- 1Department of Agricultural Microbiology, Faculty of Agriculture, Zagazig University, Zagazig, Egypt
- 2Department of Biochemistry, Faculty of Agriculture, Zagazig University, Zagazig, Egypt
- 3Department of Food Science, Faculty of Agriculture, Zagazig University, Zagazig, Egypt
- 4School of Food Science and Engineering, South China University of Technology, Guangzhou, China
- 5Department of Poultry Diseases, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt
- 6Department of Soils and Water, Faculty of Agriculture, Fayoum University, Fayoum, Egypt
- 7Molecular Cell Biology Unit, Division of Biochemistry, Department of Chemistry, Faculty of Science, Tanta University, Tanta, Egypt
- 8Biochemistry Division, Department of Chemistry, Faculty of Science, Tanta University, Tanta, Egypt
- 9Production Engineering and Mechanical Design Department, Faculty of Engineering, Menofia University, Menofia, Egypt
- 10Faculty of Control System and Robotics, Information Technologies, Mechanics and Optics (ITMO) University, Saint-Petersburg, Russia
- 11Plant Production Department (Horticulture-Pomology), Faculty of Agriculture, Saba Basha, Alexandria University, Alexandria, Egypt
- 12Biology Department, College of Science, King Khalid University, Abha, Saudi Arabia
- 13Department of Biology, College of Science, United Arab Emirates University, Al Ain, United Arab Emirates
- 14Department of Chemistry, College of Science, United Arab Emirates University, Al Ain, United Arab Emirates
- 15Faculty of Medicine, University of Debrecen, Debrecen, Hungary
- 16Harry Butler Institute, Murdoch University, Perth, WA, Australia
- 17Food Microbiology and Biotechnology Laboratory, Food and Nutritional Science Program, North Carolina A&T State University, Greensboro, NC, United States
Garlic (Allium sativum L.) is a widely abundant spice, known for its aroma and pungent flavor. It contains several bioactive compounds and offers a wide range of health benefits to humans, including those pertaining to nutrition, physiology, and medicine. Therefore, garlic is considered as one of the most effective disease-preventive diets. Many in vitro and in vivo studies have reported the sulfur-containing compounds, allicin and ajoene, for their effective anticancer, anti-diabetic, anti-inflammatory, antioxidant, antimicrobial, immune-boosting, and cardioprotective properties. As a rich natural source of bioactive compounds, including polysaccharides, saponins, tannins, linalool, geraniol, phellandrene, β-phellandrene, ajoene, alliin, S-allyl-mercapto cysteine, and β-phellandrene, garlic has many therapeutic applications and may play a role in drug development against various human diseases. In the current review, garlic and its major bioactive components along with their biological function and mechanisms of action for their role in disease prevention and therapy are discussed.
1 Introduction
Garlic (Allium sativum L.) is a member of the Liliaceae family (1). The genus Allium contain hundreds of species, including garlic, onion, shallot, leek and chive, that are known for their physiologically active secondary metabolites (2). Garlic is an ancient crop that is native to Central and South Asia, and northeastern Iran (3). Garlic has compact bulbs, and each bulb consists of several bulblets/cloves that are covered with a white skin (4). Its products, including garlic oil macerates, essential oils (EOs), garlic powder, and aged garlic extract (5) exhibit a noteworthy therapeutic impact (6). Thus, they are economically important due to their nutraceutical characteristics and health advantages (7).
The raw garlic-derived compounds contain organosulfur components susceptible to oxidation, volatilization, and deterioration when exposed to unfavorable conditions such as light, oxygen, and high temperatures (8). These components are also thermodynamically unstable. Encapsulation methods utilizing a range of wall materials and technologies were implemented to enhance the stability and suitability of the bioactive constituents in garlic for use in the food industry. The principal organosulfur components found in garlic essential oil are as follows: diallyl sulfide (DAS), dimethyl trisulfide, diallyl tetrasulfide, methyl disulfide, allyl methyl trisulfide, diallyl trisulfide, allyl disulfide, and allyl methyl tetrasulfide (9). Aqueous and alcoholic garlic extract (GE) contain S-allyl-mercapto cysteine (SAMC), S-methyl-l-cysteine, S-propenyl-l-cysteine, and S-allyl-cysteine, all of which are derived from γ-glutamyl-S-allyl-L-cysteines (10).
This comprehensive review provides recent experimental and clinical reports. It will encompass an analysis of the safety profile, metabolism pathway, bioavailability, biological and therapeutic effects, food-related applicability, adulteration detection methods, potential toxicities, and bioactive compounds derived from garlic. We believe that by publishing this review paper, more people will be interested in garlic and that it will provide up-to-date scientific data for enhanced garlic use in human health as well as illness management.
2 Methodology
2.1 Study design
The study reviews relevant and current literature on bioavailability, biological and therapeutic effects, food-related applicability, adulteration detection methods, potential toxicities, and bioactive compounds derived from garlic.
2.2 Data collection
Data were collected from related past and recent textbooks, proceedings, and research articles using PubMed, Scopus, Google Scholar, and Web of Science. The keywords were Allium sativum, bioactive compounds, functional foods, human health, human diseases, mechanisms of action, and toxicity.
All kinds of related articles and abstracts were included. Criteria were set to studies published in the last five years as much as possible. Following the outlined searches, articles were chosen based on their relevance to the objective of this review. Articles providing information in clear relation to garlic active compounds and their pharmacological effects, with a clear indication of their action mechanisms, were also included. A total of 235 articles were included in this review.
3 Botanical description
Garlic is an herbaceous plant with a fragrant stem segregated into 6 to 12 bulblets named garlic cloves, linked by a fine shell to create the garlic head (11). The roots of garlic emerge from the disc’s basal part and can grow to a depth of at least 80 cm closer to the plant’s base. Its leaves are morphologically long, narrow, and flat; however, the tip is cylindrical and sharp (12). The flowers are tiny and whitish purple.
Garlic cultivation necessitates thick clay soil, humus, and enough water (13). A 70–80 cm height characterizes the garlic plant; several leaves are 13–15, the weight of the bulb is 50 g with a diameter of 5 cm, the number of teeth is 12–13, the period of growth in sowing winter is 250 days, and 40-day delay (14). Table 1 summarizes the different parts of garlic, its active compounds, and their effects.
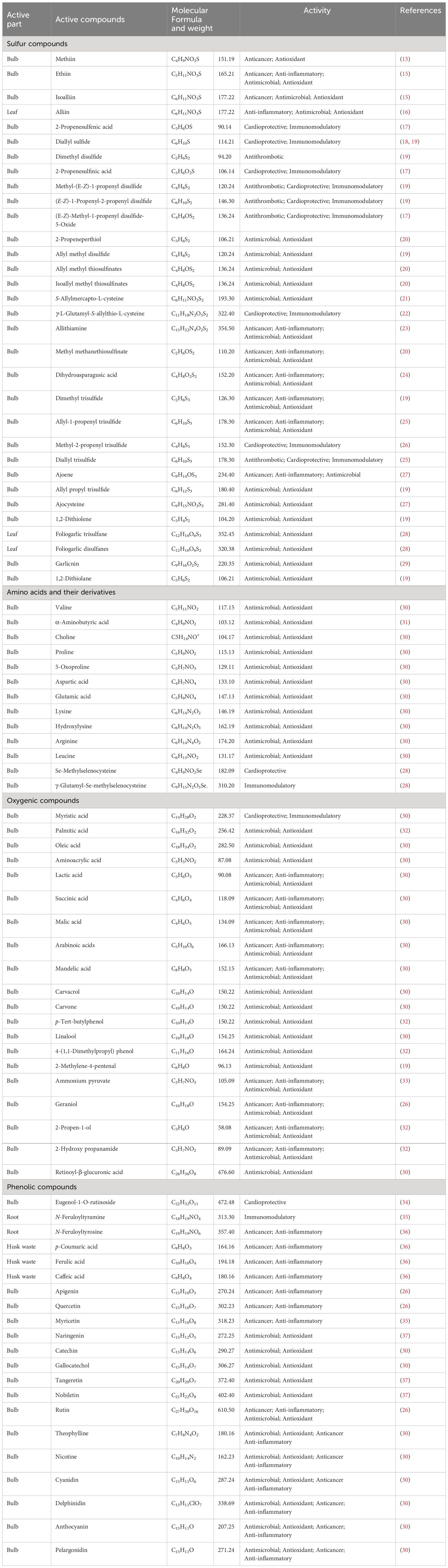
Table 1 The active compounds and their biological activities associated with different garlic parts.
4 Phytochemical compounds in garlic
Approximately 184 diverse types of active secondary metabolites are found in garlic (38). Almost 90% of these metabolites are characterized as the following: 70 organosulfur, 29 saponins, 16 flavonoids, 12 amino acids, and phenyl prostanoids 10 for each of phenyl prostanoids, 7 alkaloids, in addition to 3 compounds of fatty acids, and selenium derivatives (38). In leaves, the active secondary metabolites represent nine compounds, while in roots and husk wastes, there are 6 and 3 compounds, respectively. In general, there are 166 plant chemicals in bulbs and cloves (39).
4.1 Bioactive compounds of garlic
Due to its many phytochemicals, minerals, and dietary fiber, garlic is considered a valuable spice. Moderate amounts of selenium, calcium, magnesium, manganese, iron, vitamins A, C, and B complex, and sodium are also present. Potassium, zinc, sulfur, and phosphorus are all found in substantial concentrations (40). Dietary fiber comprises approximately 1.5% of the garlic bulb’s composition, followed by protein (2%), organosulfur substances (2.3%), carbohydrates (28%), and water (65%) (41).
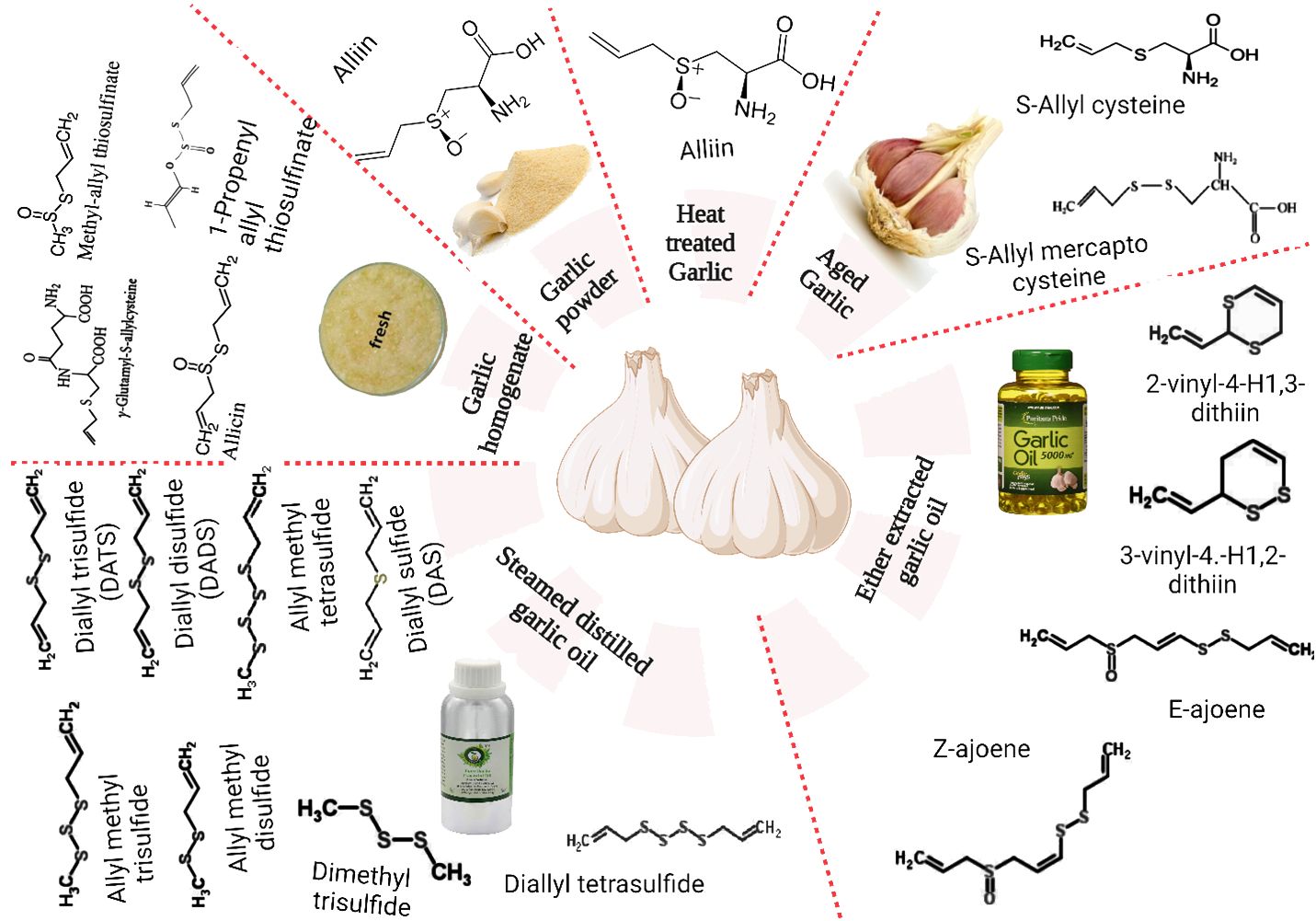
The principal bioactive components of these substances have recently sparked widespread scientific attention. These include a variety of substances such as phenols, enzymes (myrosinase, allinase, peroxidase), polysaccharides, saponins, tannins, linalool, geraniol, phellandrene, β-phellandrene, ajoene, alliin, SAMC, and β-phellandrene. A limited selection of the recognized polyphenolic constituents found in the matrix of garlic include apigenine, kaempferol-3-O-glucoside-7-O-alpha-l-rhamnoside, kaempferol 3,7-di-O-rhamnoside, luteoline, and kaempferol-3-O-glucoside (42). Furthermore, it comprises 17 amino acids, of which 8 are primary (43). Although intact garlic typically comprises advantageous constituents, further analysis has revealed the presence of additional substances, including DAS, dithiine, diallyl disulfide (DADS), allicin, and ajoene, which are the result of diverse chemical reactions occurring during pulverizing or chopping (44). Figure 1 depicts the major water-soluble and oil-soluble sulfur compounds present in garlic.
The primary chemical composition and profiles of bioactive components in garlic are significantly influenced by various factors, such as genotype, irrigation, temperature, light, and fertilization during the pre-harvest period; harvest and post-harvest conditions, ventilation, relative humidity, and temperature (45). As illustrated in Figure 1, dehydrated or whole garlic contains organosulfur components such as γ-glutamylcysteine and alliin (S-allylcysteine sulfoxide). These components are biogenic facilitators for generating additional sulfur byproducts throughout garlic production and heating (46). Alliinase enzymes (EC 4.4.1.14), which are initially limited to the cells of the vascular bundle sheath and rapidly convert alliin (which is categorized in mesophyll cells) to allicin, are released during cutting, crushing, or timidity (47).
4.2 Allicin contents
Allicin, the principal S-molecule in garlic, comprises approximately 70–80% of its bioactive compounds and is accountable for imparting its distinctive aroma (48). Allicin rapidly decomposes and generates other stable organosulfur constituents, including DADS, diallyl trisulfides, vinyldithiines (3-vinyl-4H-1,2-dithiin and 2-vinyl-4H-1,3-dithiin), DAS, and ajoenes (49). These constituents are sensitive to organic solvents, oxygen, temperature, and pH. The various physiological and biological attributes of garlic, including its antidiabetic, anti-cancer, anti-inflammatory, antiobesity, antioxidant, antifungal, antibacterial, and immunomodulatory properties, can be primarily attributed to the bioactive constituents present in its matrix. These include organosulfur compounds (e.g., allicin, DAS, allyl propyl disulfide, diallyl polysulfides, vinyldithiins, DADS, and diallyl trisulfide) (49).
4.3 E- and z-ajoene content
Ajoene is a group of organosulfur compounds with garlic-like characteristics (45). The biological activities of garlic are linked to various compounds, including ajoene, produced through allicin breakdown and non-enzymatic repair (50). In cancer cells, it has been shown triggering apoptosis (51); thus having an anti-cancer impact. Ajoene has been demonstrated to decrease the proliferation of lung cancer cells in some studies (52). Ajoene is also an antifungal medication that is safe and successful in treating chromoblastomycosis and dermatophytosis (53). Its inhibitory dose can be below 20 µg/ml (54, 55). Choi et al. (56) employed ajoene at 3 µg/ml extract concentrations to boost macrophage antimycobacterial activity. Ajoene can also disrupt biological machinery and is lethal to cancer cells at low µM concentrations (57).
5 Bioavailability of active compounds after metabolism
Allicin and other biological sulfur compounds found in garlic are believed to be the primary pharmacologically active components of the matrix due to their potent anticancer, antibacterial, and antioxidant properties. Absorption in the intestines is a critical factor in the health-promoting effects of the biological components in garlic. The absorption rate significantly influences the availability of these compounds (58).
Investigating how compounds are absorbed into the body, particularly in relation to food, is a fascinating trend in research across different fields (58). Based on in vitro experiments concerning the potential of raw garlic and its preparation in cancer treatment, in vivo studies and clinical trials have been initiated, albeit their contradictory results (59). Indeed, variations exist regarding the bioavailability of the sulfur-containing primary groups when comparing fresh garlic to specific culinary and medicinal preparations that incorporate garlic. In vivo research investigated the bioavailability of allicin from 13 garlic supplements and 9 meals in 13 participants by quantifying allyl methyl sulfide, the principal garlic metabolite, in their breath. The bioavailability of allicin supplements was found to be higher than freshly pulverized garlic (59).
Allicin is responsible for the majority of the pharmacological effects of raw garlic. When inhibiting enzymes in the gastrointestinal tract, it is swiftly biotransformed (half-life <1 min) to allyl mercaptan. Upon consuming a substantial quantity (25 g) of crushed fresh garlic, allicin and its metabolites have been detected in the urine, feces, and blood (60). Allicin is metabolized into secondary compounds, including E-ajoene, 2-ethenyl-4H-1, 3-dithiin, and DADS, much like how rapidly it exited the body following intravenous infusion (61).
