- 1Department of Burns and Plastic Surgery, The Forth Medical Center of Chinese PLA General Hospital, Beijing, China
- 2Department of Medical Service, The Sixth Medical Center of Chinese PLA General Hospital, Beijing, China
- 3Senior Department of Ophthalmology, The Third Medical Center of Chinese PLA General Hospital, Beijing, China
Coronavirus disease 19 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is affecting the world with a surge in cases. A variety of autoimmune diseases occur after SARS-CoV-2 infection or vaccination, of which IgG4-related disease (IgG4-RD) is an important type. IgG4-RD can involve multiple organs of the body. The ocular manifestation of IgG4-RD is called IgG4-related ophthalmic disease (IgG4-ROD). We herein report a patient diagnosed with IgG4-ROD. The patient developed ptosis and vision loss after SARS-CoV-2 vaccination, and the symptoms worsened after SARS-CoV-2 infection. After excluding other diseases like myasthenia gravis and Eaton-Lambert syndrome that may cause ptosis, the diagnosis of IgG4-ROD was confirmed by pathological examination. We discussed the predisposing factors, diagnosis and treatment of this patient to provide a more empirical and theoretical basis for clinical diagnosis and treatment. We conducted a literature review of previously reported cases of IgG4-RD following SARS-CoV-2 infection or vaccination. We retrieved a total of 9 cases, of which 5 developed symptoms after vaccination and 4 after infection. Demographic and clinical characteristics were summarized. In conclusion, our case represents the first case of proven IgG4-ROD after COVID-19 vaccination. We believe that IgG4-ROD and SARS-CoV-2 infection or vaccination are closely related, and the immune system disorder caused by SARS-CoV-2 infection or vaccination may be a key factor in the pathogenesis of IgG4-RD. But for now, there is no direct evidence that there is a causal relationship between SARS-CoV-2 infection or vaccination and IgG4-ROD, which still needs more research and exploration to confirm.
1 Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a highly contagious coronavirus, which causes an acute respiratory disease called coronavirus disease 19 (COVID-19) (1). COVID-19 does not only cause inflammatory symptoms such as fever and cough but also has a huge impact on the immune system. Vaccination is one of the most effective interventions to substantially reduce severe disease and death due to SARS-CoV-2 infection. At present, there is some evidence that a variety of different autoantibodies appear in patients after SARS-CoV-2 infection or vaccination, and a number of them develop autoimmune diseases, such as autoimmune hemolytic anemia, Guillain‐Barre syndrome, and so on (2–4).
IgG4-related disease (IgG4-RD) is an autoimmune disease with undetermined pathogenesis, in which multiple organs and tissues can be involved (5). IgG4-RD is characterized by infiltration of IgG4-immunopositive plasmacytes and elevated serum IgG4 concentration accompanied by enlargement and mass in various organs (6). Several previous case reports had reported the development of IgG4-RD after SARS-CoV-2 infection or vaccination, patients showed different symptoms. The ocular manifestation of IgG4-RD is called IgG4-related ophthalmic disease (IgG4-ROD). IgG4-ROD most commonly affects the lacrimal gland, but orbital soft tissue, orbital nerves, sclera, choroid, and orbital adnexa can also be involved. It presents insidiously, with symptoms of painless proptosis, eyelid swelling, decreased vision with tearing, pain, redness, and photopsia (7). IgG4-ROD may involve a single structure or multiple structures within the orbit, and may also exhibit bilateral disease (8).
There are currently no internationally standardized diagnostic criteria for IgG4-ROD. The most recent diagnostic criteria proposed by Japanese researchers in 2015 are: (1) Imaging studies show enlargement of the lacrimal gland, trigeminal nerve, or extraocular muscle as well as masses, enlargement, or hypertrophic lesions in various ophthalmic tissues; (2) Histopathologic examination shows marked lymphocyte and plasmacyte infiltration, and sometimes fibrosis. A germinal center is frequently observed. IgG4+ plasmacytes are found and satisfy the following criteria: ratio of IgG4+ cells to IgG4+ cells of 40% or above, or more than 50 IgG4+ cells per high-power field (×400); (3) Blood test shows elevated serum IgG4 (≥135 mg/dl). Diagnosis is classified as ‘‘definitive’’ when (1), (2), and (3) are satisfied; ‘‘probable’’ when (1) and (2) are satisfied; and ‘‘possible’’ when (1) and (3) are satisfied (9). In terms of treatment, intravenous glucocorticoid therapy is the first-line treatment for IgG4-ROD, which is effective and well-tolerated (10). Rituximab is also a treatment option for some refractory patients (11).
