- 1State Key Laboratory of Animal Nutrition and Feeding, Institute of Animal Sciences, Chinese Academy of Agricultural Sciences, Beijing, China
- 2Precision Livestock and Nutrition Unit, Gembloux Agro-Bio Tech, University of Liège, Gembloux, Belgium
- 3School of Agriculture and Food Science, University College Dublin, Dublin, Ireland
- 4Peking Centre Technology Co., LTD, Beijing, China
Introduction: The evolution of nutritional strategies to improve the gut health and microbiota profiles of early-weaned piglets is essential to reduce diarrhoea caused by weaning stress. Therefore, the aim of this study was to determine the effects of dietary supplementation of Qi-Weng-Huangbo powder, a traditional herbal medicine consisting of a mixture of Pulsatilla chinensis, Chinese Schneid and Astragalus extracts (PCE), on the growth performance, diarrhoea rate, immune function and intestinal health of weaned piglets.
Methods: 162 piglets were randomly assigned to the CON group (no PCE added), the PCEL group (300 mg/kg PCE) and the PCEH group (500 mg/kg PCE) at the end of the third week post farrowing. There were 9 replicates of each group with 6 pigs per replicate. The experiment lasted for 28 days and sampling was performed on the final day.
Results: The results showed that the PCE diet increased the average daily gain (ADG) and final body weight (BW) compared to the CON group. Both supplemented doses of PCE reduced the faecal scores of piglets, and the diarrhoea rate in the PCEL group was significantly lower than that in the CON group. The application of PCE diets promoted the development of the spleen in piglets and up-regulated serum immunoglobulin concentrations to enhance immune function, which was also reflected in the down-regulated gene expression of the colonic TLR/MyD88/NF-κB pathway. Supplementation with PCE improved intestinal morphology, and all doses of PCE significantly increased villus height (VH) in the ileum, whereas colonic crypt depth (CD) was significantly lower in the PCEH group than in the CON group. The PCEH diet significantly increased the levels of valeric and isovaleric acid in the colon content. Dietary PCEH also improved the colonic microbial community profile, reflected by a significant increase in Shannon’s index compared with CON group. The abundance of Veillonellaceae and Rhodospirillales was significantly increased in the PCEH group at the family level.
Discussion: In conclusion, dietary PCE reduced diarrhoea rates, improved growth performance and enhanced immune function in weaned piglets. These improvements were potentially supported by altered ileum and colonic morphology, elevated colonic VFA levels, and modulation of colonic microbial profiles.
Introduction
Early weaning of piglets, typically occurring at 3 to 4 weeks of life, is a widely employed strategy aimed at maximizing economic gains through the efficient repopulation of sows (1). However, this practice induces weaning stress as a consequence of separation from the sow and dietary alterations, thereby instigating disruptions in the microbial composition and metabolic processes within the piglet’s gastrointestinal tract (2, 3). Consequently, the structural and functional integrity of the gut may be compromised, leading to the atrophy of intestinal villi and heightened mucosal permeability (4). These detrimental effects ultimately result in a diminished immune response and the manifestation of diarrheal symptoms in piglets. In the global context of the ban on antibiotics in feed, concerns regarding bioresistance have elevated the significance of these threats (5). Thus, it is imperative to employ nutritional strategies to enhance piglet gut health during the early weaning period.
Qi-weng-Huangbo powder, the name comes from the Chinese pronunciation of the abbreviated names of three Chinese herbs, comprising a mixture of Pulsatilla chinensis, Chinese Schneid, and Astragalus extract (PCE), serves as a traditional Chinese herbal compound (6). Pulsatilla chinensis, a perennial herb belonging to the Pulsatilla genus in the buttercup family, is utilized in traditional Chinese medicine for its efficacy in treating bacterial infections (7). The root of this herb contains Pulsatilla chinensis saponin, which serves as its primary active ingredient. Additionally, Chinese Schneid and Astragalus are sourced from the bark and root of their respective plants. The active component of Chinese Schneid is berberine hydrochloride, whereas Astragalus contains astragalus polysaccharide as its active ingredient (8, 9). Recent pharmacological research has elucidated the modulatory effects of saponins derived from Pulsatilla chinensis on various biological processes, including apoptosis, immunomodulation, neuroprotection, and anticancer mechanisms (10). Previous investigations have demonstrated that the administration of Pulsatilla chinensis extract can effectively restore the integrity of the colonic mucosal barrier in rats by preserving the homeostasis and diversity of the intestinal microbiota (11). Furthermore, the therapeutic potential of Pulsatilla chinensis extract in the treatment of colitis has been attributed to its ability to enhance the levels of colonic volatile fatty acids (12). Berberine hydrochloride exhibits anti-inflammatory and immunosuppressive properties, while astragalus polysaccharide has been found to enhance animal growth performance by mitigating oxidative stress (13).
In recent years, there has been increasing utilization of single or combined herbs as feed additives, whereby the immune function of weaned piglets has been enhanced through the administration of astragalus and ginseng polysaccharides for instance (14). Egg quality in laying hens has also been improved by employing cinnamon and rosemary essential oils, while antioxidant activity in ducks has been elevated through the utilization of Pulsatilla chinensis and Eucommia extracts (15, 16). Additionally, a number of studies have been conducted to investigate the potential of Pulsatilla chinensis extracts in ameliorating intestinal inflammation and mitigating LPS challenge-induced intestinal damage in piglets. Nevertheless, there remains a lack of comprehensive research examining the impact of traditional herbal formulations on growth performance, diarrhoea rate, and intestinal health in weaned piglets (17). As such, the objective of this study was to assess the effects of applying PCE on the immune function, intestinal development, and colonic microbial profiles in weaned piglets fed varying dosages.
Materials and methods
The Animal Ethics Committee of the Institute of Animal Sciences, Chinese Academy of Agricultural Sciences approved the experimental protocol (Ethics Approval Code: IAS2022-155).
Experimental design and treatments
The Qi-Weng-Huangbo powder, sourced from Peaking Centre Technology Co., Ltd, consisted of Pulsatilla chinensis, Chinese Schneid, and Astragalus in the ratio 4:3:3 respectively. The preparation process involved boiling the mixture for three hours to obtain a solution, followed by filtration and subsequent drying of the filtrate. Starch was then added to the resulting product, at a weight equivalent to 50% of the original herb, and thoroughly mixed to yield the Qi-Weng-Huangbo powder.
A total of 162 Yorkshire x (Duroc x Landrace) piglets, with similar body weights, were randomly allocated into three groups consisting of nine replicates of six pigs each, with an equal distribution of females and males, weaning in the last day of the third week. The control group (CON) was fed a basal diet and the low-dose group (PCEL) received a basal diet supplemented with 300 mg/kg of PCE, while the high-dose group (PCEH) received a basal diet supplemented with 500 mg/kg of PCE.
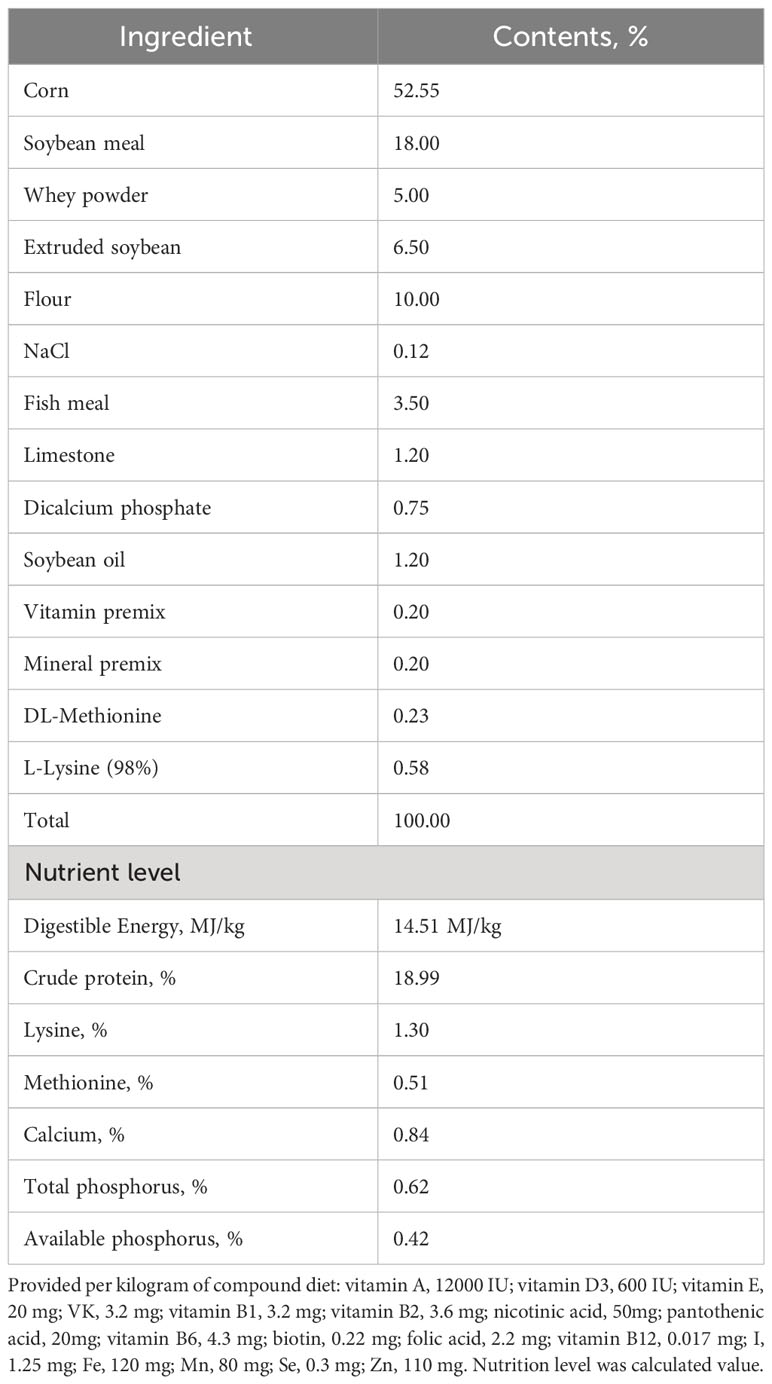
The animal experiment was conducted at the Dabeinong Research Centre Farm in Tangshan, China, with unrestricted access to water and feed. The basal diet used in the experiment (as outlined in Table 1) adhered to the nutritional requirements recommended by the National Research Council. The animals were housed in an environment maintained at a temperature of 30°C and a relative humidity of 70%, with a daily light period of 12 hours. To ensure the validity of the data, the use of antibiotics was strictly prohibited throughout the duration of the experiment. The experiment spanned a total of 28 days.