Furthermore, approximately 30 min after ingestion, the bioavailability of allicin from enteric-coated tablet formulations varied between 36 and 104%. However, when inhaled with a protein-rich meal, the bioavailability decreased from 22 to 57% (59). The bioavailability of garlic capsules was 26–109% lower than that of non-enteric-coated tablets (59). Furthermore, the cysteine protein derivative interacts quantitatively with allicin at the temperature of the host gut to generate two equivalents of SAMC. This occurs when cysteine produced from ingested food protein interacts with allicin in garlic supplements in the gastrointestinal environment (59).
In an in vivo experiment using mice, the principal metabolites allyl methyl sulfone and allyl methyl sulfoxide were detected in the liver, stomach, plasma, and urine after DADS (200 mg/kg) was administered orally (62). Metabolic analysis of DADS radioactively tagged in mouse livers revealed that 70% of the radioactive substance was present in the cytoplasm; however, it did not exist in the form of DADS. DADS constituted 80% of the radioactivity (63). This research indicates that sulfur compounds derived from the metabolism of allicin are primarily responsible for physiological effects. One of the chemical compounds generated by these substances is hydrogen sulfide, which functions as a signal transduction substance within the human body (64).
Aged garlic extract is composed primarily of water-soluble organosulfur compounds, including S-allyl cysteine (SAC) and SAMC, which have a distinct pharmacokinetic profile compared to oil-soluble organosulfur elements (65). In addition, garlic is rapidly absorbed from the gastrointestinal tract, and SAC has a half-life of over 10 h and a renal clearance of over 30 h in humans. Based on SAC’s efficacy and safety assessment, it appears to significantly impact the biological characteristics (66). Effective in vitro extraction methods can reduce potential toxicity while enhancing the bioavailability of garlic. During the extraction of aged garlic extract, the garlic matrix’s undesirable constituents are transformed into S-components securely and consistently. Several toxicological investigations have also confirmed the safety of aged garlic extract (67).
It is noteworthy that although organic S-compounds (OSCs) have a low bioavailability in the host body, research indicates that incorporating garlic into one’s daily diet can provide sufficient quantities to support their biological effects. Aryl and methyl are the two most prevalent forms of OSCs identified in garlic extract (68). SAC was found to have a relatively high bioavailability in the blood plasma, kidneys, and liver of primary animal experiments involving canine and mouse models exposed to aqueous garlic extracts (69). Garlic yielded 570 μg of DADS, 2.5 mg of allicin, and 60 μg of SAC, all of which are equivalent to 1000 μg of diallyl trisulfides (69).
After ingestion, the purified allicin was not detected in the blood, urine, or feces (70). An element that contributes to the diminished biological accessibility of allicin is its tendency to form bonds with fatty acids and proteins across plasma membranes (71). Some hypotheses regarding these barriers encompassed the challenge of allicin evading the digestive tract via its interaction with the intestinal mucosa and its oxidative reaction with red blood cells (71). Furthermore, it was characterized by its high reactivity and rapid conversion to allyl mercaptan (AM) (71).
A simulated digestion method was employed to investigate the impact of temperature, duration, pH, and neutralization after acidification on thiosulfate (TS) synthesis from garlic powder and cloves (60). An optimal pH range of 4.5-5.0 was identified as the setting for synthesizing allicin, allyl 1-propenyl, and 1-propenyl allyl (72). The best formations of allyl methyl+methylallyl and 1-propenyl methyl+methyl 1-propenyl occurred between pH 6.5-7.0. Dimethyl generated thiosulfinate at pH 5.5 and no TS below pH 3.6 (72). However, the pH of the intestines and stomach did not significantly influence the bioavailability or gastrointestinal metabolism of allicin. Although overall temperature had a negligible impact on the production of TS, the allicin experienced the most excellent disintegration and degradation at 35°C (72). Hydrolyzing allicin and alliin produced water-soluble compounds with enhanced antioxidant, bioavailability, and stability properties (72).
It is noteworthy to mention that the abrasive sensation experienced during garlic consumption can be attributed to the vasodilatory effects of DADS and allicin, which are produced by allyl isothiocyanate neurons stimulated by capsaicin neural terminals (70). In addition, increasing the levels of bioavailable substances, including DAS, ajoene, diallyl trisulfide, DADS, and SAC, and altering the expression of the DMT1 mRNA gene, garlic consumption can enhance iron absorption from carbonyl iron (73). Shallots and leeks have also been proposed to increase iron bioavailability in cereals and legumes (74).
Evaluations of sulfur constituents in different garlic substrates via in vitro digestion revealed that garlic powder exhibited the most significant accessibility, followed by garlic cloves. Nevertheless, there was a reduction in allicin availability when it was ingested alongside high-protein meals due to the subsequent delayed gastric emptying (75). Diverse organic sulfides may be produced from garlic through its multifaceted processing. The organic sulfides in question demonstrate an extensive array of significant biological and therapeutic attributes (75). Additional research into the bioavailability of various organic sulfides is crucial for expanding the pharmaceutical and health product industries involving garlic.
6 Biological activities and nutraceutical properties of garlic
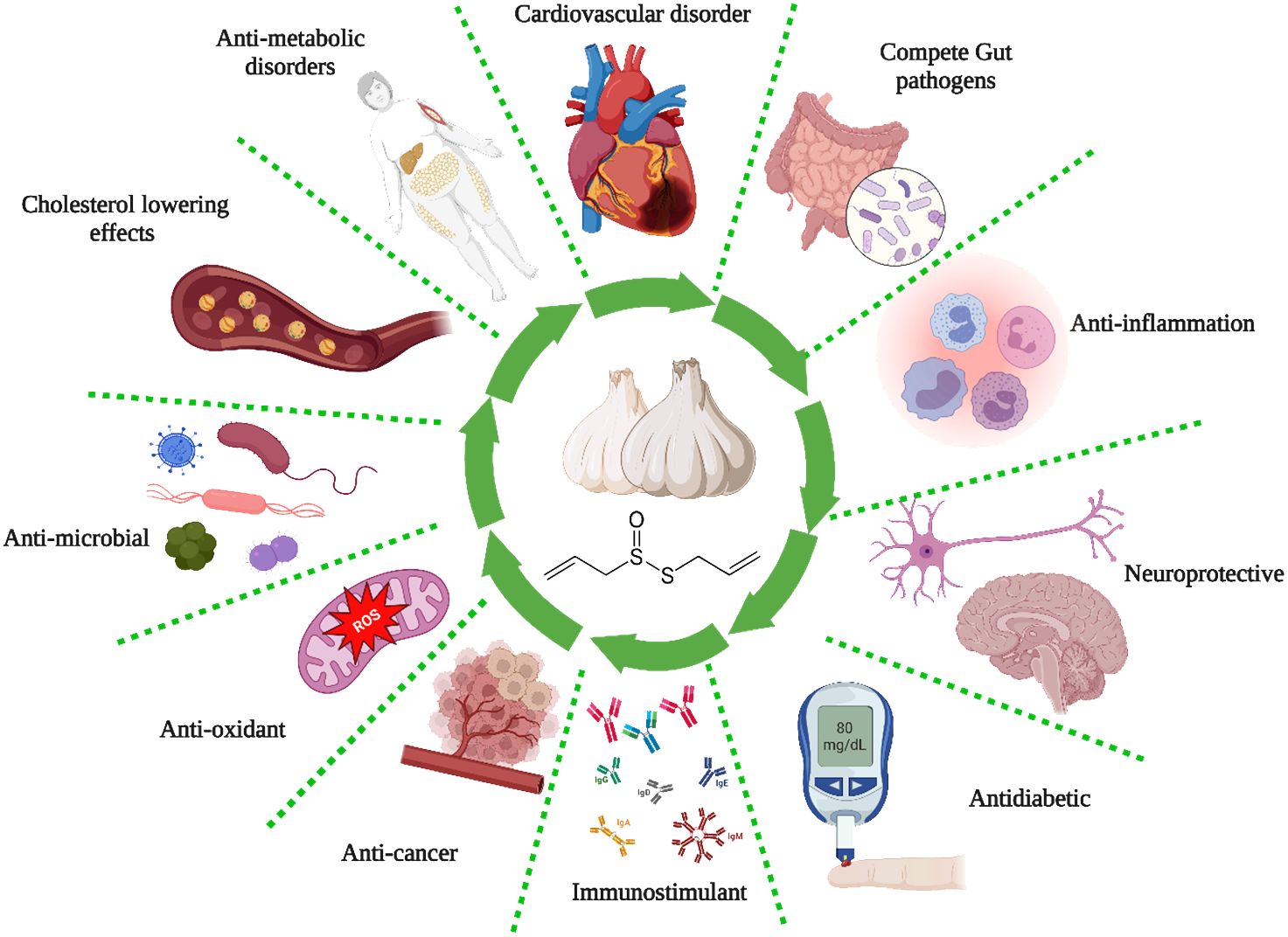
The ancient Egyptians well-documented the antiquity of garlic as a remedy for respiratory and gastrointestinal ailments. They also incorporated it into a diverse array of nutritional and therapeutic products (76). Louis Pasteur was the initial proponent of the antibacterial properties of garlic in 1858. The enzymatic activity of alliin alkyl-sulfonate-lyase transforms the inactive constituent of garlic, alliin (a sulfoxide derivative of the cysteine amino acid), into allicin (diallyl thiosulfinate), which is the bioactive element accountable for the antibacterial properties of garlic (76). Table 2 and Figure 2 summarize the different biological activities of garlic and garlic products.
6.1 Antimicrobial activity of garlic
The minimum inhibitory concentration (MIC), minimum sensitivity, and minimum bactericidal concentration (MBC) assays are frequently employed to quantify and communicate the antimicrobial properties of garlic and allicin (134). The antibacterial attributes of allicin found in garlic have been acknowledged for a considerable duration. Extensive research was conducted between 1944 and 1970 on bioeffects of garlic. These studies revealed that garlic extract exhibited exceptional antibacterial properties against Gram-positive and Gram-negative bacteria and specific medically significant fungi (135, 136). A comprehensive understanding of allicin’s antibacterial properties is still elusive; nevertheless, it has been established that it influences the free amino acid L-cysteine by generating allyl-disulfide species (136). Using the microdilution method, an in vitro study examined the antibacterial properties of synthetic allicin against 38 isolates of methicillin-resistant and methicillin-sensitive staphylococci (137).
Perez-Giraldo et al. (137) also assessed the effect of allicin on biofilm formation. The outcomes were indistinguishable between methicillin-sensitive and methicillin-resistant bacteria, and it was determined that all cultures examined demonstrated considerable sensitivity to allicin, as indicated by MIC values ranging from 0.25 to 16 mg/L. In an in vitro study using five strains of Staphylococcus epidermidis, it was reported that adding half the MIC of allicin substantially affected strain proliferation and biofilm formation (137). According to these findings, allicin might play a beneficial function in preventing the attachment of these microorganisms to medical equipment (137). The administration of allicin (128 µg/mL) substantially impacts the synthesis of extracellular polysaccharide components and their ability to adhere to surfaces, compared to a saline control group (138).
Allicin is also notable for its ability to prevent the growth of Pseudomonas aeruginosa biofilms and the quorum-sensing-regulated generation of virulence components by reducing the expression of specific enzymes and toxins (elastase and exotoxin A) (139). Thus, allicin may be a viable option for inhibiting the formation and development of P. aeruginosa biofilms (139). Research conducted by Shuford et al. (140) evaluated the efficacy of garlic extract as an anti-biofilm agent against both the sessile and planktonic strains of Candida albicans. They reported that adding fresh aqueous garlic extract may inhibit C. albicans biofilm development during the planktonic, adhesive, and sessile phases. Zhai et al. (141) analyzed the application of allicin with anti-biofilm properties. A scanning electron microscopy investigation into the impact of allicin and vancomycin on the growth of S. epidermidis biofilms was identified as the cause of aggregation and biofilm formation (141). In contrast to the individual effects of allicin and vancomycin, the results demonstrated that the combined effect of the two agents inhibits S. epidermidis growth, attachment, and biofilm formation on the outermost layer of a prosthesis (141).
The potential of garlic and its sulfur constituents as antiviral agents has been demonstrated in vivo. Globally speaking, the 2019 coronavirus disease epidemic (COVID-19) is the most critical public health crisis. As a result of the social distancing measures imposed by COVID-19, psychological, financial, and social welfare have undergone substantial transformations in recent years. Lung tissue inflammatory responses and pro-inflammatory pathways (such as IL-6 and IL-1) are primarily activated by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-caused COVID-19 pandemic (142). Viral expression of the angiotensin-converting enzyme 2 (ACE2) receptor can potentially induce harm to critical organs and tissues.
Furthermore, the dysregulation of the ACE2/angiotensin-(1–7)/mas axis and the renin-angiotensin system after SARS-CoV-2 infection worsens comorbidities and multi-organ lesions (143). A cure for the fundamental ailment that has initiated this epidemic remains unknown. Drugs such as hydroxychloroquine, remdesivir, umifenovir, favipiravir, ribavirin, lopinavir/ritonavir, and hydroxychloroquine, among others, may be administered as therapeutic interventions unless explicitly prohibited. In addition to dietary therapy and herbal medication, supportive treatments may effectively prevent the spread of COVID-19 (142, 143).
Certain foods and herbs contain bioactive substances with antioxidant, antimicrobial, and immunomodulatory properties that may enhance the function and quantity of natural killer cells, macrophages, lymphocytes, and cytokine suppressors (144). As a result, these substances may aid in pre- and post-exposure prophylaxis. As a result, herbal remedies alleviate the adverse effects of antivirals by reducing the necessary dosage and synergistically improving treatment and outcomes by reducing respiratory symptoms and inflammatory reactions (144). The numerous health benefits of garlic can be attributed to its sulfur-containing phytochemicals. These include preventing cardiac disease, regulating the immune system, lowering blood sugar levels, combating cancer, and reducing inflammation. The most prevalent sulfur compounds in garlic account for 82% of the total. These include allicin, diallyl (di and tri-) sulfide, ajoenes (E- and Z-ajoene), S-allyl cysteine sulfoxide, and vinyldithiins (2-vinyl-(4H)-1,3-dithiin, 3-vinyl-(4H)-1,2-dithiin). Garlic, apart from alliin, is known to comprise SAC, N-acetylcysteine, and S-allyl-mercapto cysteine, among other organosulfur compounds (145). Garlic has been found to possess antiviral properties against a range of viruses, including influenza B, HIV (type 1), herpes simplex virus (types 1 and 2), vesicular stomatitis virus, coxsackievirus, and gammaretrovirus (146).
Recent research has identified the positions of specific amino acids (Thr26, Asn119, Thr24) in the enzyme’s active site of the serine-like protease Mpro [chymotrypsin-like protease (3CLpro)] of SARS-primary CoV-2 (e.g., 2GTB and 6 LU7) (147). A significant degree of structural homology (96.0%) was observed between Mpro from SARS-CoV types 1 and 2. By impeding viral polyprotein cleavage, SARS-CoV-2 infection rates can be significantly diminished because viral polyprotein cleavage is essential for effective protein synthesis and viral replication (147).
In an in silico analysis of the inhibitory activity of garlic, seven onion-specific compounds (OSCs) were identified as resistant to SARS-CoV-2 (148). These compounds included S-allymercapto-cysteine, S-propyl-L-cysteine, S-(allyl/methyl/ethyl/propyl)-cysteine, and alliin. A molecular docking investigation demonstrated that, among the various cysteine sulphoxides, garlic alliin exhibited the most potent antiviral activity against COVID-19. SARS-CoV-2 could be eradicated by utilizing this bioactive component in isolation or combined with the widely recognized primary treatment medication (148).
The phytochemicals of black pepper, black cumin, and ginger exhibited a comparable inhibitory effect upon isolation (148). Garlic clove extract exhibited a significant inhibitory effect on the growth of SARS-CoV-1 in vivo at a concentration of 0.1 mL. This effect was likely mediated by the extract’s ability to impede the synthesis of genetic material and structural proteins (149). By inhibiting the protease found in SARS-CoV-1, quercetin might also impede the viral attachment phase of replication (150). Flavonoids (e.g., quercetin) and organosulfur compounds (e.g., allicin) present in aqueous extracts and essential oils of garlic may account for the decreased viral infection rate induced by SARS-CoV-2 through their interaction with the protease Mpro. Encapsulation is a technique to safeguard bioactive compounds’ functionality and oxidative stability (149, 150). Simultaneously, it permits the controlled release and distribution of these compounds to their designated sites of action within the body in the form of medication particles measuring microns or nanometers in size. Finally, to mitigate the transmission of COVID-19 among specific populations, it may be necessary to restrict their intake of foods containing free or encapsulated garlic bioactive ingredients to a moderate degree (150).
6.2 The potential anti-inflammatory effects of garlic
Garlic and its derived beneficial components have been found to induce suppression in numerous physiological disorders, such as cardiovascular complications, oxidative stress, cancer proliferation, and immunological dysfunction. NO2 and prostaglandin E-2 production were suppressed in lipopolysaccharide-stimulated RAW 264.7 macrophages by garlic ethyl linoleate (83). This was achieved through the downregulation of inducible NO synthase expression and the upregulation of heme oxygenase-1 expression. Garlic’s potential utility lies in its ability to augment natural killer cells, stimulate interferon-gamma synthesis, tumor necrosis factor-alpha, and interleukin-2, and counteract the immune response suppression associated with an elevated cancer risk (151). Furthermore, allicin can potentially mitigate the inflammatory response initiated by schistosome infection in BALB/c rodents, thus establishing itself as a feasible adjuvant therapy (84).
Furthermore, several research studies have indicated that individuals who are overweight or obese and suffer from osteoarthritis may benefit from garlic supplements as they reduce their resistance (85). Therefore, garlic effectively inhibited inflammatory pathways in both in vitro and in vivo investigations through the reduction of inflammatory mediators (including interleukin (IL)-1, tumor necrosis factor-alpha (TNF-α), and NO). Garlic exhibits potential as a therapeutic intervention for inflammatory conditions, including arthritis in humans, since its toxicity is minimal or non-existent (151). Figure 3 depicts the anti-inflammatory effects of garlic.
