
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Immunol. , 05 January 2024
Sec. Immunological Tolerance and Regulation
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1334231
This article is part of the Research Topic Immunology at the feto-maternal interface View all 19 articles
Systemic autoimmune diseases such as systemic lupus erythematosus (SLE), antiphospholipid syndrome (APS), and Sjögren’s disease (SjD) affect predominantly women of childbearing age. SLE is a heterogeneous autoimmune disease characterized by interferon upregulation, production of autoantibodies and systemic symptoms (1). Patients with APS have thrombosis and/or pregnancy morbidity associated with persistent positivity of antiphospholipid antibodies (aPL) (2), whereas SjD is characterized by exocrine glandular lymphocytic infiltration, sicca symptoms, extra glandular manifestations such as arthritis, and sometimes presence of auto-antibodies, especially against SSA and SSB (3). These autoimmune diseases have been associated with a higher risk of adverse pregnancy outcomes (APO) like intrauterine fetal death, fetal growth restriction (FGR), preterm birth, low birth weight, and preeclampsia (2–4). Type I interferon signature and complement activation in peripheral blood have been linked to this increased APO risk (5–7).
The menstrual cycle is governed by a sophisticated interaction involving endometrial cells, immune cells, cytokines and sex hormones (8). It is known that the endometrial immune environment prepares to accept the embryo and facilitates implantation (9). Receptive endometrium is characterized by a pro-inflammatory response, complement activation, and an adequate interaction between extracellular vesicles, endometrial epithelial cells and the blastocyst (10). Therefore, an optimal balance between pro-inflammatory factors and the tolerogenic adaptive immune response in the endometrial tissue is pivotal for proper embryo implantation (11, 12).
Preconceptional disease activity can predict APO in patients with systemic autoimmune diseases, which stresses the importance of remission prior to conception. However, not all APO can be predicted by these disease-related factors (7, 13). Local immune changes in the endometrial environment might also contribute to this increased risk for APO. Endometrial immune profiling is a new method to analyze the immune cell distribution and cytokine production in endometrial tissue samples or menstrual blood. Different techniques are used such as multiparameter flow cytometry or gene expression analysis (14). It has already been suggested as a new screening strategy for personalized care for couples with repeated embryo implantation failures using assisted-reproductive therapy (ART) (15). In this paper, we describe the endometrial immune environment, the method of endometrial immune profiling and hypothesize that this technique might be a valuable tool to assess local immune changes that possibly play a role in development of APO in women with systemic autoimmune diseases.
Immune cells in the endometrium/decidua are essential during implantation and placentation and immune imbalances have been associated with subsequent placental development failure (16). Uterine natural killer cells (uNK), CD4+ T regulatory cells and different subsets of CD68+ macrophages play a pivotal role in decidualization (maturation of the endometrium) and immune tolerance maintenance throughout pregnancy (12).
Early in pregnancy, uNK account for 70% of decidual lymphocytes and are involved in decidualization, trophoblast cell invasion, uterine vascular remodeling and immune tolerance (17). Notably, using a bioinformatics approach, it has been shown that impaired uNK function and defective endometrium maturation during early pregnancy preceded the development of preeclampsia (18). Decidua natural killer cells (dNK) express an inhibitor profile during gestation regulated by maternal HLA-C alleles through inhibitory receptors such as killer cell immunoglobulin-like receptors (KIRs), natural killer cell receptor NKG2A, and leukocyte immunoglobulin-like receptor B1 (LILRB1) (17). Interestingly, higher endometrial levels of transforming growth factor-beta (TGF-ß) have been associated with dNK-impaired maturation in patients with preeclampsia (19).
