- 1Department of Abdominal Surgery, University Medical Center Ljubljana, Ljubljana, Slovenia
- 2Department of Surgery, Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia
- 3Department of Experimental Oncology, Institute of Oncology Ljubljana, Ljubljana, Slovenia
- 4Faculty of Health Sciences, University of Primorska, Izola, Slovenia
- 5Faculty of Health Sciences, University of Ljubljana, Ljubljana, Slovenia
Electrochemotherapy is a novel, locoregional therapy that is used to treat cutaneous and deep-seated tumors. The electric pulses used in electrochemotherapy increase the permeability of the cell membranes of the target lesion and thus enhance the delivery of low-permeant cytotoxic drugs to the cells, leading to their death. It has also been postulated that electrochemotherapy acts as an in situ vaccination by inducing immunogenic cell death. This in turn leads to an enhanced systemic antitumor response, which could be further exploited by immunotherapy. However, only a few clinical studies have investigated the role of combined treatment in patients with melanoma, breast cancer, hepatocellular carcinoma, and cutaneous squamous cell carcinoma. In this review, we therefore aim to review the published preclinical evidence on combined treatment and to review clinical studies that have investigated the combined role of electrochemotherapy and immunotherapy.
1 Introduction
Electrochemotherapy is an emerging, locoregional ablative therapy that uses electric pulses to increase the permeability of cell membranes to facilitate the entry of chemotherapeutic agents into cells. The most commonly used chemotherapeutic agents are bleomycin and cisplatin (1). The first clinical applications of electrochemotherapy were published in the 1990s, and with the accumulation of results from multiple studies, standard operating procedures (SOPs) were published in 2006, followed by an updated SOP for electrochemotherapy in 2018 (2, 3). Indeed, electrochemotherapy is nowadays predominantly used in the treatment of cutaneous tumors, however, several studies have been published to date on the treatment of deep-seated tumors (4–7).
In addition to electrochemotherapy, other ablation therapies are also widely used to treat various cutaneous and deep-seated malignant and benign tumors. The most common ablation therapies include cryoablation, radiofrequency ablation (RFA), microwave ablation (MWA), irreversible electroporation and electrochemotherapy as mentioned earlier (1, 8). Initially, the response to ablation therapies was attributed only to local elimination of tumor by various mechanisms of chemical and physical effects. Recently, however, it has been recognized that ablation promotes tumor antigen release, increases tumor antigenicity and accordingly triggers a systemic antitumor immune response that can then be fully exploited in combination with immune-checkpoint inhibitors (ICIs) (9).
Immunotherapy with ICIs has revolutionized cancer treatment, providing patients with significant improvements in survival and quality of life (10). Essentially, immune checkpoints (the best known are CTLA-4 and PD-1) are receptors expressed on the surface of T-cells and are responsible for negatively regulating the T-cell mediated immune response during the cancer immune-editing process. Accordingly, ICIs suppress these receptors in order to reactivate the immune response against tumor cells (11). ICIs are now approved for the treatment of numerous cancer types, including advanced melanoma, lung cancer, head and neck squamous cell carcinoma, hepatocellular carcinoma (HCC), Merkel cell carcinoma, urothelial and renal carcinoma, Hodgkin’s lymphoma, triple-negative breast cancer, cervical and endometrial carcinoma, and gastric cancer. Although ICIs are generally very effective, a relatively high percentage of patients still do not respond to treatment (11, 12). Thus, ongoing research into new treatment strategies is mandated to address this problem.
In this review article, we aim to discuss electroporation and electrochemotherapy in general, evaluate the mechanisms of the synergistic effect of combined electrochemotherapy and immunotherapy, and review the published clinical applications of combined treatments.
2 Electrochemotherapy – mechanisms of action
Electrochemotherapy is now used throughout Europe, particularly for skin tumors of various histologies. The reason for its relatively rapid translation from bench to bedside was its efficacy and, more importantly, in the early stages, the known mechanisms of action (13). The application of electric pulses in the kV range with a duration of microseconds leads to structural changes in the cell membrane that enable the transport of molecules in and out of the cells. Immediately after the application of the electric pulses to the target tissue, the changes begin to close or return to their previous organization. The application of such pulses is used in so-called reversible electroporation for the administration of chemicals, e.g. in electrochemotherapy with bleomycin or cisplatin, calcium electroporation, where the main goal of the therapies is cell death, and for the administration of nucleic acids in gene electrotransfer, where the goal is to obtain expression of therapeutic transgene. When multiple electric pulses are delivered to the tissue, this can lead to irreversible electroporation, in which severe disruption of the cell membrane leads to irreparable damage to the cell and thus to cell death, without the need for a cytotoxic drug as in electrochemotherapy (14). The drugs used in electrochemotherapy are bleomycin and cisplatin, which are hydrophilic drugs with hindered transport across the cell membrane. Therefore, electroporation of the cell membrane, i.e. the application of electric pulses, enables the transport of these two cytotoxic drugs into the cells (13). The effectiveness of cytostatics, which are widely used in the treatment of cancer, is therefore only enhanced by introducing more cytostatics into the cells of the tumors. The effect is only present in the tissue in which the electric pulses were delivered. The enhanced effect of cytostatic drugs is therefore limited to the treated area. As efficacy is potentiated in this way, the drug concentration applied is low and does not cause any systemic or local undesirable side effects (15).
As noted, the main mechanism of electrochemotherapy is the delivery of cytotoxic drugs to tumor cells exposed to the electric field (13). In addition to the cytotoxic effect on the tumor cells, electrochemotherapy also affects tumor blood vessels by electric pulses alone and the delivery of the drug to the tumor vasculature, especially to the endothelial cells (16). Essentially, the application of electric pulses leads to an immediate vasoconstriction of the tumor vessels (i.e. vascular lock effect) that lasts for several hours. This in turn is followed by a delayed vascular disrupting effect (17). The cumulative effect on tumor blood vessels is further enhanced by the addition of bleomycin (18). Electroporation also increases the permeability of the affected endothelial cells which in turn take up bleomycin and eventually die when they begin to divide. Since endothelial cells divide rapidly, the vascular disruptive effect of electrochemotherapy is rapid and the destruction of the tumor vasculature resulting in a permanent reduction in blood flow to the tumor becomes pronounced on the order of hours and days. Moreover, it has been shown that electrochemotherapy has a selective vascular disrupting effect on tumor vasculature, without affecting normal blood vessels which surround the tumor (18–20).
