- 1College of Acupuncture and Massage, Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
- 2Department of Neurology, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
Background: Ankylosing spondylitis (AS) is a rheumatic and autoimmune disease associated with a chronic inflammatory response, mainly characterized by pain, stiffness, or limited mobility of the spine and sacroiliac joints. Severe symptoms can lead to joint deformity, destruction, and even lifelong disability, causing a serious burden on families and society as a whole. A large number of clinical studies have been published on AS over the past 20 years. This study aimed to summarize the current research status and global trends relating to AS clinical trials through a bibliometric analysis.
Methods: The Web of Science Core Collection database was searched for publications related to AS clinical trials published between January 2003 and June 2023. Bibliometric analysis and web visualization were performed using CiteSpace, VOSviewer, and a bibliometric online analysis platform (https://bibliometric.com), which included the number of publications, citations, countries, institutions, journals, authors, references, and keywords.
Results: 1,212 articles published in 201 journals from 65 countries were included in this study. The number of publications related to AS clinical trials is increasing annually. The United States and the Free University of Berlin, the countries and institutions, respectively, that have published the most articles on AS, have made outstanding contributions to this field. The author with the most published papers and co-citations over the period covered by the study was Desiree Van Der Heijde. The journal with the most published and cited articles was Annals of the Rheumatic Diseases. The keywords: “double-blind,” “rheumatoid arthritis,” “efficacy,” “placebo-controlled trial,” “infliximab,” “etanercept,” “psoriatic arthritis” and “therapy” represent the current research hotspots regarding AS.
Discussion: This is the first study to perform a bibliometric analysis and visualization of AS clinical trial publications, providing a reliable research focus and direction for clinicians. Future studies in the field of AS clinical trials should focus on placebo-controlled trials of targeted therapeutic drugs.
1 Introduction
Ankylosing spondylitis (AS), also known as axial spondyloarthritis (1, 2), is an immune-mediated, chronic inflammatory disease that mainly affects the spinal and sacroiliac joints (3). The clinical symptoms include pain, stiffness, and limited movement (4). The cause of the disease is unclear, with clinical studies having found that the prevalence of this disease is approximately two to three times greater in men than it is in women (5), and that it is most heavily related to genetics, infection, and trauma. According to statistics, the heritability of the disease is greater than 90% (1) and is mostly related to HLA-B27 genetic factors. In addition to spinal and peripheral joint involvement, ulcerative colitis, uveitis, and psoriasis have also been shown to occur clinically (6). The disability rate associated with this disease is high, seriously affecting the quality of life of patients and causing significant social and economic burdens. Nonsteroidal anti-inflammatory drugs and tumor necrosis factor inhibitors are commonly used for the clinical treatment of this disease (7–9). Recent studies have focused on interleukin inhibitors as a new type of targeted therapeutic drug (10).
Clinical trials, also commonly referred to as randomized controlled trials (RCT), are currently the most powerful method for evaluating interventions in clinical practice. They are scientific research activities used to determine the effectiveness and safety of a given treatment (11). Clinical trials encompass a rigorous study design and provide solid evidence based on reliable results that can be used to guide clinical treatment practices (12). Since the discovery of the first biological agent for the treatment of AS, many clinical trial articles have been published in this field (13), exploring the single and combined effects of various treatments. This study identifies the next research direction in this area by integrating and analyzing the results of these clinical trials.
Bibliometric analysis is used for the quantitative analysis of research hotspots and global trends in a specific field (14). Analysis software is used to perform bibliometric analysis of the relevant countries, authors, journals, and keywords in specific research fields (15). Although many reviews have been published on the different treatment approaches for AS, there remains a lack of a systematic bibliometric analysis of AS clinical trials. Thus, this study aims to review articles published on AS over the past 20 years to help clinicians gain a better understanding of the relevant research hotspots and frontiers in this field.
2 Materials and methods
2.1 Data sources and search strategies
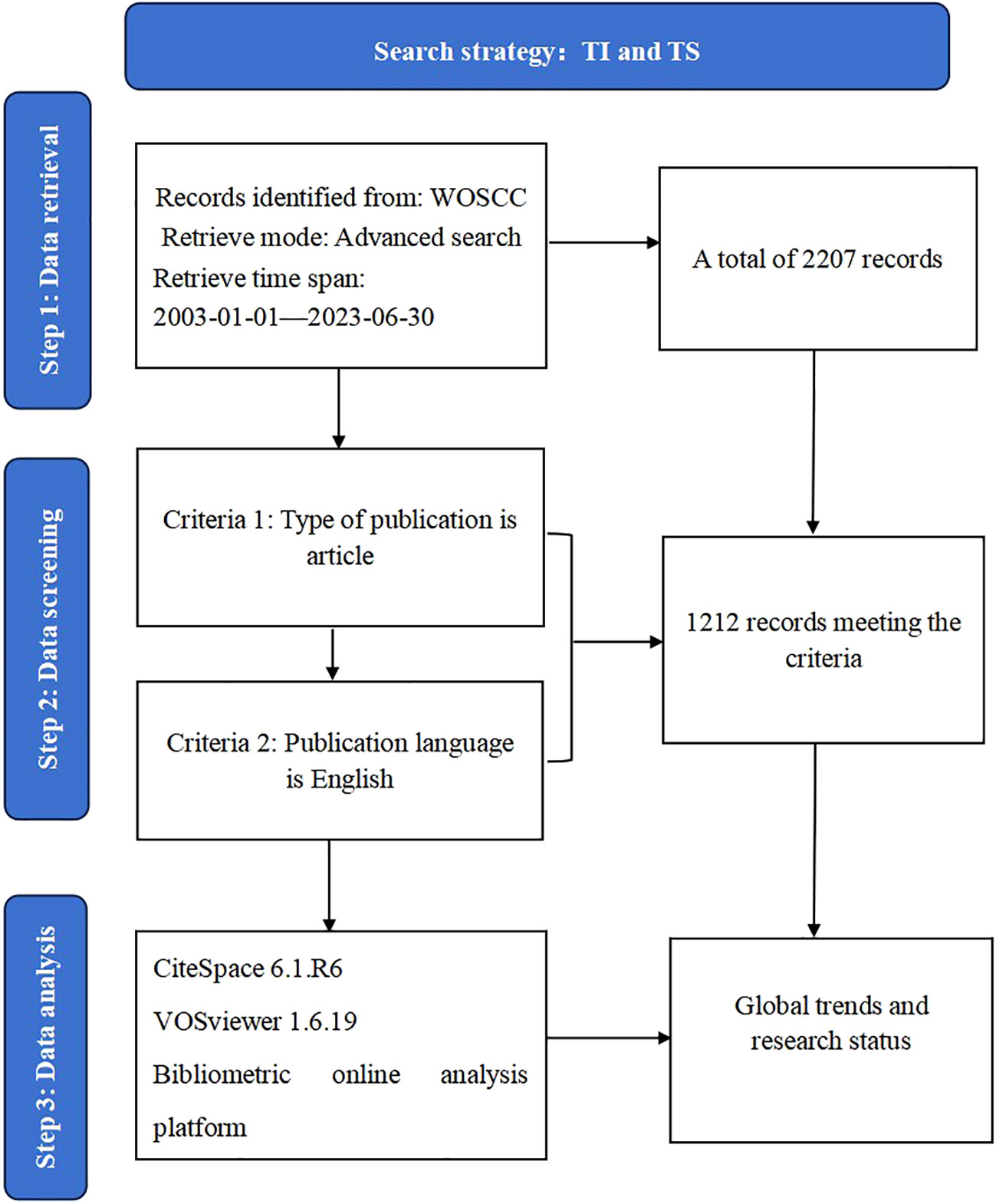
The articles in this study were indexed from the Web of Science Core Collection (WoSCC) database, which contains more than 10,000 journals and is one of the most commonly used and authoritative databases for bibliometric analysis (16). The search strategy used was as follows (17): TI = (“Spondyloarthritis Ankylopoetica*”) OR TI = (“Ankylosing Spondylitis*”) OR TI = (“Ankylosing Spondylarthritis*”) OR TI= (“Spondylarthritis Ankylopoetica*”) OR TI= (“Spondyloarthritides, Ankylosing*”) OR TI= (“Ankylosing Spondyloarthritis*”) OR TI= (“Ankylosing Spondyloarthritides*”) OR TI= (“Spondylitis Ankylopoetica*”). AND TS= (“Clinical Trial”OR “Controlled Clinical Trial”OR “Randomized Controlled Trial”). The search period covered was from January 1, 2003, to June 30, 2023. The document type was simultaneously limited to “article,” and our search was limited to articles published in English. A flowchart describing how publications were selected is presented in Figure 1.
2.2 Data extraction and analysis
CiteSpace(6.2.R4) is a visualization software developed by Dr. Chaomei Chen of Drexel University in Philadelphia, Pennsylvania, USA, which analyzes the strongest citation bursts of references and keywords to identify relevant hotspots and trends in a given research field. VOSviewer(v1.6.19) is a quantitative analysis software developed by Nees Jan van Eck and Ludo Waltman at Leiden University in the Netherlands that constructs visual network maps. This study used VOSviewer to analyze collaborative networks between countries, institutions, journals, and authors, identify reference co-citations, and map keyword overlay visualization. Each node represented a specific parameter; the larger the node, the heavier the parameter. Different clusters have differently colored nodes and lines. The thicker the connection line between the nodes, the stronger the link. The distribution of cooperation between countries was analyzed using the online analysis platform at https://bibliometric.com.
2.3 Research ethics
Ethical approval was not required because no patients or animals were included in this study.
