- 1Department of Traditional Chinese Medicine, Children’s Hospital, Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Zhejiang, Hangzhou, China
- 2Department of Nephrology, Children’s Hospital, Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Zhejiang, Hangzhou, China
Aim: This study aimed to systematically compare the efficacy of various immunosuppressive agents in treating pediatric frequently relapsing or steroid-dependent nephrotic syndrome (FRSDNS).
Methods: We conducted systematic searches of PubMed, Embase, the Cochrane Library, and the Web of Science up to May 23, 2023. Outcome measures included relapses within 1 year, mean cumulative exposure to corticosteroids, patients with treatment failure at 1 year, relapse-free survival during 1 year, and adverse events. The quality of the included studies was evaluated using the modified Jadad scale, the Methodological Index for Non-Randomized Studies (MINORS), and the modified Newcastle-Ottawa Scale (NOS).
Results: Rituximab was found to be the most likely (92.44%) to be associated with the fewest relapses within 1 year and was also most likely (99.99%) to result in the lowest mean cumulative exposure to corticosteroids. Rituximab had the highest likelihood (45.98%) of being associated with the smallest number of patients experiencing treatment failure at 1 year. CsA was most likely (57.93%) to achieve the highest relapse-free survival during 1 year, followed by tacrolimus (26.47%) and rituximab (30.48%). Rituximab showed no association with serious side effects and had comparable adverse effects to ofatumumab and tacrolimus.
Conclusion: Rituximab may be the most favorable immunosuppressive agent for treating pediatric FRSDNS. Nephrologists should consider this drug, along with their clinical experience, patient characteristics, and cost considerations, when choosing a treatment approach.
Introduction
Nephrotic syndrome plays a significant role in the progression to end-stage kidney disease (1), characterized by several key features, including edema, proteinuria, low blood albumin levels, and elevated lipid levels in the blood (2). As the most common glomerular disease affecting children, nephrotic syndrome impacts between two and five children per one hundred thousand each year (3). Corticosteroids serve as the primary treatment for idiopathic nephrotic syndrome (4), however, it is estimated that around 50% of children diagnosed with steroid-sensitive nephrotic syndrome (SSNS) will experience frequent relapsing or steroid-dependent nephrotic syndrome (FRSDNS) (5). Immunosuppressive drugs have proven effective in treating children with FRSDNS (6–8).
Immunosuppressive medications used to treat pediatric FRSDNS include rituximab, cyclophosphamide, tacrolimus, and mycophenolate mofetil (MMF). A recent study showed that children with FRSDNS who were treated with rituximab experienced improved one-year relapse-free survival, fewer relapses, and reduced cumulative corticosteroid exposure compared to those treated with cyclophosphamide and tacrolimus (9). In pediatric steroid-dependent nephrotic syndrome (SDNS), treatment with MMF/dexamethasone (DEX) has been shown to be more effective than cyclosporine A (CsA) in reducing relapses and cumulative corticosteroid dosage (10). According to another study, children with SDNS treated with ofatumumab and rituximab experienced similar rates of relapses and adverse events (11). A 2019 network meta-analysis aimed at identifying the best immunosuppressive drug for children with FRSDNS suggested that cyclophosphamide might initially be the most suitable option (6). Nonetheless, the study was limited by its inclusion of placebo and no treatment as controls, insufficient direct comparisons between immunosuppressive drugs, and neglect of recent literature updates.
To address these research gaps, our network meta-analysis aimed to systematically assess and rank the effectiveness of different immunosuppressive drugs in treating pediatric FRSDNS, drawing on the latest direct comparison evidence among these agents.
Methods
Search strategy
Four electronic databases (PubMed, Embase, Cochrane Library, and Web of Science) were systematically searched by two independent investigators (JY Chen, Y Zhang) from database inception until May 23, 2023. Discrepancies were addressed via discussion. The following English search terms were applied: “Immunosuppressive Agents” OR “Agents, Immunosuppressive” OR “Immunosuppressants” OR “Immunosuppressive Agent” OR “Agent, Immunosuppressive” OR “Immunosuppressant” OR “Immunosuppressive drugs” OR “Cyclophosphamide” OR “Cyclosporine” OR “Cyclosporine A” OR “Tacrolimus” OR “Mycophenolate mofetil” OR “MMF” OR “Rituximab” OR “Cyclosporin” OR “Azathioprine” OR “Mizoribine” OR “Vincristine” OR “Calcineurin inhibitors” OR “Mycophenolic sodium” OR “MPS” AND “Nephrotic Syndrome” OR “Nephrotic Syndromes” OR “Syndrome, Nephrotic” OR “Steroid-Dependent Nephrotic Syndrome” OR “Nephrotic Syndrome, Steroid-Dependent” OR “Steroid Dependent Nephrotic Syndrome” OR “Steroid-Dependent Nephrotic Syndromes” OR “Childhood Idiopathic Nephrotic Syndrome” OR “Pediatric Idiopathic Nephrotic Syndrome” OR “Multi-Drug Resistant Nephrotic Syndrome” OR “Multi Drug Resistant Nephrotic Syndrome” OR “Steroid-Sensitive Nephrotic Syndrome” OR “Nephrotic Syndrome, Steroid-Sensitive” OR “Steroid Sensitive Nephrotic Syndrome” OR “Steroid-Sensitive Nephrotic Syndromes” OR “Syndrome, Steroid-Sensitive Nephrotic” OR “Steroid-Resistant Nephrotic Syndrome” OR “Nephrotic Syndrome, Steroid-Resistant” OR “Steroid Resistant Nephrotic Syndrome” OR “Steroid-Resistant Nephrotic Syndromes” OR “Frequently Relapsing Nephrotic Syndrome” OR “Frequent relapses or steroid dependence nephrotic syndrome” OR “FRSDNS”. Initial screening of the retrieved studies was performed by reviewing titles and abstracts, followed by full-text evaluations. This systematic review and network meta-analysis adhered to the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension statement for network meta-analyses.
Eligibility criteria
The inclusion criteria were structured according to the Population, Intervention, Comparator, Outcome, Study design (PICOS) framework, starting with: (1) studies focusing on children diagnosed with FRSDNS (Population; (2) studies that investigated the treatment of pediatric FRSDNS with various immunosuppressive drugs, including rituximab, cyclophosphamide, tacrolimus, MMF, CsA, and ofatumumab (Intervention and Comparator); (3) studies on at least one of the following outcomes: relapses within 1 year, mean cumulative exposure to corticosteroids (mg/kg/year), patients with treatment failure at 1 year, relapse-free survival during 1 year, and adverse events (Outcome); (4) clinical controlled trials and cohort studies (Study design); (5) studies published in English language.
Exclusion criteria: (1) studies involving animal experiments; (2) studies using placebo or prednisone as the control group; (3) studies with fewer than 10 participants; (4) studies focused on comparing different doses of the same drug; (5) studies examining different methods of medication administration; (6) studies unrelated to the topic; (7) case reports, editorial materials, meeting abstracts, protocols, guidelines, expert consensus, reviews, and meta-analyses.