Growth performance
The study collected and recorded body weight data and feed intake per treatment using an automated system. The body weights of all piglets were measured on the first day and 28th day of the trial to determine the initial body weight (Inital BW), final body weight (Final BW), and daily feed intake (ADFI). The feed conversion ratio (FCR) was calculated as ADFI/Final BW - Inital BW.
Faecal scores and diarrhoea rates
Faecal scoring was conducted to assess shape and water content, with scores ranging from 0 to 5. Diarrhoea was identified when the faecal score exceeded 2. The scoring scale employed in this study encompassed the following categories: a score of 0 denoted normal solid faeces, a score of 1 indicated somewhat moist faeces that still maintained a solid shape, a score of 2 signified faeces that were beginning to lose their solid consistency, a score of 3 represented the onset of diarrhoea characterized by the absence of solid form, a score of 4 indicated a more severe form of diarrhoea where the faeces exhibited a runny consistency, and finally, a score of 5 denoted faeces that were completely watery, representing the most severe manifestation of diarrhoea. The total number of diarrhoeic piglets was recorded daily throughout the experimental period. The diarrhoea rate was calculated as: number of diarrhoeic pigs/(total number of pigs × number of days) × 100%.
Sample collection
On day 28, sample collection was conducted, whereby one male piglet was randomly chosen from each replicate. The piglets were weighed, and blood samples were extracted from the jugular vein. The serum was obtained through low-temperature centrifugation (9,000 × g) for 10 minutes and subsequently stored in a freezer at -20°C. Ileum and colon samples measuring 1 cm in length were sectioned at identical locations, gently rinsed with PBS, and promptly submerged in a 10% formalin solution, prior to dehydration. The colonic mucosa and colonic content were placed into separate sterile tubes and rapidly frozen in liquid nitrogen and stored in a -80°C freezer for subsequent index analysis. The heart, liver, spleen, lungs, and kidneys were individually weighed and used to calculate the organ index, which is determined by dividing the organ weight by the weight of the pig and multiplying it by 100%.
Determination of immunoglobulin
The levels of immunoglobulin A (IgA), immunoglobulin G (IgG), and immunoglobulin M (IgM) in the serum were assessed using Enzyme-Linked Immunosorbent Assay (ELISA) kits provided by MLBIO (Shanghai, China). All experimental procedures were conducted in strict accordance with the manufacturer’s instructions. The data exhibited an intra-batch coefficient of variation (CV) of less than 5% and an inter-batch CV of less than 8%.
Morphology of the ileum and colon
The intestinal samples were immersed in a 10% formalin solution for a duration of 24 hours to ensure fixation. Subsequently, the intestinal segments underwent dehydration and were embedded in paraffin wax. Tissue slices measuring 5 µm in thickness were then prepared using a microtome and subjected to staining with haematoxylin and eosin. The resulting sections were examined and captured using a Nikon 80i microscope, and the software Image J was employed to analyse the intestinal morphology. Villus height (VH) was determined as the measurement from the apex of the villus to the junction of the adjacent crypt, while crypt depth (CD) was defined as the extent of the depression between neighbouring villi. Ten representative data points from each sample were selected for statistical analysis.
Analysis of volatile fatty acids
The analysis of VFAs involved loading approximately 1 g of colonic content into a 10 mL centrifuge tube, thoroughly mixing it with 5 mL of distilled water, and then centrifuging it at 9,000 × g for 10 min after 1 h at 4°C. Following this, 900 μL of the supernatant was combined with 100 μL of 25% metaphosphoric acid and allowed to stand for 2 h at 0°C. After another round of centrifugation for 10 min, the resulting supernatant was subjected to gas chromatography (Agilent 7890) for the analysis.
RNA isolation and real-time quantitative PCR
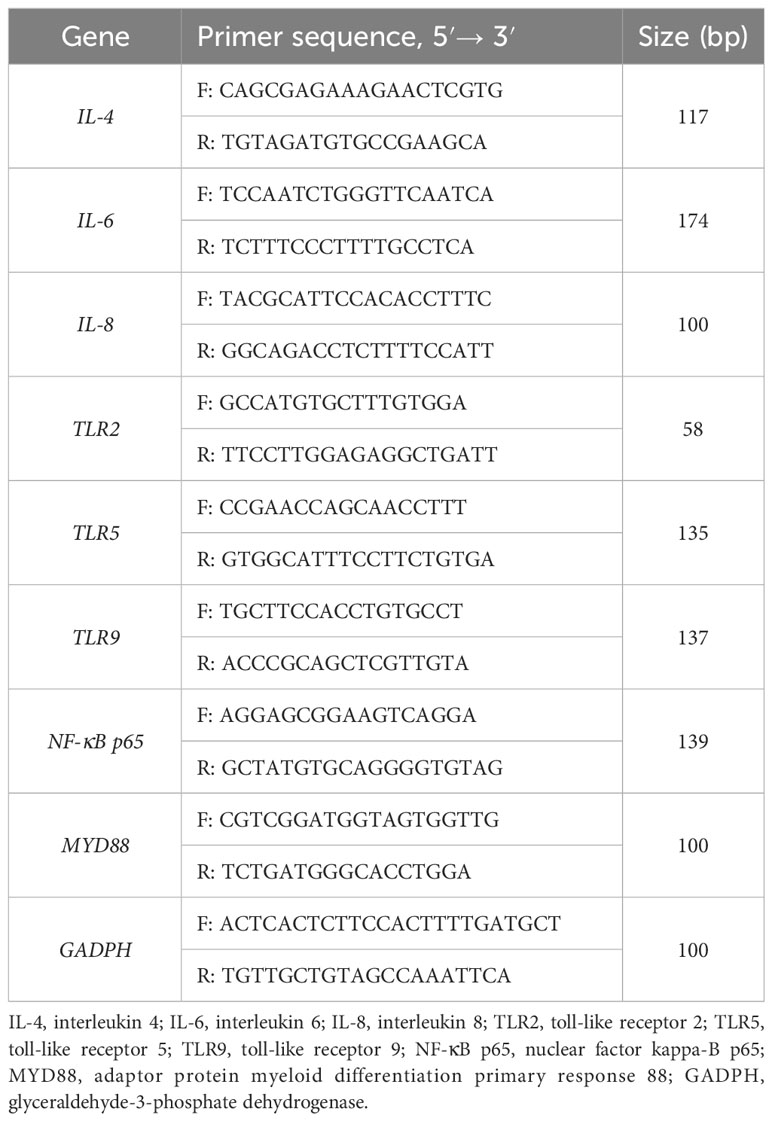
Total colon RNA was extracted using Trizol reagent (Invitrogen, San Diego, USA), and the concentration and purity of each RNA sample were assessed using a NanoDrop spectrophotometer (ND-2000, Thermo Scientific, Wilmington, USA). The integrity of the RNA was evaluated by 1% agarose gel electrophoresis. Reverse transcription was performed on 1 μg of total RNA using the PrimeScript® RT kit (RR047A, TaKaRa, Japan). Subsequently, gene expression was determined by analysing 1 μg of total RNA with TB Green Premix Ex Taq (RR820A, Takara, Japan) on an ABI 7500 real-time PCR system (Thermo Scientific, Wilmington, USA) for RT-PCR analysis. The reaction program consisted of pre-denaturation at 95°C for 10 s, followed by denaturation at 95°C for 5 s, and annealing and extension at 60°C for 40 s. Each reaction was replicated in three wells, and the primer sequences are presented in Table 2. The amplification efficiency of the primers was determined by employing a standard curve. The specificity of the amplification products was confirmed through melting curves. The expression of target genes was standardised using the geometric mean of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression. The relative expression levels of each target gene were assessed using the 2-ΔΔct method.
16S sequencing of colonic microorganisms
Total genomic DNA from the contents of the colon was extracted using the E.Z.N.A. Soil DNA Kit (Omega Bio-Tek, Norcross, CA, USA). Extracted DNA was assessed for both quantity and purity using a NanoDrop spectrophotometer (ND-2000, Thermo Scientific, Wilmington, USA). The integrity of the DNA was determined through 1% agarose gel electrophoresis, followed by PCR amplification of the bacterial 16S rRNA gene using specific primers 338F and 806R (GeneAmp9700 produced by ABI) to target the V3-V4 variable region. The amplification program was described as follows: the reaction at 95°C for 3 min was followed by 27 cycles (95°C for 30 s, 55°C for 30 s of annealing, and 72°C for 30 s), and the final step was an extension at 72°C for 10 min, while the PCR was performed in 20 μL of a mixed system containing 10 ng of template DNA. The resulting PCR products were then recovered on a 2% agarose gel and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) before quantification using the QuantiFluor™-ST assay (Promega, USA).
The purified amplification products were combined in equimolar proportions and underwent paired-end sequencing (2 × 300) on an Illumina MiSeq PE300 platform (Illumina, San Diego, CA, USA). The raw sequences were subjected to quality screening using Quantitative Insights Into Microbial Ecology 2 (QIIME2) software. Subsequently, the demultiplexed sequences of each sample were filtered, trimmed, denoised, and merged. Chimeric sequences were identified and eliminated to generate a table containing characteristics of amplicon sequence variation. Based on the 338F/806R primers, the database obtained in the previous step was trimmed to the V3-V4 region to obtain the species classification table.
Data analysis
All data were expressed as the mean and total standard error of the mean, and data were counted and analysed in replicates. Data were analysed by one-way ANOVA using the software SPSS 22.0 (SPSS. Inc., Chicago, USA). Statistical differences in the data were considered to be present when P < 0.05.
