- 1Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria
- 2Multiple Sclerosis Center and Neurologia I U, Department of Neuroscience and Mental Health, AOU Città della Salute e della Scienza di Torino, Torino, Italy
- 3Department of Clinical Biochemistry, Regional Hospital in Horsens, Horsens, Denmark
- 4Department of Neurology, University Medical Centre Ljubljana, Ljubljana, Slovenia
- 5Section of Neurology, Department of Medicine and Surgery, University of Perugia, Perugia, Italy
- 6FH Campus Wien, University of Applied Sciences, Vienna, Austria
- 7Department of Neurology and Neurophysiology, Amsterdam Neuroscience, Amsterdam UMC, Location VUMC, Amsterdam, Netherlands
- 8Cliniques Universitaires Saint-Luc, UCLouvain, Brussels, Belgium
- 9Sygehus Sønderjylland, Department of Regional Health Research, University Hospital of Southern Denmark, Hadersleben, Denmark
- 10Department of Neurology, Klinikum Ottakring, Vienna, Austria
- 11Department of Clinical Neuroscience, Institute of Neuroscience and Physiology at Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
- 12Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia
- 13Neurochemistry Laboratory, Department of Clinical Chemistry, Amsterdam UMC, Location Vrije Universiteit, Amsterdam Neuroscience, Amsterdam, Netherlands
- 14Neurology Unit Department of Translational Medicine, Maggiore della Carità University Hospital, Novara, Italy
Background: Kappa free light chains (κ-FLC) in the cerebrospinal fluid (CSF) are an emerging biomarker in multiple sclerosis (MS).
Objective: To investigate whether κ-FLC index has similar diagnostic value in patients with primary progressive multiple sclerosis (PPMS) compared to oligoclonal bands (OCB).
Methods: Patients with PPMS were recruited through 11 MS centres across 7 countries. κ-FLC were measured by immunonephelometry/-turbidimetry. OCB were determined by isoelectric focusing and immunofixation.
Results: A total of 174 patients (mean age of 52±11 years, 51% males) were included. κ-FLC index using a cut-off of 6.1 was positive in 161 (93%) and OCB in 153 (88%) patients.
Conclusion: κ-FLC index shows similar diagnostic sensitivity than OCB in PPMS.
Introduction
Diagnosis of multiple sclerosis (MS) requires the combination of clinical signs and symptoms with paraclinical findings obtained by magnetic resonance imaging and cerebrospinal fluid (CSF) analysis (1). Evidence of intrathecal immunoglobulin G (IgG) synthesis in the CSF, although not specific for MS (2), substitutes for dissemination in time according to current diagnostic criteria and increases diagnostic certainty (1). The gold standard to determine intrathecal IgG synthesis is the detection of CSF-restricted oligoclonal bands (OCB) (3). However, advances in laboratory methods brought up κ-free light chains (FLC) as new CSF biomarker, which are produced in excess over intact immunoglobulins and also accumulate in CSF in case of central nervous system-derived inflammation (4). A large body of evidence showed high diagnostic accuracy of κ-FLC index in MS with sensitivity and specificity of approximately 90% similar to OCB (5). Studies were largely confined to patients with relapsing-remitting MS, i.e. studies on the diagnostic value of κ-FLC index in patients with primary progressive MS (PPMS) are still lacking, which is why we performed the present work.
Methods
From a recent meta-analysis (5), we (HH, KB) identified those studies which included patients with PPMS (n=11), contacted the first and/or corresponding authors and invited them to contribute source data of PPMS patients for the present analysis. The following data were requested: age, sex, diagnosis, applied diagnostic criteria, OCB results (positive/negative), albumin quotient (Qalb), CSF and serum κ-FLC concentration, κ-FLC index, laboratory method used for κ-FLC detection and, if available, further CSF results (red blood and white blood cell (WBC) count, QIgG, IgG index).
