- Department of Hepatobiliary Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Guangdong Provincial Clinical Research Center for Cancer, Guangzhou, China
Background: Intrahepatic cholangiocarcinoma (iCCA) is a highly aggressive cancer with a dismal prognosis and few effective therapeutic approaches. This study aimed to investigate the efficacy, safety, and predictive biomarkers of hepatic arterial infusion chemotherapy (FOLFOX-HAIC) in combination with lenvatinib and PD-1 inhibitor for patients with advanced iCCA.
Methods: Locally advanced or metastatic iCCA patients receiving the triple combination therapy of lenvatinib, PD-1 inhibitor, and FOLFOX-HAIC were included in this retrospective study. Primary endpoint was the progression-free survival, evaluated using the RECIST criterion. The secondary endpoints included overall survival, objective response rate, and safety. Whole exome and RNA sequencing of tumor biopsy tissues were performed for biomarker exploration.
Results: Between May, 2019 and December 2022, a total of 46 patients were included in this study. The primary endpoint showed a median progression-free survival of 9.40 months (95% CI: 5.28-13.52), with a 6-month progression-free survival rate of 76.1%. The median overall survival was 16.77 months (95% CI, 14.20-19.33), with an objective response rate of 47.8% and disease control rate of 91.3% per RECIST. In addition, 4.3% and 8.7% of patients achieved complete response of all lesions and intrahepatic target lesions per mRECIST, respectively. The most common treatment-related adverse events were neutropenia, thrombocytopenia, elevated aspartate aminotransferase and alanine aminotransferase level. Furthermore, integrated analysis of genetic, transcriptomic, and immunohistochemistry data revealed that pre-existing immunity (high expression level of immune-related signatures and intra-tumoral CD8+ T cell density) in baseline tumor tissues was associated with superior clinical benefits. However, the evaluation of tumor mutation burden did not show potential predictive value in this triple combination.
Conclusion: FOLFOX-HAIC in combination with lenvatinib and PD-1 inhibitor demonstrated a promising antitumor activity with manageable safety profiles in patients with advanced iCCA. Moreover, our study also revealed new perspectives on potential biomarkers for clinical efficacy.
Introduction
Intrahepatic cholangiocarcinoma (iCCA) is the second most common primary hepatic malignancy after hepatocellular carcinoma (HCC), accounting for approximately 10-20% of all newly diagnosed hepatobiliary neoplasms (1–3). Despite the improvements in surveillance of iCCA, the majority of patients are diagnosed at advanced disease stage, and the prognosis of untreated patients is extremely poor, with a median overall survival of 3-6 months (4, 5). Gemcitabine plus cisplatin (GemCis) is currently recommended as a standard treatment regimen for locally advanced and/or metastatic iCCA, which yields a median overall survival of 11.7 months (6, 7). However, treatment options and efficacy remain limited by chemo-refractory, while studies on potential systemic treatments are less well described and of limited effectiveness (8, 9).
Cancer immunotherapy with immune checkpoint inhibitors (ICIs), most notably anti-PD-1/PD-L1 antibodies, have gained success in a spectrum of advanced malignancies, including HCC (10, 11). Recent evidence suggested that chemotherapy in combination with ICIs, such as durvalumab plus GemCis, or camrelizumab combined with gemcitabine plus oxaliplatin (GEMOX), significantly improved the clinical outcomes in advanced biliary tract cancers (BTCs) (12, 13). Lenvatinib is an oral tyrosine kinase inhibitor (TKI) that targets vascular endothelial growth factor receptor (VEGFR) and fibroblast growth factor receptor (FGFR) (14), which reverts VEGF-driven immunosuppression and thereby augments the antitumor activity of PD-1 inhibitor (15). Several preliminary studies further showed that lenvatinib and PD-1 inhibitors displayed synergistic antitumor activity in patients with advanced iCCA (16, 17).
Besides the systemic treatments, locoregional therapies such as radioembolization, transarterial chemoembolization (TACE), and hepatic arterial infusion chemotherapy (HAIC), have also been performed in the treatment for patients with unresectable iCCA. Previous studies showed that hepatic arterial infusion chemotherapy with oxaliplatin, 5-fluorouracil, and leucovorin (FOLFOX-HAIC) yielded superior clinical outcomes to TACE for advanced iCCA (18). By achieving more extensive tumor necrosis and releasing tumor antigens, FOLFOX-HAIC could induce anti-tumor immune response and exert a synergistic anticancer effect with PD-1 inhibitors (19, 20).
Considering the different anti-malignancy mechanisms and the synergistic effects of TKIs, ICIs, and FOLFOX-HAIC, we hypothesize that locoregional treatment combined with systemic therapy might be suitable as a novel treatment option for locally advanced and/or metastatic iCCA. Although efforts have been made to reveal specific genetic and immunologic characteristics to determine the prognostic values for immune-combined therapy, favorable biomarkers are still lacking. Herein, we conducted this retrospective study to evaluate the efficacy, safety, and potential predictive biomarkers of FOLFOX-HAIC in combination with lenvatinib and PD-1 inhibitor for advanced iCCA.
Materials and methods
Study design and patients
This was a retrospective study assessing the efficacy and safety of FOLFOX-HAIC in combination with lenvatinib and PD-1 inhibitor as a first-line treatment for advanced iCCA. The trial was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Sun Yet-sen University Cancer Center. Written informed consent was provided by all patients before treatment.
Consecutive iCCA patients receiving FOLFOX-HAIC, lenvatinib and PD1 inhibitor as first-line treatment at Sun Yet-sen University Cancer Center between May, 2019 and December, 2022 were identified. Eligible patients were 18 years or older and diagnosed with unresectable iCCA according to the American Association for the Study of Liver Diseases practice guidelines (21, 22). Other key eligibility criteria for inclusion included the following: no previous systemic treatments for iCCA; Eastern Cooperative Oncology Group performance status (ECOG-PS) of 0-1; Child-Pugh class A liver function; at least one measurable tumor lesions according to the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 (23); and adequate organ function: neutrophil count >1.2×109 per L, platelet cell count ≥ 75×109 per L, total bilirubin ≤ 30 mmol/L, albumin ≥ 30 g/L, ALT and AST ≤ 5 times upper limit of normal range, and creatinine clearance rate of ≤ 1.5 times the upper limit of the normal range. Exclusion criteria included the following: combined with other malignant tumors; deficient blood supply of tumor indicated from CT or MRI arterial phase; incomplete medical information; and loss to follow-up.
Treatment protocol
Eligible patients received lenvatinib orally once daily (8mg or 12mg for body weight < 60kg or ≥ 60kg, respectively), as well as a PD-1 inhibitor intravenously every 3 weeks. Every patient was informed of the clinical efficacy, adverse events and cost of each PD-1 inhibitor. The final decision was principally made by patients based on their financial conditions and safety considerations. Five different PD-1 inhibitors were utilized based on patient preference (sintilimab 200 mg, toripalimab 240 mg, camrelizumab 200 mg, pembrolizumab 200 mg, tislelizumab 200 mg). FOLFOX-HAIC was performed every 3 weeks as described in our previous study: a catheter/microcatheter was placed in the main feeding hepatic artery, and then the following regimen was administered via the hepatic artery (24): oxaliplatin 85 mg/m2, leucovorin 400 mg/m2, 5-fluorouracil 400mg/m2 on Day 1, and 5-fluorouracil 2400 mg/m2 over 24 hours on Days 1-2. Once FOLFOX-HAIC intolerance occurred, or at end of 6 cycles, the other treatments (lenvatinib and/or PD-1 inhibitors) continued as maintenance therapy until disease progression, deaths or intolerable toxicities.
Data collection and assessment
Clinical information, laboratory and radiological data were retrospectively collected via the medical records. Tumor response assessment was conducted based on computed tomography (CT) and magnetic resonance imaging (MRI). All radiological data were independently assessed by two radiologists according to RECIST criteria. If there was a controversy, the final judgment was made by another more experienced radiologist.
The primary endpoint was progression-free survival (PFS), which was defined as the interval from treatment initiation to disease progression according to RECIST criteria or death, whichever occurred first. The secondary endpoints included overall survival (OS, defined as the interval from treatment initiation to death from any cause), objective response rate [ORR, defined as the proportion of patients of complete response (CR) or partial response (PR) based on RECIST criteria], disease control rate [DCR, defined as the proportion of patients with CR/PR plus stable disease (SD)], and safety. Treatment-related adverse events assessments were conducted according to the National Cancer Institute Common Terminology Criteria for Adverse Events v4.03.
Biomarker exploration
To identify potential biomarkers, biopsy samples of tumor tissues were collected and frozen before treatments. Whole-genome transcriptome profiling of biopsy tissues was performed by whole exome sequencing (WES) and RNA sequencing (RNAseq). Briefly, DNA and RNA in tumor biopsy tissues were extracted. Then library preparation, whole exome and RNA sequencing were performed following the standard protocol according to the manufacturer’s instructions. Somatic mutations, including point mutations, small insertions, and deletions, were identified. Tumor mutation burden (TMB) measurement was considered single nucleotide variants, insertions and deletions in the coding region. TMB high was defined as the top 50% value. Pre-treatment tumor biopsies were stored in formalin-fixed paraffin-embedded blocks. Baseline tumor-infiltrating CD8+ T cells were evaluated by immunohistochemistry (IHC) staining.
Statistical analysis
Baseline characteristics and efficacy data were calculated with appropriate methods including the Student’s t-test, the Mann-Whitney U-test, the Chi-square test, or Fisher’s exact test. OS and PFS with associated 95% confidence intervals (CIs) were analyzed using the Kaplan-Meier method, and differences in the survival curves were analyzed using the log-rank test. A p-value<0.05 was considered statistically significant. SPSS and GraphPad Prism were used for statistical analysis and bioinformatic analysis of the RNA-seq and WES data was performed using R 4.2.2 software.
