
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol. , 11 January 2024
Sec. T Cell Biology
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1343718
This article is part of the Research Topic Community Series in the Role of CD1- and MR1-restricted T cells in Immunity and Disease, volume II View all 18 articles
Invariant natural killer T (iNKT) cells, a subset of unconventional T cells that recognize glycolipid antigens in a CD1d-dependent manner, are crucial in regulating diverse immune responses such as autoimmunity. By engaging with CD1d-expressing non-immune cells (such as intestinal epithelial cells and enterochromaffin cells) and immune cells (such as type 3 innate lymphoid cells, B cells, monocytes and macrophages), iNKT cells contribute to the maintenance of immune homeostasis in the intestine. In this review, we discuss the impact of iNKT cells and CD1d in the regulation of intestinal inflammation, examining both cellular and molecular factors with the potential to influence the functions of iNKT cells in inflammatory bowel diseases such as Crohn’s disease and ulcerative colitis.
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the intestine stemming from an imbalanced immune response to gut microflora rather than infectious causes. IBD manifests primarily as two forms: Crohn’s disease (CD) and ulcerative colitis (UC). Whereas CD affects the entire digestive tract, UC primarily involves inflammation in the colon and rectum (1, 2). Environmental factors such as a high-fat diet (HFD) and hormonal substances contribute to the increasing incidence of IBD in developed countries (1, 2). Although various immune cells have been implicated in the pathogenesis of IBD, we focus here on natural killer T (NKT) cells, a subset of innate-like T cells that play a crucial role in maintaining intestinal integrity. Unlike conventional T cells, NKT cells co-expressing NK cell receptors and T cell receptors (TCRs) respond to glycolipid antigens (Ags) presented by the major histocompatibility complex class I-related protein CD1d. CD1d-restricted NKT cells mainly consist of type I invariant NKT (iNKT) and type II NKT cells that react to glycolipids, α-galactosylceramide (α-GalCer) and sulfatide, respectively (3–5). Human iNKT cells include mainly CD4+, CD8+, and CD4-CD8- double-negative (DN) subsets, whereas murine iNKT cells consist of CD4+ and DN subsets. Moreover, peripheral blood iNKT cells occupy 0.1-0.2% and 1-2% of T cells in humans and mice, respectively. Type I iNKT cells are less abundant than type II NKT cells in human liver, but opposite relative frequencies are observed in mouse liver (6, 7).
NKT cells can produce various cytokines including IFN-γ, IL-4, IL-10, IL-13, and IL-17 (8–10). Moreover, iNKT cells are functionally categorized based on their preferential expression of transcription factors: T-bet for iNKT1, GATA3 and PLZF for iNKT2, RORγt for iNKT17, and E4BP4 for iNKT10 cells (11, 12). iNKT1 cells are predominant in the spleen and the liver, whereas iNKT2 cells predominantly localize to mesenteric lymph nodes (mLNs) (5, 11). IL-10-expressing iNKT10 cells are predominant in the visceral adipose tissue (VAT) as well as in the colonic lamina propria (LP) (13, 14). Whereas B6 mice contain a prominent population of iNKT1 cells in the thymus, Balb/c mice contain higher frequencies of iNKT2 cells in the thymus and mLNs (5). It was recently reported that non-immune cells (i.e., intestinal epithelial cells (IECs) and serotonin-secreting enterochromaffin cells) constitutively express CD1d molecules that can present glycolipid Ags (15–17). Although the canonical role of CD1d in lipid Ag presentation is well-characterized in immune cells such as dendritic cells (DCs), increasing evidence also shows non-canonical CD1d functions in CD1d-expressing mononuclear cells, including type 3 innate lymphoid cells (ILC3s) and IECs. Besides their capacity to present lipid Ag, CD1d molecules can intrinsically signal cells to become activated and produce cytokines (17–19). Emerging evidence indicates that intrinsic CD1d signaling in intestinal cells (i.e., tuft cells, Paneth cells, enterocytes, goblet cells, and neuroendocrine cells) has clinical implications in IBD. The quantity and phenotype of iNKT cells are altered in IBD patients compared with healthy individuals (13, 20). Moreover, since iNKT cells can produce pro- and anti-inflammatory cytokines, iNKT cell-elicited immune responses may be protective or harmful. This mini-review will focus on the immunomodulatory roles of CD1d and iNKT cells in two primary forms of IBD, CD and UC.
In CD, exaggerated T helper (Th)1 and Th17 immune responses characterize pathogenic lesions across the gastrointestinal tract (1, 2). CD patients exhibit a significant reduction in iNKT cell numbers in peripheral blood and intestinal tissues (20). Likewise, the acute mouse IBD model induced with dextran sulfate sodium (DSS) is characterized by increased Th1 and Th17 immune responses (21). iNKT cell-deficient mice display increased colitis severity in experimental models, indicating a regulatory role for colonic iNKT cells, particularly those producing anti-inflammatory cytokines like IL-9 and IL-10 (13, 22). Recently, we have demonstrated that IL-4- and IL-9-producing iNKT cells are significantly increased in the mLNs during IFN-γ-mediated intestinal inflammation after DSS treatment (23). Furthermore, consistent with our report, the prevalence of anti-inflammatory iNKT10 cells is elevated in CD patients compared to healthy individuals, suggesting the existence of regulatory pathways that compensate for the production of harmful pro-inflammatory cytokines in the intestine of CD patients (13).
In contrast to CD patients, IL-13-producing cell populations in the LP of UC patients contain CD1d-restricted type II NKT cells but not iNKT cells (24). Overexpression of CD1d in a type II NKT TCR transgenic (Tg) mouse model (CD1dTg/24αβTg) forces the negative selection of type II NKT cells, consequently reducing the frequency of type II NKT cells (25). Thus, type II NKT cell-deficient CD1dTg/24αβTg mice spontaneously develop colitis, indicating that type II NKT cells play essential roles in maintaining intestinal homeostasis (25). However, LP mononuclear cells (including type II NKT cells) from UC patients express high levels of CD161 and IL-13Rα and produce high amounts of IL-13. Upon stimulation with sulfatide glycolipids, these cells exhibit augmented cytotoxic activity against IECs (26). In addition, a methionine-choline-deficient (MCD) diet lowers the type II NKT population in both the LP and mLNs, ultimately suppressing DSS-induced colitis (27). These findings indicate that type II NKT cells can be either protective or pathogenic in UC. Since IFN-γ produced during intestinal inflammation contributes to the relative distribution of type I and II NKT cells (28, 29), the balance of these subsets plays a significant role in regulating IBD.
