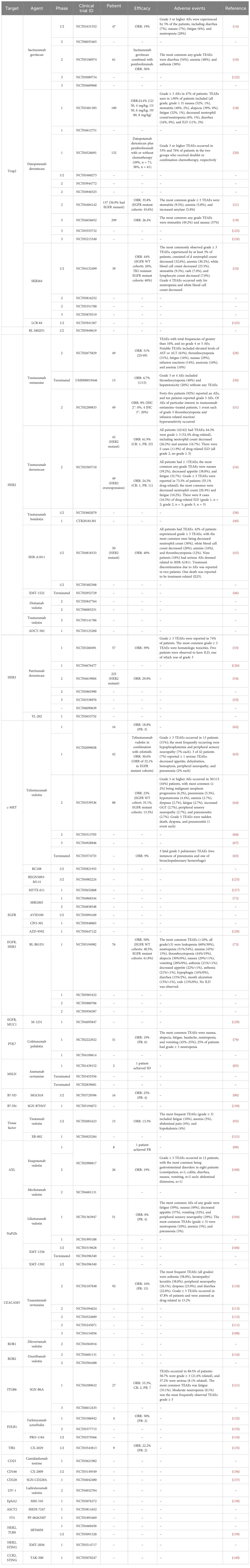
- 1The Affiliated Hospital of Qingdao University, Qingdao University, Qingdao, China
- 2Qingdao Cancer Institute, Qingdao, China
- 3School of Life Sciences, Tsinghua University, Beijing, China
Despite the emergence of molecular targeted therapy and immune checkpoint inhibitors as standard first-line treatments for non-small cell lung cancer (NSCLC), their efficacy in some patients is limited by intrinsic and acquired resistance. Antibody-drug conjugates (ADCs), a revolutionary class of antitumor drugs, have displayed promising clinical outcomes in cancer treatment. In 2022, trastuzumab deruxtecan (Enhertu) was approved for treating HER2-mutated NSCLC, thereby underscoring the clinical value of ADCs in NSCLC treatment strategies. An increasing number of ADCs, focusing on NSCLC, are undergoing clinical trials, potentially positioning them as future treatment options. In this review, we encapsulate recent advancements in the clinical research of novel ADCs for treating NSCLC. Subsequently, we discuss the mechanisms of action, clinical efficacy, and associated limitations of these ADCs.
1 Introduction
Lung cancer, known as the most common thoracic malignancy, is the leading cause of cancer-related deaths worldwide, revealing a 5-year survival rate of only 10%-20% (1). NSCLC accounts for approximately 85% of lung cancer cases, establishing it as the dominant subtype (2). In recent years, the introduction of targeted therapies and immunotherapy has significantly reshaped the treatment landscape for NSCLC. In 2003, the approval of gefitinib by the Food and Drug Administration (FDA), as the first molecular targeted drug for NSCLC treatment, led the way for the development of potent inhibitors such as EGFR, ALK, RET, and KRAS (3, 4). Nonetheless, a marginal section of patients (25%) benefit from these targeted therapies, whilst drug resistance remains a challenge (5). For most patients with driver-gene-negative NSCLC, immune checkpoint inhibitor (ICI) has surpassed combination chemotherapy as the primary approach (3). However, the effectiveness of ICIs in treating metastatic NSCLC patients remains underwhelming, with a median overall survival (mOS) of less than 3 years. Overcoming drug resistance presents continual obstacles (6, 7). ADCs, an emerging class of antineoplastic drugs, mainly encompass three components: antibody, linker, and cytotoxic payload. This therapeutic approach conveys anti-tumor effects via targeted delivery of cytotoxic drugs into tumor cells, earning it the nickname of a ‘magic bullet’ (8). Currently, ADC development is advancing rapidly. To date, the FDA has approved 12 ADCs for tumor treatment, with 9 of them receiving approval since 2017 (9). Importantly, trastuzumab deruxtecan is the only ADC used to treat HER2-mutated NSCLC, signifying an innovative approach to using ADCs in targeted therapy for NSCLC (10). In this review, we summarize recent advancements in the research of ADCs for the treatment of NSCLC, with a focus on aspects including the mechanisms of action, clinical efficacy, and limitations.
2 Trop2-targeted ADCs
Trophoblast cell surface antigen 2 (Trop2), a type I cell surface glycoprotein, shows limited expression in normal tissues but over-expression in various types of tumors, including breast cancer, NSCLC, pancreatic cancer, and other tumors. In NSCLC, over-expressed Trop2 is associated with lymph node metastasis and poor OS (11). There have been several clinical trials to evaluate the therapeutic potential of Trop2-targeted ADCs in NSCLC.
2.1 Sacituzumab govitecan (Trodelvy)
Sacituzumab govitecan is a Trop2-targeted ADC composed of hRS7, an anti-Trop2 monoclonal antibody, connecting to the irinotecan metabolite (SN-38) via a cleavable CL2A carbonate linker with a DAR of 7.6. Preclinical studies demonstrated that sacituzumab govitecan can selectively bind to Trop2+ tumor cells, causing double-stranded DNA breaks and tumor cell death by topoisomerase I and bystander effect. Sacituzumab govitecan exhibits potent antitumor effects in vitro and in vivo. Furthermore, it is well tolerated in monkeys at clinically relevant doses (12, 13). Sacituzumab govitecan is currently the only Trop2-targeted ADC approved by the FDA for the treatment of metastatic triple-negative breast cancer (mTNBC) patients who have received at least two prior therapies for metastatic disease. In a first-in-human (FIH) 1/2 clinical trial of sacituzumab govitecan (NCT01631552), an ORR of 19% was observed among 47 evaluated NSCLC patients. The 8 and 10 mg/kg doses every 21 days were selected as the recommended phase 2 dose (RP2D). Notably, neutropenia was reported by 43% of the patients. Grade 3 or higher AEs were experienced by 5% of the patients, including diarrhea (7%), nausea (7%), fatigue (6%), and neutropenia (28%) (14, 15). Currently, there are several ongoing clinical trials aimed at evaluating the clinical activity of sacituzumab govitecan as a single agent or in combination with other antitumor agents for patients with NSCLC. In a trial (NCT05186974) with sacituzumab govitecan combined with first-line therapy (pembrolizumab or platinum agent), preliminary data demonstrated an ORR of 56% among 61 patients with advanced or metastatic NSCLC that receiving treatment of combination of sacituzumab govitecan and pembrolizumab (16). In addition, three clinical trials (NCT06055465, NCT05089734, and NCT05609968) in patients with refractory or advanced metastatic NSCLC are ongoing.
2.2 Datopotamab deruxtecan
Datopotamab deruxtecan, jointly developed by Daiichi Sankyo and AstraZeneca, aims at treating metastatic breast cancer and metastatic NSCLC. It consists of an anti-Trop2 monoclonal antibody, an enzymatically cleavable tetrapeptide linker, and the exatecan derivative (DXd) (17). Unlike sacituzumab govitecan and other deruxtecan-containing ADCs, datopotamab deruxtecan has a lower DAR. This design is based on preclinical studies suggesting that datopotamab deruxtecan with a DAR of 4 offers enhanced tolerability and a broader therapeutic window in cynomolgus monkeys, in contrast to those with higher DARs. The mechanism of action (MOA) of datopotamab deruxtecan includes inhibition of DNA topoisomerase I and bystander effects (17). Datopotamab deruxtecan is currently undergoing phase 3 clinical trials. The safety, tolerability, and preliminary efficacy of datopotamab deruxtecan have been evaluated in a phase 1 clinical trial (NCT03401385) involving patients with advanced solid tumors. A total of 180 patients with NSCLC received datopotamab deruxtecan, and the treatment of datopotamab deruxtecan showed both antitumor activity and safety. The blinded independent central review determined the ORR as follows: 4 mg/kg - 24% (12/50), 6 mg/kg - 26% (13/50), and 8 mg/kg - 24% (19/80). Among the patients, 47% experienced grade 3 or worse TEAEs, with the most common being nausea, stomatitis, alopecia, and fatigue (18). Notably, the 6 mg/kg dose demonstrated better tolerability, greater effectiveness, and lower AEs, establishing it as the recommended dosage for future development. As a result, it has been recommended for use in subsequent clinical trials. Based on the notable clinical value exhibited by datopotamab deruxtecan in this trial, further evaluation is being conducted through the TROPION-LUNG program. This extensive clinical development initiative aims to assess the effectiveness and safety of datopotamab deruxtecan, either as a monotherapy or in combination with other antitumor agents, specifically targeting Trop2+ NSCLC (11). TROPION-Lung01 (NCT04656652) is a phase 3 clinical trial that assesses the effectiveness and safety of datopotamab deruxtecan when compared to docetaxel (DTX) in individuals with advanced or metastatic NSCLC who have received previous treatment. The study enrolled a total of 604 patients, with 299 in the datopotamab deruxtecan group and 305 in the DTX group. The observed ORR was significantly higher in the datopotamab deruxtecan group (26.4%) compared to the DTX group (12.8%). It is noteworthy that the datopotamab deruxtecan group had a higher occurrence of grade ≥ 3 treatment-related interstitial lung disease (ILD) compared to the DTX group, with rates of 3.4% and 1.4% respectively. Stomatitis (49.2%) and nausea (37%) were the most frequently reported TEAEs in the datopotamab deruxtecan group (19). TROPION-Lung02 (NCT04526691) is a phase 1 clinical trial evaluating the efficacy of datopotamab deruxtecan plus pembrolizumab in patients with advanced NSCLC with or without chemotherapy. As of April 2023, a 38% ORR was observed in 61 patients with advanced NSCLC who received datopotamab deruxtecan plus pembrolizumab treatment. Additionally, the ORR for patients receiving platinum-based chemotherapy in combination with datopotamab deruxtecan and pembrolizumab (n = 71) was 49%. In terms of safety, grade 3 or higher TEAEs occurred in 53% and 76% of patients in the two groups who received doublet or combination chemotherapy, respectively (20). The TROPION-Lung05 (NCT04484142), phase 2 clinical trial, aims to examine both the effectiveness and safety of datopotamab deruxtecan in individuals with advanced or metastatic NSCLC who have actionable genomic alterations. Out of 137 patients who received datopotamab deruxtecan treatment, 56.9% had EGFR mutants. As of December 2022, the observed ORR was 35.8%. Patients with EGFR mutants showed a comparable response, with an ORR of 43.6%. The most common grade ≥ 3 TEAEs were stomatitis (9.5%), anemia (5.8%), and increased amylase (5.8%) (21). Other TROPION-Lung clinical trials (TROPION-Lung07 and TROPION-Lung08) are ongoing.
2.3 SKB264
In SKB264, an anti-Trop2 antibody (hRS7) is conjugated to a topoisomerase I inhibitor (KL610023, belotecan-derived) via an sulfonyl pyrimidine-CL2A-carbonate linker, resulting in a DAR of 7.4 (22). SKB264 shares a similar MOA with sacituzumab govitecan and datopotamab deruxtecan, exerting anti-tumor effects through the inhibition of topoisomerase I and bystander effects. Preclinical studies have demonstrated the remarkable efficacy of SKB264 in the nonclinical Trop2-expressing patient-derived xenografts (PDX) models, with an acceptable safety profile and an excellent therapeutic window in animal studies (23). A phase 1/2 clinical study (NCT04152499) is evaluating the clinical activity of SKB264 in patients with solid tumors who have shown resistance to standard therapies. In the phase 2 expansion cohort, an ORR of 44% was observed among the 39 NSCLC patients. It is worth noting that patients with tyrosine kinase inhibitors (TKIs)-resistant EGFR mutants appear to be more responsive to SKB264 than patients with EGFR WT. Among the EGFR WT group, the ORR was 26% (5/19), while the TKI-resistant EGFR mutant group demonstrated an ORR of 60% (12/20). The most frequently observed grade ≥ 3 TEAEs, experienced by at least 5% of patients, consisted of neutrophil count decreased (32.6%), anemia (30.2%), white blood cell count decreased (23.3%), stomatitis (9.3%), rash (7.0%), and lymphocyte count decreased (7.0%) (24). Grade 4 TEAEs occurred only for neutropenia and white blood cell count decreased. According to the positive result of SKB264 in the EGFR mutant group, a phase 3 clinical trial (NCT05870319) in patients with EGFR-mutated NSCLC has been initiated to further determine the clinical activity of SKB264. In addition, two clinical studies (NCT05816252 and NCT05351788) aiming to investigate SKB264 in patients with advanced NSCLC are ongoing.