κ-FLC assay and calculation of intrathecal κ-FLC synthesis
κ-FLC concentrations in CSF and serum were determined by each centre as specified in the previous publications (5) either by nephelometry or turbidimetry using a serum κ-FLC immunoassay (N Latex [Siemens] or Freelite [Binding Site]) according to the manufacturers’ instructions (6, 7).
Intrathecal synthesis of κ-FLC was determined by following formula considering serum κ-FLC concentrations and blood-CSF-barrier function.
A κ-FLC index >6.1 denoted presence of intrathecal κ-FLC synthesis (termed ‘positive’), a κ-FLC index ≤6.1 denoted absence of intrathecal synthesis (termed ‘negative’) (5).
Statistics
Statistical analysis was performed by SPSS 27.0 (SPSS Inc, Chicago, IL, USA). Data are displayed as number (frequencies) and median (interquartile range), as appropriate. Group comparisons of categorical variables were done by χ2 test and of metric variables by Mann-Whitney-U test. Correlation analyses were performed using Spearman correlation coefficient (r). Concordance between dichotomous results, e.g. of κ-FLC index and OCB, was calculated as the number of positives revealed by both methods and the number of negatives revealed by both methods, related to the total number of patients, and expressed as percentage. Agreement between dichotomous results of κ-FLC index and OCB was also assessed by the adjusted kappa statistics, which considers the unequal distribution of OCB positive and negative test results in our cohort (as we included only PPMS patients) (8). A two-tailed p-value<0.05 was considered statistically significant.
Results
A total of 174 PPMS patients with a mean age of 52±11 years and a balanced sex ratio (51% males) were recruited through 11 MS centres across 7 countries (Figure 1A). Demographics, main clinical characteristics and CSF findings are given in Table 1.
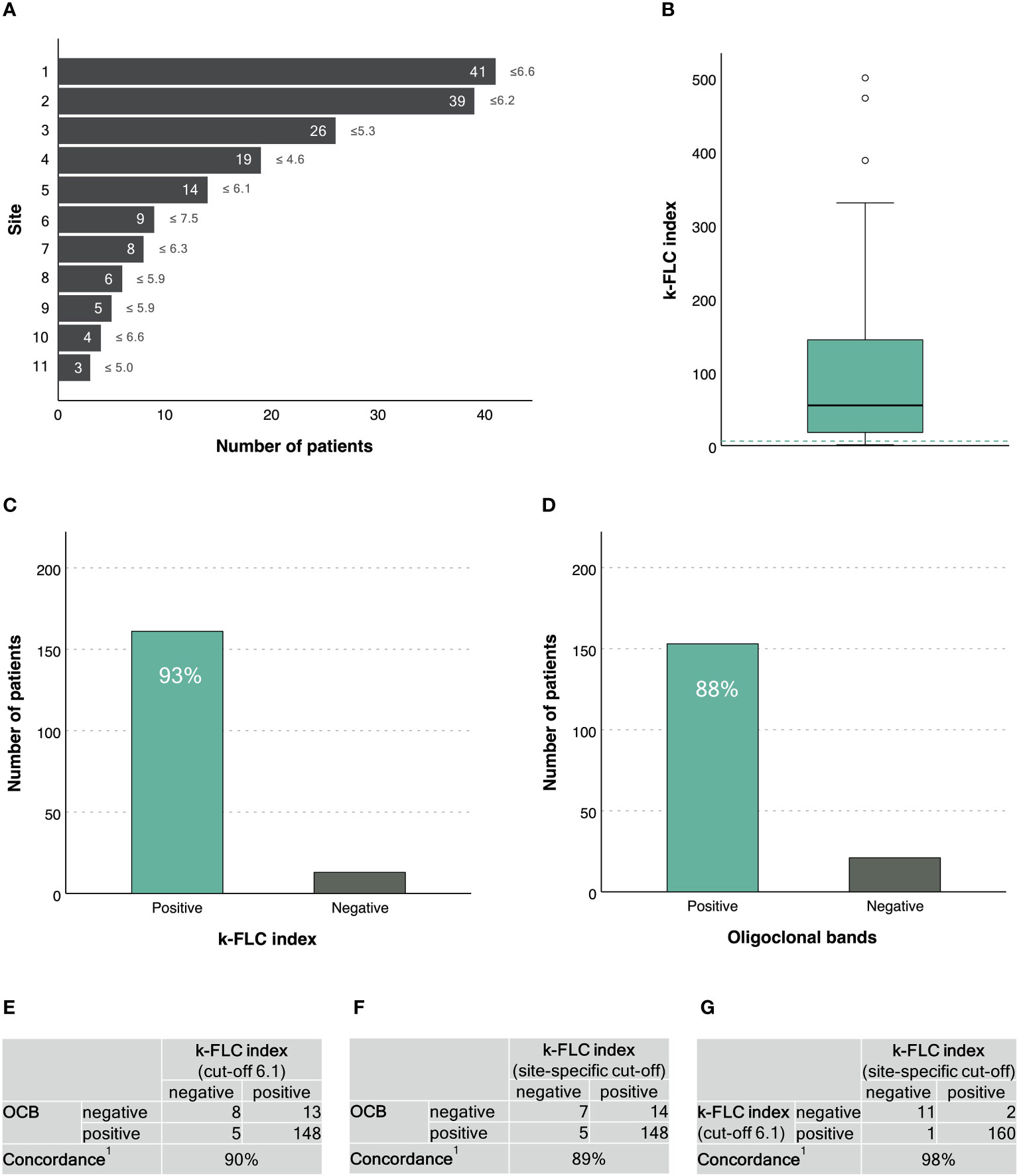