We herein report a patient diagnosed with IgG4-ROD. The patient developed ptosis and vision loss after SARS-CoV-2 vaccination, and the symptoms worsened after SARS-CoV-2 infection. We admitted her to the ophthalmic ward of the Third Medical Center of Chinese PLA General Hospital. We recorded the changes in her condition and reviewed the previous literature.
2 Case description
The patient, a previously healthy 22-year-old female, received an inactivated COVID-19 vaccine in May 2021 and reported fever within the next few days. After a month, the patient noticed a slight ptosis in the left eye and a mild loss of vision in the right eye, and these symptoms gradually worsened. In January 2022, the patient felt that the above symptoms had progressed to the point where they were affecting her life and went to the local hospital. Orbital CT showed soft tissue swelling in the left lacrimal gland region. The funduscopy examination revealed macular edema in the right eye. Fundus fluorescein angiography showed tortuous fundus veins in the right eye, anastomosed vessels in the optic disc and retina, and extensive peripheral retinal vessel dilation and leakage. During hospitalization, compound anisodine was injected into the left superficial temporal artery, together with oral mecobalamin, Mongolian medicine, acupuncture, and electromagnetic therapy, the patient felt that the symptoms were worse than before.
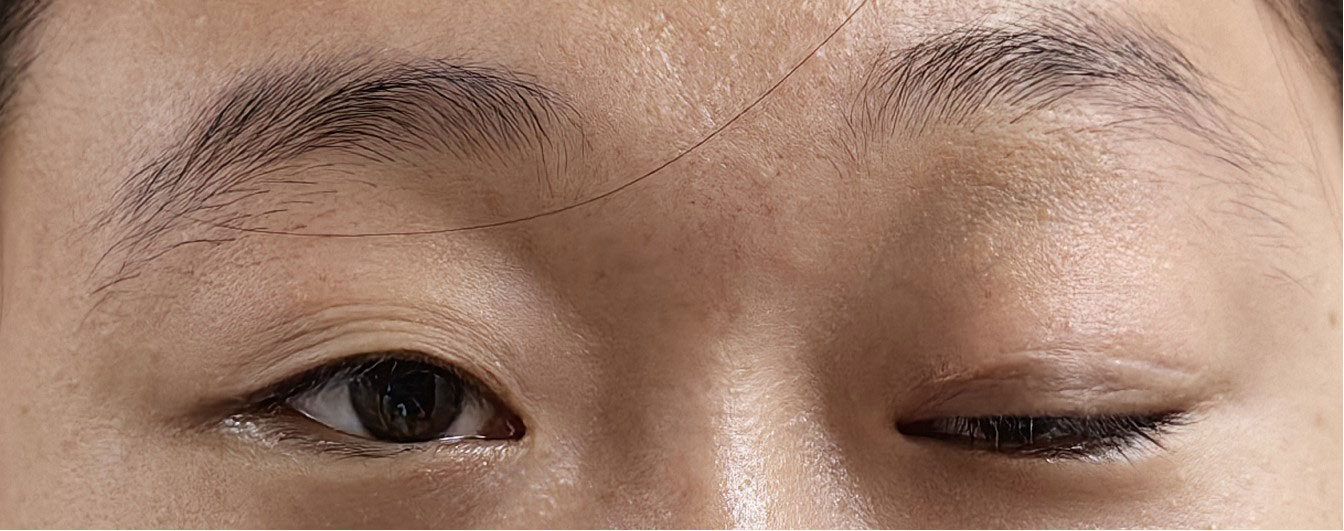
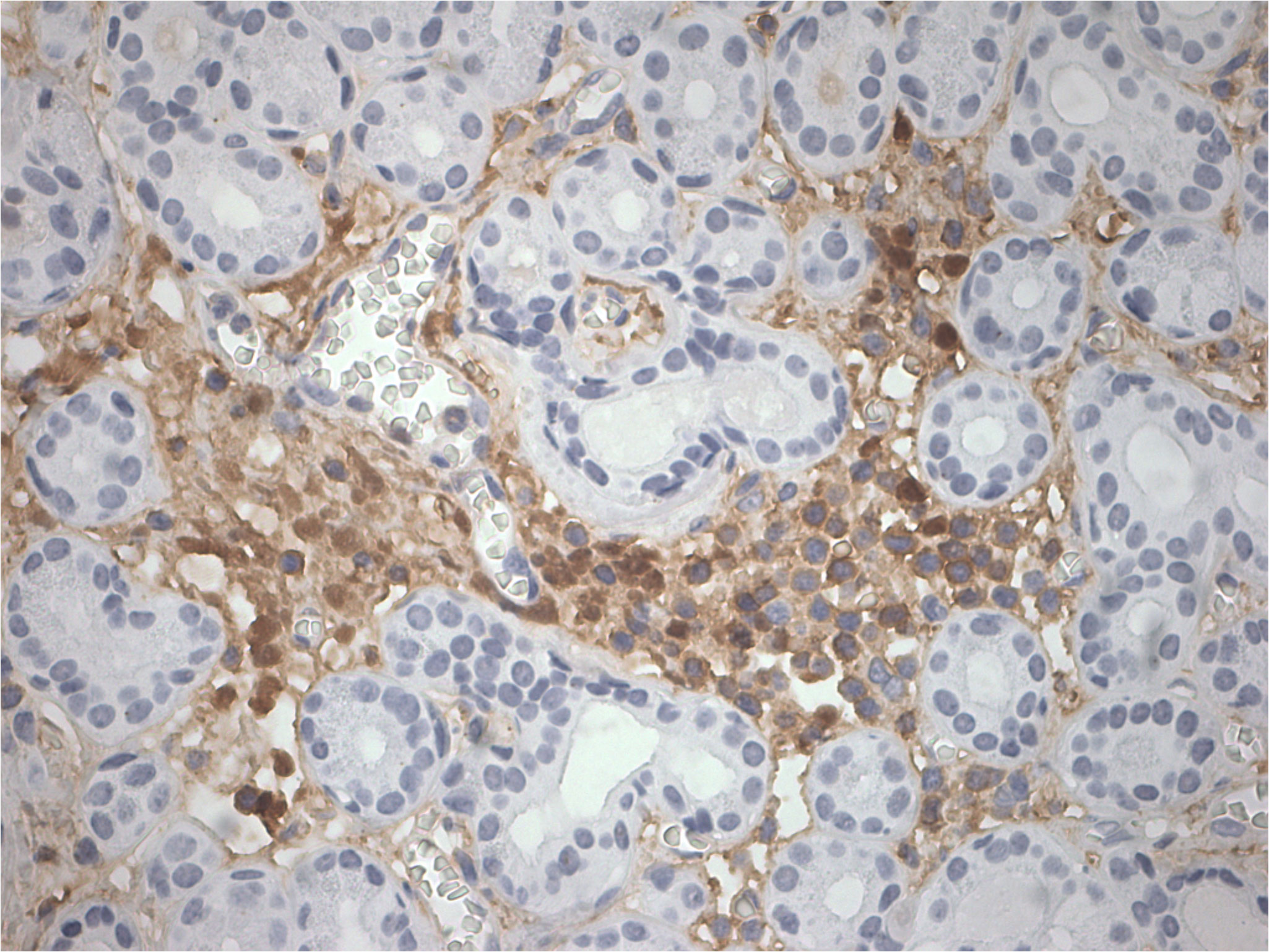
The patient was admitted to our hospital in August 2022. After admission, the visual acuity of the right eye was 0.3 and the left eye was 0.8, the ptosis of the left eye covered the pupil more than 1/2 (Figure 1). Initially, we considered a diagnosis of myasthenia gravis and central retinal vein occlusion in the right eye. Serial