Results
Baseline characteristics
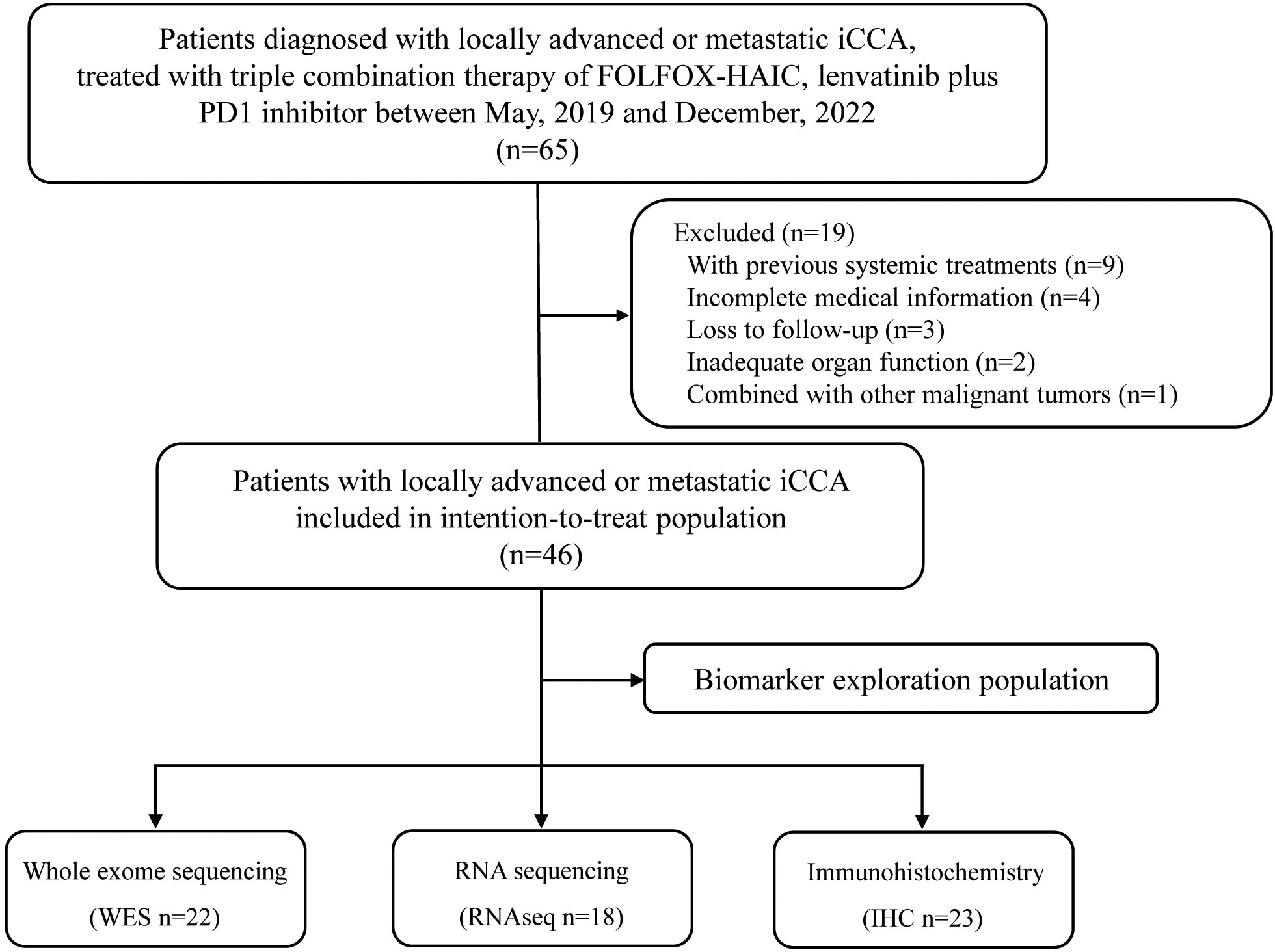
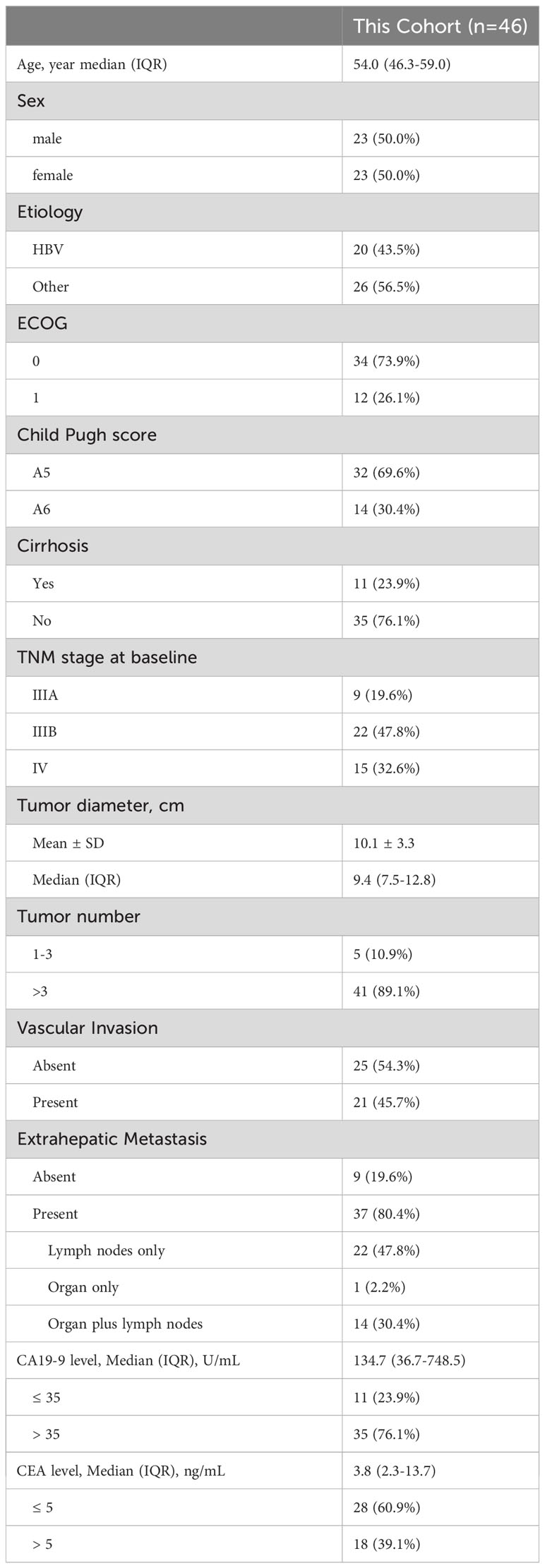
Between May, 2019 and December 2022, 65 consecutive patients who received triple combination therapy of FOLFOX-HAIC, lenvatinib plus PD-1 inhibitor were identified, and 46 iCCA patients met the criteria for inclusion in this study (Figure 1). The baseline demographic and clinical characteristics of enrolled patients are summarized in Table 1. Of the 46 patients included in this study, 23 (50.0%) were male, with a median age of 54.0 years (interquartile range, IQR: 46.3-59.0). In addition, all patients were diagnosed with locally advanced or metastatic diseases, including 80.4% with extrahepatic spread, and 45.7% of patients with macrovascular invasion. Moreover, 41 (89.1%) patients had multiple hepatic lesions and the median size of the maximum lesion was 9.4 cm (IQR: 7.5-12.8). Seventy-six percent of patients had elevated levels of carbohydrate antigen 19-9 (CA19-9, >35 U/mL).
Treatment
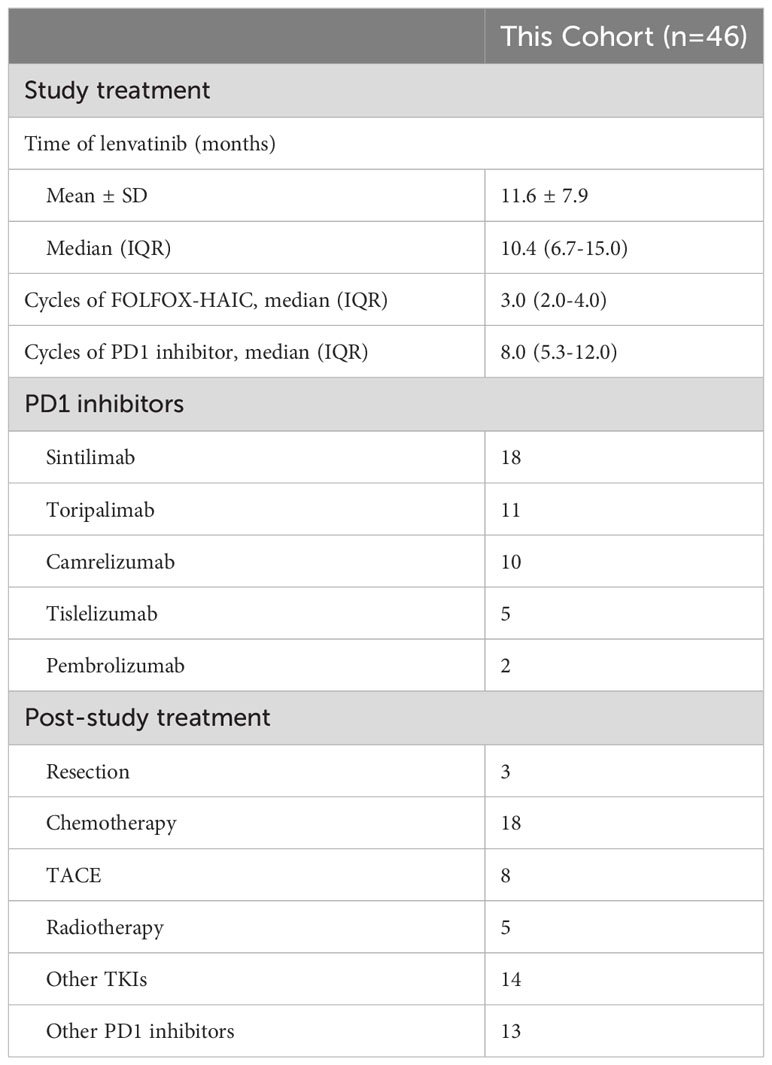
Treatment administration is listed in Table 2. Forty-six enrolled patients were treated with a total of 149 cycles of FOLFOX-HAIC [median (IQR): 3.0 (2.0-4.0)]. In addition, the median treatment cycles of PD-1 inhibitors and duration of lenvatinib were 8.0 (IQR: 5.3-12.0) and 10.4 (IQR: 6.7-15.0) months, respectively. At the date of data cutoff, 7 (15.2%) patients continued maintenance therapy with lenvatinib or PD-1 inhibitors, and were still free from disease progression. After termination of the study treatments, 32 patients received the second-line treatments, including systemic chemotherapy, curative surgical resection, TACE, radiotherapy, and other TKIs plus PD-1 inhibitors. Notably, radical resection was done in three patients with locally advanced iCCA after shrinkage of primary tumor and downgrading, all three of whom survived.