Other maternal leukocytes are also involved in decidualization processes and induce immune tolerance of the semi-allogenic fetus during early stages of pregnancy. It has been shown that levels of CD4+ CD25+ Foxp3+ T regulatory cells in peripheral blood fluctuate during the menstrual cycle, possibly preparing for pregnancy (20). During pregnancy, CD4+ T regulatory cells support maternal vascular adaptation and prevent placental inflammatory pathology (21). A lower number of these cells and/or impaired function have been associated with defective placentation and subsequent development of preeclampsia (22). The local T helper cell phenotype varies throughout pregnancy. During the implantation window, a Th1 response is crucial in inducing low-grade inflammation, immune cell recruitment, and tissue remodeling (23). Later on, production of IL-4, IL-5, IL-10 and IL-13 is promoted by a shift towards Th2 phenotype induced by decidual dendritic cells and the increased production of human chorionic gonadotropin (hCG), progesterone and estradiol during pregnancy (24). T helper cell imbalances with a predominant Th1 phenotype and a lower count of IL-10 producing CD8+ T cells have been associated with implantation failure and pregnancy loss (25). Interestingly, Th17 phenotype polarization and lower levels of Foxp3 T regulatory cells have been identified in blood from women with early onset preeclampsia compared with normotensive controls (26). Decidual CD8+ T cells are involved in antiviral protection and fetal tolerance due to an immunosuppressive phenotype through T-cell immunoglobulin mucin-3 (Tim-3), programmed cell death 1 (PD-1), and Cytotoxic T-Lymphocyte associated protein 4 (CTLA-4) inhibitory pathways. Notably, blockage of these pathways was correlated with fetal loss in animal models (27, 28).
A higher proportion of CD20+ B cells has been identified in decidua from patients with recurrent pregnancy loss compared to controls (29). Although B cells make up a small proportion of decidual lymphocytes, a regulatory B cell subset has been linked to fetal tolerance and Th1 suppression, preventing allogeneic responses against the fetus through IL-10 production (24).
Endometrial CD68+ macrophages also play an essential role in the menstrual cycle, restoring the endometrial integrity in preparation for pregnancy (30). Alterations in proportions of endometrial classical activated macrophages (M1) and alternatively activated macrophages (M2) have been described as predictors of implantation failure in patients under ART (31). Notably, a higher prevalence of CD163+ M2 type macrophages has been described during the proliferative phase in endometrial tissue, and was related to adverse implantation outcomes (31).
In conclusion, a complex immunological endometrial environment is linked to decidualization and subsequent healthy pregnancy development in the general population, and immune imbalances have been detected in patients with APO, especially preeclampsia, fetal loss and infertility. Nevertheless, there is no information concerning endometrial immune imbalances in patients with SLE, APS or SjD.
Lédée et al. proposed endometrial immune profiling based on RT-qPCR analysis of endometrial biopsies, collected by aspiration during mid-luteal phase, focusing on CD56+ uNK levels, Interleukin-18/Tumor necrosis factor-like weak inducer of apoptosis (IL-18/TWEAK) and Interleukin 15/Fibroblast growth factor-inducible molecule (IL-15/Fn-14) mRNA ratios as key factors involved in uterine embryo receptivity (14, 15). The IL-18/TWEAK mRNA ratio reflects the Th1/Th2 balance and local angiogenesis, while the IL-15/Fn-14 mRNA ratio reflects uNK maturation. An overactive endometrial immune environment in biopsies during mid-luteal phase was established in the presence of high ratios or high uNK count, while low endometrial immune activation corresponded to low ratios and/or low uNK count (15).
This method was used in infertility patients as a preconception tool to assess suitability of the uterine immunological environment for embryo implantation, and results were used for personalized treatment decisions (32). Treatment modifications differed between patients classified as overactive and those with low endometrial immune activation levels, but overall an increase in live birth rate was achieved in analyzed and treated patients compared to non-analyzed (32). In brief, overactive patients received high-dose luteal hormonal support, oral estradiol supplementation, 20mg of corticosteroids, and vitamin E. At the same time, in those with low endometrial immune activation levels, an endometrial scratching or other local injury was performed and accompanied by hCG supplementation and standard-dose luteal hormonal support (32).
In subsequent studies by Lédée et al., higher pregnancy rates were achieved in patients under ART with a history of repeated implantation failures or recurrent pregnancy loss after endometrial immune profiling assessment (14). Patients were classified as having no dysregulation, low immune activation, over immune activation or mixed profile and received personalized treatment accordingly. Those patients with over-immune activation or mixed profile received immunotherapy with corticosteroids adjunction or low molecular weight heparin in case of resistance to corticosteroids. In contrast, those with low immune activation received endometrial scratching (14). Overall, some infertility patients undergo uterine immune dysregulations and immune profiling might aid in optimizing care and outcomes in these patients (14, 33).