tests were performed to identify the differential diagnosis. Electromyography showed that repetitive nerve electrical stimulation did not show characteristic changes. The fatigue test and the neostigmine test were negative. Serum specific antibodies for neuromuscular disease were all negative. The diagnosis of neuromuscular diseases like myasthenia gravis and Eaton-Lambert syndrome can be ruled out. However, we noted that serum IgG4 was 280 mg/dl and ocular ultrasound suggested uneven enlargement of the left lacrimal gland and fullness of the right lacrimal gland. To investigate the presence of neuropathy, an MRI of the head was performed, which revealed the optic nerve in the right orbit was slightly thinner than normal with hyperintensity on T2WI and abnormal enhancement of the optic nerve and its surrounding sheath. On August 23, 2022, a biopsy of the left lacrimal gland, orbital septal tissue, and orbital fat was performed by us. The result of histopathology was inflammatory pseudotumor. Immunohistochemical showed that the ratio of IgG4+ plasmacytes to IgG+ plasmacytes was more than 40% and more than 50 IgG4+ cells per high-power field (×400) (Figure 2). We performed a staining of the CD4 cells (Supplementary Figure 1) and CD8 (Supplementary Figure 2) cells in the biopsy. This patient satisfied diagnostic criteria (1), (2), and (3) for a definite diagnosis of IgG4-ROD. After diagnosis, the patient was given high-dose intravenous methylprednisolone pulse therapy and tacrolimus. After five days of treatment, the patient reported opening the eye more easily, but there was no significant change in the ptosis and vision loss. The patient asked to be discharged. After discharge, the patient continued to take methylprednisolone orally until November 2022 and tacrolimus orally until August 2023.
