: 10.3389/fonc.2021.646979
33. Zhang Q, Liu X, Wei S, Zhang L, Tian Y, Gao Z, et al. Lenvatinib plus PD-1 inhibitors as first-line treatment in patients with unresectable biliary tract cancer: A single-arm, open-label, phase II study. Front Oncol. (2021) 11:751391. doi: 10.3389/fonc.2021.751391
34. Chiang NJ, Tan KT, Bai LY, Hsiao CF, Huang CY, Hung YP, et al. Impaired chromatin remodeling predicts better survival to modified gemcitabine and S-1 plus nivolumab in advanced biliary tract cancer: A phase II T1219 study. Clin Cancer Res. (2022) 28:4248–57. doi: 10.1158/1078-0432.CCR-22-1152
35. Cousin S, Cantarel C, Guegan JP, Mazard T, Gomez-Roca C, Metges JP, et al. Regorafenib-avelumab combination in patients with biliary tract cancer (REGOMUNE): a single-arm, open-label, phase II trial. Eur J Cancer. (2022) 162:161–9. doi: 10.1016/j.ejca.2021.11.012
36. Ding X, Li G, Sun W, Shen Y, Teng Y, Xu Y, et al. Sintilimab combined with lenvatinib for advanced intrahepatic cholangiocarcinoma in second-line setting-A multi-center observational study. Front Oncol. (2022) 12:907055. doi: 10.3389/fonc.2022.907055
37. Doki Y, Ueno M, Hsu CH, Oh DY, Park K, Yamamoto N, et al. Tolerability and efficacy of durvalumab, either as monotherapy or in combination with tremelimumab, in patients from Asia with advanced biliary tract, esophageal, or head-and-neck cancer. Cancer Med. (2022) 11:2550–60. doi: 10.1002/cam4.4593
38. Dong X, Zhang Z, Zhang Q, Chen L, Cao G, Liu C, et al. Triple therapy in biliary tract cancers: GemOX plus immune checkpoint inhibitor in combination with lenvatinib or NGS-guided targeted therapy. J Cancer Res Clin Oncol. (2023) 149:1917–27. doi: 10.1007/s00432-022-04166-z
39. Kim H, Kim R, Jo H, Kim HR, Hong J, Ha SY, et al. Expression of PD-L1 as a predictive marker of sensitivity to immune checkpoint inhibitors in patients with advanced biliary tract cancer. Therap Adv Gastroenterol. (2022) 15:17562848221117638. doi: 10.1177/17562848221117638
40. Li W, Wang Y, Yu Y, Li Q, Wang Y, Zhang C, et al. Toripalimab in advanced biliary tract cancer. Innovation (Camb). (2022) 3:100255. doi: 10.1016/j.xinn.2022.100255
41. Oh DY, Lee KH, Lee DW, Yoon J, Kim TY, Bang JH, et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, single-centre, phase 2 study. Lancet Gastroenterol Hepatol. (2022) 7:522–32. doi: 10.1016/S2468-1253(22)00043-7
42. Shi C, Li Y, Yang C, Qiao L, Tang L, Zheng Y, et al. Lenvatinib plus programmed cell death protein-1 inhibitor beyond first-line systemic therapy in refractory advanced biliary tract cancer: A real-world retrospective study in China. Front Immunol. (2022) 13:946861. doi: 10.3389/fimmu.2022.946861
43. Tan S, Yu J, Huang Q, Zhou N, Gou H. PD-1 inhibitors plus nab-paclitaxel-containing chemotherapy for advanced gallbladder cancer in a second-line setting: A retrospective analysis of a case series. Front Oncol. (2022) 12:1006075. doi: 10.3389/fonc.2022.1006075
44. Zuo B, Yang X, Yang X, Bian J, Long J, Wang D, et al. A real-world study of the efficacy and safety of anti-PD-1 antibodies plus lenvatinib in patients with advanced gallbladder cancer. Cancer Immunol Immunother. (2022) 71:1889–96. doi: 10.1007/s00262-021-03121-0
45. Jeong SY, Hong JY, Park JO, Park YS, Lim HY, Jang JY, et al. The efficacy of immune checkpoint inhibitors in biliary tract cancer with KRAS mutation. Therap Adv Gastroenterol. (2023) 16:17562848231170484. doi: 10.1177/17562848231170484
46. Jin S, Zhao R, Zhou C, Zhong Q, Shi J, Su C, et al. Feasibility and tolerability of sintilimab plus anlotinib as the second-line therapy for patients with advanced biliary tract cancers: An open-label, single-arm, phase II clinical trial. Int J Cancer. (2023) 152:1648–58. doi: 10.1002/ijc.34372
47. Shi GM, Huang XY, Wu D, Sun HC, Liang F, Ji Y, et al. Toripalimab combined with lenvatinib and GEMOX is a promising regimen as first-line treatment for advanced intrahepatic cholangiocarcinoma: a single-center, single-arm, phase 2 study. Signal Transduct Target Ther. (2023) 8:106. doi: 10.1038/s41392-023-01317-7
48. Wang Y, Xun Z, Yang X, Wang Y, Wang S, Xue J, et al. Local-regional therapy combined with toripalimab and lenvatinib in patients with advanced biliary tract cancer. Am J Cancer Res. (2023) 13:1026–37.
