- 1Department of Dermatology, Southwest Hospital, Army Medical University, Chongqing, China
- 2School of Medicine, Chongqing University, Chongqing, China
- 3Department of Pathophysiology, Army Medical University, Chongqing, China
Chronic urticaria (CU) is one of the most common dermatological diseases and has a significant impact on the quality of life of patients. However, the pathogenesis of this disease remains unclear. Autoimmunity in chronic spontaneous urticaria (CSU) has received considerable attention and has been studied previously. Atopy is an important characteristic of CU; however, it has not been fully recognized. Atopy predisposes individuals to immune responses to allergens, leading to type 2 inflammation and immunoglobulin E (IgE) overproduction. Compared with healthy individuals, patients with CU have a higher proportion of atopy, and an atopic background is correlated with the clinical characteristics of CU. The total IgE levels in patients with CU is significantly higher than those in healthy individuals. Although its level is not higher than that in classic allergic diseases, it is closely related to CU. Exogenous allergens, auto-allergens, and specific IgEs, which are closely related to atopy, have been reported, and their roles in CU pathogenesis are also being studied. Local and systemic atopic inflammation is present in patients with CU. This review summarizes the current knowledge regarding atopy and CU, speculating that there are CU subtypes, such as atopic CSU or atopic chronic inducible urticaria (CIndU) and that atopy may be involved in the pathogenesis of CU. These findings provide a new perspective for a comprehensive understanding of the clinical features of CU and further research regarding its pathogenesis.
1 Introduction
Chronic urticaria (CU) is a pruritic skin disease defined as the occurrence of evanescent wheals, angioedema, or both for more than six weeks. It can be divided into chronic spontaneous urticaria (CSU) and chronic inducible urticaria (CIndU) (1, 2). Due to its long course and recurrent symptoms, CU imposes a great burden on patients, patient families, healthcare systems, and society as a whole (3). The pathogenesis of CU is not fully understood. Since the 1990s, several studies have focused on autoimmunity in CSU, identifying a potential autoimmune etiology in up to 50% of patients with this condition (4). However, the autoimmune theory does not fully explain the pathogenesis of CSU and is less relevant for CIndU. A large proportion of CU cases are of unknown etiology, and studies regarding the etiology and mechanism of CIndU are rare. Atopy is a predisposition to immunological responses to allergens, leading to type 2 inflammation and immunoglobulin E (IgE) overproduction (5). Patients with atopy often present with allergen-specific IgE, elevated total IgE levels, or a definite history of atopic diseases (6). Compared to other typical allergic diseases (such as atopic dermatitis (AD), allergic conjunctivitis, allergic rhinitis (AR), and asthma), atopy is often considered less important in the setting of CU. However, increasing evidence has shown that atopy is a predisposing factor for CU. Targeting free IgE and its receptor with antibodies provides an effective treatment for CSU and most subtypes of CIndU (7), resulting in a strong association between atopy and CU. This review focuses on the research progress of atopy in the clinical aspects, etiology, and pathophysiology of CU.
2 High prevalence of atopy in CU
Individuals with atopy frequently have one or more atopic disorders, such as AR, asthma, AD, and allergic conjunctivitis. Patients with CU commonly present with atopic diseases. Nassif (8) reported that 82 of 85 patients with CU had a personal and/or familial atopic status (asthma, eczema, or AR/allergic conjunctivitis history), suggesting that CU may be part of the atopic diathesis, in addition to asthma, eczema, and AR/allergic conjunctivitis. To further reveal the relationship between atopic disorders and CU, Shalom et al. (9) conducted a large cross-sectional study of 11,271 patients with CU and 67,216 controls, revealing that 19.9%, 10.8%, and 9.8% of patients with CU had AR, asthma, and AD, respectively, compared to 10.1%, 6.5%, and 3.7% of controls (all significantly different). Another population-based retrospective cohort study from Taiwan included 9,332 patients with CU and 37,328 controls, and reported that CU was significantly associated with AD and AR (10). Similarly, a study of 1,108,833 adolescents from Israel found that individuals with CSU were significantly more likely to have allergic diseases, including food allergy (odds ratio (OR): 7.31, 95% confidence interval (CI): 6.13-8.72), AR (OR: 2.9, 95% CI: 2.71-3.11), AD (OR: 2.35, 95% CI: 2.03-2.72), and asthma (OR: 1.46, 95% CI: 1.35-1.57) (11). Kitsioulis et al. reported that children with a history of an early diagnosis of AD were at an increased risk of later CSU occurrence (OR: 2.923, 95% CI: 1.647-5.189, p < 0.001) (12). Taken together, these studies suggest a significant association between CU and atopic diseases.
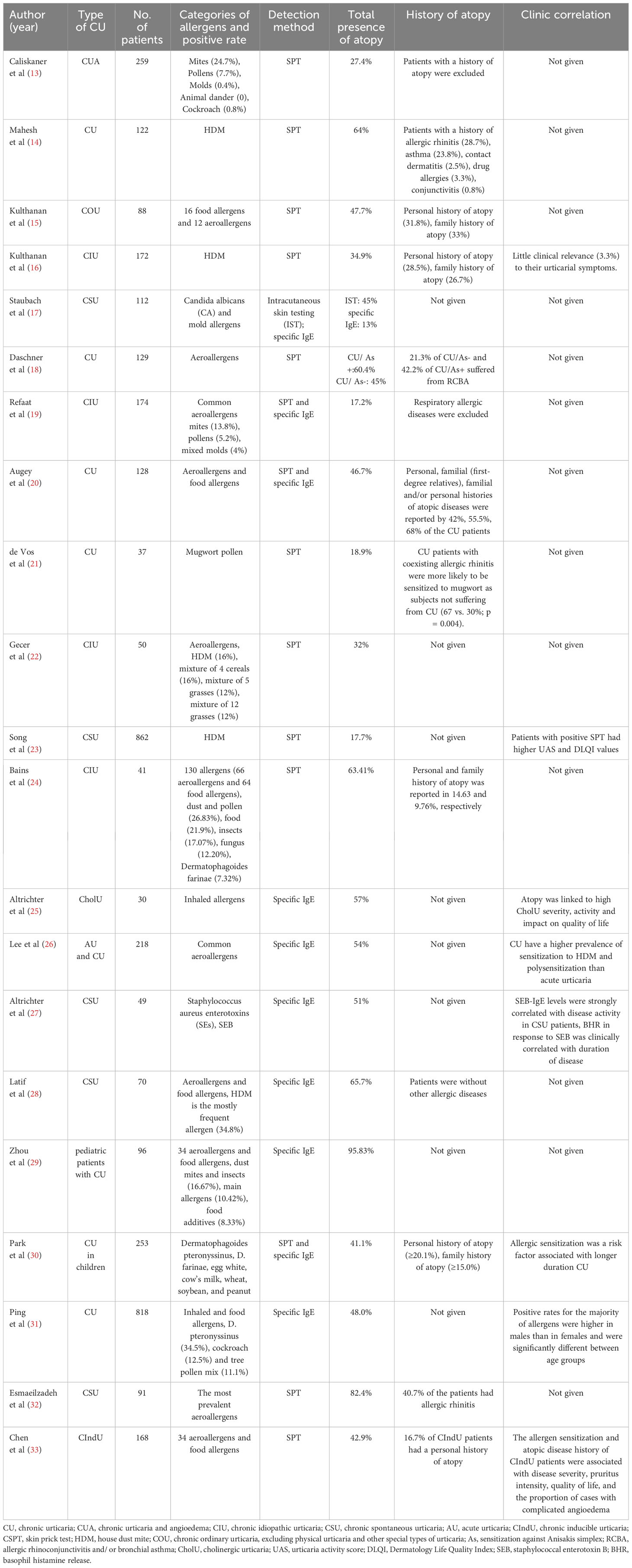
In addition to the concomitant presence of atopic diseases, sensitization to allergen-specific IgE, which is an important indicator of atopy, is also a concern in patients with CU. The positive sensitization rate of patients with CU to various allergens according to the skin prick test (SPT) or serum-specific IgE test ranged from 17.2–95.83% (Table 1), which was significantly higher than that of healthy individuals (13–33). Among the allergens identified in patients with CU, house dust mites (HDM) are often the most common (13, 14, 16, 26, 29), followed by weed (mugwort and ragweed) and tree pollen, cereal pollen, cockroaches, weeds, cat hair, rat, and Candida albicans and mixed molds (17, 19, 21, 22, 31, 32). In addition, an association between CU and Anisakis simplex (As) sensitization has been reported, and the sensitization rates to pollen, dander, and mold in patients with CU with sensitization to As (CU/As+) were significantly higher than those in patients without As sensitization (CU/As-) (18). Multiple allergen sensitization is more common than single allergen sensitization in patients with CU, and sensitization to one allergen in patients with CU may lead to subsequent sensitization to other allergens (18, 34).
The rates of allergen sensitization were significantly higher in patients with CU, regardless of the presence or absence of a history of atopy (14, 21). Among patients with CU without comorbid atopic disorders, the proportion of patients sensitized to exogenous allergens is high. Caliskaner et al. (13) reported that 27.4% of patients with CU without AR and/or asthma are sensitized to one or more inhaled allergens, which is significantly higher than that in healthy controls (7%). In addition, there are differences in the prevalence of atopy among the different CU subtypes. Cholinergic urticaria (CholU) and cold urticaria have higher rates of atopic predisposition, with the highest reported rates of 57% and 89.3%, respectively (25, 35, 36). In a recent atopy analysis of CIndU subtypes, including CholU, symptomatic dermatographism, cold contact urticaria, and heat contact urticaria, no significant differences in the SPT-positive rates were observed between these four subtypes, though CholU had the highest rate of atopic history (57.1%) (33). No comparative studies regarding the proportions of CholU and CSU atopy have been reported, and the prevalence of atopy in other CU subtypes requires further investigation.
3 Clinical relevance of allergen sensitization to CU
Sensitization to allergens is a hallmark of atopy; however, its clinical relevance to CU has not been adequately studied, and the existing findings are controversial. As previous studies have found that positive allergens are not typically associated with CU symptoms and avoidance of these allergens does not prevent recurrence, CU is not considered an allergic disease (20).