Data extraction and quality assessment
Two investigators (JY Chen, XA Wang) extracted the following data independently from eligible studies: first author, year of publication, country, study design, population, intervention, immunosuppressive agents, sample size, male/female, age (years), proteinuria, urine protein/creatine ratio, albumin (g/dL), follow-up time (months), and outcome. Disagreements were settled by another investigator (Y Zhang).
Quality assessment
The quality of randomized controlled trials was assessed using the modified Jadad scale, which evaluates four key aspects: the generation of random sequences, concealment of randomization, the methodology of blinding, and the withdrawals and dropouts (12). The modified Jadad scale has a total possible score of 7 points. Studies scoring between 1 and 3 points are considered to be of low quality, while those achieving a score of 4 to 7 points are classified as high quality. The quality of non-randomized controlled trials was evaluated using the Methodological Index for Non-Randomized Studies (MINORS), which has 12 items for comparative studies, with each item scoring 0-2 points (0: not reported; 1: reported but inadequate; 2: reported and adequate) (13). The quality of cohort studies was assessed using the modified Newcastle-Ottawa Scale (NOS), which evaluates three primary aspects: selection of the population, comparability between groups, and measurement of outcomes. The total score on this scale can reach up to 9 points. Studies scoring between 0 and 3 points were considered low quality, those with 4 to 6 points were deemed medium quality, and studies achieving 7 to 9 points were categorized as high quality (14).
Statistical analysis
Using Gemtc 1.0.1 package from R 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria), a Bayesian framework and Monte Carlo Markov Chain (MCMC) model were constructed for network meta-analysis. The model has 4 chains, 20000 initial iterations, and 50000 updated iterations with a step size of 1. When there was a network relationship, consistency and inconsistency were detected in the direct and indirect comparisons. When the difference in the Deviation Information Criterion (DIC) between the results of consistency and inconsistency detection was less than 5, it indicated that the strength of the direct and indirect evidence was consistent. The heterogeneity test was conducted for the effect size of each outcome. If the heterogeneity statistic I2 ≥ 50%, a random-effects model was used for analysis. Otherwise, a fixed-effects model was utilized. Network plots, forest plots, league tables, and rank probabilities were depicted for each outcome. For network plots, nodes represented different immunosuppressive agents, while the lines linking these nodes represented the direct comparisons between the agents. The size of nodes and the thickness of lines showed the sample size and the number of studies included for comparison, respectively. Weighted mean differences (WMDs) along with 95% credibility intervals (CrIs) were used to present measurement data. For enumeration data, odds ratios (ORs) and their corresponding 95% credibility intervals (95%CrIs) were calculated.
Results
Study selection
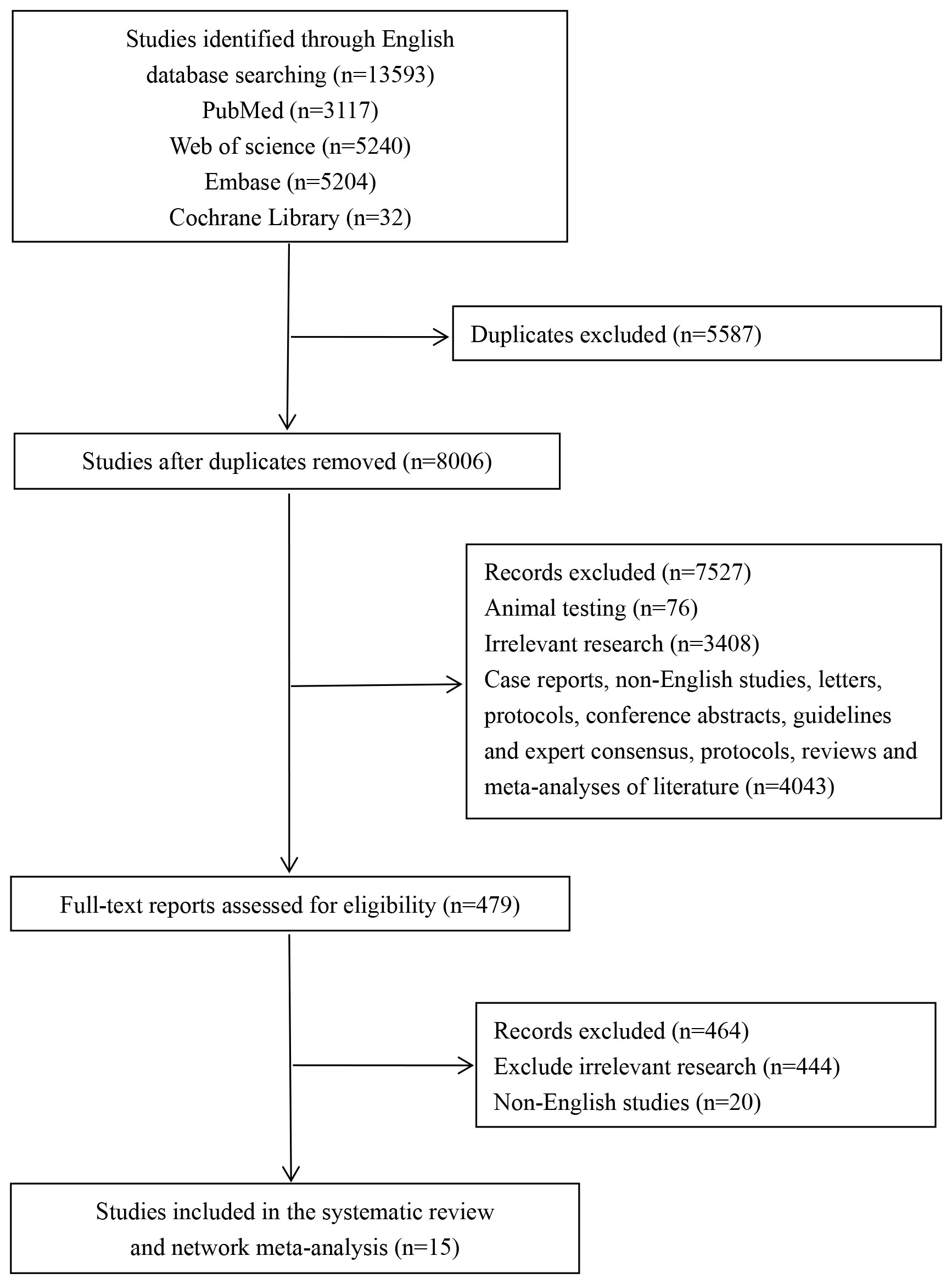
Through systematic searches across four databases, a total of 13,593 studies were retrieved, including 3,117 from PubMed, 5,240 from Embase, 5,204 from the Cochrane Library, and 32 from Web of Science. After excluding 5,587 duplicates and 7,527 studies based on their titles and abstracts, 479 studies remained for full-text evaluation. Eventually, 15 studies (9–11, 15–26) were included in this systematic review and network meta-analysis. The study selection process is depicted in Figure 1.