Unfortunately, the patient was infected with COVID-19 in December 2022. Although the patient remained on oral tacrolimus, the symptoms of ptosis in her left eye and vision loss in her right eye worsened after infection, and she was readmitted in August 2023. The visual acuity of the right eye was 0.15 and the left eye was 1.0, the ptosis of the left eye covered the entire pupil. The serum IgG4 was 240 mg/dl. Given the poor response to steroids therapy and the patient’s request for surgery to improve ptosis, we performed ptosis correction of left eye and bilateral double eyelid plasty on August 15, 2023 (Figure 3). During the ptosis correction, we performed a pathologic examination of a piece of the levator palpebrae superioris muscle, which showed fibrosis. The patient recovered well after the operation, and the eyelid margins of both eyes were symmetrical.
3 Discussion
The SARS-CoV-2, responsible for COVID-19, has spread across the world. The clinical manifestations of COVID-19 vary greatly, but it is worth noting that some patients can develop long-term complications, including the respiratory system, cardiovascular system, and nervous system (12). There is growing evidence that COVID-19 can cause autoimmune diseases. It has been suggested that common pathogenetic and clinical-radiological aspects between hyperinflammatory diseases and COVID-19 may indicate that SARS-CoV-2 may be a trigger for rapid autoimmune or autoinflammatory dysregulation (13). Several mechanisms, including molecular mimicry, bystander activation of T-cells, and epitope spreading, as for explaining how viral infection can trigger a response leading to autoimmune disease (14).
As the most important means to prevent SARS-CoV-2 infection, the COVID-19 vaccine has been widely used worldwide. Although it is well tolerated by most people, adverse reactions after vaccination should not be ignored. Vaccines induce adaptive immune responses to exert their protective effects, but may induce a hyperinflammatory state. A few cases of new or recurrent autoimmune disease have been reported after COVID-19 vaccination. Pichi et al. reported nine eyes presenting with ocular adverse events after the first inoculation of inactivated COVID-19 vaccine (15). It is assumed that COVID-19 vaccine can trigger autoimmunity through molecular mimicry, the production of particular autoantibodies, and the role of certain vaccine adjuvants (16).
We conducted a literature review of previously reported cases of IgG4-RD following SARS-CoV-2 infection or vaccination (Table 1) (17–25). We retrieved a total of 9 cases, of which 5 developed symptoms after vaccination and 4 after infection. Of the vaccinated patients, 4 received mRNA vaccine, including 3 who received the mRNA BNT162b2 vaccine. The interval between the onset of IgG4-RD symptoms and SARS-CoV-2 infection or vaccination ranged from one day to three and a half months. IgG4-RD can affect a wide range of organs, and patients can be clinically manifested as hepatopathy, lung disease, pancreatitis, sialadenitis, rhinosinusitis, lymph node enlargement, and parotid enlargement. After surgery and prednisone therapy, most patients’ symptoms were relieved or even recovered.
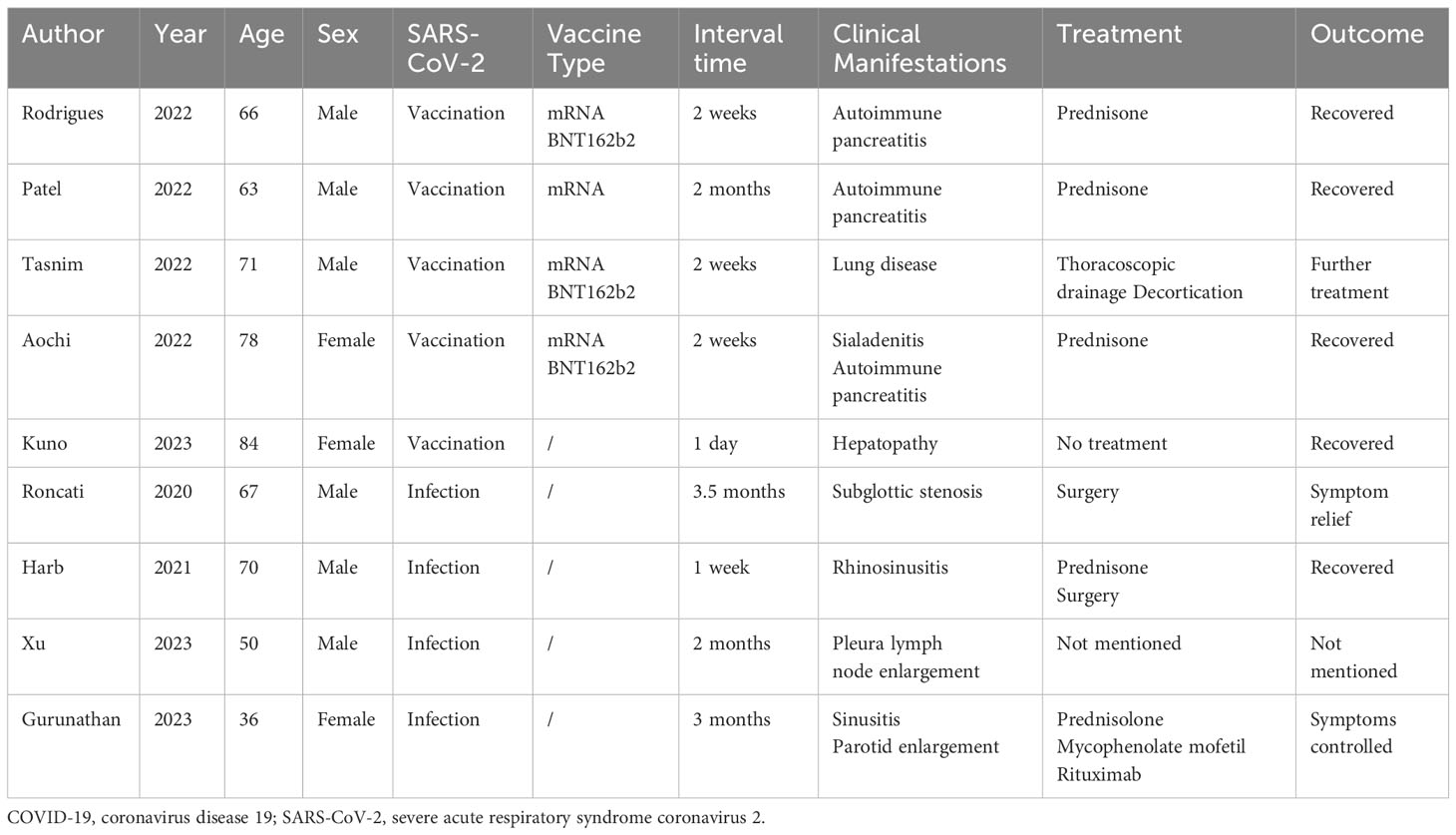
Table 1 Demographic and clinical characteristics of previously reported IgG4-related disease following COVID-19.
