- 1Department of Pediatrics, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea
- 2Department of Pediatrics, School of Medicine, Kyungpook National University, Daegu, Republic of Korea
- 3Crohn’s and Colitis Association in Daegu-Gyeongbuk (CCAiD), Daegu, Republic of Korea
- 4Division of Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, Yonsei University College of Medicine, Severance Children’s Hospital, Seoul, Republic of Korea
- 5Department of Pediatrics, Pusan National University Children’s Hospital, Pusan National University School of Medicine, Yangsan, Republic of Korea
- 6Department of Pediatrics, Jeonbuk National University Hospital, Jeonbuk National University Medical School, Jeonju, Republic of Korea
- 7Department of Pediatrics, Jeju National University Hospital, Jeju, Republic of Korea
- 8Department of Pediatrics, Dankook University College of Medicine, Cheonan, Republic of Korea
- 9Department of Pediatrics, Korea University College of Medicine, Korea University Guro Hospital, Seoul, Republic of Korea
- 10Department of Pediatrics, Ajou University School of Medicine, Suwon, Republic of Korea
- 11Department of Pediatrics, Daegu Catholic University School of Medicine, Daegu, Republic of Korea
- 12Department of Pediatrics, Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Republic of Korea
- 13Department of Pediatrics, Chungnam National University Hospital, Chungnam National University College of Medicine, Daejeon, Republic of Korea
- 14Department of Pediatrics, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Republic of Korea
- 15Department of Pediatrics, Daejeon Eulji Medical Center, Eulji University, Daejeon, Republic of Korea
- 16Department of Pediatrics, Korea University Medical Center Anam Hospital, Seoul, Republic of Korea
- 17Department of Pediatrics, Gyeongsang National University College of Medicine, Jinju, Republic of Korea
- 18Institute of Medical Science, Gyeongsang National University, Jinju, Republic of Korea
- 19Department of Pediatrics, Gyeongsang National University Changwon Hospital, Changwon, Republic of Korea
- 20Department of Pediatrics, Chung-Ang University Hospital, Chung-Ang University, College of Medicine, Seoul, Republic of Korea
- 21Department of Pediatrics, Chungbuk National University College of Medicine, Chungju, Republic of Korea
- 22Department of Pediatrics, Yeungnam University School of Medicine, Daegu, Republic of Korea
- 23Department of Pediatrics, Keimyung University School of Medicine, Daegu, Republic of Korea
- 24Department of Pediatrics, Chung-Ang University, Gwangmyeong Hospital, Gwangmyeong, Republic of Korea
Background and aims: Favourable clinical data were published on the efficacy of CT-P13, the first biosimilar of infliximab (IFX), in pediatric inflammatory bowel disease (IBD); however, few studies have compared the effect on endoscopic healing (EH) and drug retention rate between the IFX originator and CT-P13. Therefore, we aimed to compare EH and the drug retention rate between the IFX originator and CT-P13.
Methods: Children with Crohn’s disease (CD) and ulcerative colitis (UC)/IBD-unclassified (IBD-U) at 22 medical centers were enrolled, with a retrospective review conducted at 1-year and last follow-up. Clinical remission, EH and drug retention rate were evaluated.
Results: We studied 416 pediatric patients with IBD: 77.4% had CD and 22.6% had UC/IBD-U. Among them, 255 (61.3%) received the IFX originator and 161 (38.7%) received CT-P13. No statistically significant differences were found between the IFX originator and CT-P13 in terms of corticosteroid-free remission and adverse events. At 1-year follow-up, EH rates were comparable between them (CD: P=0.902, UC: P=0.860). The estimated cumulative cessation rates were not significantly different between the two groups. In patients with CD, the drug retention rates were 66.1% in the IFX originator and 71.6% in the CT-P13 group at the maximum follow-up period (P >0.05). In patients with UC, the drug retention rates were 49.8% in the IFX originator and 56.3% in the CT-P13 group at the maximum follow-up period (P >0.05).
Conclusions: The IFX originator and CT-P13 demonstrated comparable therapeutic response including EH, clinical remission, drug retention rate and safety in pediatric IBD.
1 Introduction
Inflammatory bowel disease (IBD) is a chronic gastrointestinal disorder, and the main subtypes include Crohn’s disease (CD) and ulcerative colitis (UC), with approximately 25% of cases diagnosed in children and adolescents (1). Pediatric-onset IBD typically manifests with a more severe phenotype than adult-onset IBD and is associated with an aggressive and complicated disease course (1, 2). The outcomes and prognosis of pediatric IBD significantly improved since the introduction of biologic agents as main therapeutic options approximately 20 years ago (3–6).
Owing to the severe and aggressive disease course, biologic agents are recommended for pediatric patients with IBD after risk stratification, and top–down therapy is recommended, particularly for patients with moderate-to-severe CD (7, 8). The first biologic agent introduced for the treatment of patients with IBD was infliximab (IFX), which targets tumour necrosis factor-α (TNF-α) and has significantly improved patient outcomes. Anti-TNF agents can induce clinical remission and endoscopic healing (EH), which is associated with a reduced need for hospitalization and surgery and, consequently, an improved quality of life (9, 10).
CT-P13 (Remsima, Celltrion, Incheon, South Korea; Inflectra, Pfizer, NY, USA) is the first biosimilar monoclonal antibody to reference IFX that is approved for use in all indications but is significantly more cost-efficient (11, 12). CT-P13 and IFX originator (Remicade, Janssen Biotech, Horsham) show comparable binding affinities to the monomeric and trimeric forms of human TNF-α and comparable TNF-α neutralising and cytotoxic activities (13). While preclinical comparative studies have confirmed a high degree of similarity and equivalent efficacy between the IFX originator and CT-P13 (14–19), concerns have been raised regarding drawing conclusions from rheumatoid arthritis and ankylosing spondylitis studies without direct clinical evidence in IBD (20).
Recently, favourable clinical data have been published on the efficacy of CT-P13 in pediatric IBD (15, 17, 21–25). However, these studies have not compared long-term clinical efficacy based on EH and durability between the IFX originator and CT-P13 in pediatric IBD. Therefore, we aimed to compare clinical outcomes including clinical remission, EH and drug retention rate of the IFX originator and CT-P13 based on data from a large observational cohort study of patients with CD and UC/IBD-unclassified (IBD-U).
2 Materials and methods
2.1 Patients and data collection
This retrospective observational study was conducted at the Department of Pediatrics of 22 centers in South Korea (see affiliations) between March 2015 and August 2022. Children and adolescents with IBD who had been treated with either the IFX originator or CT-P13 at age <19 years and followed for at least one year after initiation of IFX treatment were included. Patients who changed anti-TNF agents between IFX originator and CT-P13 and those with missing data on baseline clinicodemographics were excluded. CD, UC and IBD-U were diagnosed according to the revised Porto criteria of the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (2). The choice between IFX originator and CT-P13 was made by patients and caregivers based on price and preference.
The study was approved by the Institutional Review Board of Kyungpook National University Chilgok Hospital and all other participating centers. Informed consent was waived because of the retrospective nature of the study.