3 HER2-targeted ADCs
Human epidermal growth factor receptor 2 (HER2), a member of the epidermal growth factor receptor family, can initiate various oncogenic signaling pathways (MAPK, PI3K, AKT, and PKC), provoking abnormal cell proliferation and encouraging tumorigenesis (25). HER2 is overexpressed across various tumor types. The remarkable success of HER2-targeted therapies in treating HER2+ breast cancer encourages us to explore their potential beyond breast cancer. Recent studies demonstrated that NSCLC, closely correlated with abnormal HER2, may also exhibit potential for suitability towards HER2-targeted agents (26, 27). Moreover, receptor ubiquitination and internalization induced by HER2 amplification or mutant offer a mechanistic foundation for employing HER2-targeted ADCs in the treatment of NSCLC (28).
3.1 Trastuzumab emtansine (Kadcyla)
Trastuzumab emtansine, the first HER2-targeted ADC to be developed, consists of trastuzumab, connecting to the microtubule inhibitor emtansine (DM1) via a non-cleavable linker with a DAR of 3.5 (29). FDA has approved trastuzumab emtansine in treating advanced HER2+ breast cancer patients who have previously received trastuzumab and taxane therapies, either as monotherapy or in combination. Recent encouraging clinical results have demonstrated the therapeutic potential of trastuzumab emtansine for the treatment of HER2+ NSCLC. In a phase 2 clinical study (NCT02675829) involving 49 patients with HER2 amplification or mutant, trastuzumab emtansine showed an ORR of 51% (25/49) and a median progression-free survival (mPFS) of 5 months with good tolerability. According to these positive results, the NCCN has recommended trastuzumab emtansine as the only preferred 2L treatment option for metastatic HER2-mutated NSCLC (28). However, another phase 2 clinical trial (UMI000019446) was terminated because of the limited efficacy of trastuzumab emtansine in patients with HER2+ relapsed NSCLC. In this setting, 15 patients (33% with IHC3+, 20% with IHC2+, and 47% with exon 20 mutant) received trastuzumab emtansine with an RP2D of 3.6 mg/kg every three weeks. Only one patient with mutant, accounting for 6.7% (1/15), achieved PR (30). In a phase 2 trial (NCT02289833), trastuzumab emtansine was administered to 49 patients with HER2+ NSCLC (29 with IHC2+ and 20 with IHC3+). No treatment responses were observed in the IHC2+ group, whereas 4 patients in the IHC3+ cohort achieved partial response (PR). This result suggested the selective activity of trastuzumab emtansine in NSCLC with a high HER2 level (31). In summary, not all patients with HER2+ NSCLC benefit from trastuzumab emtansine treatment. The mechanisms of treatment resistance include the disruption of trastuzumab-mediated effects, abnormal changes in trafficking/metabolism, and impairment of lysine-MCC-DM-1-mediated cytotoxicity.
3.2 Trastuzumab deruxtecan (Enhertu)
Trastuzumab deruxtecan, an anti-HER2 ADC developed by Daiichi Sankyo and AstraZeneca, is composed of trastuzumab linked with topoisomerase I inhibitor (Deruxtecan, DXd) via a hydrolyzable tetrapeptide linker with a DAR of 8 (32). Owing to its highly permeable payload, trastuzumab deruxtecan exhibits a pronounced bystander effect, which allows it to keep potent antitumor activity even in HER2low tumor cells (33). In 2022, the FDA granted accelerated approval to trastuzumab deruxtecan for HER2-mutated NSCLC. This approval is derived from the significant therapeutic effect observed in the phase 2 DESTINY-Lung01 trial (NCT03505710). The trial involved 91 patients with advanced HER2+ NSCLC, who received RP2D at 6.4mg/kg every three weeks and not respond to previous treatments, which included 42 patients with HER2 mutant and 49 patients with HER2 overexpression. For the HER2 mutant group, one achieved complete response (CR) (2.4%), and 25 achieved PR (59.5%) totaling an ORR of 61.9% (34). Trastuzumab deruxtecan showcased an advantageous anti-tumor effect in this category, denoted as significant. In contrast, the cohort showing HER2 overexpression exhibited substantial toxicity and relatively reduced efficacy, with an ORR of 24.5%. Only one of these 49 patients reached CR, with 11 achieving PR, and the mPFS of 5.4 months (35). Among the 91 patients treated with trastuzumab deruxtecan, 88 reported AEs, including 42 instances of grade 3 and above AEs and two documented fatalities. Nausea, neutropenia, and ILD were the most frequent AEs. Drug-related ILD was reported at 26% across all grades and 6.6% for grade 3 and above, respectively (36). Presently, six active clinical trials involving trastuzumab deruxtecan are underway for NSCLC patients, including NCT05048797, a phase 3 study designed to assess both the efficacy and safety of trastuzumab deruxtecan in NSCLC patients with HER2 exon 19 or 20 mutant.
3.3 Trastuzumab botidotin
Trastuzumab botidotin is designed for the treatment of HER2+ solid malignancies. Its synthesis involves conjugating trastuzumab to duostatin-5 (an auristatin derivative) using a protease-cleavable linker with a DAR of 2. In preclinical studies, trastuzumab botidotin demonstrated better tumor growth inhibition than trastuzumab emtansine at a dose of 3 mg/kg in PDX models (37). The initial clinical trial (NCT03602079) of trastuzumab botidotin incorporated 35 patients suffering from locally advanced or metastatic solid tumors, including NSCLC, that were HER2+ or HER2-amplified. Preliminary data demonstrated promising anticancer efficacy at the dosages of 3.6 mg/kg and 4.8 mg/kg. Among 27 evaluable patients, which included NSCLC cases, 7 showed PR, contributing an ORR of 36%. The most frequently observed TEAEs included keratitis, decreased appetite, and dry eye, alongside blurred vision and others. Ocular toxicity was particularly prominent. The onset rate of ocular toxicity marked 80% in the 3.6 mg/kg therapy group, whereas the 4.8 mg/kg group reported an 83% incidence rate (38). Three patients exhibited more severe whorl pattern epitheliopathy, indicating limbal stem cell deficiency (LSCD), which necessitated the cessation of treatment (39). Ocular toxicity was also reported in another clinical trial of trastuzumab botidotin (CTR20181301). A total of 81 patients with advanced solid tumors received trastuzumab botidotin treatment, resulting in objective partial tumor responses observed in 43 patients, reflecting an ORR of 53% (40). The most frequent TEAEs, at grades 3 or above, comprised of corneal epitheliopathy (30.9%), blurred vision (18.5%), dry eyes (7.4%), and peripheral sensory neuropathy (6.2%) (41).
3.4 SHR-A1811
SHR-A1811 is a HER2-targeted ADC composed of trastuzumab via a cleavable linker and a novel topoisomerase I inhibitor payload (SHR9265, exatecan derivative), with a DAR of 5.7. In preclinical studies, SHR-A1811 showed growth inhibition and antitumor activity in breast cancer and gastric cancer cell lines with different HER2 expression levels (high, medium, and low). Moreover, treated cynomolgus monkeys did not exhibit any deaths or lung injuries within 42 days, indicating a good safety profile (42). NCT04818333 is a phase 1/2 trial evaluating the clinical activity of SHR-A1811 in patients with advanced HER2-mutated NSCLC. A total of 50 patients were enrolled, all of whom had received prior treatment including HER2-targeted TKIs (66%), ICI (68%), and anti-angiogenic drugs (78%). Overall, the ORR was 40%. All patients experienced TEAEs. Grade ≥ 3 TEAEs were observed in 42% of patients, with the most common being decreased neutrophil count (30%), white blood cell count decreased (20%), anemia (16%), and thrombocytopenia (12%). Among the patients, nine (18%) experienced severe AEs that might be associated with SHR-A1811. Two patients had to discontinue treatment due to AEs, and one patient died from treatment-related ILD (43). Additionally, NCT05482568 is an ongoing phase 1/2 clinical trial recruiting patients with advanced NSCLC. It aims to assess the effectiveness of SHR-A1811 when used in combination with either pyrotinib or SHR-1316.
3.5 XMT-1522
XMT-1522 is an anti-HER2 ADC made up of the monoclonal antibody HT-19 conjugated with the AF-HPA (auristatin-derivative) payload. It utilizes a cysteine linkage containing biodegradable hydrophilic polymer, with a DAR of 12. AF-HPA and its intracellular metabolite auristatin F (AF) are potent tubulin polymerization inhibitors used to kill tumor cells (44). In preclinical studies, XMT-1522 demonstrated antitumor activity in trastuzumab emtansine-resistant HER2+ breast cancer and gastric cancer cell lines as well as trastuzumab emtansine-resistant PDX models (45). In the primary phase 1 clinical trial (NCT02952729) of XMT-1522, a cohort of 19 participants was enrolled, including individuals with HER2+ NSCLC. Administered doses of 16 or 21.3 mg/m2 led to one patient experiencing PR and four others achieved stable disease (SD), thus yielding a disease control rate (DCR) of 83% (5/6) (46). However, the further development of XMT-1522 was terminated due to a grade 5 TEAEs, resulting in a patient’s death at dose level 7 (47).
4 HER3-targeted ADCs
As a member of the EGFR family, human epidermal growth factor receptor 3 (HER3) is found to be abnormally expressed in various malignancies, including NSCLC (48). It triggers the phosphorylation of receptor tyrosine residues by forming homodimers or heterodimers with other EGFR members, thereby activating multiple signaling pathways such as PI3K/AKT and MAPK, leading ultimately to oncogenesis (49). Furthermore, HER3 plays a crucial role in resisting EGFR TKIs and HER2-targeted antibodies (50, 51). This underlines HER3’s potential as a promising therapeutic target for ADC.
4.1 Patritumab deruxtecan
Patritumab deruxtecan is an ADC formed by conjugating a humanized anti-HER3 monoclonal antibody (patritumab) to a topoisomerase I inhibitor payload (DXd, exatecan derivative) via a cleavable tetrapeptide linker, with DAR of 4 (52). Preclinical studies indicated that patritumab deruxtecan exhibited robust anti-tumor efficacy in the PDX model overexpressing HER3 via DXd-mediated DNA damage and apoptosis without significant safety concerns (52). In the phase 1 clinical trial (NCT03260491), patritumab deruxtecan demonstrated significant clinical efficacy, which it granted breakthrough therapy designation by the FDA for the treatment of patients with metastatic or locally advanced, EGFR-mutated NSCLC. This trial involved patients diagnosed with locally advanced or metastatic EGFR-driven NSCLC, who had previously undergone treatment with EGFR TKI and platinum-based chemotherapy. Of the 57 patients receiving patritumab deruxtecan, the ORR was 39%, with a mPFS of 8.2 months, and a median duration of response (mDOR) of 6.9 months. Intriguingly, responsiveness was also observed in patients exhibiting resistance to EGFR TKI. It’s noteworthy that nearly all patients reported TEAEs (96%), with 74% experiencing TEAEs of grade 3 or higher. ILD was observed in 5 patients, one of which was of grade 3 (53). Given the encouraging findings of the U31402-A-U102 investigation, the HERTHENA-Lung program was initiated to more thoroughly assess the safety and effectiveness of patritumab deruxtecan in patients bearing EGFR-mutated NSCLC. HERTHENA-Lung01 is a Phase 2 clinical trial (NCT04619004) aimed at evaluating the anti-tumor activity of patritumab deruxtecan in subjects with metastatic or locally advanced NSCLC and an activating EGFR mutant (exon 19 deletion or L858R). As of May 2023, a total of 225 patients have received patritumab deruxtecan treatment, with an ORR of 29.8%, mDOR of 6.4 months, mPFS of 5.5 months, and mOS of 11.9 months. Efficacy has been observed in patients with different HER3 expression levels, different EGFR TKI resistance mechanisms, and those with brain metastases (54). Additionally, the HERTHENA-Lung02 trial (NCT05338970), a Phase 3 trial, is currently ongoing in EGFR-mutated NSCLC patients who have progressed on an EGFR TKI (55).
5 c-MET-targeted ADCs
c-MET (Mesenchymal-epithelial transition factor), also known as hepatocyte growth factor receptor, is a receptor tyrosine-protein kinase encoded by the MET gene. Upon ligand binding, c-MET can initiate several signaling pathways including PI3K/AKT and MAPK. These pathways are associated with tumor cellular processes such as proliferation, migration, and invasion (56). In NSCLC, variant forms of c-MET have been observed, including mutant, amplifications, and overexpression (57–59). Notably, a correlation has been established between c-MET amplification and resistance to multiple TKIs (60). Consequently, c-MET has been identified as a promising target in ADC development.
