
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 01 March 2024
Sec. Cancer Immunity and Immunotherapy
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1302903
This article is part of the Research Topic Tertiary lymphoid structures (TLS) in the tumor immune microenvironment View all 15 articles
A correction has been applied to this article in:
Corrigendum: Intratumor tertiary lymphatic structure evaluation predicts the prognosis and immunotherapy response of patients with colorectal cancer
 Huijing Feng1†
Huijing Feng1† Siyuan Zhang2,3,4,5,6†
Siyuan Zhang2,3,4,5,6† Qiuru Zhou2,3,4,5,6,7
Qiuru Zhou2,3,4,5,6,7 Fei Han8
Fei Han8 Gang Du9
Gang Du9 Lin Wang10
Lin Wang10 Xuena Yang2,3,4,5,6
Xuena Yang2,3,4,5,6 Xiying Zhang2,3,4,5,6
Xiying Zhang2,3,4,5,6 Wenwen Yu2,3,4,5,6
Wenwen Yu2,3,4,5,6 Feng Wei2,3,4,5,6,11
Feng Wei2,3,4,5,6,11 Xishan Hao2,3,4,5,11
Xishan Hao2,3,4,5,11 Xiubao Ren2,3,4,5,6,11,12*
Xiubao Ren2,3,4,5,6,11,12* Hua Zhao2,3,4,5,6,11*
Hua Zhao2,3,4,5,6,11*Background: Immune checkpoint therapy, involving the programmed cell death 1 (PD-1) monoclonal antibody, has revolutionized the treatment of cancer. Tertiary lymphatic structure (TLS) serves as an immune indicator to predict the efficacy of PD-1 antibody therapy. However, there is no clear result whether the distribution, quantity, and maturity of TLS can be effective indicators for predicting the clinical efficacy of anti-PD1 immunotherapy in patients with colorectal cancer (CRC).
Methods: Fifty-seven patients who underwent surgical resection and thirty-nine patients who received anti-PD-1 immunotherapy were enrolled in this retrospective study. Immunohistochemical staining and multiple fluorescence immunohistochemistry were used to evaluate the mismatch repair (MMR) subtypes and TLS distribution, quantity, and maturity, respectively.
Results: A comprehensive patient score system was built based on TLS quantity and maturity. We found that the proportion of patients with score >1 was much higher in the deficient mismatch repair(dMMR) group than in the proficient mismatch repair(pMMR) group, and this difference was mainly due to intratumoral TLS. Patient score, based on the TLS evaluation of whole tumor, peritumor, or intratumor, was used to evaluate the efficacy of anti-PD1 immunotherapy. Based only on the intratumor TLS evaluation, the proportion of patients with a score >1 was higher in the response (PR + CR) group than in the non-response (PD) group. Multivariate analysis revealed that patient scores were positively correlated with the clinical efficacy of immunotherapy. Further analysis of immune-related progression-free survival was performed in patients with CRC who received anti-PD-1 immunotherapy. Patients with score >1 based on the intratumor TLS evaluation had significantly better survival.
Conclusions: These results suggest that the patient score based on intratumor TLS evaluation may be a good immune predictive indicator for PD-1 antibody therapy in patients with CRC.
Colorectal cancer (CRC) is a malignant tumor of the colon or rectum that is characterized by poor prognosis and high metastasis (1). According to global cancer statistics, CRC ranked third and second in terms of cancer incidence and mortality, respectively, in 2020 (2). Early CRC can be treated with radical surgical resection; however, surgery and adjuvant chemotherapy are not ideal for the treatment of advanced CRC (1). In recent years, novel immune checkpoint inhibitors (ICIs) have emerged as key therapeutics for patients with metastatic CRC with mismatch repair-deficient (dMMR) and -proficient (pMMR) subtypes according to MMR gene status (3). Although programmed cell death 1 (PD-1) inhibitors have been approved as the first-line treatment for advanced CRC with dMMR as a predictive biomarker for PD-1 ICIs, less than half of the patients with dMMR CRC respond favorably to anti-PD-1 therapy (3). Therefore, exploring the much accurate immune predictive indicators for PD-1 antibody therapy is necessary to guide treatment more accurately in patients with CRC with different MMR subtypes.
Tertiary lymphoid structures (TLSs), also known as ectopic lymphoid organs, develop in non-lymphoid tissues at sites of chronic inflammation, including tumors (4). Tumor-infiltrating lymphocytes, which may originate from the aggregation of ectopic immune cells at the tumor site, have drawn considerable interest because they play an important role in improving anti-tumor immunity (5, 6). Increasing evidence indicates that the presence and maturity of TLSs are correlated with tumor prognosis and can serve as novel prognostic biomarkers (7–10). Furthermore, TLSs can predict the responses to anti-PD‐1 immunotherapy and might be a target of PD‐1 blockade in several tumors including esophageal carcinoma, bladder cancer, melanoma and head and neck squamous cell carcinoma (HNSCC) (7–9, 11). It has also been demonstrated that high PD-1 expression in the invasive margin of patients was significantly associated with the presence of TLSs, which implies that targeting PD-1 in the immune context might be more effective (12). A recent study in mouse models of spontaneous multi-organ metastasis in MSI-H CRC tumors showed that ICIs of anti-PD-1 treatment significantly reduced the growth of primary tumors and liver metastases, and therapy efficacy correlated with the formation of TLSs in ICI-responding tumors. However, the utility of TLSs as predictive biomarkers for anti-PD-1 treatment of CRC remains unclear (13).
Efficacy of tumor immunotherapy is closely related to the MMR genotype. Greco et al. reported that patients with dMMR CRC have higher objective response rates and longer progression-free survival (PFS) after receiving immunotherapy than patients with pMMR (14). A recent study reported that patients with dMMR bladder cancer with increased tumor-resident memory T cells (TRM) infiltration contributing to TLS formation had improved response rates to neoadjuvant chemotherapy (15). However, the relationship between MMR status and TLS remain unclear.
In this study, we aimed to assess the correlation between MMR status and TLSs in CRC and explore TLSs as predictive biomarkers for anti-PD-1 immunotherapy to facilitate more personalized treatment of patients with CRC with different MMR subtypes.