Marron, et al. performed endometrial analysis of luteal-phase biopsies focusing on lymphocyte subsets with a flow cytometry panel assessing natural killer cells (NKs) subsets (uNK, peripheral NKs and T NKs), CD19+ B lymphocytes, CD8+ T lymphocytes, CD4+ T lymphocytes and their Th1, Th2, Th17 and regulatory subsets. Notably, higher concentrations of peripheral NKs (CD16+, CD56dim) and CD19+ B lymphocytes were identified in patients with a history of infertility or recurrent pregnancy loss compared to healthy controls (34). Furthermore, commercial endometrial immune profiling tests using endometrial tissue samples have been introduced in clinical practice for patients under ART (35). In those patients, a high expression of B-cell CLL/Lymphoma 6 (BCL6) by immunohistochemistry was identified as a marker for implantation failure or pregnancy loss (36).
Assessment of uNK, plasma cells, CD68+ macrophages, CXC-motif ligand 1 (CXCL1), CXC-motif receptor 2 (CXCR2), syndecan-1 and Vascular Endothelial Growth Factor A (VEGF-A) with immunohistochemistry has been used to map the endometrial immune environment in infertility patients with endometriosis. Interestingly, more macrophages were found in patients with endometriosis than those without and macrophage count was negatively correlated with uNK count in these patients (37).
So different techniques are described to assess the endometrium immune balance. We acknowledge that sample collection is an issue of matter since endometrial biopsy is an invasive procedure with inherent risks. Recent studies confirmed that menstrual blood collected during the first twenty-four hours of menstruation resemble the endometrial environment and can be used as an alternative, easier-to-get sample (38, 39). Recently, multiparameter flow cytometry panels have analyzed lymphocyte subpopulations from menstrual blood samples. Notably, subpopulations differed from peripheral blood samples and between patients with recurrent pregnancy loss and healthy controls (39, 40). Although it has been described that the cytokine profile of menstrual blood differed from the peripheral blood with a higher expression of IL-6, IL-1ß and CXCL8 in healthy controls, there is no information concerning cytokine profile in menstrual blood in patients with systemic autoimmune diseases (41). Therefore, we hypothesize that menstrual blood immunophenotyping might provide valuable insight prior to conception in those patients (34).
“The European League Against Rheumatism (EULAR) recommendations for women’s health and pregnancy in patients with SLE or APS” encourage risk stratification before pregnancy considering disease activity, comorbidities, autoantibody profile and medication use as an essential tool to improve pregnancy outcomes in these patients (13). Nevertheless, a high proportion of patients still develop APO. Although different pathologic mechanisms have been associated with APO development, the specific chain of events preceding APO is not fully understood. Moreover, associations between disease activity, and endometrial immune changes are unknown.
A better understanding of pathophysiology, including new valid biomarkers, developing clinical instruments for obstetric risk assessment and new personalized interventions are essential to improve pregnancy outcomes in these patients. Given its association with APO development, endometrial immune profiling might be a new tool for preconceptional assessment in patients with systemic autoimmune diseases such as SLE, APS and SjD.
We hypothesize that non-optimal endometrial immune imbalances might be related to APO in women with autoimmune diseases. In Figure 1, we propose a methodology combining endometrial immune profiling with assessment of clinical data, peripheral blood cells and interferon signature analysis for SLE, APS and SjD patients. We aim for a new approach combining previously described preconception risk factors and immunological changes in the endometrium (5, 7, 42). Briefly, we suggest assessing T cell, uNK, monocyte/macrophages and B cells in peripheral and menstrual blood as the principal immune cells involved in early placentation and widely linked with the pathogenesis of these autoimmune diseases (43, 44).