Exposure to mucosa-associated microbiota drives pro-inflammatory activation of colonic iNKT cells (mostly CD161+ cells) from IBD patients through TCR-dependent and -independent mechanisms, consequently breaking the epithelial barrier integrity (30). The presentation of commensal-derived glycolipid Ags and exogenous glycolipids by CD11c+ cells, such as DCs, controls the homeostasis and activation of intestinal iNKT cells (31). Furthermore, Wingender et al. demonstrated that intragastric injection of Sphingomonas yanoikuyae containing glycolipid Ags activates intestinal iNKT cells with a hyporesponsive phenotype and increases the relative frequency of Vβ7+ iNKT cells in germ-free mice (32). Repeated intraperitoneal (i.p.) injection of either α-GalCer or its more potent analog (7DW8-5) (33, 34) into DSS-treated mice effectively prevents colitis development. Moreover, repeated i.p. injection of α-GalCer inhibits the development of cholangitis complicated by colitis in outbred CD-1 mice by reducing Th1-dominant responses (35). Since repeated i.p. or intravenous (i.v.) injection of α-GalCer increases iNKT10 differentiation (10, 12), it will be worthwhile to investigate whether resolution of colitis by repeated i.p. α-GalCer injection correlates with an increase in colonic iNKT10 cells. Furthermore, repeated α-GalCer challenge induces memory-like cMAF+ iNKT cells and IL-10-expressing adipose tissue iNKT1 cells, suggesting that iNKT10 cells may differentiate from iNKT1 cells (14). On the other hand, α-GalCer-like glycolipid produced by the commensal bacterium Bacteroides fragilis (B. fragilis) stimulates splenic iNKT cells to secrete high amounts of IL-10 in Balb/c mice enriched for iNKT2 cells. Upon stimulation with these glycolipids, most iNKT10 cells simultaneously secrete IL-13 but not IFN-γ (36). Fecal microbiota transplantation (enriched for Lactobacillaceae and Bifidobacteriaceae) suppresses DSS-induced colitis, which is associated with increased iNKT10 cells (37, 38). However, the origin and differentiation of colonic iNKT10 cells during colitis remains unclear. Oral administration of α-GalCer specifically up-regulates expression of TCR engagement markers (e.g., Nur77) in iNKT cells of the mLNs but not the spleen, liver, and thymus and its up-regulated expression selectively occurs in iNKT2 rather than iNKT1 populations in the mLNs (5), supporting the notion that optimal glycolipid administration frequency and route are important factors in treating colitis. Injection of OCH, a derivative of α-GalCer with Th2 selective activity, prevents DSS-induced colitis, which correlates with reduced Th1/Th2 cytokine ratios and increased IL-10 production (39). Treatment with an α-GalCer derivative containing polar functional groups (Bz amide) attenuates DSS-induced colitis, accompanied by expansion and activation of Th2- and Th17-biased iNKT cells rather than Th1-biased iNKT cells (40). Glycolipid Ags derived from the intestinal microbe B. fragilis (called GSL-Bf717 or BfaGCs) inhibit iNKT cell activation through competitive binding of their sphinganine branches with CD1d, resulting in protection against oxazolone (Oxa)-induced colitis, an experimental model mimicking UC (41–43). Additional studies have provided evidence that CD1d-dependent pathogenic iNKT cell activation by commensal-derived glycolipid Ags can be inhibited by competitive CD1d binding with globotriaosylceramide (44) and α-lactosylceramide (45) during iNKT cell-mediated Oxa-induced colitis.
CD1d expression on IECs is significantly decreased in both CD and UC patients compared with healthy controls, suggesting a unique role in controlling intestinal immune responses (46). Exposure of environmental oxazoles derived from either diet or microbes to IECs can play pathogenic roles in colonic inflammation by inhibiting IEC-mediated and CD1d-dependent IL-10 production and promote iNKT cell-derived IL-13 production (47). Lipid-mediated CD1d ligation triggers epithelial cell-derived endogenous IL-10 production, consequently decreasing epithelial permeability induced by IFN-γ (48). Furthermore, one study demonstrated that IECs produce high amounts of IL-10 via CD1d engagement-induced STAT3 activation, suppressing Oxa-induced iNKT cell-mediated colitis (17). These studies suggest that the cross-talk between iNKT cells and CD1d-expressing IECs activated by CD1d-intrinsic signaling controls intestinal homeostasis.
In addition to its effects on IECs, CD1d engagement on various other cell types can induce cell-intrinsic signaling. In mLN B cells, CD1d expression is associated with enhanced IL-10 production under chronic intestinal inflammatory conditions (49). Since IL-10-producing B cells (B10) suppress experimental arthritis in a CD1d-dependent manner (50), it will be interesting to investigate whether iNKT cells cross-talk with B10 cells to regulate colitis. Glycolipid-mediated ligation of CD1d on human monocytes directly triggers marked NFκB activation and excessive IL-12 production, indicating that CD1d-mediated signal transduction is required to induce optimal Th1 responses to glycolipid Ag stimulation (51). Macrophage-specific CD1d deletion alleviates colonic inflammation, highlighting CD1d-intrinsic activating effects on NFκB in macrophages (19). CD1d-deficient macrophages exhibit metabolic reprogramming into an inflammatory phenotype through CD36 internalization and lipid uptake. In addition, mice reconstituted with CD1d-deficient macrophages exhibit increased susceptibility to LPS-induced inflammation (52). Engagement of CD1d on ILC3s by NKT cells drives IL-22 secretion in the mLNs (18). In addition, CD1d-dependent engagement of enterochromaffin cells by iNKT cells triggers the former cells to release peripheral serotonin (5-HT), thereby regulating intestinal hemostasis (16). Finally, a recent study showed that intrinsic hepatocyte-specific CD1d signaling induced by tyrosine phosphorylation of the CD1d cytoplasmic tail protects against hepatocyte apoptosis in mice with non-alcoholic steatohepatitis induced by an HFD or MCD diet (53).
iNKT cells are thought to play protective or pathogenic roles in colitis through their activation via TCR- or cytokine-mediated signaling pathways. Furthermore, various CD1d-expressing cells (e.g., B cells, ILC3s, and macrophages) that can interact with iNKT cells also appear to play protective or pathogenic roles in colitis by producing cytokines (e.g., IL-22 and IL-10) via CD1d-intrinsic signaling. Thus, it will be important to explore how CD1d-intrinsic signaling modulates the function of NKT cells and whether such interactions contribute to colitis development.