3 Results
3.1 Annual growth trends in publications
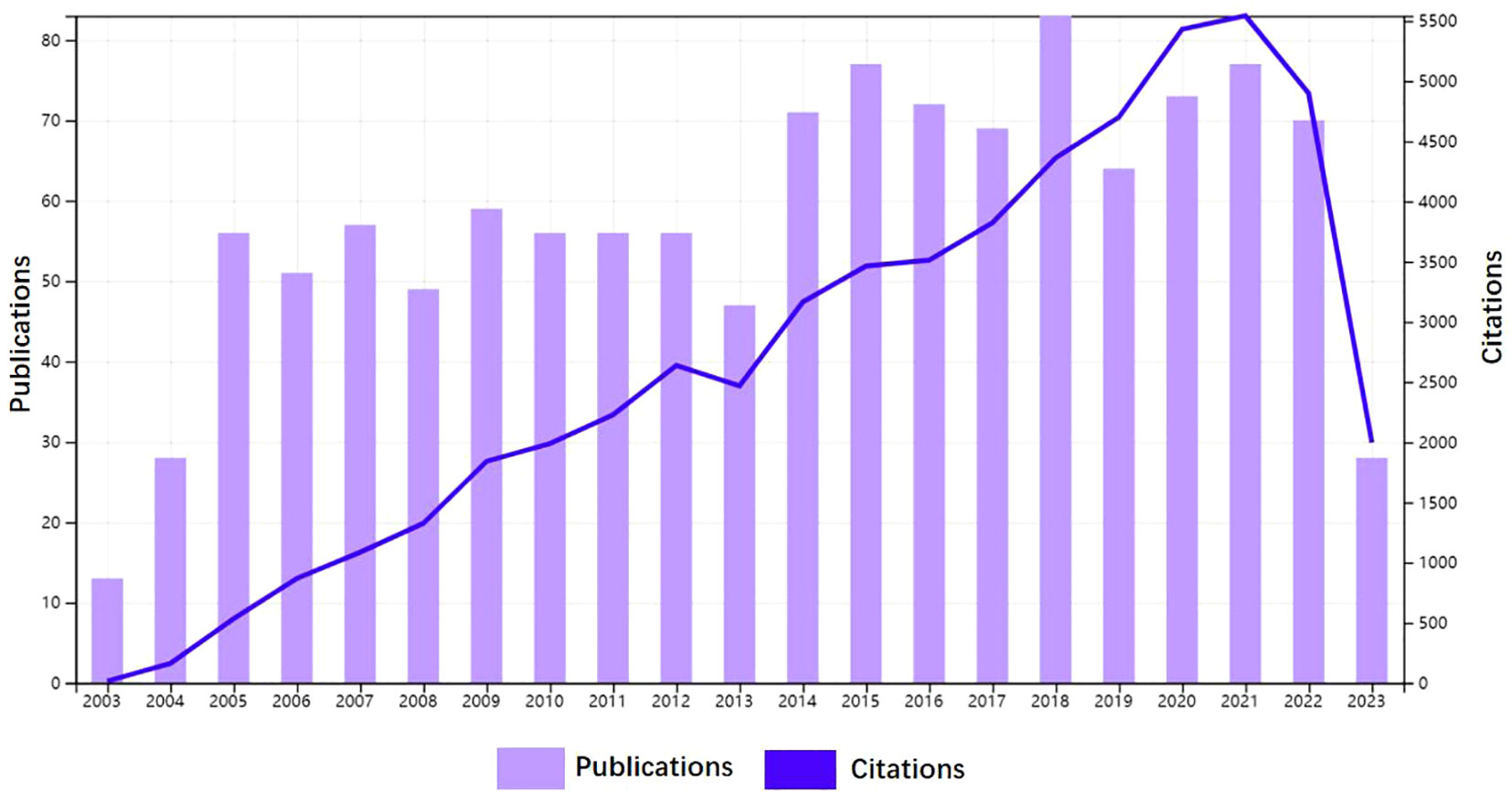
From January 1, 2003, to June 30, 2023, 1,212 clinical trial publications on AS were retrieved from the WOSCC database. Figure 2 shows the number of clinical trial publications published on AS in the past 20 years. Although there were slight fluctuations between 2013 and 2019, the overall trend is of a steady increase, indicating that clinical trial research on AS has received increasing attention from researchers. The number of publications peaked in 2018 with 83 articles published and 4,371 citations made. The publications included in this study received 56,425 citations in total, with an average citation frequency of 46.33. Between 2003 and 2023, the H-index for academic fields is 114.
3.2 Distribution of countries/regions and institutions
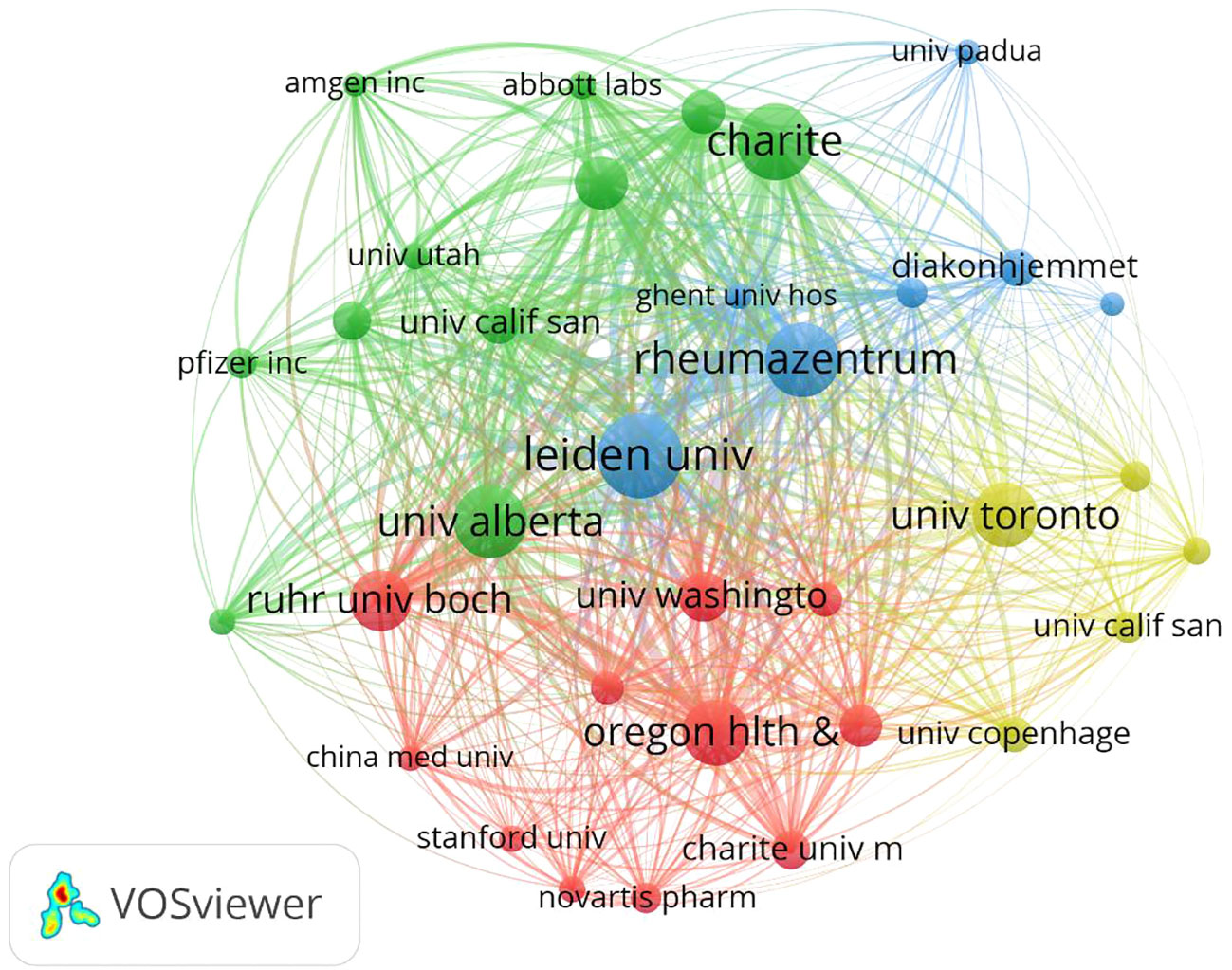
Table 1 shows the top 10 most prolific countries and institutions in the field of AS clinical trials, as well as their total number of publications (NP), total citations (NC), average citations (AC), and H-index scores. Among these countries, the United States had the most published papers (NP= 385, 21.28%), followed by Germany (NP=318, 17.58%). The country with the highest number of citations was Germany (NC=27,066), followed by the United States (NC=24,454) and the Netherlands (NC= 19,034). The country with the highest average number of citations was the Netherlands (AC=88.94), followed by France (AC=85.31) and Germany (AC=85.11). It is worth noting that among the 10 most productive countries, the United States had a significantly higher NP than Germany and the Netherlands, but a relatively low AC. By comparison, China’s NP is approximately the same as that of Italy and Belgium, while China’s NC and AC are both relatively low. Figure 3A shows the number of annual publications in the top 10 most productive countries, demonstrating a steady increase in the number of publications relating to AS clinical trials in each country. Figure 3B shows the proportion of publications in the 10 most productive countries in the past 20 years. Together, the three most productive countries the United States, Germany, and the Netherlands account for more than half of the publications in this area. Figure 3C represents a map of international cooperation between countries, and shows that the United States works most closely with Germany and Canada. Citation relationships between countries were analyzed using VOSviewer (Figure 3D). Only countries with at least five publications were included in the analysis. Of the 43 countries and territories that reached this threshold, the top five based on Total Link Strength (TLS) rankings were The United States (TLS=937), Germany (TLS=862), the Netherlands (TLS=646), Canada (TLS=566), and the United Kingdom (TLS=483). Table 1 summarizes the 10 most influential institutions in this area. The institution with the highest NP value was the Free University of Berlin (NP=184), followed by Charite Universitatsmedizin Berlin (NP=179) and Humboldt University of Berlin (NP=179). Of the top 10 institutions, the top five were all from Germany, followed by three from France and two from the Netherlands. VOSviewer was used to analyze the citation relationships between institutions (Figure 4). Only the institutions with at least 20 publications were included in the analysis. Of the 34 that reached this threshold, the top five with the highest TLS rankings were Rheumazentrum Ruhrgebiet (TLS=4043), Leiden University (TLS=3992), Charite Universitatsmedizin Berlin (TLS=3194), the University of Alberta (TLS=3046), and Oregon Health Science University (TLS=2603).