5.1 Telisotuzumab vedotin
Telisotuzumab vedotin is a novel ADC produced by conjugating a humanized anti-c-MET monoclonal antibody (ABT-700) to a microtubule inhibitor (MMAE) via a cleavable linker and has a DAR of 3.1 (61). In preclinical studies, telisotuzumab vedotin demonstrated significant tumor growth inhibition and regression in cell lines and PDX models with c-MET overexpression or MET amplification, by targeted delivery of toxins (61). The FIH clinical trial of telisotuzumab vedotin (NCT02099058) was performed on patients with advanced solid tumors showing c-MET overexpression, aiming to evaluate the drug’s safety, tolerability, pharmacokinetics, and maximum tolerable dose. Notably, the results revealed that the response was confined to NSCLC patients. Among the 16 c-MET+ NSCLC patients treated with telisotuzumab vedotin, 3 demonstrated a PR, mPFS of 5.7 months, and mDOR of 4.8 months. The RP2D was established at 2.7 mg/kg every 21 days (62). In the following phase 1b trial, the combined efficacy of telisotuzumab vedotin and erlotinib was assessed. The trial involved 42 patients, yielding an overall ORR of 30.6%, and a mPFS of 5.9 months. Among the patients with EGFR mutant (n = 28), the ORR was 32.1% (63). However, in a different phase 2 clinical trial (NCT03539536), telisotuzumab vedotin showed limited efficacy in patients with EGFR mutant. In this trial, telisotuzumab vedotin was used as monotherapy. The ORR was 35.1% for the EGFR WT cohort, while the EGFR mutant cohort had an ORR of 13.3% (64). Overall, for the treatment of c-MET+ NSCLC patients with EGFR mutant, the combination of telisotuzumab vedotin and erlotinib might prove more beneficial. Furthermore, the occurrence of TEAEs should be given due attention. A phase 2 clinical trial (NCT03574753) in patients with c-MET+ NSCLC, recording an ORR of 9% (2/23), along with 3 fatal grade 5 pulmonary TEAEs (two instances of pneumonia and one of bronchopulmonary hemorrhage) (65). In addition, multiple telisotuzumab vedotin clinical trials (NCT05513703, NCT04928846) are currently underway for patients with advanced/metastatic NSCLC (66, 67).
6 EGFR-targeted ADCs
Epidermal growth factor receptor (EGFR), a member of the EGFR family, promotes tumor cell proliferation by activating downstream PI3K/AKT, MAPK, and JAK/STAT signaling pathways. Many malignant tumors, including NSCLC, have been found to carry mutants and amplifications in the EGFR gene (68). Presently, therapy targeting EGFR has been sanctioned as the first-line standard for NSCLC with EGFR mutant (69). Nonetheless, the vast preponderance of therapies targeting EGFR unavoidably engenders resistance, which necessitates the extension of EGFR-targeted therapies, with ADC emerging as a potential candidate (70).
6.1 MRG003
MRG003, an EGFR-targeted ADC, consists of an anti-EGFR monoclonal antibody conjugated to a microtubule-disrupting compound, MMAE (71). The safety and antitumor activity of MRG003 patients with either advanced or metastatic solid malignancies were evaluated in a phase 1 clinical trial (NCT04868344). Of 61 patients, 9 (14.7%) achieved PR and 17 (27.8%) documented SD. The recommended dose was determined as 2.5 mg/kg. TEAEs occurred in 89% of the participants, with the majority experiencing grade 1 or 2 AEs. 19 patients (31%) reported grade 3 or higher TEAEs, including hyponatremia, leukocytopenia, neutropenia, elevated aspartate aminotransferase levels, and febrile neutropenia (71). In conclusion, MRG003 administration demonstrated therapeutic potential in patients with EGFR+ solid malignancies. Furthermore, a phase 2 study (NCT04838548) examining the efficacy and safety of MRG003 in patients with EGFR+ advanced NSCLC is currently ongoing. The sustained promising tumor activity of MRG003 justifies further anticipation.
6.2 BL-B01D1
BL-B01D1 is a bispecific ADC targeting EGFR and HER3, which induces cell cycle arrest in the S phase and subsequent apoptosis, leading to kill EGFR+ and/or HER3+ tumor cells. It is comprised of a bispecific antibody against EGFR/HER3 (SI-B001), a cathepsin B cleavable linker, and a novel topoisomerase I inhibitor (Ed-04), with a DAR of 8. Preclinical studies have shown that BL-B01D1 exhibits tumor suppressive effects in PDX models using human colorectal cancer cell lines and pancreatic cancer cell lines (72). In an FIH phase 1 clinical trial (NCT05194982), 76 NSCLC patients were evaluable for efficacy. The ORR in the subset of 34 NSCLC patients with EGFR mutant was observed to be 61.8% (CR: 15, PR: 6), while in the subgroup of 42 NSCLC patients with EGFR WT, the ORR was 40.5% (CR: 7, PR: 10). The most frequent TEAEs (>10%, all grade/≥ G3) were leukopenia (60%/30%), neutropenia (51%/34%), anemia (45%/15%), thrombocytopenia (44%/19%), alopecia (30%/0%), nausea (29%/<1%), vomiting (28%/0%), asthenia (21%/<1%), decreased appetite (22%/<1%), asthenia (21%/<1%), hypophagia (16%/0%), diarrhea (15%/2%), mouth ulceration (15%/<1%), rash (13%/0%). No cases of ILD were observed (73). In addition, there are ongoing clinical trials of BL-B01D1 as a single agent or combination therapy for metastatic or unresectable, advanced or metastatic NSCLC (NCT05983432, NCT05880706, and NCT05956587).
7 PTK7-targeted ADCs
The protein tyrosine kinase 7 (PTK7), also known as colon carcinoma kinase 4 (CCK4), is a receptor protein tyrosine kinase (74). Although PTK7 lacks catalytic activity within its kinase domain, it plays significant roles in canonical and non-canonical Wnt, as well as VEGF signaling (75). Additionally, PTK7 is highly expressed in diverse cancer cells, particularly NSCLC. The abnormal expression of PTK7, associated with multiple adverse prognoses, suggests its potential as a therapeutic target for NSCLC (76).
7.1 Cofetuzumab pelidotin
Cofetuzumab pelidotin is an ADC consisting of the anti-PTK7 monoclonal antibody cofetuzumab, conjugated to the microtubule inhibitor (Aur0101) through a cleavable linker, exhibiting a DAR of 4. Upon binding and internalization into PTK7-expressing cells, cofetuzumab pelidotin undergoes cleavage by intracellular proteases, leading to the release of the auristatin payload. This disrupts microtubules, induces G2-M phase cell cycle arrest, and triggers cell apoptosis, ultimately resulting in the death of cancer cells (77, 78). Preclinical studies have shown that treatment with cofetuzumab pelidotin leads to sustained regression of tumors in PDX models derived from patient samples, and it exhibits stronger anti-tumor activity compared to standard chemotherapy (77). A phase 1 clinical study (NCT02222922) involving patients with advanced solid tumors reported that neutropenia of grade 3 or above was experienced by 25% of participants. Two patients encountered dose-limiting toxicities, presenting as a grade 3 headache and fatigue. Antitumor activity was observed in treated NSCLC patients, where 6 out of 31 achieved PR, thus indicating an ORR of 19%. The RP2D was 2.8 mg/kg every 3 weeks. It is worth mentioning that patients with moderate or high expression levels of PTK7 were more responsive (79). Another ongoing phase 1 clinical trial (NCT04189614) is further investigating the effectiveness and safety of cofetuzumab pelidotin in patients with recurring PTK7+ NSCLC.
8 MSLN-targeted ADCs
Mesothelin (MSLN), a membrane-bound glycoprotein, is typically expressed at low levels in normal tissues. Conversely, there is observed overexpression of MSLN in various tumor cell types, including NSCLC (80). Overexpressed MSLN can stimulate resistance to apoptosis by activating NFκB, MAPK, and PI3K signaling pathways. This leads to enhanced cell proliferation, migration, and metastasis via the induction of MMP7 and MMP9 activation and expression (81–83). Hence, MSLN emerges as a promising potential therapeutic target for NSCLC.
8.1 Anetumab ravtansine
Anetumab ravtansine is an MSLN-targeted ADC, composed of a monoclonal antibody (MF-T) coupled with a microtubule inhibitor (DM4) via a reducible disulfide linker, exhibiting a DAR of 3.2 (84). The FIH study (NCT01439152) involving 148 patients with advanced or metastatic solid tumors, encompassing mesothelioma, ovarian, pancreatic, NSCLC, and breast cancers, SD was reported in 66 cases, including one with NSCLC. Additionally, PR was observed in 11 patients, and a CR was noted in one patient. The RP2D and schedule of anetumab ravtansine was determined as 6.5 mg/kg every three weeks or 2.2 mg/kg per week (85). Subsequently, two distinct clinical trials (NCT03455556, NCT02839681) were planned to determine the efficacy of anetumab ravtansine in advanced MSLN+ NSCLC patients. However, these trials were prematurely terminated due to slow patient recruitment and insufficient accrual.
9 B7-H3-targeted ADCs
B7-H3, also referred as CD276, is a transmembrane glycoprotein and belongs to the B7 ligand family. Although expression levels of B7-H3 are minimal in normal tissues, they markedly increase in a plethora of malignant tumors, including NSCLC, which correlates with poor prognosis (86). B7-H3 can instigate the migration and invasion of tumor cells, thereby escalating the progression of cancer (87). Furthermore, B7-H3 exerts immunosuppressive effects by promoting the infiltration of regulatory T cells within tumor tissues (88).
9.1 MGC018
MGC018 is an ADC composed of a B7-H3-targeted monoclonal antibody conjugated to a DNA-alkylating payload (duocarmycin) via a protease-cleavable linker with a DAR of 2.7. Its MOA includes payload-mediated DNA damage and bystander effects. In preclinical studies, antitumor activity was observed in PDX models. In addition, it showed good pharmacokinetics and safety in cynomolgus monkeys (89). In a phase 1/2 study (NCT03729596) involving 115 patients with advanced solid tumors, MGC018 demonstrated manageable safety and noticeable efficacy. Out of 16 evaluable patients with NSCLC, 4 patients achieved PR, with an ORR of 25%. The RP2D was determined as 3 mg/kg (90).
10 Tissue factor-targeted ADCs
Tissue factor, a transmembrane glycoprotein, plays a crucial role in the coagulation cascade and hemostasis under normal conditions. High expression of tissue factor is observed in various malignant tumors, including NSCLC. Its abnormal expression is strongly associated with tumor growth, enhanced metastasis, and poor prognosis (91).
10.1 Tisotumab vedotin (Tivdak)
Tisotumab vedotin is composed of the anti-tissue factor, monoclonal antibody (TF-011) conjugated to the payload MMAE via a protease-cleavable linker, with a DAR of 4.1 (92). Its main MOA in vivo is auristatin-mediated tumor cell killing. In addition, tisotumab vedotin has shown excellent anti-tumor activity in PDX models derived from solid cancer patients with different tissue factor expression levels, including models that showed tissue factor expression in only 25% to 50% of the tumor cells (92). In 2021, tisotumab vedotin has been approved by the FDA for treating patients with recurrent or metastatic cervical cancer. The FIH clinical trial of tisotumab vedotin (NCT02001623) was conducted in patients with advanced solid tumors. The RP2D was 2.0 mg/kg every three weeks. During the dose-expansion phase of the trial, a 13.3% ORR was recorded in 2 out of 15 patients diagnosed with NSCLC. The most frequent TEAEs (grade ≥ G3) included fatigue (10%), anemia (5%), abdominal pain (4%), and hypokalemia (4%). Notably, 69% of patients experienced epistaxis, potentially due to an impairment in tissue factor-mediated coagulation (93).
11 AXL-targeted ADCs
AXL is a transmembrane receptor tyrosine kinase and forms part of the TAM family (94). Upon activation, AXL stimulates several oncogenic signaling pathways such as PI3K and JAK/STAT (95). It plays an important role in promoting invasion and migration of tumor cells. Moreover, there is a correlation between AXL activation with resistance to EGFR-targeted treatments in the NSCLC (96).
11.1 Enapotamab vedotin
Enapotamab vedotin is an ADC composed of AXL-targeted monoclonal antibody (AXL-107) and a microtubule disrupting agent, MMAE, connected by a protease-cleavable linker (97). In preclinical studies, enapotamab vedotin exhibited significant single-agent activity in PDX NSCLC models expressing AXL, EGFR mutant, and EGFR inhibitor resistance (98). The FIH clinical trial of enapotamab vedotin (NCT02988817) was conducted with patients bearing relapsed or refractory solid tumors, recruiting a total of 47 patients, including 8 diagnosed with NSCLC. The preliminary data indicated that PR was observed in 3 patients, one of whom was diagnosed with NSCLC. The RP2D was determined as 2.2 mg/kg every three weeks (99). Phase 2a (expansion phase) of this study, included 26 patients with NSCLC, void of sensitizing EGFR mutant (EGFR WT) or ALK rearrangements (ALK-). The trial recorded an ORR of 19%, along with a DCR of 50%. 75% (9 of 12) evaluable fresh biopsies tested positive for AXL tumor cell staining (100).