CX3CR1hi mononuclear phagocytes guide non-invasive Salmonellae to the mLNs where these organisms induce both pathogen-specific T cell responses and IgA antibodies (54). During naive CD4+ T cell transfer-mediated colitis, CD4+ T cells are in close contact with colonic CX3CR1+ phagocytes that present bacterial-derived Ags (55). Interestingly, embryonic CX3CR1+ macrophages are essential for the localization and proliferation of colonic iNKT cells (particularly iNKT17) in their local environment during early life (56). In this regard, it will be worthwhile to further investigate whether CX3CR1+ macrophages contribute to iNKT cell-mediated host defenses during enteropathogen-induced colitis. Compared with macrophage recruitment, the effect of iNKT cells on neutrophil recruitment remains unclear. For example, the expression of neutrophil-attracting chemokines (i.e., CXCL1, CXCL2, and CXCL3) decreases in the colon of CD1d-/- mice, along with reduced pathogenic neutrophil infiltration (57). In contrast, more neutrophils are recruited to the colon in DSS-treated iNKT cell-deficient Jα18-/- mice and these cells possess a pronounced anti-inflammatory phenotype (58). Since neutrophils are classified into two groups based on their functional differences (59), pro-inflammatory N1 (e.g., TNF-α-secreting) and anti-inflammatory N2 neutrophils (e.g., TGF-β-secreting), further studies are needed to clarify the role of iNKT cells on neutrophil polarization (N1 vs. N2) during colitis development.
Blockade of IL-25 signaling is an effective strategy to prevent intestinal inflammation in Oxa-induced colitis. This protection is achieved by inhibiting IL-13 production by ILC2s and NKT cells (60). After helminth infection, tuft cell-derived IL-25 constitutively activates ILC2s to produce IL-13, followed by the differentiation of tuft and goblet cells (61). Moreover, Tritrichomonas-generated succinate triggers tuft cell hyperplasia via induction of the tuft cell-IL-25-ILC2-IL-13 axis (62). Lucas et al. demonstrated that IL-25 production from thymic tuft cells skews iNKT cells towards the iNKT2 phenotype in the thymus (63). Moreover, in addition to their interactions within the thymus, the interaction among tuft cells, ILC2s, and iNKT2 cells in the peripheral tissues, particularly the intestine, requires clarification.
iNKT cells (particularly IL-9-producing iNKT cells) also up-regulate IL-22-producing ILC3s in the mLNs, ultimately preventing DSS-induced colitis under IFN-γ-dysregulated conditions (23), indicating that iNKT cells and ILC3s counter-regulate IFN-γ-mediated intestinal inflammation. Moreover, IL-22 increases intestinal barrier function and anti-microbial peptide production (64, 65), and iNKT cells trigger ILC3s to produce IL-22 in the intestine (18), indicating that secretion of IL-22 by ILC3s following their interaction with iNKT cells might be effective in preventing colitis development.
A previous study demonstrated that HFD feeding increases susceptibility of mice to DSS-induced colitis via a concurrent increase in CD1d-unrestricted NKT cells (mostly CD8+ and DN T cells) and a decrease in Tregs (66). The severity of intestinal inflammation in transgenic mice expressing human IL-15 in IECs closely correlates with increased numbers of LP CD8+ NKT cells and increased levels of Th1-type cytokines such as IFN-γ and TNF-α (67). We have previously reported that CD1d-independent CD8+ NKT cells are critical effectors that exacerbate DSS-induced colitis in Yeti mice characterized by enhanced stability of IFN-γ mRNA transcripts. In contrast, through cooperation with Tregs, iNKT cells are required to control CD1d-independent CD8+ NKT cell-mediated pathogenesis during DSS-induced colitis (68). Pro-inflammatory DCs induce the differentiation of pathogenic Foxp3−CD25+CD4+ T cells with Th1 and Th17 phenotypes and antagonize Treg differentiation in the mLNs of CD1d−/− Yeti mice lacking iNKT cells (69). iNKT cell-primed Tregs produce IL-10 in the presence of bacterial diacylglycerols and show an enhanced suppressive capacity, which provides support for the iNKT-Treg axis in regulating colitis (70). However, the regulatory effects of iNKT cells are restricted to the intestine but not to the spleen during DSS-induced colitis in Yeti mice (68), consistent with the dominance of iNKT2 cells in the intestine.
Short-chain fatty acid (SCFA)-producing bacteria (e.g., Faecalibacterium, Lachnospiraceae, Veillonella Gemmiger, and Prevotella) help to maintain IL-10 production by iNKT cells (13). However, a previous study demonstrated that Acetatifactor muris (A. muris) belonging to Firmicutes is exclusively detected in the fecal samples of NOD2−/−CD1d−/− mice lacking iNKT cells, compared to NOD2−/− mice with altered intestinal microbiota. Moreover, oral gavage of A. muris promotes colitis in DSS-treated WT mice (71). In addition, increased colonization of pathogenic bacteria (e.g., Pseudomonas aeruginosa and Staphylococcus aureus) in the intestine of CD1d−/− mice has been implicated in reduced release of Paneth cell-derived lysozyme, a potent anti-microbial protein (72). These anti-bacterial effects in WT mice are enhanced following in vivo iNKT cell activation by α-GalCer (72).
In DSS-induced colitis, WT B6 mice that received B6 CD1d KO-derived cecal contents show increased sensitivity to colitis compared with control WT B6 mice injected orally with WT B6-derived cecal contents. Furthermore, an increase in particular segmented filamentous bacteria and a decrease in Akkermansia muciniphila (A. muciniphila) closely correlates with increased colitis sensitivity in WT B6 mice that received B6 CD1d KO-derived cecal contents (73). Although oral administration of A. muciniphila plays a protective role in the Oxa-induced colitis model and A. muciniphila stimulates macrophages to release IL-10 (74), it remains unclear whether IL-10 production by Akkermansia-stimulated macrophages can induce iNKT10 differentiation in the colon during UC. Thus, it will be important to perform further studies on the protective mechanism of Akkermansia in colitis.