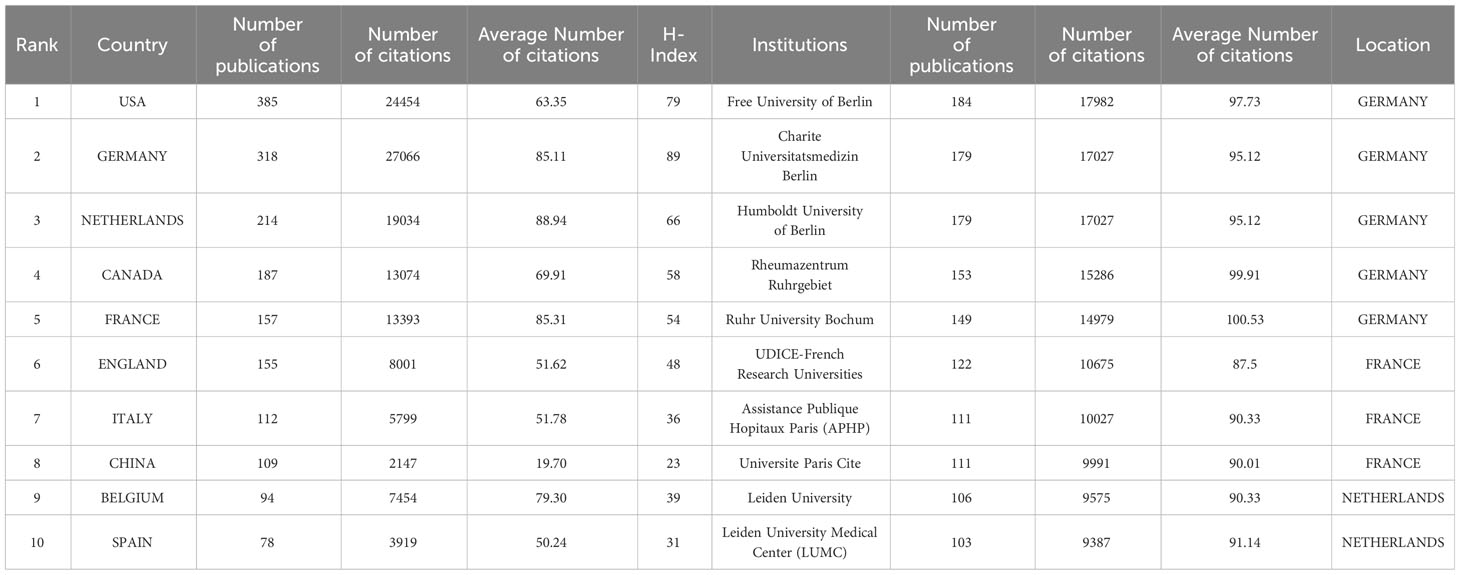
Table 1 Top 10 countries or regions and institutions that have contributed to clinical trial research for AS.
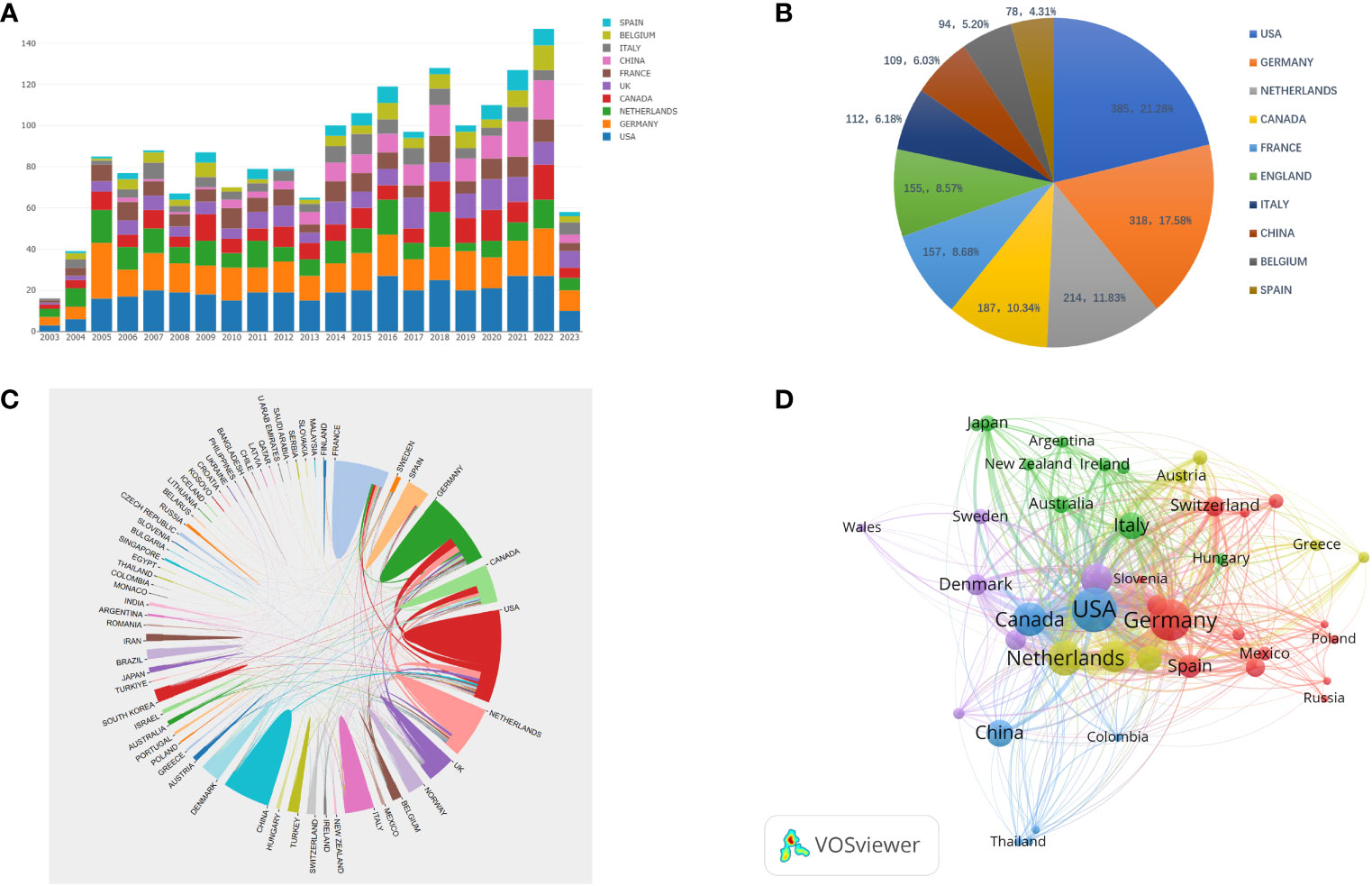
Figure 3 (A) Annual publishing trends in the top 10 countries from 2003 to 2023; (B) The proportion of the 10 most productive countries in the field of AS clinical trials over the past 20 years; (C) A visual map of international cooperation in countries and regions; (D) Network visualization showing relationships between countries.
3.3 Authors and co-citation authors
The analysis of the 1,212 articles included in this analysis showed that 5,448 authors contributed to the publication of the included AS clinical trial articles. Table 2 shows the top 10 most productive and co-cited authors in the field of AS clinical trials. Desiree Van Der Heijde has been the most productive author, with 126 articles and 15,139 citations, followed by Joachim Sieper (NP=85, NC=9115) and Juergen Braun (NP=80, NC=11276). Van Der Heijde was also the most-cited author, with 627 citations, and ranked second in terms of centrality (0.06). Xenofon Baraliakos was the author with the highest centrality ranking (0.11). The minimum number of author publications was set to 15, and 27 authors were selected for the author co-analysis using VOSviewer. Co-author analysis (Figure 5A) grouped the authors into four clusters. The red cluster (eight authors) represents the largest cluster of co-authors. Subsequently, author citation analysis was performed using the VOSviewer software. The overlay visualization (Figure 5B) shows that Sieper and Braun are the authors most associated with early stage clinical trial studies. Van Der Heijde is the most influential author in the mid-stage, while Atul Deodhar and Baraliakos are the emerging authors with regard to the clinical trial studies published in recent years. The top three authors based on TLS were Van Der Heijde (TLS = 2,933), Sieper (TLS = 2,835), and Braun (TLS = 2,254).
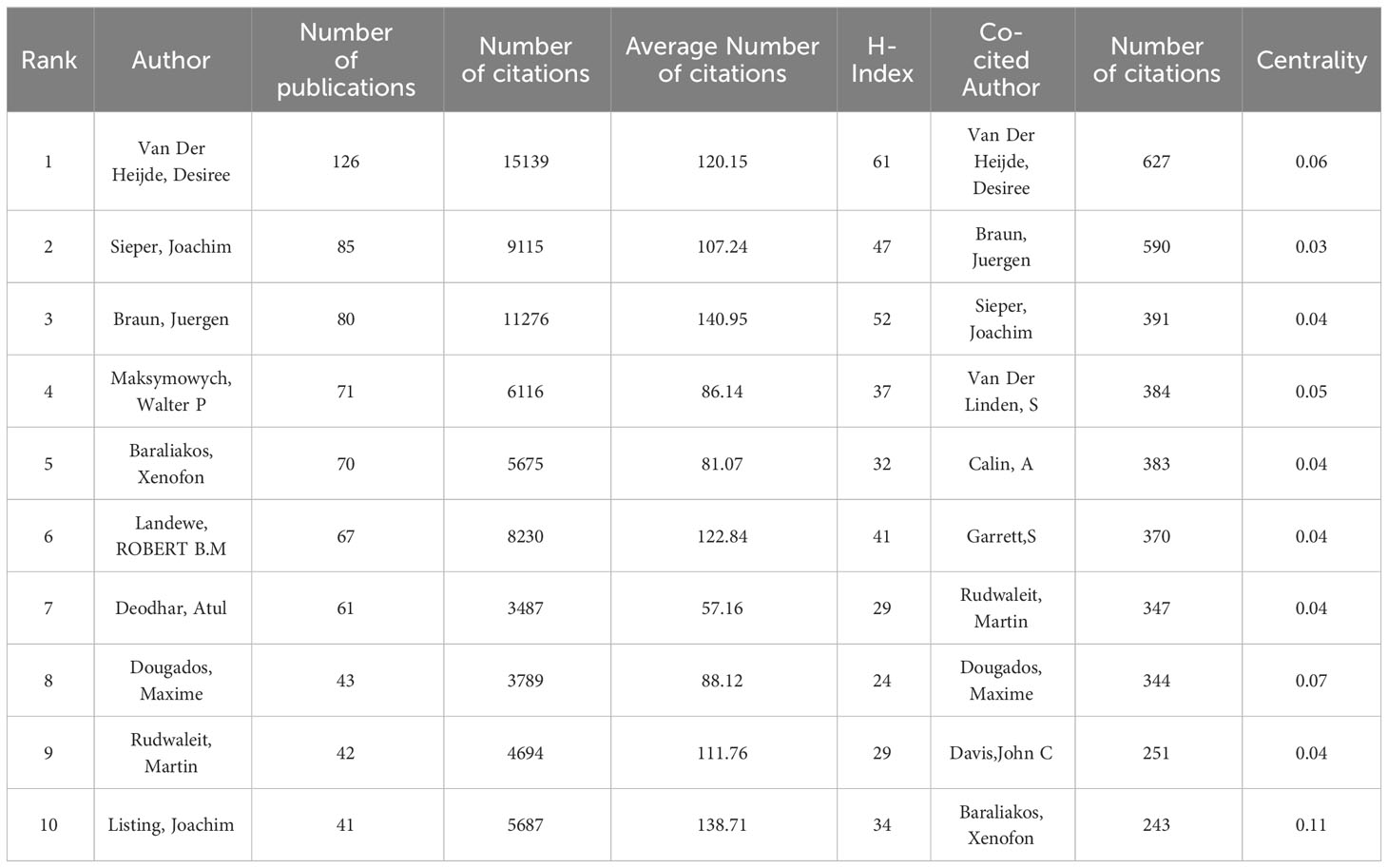
Table 2 The top 10 most productive authors and co-cited authors who contributed to AS clinical trial research.