Efficacy
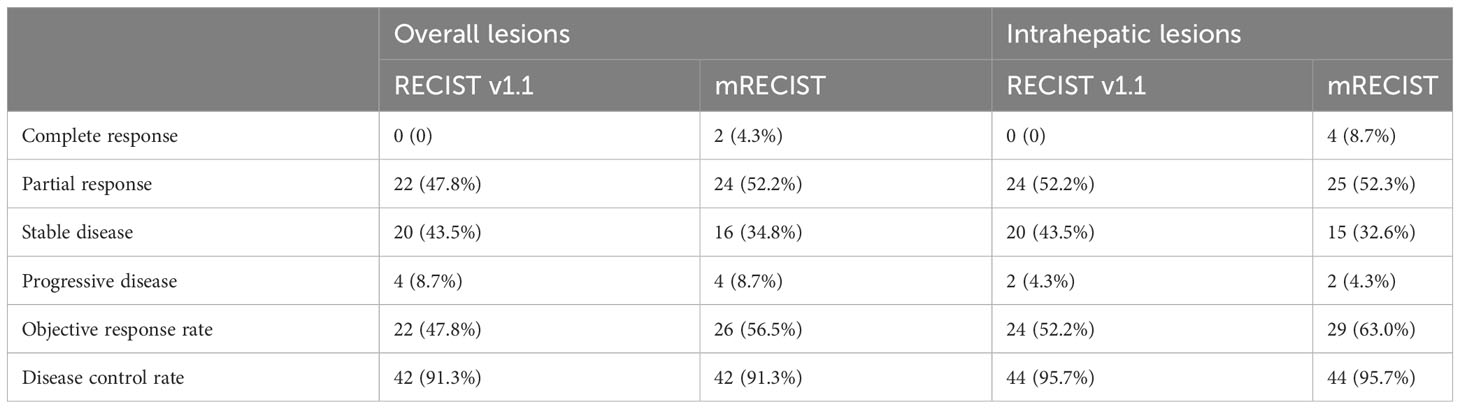
At the data cutoff for the analysis (October 1, 2023), all 46 patients completed follow-up, and a total of 37 patients had experienced disease progression or died. In regards to the primary endpoint, the median PFS was 9.4 months (95% CI: 5.28-13.52, Figure 2A), with a 6-month PFS rate of 76.1%. The median OS was 16.77 months (95% CI, 14.20-19.33, Figure 2B), with a 12-month OS rate of 69.3%. The tumor responses are showed in Table 3. Among the 46 examined patients who underwent tumor response evaluation with radiologic imaging, 22 (47.8%) of patients achieved partial response according to RECIST v1.1, 20 (43.5%) had stable disease, 4 (8.7%) experienced progressive disease, with an ORR of 47.8% and DCR of 91.3%. In addition, based on the mRECIST criteria, two patients achieved complete responses, the ORR and DCR were 56.5% and 91.3%, respectively.
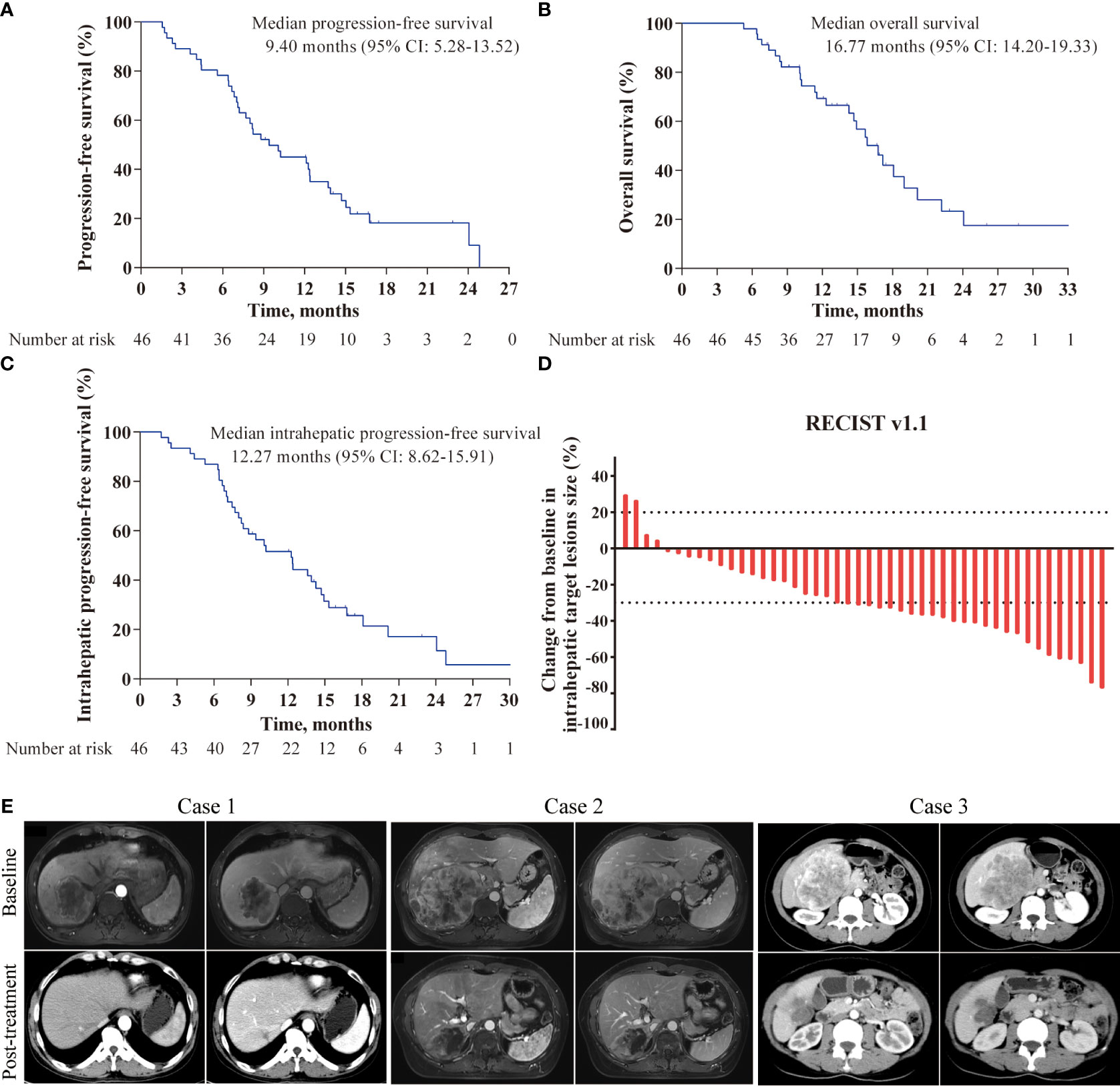
Figure 2 Kaplan-Meier curves and characteristics of tumor response in the study. (A) Kaplan-Meier survival curves of progression-free survival; (B) Kaplan-Meier survival curves of overall survival; (C) Kaplan-Meier survival curves of intrahepatic progression-free survival; (D) Best percentage changes from baseline in size of the intrahepatic target lesions; (E) Representative images of three patients responded to the triple combination therapy.
Given that FOLFOX-HAIC is a locoregional therapy and has better control for liver lesions, tumor responses of intrahepatic target lesions were sequentially tested. The best response rate of intrahepatic lesions was 52.2% per RECIST and 63.0% per mRECIST, with a DCR of 95.7%, including four patients experienced complete response based on mRECIST criteria. The median intrahepatic PFS was 12.27 months (95%CI, 8.62-15.91, Figure 2C). A waterfall plot was constructed to show the changes from baseline in the intrahepatic target lesions (Figure 2D). In addition, contrast-enhanced CT or MRI scans of three representative patients who received this triple combination therapy are shown in Figure 2E.