6.3 Garlic potential as an antioxidant and anticancer agents
According to a growing body of research, garlic exerts potent antioxidant effects. Aged garlic extract possesses greater antioxidant properties than fresh garlic and other available garlic supplements (28). The antioxidant activity of water-soluble organosulfur compounds such as SAC and SAMC is high. SAC and SAMC are the principal organosulphur components of aged garlic extract (152). DADS, DAS, and diallyl trisulfide are fat-soluble allyl sulfides with antioxidant potential (153). Raw garlic showed greater antioxidant activity than cooked garlic, according to a study that examined the antioxidant capabilities of raw and cooked garlic (154). The antioxidant capacity of stir-fried garlic was also greater (carotene bleaching), indicating that processing may affect the garlic’s antioxidant properties (154).
The ethanolic extract possesses more robust antioxidant activity than raw garlic, 2,2-diphenyl-1-picrylhydrazyl (DPPH), and oxygen radical absorption capacity (ORAC) confirmed these results (155). Additionally, DPPH, 2,2'-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS), ferric reducing antioxidant power, H2O2 scavenging, and Fe2+ chelating assays revealed that aged garlic extract possessed greater antioxidant properties than fresh garlic (156). Interestingly, single-clove garlic extract contained a higher concentration of phenolic compounds and exhibited more antioxidant activity than multi-clove garlic extract (129). In addition, heat treatment improved the antioxidant activity of black garlic, with the peak antioxidant capacity occurring on the 21st day of processing (157, 158). Moreover, the increased pressure enhanced the garlic paste’s antioxidant qualities (159). During fermentation, however, “Laba” garlic, a traditional Chinese garlic product, loses its antioxidant properties (160). Garlic saponins have been found to prevent H2O2-induced growth inhibition and DNA damage in C2C12 myoblasts generated from mice (78).
Garlic and its active constituents (phenols and saponins) are antioxidants (161). Various processing procedures also modulated the antioxidant activity of garlic (45). The antioxidant activity of raw garlic was greater than that of cooked garlic, and the antioxidant activity of raw garlic was more than that of cooked garlic (154). The potency of fermented garlic, such as black garlic, is greater than that of raw garlic (162). In addition, the cellular experiment demonstrated that the mechanism of garlic’s antioxidative effect might include the modulation of the Nrf-2-ARE pathway and the augmentation of antioxidant enzyme activity (78).
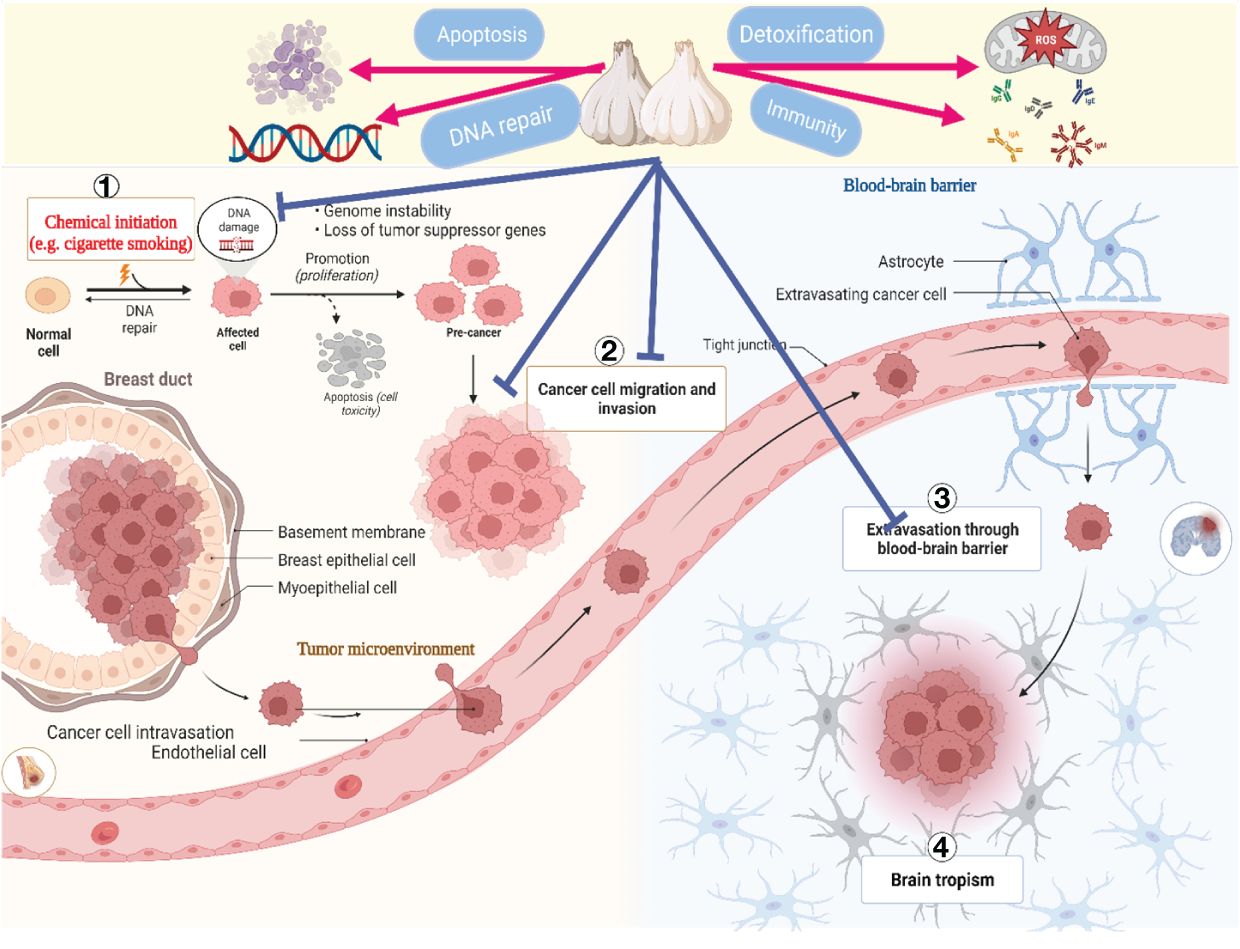
Even though cancer is a significant contributor to mortality worldwide, several spices and foods including ginger, berries, tomatoes, and cruciferous vegetables have been shown to possess potent anticancer properties (163). Recent research has demonstrated that garlic and its bioactive constituents inhibit the development of numerous types of cancer, including those of the gastrointestinal tract, colon, lungs, and urinary tract (102). Daily, individuals frequently encounter numerous carcinogenic substances (164). According to an in vitro study, garlic’s sulfur-containing compounds inhibited the activation of cancer-causing substances (165). Furthermore, the production of nitrosamines, typical carcinogens generated during heating and storage, could be inhibited by garlic and the organic allyl sulfides it produces (166).
Furthermore, the allyl sulfides in garlic inhibit DNA alkylation, an early step in developing nitrosamine carcinogenesis (167). Cancer cells are primarily characterized by their uncontrolled proliferation, a distinctive feature of the ailment. Previous studies (168) have documented the high sensitivity of various human cancer cell lines to the antiproliferative effects of unrefined garlic extract, including colon (Caco-2), prostate (PC-3), breast (MCF-7), and liver (HepG2) cancer cells (168). By inhibiting the phosphorylation of Cdc25C and Cdc2, they are increasing the expression of p21WAF1 and decreasing the phosphorylation of Cyclin B1 (168, 169). Garlic extract (107, 170) improved checkpoint kinase two and G2/M-phase cell modification. Research has demonstrated that whether in its unprocessed or ground state, garlic can upregulate the activity of apoptosis-related genes, including hypoxia-inducible factor 1, proto-oncogene c-jun, and aryl hydrocarbon receptor (118, 171).
Additionally, the aged garlic extract inhibited the motility, development, and tube formation of ECV304 endothelial cells, altering the cellular composition of rat lungs (79). Therefore, garlic and its bioactive constituents have the potential to serve as a beneficial strategy in the prevention and management of specific types of cancer (172, 173). Primary anticancer strategies include initiating apoptosis, inhibiting the growth and division of cancer cells, regulating the metabolism of carcinogenic agents, limiting the formation of new blood vessels, and minimizing invasion and metastasis (174, 175).
Figure 4 illustrates the process by which garlic’s bioactive components inhibit the growth of cancer cells.
6.4 The potential immunomodulatory effects of garlic
Garlic and its derived bioactive constituents, including organosulfur compounds, may enhance immune system performance via pathways such as oxidative stress, interleukins, immunological and inflammatory response, and others, according to numerous in vitro and in vivo experimental studies (176). Upon exposure to polysaccharides derived from garlic, RAW 264.7 macrophages exhibit distinct variations in tumor necrosis factor-α, IL-10, IL-6, and interferon-γ expression. These polysaccharides possess notable immunomodulatory properties (177).
Fresh garlic polysaccharides exhibit a more pronounced immunomodulatory impact than black garlic polysaccharides (93). This effect results from fructan degradation associated with the process (177). In an in vivo study, Li et al. (177) showed that by administering garlic oil to Wistar rats 30 min before diazinon, a range of immunological parameters were restored to normalcy. These parameters included the serum total immunoglobulin level and T-cell subtype CD4+. Additionally, research has demonstrated that administering aged garlic extract to individuals may reduce the severity of specific viral infections, such as the common cold, and strengthen the immune system (93). This indicates that garlic’s primary immune-modulating components are polysaccharides (93).
7 Therapeutical application of bioactive compounds in garlic
7.1 Anti-hypertensive function
Garlic may decrease oxidative stress, inhibit the angiotensin-converting enzyme, and increase the production of NO and H2S, all of which contribute to blood pressure reduction (178, 179). By increasing NO production, aged garlic extract induced endothelial-dependent vasodilation in individual rat aortic rings, according to one study. Moreover, L-arginine was crucial for NOS-mediated NO production in aged garlic extract (178). Additionally, it was demonstrated that S-1-propylene cysteine was the principal antihypertensive component in the aged garlic extract. It was demonstrated that administration of S-1-propylenecysteine decreased the systolic blood pressure of rats with spontaneous hypertension but had no effect on control rats (180). In an additional investigation, nitrites present in fermented garlic extract (FGE) were metabolized to NO through the action of Bacillus subtilis (180).
Furthermore, NO decreased systolic blood pressure in rodents with spontaneous hypertension via the protein kinase G (PKG)-soluble guanylyl cyclase (sGC)-cyclic guanosine monophosphate (cGMP) signaling pathway (181). By downregulating vascular endothelial cell adhesion molecule-1 and matrix metalloproteinase-9 (MMP-9) and upregulating PKG and endothelial nitric oxide synthase (eNOS), FGE decreased blood pressure in rats with pulmonary hypertension induced by monocrotaline (181). Furthermore, the efficacy of captopril in reducing hypertension and angiotensin-converting enzyme (ACE) in rodents was improved through the combination of captopril with garlic and its bioactive component, alliin (182). Systolic and diastolic blood pressures of 44 hypertensive participants registered for a double-blind, placebo-controlled study experienced a substantial reduction when garlic was prepared with enzymatic browning (183).
7.2 The impact of garlic on kidney and liver functions
Recent in vivo studies have demonstrated garlic’s effective reduction of nephrotoxicity (184). After being exposed to garlic’s aqueous component, the kidneys of diabetic rats showed a reduction in oxidative stress (132). In addition, the water-based element of garlic improved the levels of renal plasma biochemical markers in Wistar rat models that were affected by alloxan (109). In addition, studies have shown that diallyl trisulfide can activate the Nrf2-ARE pathway in rats, which helps protect kidney tissue from oxidative stress caused by arsenic (174).
Numerous studies have indicated that various natural compounds, such as garlic, can positively impact liver function (185). In an in vivo study, researchers discovered that a natural extract derived from black garlic significantly reduced the harmful effects of tert-butyl hydroperoxide on rat clone-9 hepatocytes (112). This extract effectively countered oxidative stress, lipid peroxidation, inflammation, and apoptosis, showcasing its potential as a valuable therapeutic agent. Ascorbic acid and garlic effectively prevented liver damage caused by Cd in an albino mouse model (112). An in vivo study using a male rabbit model found that a single clove of garlic provided more excellent protection against acute liver damage induced by CCl4 compared to multiple cloves of garlic (129).
Furthermore, it has been proposed that the active compounds found in garlic, specifically S-methyl-l-cysteine, DADS, and DAS may have potential applications in treating and preventing liver damage caused by ethanol. A study found that DADS in garlic essential oil reduced the effects of nonalcoholic fatty liver disease caused by a high-fat diet in rats (186). Through its effects on cytochrome P450 2E1, DADS enhanced antioxidant capacity and decreased pro-inflammatory cytokine production in liver tissue (186). Lai et al. (130) reported that fermented garlic extract, shows potential as a treatment for chronic hepatic diseases (130).
In a study by Kim et al. (187), a randomized, double-blind, placebo-controlled trial was conducted to examine the impact of fermented garlic extract on hepatic activity in 36 individuals with moderately elevated blood gamma-glutamyl transpeptidase concentrations. Based on the results, no unexpected outcomes were linked to the increased alanine aminotransferase levels and gamma-glutamyl transpeptidase. Therefore, garlic has the potential to treat either condition effectively.
7.3 The impact of garlic on the digestive system
Garlic has been extensively studied for its positive impact on digestive processes. In a study conducted in a laboratory setting, it was found that black garlic extract has the potential to enhance bowel movements. This is achieved by promoting bowel emptying and stimulating peristaltic action. An in vitro study found that the aquatic fraction of black garlic had a more significant impact on improving gastrointestinal function in the small intestine than the n-butanol and ethyl acetate mixture (116). Additionally, therapy involving cabbage and garlic extract showed a decrease in the severity of gastric ulcers and a reduction in gastric juice volume, stomach acid levels, microbial count, and histological alterations. This type of treatment improved stomach acidity in rat models (188).
In vivo rat models, aged garlic extract effectively reduced the development of ulcers caused by indomethacin. This was achieved by increasing the levels of prostaglandin E-2 and glutathione while also reducing oxidative stress in the stomach tissue of the rats (90). Like a pharmacologist, DADS was found to suppress the expression of signal transducer and transcription 1 (STAT-1) activator in interferon-induced intestinal cells (117).
Additionally, DAS was observed to suppress the expression of interferon-inducible protein-10, interleukin-6, and nitric oxide. In a mouse model, the colitis caused by dinitrobenzene sulfonic acid was made worse by including DAS and DADS (117). Raw garlic has been shown to positively impact microbial urease function and Helicobacter pylori levels in the stomachs of patients (121).
7.4 The impact of garlic on cardiovascular functioning
In recent years, there has been a significant increase in the death rate from cardiovascular illnesses (189). There has been a recent surge in interest in utilizing natural crops to support optimal heart health, and garlic stands out as one of the most promising choices (190). Eating garlic powder has been shown to reduce several risk factors for cardiovascular disease, including total cholesterol, blood pressure, and low-density lipoprotein cholesterol (191).
Garlic has the potential to offer various health benefits in this field. These include an increase in the production of NO and H2S, a reduction in oxidative stress, and the ability to inhibit the angiotensin-converting enzyme, which can help lower blood pressure (117). Research conducted by Takashima et al. (178) suggests that aged garlic extract may have the potential to stimulate NO synthesis and promote endothelial-dependent vasodilation in the aortic rings of rats. It was found that the L-arginine in aged garlic extract plays a crucial role in NOS-mediated NO production (117).
Garlic has the potential to support the maintenance of the heart. Garlic when combined with allicin, the rat model showed enhanced antihypertensive and ACE-lowering effects of captopril (182). In an animal model of insulin resistance and obesity, garlic extract effectively reduced cardiac and mitochondrial dysfunction (192). When allicin comes into contact with thiols, it transforms organic diallyl polysulfide. This compound plays a crucial role in supplying H2S to support the proper functioning of the heart (193). Given these discoveries, garlic has been the focus of numerous clinical trials exploring its potential in treating hyperlipidemia, hypertension, and cardiovascular disease. Garlic may exert its effects through various mechanisms, including modulation of NO and H2S production, reduction of oxidative stress, and inhibition of angiotensin-converting enzyme activity (193).
7.5 Anti-diabetic activity
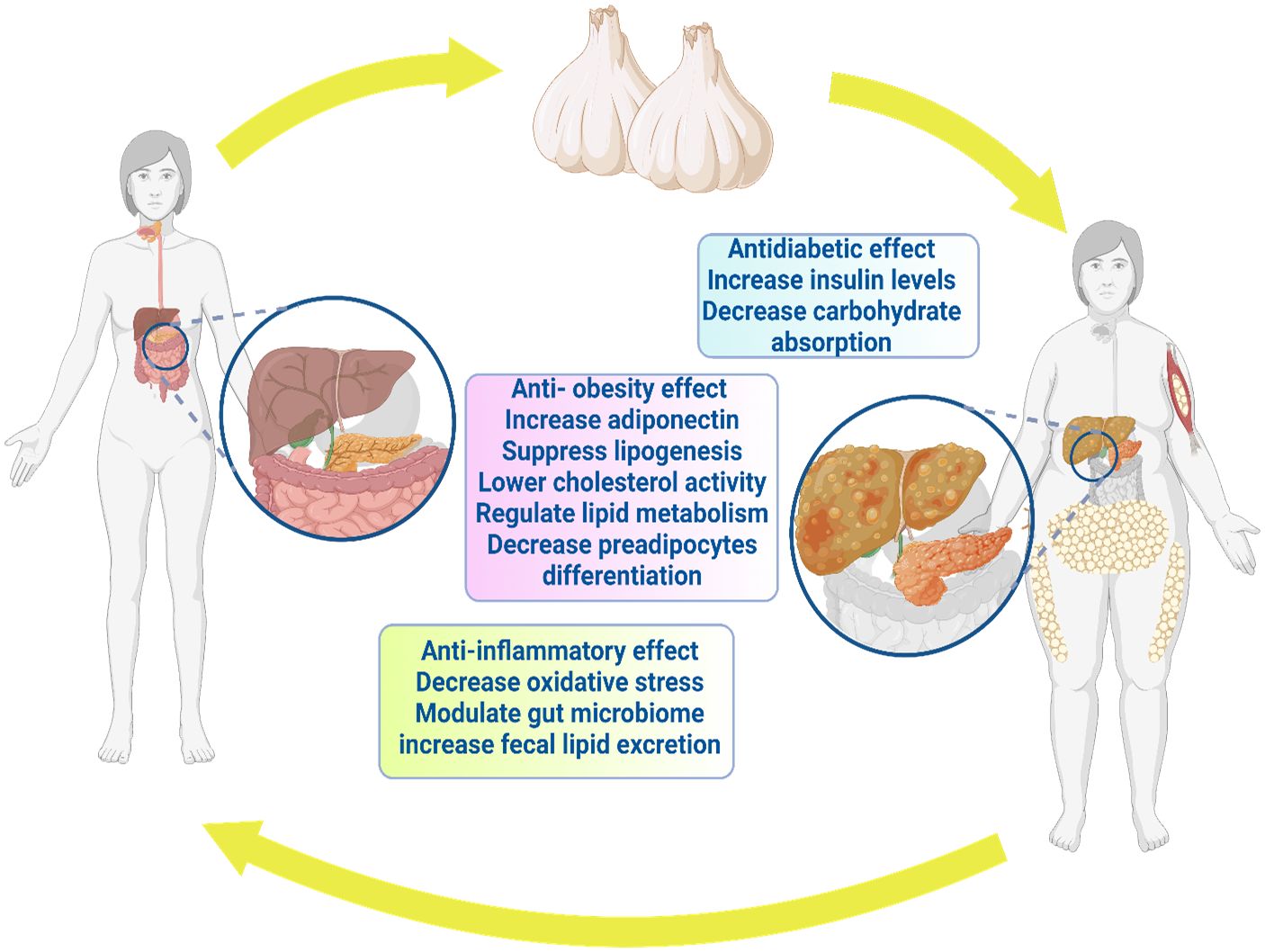
Regarding diabetes, garlic prevented pancreatic cell damage and associated pathological alterations (194). Moreover, diabetes-linked retinopathy risk was significantly reduced through garlic (195). Al-Brakati (119) addressed significant improvement in retinal tissue after seven weeks of oral administration of raw garlic. Moreover, Thomson et al. (196) noticed the favorable modulatory effect of garlic on body weight and blood glucose level. Aged garlic extract pointed out that antidiabetic behavior correlated with dosage (196). Garlic supplements showed reduced fructosamine and glycosylated hemoglobin in type 2 diabetes patients (174). Consistently, garlic has shown potential in treating type 2 diabetes (197). In conclusion, garlic can be a candidate for protecting and treating diabetes and diabetic disorders (198). Figure 3 depicts the antidiabetic benefits of garlic.