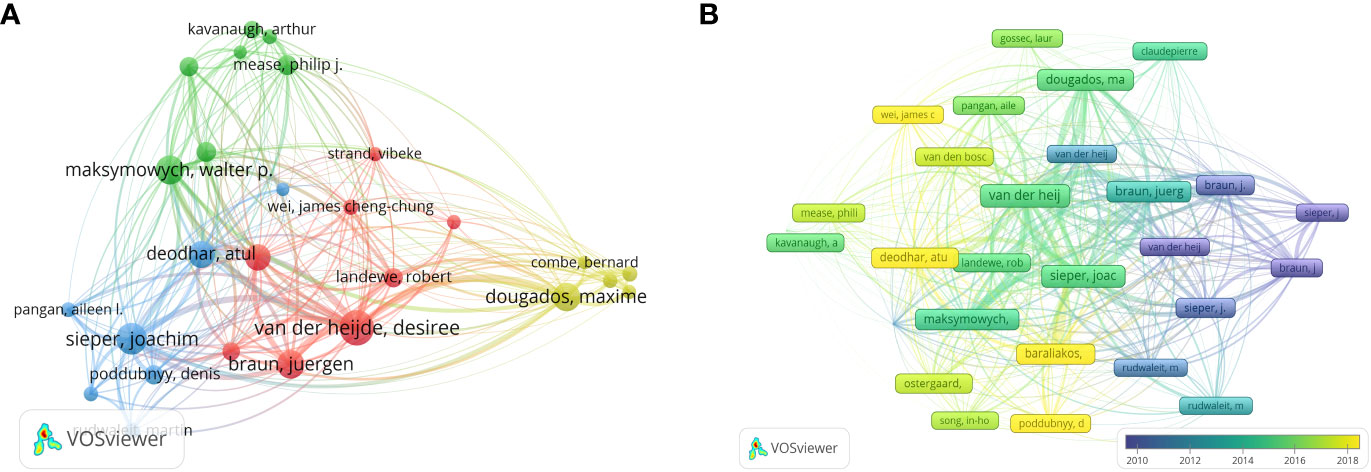
Figure 5 (A) Network visualization of co-authored analysis. (B) Author citation analysis overlay visualization based on VOSviewer. Purple nodes represent authors involved in earlier studies in the field, while yellow nodes represent authors engaged in later studies.
3.4 Journals and co-cited journals
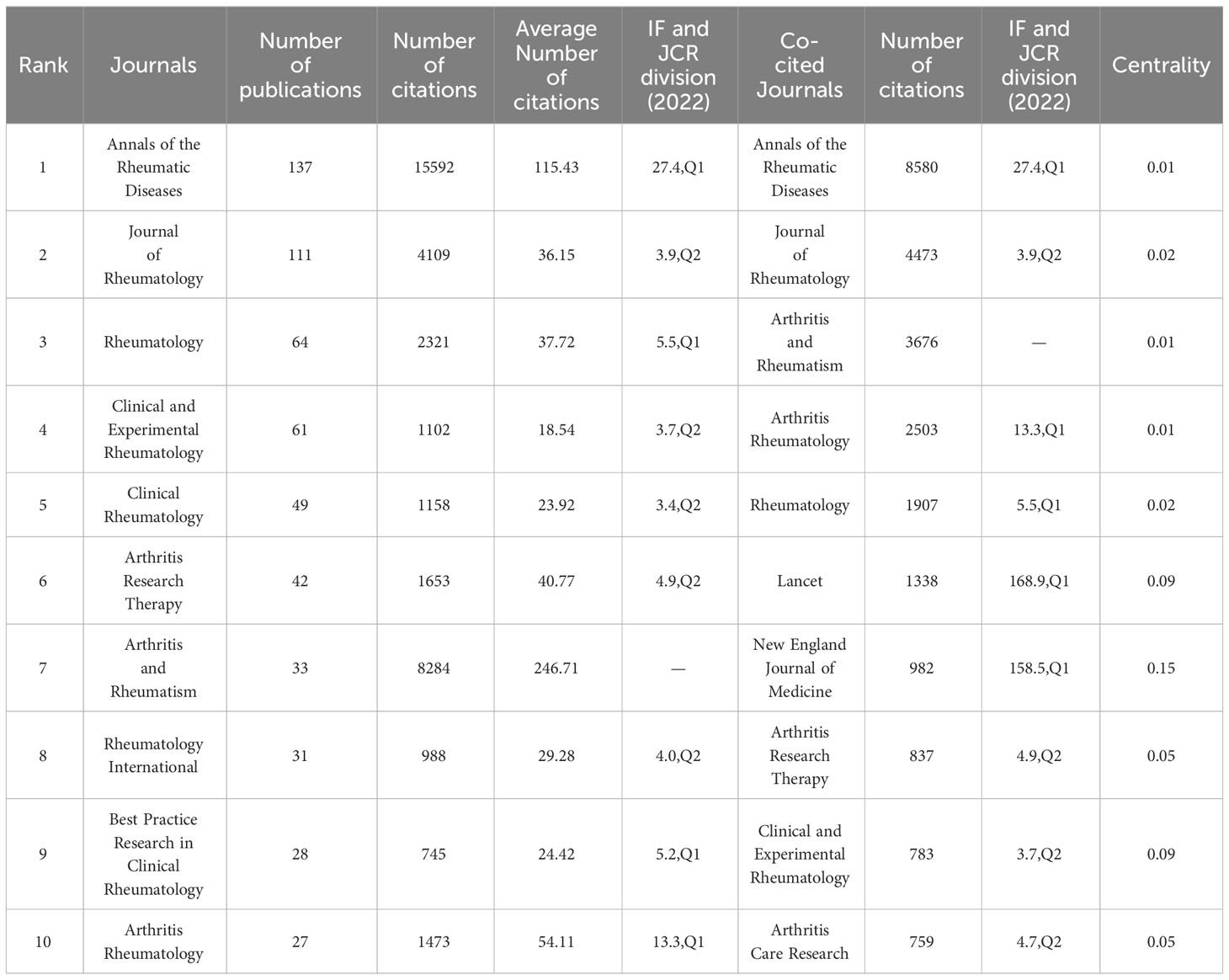
A total of 1,212 articles related to clinical trials on AS were published in 251 journals. Table 3 shows the top 10 journals and top 10 co-citations. The journal with the most published papers was Annals of the Rheumatic Diseases (n=137, 23.50%), which was also the most cited journal (NC=15,592), followed by the Journal of Rheumatology (n=111,10.04%) and Rheumatology (n=64,10.98%). Annals of the Rheumatic Diseases (NC=8,580) was also the most commonly cited journal, followed by the Journal of Rheumatology (NC=4,473). In addition, four of the top ten co-cited journals had an impact factor (IF) greater than 10, with the Lancet Journal having the highest IF, followed by the New England Journal of Medicine. Figure 6A shows the co-citation analysis of journals using the VOSviewer software. Only journals with at least 60 published articles were included, and 70 journals were selected for the analysis. The top three biggest journals in terms of TLS were Annals of the Rheumatic Diseases (TLS = 213,896), Journal of Rheumatology (TLS = 124,400), and Arthritis and Rheumatism (TLS = 114,740). The dual map overlay of journals (Figure 6B) shows three main citation pathways: Papers published in Health/Nursing/Medicine, Sports/Rehabilitation/Sport, and Molecular/Biology/Genetics journals were often cited by papers published in Medicine/Medical/Clinical journals.
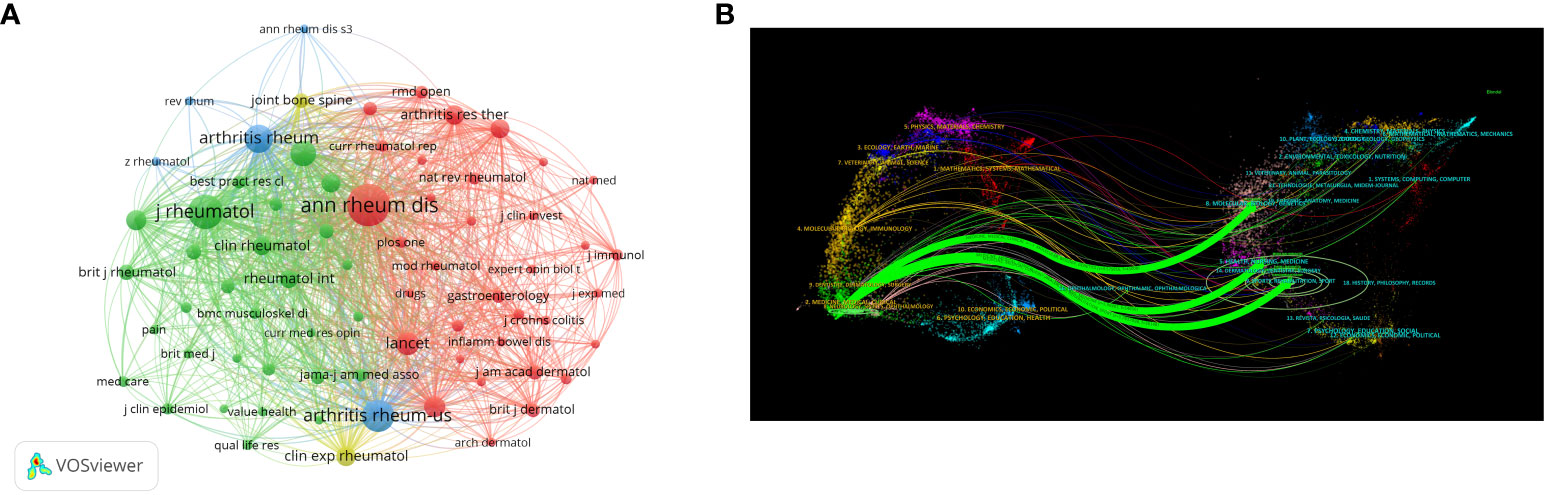
Figure 6 (A) Web visualization of journal co-citation analysis. (B) The dual map overlay of journals.
