
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol., 14 December 2023
Sec. Cytokines and Soluble Mediators in Immunity
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1326667
This article is part of the Research TopicThe immunological regulation of extracellular vesicles on chronic diseasesView all 12 articles
Lung cancer is a chronic wasting disease with insidious onset and long treatment cycle. Exosomes are specialized extracellular vesicles, at first exosomes were considered as a transporter of cellular metabolic wastes, but recently many studies have identified exosomes which contain a variety of biologically active substances that play a role in the regulation of cellular communication and physiological functions. Exosomes play an important role in the development of lung cancer and can promote metastasis through a variety of mechanisms. However, at the same time, researchers have also discovered that immune cells can also inhibit lung cancer through exosomes. In addition, researchers have discovered that some specific miRNAs in exosomes can be used as markers for early diagnosis of lung cancer. Engineering exosomes may be one of the strategies to enhance the clinical translational application of exosomes in the future, for example, strategies such as modifying exosomes to enhance targeting or utilizing exosomes as carriers for drug delivery have been explored. but more studies are needed to verify the safety and efficacy. This article reviews the latest research on exosomes in the field of lung cancer, from the mechanism of lung cancer development, the functions of immune cell-derived exosomes and tumor-derived exosomes, to the early diagnosis of lung cancer.
Cancer is essentially a disease of uncontrolled growth, unlimited proliferation and metastasis to distant sites, with an insidious onset and a long treatment period (1, 2). The American Cancer Society reported in 2020 that lung cancer currently ranks second in new cancer cases and first in cancer-related deaths worldwide (3, 4). Lung cancer is mainly categorized into two types: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). NSCLC is a predominant type of lung cancer, accounting for approximately 85% to 90% of lung cancer cases. In contrast to SCLC, NSCLC typically exhibits slower growth with better prognosis compared to SCLC (5).
Almost all cell types can secrete extracellular vesicles (EVs). Currently, there is no gold standard for classifying extracellular vesicles (EVs). They can be categorized into microvesicles, exosomes, apoptotic bodies, and more. Different types of EVs exhibit distinct biological characteristics and origins. For instance, microvesicles, ranging in size from 100-1000nm, are directly secreted from the cellular membrane. Apoptotic bodies, generated during cell apoptosis, typically have a size of 50-500nm (6). Various EVs carry different surface markers, allowing for isolation and purification. The biogenesis of exosomes involves the inward budding of the plasma membrane and the formation of intraluminal vesicles (ILVs) within multivesicular bodies (MVBs). Through MVB fusion with the plasma membrane and exocytosis, ILVs are eventually released as exosomes (7).
Exosomes are specialized extracellular vesicles produced by the endocytosis pathway with a lipid bilayer closed structure, with diameters ranging from 30-150 nm, and are widely distributed in body fluids including urine, saliva, plasma, cerebrospinal fluid, and bile (7, 8). They contain a variety of biologically active substances, including proteins, DNA, microRNA, lipids, etc (Figure 1). Researchers have conducted extensive research to verify their biological functions (9). Cell-to-cell communication can be realized through exosomes, which have also been found to be involved in various physiopathological processes and intracellular mechanism regulation (10).