However, this may not be accurate. Recurrent urticaria associated with dietary allergies is frequently diagnosed as CU, and previous studies have shown that IgE sensitization via wheat and barley allergens is a causative factor for CU (37, 38). Allergen immunotherapy, a treatment that induces tolerance to specific allergens, has been reported to relieve urticaria and respiratory symptoms in patients with concomitant CU and respiratory allergies (39, 40). In contrast, the exacerbation of urticaria symptoms during HDM immunotherapy for CU has been reported (41). Acetylsalicylic acid sensitization has been reported to cause CU via desensitization (42). These reports suggest that allergens may be causative or deteriorating factors in patients with CU.
Increasing evidence suggests that allergen sensitization is clinically relevant to CU. As summarized in Table 1, Kulthanan et al. reported clinical correlations between allergen sensitization and urticaria symptoms (16), and Song et al. (23) reported more conclusive evidence. This study investigated the correlation between HDM IgE sensitization and clinical status in 862 patients with CSU and reported that the disease activity of patients with HDM sensitization was significantly higher than that of non-sensitized patients. Furthermore, 23 patients with strong sensitization to HDM had significantly aggravated urticaria symptoms when they continued to live in a room with high HDM density, while their symptoms significantly improved when the HDM exposure was avoided (23). Altrichter et al. (27) reported that levels of staphylococcal enterotoxin B (SEB)-specific IgE were strongly associated with disease activity in patients with CSU, while the degree of basophil histamine release (BHR) induced by SEB was clinically correlated with disease duration. In addition, a retrospective cohort study including 4,552 patients with CU reported that HDM sensitization is an important factor affecting the remission of CU (43). Another Korean study confirmed that children sensitized to common inhaled and food allergens had a longer natural course of CU (30), indicating that allergen sensitization may be related to the poor prognosis of CU. In a recent study regarding atopy and CIndU, patients with CIndU and allergen sensitization or a history of atopic disease had a longer natural history than those non-atopic individuals, though the difference was not significant (33).
The distribution of allergen sensitization in patients with urticaria appears to be related to sex. Kulthanan et al. (16) reported that HDM sensitization was more common in male patients with CU than in female patients, and Ping et al. demonstrated the same sex distribution difference in atopy in patients with CU in a larger sample study (31). CU and acute urticaria had different allergen sensitization spectra; however, the reason for this phenomenon remains unclear (31). Some CU subtypes are more closely associated with atopy. Patients with an atopic predisposition had higher CholU severity, activity, and impact on quality of life, as well as different comorbidity profiles and seasonal exacerbation patterns compared to patients without an atopic predisposition (25). In addition, sweat allergy, a type I hypersensitivity reaction to sweat components, is observed in patients with CholU and AD (44). Sweat allergy is closely related to the clinical manifestations and treatment response of CholU and is a key factor in the CholU classification (45).
4 High total IgE and its clinical relevance in CU
Atopy is the familial or individual tendency to overproduce IgE antibodies. Therefore, elevated serum total IgE levels are generally considered important markers of atopy (46). Elevated total serum IgE levels in patients with CU were first reported by Greaves et al. approximately 50 years ago (47). Subsequently, an increasing number of studies have confirmed this phenomenon (48). A high proportion (18-82%) of patients with CSU have elevated serum total IgE levels, though these levels were lower than those in patients with classic allergic diseases, such as AD (48–50). The IgE level of patients with CSU and AD are significantly higher than those of patients with CSU without AD (51). In addition, another study reported that the expression of IgE high-affinity receptors (FcϵRI) on basophils of patients with CIndU, regardless of the subtype, is significantly increased and correlated with the response to omalizumab treatment (52). Although it was not observed that the total serum IgE level was significantly higher in patients with CIndU than in healthy individuals, the total serum IgE level was higher in patients resistant to antihistamines (52). More studies regarding the total serum IgE levels in patients with CIndU and its subtypes are needed.
The total IgE levels vary considerably among different types of CSU. Autoimmunity plays an important role in the pathogenesis of CSU, which is divided into autoimmune CSU (aiCSU) and non-autoimmune CSU. aiCSU can be further divided into type I (also known as auto-allergic CSU) and type IIb aiCSU (53). The presence of auto-IgE is a hallmark of auto-allergic CSU, and studies have shown that the total IgE level and the proportion of atopy in patients with CSU and auto-IgE are significantly higher than those patients with CSU without auto-IgE (54–56). In contrast, patients with type IIb aiCSU typically have very low levels of total IgE and often have a positive autologous serum skin test (ASST) or positive thyroid autoantibodies (57). Some patients have positive ASST and SPT, though the total IgE level in these patients was lower than that in patients with negative ASST and positive SPT (23).
The correlation between the total IgE level and the disease characteristics of patients with CSU is unclear. Elevated serum total IgE levels in patients with CSU are positively correlated with disease severity and the natural course of the disease (49). Choi et al. reported that total IgE levels are significantly and positively correlated with the Urticaria Activity Score (UAS) and Chronic Urticaria Quality of Life Questionnaire score (58). However, a cluster study reported no significant correlation between serum total IgE levels and CU remission or relapse (43). This association may differ in different patient populations. Kocaturk et al. reported that symptoms were more severe in pregnant patients with CSU with low total IgE levels (59). Although patients with type IIb aiCSU usually have low total IgE levels, they are commonly characterized by a high degree of disease severity (60). Therefore, the relationship between total IgE levels and the severity of CSU disease is controversial and only consistent with specific circumstances.
The relationship between total serum IgE levels and treatment response in patients with CSU is of great interest. Previous studies have not found a correlation between serum total IgE levels and response to antihistamines in patients with CSU (51, 61). However, patients with CIndU with higher total IgE levels are resistant to antihistamine treatment (52). The total IgE levels are associated with cyclosporine treatment outcomes, as patients with high total IgE levels have a poor response to cyclosporine treatment (62). The relationship between total IgE levels and omalizumab treatment has been widely reported, and includes treatment response (63), onset time (64), and relapse after drug withdrawal (65). Overall, the total IgE level can be used as a reference for administering omalizumab or cyclosporine for the treatment of refractory CSU as a high level of total IgE predicts that the patient is likely to respond well to omalizumab and be resistant to cyclosporine.
Free IgE cannot be distinguished from complex IgE in the circulation using conventional methods (66). Serum-free IgE levels are more predictive of atopic status than total IgE levels (46). Jang et al. (67) recently developed a novel assay to measure serum-free IgE levels in patients with CSU. Patients with CSU with atopy had significantly higher serum free IgE levels than those without atopy (67), while no associations were noted with UAS, urticaria duration, or response to omalizumab. However, patients with elevated levels of Der p-specific IgE and its ratio to the level of serum total free IgE have favorable responses to omalizumab (67). Therefore, the clinical relationship between serum-free IgE levels and CSU requires further confirmation.
5 Exogenous allergens and auto-allergens in CU
Allergen sensitization is one of the most prominent signs of atopy. Similar to classic type I hypersensitivity involving exogenous allergens, mechanisms of mast cell (MC) activation and degranulation may also be present in the setting of CU. Specific targets of IgE-induced MC degranulation in patients with CU may be exogenous or endogenous.
IgE targeting classic exogenous allergens, such as aeroallergens and food allergens, has been detected in some patients with CSU; however, its pathogenic function remains unclear and controversial (15, 21, 68). Most exogenous allergen exposures do not directly induce CU symptoms, and specific IgE levels are not believed to be related to CU symptoms. However, Altrichter et al. reported that specific IgE against SEB are common and functional in patients with CSU, as determined using the BHR test (27). However, the proportion of histamine release induced by SEB-specific IgE among the total amount of histamine released in patients with CSU is very low and not enough to induce the occurrence and recurrence of CSU symptoms, suggesting that either the occurrence of CSU requires the simultaneous stimulation of multiple exogenous allergen-specific IgEs or that these specific IgEs trigger inflammation by acting on inflammatory cells other than MCs (69, 70). In addition, more than 50% of the patients with CU tested positive for nasal Staphylococcus aureus, which may be the underlying cause of SEB sensitization (71, 72). Overall, more research regarding the involvement of exogenous allergens in the pathogenesis of CU is necessary.