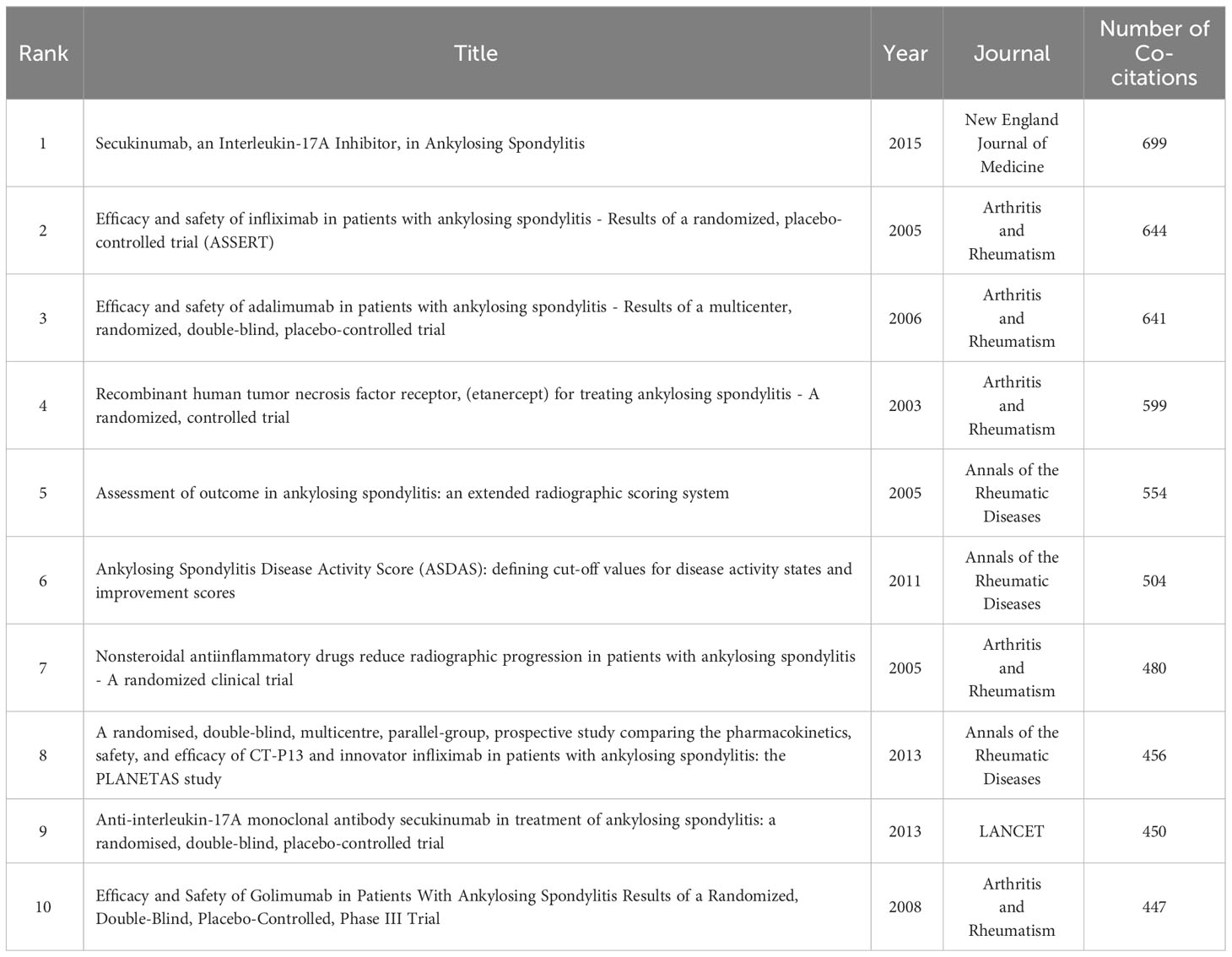
3.5 Co-cited references and references burst
Table 4 summarizes the 10 most frequently cited articles in the field of AS clinical trials. All 10 articles were published between 2003 and 2015, mainly in two journals: Arthritis and Rheumatism and Annals of the Rheumatic Diseases. Figure 7A presents an analysis of the co-citations of references using CiteSpace software. The visual co-citation network contained 300 nodes and 1,601 links. This figure shows the first author and release time of the top 10 most-cited references. The 25 references with the strongest citation bursts are also shown in Figure 7B. The first reference with the strongest citation explosion (intensity = 36.53) appeared in 2003 and the most recent reference with the strongest citation explosion (intensity = 19.87) appeared in 2020.
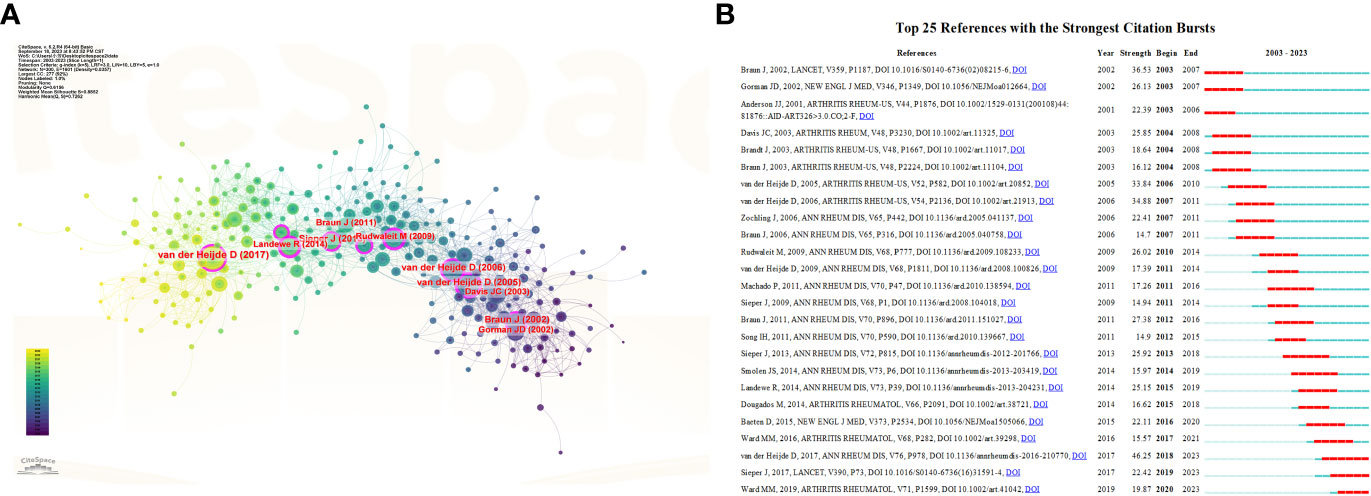
Figure 7 (A) Visual analysis of co-cited references in the field of AS clinical trials. (B) Cite the top 25 references with the most outbreaks.
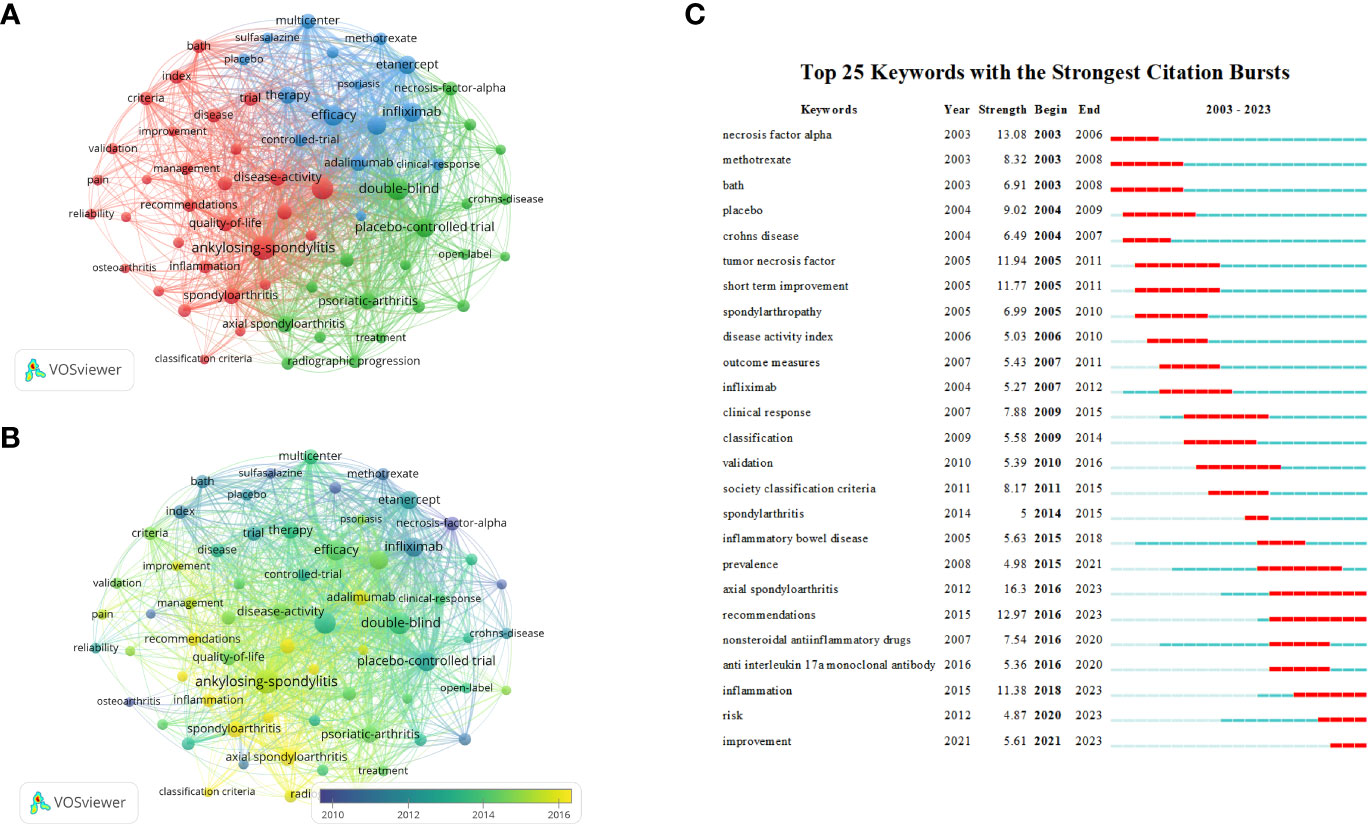
Figure 8 (A) Web visualization of the keywords in the AS clinical trials field. (B) AS clinical trials in the field of keyword overlay visualization. (C) The top 25 references with the most outbreaks cited.