The kynurenic acid-GPR35 signaling pathway ameliorates DSS-induced colonic injury and inflammation by suppressing NLRP3-dependent IL-1β production in macrophages (75). Peripheral iNKT cells express high surface levels of GPR35, and the specific activation of GPR35 by its ligand (kynurenic acid) significantly reduces the release of IL-4 but not IFN-γ by α-GalCer-activated human iNKT cells (76). In addition, GPR65 gene expression in intestinal tissues is significantly up-regulated in both CD and UC patients compared with healthy controls (77). Moreover, the progression of DSS-induced colitis is aggravated by GPR65 deficiency, which supports the protective role of GPR65 in this model (77). iNKT cells expressing high levels of GPR65 play a pivotal role in suppressing autoimmune disease. For example, GPR65-deficient mice are more sensitive to experimental autoimmune encephalomyelitis in an iNKT cell-dependent manner (78). Collectively, these studies suggest that G protein-coupled receptors such as GPR35 and GPR65 play essential roles in IBD protection through iNKT cells.
Butyrate, one of the SCFAs produced by microbiota, suppresses iNKT cell production of both IFN-γ and IL-4 via inhibiting class I histone deacetylases (79). In addition, palmitic acid (C16:0), a saturated fatty acid found in animal and vegetable fats, reduces the levels of both IFN-γ and IL-4 via inositol-requiring enzyme 1α (IRE1α) (80). Moreover, palmitic acid directly triggers NK1.1-negative iNKT cells to produce anti-inflammatory cytokines (e.g., IL-10) via IRE1α-X-box binding protein 1 (81). Thus, combining butyrate and palmitic acid as IFN-γ/IL-4 dual antagonists might represent a promising strategy to induce iNKT cell-mediated suppression in Th1 cell-mediated CD and Th2 cell-mediated UC.
The studies reviewed here identify iNKT cells as promising targets for designing IBD immune therapies (Figure 1 and Table 1). Although emerging evidence shows the immunological functions of CD1d and iNKT cell subsets (iNKT1, iNKT2, iNKT17, and iNKT10), little is known about their contribution to protection/pathogenesis against CD and UC. Further investigations are needed to explore the precise immunoregulatory mechanisms of intestinal iNKT subsets during IBD. Finally, it will be important to develop new tools to selectively activate or inhibit iNKT cells in the intestine.
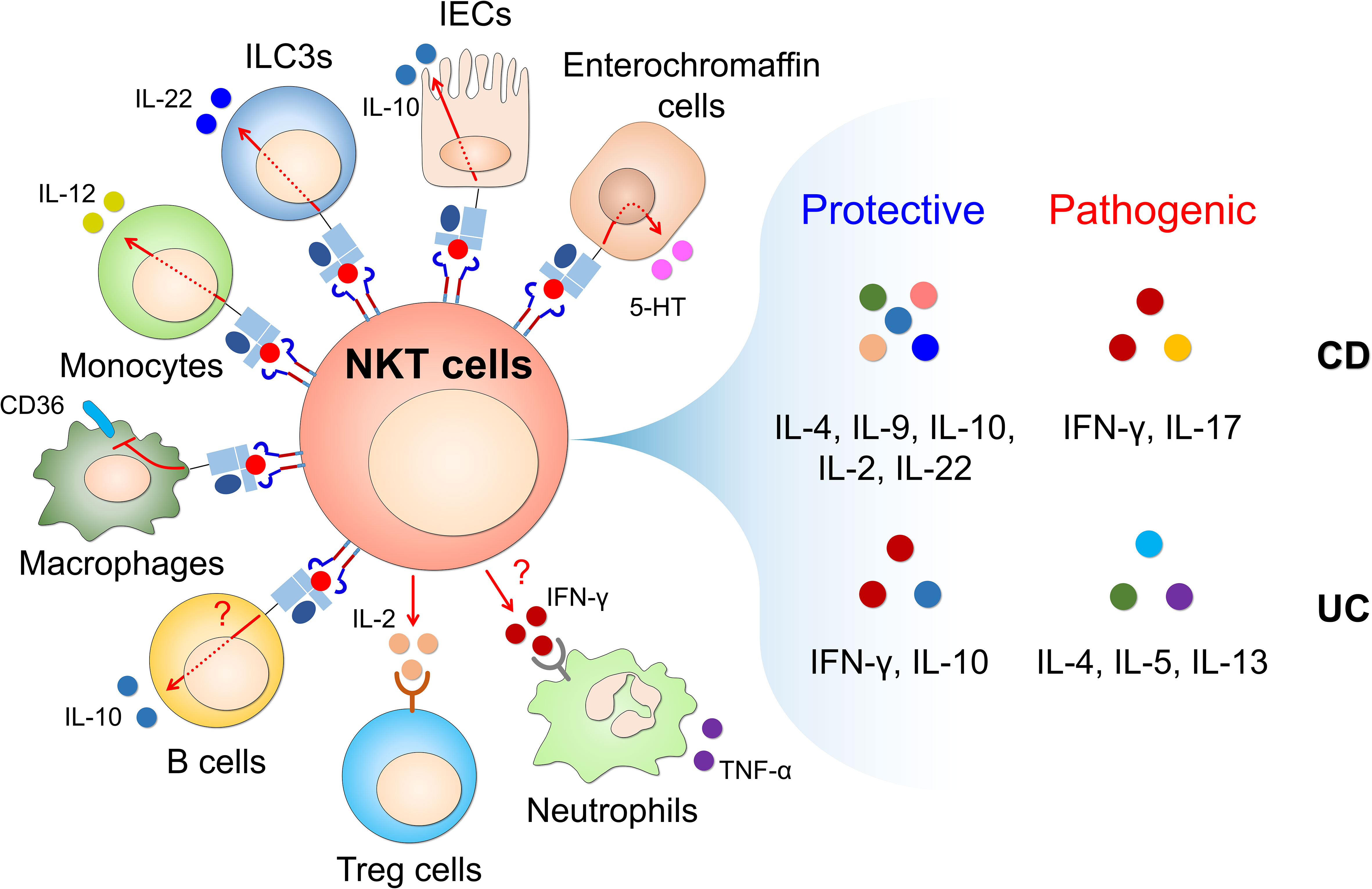
Figure 1 CD1d-restricted NKT cells interact with CD1d-expressing cells to produce NKT cell-derived soluble factors that regulate intestinal inflammatory responses. Glycolipid-mediated CD1d engagement triggers the activation of CD1d-expressing non-immune cells (such as IECs and enterochromaffin cells) and immune cells (such as ILC3s, B cells, monocytes and macrophages). This activation occurs through CD1d-intrinsic signaling in non-immune and immune cells and TCR/cytokine-dependent mechanisms in NKT cells. In addition, NKT cells interact with Tregs and neutrophils in a cytokine-dependent manner. Through this cross-talk with diverse cell types, CD1d-restricted NKT cells rapidly produce T helper (Th)1 cytokines (e.g., IFN-γ), Th2 cytokines (e.g., IL-4, IL-5, IL-9, and IL-13), Th17 cytokines (e.g., IL-17 and IL-22), and regulatory cytokines (e.g., IL-10) upon stimulation with various stimuli (e.g., cytokines, chemokines, toll-like receptor (TLR) ligands, fatty acids, and glycolipids), which can elicit either protective or pathogenic effects in CD and UC. CD, Crohn’s disease; UC, ulcerative colitis; IECs, intestinal epithelial cells; ILC3s, type 3 innate lymphoid cells; 5-HT, 5-hydroxytryptamine.