The third, very important mechanism is the induction of the immune response by inducing immunogenic tumor cell death, which can trigger the local immune response (21). The first studies have shown that some immune response is induced after electrochemotherapy of tumors in mice (22). The study on sarcoma-bearing mice then tested whether the tumor response depends on the presence of the immune response. The study showed that the tumor response was lower in immunodeficient mice and, more importantly, that the absence of the immune response abrogated the complete tumor response. This suggests that an immune response must be present for complete eradication of tumors and that electrochemotherapy itself must play some role in eliciting the response (23). In addition, a comparison of tumor responses according to differences in tumor immunogenicity showed that more immunogenic tumors responded better than less immunogenic tumors (24).
As the importance of immunogenic cell death in cancer therapy has been recognized (25), the role of electrochemotherapy as well as other ablative therapies in eliciting immunogenic cell death leading to a local and systemic immune response has been investigated (26). Various ablative therapies such as ionizing radiation, RFA, cryotherapy, and irreversible electroporation, have been found to induce a local immune response by triggering immunogenic cell death (27). This has also prompted researchers to investigate immunogenic cell death by electroporation itself and specifically by electrochemotherapy (28, 29). Essentially, immunogenic cell death is characterized by the release of damage-associated molecular patterns molecules (DAMPs). These molecules are normally present intracellularly and cannot penetrate cell membranes. However, when they are released from cells (e.g. in trauma or other states of increased cell permeability), they can boost innate and adaptive immune response by activating other cells involved in the immune response (30). In addition, other cytokines, chemokines and inflammatory markers are often released along with DAMPs (31). In the early studies on electroporation, DAMPs (especially ATP) were used as indicators of successful permeabilization of the cell membrane (32). Therefore, in a recent study, Polajzer et al. investigated if and when specific DAMPs (i.e. ATP, calreticulin, nucleic acids and uric acids) are released as a consequence of electroporation itself. They showed that the release of DAMPs increased with increasing pulse amplitude, while the concentration of most DAMPs correlated strongly with cell death, suggesting that DAMPs may serve as markers for the prediction of cell death (28).
In our study by Ursic et al. (29), we found that several parameters indicated that the immune response was triggered by electrochemotherapy with either bleomycin, cisplatin or carboplatin. It was found that there were differences in the activation of the immune response between the tumors and the drugs used for electrochemotherapy. In particular, electrochemotherapy was more effective when the tumors were more immunogenic (CT 26 murine colorectal carcinoma) than when they were less immunogenic (4T1 murine mammary carcinoma and B16F10 murine melanoma). In continuation of this study, we supported these data by an in vitro assessment of immunologically important changes in tumor cells after electrochemotherapy with different inhibitory concentrations of the drugs leading to different degrees of cell death. We evaluated in murine tumor cell lines B16F10, 4T1, and CT26 whether electrochemotherapy triggered changes in immunogenic cell death DAMPs: calreticulin, ATP, high mobility group box 1 (HMGB1), and four immunologically important cellular markers: MHCI, MHC II, PD-L1 and CD40. Again, similar to the in vivo results, electrochemotherapy with all three chemotherapeutic agents tested induced DAMPs, but the induced DAMP signature was cell line and chemotherapeutic concentration specific. Similarly, electrochemotherapy altered the expression of MHC I, MHC II, PD-L1 and CD40, which was also cell line and chemotherapeutic concentration specific (33). The data obtained from in vitro and in vivo studies have shown that electrochemotherapy indeed induces immunogenic cell death and is therefore a suitable candidate for combination with ICIs (34). Nevertheless, further studies in this direction are needed, as understanding the biological factors that influence tumor response to electrochemotherapy will enable better treatment planning and combination with immunotherapy (35).
3 Intersection of electrochemotherapy and immunotherapy