Results
Growth performance and organ index
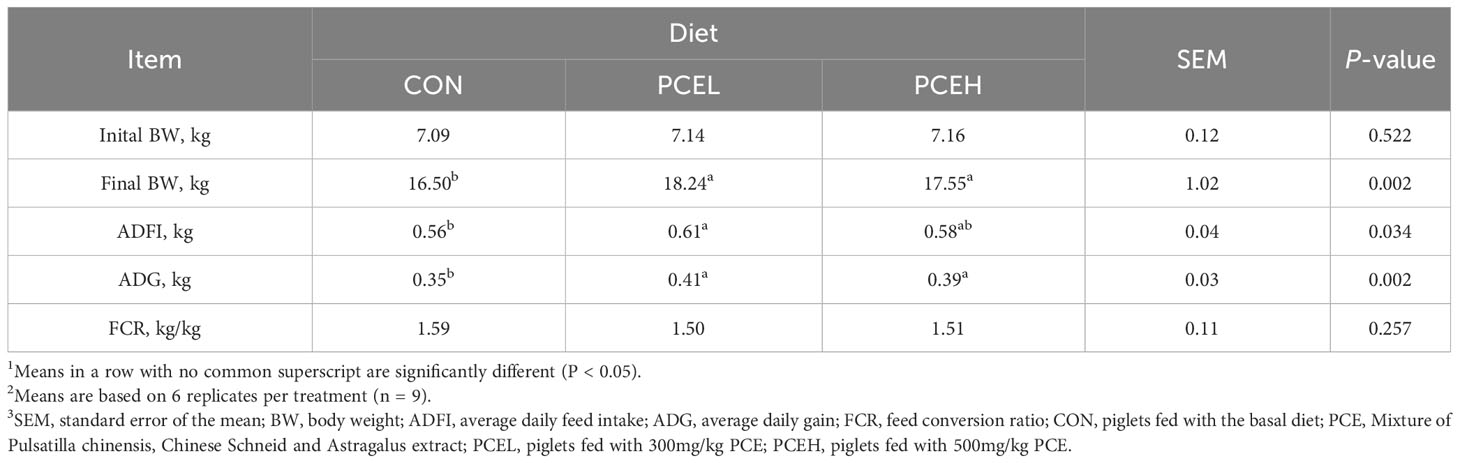
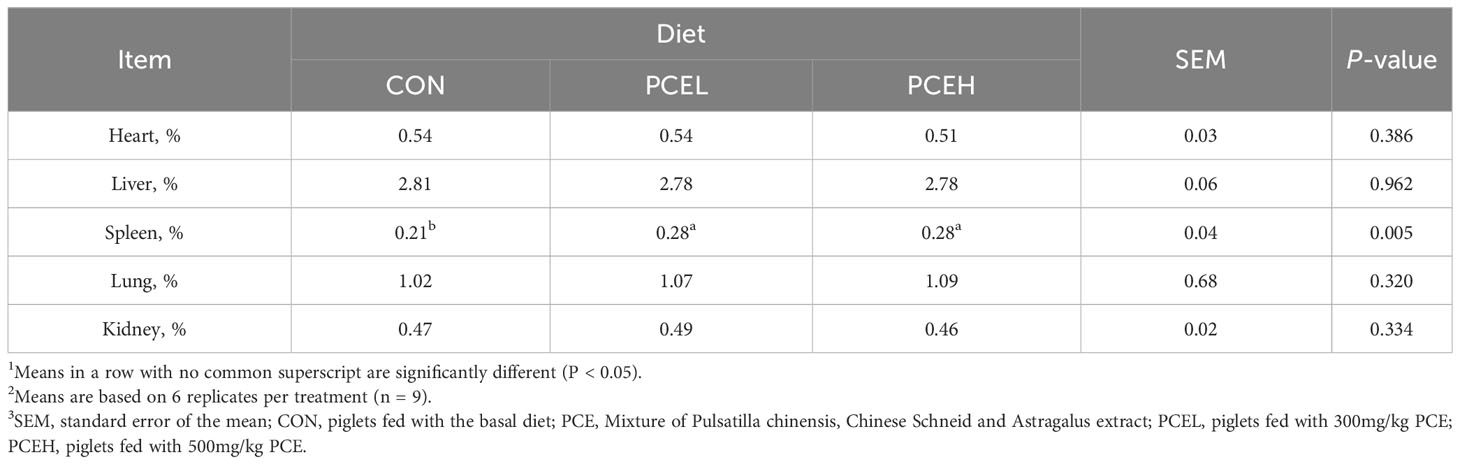
Table 3 presents the findings regarding the impact of various doses of PCE on the performance of weaned piglets. The results indicated that all doses of PCE had a significant positive effect on ADG (P = 0.002) and final BW (P = 0.002) of the piglets. Furthermore, the inclusion of PCEL in the diet resulted in a significant increase in ADFI compared to the CON group (P = 0.034). Additionally, Table 4 demonstrated that the administration of extracts in the diet had a significant effect on the organ indexes of the spleen in piglets (P = 0.005), while the organ indexes of the heart, liver, lungs, and kidneys did not exhibit significant changes (P > 0.05).
Faecal scores and diarrhoea rates
Figure 1 provides evidence supporting the impact of PCE on the faecal scores and diarrhoea rates of piglets. The data clearly demonstrated that PCE significantly decreased the faecal scores of piglets (P < 0.001) and exhibited a dose-dependent positive effect. Furthermore, the diarrhoea rate in the PCEL group was significantly lower compared to the CON group (P = 0.017).
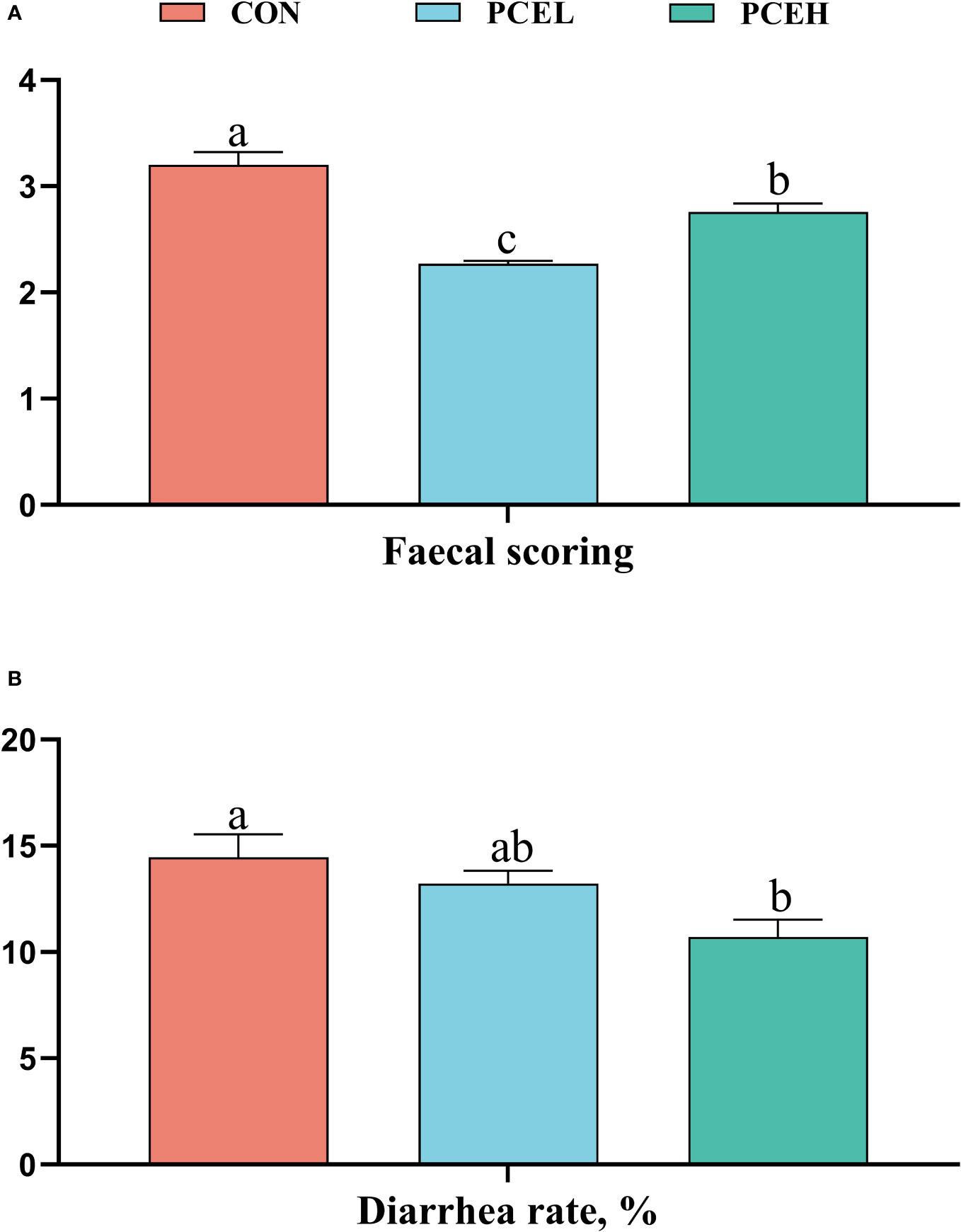
Figure 1 Effect of dietary PCE on faecal scoring (A) and diarrhoea rate (B) of the weaned piglets. Means with no common superscripts differ significantly (P < 0.05). CON, piglets fed with the basal diet; PCE, Mixture of Pulsatilla chinensis, Chinese Schneid and Astragalus extract; PCEL, piglets fed with 300mg/kg PCE; PCEH, piglets fed with 500mg/kg PCE.
Serum immunoglobulins
The influence of PCE on the serum immunoglobulins of weaned piglets is presented in Figure 2. The PCEL group exhibited a significant increase in the levels of serum IgA, IgG, and IgM in piglets compared to the CON group (P < 0.05). Additionally, the PCEH group demonstrated a significant beneficial effect on IgM (P = 0.036).