SL: Funding acquisition, Writing – original draft, Writing – review & editing. HP: Funding acquisition, Writing – original draft, Writing – review & editing. LVK: Writing – review & editing. SH: Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1I1A1A01054418 to SL; NRF-2021R1I1A1A01051465 to HP; NRF-2022R1A2C1009590 to SH).
LVK is a member of the scientific advisory board of Isu Abxis Co., Ltd. (Republic of Korea).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Roda G, Chien Ng S, Kotze PG, Argollo M, Panaccione R, Spinelli A, et al. Crohn's disease. Nat Rev Dis Primers (2020) 6(1):22. doi: 10.1038/s41572-020-0156-2
2. Kobayashi T, Siegmund B, Le Berre C, Wei SC, Ferrante M, Shen B, et al. Ulcerative colitis. Nat Rev Dis Primers (2020) 6(1):74. doi: 10.1038/s41572-020-0205-x
3. Van Kaer L, Postoak JL, Wang C, Yang G, Wu L. Innate, innate-like and adaptive lymphocytes in the pathogenesis of MS and EAE. Cell Mol Immunol (2019) 16(6):531–9. doi: 10.1038/s41423-019-0221-5
4. Park HJ, Lee SW, Park YH, Kim TC, Van Kaer L, Hong S. CD1d-independent NK1.1(+) Treg cells are IL2-inducible Foxp3(+) T cells co-expressing immunosuppressive and cytotoxic molecules. Front Immunol (2022) 13:951592. doi: 10.3389/fimmu.2022.951592
5. Lee YJ, Wang H, Starrett GJ, Phuong V, Jameson SC, Hogquist KA. Tissue-specific distribution of iNKT cells impacts their cytokine response. Immunity (2015) 43(3):566–78. doi: 10.1016/j.immuni.2015.06.025
6. Nowak M, Schmidt-Wolf IG. Natural killer T cells subsets in cancer, functional defects in prostate cancer and implications for immunotherapy. Cancers (Basel) (2011) 3(3):3661–75. doi: 10.3390/cancers3033661
7. Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol (2013) 13(2):101–17. doi: 10.1038/nri3369
8. Lee SW, Park HJ, Van Kaer L, Hong S. Roles and therapeutic potential of CD1d-Restricted NKT cells in inflammatory skin diseases. Front Immunol (2022) 13:979370. doi: 10.3389/fimmu.2022.979370
9. Park HJ, Lee SW, Hong S. Regulation of allergic immune responses by microbial metabolites. Immune Netw (2018) 18(1):e15. doi: 10.4110/in.2018.18.e15
10. Park HJ, Kim TC, Park YH, Lee SW, Jeon J, Park SH, et al. Repeated alpha-GalCer Administration Induces a Type 2 Cytokine-Biased iNKT Cell Response and Exacerbates Atopic Skin Inflammation in V alpha 14(Tg) NC/Nga Mice. Biomedicines (2021) 9(11):1619. doi: 10.3390/biomedicines9111619
11. Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol (2013) 14(11):1146–54. doi: 10.1038/ni.2731
12. Sag D, Krause P, Hedrick CC, Kronenberg M, Wingender G. IL-10-producing NKT10 cells are a distinct regulatory invariant NKT cell subset. J Clin Invest (2014) 124(9):3725–40. doi: 10.1172/JCI72308
13. Burrello C, Strati F, Lattanzi G, Diaz-Basabe A, Mileti E, Giuffre MR, et al. IL10 secretion endows intestinal human iNKT cells with regulatory functions towards pathogenic T lymphocytes. J Crohns Colitis (2022) 16(9):1461–74. doi: 10.1093/ecco-jcc/jjac049
14. Kane H, LaMarche NM, Ni Scannail A, Garza AE, Koay HF, Azad AI, et al. Longitudinal analysis of invariant natural killer T cell activation reveals a cMAF-associated transcriptional state of NKT10 cells. Elife (2022) 11:e76586. doi: 10.7554/eLife.76586
15. Iwabuchi K, Van Kaer L. Editorial: role of CD1- and MR1-restricted T cells in immunity and disease. Front Immunol (2019) 10:1837. doi: 10.3389/fimmu.2019.01837
16. Luo J, Chen Z, Castellano D, Bao B, Han W, Li J, et al. Lipids regulate peripheral serotonin release via gut CD1d. Immunity (2023) 56(7):1533–47.e7. doi: 10.1016/j.immuni.2023.06.001
17. Olszak T, Neves JF, Dowds CM, Baker K, Glickman J, Davidson NO, et al. Protective mucosal immunity mediated by epithelial CD1d and IL-10. Nature (2014) 509(7501):497–502. doi: 10.1038/nature13150
18. Saez de Guinoa J, Jimeno R, Farhadi N, Jervis PJ, Cox LR, Besra GS, et al. CD1d-mediated activation of group 3 innate lymphoid cells drives IL-22 production. EMBO Rep (2017) 18(1):39–47. doi: 10.15252/embr.201642412
19. Cui S, Wang C, Bai W, Li J, Pan Y, Huang X, et al. CD1d1 intrinsic signaling in macrophages controls NLRP3 inflammasome expression during inflammation. Sci Adv (2020) 6(43):eaaz7290. doi: 10.1126/sciadv.aaz7290
20. Grose RH, Thompson FM, Baxter AG, Pellicci DG, Cummins AG. Deficiency of invariant NK T cells in Crohn's disease and ulcerative colitis. Dig Dis Sci (2007) 52(6):1415–22. doi: 10.1007/s10620-006-9261-7
21. Eichele DD, Kharbanda KK. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J Gastroenterol (2017) 23(33):6016–29. doi: 10.3748/wjg.v23.i33.6016
22. Kim HS, Chung DH. IL-9-producing invariant NKT cells protect against DSS-induced colitis in an IL-4-dependent manner. Mucosal Immunol (2013) 6(2):347–57. doi: 10.1038/mi.2012.77
23. Park HJ, Lee SW, Van Kaer L, Hong S. CD1d-dependent iNKT cells control DSS-induced colitis in a mouse model of IFNgamma-mediated hyperinflammation by increasing IL22-secreting ILC3 cells. Int J Mol Sci (2021) 22(3):1250. doi: 10.3390/ijms22031250
24. Fuss IJ, Heller F, Boirivant M, Leon F, Yoshida M, Fichtner-Feigl S, et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest (2004) 113(10):1490–7. doi: 10.1172/JCI19836