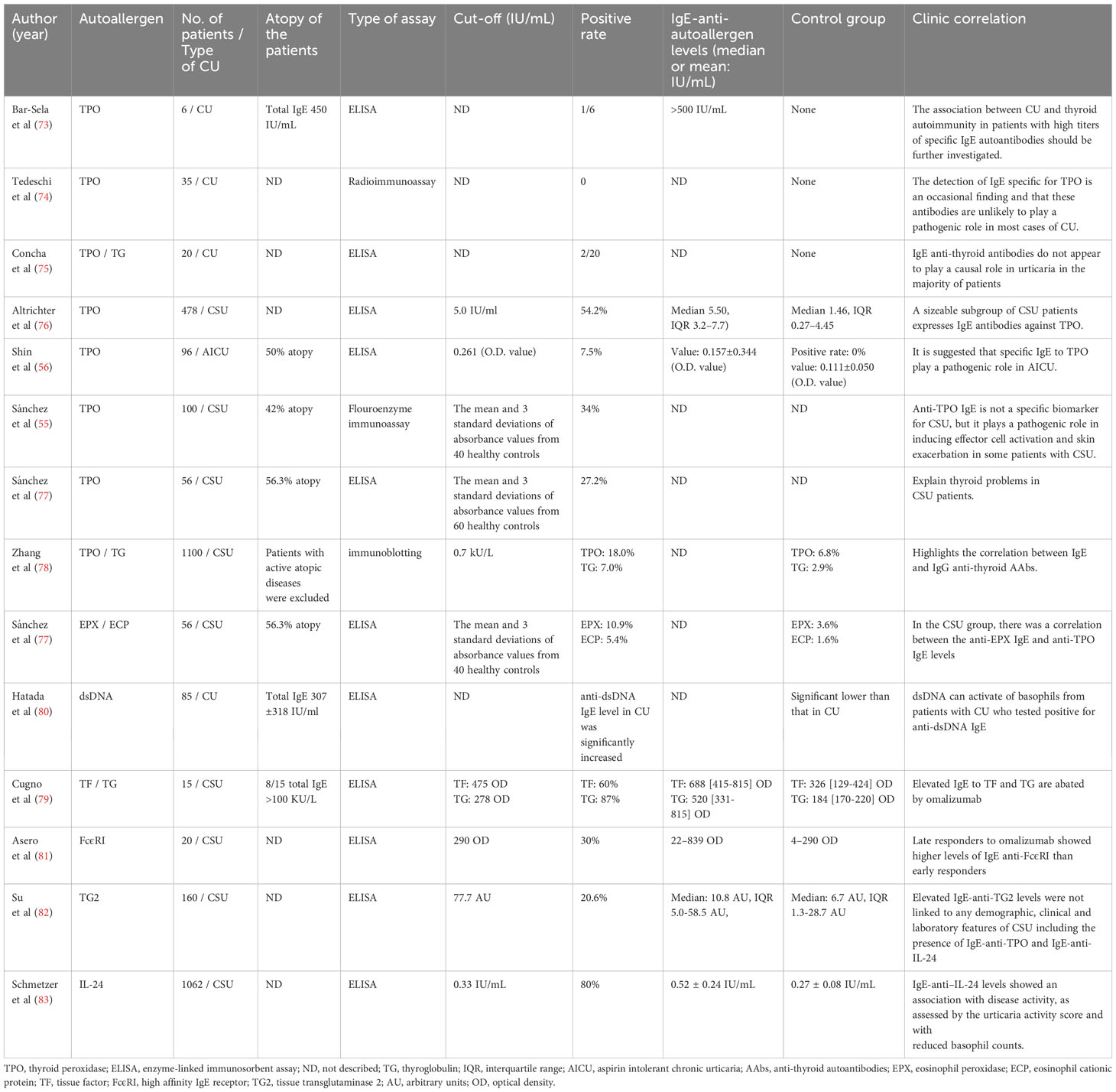
Auto-allergens, also known as endogenous allergens, have attracted increasing attention in research regarding the pathogenesis of CU. Bar-Sela et al. detected IgE autoantibodies against thyroid peroxidase (TPO) in patients with CU (73), and several other studies have detected IgE-type antithyroid autoantibodies (AAbs), including anti-TPO IgE and anti-thyroglobulin IgE, in different populations and races of patients with CSU (55, 56, 74–79). In 2019, Sanchez et al. confirmed the pathogenic role of anti-TPO IgE in patients with CSU using in vitro and in vivo tests (55). Sanchez et al. reported that peripheral blood basophil CD203c expression was significantly increased in patients with anti-TPO IgE-positive CSU after exposure to TPO as well as significant positive responses in intradermal and SPT with TPO (55). Urticarial wheals are also induced by transferring serum containing anti-TPO IgE to healthy controls (55). A study of patients with urticaria intolerant to aspirin reported similar results (56). In addition to thyroid-related autoantigens and IgE, more autoantigens and specific IgEs associated with CU have been identified (Table 2). Eosinophil cationic protein and eosinophil peroxidase (EPX)-specific IgE antibodies have been observed in patients with severe CSU (77). The extracellular domain of TPO shares approximately 45% similarity with the myeloperoxidase of eosinophils (84). An IgE cross-reaction between EPX and TPO and IgE sensitization to EPX preceded sensitization to TPO (77). Kashiwakura et al. reported that the anti-dsDNA IgE levels in patients with CU were significantly higher than those in healthy individuals, and its role in the pathogenesis of CU was confirmed by the basophil activation test (80). Cugno et al. first reported that tissue factor (TF)-specific IgE antibodies were elevated in patients with CSU and that these antibodies could functionally mediate the release of leukotriene C4 (LTC4) from peripheral blood basophils upon TF stimulation (79). Asero et al. detected the levels of IgE autoantibodies to FcϵRI exceeding the upper normal limit in six patients (30%) via sandwich enzyme-linked immunosorbent assay. Therefore, FcϵRI may be a novel auto-allergen in patients with CSU (81). Most recently, Su et al. reported that one in five patients with CSU has serum IgE specific for tissue transglutaminase 2 (TG2), an autoantigen. Functional assays were used to confirm that TG2- and TG2-specific IgE can induce the activation and degranulation of human skin MCs (82). In contrast to other studies, Schmetzer et al. detected over 200 autoantigens recognized by IgE in the serum of patients with CSU using microarray analyses, of which eight autoantigens were accessible in the skin, including IL-24. Among the autoantigen-specific IgE antibodies, IL-24 has the highest level of specific IgE in the serum (83) and can induce the degranulation of sensitized MCs from the serum of patients with CSU, but not in MCs incubated with serum from healthy control patients (83). As an increasing number of autoantigens and corresponding IgEs have been identified, their clinical value in CU has attracted attention. Although anti-TPO IgE is not a specific biomarker for CSU, it plays a pathogenic role in inducing effector cell activation and skin exacerbation in some patients with CSU (55). Elevated IgE anti-FcϵRI in patients with CSU may be associated with late-and non-responses to omalizumab treatment (81, 85), which serve as predictors of response to omalizumab. Anti–IL-24 IgE levels are associated with disease activity (83). However, other studies have reported no clinical relevance of autoantigens or the corresponding IgE levels. Therefore, additional studies are needed to identify the clinical value of autoantigens and their corresponding IgEs in patients with CU.
Few studies regarding the roles of exogenous or endogenous allergens in patients with CIndU have been reported. Hide et al. initially reported that more than half of the basophils from patients with CholU had increased histamine release when stimulated with semi-purified sweat antigens, though basophils from healthy individuals did not (86). They further found that the serum levels of specific IgE against MGL_1304, the major allergen in human sweat, were significantly higher in patients with CholU than in healthy controls (87). MGL_1304 is secreted by Malassezia globosa and induces the release of histamine from human basophils (88). Auto-allergy is considered an important mechanism of CIndU. In some patients, such as those with solar urticaria, relevant autoantigens have been identified and characterized using IgE (89). In addition, some factors of CIndU, including those associated with cold contact urticaria, symptomatic dermographism, and solar urticaria, are transferable, and the transferable factors in the serum may be specific IgE against a currently unknown auto-allergen (90–92). In CIndU, an appropriate trigger may lead to the production of auto-allergens that increase specific IgE levels, resulting in MC degranulation (93).
6 IgE in the pathogenesis of CU
The pathogenesis of CU remains unclear. IgE involved in atopy in patients with CU includes endogenous and exogenous allergen-specific IgE and elevated total IgE (or free IgE) levels. Exogenous allergens, auto-allergens, and their specific IgEs are mainly involved in the pathogenesis of CU through classic type I hypersensitivity (94). IgE is traditionally believed to induce MC sensitization rather than activation. However, basophil CD63 expression was significantly elevated in patients with CSU sensitized to allergen-specific IgE, even in the absence of allergen exposure (95). In addition, several studies conducted in the past twenty years have reported that high concentrations of IgE or sensitization with IgE alone may induce a variety of reactions without allergens in human basophils, human MCs, human cell lines, bone marrow-derived MCs, and rat basophilic leukemia cells (RBL-2H3) (96–101). These findings provide new insights regarding the mechanism underlying atopy observed in patients with CU.
Several mechanisms of atopy in patients with CU have been proposed (Figure 1). First, IL-3 may play a pro-inflammatory role by enhancing the action of IgE (100). Hide et al. reported that basophil activation induced by high concentrations of IgE is enhanced by physiological concentrations of interleukin (IL)-3 (101), and IgE binding alone can positively regulate the expression of FcϵRI on the surface of MCs by inducing autocrine IL-3, promoting MC survival (102). In the second proposed mechanism, highly cytokinergic IgE (HC-IgE) directly activates MCs or basophils independent of allergens (103). Mouse NS1 hybridoma SPE-7 IgE is an anti-dinitrophenyl antibody and is the most potent HC-IgE discovered to date (104). Bax et al. reported that HC-IgE can self-crosslink to form trimers or multimers that activate MCs (103, 104). Thirdly, IgE is highly glycosylated, and its level of glycosylation has an important effect on its lipophilicity (105). IgE with high lipophilicity expresses polyreactivity to various auto-allergens including dsDNA, TG, TF, TPO, and other histamine-releasing factors, inducing MC degranulation (106). HC-IgE is also related to higher lipophilicity, promoting IgE aggregation and stacking and inducing crosslinking with FcεRI without antigen binding (103).