Characteristics of the included studies
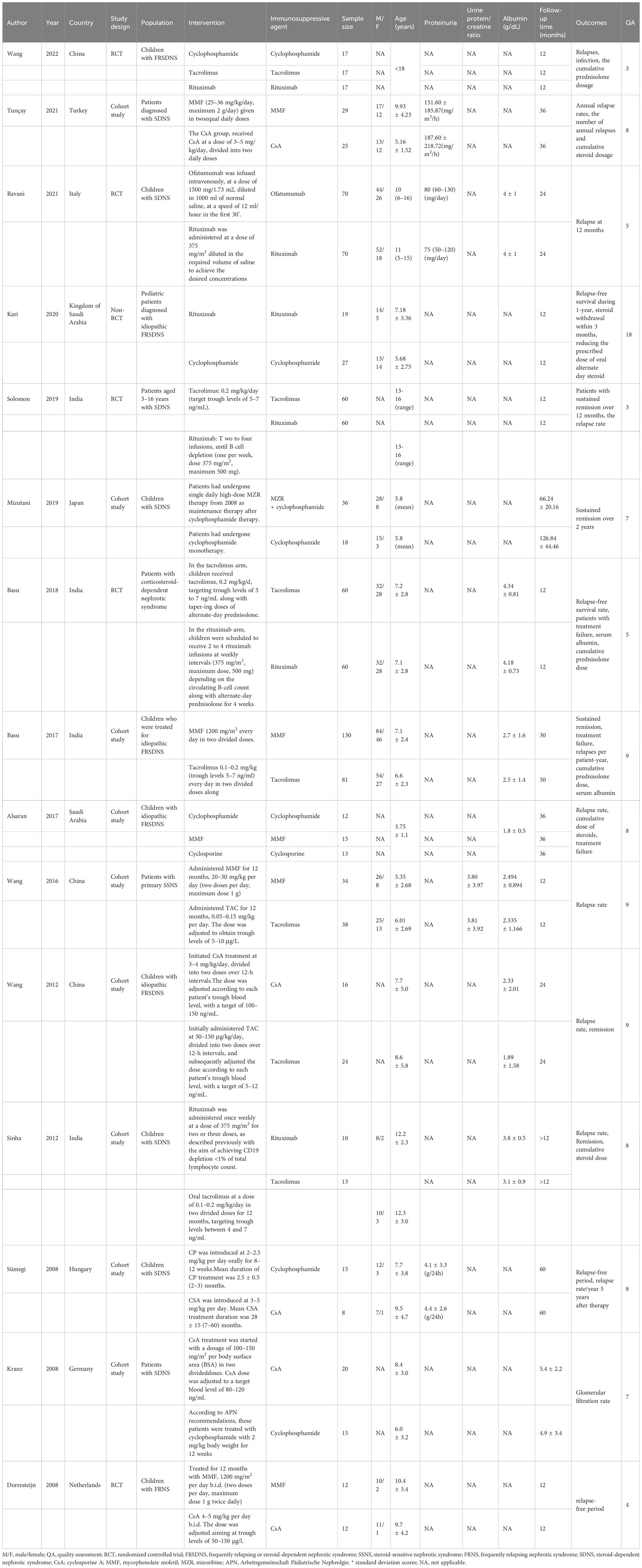
The 15 eligible studies (9–11, 15–26) included 1053 pediatric patients with FRSDNS. The year of publication covered 2008 to 2022, and 9 countries were involved. A total of 6 immunosuppressive agents were assessed in these studies: rituximab, cyclophosphamide, tacrolimus, MMF, CsA, and ofatumumab. There were 5 randomized controlled trials, 1 non-randomized controlled trial, and 9 cohort studies. The quality assessment results indicated that 12 studies were of high quality, 2 were of low quality, and one study achieved a MINORS score of 18 points. The characteristics of the included studies are detailed in Table 1.
Network analysis
Relapses within 1 year
Data on relapses within 1 year were assessed in 6 studies (9, 10, 17, 18, 21, 22), involving 402 patients and 5 immunosuppressive agents (cyclophosphamide, rituximab, tacrolimus, MMF, and CsA). Cyclophosphamide was a more frequently used agent, and a relatively greater number of studies directly compared CsA and cyclophosphamide (Figure 2A). The forest plot revealed that children who received rituximab treatment experienced significantly fewer relapses within 1 year compared to those treated with cyclophosphamide (pooled WMD=-0.70, 95%CrI: -1.10, -0.25) (Figure 3A). According to the league table, CsA (pooled WMD=0.94, 95%CrI: 0.50, 1.38), cyclophosphamide (pooled WMD=0.70, 95%CrI: 0.34, 1.05) and MMF (pooled WMD=0.55, 95%CrI: 0.18, 0.91) were associated with significantly more relapses within 1 year, as compared with rituximab (Table 2). The rank probabilities indicated that rituximab had the highest likelihood (92.44%) to be associated with the fewest relapses within 1 year (Table 3).