Safety
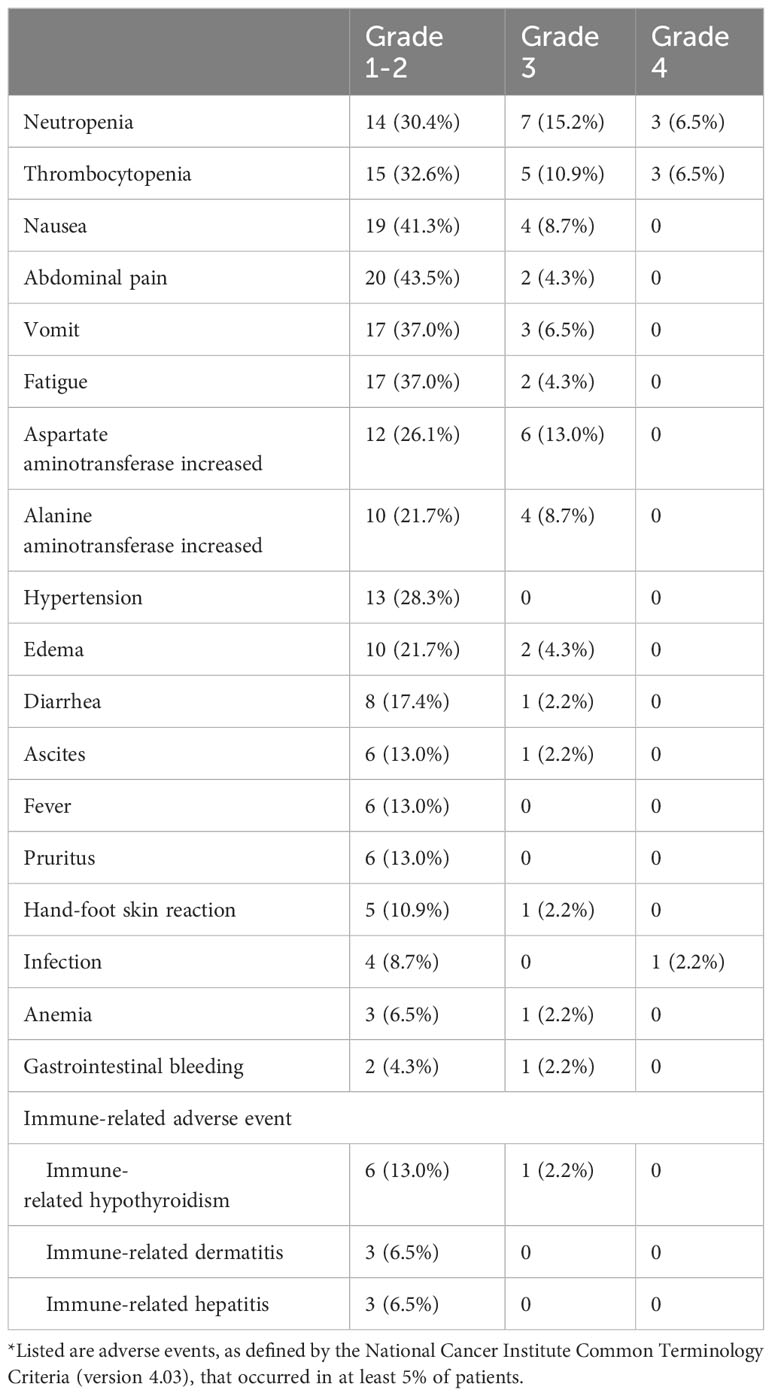
Treatment-related deaths did not occur in this study, and treatment-related adverse events (TRAEs), which occurred in at least 5.0% of patients, are shown in Table 4. The common TRAEs were neutropenia, thrombocytopenia, abdominal pain, nausea, fatigue, vomiting, and elevated ALT and AST levels. No grade 5 TRAEs were observed in this study, while 95.7% (44/46), 47.8% (22/46), and 10.9% (5/46) of patients experienced grades 1-2, 3, and 4 TRAEs, respectively. In present study, the most common grade 3 or higher TRAEs included neutropenia (n=10, 21.7%), thrombocytopenia (n=8, 17.4%), increased AST levels (n=6, 13.0%), and increased ALT levels (n=4, 8.7%). Additionally, immune-related adverse events of any grade were observed in 13 (28.3%) participants, including hepatitis (n=3), dermatitis (n=3) and hypothyroidism (n=7), which could be alleviated and eliminated by treatment interruption or dose modification.
Overview of the genomic mutation spectrum
In the prespecified exploratory analyses, we first evaluated the association between genomic alteration and clinical response to this triple combination therapy in 22 patients with adequate pre-treatment tumor biopsies for WES (10 responders defined as having a complete and partial response, and 12 non-responders defined as having a stable and progressive disease). Figure 3A depicted the genetic alterations and frequencies in the entire cohort, including the most commonly altered genes and cancer-related pathways. Unfortunately, none of the genomic mutation or pathway alteration exhibited significant associations with clinical response or survival in univariable analysis (data not shown). We next analyzed the clinical relevance of TMB in the 22 patients with available data. According to the median split, there was no significant correlation between the level of TMB and clinical response, intrahepatic PFS, or OS (Figures 3B–D).
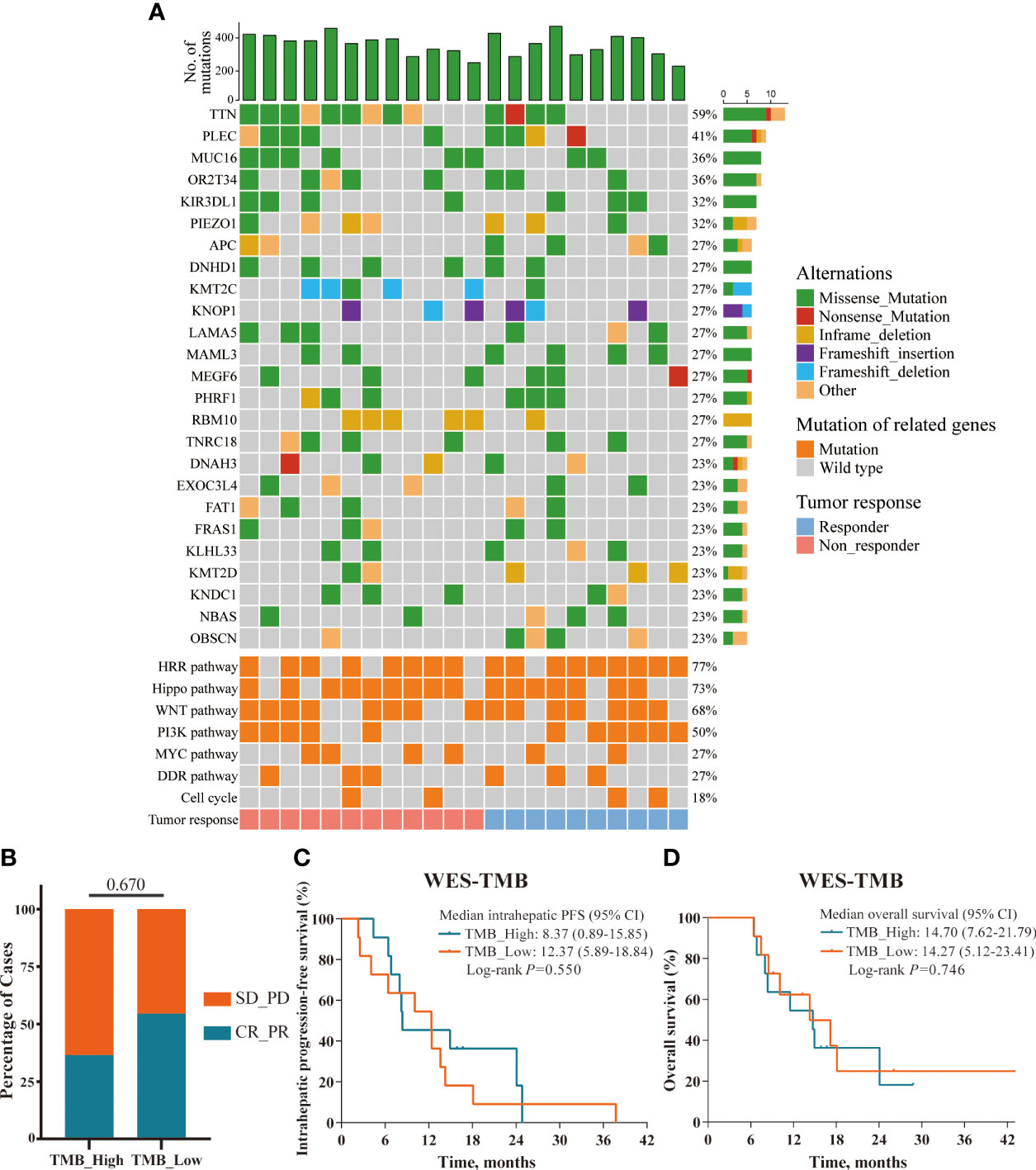
Figure 3 Overview of the genomic mutation spectrum. (A) Overview of the genomic mutation spectrum and pathway alterations in the WES cohort (responders, n=10; non-responders, n=12); (B–D) TMB status presented no significant correlations with ORR, intrahepatic PFS, and OS; defined by median split.