25. Liao CM, Zimmer MI, Shanmuganad S, Yu HT, Cardell SL, Wang CR. dysregulation of CD1d-restricted type ii natural killer T cells leads to spontaneous development of colitis in mice. Gastroenterology (2012) 142(2):326–34.e1-2. doi: 10.1053/j.gastro.2011.10.030
26. Fuss IJ, Joshi B, Yang Z, Degheidy H, Fichtner-Feigl S, de Souza H, et al. IL-13Ralpha2-bearing, type II NKT cells reactive to sulfatide self-antigen populate the mucosa of ulcerative colitis. Gut (2014) 63(11):1728–36. doi: 10.1136/gutjnl-2013-305671
27. Sagami S, Ueno Y, Tanaka S, Fujita A, Niitsu H, Hayashi R, et al. Choline deficiency causes colonic type II natural killer T (NKT) cell loss and alleviates murine colitis under type I NKT cell deficiency. PLoS One (2017) 12(1):e0169681. doi: 10.1371/journal.pone.0169681
28. Liao CM, Zimmer MI, Wang CR. The functions of type I and type II natural killer T cells in inflammatory bowel diseases. Inflammation Bowel Dis (2013) 19(6):1330–8. doi: 10.1097/MIB.0b013e318280b1e3
29. Hashimoto M, Hiwatashi K, Ichiyama K, Morita R, Sekiya T, Kimura A, et al. SOCS1 regulates type I/type II NKT cell balance by regulating IFNgamma signaling. Int Immunol (2011) 23(3):165–76. doi: 10.1093/intimm/dxq469
30. Burrello C, Pellegrino G, Giuffre MR, Lovati G, Magagna I, Bertocchi A, et al. Mucosa-associated microbiota drives pathogenic functions in IBD-derived intestinal iNKT cells. Life Sci Alliance (2019) 2(1):e201800229. doi: 10.26508/lsa.201800229
31. Saez de Guinoa J, Jimeno R, Gaya M, Kipling D, Garzon MJ, Dunn-Walters D, et al. CD1d-mediated lipid presentation by CD11c(+) cells regulates intestinal homeostasis. EMBO J (2018) 37(5):e97537. doi: 10.15252/embj.201797537
32. Wingender G, Stepniak D, Krebs P, Lin L, McBride S, Wei B, et al. Intestinal microbes affect phenotypes and functions of invariant natural killer T cells in mice. Gastroenterology (2012) 143(2):418–28. doi: 10.1053/j.gastro.2012.04.017
33. Saubermann LJ, Beck P, De Jong YP, Pitman RS, Ryan MS, Kim HS, et al. Activation of natural killer T cells by alpha-galactosylceramide in the presence of CD1d provides protection against colitis in mice. Gastroenterology (2000) 119(1):119–28. doi: 10.1053/gast.2000.9114
34. Lee C, Hong SN, Kim YH. A glycolipid adjuvant, 7DW8-5, provides a protective effect against colonic inflammation in mice by the recruitment of CD1d-restricted natural killer T cells. Intest Res (2020) 18(4):402–11. doi: 10.5217/ir.2019.00132
35. Numata Y, Tazuma S, Ueno Y, Nishioka T, Hyogo H, Chayama K. Therapeutic effect of repeated natural killer T cell stimulation in mouse cholangitis complicated by colitis. Dig Dis Sci (2005) 50(10):1844–51. doi: 10.1007/s10620-005-2949-2
36. Cameron G, Nguyen T, Ciula M, Williams SJ, Godfrey DI. Glycolipids from the gut symbiont Bacteroides fragilis are agonists for natural killer T cells and induce their regulatory differentiation. Chem Sci (2023) 14(29):7887–96. doi: 10.1039/d3sc02124f
37. Strati F, Pujolassos M, Burrello C, Giuffre MR, Lattanzi G, Caprioli F, et al. Antibiotic-associated dysbiosis affects the ability of the gut microbiota to control intestinal inflammation upon fecal microbiota transplantation in experimental colitis models. Microbiome (2021) 9(1):39. doi: 10.1186/s40168-020-00991-x
38. Burrello C, Garavaglia F, Cribiu FM, Ercoli G, Lopez G, Troisi J, et al. Therapeutic faecal microbiota transplantation controls intestinal inflammation through IL10 secretion by immune cells. Nat Commun (2018) 9(1):5184. doi: 10.1038/s41467-018-07359-8
39. Ueno Y, Tanaka S, Sumii M, Miyake S, Tazuma S, Taniguchi M, et al. Single dose of OCH improves mucosal T helper type 1/T helper type 2 cytokine balance and prevents experimental colitis in the presence of valpha14 natural killer T cells in mice. Inflammation Bowel Dis (2005) 11(1):35–41. doi: 10.1097/00054725-200501000-00005
40. Inuki S, Hirata N, Kashiwabara E, Kishi J, Aiba T, Teratani T, et al. Polar functional group-containing glycolipid CD1d ligands modulate cytokine-biasing responses and prevent experimental colitis. Sci Rep (2020) 10(1):15766. doi: 10.1038/s41598-020-72280-4
41. An D, Oh SF, Olszak T, Neves JF, Avci FY, Erturk-Hasdemir D, et al. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell (2014) 156(1-2):123–33. doi: 10.1016/j.cell.2013.11.042
42. Oh SF, Praveena T, Song H, Yoo JS, Jung DJ, Erturk-Hasdemir D, et al. Host immunomodulatory lipids created by symbionts from dietary amino acids. Nature (2021) 600(7888):302–7. doi: 10.1038/s41586-021-04083-0
43. Song X, Zhang H, Zhang Y, Goh B, Bao B, Mello SS, et al. Gut microbial fatty acid isomerization modulates intraepithelial T cells. Nature (2023) 619(7971):837–43. doi: 10.1038/s41586-023-06265-4
44. Pereira CS, Sa-Miranda C, De Libero G, Mori L, Macedo MF. Globotriaosylceramide inhibits iNKT-cell activation in a CD1d-dependent manner. Eur J Immunol (2016) 46(1):147–53. doi: 10.1002/eji.201545725
45. Lai AC, Chi PY, Thio CL, Han YC, Kao HN, Hsieh HW, et al. alpha-lactosylceramide protects against iNKT-mediated murine airway hyperreactivity and liver injury through competitive inhibition of cd1d binding. Front Chem (2019) 7:811. doi: 10.3389/fchem.2019.00811