Fifty-seven patients with CRC who underwent surgical resection between 2016 and 2019 at the Tianjin Medical University Cancer Hospital were enrolled in this retrospective study. Pathological TNM staging was based on the 8th edition of the Union for International Cancer Control TNM classification. Formalin-fixed paraffin-embedded tumor tissue samples of these patients were collected for subsequent multiple immunofluorescence staining, in which 19 patients were dMMR positively expressing mismatch repair proteins, such as MLH1, PMS2, MSH2, and MSH6, and the 38 patients had pMMR-matched basic clinicopathological features with the former. None of the patients had received any therapy before surgery. Thirty-nine patients, comprising 10 dMMR patients and 29 pMMR patients, who received anti-PD1 immunotherapy between 2015 and 2021 at the Shanxi Provincial Cancer Hospital were enrolled in this study. None of the 39 patients ever underwent surgery or other treatments before pathological puncture biopsy.
Specimens from all patients were approved by the Ethics Committee of the Tianjin Medical University Cancer Institute and Hospital (Ek2020214) and the Shanxi Provincial Cancer Hospital (SBQLL-2022-028).
A PerkinElmer Opal 7-color Technology Kit (NEL81001KT) was used to conduct immunofluorescence staining according to the manufacturer’s protocol. Antibodies against CD20 (1:800, ab9475, Abcam), CD21 (1:800, ab75985, Abcam), CK (1:800, ab215838, Abcam), BCL-6 (1:100, NBP3-07540, NOVUS), and GP2(1:400, D277-3, MBL) were used. Then, 4’,6-diamidino-2-phenylindole was used to stain the nuclei after completing all the staining cycles. An Automated Quantitative Pathology Imaging System (Vectra Polaris) was used to scan and visualize the stained slides, and the inForm image analysis software (v2.4.4; PerkinElmer) was used for quantification and scoring.
The method of evaluating TLS quantity and maturity was as follow:
Firstly, according to HE staining results, wax blocks with both normal and tumor tissues were selected to slice. Secondly, mIHC was performed to determinate the number and maturity of TLS.
We collected all TLSs of every tumor section and randomly collected three to five fields from areas outside the TLSs. The early TLS (Grade 1 TLS) was characterized by dense lymphocytic aggregates without CD21 and Bcl-6 expression; primary follicle-like TLS(Grade 2 TLS) was characterized by lymphocytic clusters with central network CD21 expression, but no GC reaction (Bcl-6-); and secondary follicle-like TLS (Grade 3 TLS) was characterized by lymphocytic clusters with GC reaction (CD20+Bcl-6+).
Statistical analyses were performed using SPSS 23.0 and GraphPad Prism (v.9.0). Shapiro Wilktest was used to test the normality of continuous variables, and the data normal distributing was described by mean ± standard deviation; data not subject to normal distribution was described by median and quartile. Comparisons of unpaired numerical variables between the two groups were assessed using Student’s t-test or Mann–Whitney test. X2 tests or Fisher’s exact test were used for comparisons between groups. Statistical significance was set at P value <0.05. When comparing the prognostic differences between the two subgroups after combining TLS quantity and maturity, P value and HR ratio were calculated using the log-rank test in GraphPad Prism software.