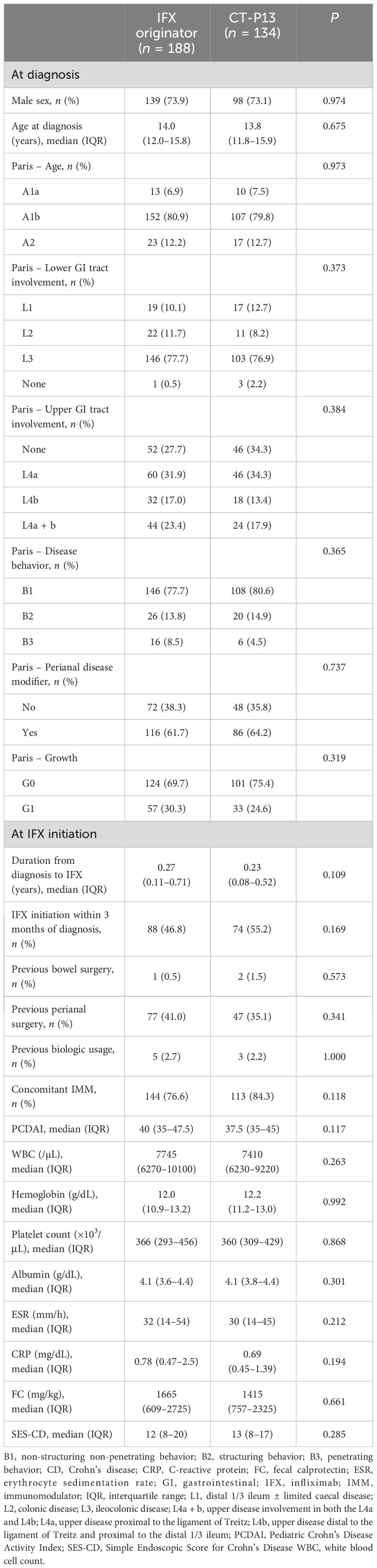
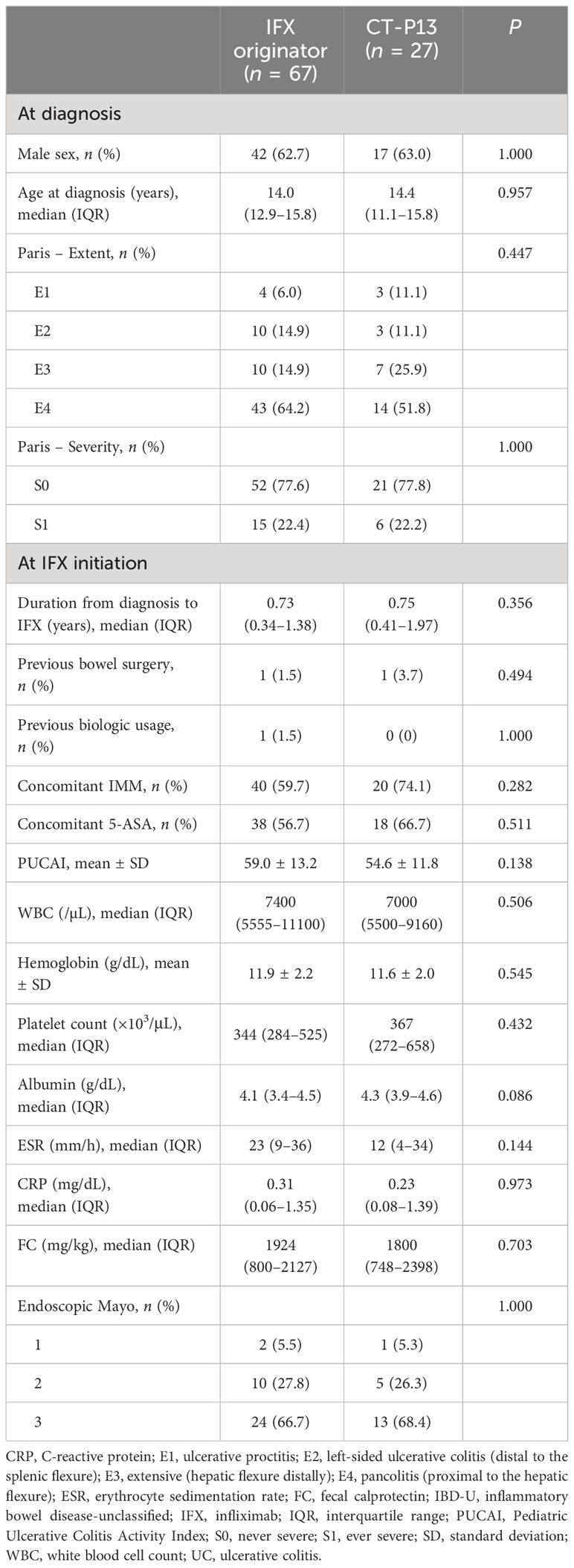
Baseline clinicodemographics including sex, age at diagnosis and disease phenotype were collected at diagnosis, and the duration from diagnosis to IFX start, previous bowel and perianal surgery, previous biologic usage and concomitant immunomodulator were collected at IFX initiation. The disease phenotype at diagnosis was classified according to the Paris classification (26). Disease activity scores including the Pediatric Crohn’s Disease Activity Index (PCDAI) for CD and the Pediatric Ulcerative Colitis Activity Index (PUCAI) for UC and IBD-U were also collected, as well as laboratory findings, such as white blood cell (WBC) count, hemoglobin, platelet count, serum albumin, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and fecal calprotectin (FC). The centers participating in this study are tertiary hospitals in South Korea, and each laboratory conducts total quality management to minimize inter-institutional errors and maintain test quality. Through this, thorough quality control is carried out to minimize errors in pre-analytical, analytical, and post-analytical stage, and SI units are used to ensure consistency of units.
Endoscopic scores including the Simple Endoscopic Score for Crohn’s Disease (SES-CD) for patients with CD and the endoscopic Mayo score for patients with UC were collected. Disease activity scores, laboratory findings and endoscopic scores were also collected during the 1-year treatment with IFX. The Mucosal Inflammation Non-invasive index (MINI) was also calculated at 1-year treatment with IFX (27). For CD patients, dose intensification with shortened IFX dosing intervals was allowed by Korean insurance coverage only if secondary loss of response (LOR) was suspected, i.e., if clinical symptoms worsened and CRP or FC levels increased significantly at two consecutive visits, necessitating dose intensification or a switch of therapy. All patients were treated according to the same treatment strategy at the time (28, 29). For the drug retention rate analysis, factors such as whether the patient was continuously on IFX and the maximum follow-up period while on IFX were also collected.
2.2 Endpoints, group allocation and definitions
The primary endpoint of this study was EH at 1-year treatment with IFX, and the secondary endpoint was corticosteroid-free remission (CSFR) 1 year after treatment with IFX and the drug retention rate during IFX treatment. The tertiary endpoint was adverse events during IFX treatment. Patients were divided into two groups according to whether they had received the IFX originator or CT-P13, and variables were compared separately between the groups among patients with CD and UC/IBD-U.
EH was defined as an SES-CD of <3 for patients with CD and an endoscopic Mayo score of <2 for patients with UC. CSFR was defined as a PCDAI of <10 in patients with CD and PUCAI of <10 in patients with UC while not requiring any treatment with corticosteroids. Drug retention rate was defined as probability of retention with IFX treatment after treatment initiation. Patients who stopped IFX and resumed it after more than 90 days were counted as permanent discontinuations.
2.3 Statistical analysis
For statistical comparisons between groups, Student’s t test and Wilcoxon’s rank-sum test were used for continuous variables and the chi-squared test or Fisher’s exact test for categorical variables. Comparative data for continuous variables were reported as medians with interquartile range (IQR) or means with standard deviation. To derive the estimated cumulative drug cessation rate during IFX treatment, Kaplan–Meier survival analysis was used. SAS version 9.4 (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
3 Results
3.1 Baseline characteristics
In this study, a total of 458 patients were included. Forty-two patients who changed anti-TNF agents between IFX originator and CT-P13 and those with missing data on baseline clinicodemographics were excluded from the study, with 416 patients for final inclusion in the study (Figure 1). Among them, 322 (77.4%) patients were diagnosed with CD and 94 (22.6%) were diagnosed with UC/IBD-U. Men comprised 73.6% (237/322) of the CD group and 62.8% (59/94) of the UC/IBD-U group. Of the 322 patients with CD, 188 (58.4%) received IFX originator and 134 (41.6%) received CT-P13. Furthermore, 67 of the 94 patients with UC (71.3%) received IFX originator and 27 (28.7%) received CT-P13. In the comparison of baseline characteristics, no statistically significant difference was found between the IFX originator group and the CT-P13 group in neither patient with CD (Table 1) nor those with UC/IBD-U (Table 2).