46. Perera L, Shao L, Patel A, Evans K, Meresse B, Blumberg R, et al. Expression of nonclassical class I molecules by intestinal epithelial cells. Inflammation Bowel Dis (2007) 13(3):298–307. doi: 10.1002/ibd.20026
47. Iyer SS, Gensollen T, Gandhi A, Oh SF, Neves JF, Collin F, et al. Dietary and microbial oxazoles induce intestinal inflammation by modulating aryl hydrocarbon receptor responses. Cell (2018) 173(5):1123–34.e11. doi: 10.1016/j.cell.2018.04.037
48. Colgan SP, Hershberg RM, Furuta GT, Blumberg RS. Ligation of intestinal epithelial CD1d induces bioactive IL-10: critical role of the cytoplasmic tail in autocrine signaling. Proc Natl Acad Sci U S A (1999) 96(24):13938–43. doi: 10.1073/pnas.96.24.13938
49. Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity (2002) 16(2):219–30. doi: 10.1016/s1074-7613(02)00274-1
50. Oleinika K, Rosser EC, Matei DE, Nistala K, Bosma A, Drozdov I, et al. CD1d-dependent immune suppression mediated by regulatory B cells through modulations of iNKT cells. Nat Commun (2018) 9(1):684. doi: 10.1038/s41467-018-02911-y
51. Yue SC, Shaulov A, Wang R, Balk SP, Exley MA. CD1d ligation on human monocytes directly signals rapid NF-kappaB activation and production of bioactive IL-12. Proc Natl Acad Sci U S A (2005) 102(33):11811–6. doi: 10.1073/pnas.0503366102
52. Brailey PM, Evans L, Lopez-Rodriguez JC, Sinadinos A, Tyrrel V, Kelly G, et al. CD1d-dependent rewiring of lipid metabolism in macrophages regulates innate immune responses. Nat Commun (2022) 13(1):6723. doi: 10.1038/s41467-022-34532-x
53. Zhigang Lei JY, Wu Yu, Shen J, Lin S, Xue W, Mao C, et al. CD1d protects against hepatocyte apoptosis in non-alcoholic steatohepatitis. J Hepatol (2023) 23:S0168–8278. doi: 10.1016/j.jhep.2023.10.025
54. Diehl GE, Longman RS, Zhang JX, Breart B, Galan C, Cuesta A, et al. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1(hi) cells. Nature (2013) 494(7435):116–20. doi: 10.1038/nature11809
55. Rossini V, Zhurina D, Radulovic K, Manta C, Walther P, Riedel CU, et al. CX3CR1(+) cells facilitate the activation of CD4 T cells in the colonic lamina propria during antigen-driven colitis. Mucosal Immunol (2014) 7(3):533–48. doi: 10.1038/mi.2013.70
56. Gensollen T, Lin X, Zhang T, Pyzik M, See P, Glickman JN, et al. Embryonic macrophages function during early life to determine invariant natural killer T cell levels at barrier surfaces. Nat Immunol (2021) 22(6):699–710. doi: 10.1038/s41590-021-00934-0
57. Huang E, Liu R, Lu Z, Liu J, Liu X, Zhang D, et al. NKT cells mediate the recruitment of neutrophils by stimulating epithelial chemokine secretion during colitis. Biochem Biophys Res Commun (2016) 474(2):252–8. doi: 10.1016/j.bbrc.2016.04.024
58. Shen S, Prame Kumar K, Stanley D, Moore RJ, Van TTH, Wen SW, et al. Invariant natural killer T cells shape the gut microbiota and regulate neutrophil recruitment and function during intestinal inflammation. Front Immunol (2018) 9:999. doi: 10.3389/fimmu.2018.00999
59. Ohms M, Moller S, Laskay T. An attempt to polarize human neutrophils toward N1 and N2 phenotypes in vitro. Front Immunol (2020) 11:532. doi: 10.3389/fimmu.2020.00532
60. Camelo A, Barlow JL, Drynan LF, Neill DR, Ballantyne SJ, Wong SH, et al. Blocking IL-25 signalling protects against gut inflammation in a type-2 model of colitis by suppressing nuocyte and NKT derived IL-13. J Gastroenterol (2012) 47(11):1198–211. doi: 10.1007/s00535-012-0591-2
61. von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature (2016) 529(7585):221–5. doi: 10.1038/nature16161
62. Schneider C, O'Leary CE, von Moltke J, Liang HE, Ang QY, Turnbaugh PJ, et al. A metabolite-triggered tuft cell-ILC2 circuit drives small intestinal remodeling. Cell (2018) 174(2):271–84.e14. doi: 10.1016/j.cell.2018.05.014
63. Lucas B, White AJ, Cosway EJ, Parnell SM, James KD, Jones ND, et al. Diversity in medullary thymic epithelial cells controls the activity and availability of iNKT cells. Nat Commun (2020) 11(1):2198. doi: 10.1038/s41467-020-16041-x
64. Gulhane M, Murray L, Lourie R, Tong H, Sheng YH, Wang R, et al. High fat diets induce colonic epithelial cell stress and inflammation that is reversed by IL-22. Sci Rep (2016) 6:28990. doi: 10.1038/srep28990
65. Coorens M, Rao A, Grafe SK, Unelius D, Lindforss U, Agerberth B, et al. Innate lymphoid cell type 3-derived interleukin-22 boosts lipocalin-2 production in intestinal epithelial cells via synergy between STAT3 and NF-kappaB. J Biol Chem (2019) 294(15):6027–41. doi: 10.1074/jbc.RA118.007290
66. Ma X, Torbenson M, Hamad AR, Soloski MJ, Li Z. High-fat diet modulates non-CD1d-restricted natural killer T cells and regulatory T cells in mouse colon and exacerbates experimental colitis. Clin Exp Immunol (2008) 151(1):130–8. doi: 10.1111/j.1365-2249.2007.03530.x
67. Yoshihara K, Yajima T, Kubo C, Yoshikai Y. Role of interleukin 15 in colitis induced by dextran sulphate sodium in mice. Gut (2006) 55(3):334–41. doi: 10.1136/gut.2005.076000