Figure 1 κ-FLC index in patients with primary progressive MS. (A) Number of patients (and κ-FLC index cut-off points) provided per MS centre2. (B) Distribution of κ-FLC index3. (C) Prevalence of positive/negative κ-FLC index (using a cut-off of 6.1). (D) Prevalence of positive/negative OCB. (E) Agreement between κ-FLC index (using a cut-off of 6.1) and OCB. (F) Agreement between κ-FLC index (using the centre-specific cut-offs) and OCB. (G) Agreement between κ-FLC index using a cut-off of 6.1 or centre-specific cut-offs. 1 Concordance was calculated as the number of positives revealed by both methods and the number of negatives revealed by both methods, related to the total number of patients, and expressed as percentage. 2 Patients’ data were drawn out of following publications and/or recruited through following MS centres: 1 Leurs et al. Mult Scler 2020;26(8):912-923. 2 Cavalla et al. J Neuroimmunol 2020;339:577122 (n=11) and unpublished data of patients from AOU Città della Salute e della Scienza di Torino, Torino, Italy (n=28). 3 Emersic et al. Clin Chim Acta 2019;489:109-116 (n=8) and unpublished data of patients from University Medical Centre Ljubljana, Ljubljana, Slovenia (n=18). 4 Rosenstein et al. J Neurochem 2021;159(3):618-628. 5 Unpublished data of patients from Medical University of Innsbruck, Innsbruck, Austria. 6 Christiansen et al. Clin Chem Lab Med 2018;57(2):210-220. 7 Bayart et al. Acta Neurol Scand 2018;138(4):352-358. 8 Presslauer et al. Mult Scler 2016;22(4):502-10. 9 Presslauer et al. J Neurol 2008;255(10):1508-14. 10 Gaetani et al. J Neuroimmunol 2020;339:577108. 11 Vecchio et al. Sci Rep 2020;10(1):20329. 3 The horizontal, green, dashed line indicates a κ-FLC index at 6.1. FLC, free light chain; OCB, oligoclonal bands.
κ-FLC index had a median of 54.6 (IQR 17.8-144.0) (Figure 1B). κ-FLC index did not statistically significantly differ between males and females (p=0.151), did not correlate with patients’ age (r=-0.123, p=0.106), but significantly correlated with CSF WBC (r=0.23, p<0.023) and IgG index (r=0.80, p<0.001).