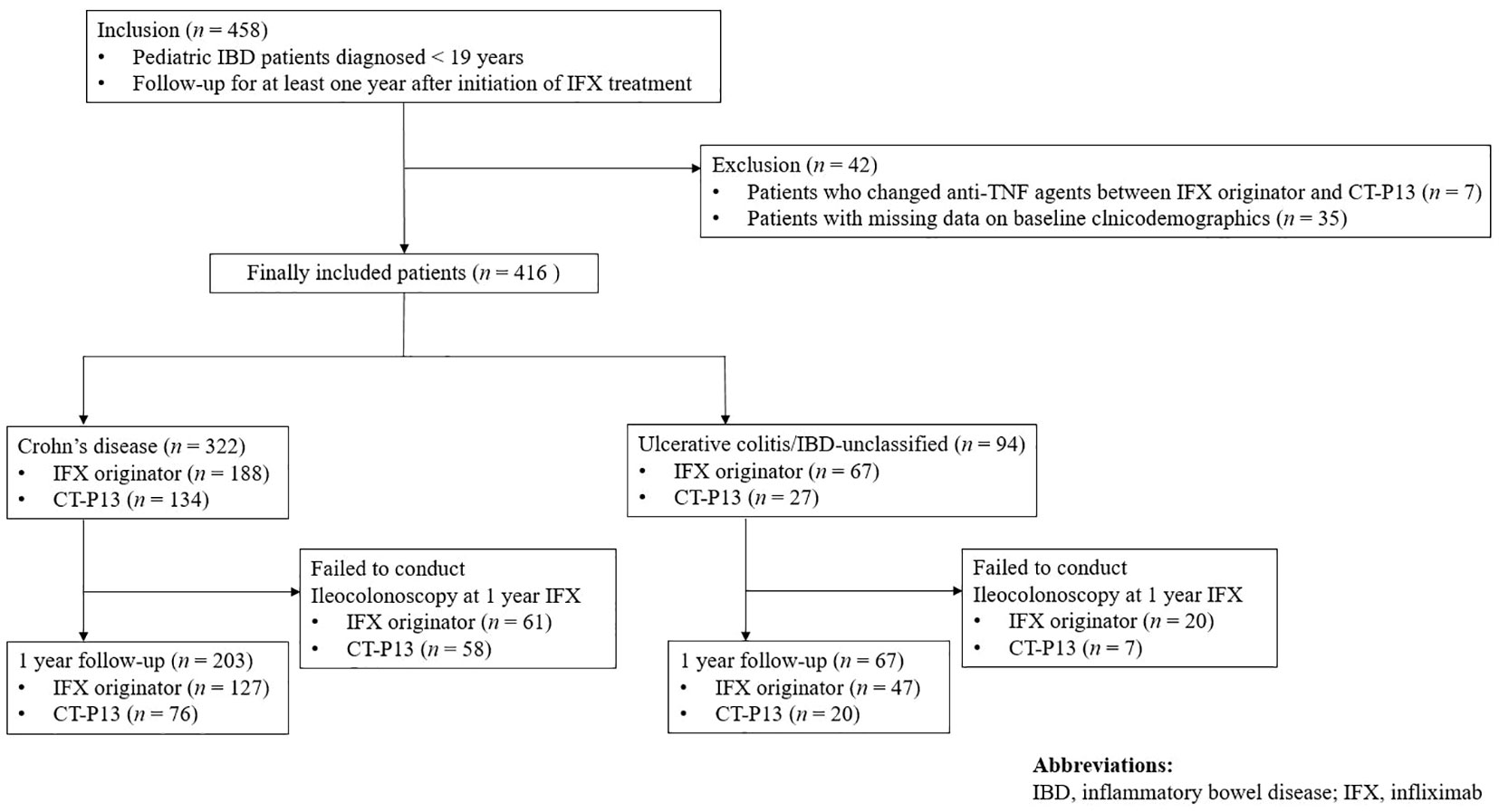
Figure 1 Flow diagram of the patient selection process. IBD, inflammatory bowel disease; IFX, infliximab.
3.2 Comparison of outcomes at 1-year follow-up between the IFX originator and CT-P13 groups
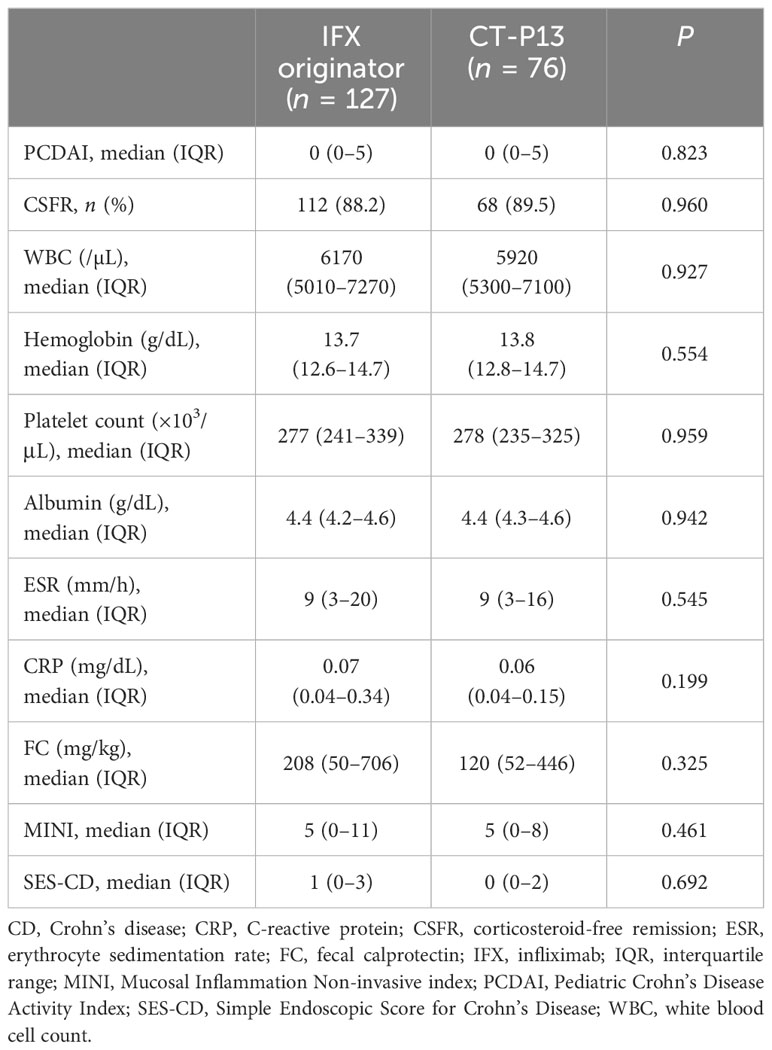
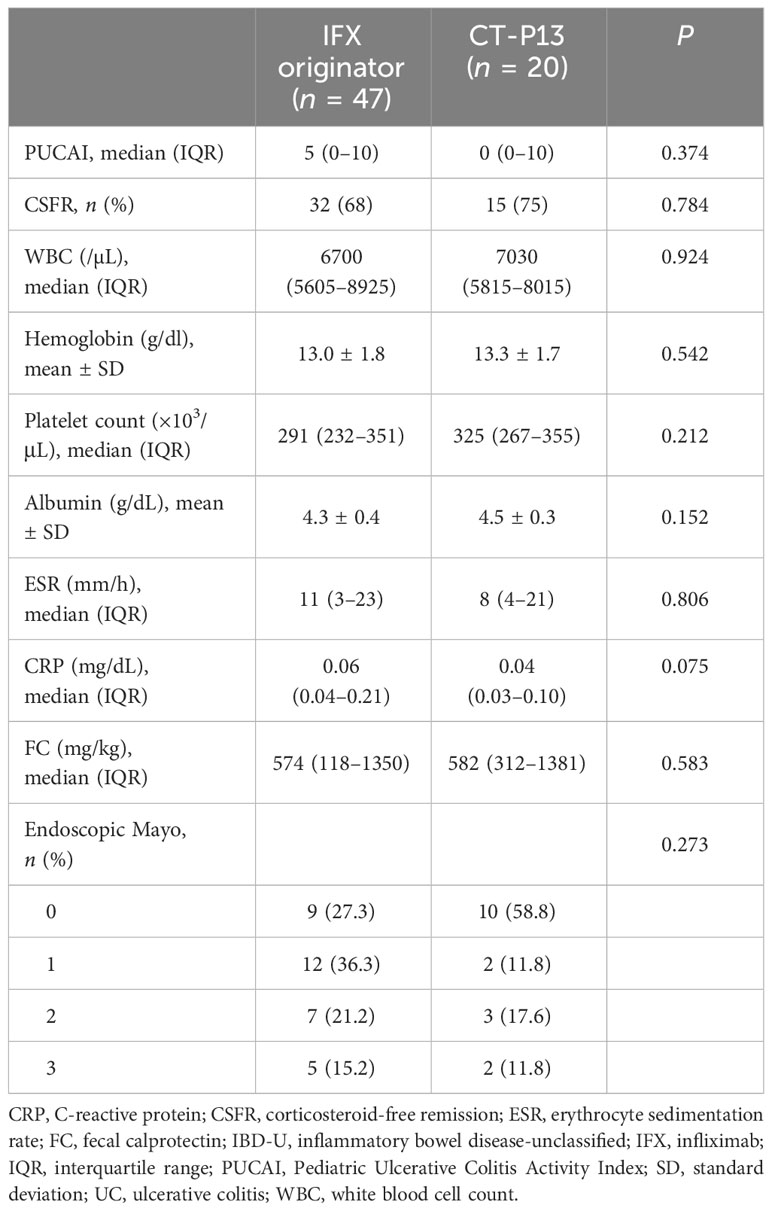
A comparison of outcomes at 1-year follow-up between the IFX originator and CT-P13 groups did not demonstrate any statistically significant difference among patients with CD (Table 3), including patients with UC/IBD-U (Table 4) . EH rates at 1-year follow-up were comparable between the two groups among patients with CD, with 73.1% (68/93) and 75.4% (43/57) of the IFX originator and CT-P13 groups achieving EH, respectively (P = 0.902) (Figure 2A). EH rates at 1-year follow-up were also comparable between the two groups among patients with UC/IBD-U, with 63.6% (21/33) and 70.6% (12/17) of the IFX originator and CT-P13 groups achieving EH, respectively (P = 0.860) (Figure 2B).
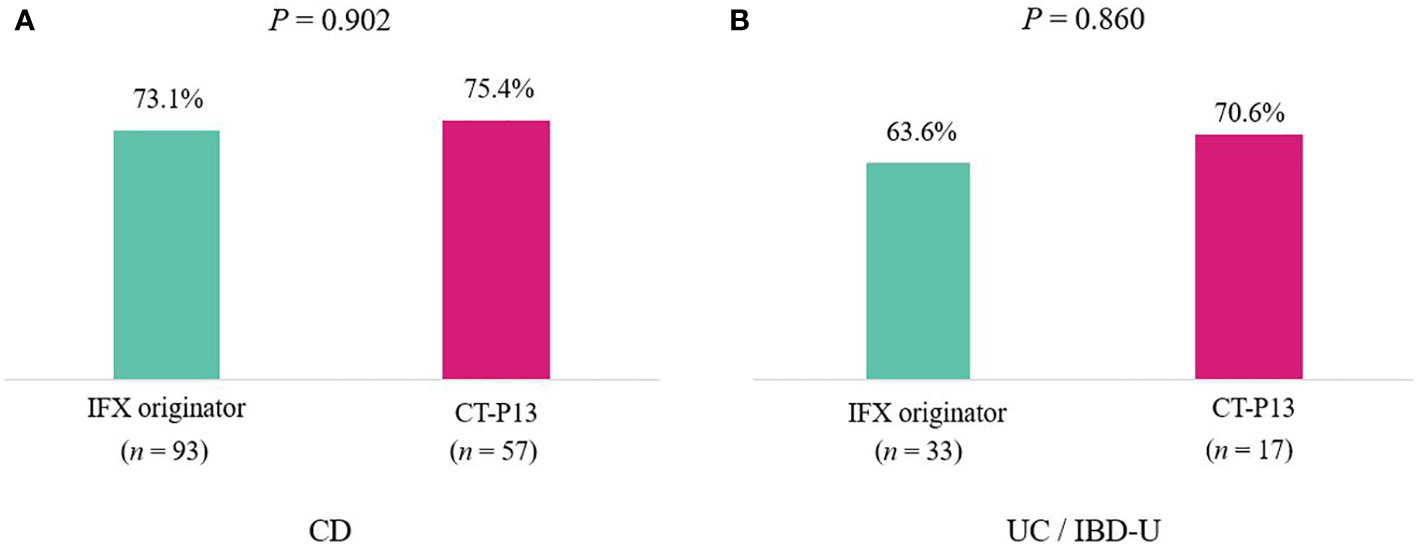
Figure 2 Endoscopic healing rates after 1 year of treatment with infliximab among (A) patients with CD (n = 150) and (B) patients with UC (n = 50). CD, Crohn’s disease; IBD-U, inflammatory bowel disease-unclassified; IFX, infliximab; UC, ulcerative colitis.
3.3 Comparison of drug retention rate between the IFX originator and the CT-P13 group
The median follow-up time for all patients with CD was 1.44 (range, 0.02–7.70) years, with 1.50 years in the IFX originator group and 1.22 years in the CT-P13 group (P = 0.20). After a median follow-up period of 1.44 years in patients with CD, approximately 10.2% (33/322) of patients discontinued treatment with IFX. According to the Kaplan–Meier survival analysis, the 1-, 2- and 5-year estimated cumulative cessation rates were 6.3%, 11.1% and 31.3% for the IFX originator group and 5.6%, 8.7% and 21.4% for the CT-P13 group, respectively. Moreover, the drug retention rates were 66.1% at the maximum follow-up period of 5.8 years in the IFX originator group and 71.6% at 5.0 years in the CT-P13 group. No statistical significance was found between the IFX originator and CT-P13 groups (P = 0.36) (Figure 3A).