In our literature review, we found four patients who developed IgG4-RD after mRNA vaccination, whereas the patient we report here received an inactivated vaccine. Based on the available evidence, it is not clear whether different COVID-19 vaccine types have an effect on IgG4-RD pathogenesis. The mRNA in mRNA vaccines can present as either an antigen or an adjuvant, and can be recognized and bound by endosomal Toll-like receptors (TLRs) and inflammasomes to induce inflammation and immune responses (26). It has been found that TLRs play an important role in the pathogenesis of IgG4-RD. M2 macrophages expressing TLR7 in diseased organs recognize the COVID-19 mRNA or their own RNAs released by stressed or injured cells and promote the production of a variety of fibrotic cytokines through TLR7/IRAK4/NF-κB signaling, leading to severe fibrosis in diseased organs (27). Furthermore, after injection of the COVID-19 mRNA vaccine, the mRNA enters the muscle cells and the ribosomes perform cellular translation to produce the spike protein (16). Multiple amino acid fragments on the SARS-CoV-2 spike protein have been identified as molecular mimics of human proteins, which can induce the immune system to produce autoantibodies (28). Although the inactivated COVID-19 vaccine cannot be translated in vivo like the mRNA vaccine, it carries the spike protein itself. When individuals are exposed to antigens for prolonged period of time, the humoral immune response eventually switches to IgG4. The proportion of IgG4 in immunoglobulin would increase significantly after multiple vaccination or breakthrough infection with SARS-CoV-2 (29). Recently revealed a novel subset of IL-10+LAG3+ T follicular cells infiltrating the affected organs of IgG4-RD patients, it may play an important role in driving IgG4 class switching (30). CD4+ helper T cells are the most abundant cells in tissues affected by IgG4-RD and are thought to be the driver of IgG4-RD pathogenesis (31). Under some unknown conditions, CD4+ helper T cells are activated in response to long-standing COVID-19 spike protein or other antigenic stimulation, thereby promoting the differentiation of IL-10+LAG3+ T follicular cells, driving IgG4 class switching.
IgG4-RD is an immune-mediated fibro-inflammatory disease, which usually leads to tumefactive lesions and fibrosis (32, 33). We considered the following two possible causes of this patient’s ptosis: the first was the compression of the oculomotor nerve by the swelling of the orbital soft tissue caused by IgG4-ROD, and the second was the fibrosis of the levator muscle of the upper eyelid caused by IgG4-ROD. It has been suggested that serum IgG4 levels are useful in assessing dynamic disease severity and monitoring relapse (34). This is essential for physicians to treat IgG4-RD clinically. Kobak et al. reported a patient with IgG4-RD, who had a good response to treatment, but eventually causing IgG4-RD recurrence after infection with SARS-CoV-2 (35). Relapse of IgG4-RD is not only secondary to COVID-19 but may also be secondary to mRNA vaccination (36). Egashira et al. reported the development of cerebral venous sinus thrombosis in a patient with IgG4-ROD during recovery from COVID-19 (37). Cerebral venous sinus thrombosis is an important complication of COVID-19, which suggests that IgG4-ROD patients are more likely to have complications of COVID-19. A prospective study reported that serum IgG4 levels may predict the prognosis of COVID-19, a concentration of serum IgG4>700 mg/dl and an IgG4/IgG1 ratio>0.05 were associated with a significantly increased mortality at 30 days (38).