Transcriptome analyses in responders and non-responders
To further assess the impact of tumor immune microenvironment (TIME) on clinical response to this triple combination therapy, we performed transcriptome analyses in 18 patients (9 responders, 9 non-responders). Genome-wide differentially expressed gene analyses identified multiple genes representing pre-existing antitumor immunity as the top features associated with tumor response, including those immune cell-related genes (PTPRC, CD3E, CD8A, MS4A1, and IGHG4); cytotoxic T cell signature (IFNG, GZMH, GZMK, KLRD1, and IRF1); chemokines (CXCL13, CXCL9, CCL17, and CCL22), and immune checkpoint target genes (CD274, CTLA4, LAG3, and TIGIT, Figure 4A). Gene Ontology (GO) enrichment analyses further confirmed that multiple adaptive and innate immunity pathways, including lymphocyte activation, B cell mediated immunity, T cell proliferation, interferon-gamma production and T cell receptor signaling pathway, were significantly enriched in patients that responded to this triple combination (Figure 4B). We obtained the similar results in the REACTOME pathway analyses, which were also concentrated in adaptive immunity and chemokine signaling pathways (Figure 4C). We next assessed the expression levels of several gene signatures representing pre-existing antitumor immunity and evaluated their association with the clinical outcomes. Notably, we observed that the expression scores of six gene signatures were significantly higher in responders than that in non-responders, namely chemokines (p = 0.004), MHC class II signature (p = 0.006), cytotoxic activity signature (p = 0.008), co-inhibition signature (p = 0.024), effector T cell (p = 0.031) and co-stimulation signature (p = 0.040; Figures 5A, B). Furthermore, we observed that iCCA patients with higher expression levels of chemokine, cytotoxic activity, and co-inhibition signatures exhibited improved ORRs (chemokine: 88.9% vs. 11.1%, p = 0.003; cytotoxic activity: 88.9% vs. 11.1%, p = 0.003; co-inhibition: 77.8% vs. 22.2%, p = 0.057) and superior OS (chemokine: 18.10 vs. 8.37 months, p = 0.017; cytotoxic activity: 18.10 vs. 8.37 months, p = 0.017; co-inhibition: 18.10 vs. 11.53 months, p=0.030; Figures 5C, D).
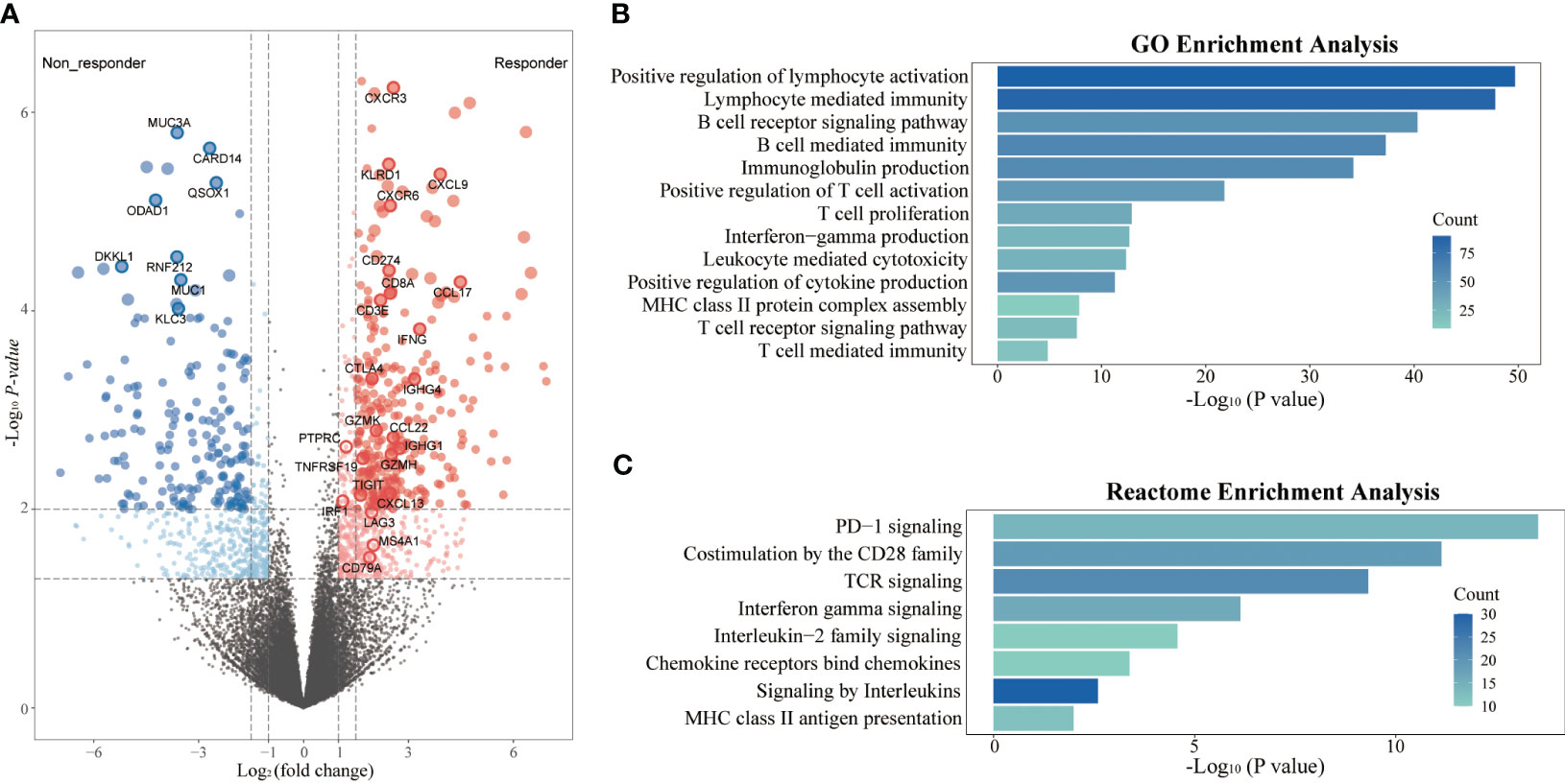
Figure 4 Transcriptome analyses in responders and non-responders. (A) Differential expression genes between responders (n=9) and non-responders (n=9) in the RNAseq cohort; (B) GO enrichment analysis of differential expression genes; (C) REACTOME pathway enrichment analysis of differential expression genes.
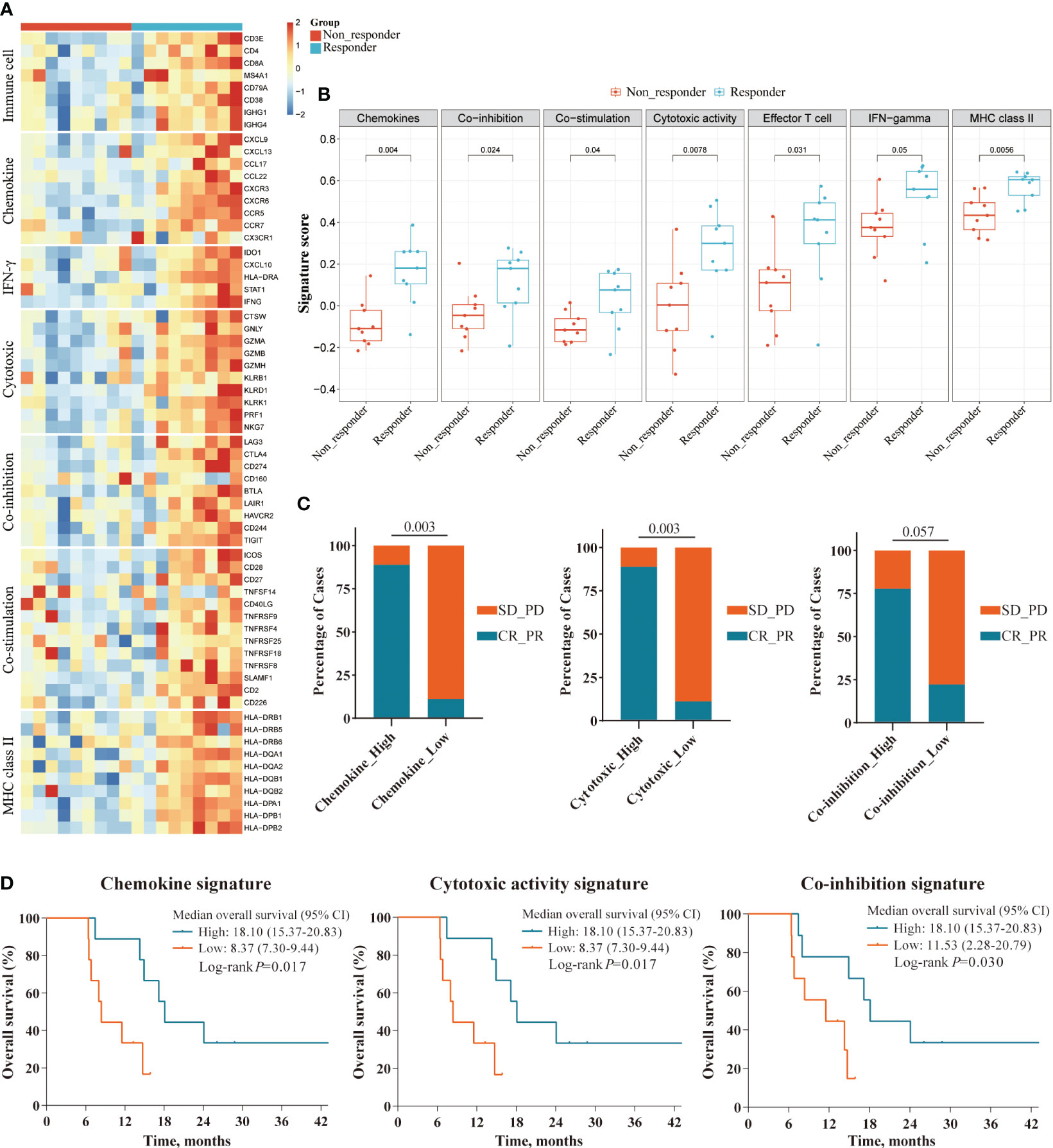
Figure 5 Survival analyses of immune-related gene signatures in responders and non-responders. (A) Heatmap showing the expression level of gene signatures representing pre-existing antitumor immunity between responders and non-responders in the RNAseq cohort; (B) Boxplot showing the expression scores of gene signatures between responders and non-responders in the RNAseq cohort; (C, D) Superior ORR and OS were associated with higher expression level of three gene signatures: Chemokine, Cytotoxic activity, and Co-inhibition signature, defined by the median split.