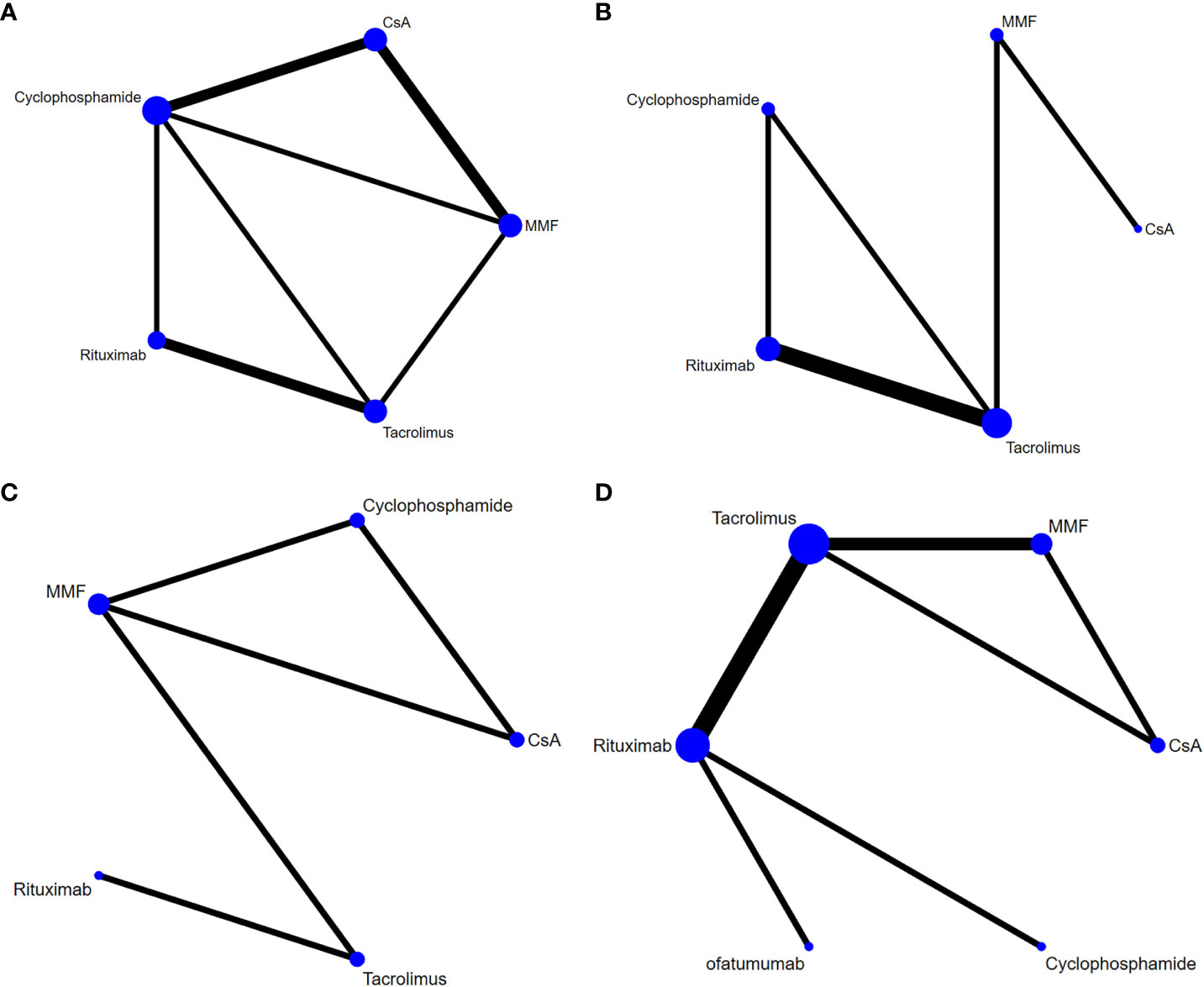
Figure 2 Network plots of different immunosuppressive agents for various outcomes in pediatric FRSDNS. (A), relapses within 1 year; (B), mean cumulative exposure to corticosteroids; (C), patients with treatment failure at 1 year; (D), relapse-free survival during 1 year. FRSDNS, frequently relapsing or steroid-dependent nephrotic syndrome; CsA; cyclosporine A; MMF, mycophenolate mofetil.
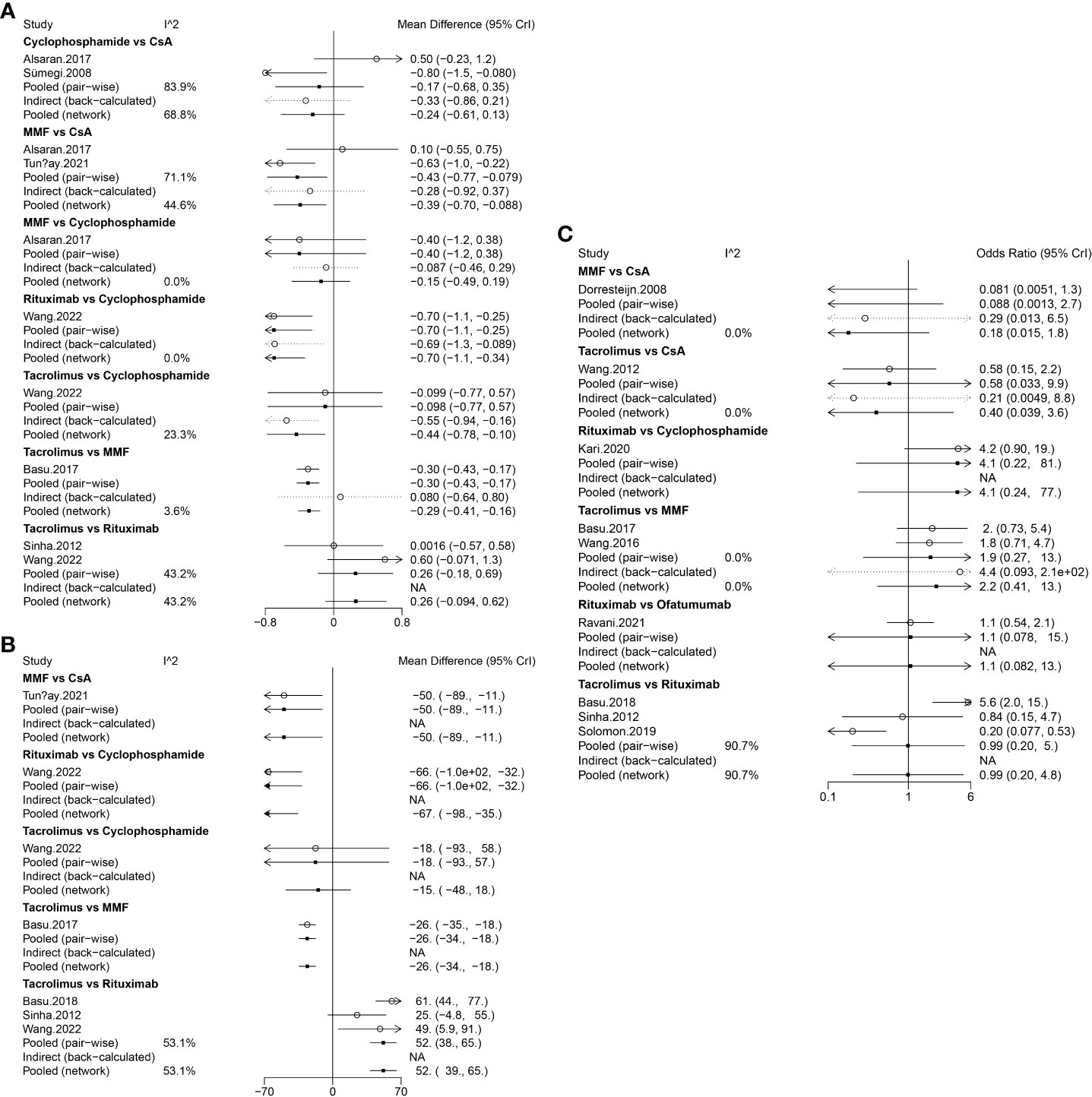
Figure 3 Forest plots of different immunosuppressive agents for various outcomes in pediatric FRSDNS. (A), relapses within 1 year; (B), mean cumulative exposure to corticosteroids; (C), relapse-free survival during 1 year. FRSDNS, frequently relapsing or steroid-dependent nephrotic syndrome; CsA; cyclosporine A; MMF, mycophenolate mofetil; CrI, credibility intervals; NA, not applicable.