As to the cause of this patient’s vision loss in the right eye, the central retinal vein occlusion was confirmed on examination. Ocular vascular embolism after COVID-19 vaccination is a rare complication (39). The exact mechanisms have yet to be ascertained. Leung et al. raised three possible mechanisms: vaccine-induced immune thrombotic thrombocytopenia, COVID-19 vaccine as a trigger in homocysteinaemia, and retinal vasculitis (40). There are a few reports of vision loss caused by central retinal vein thrombosis after COVID-19 vaccination, and vision can return to normal after timely treatment (41, 42). However, a self-controlled case series found no evidence of an association between retinal vein thrombosis and COVID-19 vaccination (43).
In conclusion, our case represents the first case of proven IgG4-ROD after COVID-19 vaccination. We believe that IgG4-ROD and SARS-CoV-2 infection or vaccination are closely related, and the immune system disorder caused by SARS-CoV-2 infection or vaccination may be a key factor in the pathogenesis of IgG4-RD. But for now, there is no direct evidence that there is a causal relationship between SARS-CoV-2 infection or vaccination and IgG4-ROD, which still needs more research and exploration to confirm.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
PZ: Writing – original draft. QWu: Writing – review & editing. XX: Writing – review & editing. MC: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Thanks to Rui Ma for providing valuable insights and suggestions in the completion of the article. Thanks to Qi Wang and Xinji Yang for providing important comments and guidance in the revision of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1303589/full#supplementary-material
Supplementary Figure 1 | CD4 cells (×100).
Supplementary Figure 2 | CD8 cells (×100).
Abbreviations
COVID-19, Coronavirus disease 19; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; IgG4-RD, IgG4-related disease; IgG4-ROD, IgG4-related ophthalmic disease; TLRs, Toll-like receptors.
References
1. Hu B, Guo H, Zhou P, Shi Z-L. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol (2020) 19:141–54. doi: 10.1038/s41579-020-00459-7
2. Yazdanpanah N, Rezaei N. Autoimmune complications of COVID-19. J Med Virol (2021) 94:54–62. doi: 10.1002/jmv.27292
3. Dobrowolska K, Zarębska-Michaluk D, Poniedziałek B, Jaroszewicz J, Flisiak R, Rzymski P. Overview of autoantibodies in COVID-19 convalescents. J Med Virol (2023) 95:e28864. doi: 10.1002/jmv.28864
4. Mahroum N, Lavine N, Ohayon A, Seida R, Alwani A, Alrais M, et al. COVID-19 vaccination and the rate of immune and autoimmune adverse events following immunization: insights from a narrative literature review. Front Immunol (2022) 13:872683. doi: 10.3389/fimmu.2022.872683
5. Lanzillotta M, Mancuso G, Della-Torre E. Advances in the diagnosis and management of IgG4 related disease. BMJ (2020) 369:m1067. doi: 10.1136/bmj.m1067
6. Umehara H, Okazaki K, Kawa S, Takahashi H, Goto H, Matsui S, et al. The 2020 revised comprehensive diagnostic (RCD) criteria for IgG4-RD. Mod Rheumatol (2021) 31:529–33. doi: 10.1080/14397595.2020.1859710
7. Derzko-Dzulynsky L. IgG4-related disease in the eye and ocular adnexa. Curr Opin Ophthalmol (2017) 28:617–22. doi: 10.1097/icu.0000000000000427
8. Wallace ZS, Deshpande V, Stone JH. Ophthalmic manifestations of IgG4-related disease: Single-center experience and literature review. Semin Arthritis Rheum (2014) 43:806–17. doi: 10.1016/j.semarthrit.2013.11.008
9. Goto H, Takahira M, Azumi A. Diagnostic criteria for IgG4-related ophthalmic disease. Jpn J Ophthalmol (2014) 59:1–7. doi: 10.1007/s10384-014-0352-2