After the initial preclinical studies, an alliance between electrochemotherapy and immunotherapy was expected. We postulated that electrochemotherapy can be considered an in situ vaccination inducing immunogenic cell death for adjuvant immunotherapy with ICIs (Figure 1) (36). By definition, in situ vaccination refers to any approach which exploits tumor associated antigens (TAAs) in the vicinity of the tumor to induce a TAA-specific systemic adaptive immune response (37). Specifically, the vaccination effect of electrochemotherapy was demonstrated in a study by Calvet et al. (21). In their study, CT26 murine colon cancer cells were initially successfully treated in vitro with electrochemotherapy with bleomycin resulting in their immunogenic cell death characterized by the release of DAMPs. Subsequently, injection of dying electrochemotherapy-treated cells into an immunocompetent mouse elicited an immune response that ultimately prevented the growth of viable cancer cells in the treated mouse. In their review article, CY Calvet and LM Mir pointed out the possible combination of electrochemotherapy with ICIs, and we have postulated that gene electrotransfer of a plasmid encoding the cytokine interleukin 12 (IL-12) may also be effective in combination with electrochemotherapy (29, 38). Both approaches were subsequently tested in several preclinical studies (34).
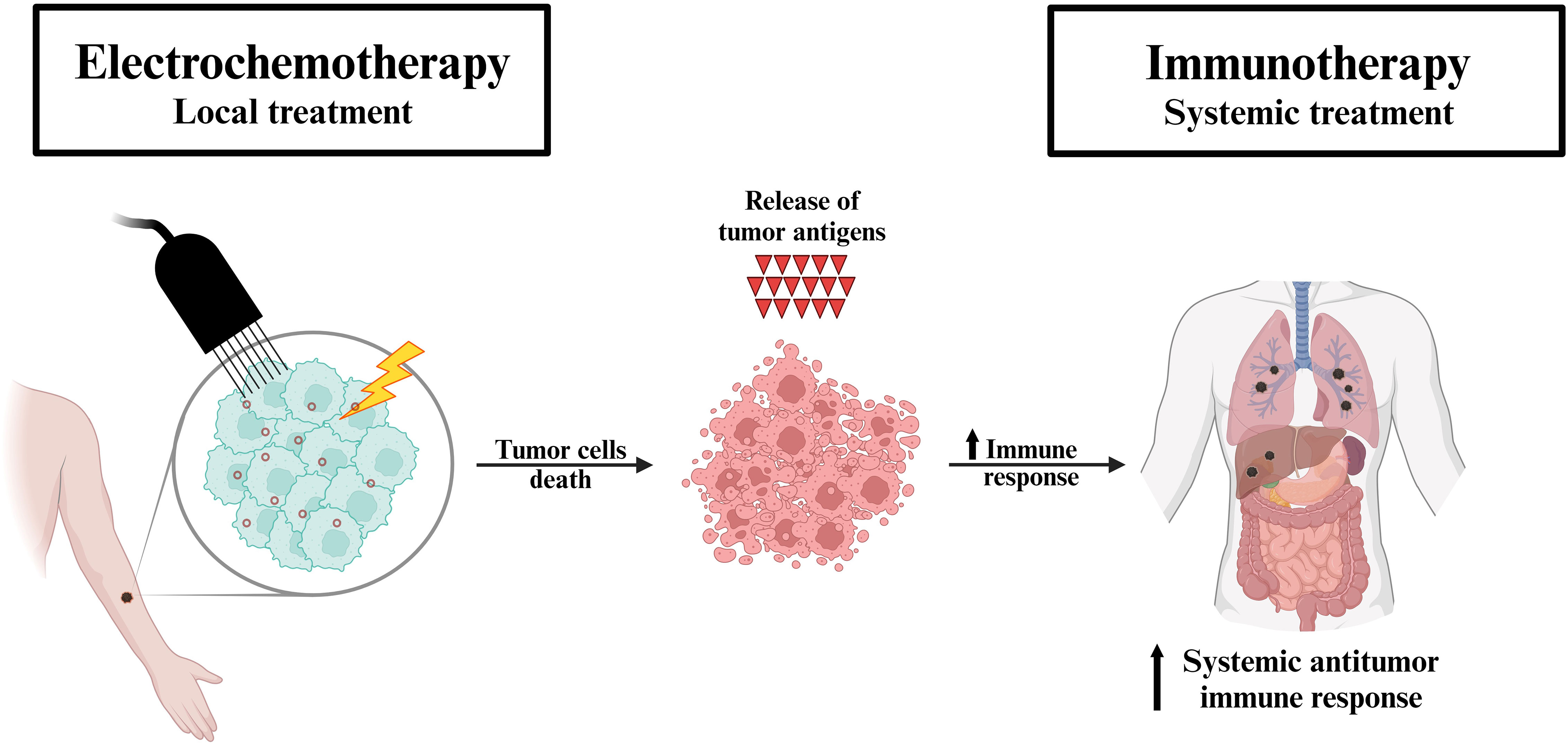
Figure 1 Proposed mechanism of the synergistic effect of electrochemotherapy and immunotherapy. Electrochemotherapy (local treatment) induces the immune system through immunogenic cell death (i.e. in situ vaccination). The tumor antigens released by the destroyed cells trigger a systemic antitumor immune response, which in turn is further exploited by immunotherapy (systemic treatment) in patients with metastatic disease.
3.1 Animal studies
The combination of electrochemotherapy and IL-12 immunotherapy using gene electrotransfer has been shown to be effective in the treatment of mouse tumors. The study provided evidence that adjuvant gene electrotransfer of the IL-12 plasmid can improve tumor response to electrochemotherapy. This was particularly evident in less immunogenic tumors such as B16F10 melanoma, where the efficacy of electrochemotherapy was lower than in more immunogenic tumors, such as CT26 colon carcinoma. In contrast, in more immunogenic tumors, the efficacy of electrochemotherapy was more pronounced, and the contribution of adjuvant IL-12 therapy was less noticeable (29). In veterinary oncology, different routes of administration for IL-12 gene transfer (peritumoral vs. intratumoral) have been investigated in clinical studies combining electrochemotherapy and immunotherapy with IL-12 (39). In a recent clinical trial in canine mastocytoma tumors, we showed that the same technology, i.e. electroporation, allows simultaneous delivery of drugs and genes to the tumors and produces a better antitumor response than when electrochemotherapy and peritumoral IL-12 gene electrotransfer were subsequently administered. Tumor control and survival of dogs after combined concomitant electrochemotherapy and IL-12 gene electrotransfer were improved and prolonged, respectively (40). In addition, a study on sarcoma bearing mice showed that adjuvant intramuscular mIL-12 application after initial electrochemotherapy is dose dependent and dependent on the amount of IL-12 in the system, achieving better results in immunocompetent mice (24).
To translate this therapeutic approach to human oncology, we recently conducted a phase I clinical trial testing IL-12-encoding plasmid gene electrotransfer to basal cell carcinoma tumors. The study protocol has been published (41), the study has now been completed and the results are currently being analyzed. In further clinical trials, we intend to combine electrochemotherapy with IL-12 gene electrotransfer to investigate a possible interaction and exploitation of the in situ vaccination effect of electrochemotherapy. The interaction of electrochemotherapy with the ICI pembrolizumab has already been demonstrated in melanoma patients, as further described in this manuscript. The combination was not tested in the framework of a designed clinical trial with a defined treatment schedule. Electrochemotherapy was added concomitantly or adjuvant to the treatment with pembrolizumab for the treatment of cutaneous metastases (42).
4 Electrochemotherapy in clinical practice
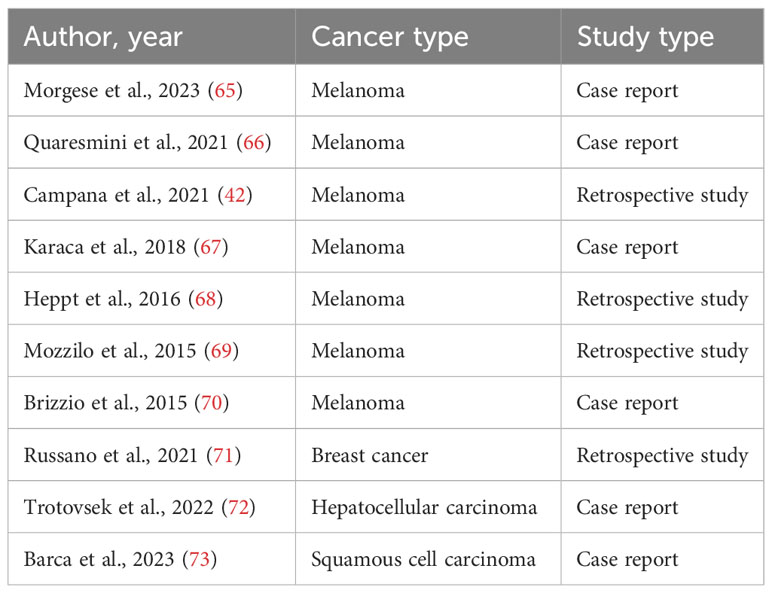
Electrochemotherapy has been recognized as safe and effective treatment method for various types of cancer (7). However, in this section we will briefly discuss the clinical evidence with electrochemotherapy alone in the treatment of melanoma, breast cancer, HCC, and cutaneous squamous cell carcinoma (SCC), as the combined treatment approaches with electrochemotherapy and immunotherapy have only been published for the cancers to date.
