- 1Division of Immunology, Department of Biology, University of Konstanz, Konstanz, Germany
- 2Department of Dermatology, University Hospital Zürich (USZ), Zürich, Switzerland
- 3Faculty of Medicine, University of Zürich (UZH), Zürich, Switzerland
- 4Biotechnology Institute Thurgau at the University of Konstanz, Kreuzlingen, Switzerland
A Corrigendum on:
Immunoproteasome inhibition attenuates experimental psoriasis
by del Rio Oliva M, Mellett M and Basler M (2022) Front. Immunol. 13:1075615. doi: 10.3389/fimmu.2022.1075615
In the published article, there was an error in Figure 6 as published. In Figure 6B the representative microscopy images for CD3 IMQ vehicle and IMQ ONX 0914 were mistakenly switched and the representative image for IL-17A IMQ vehicle was incorrectly incorporated. The corrected Figure 6 and its caption appear below.
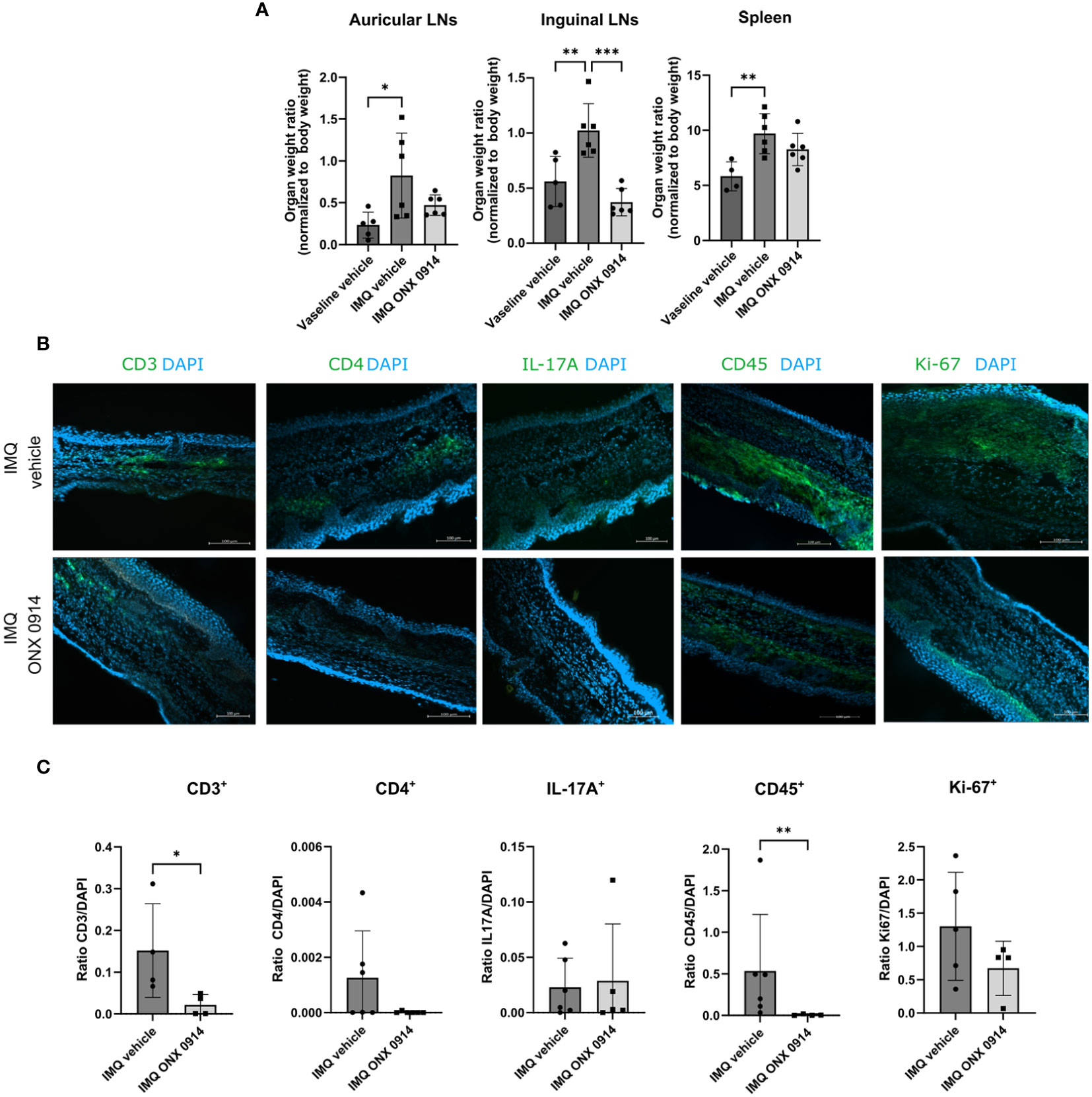
Figure 6 Immunoproteasome inhibition normalizes the weight of dLNs and ameliorates the inflammatory infiltrate in IMQ-induced psoriasis-like inflammation. IL-17A-GFP mice were treated as described in Figure 5A. (A) The dLNs and spleens were harvested after 8 days of treatment with IMQ/vaseline. On the γ-axis, the organ weight normalized to the body weight is depicted. Data (vaseline vehicle n = 4-5, IMQ vehicle, and ONX 0914 n = 6) was pooled from two independent experiments and analyzed by a one-way ANOVA followed by a Šidák test. (B) Representative images of ear cryosections that were stained with anti-CD3, anti-CD4, anti-CD45, anti-Ki67 antibodies or IL-17A (all in green), and DAPI (in blue). The scale bar is 100 μm (C) The positive signal was quantified with ImageJ. On the γ-axis, the ratio of the fluorescence signal to DAPI is depicted. Data (n = 4-6) were pooled from 2 independent experiments and statistically analyzed by unpaired t-test or Mann-Whitney test. All values represent mean ± SD. *p < 0.05, **p <0.01, and ***p < 0.001.
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: immunoproteasome inhibition, psoriasis, CARD14, imiquimod, ONX 0914
Citation: del Rio Oliva M, Mellett M and Basler M (2024) Corrigendum: Immunoproteasome inhibition attenuates experimental psoriasis. Front. Immunol. 14:1335691. doi: 10.3389/fimmu.2023.1335691
Received: 09 November 2023; Accepted: 27 December 2023;
Published: 16 January 2024.
Edited and Reviewed by:
Stefan Tukaj, University of Gdansk, PolandCopyright © 2024 del Rio Oliva, Mellett and Basler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Basler, bWljaGFlbC5iYXNsZXJAdW5pLWtvbnN0YW56LmRl
†ORCID: Marta del Rio Oliva, orcid.org/0000-0002-1015-5925
Mark Mellett, orcid.org/0000-0002-6315-167X
Michael Basler, orcid.org/0000-0002-9428-2349
 Marta del Rio Oliva
Marta del Rio Oliva Mark Mellett
Mark Mellett Michael Basler
Michael Basler