10. Hosohata K, Yang MK, Kim GJ, Choi YA, Sa H-S. Efficacy and safety of intravenous glucocorticoid therapy for IgG4-related ophthalmic disease. PloS One (2023) 18:e0284442. doi: 10.1371/journal.pone.0284442
11. Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet (2015) 385:1460–71. doi: 10.1016/s0140-6736(14)60720-0
12. Desai AD, Lavelle M, Boursiquot BC, Wan EY. Long-term complications of COVID-19. Am J Physiol-cell P (2022) 322:C1–C11. doi: 10.1152/ajpcell.00375.2021
13. Favalli EG, Ingegnoli F, De Lucia O, Cincinelli G, Cimaz R, Caporali R. COVID-19 infection and rheumatoid arthritis: Faraway, so close! Autoimmun Rev (2020) 19:102523. doi: 10.1016/j.autrev.2020.102523
14. Hussein HM, Rahal EA. The role of viral infections in the development of autoimmune diseases. Crit Rev Microbiol (2019) 45:394–412. doi: 10.1080/1040841x.2019.1614904
15. Pichi F, Aljneibi S, Neri P, Hay S, Dackiw C, Ghazi NG. Association of ocular adverse events with inactivated COVID-19 vaccination in patients in abu dhabi. JAMA Ophthalmol (2021) 139:1131–5. doi: 10.1001/jamaophthalmol.2021.3477
16. Chen Y, Xu Z, Wang P, Li XM, Shuai ZW, Ye DQ, et al. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology (2022) 165:386–401. doi: 10.1111/imm.13443
17. Xu YG, Zheng Y, Qiu YY. A rare case of IgG4-associated disease caused by COVID-19: Case report and literature review. Int J Rheum Dis (2023) 00:1–4. doi: 10.1111/1756-185x.14863
18. Patel AH. Acute Liver Injury and IgG4-related Autoimmune Pancreatitis following mRNA based COVID-19 vaccination. Hepatol Forum (2022) 3:97–9. doi: 10.14744/hf.2022.2022.0019
19. Roncati L, Bergonzini G, Lusenti B, Nasillo V, Paolini A, Zanelli G, et al. High density of IgG4-secreting plasma cells in the fibrotic tissue from a surgically resected tracheal ring impaired by complex subglottic stenosis post-tracheostomy as immune expression of a Th2 response due to severe COVID-19. Ann Hematol (2020) 100:2659–60. doi: 10.1007/s00277-020-04231-y
20. Tasnim S, Al-Jobory O, Hallak A, Bharadwaj T, Patel M. IgG4 related pleural disease: Recurrent pleural effusion after COVID-19 vaccination. Respirol Case Rep (2022) 10:e01026. doi: 10.1002/rcr2.1026
21. Aochi S, Uehara M, Yamamoto M. IgG4-related Disease Emerging after COVID-19 mRNA Vaccination. Intern Med (2023) 62:1547–51. doi: 10.2169/internalmedicine.1125-22
22. Kuno M, Sawa N, Mizuno H, Oba Y, Ikuma D, Sekine A, et al. Immunoglobulin G4-related hepatopathy after COVID-19 vaccination. Intern Med (2023) 62:2139–43. doi: 10.2169/internalmedicine.1634-23
23. Gurunathan R, Dhanasekaran P, Devaprasad D, Jacob SSK, Abraham BK. Possible ambiguity in interpretation between immunoglobulin G4-related disease (IgG4-RD) and sarcoidosis in a post-COVID-19 pandemic. Cureus (2023) 15(4):e38124. doi: 10.7759/cureus.38124
24. Harb AA, Chen Y, Ben-Ami JR, Francke M, Hur C, Turk AT, et al. Acute vision loss from igG4-related and bacterial rhinosinusitis after COVID-19. JAMA Otolaryngol Head Neck Surg (2021) 147:914–5. doi: 10.1001/jamaoto.2021.2121
25. Rodrigues T, Komanduri S. Seronegative type I autoimmune pancreatitis with immunoglobulin G4-related disease triggered by the Pfizer-BioNTech COVID-19 vaccine. Gastrointest Endosc (2022) 95:AB36. doi: 10.1016/j.gie.2022.04.130
26. Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol (2021) 21:195–7. doi: 10.1038/s41577-021-00526-x
27. Chinju A, Moriyama M, Kakizoe-Ishiguro N, Chen H, Miyahara Y, Haque ASMR, et al. CD163+ M2 macrophages promote fibrosis in igG4-related disease via toll-like receptor 7/interleukin-1 receptor–associated kinase 4/NF-κB signaling. Arthritis Rheumatol (2022) 74:892–901. doi: 10.1002/art.42043
28. Nunez-Castilla J, Stebliankin V, Baral P, Balbin CA, Sobhan M, Cickovski T, et al. Potential autoimmunity resulting from molecular mimicry between SARS-coV-2 spike and human proteins. Viruses (2022) 14:1415. doi: 10.3390/v14071415