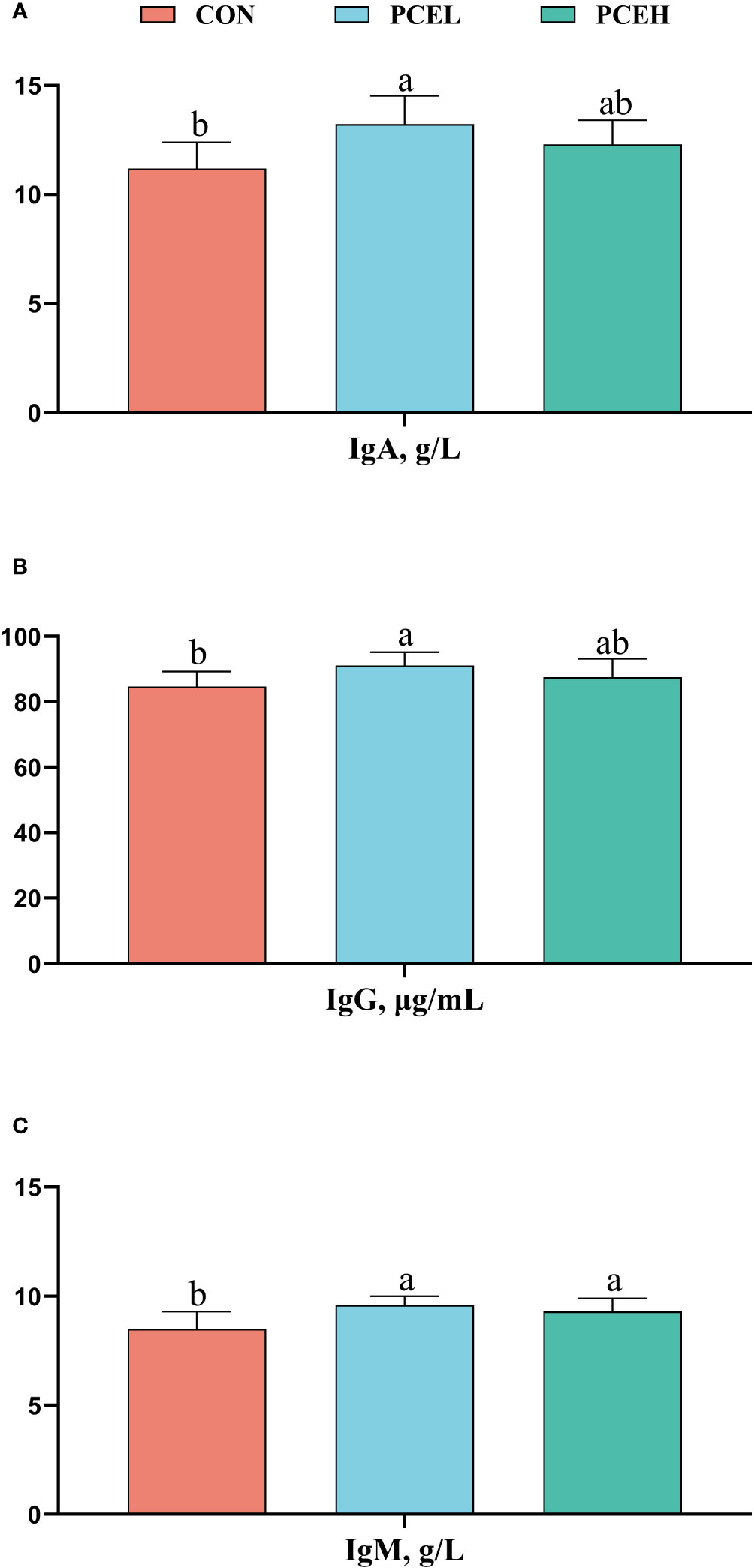
Figure 2 Effects of dietary PCE on serum immunoglobulin A (A), immunoglobulin G (B), and immunoglobulin M (C) concentrations of the weaned piglets. Means with no common superscripts differ significantly (P < 0.05). Ig, immunoglobulin; CON, piglets fed with the basal diet; PCE, Mixture of Pulsatilla chinensis, Chinese Schneid and Astragalus extract; PCEL, piglets fed with 300mg/kg PCE; PCEH, piglets fed with 500mg/kg PCE.
Morphometric analysis of the ileum and colon
The findings presented in Figure 3 illustrate the impact of diet on the intestinal morphology of weaned piglets. Specifically, the administration of PCE at a dosage of 500 mg/kg resulted in a significant increase in ileal, as well as a decrease in colonic CD, when compared to the normal diet (P < 0.05). Furthermore, the PCEL group exhibited significantly higher ileal compared to the CON group (P < 0.05).
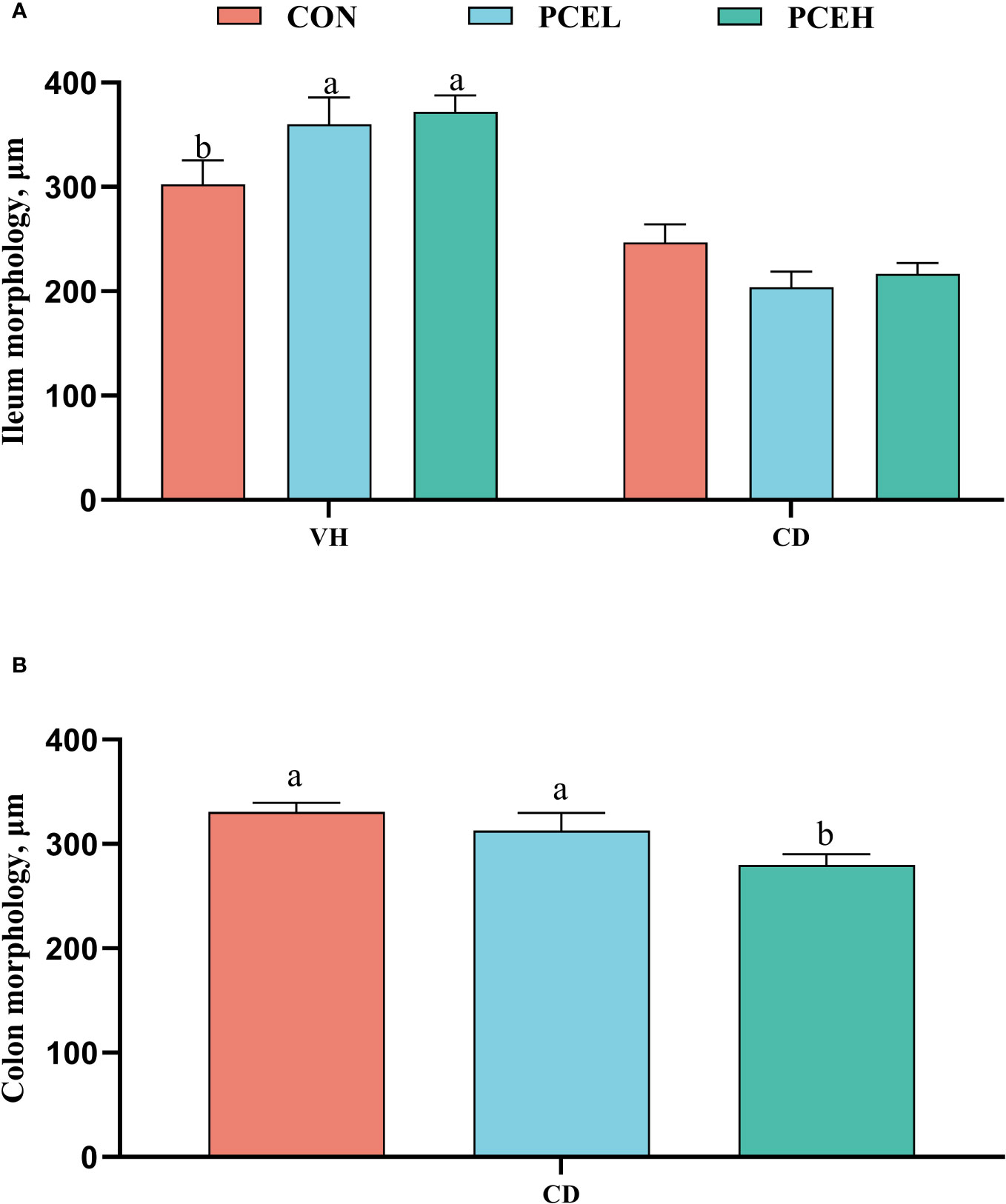
Figure 3 Effects of dietary PCE on morphology of the ileum (A) and colon (B) of the weaned piglets. Means with no common superscripts differ significantly (P < 0.05). VH, villus height; CD, crypt depth; CON, piglets fed with the basal diet; PCE, Mixture of Pulsatilla chinensis, Chinese Schneid and Astragalus extract; PCEL, piglets fed with 300mg/kg PCE; PCEH, piglets fed with 500mg/kg PCE.
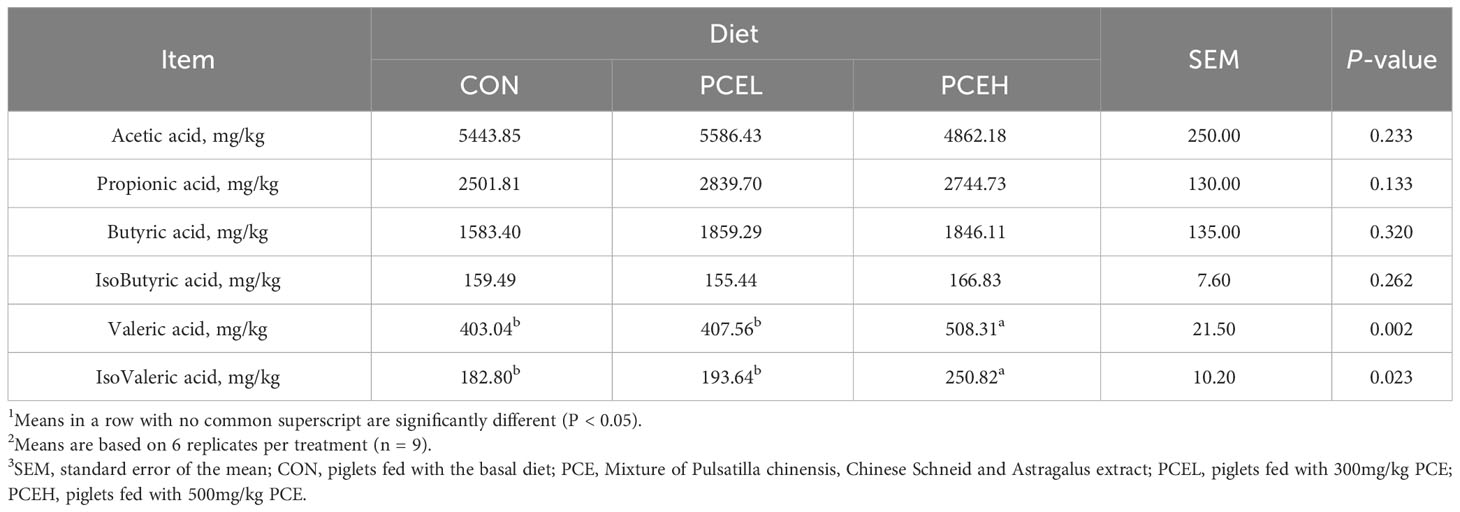
Volatile fatty acids concentration of the colonic content
The effect of PCE diets on the concentration of VFAs in the colonic content of weaned piglets is presented in Table 5. Concentrations of valeric acid and isovaleric acid were significantly higher (P < 0.05) in the PCEH group compared to the CON group. However, the low-dose PCE diet did not show a trend towards significant changes.