68. Lee SW, Park HJ, Cheon JH, Wu L, Van Kaer L, Hong S. iNKT cells suppress pathogenic NK1.1(+)CD8(+) T cells in DSS-induced colitis. Front Immunol (2018) 9:2168. doi: 10.3389/fimmu.2018.02168
69. Lee SW, Park HJ, Van Kaer L, Hong S. Opposing Roles of DCs and iNKT Cells in the Induction of Foxp3 Expression by MLN CD25(+)CD4(+) T Cells during IFNgamma-Driven Colitis. Int J Mol Sci (2022) 23(23):15316. doi: 10.3390/ijms232315316
70. Venken K, Decruy T, Aspeslagh S, Van Calenbergh S, Lambrecht BN, Elewaut D. Bacterial CD1d-restricted glycolipids induce IL-10 production by human regulatory T cells upon cross-talk with invariant NKT cells. J Immunol (2013) 191(5):2174–83. doi: 10.4049/jimmunol.1300562
71. Lee C, Hong SN, Paik NY, Kim TJ, Kim ER, Chang DK, et al. CD1d modulates colonic inflammation in NOD2-/- mice by altering the intestinal microbial composition comprising acetatifactor muris. J Crohns Colitis (2019) 13(8):1081–91. doi: 10.1093/ecco-jcc/jjz025
72. Nieuwenhuis EE, Matsumoto T, Lindenbergh D, Willemsen R, Kaser A, Simons-Oosterhuis Y, et al. Cd1d-dependent regulation of bacterial colonization in the intestine of mice. J Clin Invest (2009) 119(5):1241–50. doi: 10.1172/JCI36509
73. Selvanantham T, Lin Q, Guo CX, Surendra A, Fieve S, Escalante NK, et al. NKT cell-deficient mice harbor an altered microbiota that fuels intestinal inflammation during chemically induced colitis. J Immunol (2016) 197(11):4464–72. doi: 10.4049/jimmunol.1601410
74. Qu S, Fan L, Qi Y, Xu C, Hu Y, Chen S, et al. Akkermansia muciniphila alleviates dextran sulfate sodium (DSS)-induced acute colitis by NLRP3 activation. Microbiol Spectr (2021) 9(2):e0073021. doi: 10.1128/Spectrum.00730-21
75. Zheng X, Hu M, Zang X, Fan Q, Liu Y, Che Y, et al. Kynurenic acid/GPR35 axis restricts NLRP3 inflammasome activation and exacerbates colitis in mice with social stress. Brain Behav Immun (2019) 79:244–55. doi: 10.1016/j.bbi.2019.02.009
76. Fallarini S, Magliulo L, Paoletti T, de Lalla C, Lombardi G. Expression of functional GPR35 in human iNKT cells. Biochem Biophys Res Commun (2010) 398(3):420–5. doi: 10.1016/j.bbrc.2010.06.091
77. Marie MA, Sanderlin EJ, Satturwar S, Hong H, Lertpiriyapong K, Donthi D, et al. GPR65 (TDAG8) inhibits intestinal inflammation and colitis-associated colorectal cancer development in experimental mouse models. Biochim Biophys Acta Mol Basis Dis (2022) 1868(1):166288. doi: 10.1016/j.bbadis.2021.166288
78. Wirasinha RC, Vijayan D, Smith NJ, Parnell GP, Swarbrick A, Brink R, et al. GPR65 inhibits experimental autoimmune encephalomyelitis through CD4(+) T cell independent mechanisms that include effects on iNKT cells. Immunol Cell Biol (2018) 96(2):128–36. doi: 10.1111/imcb.1031
79. Lee S, Koh J, Chang Y, Kim HY, Chung DH. Invariant NKT cells functionally link microbiota-induced butyrate production and joint inflammation. J Immunol (2019) 203(12):3199–208. doi: 10.4049/jimmunol.1801314
80. Ko JS, Koh JM, So JS, Jeon YK, Kim HY, Chung DH. Palmitate inhibits arthritis by inducing t-bet and gata-3 mRNA degradation in iNKT cells via IRE1alpha-dependent decay. Sci Rep (2017) 7(1):14940. doi: 10.1038/s41598-017-14780-4
81. LaMarche NM, Kane H, Kohlgruber AC, Dong H, Lynch L, Brenner MB. Distinct iNKT cell populations use IFNgamma or ER stress-induced IL-10 to control adipose tissue homeostasis. Cell Metab (2020) 32(2):243–58.e6. doi: 10.1016/j.cmet.2020.05.017
82. von Gerichten J, Lamprecht D, Opalka L, Soulard D, Marsching C, Pilz R, et al. Bacterial immunogenic alpha-galactosylceramide identified in the murine large intestine: dependency on diet and inflammation. J Lipid Res (2019) 60(11):1892–904. doi: 10.1194/jlr.RA119000236
83. Ennamorati M, Vasudevan C, Clerkin K, Halvorsen S, Verma S, Ibrahim S, et al. Intestinal microbes influence development of thymic lymphocytes in early life. Proc Natl Acad Sci U S A (2020) 117(5):2570–8. doi: 10.1073/pnas.1915047117
84. Wieland Brown LC, Penaranda C, Kashyap PC, Williams BB, Clardy J, Kronenberg M, et al. Production of alpha-galactosylceramide by a prominent member of the human gut microbiota. PLoS Biol (2013) 11(7):e1001610. doi: 10.1371/journal.pbio.1001610
85. Ito Y, Vela JL, Matsumura F, Hoshino H, Tyznik A, Lee H, et al. Helicobacter pylori cholesteryl alpha-glucosides contribute to its pathogenicity and immune response by natural killer T cells. PLoS One (2013) 8(12):e78191. doi: 10.1371/journal.pone.0078191
Keywords: invariant NKT cells, inflammatory bowel diseases, CD1d, glycolipid antigens, commensal bacteria, short-chain fatty acids
Citation: Lee SW, Park HJ, Van Kaer L and Hong S (2024) Role of CD1d and iNKT cells in regulating intestinal inflammation. Front. Immunol. 14:1343718. doi: 10.3389/fimmu.2023.1343718
Received: 24 November 2023; Accepted: 26 December 2023;
Published: 11 January 2024.
Edited by:
Hyun Park, National Cancer Institute (NIH), United StatesReviewed by:
Federica Facciotti, University of Milano Bicocca, ItalyCopyright © 2024 Lee, Park, Van Kaer and Hong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seokmann Hong, c2hvbmdAc2Vqb25nLmFjLmty
†These authors contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.