4.1 Melanoma
Superficially metastatic melanoma is one of the most common indications for the treatment with electrochemotherapy (7). The first clinical studies of electrochemotherapy in the treatment of malignant melanoma were published almost 30 years ago. In 1995, our group published the first experiences with electrochemotherapy with intravenous bleomycin in melanoma patients. A complete response was achieved in 22 out of 24 treated nodules (43). Since then, many clinical studies have investigated the efficacy of electrochemotherapy in melanoma patients (44). Literature review published in 2019 included 9 case series of electrochemotherapy in the treatment of melanoma that were carried out after the publication of the ESOPE guidelines. The complete response rate ranged from 20% to 50%, while the objective response rate ranged from 60% to 100%. Electrochemotherapy had a low toxicity profile and few minor side effects, mostly in the form of local pain, erythema, and ulceration (4). Furthermore, it was shown that multiple applications of electrochemotherapy with intravenously injected bleomycin can lead to regression of even untreated skin melanoma metastases in transit. In four consecutive treatment sessions, 224 tumor nodules were treated. Although not all metastases were treated, even the untreated metastases did not progress after 9 months, suggesting an induction of locoregional or even systemic immune response (45). In addition, a recent analysis by the pan-European International Network for Sharing Practice in Electrochemotherapy (InspECT) reported an overall response rate of 82% and complete response rate of 64% in patients with melanoma. In contrast to previously published studies, they included prospectively uploaded data from 28 European centers that regularly use electrochemotherapy (46). The results of several published studies demonstrating the high efficacy, safety, and limited toxicity of electrochemotherapy have led to its inclusion in the most recent ESMO melanoma guidelines (47).
4.2 Breast cancer
Breast cancer is the most common neoplasm and the leading cause of cancer death in women worldwide (48). It is also the most common malignancy to metastasize to skin in women. Small single metastases can be removed surgically, while larger or multiple lesions are more challenging to treat (49, 50). For breast cancer patients who present with skin metastases, one of the treatment options is also electrochemotherapy. It is a safe and effective treatment to manage such lesions. The advantage of electrochemotherapy is that it can be performed in previously irradiated areas and can be repeated multiple times. Additionally, other systemic therapies can be concomitantly applied. The first report describing the effectiveness of electrochemotherapy in breast cancer patients was published in 1996. In a phase I/II trial, cutaneous and subcutaneous tumors were treated with electrochemotherapy. Among those patients, one metastatic breast adenocarcinoma patient, with two nodules, was also included. Both nodules showed complete responses after the treatment (51). Since then several studies have demonstrated a high response rate in providing local tumor control, with an overall response rate up to 60 - 80% and a complete response rate up to 60% (46, 52, 53).
There are various histologic types of breast cancer that differ in microscopic appearance and receptor expression, leading to differences in response to the treatment. Recently, a multicentric study investigating the efficacy of electrochemotherapy in breast cancer patients with different receptor statuses was published (54). The study demonstrated that electrochemotherapy is equally effective in the treatment of breast cancer metastases, regardless of receptor status. The response and local tumor control were better in multiple smaller lesions than in larger lesions, as previously observed. Local progression-free survival was, however, significantly lower in triple-negative type, probably due to the more aggressive cancer type and smaller choice of systemic treatments (54).
4.3 Hepatocellular carcinoma
A pilot study investigating the role of electrochemotherapy in the treatment of HCC was published in 2018 (55). The study included patients with unresectable HCCs located near large blood vessels where other ablative therapies are not efficient due to the heat sink effect. Ultimately, 10 patients with 17 lesions were enrolled in the study. Electrochemotherapy with bleomycin was performed in the setting of open surgery. The treatment proved to be feasible, safe, and effective, as a complete response was achieved in 88% of treated lesions (55). The promising results were confirmed in the subsequent phase II study, which enrolled 24 patients with 32 lesions. The complete response rate and partial response rate per treated lesion were 84% and 12.5%, respectively. Again, the treatment proved safe, as no major postoperative complications were observed. Only 16% of patients developed ascites due to transient liver dysfunction, which resolved spontaneously or with diuretic therapy (56).
4.4 Cutaneous squamous cell carcinoma
The first clinical study on electrochemotherapy in the treatment of SCC was published by Mir et al. in 1991 (57). Since then, several studies have investigated the efficacy of electrochemotherapy in the treatment of cutaneous SCC. A prospective European EURECA study of 47 patients with cutaneous SCC showed a 55% complete response rate at 2-month follow-up after electrochemotherapy. In addition, 24% of patients achieved a partial response rate, 15% had stable disease and only 4% experienced progression during treatment (58). In 2017, a retrospective study of 22 patients with cutaneous SCC treated with electrochemotherapy showed a complete response rate of 22% and a partial response rate of 59%, thus achieving a similar objective response rate to the EURECA study (59). Furthermore, a recent analysis of the InspECT registry included 162 patients with cutaneous SCC. The complete and partial response rates were 62% and 21% respectively. Better results were achieved in patients with primary and smaller (< 3 cm) tumors using intravenously administered bleomycin (60).
5 Combining electrochemotherapy and immunotherapy in clinical practice
Immunotherapy with ICIs has attracted substantial and broad interest since 2011, when the first ICI drug was approved by the FDA (61). Between 2015 and 2017, the number of trials with PD-1 and PD-L1 inhibitors increased from 215 to more than 1500 trials (62). A recent cross-sectional study estimated that 38.5% of cancer patients in the United States are eligible for ICI therapy (63). Electrochemotherapy is used in more than 180 centers around the world, mostly for the treatment of cutaneous tumors due to their accessibility (64). Thus, we provide an overview of cancers for which clinical reports of combined treatment with electrochemotherapy and immunotherapy with ICIs have been published. The studies are summarized in Table 1 and explained in the following text.
5.1 Melanoma
The first case of a combined approach using electrochemotherapy and ICIs for melanoma was published in 2015. Initially, a patient with multiple cutaneous melanoma metastases had a partial response after 3 cycles of electrochemotherapy. Subsequently, a complete response was observed in all lesions after treatment with ipilimumab (70). Similarly, a 2016 retrospective study suggested that combined treatment with ipilimumab followed by electrochemotherapy was feasible and resulted in an increased therapeutic response of skin lesions. Thus, a local objective response was observed in 67%, while a systemic response was observed in 33% of patients with metastatic melanoma. Furthermore, T-regulatory cell counts decreased in all responders and were significantly lower than in non-responders at 3 months (69). Another retrospective study investigated the role of combined electrochemotherapy and immunotherapy with ipilimumab or PD-1 inhibitors. Authors reported objective local and systemic response rates of 67% and 22%, respectively. However, severe systemic adverse effects were observed in 25% of patients receiving ipilimumab (68). In a recent case report, a patient with metastatic melanoma progressed during the treatment with pembrolizumab with scalp metastases. These were treated with two cycles of electrochemotherapy with bleomycin, achieving partial response. Nearly complete response was eventually obtained after the treatment with ipilimumab and was present at 6-months follow-up (65). Two other case reports also demonstrated beneficial effects of combined treatment with electrochemotherapy and ICIs (66, 67).