Inflammatory factor-related gene expression
Figure 4 demonstrates a significant downregulation of gene expression levels of the pro-inflammatory factor interleukin 6 (IL-6) in the colon of piglets in the PCEL and PCEH groups, as compared to CON piglets. Additionally, the gene expression levels of toll-like receptor 5 (TLR5), adaptor protein myeloid differentiation primary response 88 (MyD88), and nuclear factor kappa-B p65 (NF-κB p65) were also significantly downregulated (P < 0.05). These findings suggested that the application of PCE lead to the downregulation of gene expression in the TLR/MyD88/NF-κB pathway.
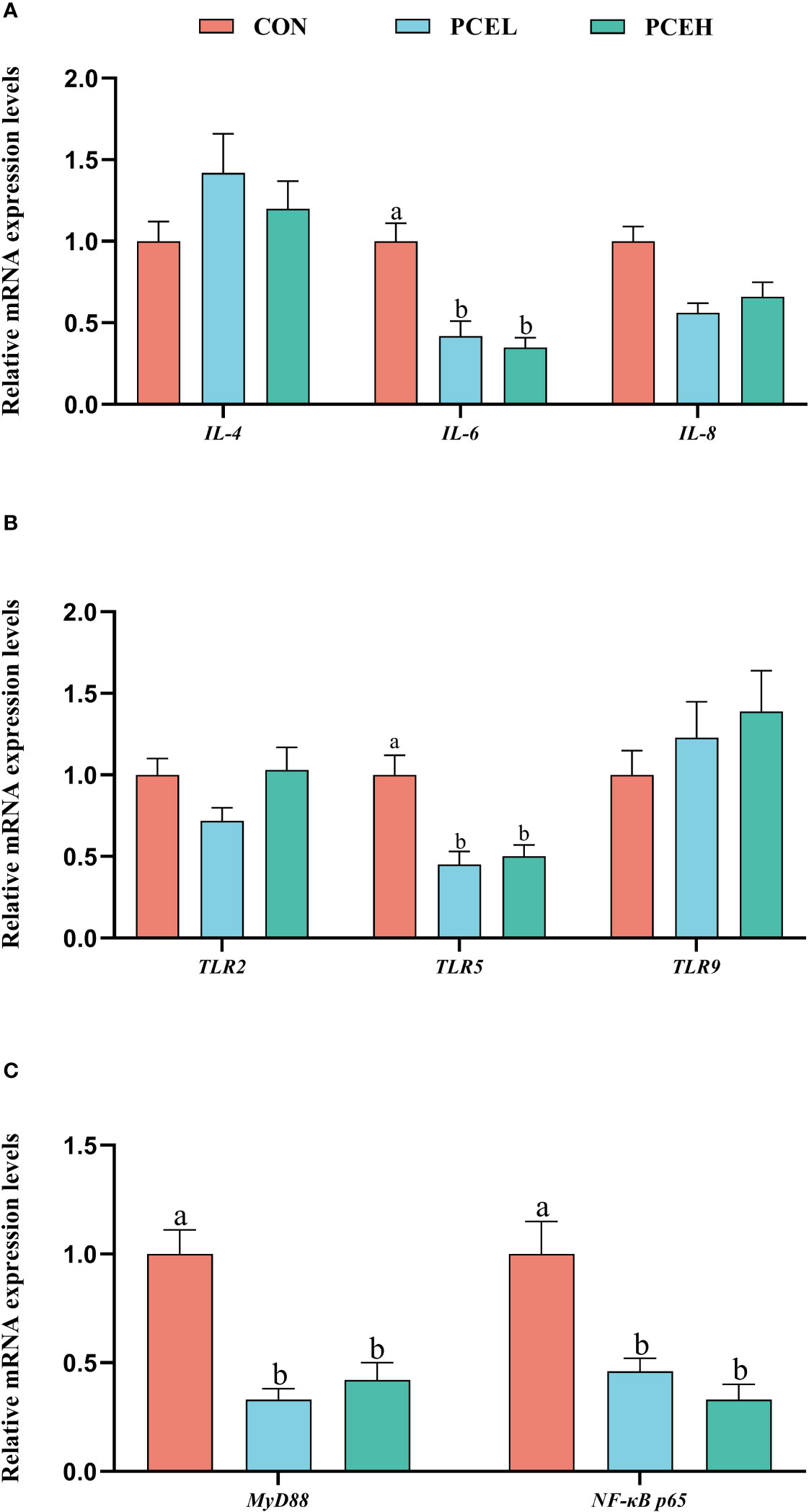
Figure 4 Effects of dietary PCE on the gene expression levels of immune cytokines and TLR/MyD88/NF-κB pathway in the colon of the weaned piglets. Means with no common superscripts differ significantly (P < 0.05). IL, interleukin; TLR, toll-like receptor; NF-κB p65, nuclear factor kappa-B p65; MYD88, adaptor protein myeloid differentiation primary response 88; CON, piglets fed with the basal diet; PCE, Mixture of Pulsatilla chinensis, Chinese Schneid and Astragalus extract; PCEL, piglets fed with 300mg/kg PCE; PCEH, piglets fed with 500mg/kg PCE.
Colonic microbial profiles
In order to assess the impact of dietary PCE on colonic microorganisms, we conducted high-throughput sequencing of colonic content in weaned piglets using 16s rRNA. Based on the Venn diagram (Figure 5A), the CON, PCEL, and PCEH groups exhibited 631, 646, and 649 operational taxonomic units (OTUs) respectively, with 532 OTUs shared among all three colon microbial communities. To analyse beta diversity, we employed unweighted Unifrac distance PCoA analysis to demonstrate that the dietary treatments did not lead to a more distinct separation of colonic microbiota (Figure 5B). The alpha-diversity of colonic microorganisms was evaluated using Ace, Chao, Shannon, and Coverage indices (Figure 5C), with the Shannon index demonstrating a significant increase in the PCEH group compared to the control group. These findings indicate that the high dose PCE diet has a positive impact on the diversity of colonic microbial populations in piglets.
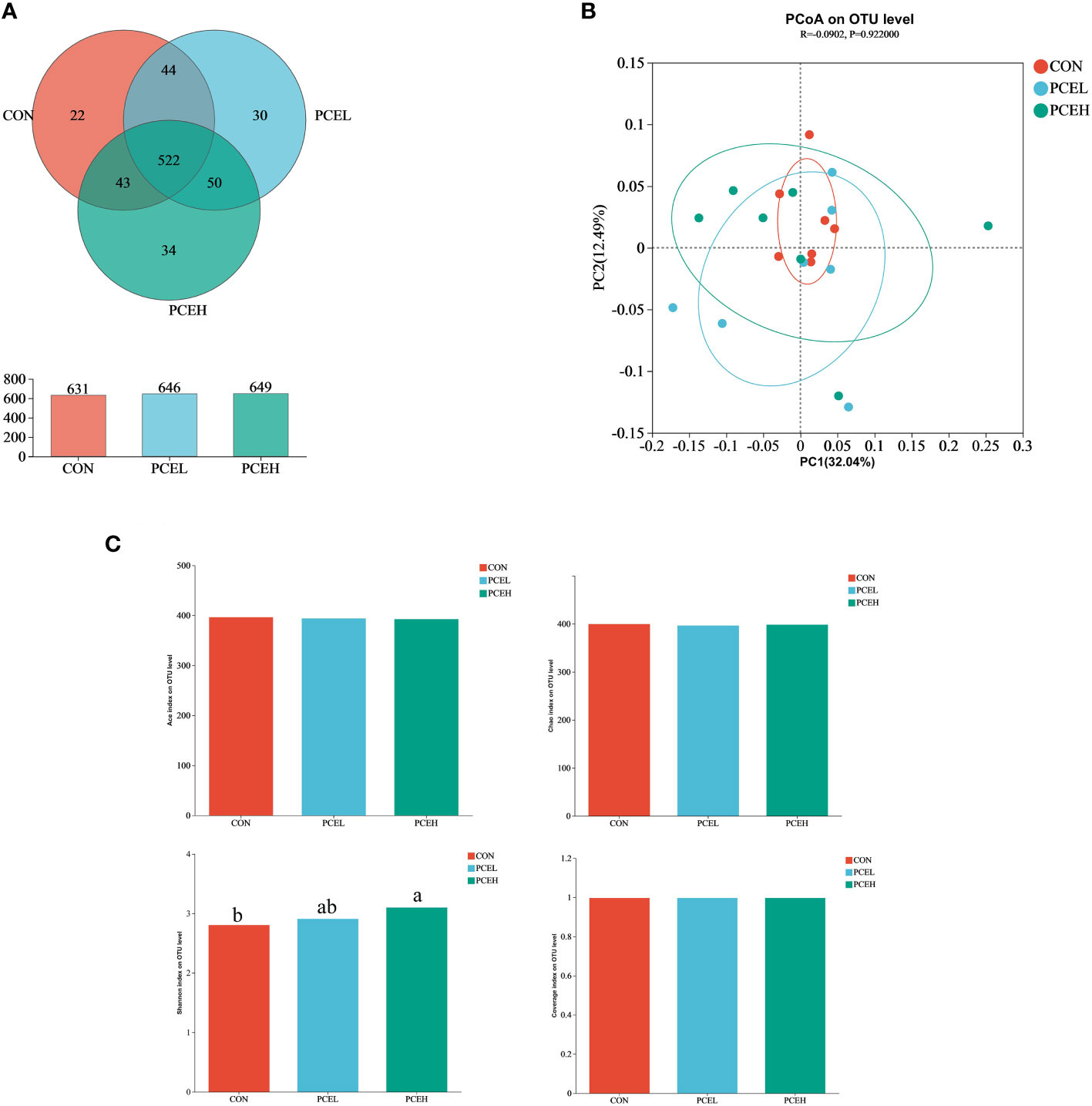
Figure 5 Effects of dietary PCE on colonic microbiota diversity of the weaned piglets. (A) Venn diagram based on the number of OTUs per group; (B) PCoA based on unweighted Unifrac distance; (C) α diversity based on the Ace, Chao1, Shannon and Coverage indices. Means with no common superscripts differ significantly (P < 0.05). CON, piglets fed with the basal diet; PCE, Mixture of Pulsatilla chinensis, Chinese Schneid and Astragalus extract; PCEL, piglets fed with 300mg/kg PCE; PCEH, piglets fed with 500mg/kg PCE.