Using a cut-off of 6.1, κ-FLC index was denoted positive in 161 (93%) patients (Figure 1C). OCB were positive in 153 (88%) patients (Figure 1D). Concordance between κ-FLC index and OCB was reached in 90% of cases (Figure 1E). The adjusted kappa statistics revealed a substantial agreement (0.79).
The different MS centres used different cut-off points for κ-FLC index to denote a positive result ranging from 4.6 to 7.5 (Figure 1A). Applying site-specific cut-off points also revealed a (median) diagnostic sensitivity for κ-FLC index of 93%. The concordance with OCB was 89% (Figure 1F), and the adjusted kappa statistics showed a substantial agreement (0.78).
The agreement between κ-FLC index using site-specific cut-off points or a fixed cut-off at 6.1 reached 98% (Figure 1G). Neither the type of platform (nephelometry vs. turbidimetry) nor the type of immunoassay (N Latex vs. Freelite) did impact on diagnostic sensitivity (χ2, p<0.415 and 0.777).
Discussion
In this study, we demonstrated that κ-FLC index reaches a diagnostic sensitivity of 93% in patients with PPMS which is similar to OCB. Even though a multitude of studies have already reported a comparable diagnostic accuracy of κ-FLC index and OCB (5), these studies were largely confined to patients with a relapsing-remitting disease course. Providing evidence on the performance of κ-FLC index in PPMS is of high clinical importance, as it has been suggested to include intrathecal κ-FLC synthesis into the next revision of MS diagnostic criteria (9).
We observed that there was no difference in diagnostic sensitivity of κ-FLC index whether a fixed cut-off of 6.1 –as retrieved by a recent meta-analysis (5)– or each the site-specific cut-off points were used. This might be due to the fact that the site-specific cut-off points were close to 6.1 (ranging from 4.6 to 7.5) and that the vast majority of MS patients shows considerably higher κ-FLC indices (approximately 95% of patients with positive κ-FLC index had values of 10 or higher).
We did not observe any impact of type of platform or assay on κ-FLC index positivity. This is in line with two prior large analyses (5, 10) and might be explained by a smaller laboratory variation due to using the CSF/serum κ-FLC ratio within the index rather than absolute concentrations. Furthermore, we compared the positivity rate of κ-FLC index and as the majority of patients show considerably elevated κ-FLC index values, a certain variation does not change classification in positive or negative.
There are some limitations of the study. This is a retrospective study with all the inherent limitations of such a design. The analytic performance of OCB detection probably differed between centres, as different methods (e.g. commercial versus in-house assays) were applied; and the interpretation of results is rater-dependent (4). It cannot be excluded that these differences might have influenced diagnostic sensitivity of OCB. We would like to state that one of the advantages of κ-FLC is the reliable and rater-independent determination which should overcome technical difficulties of OCB.
Altogether, we provide profound evidence that κ-FLC index and OCB show similar diagnostic performance in patients with PPMS and, thereby, filled the prior scientific and practical gap in this field. Determination of κ-FLC index is a fast, cost-effective and – as already mentioned above – a rater-independent method. By showing high diagnostic performance independent of the initial MS disease courses, it might serve as alternative to OCB testing in patients with suspected MS (5, 8).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by local ethic committees of participating centres. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired as part of previous studies for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
HH: Conceptualization, Data curation, Formal analysis, Writing – original draft. KB: Data curation, Writing – review & editing. PC: Data curation, Writing – review & editing. MC: Data curation, Writing – review & editing. AE: Data curation, Writing – review & editing. MD: Data curation, Writing – review & editing. LG: Data curation, Writing – review & editing. MH: Data curation, Writing – review & editing. CL: Data curation, Writing – review & editing. DM: Data curation, Writing – review & editing. Vv: Data curation, Writing – review & editing. TP: Data curation, Writing – review & editing. SP: Data curation, Writing – review & editing. IR: Data curation, Writing – review & editing. UR: Data curation, Writing – review & editing. CS: Data curation, Writing – review & editing. CT: Data curation, Writing – review & editing. DV: Data curation, Writing – review & editing. MV: Conceptualization, Writing – review & editing. FD: Writing – review & editing.