7.6 Anti-obesity activity
Garlic and sulfur-containing compounds can help lose weight by inhibiting 3T3-L1 adipocytes and acetyl-CoA carboxylase (ACC-1) (120, 199). Lee et al. (200) addressed an activation in adenosine monophosphate-activated protein kinase (AMPK)I in addition to carnitine palmitoyl transferase (CPT-1). Consistently (201), found an increase in UCP-1 through Kruppel-like factor 15 (KLF15), linked to brown adipocytes that represent a crucial phenotype for protection against obesity. Furthermore, in the diet-induced obesity model, fermented garlic (250 and 500 mg/kg) revealed lowered body weight with underlying decreased cholesterol levels. Garlic oil reduced body weight and white adipose tissue quantity in the high-fat diet model (201). El-Demerdash et al. (202) observed an improved O2 consumption during the night with interesting increased energy expenditure during the daytime. Similarly, garlic extract in water was linked to hypoglycemic impact (202).
Sanie-Jahromi et al. (203) noted significant hypoglycemic impact among garlic compounds, alliin, allyl sulfide, and glibenclamide (203). Garlic oil (10 mg/kg i.p.) and diallyl trisulfides promoted both sensitivity and secretion of insulin in diabetic rat models (28, 202, 204). In high-fat diet mice, lactic acid bacteria fermented garlic extract (LABFGE) showed an anti-obesity property with significantly decreased body weight and adipose tissue (113). LABFGE's ability to reduce lipogenesis is attributed to a decrease in the expression of lipogenic proteins (113). Methanolic extract of black garlic (MEBG) reversed the weight gain of a high-fat diet (205). MEBG modulated the network of adipogenesis, adipokine biosynthesis, and lipolysis, such as upregulation of AMPK, FOXO1, and adiponectin, whereas MEBG downregulated CD36 and TNF-α in HFD rats (115). Therefore, we could address that garlic and garlic-derived compounds have a potential role as anti-obesity and can reduce lipogenesis. The anti-obesity properties of garlic are illustrated in Figure 3.
7.7 Cardiovascular and neuroprotection
In recent years, cardiovascular disease has been the leading cause of fatality (190, 191). Recently, interest in using natural crops to support cardiac health has increased, and garlic is among the most promising candidates (190). Several cardiovascular disease risk factors, including low-density lipoprotein cholesterol, total cholesterol, and blood pressure, are reduced by consuming garlic powder (194). Potential prospective health benefits of garlic in this domain include the following: (1) increased production of NO and H2S; (2) reduced oxidative stress; and (3) inhibition of the angiotensin-converting enzyme, which ultimately results in reduced blood pressure (117).
Takashima et al. (178) conducted research suggesting that administering matured garlic extract to rodents could induce endothelial-dependent vasodilation in the aortic rings by stimulating NO synthesis. In addition, the L-arginine in aged garlic extract was found to be crucial for NOS-mediated NO production (178). Furthermore, adding garlic and allicin enhanced captopril’s antihypertensive and ACE-lowering effects in the rodent model (182). An additional organ that garlic may aid in maintaining is the heart. Cardiovascular and mitochondrial dysfunction were mitigated by garlic extract in an animal model of obesity and insulin resistance (192). Allicin was readily converted to organic diallyl polysulfide upon exposure to thiols; this compound supplied H2S to sustain the heart’s activity (203). In light of these findings, garlic has been the subject of numerous clinical trials regarding its potential to treat hyperlipidemia, hypertension, and cardiovascular diseases (203). Potential principal mechanisms of action for garlic include alterations in the production of NO and H2S, a reduction in oxidative stress, and a decrease in the activity of angiotensin-converting enzyme (203).
It was reported that an aged garlic extract-carbohydrate derivative, N-α-(1-deoxy-D-fructos-1-yl)-L-arginine, could reduce neurological inflammation in a mouse model of BV-2 microglial cells activated lipopolysaccharide by regulating the expression of numerous oxidative stress-related protein targets and inhibiting NO production (192, 193). Ho and Su (206) suggested that the anti-neuritis effect of garlic against lipopolysaccharide-activated BV-2 microglial cells (206) was due to its organosulfur components. Supplementation with garlic in pregnant and lactating rats reduced blood and brain Pb concentrations and prevented Pb-induced neuronal loss during hippocampal development (207).
Utilizing the Basso, Beattie, and Bresnahan (BBB) scoring system, the neurological impact of aging on a spinal cord I/R model of rats was evaluated (208). Significantly higher BBB scores were observed in the aged garlic extract group compared to the I/R group, suggesting that aged garlic extract exerted a substantial neuroprotective effect. Aged garlic extract also increased levels of glutamate decarboxylase and vesicular glutamate transporter 1 in the hippocampus, which enhanced working memory in rodents, additionally, it reduced the loss of cholinergic neurons (208).
Furthermore, research has demonstrated that a garlic ethanol extract can enhance memory (88). Garlic treatment increased the concentrations of Na+/K+ ATPase, Ca2+ ATPase, and glutamine synthetase in the brains of diabetic Wistar rats (209). In addition, the detrimental effects of monosodium glutamate on working memory were effectively counterbalanced by the fermented garlic ethanol extract (210). Z-ajoene has demonstrated advantageous effects in the CA1 region of the hippocampus by decreasing lipid peroxidation and mitigating delayed neuronal mortality and gliosis induced by I/R stimulation (111). SAC ameliorated rat cognitive impairment due to reduced acetylcholinesterase activity, neuroinflammation, astrogliosis, and oxidative stress (110). The neuroprotective effects of garlic primarily target the hippocampus, as demonstrated by both in vivo and in vitro studies. Substantial evidence indicates that organic sulfur compounds play a vital function in protecting neurons (110). The neuroprotective properties of garlic are illustrated in Figure 5.
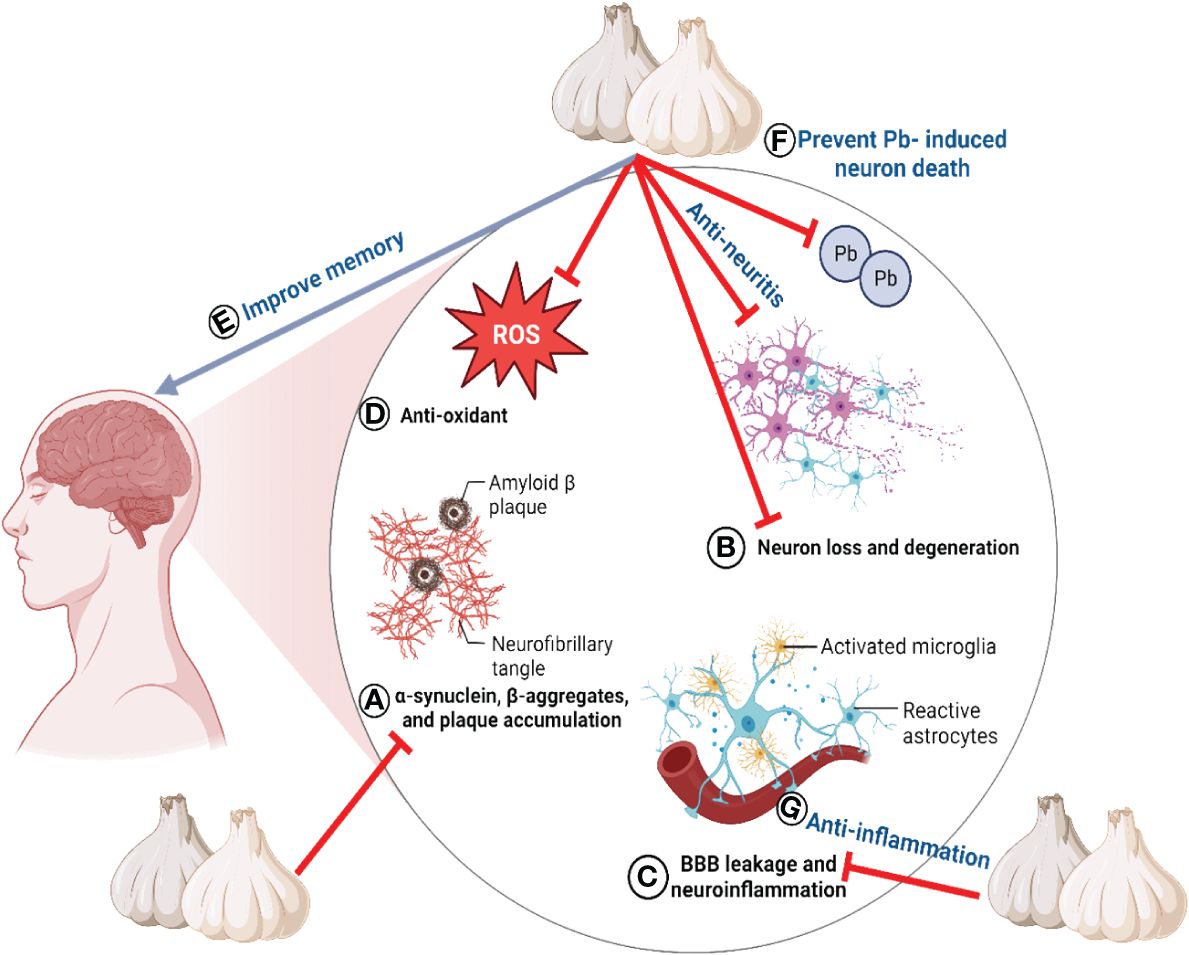
Figure 5 The neuroprotective properties of garlic. All lables (A–G) are the sequence of events/properties of garlic toward neuroprotection.
8 Safety of garlic bioactive compounds and their derivatives
8.1 Suggested dosage
The elderly are advised to consume four grams of fresh garlic, seven and a half grams of matured garlic extract, or one dried garlic powder tablet three or four times daily (211). In their study, Rana et al. (212) demonstrated that administering garlic orally or intraperitoneally to rats at a 50 mg/kg dose had no discernible impact on liver and lung tissue. However, rats consuming garlic daily at doses of 250, 500, and 1000 mg/kg developed acute alterations in liver and lung tissue, indicating the presence of dose-dependent toxicity (212). Upon light microscopy and ultrastructural analysis, morphological abnormalities in the liver were observed at a daily dosage of 1000 mg/kg garlic. In contrast, auto-antioxidant levels were significantly reduced at 500 and 1000 mg/kg garlic concentrations without affecting lipid peroxidation levels. Histological analysis further demonstrated the presence of nonspecific focal injury on hepatocytes (212).
A study by Mikaili et al. (213) demonstrated that biological and histological markers in male and female rodents were altered, and development was halted at 300 and 600 mg of garlic bulb extract. Asdaq and Inamdar (214) showed that the combination of 250 mg/kg of garlic and hydrochlorothiazide offers cardioprotective and synergistic effects against fructose and isoproterenol toxicity. Antioxidant enzyme activity was substantially increased in the presence of propranolol and 250 mg/kg garlic during ischemia-induced injury (214).
8.2 Hazards and adverse reactions
The US Food and Drug Administration (FDA) has determined that garlic is safe for human consumption; however, sensitive stomachs may experience distress when consuming large quantities of garlic. Randomized controlled trials examining the safety of garlic documented the following adverse effects: insomnia, dizziness, emesis, tachycardia, headache, flushing, mild orthostatic hypotension, sweat, discomfort, foul body odor, and defecation (212). When consumed in excess on an empty stomach, fresh garlic can cause intestinal flora to shift, flatulence, and bloating. The topical application of fresh garlic also led to the development of excruciating lesions, rashes, and burns (215).
In contrast to the prevailing notion that garlic does not affect medication metabolism, recent studies involving healthy volunteers indicated that its antithrombotic properties might result in an inconsistent effect of garlic on the pharmacokinetics of anticoagulants and protease inhibitors (216). As it has been shown to prolong bleeding in one individual with an epidural spontaneous hematoma, many surgeons advise against consuming large quantities of garlic 7–10 days before surgery (216).
Long-term, high-dose exposure to raw garlic in animals results in anemia and weight loss due to the lysis of red blood cells (RBCs), whereas 5 mL/kg of raw garlic liquid injected into animals induces gastrointestinal damage and mortality. Spermatogenesis is inhibited in rodents when 50 milligrams of garlic powder are administered daily for an extended period (212). This results in decreased sialic acid levels in the testes, the bladder, and seminal vesicles and decreased Leydig cell activity (212). As evidenced by the formation of Heinz bodies in RBCs and the development of methemoglobinemia, oxidative hemolysis is the principal toxicological mechanism by which sulfur compounds derived from Allium (217). Initial clinical manifestations included depression, appetite loss, vomiting, abdominal discomfort, anemia manifested by pallid mucous membranes, diarrhea, jaundice, lethargy, accelerated heart and respiratory rates, and hemoglobinuria (218). The onset of symptoms associated with garlic poisoning can vary significantly, spanning from one day to several weeks following ingestion (218).
Anticoagulant and fibrinolytic properties, in addition to a significant decrease in platelet production and conflicting effects on fibrinolytic effectiveness, have been documented in garlic, as reported in prior studies (219). Upon oral ingestion, dehydrated raw garlic powder damages the stomach mucosa promptly, as demonstrated by Chen et al. (220). However, experimental mice’s intestinal mucosa is protected by the sulfur-free compound aged garlic extract, as reported by Yüncü et al. (221). Research studies have established that while garlic is deemed safe when consumed in moderation amounts, therapeutic doses may induce gastrointestinal distress, and excessive quantities have been linked to liver harm (188). Allicin’s potential toxicity could be attributed to its capacity to traverse cellular membranes and interact with cellular thiols, such as glutathione or cysteine residues in proteins (132), as well as enzymes incorporating reactive cysteine (222). Rana et al. (212) discovered that allicin or garlic powder at a concentration of 200 mg/mL could potentially cause severe cell injury in the livers of isolated rats (212).
9 Food application of garlic bioactive compounds and their derivatives
Food illness and decay may result from pathogenic bacteria or other microorganisms. Antibiotics are frequently employed in the fight against these microorganisms. Antioxidants are gaining popularity as a method of preventing nutrient losses and extending the storage life of foods by preventing oxidation. However, excessive antibiotic use may lead to the emergence of antibiotic resistance (142). The majority of artificial antimicrobial agents authorized for use as food preservatives by regulatory bodies, based on studies conducted by Gutiérrez-del-Ro et al. (223), pose a risk to consumer health.
Sulfites, a class of thiol compounds used as commercial food preservatives, have been associated with numerous adverse nutritional effects, including ingesting thiamine, commonly referred to as vitamin B1. Therefore, preservatives derived from naturally generated antimicrobial compounds discovered in microorganisms, plants, and animals are widely employed. Antimicrobials prevent the spoilage of food and impede the transmission of disease (224).
Many individuals use natural and safer preservatives to impart an “organic” or “green” appearance to their food. Investigations have been directed toward the potential use of essential oils derived from botanicals as food preservatives (225). Garlic in food storage extends the expiration life by six days when refrigerated and slows the rate of food spoilage caused by microorganisms, according to recent studies (226). The utilization of garlic paste extended the freshness of Nile tilapia by over three days, according to research conducted by Kombat et al. (227).
Research on extending the shelf life of ready-to-eat (RTE) products has primarily focused on utilizing natural organic essential oils (EOs) and coatings. The extraction of plants EOs results in low harvests and high costs (228, 229). Therefore, in culinary applications, plant aqueous extracts may function as acceptable alternatives to natural essential oils (230). Diao et al. (231) investigated the preservation effect of carboxymethyl chitosan (CMCS) ultrasonicated coating modified with garlic aqueous extracts on RTE fragrant poultry. By employing ultrasonic processing, CMCS, and garlic aqueous extracts were transformed into a rudimentary nano-coating solution that satisfies the safety requirements for human consumption. Utilizing the ultrasonicated coating method devised with garlic aqueous extracts-CMCS may be advantageous for RTE poultry meat (231).