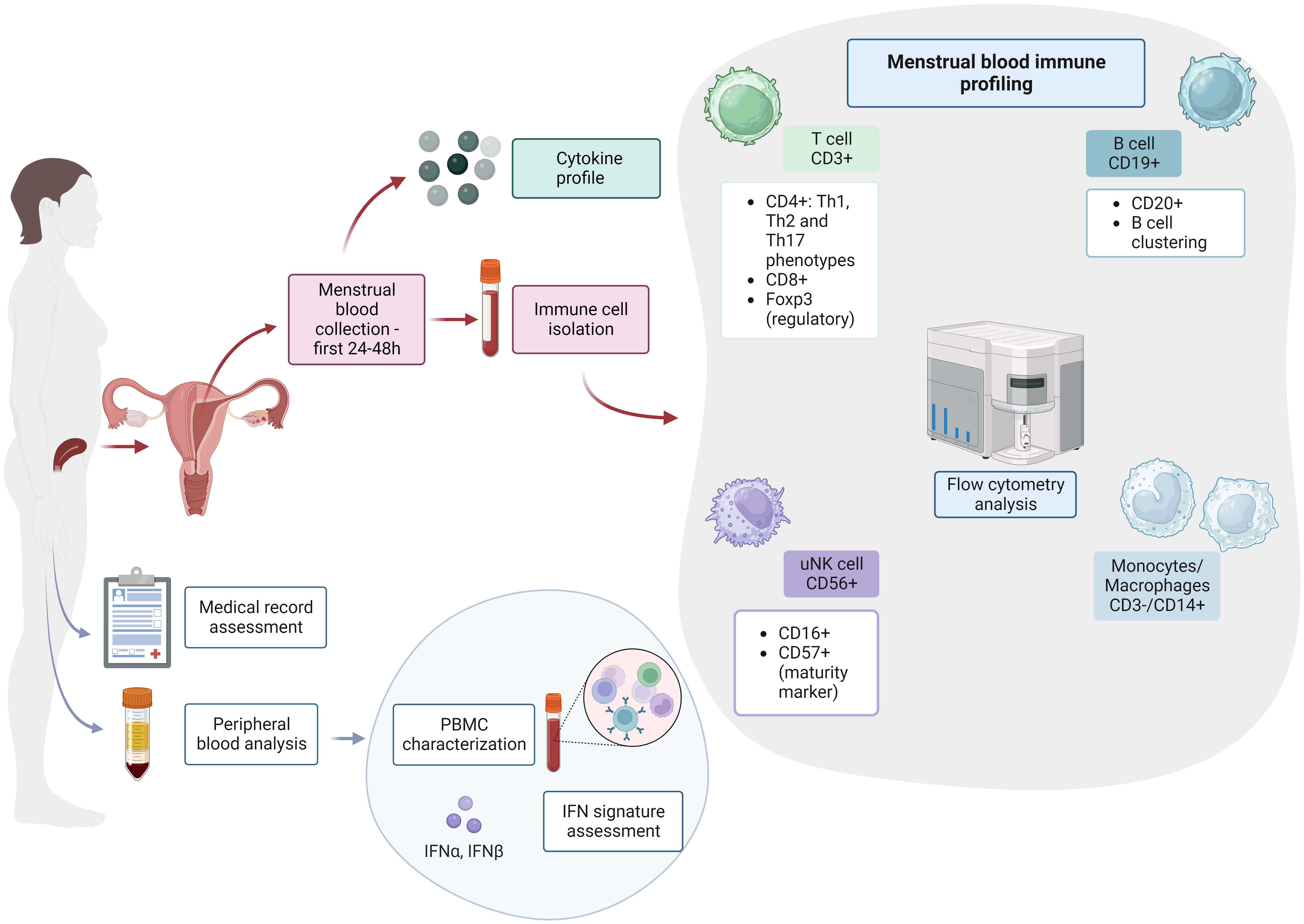
Figure 1 Endometrial immune profiling in patients with systemic autoimmune diseases. PBMC, peripheral blood mononuclear cell; IFN, interferon; T cell, T lymphocytes; B cell, B lymphocytes; uNK, uterine natural killer cells. This figure was created with BioRender.com.
In conclusion, there is a lack of evidence regarding the endometrial immune environment in women with systemic autoimmune diseases in relation to APO. Due to the systemic pro-inflammatory state in these women, we expect an altered endometrial immune environment that can influence implantation and decidualization processes. The clinical value of endometrial immune profiling, in combination with previously used parameters for obstetric risk assessment, should be investigated in future studies. In particular, the use of menstrual blood seems to be a promising new and non-invasive technique, given the close resemblance with the endometrium biopsies.