Funding
The authors declare no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
HH has participated in meetings sponsored by, received speaker honoraria or travel funding from Bayer, Biogen, Celgene, Merck, Novartis, Sanofi-Genzyme, Siemens, Teva, and received honoraria for acting as consultant for Biogen, Celgene, Novartis and Teva. He is associate editor of Frontiers in Neurology. KB has participated in meetings sponsored by and received travel funding or speaker honoraria from Roche, Teva, Merck, Biogen, Sanofi. PC has received research funding and speaker fees from Merck Serono, Roche, Novartis, Biogen, Sanofi. AE has participated in meetings sponsored by Novartis. MDF participated on advisory boards for and received speaker or writing honoraria, funding for travelling and research support from Alexion, Bayer, Biogen Idec, Sanofi, Siemens Healthineers, Merck, Mylan, Novartis, Roche, Teva and Viatris. LG participated on advisory boards for, and received writing honoraria and travel grants from Almirall, Biogen, Euroimmun, Fujirebio, Merck, Mylan, Novartis, Roche, Sanofi, Siemens Healthineers, and Teva. DM has participated in meetings sponsored by Siemens. VVP has received travel grants from Merck, Biogen, Sanofi and Roche. His institution has received research grants and consultancy fees from Roche, Biogen, Sanofi, Merck, Bristol Meyer Squibb, Janssen, Almirall and Novartis Pharma. TP has received research grant support and travel support from Biogen Idec, Merck Serono, Novartis, Bayer Schering, Sanofi-Aventis, Roche, and Genzyme. SP had received travel funding and speaker honoraria from Bayer, Biogen, Merck, Novartis, Sanofi-Genzyme, Teva, The Binding Site. IR has received compensation for lectures from Biogen. UR has participated in meetings sponsored by or received honoraria for advisor/speaker for Bayer, Biogen, Janssen, Lek, Merck, Novartis, Roche, Sanofi-Genzyme, Teva. His institution has received research support from Biogen and Novartis. CS has participated in meetings sponsored by Siemens. CT has a collaboration contract with ADx Neurosciences, Quanterix and Eli Lilly, performed contract research or received grants from AC-Immune, Axon Neurosciences, Bioconnect, Biogen, Bioorchstra, Brainstorm Therapeutics, Celgene, EIP Pharma, Eisai, Novo Nordisk, PeopleBio, Roche, Toyama, Vivoryon. She serves on editorial boards of Medidact Neurologie/Springer, Alzheimer Research and Therapy, Neurology: Neuroimmunology & Neuroinflammation, and is editor of a Neuromethods book Springer. Research of CET is supported by the European Commission Marie Curie International Training Network, grant agreement No 860197 MIRIADE, Innovative Medicines Initiatives 3TR Horizon 2020, grant no 831434and JPND bPRIDE, National MS Society Progressive MS alliance and Health Holland, the Dutch Research Council ZonMW, Alzheimer Drug Discovery Foundation, The Selfridges Group Foundation, Alzheimer Netherlands, Alzheimer Association. CT is recipient of ABOARD, which is a public-private partnership receiving funding from ZonMW #73305095007 and Health~Holland, Topsector Life Sciences & Health PPP-allowance; #LSHM20106. ABOARD also receives funding from Edwin Bouw Fonds and Gieskes-Strijbisfonds. DV received travel grants from Merck, Sanofi-Genzyme, Almirall and Novartis and research grants from Merck. MV has received research funding and speaker fees from Merck Serono, Roche, Novartis, Biogen, Sanofi. FD has participated in meetings sponsored by or received honoraria for acting as an advisor/speaker for Alexion, Almirall, Biogen, Celgene-BMS, Genzyme-Sanofi, Horizon, Merck, Novartis Pharma, Roche, and Teva. His institution has received research grants from Biogen and Genzyme Sanofi. He is section editor of the MSARD Journal Multiple Sclerosis and Related Disorders and review editor of Frontiers Neurology.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol (2018) 17(2):162–73. doi: 10.1016/S1474-4422(17)30470-2
2. Deisenhammer F, Bartos A, Egg R, Gilhus NE, Giovannoni G, Rauer S, et al. Guidelines on routine cerebrospinal fluid analysis. Rep an EFNS task force Eur J Neurol (2006) 13(9):913–22.