Garlic essential oil derives its antibacterial properties from its substantial concentration of organosulfur compounds. Primarily present in garlic essential oil, allicin is susceptible to degradation when exposed to acidity and temperature shocks. Encapsulation may enhance the stability and solubility of the substance in water-based foods. Additionally, encapsulation obscures the foul, pungent odor of pure garlic EOs (232). A bioactive component in liquid, solid, or gaseous state is encapsulated within a matrix of inert material via nanoencapsulation to maintain coated items. Nanoencapsulation can potentially enhance the sensory attributes of products through the augmentation of bioactive chemical stability and the obfuscation of offensive flavors and aromas (233).
Phospholipids and naturally active phytochemicals that are structurally bound are produced when phosphatidylcholine (or another hydrophilic polar head group) reacts with plant extracts in an aprotic solvent to form phytosomes. The findings of Nazari et al. (58) indicated that garlic essential oil nanophytosomes have the potential to exhibit desirable physicochemical characteristics, particularly when incorporated into acidic food products (58). Youssef et al. (234) also created bio-nano composites from Arabic gum (AG), carboxymethyl cellulose (CMC), titanium dioxide (TiO2-NPs), and gelatin (GL) using a solution casting technique, with and without varying volumes of garlic extract. The ability of fresh Nile tilapia fillets to retain their quality in the presence of various pollutants was examined (234). According to the findings, garlic extract-incorporated CMC/AG/GL/GE–TiO2 bio-nanocomposites demonstrated favorable thermal, mechanical, and morphological characteristics (234). The application of CMC/AG/GL/GE – TiO2 bio-nanocomposites containing 4% and 8% garlic extract on Nile tilapia fillets inhibited bacterial growth, reduced water loss during cold storage for 21 days (compared to uncoated samples), and extended the shelf life by one week (234).
By utilizing nano-coating technology, it is possible to extend the freshness of food significantly. To extend the shelf life of various products, antimicrobial and antioxidant properties are generated through the combination of hydrophilic and lipophilic substances, such as plant extracts or other carbohydrates, with metal nanoparticles, including zinc oxide, copper oxide, titanium dioxide, silver, and gold nanoparticles (235).
10 Conclusions
The potential of garlic in treating various chronic or acute conditions has been the subject of extensive research, as elaborated upon in the current review. The substantial abundance of phytochemicals present enhances many physiological processes. Protein, tryptophan, selenium, manganese, vitamins B1 and B6, and tryptophan are some nutrients found in garlic. The elements above synergize against a diverse range of health-threatening agents. However, further research is required to determine their exact mechanisms of action. Sulfoxide, s-methyl-1-cysteine, linalool, geraniol, and citral are a few of the sulfur-containing compounds found in garlic that have been associated with beneficial effects on health. Thiosulfinates and cysteine sulfoxides are two organic sulfur compounds associated with pharmacological activity. Garlic is increasingly recognized and utilized as fresh garlic juice, oil, garlic extract, and garlic powder, becoming increasingly popular as a natural remedy for various physiological disorders. Due to the unique amalgamation of bioactive constituents in Allium vegetables, their incorporation into our daily diet is imperative. Studies conducted in vitro and in vivo have demonstrated that garlic is beneficial in combating a range of metabolic disorders.
Garlic’s hypocholesterolemic, hypoglycemic, anticoagulant, antifungal, antioxidant, and antithrombotic attributes render it a multipurpose sustenance and medicinal substance. It is necessary to isolate and define garlic’s relative compounds and conduct additional research into its biological functions. Additional research is necessary to comprehend garlic’s mechanisms of action fully. Additional investigation is warranted to ascertain the potential impact of processing techniques such as fermentation and heat on garlic, as such knowledge could compromise the herb’s efficacy and safety. Further human clinical trials are required to validate the health benefits of garlic, with an emphasis on assessing its potential safety and adverse effects.
Author contributions
ME-S: Writing – original draft, Formal analysis, Data curation. AS: Writing – original draft, Formal analysis, Data curation. SK: Writing – original draft, Software. HS: Writing – original draft, Resources. TAE-M: Writing – original draft, Software, Data curation. SA: Writing – original draft, Software, Formal analysis. ME: Writing – original draft, Software. SE: Writing – original draft, Software, Data curation. WM: Writing – original draft, Software, Resources. AAH: Writing – original draft, Software, Data curation. BM: Writing – original draft, Data curation. NA: Writing – original draft, Data curation. AAl: Writing – original draft, Software. ME-T: Writing – original draft, Software. SAQ: Writing – review & editing, Formal analysis. KE-T: Writing – review & editing, Investigation. SI: Writing – review & editing, Supervision, Resources.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This project was funded by the Khalifa Center for Biotechnology and Genetic Engineering-UAEU (Grant number: 12R028) to SAQ. The authors are grateful to the Deanship of Scientific Research at King Khalid University for funding this work through the large group research project under grant number (R.G.P.2/326/45).
Acknowledgments
This publication was made possible by grant number NC.X-267-5-12-170-1 from the National Institute of Food and Agriculture (NIFA) and the Department of Family and Consumer Sciences and the Agriculture Research Station at North Carolina Agriculture and Technical State University (Greensboro, NC, USA 27411). This work was also supported, in part, by 1890 Capacity Building Program grant no. (2020-38821-31113/project accession no. 021765). The work was also financially supported by Abu Dhabi Award for Research Excellence-Department of Education and Knowledge (Grant number: 21S105); and UAEU program of Advanced Research (Grant number: 21S169) to KE-T.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Harris JC, Cottrell SL, Plummer S, Lloyd D. Antimicrobial properties of Allium sativum (garlic). Appl Microbiol Biotechnol. (2001) 57:282–6. doi: 10.1007/s002530100722
2. Ramírez-Concepción HR, Castro-Velasco LN, Martínez-Santiago E. Efectos terapéuticos del ajo (Allium sativum). Rev Salud y Administración. (2016) 3:39–47.
3. Li H-J, Jiang Y, Li P. Chemistry, bioactivity and geographical diversity of steroidal alkaloids from the Liliaceae family. Nat Prob Rep. (2006) 23:735–52. doi: 10.1039/b609306j
4. Li J, Dadmohammadi Y, Abbaspourrad A. Flavor components, precursors, formation mechanisms, production and characterization methods: Garlic, onion, and chili pepper flavors. Crit Rev Food Sci Nutr. (2022) 62:8265–87. doi: 10.1080/10408398.2021.1926906
5. Lu M, Pan J, Hu Y, Ding L, Li Y, Cui X, et al. Advances in the study of vascular related protective effect of garlic (Allium sativum) extract and compounds. J Nutr Biochem. (2024) 124:109531. doi: 10.1016/j.jnutbio.2023.109531
6. Imaizumi VM, Laurindo LF, Manzan B, Guiguer EL, Oshiiwa M, Otoboni AMMB, et al. Garlic: A systematic review of the effects on cardiovascular diseases. Crit Rev Food Sci Nutr. (2023) 63:6797–819. doi: 10.1080/10408398.2022.2043821
7. Ansary J, Forbes-Hernández TY, Gil E, Cianciosi D, Zhang J, Elexpuru-Zabaleta M, et al. Potential health benefit of garlic based on human intervention studies: A brief overview. J Antioxid Act. (2020) 9:619. doi: 10.3390/antiox9070619
8. Tavares L, Barros H, Vaghetti JCP, Noreña C. Microencapsulation of garlic extract by complex coacervation using whey protein isolate/chitosan and gum Arabic/chitosan as wall materials: Influence of anionic biopolymers on the physicochemical and structural properties of microparticles. Food Bioprocess Technol. (2019) 12:2093–106. doi: 10.1007/s11947-019-02375-y
9. Hu G, Cai K, Li Y, Hui T, Wang Z, Chen C, et al. Significant inhibition of garlic essential oil on benzo [a] pyrene formation in charcoal-grilled pork sausages relates to sulfide compounds. Food Res Int. (2021) 141:110127. doi: 10.1016/j.foodres.2021.110127
10. Ozma MA, Khodadadi E, Pakdel F, Kamounah FS, Yousefi M, Yousefi B, et al. Baicalin, a natural antimicrobial and anti-biofilm agent. J Herb Med. (2021) 27:100432. doi: 10.1016/j.hermed.2021.100432
11. Espinoza T, Valencia E, Albarrán M, Díaz D, Quevedo RA, Díaz O, et al. Garlic (Allium sativum L) and its beneficial properties for health: a review. Agroind Sci. (2020) 10:103–15. doi: 10.17268/agroind.sci.2020.01.14
12. Kıraç H, Dalda Şekerci A, Coşkun ÖF, Gülşen O. Morphological and molecular characterization of garlic (Allium sativum L.) genotypes sampled from Turkey. Genet Resour Crop Evol. (2022) 69:1833–41. doi: 10.1007/s10722-022-01343-4
13. Totić I, Čanak S. Production and economic specificities in growing of different garlic varieties. Ekonomika poljoprivrede. (2014) 61:915–28. doi: 10.5937/ekoPolj1404915T
14. Wu C, Wang M, Dong Y, Cheng Z, Meng H. Growth, bolting and yield of garlic (Allium sativum L.) in response to clove chilling treatment. Sci Hortic. (2015) 194:43–52. doi: 10.1016/j.scienta.2015.07.018
15. Dai X, Yu H, Zhu L, Yu Z. S-alk (en) ylcysteine sulfoxides biosynthesis and free amino acids profile in different parts of postharvest chive (Allium schoenoprasum L.). Sci Hortic. (2022) 303:111191. doi: 10.1016/j.scienta.2022.111191
16. Manpreet K, Priyanshi G, Reena G. Phytochemical analysis, antioxidant and antimicrobial activity assessment of Allium sativum (garlic) extract. Res J Biotech. (2021) 16:134–40.
17. Block E, Dane AJ, Thomas S, Cody RB. Applications of direct analysis in real-time mass spectrometry (DART-MS) in Allium chemistry. 2-Propenesulfenic and 2-propenesulfinic acids, diallyl trisulfane S-oxide, and other reactive sulfur compounds from crushed garlic and other Alliums. J Agric Food Chem. (2010) 58:4617–25. doi: 10.1021/jf1000106
18. Wargovich MJ. Diallyl sulfide, a flavor component of garlic (Allium sativum), inhibits dimethyihydrazine-induced colon cancer. Carcinogenesis. (1987) 8:487–9. doi: 10.1093/carcin/8.3.487
19. Üstüner T, Kordali S, Bozhüyük AU. Herbicidal and fungicidal effects of Cuminum cyminum, Mentha longifolia and Allium sativum essential oils on some weeds and fungi. Rec Nat Prod. (2018) 12:619–29. doi: 10.25135/rnp.80.18.05.106
20. Block E, Dethier B, Bechand B, Cotelesage JJ, George GN, Goto K, et al. Ajothiolanes: 3, 4-dimethylthiolane natural products from garlic (Allium sativum). J Agric Food Chem. (2018) 66:10193–204. doi: 10.1021/acs.jafc.8b03638
21. Yi LV, Kwok-Fai SO, Nai-Kei WO, Jia XI. Anti-cancer activities of S-allylmercaptocysteine from aged garlic. Chin J Nat Med. (2019) 17:43–9. doi: 10.1016/S1875–5364(19)30008–1
22. Yu X, Lim CY, Dong B, Hadinoto K. Development of magnetic solid phase extraction platform for the purification of bioactive γ-glutamyl peptides from garlic (Allium sativum). LWT. (2020) 127:109410. doi: 10.1016/j.lwt.2020.109410
23. Ozma MA, Abbasi A, Ahangarzadeh Rezaee M, Hosseini H, Hosseinzadeh N, Sabahi S, et al. A critical review on the nutritional and medicinal profiles of garlic’s (Allium sativum L.) Bioactive Compounds. Food Rev Int. (2023) 39:6324–61. doi: 10.1080/87559129.2022.2100417
24. Venditti A, Bianco A. Sulfur-containing secondary metabolites as neuroprotective agents. Curr Med Chem. (2020) 27:4421–436. doi: 10.2174/0929867325666180912105036
25. Tocmo R, Liang D, Lin Y, Huang D. Chemical and biochemical mechanisms underlying the cardioprotective roles of dietary organopolysulfides. Front Nutr. (2015) 2:1. doi: 10.3389/fnut.2015.00001
26. Balqis B, Widodo W, Lukiati B, Amin M. Active compounds with antioxidant potential in boiled local Papua-Indonesian garlic. AIP Conf Proc. (2018) 2019:020012. doi: 10.1063/1.5061848
27. El-Fiki A, Adly M. Morphological, molecular, and organosulphur compounds characterization in irradiated garlic (Allium sativum) by GC–MS and SCoT markers. J Radiat Res Appl Sci. (2020) 13:61–70. doi: 10.1080/16878507.2019.1697079
28. Suleria HA, Butt MS, Khalid N, Sultan S, Raza A, Aleem M, et al. Garlic (Allium sativum): diet based therapy of 21st century–a review. Asian Pac J Trop Dis. (2015) 5:271–8. doi: 10.1016/S2222–1808(14)60782–9
29. Nohara T, Ono M, Nishioka N, Masuda F, Fujiwara Y, Ikeda T, et al. New cyclic sulfides, garlicnins I2, M, N, and O, from Allium sativum. J Nat Med. (2018) 72:326–31. doi: 10.1007/s11418–017-1133–2
30. Maccelli A, Cesa S, Cairone F, Secci D, Menghini L, Chiavarino B, et al. Metabolic profiling of different wild and cultivated Allium species based on high-resolution mass spectrometry, high-performance liquid chromatography-photodiode array detector, and color analysis. J Mass Spectrom. (2020) 55:e4525. doi: 10.1002/jms.4525
31. Cong Y, Wu Y, Shen S, Liu X, Guo J. A structure-activity relationship between the veratrum alkaloids on the antihypertension and DNA damage activity in mice. Chem Biodivers. (2020) 17:e1900473. doi: 10.1002/cbdv.201900473
32. Padmini R, Uma Maheshwari V, Saravanan P, Woo Lee K, Razia M, Alwahibi MS, et al. Identification of novel bioactive molecules from garlic bulbs: a special effort to determine the anticancer potential against lung cancer with targeted drugs. Saudi J Biol Sci. (2020) 27:3274–89. doi: 10.1016/j.sjbs.2020.09.041
33. Avgeri I, Zeliou K, Petropoulos SA, Bebeli PJ, Papasotiropoulos V. Lamari FN.Variability in bulb organosulfur compounds, sugars, phenolics, and pyruvate among greek garlic genotypes: association with antioxidant properties. Antioxidants. (2020) 9:967. doi: 10.3390/antiox9100967
34. De Falco B, Bonanomi G, Lanzotti V. Dithiosulfinates and sulfoxides with antifungal activity from bulbs of Allium sativum L. var. Voghiera. Nat Prod Commun. (2018) 13:1163–1166. doi: 10.1177/1934578X1801300916
35. Akinwande BA, Olatunde SJ. Comparative evaluation of the mineral profile and other selected components of onion and garlic. Int Food Res J. (2015) 22:332–6.