JF: Conceptualization, Methodology, Writing – original draft. JP: Writing – review & editing. SH: Writing – review & editing. HB: Writing – review & editing. JW: Writing – review & editing. KdL: Conceptualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. JF is a recipient of a doctoral scholarship from Ministerio de Ciencia, Tecnología e Innovación (MinCiencias, Colombia), (906-2021).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Dorner T, Furie R. Novel paradigms in systemic lupus erythematosus. Lancet (2019) 393:2344–58. doi: 10.1016/S0140-6736(19)30546-X
2. Barbhaiya M, Zuily S, Naden R, Hendry A, Manneville F, Amigo M-C, et al. 2023 ACR/EULAR antiphospholipid syndrome classification criteria. Arthritis Rheumatol (2023) 82(10):1258–70. doi: 10.1002/art.42624
3. Chan T-M, Wu C-E, Yu H-H, Hsiao C-Y, Su T-H, Chen C-B, et al. Fetal-neonatal and maternal outcomes in women with Sjögren syndrome: a population-based registry linkage study. Rheumatology (2023) 62:2820–8. doi: 10.1093/rheumatology/keac711
4. Bundhun PK, Soogund MZ, Huang F. Impact of systemic lupus erythematosus on maternal and fetal outcomes following pregnancy: A meta-analysis of studies published between years 2001-2016. J Autoimmun (2017) 79:17–27. doi: 10.1016/j.jaut.2017.02.009
5. Andrade D, Kim M, Blanco LP, Karumanchi SA, Koo GC, Redecha P, et al. Interferon-α and angiogenic dysregulation in pregnant lupus patients who develop preeclampsia. Arthritis Rheumatol (2015) 67:977–87. doi: 10.1002/art.39029
6. Xourgia E, Tektonidou MG. Type I interferon gene expression in antiphospholipid syndrome: Pathogenetic, clinical and therapeutic implications. J Autoimmun (2019) 104:102311. doi: 10.1016/j.jaut.2019.102311
7. Fierro JJ, Prins JR, Verstappen GM, Bootsma H, Westra J, de Leeuw K. Preconception clinical factors related to adverse pregnancy outcomes in patients with systemic lupus erythematosus or primary Sjögren’s syndrome: a retrospective cohort study. RMD Open (2023) 9(3):e003439. doi: 10.1136/rmdopen-2023-003439
8. Oertelt-Prigione S. Immunology and the menstrual cycle. Autoimmun Rev (2012) 11:A486–92. doi: 10.1016/j.autrev.2011.11.023
9. Liu S, Diao L, Huang C, Li Y, Zeng Y, Kwak-Kim JYH. The role of decidual immune cells on human pregnancy. J Reprod Immunol (2017) 124:44–53. doi: 10.1016/j.jri.2017.10.045
10. Altmäe S, Koel M, Võsa U, Adler P, Suhorutšenko M, Laisk-Podar T, et al. Meta-signature of human endometrial receptivity: a meta-analysis and validation study of transcriptomic biomarkers. Sci Rep (2017) 7:10077. doi: 10.1038/s41598-017-10098-3
11. Shima T, Sasaki Y, Itoh M, Nakashima A, Ishii N, Sugamura K, et al. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J Reprod Immunol (2010) 85:121–9. doi: 10.1016/j.jri.2010.02.006
12. Ashary N, Tiwari A, Modi D. Embryo implantation: war in times of love. Endocrinology (2018) 159:1188–98. doi: 10.1210/en.2017-03082
13. Andreoli L, Bertsias GK, Agmon-Levin N, Brown S, Cervera R, Costedoat-Chalumeau N, et al. EULAR recommendations for women's health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann Rheum Dis (2017) 76:476–485. doi: 10.1136/annrheumdis-2016-209770
14. Lédée N, Petitbarat M, Prat-Ellenberg L, Dray G, Cassuto GN, Chevrier L, et al. Endometrial immune profiling: A method to design personalized care in assisted reproductive medicine. Front Immunol (2020) 11:1032. doi: 10.3389/fimmu.2020.01032
15. Lédée N, Petitbarat M, Chevrier L, Vitoux D, Vezmar K, Rahmati M, et al. The uterine immune profile may help women with repeated unexplained embryo implantation failure after in vitro fertilization. Am J Reprod Immunol (2016) 75:388–401. doi: 10.1111/aji.12483