The largest retrospective study to date investigating the combined role of electrochemotherapy and immunotherapy was published in 2021 by Campana et al. Furthermore, outcomes of patients who received pembrolizumab alone or in combination with electrochemotherapy were compared. A higher local objective response rate, longer 1-year progression-free survival, and longer overall survival in patients who received combined treatment were observed, while no serious adverse events were reported (42). In summary, several studies have demonstrated the beneficial effects of combined treatment with electrochemotherapy and ICIs for the treatment of advanced metastatic melanoma. However, these studies could not prove but could only indicate the in situ vaccination effect of electrochemotherapy, which enhances the systemic response to ICIs. Therefore, further translational studies are needed to investigate this effect (35). In addition, randomized controlled trials with larger numbers of patients are warranted to compare the efficacy of combined electrochemotherapy and immunotherapy with immunotherapy alone. Currently, a phase 2 multicenter, non-randomized study is enrolling patients with metastatic melanoma to determine whether concurrent treatment with pembrolizumab and electrochemotherapy is safe and can improve local and systemic response rates (ClinicalTrials.gov; ID: NCT03448666).
5.2 Breast cancer
To date, only one report has demonstrated the efficacy of electrochemotherapy combined with immunotherapy in breast cancer patients (71). In that study, 55 metastatic breast cancer patients were treated with electrochemotherapy 78 months after the primary breast cancer diagnosis (range 14–441 months). At the time of electrochemotherapy, 27% of patients did not receive any concomitant therapy, 27% received chemotherapy, 25% hormonal therapy, 5% immunotherapy, and 14% combined therapy (5 chemotherapy + immunotherapy, 5% hormonal therapy + immunotherapy). Overall, a complete response rate was observed in 64% of patients, while 22% of patients had a partial response and 14% had stable disease. Regarding the concomitant treatment, the efficacy of electrochemotherapy combined with immunotherapy was almost 100%, while when electrochemotherapy was combined with chemotherapy the complete response was lower (67%). The progression-free survival and overall survival at 24 months were higher in patients who were treated with electrochemotherapy combined with immunotherapy compared to other treatments. Unfortunately, at 36 months this benefit was no longer observed. The study showed the efficacy of electrochemotherapy combined with immunotherapy in providing a local tumor control rate, as well as short-term progression-free and overall survival benefits. However, it should be noted that only three patients in this study received combined treatment with electrochemotherapy and immunotherapy, therefore larger studies are needed to examine the combined treatment in breast cancer patients (71).
5.3 Hepatocellular carcinoma
To our knowledge, only one case of combined treatment with electrochemotherapy and immunotherapy in a patient with HCC has been published (72). A 43-year-old patient with multifocal HCC in both lobes of the liver and cirrhosis was initially treated with 24 cycles of bevacizumab and atezolizumab. Subsequently, electrochemotherapy with bleomycin was used for the remaining two lesions in segment 3 of the liver, and a complete response was observed 3 months after treatment. However, because the patient had only two lesions at the time of electrochemotherapy and the follow-up period was relatively short, the synergistic role of both treatments could not be fully determined (72). Therefore, further studies are needed to investigate the combined role of electrochemotherapy and immunotherapy for HCC (74).
5.4 Cutaneous squamous cell carcinoma
Recently, Barca et al. have published a case of combined treatment of electrochemotherapy and immunotherapy in a patient with advance-stage cutaneous SCC (73). An 80-year-old patient with a large, bleeding, frontotemporal cutaneous SCC was initially treated with two cycles of electrochemotherapy. As early disease progression was detected, the patient underwent experimental treatment with 24 cycles of cemiplimab followed by a further two cycles of electrochemotherapy. Eventually, remission of the disease on the skin side was demonstrated with multiple incisional biopsies. Unfortunately, the disease progressed to the orbital cavity and patient died after four months of combined treatment with electrochemotherapy and immunotherapy. The authors concluded that the combined treatment was effective in both controlling disease progression and alleviating patient’s symptoms.
6 Conclusions
Electrochemotherapy is a highly effective, locoregional, ablative therapy that is predominantly used for the treatment of cutaneous tumors and, more recently, for the treatment of deep-seated tumors. Electric pulses applied to the target lesion temporarily increase the permeability of the cell membrane and subsequently enhance the delivery of low-permeant cytotoxic drugs to the tumor cells, leading to their death. In addition, electrochemotherapy could be considered an in situ vaccination, as it induces an immune response through immunogenic cell death, which in turn can trigger the efficacy of immunotherapy. The clinical evidence for combined treatment with electrochemotherapy and immunotherapy is still lacking. To date, only a few retrospective studies on patients with melanoma and breast cancer and a case reports on a patient with HCC and cutaneous SCC have shown a beneficial role of the combined treatment in the short term.
In summary, combined treatment with electrochemotherapy and immunotherapy could improve local tumor control and boost the systemic antitumor response. Moreover, electrochemotherapy as a form of in situ vaccination could also broaden the applicability of immunotherapy in different cancer types. Nevertheless, further randomized controlled trials with a larger number of patients and a longer follow-up period are warranted to better investigate the (positive) role of the combined treatment. Furthermore, additional preclinical and translational studies are needed to better explain the underlying mechanism of the combined treatment.
Author contributions
BH: Conceptualization, Writing – original draft. MO: Writing – original draft. BT: Writing – review & editing. MC: Conceptualization, Supervision, Writing – review & editing. TJ: Writing – review & editing. GS: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. MD: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Slovenian Research and Innovation Agency (Program no. P3-0003).