At the phylum level, statistical analysis revealed that the predominant phyla, Firmicutes, Bacteroidetes, and Actinobacteriota accounted collectively for over 95% of the overall microbial community (Figure 6A). At the family level, it is evident that the top five most dominant families, namely Clostridiaceae, Peptostreptococcaceae, Erysipelotrichaceae, Prevotellaceae, and Ruminococcaceaae, collectively accounted for at least 60% or more of the overall microbial community (Figure 6B). When considering microorganisms at the genus level, the primary dominant genera were identified as Clostridium sensu stricto 1, Terrisporobacter, Turicibacter, Subdoligranulum, Prevotella, and Lactobacillus (Figure 6C). The results of the Wilcoxon differentiation analysis revealed a statistically significant increase in the abundance of Veillonellaceae and Rhodospirillales at the family level in the PCEH group compared to the CON group (Figure 6D). Furthermore, at the genus level, the abundance of Rhodospirillales was significantly higher in the PCEH group compared to the CON group, while the abundance of Eubacterium ventriosum was significantly lower in the PCEH group compared to the CON group (Figure 6E).
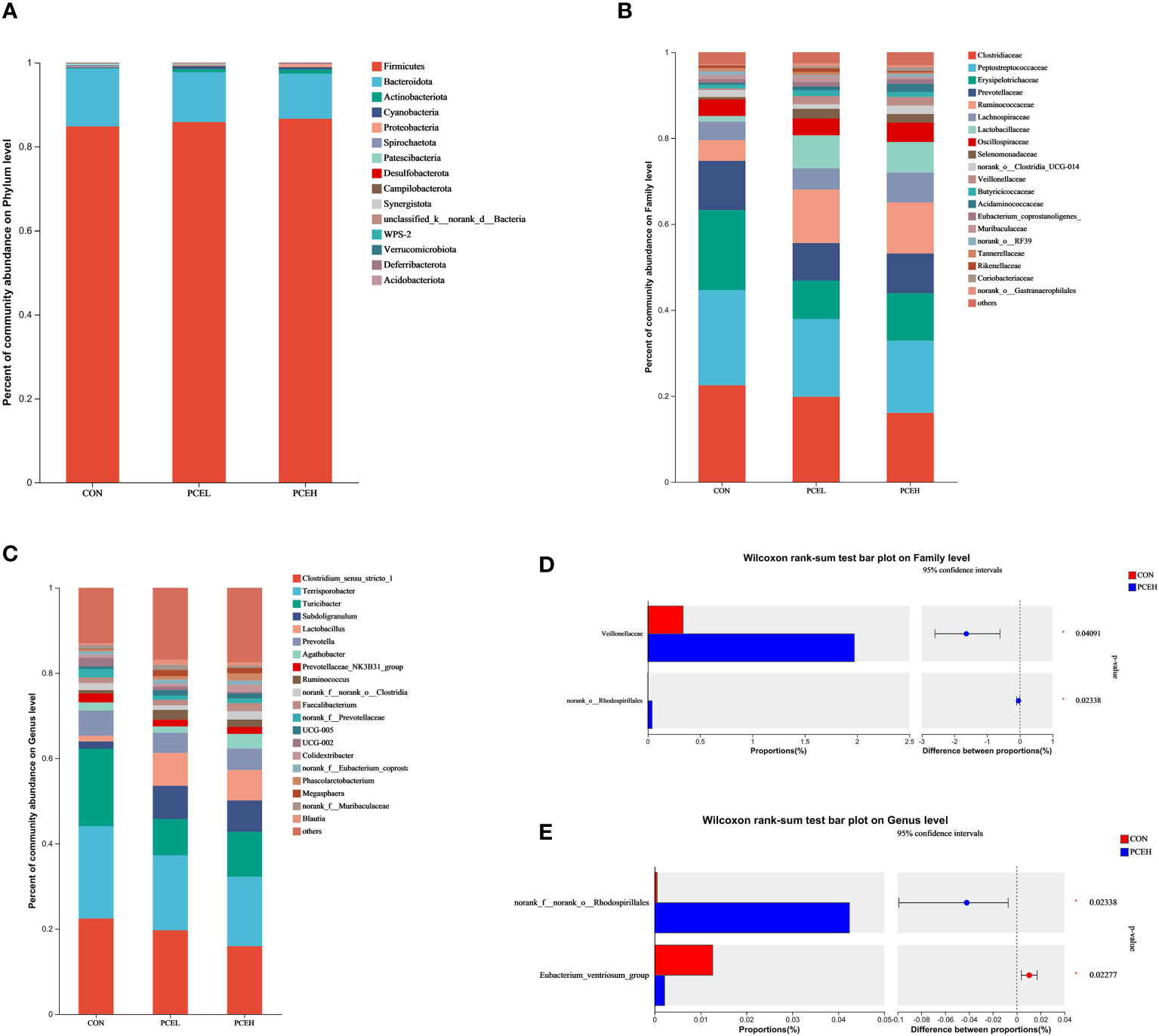
Figure 6 Effects of dietary PCE on colonic microbiota composition of the weaned piglets. (A) Microbial composition at the phylum level; (B) Microbial composition at the family level; (C) Microbial composition at the genus level; (D) Relative abundances at the family level; (E) Relative abundances at the genus level; * indicates significant difference (P < 0.05). CON, piglets fed with the basal diet; PCE, Mixture of Pulsatilla chinensis, Chinese Schneid and Astragalus extract; PCEL, piglets fed with 300mg/kg PCE; PCEH, piglets fed with 500mg/kg PCE.
Discussion
Previous research has demonstrated that the utilization of plant extracts has the potential to enhance animal performance through the modulation of gut health and the improvement of immune function (18). For instance, the administration of Gynura procumbens, Rehmannia glutinosa, and Scutellaria baicalensis has been found to ameliorate diarrhoea in weaned piglets, consequently leading to enhanced growth performance (19). Additionally, the incorporation of Camellia sinensis and Punica granatum extracts into the drinking water has been shown to effectively counteract E. coli infections, thereby preserving the intestinal health of weaned piglets (20). The stress experienced during the weaning process can lead to diarrhoea, compromised immune function, and hindered growth in piglets (21). In line with previous research, the utilization of PCE diets resulted in enhanced ADG and ADFI among weaned piglets, which was directly associated with a reduction in the incidence of diarrhoea. The spleen, an essential immune organ in animals, plays a crucial role in modulating lymphatic and immune-related cytokines to exert anti-inflammatory effects (22). The organ index of the spleen serves as an indicator of the potential immune function of the animal (23). The present study substantiates the efficacy of the PCE extract in enhancing the spleen organ index in piglets, aligning with previous research on the utilization of herbal remedies to ameliorate the inflammatory response in weaned piglets (24).
The compromised immune system of early weaned piglets, owing to the ongoing development of their immune organs and weakened immune function, renders them susceptible to diseases (25). Maternal antibody immunity, encompassing the transmission of an animal’s immunoglobulins from the mother to the offspring via the placenta and breast milk, plays a pivotal role in bolstering the immune defence of piglets in early life (26). The cessation of immunoglobulin consumption following weaning increases the susceptibility of the piglet’s intestinal tract to infection by pathogenic microorganisms (27). As a rule, the IgG and IgA are frequently employed as markers to assess the impact of maternally acquired antibodies on the immune function of the offspring, whereas IgM serves as the primary defence against infections in the early stages of humoral immune response (28, 29). The immunomodulatory properties of plant extracts have been observed to activate immune function in weaned piglets (30). Our findings indicate that the administration of plant extract-containing PCE diets resulted in an increase in serum IgA, IgG, and IgM levels in weaned piglets. Furthermore, a dose-dependent effect was observed, suggesting that higher doses of PCE positively influenced the immune function of weaned piglets.
It is important to note that intestinal mucosal immune function plays a crucial role in protecting the intestine against pathogenic microorganisms (31). Past research has demonstrated that piglets, when exposed to immunological challenges and stress following weaning, experience the infiltration of endotoxins through the compromised intestinal barrier into the bloodstream. This infiltration triggers an elevation in the secretion of cytokines, including interleukin 1β (IL-1β) and interleukin 6 (IL-6), through the activation of the NF-κB signaling pathway (32). Notably, the administration of Pulsatilla chinensis, Chinese Schneid, and Astragalus extracts has been found to mitigate the inflammatory responses induced by endotoxins by inhibiting the reduction in cytokine secretion in various inflammatory models (33–35). Toll-like receptors (TLRs) serve as initiators of innate immunity by recognizing lipopolysaccharides, subsequently initiating a cascade of cellular signals involving MYD88 and ultimately activating the NF-κB signalling pathway (36). NF-κB, in turn, governs the inflammatory response by binding to nucleotide sequences located upstream of pro-inflammatory cytokine and chemokine genes (37). Our study revealed that PCE resulted in the downregulation of gene expression related to the colonic TLR5 and MyD88/NF-κB pathways, ultimately leading to a decrease in levels of the proinflammatory factor IL-6. Hence, it is plausible that PCE exerts regulatory effects on the inflammatory response in weaned piglets via the TLR/MyD88/NF-κB pathway, as evidenced by the downregulation of pro-inflammatory cytokine gene expression level by PCE.
The establishment of a well-developed intestinal epithelium and a beneficial intestinal microbial community are crucial determinants in safeguarding animals against pathogenic bacterial infections (38). Intestinal villus height and crypt depth serve as indicators of intestinal nutrient absorption capacity and overall intestinal health, respectively (39). In contrast, low feed intake of piglets after early weaning adversely affects villus height and leads to frequent diarrhoea (40). The greater ease at which toxins enter the bloodstream as a result of morphological damage to the intestinal epithelium leads to the occurrence of a systemic inflammatory response (41). The inclusion of astragalus polysaccharide and berberine hydrochloride in the diet resulted in notable changes in the intestinal morphology of the animals, particularly in terms of villus height and crypt depth (42, 43). This observation implies that the active constituents present in these plants convey a beneficial impact on the gastrointestinal well-being and digestive health of piglets. The application of PCE in piglets resulted in an observed increase in ileal VH and a decrease in colonic CD, the ADFI of piglets from PCE groups was also enhanced. This suggests that PCE has the potential to enhance intestinal absorptive capacity and improve colonic health. It is worth noting that the increase in piglet feed intake might also indirectly reflect the strength. The intestinal flora, a crucial element of the intestinal barrier, exerts an immunosuppressive effect by secreting anti-inflammatory substances, with VFA being the main fermentation product (44). VFAs play a role in maintaining a dynamic equilibrium of the intestinal flora by reducing the pH, thereby enhancing the host’s resistance to pathogens and preventing adverse inflammatory responses (45). The combination of Astragalus polysaccharide and Codonopsis polysaccharide has been shown to re-establish immune homeostasis by increasing butyric, valeric, and isovaleric acid levels in the faeces of DSS-induced ulcerative colitis mice and restoring the composition of the intestinal flora (46). The supplementation of berberine hydrochloride has demonstrated the ability to enhance the content of VFAs as colonic metabolites through the modulation of the intestinal flora (47). Specifically, the administration of 500 mg/kg of a plant crude extract (PCE) resulted in elevated levels of valeric acid and isovaleric acid within the colonic content of weaned piglets. This increase in VFAs may be directly associated with the observed decrease in piglet diarrhoea rates and the subsequent enhancement of production performance.