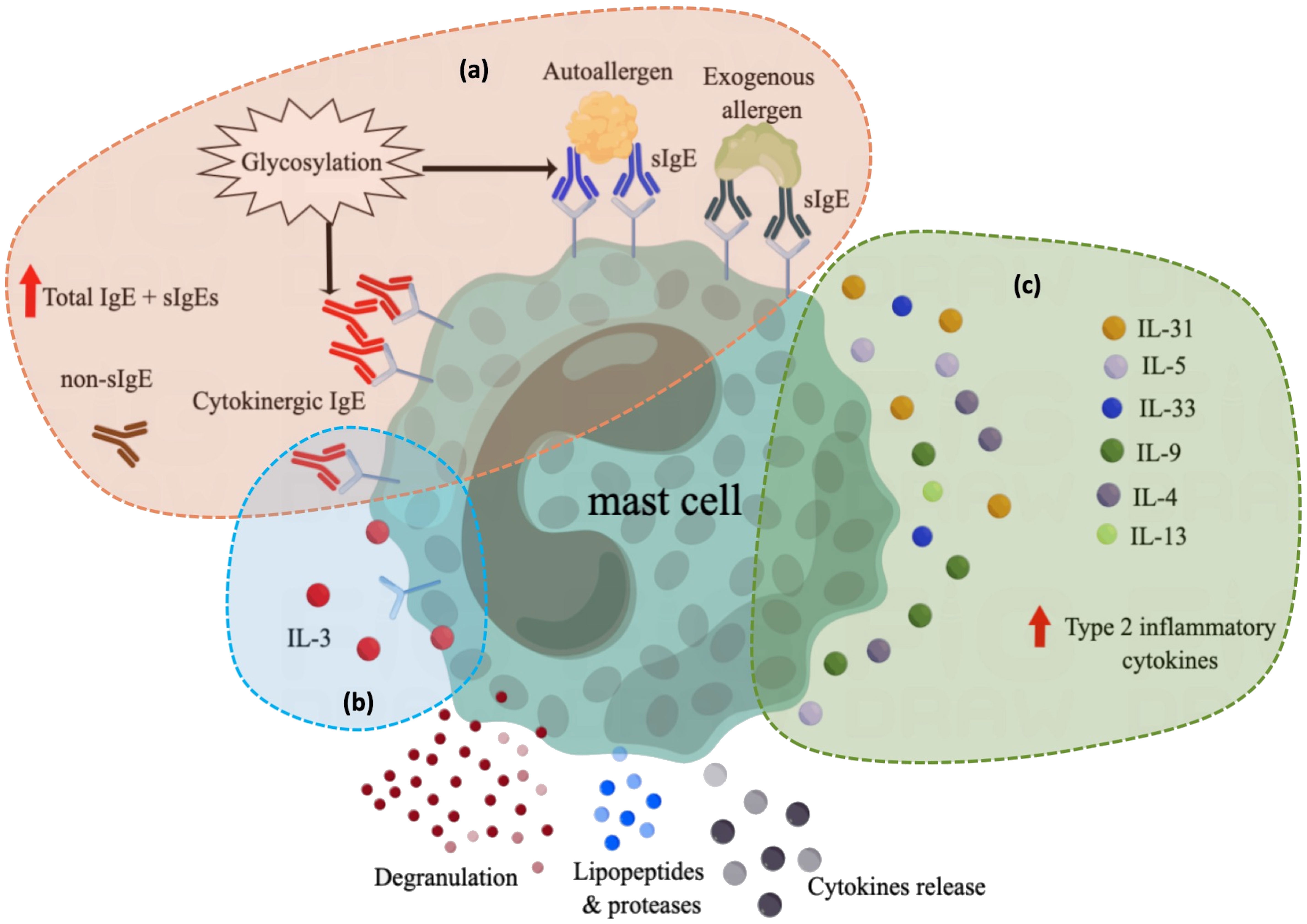
Figure 1 Overview of current understanding of the role of atopy in the pathogenesis of CU. (A) In atopic conditions, elevated total IgE levels or the production of various specific IgE induce mast cell activation through different mechanisms, including exogenous allergen-specific IgE and autoallergen-specific IgE, which can activate mast cells by binding to allergens. cytokinergic IgE can activate mast cells through self-cross-linking. Both autoallergen-specific IgE and cytokinergic IgE are modified by glycosylation, which affects their lipophilicity and regulates their binding ability. Although the function of non-specific IgE is unknown, their high levels in atopic contexts may also contribute to mast cell activation or instability. Antibody in green: specific IgE against exogenous allergens; Antibody in blue: specific IgE against autoantigens; Antibody in red: cytokinergic IgE; Antibody in brown: non-specific IgE that has not yet been identified as a specific allergen and whose function is not well defined. (B) After IgE binding alone, the autocrine IL-3 secreted by mast cells can positively regulate the survival and function of mast cells; (C) Mast cell reactivity is increased in the context of type 2 inflammation. sIgE, specific IgE for exogenous and autoallergens; non-sIgE, nonspecific IgE of unknown function.
Most previous studies have focused on the activation phenomenon and crosslinking mechanism of IgE, though studies regarding the intracellular molecular mechanism underlying HC-IgE are rare. HC-IgE induces a series of reactions observed upon IgE-mediated allergen stimulation, such as Ca2+ mobilization and phosphorylation of PI3K and MAPK, despite some differences in intensity or time course. These findings suggest that the two may share common signaling pathways (96); however, the similarities require further research. Other studies (107, 108) reported inconsistent miRNA and mRNA profiles between sensitized and activated MCs, suggesting that the intracellular signaling pathways downstream of FcϵRI may differ. Therefore, atopy in patients with elevated IgE levels or sensitization to IgE alone may affect the function and activity of MCs or basophils via multiple pathways, which may contribute to the onset and recurrence of CU symptoms.
7 Atopic inflammation in CU
Atopic inflammation, mainly type 2 inflammation, is caused by type 2 immune dysregulation, which is characterized by the expansion of Th2 cells and eosinophils and the excessive production of related cytokines, such as IL-4, IL-13, IL-5, IL-9, IL-31, and IL-33 (109). An increasing number of studies have shown that CU is not a simple histamine-mediated disease, but an inflammatory disease (110). In addition to MCs, activation of a variety of inflammatory cells and changes in a large number of inflammatory mediators are involved, and there is increasing evidence that the inflammatory response to CU is biased toward type 2 inflammation (110).
The inflammatory response to CU can be broadly divided into local and systemic responses. Locally, a particularly pronounced type 2 inflammation pattern has been observed at the wheal lesion sites (111, 112). Histological studies have reported that CU wheals are peri-vascularly infiltrated by mononuclear cells and eosinophils (113, 114), which differs from the pathological appearance of wheal-like lesions in the setting of urticarial vasculitis (115). The infiltration of eosinophils in CSU typically manifests as activation, suggesting a molecular immunopathology similar to the late-phase reaction of cutaneous allergies (113). Immunohistochemical studies suggest an increase in the number of IL-5-positive cells, a chemotactic factor for eosinophils, in the skin lesions of patients with CSU (116). Likewise, IL-33-positive cells, such as macrophages, MCs, endothelial cells, and fibroblasts, are also significantly increased in the dermis of CSU skin lesions (117), while IL-33 plays an important role in type 2 immunity and allergic diseases (118).
In systemic inflammation, multiple type 2 inflammatory mediators are significantly increased in the serum and plasma of patients with CU. As a key cytokine in atopy and a marker of type 2 inflammation, IL-4 is significantly increased in the serum of patients with atopy (119). Some studies have detected significantly increased IL-4 levels in patients with CU (120–122), though these studies did not further clarify whether the patients had atopy. In addition, if patients with atopic CU were divided into separate groups, the IL-4 levels may be even higher. IL-13 shares a receptor subunit with IL-4 and is a pathogenic cytokine involved in Th2 inflammation (123). A previous study of our team reported that the plasma levels of IL-13 in patients with CSU were significantly higher than those in healthy controls (124). Caproni et al. and Bae et al. also demonstrated that the levels of IL-13 in the peripheral blood of patients with CU were higher than those in healthy individuals (125, 126). The roles of IL-9 and T helper 9 (Th9) Cells in allergic diseases have received increasing attention, and IL-9 is recognized as an important factor in type 2 inflammation (127). A pilot study reported elevated serum IL-9 levels in patients with CSU (128). In addition to its high expression in local skin lesions, IL-5 is also significantly more abundant in the serum of patients with CU (129). The serum levels of IL-31, which also promotes Th2 inflammation, were higher in patients with CSU than in healthy controls, but lower than those in patients with AD (130, 131). Based on the analysis of local and systemic inflammatory activation, CU effector cells, such as MCs and basophils, have reduced activation thresholds and higher reactivity in the context of atopic inflammation.
8 Biologics in atopic diseases and CU
In recent years, the development of new drugs, especially biologics, for the treatment of CU has reflected the importance of atopy in the setting of CU. As an important factor in allergic diseases, IgE is a diagnostic biomarker and a potential therapeutic target for the treatment of atopic diseases (132). Omalizumab, a monoclonal antibody (mAb) targeting free IgE, was initially approved for the treatment of allergic asthma and was found to elicit a good therapeutic response in patients with refractory CSU. Omalizumab has now been approved as a second-line treatment for patients with CSU who are resistant to H1 antihistamines and is an effective treatment for refractory CIndU (133). Ligelizumab, a next-generation humanized monoclonal anti-IgE antibody, has more than 40 times greater affinity for IgE than omalizumab. A phase 2 trial showed that ligelizumab was more effective than omalizumab for the treatment of CSU; however, this advantage was not confirmed in a phase 3 trial (134). Ligelizumab has also been studied in other allergic conditions, such as food allergies and asthma, though it is not more effective than omalizumab for the treatment of severe asthma (135, 136). UUB-221, an IgE neutralizing mAb distinct from omalizumab and llizumab, exhibits CD23-mediated IgE downregulation and relieves urticaria symptoms (137). Biologics targeting type 2 inflammatory mediators for the treatment of CU have also been reported. Dupilumab, an iconic biological agent for type 2 inflammation that targets the IL-4 and IL-13 signaling pathways (138), has been suggested to successfully treat patients with refractory CU who are refractory to omalizumab in case reports and case series (139–141). Two phase 3 trials (LIBERTY-CUPID Studies A and B, NCT03749135) that evaluate the efficacy of dupilumab for the treatment of CSU that was uncontrolled by standard-of-care antihistamines (CUPID Study A) and or by standard-of-care antihistamines and omalizumab (CUPID Study B) have been completed. The partial results suggest that dupilumab elicits clinical improvement in patients with CSU with H1 antihistamine resistance and is well-tolerated. The use of dupilumab for the treatment of CSU in adults and adolescents was recently accepted for Food and Drug Administration review in the United States. In addition, a phase 3 trial was completed to evaluate the use of dupilumab in CholU (NCT03749148); however, the results have not been published. Dupilumab is an effective and safe “off-label” treatment for CholU (142). Other type 2 inflammatory factors may also be potential targets for CU treatment. Humanized anti-IL-5 mAb reslizumab has been reported as effective in a patient with CSU and cold urticaria (143). Ixarelimab, a fully human mAb that simultaneously inhibits IL-31 and oncostatin M, is being trialed in pruritic diseases including CSU (NCT03858634). Overall, based on the overlap between CU and atopic diseases and the role of atopy in the pathogenesis of CU, an increasing number of biologics targeting atopic diseases will be developed for the treatment of CU.
9 Conclusion
As CU is not considered an atopic disease, the relationship between atopy and CU is often overlooked. However, the prevalence of atopy in patients with CU is high and correlates with the clinical characteristics of the disease. An increasing number of studies have found more specific IgEs against various auto-allergens and some exogenous allergens in patients with CU. The clinical value of total IgE in patients with CU is being further explored. Therefore, IgE is considered a critical and promising therapeutic target for CU and other atopic diseases (144). Additionally, an increasing number of studies have shown that CU is associated with a certain degree of type 2 inflammatory activation, whether local or systemic, which is a common phenomenon in other atopic diseases. The effectiveness of targeted therapies for type 2 inflammation in the treatment of CU is gradually increasing. Therefore, by comprehensively summarizing the existing research progress, the role of atopy in the pathogenesis of CU can be estimated (Figure 1). In summary, although the available evidence is insufficient to support atopy as a direct trigger for CU, the presence of atopy increases the risk of CU and induces MCs to become more active, aggravating CU symptoms.