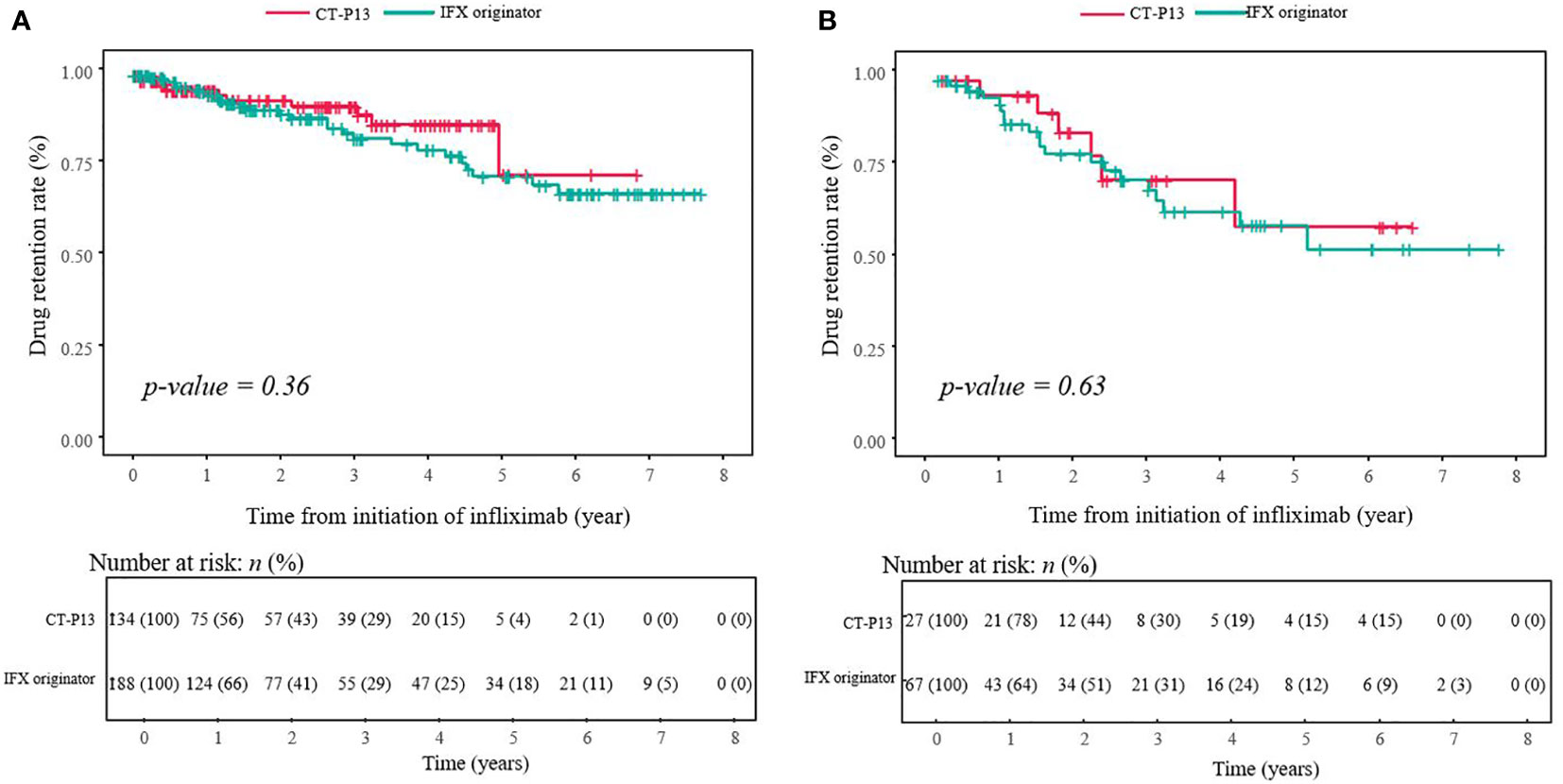
Figure 3 Drug retention curve during treatment with infliximab among (A) patients with CD (n = 322) and (B) patients with UC (n = 94). CD, Crohn’s disease; IBD-U, inflammatory bowel disease-unclassified; IFX, infliximab; UC, ulcerative colitis.
After a median follow-up period of 1.85 years, 24.5% (23/94) of patients with UC/IBD-U discontinued treatment with IFX. The median follow-up time for all patients with UC was 1.85 (range, 0.02–7.87) years with 2.01 years in the IFX originator group and 1.84 years in the CT-P13 group (P = 0.97). After a median follow-up period of 1.84 years, 23.4% (22/94) of patients with UC/IBD-U discontinued treatment with IFX. According to the Kaplan–Meier survival analysis, the estimated cumulative cessation rates at 1, 2 and 5 years were 15.2%, 24.3% and 50.2% for the IFX originator group and 9.8%, 22.5% and 43.7% for the CT-P13 group, respectively. In addition, the drug retention rates were 49.8% at the maximum follow-up period of 5.2 years in the IFX originator group and 56.3% at 4.9 years in the CT-P13 group. No statistical significance was observed between the IFX originator and CT-P13 group (P = 0.63) (Figure 3B).
3.4 Comparison of adverse events between the IFX originator and CT-P13 groups
In total, 105/255 (41.2%) and 70/161 (43.5%) adverse events occurred in the IFX originator and CT-P13 groups, respectively, but with no statistical significance (P = 0.718). Infusion reactions occurred in 13/255 (5.1%) and 4/161 (2.5%) of the IFX originator and CT-P13 groups, respectively (P = 0.290) (Table 5).
Serious adverse events requiring inpatient hospitalization was documented in 4/255 (1.6%) and 5/161 (3.1%) of the IFX originator and CT-P13 groups, respectively (P = 0.317). These included gastrointestinal obstructive signs and symptoms in five patients, massive gastrointestinal tract bleeding in two, tuberculosis reactivation in one, and de novo tuberculosis in one. No other serious adverse events were observed including life-threatening complications, resulting in death, persistent or significant disability/incapacity or congenital anomaly/birth defect.
4 Discussion
This multicenter study retrospectively evaluated and compared the clinical outcomes between the IFX originator and CT-P13 in pediatric patients with IBD. To the best of our knowledge, this is the first study to demonstrate equivalent EH achievement, IFX durability and drug retention rate between IFX originator and CT-P13 in a large and real-world pediatric IBD cohort. No significant differences were found in clinical remission, CSFR and biochemical remission between the IFX originator and CT-P13 groups (P > 0.05). In addition, a significant proportion of patients with CD and UC/IBD-U achieved EH with no difference between the two arms. Our results confirmed that CT-P13 was well tolerated and had a similar long-term efficacy as the IFX originator in patients with pediatric IBD.
Since the CT-P13 approval was based on the PLANETRA clinical trial results conducted in patients with rheumatoid arthritis and ankylosing spondylitis (30, 31), a steady stream of studies has been observed, which published on interchangeability between the IFX originator and CT-P13 in IBD (14, 16, 18, 19, 32). Furthermore, interest in the clinical use of CT-P13 is growing, which is expected to provide a similar clinical efficacy to IFX at a significant savings in terms of cost (33, 34). However, while a generic drug is an atomically identical copy of a reference drug, a biosimilar may not be completely identical to the originator biologic because a biologic agent is a structurally complex protein produced in vivo (20, 35). Even small changes in the cell line or laboratory conditions may result in minimal variations and differences from the reference drug. Therefore, to promote the appropriate use of CT-P13, a biosimilar of IFX, data on long-term clinical outcomes of CT-P13 in the treatment of IBD are needed. However, in pediatric patients, data on direct comparison of the clinical outcomes of the IFX originator and CT-P13 in the treatment of IBD were limited (15, 21).
To date, international guidelines indicate that CT-P13 is equivalent to the IFX originator in both adult and pediatric patients with IBD (36, 37). The interchangeability of CT-P13 with its originator has been well-established in both adult and pediatric patients with IBD (38–41). A prospective, open-label study of 143 adult patients with IBD (99 with CD, 44 with UC) who switched from the IFX originator to CT-P13 found that approximately 97% of patients remained on medication 6 months after switching, with few adverse events (38). The study also did not find differences in the disease activity, inflammatory marker levels and trough levels of IFX when comparing 6 months before and 6 months after switching from IFX originator to CT-P13. In line with the results of adult studies, several studies in pediatric patients with IBD have also demonstrated the interchangeability between the IFX originator and CT-P13 (15, 24, 42, 43). Kang et al. reported no statistically significant differences in disease activity, pharmacokinetics, immunogenicity and adverse events between the time of switching of the two drugs and during the 1-year follow-up (15).
To date, only two studies have directly compared the clinical efficacy and safety of IFX originator and CT-P13. A prospective study by Chanchlani et al., who analysed 82 children with IBD (63 with CD and 19 with UC/IBD-U), documented the lack of difference in the clinical remission rates on week 12 between the IFX originator group (68%) and the CT-P13 group (79%) (23). Moreover, Nikkonen et al. reported that 65% and 61% of the IFX originator and CT-P13 groups achieved clinical remission at 1 year, respectively (P > 0.05). In line with previous studies, the present study did not find significant differences in the disease activity scores and inflammatory marker levels at 1-year follow-up. In addition, our study showed that a significant proportion of pediatric patients with IBD maintained their CSFR after 1 year of treatment (88.6% (180/203) of patients with CD and 70.1% (47/67) of patients with UC), with no difference between the IFX originator and CT-P13-treated groups. Our results support that CT-P13 has therapeutic efficacy equivalent to the IFX originator in pediatric patients with IBD.