12 NaPi2b-targeted ADCs
Sodium-dependent phosphate transport protein 2B (NaPi2b) is encoded by SLC34A2, which has been recognized to play a significant role in the regulation of tumor development (101). Studies have indicated elevated expression of NaPi2b in diverse cancers, especially notable in lung cancer patients exhibiting TTF1 positivity along with mutants in KRAS and EGFR (102). Such characteristics make it an appealing target for the development of ADC.
12.1 Lifastuzumab vedotin
Lifastuzumab vedotin is an ADC composed of anti-NaPi2b mAb (MNIB2126A) and a potent microtubule inhibitor (MMAE). Preclinical studies demonstrated that lifastuzumab vedotin exhibited significant anti-tumor efficacy in mouse models of ovarian cancer and the NSCLC PDX model, and it also showed acceptable safety in animal studies (103). In a phase 1a clinical trial (NCT01363947) involving patients with NSCLC and platinum-resistant ovarian cancer (PROC), 4 out of 51 NSCLC patients achieved PR, resulting in an ORR of 8%. The dose of 2.4 mg/kg was established as the RP2D. Lifastuzumab vedotin has limited efficacy in patients with NSCLC but is promising in patients with PROC, with an ORR of 46%. The most common AEs of any grade were fatigue (59%), nausea (49%), decreased appetite (37%), vomiting (32%), and peripheral sensory neuropathy (29%). The most common TEAEs (grade ≥ 3) were neutropenia (10%), anemia (3%), and pneumonia (3%) (104). Additionally, another phase 1 clinical trial (NCT01995188) is ongoing, also in patients with NSCLC and PROC.
12.2 XMT-1536
XMT-1536 is an ADC composed of a humanized anti-NaPi2B antibody conjugated to the payload AF-HPA with a high DAR of 10-15. AF-HPA is a cell-permeable anti-mitotic compound that slowly metabolizes into a highly low-permeable metabolite called auristatin F (AF) within the tumor, resulting in controlled bystander killing. The antitumor effect of XMT-1536 has been observed in preclinical studies using in vivo and in vitro models of adenocarcinoma, ovarian cancer, and lung cancer (105). Pharmacokinetic analysis showed approximately proportional increases in exposure in rats and monkeys. Systemic-free AF-HPA and AF concentrations were observed to be low in all animal species. The clinical activity of XMT-1536 is being evaluated in a phase 1/2 clinical trial (NCT03319628) involving patients with NaPi2b+ ovarian cancer and NSCLC. However, a separate clinical trial (NCT04396340) was terminated (106).
13 CEACAM5-targeted ADC
Carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5), a cell surface glycoprotein, is typically expressed at low levels in the majority of normal tissues. However, its expression is significantly elevated in various tumors, notably those in the gastrointestinal tract, breast, and lung (107). Approximately 20% of patients diagnosed with NSCLC show overexpression of CEACAM5 (108). Thus, the deployment of ADCs targeting CEACAM5 could potentially exhibit promise for treating patients with NSCLC.
13.1 Tusamitamab ravtansine
Tusamitamab ravtansine is a CEACAM5-targeted ADC consisting of a humanized monoclonal antibody and a maytansinoid agent (DM4). It exerts anti-tumor activity by inhibiting tubulin polymerization through the action of DM4. Preclinical studies demonstrated the in vitro cytotoxicity and in vivo efficacy of tusamitamab ravtansine in a PDX model, as well as its safety profile in monkeys (109). A phase 2 clinical trial (NCT02187848) evaluated the efficacy and safety of tusamitamab ravtansine in patients with CEACAM5+ non-squamous NSCLC. This study enrolled a total of 92 individuals, of which 28 exhibited moderate IHC expression and 64 had high expression. The respective ORRs for moderate and high expression stood at 7.1% and 20.3%. Grade 3 or greater TEAEs occurred in 47.8% of patients, with 15.2% of them being assessed as drug-related (110). Additionally, tusamitamab ravtansine is currently under investigation in several ongoing clinical trials involving NSCLC patients, namely NCT04394624, NCT04524689, NCT05245071, and NCT04154956 (108, 111–113).
14 ROR2-targeted ADCs
Tyrosine-protein kinase transmembrane receptor (ROR2), a transmembrane protein receptor, is a member of the tyrosine kinase-like orphan receptor family. Despite lacking kinase function, it interacts with the non-canonical Wnt signalling (114). Research has demonstrated that ROR2 is significantly expressed in a range of malignant tumors, including NSCLC, and correlated with poor prognosis. It could potentially serve as a target for NSCLC treatment (115).
14.1 Ozuriftamab vedotin
Ozuriftamab vedotin is a novel ADC that consists of an anti-ROR2 monoclonal antibody conjugated to MMAE via a cleavable linker. Preclinical data suggest targeting ROR2 may result in antitumor activities in various tumor types, such as NSCLC. Multiple clinical trials for NSCLC are currently underway for ozuriftamab vedotin. The phase 2 clinical trial, NCT04681131, aims to assess the clinical efficacy of ozuriftamab vedotin as a single agent or in combination with nivolumab in patients with advanced solid tumors, including NSCLC (116). NCT03504488 is a phase 1/2 clinical trial evaluating the safety and efficacy of ozuriftamab vedotin in patients with NSCLC, TNBC, head and neck cancer, and melanoma.
15 ITGB6
Integrin subunit beta 6 (ITGB6) is an integrin protein heterodimer composed of an αv subunit and a β6 subunit. Normally, the expression of ITGB6 is low or absent in the epithelial cells of healthy tissues. However, its expression is increased during tissue repair and embryogenesis (117). Furthermore, studies have confirmed the overexpression of ITGB6 in various cancers, including NSCLC, which is associated with poor prognosis (118). Failure of ITGB6-based signaling mechanisms can result in abnormal cell division, adhesion, and migration, consequently contributing to tumorigenesis and metastasis (119).
15.1 SGN-B6A
SGN-B6A is an ITGB6-targeted ADC with MMAE as the payload. SGN-B6A exhibits anti-tumor activity through MMAE-mediated cytotoxicity, bystander effects, and immunogenic cell death. In preclinical studies, the antibody component of SGN-B6A specifically targets ITGB6, without binding to other members of the alpha-V family, and exhibits in vivo activity in models of NSCLC, pancreatic cancer, pharyngeal cancer, and bladder cancer (120). FIH phase 1 clinical trial (NCT04389632) is assessing the clinical activity of SGN-B6A in patients with advanced solid tumors. Out of the 27 patients with NSCLC who received treatment, 2 achieved CR and 7 achieved PR, resulting in an ORR of 33.3%. TEAEs occurred in 88.5% of patients: 50.7% were grade ≥ 3 (21.6% related), and 37.2% were serious (8.1% related). The most common TEAEs was fatigue (35.1%). Moderate neutropenia (8.1%) was the most frequently observed TEAEs grade ≥ 3 (121). Furthermore, another phase 3 clinical trial (NCT06012435) to evaluate the efficacy of SGN-B6A in patients with previously treated NSCLC is ongoing.
16 Conclusions and perspectives
ADCs, that are able to combine targeted therapy and cytotoxic chemotherapy, have demonstrated promising antitumor efficacy in preclinical and clinical trials, introducing a new treatment modality for advanced NSCLC patients (Table 1). Compared to conventional molecular targeted agents, ADCs offer an improved therapeutic index and have demonstrated more favorable clinical outcomes in certain NSCLC clinical trials (NCT04152499) (24, 141, 142). Despite the promising potential of ADCs in NSCLC therapy, the issue of drug resistance poses a significant challenge. For instance, telisotuzumab vedotin exhibits promising clinical activity for c-MET-positive NSCLC patients yet it provides limited clinical efficacy, with an ORR of merely 13.3%, in NSCLC patients with mutant EGFR (62, 64). EGFR mutations may be a one way through which cancer cells can escape the cytotoxic effects of telisotuzumab vedotin. Resistance to ADC may occur via multiple mechanisms: loss of internalization pathways preventing ADC internalization and transport; reduced lysosomal proteolysis or loss of lysosomal transporter function restraining linker cleavage and payload release within tumor cells; the upregulation of ATP-binding cassette transporter causing the direct transport and efflux of payload; the inactivation of pro-apoptotic proteins (Bak and Bax) or overexpression of anti-apoptotic proteins (Bcl-2 and Bcl-XL) leading to dysregulation of apoptotic pathways (142, 143). The mechanisms contributing resistance in NSCLC need further investigation.
Combinations with other antitumor agents or use of multi-specific ADCs targeting different antigens may be alternative approaches to overcome resistance in NSCLC treatments (Figure 1). For example, the combination of telisotuzumab vedotin with erlotinib resulted in an ORR of 32.1% among NSCLC patients with EGFR mutation, significantly surpassing the ORR observed with telisotuzumab vedotin monotherapy (63). The combination of ADCs with immune checkpoint inhibitors (ICIs) has also exhibited powerful tumor-killing activity. For instance, the combination of sacituzumab govitecan and pembrolizumab showed promising clinical activity as a first-line treatment for metastatic NSCLC (Figure 1). The next aspect to consider for future research is how to optimize the risk-benefit profiles of ADCs in NSCLC patients. Some target antigens for NSCLC ADCs, such as Trop2, are widely expressed in normal tissues, which potentially leads to excessive exposure of normal tissues and can result in unmanageable toxicity. Therefore, the ongoing quest to discover more effective and safer ADCs that have advantageous tumor-specificity in NSCLC remains a critical focus for future research.
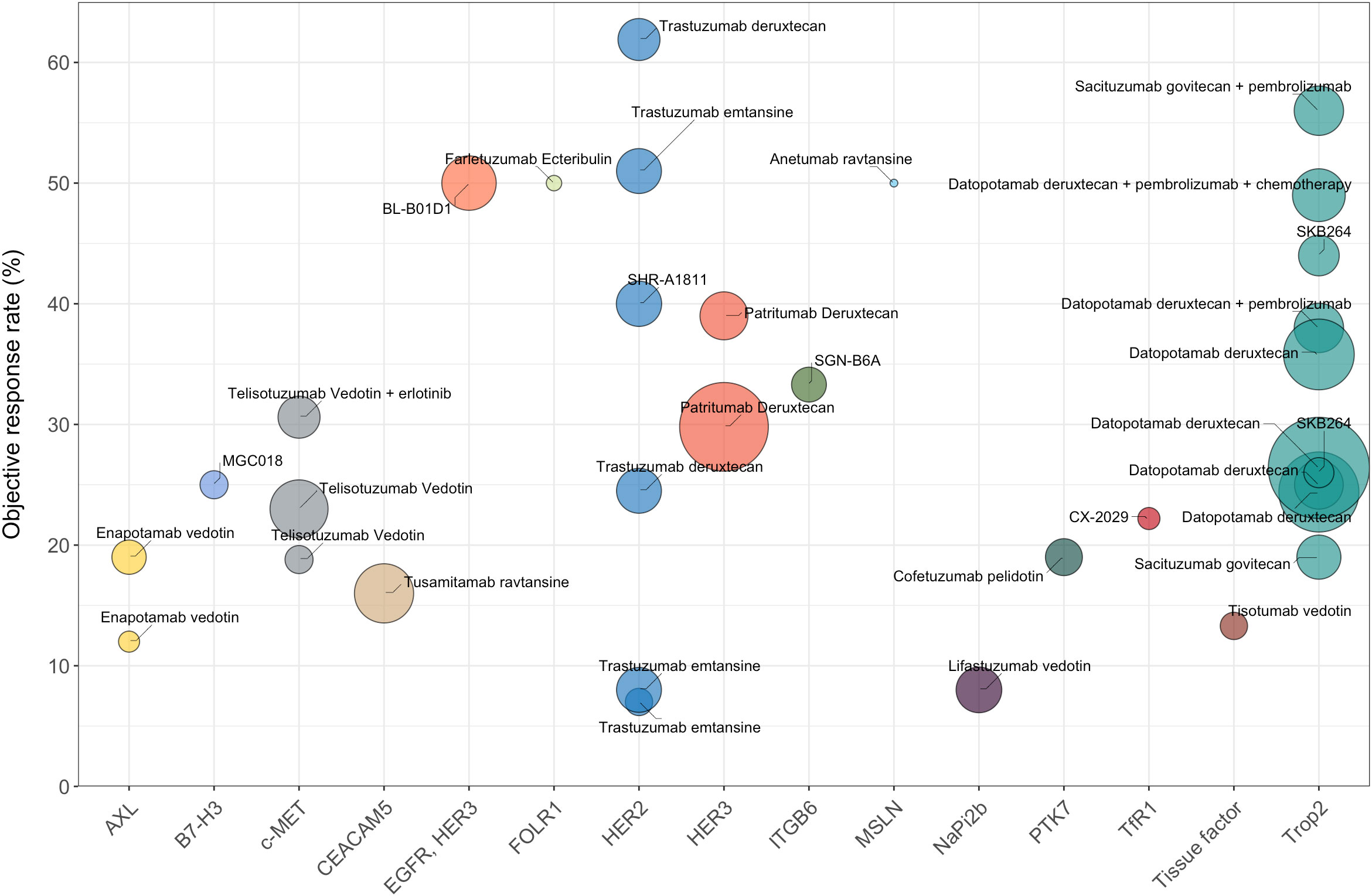
Figure 1 The efficacy data (ORR) of different ADCs for the treatment of NSCLC (limited to ADCs with published clinical results). Trastuzumab deruxtecan, as a monotherapy, has the highest ORR of 61.9%. Sacituzumab govitecan combined with pembrolizumab demonstrates an ORR of 56%. Bispecific ADC serves as another promising therapeutic modality, with BL-B0D1 demonstrating an ORR of 50% in NSCLC patients. The fill color of the circle represents different targets for NSCLC. The sizes of the circles represent the number of evaluable patients with NSCLC.