Based on the available evidence and progress, several clinical statements can be made. In contrast to aiCSU, clinical subtypes of CU, such as atopic CSU or atopic CIndU, may be recognized; however, more clinical studies are needed to clarify the characteristics of these subtypes. In addition, cross-antigens between exogenous allergens and CU auto-allergens or a correlation between exogenous allergens and CU auto-allergens may exist. In the future, the presence of auto-allergens may be confirmed by testing for common exogenous allergens, and specific immunotherapy of common allergens may alleviate the symptoms of atopic CU caused by specific IgEs of auto-allergens. An understanding of the effect of IgE sensitization or type 2 inflammation on the pathogenesis of urticaria, such as in MCs and basophils, may allow for the discovery of therapies (such as omalizumab, allergen-specific immunotherapy, dupilumab, and Jak1 inhibitors) that target IgE sensitization and type 2 inflammatory factors in the setting of CU.
Author contributions
QC: Conceptualization, Investigation, Writing – original draft. XY: Data curation, Investigation, Writing – original draft. BN: Conceptualization, Supervision, Validation, Writing – review & editing. ZS: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (No.82003359).
Acknowledgments
We would like to thank Ms. Liu Wenying and Ms. Zhai ZhiFang for adding the literatures and revising the language of the full text. Thanks to Ms. Li Shifei for helping to draw the picture in this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zuberbier T, Abdul Latiff AH, Abuzakouk M, Aquilina S, Asero R, Baker D, et al. The international EAACI/GA²LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy (2022) 3:734–66. doi: 10.1111/all.15090
2. Kolkhir P, Giménez-Arnau AM, Kulthanan K, Peter J, Metz M, Maurer M. Urticaria. Nat Rev Dis Primers (2022) 1:61. doi: 10.1038/s41572-022-00389-z
3. Gonçalo M, Gimenéz-Arnau A, Al-Ahmad M, Ben-Shoshan M, Bernstein JA, Ensina LF, et al. The global burden of chronic urticaria for the patient and society. Br J Dermatol (2021) 2:226–36. doi: 10.1111/bjd.19561
4. Bracken SJ, Abraham S, MacLeod AS. Autoimmune theories of chronic spontaneous urticaria. Front Immunol (2019) 10:627. doi: 10.3389/fimmu.2019.00627
5. Diaz-Cabrera NM, Sánchez-Borges MA, Ledford DK. Atopy: A collection of comorbid conditions. J Allergy Clin Immunol Pract (2021) 11:3862–6. doi: 10.1016/j.jaip.2021.09.002
7. Wedi B, Traidl S. Anti-igE for the treatment of chronic urticaria. Immunotargets Ther (2021) 10:27–45. doi: 10.2147/itt.S261416
8. Nassif A. Is chronic urticaria an atopic condition? Eur J Dermatol (2007) 6:545–6. doi: 10.1684/ejd.2007.0279
9. Shalom G, Magen E, Dreiher J, Freud T, Bogen B, Comaneshter D, et al. Chronic urticaria and atopic disorders: a cross-sectional study of 11 271 patients. Br J Dermatol (2017) 4:e96–e7. doi: 10.1111/bjd.15347
10. Chiu HY, Muo CH, Sung FC. Associations of chronic urticaria with atopic and autoimmune comorbidities: a nationwide population-based study. Int J Dermatol (2018) 7:822–9. doi: 10.1111/ijd.14000
11. Rosman Y, Hershko AY, Meir-Shafrir K, Kedem R, Lachover-Roth I, Mekori YA, et al. Characterization of chronic urticaria and associated conditions in a large population of adolescents. J Am Acad Dermatol (2019) 1:129–35. doi: 10.1016/j.jaad.2019.02.034
12. Kitsioulis NA, Papadopoulos NG, Kostoudi S, Manousakis E, Douladiris N, Xepapadaki P. Assessment of atopic dermatitis as a risk factor for chronic spontaneous urticaria in a pediatric population. Allergy Asthma Proc (2018) 6:445–8. doi: 10.2500/aap.2018.39.4166
13. Caliskaner Z, Ozturk S, Turan M, Karaayvaz M. Skin test positivity to aeroallergens in the patients with chronic urticaria without allergic respiratory disease. J Investig Allergol Clin Immunol (2004) 1:50–4.
14. Mahesh PA, Kushalappa PA, Holla AD, Vedanthan PK. House dust mite sensitivity is a factor in chronic urticaria. Indian J Dermatol Venereol Leprol (2005) 2:99–101. doi: 10.4103/0378-6323.13993
15. Kulthanan K, Jiamton S, Rutnin NO, Insawang M, Pinkaew S. Prevalence and relevance of the positivity of skin prick testing in patients with chronic urticaria. J Dermatol (2008) 6:330–5. doi: 10.1111/j.1346-8138.2008.00477.x
16. Kulthanan K, Wachirakaphan C. Prevalence and clinical characteristics of chronic urticaria and positive skin prick testing to mites. Acta Derm Venereol (2008) 6:584–8. doi: 10.2340/00015555-0546
17. Staubach P, Vonend A, Burow G, Metz M, Magerl M, Maurer M. Patients with chronic urticaria exhibit increased rates of sensitisation to Candida albicans, but not to common moulds. Mycoses (2009) 4:334–8. doi: 10.1111/j.1439-0507.2008.01601.x
18. Daschner A, Rodero M, De Frutos C, Valls A, Cuéllar C. Chronic urticaria is associated with a differential helminth-arthropod-related atopy phenotype. J Dermatol (2010) 9:780–5. doi: 10.1111/j.1346-8138.2010.00869.x
19. Refaat M, Ossman EN, Farres MN, El-Khodeery MM, Arafa N, Attia M. Assessment of the role of aeroallergens in patients with chronic urticaria. Rev Francaise D Allergologie - Rev FR ALLERGOL (2010) 50:394–7. doi: 10.1016/j.reval.2009.11.009
20. Augey F, Gunera-Saad N, Bensaid B, Nosbaum A, Berard F, Nicolas JF. Chronic spontaneous urticaria is not an allergic disease. Eur J Dermatol (2011) 3:349–53. doi: 10.1684/ejd.2011.1285
21. de Vos G, Kravvariti E, Collins J, Tavdy A, Nazari R, Hudes G, et al. Increased allergic sensitization to mugwort in chronic urticaria. Dermatology (2012) 2:141–6. doi: 10.1159/000342356
22. Gecer E, Erdem T. Aeroallergen prick skin test and autologous serum skin test results in patients with chronic urticaria and their comparison. Ann Dermatol (2012) 4:472–4. doi: 10.5021/ad.2012.24.4.472
23. Song Z, Zhai Z, Zhong H, Zhou Z, Chen W, Hao F. Evaluation of autologous serum skin test and skin prick test reactivity to house dust mite in patients with chronic spontaneous urticaria. PloS One (2013) 5:e64142. doi: 10.1371/journal.pone.0064142
24. Bains P, Dogra A. Skin prick test in patients with chronic allergic skin disorders. Indian J Dermatol (2015) 2:159–64. doi: 10.4103/0019-5154.152513
25. Altrichter S, Koch K, Church MK, Maurer M. Atopic predisposition in cholinergic urticaria patients and its implications. J Eur Acad Dermatol Venereol (2016) 12:2060–5. doi: 10.1111/jdv.13765
26. Lee JB, Lee SH, Han MY, Yoon JW. Allergen sensitization and vitamin D status in young Korean children with urticaria. Allergy Asthma Respir Dis (2017) 3:153. doi: 10.4168/aard.2017.5.3.153
27. Altrichter S, Hawro T, Liedtke M, Holtappels G, Bachert C, Skov PS, et al. In chronic spontaneous urticaria, IgE against staphylococcal enterotoxins is common and functional. Allergy (2018) 7:1497–504. doi: 10.1111/all.13381
28. Mohamed Abdel Latif O. Specific igE for aero and food allergens in adul t chronic urticaria patients without other allergic diseases. Int J Immunol (2018) 2:25. doi: 10.11648/j.iji.20180602.11
29. Zhou Y, Sheng M, Chen M. Detection and allergen analysis of serum IgE in pediatric patients with chronic urticaria. Pak J Med Sci (2018) 2:385–9. doi: 10.12669/pjms.342.14681
30. Park H, Lee JY, Song A, Jung M, Kim M, Sohn I, et al. Natural course and prognostic factors of chronic urticaria in Korean children: A single center experience. Asian Pac J Allergy Immunol (2019) 1:19–24. doi: 10.12932/ap-151117-0197
31. Ping JD, Zhao JW, Sun XX, Wu F, Jiang ZY, Cheng Z, et al. Prevalence of allergen sensitization among 1,091 patients with urticaria. Exp Ther Med (2020) 3:1908–14. doi: 10.3892/etm.2019.8367
32. Esmaeilzadeh H, Eskandarisani M, Nabavizadeh H, Alyasin S, Vali M, Mortazavi N. Investigating the association of atopy and aeroallergen sensitization and chronic spontaneous urticaria. Postepy Dermatol Alergol (2022) 1:121–5. doi: 10.5114/ada.2022.113805
33. Chen QQ, Yang X, Wang H, Li J, Zhang M, Song Z. Clinical analysis of allergen reactivity and atopic disease history in 168 patients with chronic inducible urticaria. Chin J Dermatol (2023) 6:496–503. doi: 10.35541/cjd.20220791
34. Chong AC, Chwa WJ, Ong PY. Aeroallergens in atopic dermatitis and chronic urticaria. Curr Allergy Asthma Rep (2022) 7:67–75. doi: 10.1007/s11882-022-01033-2
35. Syrigos N, Psarros F, Tziotou M, Syrigou E. The rate of atopy in patients with cold-induced urticaria. J Investig Allergol Clin Immunol (2017) 139:AB246. doi: 10.1016/j.jaci.2016.12.793