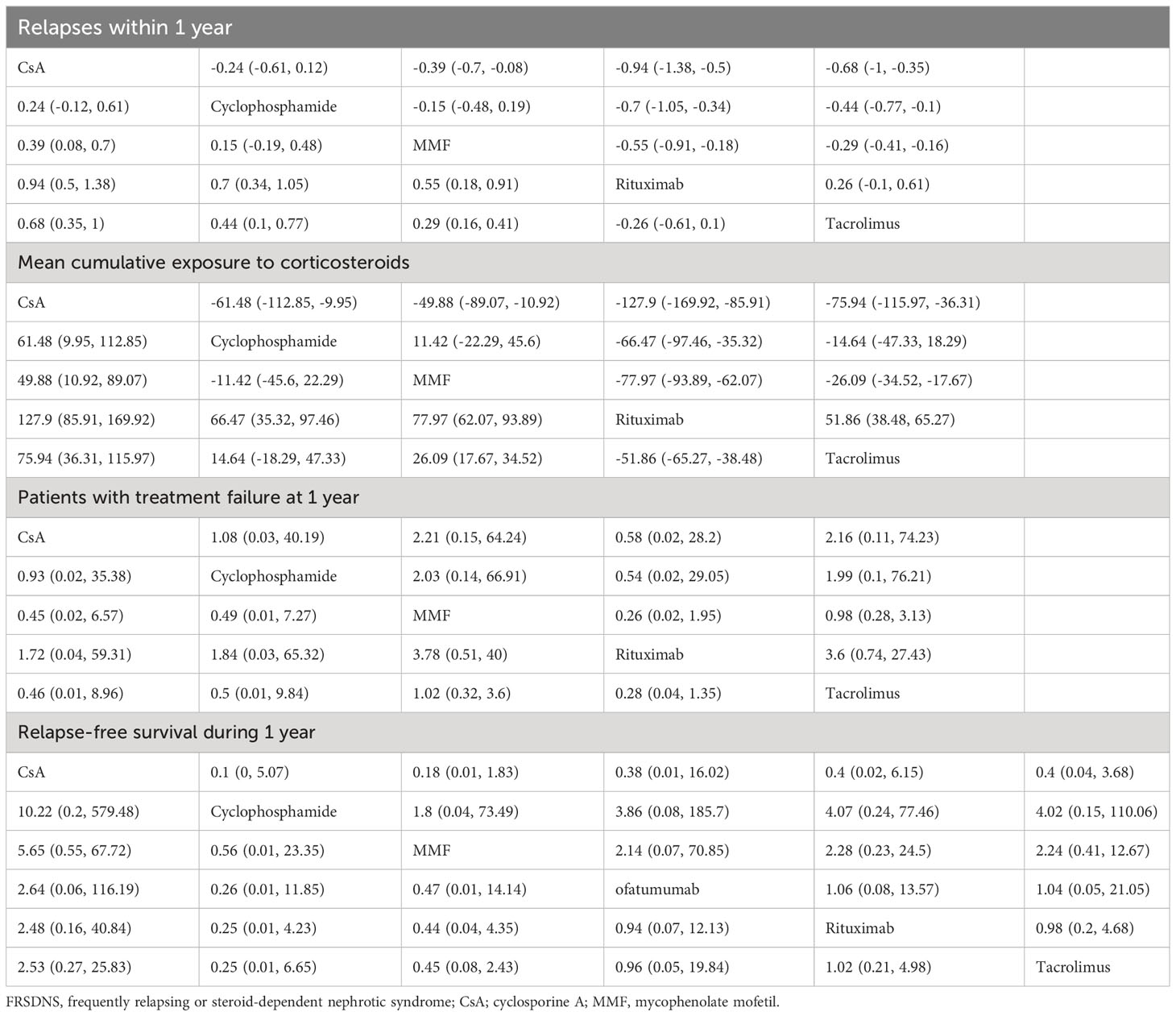
Table 2 League table of different immunosuppressive agents for various outcomes in pediatric FRSDNS.
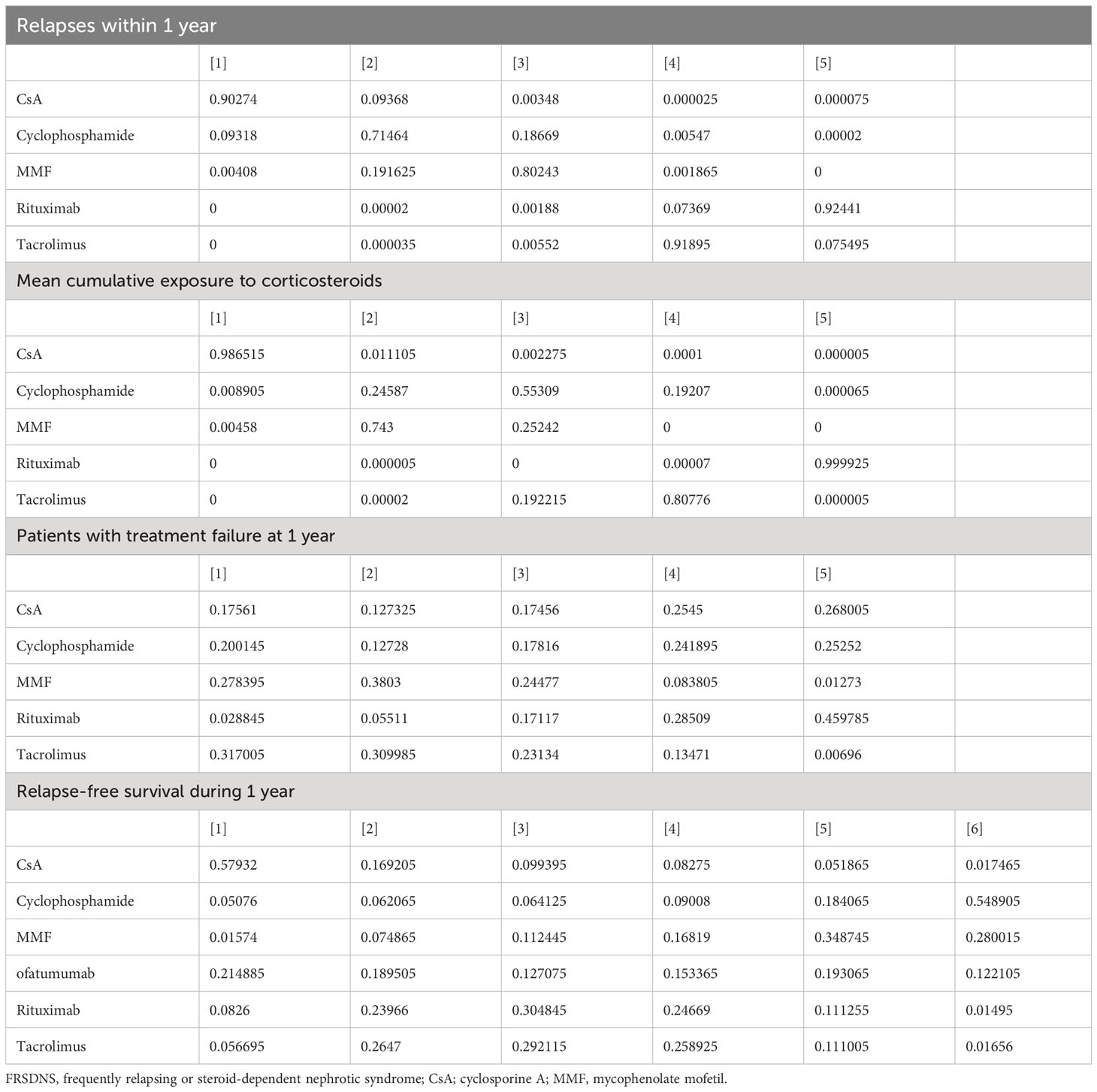
Table 3 Rank probabilities of different immunosuppressive agents for various outcomes in pediatric FRSDNS.