3.6 Analysis of the top 10 most-cited articles
By analyzing the most cited articles in the field, the center and focus of research can be identified, thereby assisting beginners in the field to quickly understand the research process and direction. Based on the data available in Table 4, the intervention measures, treatment methods, duration, and main outcome indicators of the first 10 highly cited clinical trials were analyzed in detail (Table 5). Studies ranged from earlier ones on early nonsteroidal anti-inflammatory drugs and tumor necrosis factor inhibitors to more recent ones on interleukin-17A inhibitors, with more than half of trials using placebo-controlled interventions. The treatment was mostly subcutaneous injection. Almost all of the studies assessed clinical symptom improvement according to the Assessment of Spondyloarthritis International Society 20.
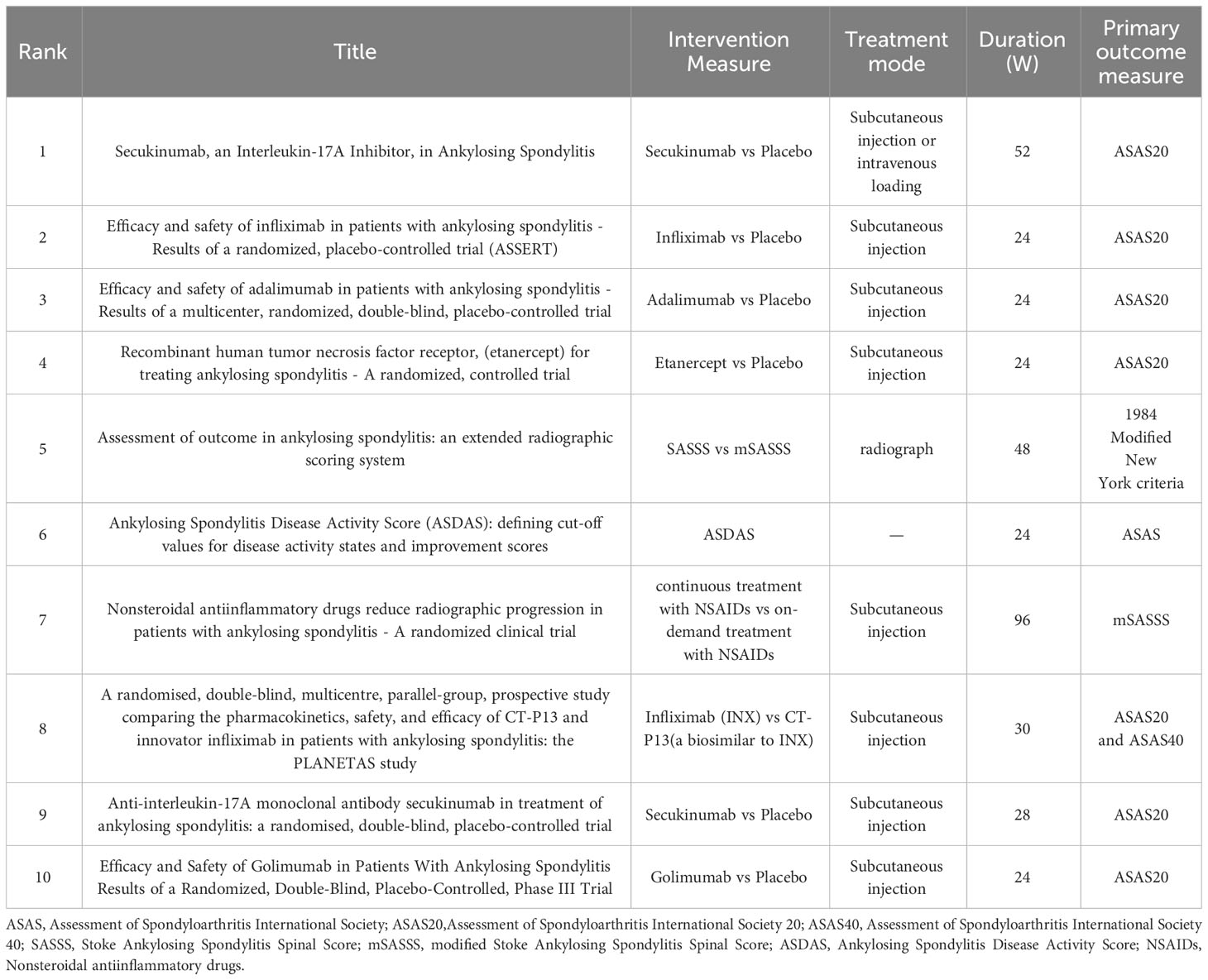
Table 5 AS clinical trial study of the top 10 most frequently cited articles treatment measures analysis.
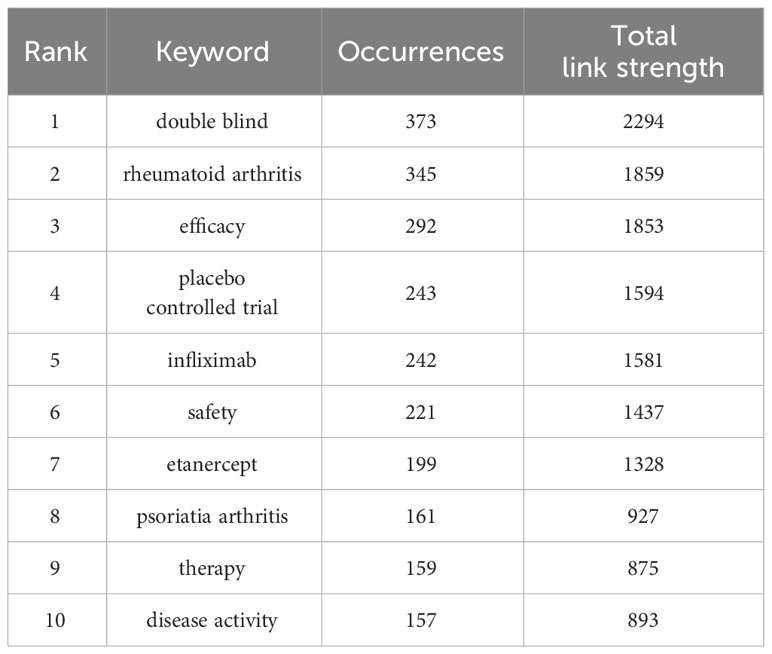
3.7 Keywords co-occurrence and burst analysis
Table 6 summarizes the top 10 keywords occurring most frequently, among which “double blind” and “placebo controlled trial” are keywords related to clinical trials, while excluding keywords related to ankylosing spondylitis clinical trials. The most frequently occurring keywords were “rheumatoid arthritis,” “efficacy,” “infliximab,” “etanercept,” “psoriatia arthritis,” and “therapy.” We used the VOSviewer software to perform keyword co-occurrence analysis, which can identify key points and research frontiers in the field of AS clinical trials. VOSviewer searched for a total of 3,210 keywords, of which 66 appeared more than 30 times, and 65 were screened after combining repeated keywords. A cluster analysis was then performed using the extracted keywords. As shown in Figure 8A, three clusters of different colors were obtained. Red represents the largest cluster, which contained 30 keywords associated with “ankylosing spondylitis,” “arthritis,” “bath,” “controlled trial,” “disease activity,” “rheumatoid arthritis,” “inflammation,” “quality of life,” “index,” “pain,” “psoriatic arthritis” and “risk.” The green cluster was the second-largest cluster, containing 20 keywords. These keywords mainly included “active ankylosing spondylitis,” “anti-TNF therapy,” “antitumor necrosis factor,” “crohns disease,” “psoriatic arthritis,” “axial spondyloarthritis,” “nonsteroidal anti-inflammatory drugs,” “radiographic progression” “infliximab,” “methotrexate,” “necrosis factor therapy” and “monoclonal antibody.” Blue was the third largest cluster, with a total of 15 keywords extracted, including “clinical response,” “etanercept,” “multicenter,” “remission,” “efficacy” and “safety.” Figure 8B presents a superimposed visualization of keywords, showing the changes that took place in terms of the keywords used over time. Purple nodes indicate early hotspots, while yellow nodes indicate emerging hotspots. The keywords gradually changed from “necrosis factor alpha,” “methotrexate,” “sulfasalazine,” and “tumor necrosis factor” to “inflammation” and “psoriatic,” “arthritis,” “axial spondyloarthritis,” and “remission.” Figure 8C shows the results of an analysis performed using the CiteSpace software, showing the top 25 keywords with the strongest reference bursts. The keyword “axial spondyloarthritis” has the strongest explosive power (intensity = 16.3) and is also the keyword with the longest eruption duration, ranging from 2003 to 2006. The earliest keyword for the outbreak was “necrosis factor alpha,” while “inflammation,” “risk” and “improvement” were the latest keywords added in recent years. Figures 8B, C that demonstrate that “inflammation,” “psoriatic arthritis” and “axial spondyloarthritis” were the keywords that occurred most frequently recently, indicating that these keywords may represent the current hotspot and future trend regarding clinical trial research on AS.
4 Discussion
This study represents the first bibliometric analysis of AS clinical trials that has been implemented to date over the last 20 years. This study mainly used CiteSpace and VOSviewer to analyze 1,212 articles selected from the Web of Science Core Collection. This included the number of global publications focusing on AS clinical trials, the countries or regions where these publications originated, the level of cooperation between institutions and authors, references, and keywords. Together, this enabled us to create a more in-depth analysis of the research hotspots and future development trends in the field of AS clinical trials.
4.1 Overview of development in the field of AS clinical trials
Over the past two decades, publications in the field of AS clinical trials have shown a steady upward annual trend (mainly divided into two phases), which began to plateau after a surge in 2005 and 2014, respectively. This may be related to the approval of tumor necrosis factor inhibitors (18) and interleukin-17A inhibitors (19, 20) for clinical use. The number of publications peaked in 2018, when nearly 70% of the articles published in the prior decade were released. This indicated a growing interest in AS among clinical trial practitioners.