36. Alangari AA, Twarog FJ, Shih MC, Schneider LC. Clinical features and anaphylaxis in children with cold urticaria. Pediatrics (2004) 4:e313–7. doi: 10.1542/peds.113.4.e313
37. Xu YY, Jiang NN, Wen LP, Li H, Yin J. Wheat allergy in patients with recurrent urticaria. World Allergy Organ J (2019) 2:100013. doi: 10.1016/j.waojou.2019.100013
38. Curioni A, Santucci B, Cristaudo A, Canistraci C, Pietravalle M, Simonato B, et al. Urticaria from beer: an immediate hypersensitivity reaction due to a 10-kDa protein derived from barley. Clin Exp Allergy (1999) 29:407–13. doi: 10.1046/j.1365-2222.1999.00491.x
39. Kasperska-Zajac A, Brzoza Z. Remission of chronic urticaria in the course of house dust mite immunotherapy–mere coincidence or something more to it? Vaccine (2009) 52:7240–1. doi: 10.1016/j.vaccine.2009.08.076
40. August PJ, O’Driscoll J. Urticaria successfully treated by desensitization with grass pollen extract. Br J Dermatol (1989) 3:409–10. doi: 10.1111/j.1365-2133.1989.tb04168.x
41. Kasperska-Zajac A. Exacerbation of chronic urticaria in the course of house dust mite immunotherapy. Hum Vaccin (2011) 4:417–8. doi: 10.4161/hv.7.4.14236
42. Puźniakowska KM, Gawinowska M, Chełmińska M. Confirming acetylsalicylic acid hypersensitivity as a cause of chronic urticaria by desensitization. Postepy Dermatol Alergol (2019) 3:374–5. doi: 10.5114/ada.2019.85645
43. Ye YM, Yoon J, Woo SD, Jang JH, Lee Y, Lee HY, et al. Clustering the clinical course of chronic urticaria using a longitudinal database: effects on urticaria remission. Allergy Asthma Immunol Res (2021) 3:390–403. doi: 10.4168/aair.2021.13.3.390
44. Takahagi S, Tanaka A, Hide M. Sweat allergy. Allergol Int (2018) 4:435–41. doi: 10.1016/j.alit.2018.07.002
45. Fukunaga A, Washio K, Hatakeyama M, Oda Y, Ogura K, Horikawa T, et al. Cholinergic urticaria: epidemiology, physiopathology, new categorization, and management. Clin Auton Res (2018) 1:103–13. doi: 10.1007/s10286-017-0418-6
46. Woo SD, Yang EM, Jang J, Lee Y, Shin YS, Ye YM, et al. Serum-free immunoglobulin E: A useful biomarker of atopy and type 2 asthma in adults with asthma. Ann Allergy Asthma Immunol (2021) 1:109–15.e1. doi: 10.1016/j.anai.2021.03.023
47. Greaves MW, Plummer VM, McLaughlan P, Stanworth DR. Serum and cell bound IgE in chronic urticaria. Clin Allergy (1974) 3:265–71. doi: 10.1111/j.1365-2222.1974.tb01384.x
48. Altrichter S, Fok JS, Jiao Q, Kolkhir P, Pyatilova P, Romero SM, et al. Total igE as a marker for chronic spontaneous urticaria. Allergy Asthma Immunol Res (2021) 2:206–18. doi: 10.4168/aair.2021.13.2.206
49. Kessel A, Helou W, Bamberger E, Sabo E, Nusem D, Panassof J, et al. Elevated serum total IgE–a potential marker for severe chronic urticaria. Int Arch Allergy Immunol (2010) 3:288–93. doi: 10.1159/000314370
50. Hu Y, Liu S, Liu P, Mu Z, Zhang J. Clinical relevance of eosinophils, basophils, serum total IgE level, allergen-specific IgE, and clinical features in atopic dermatitis. J Clin Lab Anal (2020) 6:e23214. doi: 10.1002/jcla.23214
51. de Montjoye L, Darrigade AS, Giménez-Arnau A, Herman A, Dumoutier L, Baeck M. Correlations between disease activity, autoimmunity and biological parameters in patients with chronic spontaneous urticaria. Eur Ann Allergy Clin Immunol (2021) 2:55–66. doi: 10.23822/EurAnnACI.1764-1489.132
52. Giménez-Arnau AM, Ribas-Llauradó C, Mohammad-Porras N, Deza G, Pujol RM, Gimeno R. IgE and high-affinity IgE receptor in chronic inducible urticaria, pathogenic, and management relevance. Clin Transl Allergy (2022) 2:e12117. doi: 10.1002/clt2.12117
53. He L, Yi W, Huang X, Long H, Lu Q. Chronic urticaria: advances in understanding of the disease and clinical management. Clin Rev Allergy Immunol (2021) 3:424–48. doi: 10.1007/s12016-021-08886-x
54. Sánchez J, Sánchez A, Cardona R. Clinical characterization of patients with chronic spontaneous urticaria according to anti-TPO igE levels. J Immunol Res (2019) 11:4202145. doi: 10.1155/2019/4202145
55. Sánchez J, Sánchez A, Cardona R. Causal relationship between anti-TPO igE and chronic urticaria by in vitro and in vivo tests. Allergy Asthma Immunol Res (2019) 11(1):29–42. doi: 10.4168/aair.2019.11.1.29
56. Shin YS, Suh DH, Yang EM, Ye YM, Park HS. Serum specific igE to thyroid peroxidase activates basophils in aspirin intolerant urticaria. J Korean Med Sci (2015) 6:705–9. doi: 10.3346/jkms.2015.30.6.705
57. Kolkhir P, Kovalkova E, Chernov A, Danilycheva I, Krause K, Sauer M, et al. Autoimmune chronic spontaneous urticaria detection with igG anti-TPO and total igE. J Allergy Clin Immunol Pract (2021) 11:4138–46.e8. doi: 10.1016/j.jaip.2021.07.043
58. Choi WS, Lim ES, Ban GY, Kim JH, Shin YS, Park HS, et al. Disease-specific impairment of the quality of life in adult patients with chronic spontaneous urticaria. Korean J Intern Med (2018) 1:185–92. doi: 10.3904/kjim.2015.195
59. Kocatürk E, Thomsen SF, Al-Ahmad M, Gimenez Arnau AM, Conlon N, Şavk E, et al. Total IgE levels are linked to the course of chronic spontaneous urticaria during pregnancy. J Allergy Clin Immunol Pract (2023) 1:350–3. doi: 10.1016/j.jaip.2022.10.018
60. Kolkhir P, Muñoz M, Asero R, Ferrer M, Kocatürk E, Metz M, et al. Autoimmune chronic spontaneous urticaria. J Allergy Clin Immunol (2022) 6:1819–31. doi: 10.1016/j.jaci.2022.04.010
61. Magen E, Mishal J, Zeldin Y, Schlesinger M. Clinical and laboratory features of antihistamine-resistant chronic idiopathic urticaria. Allergy Asthma Proc (2011) 6:460–6. doi: 10.2500/aap.2011.32.3483
62. Santiago L, Ferreira B, Ramos L, Gonçalo M. IgE levels are negatively correlated with clinical response to ciclosporin in chronic spontaneous urticaria. Br J Dermatol (2019) 1:199–200. doi: 10.1111/bjd.17005
63. Asero R, Ferrucci S, Casazza G, Marzano AV, Cugno M. Total IgE and atopic status in patients with severe chronic spontaneous urticaria unresponsive to omalizumab treatment. Allergy (2019) 8:1561–3. doi: 10.1111/all.13754
64. Marzano AV, Genovese G, Casazza G, Fierro MT, Dapavo P, Crimi N, et al. Predictors of response to omalizumab and relapse in chronic spontaneous urticaria: a study of 470 patients. J Eur Acad Dermatol Venereol (2019) 5:918–24. doi: 10.1111/jdv.15350
65. Ertas R, Ozyurt K, Ozlu E, Ulas Y, Avci A, Atasoy M, et al. Increased IgE levels are linked to faster relapse in patients with omalizumab-discontinued chronic spontaneous urticaria. J Allergy Clin Immunol (2017) 6:1749–51. doi: 10.1016/j.jaci.2017.08.007
66. Gergen PJ, Arbes SJ Jr., Calatroni A, Mitchell HE, Zeldin DC. Total IgE levels and asthma prevalence in the US population: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol (2009) 3:447–53. doi: 10.1016/j.jaci.2009.06.011
67. Jang JH, Yang EM, Lee Y, Ye YM, Moon J, Ryu MS, et al. Increased serum free IgE levels in patients with chronic spontaneous urticaria (CSU). World Allergy Organ J (2022) 2:100629. doi: 10.1016/j.waojou.2022.100629
68. Wong MM, Keith PK. Presence of positive skin prick tests to inhalant allergens and markers of T2 inflammation in subjects with chronic spontaneous urticaria (CSU): a systematic literature review. Allergy Asthma Clin Immunol (2020), 72. doi: 10.1186/s13223-020-00461-x
69. Leung DY, Harbeck R, Bina P, Reiser RF, Yang E, Norris DA, et al. Presence of IgE antibodies to staphylococcal exotoxins on the skin of patients with atopic dermatitis. Evidence for a new group of allergens. J Clin Invest (1993) 3:1374–80. doi: 10.1172/jci116711
70. Park KD, Pak SC, Park KK. The pathogenetic effect of natural and bacterial toxins on atopic dermatitis. Toxins (Basel) (2016) 1:3. doi: 10.3390/toxins9010003
71. Ertam I, Biyikli SE, Yazkan FA, Aytimur D, Alper S. The frequency of nasal carriage in chronic urticaria patients. J Eur Acad Dermatol Venereol (2007) 6:777–80. doi: 10.1111/j.1468-3083.2006.02083.x
72. Sharma AD. Role of nasal carriage of staphylococcus aureus in chronic urticaria. Indian J Dermatol (2012) 3:233–6. doi: 10.4103/0019-5154.96211
73. Bar-Sela S, Reshef T, Mekori YA. IgE antithyroid microsomal antibodies in a patient with chronic urticaria. J Allergy Clin Immunol (1999) 6:1216–7. doi: 10.1016/s0091-6749(99)70204-6
74. Tedeschi A, Lorini M, Asero R. Anti-thyroid peroxidase IgE in patients with chronic urticaria. J Allergy Clin Immunol (2001) 3:467–8. doi: 10.1067/mai.2001.117792
75. Concha LB, Chang CC, Szema AM, Dattwyler RJ, Carlson HE. IgE antithyroid antibodies in patients with Hashimoto’s disease and chronic urticaria. Allergy Asthma Proc (2004) 5:293–6.