Mean cumulative exposure to corticosteroids
Five studies (9, 10, 18, 22, 23) including 459 patients assessed 5 immunosuppressive agents (cyclophosphamide, rituximab, tacrolimus, MMF, and CsA) in terms of the mean cumulative exposure to corticosteroids. A larger number of studies focused on the direct comparison between rituximab and tacrolimus, with a greater number of children receiving treatment with tacrolimus (Figure 2B). The forest plot illustrated that the mean cumulative exposure to corticosteroids was significantly lower in children treated with rituximab compared to those in the cyclophosphamide group (pooled WMD=-66.00, 95%CrI: -100.00, -32.00); patients treated with tacrolimus experienced a significantly higher mean cumulative exposure to corticosteroids compared to those receiving rituximab (pooled WMD=52.00, 95%CrI: 38.00, 65.00) (Figure 3B). The league table showed that the CsA (pooled WMD=127.90, 95%CrI: 85.91, 169.92), cyclophosphamide (pooled WMD=66.47, 95%CrI: 35.32, 97.46), and MMF (pooled WMD=77.97, 95%CrI: 62.07, 93.89) groups had significantly greater mean cumulative exposure to corticosteroids than the rituximab group (Table 2). According to the rank probabilities, patients treated with rituximab were the most likely, with a 99.99% probability, to have the lowest mean cumulative exposure to corticosteroids (Table 3).
Patients with treatment failure at 1 year
Treatment failure at 1 year was estimated in 3 studies (21–23) with 371 patients, and 5 immunosuppressive agents were compared: cyclophosphamide, rituximab, tacrolimus, MMF, and CsA. MMF was a more commonly applied drug (Figure 2C). The league table indicated that there was no significant difference in the number of patients experiencing treatment failure at 1 year between the groups treated with cyclophosphamide, rituximab, tacrolimus, MMF, and CsA (Table 2). The rank probabilities suggested that rituximab had the greatest likelihood (45.98%) to be associated with the smallest number of patients with treatment failure at 1 year (Table 3).
Relapse-free survival during 1 year
Nine studies (11, 15, 18–20, 22, 23, 25, 26) with 796 patients evaluated relapse-free survival during 1 year after treatment with 6 immunosuppressive agents (cyclophosphamide, rituximab, tacrolimus, MMF, CsA, and ofatumumab). Tacrolimus was administered to a larger number of children, and a higher number of studies directly compared tacrolimus with rituximab (Figure 2D). The forest plot (Figure 3C) and league table (Table 2) exhibited no significant difference in relapse-free survival during 1 year among the 6 medications. The rank probabilities indicated that, among children, those treated with CsA had the highest likelihood (57.93%) of achieving the greatest relapse-free survival over 1 year, followed closely by rituximab (30.48%) and tacrolimus (26.47%) (Table 3).
Adverse events
In the study of Wang et al. (9), 108 adverse events were recorded within 1 year: 24 in the rituximab group, 34 in the tacrolimus group, and 50 in the cyclophosphamide group (P = 0.327). The difference was primarily caused by infection. The incidence of infection with cyclophosphamide was almost twice that of rituximab and tacrolimus (P = 0.001). Ofatumumab and rituximab had similar adverse effects according to Ravani et al. (11). Both agents did not bring side effects during infusion, with most limited to pruritus or erythema. Wang et al. (20) illustrated no significant difference in the incidence of adverse events, such as infection, gastrointestinal symptoms, acute kidney injury, and neutropenia between patients using tacrolimus and MMF. The most common adverse event was infection, which was found in four (11.8%) MMF patients and three (7.89%) tacrolimus patients.
Discussion
This updated systematic review and network meta-analysis comprehensively assessed and compared the effectiveness of different immunosuppressive agents in pediatric patients with FRSDNS by incorporating studies with head-to-head comparisons of these agents. Rituximab emerged as a potentially superior medication in terms of reducing relapses within 1 year, minimizing mean cumulative corticosteroid exposure, and reducing treatment failure rates at 1 year. Furthermore, children with FRSDNS treated with rituximab may experience better relapse-free survival over the course of 1 year. These findings have implications for clinicians when selecting immunosuppressive agents for the treatment of pediatric FRSDNS and suggest that rituximab should be considered due to its favorable effectiveness.
A review by Larkins et al. (7) assessed non-corticosteroid immunosuppressive drugs in pediatric SSNS and reported that rituximab was an important adjunctive therapy for SDNS, and greatly reduces recurrences in children with relapsing SSNS. For FRSDNS in children, Tan et al. (6) performed a network meta-analysis for efficacy and acceptability of immunosuppressive medications in FRSDNS among children, and concluded that cyclophosphamide may be a preferable agent; chlorambucil and rituximab may also be suitable in treating FRSDNS. The above meta-analysis used placebo and no treatment as common comparators, and merely 8 out of 26 studies provided head-to-head comparisons of different immunosuppressive agents, which indicated insufficient evidence of direct comparison between immunosuppressive drugs. Besides, only the relapse rate was evaluated for efficacy. The present network meta-analysis utilized the latest direct comparison of various immunosuppressive agents to assess and rank the effectiveness of these agents and illustrated that considering relapses within 1 year, the mean cumulative exposure to corticosteroids, the number of patients with treatment failure at 1 year, and relapse-free survival during 1 year, rituximab may be the most favorable immunosuppressive drug for children with FRSDNS. Although CsA was most likely to be associated with the highest relapse-free survival during 1 year, no significant difference was found between rituximab and CsA. Rituximab is identified as a standard treatment for childhood-onset complicated FRSDNS (27, 28). A previous review showed the efficacy of rituximab in complicated FRSDNS (29). Rituximab, a monoclonal anti-CD20 antibody, has been shown in several studies to prolong clinical remission and reduce glucocorticoid dosages in children with FRSDNS (30, 31). Rituximab therapy causes B cell depletion by apoptosis, antibody-dependent cellular cytotoxicity or phagocytosis, reducing interactions between B cells and T cells, which perhaps prevents relapses in patients with FRSDNS. Besides, rituximab may improve the number and function of regulatory T (Treg) cells which have been demonstrated to cause remission in individuals with nephrotic syndrome, thus bringing better prognosis to patients (32, 33). Rituximab has been considered a potential substitute for cyclophosphamide and calcineurin inhibitors in SDNS (34). Recent evidence showed an advantageous impact of rituximab in the treatment of FRSDNS, reducing the need for steroids and other immunosuppressive agents to the lowest possible level (35, 36).