Author contributions
XL: Conceptualization, Funding acquisition, Resources, Writing – original draft. JD: Writing – original draft, Writing – review & editing. RZ: Writing – review & editing. JXi: Writing – review & editing. YW: Writing – review & editing. WC: Writing – review & editing. BL: Writing – review & editing. DX: Writing – review & editing. JXu: Resources, Writing – review & editing. MZ: Writing – review & editing, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Natural Science Foundation of Shandong Province Grants ZR2022QH201 (XL) and the National Natural Science Foundation of China Grants 32300788 (XL).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin (2017) 67(1):7–30. doi: 10.3322/caac.21387
3. Wang M, Herbst RS, Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med (2021) 27(8):1345–56. doi: 10.1038/s41591-021-01450-2
4. Dyer O. Fda announces fast track approval of new drug for lung cancer. Bmj (2003) 326(7397):1004. doi: 10.1136/bmj.326.7397.1004/d
5. Boumahdi S, de Sauvage FJ. The great escape: tumour cell plasticity in resistance to targeted therapy. Nat Rev Drug Discov (2020) 19(1):39–56. doi: 10.1038/s41573-019-0044-1
6. Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ, et al. Five-year overall survival for patients with advanced non-Small-cell lung cancer treated with pembrolizumab: results from the phase I keynote-001 study. J Clin Oncol (2019) 37(28):2518–27. doi: 10.1200/jco.19.00934
7. Bie F, Tian H, Sun N, Zang R, Zhang M, Song P, et al. Research progress of anti-pd-1/pd-L1 immunotherapy related mechanisms and predictive biomarkers in nsclc. Front Oncol (2022) 12:769124. doi: 10.3389/fonc.2022.769124
8. Strebhardt K, Ullrich A. Paul ehrlich's magic bullet concept: 100 years of progress. Nat Rev Cancer (2008) 8(6):473–80. doi: 10.1038/nrc2394
9. Coleman N, Yap TA, Heymach JV, Meric-Bernstam F, Le X. Antibody-drug conjugates in lung cancer: dawn of a new era? NPJ Precis Oncol (2023) 7(1):5. doi: 10.1038/s41698-022-00338-9
10. Fda gives nod to T-dxd for her2-mutant nsclc. Cancer Discov (2022) 12(10):2224. doi: 10.1158/2159-8290.Cd-nb2022-0053
11. Liu X, Deng J, Yuan Y, Chen W, Sun W, Wang Y, et al. Advances in trop2-targeted therapy: novel agents and opportunities beyond breast cancer. Pharmacol Ther (2022) 239:108296. doi: 10.1016/j.pharmthera.2022.108296
12. Fenn KM, Kalinsky K. Sacituzumab govitecan: antibody-drug conjugate in triple-negative breast cancer and other solid tumors. Drugs Today (Barc) (2019) 55(9):575–85. doi: 10.1358/dot.2019.55.9.3039669
13. Cardillo TM, Govindan SV, Sharkey RM, Trisal P, Goldenberg DM. Humanized anti-trop-2 igg-sn-38 conjugate for effective treatment of diverse epithelial cancers: preclinical studies in human cancer xenograft models and monkeys. Clin Cancer Res an Off J Am Assoc Cancer Res (2011) 17(10):3157–69. doi: 10.1158/1078-0432.CCR-10-2939
14. Heist RS, Guarino MJ, Masters G, Purcell WT, Starodub AN, Horn L, et al. Therapy of advanced non-small-cell lung cancer with an sn-38-anti-trop-2 drug conjugate, sacituzumab govitecan. J Clin Oncol (2017) 35(24):2790–7. doi: 10.1200/jco.2016.72.1894
15. Starodub AN, Ocean AJ, Shah MA, Guarino MJ, Picozzi VJ Jr., Vahdat LT, et al. First-in-human trial of a novel anti-trop-2 antibody-sn-38 conjugate, sacituzumab govitecan, for the treatment of diverse metastatic solid tumors. Clin Cancer Res (2015) 21(17):3870–8. doi: 10.1158/1078-0432.Ccr-14-3321
16. Gilead. Gilead’s Phase 2 Evoke-02 Study of Trodelvy (Sacituzumab Govitecan-Hziy) in Combination with Keytruda (Pembrolizumab) Demonstrates Promising Clinical Activity in First-Line Metastatic Non-Small Cell Lung Cancer (2023). Available at: https://www.gilead.com/news-and-press/press-room/press-releases/2023/9/gileads-phase-2-evoke02-study-of-trodelvy-sacituzumab-govitecanhziy-in-combination-with-keytruda-pembrolizumab-demonstrates-promising-clinica.
17. Okajima D, Yasuda S, Maejima T, Karibe T, Sakurai K, Aida T, et al. Datopotamab deruxtecan, a novel trop2-directed antibody-drug conjugate, demonstrates potent antitumor activity by efficient drug delivery to tumor cells. Mol Cancer Ther (2021) 20(12):2329–40. doi: 10.1158/1535-7163.Mct-21-0206
18. Garon E, Johnson M, Lisberg A, Spira A, Yamamoto N, Heist R, et al. Ma03.02 tropion-pantumor01: updated results from the nsclc cohort of the phase 1 study of datopotamab deruxtecan in solid tumors. J Thorac Oncol (2021) 16(10):S892–S3. doi: 10.1016/j.jtho.2021.08.118
19. Ahn M, Lisberg A, Paz-Ares L, Cornelissen R, Girard N, Pons-Tostivint E, et al. Lba12 datopotamab deruxtecan (Dato-dxd) vs docetaxel in previously treated advanced/metastatic (Adv/met) non-small cell lung cancer (Nsclc): results of the randomized phase iii study tropion-lung01. Ann Oncol (2023) 34:S1305–S6. doi: 10.1016/j.annonc.2023.10.061
20. Doherty K. Datopotamab Deruxtecan Plus Pembrolizumab Displays Efficacy in Advanced Nsclc with or without Chemotherapy (2023). Available at: https://www.onclive.com/view/datopotamab-deruxtecan-plus-pembrolizumab-displays-efficacy-in-advanced-nsclc-with-or-without-chemotherapy.
21. Paz-Ares L, Ahn M, Lisberg A, Kitazono S, Cho B, Blumenschein G, et al. 1314mo tropion-lung05: datopotamab deruxtecan (Dato-dxd) in previously treated non-small cell lung cancer (Nsclc) with actionable genomic alterations (Agas). Ann Oncol (2023) 34:S755–S6. doi: 10.1016/j.annonc.2023.09.2348
22. Liu Y, Lian W, Zhao X, Diao Y, Xu J, Xiao L, et al. Skb264 adc: A first-in-human study of skb264 in patients with locally advanced unresectable/metastatic solid tumors who are refractory to available standard therapies. J Clin Oncol (2020) 38(15_suppl):TPS3659–TPS. doi: 10.1200/JCO.2020.38.15_suppl.TPS3659
23. Cheng Y, Yuan X, Tian Q, Huang X, Chen Y, Pu Y, et al. Preclinical profiles of skb264, a novel anti-trop2 antibody conjugated to topoisomerase inhibitor, demonstrated promising antitumor efficacy compared to immu-132. Front Oncol (2022) 12:951589. doi: 10.3389/fonc.2022.951589
24. Fang W, Cheng Y, Chen Z, Wang W, Yin Y, Li Y, et al. Skb264 (Trop2-adc) for the treatment of patients with advanced nsclc: efficacy and safety data from a phase 2 study. J Clin Oncol (2023) 41(16_suppl):9114–. doi: 10.1200/JCO.2023.41.16_suppl.9114
25. Robichaux JP, Elamin YY, Vijayan RSK, Nilsson MB, Hu L, He J, et al. Pan-cancer landscape and analysis of erbb2 mutations identifies poziotinib as a clinically active inhibitor and enhancer of T-dm1 activity. Cancer Cell (2019) 36(4):444–57.e7. doi: 10.1016/j.ccell.2019.09.001
26. Aksoy P, Abban CY, Kiyashka E, Qiang WT, Meneses PI. Hpv16 infection of hacats is dependent on beta 4 integrin, and alpha 6 integrin processing. Virology (2014) 449:45–52. doi: 10.1016/j.virol.2013.10.034
27. Hirsch FR, Varella-Garcia M, Franklin WA, Veve R, Chen L, Helfrich B, et al. Evaluation of her-2/neu gene amplification and protein expression in non-small cell lung carcinomas. Br J Cancer (2002) 86(9):1449–56. doi: 10.1038/sj.bjc.6600286
28. Li BT, Michelini F, Misale S, Cocco E, Baldino L, Cai Y, et al. Her2-mediated internalization of cytotoxic agents in erbb2 amplified or mutant lung cancers. Cancer Discov (2020) 10(5):674–87. doi: 10.1158/2159-8290.Cd-20-0215
29. He J, Yu SF, Yee S, Kaur S, Xu K. Characterization of in vivo biotransformations for trastuzumab emtansine by high-resolution accurate-mass mass spectrometry. MAbs (2018) 10(7):960–7. doi: 10.1080/19420862.2018.1494487
30. Hotta K, Aoe K, Kozuki T, Ohashi K, Ninomiya K, Ichihara E, et al. A phase ii study of trastuzumab emtansine in her2-positive non-small cell lung cancer. J Thorac Oncol (2018) 13(2):273–9. doi: 10.1016/j.jtho.2017.10.032
31. Peters S, Stahel R, Bubendorf L, Bonomi P, Villegas A, Kowalski DM, et al. Trastuzumab emtansine (T-dm1) in patients with previously treated her2-overexpressing metastatic non-small cell lung cancer: efficacy, safety, and biomarkers. Clin Cancer Res (2019) 25(1):64–72. doi: 10.1158/1078-0432.Ccr-18-1590
32. Gray JE, Heist RS, Starodub AN, Camidge DR, Kio EA, Masters GA, et al. Therapy of small cell lung cancer (Sclc) with a topoisomerase-I-inhibiting antibody-drug conjugate (Adc) targeting trop-2, sacituzumab govitecan. Clin Cancer Res (2017) 23(19):5711–9. doi: 10.1158/1078-0432.Ccr-17-0933
33. Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of ds-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci (2016) 107(7):1039–46. doi: 10.1111/cas.12966
34. Smit EF, Nakagawa K, Nagasaka M, Felip E, Goto Y, Li BT, et al. Trastuzumab deruxtecan (T-dxd; ds-8201) in patients with her2-mutated metastatic non-small cell lung cancer (Nsclc): interim results of destiny-lung01. J Clin Oncol (2020) 38(15_suppl):9504–. doi: 10.1200/JCO.2020.38.15_suppl.9504
35. Nakagawa K, Nagasaka M, Felip E, Pacheco J, Baik C, Goto Y, et al. Oa04.05 trastuzumab deruxtecan in her2-overexpressing metastatic non-small cell lung cancer: interim results of destiny-lung01. J Thorac Oncol (2021) 16(3):S109–S10. doi: 10.1016/j.jtho.2021.01.285
36. Li BT, Smit EF, Goto Y, Nakagawa K, Udagawa H, Mazières J, et al. Trastuzumab deruxtecan in her2-mutant non-small-cell lung cancer. N Engl J Med (2022) 386(3):241–51. doi: 10.1056/NEJMoa2112431
37. Xue T, Miao Z, Wang J, Chen G, Yan Q, Zhu T, et al. Anti-erbb2 antibody-drug conjugate and composition thereof, preparation method therefor, and application thereof. Google Patents (2019).