To explore the TLS difference between dMMR and pMMR, 96 mIHC staining samples of patients with CRC were analyzed (Table 1). TLS with CD20+CD21-BCL6- was defined as grade 1, TLS with CD20+CD21+BCL6- was defined as grade 2, and TLS with CD20+CD21+BCL6+ was defined as grade 3 (16). In addition, the GP2+ lymphoid tissue represented a payer patch that was excluded, and CK was used to differentiate the intratumoral and peritumoral regions (Figure 1C). We found that, regardless of the level of intratumor, peritumor, or whole sample, TLS quantity was higher in dMMR than in pMMR (P<0.0001, P<0.0001, P<0.0001; Figures 1A, B), while TLS maturity was higher in dMMR than in pMMR only at the intratumor level (P<0,05; Figures 1C, D).
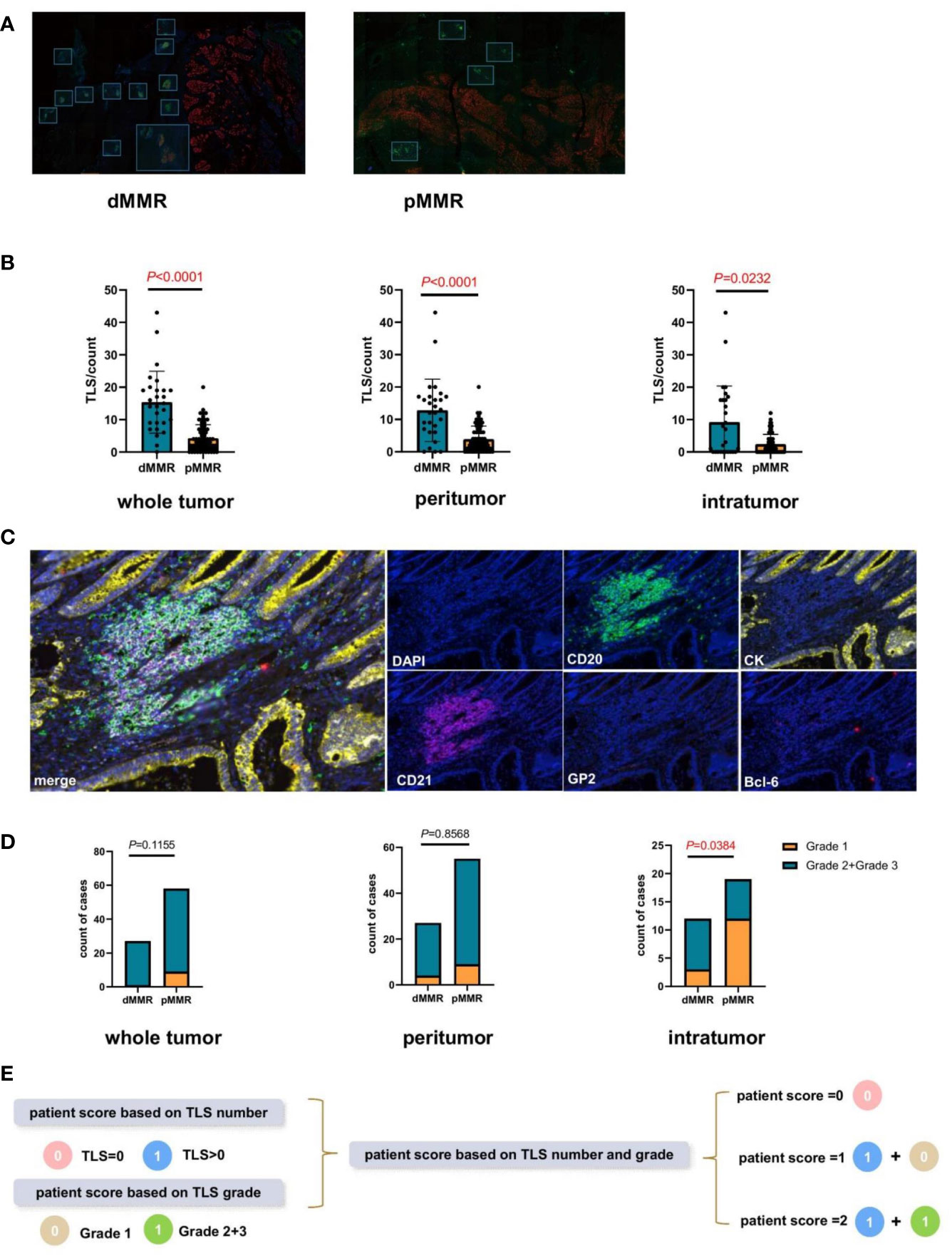
Figure 1 Comparison of the tertiary lymphatic structure (TLS) quantity and maturity between patients with dMMR and pMMR CRC (n=96). (A) Representative images of TLS number of dMMR and pMMR patient samples (magnification, ×100). The slide was stained with CK, CD21 (red), CD20 (green), Bcl-6 (red), GP2 (orange), and 4’,6-diamidino-2-phenylindole (DAPI; blue). (B) Comparison of TLS number between dMMR and pMMR patients in three levels: whole tumor (P<0.0001), peritumor (P<0.0001), and intratumor (P<0.0001) levels. (C) Representative images of TLS maturity (magnification, ×100). The slide was stained with GP2 (orange), CD21 (purple), CD20 (green), CK (yellow), Bcl-6 (pink), and DAPI (blue). Grade1-TLS, both CD21 and Bcl-6 markers were negative and GP2 was negative. Grade2-TLS, CD21 was positive and Bcl-6 and GP2 were negative. Grade3-TLS, both CD21 and Bcl-6 markers were positive and GP2 was negative. (D) Comparison of the patient proportion between dMMR and pMMR patients based on the TLS grade in the whole tumor (P=0.1155), peritumor (P=0.8568), and intratumor (P<0.05) groups, respectively. (E) Establishment of the new patient score system integrating TLS quantity and maturity grade.
Therefore, a comprehensive patient scoring system was built based on the TLS quantity and maturity. Scores based on the TLS number were defined as 0 (TLS number=0) or 1(TLS number >0). The score based on TLS maturity was defined as 0 (TLS grade 1) or 1 (TLS grade 2 or 3). Based on the sum of these two scores, the patient scores were calculated and divided into 0, 1, and 2 (Figure 1E).
The proportions of patients with different scores based on the peritumor, intratumor, and whole tumor microenvironment were analyzed separately. Based on the whole tumor, the proportion of patients with a score >1 in the dMMR group was much higher than that in the pMMR group (P=0.0459; Figure 2A). Continuing the analysis, we found that this difference was mainly due to intratumoral TLS, but not peritumoral TLS (P=0.0459, P=0.1510; Figures 2B, C).