Therapeutic options for FRSDNS depend not only on efficacy, but also on safety factors. Rituximab is often well tolerated with very low occurrences of serious adverse events, making it an appealing treatment choice in patients with autoimmune or immune-mediated diseases (34). In the current analysis, with limited data from the included studies, the qualitative description was conducted, and rituximab was shown to have no relationship with serious side effects, and seemed to have comparable adverse influences to ofatumumab and tacrolimus, which indicated that rituximab may be a safe and feasible agent for children with FRSDNS. These results necessitate future studies for validation. Additionally, for many families, the ability to achieve disease remission with a single course of intravenous treatment without the requirement for maintenance oral medication intake is the main practical reason in favor of rituximab. Of note, the total cost of therapy is a concern, especially in developing countries. In a 12-month research, the cost of two doses of rituximab was up to 20% lower than the total cost for even the generic tacrolimus formulations adopted in India (23). A study for FRSDNS in Japan confirmed the cost-effectiveness of rituximab, with the total medical expenses declining from 2923 USD to 1280 USD per month after rituximab use (37). Iorember et al. (38) reported that the annualized healthcare expenditure for the rituximab group was 197031 USD compared with 189857 USD for the CNI group (P > 0.05). This evidence may further support the clinical application of rituximab.
With the updated head-to-head comparison of different immunosuppressive agents, rituximab may be a beneficial option in treating FRSDNS. Apart from efficacy and safety, nephrologists could take this agent into account based on their clinical experience, patient characteristics, and costs in clinical practice. Some limitations should be acknowledged in result interpretation. First, most included studies were cohort studies, which may negatively affect the quality of evidence in this analysis. Second, some outcomes, such as the number of patients with treatment failure at 1 year, incorporated a relatively small number of studies and patients, probably lowering the accuracy of the results. Dosages and administration routes of immunosuppressive agents may play a role in their efficacy, which was not considered in the present work. More studies are warranted to explore this point.
Conclusion
Rituximab may be the most effective agent regarding relapses within 1 year, the mean cumulative exposure to corticosteroids, and treatment failure at 1 year; relatively better relapse-free survival during 1 year may be obtained after rituximab use in FRSDNS children. It is necessary to have future investigations to clinically verify the role of rituximab.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YuZ: Conceptualization, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing. JC: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. YaZ: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. XW: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. JW: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Traditional Chinese Medicine Science and Technology Plan Project of Zhejiang Province (No.2023ZL498).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Politano SA, Colbert GB, Hamiduzzaman N. Nephrotic syndrome. Prim Care. (2020) 47:597–613. doi: 10.1016/j.pop.2020.08.002
2. Wang CS, Greenbaum LA. Nephrotic syndrome. Pediatr Clin North Am. (2019) 66:73–85. doi: 10.1016/j.pcl.2018.08.006
3. Williams AE, Gbadegesin RA. Steroid regimen for children with nephrotic syndrome relapse. Clin J Am Soc Nephrol. (2021) 16:179–81. doi: 10.2215/cjn.19201220
4. Mattoo TK, Sanjad S. Current understanding of nephrotic syndrome in children. Pediatr Clin North Am. (2022) 69:1079–98. doi: 10.1016/j.pcl.2022.08.002
5. Downie ML, Gallibois C, Parekh RS, Noone DG. Nephrotic syndrome in infants and children: pathophysiology and management. Paediatr Int Child Health. (2017) 37:248–58. doi: 10.1080/20469047.2017.1374003
6. Tan L, Li S, Yang H, Zou Q, Wan J, Li Q. Efficacy and acceptability of immunosuppressive agents for pediatric frequently-relapsing and steroid-dependent nephrotic syndrome: A network meta-analysis of randomized controlled trials. Med (Baltimore). (2019) 98:e15927. doi: 10.1097/md.0000000000015927
7. Larkins NG, Liu ID, Willis NS, Craig JC, Hodson EM. Non-corticosteroid immunosuppressive medications for steroid-sensitive nephrotic syndrome in children. Cochrane Database Syst Rev. (2020) 4:Cd002290. doi: 10.1002/14651858.CD002290.pub5
8. Ravani P, Lugani F, Drovandi S, Caridi G, Angeletti A, Ghiggeri GM. Rituximab vs low-dose mycophenolate mofetil in recurrence of steroid-dependent nephrotic syndrome in children and young adults: A randomized clinical trial. JAMA Pediatr. (2021) 175:631–2. doi: 10.1001/jamapediatrics.2020.6150
9. Wang L, Zhu J, Xia M, Hua R, Deng F. Comparison of rituximab, cyclophosphamide, and tacrolimus as first steroid-sparing agents for complicated relapsing/steroid-dependent nephrotic syndrome in children: an evaluation of the health-related quality of life. Arch Med Sci. (2022) 18:275–8. doi: 10.5114/aoms/145587
10. Tunçay SC, Mir S, Hakverdi G. What is the best choice in steroid-dependent nephrotic syndrome: mycophenolate mofetil plus dexamethasone or cyclosporine A. Saudi J Kidney Dis Transpl. (2021) 32:1019–27. doi: 10.4103/1319-2442.338275
11. Ravani P, Colucci M, Bruschi M, Vivarelli M, Cioni M, DiDonato A, et al. Human or chimeric monoclonal anti-CD20 antibodies for children with nephrotic syndrome: A superiority randomized trial. J Am Soc Nephrol. (2021) 32:2652–63. doi: 10.1681/asn.2021040561
12. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4
13. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
14. Wells GA, Shea BJ, O'Connell D, Peterson J, Tugwell P. The newcastle–ottawa scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. (2000).