16. Ng S-W, Norwitz GA, Pavlicev M, Tilburgs T, Simón C, Norwitz ER. Endometrial decidualization: the primary driver of pregnancy health. Int J Mol Sci (2020) 21(11):4092. doi: 10.3390/ijms21114092
17. Xie M, Li Y, Meng Y-Z, Xu P, Yang Y-G, Dong S, et al. Uterine natural killer cells: A rising star in human pregnancy regulation. Front Immunol (2022) 13:918550. doi: 10.3389/fimmu.2022.918550
18. Rabaglino MB, Post Uiterweer ED, Jeyabalan A, Hogge WA, Conrad KP. Bioinformatics approach reveals evidence for impaired endometrial maturation before and during early pregnancy in women who developed preeclampsia. Hypertens (2015) 65:421–9. doi: 10.1161/HYPERTENSIONAHA.114.04481
19. Zhang J, Dunk CE, Shynlova O, Caniggia I, Lye SJ. TGFb1 suppresses the activation of distinct dNK subpopulations in preeclampsia. EBioMedicine (2019) 39:531–9. doi: 10.1016/j.ebiom.2018.12.015
20. Arruvito L, Sanz M, Banham AH, Fainboim L. Expansion of CD4+CD25+and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: implications for human reproduction. J Immunol (2007) 178:2572–8. doi: 10.4049/jimmunol.178.4.2572
21. Krop J, Heidt S, Claas FHJ, Eikmans M. Regulatory T cells in pregnancy: it is not all about foxP3. Front Immunol (2020) 11:1182. doi: 10.3389/fimmu.2020.01182
22. Robertson SA, Green ES, Care AS, Moldenhauer LM, Prins JR, Hull ML, et al. Therapeutic potential of regulatory T cells in preeclampsia-opportunities and challenges. Front Immunol (2019) 10:478. doi: 10.3389/fimmu.2019.00478
23. Mjösberg J, Berg G, Jenmalm MC, Ernerudh J. FOXP3+ regulatory T cells and T helper 1, T helper 2, and T helper 17 cells in human early pregnancy decidua. Biol Reprod (2010) 82:698–705. doi: 10.1095/biolreprod.109.081208
24. Colamatteo A, Fusco C, Micillo T, D’Hooghe T, de Candia P, Alviggi C, et al. Immunobiology of pregnancy: from basic science to translational medicine. Trends Mol Med (2023) 29:711–25. doi: 10.1016/j.molmed.2023.05.009
25. Ng SC, Gilman-Sachs A, Thaker P, Beaman KD, Beer AE, Kwak-Kim J. Expression of intracellular Th1 and Th2 cytokines in women with recurrent spontaneous abortion, implantation failures after IVF/ET or normal pregnancy. Am J Reprod Immunol (2002) 48:77–86. doi: 10.1034/j.1600-0897.2002.01105.x
26. Ribeiro VR, Romao-Veiga M, Romagnoli GG, Matias ML, Nunes PR, Borges VTM, et al. Association between cytokine profile and transcription factors produced by T-cell subsets in early- and late-onset pre-eclampsia. Immunology (2017) 152:163–73. doi: 10.1111/imm.12757
27. Wang S-C, Li Y-H, Piao H-L, Hong X-W, Zhang D, Xu Y-Y, et al. PD-1 and Tim-3 pathways are associated with regulatory CD8+ T-cell function in decidua and maintenance of normal pregnancy. Cell Death Dis (2015) 6:e1738. doi: 10.1038/cddis.2015.112
28. Wang S, Sun F, Li M, Qian J, Chen C, Wang M, et al. The appropriate frequency and function of decidual Tim-3(+)CTLA-4(+)CD8(+) T cells are important in maintaining normal pregnancy. Cell Death Dis (2019) 10:407. doi: 10.1038/s41419-019-1642-x
29. Kavvadas D, Karachrysafi S, Anastasiadou P, Kavvada A, Fotiadou S, Papachristodoulou A, et al. Immunohistochemical evaluation of CD3, CD4, CD8, and CD20 in decidual and trophoblastic tissue specimens of patients with recurrent pregnancy loss. Clin Pract (2022) 12:177–93. doi: 10.3390/clinpract12020022
30. Ma H, Cai S, Yang L, Wang L, Ding J, Li L, et al. How do pre-pregnancy endometrial macrophages contribute to pregnancy? J Reprod Immunol (2022) 154:103736. doi: 10.1016/j.jri.2022.103736
31. Diao L, Cai S, Huang C, Li L, Yu S, Wang L, et al. New endometrial immune cell-based score (EI-score) for the prediction of implantation success for patients undergoing IVF/ICSI. Placenta (2020) 99:180–8. doi: 10.1016/j.placenta.2020.07.025