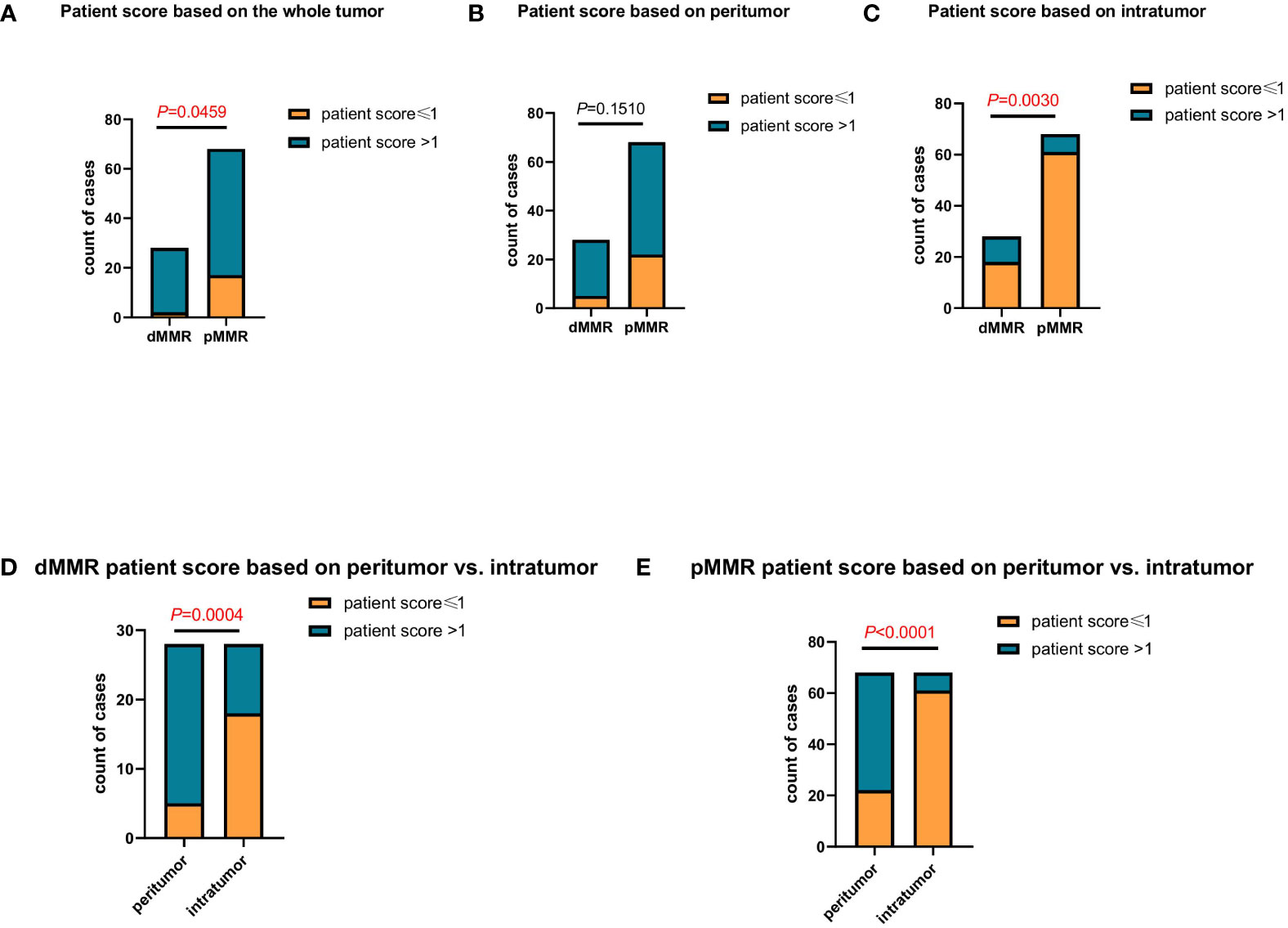
Figure 2 Comparing the differences in patient scores between patients with dMMR and pMMR CRC (n=96). (A–C) Comparison of patient score between patients with dMMR and pMMR CRC at the whole tumor (P<0.05) (A), peritumor (P=0.1510) (B), and intratumor (P<0.005) (C) levels. (D, E) Comparison of patient score at the peritumor and intratumor levels between patients with dMMR (P<0.005) (D) and pMMR (P<0.0001) (E) CRC, respectively.
In addition, we compared the proportion of peritumor and intratumor patient scores between the dMMR and pMMR groups. In both the dMMR and pMMR groups, the proportion of patients with a score >1 based on the intratumor was much lower than that in the peritumor group (P=0.004, P<0.0001; Figures 2D, E). Since the proportion of patients scoring >1 based on intratumor TLS was very similar to the previously reported clinical response rate (17), it indicated that the patient score based on intratumor TLS might be an effective indicator in anti-PD1 immunotherapy.
In the present study, 39 patients with CRC received anti-PD-1 immunotherapy were enrolled to further validate the predictive role of the TLS score in the efficacy of PD-1 inhibitor therapy (Table 2). The patients were divided into two groups according to their therapeutic responses. Patients with a partial response (PR) and complete response (CR) were assigned to the response group, and those with progressive disease (PD) were assigned to the non-responsive group. The results showed that based on the intratumor TLS score, the proportion of patients with a score >1 in the PR+CR group was much higher than that in the PD group (P<0.0001; Figure 3A). Furthermore, we continued to evaluate the treatment response in patients with dMMR and pMMR based on TLS scores, and the data showed similar results to those of all patients. Based only on the intratumor TLS score, the proportion of patients with a score >1 was higher in the response group than in the non-response group (P=0.0384, P=0.0001; Figures 3B, C).
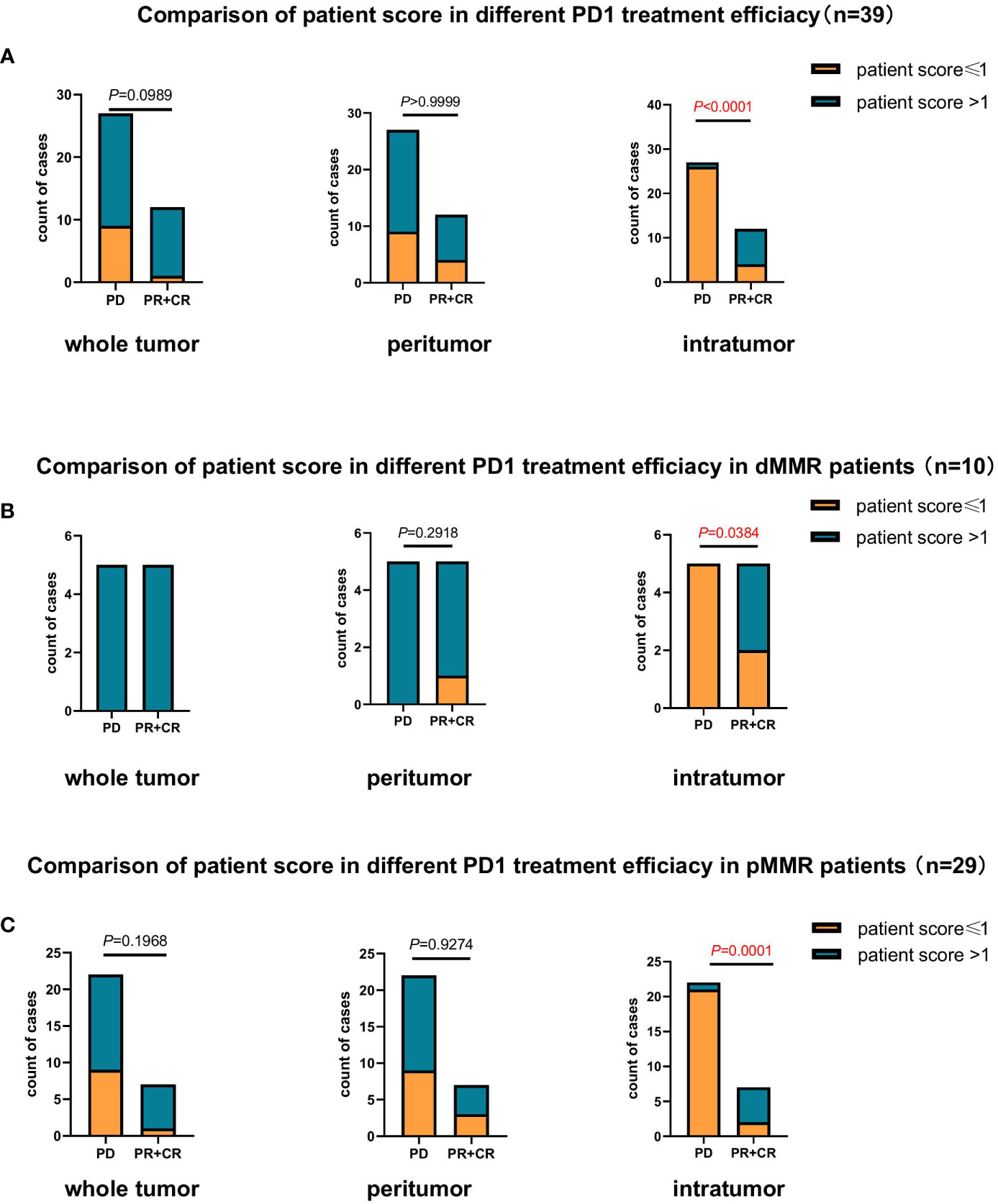
Figure 3 Comparison of the association between patient scores based on TLS quantity and maturity and response to anti-PD1 therapy (n=39). (A) Comparison of the association between patient score and response to anti-PD1 therapy in patients with CRC (n=39) at the whole tumor (P=0.0989), peritumor (P>0.9999), and intratumor (P<0.0001) levels. (B) Comparison of the association between patient score and response to anti-PD1 therapy in patients with dMMR CRC (n=10) at the whole tumor, peritumor (P=0.2918) and intratumor (P<0.05) levels. (C) Comparison of the association between patient score and response to anti-PD1 therapy in patients with pMMR CRC (n=29) at the whole tumor (P=0.1968), peritumor (P=0.9274), and intratumor (P=0.0001) levels.