With an expanding array of therapeutic options, the goal of IBD treatment has shifted from symptom control to EH (7, 44). In the Selecting Therapeutic Targets in Inflammatory Bowel Disease-II study, EH is accepted as a long-term prognostic target for IBD treatment and reduces the risk of bowel damage (44, 45). Therefore, the difference in achieving EH and clinical remission in patients with IBD treated with IFX originator and CT-P13 must be evaluated. This study is significant in that it is the first to demonstrate the lack of differences between the two agents in achieving EH and drug retention rate in pediatric patients with IBD.
Current available literature data revealed no substantial difference in trough levels between the IFX originator and CT-P13 groups (15, 24, 43, 46). Serum IFX trough concentration is an important factor that influences the achievement of EH (odds ratio 1.479, 95% confidence interval 1.176–1.860, P < 0.001). In the light of these results, the lack of difference in EH achievement at 1 year of treatment between the IFX originator and CT-P13 arms is consistent. In the present study, EH was achieved in 73.1% of the patients in the IFX originator vs. 75.4% in the CT-P13 group (P = 0.902) in patients with CD and 63.6% in the IFX originator vs. 70.6% in the CT-P13 group (P = 0.860) in patients with UC/IBD-U. In addition, the MINI index was proposed by Martinus et al., a tool for the non-invasive assessment of mucosal inflammation in pediatric patients with CD using stool, FC, ESR and CRP (27). The study demonstrated that 78% of patients with MINI index scores <8 achieved EH, and scores of <6 had a positive predictive value of 86% for EH. In the CD cohort of our study, both the IFX and CT-P13 groups had a median MINI index of 5 at 1 year, with no difference between the two groups. This study was the first to demonstrate the lack of difference in the MINI index between the IFX originator and CT-P13 groups.
In the present study, CT-P13 is not inferior to the IFX originator in terms of IFX durability. According to the Kaplan–Meier curve, no significant difference was found in the estimated cumulative cessation rate between the IFX originator and CT-P13 groups. The durability rates of IFX were 88.2% at a median follow-up of 1.4 years in CD and 73.4% at a median follow-up of 1.85 years in UC. In patients with CD, the drug retention rates were 66.1% in the IFX originator and 71.6% in the CT-P13 group at the maximum follow-up period (P > 0.05). In patients with UC, the drug retention rate was 49.8% in the IFX originator and 56.3% in the CT-P13 group at the maximum follow-up period (P > 0.05). In addition, the proportions of patients with CD who remained on IFX maintenance therapy for 1 year were 93.7% in the IFX originator group and 94.4% in the CT-P13 group, and 84.8% and 90.2%, respectively, in patients with UC. A previous study in pediatric patients with IBD reported no statistically significant difference in the proportion of patients on maintenance therapy at 1 year: 65% for the IFX originator and 61.1% for the CT-P13 group (46).
The retention rate of treatment with biologic agents, or the probability of treatment persistence with the same biologic agents provides an index of overall drug effectiveness, patient satisfaction and treatment compliance. Our results were consistent with those of a previous study, revealing no difference in IFX drug retention rate between the IFX originator and CT-P13 groups (P > 0.05); however, the IFX durability rate was higher in our study than in the previous study. One possible explanation for the discrepancy between the results of the present study and of the former study is the higher rate of combination therapy with immunomodulators in the present study (47–50). In the present study, the proportions of combination therapy were 72.2% (184/255) in the IFX originator group and 82.6% (133/161) in the CT-P13 group, compared with 34.8% (8/23) and 53.6% (15/28), respectively, in the previous study. Furthermore, a large proportion of the patients in the present study had an early initiation of IFX strategy (49). In the present study, the median times from diagnosis to IFX initiation were 0.27 years in the IFX originator group and 0.23 years in the CT-P13 group for patients with CD and 0.77 years and 0.73 years for patients with UC, respectively.
The retrospective design of this study is a major limitation because it introduces the potential for confounding factors and bias that may affect the study results. However, all patients visited the outpatient clinic at regular intervals, allowing for a consistent clinical assessment of the therapeutic effects and adverse event monitoring. Second, laboratory results for trough concentrations and immunogenicity, which renders it possible to predict clinical outcomes and identify the cause of insufficient clinical response, were lacking. However, as mentioned above, previous studies have demonstrated the statistical similarity of the pharmacokinetics of the IFX originator and CT-P13; thus, it is unlikely to have affected our results. Third, our data were hospital-based rather than population-based; therefore, patients with a higher severity were included in the cohort, and a referral center bias cannot be excluded. However, most pediatric patients with IBD in Korea are referred to IBD specialists at university hospitals. Therefore, our data may be representative of the characteristics of pediatric patients with IBD in Korea. Despite these limitations, our data provide meaningful information that reflects the experience of long-term treatment with IFX originator and CT-P13 in a large, real-world cohort of pediatric patients with IBD.
In conclusion, the IFX originator and its biosimilar CT-P13 exhibited comparable therapeutic response including EH and clinical remission, durability and safety in pediatric patients with IBD. Our results are significant in that this is the first study to directly compare clinical outcomes, including EH at 1 year after treatment in pediatric patients with IBD treated with an IFX originator and CT-P13.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Kyungpook National University Chilgok Hospital and all other participating centers. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
ESK: Data curation, Formal analysis, Investigation, Visualization, Writing – original draft. SC: Data curation, Formal analysis, Investigation, Writing – original draft. B-HC: Data curation, Writing – review & editing. SP: Data curation, Writing – review & editing. YJL: Data curation, Writing – review & editing. SJS: Data curation, Writing – review & editing. SCK: Data curation, Writing – review & editing. KSK: Data curation, Writing – review & editing. KL: Data curation, Writing – review & editing. JOS: Data curation, Writing – review & editing. YBK: Data curation, Writing – review & editing. SJH: Data curation, Writing – review & editing. YML: Data curation, Writing – review & editing. HJK: Data curation, Writing – review & editing. SYC: Data curation, Writing – review & editing. JuYK: Data curation, Writing – review & editing. YL: Data curation, Writing – review & editing. J-SP: Data curation, Writing – review & editing. JaYK: Data curation, Writing – review & editing. DYY: Data curation, Writing – review & editing. JHL: Data curation, Writing – review & editing. K-HC: Data curation, Writing – review & editing. H-JJ: Data curation, Writing – review & editing. ISJ: Data curation, Writing – review & editing. BK: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2021R1A2C1011004).