Various saponins have demonstrated the ability to enhance microbial diversity (48). Additionally, Astragalus polysaccharide and berberine hydrochloride have been found to strengthen the intestinal barrier’s function by intervening to augment intestinal microbial diversity and facilitate the proliferation of advantageous bacteria (49). A robust gut microbial community co-evolves with the immune system, thereby impeding the infiltration of detrimental pathogens within the gut (50). Consequently, the enhancement of microbial diversity through the supplementation of dietary PCE proves advantageous for gut health. At the family level, piglets in the PCEH group demonstrated a heightened prevalence of the colonic microorganisms Veillonellaceae and Rhodospirillales. Similarly, at the genus level, the PCEH group exhibited a greater relative abundance of Rhodospirillales compared to the control group. Conversely, the relative abundance of Eubacterium ventriosum displayed an inverse pattern. Veillonellaceae primarily functions in lactic acid metabolism and enhances fat absorption by facilitating the dihydroxylation of bile acids (51). Rhodospirillales, a significant family of α-Ascomycetes, has been demonstrated to exert a beneficial influence on the modulation of the inflammatory response. Notably, the presence of Rhodospirillales was observed to be diminished in the intestinal microflora of afflicted mice in both a mouse model of colon cancer and a model of hypoxia-induced pulmonary hypertension (52, 53). Eubacterium ventriosum, a cluster of microorganisms closely linked to hindgut inflammation, exhibited a decrease in relative abundance in the gut of type 2 diabetic rats following treatment with extracts of Astragalus and Rhizoma coptidis (54). Furthermore, it was observed that the abundance of Eubacterium ventriosum exhibited a negative correlation with the levels of docosapentaenoic acid, a gut metabolite linked to inflammatory bowel disease (55). Consequently, the introduction of dietary PCE supplementation increases the diversity of the colonic microbiota and mitigates colonic inflammation and the immune response by facilitating the up-regulation of Veillonellaceae and Rhodospirillales at the family level, as well as inducing alterations in Rhodospirillales and Eubacterium ventriosum at the genus level.
Conclusion
In summary, the findings of this study indicate that the inclusion of PCE in the diet resulted in a reduction in the incidence of diarrhoea and enhanced growth performance in weaned piglets. Furthermore, PCE exhibited efficacy as a supplement by enhancing the immune function of weaned piglets, potentially attributed to improvements in jejunal and colonic morphology, increased levels of intestinal VFA, and led to compositional alterations in colonic microorganisms. Notably, the dosage of 500 mg/kg demonstrated superior effects.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA1044009.
Ethics statement
The animal study was approved by The Animal Ethics Committee of the Institute of Animal Sciences, Chinese Academy of Agricultural Sciences approved the experimental protocol (Ethics Approval Code: IAS2022-155). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
CX: Data curation, Writing – original draft. KL: Investigation, Writing – review & editing. CT: Investigation, Writing – review & editing. ZW: Project administration, Writing – review & editing. JL: Writing – review & editing. SZ: Writing – review & editing. LL: Writing – review & editing. HL: Project administration, Writing – review & editing. RZ: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Natural Science Foundation of China (32102582), the Youth innovation of Chinese Academy of Agricultural Sciences (Y2023QC09), and the Agricultural Science and Technology Innovation Program (ASTIPIAS07).
Conflict of interest
Author HL was employed by Peking Centre Technology Co., LTD.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ADFI, average daily feed intake; ADG, average daily gain; BW, body weight; CD, crypt depth; CON, piglets fed with the basal diet; FCR, feed conversion ratio; IL-1β, interleukin 1β; IL-6, interleukin 6; IgA, Immunoglobulin A; IgG, Immunoglobulin G; IgM, Immunoglobulin M; PCE, Mixture of Pulsatilla chinensis, Chinese Schneid and Astragalus extract; PCEL, piglets fed with 300mg/kg PCE; PCEH, piglets fed with 500mg/kg PCE; MYD88, adaptor protein myeloid differentiation primary response 88; NF-κB p65, nuclear factor kappa-B p65; OUT, operational taxonomic unit; TLR, toll-like receptor; VFA, volatile fatty acid.
References
1. Qiao L, Dou X, Song X, Chang J, Zeng X, Zhu L, et al. Replacing dietary sodium selenite with biogenic selenium nanoparticles improves the growth performance and gut health of early-weaned piglets. Anim Nutr (2023):99–113. doi: 10.1016/j.aninu.2023.08.003
2. Cao S, Hou L, Sun L, Gao J, Gao K, Yang X, et al. Intestinal morphology and immune profiles are altered in piglets by early-weaning. Int Immunopharmacol (2022) 105:108520. doi: 10.1016/j.intimp.2022.108520
3. Ramirez BC, Hayes MD, Condotta I, Leonard SM. Impact of housing environment and management on pre-/post-weaning piglet productivity. J Anim Sci (2022) 100:skac142. doi: 10.1093/jas/skac142
4. Zhang Y, Liu H, Yue Z, Tan P, Sun M, Ji L, et al. Wickerhamomyces anomalus relieves weaning diarrhea via improving gut microbiota and redox homeostasis using a piglet model. Food Funct (2022) 13:11223–35. doi: 10.1039/d2fo01861f
5. Kim K, Song M, Liu Y, Ji P. Enterotoxigenic Escherichia coli infection of weaned pigs: Intestinal challenges and nutritional intervention to enhance disease resistance. Front Immunol (2022) 13:885253. doi: 10.3389/fimmu.2022.885253
6. Liang J, Huang X, Ma G. Antimicrobial activities and mechanisms of extract and components of herbs in East Asia. RSC Adv (2022) 12:29197–213. doi: 10.1039/d2ra02389j
7. Zhong J, Tan L, Chen M, He C. Pharmacological activities and molecular mechanisms of Pulsatilla saponins. Chin Med (2022) 17:59. doi: 10.1186/s13020-022-00613-8
8. Li X, Qu L, Dong Y, Han L, Liu E, Fang S, et al. A review of recent research progress on the astragalus genus. Molecules (2014) 19:18850–80. doi: 10.3390/molecules191118850
9. Cheng H, Liu J, Tan Y, Feng W, Peng C. Interactions between gut microbiota and berberine, a necessary procedure to understand the mechanisms of berberine. J Pharm Anal (2022) 12:541–55. doi: 10.1016/j.jpha.2021.10.003
10. Li YH, Zou M, Han Q, Deng LR, Weinshilboum RM. Therapeutic potential of triterpenoid saponin anemoside B4 from Pulsatilla chinensis. Pharmacol Res (2020) 160:105079. doi: 10.1016/j.phrs.2020.105079
11. Liu Y, Zhou M, Yang M, Jin C, Song Y, Chen J, et al. Pulsatilla chinensis saponins ameliorate inflammation and DSS-induced ulcerative colitis in rats by regulating the composition and diversity of intestinal flora. Front Cell Infect Microbiol (2021) 11:728929. doi: 10.3389/fcimb.2021.728929
12. Li Z, Song Y, Xu W, Chen J, Zhou R, Yang M, et al. Pulsatilla chinensis saponins improve SCFAs regulating GPR43-NLRP3 signaling pathway in the treatment of ulcerative colitis. J Ethnopharmacol (2023) 308:116215. doi: 10.1016/j.jep.2023.116215
13. Fu K, Lv X, Li W, Wang Y, Li H, Tian W, et al. Berberine hydrochloride attenuates lipopolysaccharide-induced endometritis in mice by suppressing activation of NF-kappaB signal pathway. Int Immunopharmacol (2015) 24:128–32. doi: 10.1016/j.intimp.2014.11.002
14. Wang K, Zhang H, Han Q, Lan J, Chen G, Cao G, et al. Effects of astragalus and ginseng polysaccharides on growth performance, immune function and intestinal barrier in weaned piglets challenged with lipopolysaccharide. J Anim Physiol Anim Nutr (Berl) (2020) 104:1096–105. doi: 10.1111/jpn.13244
15. Bai L, Song X, Fu Y, Chen S, Tian Y, Jia R, et al. Effects of a mixed extract of Cortex Fraxini, Pulsatilla chinensis, and Eucommia ulmoides on immunity and antioxidant activity in hemp ducks. Livestock Sci (2019) 221:63–9. doi: 10.1016/j.livsci.2019.01.009
16. Abo Ghanima MM, Elsadek MF, Taha AE, Abd El-Hack ME, Alagawany M, Ahmed BM, et al. Effect of housing system and rosemary and cinnamon essential oils on layers performance, egg quality, haematological traits, blood chemistry, immunity, and antioxidant. Anim (Basel) (2020):245. doi: 10.Doi:10.3390/ani10020245
17. Xiao K, Cao ST, Jiao le F, Lin FH, Wang L, Hu CH. Anemonin improves intestinal barrier restoration and influences TGF-beta1 and EGFR signaling pathways in LPS-challenged piglets. Innate Immun (2016) 22:344–52. doi: 10.1177/1753425916648223
18. Tsiplakou E, Pitino R, Manuelian CL, Simoni M, Mitsiopoulou C, De Marchi M, et al. Plant feed additives as natural alternatives to the use of synthetic antioxidant vitamins in livestock animal products yield, quality, and oxidative status: A review. Antioxidants (2021) 10:780. doi: 10.3390/antiox10050780
19. Jeong JS, Kim IH. Effect of probiotic bacteria-fermented medicinal plants (G ynura procumbens, R ehmannia glutinosa, S cutellaria baicalensis) as performance enhancers in growing pigs. Anim Sci J (2015) 86:603–9. doi: 10.1111/asj.12331
20. Bontempo V, Jiang XR, Cheli F, Lo Verso L, Mantovani G, Vitari F, et al. Administration of a novel plant extract product via drinking water to post-weaning piglets: effects on performance and gut health. Animal (2014) 8:721–30. doi: 10.1017/S175173111400041X