32. Lédée N, Prat-Ellenberg L, Chevrier L, Balet R, Simon C, Lenoble C, et al. Uterine immune profiling for increasing live birth rate: A one-to-one matched cohort study. J Reprod Immunol (2017) 119:23–30. doi: 10.1016/j.jri.2016.11.007
33. Lédée N, Petitbarat M, Prat-Ellenberg L, Dray G, Cassuto GN, Chevrier L, et al. The uterine immune profile: A method for individualizing the management of women who have failed to implant an embryo after IVF/ICSI. J Reprod Immunol (2020) 142:103207. doi: 10.1016/j.jri.2020.103207
34. Marron K, Harrity C. Endometrial lymphocyte concentrations in adverse reproductive outcome populations. J Assist Reprod Genet (2019) 36:837–46. doi: 10.1007/s10815-019-01427-8
35. Kliman HJ, Frankfurter D. Clinical approach to recurrent implantation failure: evidence-based evaluation of the endometrium. Fertil Steril (2019) 111:618–28. doi: 10.1016/j.fertnstert.2019.02.011
36. Almquist LD, Likes CE, Stone B, Brown KR, Savaris R, Forstein DA, et al. Endometrial BCL6 testing for the prediction of in vitro fertilization outcomes: a cohort study. Fertil Steril (2017) 108:1063–9. doi: 10.1016/j.fertnstert.2017.09.017
37. Freitag N, Baston-Buest DM, Kruessel J-S, Markert UR, Fehm TN, Bielfeld AP. Eutopic endometrial immune profile of infertility-patients with and without endometriosis. J Reprod Immunol (2022) 150:103489. doi: 10.1016/j.jri.2022.103489
38. Van Der Molen RG, Schutten JHF, Van Cranenbroek B, Ter Meer M, Donckers J, Scholten RR, et al. Menstrual blood closely resembles the uterine immune micro-environment and is clearly distinct from peripheral blood. Hum Reprod (2014) 29:303–14. doi: 10.1093/humrep/det398
39. Vomstein K, Egerup P, Kolte AM, Behrendt-Møller I, Boje AD, Bertelsen M-L, et al. Biopsy-free profiling of the uterine immune system in patients with recurrent pregnancy loss and unexplained infertility. Reprod BioMed Online (2023) 47:103207. doi: 10.1016/j.rbmo.2023.03.018
40. Marron K, Harrity C. Potential utility of a non-invasive menstrual blood immunophenotype analysis in reproductive medicine. Reprod Fertil (2022) 3:255–61. doi: 10.1530/RAF-22-0047
41. Crona Guterstam Y, Strunz B, Ivarsson MA, Zimmer C, Melin A-S, Jonasson AF, et al. The cytokine profile of menstrual blood. Acta Obstet Gynecol Scand (2021) 100:339–46. doi: 10.1111/aogs.13990
42. Walter IJ, Klein Haneveld MJ, Lely AT, Bloemenkamp KWM, Limper M, Kooiman J. Pregnancy outcome predictors in antiphospholipid syndrome: A systematic review and meta-analysis. Autoimmun Rev (2021) 20:102901. doi: 10.1016/j.autrev.2021.102901
43. Vrzić Petronijević S, Vilotić A, Bojić-Trbojević Ž, Kostić S, Petronijević M, Vićovac L, et al. Trophoblast cell function in the antiphospholipid syndrome. Biomedicines (2023) 11(10):2681. doi: 10.3390/biomedicines11102681
Keywords: endometrial immune profiling, systemic lupus erythematosus, antiphospholipid syndrome, Sjögren's disease, pregnancy outcomes
Citation: Fierro JJ, Prins JR, Henning S, Bootsma H, Westra J and de Leeuw K (2024) Endometrial immune profiling as a new tool for preconceptional assessment in patients with systemic autoimmune diseases. Front. Immunol. 14:1334231. doi: 10.3389/fimmu.2023.1334231
Received: 06 November 2023; Accepted: 15 December 2023;
Published: 05 January 2024.
Edited by:
Marie-Pierre Piccinni, University of Florence, ItalyReviewed by:
Kailash Singh, Uppsala University, SwedenCopyright © 2024 Fierro, Prins, Henning, Bootsma, Westra and de Leeuw. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan J. Fierro, ai5qLmZpZXJyby5tYXJ0aW5lekB1bWNnLm5s
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.