Conflict of interest
For the past 3 years, BK has served as a speaker for Celltrion, Janssen, Abbvie, Takeda, Yuhan and Samsung Bioepis and has received research funding from Celltrion.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rosen MJ, Dhawan A, Saeed SA. Inflammatory bowel disease in children and adolescents. JAMA Pediatr (2015) 169:1053–60. doi: 10.1001/jamapediatrics.2015.1982
2. Levine A, Koletzko S, Turner D, Escher JC, Cucchiara S, de Ridder L, et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr (2014) 58:795–806. doi: 10.1097/MPG.0000000000000239
3. Walters TD, Kim MO, Denson LA, Griffiths AM, Dubinsky M, Markowitz J, et al. Increased effectiveness of early therapy with anti-tumor necrosis factor-α vs an immunomodulator in children with Crohn's disease. Gastroenterology (2014) 146:383–91. doi: 10.1053/j.gastro.2013.10.027
4. Hyams J, Crandall W, Kugathasan S, Griffiths A, Olson A, Johanns J, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn's disease in children. Gastroenterology (2007) 132:863–73. doi: 10.1053/j.gastro.2006.12.003
5. Kang B, Choi SY, Kim HS, Kim K, Lee YM, Choe YH. Mucosal healing in pediatric patients with moderate-to-severe luminal Crohn's disease under combined immunosuppression: escalation versus early treatment. J Crohns Colitis (2016) 10:1279–86. doi: 10.1093/ecco-jcc/jjw086
6. Kang B, Choe YH. Early biologic treatment in pediatric Crohn's disease: catching the therapeutic window of opportunity in early disease by treat-to-target. Pediatr Gastroenterol Hepatol Nutr (2018) 21:1–11. doi: 10.5223/pghn.2018.21.1.1
7. van Rheenen PF, Aloi M, Assa A, Bronsky J, Escher JC, Fagerberg UL, et al. The medical management of pediatric Crohn's disease: an ECCO-ESPGHAN guideline update. J Crohns Colitis (2020). doi: 10.1093/ecco-jcc/jjaa161
8. Turner D, Ruemmele FM, Orlanski-Meyer E, Griffiths AM, de Carpi JM, Bronsky J, et al. Management of pediatric ulcerative colitis, part 1: ambulatory care-an evidence-based guideline from European Crohn's and colitis organization and European society of pediatric gastroenterology, hepatology and nutrition. J Pediatr Gastroenterol Nutr (2018) 67:257–91. doi: 10.1097/MPG.0000000000002035
9. Jeuring SF, van den Heuvel TR, Liu LY, Zeegers MP, Hameeteman WH, Romberg-Camps MJ, et al. Improvements in the long-term outcome of Crohn's disease over the past two decades and the relation to changes in medical management: results from the population-based IBDSL cohort. Am J Gastroenterol (2017) 112:325–36. doi: 10.1038/ajg.2016.524
10. Kang B, Choi SY, Choi YO, Lee SY, Baek SY, Sohn I, et al. Infliximab trough levels are associated with mucosal healing during maintenance treatment with infliximab in pediatric Crohn's disease. J Crohns Colitis (2019) 13:189–97. doi: 10.1093/ecco-jcc/jjy155
11. Organization site European Medicines Agency. Committee for medicinal products for human use (CHMP). Assessment report: Inflectra (infliximab) (2013). Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002778/WC500151490.pdf (Accessed August 3,2018).
12. Organization site World Health Organization. Expert Committee on Biological Standardization Geneva, 17 to 20 October 2017. Report on a Collaborative Study for Proposed 1st International Standard for Infliximab (2017). Available at: http://www.who.int/biologicals/expert_committee/BS2323_Infliximab_ECBS_2017_V.6.1.pdf (Accessed August 3, 2018).
13. Mantzaris GJ. Anti-TNFs: originators and biosimilars. Dig Dis (2016) 34:132–9. doi: 10.1159/000443128
14. Bergqvist V, Kadivar M, Molin D, Angelison L, Hammarlund P, Olin M, et al. Switching from originator infliximab to the biosimilar CT-P13 in 313 patients with inflammatory bowel disease. Therap Adv Gastroenterol (2018) 11:1756284818801244. doi: 10.1177/1756284818801244
15. Kang B, Lee Y, Lee K, Choi YO, Choe YH. Long-term outcomes after switching to CT-P13 in pediatric-onset inflammatory bowel disease: A single-center prospective observational study. Inflammation Bowel Dis (2018) 24:607–16. doi: 10.1093/ibd/izx047
16. Goll GL, Jørgensen KK, Sexton J, Olsen IC, Bolstad N, Haavardsholm EA, et al. Long-term efficacy and safety of biosimilar infliximab (CT-P13) after switching from originator infliximab: open-label extension of the NOR-SWITCH trial. J Intern Med (2019) 285:653–69. doi: 10.1111/joim.12880
17. van Hoeve K, Dreesen E, Hoffman I, Van Assche G, Ferrante M, Gils A, et al. Efficacy, pharmacokinetics, and immunogenicity is not affected by switching from infliximab originator to a biosimilar in pediatric patients with inflammatory bowel disease. Ther Drug Monit (2019) 41:317–24. doi: 10.1097/FTD.0000000000000601
18. Ye BD, Pesegova M, Alexeeva O, Osipenko M, Lahat A, Dorofeyev A, et al. Efficacy and safety of biosimilar CT-P13 compared with originator infliximab in patients with active Crohn's disease: an international, randomised, double-blind, phase 3 non-inferiority study. Lancet (2019) 393:1699–707. doi: 10.1016/S0140-6736(18)32196-2
19. Meyer A, Rudant J, Drouin J, Coste J, Carbonnel F, Weill A. The effectiveness and safety of infliximab compared with biosimilar CT-P13, in 3112 patients with ulcerative colitis. Aliment Pharmacol Ther (2019) 50:269–77. doi: 10.1111/apt.15323
20. Ben-Horin S, Vande Casteele N, Schreiber S, Lakatos PL. Biosimilars in inflammatory bowel disease: facts and fears of extrapolation. Clin Gastroenterol Hepatol (2016) 14:1685–96. doi: 10.1016/j.cgh.2016.05.023
21. Dipasquale V, Pellegrino S, Ventimiglia M, Cucinotta U, Citrano M, Graziano F, et al. Real-life experience of infliximab biosimilar in pediatric-onset inflammatory bowel disease: data from the Sicilian Network for Inflammatory Bowel Disease. Eur J Gastroenterol Hepatol (2022) 34:1007–14. doi: 10.1097/MEG.0000000000002408
22. Gervais L, McLean LL, Wilson ML, Cameron C, Curtis L, Garrick V, et al. Switching from originator to biosimilar infliximab in paediatric inflammatory bowel disease is feasible and uneventful. J Pediatr Gastroenterol Nutr (2018) 67:745–8. doi: 10.1097/MPG.0000000000002091
23. Chanchlani N, Mortier K, Williams LJ, Muhammed R, Auth MKH, Cosgrove M, et al. Use of infliximab biosimilar versus originator in a pediatric United Kingdom inflammatory bowel disease induction cohort. J Pediatr Gastroenterol Nutr (2018) 67:513–9. doi: 10.1097/MPG.0000000000002011
24. Sieczkowska J, Jarzębicka D, Banaszkiewicz A, Plocek A, Gawronska A, Toporowska-Kowalska E, et al. Switching between infliximab originator and biosimilar in paediatric patients with inflammatory bowel disease. Preliminary Observations. J Crohns Colitis (2016) 10(2):127–32. doi: 10.1093/ecco-jcc/jjv233
25. McClinchie MG, Lakhani A, Abdel-Rasoul M, McNicol M, Shkhkhalil AK, Boyle BB, et al. Similar growth outcomes in children with inflammatory bowel disease initiated on infliximab originator or biosimilar. J Pediatr Gastroenterol Nutr (2023) 77:499–504. doi: 10.1097/MPG.0000000000003890
26. Levine A, Griffiths A, Markowitz J, Wilson DC, Turner D, Russell RK, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflammation Bowel Dis (2011) 17:1314–21. doi: 10.1002/ibd.21493