36. Kallel F, Driss D, Bouaziz F, Belghith L, Zouari-Ellouzi S, Haddar A, et al. Polysaccharide from garlic straw: extraction, structural data, biological properties and application to beef meat preservation. RSC Adv. (2015) 5:6728–41. doi: 10.1039/C4RA11045E
37. Liang G, Li N, Ma L, Qian Z, Sun Y, Shi L, et al. Effect of quercetin on the transport of ritonavir to the central nervous system in vitro and in vivo. Acta Pharm. (2016) 66:97–107. doi: 10.1515/acph-2016–0009
38. Ahmed SST, Fahim JR, Abdelmohsen UR. Chemical and biological studies on Allium sativum L. (1952–2020): a comprehensive review. J Adv BioMed Pharm Sci. (2022) 5:1–22. doi: 10.21608/JABPS.2021.90667.1137
39. Bencheikh N, Elbouzidi A, Kharchoufa L, Ouassou H, Alami Merrouni I, Mechchate H, et al. Inventory of medicinal plants used traditionally to manage kidney diseases in north-eastern Morocco: Ethnobotanical fieldwork and pharmacological evidence. Plants. (2021) 10:1966. doi: 10.3390/plants10091966
40. Agarwal KC. Therapeutic actions of garlic constituents. Med Res Rev. (1996) 16:111–24. doi: 10.1002/(SICI)1098–1128(199601)16:1<111::AID-MED4>3.0.CO;2–5
41. Oosthuizen CB, Reid AM, Lall N. Garlic (Allium sativum) and its associated molecules, as medicine. In: Lall N. (Eds) Medicinal Plants for Holistic Health and Well-being. Academic Press (2018). p. 277–95. doi: 10.1016/B978–0-12–812475–8.00009–3
42. Dziri S, Hassen I, Fatnassi S, Mrabet Y, Casabianca H, Hanchi B, et al. Phenolic constituents, antioxidant and antimicrobial activities of rosy garlic (Allium roseum var. odoratissimum). (2012) 4:423–32. doi: 10.1016/j.jff.2012.01.010
43. Yamaguchi Y, Hirata Y, Saito T, Kumagai H. Combined effects of amino acids in garlic and buna-shimeji (Hypsizygus marmoreus) on suppression of CCl4-induced hepatic injury in rats. Foods (2021) 10:1491. doi: 10.3390/foods10071491
44. Subroto E, Cahyana Y, Tensiska M, Lembong F, Filianty E, Kurniati E, et al. Bioactive compounds in garlic (Allium sativum L.) as a source of antioxidants and its potential to improve the immune system: a review. Food Res. (2021) 5:1–11. doi: 10.26656/fr.2017.5(6).042
45. Martins N, Petropoulos S, Ferreira IC. Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre-and post-harvest conditions: a review. Food Chem. (2016) 211:41–50. doi: 10.1016/j.foodchem.2016.05.029
46. Yoshimoto N, Saito K. Biosynthesis of S-alk (en) yl-L-cysteine sulfoxides in Allium: retro perspective. In: De Kok LJ, Hawkesford MJ, Haneklaus SH, Schnug E (Eds) Sulfur Metabolism in Higher Plants-Fundamental, Environmental and Agricultural Aspects, Proceedings of the International Plant Sulfur Workshop. Springer, Cham. (2017). p. 49–60. doi: 10.1007/978-3-319-56526-2_5
47. Aziz M, Karboune S. Natural antimicrobial/antioxidant agents in meat and poultry products as well as fruits and vegetables: a review. Crit Rev Food Sci Nutr. (2018) 58:486–511. doi: 10.1080/10408398.2016.1194256
48. Salehi B, Zucca P, Orhan IE, Azzini E, Adetunji CO, Mohammed SA, et al. Allicin and health: a comprehensive review. Trends Food Sci Technol. (2019) 86:502–16. doi: 10.1016/j.tifs.2019.03.003
49. Sabahi S, Abbasi A, Mortazavi SA. Characterization of cinnamon essential oil and its application in Malva sylvestris seed mucilage edible coating to the enhancement of the microbiological, physicochemical and sensory properties of lamb meat during storage. J Appl Microbiol. (2022) 133:488–502. doi: 10.1111/jam.15578
50. Kasim WAE-A, Nessem AA, Gaber A. Alleviation of drought stress in Vicia faba by seed priming with ascorbic acid or extracts of garlic and carrot. Egypt J Bot. (2017) 57:45–59. doi: 10.21608/ejbo.2017.831.1057
51. Kaschula CH, Hunter R, Cotton J, Tuveri R, Ngarande E, Dzobo K, et al. The garlic compound ajoene targets protein folding in the endoplasmic reticulum of cancer cells. Mol Carcinog. (2016) 55:1213–28. doi: 10.1002/mc.22364
52. Wang Y, Guan M, Zhao X, Li X. Effects of garlic polysaccharide on alcoholic liver fibrosis and intestinal microflora in mice. Pharm Biol. (2018) 56:325–32. doi: 10.1080/13880209.2018.1479868
53. Thomaz L, Apitz-Castro R, Marques AF, Travassos LR, Taborda CP. Experimental paracoccidioidomycosis: alternative therapy with ajoene, compound from Allium sativum, associated with sulfamethoxazole/trimethoprim. Med Mycol. (2008) 46:113–8. doi: 10.1080/13693780701651681
54. Naganawa R, Iwata N, Ishikawa K, Fukuda H, Fujino T, Suzuki A. Inhibition of microbial growth by ajoene, a sulfur-containing compound derived from garlic. Appl Environ Microbiol. (1996) 62:4238–42. doi: 10.1128/aem.62.11.4238-4242
55. Yoshida S, Kasuga S, Hayashi N, Ushiroguchi T, Matsuura H, Nakagawa S. Antifungal activity of ajoene derived from garlic. Appl Environ Microbiol. (1987) 53:615–7. doi: 10.1128/aem.53.3.615-617.1987
56. Choi JA, Cho SN, Lim YJ, Lee J, Go D, Kim SH, et al. Enhancement of the antimycobacterial activity of macrophages by ajoene. Innate Immun. (2018) 24:79–88. doi: 10.1177/1753425917747975
57. Siyo V, Schäfer G, Hunter R, Grafov A, Grafova I, Nieger M, et al. The cytotoxicity of the ajoene analogue BisPMB in WHCO1 oesophageal cancer cells is mediated by CHOP/GADD153. Molecules. (2017) 22:892. doi: 10.3390/molecules22060892
58. Nazari M, Ghanbarzadeh B, Kafil HS, Zeinali M, Hamishehkar H. Garlic essential oil nanophytosomes as a natural food preservative: Its application in yogurt as food model. Colloids Interface Sci Commun. (2019) 30:100176. doi: 10.1016/j.colcom.2019.100176
59. Lawson LD, Hunsaker SM. Allicin bioavailability and bioequivalence from garlic supplements and garlic foods. Nutrients. (2018) 10:812. doi: 10.3390/nu10070812
60. Lawson LD, Ransom DK, Hughes BG. Inhibition of whole blood platelet-aggregation by compounds in garlic clove extracts and commercial garlic products. Thromb Res. (1992) 65:141–56. doi: 10.1016/0049–3848(92)90234–2
61. Freeman F, Kodera Y. Garlic chemistry: stability of S-(2-propenyl)-2-propene-1-sulfinothioate (allicin) in blood, solvents, and simulated physiological fluids. J Agrie Food Chem. (1995) 43:2332–8. doi: 10.1021/jf00057a004
62. Germain E, Auger J, Ginies C, Siess MH, Teyssier C. In vivo metabolism of diallyl disulphide in the rat: identification of two new metabolites. Xenobiotica. (2002) 32:1127–38. doi: 10.1080/0049825021000017902
63. Lawson LD, Hughes BG. Characterization of the formation of allicin and other thiosulfinates from garlic. Planta Med. (1992) 58:345–50. doi: 10.1055/s-2006–961482
64. Lefer DJ. A new gaseous signaling molecule emerges: cardioprotective role of hydrogen sulfide. Proc Natl Acad Sci U S A. (2007) 104:17907–8. doi: 10.1073/pnas.0709010104
65. Ried K, Travica N, Sali A. The effect of kyolic aged garlic extract on gut microbiota, inflammation, and cardiovascular markers in hypertensives: The GarGIC Trial. Front Nutr. (2018) 5:122. doi: 10.3389/fnut.2018.00122
66. Asdaq SMB, Challa O, Alamri AS, Alsanie WF, Alhomrani M, Almutiri AH, et al. Cytoprotective potential of aged garlic extract (AGE) and its active constituent, S-allyl-l-cysteine, in presence of carvedilol during isoproterenol-induced myocardial disturbance and metabolic derangements in rats. Molecules. (2021) 26:3203. doi: 10.3390/molecules26113203
67. Sabahi S, Homayouni Rad A, Aghebati-Maleki L, Sangtarash N, Ozma MA, Karimi A, et al. Postbiotics as the new frontier in food and pharmaceutical research. Crit Rev Food Sci Nutr. (2023) 63:8375–402. doi: 10.1080/10408398.2022.2056727
68. Sujithra K, Srinivasan S, Indumathi D, Vinothkumar V. Allyl methyl sulfide, an organosulfur compound alleviates hyperglycemia mediated hepatic oxidative stress and inflammation in streptozotocin-induced experimental rats. BioMed Pharmacother. (2018) 107:292–302. doi: 10.1016/j.biopha.2018.07.162
69. Song G, Sumit B, Guangyi Y, Arijita D, Ming H. Oral bioavailability challenges of natural products used in cancer chemoprevention. Prog Chem. (2013) 25:1553. doi: 10.7536/PC130729
70. Rahman MS. Allicin and other functional active components in garlic: Health benefits and bioavailability. Int J Food Prop. (2007) 10:245–68. doi: 10.1080/10942910601113327
71. Marchese A, Barbieri R, Sanches-Silva A, Daglia M, Nabavi SF, Jafari NJ, et al. Antifungal and antibacterial activities of allicin: A review. Trends Food Sci Technol. (2016) 52:49–56. doi: 10.1016/j.tifs.2016.03.010
72. Abourashed EA. Bioavailability of plant-derived antioxidants. Antioxidants. (2013) 2:309–25. doi: 10.3390/antiox2040309
73. Nahdi A, Hammami I, Brasse-Lagnel C, Pilard N, Hamdaoui MH, Beaumont C, et al. Influence of garlic or its main active component diallyl disulfide on iron bioavailability and toxicity. Nutr Res. (2010) 30:85–95. doi: 10.1016/j.nutres.2010.01.004
74. Luo Y, Xie W, Hao Z, Jin X, Wang Q. Use of shallot (Allium ascalonicum) and leek (Allium tuberosum) to improve the in vitro available iron and zinc from cereals and legumes. CYTA - J Food. (2014) 12:195–8. doi: 10.1080/19476337.2013.814168
75. Torres-Palazzolo CA, Ramírez DA, Beretta VH, Camargo AB. Matrix effect on phytochemical bioaccessibility. The case of organosulfur compounds in garlic preparations. LWT. (2021) 136:110301. doi: 10.1016/j.lwt.2020.110301
76. Ashfaq F, Ali Q, Haider MA, Hafeez MM, Malik A. Therapeutic activities of garlic constituent phytochemicals. Biol Clin Sci Res J. (2021) 2021:53. doi: 10.54112/bcsrj.v2021i1.53
77. Liu J, Guo W, Yang M, Liu L, Huang S, Tao L, et al. Investigation of the dynamic changes in the chemical constituents of Chinese “laba” garlic during traditional processing. RSC Adv. (2018) 8:41872–83. doi: 10.1039/C8RA09657K
78. Kang JS, Kim SO, Kim GY, Hwang HJ, Kim BW, Chang YC, et al. An exploration of the antioxidant effects of garlic saponins in mouse-derived C2C12 myoblasts. Int J Mol Med. (2016) 37:149–56. doi: 10.3892/ijmm.2015.2398
79. Matsuura N, Miyamae Y, Yamane K, Nagao Y, Hamada Y, Kawaguchi N, et al. Aged garlic extract inhibits angiogenesis and proliferation of colorectal carcinoma cells. J Nutr. (2006) 136:842S–6S. doi: 10.1093/jn/136.3.842S
80. Jikihara H, Qi G, Nozoe K, Hirokawa M, Sato H, Sugihara Y, et al. Aged garlic extract inhibits 1, 2-dimethylhydrazine-induced colon tumor development by suppressing cell proliferation. Oncol Rep. (2015) 33:1131–40. doi: 10.3892/or.2014.3705
81. Nasr AY, Saleh HA. Aged garlic extract protects against oxidative stress and renal changes in cisplatin-treated adult male rats. Cancer Cell Int. (2014) 14:92. doi: 10.1186/s12935-014-0092-x
82. Tabari MA, Ebrahimpour S. Effect of aged garlic extract on immune responses to experimental fibrosarcoma tumor in BALB/c mice. Indian J Cancer. (2014) 51:609–13. doi: 10.4103/0019–509X.175359
83. Park SY, Seetharaman R, Ko MJ, Kim DY, Kim TH, Yoon MK, et al. Ethyl linoleate from garlic attenuates lipopolysaccharide-induced pro-inflammatory cytokine production by inducing heme oxygenase-1 in RAW264. 7 cells. Int Immunopharmacol. (2014) 19:253–61. doi: 10.1016/j.intimp.2014.01.017
84. Metwally DM, Al-Olayan EM, Alanazi M, Alzahrany SB, Semlali A. Antischistosomal and anti-inflammatory activity of garlic and allicin compared with that of praziquantel in vivo. BMC Complement Altern Med. (2018) 18:1–11. doi: 10.1186/s12906-018-2191-z
85. Dehghani S, Alipoor E, Salimzadeh A, Yaseri M, Hosseini M, Feinle-Bisset C, et al. The effect of a garlic supplement on the pro-inflammatory adipocytokines, resistin and tumor necrosis factor-alpha, and on pain severity, in overweight or obese women with knee osteoarthritis. Phytomedicine. (2018) 48:70–5. doi: 10.1016/j.phymed.2018.04.060
86. Zamani Taghizadeh Rabe S, Ghazanfari T, Siadat Z, Rastin M, Zamani Taghizadeh Rabe S, Mahmoudi M. Anti-inflammatory effect of garlic 14-kDa protein on LPS-stimulated-J774A.1 macrophages. Immunopharmacol Immunotoxicol. (2015) 37:158–64. doi: 10.3109/08923973.2015.1005229
87. Zhou H, Qu Z, Mossine VV, Nknolise DL, Li J, Chen Z, et al. Proteomic analysis of the effects of aged garlic extract and its FruArg component on lipopolysaccharide-induced neuroinflammatory response in microglial cells. PloS One. (2014) 9:e113531. doi: 10.1371/journal.pone.0113531
88. Thorajak P, Pannangrong W, Welbat JU, Chaijaroonkhanarak W, Sripanidkulchai K, Sripanidkulchai B. Effects of aged garlic extract on cholinergic, glutamatergic and GABAergic systems with regard to cognitive impairment in Aβ-induced rats. Nutrients. (2017) 9:686. doi: 10.3390/nu9070686
89. Badr GM, Al-Mulhim JA. The protective effect of aged garlic extract on nonsteroidal anti-inflammatory drug-induced gastric inflammations in male albino rats. Evid Based Complement Alternat Med. (2014) 2014:759642. doi: 10.1155/2014/759642
90. El-Ashmawy NE, Khedr EG, El-Bahrawy HA, Selim HM. Gastroprotective effect of garlic in indomethacin induced gastric ulcer in rats. Nutrition. (2016) 32:849–54. doi: 10.1016/j.nut.2016.01.010
91. Shi L, Lin Q, Li X, Nie Y, Sun S, Deng X, et al. Alliin, a garlic organosulfur compound, ameliorates gut inflammation through MAPK-NF-κB/AP-1/STAT-1 inactivation and PPAR-γ activation. Mol Nutr Food Res. (2017) 61:1601013. doi: 10.1002/mnfr.201601013
92. Ho XL, Tsen SY, Ng MY, Lee WN, Low A, Loke WM. Aged garlic supplement protects against lipid peroxidation in hypercholesterolemic individuals. J Med Food. (2016) 19:931–7. doi: 10.1089/jmf.2016.3693
93. Percival SS. Aged garlic extract modifies human immunity. J Nutr. (2016) 146:433S–6S. doi: 10.3945/jn.115.210427
94. Fratianni F, Riccardi R, Spigno P, Ombra MN, Cozzolino A, Tremonte P, et al. Biochemical characterization and antimicrobial and antifungal activity of two endemic varieties of garlic (Allium sativum L.) of the campania region, southern Italy. J Med Food. (2016) 19:686–91. doi: 10.1089/jmf.2016.0027
95. Nazzaro F, Polito F, Amato G, Caputo L, Francolino R, D’Acierno A, et al. Chemical composition of essential oils of bulbs and aerial parts of two cultivars of Allium sativum and their antibiofilm activity against food and nosocomial pathogens. Antibiotics. (2022) 11:724. doi: 10.3390/antibiotics11060724
96. Vargas-Ortiz MA, San Martín-Hernández C, Angulo-Escalante MÁ, Muy-Rangel MD, Quintana-Obregón EA. GARLIC (Allium sativum L.) essential oil against growth and aflatoxin production of Aspergillus parasiticus. Trop Subtrop Agroecosystems. (2020) 23:1–6. doi: 10.56369/tsaes.3150
97. Gudalwar BR, Nimbalwar MG, Panchale WA, Wadekar AB, Manwar JV, Bakal RL. Allium sativum, a potential phytopharmacological source of natural medicine for better health. GSC Adv Res Rev. (2021) 6:220–32. doi: 10.30574/gscarr.2021.6.3.0049
98. Kour J, Kour K, Bansal V, Sharma R, Sharma K, Elshikh MS, et al. Bioactive compounds in garlic. In: Wani S. A., Singh A., Kumar P. (Eds) Spice Bioactive Compounds, 1st ed. Boca Raton, FL, USA: CRC Press (2022). p. 31–44.
99. Mansingh DP, Dalpati N, Sali VK, Vasanthi AHR. Alliin the precursor of allicin in garlic extract mitigates proliferation of gastric adenocarcinoma cells by modulating apoptosis. Pharmacogn Mag. (2018) 14:s84–91. doi: 10.4103/pm.pm_342_17
100. Jiang X, Zhu X, Huang W, Xu H, Zhao Z, Li S, et al. Garlic-derived organosulfur compound exerts antitumor efficacy via activation of MAPK pathway and modulation of cytokines in SGC-7901 tumor-bearing mice. Int Immunopharmacol. (2017) 48:135–45. doi: 10.1016/j.intimp.2017.05.004
101. Wei Z, Shan Y, Tao L, Liu Y, Zhu Z, Liu Z, et al. Diallyl trisulfides, a natural histone deacetylase inhibitor, attenuate HIF-1α synthesis, and decreases breast cancer metastasis. Mol Carcinog. (2017) 56:2317–31. doi: 10.1002/mc.22686
102. Xu YS, Feng JG, Zhang D, Zhang B, Luo M, Su D, et al. S-allylcysteine, a garlic derivative, suppresses proliferation and induces apoptosis in human ovarian cancer cells in vitro. Actal Pharmacol Sin. (2014) 35:267–74. doi: 10.1038/aps.2013.176
103. Zhang Y, Li HY, Zhang ZH, Bian HL, Lin G. Garlic−derived compound S−allylmercaptocysteine inhibits cell growth and induces apoptosis via the JNK and p38 pathways in human colorectal carcinoma cells. Oncol Lett. (2014) 8:2591–6. doi: 10.3892/ol.2014.2579
104. Xiao J, Xing F, Liu Y, Lv Y, Wang X, Ling MT, et al. Garlic-derived compound S-allylmercaptocysteine inhibits hepatocarcinogenesis through targeting LRP6/Wnt pathway. Acta Pharm Sin B. (2018) 8:575–86. doi: 10.1016/j.apsb.2017.10.003
105. Wang W, Cheng J, Zhu Y. The JNK signaling pathway is a novel molecular target for S-propargyl-L-cysteine, a naturally-occurring garlic derivatives: link to its anticancer activity in pancreatic cancer in vitro and in vivo. Curr Cancer Drug Targets. (2015) 15:613–23. doi: 10.2174/1568009615666150602143943
106. Bagul M, Kakumanu S, Wilson TA. Crude garlic extract inhibits cell proliferation and induces cell cycle arrest and apoptosis of cancer cells in vitro. J Med Food. (2015) 18:731–7. doi: 10.1089/jmf.2014.0064
107. Shin SS, Song JH, Hwang B, Noh DH, Park SL, Kim WT, et al. HSPA6 augments garlic extract-induced inhibition of proliferation, migration, and invasion of bladder cancer EJ cells; Implication for cell cycle dysregulation, signaling pathway alteration, and transcription factor-associated MMP-9 regulation. PloS One. (2017) 12:e0171860. doi: 10.1371/journal.pone.0171860
108. Gatt ME, Strahilevitz J, Sharon N, Lavie D, Goldschmidt N, Kalish Y, et al. A randomized controlled study to determine the efficacy of garlic compounds in patients with hematological malignancies at risk for chemotherapy-related febrile neutropenia. Integr Cancer Ther. (2015) 14:428–35. doi: 10.1177/1534735415588928
109. Aprioku J, Amah-Tariah F. Garlic (Allium sativum L.) protects hepatic and renal toxicity of alloxan in rats. J Pharm Res Int. (2017) 17:1–7. doi: 10.9734/JPRI/2017/34909
110. Zarezadeh M, Baluchnejadmojarad T, Kiasalari Z, Afshin-Majd S, Roghani M. Garlic active constituent s-allyl cysteine protects against lipopolysaccharide-induced cognitive deficits in the rat: Possible involved mechanisms. Eur J Pharmacol. (2017) 795:13–21. doi: 10.1016/j.ejphar.2016.11.051
111. Yoo DY, Kim W, Nam SM, Yoo M, Lee S, Yoon YS, et al. Neuroprotective effects of Z-ajoene, an organosulfur compound derived from oil-macerated garlic, in the gerbil hippocampal CA1 region after transient forebrain ischemia. Food Chem Toxicol. (2014) 72:1–7. doi: 10.1016/j.fct.2014.06.023
112. Lee KC, Teng CC, Shen CH, Huang WS, Lu CC, Kuo HC, et al. Protective effect of black garlic extracts on tert-Butyl hydroperoxide-induced injury in hepatocytes via a c-Jun N-terminal kinase-dependent mechanism. Exp Ther Med. (2018) 15:2468–74. doi: 10.3892/etm.2018.5719
113. Lee H-S, Lim W-C, Lee S-J, Lee S-H, Lee J-H, Cho H-Y. Antiobesity effect of garlic extract fermented by Lactobacillus plantarum BL2 in diet-induced obese mice. J Med Food. (2016) 19:823–9. doi: 10.1089/jmf.2016.3674
114. Yang C, Li L, Yang L, Lǚ H, Wang S, Sun G. Anti-obesity and hypolipidemic effects of garlic oil and onion oil in rats fed a high-fat diet. Nutr Metab. (2018) 15:43. doi: 10.1186/s12986-018-0275-x
115. Chen Y-C, Kao T-H, Tseng C-Y, Chang W-T, Hsu C-L. Methanolic extract of black garlic ameliorates diet-induced obesity via regulating adipogenesis, adipokine biosynthesis, and lipolysis. J Funct Foods. (2014) 9:98–108. doi: 10.1016/j.jff.2014.02.019
116. Chen Y-A, Tsai J-C, Cheng K-C, Liu K-F, Chang C-K, Hsieh C-W. Extracts of black garlic exhibits gastrointestinal motility effect. Food Res Int. (2018) 107:102–9. doi: 10.1016/j.foodres.2018.02.003
117. Fasolino I, Izzo AA, Clavel T, Romano B, Haller D, Borrelli F. Orally administered allyl sulfides from garlic ameliorate murine colitis. Mol Nutr Food Res. (2015) 59:434–42. doi: 10.1002/mnfr.201400347
118. Charron CS, Dawson HD, Albaugh GP, Solverson PM, Vinyard BT, Solano-Aguilar GI, et al. A single meal containing raw, crushed garlic influences expression of immunity-and cancer-related genes in whole blood of humans. J Nutr. (2015) 145:2448–55. doi: 10.3945/jn.115.215392
119. Al-Brakati A. Protective effect of garlic against diabetic retinopathy in adult albino rats. Res J Pharm Biol Chem Sci. (2016) 7:2748–59.