38. Liu Y, Lian W, Zhao X, Qi W, Xu J, Xiao L, et al. A first in-human study of A166 in patients with locally advanced/metastatic solid tumors which are her2-positive or her2-amplified who did not respond or stopped responding to approved therapies. J Clin Oncol (2020) 38(15_suppl):1049–. doi: 10.1200/JCO.2020.38.15_suppl.1049
39. Sharma A, Riaz KM, Gill MS, Patnaik A, Ulahannan SV, Wang JS, et al. Reversible her2 antibody-drug conjugate-induced ocular toxicity. Can J Ophthalmol (2022) 57(2):118–26. doi: 10.1016/j.jcjo.2021.02.028
40. Zhang J, Liu R, Gao S, Li W, Chen Y, Meng Y, et al. Phase I study of A166, an antibody-Drug conjugate in advanced her2-expressing solid tumours. NPJ Breast Cancer (2023) 9(1):28. doi: 10.1038/s41523-023-00522-5
41. Hu X, Zhang J, Liu R, Gao S, Qing Y, Yi S, et al. Phase I study of A166 in patients with her2-expressing locally advanced or metastatic solid tumors. J Clin Oncol (2021) 39(15_suppl):1024. doi: 10.1200/JCO.2021.39.15_suppl.1024
42. Zhang T, You L, Xu J, Yin J, Qu B, Mao Y, et al. Abstract lb031: shr-A1811, a novel anti-her2 adc with superior bystander effect, optimal dar and favorable safety profiles. Cancer Res (2023) 83(8_Supplement):LB031–LB. doi: 10.1158/1538-7445.Am2023-lb031
43. Lu S, Jian H, Hong W, Song Z, Yang N, Hu S, et al. Abstract ct204: safety, tolerability, pharmacokinetics, and efficacy of shr-A1811, an antibody-drug conjugate, in patients with advanced her2-mutant non-small cell lung cancer (Nsclc): A multicenter, open-label, phase 1/2 study. Cancer Res (2023) 83(8_Supplement):CT204–CT. doi: 10.1158/1538-7445.Am2023-ct204
44. Clardy SM, Yurkovetskiy A, Yin M, Gumerov D, Xu L, Ter-Ovanesyan E, et al. Abstract 754: unique pharmacologic properties of dolaflexin-based adcs—a controlled bystander effect. Cancer Res (2018) 78(13_Supplement):754. doi: 10.1158/1538-7445.Am2018-754
45. Le Joncour V, Martins A, Puhka M, Isola J, Salmikangas M, Laakkonen P, et al. A novel anti-her2 antibody-drug conjugate xmt-1522 for her2-positive breast and gastric cancers resistant to trastuzumab emtansine. Mol Cancer Ther (2019) 18(10):1721–30. doi: 10.1158/1535-7163.Mct-19-0207
46. Hamilton EP, Barve MA, Bardia A, Beeram M, Bendell JC, Mosher R, et al. Phase 1 dose escalation of xmt-1522, a novel her2-targeting antibody-drug conjugate (Adc), in patients (Pts) with her2-expressing breast, lung and gastric tumors. J Clin Oncol (2018) 36(15_suppl):2546. doi: 10.1200/JCO.2018.36.15_suppl.2546
47. Therapeutics M. Mersana therapeutics announces partial clinical hold for xmt-1522 clinical trial. Press Release (2018) 19.
48. Ocana A, Vera-Badillo F, Seruga B, Templeton A, Pandiella A, Amir E. Her3 overexpression and survival in solid tumors: A meta-analysis. J Natl Cancer Inst (2013) 105(4):266–73. doi: 10.1093/jnci/djs501
49. Campbell MR, Amin D, Moasser MM. Her3 comes of age: new insights into its functions and role in signaling, tumor biology, and cancer therapy. Clin Cancer Res (2010) 16(5):1373–83. doi: 10.1158/1078-0432.Ccr-09-1218
50. Watanabe S, Yonesaka K, Tanizaki J, Nonagase Y, Takegawa N, Haratani K, et al. Targeting of the her2/her3 signaling axis overcomes ligand-mediated resistance to trastuzumab in her2-positive breast cancer. Cancer Med (2019) 8(3):1258–68. doi: 10.1002/cam4.1995
51. Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. Met amplification leads to gefitinib resistance in lung cancer by activating erbb3 signaling. Science (2007) 316(5827):1039–43. doi: 10.1126/science.1141478
52. Hashimoto Y, Koyama K, Kamai Y, Hirotani K, Ogitani Y, Zembutsu A, et al. A novel her3-targeting antibody-drug conjugate, U3-1402, exhibits potent therapeutic efficacy through the delivery of cytotoxic payload by efficient internalization. Clin Cancer Res (2019) 25(23):7151–61. doi: 10.1158/1078-0432.Ccr-19-1745
53. Jänne PA, Baik C, Su WC, Johnson ML, Hayashi H, Nishio M, et al. Efficacy and safety of patritumab deruxtecan (Her3-dxd) in egfr inhibitor-resistant, egfr-mutated non-small cell lung cancer. Cancer Discov (2022) 12(1):74–89. doi: 10.1158/2159-8290.Cd-21-0715
54. Yu HA, Goto Y, Hayashi H, Felip E, Yang JC-H, Reck M, et al. Herthena-lung01, a phase ii trial of patritumab deruxtecan (Her3-dxd) in epidermal growth factor receptor–mutated non–small-cell lung cancer after epidermal growth factor receptor tyrosine kinase inhibitor therapy and platinum-based chemotherapy. J Clin Oncol (2023) 00:1–13.. doi: 10.1200/jco.23.01476
55. Mok TSK, Wu YL, Nishio M, Reck M, Wu E, Sternberg DW, et al. 1195tip herthena-lung02: A randomized phase iii study of patritumab deruxtecan vs platinum-based chemotherapy in locally advanced or metastatic egfr-mutated nsclc after progression with a third-generation egfr tki. Ann Oncol (2022) 33:S1095. doi: 10.1016/j.annonc.2022.07.1318
56. Ko B, He T, Gadgeel S, Halmos B. Met/hgf pathway activation as a paradigm of resistance to targeted therapies. Ann Transl Med (2017) 5(1):4. doi: 10.21037/atm.2016.12.09
57. Salgia R. Met in lung cancer: biomarker selection based on scientific rationale. Mol Cancer Ther (2017) 16(4):555–65. doi: 10.1158/1535-7163.Mct-16-0472
58. Awad MM, Oxnard GR, Jackman DM, Savukoski DO, Hall D, Shivdasani P, et al. Met exon 14 mutations in non-small-cell lung cancer are associated with advanced age and stage-dependent met genomic amplification and C-met overexpression. J Clin Oncol (2016) 34(7):721–30. doi: 10.1200/jco.2015.63.4600
59. Schildhaus HU, Schultheis AM, Rüschoff J, Binot E, Merkelbach-Bruse S, Fassunke J, et al. Met amplification status in therapy-naïve adeno- and squamous cell carcinomas of the lung. Clin Cancer Res (2015) 21(4):907–15. doi: 10.1158/1078-0432.Ccr-14-0450
60. Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to egfr inhibitors. Sci Transl Med (2011) 3(75):75ra26. doi: 10.1126/scitranslmed.3002003
61. Wang J, Anderson MG, Oleksijew A, Vaidya KS, Boghaert ER, Tucker L, et al. Abbv-399, a C-met antibody-drug conjugate that targets both met-amplified and C-met-overexpressing tumors, irrespective of met pathway dependence. Clin Cancer Res (2017) 23(4):992–1000. doi: 10.1158/1078-0432.Ccr-16-1568
62. Strickler JH, Weekes CD, Nemunaitis J, Ramanathan RK, Heist RS, Morgensztern D, et al. Dose-escalation and -expansion study of telisotuzumab vedotin, an antibody-drug conjugate targeting C-met, in patients with advanced solid tumors. J Clin Oncol (2018) 36(33):3298–306. doi: 10.1200/jco.2018.78.7697
63. Camidge DR, Barlesi F, Goldman JW, Morgensztern D, Heist R, Vokes E, et al. Phase ib study of telisotuzumab vedotin in combination with erlotinib in patients with C-met protein-expressing non-small-cell lung cancer. J Clin Oncol (2023) 41(5):1105–15. doi: 10.1200/jco.22.00739
64. Camidge DR, Moiseenko F, Cicin I, Horinouchi H, Filippova E, Bar J, et al. Oa15.04 telisotuzumab vedotin (Teliso-V) monotherapy in patients with previously treated C-met<Sup>+</sup> advanced non-small cell lung cancer. J Thorac Oncol (2021) 16(10):S875. doi: 10.1016/j.jtho.2021.08.085
65. Waqar SN, Redman MW, Arnold SM, Hirsch FR, Mack PC, Schwartz LH, et al. A phase ii study of telisotuzumab vedotin in patients with C-met-positive stage iv or recurrent squamous cell lung cancer (Lung-map sub-study S1400k, nct03574753). Clin Lung Cancer (2021) 22(3):170–7. doi: 10.1016/j.cllc.2020.09.013
66. Horinouchi H, Shibata Y, Looman J, Sui Y, Noon E, Lu S. Phase 2 study of telisotuzumab vedotin (Teliso-V) monotherapy in patients with previously untreated met-amplified locally advanced/metastatic non-squamous non-small cell lung cancer (Nsq nsclc). J Clin Oncol (2023) 41(16_suppl):TPS9149–TPS. doi: 10.1200/JCO.2023.41.16_suppl.TPS9149
67. Lugini A, Goldman JW, Tanizaki J, Akamatsu H, Xia S, Ratajczak C, et al. 1501tip a phase iii global study of telisotuzumab vedotin versus docetaxel in previously treated patients with C-met overexpressing, egfr wildtype, locally advanced/metastatic nonsquamous nsclc (Telimet nsclc-01). Ann Oncol (2023) 34:S845. doi: 10.1016/j.annonc.2023.09.2532
68. Kato S, Okamura R, Mareboina M, Lee S, Goodman A, Patel SP, et al. Revisiting epidermal growth factor receptor (Egfr) amplification as a target for anti-egfr therapy: analysis of cell-free circulating tumor DNA in patients with advanced Malignancies. JCO Precis Oncol (2019) 3. doi: 10.1200/po.18.00180
69. Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, egfr-mutated advanced nsclc. N Engl J Med (2020) 382(1):41–50. doi: 10.1056/NEJMoa1913662
70. Zhang G, Li X, Chen Q, Li J, Ruan Q, Chen YH, et al. Cd317 activates egfr by regulating its association with lipid rafts. Cancer Res (2019) 79(9):2220–31. doi: 10.1158/0008-5472.Can-18-2603
71. Qiu M-Z, Zhang Y, Guo Y, Guo W, Nian W, Liao W, et al. Evaluation of safety of treatment with anti–epidermal growth factor receptor antibody drug conjugate mrg003 in patients with advanced solid tumors: A phase 1 nonrandomized clinical trial. JAMA Oncol (2022) 8(7):1042–6. doi: 10.1001/jamaoncol.2022.0503
72. Wan W, Zhao S, Zhuo S, Zhang Y, Chen L, Li G, et al. Abstract 2642: bl-B01d1, a novel egfr×Her3-targeting adc, demonstrates robust anti-tumor efficacy in preclinical evaluation. Cancer Res (2023) 83(7_Supplement):2642. doi: 10.1158/1538-7445.Am2023-2642
73. Zhang L, Ma Y, Zhao Y, Fang W, Zhao H, Huang Y, et al. Bl-B01d1, a first-in-class egfrxher3 bispecific antibody-drug conjugate (Adc), in patients with locally advanced or metastatic solid tumor: results from a first-in-human phase 1 study. J Clin Oncol (2023) 41(16_suppl):3001. doi: 10.1200/JCO.2023.41.16_suppl.3001
74. Boudeau J, Miranda-Saavedra D, Barton GJ, Alessi DR. Emerging roles of pseudokinases. Trends Cell Biol (2006) 16(9):443–52. doi: 10.1016/j.tcb.2006.07.003
75. Katoh M. Antibody-drug conjugate targeting protein tyrosine kinase 7, a receptor tyrosine kinase-like molecule involved in wnt and vascular endothelial growth factor signaling: effects on cancer stem cells, tumor microenvironment and whole-body homeostasis. Ann Transl Med (2017) 5(23):462. doi: 10.21037/atm.2017.09.11