76. Altrichter S, Peter HJ, Pisarevskaja D, Metz M, Martus P, Maurer M. IgE mediated autoallergy against thyroid peroxidase–a novel pathomechanism of chronic spontaneous urticaria? PloS One (2011) 4:e14794. doi: 10.1371/journal.pone.0014794
77. Sánchez J, Sánchez A, Munera M, Garcia E, Lopez JF, Velásquez-Lopera M, et al. Presence of igE autoantibodies against eosinophil peroxidase and eosinophil cationic protein in severe chronic spontaneous urticaria and atopic dermatitis. Allergy Asthma Immunol Res (2021) 5:746–61. doi: 10.4168/aair.2021.13.5.746
78. Zhang L, Qiu L, Wu J, Qi Y, Wang H, Qi R, et al. IgE and igG anti-thyroid autoantibodies in chinese patients with chronic spontaneous urticaria and a literature review. Allergy Asthma Immunol Res (2022) 1:131–42. doi: 10.4168/aair.2022.14.1.131
79. Cugno M, Asero R, Ferrucci S, Lorini M, Carbonelli V, Tedeschi A, et al. Elevated IgE to tissue factor and thyroglobulin are abated by omalizumab in chronic spontaneous urticaria. Allergy (2018) 12:2408–11. doi: 10.1111/all.13587
80. Hatada Y, Kashiwakura J, Hayama K, Fujisawa D, Sasaki-Sakamoto T, Terui T, et al. Significantly high levels of anti-dsDNA immunoglobulin E in sera and the ability of dsDNA to induce the degranulation of basophils from chronic urticaria patients. Int Arch Allergy Immunol (2013) 161:154–8. doi: 10.1159/000350388
81. Asero R, Marzano AV, Ferrucci S, Lorini M, Carbonelli V, Cugno M. Co-occurrence of IgE and IgG autoantibodies in patients with chronic spontaneous urticaria. Clin Exp Immunol (2020) 3:242–9. doi: 10.1111/cei.13428
82. Su H, Kolkhir P, Scheffel J, Xiang YK, Yao X, Maurer M, et al. One in five patients with chronic spontaneous urticaria has IgE to tissue transglutaminase 2. Allergy (2023) 78:2537–9. doi: 10.1111/all.15734
83. Schmetzer O, Lakin E, Topal FA, Preusse P, Freier D, Church MK, et al. IL-24 is a common and specific autoantigen of IgE in patients with chronic spontaneous urticaria. J Allergy Clin Immunol (2018) 3:876–82. doi: 10.1016/j.jaci.2017.10.035
84. Haapala AM, Hyöty H, Parkkonen P, Mustonen J, Soppi E. Antibody reactivity against thyroid peroxidase and myeloperoxidase in autoimmune thyroiditis and systemic vasculitis. Scand J Immunol (1997) 1:78–85. doi: 10.1046/j.1365-3083.1997.d01-90.x
85. Maronese CA, Ferrucci SM, Moltrasio C, Lorini M, Carbonelli V, Asero R, et al. IgG and igE autoantibodies to igE receptors in chronic spontaneous urticaria and their role in the response to omalizumab. J Clin Med (2023) 1:378. doi: 10.3390/jcm12010378
86. Takahagi S, Tanaka T, Ishii K, Suzuki H, Kameyoshi Y, Shindo H, et al. Sweat antigen induces histamine release from basophils of patients with cholinergic urticaria associated with atopic diathesis. Br J Dermatol (2009) 2:426–8. doi: 10.1111/j.1365-2133.2008.08862.x
87. Hiragun M, Hiragun T, Ishii K, Suzuki H, Tanaka A, Yanase Y, et al. Elevated serum IgE against MGL_1304 in patients with atopic dermatitis and cholinergic urticaria. Allergol Int (2014) 1:83–93. doi: 10.2332/allergolint.13-OA-0611
88. Hiragun T, Ishii K, Hiragun M, Suzuki H, Kan T, Mihara S, et al. Fungal protein MGL_1304 in sweat is an allergen for atopic dermatitis patients. J Allergy Clin Immunol (2013) 3:608–15.e4. doi: 10.1016/j.jaci.2013.03.047
89. Lugović Mihić L, Bulat V, Situm M, Cavka V, Krolo I. Allergic hypersensitivity skin reactions following sun exposure. Coll Antropol (2008) 32:153–7.
90. Murphy GM, Zollman PE, Greaves MW, Winkelmann RK. Symptomatic dermographism (factitious urticaria)–passive transfer experiments from human to monkey. Br J Dermatol (1987) 6:801–4. doi: 10.1111/j.1365-2133.1987.tb04898.x
91. Newcomb RW, Nelson H. Dermographia mediated by immunoglobulin E. Am J Med (1973) 2:174–80. doi: 10.1016/0002-9343(73)90221-0
92. Gruber BL, Baeza ML, Marchese MJ, Agnello V, Kaplan AP. Prevalence and functional role of anti-IgE autoantibodies in urticarial syndromes. J Invest Dermatol (1988) 2:213–7. doi: 10.1111/1523-1747.ep12462239
93. Maurer M, Metz M, Brehler R, Hillen U, Jakob T, Mahler V, et al. Omalizumab treatment in patients with chronic inducible urticaria: A systematic review of published evidence. J Allergy Clin Immunol (2018) 2:638–49. doi: 10.1016/j.jaci.2017.06.032
94. Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med (2012) 5:693–704. doi: 10.1038/nm.2755
95. Chen Q, Zhai Z, Xu J, Chen W, Chen S, Zhong H, et al. Basophil CD63 expression in chronic spontaneous urticaria: correlation with allergic sensitization, serum autoreactivity and basophil reactivity. J Eur Acad Dermatol Venereol (2017) 3:463–8. doi: 10.1111/jdv.13912
96. Kalesnikoff J, Huber M, Lam V, Damen JE, Zhang J, Siraganian RP, et al. Monomeric IgE stimulates signaling pathways in mast cells that lead to cytokine production and cell survival. Immunity (2001) 6:801–11. doi: 10.1016/s1074-7613(01)00159-5
97. Oka T, Hori M, Tanaka A, Matsuda H, Karaki H, Ozaki H. IgE alone-induced actin assembly modifies calcium signaling and degranulation in RBL-2H3 mast cells. Am J Physiol Cell Physiol (2004) 2:C256–63. doi: 10.1152/ajpcell.00197.2003
98. Pandey V, Mihara S, Fensome-Green A, Bolsover S, Cockcroft S. Monomeric IgE stimulates NFAT translocation into the nucleus, a rise in cytosol Ca2+, degranulation, and membrane ruffling in the cultured rat basophilic leukemia-2H3 mast cell line. J Immunol (2004) 7:4048–58. doi: 10.4049/jimmunol.172.7.4048
99. Cruse G, Kaur D, Yang W, Duffy SM, Brightling CE, Bradding P. Activation of human lung mast cells by monomeric immunoglobulin E. Eur Respir J (2005) 5:858–63. doi: 10.1183/09031936.05.00091704
100. Matsuda K, Piliponsky AM, Iikura M, Nakae S, Wang EW, Dutta SM, et al. Monomeric IgE enhances human mast cell chemokine production: IL-4 augments and dexamethasone suppresses the response. J Allergy Clin Immunol (2005) 6:1357–63. doi: 10.1016/j.jaci.2005.08.042
101. Yanase Y, Matsuo Y, Kawaguchi T, Ishii K, Tanaka A, Iwamoto K, et al. Activation of human peripheral basophils in response to high igE antibody concentrations without antigens. Int J Mol Sci (2018) 20:1. doi: 10.3390/ijms20010045
102. Kohno M, Yamasaki S, Tybulewicz VL, Saito T. Rapid and large amount of autocrine IL-3 production is responsible for mast cell survival by IgE in the absence of antigen. Blood (2005) 5:2059–65. doi: 10.1182/blood-2004-07-2639
103. Bax HJ, Bowen H, Dodev TS, Sutton BJ, Gould HJ. Mechanism of the antigen-independent cytokinergic SPE-7 IgE activation of human mast cells in vitro. Sci Rep (2015) 5:9538. doi: 10.1038/srep09538
104. Bax HJ, Bowen H, Beavil RL, Chung R, Ward M, Davies AM, et al. IgE trimers drive SPE-7 cytokinergic activity. Sci Rep (2017) 1:8164. doi: 10.1038/s41598-017-08212-6
105. Shade KC, Conroy ME, Washburn N, Kitaoka M, Huynh DJ, Laprise E, et al. Sialylation of immunoglobulin E is a determinant of allergic pathogenicity. Nature (2020) 7811:265–70. doi: 10.1038/s41586-020-2311-z
106. Kashiwakura J, Okayama Y, Furue M, Kabashima K, Shimada S, Ra C, et al. Most highly cytokinergic igEs have polyreactivity to autoantigens. Allergy Asthma Immunol Res (2012) 6:332–40. doi: 10.4168/aair.2012.4.6.332
107. Jayapal M, Tay HK, Reghunathan R, Zhi L, Chow KK, Rauff M, et al. Genome-wide gene expression profiling of human mast cells stimulated by IgE or FcepsilonRI-aggregation reveals a complex network of genes involved in inflammatory responses. BMC Genomics (2006) 7:210. doi: 10.1186/1471-2164-7-210
108. Just J, Munk Ipsen P, Kruhøffer M, Lykkemark S, Skjold T, Schiøtz PO, et al. Human mast cell sensitization with igE increases miRNA-210 expression. Int Arch Allergy Immunol (2019) 2:102–7. doi: 10.1159/000496513
109. Fahy JV. Type 2 inflammation in asthma–present in most, absent in many. Nat Rev Immunol (2015) 1:57–65. doi: 10.1038/nri3786
110. Zhou B, Li J, Liu R, Zhu L, Peng C. The role of crosstalk of immune cells in pathogenesis of chronic spontaneous urticaria. Front Immunol (2022) 13. doi: 10.3389/fimmu.2022.879754
111. Ying S, Kikuchi Y, Meng Q, Kay AB, Kaplan AP. TH1/TH2 cytokines and inflammatory cells in skin biopsy specimens from patients with chronic idiopathic urticaria: comparison with the allergen-induced late-phase cutaneous reaction. J Allergy Clin Immunol (2002) 4:694–700. doi: 10.1067/mai.2002.123236
112. Church MK, Kolkhir P, Metz M, Maurer M. The role and relevance of mast cells in urticaria. Immunol Rev (2018) 1:232–47. doi: 10.1111/imr.12632
113. Toyoda M, Maruyama T, Morohashi M, Bhawan J. Free eosinophil granules in urticaria: a correlation with the duration of wheals. Am J Dermatopathol (1996) 1:49–57. doi: 10.1097/00000372-199602000-00008
114. English JS, Murphy GM, Winkelmann RK, Bhogal B. A sequential histopathological study of dermographism. Clin Exp Dermatol (1988) 5:314–7. doi: 10.1111/j.1365-2230.1988.tb00712.x
115. Marzano AV, Maronese CA, Genovese G, Ferrucci S, Moltrasio C, Asero R, et al. Urticarial vasculitis: Clinical and laboratory findings with a particular emphasis on differential diagnosis. J Allergy Clin Immunol (2022) 4:1137–49. doi: 10.1016/j.jaci.2022.02.007
116. Altrichter S, Frischbutter S, Fok JS, Kolkhir P, Jiao Q, Skov PS, et al. The role of eosinophils in chronic spontaneous urticaria. J Allergy Clin Immunol (2020) 6:1510–6. doi: 10.1016/j.jaci.2020.03.005
117. Kay AB, Clark P, Maurer M, Ying S. Elevations in T-helper-2-initiating cytokines (interleukin-33, interleukin-25 and thymic stromal lymphopoietin) in lesional skin from chronic spontaneous (‘idiopathic’) urticaria. Br J Dermatol (2015) 5:1294–302. doi: 10.1111/bjd.13621
118. Drake LY, Kita H. IL-33: biological properties, functions, and roles in airway disease. Immunol Rev (2017) 1:173–84. doi: 10.1111/imr.12552
119. Ricci M. IL-4: a key cytokine in atopy. Clin Exp Allergy (1994) 9:801–12. doi: 10.1111/j.1365-2222.1994.tb01803.x
120. Ferrer M, Luquin E, Sanchez-Ibarrola A, Moreno C, Sanz ML, Kaplan AP. Secretion of cytokines, histamine and leukotrienes in chronic urticaria. Int Arch Allergy Immunol (2002) 3:254–60. doi: 10.1159/000066772
121. Mohamed RW, Fathy A, el-Sayed AE. Increased circulating FcepsilonRII-bearing B-lymphocytes and serum levels of IL-4 in non-autoreactive chronic idiopathic urticaria. Egypt J Immunol (2003) 2:9–18.