Subsequently, we assessed the abundance of immune cells to further investigate the TIME using xCell deconvolution analysis (25). And we observed that a higher presence of several immune subsets, including CD8+ and CD4+ T cells, B cells and dendritic cells, also seemed to be associated with better response (Figure 6A). Further survival analysis showed that high levels of tumor-infiltrating lymphocytes (TILs) were associated with markedly longer survival outcomes in OS (CD4 naïve T cells: 24.07 vs. 8.00 months, p < 0.001; CD8 T cells: 24.07 vs. 8.37 months, p=0.004; Figure 6B) and intrahepatic PFS (CD4 naïve T cells: 18.10 vs. 6.83 months, p=0.002; CD8 T cells: 18.10 vs. 8.00 months, p < 0.001; Figure 6C).
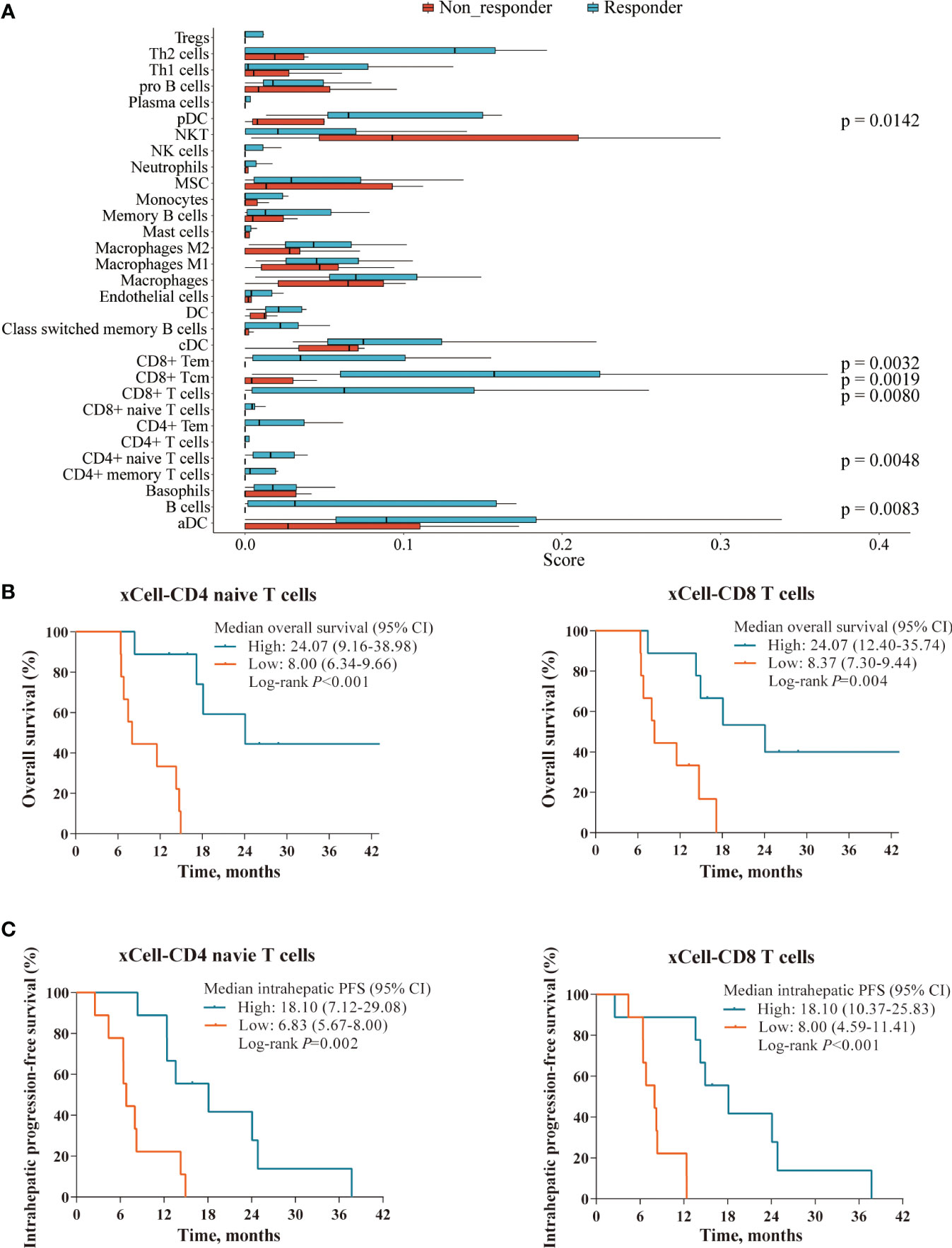
Figure 6 Immune cell profile analyses in the RNAseq cohort. (A) Boxplot showing the abundance of several immune cells composition between responders (n=9) and non-responders (n=9) in the RNAseq cohort, deconvolved by xCell algorithm. (B, C) Superior OS and intrahepatic PFS were associated with higher levels of tumor-infiltrating lymphocytes defined xCell deconvolution analysis: CD4 naïve T cells (left panel) and CD8 T cells (right panel).
We next analyzed the density of tumor-infiltrating CD8+ T cells and their association with clinical outcomes (IHC cohort: 12 responders and 11 non-responders). Consistent with our genomic findings, IHC analysis of 23 tumor samples showed that responders exhibited a higher density of tumor-infiltrating CD8+ T cells than non-responders (p = 0.0013, Figures 7A, B). Patients with a higher density of tumor-infiltrating CD8+ T cells exhibited improved ORR (83.3% vs. 18.2%, p = 0.003), OS (17.17 vs. 10.07 months, p = 0.005) and intrahepatic PFS (13.90 vs. 6.67 months, p < 0.001; Figures 7C–E).
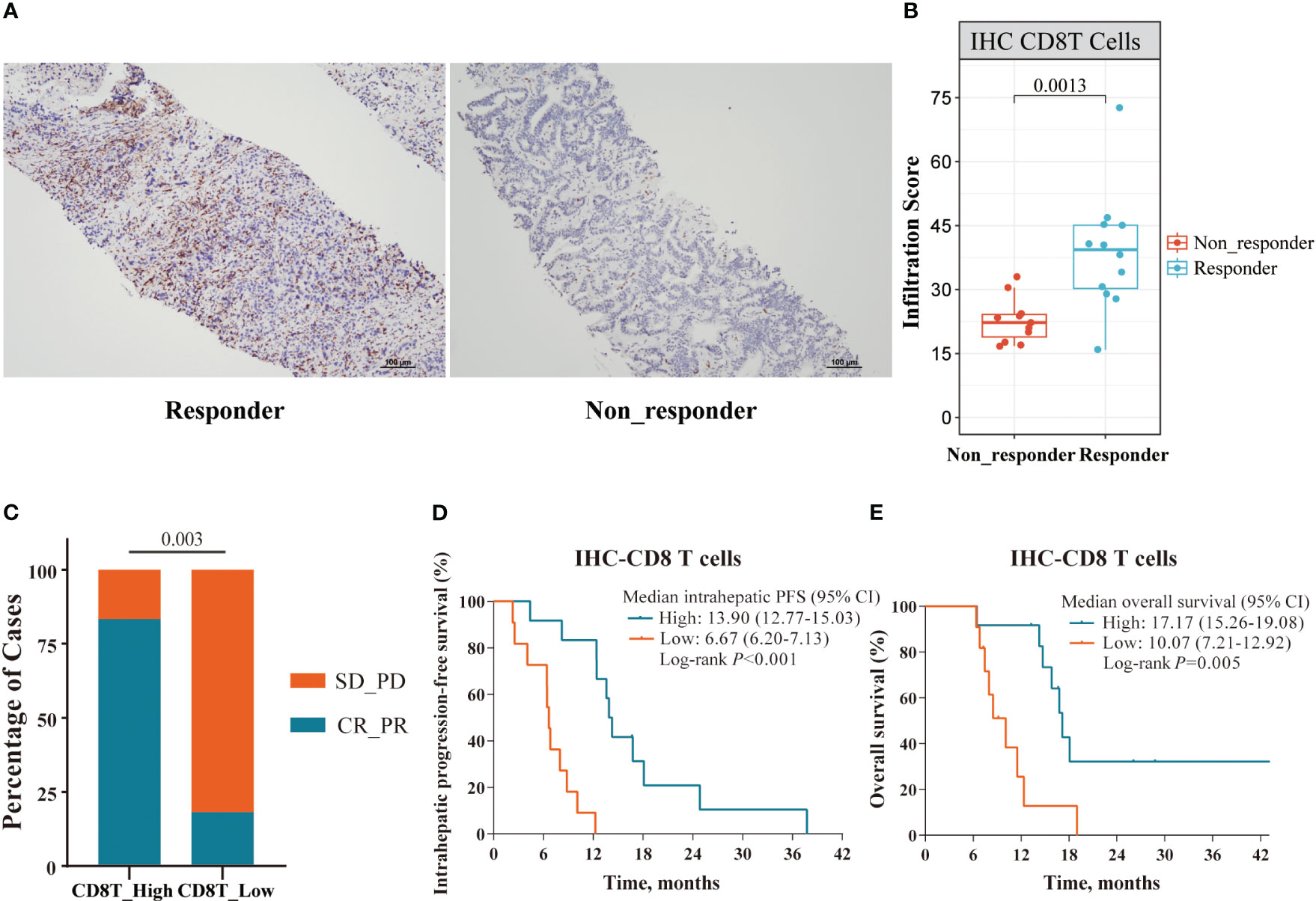
Figure 7 Immune cell profile analyses in the IHC cohort. (A) Representative IHC staining images of responder and non-responder; (B) The Boxplot showing the significant differences with a density of tumor-infiltrating CD8+ T cells between responders (n=12) and non-responders (n=11) in the IHC cohort; (C–E) Superior ORR, intrahepatic PFS and OS were associated with higher density of tumor-infiltrating CD8+ T cells, defined by median split.