From a country or regional perspective, the top three most productive countries in the field of AS clinical trials are the United States, Germany, and the Netherlands, with these three countries also contributing the most to the NC, AC, and H indices. It is worth noting that although China ranks 7th in terms of NP, its NC, AC, and H-index are the lowest among the top 10 most productive countries. This indicates that there is currently an imbalance between the number and quality of clinical trials in the AS field in China. Clinicians in China should pay close attention to current research hotspots, while also placing additional importance on ongoing scientific innovation, and strengthening cooperation with other countries to improve the existing quality of clinical trial articles. Of the 10 most productive institutions, the top five are from Germany, followed by France and the Netherlands. These are also notably the top three countries in terms of AC contribution, which clearly demonstrates that establishing world-class academic institutions is key to solving any existing imbalance in resource development. The presence of such institutions can also improve a country’s scientific research, innovation ability, and academic status.
Of the top 10 most prolific authors, Desiree Van Der Heijde has been the author that has made the most contributions in the field of AS clinical trials over the past 20 years. Van Der Heijde is a member of the Leiden University Medical Center (LUMC), whose team focuses on research in rheumatology, particularly in the treatment of spinal arthritis. Van Der Heijde has led several clinical trials for AS (21–23), and has developed definitions (24), classifications (25), and guidelines for spinal arthritis (26). Van Der Heijde’s team have also validated and improved the classification criteria for axial spinal arthritis (27), helping clinicians to diagnose this disease more accurately. Joachim Sieper and Juergen Braun have been the second most prolific authors after Van Der Heijde; they are both rheumatologists at the Charite Universitatsmedizin Berlin and Rheumazentrum Ruhrgebiet, respectively. Both authors contributed significantly to early AS clinical trials and have collaborated together many times. Collaboration has notably always been an important avenue for scientific research. Sieper and Braun jointly published an article entitled “Ankylosing spondylitis” in the Lancet in 2007 (3); systematically describing the pathogenesis and treatment of AS and pointing out that while tumor necrosis factor blockers are good news for refractory patients, nonsteroidal anti-inflammatory drug therapy and physical therapy remain the preferential long-term treatment options for conventional AS patients. Two recent studies have provided a more detailed description of the structure and function of HLA-B27 (28), suggesting that HLA-B27 may confer evolutionary advantages associated with infection prevention. A joint article published in the New England Journal of Medicine in 2015 reported that a subcutaneous dose of 150mg of secukinumab was shown to significantly improve the signs and symptoms of AS patients after 16 weeks of treatment, indicating a potentially promising direction for subsequent clinical treatment (29).
An analysis of journals and co-cited journals can provide a reliable reference source for researchers’ contributions (30). Of the top 10 most productive journals, Annals of the Rheumatic Diseases and the Journal of Rheumatology are the two most productive specialized journals in the field of rheumatology. Both have published numerous clinical trial articles on the treatment of AS. Annals of the Rheumatic Diseases in particular has the highest NC and AC and is far ahead of other journals in this field. The IF of this journal has also been steadily improving and more valuable research results are expected to be published in the future.
4.2 Research hotspots and development trends of AS in clinical trials
The top 10 cited references were published in the top journals of rheumatism and immunity, with most of them having been written by the teams of Van Der Heijde and Sieper (31, 32), a finding which is consistent with the results of our previous studies. As shown in Table 4, most articles (33–35) covered in this study centered on clinical trials focused on the effect of tumor necrosis factor inhibitors on AS, with the research time having been concentrated in the early stages. These findings suggest that tumor necrosis factor inhibitors such as adalimumab, infliximab, and golimumab are more effective and better tolerated in the majority of AS patients than traditional rheumatic immune agents such as nonsteroidal anti-inflammatory drugs and glucocorticoids. These clinical trials have laid the foundation for future drug promotion and related research. The majority of the most-cited studies in the past decade have focused on Secukinumab, an interleukin-17A inhibitor (29, 36), which found that this drug rapidly reduced clinical symptoms in patients with AS and can operate as a complementary and alternative treatment for tumor necrosis factor inhibitors.
Interleukin-17 (IL-17) is a pro-inflammatory cytokine derived mainly from TCD4+T cells and helper T subtype 17 cells (37). Studies have found that it is related to matrix destruction, tissue inflammation, and autoimmunity (10, 37). Compared with healthy subjects, the number of TH17 cells in AS patients was significantly increased, and IL-17 in serum and joints were also significantly increased (38). Six IL-17 family members range from A to F. IL-17A, as the first member studied (39), was found to increase the expression of receptor activator of NF-κB ligand in osteoblasts (40), indicating that IL-17A plays an important role in bone remodeling. More studies have found that IL-17A can also increase the expression of adhesion molecules in endothelial cells by stabilizing the chemokines induced by tumor necrosis factor, thus promoting the aggregation of granulocytes to the inflammatory site (41, 42). IL-17, as a potential therapeutic target, plays an important role in immune system diseases. Additionally, no significant adverse reactions and infections have been found in patients since the clinical approval of IL-17A inhibitors. All these findings indicate that the in-depth mining of IL-17 has increasingly become the focus of AS research.
Keywords are the key to retrieving the required articles for a comprehensive analysis. They represent the core and essence of an article, and can best reflect the relevant research hotspots in a specific field. This study also analyzed the evolution of keywords in the field of AS clinical trials over time through superposition visualization, which, to a certain extent, can better reflect changes in research hotspots, thus guiding the future direction of this research. Among the top 10 most-cited keywords, “double-blind” and “placebo-controlled trial” are the most commonly used methods in clinical trials in this field; this result is also consistent with the analysis of interventions in the top 10 most-cited articles displayed in Table 5. In clinical trials, placebos not only have no therapeutic effect on the observed disease, but can also simulate the possible adverse effects of the experimental drug (43). Therefore, it is very important to establish a placebo group in controlled clinical trials (44), a format which is increasingly being chosen by most clinical workers. Rheumatoid and psoriatic arthritis are the two diseases most closely related to AS, and their treatment methods are similar. “Infliximab” and “etanercept,” two tumor necrosis factor inhibitors (45, 46), are mostly used in clinical trials and have been shown to be both effective and well tolerated. According to the combined keyword cluster, “efficacy” and “safety” are keywords closely related to RCT, which indicates that the development of evidence-based medicine for AS is becoming more and more mature (47). As can be seen from the superimposed visual analysis, research in AS clinical trials has shifted from focusing on drug effectiveness to focusing on drug tolerance. Combined with the keyword outbreak analysis, “inflammation,” “risk” and “improvement” have been the emerging keywords in recent years. This indicates that clinicians are now more focused on the long-term quality of life of patients (48–50). Through the superimposed visual analysis of keywords, combined with the analysis of highly cited articles displayed in Table 4 and Table 5 in the past 10 years, it can be predicted that “placebo-controlled trial,” “double-blind,” “infliximab,” “improvement,” and other keywords may still break out in the future. However, “Interleukin-17” and “Secukinumab” will be the keywords for future outbreaks in this field. Based on the above analysis of various keywords, it can be seen that future research hotspots in the field of AS clinical trials will continue focus on the development of immunotherapy drugs for the treatment of this disease. This is especially the case with regard to randomized controlled trials studying the effects of tumor necrosis factor inhibitors and interleukin inhibitors. However, researchers will also continue to pay more attention to the long-term effectiveness of drugs in improving the clinical symptoms of patients and reducing the recurrence rate of AS, to enable patients to continue to pursue a higher quality of life.
5 Limitations
It is important to note that this study possesses some limitations. Although Web of Science is one of the most authoritative literature search tools, it does not cover all of the literature related to this study. In addition, we only searched for articles written in English and ignored those published in other languages, which may have led to a bias in the results.
6 Conclusion
To the best of our knowledge, this study represents the first bibliometric analysis in the field of AS clinical trials. It identifies current research hotspots and directions in this field, and aims to predict future research developments, which may provide valuable information for future clinicians. Future studies should focus on the continued exploration of tumor necrosis factor and interleukin inhibitors and the conducting of placebo-controlled trials.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
WZ: Writing – original draft. ML: Data curation, Formal analysis, Writing – original draft. XL: Writing – original draft. XW: Data curation, Formal analysis, Writing – original draft. YL: Supervision, Writing – review & editing. JY: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (Grant No.82174491).
Acknowledgments
We appreciate the data availability that was present via the Web of Science Core Ensemble Data, as well as the cooperation of all authors.