122. Hong GU, Ro JY, Bae Y, Kwon IH, Park GH, Choi YH, et al. Association of TG2 from mast cells and chronic spontaneous urticaria pathogenesis. Ann Allergy Asthma Immunol (2016) 3:290–7. doi: 10.1016/j.anai.2016.06.026
123. Gandhi NA, Bennett BL, Graham NM, Pirozzi G, Stahl N, Yancopoulos GD. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discovery (2016) 1:35–50. doi: 10.1038/nrd4624
124. Chen Q, Zhong H, Chen WC, Zhai Z, Zhou Z, Song Z, et al. Different expression patterns of plasma Th1-, Th2-, Th17- and Th22-related cytokines correlate with serum autoreactivity and allergen sensitivity in chronic spontaneous urticaria. J Eur Acad Dermatol Venereol (2018) 3:441–8. doi: 10.1111/jdv.14541
125. Caproni M, Cardinali C, Giomi B, Antiga E, D’Agata A, Walter S, et al. Serological detection of eotaxin, IL-4, IL-13, IFN-gamma, MIP-1alpha, TARC and IP-10 in chronic autoimmune urticaria and chronic idiopathic urticaria. J Dermatol Sci (2004) 1:57–9. doi: 10.1016/j.jdermsci.2004.07.006
126. Bae Y, Izuhara K, Ohta S, Ono J, Hong GU, Ro JY, et al. Periostin and interleukin-13 are independently related to chronic spontaneous urticaria. Allergy Asthma Immunol Res (2016) 5:457–60. doi: 10.4168/aair.2016.8.5.457
127. Hauber HP, Bergeron C, Hamid Q. IL-9 in allergic inflammation. Int Arch Allergy Immunol (2004) 1:79–87. doi: 10.1159/000078384
128. Ciprandi G, De Amici M, Legoratto S, Giunta V, Vignini M, Borroni G. Serum IL-9 levels in patients with spontaneous urticaria: a preliminary study. J Investig Allergol Clin Immunol (2012) 3:232–4.
129. Serhat Inaloz H, Ozturk S, Akcali C, Kirtak N, Tarakcioglu M. Low-dose and short-term cyclosporine treatment in patients with chronic idiopathic urticaria: a clinical and immunological evaluation. J Dermatol (2008) 5:276–82. doi: 10.1111/j.1346-8138.2008.00466.x
130. Raap U, Wieczorek D, Gehring M, Pauls I, Ständer S, Kapp A, et al. Increased levels of serum IL-31 in chronic spontaneous urticaria. Exp Dermatol (2010) 5:464–6. doi: 10.1111/j.1600-0625.2010.01067.x
131. Chaowattanapanit S, Choonhakarn C, Salao K, Winaikosol K, Julanon N, Wongjirattikarn R, et al. Increased serum IL-31 levels in chronic spontaneous urticaria and psoriasis with pruritic symptoms. Heliyon (2020) 12:e05621. doi: 10.1016/j.heliyon.2020.e05621
132. Licari A, Marseglia G, Castagnoli R, Marseglia A, Ciprandi G. The discovery and development of omalizumab for the treatment of asthma. Expert Opin Drug Discovery (2015) 9:1033–42. doi: 10.1517/17460441.2015.1048220
133. Jeong SH, Lim DJ, Chang SE, Kim KH, Kim KJ, Park EJ. Omalizumab on chronic spontaneous urticaria and chronic inducible urticaria: A real-world study of efficacy and predictors of treatment outcome. J Korean Med Sci (2022) 27:e211. doi: 10.3346/jkms.2022.37.e211
134. Maurer M, Giménez-Arnau AM, Sussman G, Metz M, Baker DR, Bauer A, et al. Ligelizumab for chronic spontaneous urticaria. N Engl J Med (2019) 14:1321–32. doi: 10.1056/NEJMoa1900408
135. Wood RA, Chinthrajah RS, Eggel A, Bottoli I, Gautier A, Woisetschlaeger M, et al. The rationale for development of ligelizumab in food allergy. World Allergy Organ J (2022) 9:100690. doi: 10.1016/j.waojou.2022.100690
136. Trischler J, Bottoli I, Janocha R, Heusser C, Jaumont X, Lowe P, et al. Ligelizumab treatment for severe asthma: learnings from the clinical development programme. Clin Transl Immunol (2021) 3:e1255. doi: 10.1002/cti2.1255
137. Kuo BS, Li CH, Chen JB, Shiung YY, Chu CY, Lee CH, et al. IgE-neutralizing UB-221 mAb, distinct from omalizumab and ligelizumab, exhibits CD23-mediated IgE downregulation and relieves urticaria symptoms. J Clin Invest (2022) 15:e157765. doi: 10.1172/jci157765
138. Muñoz-Bellido FJ, Moreno E, Dávila I. Dupilumab: A review of present indications and off-label uses. J Investig Allergol Clin Immunol (2022) 2:97–115. doi: 10.18176/jiaci.0682
139. Lee JK, Simpson RS. Dupilumab as a novel therapy for difficult to treat chronic spontaneous urticaria. J Allergy Clin Immunol Pract (2019) 5:1659–61.e1. doi: 10.1016/j.jaip.2018.11.018
140. Ferrucci S, Benzecry V, Berti E, Asero R. Rapid disappearance of both severe atopic dermatitis and cold urticaria following dupilumab treatment. Clin Exp Dermatol (2020) 3:345–6. doi: 10.1111/ced.14081
141. Föhr J, Herbst M, Jahn S. Treatment of simultaneously occurring urticaria and atopic dermatitis with dupilumab. Hautarzt (2021) 3:249–51. doi: 10.1007/s00105-020-04675-3
142. Sirufo MM, Catalogna A, Raggiunti M, De Pietro F, Ginaldi L, De Martinis M. Cholinergic urticaria, an effective and safe “Off label” Use of dupilumab: A case report with literature review. Clin Cosmet Investig Dermatol (2022) 15:253–60. doi: 10.2147/ccid.S343462
143. Maurer M, Altrichter S, Metz M, Zuberbier T, Church MK, Bergmann KC. Benefit from reslizumab treatment in a patient with chronic spontaneous urticaria and cold urticaria. J Eur Acad Dermatol Venereol (2018) 3:e112–e3. doi: 10.1111/jdv.14594
Keywords: chronic urticaria, chronic spontaneous urticaria, chronic inducible urticaria, atopy, IgE, mast cell
Citation: Chen Q, Yang X, Ni B and Song Z (2024) Atopy in chronic urticaria: an important yet overlooked issue. Front. Immunol. 15:1279976. doi: 10.3389/fimmu.2024.1279976
Received: 19 August 2023; Accepted: 22 January 2024;
Published: 06 February 2024.
Edited by:
Angelo Valerio Marzano, University of Milan, ItalyReviewed by:
Carlo Alberto Maronese, IRCCS Ca ‘Granda Foundation Maggiore Policlinico Hospital, ItalyMichael Makris, “Attikon” University Hospital, Greece
Copyright © 2024 Chen, Yang, Ni and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiqiang Song, ZHJzb25nenFAaG90bWFpbC5jb20=; Bing Ni, bmliaW5neGlAMTI2LmNvbQ==
†These authors share first authorship
 Qiquan Chen
Qiquan Chen Xianjie Yang
Xianjie Yang Bing Ni
Bing Ni Zhiqiang Song
Zhiqiang Song