76. Chen R, Khatri P, Mazur PK, Polin M, Zheng Y, Vaka D, et al. A meta-analysis of lung cancer gene expression identifies ptk7 as a survival gene in lung adenocarcinoma. Cancer Res (2014) 74(10):2892–902. doi: 10.1158/0008-5472.Can-13-2775
77. Damelin M, Bankovich A, Bernstein J, Lucas J, Chen L, Williams S, et al. A ptk7-targeted antibody-drug conjugate reduces tumor-initiating cells and induces sustained tumor regressions. Sci Transl Med (2017) 9(372). doi: 10.1126/scitranslmed.aag2611
78. Maderna A, Doroski M, Subramanyam C, Porte A, Leverett CA, Vetelino BC, et al. Discovery of cytotoxic dolastatin 10 analogues with N-terminal modifications. J Med Chem (2014) 57(24):10527–43. doi: 10.1021/jm501649k
79. Maitland ML, Sachdev JC, Sharma MR, Moreno V, Boni V, Kummar S, et al. First-in-human study of pf-06647020 (Cofetuzumab pelidotin), an antibody-drug conjugate targeting protein tyrosine kinase 7, in advanced solid tumors. Clin Cancer Res (2021) 27(16):4511–20. doi: 10.1158/1078-0432.Ccr-20-3757
80. Hassan R, Thomas A, Alewine C, Le DT, Jaffee EM, Pastan I. Mesothelin immunotherapy for cancer: ready for prime time? J Clin Oncol (2016) 34(34):4171–9. doi: 10.1200/jco.2016.68.3672
81. Bharadwaj U, Marin-Muller C, Li M, Chen C, Yao Q. Mesothelin confers pancreatic cancer cell resistance to tnf-Α-induced apoptosis through akt/pi3k/nf-Κb activation and il-6/mcl-1 overexpression. Mol Cancer (2011) 10:106. doi: 10.1186/1476-4598-10-106
82. Chen SH, Hung WC, Wang P, Paul C, Konstantopoulos K. Mesothelin binding to ca125/muc16 promotes pancreatic cancer cell motility and invasion via mmp-7 activation. Sci Rep (2013) 3:1870. doi: 10.1038/srep01870
83. Servais EL, Colovos C, Rodriguez L, Bograd AJ, Nitadori J, Sima C, et al. Mesothelin overexpression promotes mesothelioma cell invasion and mmp-9 secretion in an orthotopic mouse model and in epithelioid pleural mesothelioma patients. Clin Cancer Res (2012) 18(9):2478–89. doi: 10.1158/1078-0432.Ccr-11-2614
84. Golfier S, Kopitz C, Kahnert A, Heisler I, Schatz CA, Stelte-Ludwig B, et al. Anetumab ravtansine: A novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect. Mol Cancer Ther (2014) 13(6):1537–48. doi: 10.1158/1535-7163.Mct-13-0926
85. Hassan R, Blumenschein GR Jr., Moore KN, Santin AD, Kindler HL, Nemunaitis JJ, et al. First-in-human, multicenter, phase I dose-escalation and expansion study of anti-mesothelin antibody-drug conjugate anetumab ravtansine in advanced or metastatic solid tumors. J Clin Oncol (2020) 38(16):1824–35. doi: 10.1200/jco.19.02085
86. Nygren MK, Tekle C, Ingebrigtsen VA, Fodstad O. B7-H3 and its relevance in cancer; immunological and non-immunological perspectives. Front Biosci (Elite Ed) (2011) 3(3):989–93. doi: 10.2741/e304
87. Chen YW, Tekle C, Fodstad O. The immunoregulatory protein human B7h3 is a tumor-associated antigen that regulates tumor cell migration and invasion. Curr Cancer Drug Targets (2008) 8(5):404–13. doi: 10.2174/156800908785133141
88. Jin Y, Zhang P, Li J, Zhao J, Liu C, Yang F, et al. B7-H3 in combination with regulatory T cell is associated with tumor progression in primary human non-small cell lung cancer. Int J Clin Exp Pathol (2015) 8(11):13987–95.
89. Scribner JA, Brown JG, Son T, Chiechi M, Li P, Sharma S, et al. Preclinical development of mgc018, a duocarmycin-based antibody-drug conjugate targeting B7-H3 for solid cancer. Mol Cancer Ther (2020) 19(11):2235–44. doi: 10.1158/1535-7163.Mct-20-0116
90. Shenderov E, Mallesara G, Wysocki P, Xu W, Ramlau R, Weickhardt A, et al. 620p mgc018, an anti-B7-H3 antibody-drug conjugate (Adc), in patients with advanced solid tumors: preliminary results of phase I cohort expansion. Ann Oncol (2021) 32:S657–S9. doi: 10.1016/j.annonc.2021.08.1133
91. Hisada Y, Mackman N. Tissue factor and cancer: regulation, tumor growth, and metastasis. Semin Thromb Hemost (2019) 45(4):385–95. doi: 10.1055/s-0039-1687894
92. Breij EC, de Goeij BE, Verploegen S, Schuurhuis DH, Amirkhosravi A, Francis J, et al. An antibody-drug conjugate that targets tissue factor exhibits potent therapeutic activity against a broad range of solid tumors. Cancer Res (2014) 74(4):1214–26. doi: 10.1158/0008-5472.Can-13-2440
93. de Bono JS, Concin N, Hong DS, Thistlethwaite FC, Machiels J-P, Arkenau H-T, et al. Tisotumab vedotin in patients with advanced or metastatic solid tumours (Innovatv 201): A first-in-human, multicentre, phase 1–2 trial. Lancet Oncol (2019) 20(3):383–93. doi: 10.1016/S1470-2045(18)30859-3
94. Zhu C, Wei Y, Wei X. Axl receptor tyrosine kinase as a promising anti-cancer approach: functions, molecular mechanisms and clinical applications. Mol Cancer (2019) 18(1):153. doi: 10.1186/s12943-019-1090-3
95. Gay CM, Balaji K, Byers LA. Giving axl the axe: targeting axl in human Malignancy. Br J Cancer (2017) 116(4):415–23. doi: 10.1038/bjc.2016.428
96. Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise T, et al. Activation of the axl kinase causes resistance to egfr-targeted therapy in lung cancer. Nat Genet (2012) 44(8):852–60. doi: 10.1038/ng.2330
97. Boshuizen J, Koopman LA, Krijgsman O, Shahrabi A, van den Heuvel EG, Ligtenberg MA, et al. Cooperative targeting of melanoma heterogeneity with an axl antibody-drug conjugate and braf/mek inhibitors. Nat Med (2018) 24(2):203–12. doi: 10.1038/nm.4472
98. Koopman LA, Terp MG, Zom GG, Janmaat ML, Jacobsen K, Gresnigt-van den Heuvel E, et al. Enapotamab vedotin, an axl-specific antibody-drug conjugate, shows preclinical antitumor activity in non-small cell lung cancer. JCI Insight (2019) 4(21). doi: 10.1172/jci.insight.128199
99. Ameratunga M, Harvey RD, Mau-Sørensen M, Thistlethwaite F, Forssmann U, Gupta M, et al. First-in-human, dose-escalation, phase (Ph) I trial to evaluate safety of anti-axl antibody-drug conjugate (Adc) enapotamab vedotin (Enav) in solid tumors. J Clin Oncol (2019) 37(15_suppl):2525. doi: 10.1200/JCO.2019.37.15_suppl.2525
100. Ramalingam S, Lopez J, Mau-Sorensen M, Thistlethwaite F, Piha-Paul S, Gadgeel S, et al. Oa02.05 first-in-human phase 1/2 trial of anti-axl antibody–Drug conjugate (Adc) enapotamab vedotin (Enav) in advanced nsclc. J Thorac Oncol (2019) 14(10):S209. doi: 10.1016/j.jtho.2019.08.414
101. Jiang Z, Hao Y, Ding X, Zhang Z, Liu P, Wei X, et al. The effects and mechanisms of slc34a2 on tumorigenicity in human non-small cell lung cancer stem cells. Tumour Biol (2016) 37(8):10383–92. doi: 10.1007/s13277-016-4928-y
102. Heynemann S, Yu H, Churilov L, Rivalland G, Asadi K, Mosher R, et al. Napi2b expression in a large surgical non-small cell lung cancer (Nsclc) cohort. Clin Lung Cancer (2022) 23(2):e90–e8. doi: 10.1016/j.cllc.2021.11.005
103. Lin K, Rubinfeld B, Zhang C, Firestein R, Harstad E, Roth L, et al. Preclinical development of an anti-napi2b (Slc34a2) antibody–drug conjugate as a therapeutic for non–small cell lung and ovarian cancers. Clin Cancer Res (2015) 21(22):5139–50. doi: 10.1158/1078-0432.Ccr-14-3383
104. Gerber DE, Infante JR, Gordon MS, Goldberg SB, Martín M, Felip E, et al. Phase ia study of anti-napi2b antibody–drug conjugate lifastuzumab vedotin dnib0600a in patients with non–small cell lung cancer and platinum-resistant ovarian cancer. Clin Cancer Res (2020) 26(2):364–72. doi: 10.1158/1078-0432.Ccr-18-3965
105. Bodyak ND, Mosher R, Yurkovetskiy AV, Yin M, Bu C, Conlon PR, et al. The dolaflexin-based antibody-drug conjugate xmt-1536 targets the solid tumor lineage antigen slc34a2/napi2b. Mol Cancer Ther (2021) 20(5):896–905. doi: 10.1158/1535-7163.Mct-20-0183
106. Richardson DL, Barve MA, Strauss JF, Ulahannan SV, Moore KN, Hamilton EP, et al. Phase I expansion study of xmt-1536, a novel napi2b-targeting antibody-drug conjugate (Adc): preliminary efficacy, safety, and biomarker results in patients with previously treated metastatic ovarian cancer (Oc) or non-small cell lung cancer (Nsclc). J Clin Oncol (2020) 38(15_suppl):3549. doi: 10.1200/JCO.2020.38.15_suppl.3549
107. Camacho-Leal P, Zhai AB, Stanners CP. A co-clustering model involving alpha5beta1 integrin for the biological effects of gpi-anchored human carcinoembryonic antigen (Cea). J Cell Physiol (2007) 211(3):791–802. doi: 10.1002/jcp.20989
108. Johnson ML, Chadjaa M, Yoruk S, Besse B. Phase iii trial comparing antibody-drug conjugate (Adc) sar408701 with docetaxel in patients with metastatic non-squamous non-small cell lung cancer (Nsq nsclc) failing chemotherapy and immunotherapy. J Clin Oncol (2020) 38(15_suppl):TPS9625–TPS. doi: 10.1200/JCO.2020.38.15_suppl.TPS9625
109. Decary S, Berne PF, Nicolazzi C, Lefebvre AM, Dabdoubi T, Cameron B, et al. Preclinical activity of sar408701: A novel anti-ceacam5-maytansinoid antibody-drug conjugate for the treatment of ceacam5-positive epithelial tumors. Clin Cancer Res (2020) 26(24):6589–99. doi: 10.1158/1078-0432.Ccr-19-4051
110. Gazzah A, Ricordel C, Cousin S, Cho BC, Calvo E, Kim TM, et al. Efficacy and safety of the antibody-drug conjugate (Adc) sar408701 in patients (Pts) with non-squamous non-small cell lung cancer (Nsq nsclc) expressing carcinoembryonic antigen-related cell adhesion molecule 5 (Ceacam5). J Clin Oncol (2020) 38(15_suppl):9505. doi: 10.1200/JCO.2020.38.15_suppl.9505
111. Cousin S, Soufflet C. Ep08.02-030 tusamitamab ravtansine in patients with nsq nsclc and negative or moderate ceacam5 expression tumors and high circulating cea. J Thorac Oncol (2022) 17(9):S411. doi: 10.1016/j.jtho.2022.07.712
112. Paz-Ares L, Parakh S, Park J, Rojas C, Orlandi F, Veillon R, et al. 75tip open-label, phase ii study of tusamitamab ravtansine (Sar408701) in combination with pembrolizumab and with pembrolizumab + Platinum-based chemotherapy +/– pemetrexed in patients with ceacam5-positive nonsquamous nsclc (Carmen-lc05). Ann Oncol (2022) 33:S65–S6. doi: 10.1016/j.annonc.2022.02.084