120. Yoneda T, Kojima N, Matsumoto T, Imahori D, Ohta T, Yoshida T, et al. Construction of sulfur-containing compounds with anti-cancer stem cell activity using thioacrolein derived from garlic based on nature-inspired scaffolds. Org Biomol Chem. (2022) 20:196–207. doi: 10.1039/D1OB01992A
121. Zardast M, Namakin K, Kaho JE, Hashemi SS. Assessment of antibacterial effect of garlic in patients infected with Helicobacter pylori using urease breath test. Avicenna J Phytomed. (2016) 6:495–501.
122. Sultana MR, Bagul PK, Katare PB, Anwar Mohammed S, Padiya R, Banerjee SK. Garlic activates SIRT-3 to prevent cardiac oxidative stress and mitochondrial dysfunction in diabetes. Life Sci. (2016) 164:42–51. doi: 10.1016/j.lfs.2016.08.030
123. Khatua TN, Borkar RM, Mohammed SA, Dinda AK, Srinivas R, Banerjee SK. Novel sulfur metabolites of garlic attenuate cardiac hypertrophy and remodeling through induction of Na+/K+-ATPase expression. Front Pharmacol. (2017) 8:18. doi: 10.3389/fphar.2017.00018
124. Siddiqui NA, Haider S, Misbah-ur-Rehman M, Perveen T. Role of herbal formulation of garlic on lipid profile in patients with type 2 diabetes related dyslipidemia. Pak Heart J. (2016) 49:146–50. doi: 10.47144/phj.v49i4.1159
125. Mohamed EH, Baiomy AA, Ibrahim ZS, Soliman MM. Modulatory effects of levamisole and garlic oil on the immune response of Wistar rats: Biochemical, immunohistochemical, molecular and immunological study. Mol Med Rep. (2016) 14:2755–63. doi: 10.3892/mmr.2016.5551
126. Qiu S, Chen J, Qin T, Hu Y, Wang D, Fan Q, et al. Effects of selenylation modification on immune-enhancing activity of garlic polysaccharide. PloS One. (2014) 9:e86377. doi: 10.1371/journal.pone.0086377
127. Suddek GM. Allicin enhances chemotherapeutic response and ameliorates tamoxifen-induced liver injury in experimental animals. Pharm Biol. (2014) 52:1009–14. doi: 10.3109/13880209.2013.876053
128. Saud SM, Li W, Gray Z, Matter MS, Colburn NH, Young MR, et al. Diallyl disulfide (DADS), a constituent of garlic, inactivates NF-κB and prevents colitis-induced colorectal cancer by inhibiting GSK-3β. Cancer Prev Res. (2016) 9:607–15. doi: 10.1158/1940–6207.CAPR-16–0044
129. Naji KM, Al-Shaibani ES, Alhadi FA, Al-Soudi SA, D’souza MR. Hepatoprotective and antioxidant effects of single clove garlic against CCl4-induced hepatic damage in rabbits. BMC Complement Altern Med. (2017) 17:1–12. doi: 10.1186/s12906–017-1916–8
130. Lai YS, Chen WC, Ho CT, Lu KH, Lin SH, Tseng HC, et al. Garlic essential oil protects against obesity-triggered nonalcoholic fatty liver disease through modulation of lipid metabolism and oxidative stress. J Agric Food Chem. (2014) 62:5897–906. doi: 10.1021/jf500803c
131. Ko JW, Park SH, Lee IC, Lee SM, Shin IS, Kang SS, et al. Protective effects of garlic oil against 1, 3-dichloro-2-propanol-induced hepatotoxicity: Role of CYP2E1 and MAPKs. Mol Cell Toxicol. (2016) 12:185–95. doi: 10.1007/s13273–016-0023–0
132. Nasiri A, Ziamajidi N, Abbasalipourkabir R, Goodarzi MT, Saidijam M, Behrouj H, et al. Beneficial effect of aqueous garlic extract on inflammation and oxidative stress status in the kidneys of type 1 diabetic rats. Indian J Clin Biochem. (2017) 32:329–36. doi: 10.1007/s12291–016-0621–6
133. Miltonprabu S, Sumedha NC, Senthilraja P. RETRACTED: Diallyl trisulfide, a garlic polysulfide protects against As-induced renal oxidative nephrotoxicity, apoptosis and inflammation in rats by activating the Nrf2/ARE signaling pathway. Int Immunopharmacol. (2017) 50:107–20. doi: 10.1016/j.intimp.2017.06.011
134. Wallock-Richards D, Doherty CJ, Doherty L, Clarke DJ, Place M, Govan JR, Campopiano DJ. Garlic revisited: Antimicrobial activity of allicin-containing garlic extracts against Burkholderia cepacia complex. PLoS One. (2014) 9:e112726. doi: 10.1371/journal.pone.0112726
135. Karimi N, Jabbari V, Nazemi A, Ganbarov K, Karimi N, Tanomand A, et al. Thymol, cardamom and Lactobacillus plantarum nanoparticles as a functional candy with high protection against Streptococcus mutans and tooth decay. Microb Pathog. (2020) 148:104481. doi: 10.1016/j.micpath.2020.104481
136. Ghorbani Tajani A, Bisha B. Effect of food matrix and treatment time on the effectiveness of grape seed extract as an antilisterial treatment in fresh produce. Microorganisms. (2023) 11:1029. doi: 10.3390/microorganisms11041029
137. Pérez-Giraldo C, Cruz-Villalón G, Sánchez-Silos R, Martínez-Rubio R, Blanco MT, Gómez-García AC. In vitro activity of allicin against Staphylococcus epidermidis and influence of subinhibitory concentrations on biofilm formation. J Appl Microbiol. (2003) 95:709–11. doi: 10.1046/j.1365-2672.2003.02030.x
138. Cutler RR, Wilson P. Antibacterial activity of a new, stable, aqueous extract of allicin against methicillin-resistant Staphylococcus aureus. Br J BioMed Sci. (2004) 61:71–4. doi: 10.1080/09674845.2004.11732646
139. Lihua L, Jianhuit W, Jialini Y, Yayin L, Guanxin L. Effects of allicin on the formation of Pseudomonas aeruginosa biofilm and the production of quorum-sensing controlled virulence factors. Pol J Microbiol. (2013) 62:243–51. doi: 10.33073/pjm-
140. Shuford JA, Steckelberg JM, Patel R. Effects of fresh garlic extract on Candida albicans biofilms. Antimicrob Agents Chemother. (2005) 49:473. doi: 10.1128/AAC.49.1.473.2005
141. Zhai H, Pan J, Pang E, Bai B. Lavage with allicin in combination with vancomycin inhibits biofilm formation by Staphylococcus epidermidis in a rabbit model of prosthetic joint infection. PloS One. (2014) 9:e102760. doi: 10.1371/journal.pone.0102760
142. Montazersaheb S, Hosseiniyan Khatibi SM, Hejazi MS, Tarhriz V, Farjami A, Ghasemian Sorbeni F, et al. Ghasemnejad T. COVID-19 infection: An overview on cytokine storm and related interventions. Virol J. (2022) 19:92. doi: 10.1186/s12985-022-01814-1
143. Hashemifesharaki R, Gharibzahedi SMT. Future nutrient-dense diets rich in vitamin D: a new insight toward the reduction of adverse impacts of viral infections similar to COVID-19. Nutrire. (2020) 45:19. doi: 10.1186/s41110–020-00122–4
144. Panyod S, Ho CT, Sheen LY. Dietary therapy and herbal medicine for COVID-19 prevention: A review and perspective. J Tradit Complement Med. (2020) 10:420–7. doi: 10.1016/j.jtcme.2020.05.004
145. El-Saber Batiha G, Magdy Beshbishy A G, Wasef L, Elewa YHA, A Al-Sagan A, Abd El-Hack ME, et al. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): A review. Nutrients. (2020) 12:872. doi: 10.3390/nu12030872
146. Chakraborty D, Majumder A. Garlic (Lahsun)–an immunity booster against SARS-CoV-2. Biotica Res Today. (2020) 2:755–7.
147. Liu X, Wang XJ. Genomics. Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicines. J Genet Genomics. (2020) 47:119–21. doi: 10.1016/j.jgg.2020.02.001
148. Rajagopal K, Byran G, Jupudi S, Vadivelan R. Activity of phytochemical constituents of black pepper, ginger, and garlic against coronavirus (COVID-19): an in silico approach. Int J Health Allied Sci. (2020) 9:43–50. doi: 10.4103/ijhas.IJHAS_55_20
149. Pandey P, Khan F, Kumar A, Srivastava A, Jha NK. Screening of potent inhibitors against 2019 novel coronavirus (Covid-19) from Allium sativum and Allium cepa: an in silico approach. Biointerface Res Appl Chem. (2021) 11:7981–93. doi: 10.33263/BRIAC111.79817993
150. Chen L, Li J, Luo C, Liu H, Xu W, Chen G, et al. Binding interaction of quercetin-3-β-galactoside and its synthetic derivatives with SARS-CoV 3CLpro: Structure–activity relationship studies reveal salient pharmacophore features. Bioorg Med Chem. (2006) 14:8295–306. doi: 10.1016/j.bmc.2006.09.014
151. Upadhyay RK. Garlic: a potential source of pharmaceuticals and pesticides: A review. Int J Green Pharm. (2016) Suppl 10:S1–28. doi: 10.22377/ijgp.v10i1.609
152. Isbilen O, Volkan E. Allium Species in the Fight Against Cancer. Reno, NV, USA: MedDocs Publisher LLC (2020) p. 1–11.
153. Ichikawa M, Yoshida J, Ide N, Sasaoka T, Yamaguchi H, Ono K. Tetrahydro-β-carboline derivatives in aged garlic extract show antioxidant properties. J Nutr. (2006) 136:726S–31S. doi: 10.1093/jn/136.3.726S
154. Locatelli DA, Nazareno MA, Fusari C, Camargo AB. Cooked garlic and antioxidant activity: Correlation with organosulfur compound composition. Food Chem. (2017) 220:219–24. doi: 10.1016/j.foodchem.2016.10.001
155. Zakarova A, Seo JY, Kim HY, Kim JH, Shin JH, Cho KM, et al. Garlic sprouting is associated with increased antioxidant activity and concomitant changes in the metabolite profile. J Agric Food Chem. (2014) 62:1875–80. doi: 10.1021/jf500603v
156. Jang HJ, Lee HJ, Yoon DK, Ji DS, Kim JH, Lee CH. Antioxidant and antimicrobial activities of fresh garlic and aged garlic by-products extracted with different solvents. Food Sci Biotechnol. (2018) 27:219–25. doi: 10.1007/s10068–017-0246–4
157. Choi IS, Cha HS, Lee YS. Physicochemical and antioxidant properties of black garlic. Molecules. (2014) 19:16811–23. doi: 10.3390/molecules191016811
158. Zhang Z, Lei M, Liu R, Gao Y, Xu M, Zhang M. Evaluation of alliin, saccharide contents and antioxidant activities of black garlic during thermal processing. J Food Biochem. (2015) 39:39–47. doi: 10.1111/jfbc.12102
159. Hiramatsu K, Tsuneyoshi T, Ogawa T, Morihara N. Aged garlic extract enhances heme oxygenase-1 and glutamate-cysteine ligase modifier subunit expression via the nuclear factor erythroid 2–related factor 2–antioxidant response element signaling pathway in human endothelial cells. Nutr Res. (2016) 36:143–9. doi: 10.1016/j.nutres.2015.09.018
160. Eroman Unni L, Chauhan OP, Raju PS. High pressure processing of garlic paste: effect on the quality attributes. Int J Food Sci. (2014) 49:1579–85. doi: 10.1111/ijfs.12456
161. Sari YP, Anwar MS. Antioxidant activity of single bulb garlic callus (Allium sativum L.) extract with in vitro method. AIP Conf. (2022) 2668:020004. doi: 10.1063/5.0112192
162. Kimura S, Tung Y-C, Pan M-H, Su N-W, Lai Y-J, Cheng K-C. Black garlic: A critical review of its production, bioactivity, and application. J Food Drug Anal. (2017) 25:62–70. doi: 10.1016/j.jfda.2016.11.003
163. Rad AH, Aghebati-Maleki L, Kafil HS, Abbasi A. Molecular mechanisms of postbiotics in colorectal cancer prevention and treatment. Crit Rev Food Sci Nutr. (2021) 61:1787–803. doi: 10.1080/10408398.2020.1765310
164. Smith MT, Guyton KZ, Gibbons CF, Fritz JM, Portier CJ, Rusyn I, et al. Key characteristics of carcinogens as a basis for organizing data on mechanisms of carcinogenesis. Environ Health Perspect. (2016) 124:713–21. doi: 10.1289/ehp.1509912
165. Rad AH, Maleki LA, Kafil HS, Zavoshti HF, Abbasi A. Postbiotics as promising tools for cancer adjuvant therapy. Adv Pharm Bull. (2021) 11:1–5. doi: 10.34172/apb.2021.007
166. Jakszyn P, Agudo A, Ibáñez R, García-Closas R, Pera G, Amiano P, et al. Development of a food database of nitrosamines, heterocyclic amines, and polycyclic aromatic hydrocarbons. J Nutr. (2004) 134:2011–4. doi: 10.1093/jn/134.8.2011
167. Nicastro HL, Ross SA, Milner JA. Garlic and onions: their cancer prevention properties. Cancer Prev Res. (2015) 8:181–9. doi: 10.1158/1940–6207.CAPR-14–0172
168. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
169. Elsalahaty MI, Alkafaas SS, Bashir AO, El-Tarabily KA, El-Saadony MT, Yousef EM. Revealing the association between vitamin D metabolic pathway gene variants and lung cancer risk: a systematic review and meta-analysis. Front Genet. (2024) 15:1302527. doi: 10.3389/fgene.2024.1302527
170. Diab T, AlKafaas SS, Shalaby TI, Hessien M. Dexamethasone simulates the anticancer effect of nano-formulated paclitaxel in breast cancer cells. Bioorg Chem. (2020) 99:103792. doi: 10.1016/j.bioorg.2020.103792
171. Alkafaas SS, Loutfy SA, Diab T, Hessien M. Vasopressin induces apoptosis but does not enhance the antiproliferative effect of dynamin 2 or PI3K/Akt inhibition in luminal A breast cancer cells. Med Oncol. (2022) 40:35. doi: 10.1007/s12032–022-01889–4
172. Diab T, Alkafaas SS, Shalaby TI, Hessien M. Paclitaxel nanoparticles induce apoptosis and regulate txr1, cyp3a4 and cyp2c8 in breast cancer and hepatoma cells. Anticancer Agents Med Chem. (2020) 20:1582–91. doi: 10.2174/1871520620666200504071530
173. Alkafaas SS, Diab T, Shalaby T, Hessien M. Dexamethasone improves the responsiveness of hepatoma cells for both free and solvent containing paclitaxel in vitro. Egypt J Biochem Mol Biol. (2019) 37:95–110. doi: 10.21608/ejb.2019.63581
174. Shang A, Cao SY, Xu XY, Gan RY, Tang GY, Corke H, et al. Bioactive compounds and biological functions of garlic (Allium sativum L.). Foods. (2019) 8:246. doi: 10.3390/foods8070246
175. Alkafaas SS, Elsalahaty MI, Ismail DF, Radwan MA, Elkafas SS, Loutfy SA, et al. The emerging roles of sphingosine 1-phosphate and SphK1 in cancer resistance: a promising therapeutic target. Cancer Cell Int. (2024) 24:89. doi: 10.1186/s12935–024-03221–8
176. Imran M, Nadeem M, Saeed F, Imran A, Khan MR, Khan MA, et al. Immunomodulatory perspectives of potential biological spices with special reference to cancer and diabetes. Food Agric Immunol. (2017) 28:543–72. doi: 10.1080/09540105.2016.1259293
177. Li M, Yan YX, Yu QT, Deng Y, Wu DT, Wang Y, et al. Comparison of immunomodulatory effects of fresh garlic and black garlic polysaccharides on RAW 264.7 macrophages. J Food Sci. (2017) 82:765–71. doi: 10.1111/1750–3841.13589
178. Takashima M, Kanamori Y, Kodera Y, Morihara N, Tamura K. Aged garlic extract exerts endothelium-dependent vasorelaxant effect on rat aorta by increasing nitric oxide production. Phytomedicine. (2017) 24:56–61. doi: 10.1016/j.phymed.2016.11.016
179. Abbasi A, Rad AH, Ghasempour Z, Sabahi S, Kafil HS, Hasannezhad P, et al. Shahbazi N.The biological activities of postbiotics in gastrointestinal disorders. Crit Rev Food Sci Nutr. (2022) 62:5983–6004. doi: 10.1080/10408398.2021.1895061
180. Ushijima M, Takashima M, Kunimura K, Kodera Y, Morihara N, Tamura K. Effects of S-1-propenylcysteine, a sulfur compound in aged garlic extract, on blood pressure and peripheral circulation in spontaneously hypertensive rats. J Pharm Pharmacol. (2018) 70:559–65. doi: 10.1111/jphp.12865
181. Park BM, Chun H, Chae SW, Kim SH. Fermented garlic extract ameliorates monocrotaline-induced pulmonary hypertension in rats. J Funct Foods. (2017) 30:247–53. doi: 10.1016/j.jff.2017.01.024
182. Asdaq SM, Inamdar MN. Potential of garlic and its active constituent, S-allyl cysteine, as antihypertensive and cardioprotective in presence of captopril. Phytomedicine. (2010) 17:1016–26. doi: 10.1016/j.phymed.2010.07.012
183. Han CH, LIu JC, Chen KH, Lin YS, Chen CT, Fan CT, et al. Antihypertensive activities of processed garlic on spontaneously hypertensive rats and hypertensive humans. Bot Stud. (2011) 52:277–83.