15. Dorresteijn EM, Kist-van Holthe JE, Levtchenko EN, Nauta J, Hop WC, van der Heijden AJ. Mycophenolate mofetil versus cyclosporine for remission maintenance in nephrotic syndrome. Pediatr Nephrol. (2008) 23:2013–20. doi: 10.1007/s00467-008-0899-6
16. Kranz B, Vester U, Büscher R, Wingen AM, Hoyer PF. Cyclosporine-A-induced nephrotoxicity in children with minimal-change nephrotic syndrome: long-term treatment up to 10 years. Pediatr Nephrol. (2008) 23:581–6. doi: 10.1007/s00467-007-0709-6
17. Sümegi V, Haszon I, Bereczki C, Papp F, Túri S. Long-term follow-up after cyclophosphamide and cyclosporine-A therapy in steroid-dependent and -resistant nephrotic syndrome. Pediatr Nephrol. (2008) 23:1085–92. doi: 10.1007/s00467-008-0771-8
18. Sinha A, Bagga A, Gulati A, Hari P. Short-term efficacy of rituximab versus tacrolimus in steroid-dependent nephrotic syndrome. Pediatr Nephrol. (2012) 27:235–41. doi: 10.1007/s00467-011-1997-4
19. Wang W, Xia Y, Mao J, Chen Y, Wang D, Shen H, et al. Treatment of tacrolimus or cyclosporine A in children with idiopathic nephrotic syndrome. Pediatr Nephrol. (2012) 27:2073–9. doi: 10.1007/s00467-012-2228-3
20. Wang J, Mao J, Chen J, Fu H, Shen H, Zhu X, et al. Evaluation of mycophenolate mofetil or tacrolimus in children with steroid sensitive but frequently relapsing or steroid-dependent nephrotic syndrome. Nephrol (Carlton). (2016) 21:21–7. doi: 10.1111/nep.12537
21. Alsaran K, Mirza K, Al-Talhi A, Al-Kanani E. Experience with second line drugs in frequently relapsing and steroid dependent childhood nephrotic syndrome in a large Saudi center. Int J Pediatr Adolesc Med. (2017) 4:66–70. doi: 10.1016/j.ijpam.2017.03.002
22. Basu B, Babu BG, Mahapatra TK. Long-term efficacy and safety of common steroid-sparing agents in idiopathic nephrotic children. Clin Exp Nephrol. (2017) 21:143–51. doi: 10.1007/s10157-016-1266-8
23. Basu B, Sander A, Roy B, Preussler S, Barua S, Mahapatra TKS, et al. Efficacy of rituximab vs tacrolimus in pediatric corticosteroid-dependent nephrotic syndrome: A randomized clinical trial. JAMA Pediatr. (2018) 172:757–64. doi: 10.1001/jamapediatrics.2018.1323
24. Mizutani A, Fujinaga S, Sakuraya K, Hirano D, Shimizu T. Positive effects of single-daily high-dose mizoribine therapy after cyclophosphamide in young children with steroid-dependent nephrotic syndrome. Clin Exp Nephrol. (2019) 23:244–50. doi: 10.1007/s10157-018-1628-5
25. Solomon N, Lalayiannis AD. Rituximab is more effective than tacrolimus in steroid-dependent nephrotic syndrome. Arch Dis Child Educ Pract Ed. (2019) 104:279–80. doi: 10.1136/archdischild-2018-316537
26. Kari JA, Alhasan KA, Albanna AS, Safdar OY, Shalaby MA, Böckenhauer D, et al. Rituximab versus cyclophosphamide as first steroid-sparing agent in childhood frequently relapsing and steroid-dependent nephrotic syndrome. Pediatr Nephrol. (2020) 35:1445–53. doi: 10.1007/s00467-020-04570-y
27. Iijima K, Sako M, Kamei K, Nozu K. Rituximab in steroid-sensitive nephrotic syndrome: lessons from clinical trials. Pediatr Nephrol. (2018) 33:1449–55. doi: 10.1007/s00467-017-3746-9
28. Kallash M, Smoyer WE, Mahan JD. Rituximab use in the management of childhood nephrotic syndrome. Front Pediatr. (2019) 7:178. doi: 10.3389/fped.2019.00178
29. Iijima K, Sako M, Nozu K. Rituximab for nephrotic syndrome in children. Clin Exp Nephrol. (2017) 21:193–202. doi: 10.1007/s10157-016-1313-5
30. Iijima K, Sako M, Nozu K, Mori R, Tuchida N, Kamei K, et al. Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. (2014) 384:1273–81. doi: 10.1016/s0140-6736(14)60541-9
31. Ravani P, Rossi R, Bonanni A, Quinn RR, Sica F, Bodria M, et al. Rituximab in children with steroid-dependent nephrotic syndrome: A multicenter, open-label, noninferiority, randomized controlled trial. J Am Soc Nephrol. (2015) 26:2259–66. doi: 10.1681/asn.2014080799
32. Stasi R, Cooper N, Del Poeta G, Stipa E, Laura Evangelista M, Abruzzese E, et al. Analysis of regulatory T-cell changes in patients with idiopathic thrombocytopenic purpura receiving B cell-depleting therapy with rituximab. Blood. (2008) 112:1147–50. doi: 10.1182/blood-2007-12-129262
33. Le Berre L, Bruneau S, Naulet J, Renaudin K, Buzelin F, Usal C, et al. Induction of T regulatory cells attenuates idiopathic nephrotic syndrome. J Am Soc Nephrol. (2009) 20:57–67. doi: 10.1681/asn.2007111244
34. Kattah AG, Fervenza FC, Roccatello D. Rituximab-based novel strategies for the treatment of immune-mediated glomerular diseases. Autoimmun Rev. (2013) 12:854–9. doi: 10.1016/j.autrev.2012.09.002
35. Webb H, Jaureguiberry G, Dufek S, Tullus K, Bockenhauer D. Cyclophosphamide and rituximab in frequently relapsing/steroid-dependent nephrotic syndrome. Pediatr Nephrol. (2016) 31:589–94. doi: 10.1007/s00467-015-3245-9
36. Hogan J, Deschenes G. How to improve response to rituximab treatment in children with steroid-dependent nephrotic syndrome: answer to Drs. Fujinaga and Nishino. Pediatr Nephrol. (2019) 34:361–2. doi: 10.1007/s00467-018-4133-x
37. Takura T, Takei T, Nitta K. Cost-effectiveness of administering rituximab for steroid-dependent nephrotic syndrome and frequently relapsing nephrotic syndrome: A preliminary study in Japan. Sci Rep. (2017) 7:46036. doi: 10.1038/srep46036
Keywords: immunosuppressive agent, rituximab, frequently relapsing/steroid dependent nephrotic syndrome, children, network meta-analysis
Citation: Zhu Y, Chen J, Zhang Y, Wang X and Wang J (2024) Immunosuppressive agents for frequently relapsing/steroid-dependent nephrotic syndrome in children: a systematic review and network meta-analysis. Front. Immunol. 15:1310032. doi: 10.3389/fimmu.2024.1310032
Received: 09 October 2023; Accepted: 06 February 2024;
Published: 23 February 2024.
Edited by:
Giuseppe Murdaca, University of Genoa, ItalyReviewed by:
Devis Benfaremo, Marche Polytechnic University, ItalyAndrea Angeletti, Giannina Gaslini Institute (IRCCS), Italy
Copyright © 2024 Zhu, Chen, Zhang, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingjing Wang, amluZ2ppbmd3X3pqQG91dGxvb2suY29t
 Yu Zhu1
Yu Zhu1 Junyi Chen
Junyi Chen Yao Zhang
Yao Zhang Jingjing Wang
Jingjing Wang