Multiple clinical, pathological, and immune characteristics were investigated to evaluate their impact on the clinical response to anti-PD1 immunotherapy. The results revealed that Patient scores were positively correlated with clinical efficacy in the 39 patients and pMMR group (P=0.004, P=0.012, Tables 3, 4). Multivariate analysis was not performed in the dMMR group because of the small number of enrolled patients.
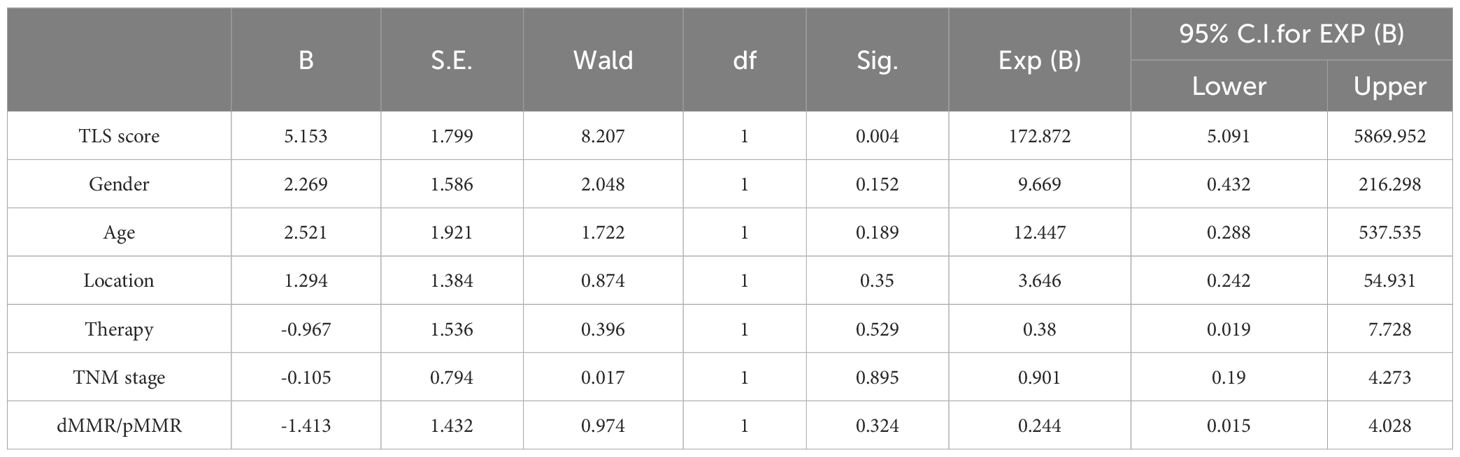
Table 3 Multivariate clinical pathological characteristics and immune characteristics affecting response of anti-PD1 immunotherapy in 39 pMMR patients.
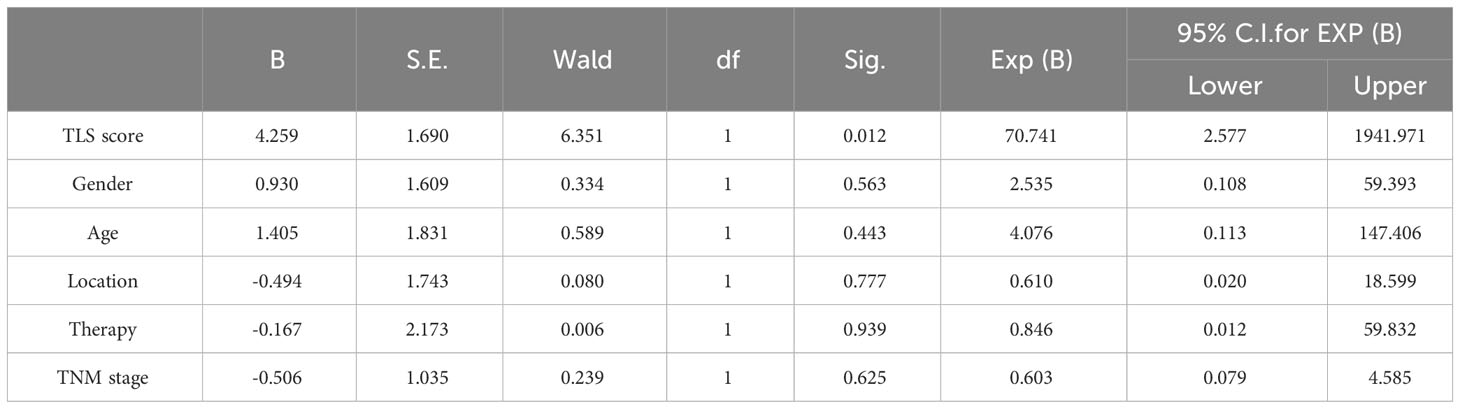
Table 4 Multivariate clinical pathological characteristics and immune characteristics affecting response of anti-PD1 immunotherapy in 29 pMMR patients.