113. Cho BC, Aguado de la Rosa C, Vilà L, Isla D, Oliveira J, de Castro Carpeno J, et al. 1364tip phase ii single-arm trial of safety, antitumor activity, and pharmacokinetics of tusamitamab ravtansine (Sar408701) plus ramucirumab in ceacam5-positive, metastatic, non-squamous, non-small cell lung cancer progressing on platinum-based chemotherapy and immunotherapy. Ann Oncol (2021) 32:S1034–S5. doi: 10.1016/j.annonc.2021.08.1965
114. Menck K, Heinrichs S, Baden C, Bleckmann A. The wnt/ror pathway in cancer: from signaling to therapeutic intervention. Cells (2021) 10(1). doi: 10.3390/cells10010142
115. Lu C, Wang X, Zhu H, Feng J, Ni S, Huang J. Over-expression of ror2 and wnt5a cooperatively correlates with unfavorable prognosis in patients with non-small cell lung cancer. Oncotarget (2015) 6(28):24912–21. doi: 10.18632/oncotarget.4701
116. Alexander M, Starodub A. 77tip a phase I/ii dose escalation and dose expansion study of ozuriftamab vedotin (Ba3021) alone and in combination with nivolumab in patients with advanced solid tumors including non-small cell lung cancer. J Thorac Oncol (2023) 18(4):S84. doi: 10.1016/S1556-0864(23)00331-3
117. Breuss JM, Gallo J, DeLisser HM, Klimanskaya IV, Folkesson HG, Pittet JF, et al. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci (1995) 108(Pt 6):2241–51. doi: 10.1242/jcs.108.6.2241
118. Böger C, Kalthoff H, Goodman SL, Behrens HM, Röcken C. Integrins and their ligands are expressed in non-small cell lung cancer but not correlated with parameters of disease progression. Virchows Arch (2014) 464(1):69–78. doi: 10.1007/s00428-013-1506-1
119. Brzozowska E, Deshmukh S. Integrin alpha V beta 6 (Αvβ6) and its implications in cancer treatment. Int J Mol Sci (2022) 23(20). doi: 10.3390/ijms232012346
120. Lyon RP, Jonas M, Frantz C, Trueblood ES, Yumul R, Westendorf L, et al. Sgn-B6a: A new vedotin antibody-drug conjugate directed to integrin beta-6 for multiple carcinoma indications. Mol Cancer Ther (2023) 22(12):1444–53. doi: 10.1158/1535-7163.Mct-22-0817
121. Hollebecque A, Lopez JS, Piha-Paul SA, Dowlati A, Patnaik A, Galvao V, et al. Sgn-B6a, an integrin beta-6 (Itgb6)-targeted antibody-drug conjugate (Adc), in patients with advanced solid tumors: updated results from a phase 1 study (Sgnb6a-001). J Clin Oncol (2023) 41(16_suppl):3024. doi: 10.1200/JCO.2023.41.16_suppl.3024
122. Garassino MC, Reznick D, Liu SY, Reinmuth N, Girard N, Marinis Fd, et al. Evoke-01: A phase 3 study of sacituzumab govitecan (Sg) versus docetaxel in patients with non–small cell lung cancer (Nsclc) progressing on or after platinum-based chemotherapy and checkpoint inhibitors. J Clin Oncol (2022) 40(16_suppl):TPS9149–TPS. doi: 10.1200/JCO.2022.40.16_suppl.TPS9149
123. Okamoto I, Kuyama S, Girard N, Lu S, Franke FA, Pan E, et al. 1505tip tropion-lung07: A phase iii trial of datopotamab deruxtecan (Dato-dxd) plus pembrolizumab (Pembro) with or without platinum chemotherapy (Pt-ct) as first-line (1l) therapy in advanced/metastatic (Adv/met) non-small cell lung cancer (Nsclc) with pd-L1 expression. Ann Oncol (2023) 34:S847–S8. doi: 10.1016/j.annonc.2023.09.2536
124. Levy BP, Felip E, Reck M, Yang JC, Cappuzzo F, Yoneshima Y, et al. Tropion-lung08: phase iii study of datopotamab deruxtecan plus pembrolizumab as first-line therapy for advanced nsclc. Future Oncol (2023) 19(21):1461–72. doi: 10.2217/fon-2023-0230
125. Drilon AE, Awad MM, Gadgeel SM, Villaruz LC, Sabari JK, Perez J, et al. A phase 1/2 study of regn5093-M114, a metxmet antibody-drug conjugate, in patients with mesenchymal epithelial transition factor (Met)-overexpressing nsclc. J Clin Oncol (2022) 40(16_suppl):TPS8593–TPS. doi: 10.1200/JCO.2022.40.16_suppl.TPS8593
126. Janne PA, Mostillo J, Shrestha P, Zhang R, Fan P-D, Cantero F. Phase 1 study of patritumab deruxtecan (Her3-dxd; U3-1402) in combination with osimertinib in patients with advanced egfr-mutated nsclc. J Clin Oncol (2022) 40(16_suppl):TPS3161–TPS. doi: 10.1200/JCO.2022.40.16_suppl.TPS3161
127. Spira AI, Johnson ML, Blumenschein GR, Burns TF, Thompson JR, Deshpande A, et al. Phase 1 multicenter dose escalation and dose expansion study of antibody-drug conjugate (Adc) mytx-011 in subjects with non-small cell lung cancer. J Clin Oncol (2023) 41(16_suppl):TPS9147–TPS. doi: 10.1200/JCO.2023.41.16_suppl.TPS9147
128. Aggarwal C, Azzoli CG, Spira AI, Solomon BJ, Le X, Rolfo C, et al. Egret: A first-in-human study of the novel antibody-drug conjugate (Adc) azd9592 as monotherapy or combined with other anticancer agents in patients (Pts) with advanced solid tumors. J Clin Oncol (2023) 41(16_suppl):TPS3156–TPS. doi: 10.1200/JCO.2023.41.16_suppl.TPS3156
129. Zutshi A, Neuteboom B, Kumar S, Sloot W, Knuehl C, Dotterweich J, et al. Abstract 5423: translational pk/pd/efficacy modeling and efficacious human dose prediction for a first-in-class muc1-egfr (M1231) bispecific antibody drug conjugate. Cancer Res (2022) 82(12_Supplement):5423. doi: 10.1158/1538-7445.Am2022-5423
130. Patnaik A, Lakhani NJ, Xu P, Nazarenko NN, Call JA. Phase 1 study of sgn-B7h4v, a novel, investigational vedotin antibody–drug conjugate directed to B7-H4, in patients with advanced solid tumors (Sgnb7h4v-001, trial in progress). J Clin Oncol (2022) 40(16_suppl):TPS3155–TPS. doi: 10.1200/JCO.2022.40.16_suppl.TPS3155
131. Ulahannan S, Johnson ML, Park H, Vandross A, Uttamsingh S, Li J, et al. A phase 1 study of the anti-tissue factor antibody-drug conjugate xb002 in patients with advanced solid tumors (Jewel-101): initial results from the dose-escalation stage. Eur J Cancer (2022) 174:S92–S3. doi: 10.1016/S0959-8049(22)01043-7
132. Shimizu T, Fujiwara Y, Yonemori K, Koyama T, Sato J, Tamura K, et al. First-in-human phase 1 study of morab-202, an antibody–drug conjugate comprising farletuzumab linked to eribulin mesylate, in patients with folate receptor-Α–positive advanced solid tumors. Clin Cancer Res (2021) 27(14):3905–15. doi: 10.1158/1078-0432.Ccr-20-4740
133. Planchard D, Paz-Ares L, Spira A, Felip E, McCune S, Cascella T, et al. 75tip a multicenter, open-label, phase ii trial evaluating the safety and efficacy of folate receptor alpha (Frα) antibody-drug conjugate (Adc) farletuzumab ecteribulin (Fzec*) in patients with previously treated, metastatic non-small cell lung cancer (Nsclc) adenocarcinoma (Ac). J Thorac Oncol (2023) 18(4):S83–S4. doi: 10.1016/S1556-0864(23)00329-5
134. Call JA, Orr DW, Anderson IC, Richardson DL, Chen Z, Ma S, et al. Phase 1/2 study of pro1184, a novel folate receptor alpha-directed antibody-drug conjugate, in patients with locally advanced and/or metastatic solid tumors. J Clin Oncol (2023) 41(16_suppl):TPS3157–TPS. doi: 10.1200/JCO.2023.41.16_suppl.TPS3157
135. Johnson M, El-Khoueiry A, Hafez N, Lakhani N, Mamdani H, Rodon J, et al. First-in-human study of the probody therapeutic cx-2029 in adults with advanced solid tumor Malignancies. Clin Cancer Res (2021) 27(16):4521–30. doi: 10.1158/1078-0432.Ccr-21-0194
136. Boni V, III HAB, Liu JF, Spira AI, Arkenau H-T, Fidler MJ, et al. Cx-2009, a cd166-directed probody drug conjugate (Pdc): results from the first-in-human study in patients (Pts) with advanced cancer including breast cancer (Bc). J Clin Oncol (2020) 38(15_suppl):526. doi: 10.1200/JCO.2020.38.15_suppl.526
137. Patnaik A, Meric-Bernstam F, Lima CMSPR, Robert F, Dowlati A, Kindler HL, et al. Sgn228-001: A phase I open-label dose-escalation, and expansion study of sgn-cd228a in select advanced solid tumors. J Clin Oncol (2020) 38(15_suppl):TPS3652–TPS. doi: 10.1200/JCO.2020.38.15_suppl.TPS3652
138. Ernstoff MS, Ma WW, Tsai FY-C, Munster PN, Zhang T, Kamoun W, et al. A phase 1 study evaluating the safety, pharmacology and preliminary activity of mm-310 in patients with solid tumors. J Clin Oncol (2018) 36(15_suppl):TPS2604–TPS. doi: 10.1200/JCO.2018.36.15_suppl.TPS2604
139. Klempner SJ, Beeram M, Sabanathan D, Chan A, Hamilton E, Loi S, et al. 209p interim results of a phase I/ib study of sbt6050 monotherapy and pembrolizumab combination in patients with advanced her2-expressing or amplified solid tumors. Ann Oncol (2021) 32:S450. doi: 10.1016/j.annonc.2021.08.491
140. Diamond JR, Henry JT, Falchook GS, Olszanski AJ, Singh H, Leonard EJ, et al. Phase 1a/1b study design of the novel sting agonist, immune-stimulating antibody-conjugate (Isac) tak-500, with or without pembrolizumab in patients with advanced solid tumors. J Clin Oncol (2022) 40(16_suppl):TPS2690–TPS. doi: 10.1200/JCO.2022.40.16_suppl.TPS2690
141. Khongorzul P, Ling CJ, Khan FU, Ihsan AU, Zhang J. Antibody-drug conjugates: A comprehensive review. Mol Cancer Res (2020) 18(1):3–19. doi: 10.1158/1541-7786.Mcr-19-0582
142. Drago JZ, Modi S, Chandarlapaty S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat Rev Clin Oncol (2021) 18(6):327–44. doi: 10.1038/s41571-021-00470-8
143. Hafeez U, Parakh S, Gan HK, Scott AM. Antibody-drug conjugates for cancer therapy. Molecules (2020) 25(20). doi: 10.3390/molecules25204764
Glossary
Keywords: NSCLC, antibody-drug conjugate, targeted therapy, clinical outcome, mechanisms of action
Citation: Liu X, Deng J, Zhang R, Xing J, Wu Y, Chen W, Liang B, Xing D, Xu J and Zhang M (2023) The clinical development of antibody-drug conjugates for non-small cell lung cancer therapy. Front. Immunol. 14:1335252. doi: 10.3389/fimmu.2023.1335252
Received: 08 November 2023; Accepted: 28 November 2023;
Published: 11 December 2023.
Edited by:
Yuanzhi Chen, Xiamen University, ChinaReviewed by:
Xue Liu, Xiamen University, ChinaYasuaki Anami, University of Texas Health Science Center at Houston, United States
Aiko Yamaguchi, University of Texas MD Anderson Cancer Center, United States
Copyright © 2023 Liu, Deng, Zhang, Xing, Wu, Chen, Liang, Xing, Xu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiazhen Xu, MTgyNTMxOTk1MDJAMTYzLmNvbQ==; Miao Zhang, emhhbmdtaWFvQHFkdS5lZHUuY24=
 Xinlin Liu
Xinlin Liu Junwen Deng1,2
Junwen Deng1,2 Renshuai Zhang
Renshuai Zhang Bing Liang
Bing Liang Jiazhen Xu
Jiazhen Xu Miao Zhang
Miao Zhang