Discussion
To the best of our knowledge, this retrospective study is the first to report the efficacy and safety of a novel triple combination therapy with FOLFOX-HAIC, lenvatinib plus PD-1 inhibitor, which yielded a promising antitumor activity with manageable safety profiles in patients with locally advanced and metastatic iCCA.
Many efforts have been made to explore the clinical efficacy of PD-1 inhibitors in advanced BTCs with little success. Previous clinical trials showed the limited antitumor activity of ICIs monotherapy in advanced iCCA, with a median OS of 5.2-7.4 months and ORR of less than 15% (26, 27). However, TOPAZ-1 trial showed that durvalumab plus GemCis provided significant survival benefits compared to GemCis (median OS: 12.8 vs. 11.5 months) (13), which is also recommended as one of preferred first-line treatments for advanced BTCs, including iCCA. In addition, iCCA was characterized by abnormal activation of VEGF and FGFR signaling pathway (28, 29), which are the primary targets of lenvatinib. And the survival benefits were further demonstrated in a recent phase II trial combining toripalimab with lenvatinib and GEMOX, which yielded a high ORR (80%) and a median OS of 22.5 months for advanced iCCA (30). These data had provided definite evidence for the reasoning of chemotherapy, lenvatinib plus PD-1 inhibitor as a combo to treat advanced iCCA.
In our previous publication, we demonstrated lenvatinib, toripalimab and FOLFOX-HAIC exhibited encouraging antitumor activity for advanced HCC (20). These advantages were attainable due to the specific administration patterns of HAIC. FOLFOX-HAIC could deliver chemotherapeutic agents directly into tumor-associated arterial branches, and increase local drug concentrations (31), thus providing stronger antitumor efficacy and lower systemic toxicities than systemic therapies (32). In the current study, we focused on the clinical effects and safety of this similar triple combination for pure advanced iCCA.
Our results displayed potential synergistic effect and promising preliminary efficacy results of this triple combination therapy, with an overall ORR of 47.8%, DCR of 91.3%, median PFS of 9.40 months (95% CI, 5.28-13.52) and median OS of 16.77 months (95% CI, 14.20-19.33). This combination therapy resulted in a higher ORR and better mPFS for advanced iCCA than standard chemotherapy or PD-1 inhibitors plus chemotherapy, thus providing a robust antitumor effect and allowing a proportion of patients to achieve downstaging and conversion to radical resection. Notably, the patient population in this study might be considered to have a poor prognosis because all patients included in this study had stage IIIA disease or higher at baseline, presented with a higher intrahepatic tumor burden. Additionally, we demonstrated that FOLFOX-HAIC played an important role in shrinkage of hepatic lesions. The response rate of intrahepatic lesions was 52.2% per RECIST and 63.0% per mRECIST, including 4 patients achieved complete response based on mRECIST criteria.
TRAEs observed with this combination therapy are consistent with those reported in previous trials (20, 24, 33). The most common TRAEs were neutropenia, thrombocytopenia, abdominal pain, nausea, vomiting, and elevated serum ALT or AST level. Hematologic toxicity was the main grade 3 and higher TRAEs in this study, which were tolerable and manageable. Furthermore, immune-related adverse events of any grade were observed in 13 (28.3%) patients, including hepatitis, dermatitis, and hypothyroidism, which were associated with PD-1 inhibitors as reported in the previous studies (34, 35).
Although this combination significantly improved clinical outcomes, unfortunately, small proportion of patients remained unresponsive. It is therefore important to identify biomarkers to predict which patients could benefit most. By integrating transcriptomic, genetic, and IHC analyses of primary tumors, we characterized the molecular correlates of clinical response and resistance to this triple combination therapy. Surprisingly, gene sequencing result demonstrated no significant correlations of gene mutations, pathway alterations, or TMB with clinical response and survival outcomes. Moreover, there have been contradictory results about the predictive value of TMB status for immunotherapy. Exploratory analysis of prospective trials revealed that higher TMB was associated with superior survival and objective response in advanced solid tumor (36–38). However, several studies evaluating combined-immunotherapy showed that TMB status did not show any predictive value for efficacy in patients with advanced BTC, NSCLC and ESCC (39–42). It is plausible that giving chemotherapy with immunotherapy could have confounded the utility of TMB. Our results showed that neither objective response nor survival differed significantly on the basis of TMB status, highlighting that TMB may not be an effective biomarker for predicting the clinical benefit of this combination therapy.
A central finding from our study is that the presence of pre-existing immunity (higher expression of specific immune-related signatures, and intra-tumoral CD8+ T cell density) in baseline tumor tissues was associated with better clinical outcomes with this triple combination. Consistent with the features of ‘immune-hot’ tumor, immune cell-related genes, chemokines, and immune checkpoint targets were found to be upregulated in the responders. Biomarkers of inflammation and inflammatory gene signatures were indeed reported to be associated with response to immunotherapy (39, 43). Our study also observed significant correlations between immune-related signatures (higher expression level of cytotoxic activity, co-inhibition, and co-stimulation signatures) with improved clinical outcomes, suggesting the predominant TIME characteristics of responsive iCCA. It is worth mentioning that, as a crucial prognostic indicator, TILs exhibit great predictive power for survival in solid cancers (44, 45). Moreover, our study further confirmed its value by showing that a higher baseline density of CD8+ T cells in the TIME was an indicator of treatment activity, which was associated with significantly superior clinical benefits. Taken together, pre-existing antitumor immunity in baseline tumor might be predictive biomarkers for the triple combination therapy. However, confirmation in a larger study population is required to verify our preliminary results.
This study had several limitations. First, this was a retrospective and nonrandomized study with a limited sample size, for which the retrospective design and nonrandomized nature made it vulnerable to a variety of potential biases. These findings in the study needed prospective randomized controlled trials to verify. Second, the follow-up time was relatively short for OS, but was sufficient for short-term efficacy (PFS and tumor responses). Therefore, we chose PFS as the primary endpoint in this study, which could eliminate the confounding effect of subsequent therapy and reflected the efficacy more accurately. Finally, due to the relatively small number of tissue specimens, exploratory research in this study did not have strong statistical power to draw a definite conclusion on the predictive role of these candidate biomarkers.
Conclusion
In conclusion, this retrospective study provided preliminary evidence that FOLFOX-HAIC in combination with lenvatinib and PD-1 inhibitor showed a promising antitumor activity with manageable safety profiles in advanced iCCA. Moreover, our results also revealed new perspectives on potential biomarkers for clinical efficacy. These findings warrant further validation in a large randomized clinical trial.
Data availability statement
The raw data generated in this study have been deposited in the Gene Expression Omnibus (GEO) database, with the accession number as GSE255058.
Ethics statement
The studies involving humans were approved by institutional review board of Sun Yat-sen University Cancer Center. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YH: Conceptualization, Data curation, Formal Analysis, Investigation, Visualization, Writing – original draft. ZD: Data curation, Formal Analysis, Methodology, Software, Visualization, Writing – original draft. AK: Data curation, Funding acquisition, Investigation, Methodology, Writing – original draft. MH: Conceptualization, Data curation, Funding acquisition, Investigation, Writing – original draft. HL: Data curation, Formal Analysis, Methodology, Writing – original draft. ZL: Formal Analysis, Investigation, Methodology, Writing – original draft. DW: Data curation, Investigation, Methodology, Writing – original draft. LH: Data curation, Methodology, Writing – original draft. QL: Data curation, Investigation, Methodology, Writing – original draft. LX: Conceptualization, Investigation, Supervision, Validation, Writing – original draft. MS: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (No. 82272890, No. 82102985, No. 82072610), Development Planned Project in Key Areas of Guangdong Province (2019B110233002), China Postdoctoral Science Foundation (No. 2021TQ0383).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
References
1. Peery AF, Crockett SD, Murphy CC, Jensen ET, Kim HP, Egberg MD, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2021. Gastroenterology (2022) 162:621–44. doi: 10.1053/j.gastro.2021.10.017
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
3. Bertuccio P, Malvezzi M, Carioli G, Hashim D, Boffetta P, El-Serag HB, et al. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol (2019) 71:104–14. doi: 10.1016/j.jhep.2019.03.013