To further investigate the patient score on clinical efficacy prediction, survival analysis of irPFS and multivariate Cox regression analyses of clinical and immune characteristics in different patient score group was done, which verified that the irPFS of patients score>1 was longer than that of patients score ≤ 1 in pMMR group (P=0.0004; Figure 4A; Table 5). The irPFS in dMMR group did not show any difference, which may have been due to its small sample size (n=10, Figure 4A). Survival analysis of OS was also done, which verified that the OS of patients score>1 was longer than that of patients score ≤ 1 in pMMR group but not the whole 39 patients and dMMR group (P=0.0030, P=0.0576 and P=0.7760, respectively, Figure 4B). In addition, receiver operating characteristic (ROC) curve analysis was performed in pMMR patients, revealing that the patient score had good predictive ability for the clinical efficacy of PD1 treatment (AUC=0.815; Figure 4C). All the results indicated that the patient score based on the intratumor microenvironment can be used as a predictive factor for anti-PD1 immunotherapy in patients with CRC.
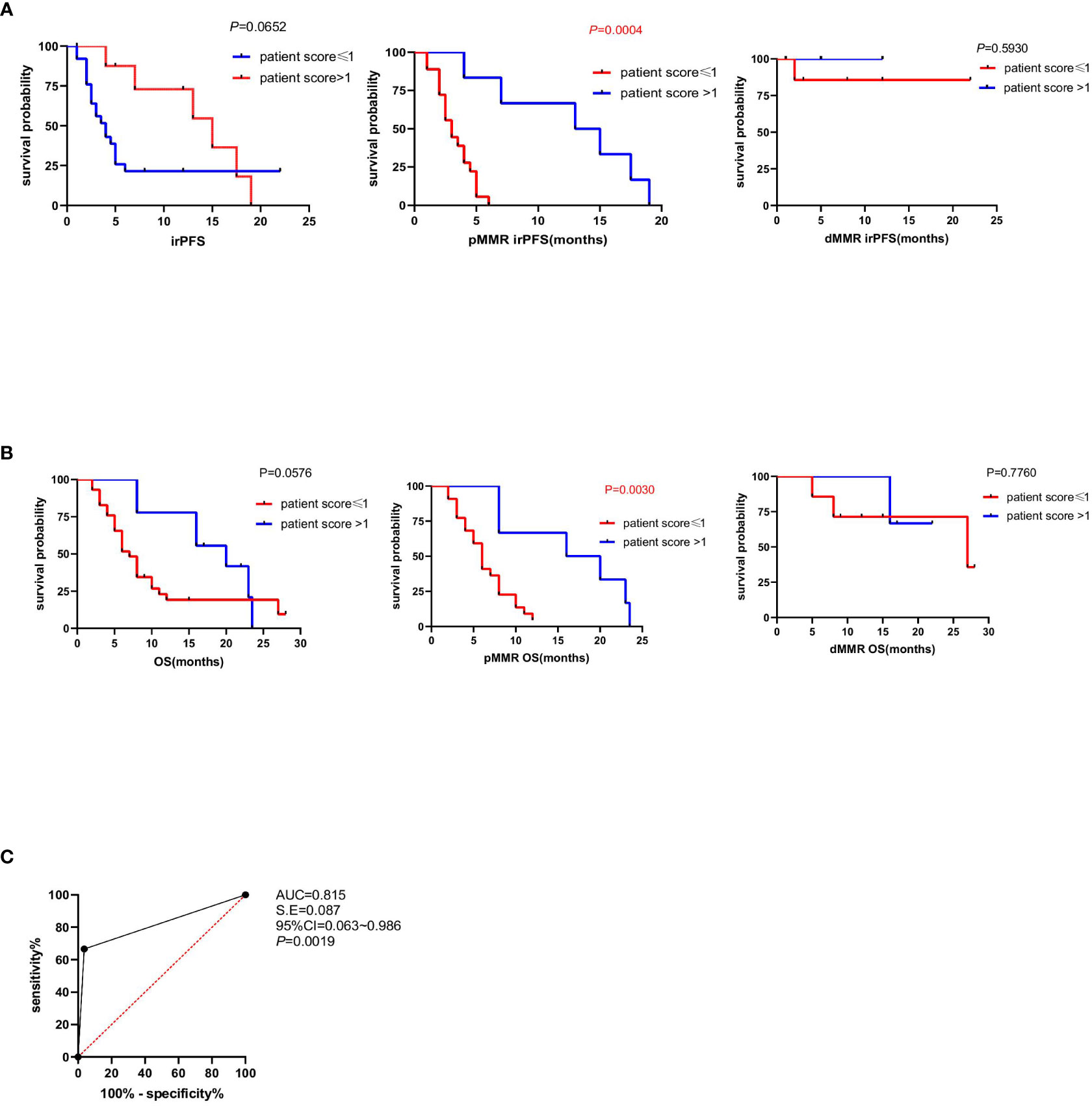
Figure 4 Association between patient score and immune-related progression-free survival (irPFS and OS). (A) Kaplan–Meier survival curves showing irPFS according to patient score of all the 39 patients, pMMR patients and dMMR patients (P=0.0652, P=0.0004, P=0.5930). P values were calculated by the log-rank test. (B) Kaplan–Meier survival curves showing OS according to patient score of all the 39 patients, pMMR patients and dMMR patients. P values were calculated by the log-rank test (P=0.0576, P=0.0030, P=0.7760). (C) The ROC curve to evaluate the predictive ability of patient score on anti-PD1 immunotherapy clinical responses (AUC=0.815).