Conflict of interest
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. (2017) 390(10089):73–84. doi: 10.1016/S0140-6736(16)31591-4
2. Sieper J, Braun J, Dougados M, Baeten D. Axial spondyloarthritis. Nat Rev Dis Primers. (2015) 1:15013. doi: 10.1038/nrdp.2015.13
3. Braun J, Sieper J. Ankylosing spondylitis. Lancet. (2007) 369(9570):1379–90. doi: 10.1016/S0140-6736(07)60635-7
4. Liu L, Yuan Y, Zhang S, Xu J, Zou J. Osteoimmunological insights into the pathogenesis of ankylosing spondylitis. J Cell Physiol (2021) 236(9):6090–100. doi: 10.1002/jcp.30313
5. Wright GC, Kaine J, Deodhar A. Understanding differences between men and women with axial spondyloarthritis. Semin Arthritis Rheumatol (2020) 50(4):687–94. doi: 10.1016/j.semarthrit.2020.05.005
6. Calabro JJ, Maltz BA. Ankylosing spondylitis. N Engl J Med (1970) 282(11):606–10. doi: 10.1056/NEJM197003122821107
7. Rausch Osthoff A-K, Niedermann K, Braun J, Adams J, Brodin N, Dagfinrud H, et al. EULAR recommendations for physical activity in people with inflammatory arthritis and osteoarthritis. Ann Rheum Dis (2018) 77(9):1251–60. doi: 10.1136/annrheumdis-2018-213585
8. Ward MM, Deodhar A, Gensler LS, Dubreuil M, Yu D, Khan MA, et al. Update of the american college of rheumatology/Spondylitis association of america/Spondyloarthritis research and treatment network recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol (2019) 71(10):1599–613. doi: 10.1002/art.41042
9. Smolen JS, Schöls M, Braun J, Dougados M, FitzGerald O, Gladman DD, et al. Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheum Dis (2018) 77(1):3–17. doi: 10.1136/annrheumdis-2017-211734
10. Schinocca C, Rizzo C, Fasano S, Grasso G, La Barbera L, Ciccia F, et al. Role of the IL-23/IL-17 pathway in rheumatic diseases: an overview. Front Immunol (2021) 12:637829. doi: 10.3389/fimmu.2021.637829
11. Wade DT. Randomized clinical trials in Clinical Rehabilitation. Clin Rehabil. (2005) 19(3):233–6. doi: 10.1191/0269215505cr871ed
12. Solomon MJ, McLeod RS. Surgery and the randomised controlled trial: past, present and future. Med J Aust (1998) 169(7):380–3. doi: 10.5694/j.1326-5377.1998.tb126809.x
13. Gensler L, Inman R, Deodhar A. The “knowns” and “unknowns” of biologic therapy in ankylosing spondylitis. Am J Med Sci (2012) 343(5):360–3. doi: 10.1097/MAJ.0b013e318251406c
14. Hassan W, Zafar M, Duarte AE, Kamdem JP, Teixeira da Rocha JB. Pharmacological Research: A bibliometric analysis from 1989 to 2019. Pharmacol Res (2021) 169:105645. doi: 10.1016/j.phrs.2021.105645
15. Chen B, Fu Y, Song G, Zhong W, Guo J. Research trends and hotspots of exercise for Alzheimer’s disease: A bibliometric analysis. Front Aging Neurosci (2022) 14:984705. doi: 10.3389/fnagi.2022.984705
16. Su Y, Ruan Z, Li S, Li Z, Chang T. Emerging trends and research foci of neuromyelitis optica spectrum disorder: a 20-year bibliometric analysis. Front Immunol (2023) 14:1177127. doi: 10.3389/fimmu.2023.1177127
17. Li X, Yu W, Jia Z, Li J, Liu Y, Yang J. Frontiers of ankylosing spondylitis research: an analysis from the top 100 most influential articles in the field. Clin Exp Med (2023) 23(7):3019–40. doi: 10.1007/s10238-023-01102-4
18. Sieper J, Braun J. New treatment options in ankylosing spondylitis: a role for anti-TNFalpha therapy. Ann Rheum Dis (2001) 60 Suppl 3(Suppl 3):iii58–61. doi: 10.1136/ard.60.90003.iii58
19. Patel DD, Lee DM, Kolbinger F, Antoni C. Effect of IL-17A blockade with secukinumab in autoimmune diseases. Ann Rheum Dis (2013) ;72(Suppl 2):ii116–123. doi: 10.1136/annrheumdis-2012-202371
20. Sanford M, McKeage K. Secukinumab: first global approval. Drugs. (2015) 75(3):329–38. doi: 10.1007/s40265-015-0359-0
21. van der Heijde D, Baraliakos X, Sieper J, Deodhar A, Inman RD, Kameda H, et al. Efficacy and safety of upadacitinib for active ankylosing spondylitis refractory to biological therapy: a double-blind, randomised, placebo-controlled phase 3 trial. Ann Rheum Dis (2022) 81(11):1515–23. doi: 10.1136/ard-2022-222608
22. Deodhar A, van der Heijde D, Sieper J, Van den Bosch F, Maksymowych WP, Kim T-H, et al. Safety and efficacy of upadacitinib in patients with active ankylosing spondylitis and an inadequate response to nonsteroidal antiinflammatory drug therapy: one-year results of a double-blind, placebo-controlled study and open-label extension. Arthritis Rheumatol (2022) 74(1):70–80. doi: 10.1002/art.41911
23. van der Heijde D, Deodhar A, Maksymowych WP, Sieper J, Van den Bosch F, Kim T-H, et al. Upadacitinib in active ankylosing spondylitis: results of the 2-year, double-blind, placebo-controlled SELECT-AXIS 1 study and open-label extension. RMD Open (2022) 8(2):e002280. doi: 10.1136/rmdopen-2022-002280
24. Navarro-Compán V, Benavent D, Capelusnik D, van der Heijde D, Landewé RB, Poddubnyy D, et al. ASAS consensus definition of early axial spondyloarthritis. Ann Rheum Dis (2023), ard–2023-224232. doi: 10.1136/ard-2023-224232
25. Zeidler H, Amor B. The Assessment in Spondyloarthritis International Society (ASAS) classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general: the spondyloarthritis concept in progress. Ann Rheum Dis (2011) 70(1):1–3. doi: 10.1136/ard.2010.135889
26. Sieper J, Rudwaleit M, Baraliakos X, Brandt J, Braun J, Burgos-Vargas R, et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis (2009) 68(Suppl 2):ii1–44. doi: 10.1136/ard.2008.104018
27. Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis (2009) 68(6):777–83. doi: 10.1136/ard.2009.108233
28. Braun J, Sieper J. Fifty years after the discovery of the association of HLA B27 with ankylosing spondylitis. RMD Open (2023) 9(3):e003102. doi: 10.1136/rmdopen-2023-003102
29. Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med (2015) 373(26):2534–48. doi: 10.1056/NEJMoa1505066
31. Davis JC, van der Heijde D, Braun J, Dougados M, Cush J, Clegg DO, et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheumatol (2003) 48(11):3230–6. doi: 10.1002/art.11325
32. MaChado P, Landewé R, Lie E, Kvien TK, Braun J, Baker D, et al. Ankylosing Spondylitis Disease Activity Score (ASDAS): defining cut-off values for disease activity states and improvement scores. Ann Rheum Dis (2011) 70(1):47–53. doi: 10.1136/ard.2010.138594
33. van der Heijde D, Dijkmans B, Geusens P, Sieper J, DeWoody K, Williamson P, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis - Results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheumatol (2005) 52(2):582–91. doi: 10.1002/art.20852
34. van der Heijde D, Kivitz A, Schiff MH, Sieper J, Dijkmans BAC, Braun J, et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis - Results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol (2006) 54(7):2136–46. doi: 10.1002/art.21913
35. Park W, Hrycaj P, Jeka S, Kovalenko V, Lysenko G, Miranda P, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis (2013) 72(10):1605–12. doi: 10.1136/annrheumdis-2012-203091
36. Baeten D, Baraliakos X, Braun J, Sieper J, Emery P, van der Heijde D, et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet. (2013) 382(9906):1705–13. doi: 10.1016/S0140-6736(13)61134-4
37. Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discovery (2012) 11(10):763–76. doi: 10.1038/nrd3794
38. Shen H, Goodall JC, Hill Gaston JS. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheumatol (2009) 60(6):1647–56. doi: 10.1002/art.24568
39. Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, et al. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity (1995) 3(6):811–21. doi: 10.1016/1074-7613(95)90070-5
40. Suzuki E, Mellins ED, Gershwin ME, Nestle FO, Adamopoulos IE. The IL-23/IL-17 axis in psoriatic arthritis. Autoimmun Rev (2014) 13(4-5):496–502. doi: 10.1016/j.autrev.2014.01.050
41. Griffin GK, Newton G, Tarrio ML, Bu D-X, Maganto-Garcia E, Azcutia V, et al. IL-17 and TNF-α sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol (Baltimore Md : 1950). (2012) 188(12):6287–99. doi: 10.4049/jimmunol.1200385
42. Fragoulis GE, Siebert S, McInnes IB. Therapeutic targeting of IL-17 and IL-23 cytokines in immune-mediated diseases. Annu Rev Med (2016) 67(1):337–53. doi: 10.1146/annurev-med-051914-021944
43. Demasi M, Jefferson T. Placebo-the unknown variable in a controlled trial. JAMA Intern Med (2021) 181(5):577–8. doi: 10.1001/jamainternmed.2020.8670
44. Holbrook A, Goldsmith C. Innovation and placebos in research: a new design of clinical trial. Lancet. (2003) 362(9401):2036–7. doi: 10.1016/S0140-6736(03)15147-1
45. Menter A, Strober BE, Kaplan DH, Kivelevitch D, Prater EF, Stoff B, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol (2019) 80(4):1029–72. doi: 10.1016/j.jaad.2018.11.057
46. Aladul MI, Fitzpatrick RW, Chapman SR. Patients’ Understanding and attitudes towards infliximab and etanercept biosimilars: result of a UK web-based survey. BioDrugs. (2017) 31(5):439–46. doi: 10.1007/s40259-017-0238-1
47. Millner JR, Barron JS, Beinke KM, Butterworth RH, Chasle BE, Dutton LJ, et al. Exercise for ankylosing spondylitis: An evidence-based consensus statement. Semin Arthritis Rheumatol (2016) 45(4):411–27. doi: 10.1016/j.semarthrit.2015.08.003
48. Tu L, Xie Y, Liao Z, Jiang Y, Lv Q, Cao S, et al. Cost of illness, quality of life, and work outcomes in active ankylosing spondylitis patients treated with adalimumab in China. Front Public Health (2020) 8:602334. doi: 10.3389/fpubh.2020.602334
49. Navarro-Compán V, Wei JCC, Van den Bosch F, Magrey M, Wang L, Fleishaker D, et al. Effect of tofacitinib on pain, fatigue, health-related quality of life and work productivity in patients with active ankylosing spondylitis: results from a phase III, randomised, double-blind, placebo-controlled trial. RMD Open (2022) 8(2):e002253. doi: 10.1136/rmdopen-2022-002253
Keywords: ankylosing spondylitis, clinical trials, bibliometric analysis, placebo-controlled trials, Citespace, VOSviewer, interleukin-17
Citation: Zhang W, Li M, Li X, Wang X, Liu Y and Yang J (2024) Global trends and research status in ankylosing spondylitis clinical trials: a bibliometric analysis of the last 20 years. Front. Immunol. 14:1328439. doi: 10.3389/fimmu.2023.1328439
Received: 26 October 2023; Accepted: 12 December 2023;
Published: 08 January 2024.
Edited by:
Xin Wu, Shanghai Changzheng Hospital, ChinaReviewed by:
Cunzhi Liu, Beijing University of Chinese Medicine, ChinaHaifa Qiao, Shaanxi University of Chinese Medicine, China
Copyright © 2024 Zhang, Li, Li, Wang, Liu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiguo Yang, c2R5YW5namlndW9AMTI2LmNvbQ==; Yuanxiang Liu, bHl4bHd0Z0AxMjYuY29t
 Wenhui Zhang
Wenhui Zhang Meng Li
Meng Li Xuhao Li
Xuhao Li Xingxin Wang
Xingxin Wang Yuanxiang Liu
Yuanxiang Liu Jiguo Yang
Jiguo Yang