3. Freedman MS, Thompson EJ, Deisenhammer F, Giovannoni G, Grimsley G, Keir G, et al. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: a consensus statement. Arch Neurol (2005) 62(6):865–70. doi: 10.1001/archneur.62.6.865
4. Hegen H, Berek K, Deisenhammer F. Cerebrospinal fluid kappa free light chains as biomarker in multiple sclerosis-from diagnosis to prediction of disease activity. Wien Med Wochenschr (2022) 172(15-16):337–45. doi: 10.1007/s10354-022-00912-7
5. Hegen H, Walde J, Berek K, Arrambide G, Gnanapavan S, Kaplan B, et al. Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis. Mult Scler (2023) 29(2):169–81. doi: 10.1177/13524585221134213
6. Velthuis HT, Knop I, Stam P, van den Broek M, Bos HK, Hol S, et al. N Latex FLC - new monoclonal high-performance assays for the determination of free light chain kappa and lambda. Clin Chem Lab Med (2011) 49(8):1323–32. doi: 10.1515/CCLM.2011.624
7. Bradwell AR, Carr-Smith HD, Mead GP, Tang LX, Showell PJ, Drayson MT, et al. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem (2001) 47(4):673–80. doi: 10.1093/clinchem/47.4.673
8. Brennan RL, Prediger DJ. Coefficient kappa: Some uses, misuses, and alternatives. Educ psychol measurement (1981) 41(3):687–99. doi: 10.1177/001316448104100307
9. Hegen H, Arrambide G, Gnanapavan S, Kaplan B, Khalil M, Saadeh R, et al. Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A consensus statement. Mult Scler (2023) 29(2):182–95. doi: 10.1177/13524585221134217
Keywords: cerebrospinal fluid, kappa free light chain, primary progressive, multiple sclerosis, diagnosis, sensitivity
Citation: Hegen H, Berek K, Cavalla P, Christiansen M, Emeršič A, Di Filippo M, Gaetani L, Hassler M, Leurs C, Milosavljevic D, van Pesch V, Petersen T, Presslauer S, Rosenstein I, Rot U, Schnabl C, Teunissen C, Vecchio D, Vercellino M and Deisenhammer F (2023) Diagnostic value of kappa free light chain index in patients with primary progressive multiple sclerosis – a multicentre study. Front. Immunol. 14:1327947. doi: 10.3389/fimmu.2023.1327947
Received: 25 October 2023; Accepted: 06 December 2023;
Published: 19 December 2023.
Edited by:
Dejan Jakimovski, University at Buffalo, United StatesReviewed by:
Tobias Zrzavy, Medical University of Vienna, AustriaMassimiliano Castellazzi, University of Ferrara, Italy
Copyright © 2023 Hegen, Berek, Cavalla, Christiansen, Emeršič, Di Filippo, Gaetani, Hassler, Leurs, Milosavljevic, van Pesch, Petersen, Presslauer, Rosenstein, Rot, Schnabl, Teunissen, Vecchio, Vercellino and Deisenhammer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Harald Hegen, aGFyYWxkLmhlZ2VuQGktbWVkLmFjLmF0
 Harald Hegen
Harald Hegen Klaus Berek
Klaus Berek Paola Cavalla
Paola Cavalla Mikael Christiansen3
Mikael Christiansen3 Massimiliano Di Filippo
Massimiliano Di Filippo Dejan Milosavljevic
Dejan Milosavljevic Vincent van Pesch
Vincent van Pesch Thor Petersen
Thor Petersen Igal Rosenstein
Igal Rosenstein Charlotte Teunissen
Charlotte Teunissen Domizia Vecchio
Domizia Vecchio Marco Vercellino
Marco Vercellino Florian Deisenhammer
Florian Deisenhammer