Acknowledgments
The authors thank Uros Jedlovcnik for his help with graphic design. Figure was created with BioRender.com (accessed on 10/11/2023). Language editing services for this manuscript were provided by company American Journal Experts.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cemazar M, Sersa G. Recent advances in electrochemotherapy. Bioelectricity (2019) 1:204–13. doi: 10.1089/bioe.2019.0028
2. Marty M, Sersa G, Garbay JR, Gehl J, Collins CG, Snoj M, et al. Electrochemotherapy – An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur J Cancer Suppl (2006) 4:3–13. doi: 10.1016/j.ejcsup.2006.08.002
3. Gehl J, Sersa G, Matthiessen LW, Muir T, Soden D, Occhini A, et al. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol (2018) 57:874–82. doi: 10.1080/0284186X.2018.1454602
4. Campana LG, Miklavčič D, Bertino G, Marconato R, Valpione S, Imarisio I, et al. Electrochemotherapy of superficial tumors – Current status: Basic principles, operating procedures, shared indications, and emerging applications. Semin Oncol (2019) 46:173–91. doi: 10.1053/j.seminoncol.2019.04.002
5. Edhemovic I, Brecelj E, Cemazar M, Boc N, Trotovsek B, Djokic M, et al. Intraoperative electrochemotherapy of colorectal liver metastases: A prospective phase II study. Eur J Surg Oncol (2020) 46:1628–33. doi: 10.1016/j.ejso.2020.04.037
6. Schipilliti FM, Onorato M, Arrivi G, Panebianco M, Lerinò D, Milano A, et al. Electrochemotherapy for solid tumors: literature review and presentation of a novel endoscopic approach. Radiol Oncol (2022) 56:285–91. doi: 10.2478/raon-2022-0022
7. Campana LG, Daud A, Lancellotti F, Arroyo JP, Davalos RV, Di Prata C, et al. Pulsed electric fields in oncology: A snapshot of current clinical practices and research directions from the 4th world congress of electroporation. Cancers (Basel) (2023) 15:3340. doi: 10.3390/cancers15133340
8. Debela DT, Muzazu SG, Heraro KD, Ndalama MT, Mesele BW, Haile DC, et al. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med (2021) 9:20503121211034370. doi: 10.1177/20503121211034366
9. Yin L, Li XY, Zhu LL, Chen GL, Xiang Z, Wang QQ, et al. Clinical application status and prospect of the combined anti-tumor strategy of ablation and immunotherapy. Front Immunol (2022) 13:965120. doi: 10.3389/fimmu.2022.965120
10. Esfahani K, Roudaia L, Buhlaiga N, Del Rincon SV, Papneja N, Miller WHJ. A review of cancer immunotherapy: from the past, to the present, to the future. Curr Oncol (2020) 27:S87–97. doi: 10.3747/co.27.5223
11. Shiravand Y, Khodadadi F, Kashani SMA, Hosseini-Fard SR, Hosseini S, Sadeghirad H, et al. Immune checkpoint inhibitors in cancer therapy. Curr Oncol (2022) 29:3044–60. doi: 10.3390/curroncol29050247
12. O’Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol (2019) 16:151–67. doi: 10.1038/s41571-018-0142-8
13. Miklavčič D, Mali B, Kos B, Heller R, Serša G. Electrochemotherapy: from the drawing board into medical practice. BioMed Eng Online (2014) 13:29. doi: 10.1186/1475-925X-13-29
14. Batista Napotnik T, Polajžer T, Miklavčič D. Cell death due to electroporation - A review. Bioelectrochemistry (2021) 141:107871. doi: 10.1016/j.bioelechem.2021.107871
15. Sersa G, Bosnjak M, Cemazar M, Heller R. Preclinical studies on electrochemotherapy. In: Miklavcic D, editor. Handbook of electroporation. Cham: Springer International Publishing (2016) p. 1511–25. doi: 10.1007/978-3-319-26779-1_45-1
16. Cemazar M, Parkins CS, Holder AL, Chaplin DJ, Tozer GM, Sersa G. Electroporation of human microvascular endothelial cells: evidence for an anti-vascular mechanism of electrochemotherapy. Br J Cancer (2001) 84:565–70. doi: 10.1054/bjoc.2000.1625
17. Groselj A, Kranjc S, Bosnjak M, Krzan M, Kosjek T, Prevc A, et al. Vascularization of the tumours affects the pharmacokinetics of bleomycin and the effectiveness of electrochemotherapy. Basic Clin Pharmacol Toxicol (2018) 123:247–56. doi: 10.1111/bcpt.13012
18. Markelc B, Sersa G, Cemazar M. Differential mechanisms associated with vascular disrupting action of electrochemotherapy: intravital microscopy on the level of single normal and tumor blood vessels. PloS One (2013) 8:e59557. doi: 10.1371/journal.pone.0059557
19. Jarm T, Cemazar M, Miklavcic D, Sersa G. Antivascular effects of electrochemotherapy: implications in treatment of bleeding metastases. Expert Rev Anticancer Ther (2010) 10:729–46. doi: 10.1586/era.10.43
20. Kanthou C, Kranjc S, Sersa G, Tozer G, Zupanic A, Cemazar M. The endothelial cytoskeleton as a target of electroporation-based therapies. Mol Cancer Ther (2006) 5:3145–52. doi: 10.1158/1535-7163.MCT-06-0410
21. Calvet CY, Famin D, André FM, Mir LM. Electrochemotherapy with bleomycin induces hallmarks of immunogenic cell death in murine colon cancer cells. Oncoimmunology (2014) 3:e28131. doi: 10.4161/onci.28131
22. Sersa G, Kotnik V, Cemazar M, Miklavcic D, Kotnik A. Electrochemotherapy with bleomycin in SA-1 tumor-bearing mice–natural resistance and immune responsiveness. Anticancer Drugs (1996) 7:785–91. doi: 10.1097/00001813-199609000-00011
23. Serša G, Miklavčič D, Čemažar M, Belehradek J, Jarm T, Mir LM. Electrochemotherapy with CDDP on LPB sarcoma: comparison of the anti-tumor effectiveness in immunocompotent and immunodeficient mice. Bioelectrochem Bioenerg (1997) 43:279–83. doi: 10.1016/S0302-4598(96)05194-X
24. Sedlar A, Dolinsek T, Markelc B, Prosen L, Kranjc S, Bosnjak M, et al. Potentiation of electrochemotherapy by intramuscular IL-12 gene electrotransfer in murine sarcoma and carcinoma with different immunogenicity. Radiol Oncol (2012) 46:302–11. doi: 10.2478/v10019-012-0044-9
25. Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol (2013) 31:51–72. doi: 10.1146/annurev-immunol-032712-100008
26. Falk H, Lambaa S, Johannesen HH, Wooler G, Venzo A, Gehl J. Electrochemotherapy and calcium electroporation inducing a systemic immune response with local and distant remission of tumors in a patient with Malignant melanoma - a case report. Acta Oncol (2017) 56:1126–31. doi: 10.1080/0284186X.2017.1290274
27. O’Brien MA, Power DG, Clover AJP, Bird B, Soden DM, Forde PF. Local tumour ablative therapies: opportunities for maximising immune engagement and activation. Biochim Biophys Acta (2014) 1846:510–23. doi: 10.1016/j.bbcan.2014.09.005
28. Polajzer T, Jarm T, Miklavcic D. Analysis of damage-associated molecular pattern molecules due to electroporation of cells in vitro. Radiol Oncol (2020) 54:317–28. doi: 10.2478/raon-2020-0047