21. Tang X, Xiong K, Fang R, Li M. Weaning stress and intestinal health of piglets: A review. Front Immunol (2022) 13:1042778. doi: 10.3389/fimmu.2022.1042778
22. Ding D, Mou D, Zhao L, Jiang X, Che L, Fang Z, et al. Maternal organic selenium supplementation alleviates LPS induced inflammation, autophagy and ER stress in the thymus and spleen of offspring piglets by improving the expression of selenoproteins. Food Funct (2021) 12:11214–28. doi: 10.1039/d1fo01653a
23. Zhang X, Zhang L, Xia K, Dai J, Huang J, Wang Y, et al. Effects of dietary selenium on immune function of spleen in mice. J Funct Foods (2022) 89:104914. doi: 10.1016/j.jff.2021.104914
24. Chen J, Hu N, Mao Y, Hu A, Jiang W, Huang A, et al. Traditional Chinese medicine prescriptions (XJZ, JSS) ameliorate spleen inflammatory response and antioxidant capacity by synergistically regulating NF-kappaB and Nrf2 signaling pathways in piglets. Front Vet Sci (2022) 9:993018. doi: 10.3389/fvets.2022.993018
25. Lauridsen C. Effects of dietary fatty acids on gut health and function of pigs pre- and post-weaning. J Anim Sci (2020) 98:skaa086. doi: 10.1093/jas/skaa086
26. Brodin P. Immune-microbe interactions early in life: A determinant of health and disease long term. Science (2022) 376:945–50. doi: 10.1126/science.abk2189
27. Luise D, Lauridsen C, Bosi P, Trevisi P. Methodology and application of Escherichia coli F4 and F18 encoding infection models in post-weaning pigs. J Anim Sci Biotechnol (2019) 10:53. doi: 10.1186/s40104-019-0352-7
28. Quesnel H, Farmer C. Review: nutritional and endocrine control of colostrogenesis in swine. Animal (2019) 13:s26–34. doi: 10.1017/S1751731118003555
29. Pierzynowska K, Woliński J, Weström B, Pierzynowski SG. Maternal immunoglobulins in infants—are they more than just a form of passive immunity? Front Immunol (2020) 11:855. doi: 10.3389/fimmu.2020.00855
30. Xing Y, Wu Y, Mao C, Sun D, Guo S, Xu Y, et al. Water extract of Artemisia ordosica enhances antioxidant capability and immune response without affecting growth performance in weanling piglets. J Anim Physiol Anim Nutr (2019) 103:1848–56. doi: 10.1111/jpn.13171
31. Zhang J, Zhu S, Ma N, Johnston LJ, Wu C, Ma X. Metabolites of microbiota response to tryptophan and intestinal mucosal immunity: A therapeutic target to control intestinal inflammation. Med Res Rev (2021) 41:1061–88. doi: 10.1002/med.21752
32. Chen J, Xu XW, Kang JX, Zhao BC, Xu YR, Li JL. Metasilicate-based alkaline mineral water confers diarrhea resistance in maternally separated piglets via the microbiota-gut interaction. Pharmacol Res (2023) 187:106580. doi: 10.1016/j.phrs.2022.106580
33. Zhang Y, Takagi N, Yuan B, Zhou Y, Si N, Wang H, et al. The protection of indolealkylamines from LPS-induced inflammation in zebrafish. J Ethnopharmacol (2019) 243:112122. doi: 10.1016/j.jep.2019.112122
34. Wang S, Tang S, Chen X, Li X, Jiang S, Li H-p, et al. Pulchinenoside B4 exerts the protective effects against cisplatin-induced nephrotoxicity through NF-κB and MAPK mediated apoptosis signaling pathways in mice. Chemico-Biological Interact (2020) 331:109233. doi: 10.1016/j.cbi.2020.109233
35. Zhong M, Yan Y, Yuan H, Rong A, Xu G, Cai F, et al. Astragalus mongholicus polysaccharides ameliorate hepatic lipid accumulation and inflammation as well as modulate gut microbiota in NAFLD rats. Food Funct (2022) 13:7287–301. doi: 10.1039/D2FO01009G
36. Le HG, Kang JM, Vo TC, Na BK. Naegleria fowleri Cathepsin B Induces a Pro-Inflammatory Immune Response in BV-2 Microglial Cells via NF-kappaB and AP-1 Dependent-MAPK Signaling Pathway. Int J Mol Sci (2022) 23:8388. doi: 10.3390/ijms23158388
37. Ramakrishnan SK, Zhang H, Ma X, Jung I, Schwartz AJ, Triner D, et al. Intestinal non-canonical NFkappaB signaling shapes the local and systemic immune response. Nat Commun (2019) 10:660. doi: 10.1038/s41467-019-08581-8
38. Xiao C, Zhang L, Zhang B, Kong L, Pan X, Goossens T, et al. Dietary sodium butyrate improves female broiler breeder performance and offspring immune function by enhancing maternal intestinal barrier and microbiota. Poult Sci (2023) 102:102658. doi: 10.1016/j.psj.2023.102658
39. Casas GA, Blavi L, Cross TL, Lee AH, Swanson KS, Stein HH. Inclusion of the direct-fed microbial Clostridium butyricum in diets for weanling pigs increases growth performance and tends to increase villus height and crypt depth, but does not change intestinal microbial abundance. J Anim Sci (2020) 98. doi: 10.1093/jas/skz372
40. Muns R, Magowan E. The effect of creep feed intake and starter diet allowance on piglets' gut structure and growth performance after weaning. J Anim Sci (2018) 96:3815–23. doi: 10.1093/jas/sky239
41. Ge P, Luo Y, Okoye CS, Chen H, Liu J, Zhang G, et al. Intestinal barrier damage, systemic inflammatory response syndrome, and acute lung injury: a troublesome trio for acute pancreatitis. BioMed Pharmacother (2020) 132:110770. doi: 10.1016/j.biopha.2020.110770
42. Yang CM, Han QJ, Wang KL, Xu YL, Lan JH. Astragalus and ginseng polysaccharides improve developmental, intestinal morphological, and immune functional characters of weaned piglets. Front Physiol (2019) 10:418. doi: 10.3389/fphys.2019.00418
43. Dehau T, Cherlet M, Croubels S, van Immerseel F, Goossens E. A high dose of dietary berberine improves gut wall morphology, despite an expansion of enterobacteriaceae and a reduction in beneficial microbiota in broiler chickens. mSystems (2023) 8:e0123922. doi: 10.1128/msystems.01239-22
44. Vigors S, O'Doherty JV, Rattigan R, McDonnell MJ, Rajauria G, Sweeney T. Effect of a laminarin rich macroalgal extract on the caecal and colonic microbiota in the post-weaned pig. Mar Drugs (2020) 18:157. doi: 10.3390/md18030157
45. Wang K, Cao G, Zhang H, Li Q, Yang C. Effects of Clostridium butyricum and Enterococcus faecalis on growth performance, immune function, intestinal morphology, volatile fatty acids, and intestinal flora in a piglet model. Food Funct (2019) 10:7844–54. doi: 10.1039/c9fo01650c
46. Tang S, Liu W, Zhao Q, Li K, Zhu J, Yao W, et al. Combination of polysaccharides from Astragalus membranaceus and Codonopsis pilosula ameliorated mice colitis and underlying mechanisms. J Ethnopharmacol (2021) 264:113280. doi: 10.1016/j.jep.2020.113280
47. Yan S, Chang J, Hao X, Liu J, Tan X, Geng Z, et al. Berberine regulates short-chain fatty acid metabolism and alleviates the colitis-associated colorectal tumorigenesis through remodeling intestinal flora. Phytomedicine (2022) 102:154217. doi: 10.1016/j.phymed.2022.154217
48. Luo Z, Xu W, Zhang Y, Di L, Shan J. A review of saponin intervention in metabolic syndrome suggests further study on intestinal microbiota. Pharmacol Res (2020) 160:105088. doi: 10.1016/j.phrs.2020.105088
49. Yue SJ, Wang WX, Zhang L, Liu J, Feng WW, Gao H, et al. Anti-obesity and gut microbiota modulation effect of astragalus polysaccharides combined with berberine on high-fat diet-fed obese mice. Chin J Integr Med (2023) 29:617–25. doi: 10.1007/s11655-021-3303-z
50. Ansaldo E, Farley TK, Belkaid Y. Control of immunity by the microbiota. Annu Rev Immunol (2021) 39:449–79. doi: 10.1146/annurev-immunol-093019-112348
51. Castonguay-Paradis S, Lacroix S, Rochefort G, Parent L, Perron J, Martin C, et al. Dietary fatty acid intake and gut microbiota determine circulating endocannabinoidome signaling beyond the effect of body fat. Sci Rep (2020) 10:15975. doi: 10.1038/s41598-020-72861-3
52. Li T, Gao X, Yan Z, Wai T-S, Yang W, Chen J, et al. Understanding the tonifying and the detoxifying properties of Chinese medicines from their impacts on gut microbiota and host metabolism: a case study with four medicinal herbs in experimental colitis rat model. Chin Med (2022) 17:1–17. doi: 10.1186/s13020-022-00673-w
53. Liu K, Zou J, Yuan R, Fan H, Hu H, Cheng Y, et al. Exploring the effect of the gut microbiome on the risk of age-related macular degeneration from the perspective of causality. Invest Ophthalmol Vis Sci (2023) 64:22. doi: 10.1167/iovs.64.7.22
54. Wei X, Tao J, Xiao S, Jiang S, Shang E, Zhu Z, et al. Xiexin Tang improves the symptom of type 2 diabetic rats by modulation of the gut microbiota. Sci Rep (2018) 8:3685. doi: 10.1038/s41598-018-22094-2
Keywords: diarrhoea, gut health, herbal medicine, immune function, weaned piglets
Citation: Xiao C, Li K, Teng C, Wei Z, Li J, Zhang S, Liu L, Lv H and Zhong R (2023) Dietary Qi-Weng-Huangbo powder enhances growth performance, diarrhoea and immune function of weaned piglets by modulating gut health and microbial profiles. Front. Immunol. 14:1342852. doi: 10.3389/fimmu.2023.1342852
Received: 22 November 2023; Accepted: 05 December 2023;
Published: 19 December 2023.
Edited by:
Yong Zhuo, Sichuan Agricultural University, ChinaReviewed by:
Yueping Chen, Nanjing Agricultural University, ChinaChunyan Fu, Shandong Academy of Agricultural Sciences, China
Hu Liu, China Agricultural University, China
Copyright © 2023 Xiao, Li, Teng, Wei, Li, Zhang, Liu, Lv and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruqing Zhong, emhvbmdydXFpbmdAY2Fhcy5jbg==; Huiyuan Lv, bHZodWl5dWFuMDUwN0AxNjMuY29t
 Chuanpi Xiao
Chuanpi Xiao Kai Li1
Kai Li1 Huiyuan Lv
Huiyuan Lv Ruqing Zhong
Ruqing Zhong