49. Yoo C, Javle MM, Verdaguer Mata H, de Braud F, Trojan J, Raoul JL, et al. Phase 2 trial of bintrafusp alfa as second-line therapy for patients with locally advanced/metastatic biliary tract cancers. Hepatology. (2023) 78:758–70. doi: 10.1097/hep.0000000000000365
50. Zhu C, Li H, Yang X, Wang S, Wang Y, Zhang N, et al. Efficacy, safety, and prognostic factors of PD-1 inhibitors combined with lenvatinib and Gemox chemotherapy as first-line treatment in advanced intrahepatic cholangiocarcinoma: a multicenter real-world study. Cancer Immunol Immunother. (2023) 72:2949–60. doi: 10.1007/s00262-023-03466-8
51. Zhu C, Xue J, Wang Y, Wang S, Zhang N, Wang Y, et al. Efficacy and safety of lenvatinib combined with PD-1/PD-L1 inhibitors plus Gemox chemotherapy in advanced biliary tract cancer. Front Immunol. (2023) 14:1109292. doi: 10.3389/fimmu.2023.1109292
52. Lei C, Peng X, Gong X, Fan Y, Wu S, Liu N, et al. Prognostic role of programmed death-ligand 1 expression in patients with biliary tract cancer: a meta-analysis. Aging (Albany NY). (2019) 11:12568–80. doi: 10.18632/aging.102588
53. Mody K, Starr J, Saul M, Poorman K, Weinberg BA, Salem ME, et al. Patterns and genomic correlates of PD-L1 expression in patients with biliary tract cancers. J Gastrointest Oncol. (2019) 10:1099–109. doi: 10.21037/jgo.2019.08.08
54. Kim H, Kim J, Byeon S, Jang KT, Hong JY, Lee J, et al. Programmed death ligand 1 expression as a prognostic marker in patients with advanced biliary tract cancer. Oncology. (2021) 99:365–72. doi: 10.1159/000514404
55. Zhang B, Liu Y, Zhou S, Jiang H, Zhu K, Wang R. Predictive effect of PD-L1 expression for immune checkpoint inhibitor (PD-1/PD-L1 inhibitors) treatment for non-small cell lung cancer: A meta-analysis. Int Immunopharmacol. (2020) 80:106214. doi: 10.1016/j.intimp.2020.106214
56. Khan M, Du K, Ai M, Wang B, Lin J, Ren A, et al. PD-L1 expression as biomarker of efficacy of PD-1/PD-L1 checkpoint inhibitors in metastatic triple negative breast cancer: A systematic review and meta-analysis. Front Immunol. (2023) 14:1060308. doi: 10.3389/fimmu.2023.1060308
57. Liu H, Ye T, Yang X, Lv P, Wu X, Zhou H, et al. Predictive and prognostic role of PD-L1 in urothelial carcinoma patients with anti-PD-1/PD-L1 therapy: A systematic review and meta-analysis. Dis Markers. (2020) 2020:8375348. doi: 10.1155/2020/8375348
58. Jiang Q, Huang J, Zhang B, Li X, Chen X, Cui B, et al. Efficacy and safety of anti-PD1/PDL1 in advanced biliary tract cancer: A systematic review and meta-analysis. Front Immunol. (2022) 13:801909. doi: 10.3389/fimmu.2022.801909
59. Torlakovic E, Lim HJ, Adam J, Barnes P, Bigras G, Chan AWH, et al. "Interchangeability" of PD-L1 immunohistochemistry assays: a meta-analysis of diagnostic accuracy. Mod Pathol. (2020) 33:4–17. doi: 10.1038/s41379-019-0327-4
60. Ahn S, Lee JC, Shin DW, Kim J, Hwang JH. High PD-L1 expression is associated with therapeutic response to pembrolizumab in patients with advanced biliary tract cancer. Sci Rep. (2020) 10:12348. doi: 10.1038/s41598-020-69366-4
61. Jöhrens K, Rüschoff J. The challenge to the pathologist of PD-L1 expression in tumor cells of non-small-cell lung cancer—An overview. Curr Oncol. (2021) 28:5227–39. doi: 10.3390/curroncol28060437
62. Goeppert B, Roessler S, Renner M, Singer S, Mehrabi A, Vogel MN, et al. Mismatch repair deficiency is a rare but putative therapeutically relevant finding in non-liver fluke associated cholangiocarcinoma. Br J Cancer. (2019) 120:109–14. doi: 10.1038/s41416-018-0199-2
Keywords: cholangiocarcinoma, programmed death ligand 1, immune checkpoint inhibitors, biomarker, immunohistochemistry
Citation: Yoon SB, Woo SM, Chun JW, Kim DU, Kim J, Park JK, So H, Chung MJ, Cho IR and Heo J (2024) The predictive value of PD-L1 expression in response to anti-PD-1/PD-L1 therapy for biliary tract cancer: a systematic review and meta-analysis. Front. Immunol. 15:1321813. doi: 10.3389/fimmu.2024.1321813
Received: 15 October 2023; Accepted: 18 March 2024;
Published: 28 March 2024.
Edited by:
Xuesong Gu, Beth Israel Deaconess Medical Center and Harvard Medical School, United StatesReviewed by:
Jianfeng Yang, Hangzhou First People’s Hospital, ChinaCopyright © 2024 Yoon, Woo, Chun, Kim, Kim, Park, So, Chung, Cho and Heo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sang Myung Woo, d3NtQG5jYy5yZS5rcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.