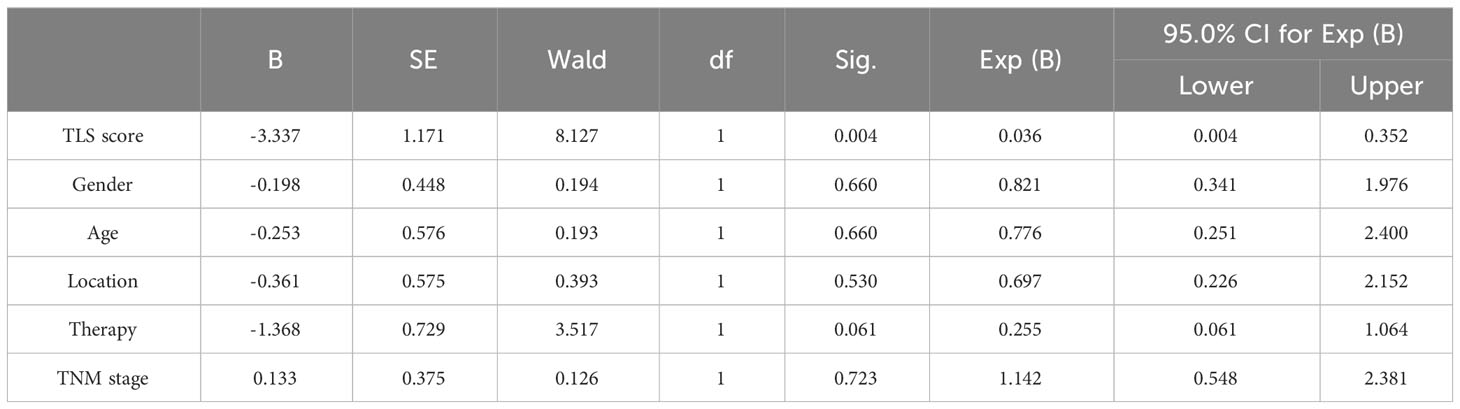
Table 5 Multivariate Cox regression analyses of clinical and immune characteristics affecting irPFS of anti-PD1 immunotherapy in 29 pMMR patients.
In this study, we first evaluated the associations between MMR typing and TLS distribution, quantity, and maturity, clinical features, and prognosis of 96 patients with stage I–IV CRC. In the analysis of TLS quantity difference of the whole tumor, peritumor, or intratumor microenvironment between patients with dMMR and pMMR CRC, the results showed that the number of TLS was much higher in dMMR than in pMMR patients, which was consistent with the results of a study on the immunomicroenvironment characteristics of urachal carcinoma (UrC). They found that the number of TLS tended to be higher in UrC tumor with dMMR (P=0.1919), as well the patients with higher TLS numbers tended to result in a much better prognosis (18). TLS maturity analysis revealed no differences in the whole tumor or peritumor between the dMMR and pMMR groups. In the intratumor-TLS analysis, the proportion of patients with grade 2+grade 3 pMMR was lower than that of patients with dMMR. The maturity of intratumoral TLS was higher in patients with dMMR than in those with pMMR. This is consistent with the findings of CRC and lung squamous cell carcinoma, in which patients from germinal centers (GCs) had a better prognosis (19, 20). These results further indicated that B cell maturity and humoral immunity play key roles in anti-tumor immune responses (16).
Based on the contribution of TLS quantity and maturity to the anti-tumor response, we used a patient scoring system (PS) to predict the clinical response of patients with CRC to PD-1 antibodies immunotherapy. Using this new score, we analyzed the relationship between TLS and MMR subtype and the results showed both in dMMR and pMMR patients, the proportion of patients score >1 based on intratumor was much lower than that peritumor. Moreover, in either the dMMR group or the pMMR group, the proportion of patients scoring >1 based on intratumoral TLS was similar to the clinical response rate that has been reported (17), and whether the patient score based on intratumoral TLS could be an effective indicator of anti-PD1 immunotherapy.
In this study, we analyzed the correlation between patient scores and clinical responses in 39 patients receiving anti-PD-1 immunotherapy and found that the proportion of patients with intratumoral TLS patient scores > 1 was much higher in the response (PR + CR) group than in the non-responders groups. Similar results were observed in 29 pMMR and 10 dMMR patients. These results suggest that the distribution of TLS affects the efficacy of immunotherapy. Previous studies defined the peritumoral TLS of breast cancer as a range within 5 mm from the invasive edge and divided it into adjacent and distal TLS according to the distance and interval between the normal breast tissue and the invasive edge. The higher the peritumoral TLS (distal TLS) density, the lower is the disease-free survival (DFS), independent of overall survival (OS), and the higher the distal TLS density, the lower is the DFS and OS (21). This is consistent with the results of the present study; however, no similar studies have been reported in CRC to date.
Further analysis of irPFS was performed in 39 patients with CRC who received anti-PD-1 immunotherapy. We found that patients with score > 1 based on the intratumor TLS evaluation had much better survival among the 29 patients with pMMR. Although the same trend was observed in the dMMR group, statistical results are not available because of the small number of cases. The specimens of the 39 patients receiving anti-PD-1 treatment were collected before immunotherapy and the sample size was limited, which might lead to a potential bias. In future research, we will collect more specimens and conduct prospective studies to verify the authenticity and accuracy of the conclusions. In conclusion, our findings indicate the patient score based on intratumor TLS evaluation as a good immune predictive indicator for the efficacy of PD-1 antibody therapy in patients with CRC.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the Ethics Committee of the Tianjin Medical University Cancer Institute and Hospital and the Ethics Committee of Shanxi Provincial Cancer Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of your previous study for which ethical approval was obtained. Written informed consent for participation from the participants or the participants’ legal guardians/next of kin was not mentioned in accordance with the national legislation and institutional requirements.
HF: Formal analysis, Methodology, Writing – original draft. SZ: Formal analysis, Methodology, Writing – original draft. QZ: Writing – review & editing. FH: Writing – review & editing. GD: Writing – review & editing. LW: Writing – review & editing. XY: Writing – review & editing. XZ: Writing – review & editing. WY: Writing – review & editing. FW: Writing – review & editing. XH: Writing – review & editing. XR: Project administration, Writing – review & editing. HZ: Project administration, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study is funded by National Natural Science Foundation of China (82372779, U20A20375), Haihe Laboratory of Cell Ecosystem Innovation Fund (22HHXBSS00004), Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-009A), and Shanxi Province “136 Revitalization Medical Project Construction Funds”.