184. Rad AH, Maleki LA, Kafil HS, Abbasi A. Postbiotics: A novel strategy in food allergy treatment. Crit Rev Food Sci Nutr. (2021) 61:492–9. doi: 10.1080/10408398.2020.1738333
185. Abbasi A, Aghebati-Maleki A, Yousefi M, Aghebati-Maleki L. Probiotic intervention as a potential therapeutic for managing gestational disorders and improving pregnancy outcomes. J Reprod Immunol. (2021) 143:103244. doi: 10.1016/j.jri.2020.103244
186. Guan M-J, Zhao N, Xie K-Q, Zeng T. Toxicology C. Hepatoprotective effects of garlic against ethanol-induced liver injury: A mini-review. Food Chem Toxicol. (2018) 111:467–73. doi: 10.1016/j.fct.2017.11.059
187. Kim H-N, Kang S-G, Roh YK, Choi M-K, Song S-W. Efficacy and safety of fermented garlic extract on hepatic function in adults with elevated serum gamma-glutamyl transpeptidase levels: A double-blind, randomized, placebo-controlled trial. Eur J Nutr. (2017) 56:1993–2002. doi: 10.1007/s00394–016-1318–6
188. Ben Hadda T, ElSawy NA, Header EA, Mabkhot YN, Mubarak MS. Effect of garlic and cabbage on healing of gastric ulcer in experimental rats. Med Chem Res. (2014) 23:5110–9. doi: 10.1007/s00044-014-1092-z
189. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. (2018) 137:e67–e492. doi: 10.1161/CIR.0000000000000558
190. Zhao CN, Meng X, Li Y, Li S, Liu Q, Tang GY, et al. Fruits for prevention and treatment of cardiovascular diseases. Nutrients. (2017) 9:598. doi: 10.3390/nu9060598
191. Kwak JS, Kim JY, Paek JE, Lee YJ, Kim HR, Park DS, et al. Garlic powder intake and cardiovascular risk factors: a meta-analysis of randomized controlled clinical trials. Nutr Res Pract. (2014) 8:644–54. doi: 10.4162/nrp.2014.8.6.644
192. Supakul L, Pintana H, Apaijai N, Chattipakorn S, Shinlapawittayatorn K, Chattipakorn N. Protective effects of garlic extract on cardiac function, heart rate variability, and cardiac mitochondria in obese insulin-resistant rats. Eur J Nutr. (2014) 53:919–28. doi: 10.1007/s00394–013-0595–6
193. Bradley JM, Organ CL, Lefer DJ. Garlic-derived organic polysulfides and myocardial protection. J Nutr. (2016) 146:403S–9S. doi: 10.3945/jn.114.208066
194. Kaur G, Padiya R, Adela R, Putcha UK, Reddy GS, Reddy BR, et al. Banerjee SK.Garlic and resveratrol attenuate diabetic complications, loss of β-cells, pancreatic and hepatic oxidative stress in streptozotocin-induced diabetic rats. Front Pharmacol. (2016) 7:360. doi: 10.3389/fphar.2016.00360
195. Jiang T, Chang Q, Cai J, Fan J, Zhang X, Xu G. Protective effects of melatonin on retinal inflammation and oxidative stress in experimental diabetic retinopathy. Oxid Med Cell Longev. (2016) 2016:3528274. doi: 10.1155/2016/3528274
196. Thomson M, Al-Qattan KK JSD, Ali M. Anti-diabetic and anti-oxidant potential of aged garlic extract (AGE) in streptozotocin-induced diabetic rats. BMC Complement Altern Med. (2016) 16:17. doi: 10.1186/s12906–016-0992–5
197. Wang J, Zhang X, Lan H, Wang W. Effect of garlic supplement in the management of type 2 Diabetes mellitus (T2DM): a meta-analysis of randomized controlled trials. Food Nutr Res. (2017) 61:1377571. doi: 10.1080/16546628.2017.1377571
198. Saikat AS, Hossain R, Mina FB, Das S, Khan IN, Mubarak MS, et al. Antidiabetic effect of garlic. Rev Bras Farmacogn. (2021) 32:1–11. doi: 10.1007/s43450-021-00193-y
199. Kim EJ, Lee DH, Kim HJ, Lee SJ, Ban JO, Cho MC, et al. Thiacremonone, a sulfur compound isolated from garlic, attenuates lipid accumulation partially mediated via AMPK activation in 3T3-L1 adipocytes. J Nutr Biochem. (2012) 23:1552–8. doi: 10.1016/j.jnutbio.2011.10.008
200. Lee CG, Rhee DK, Kim BO, Um SH, Pyo S. Allicin induces beige-like adipocytes via KLF15 signal cascade. J Nutr Biochem. (2019) 64:13–24. doi: 10.1016/j.jnutbio.2018.09.014
201. Kagawa Y, Ozaki−Masuzawa Y, Hosono T, Seki T. Garlic oil suppresses high−fat diet induced obesity in rats through the upregulation of UCP−1 and the enhancement of energy expenditure. Exp Ther Med. (2020) 19:1536–40. doi: 10.3892/etm.2019.8386
202. El-Demerdash FM, Yousef MI, Abou El-Naga N. Biochemical study on the hypoglycemic effects of onion and garlic in alloxan-induced diabetic rats. Food Chem Toxicol. (2005) 43:57–63. doi: 10.1016/j.fct.2004.08.012
203. Sanie-Jahromi F, Zia Z, Afarid M. A review on the effect of garlic on diabetes, BDNF, and VEGF as a potential treatment for diabetic retinopathy. Chin Med. (2023) 18:18. doi: 10.1186/s13020–023-00725–9
204. Staba EJ, Lash L, Staba JE. A commentary on the effects of garlic extraction and formulation on product composition. J Nutr. (2001) 131:1118S–9S. doi: 10.1093/jn/131.3.1118S
205. Wu J, Liu Y, Dou Z, Wu T, Liu R, Sui W, et al. Black garlic melanoidins prevent obesity, reduce serum LPS levels and modulate the gut microbiota composition in high-fat diet-induced obese C57BL/6J mice. Food Funct. (2020) 11:9585–98. doi: 10.1039/D0FO02379E
206. Ho S-C, Su M-S. Evaluating the anti-neuroinflammatory capacity of raw and steamed garlic as well as five organosulfur compounds. Molecules. (2014) 19:17697–714. doi: 10.3390/molecules191117697
207. Ebrahimzadeh-Bideskan A-R, Hami J, Alipour F, Haghir H, Fazel A-R, Sadeghi A. Protective effects of ascorbic acid and garlic extract against lead-induced apoptosis in developing rat hippocampus. Metab Brain Dis. (2016) 31:1123–32. doi: 10.1007/s11011–016-9837–7
208. Cemil B, Gokce EC, Kahveci R, Gokce A, Aksoy N, Sargon MF, et al. Aged garlic extract attenuates neuronal injury in a rat model of spinal cord ischemia/reperfusion injury. J Med Food. (2016) 19:601–6. doi: 10.1089/jmf.2016.0018
209. Semuyaba I, Safiriyu AA, Tiyo EA, Niurka RF. Memory improvement effect of ethanol garlic (A. sativum) extract in streptozotocin-nicotinamide induced diabetic Wistar rats is mediated through increasing of hippocampal sodium-potassium ATPase, glutamine synthetase, and calcium ATPase activities. Evid Based Complement Alternat Med. (2017) 2017:3720380. doi: 10.1155/2017/3720380
210. Nurmasitoh T, Sari DCR, Partadiredja G. The effects of black garlic on the working memory and pyramidal cell number of medial prefrontal cortex of rats exposed to monosodium glutamate. Drug Chem Toxicol. (2018) 41:324–9. doi: 10.1080/01480545.2017.1414833
212. Rana S, Pal R, Vaiphei K, Sharma SK, Ola RP. Garlic in health and disease. Nutr Res Rev. (2011) 24:60–71. doi: 10.1017/S0954422410000338
213. Mikaili P, Maadirad S, Moloudizargari M, Aghajanshakeri S, Sarahroodi S. Therapeutic uses and pharmacological properties of garlic, shallot, and their biologically active compounds. Iran J Basic Med Sci. (2013) 16:1031–48.
214. Asdaq SMB, Inamdar MN. The potential benefits of a garlic and hydrochlorothiazide combination as antihypertensive and cardioprotective in rats. J Nat Med. (2011) 65:81–8. doi: 10.1007/s11418–010-0467–9
215. Piasek A, Bartoszek A, Namieśnik J. Phytochemicals that counteract the cardiotoxic side effects of cancer chemotherapy. Postepy Hig Med Dosw. (2009) 63:142–58.
216. Rahman K, Lowe GM. Garlic and cardiovascular disease: a critical review. J Nutr. (2006) 136:736S–40S. doi: 10.1093/jn/136.3.736S
217. Salgado B, Monteiro LN, Rocha NS. Diseases TiT. Allium species poisoning in dogs and cats. J Venom Anim Toxins Incl Trop Dis. (2011) 17:4–11. doi: 10.1590/S1678–91992011000100002
218. Lee LS, Andrade AS, Flexner C. Interactions between natural health products and antiretroviral drugs: pharmacokinetic and pharmacodynamic effects. Clin Infect Dis. (2006) 43:1052–9. doi: 10.1086/507894
219. Borrelli F, Capasso R, Izzo AA. Garlic (Allium sativum L.): adverse effects and drug interactions in humans. Mol Nutr Food Res. (2007) 51:1386–97. doi: 10.1002/mnfr.200700072
220. Chen K, Xie K, Liu Z, Nakasone Y, Sakao K, Hossain A, et al. Preventive effects and mechanisms of garlic on dyslipidemia and gut microbiome dysbiosis. Nutrients. (2019) 11:1225. doi: 10.3390/nu11061225
221. Yüncü M, Eralp A, Celik A. Effect of aged garlic extract against methotrexate-induced damage to the small intestine in rats. Phytother Res. (2006) 20:504–10. doi: 10.1002/ptr.1896
222. Gruhlke MC, Nicco C, Batteux F, Slusarenko AJ. The effects of allicin, a reactive sulfur species from garlic, on a selection of mammalian cell lines. Antioxidants (2016) 6:1. doi: 10.3390/antiox6010001
223. Gutiérrez-del-Río I, Fernández J, Lombó F. Plant nutraceuticals as antimicrobial agents in food preservation: Terpenoids, polyphenols and thiols. Int J Antimicrob Agents. (2018) 52:309–15. doi: 10.1016/j.ijantimicag.2018.04.024
224. El-Sayed SM, El-Sayed HS. Antimicrobial nanoemulsion formulation based on thyme (Thymus vulgaris) essential oil for UF labneh preservation. J Mater Res Technol. (2021) 10:1029–41. doi: 10.1016/j.jmrt.2020.12.073
225. Dhifi W, Bellili S, Jazi S, Bahloul N, Mnif W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines (Basel). (2016) 3:25. doi: 10.3390/medicines3040025
226. Kannaiyan SK, Jeyakumari A, Nagalakshmi K, Venkateshwarlu G. Shelf life extension of tuna fillets using natural preservatives isolated from garlic. Fish Technol. (2014) 2014:179–86.
227. Kombat E, Bonu-Ire M, Adetunde L, Owusu-Frimpong M. Preservative effect of garlic (Allium sativum) paste on fresh Nile tilapia, Oreochromis niloticus (Cichlidae). Ghana J Sci Technol Dev. (2017) 5:1–6. doi: 10.47881/96.967x
228. El-Saadony MT, Saad AM, Elakkad HA, El-Tahan AM, Alshahrani OA, Alshilawi MS, et al. Flavoring and extending the shelf life of cucumber juice with aroma compounds-rich herbal extracts at 4°C through controlling chemical and microbial fluctuations. Saudi J Biol Sci. (2022) 29:346–54. doi: 10.1016/j.sjbs.2021.08.092
229. Saad AM, Elmassry R, Wahdan K, Hassanien M. Chickpea (Cicer arietinum) steep liquor as a leavening agent: effect on dough rheology and sensory properties of bread. Acta Period Technol. (2015) 46:91–102. doi: 10.2298/APT1546091S
230. Liu Q, Zhang M, Bhandari B, Xu J, Yang C. Effects of nanoemulsion-based active coatings with composite mixture of star anise essential oil, polylysine, and nisin on the quality and shelf life of ready-to-eat Yao meat products. Food Control. (2020) 107:106771. doi: 10.1016/j.foodcont.2019.106771
231. Diao X, Huan Y, Chitrakar B. Extending the shelf life of ready-to-eat spiced chicken meat: Garlic aqueous extracts-carboxymethyl chitosan ultrasonicated coating solution. Food Bioprocess Technol. (2020) 13:786–96. doi: 10.1007/s11947–020-02428–7
232. Gruskiene R, Bockuviene A, Sereikaite J. Microencapsulation of bioactive ingredients for their delivery into fermented milk products: a review. Molecules. (2021) 26:4601. doi: 10.3390/molecules26154601
233. Pateiro M, Gómez B, Munekata PES, Barba FJ, Putnik P, Kovačević DB, et al. Nanoencapsulation of promising bioactive compounds to improve their absorption, stability, functionality and the appearance of the final food products. Molecules. (2021) 26:1547. doi: 10.3390/molecules26061547
234. Youssef AM, El-Sayed HS, El-Nagar I, El-Sayed SM. Preparation and characterization of novel bionanocomposites based on garlic extract for preserving fresh Nile tilapia fish fillets. RSC Adv. (2021) 11:22571–84. doi: 10.1039/D1RA03819B
235. Moustafa H, El-Sayed SM, Youssef AM. Synergistic impact of cumin essential oil on enhancing of UV-blocking and antibacterial activity of biodegradable poly (butylene adipate-co-terephthalate)/clay platelets nanocomposites. J Thermoplast Compos Mater. (2023) 36:96–117. doi: 10.1177/0892705721989
Keywords: Allium sativum, bioactive compounds, functional foods, human diseases, human health, mechanisms of action, toxicity
Citation: El-Saadony MT, Saad AM, Korma SA, Salem HM, Abd El-Mageed TA, Alkafaas SS, Elsalahaty MI, Elkafas SS, Mosa WFA, Ahmed AE, Mathew BT, Albastaki NA, Alkuwaiti AA, El-Tarabily MK, AbuQamar SF, El-Tarabily KA and Ibrahim SA (2024) Garlic bioactive substances and their therapeutic applications for improving human health: a comprehensive review. Front. Immunol. 15:1277074. doi: 10.3389/fimmu.2024.1277074
Received: 13 August 2023; Accepted: 06 May 2024;
Published: 10 June 2024.
Edited by:
Wuquan Deng, Chongqing Emergency Medical Center, ChinaReviewed by:
Marcella Reale, University of Studies G. d’Annunzio Chieti and Pescara, ItalyShahzad Munir, Yunnan Agricultural University, China
Copyright © 2024 El-Saadony, Saad, Korma, Salem, Abd El-Mageed, Alkafaas, Elsalahaty, Elkafas, Mosa, Ahmed, Mathew, Albastaki, Alkuwaiti, El-Tarabily, AbuQamar, El-Tarabily and Ibrahim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Synan F. AbuQamar, c2FidXFhbWFyQHVhZXUuYWMuYWU=
 Mohamed T. El-Saadony
Mohamed T. El-Saadony Ahmed M. Saad
Ahmed M. Saad Sameh A. Korma
Sameh A. Korma Heba M. Salem
Heba M. Salem Taia A. Abd El-Mageed
Taia A. Abd El-Mageed Samar Sami Alkafaas
Samar Sami Alkafaas Mohamed I. Elsalahaty
Mohamed I. Elsalahaty Sara Samy Elkafas9,10
Sara Samy Elkafas9,10 Walid F. A. Mosa
Walid F. A. Mosa Ahmed Ezzat Ahmed
Ahmed Ezzat Ahmed Synan F. AbuQamar
Synan F. AbuQamar Khaled A. El-Tarabily
Khaled A. El-Tarabily Salam A. Ibrahim
Salam A. Ibrahim