29. Irrgang P, Gerling J, Kocher K, Lapuente D. Class switch towards non-inflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination. Sci Immunol (2022) 8:1–13. doi: 10.1126/sciimmunol.ade2798
30. Munemura R, Maehara T, Murakami Y, Koga R, Aoyagi R, Kaneko N, et al. Distinct disease-specific Tfh cell populations in 2 different fibrotic diseases: IgG4-related disease and Kimura disease. J Allergy Clin Immunol (2022) 150:440–455.e417. doi: 10.1016/j.jaci.2022.03.034
31. Maehara T, Moriyama M, Nakamura S. Pathogenesis of IgG4-related disease: a critical review. Odontology (2018) 107:127–32. doi: 10.1007/s10266-018-0377-y
32. Saitakis G, Chwalisz BK. The neurology of IGG4-related disease. J Neurol Sci (2021) 424:117420. doi: 10.1016/j.jns.2021.117420
33. Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med (2012) 366:539–51. doi: 10.1056/NEJMra1104650
34. Mizushima I, Konishi M, Sanada H, Suzuki K, Takeji A, Zoshima T, et al. Serum IgG4 levels at diagnosis can predict unfavorable outcomes of untreated patients with IgG4-related disease. Sci Rep (2021) 11:13341. doi: 10.1038/s41598-021-92814-8
35. Kobak S. May IgG4-related disease be reactivated by SARS-CoV-2 infection? Rheumatology (2022) 60:161–2. doi: 10.5114/reum.2022.115985
36. Aydın MF, Yıldız A, Oruç A, Sezen M, Dilek K, Güllülü M, et al. Relapse of primary membranous nephropathy after inactivated SARS-CoV-2 virus vaccination. Kidney Int (2021) 100:464–5. doi: 10.1016/j.kint.2021.05.001
37. Egashira S, Yoshimoto T, Tanaka K, Kamogawa N, Shiozawa M, Koge J, et al. Cerebral venous sinus thrombosis presenting transient ischemic attack after recovery from COVID-19 with Graves’ disease and IgG4-related ophthalmic disease: a case report. Rinsho Shinkeigaku (2022) 62:928–34. doi: 10.5692/clinicalneurol.cn-001788
38. Della-Torre E, Lanzillotta M, Strollo M, Ramirez GA, Dagna L, Tresoldi M. Serum IgG4 level predicts COVID-19 related mortality. Eur J Intern Med (2021) 93:107–9. doi: 10.1016/j.ejim.2021.09.012
39. Yeo S, Kim H, Lee J, Yi J, Chung Y-R. Retinal vascular occlusions in COVID-19 infection and vaccination: a literature review. Graefes Arch Clin Exp Ophthalmol (2023) 261:1793–808. doi: 10.1007/s00417-022-05953-7
40. Leung H-M, Au SC-L. Retinal vein occlusion after COVID-19 vaccination—A review. Vaccines (2023) 11:1281. doi: 10.3390/vaccines11081281
41. Bialasiewicz AA, Farah-Diab MS, Mebarki HT. Central retinal vein occlusion occurring immediately after 2nd dose of mRNA SARS-CoV-2 vaccine. Int Ophthalmol (2021) 41:3889–92. doi: 10.1007/s10792-021-01971-2
42. Endo B, Bahamon S, Martínez-Pulgarín D. Central retinal vein occlusion after mRNA SARS-CoV-2 vaccination: A case report. Indian J Ophthalmol (2021) 69:2865–6. doi: 10.4103/ijo.IJO_1477_21
Keywords: autoimmunity, case report, COVID-19, IgG4, IgG4-related ophthalmic disease, SARS-CoV-2
Citation: Zhang P, Wu Q, Xu X and Chen M (2024) A case of IgG4-related ophthalmic disease after SARS-CoV-2 vaccination: case report and literature review. Front. Immunol. 15:1303589. doi: 10.3389/fimmu.2024.1303589
Received: 28 September 2023; Accepted: 08 January 2024;
Published: 22 February 2024.
Edited by:
Mattia Bellan, University of Eastern Piedmont, ItalyReviewed by:
Loredana Frasca, National Institute of Health (ISS), ItalyIqbal Tajunisah, University of Malaya, Malaysia
Yutaka Kaneko, Yamagata University, Japan
Venkataramana Kandi, Kaloji Narayana Rao University of Health Sciences, India
Copyright © 2024 Zhang, Wu, Xu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao Xu, MTM2MjMzNDVAcXEuY29t; Minliang Chen, Y2hlbm1sQHNvaHUuY29t
†These authors have contributed equally to this work
 Peixuan Zhang
Peixuan Zhang Qian Wu
Qian Wu Xiao Xu3*
Xiao Xu3*