We sincerely appreciate our colleagues in the Department of Pathology for their help with tumor section preparation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. (2019) 394:1467–80. doi: 10.1016/S0140-6736(19)32319-0
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Li J, Wu C, Hu H, Qin G, Wu X, Bai F, et al. Remodeling of the immune and stromal cell compartment by PD-1 blockade in mismatch repair-deficient colorectal cancer. Cancer Cell. (2023) 41:1152–69.e7. doi: 10.1016/j.ccell.2023.04.011
4. Sautès-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. (2019) 19:307–25. doi: 10.1038/s41568-019-0144-6
5. Wang J, Jiang D, Zheng X, Li W, Zhao T, Wang D, et al. Tertiary lymphoid structure and decreased CD8+ T cell infiltration in minimally invasive adenocarcinoma. iScience. (2022) 25:103883. doi: 10.1016/j.isci.2022.103883
6. Tokunaga R, Naseem M, Lo JH, Battaglin F, Soni S, Puccini A, et al. B cell and B cell-related pathways for novel cancer treatments. Cancer Treat Rev. (2019) 73:10–9. doi: 10.1016/j.ctrv.2018.12.001
7. Hayashi Y, Makino T, Sato E, Ohshima K, Nogi Y, Kanemura T, et al. Density and maturity of peritumoral tertiary lymphoid structures in oesophageal squamous cell carcinoma predicts patient survival and response to immune checkpoint inhibitors. Br J Cancer. (2023) 128:2175–85. doi: 10.1038/s41416-023-02235-9
8. Zhou L, Xu B, Liu Y, Wang Z. Tertiary lymphoid structure signatures are associated with survival and immunotherapy response in muscle-invasive bladder cancer. Oncoimmunology. (2021) 10:915574. doi: 10.1080/2162402X.2021.1915574
9. Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. (2020) 577:561–5. doi: 10.1038/s41586-019-1914-8
10. Zhan Z, Shi-jin L, Yi-Ran Z, Zhi-Long L, Xiao-Xu Z, Hui D, et al. High endothelial venules proportion in tertiary lymphoid structure is a prognostic marker and correlated with anti-tumor immune microenvironment in colorectal cancer. Ann Med. (2023) 55:114–26. doi: 10.1080/07853890.2022.2153911
11. Li H, Zhu SW, Zhou JJ, Chen DR, Liu J, Wu ZZ, et al. Tertiary lymphoid structure raises survival and immunotherapy in HPV- HNSCC. J Dent Res. (2023) 102:678–88. doi: 10.1177/00220345231151685
12. Mokhtari Z, Rezaei M, Sanei MH, Dehghanian A, Faghih Z, Heidari Z, et al. Tim3 and PD-1 as therapeutic and prognostic targets in colorectal cancer: Relationship with sidedness, clinicopathological parameters, and survival. Front Oncol. (2023) 13:1069696. doi: 10.3389/fonc.2023.1069696
13. Küçükköse E, Heesters BA, Villaudy J, Verheem A, Cercel M, van Hal S, et al. Modeling resistance of colorectal peritoneal metastases to immune checkpoint blockade in humanized mice. J Immunother Cancer. (2022) 10:e005345. doi: 10.1136/jitc-2022-005345
14. Greco L, Rubbino F, Dal Buono A, Laghi L. Microsatellite instability and immune response: From microenvironment features to therapeutic actionability-lessons from colorectal cancer. Genes (Basel). (2023) 14:1169. doi: 10.3390/genes14061169
15. Jin K, Yu Y, Zeng H, Liu Z, You R, Zhang H, et al. CD103+CD8+ tissue-resident memory T cell infiltration predicts clinical outcome and adjuvant therapeutic benefit in muscle-invasive bladder cancer. Br J Cancer. (2022) 126:1581–8. doi: 10.1038/s41416-022-01725-6
16. Zhao H, Wang H, Zhao Y, Sun Q, Ren X. Tumor-resident T cells associated with tertiary lymphoid structure maturity, improve survival in patients with Stage III lung adenocarcinoma. Front Immunol. (2022) 13:877689. doi: 10.3389/fimmu.2022.877689
17. Chalabi M, Fanchi LF, Dijkstra KK, Van den Berg JG, Aalbers AG, Sikorska K, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. (2020) 26:566–76. doi: 10.1038/s41591-020-0805-8
18. Zhang X, Wang S, Nie RC, Qu C, Chen J, Yang Y, et al. Immune microenvironment characteristics of urachal carcinoma and its implications for prognosis and immunotherapy. Cancers (Basel). (2022) 14:615. doi: 10.3390/cancers14030615
19. Posch F, Silina K, Leibl S, Mündlein A, Moch H, Siebenhüner A, et al. Maturation of tertiary lymphoid structures and recurrence of Stage II and III colorectal cancer. Oncoimmunology. (2018) 7:e1378844. doi: 10.1080/2162402X.2017.1378844
20. Siliņa K, Soltermann A, Attar FM, Casanova R, Uckeley ZM, Thut H, et al. Germinal centers determine the prognostic relevance of tertiary lymphoid structures and are impaired by corticosteroids in lung squamous cell carcinoma. Cancer Res. (2018) 78:1308–20. doi: 10.1158/0008-5472.CAN-17-1987
Keywords: TLS, CRC, dMMR, pMMR, anti-PD1 immunotherapy
Citation: Feng H, Zhang S, Zhou Q, Han F, Du G, Wang L, Yang X, Zhang X, Yu W, Wei F, Hao X, Ren X and Zhao H (2024) Intratumor tertiary lymphatic structure evaluation predicts the prognosis and immunotherapy response of patients with colorectal cancer. Front. Immunol. 15:1302903. doi: 10.3389/fimmu.2024.1302903
Received: 27 September 2023; Accepted: 07 February 2024;
Published: 01 March 2024.
Edited by:
Hui Zhao, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Yongkun Wei, University of Texas MD Anderson Cancer Center, United StatesCopyright © 2024 Feng, Zhang, Zhou, Han, Du, Wang, Yang, Zhang, Yu, Wei, Hao, Ren and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiubao Ren, cmVueGl1YmFvQHRqbXVjaC5jb20=; Hua Zhao, aHVhemhhb0B0bXUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.