4. Zhang H, Yang T, Wu M, Shen F. Intrahepatic cholangiocarcinoma: Epidemiology, risk factors, diagnosis and surgical management. Cancer Lett (2016) 379:198–205. doi: 10.1016/j.canlet.2015.09.008
5. Charbel H, Al-Kawas FH. Cholangiocarcinoma: epidemiology, risk factors, pathogenesis, and diagnosis. Curr Gastroenterol Rep (2011) 13:182–7. doi: 10.1007/s11894-011-0178-8
6. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med (2010) 362:1273–81. doi: 10.1056/NEJMoa0908721
7. Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2021) 19:541–65. doi: 10.6004/jnccn.2021.0022
8. Kelley RK, Bridgewater J, Gores GJ, Zhu AX. Systemic therapies for intrahepatic cholangiocarcinoma. J Hepatol (2020) 72:353–63. doi: 10.1016/j.jhep.2019.10.009
9. Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol (2020) 17:557–88. doi: 10.1038/s41575-020-0310-z
10. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science (2018) 359:1350–5. doi: 10.1126/science.aar4060
11. Kraehenbuehl L, Weng CH, Eghbali S, Wolchok JD, Merghoub T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat Rev Clin Oncol (2022) 19:37–50. doi: 10.1038/s41571-021-00552-7
12. Chen X, Wu X, Wu H, Gu Y, Shao Y, Shao Q, et al. Camrelizumab plus gemcitabine and oxaliplatin (GEMOX) in patients with advanced biliary tract cancer: a single-arm, open-label, phase II trial. J Immunother Cancer (2020) 8. doi: 10.1136/jitc-2020-001240
13. Oh D-Y, Ruth He A, Qin S, Chen L-T, Okusaka T, Vogel A, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evidence (2022) 1. doi: 10.1056/EVIDoa2200015
14. Tohyama O, Matsui J, Kodama K, Hata-Sugi N, Kimura T, Okamoto K, et al. Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res (2014) 2014:638747. doi: 10.1155/2014/638747
15. Hack SP, Zhu AX and Wang Y. Augmenting anticancer immunity through combined targeting of angiogenic and PD-1/PD-L1 pathways: challenges and opportunities. Front Immunol (2020) 11:598877. doi: 10.3389/fimmu.2020.598877
16. Zhang Q, Liu X, Wei S, Zhang L, Tian Y, Gao Z, et al. Lenvatinib plus PD-1 inhibitors as first-line treatment in patients with unresectable biliary tract cancer: a single-arm, open-label, phase II study. Front Oncol (2021) 11:751391. doi: 10.3389/fonc.2021.751391
17. Shi C, Li Y, Yang C, Qiao L, Tang L, Zheng Y, et al. Lenvatinib plus programmed cell death protein-1 inhibitor beyond first-line systemic therapy in refractory advanced biliary tract cancer: a real-world retrospective study in China. Front Immunol (2022) 13:946861. doi: 10.3389/fimmu.2022.946861
18. Cai Z, He C, Zhao C, Lin X. Survival comparisons of hepatic arterial infusion chemotherapy with mFOLFOX and transarterial chemoembolization in patients with unresectable intrahepatic cholangiocarcinoma. Front Oncol (2021) 11:611118. doi: 10.3389/fonc.2021.611118
19. Liu WM, Fowler DW, Smith P, Dalgleish AG. Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br J Cancer (2010) 102:115–23. doi: 10.1038/sj.bjc.6605465
20. Lai Z, He M, Bu X, Xu Y, Huang Y, Wen D, et al. Lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy in patients with high-risk advanced hepatocellular carcinoma: A biomolecular exploratory, phase II trial. Eur J Cancer (2022) 174:68–77. doi: 10.1016/j.ejca.2022.07.005
21. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology (2018) 67:358–80. doi: 10.1002/hep.29086
22. Lee AJ, Chun YS. Intrahepatic cholangiocarcinoma: the AJCC/UICC 8th edition updates. Chin Clin Oncol (2018) 7:52. doi: 10.21037/cco.2018.07.03
23. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
24. Li QJ, He MK, Chen HW, Fang WQ, Zhou YM, Xu L, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: a randomized phase III trial. J Clin Oncol (2022) 40:150–60. doi: 10.1200/JCO.21.00608
25. Aran D, Hu Z and Butte AJ. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol (2017) 18:220. doi: 10.1186/s13059-017-1349-1
26. Kim RD, Chung V, Alese OB, El-Rayes BF, Li D, Al-Toubah TE, et al. A phase 2 multi-institutional study of nivolumab for patients with advanced refractory biliary tract cancer. JAMA Oncol (2020) 6:888–94. doi: 10.1001/jamaoncol.2020.0930
27. Piha-Paul SA, Oh DY, Ueno M, Malka D, Chung HC, Nagrial A, et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int J Cancer (2020) 147:2190–8. doi: 10.1002/ijc.33013
28. Javle M, Lowery M, Shroff RT, Weiss KH, Springfeld C, Borad MJ, et al. Phase II study of BGJ398 in patients with FGFR-altered advanced cholangiocarcinoma. J Clin Oncol (2018) 36:276–82. doi: 10.1200/JCO.2017.75.5009
29. Yoshikawa D, Ojima H, Iwasaki M, Hiraoka N, Kosuge T, Kasai S, et al. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer (2008) 98:418–25. doi: 10.1038/sj.bjc.6604129
30. Shi GM, Huang XY, Wu D, Sun HC, Liang F, Ji Y, et al. Toripalimab combined with lenvatinib and GEMOX is a promising regimen as first-line treatment for advanced intrahepatic cholangiocarcinoma: a single-center, single-arm, phase 2 study. Signal Transduct Target Ther (2023) 8:106. doi: 10.1038/s41392-023-01317-7
31. Bartkowski R, Berger MR, Aguiar JL, Henne TH, Dorsam J, Geelhaar GH, et al. Experiments on the efficacy and toxicity of locoregional chemotherapy of liver tumors with 5-fluoro-2'-deoxyuridine (FUDR) and 5-fluorouracil (5-FU) in an animal model. J Cancer Res Clin Oncol (1986) 111:42–6. doi: 10.1007/BF00402774
32. Ueshima K, Ogasawara S, Ikeda M, Yasui Y, Terashima T, Yamashita T, et al. Hepatic arterial infusion chemotherapy versus sorafenib in patients with advanced hepatocellular carcinoma. Liver Cancer (2020) 9:583–95. doi: 10.1159/000508724
33. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet (2018) 391:1163–73. doi: 10.1016/S0140-6736(18)30207-1
34. Wang ZX, Cui C, Yao J, Zhang Y, Li M, Feng J, et al. Toripalimab plus chemotherapy in treatment-naive, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell (2022) 40:277–288.e273. doi: 10.1016/j.ccell.2022.02.007
35. Mai HQ, Chen QY, Chen D, Hu C, Yang K, Wen J, et al. Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial. Nat Med (2021) 27:1536–43. doi: 10.1038/s41591-021-01444-0
36. Jardim DL, Goodman A, de Melo Gagliato D, Kurzrock R. The challenges of tumor mutational burden as an immunotherapy biomarker. Cancer Cell (2021) 39:154–73. doi: 10.1016/j.ccell.2020.10.001
37. Sharma P, Pachynski RK, Narayan V, Flechon A, Gravis G, Galsky MD, et al. Initial results from a phase II study of nivolumab (NIVO) plus ipilimu mab (IPI) for the treatment of metastatic castration-resistant prostat e cancer (mCRPC; CheckMate 650). J Clin Oncol 37:142. doi: 10.1200/jco.2019.37.7_suppl.142
38. Marabelle A, Fakih MG, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of Tumour Mutational Burden with Outcomes in Patients with Select Advanced Solid Tumours Treated with Pembrolizumab in Keynote-1 58. Ann Oncol 30:v477–v8. doi: 10.1093/annonc/mdz253.018
39. Zeng TM, Yang G, Lou C, Wei W, Tao CJ, Chen XY, et al. Clinical and biomarker analyses of sintilimab plus gemcitabine and cisplatin as first-line treatment for patients with advanced biliary tract cancer. Nat Commun (2023) 14:1340. doi: 10.1038/s41467-023-37030-w
40. Feng K, Liu Y, Zhao Y, Yang Q, Dong L, Liu J, et al. Efficacy and biomarker analysis of nivolumab plus gemcitabine and cisplatin in patients with unresectable or metastatic biliary tract cancers: results from a phase II study. J Immunother Cancer (2020) 8. doi: 10.1136/jitc-2019-000367
41. Garassino MC, Gadgeel S, Esteban E, Felip E, Speranza G, Domine M, et al. Patient-reported outcomes following pembrolizumab or placebo plus pemetrexed and platinum in patients with previously untreated, metastatic, non-squamous non-small-cell lung cancer (KEYNOTE-189): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol (2020) 21:387–97. doi: 10.1016/S1470-2045(19)30801-0
42. Zhu Y, Wen J, Li Q, Chen B, Zhao L, Liu S, et al. Toripalimab combined with definitive chemoradiotherapy in locally advanced oesophageal squamous cell carcinoma (EC-CRT-001): a single-arm, phase 2 trial. Lancet Oncol (2023) 24:371–82. doi: 10.1016/S1470-2045(23)00060-8
43. Sangro B, Melero I, Wadhawan S, Finn RS, Abou-Alfa GK, Cheng AL, et al. Association of inflammatory biomarkers with clinical outcomes in nivolumab-treated patients with advanced hepatocellular carcinoma. J Hepatol (2020) 73:1460–9. doi: 10.1016/j.jhep.2020.07.026
44. Goeppert B, Frauenschuh L, Zucknick M, Stenzinger A, Andrulis M, Klauschen F, et al. Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer. Br J Cancer (2013) 109:2665–74. doi: 10.1038/bjc.2013.610
Keywords: intrahepatic cholangiocarcinoma, hepatic arterial infusion chemotherapy, lenvatinib, PD-1 inhibitor, whole exome sequencing, predictive biomarkers, tumor mutation burden, tumor-infiltrating lymphocytes
Citation: Huang Y, Du Z, Kan A, He M, Li H, Lai Z, Wen D, Huang L, Li Q, Xu L and Shi M (2024) Clinical and biomarker analyses of hepatic arterial infusion chemotherapy plus lenvatinib and PD-1 inhibitor for patients with advanced intrahepatic cholangiocarcinoma. Front. Immunol. 15:1260191. doi: 10.3389/fimmu.2024.1260191
Received: 17 July 2023; Accepted: 15 January 2024;
Published: 07 February 2024.
Edited by:
Francesco Sabbatino, University of Salerno, ItalyReviewed by:
Mingyue Cai, The Second Affiliated Hospital of Guangzhou Medical University, ChinaMarco Massani, ULSS2 Marca Trevigiana, Italy
Copyright © 2024 Huang, Du, Kan, He, Li, Lai, Wen, Huang, Li, Xu and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Shi, c2hpbWluZ0BzeXN1LmVkdS5jbg==; Li Xu, eHVsaUBzeXN1Y2Mub3JnLmNu
†These authors have contributed equally to this work
 YeXing Huang
YeXing Huang ZeFeng Du
ZeFeng Du Anna Kan
Anna Kan MinKe He
MinKe He HuiFang Li
HuiFang Li ZhiCheng Lai
ZhiCheng Lai DongSheng Wen
DongSheng Wen LiChang Huang
LiChang Huang Li Xu
Li Xu Ming Shi
Ming Shi