29. Ursic K, Kos S, Kamensek U, Cemazar M, Miceska S, Markelc B, et al. Potentiation of electrochemotherapy effectiveness by immunostimulation with IL-12 gene electrotransfer in mice is dependent on tumor immune status. J Control release (2021) 332:623–35. doi: 10.1016/j.jconrel.2021.03.009
30. Roh JS, Sohn DH. Damage-associated molecular patterns in inflammatory diseases. Immune Netw (2018) 18:e27. doi: 10.4110/in.2018.18.e27
31. Chan JK, Roth J, Oppenheim JJ, Tracey KJ, Vogl T, Feldmann M, et al. Alarmins: awaiting a clinical response. J Clin Invest (2012) 122:2711–9. doi: 10.1172/JCI62423
32. Rols MP, Teissié J. Electropermeabilization of mammalian cells. Quantitative analysis of the phenomenon. Biophys J (1990) 58:1089–98. doi: 10.1016/S0006-3495(90)82451-6
33. Kesar U, Markelc B, Jesenko T, Ursic Valentinuzzi K, Cemazar M, Strojan P, et al. Effects of electrochemotherapy on immunologically important modifications in tumor cells. Vaccines (2023) 11:925. doi: 10.3390/vaccines11050925
34. Justesen TF, Orhan A, Raskov H, Nolsoe C, Gögenur I. Electroporation and immunotherapy-unleashing the abscopal effect. Cancers (Basel) (2022) 14:2876. doi: 10.3390/cancers14122876
35. Sersa G, Ursic K, Cemazar M, Heller R, Bosnjak M, Campana LG. Biological factors of the tumour response to electrochemotherapy: Review of the evidence and a research roadmap. Eur J Surg Oncol (2021) 47:1836–46. doi: 10.1016/j.ejso.2021.03.229
36. Sersa G, Teissie J, Cemazar M, Signori E, Kamensek U, Marshall G, et al. Electrochemotherapy of tumors as in situ vaccination boosted by immunogene electrotransfer. Cancer Immunol Immunother (2015) 64:1315–27. doi: 10.1007/s00262-015-1724-2
37. Hammerich L, Binder A, Brody JD. In situ vaccination: Cancer immunotherapy both personalized and off-the-shelf. Mol Oncol (2015) 9:1966–81. doi: 10.1016/j.molonc.2015.10.016
38. Calvet CY, Mir LM. The promising alliance of anti-cancer electrochemotherapy with immunotherapy. Cancer Metastasis Rev (2016) 35:165–77. doi: 10.1007/s10555-016-9615-3
39. Mukhopadhyay A, Wright J, Shirley S, Canton DA, Burkart C, Connolly RJ, et al. Characterization of abscopal effects of intratumoral electroporation-mediated IL-12 gene therapy. Gene Ther (2019) 26:1–15. doi: 10.1038/s41434-018-0044-5
40. Lampreht Tratar U, Milevoj N, Cemazar M, Znidar K, Ursic Valentinuzzi K, Brozic A, et al. Treatment of spontaneous canine mast cell tumors by electrochemotherapy combined with IL-12 gene electrotransfer: Comparison of intratumoral and peritumoral application of IL-12. Int Immunopharmacol (2023) 120:110274. doi: 10.1016/j.intimp.2023.110274
41. Groselj A, Bosnjak M, Jesenko T, Cemazar M, Markelc B, Strojan P, et al. Treatment of skin tumors with intratumoral interleukin 12 gene electrotransfer in the head and neck region: a first-in-human clinical trial protocol. Radiol Oncol (2022) 56:398–408. doi: 10.2478/raon-2022-0021
42. Campana LG, Peric B, Mascherini M, Spina R, Kunte C, Kis E, et al. Combination of pembrolizumab with electrochemotherapy in cutaneous metastases from melanoma: A comparative retrospective study from the inspECT and Slovenian cancer registry. Cancers (Basel) (2021) 13:4289. doi: 10.3390/cancers13174289
43. Rudolf Z, Štabuc B, Čemažar M, Miklavčič D, Vodovnik L, Serša G. Electrochemotherapy with bleomycin, The first clinical experience in Malignant melanoma patients. Radiol Oncol (1995) 29:229–35.
44. Mali B, Jarm T, Snoj M, Sersa G, Miklavcic D. Antitumor effectiveness of electrochemotherapy: a systematic review and meta-analysis. Eur J Surg Oncol (2013) 39:4–16. doi: 10.1016/j.ejso.2012.08.016
45. Snoj M, Cemazar M, Slekovec Kolar B, Sersa G. Effective treatment of multiple unresectable skin melanoma metastases by electrochemotherapy. Croat Med J (2007) 48:391–5.
46. Clover AJP, de Terlizzi F, Bertino G, Curatolo P, Odili J, Campana LG, et al. Electrochemotherapy in the treatment of cutaneous Malignancy: Outcomes and subgroup analysis from the cumulative results from the pan-European International Network for Sharing Practice in Electrochemotherapy database for 2482 lesions in 987 patients. Eur J Cancer (2020) 138:30–40. doi: 10.1016/j.ejca.2020.06.020
47. Michielin O, van Akkooi A, Lorigan P, Ascierto PA, Dummer R, Robert C, et al. ESMO consensus conference recommendations on the management of locoregional melanoma: under the auspices of the ESMO Guidelines Committee. Ann Oncol (2020) 31:1449–61. doi: 10.1016/j.annonc.2020.07.005
48. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin (2019) 69:7–34. doi: 10.3322/caac.21551
49. Tan AR. Cutaneous manifestations of breast cancer. Semin Oncol (2016) 43:331–4. doi: 10.1053/j.seminoncol.2016.02.030
50. De Giorgi V, Grazzini M, Alfaioli B, Savarese I, Corciova SA, Guerriero G, et al. Cutaneous manifestations of breast carcinoma. Dermatol Ther (2010) 23:581–9. doi: 10.1111/j.1529-8019.2010.01365.x
51. Heller R, Jaroszeski MJ, Glass LF, Messina JL, Rapaport DP, DeConti RC, et al. Phase I/II trial for the treatment of cutaneous and subcutaneous tumors using electrochemotherapy. Cancer (1996) 77:964–71. doi: 10.1002/(sici)1097-0142(19960301)77:5<964::aid-cncr24>3.0.co;2-0
52. Rebersek M, Cufer T, Cemazar M, Kranjc S, Sersa G. Electrochemotherapy with cisplatin of cutaneous tumor lesions in breast cancer. Anticancer Drugs (2004) 15:593–7. doi: 10.1097/01.cad.0000132234.30674.df
53. Matthiessen LW, Keshtgar M, Curatolo P, Kunte C, Grischke EM, Odili J, et al. Electrochemotherapy for breast cancer-results from the INSPECT database. Clin Breast Cancer (2018) 18:e909–17. doi: 10.1016/j.clbc.2018.03.007
54. Di Prata C, Mascherini M, Ross AM, Silvestri B, Kis E, Odili J, et al. Efficacy of electrochemotherapy in breast cancer patients of different receptor status: the INSPECT experience. Cancers (Basel) (2023) 15:3116. doi: 10.3390/cancers15123116
55. Djokic M, Cemazar M, Popovic P, Kos B, Dezman R, Bosnjak M, et al. Electrochemotherapy as treatment option for hepatocellular carcinoma, a prospective pilot study. Eur J Surg Oncol (2018) 44:651–7. doi: 10.1016/j.ejso.2018.01.090
56. Djokic M, Cemazar M, Bosnjak M, Dezman R, Badovinac D, Miklavcic D, et al. A prospective phase II study evaluating intraoperative electrochemotherapy of hepatocellular carcinoma. Cancers (Basel) (2020) 12:3778. doi: 10.3390/cancers12123778
57. Mir LM, Belehradek M, Domenge C, Orlowski S, Poddevin B, Belehradek JJ, et al. [Electrochemotherapy, a new antitumor treatment: first clinical trial]. C R Acad Sci III (1991) 313:613–8.