27. Cozijnsen MA, Ben Shoham A, Kang B, Choe BH, Choe YH, Jongsma MME, et al. Development and validation of the mucosal inflammation noninvasive index for pediatric Crohn's disease. Clin Gastroenterol Hepatol (2020) 18:133–40.e1. doi: 10.1016/j.cgh.2019.04.012
28. Ruemmele FM, Veres G, Kolho KL, Griffiths A, Levine A, Escher JC, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J Crohns Colitis (2014) 8:1179–207. doi: 10.1016/j.crohns.2014.04.005
29. Turner D, Levine A, Escher JC, Griffiths AM, Russell RK, Dignass A, et al. Management of pediatric ulcerative colitis: joint ECCO and ESPGHAN evidence-based consensus guidelines. J Pediatr Gastroenterol Nutr (2012) 55:340–61. doi: 10.1097/MPG.0b013e3182662233
30. Yoo DH, Hrycaj P, Miranda P, Ramiterre E, Piotrowski M, Shevchuk S, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis (2013) 72:1613–20. doi: 10.1136/annrheumdis-2012-203090
31. Park W, Hrycaj P, Jeka S, Kovalenko V, Lysenko G, Miranda P, et al. A randomised, double-blind, multicenter, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis (2013) 72:1605–12. doi: 10.1136/annrheumdis-2012-203091
32. Komaki Y, Yamada A, Komaki F, Micic D, Ido A, Sakuraba A. Systematic review with meta-analysis: the efficacy and safety of CT-P13, a biosimilar of anti-tumour necrosis factor-alpha agent (infliximab), in inflammatory bowel diseases. Aliment Pharmacol Ther (2017) 45:1043–57. doi: 10.1111/apt.13990
33. Epstein MS, Ehrenpreis ED, Kulkarni PM. FDA-Related Matters Committee of the American College of Gastroenterology. Biosimilars: the need, the challenge, the future: the FDA perspective. Am J Gastroenterol (2014) 109:1856–9. doi: 10.1038/ajg.2014.151
34. Jha A, Upton A, Dunlop WC, Akehurst R. The budget impact of biosimilar infliximab (Remsima®) for the treatment of autoimmune diseases in five European countries. Adv Ther (2015) 32:742–56. doi: 10.1007/s12325-015-0233-1
35. Schellekens H. Bioequivalence and the immunogenicity of biopharmaceuticals. Nat Rev Drug Discovery (2002) 1:457–62. doi: 10.1038/nrd818
36. Danese S, Fiorino G, Raine T, Ferrante M, Kemp K, Kierkus J, et al. ECCO position statement on the use of biosimilars for inflammatory bowel disease-an update. J Crohns Colitis (2017) 11:26–34. doi: 10.1093/ecco-jcc/jjw198
37. de Ridder L, Assa A, Bronsky J, Romano C, Russell RK, Afzal NA, et al. Use of biosimilars in pediatric inflammatory bowel disease: an updated position statement of the pediatric IBD porto group of ESPGHAN. J Pediatr Gastroenterol Nutr (2019) 68:144–53. doi: 10.1097/MPG.0000000000002141
38. Buer LC, Moum BA, Cvancarova M, Warren DJ, Medhus AW, Høivik ML. Switching from remicade(R) to remsima(R) is well tolerated and feasible: A prospective, open-label study. J Crohns Colitis (2017) 11:297–304. doi: 10.1093/ecco-jcc/jjw166
39. Høivik ML, Buer LCT, Cvancarova M, Warren DJ, Bolstad N, Moum BA, et al. Switching from originator to biosimilar infliximab - real world data of a prospective 18 months follow-up of a single-center IBD population. Scand J Gastroenterol (2018) 53:692–9. doi: 10.1080/00365521.2018.1463391
40. Strik AS, van de Vrie W, Bloemsaat-Minekus JPJ, Nurmohamed M, Bossuyt PJJ, Bodelier A, et al. Serum concentrations after switching from originator infliximab to the biosimilar CT-P13 in patients with quiescent inflammatory bowel disease (SECURE): an open-label, multicenter, phase 4 non-inferiority trial. Lancet Gastroenterol Hepatol (2018) 3:404–12. doi: 10.1016/S2468-1253(18)30082-7
41. Jørgensen KK, Olsen IC, Goll GL, Lorentzen M, Bolstad N, Haavardsholm EA, et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet (2017) 389:2304–16. doi: 10.1016/S0140-6736(17)30068-5
42. Sieczkowska-Golub J, Meglicka M, Plocek A, Banaszkiewicz A, Jarzębicka D, Toporowska-Kowalska E, et al. Induction therapy with biosimilar infliximab in children with Crohn disease. J Pediatr Gastroenterol Nutr (2017) 65:285–8. doi: 10.1097/MPG.0000000000001643
43. Gervais L, McLean LL, Wilson ML, Cameron C, Curtis L, Garrick V, et al. Switching from originator to biosimilar infliximab in pediatric inflammatory bowel disease is feasible and uneventful. J Pediatr Gastroenterol Nutr (2018) 67:745–8. doi: 10.1097/MPG.0000000000002091
44. Turner D, Ricciuto A, Lewis A, D'Amico F, Dhaliwal J, Griffiths AM, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology (2021) 160:1570–83. doi: 10.1053/j.gastro.2020.12.031
45. Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut (2012) 61:1619–35. doi: 10.1136/gutjnl-2012-302830
46. Nikkonen A, Kolho KL. Infliximab and its biosimilar produced similar first-year therapy outcomes in patients with inflammatory bowel disease. Acta Paediatr (2020) 109:836–41. doi: 10.1111/apa.15026
47. Grossi V, Lerer T, Griffiths A, LeLeiko N, Cabrera J, Otley A, et al. Concomitant use of immunomodulators affects the durability of infliximab therapy in children with Crohn's disease. Clin Gastroenterol Hepatol (2015) 13:1748–56. doi: 10.1016/j.cgh.2015.04.010
48. Cheng J, Hamilton Z, Smyth M, Barker C, Israel D, Jacobson K. Concomitant therapy with immunomodulator enhances infliximab durability in pediatric inflammatory bowel disease. Inflammation Bowel Dis (2017) 23:1762–73. doi: 10.1097/MIB.0000000000001212
49. Zhu P, Sun JF, Gu YF, Chen HJ, Xu MM, Li YR, et al. Combined therapy with early initiation of infliximab following drainage of perianal fistulising Crohn's disease: a retrospective cohort study. BMC Gastroenterol (2022) 22:15. doi: 10.1186/s12876-021-02078-9
Keywords: children, inflammatory bowel disease, CT-P13, endoscopic healing, durability
Citation: Kim ES, Choi S, Choe B-H, Park S, Lee YJ, Sohn SJ, Kim SC, Kang KS, Lee K, Shim JO, Kim YB, Hong SJ, Lee YM, Kim HJ, Choi SY, Kim JY, Lee Y, Park J-S, Kim JY, Yi DY, Lee JH, Choi K-H, Jang H-J, Jeong IS and Kang B (2024) Comparison of endoscopic healing and durability between infliximab originator and CT-P13 in pediatric patients with inflammatory bowel disease. Front. Immunol. 15:1284181. doi: 10.3389/fimmu.2024.1284181
Received: 28 August 2023; Accepted: 25 January 2024;
Published: 22 February 2024.
Edited by:
Maria Manuela Estevinho, Centro Hospitalar de Vila Nova de Gaia, PortugalReviewed by:
Ruggiero Francavilla, University of Bari Aldo Moro, ItalyGuohui Xue, Jiujiang First People’s Hospital, China
Copyright © 2024 Kim, Choi, Choe, Park, Lee, Sohn, Kim, Kang, Lee, Shim, Kim, Hong, Lee, Kim, Choi, Kim, Lee, Park, Kim, Yi, Lee, Choi, Jang, Jeong and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ben Kang, YmVua2FuZ0BrbnUuYWMua3I=
†These authors have contributed equally to this work and share first authorship
 Eun Sil Kim
Eun Sil Kim Sujin Choi2,3†
Sujin Choi2,3† Sowon Park
Sowon Park Yeoun Joo Lee
Yeoun Joo Lee Soon Chul Kim
Soon Chul Kim Jung Ok Shim
Jung Ok Shim Yu Bin Kim
Yu Bin Kim Suk Jin Hong
Suk Jin Hong So Yoon Choi
So Yoon Choi Dae Yong Yi
Dae Yong Yi Hyo-Jeong Jang
Hyo-Jeong Jang In Sook Jeong
In Sook Jeong Ben Kang
Ben Kang