58. Bertino G, Sersa G, De Terlizzi F, Occhini A, Plaschke CC, Groselj A, et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) project: Results of the treatment of skin cancer. Eur J Cancer (2016) 63:41–52. doi: 10.1016/j.ejca.2016.05.001
59. Di Monta G, Caracò C, Simeone E, Grimaldi AM, Marone U, Di Marzo M, et al. Electrochemotherapy efficacy evaluation for treatment of locally advanced stage III cutaneous squamous cell carcinoma: a 22-cases retrospective analysis. J Transl Med (2017) 15:82. doi: 10.1186/s12967-017-1186-8
60. Bertino G, Groselj A, Campana LG, Kunte C, Schepler H, Gehl J, et al. Electrochemotherapy for the treatment of cutaneous squamous cell carcinoma: The INSPECT experience (2008-2020). Front Oncol (2022) 12:951662. doi: 10.3389/fonc.2022.951662
61. Dobosz P, Dzieciątkowski T. The intriguing history of cancer immunotherapy. Front Immunol (2019) 10:2965. doi: 10.3389/fimmu.2019.02965
62. Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open (2019) 2:e192535. doi: 10.1001/jamanetworkopen.2019.2535
63. Haslam A, Gill J, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for immune checkpoint inhibitor drugs. JAMA Netw Open (2020) 3:e200423. doi: 10.1001/jamanetworkopen.2020.0423
64. Campana LG, Edhemovic I, Soden D, Perrone AM, Scarpa M, Campanacci L, et al. Electrochemotherapy - Emerging applications technical advances, new indications, combined approaches, and multi-institutional collaboration. Eur J Surg Oncol (2019) 45:92–102. doi: 10.1016/j.ejso.2018.11.023
65. Morgese F, De Feudis F, Balercia P, Berardi R. Potential dual synergy between electrochemotherapy and sequence of immunotherapies in metastatic melanoma: A case report. Mol Clin Oncol (2023) 18:8. doi: 10.3892/mco.2023.2604
66. Quaresmini D, Di Lauro A, Fucci L, Strippoli S, De Risi I, Sciacovelli AM, et al. Electrochemotherapy as a trigger to overcome primary resistance to anti-PD-1 treatment: A case report of melanoma of the scalp. Front Oncol (2021) 11:742666. doi: 10.3389/fonc.2021.742666
67. Karaca B, Yayla G, Erdem M, Gürler T. Electrochemotherapy with anti-PD-1 treatment induced durable complete response in heavily pretreated metastatic melanoma patient. Anticancer Drugs (2018) 29:190–6. doi: 10.1097/CAD.0000000000000580
68. Heppt MV, Eigentler TK, Kähler KC, Herbst RA, Göppner D, Gambichler T, et al. Immune checkpoint blockade with concurrent electrochemotherapy in advanced melanoma: a retrospective multicenter analysis. Cancer Immunol Immunother (2016) 65:951–9. doi: 10.1007/s00262-016-1856-z
69. Mozzillo N, Simeone E, Benedetto L, Curvietto M, Giannarelli D, Gentilcore G, et al. Assessing a novel immuno-oncology-based combination therapy: Ipilimumab plus electrochemotherapy. Oncoimmunology (2015) 4:e1008842. doi: 10.1080/2162402X.2015.1008842
70. Brizio M, Fava P, Astrua C, Cavaliere G, Savoia P. Complete regression of melanoma skin metastases after electrochemotherapy plus ipilimumab treatment: an unusual clinical presentation. Eur J Dermatol (2015) 25:271–2. doi: 10.1684/ejd.2015.2522
71. Russano F, Del Fiore P, Di Prata C, Pasqual A, Marconato R, Campana LG, et al. The role of electrochemotherapy in the cutaneous and subcutaneous metastases from breast cancer: analysis of predictive factors to treatment from an italian cohort of patients. Front Oncol (2021) 11:772144. doi: 10.3389/fonc.2021.772144
72. Trotovsek B, Hadzialjevic B, Cemazar M, Sersa G, Djokic M. Laparoscopic electrochemotherapy for the treatment of hepatocellular carcinoma: Technological advancement. Front Oncol (2022) 12:996269. doi: 10.3389/fonc.2022.996269
73. Barca I, Ferragina F, Kallaverja E, Cristofaro MG. Synergy of electrochemotherapy and immunotherapy in the treatment of skin squamous cell carcinoma of the head and neck. Oral Maxillofac Surg Cases (2023) 9:100330. doi: 10.1016/j.omsc.2023.100330
Keywords: cancer, electrochemotherapy, electroporation, immunotherapy, immune response, melanoma, breast cancer, hepatocellular cancer
Citation: Hadzialjevic B, Omerzel M, Trotovsek B, Cemazar M, Jesenko T, Sersa G and Djokic M (2024) Electrochemotherapy combined with immunotherapy – a promising potential in the treatment of cancer. Front. Immunol. 14:1336866. doi: 10.3389/fimmu.2023.1336866
Received: 11 November 2023; Accepted: 29 December 2023;
Published: 15 January 2024.
Edited by:
Yueyong Xiao, Chinese People’s Liberation Army General Hospital, ChinaReviewed by:
Badrinath Narayanasamy, Bionoxx Inc., Republic of KoreaSilvia D’Amico, National Research Council (CNR), Italy
Copyright © 2024 Hadzialjevic, Omerzel, Trotovsek, Cemazar, Jesenko, Sersa and Djokic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gregor Sersa, Z3NlcnNhQG9ua28taS5zaQ==; Mihajlo Djokic, bWloYWpsby5kam9raWNAa2Nsai5zaQ==
 Benjamin Hadzialjevic
Benjamin Hadzialjevic Masa Omerzel3
Masa Omerzel3 Blaz Trotovsek
Blaz Trotovsek Maja Cemazar
Maja Cemazar Tanja Jesenko
Tanja Jesenko Gregor Sersa
Gregor Sersa Mihajlo Djokic
Mihajlo Djokic