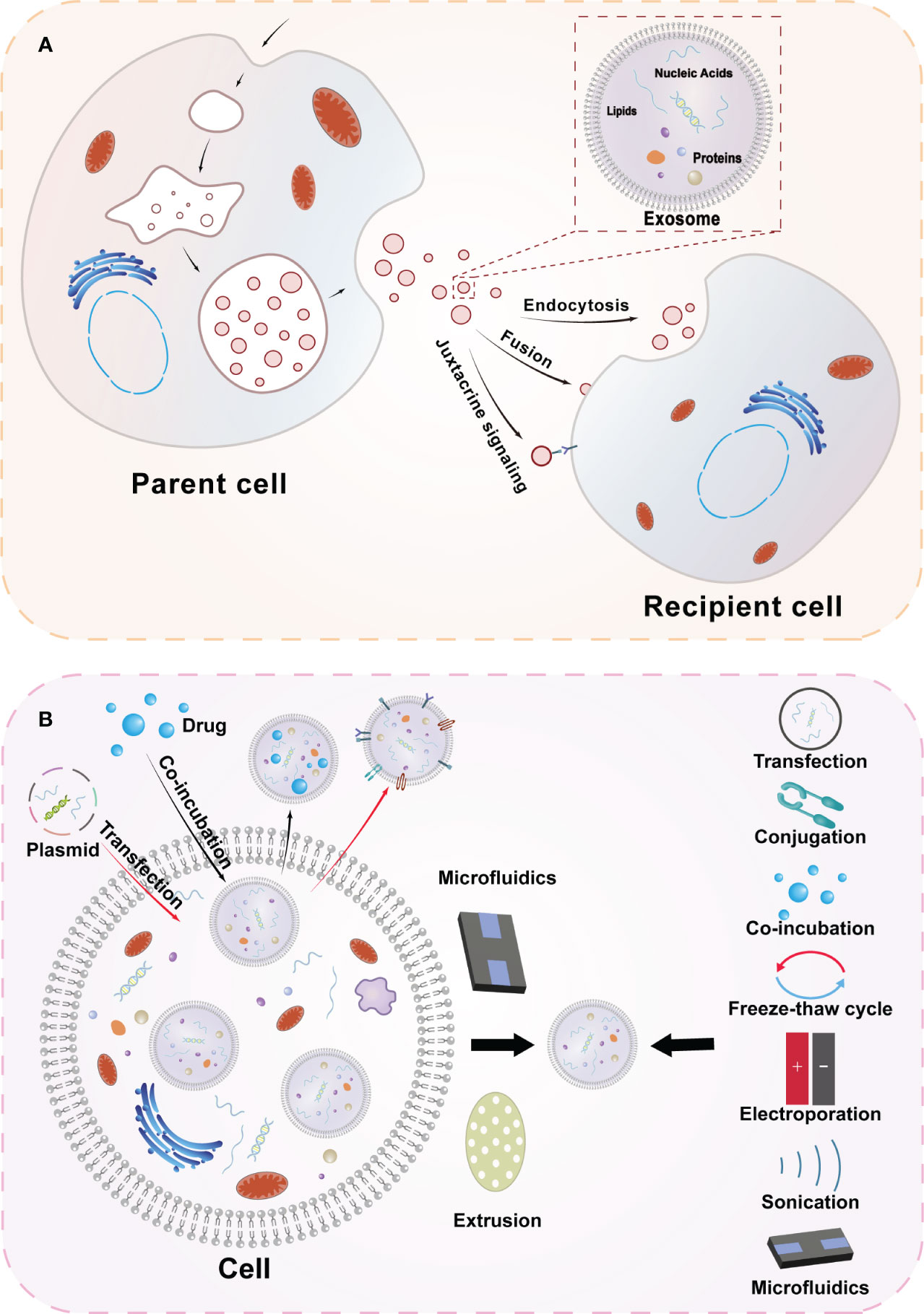
Figure 1 Overview diagram of exosome biogenesis and engineered exosomes. (A) Secretion and uptake of exosomes and the composition of exosomes. The cell membrane of the parent cell invaginates to form early endosomes, which subsequently mature into late endosomes and multivesicular vesicles, and the vesicles in the lumen of the multivesicular vesicles are exosomes secreted by the cell after membrane fusion. After exosomes are released to the outside of the cell, they can transmit information to the receptor cells in three ways(Endocytosis, Fusion, Juxtacrine signaling) to achieve the corresponding functions. (B) Extraction methods of exosomes and strategies for engineering exosomes. Engineered exosomes can be obtained by modifying exosomes at both cellular and exosome levels. At the cellular level, engineered exosomes can be obtained by processing cells through transfection and co-incubation, etc. At the exosome level, after extracting cellular exosomes through microfluidics and other techniques, information is loaded into exosomes using transfection, cojugation, freeze-thaw cycling, electroporation, sonication and other techniques.
Exosomes were first discovered and named in 1983. But for a long time afterward, exosomes were not thought to be valuable components until 2007 that it was found to have the capability of intercellular communication, and more studies have subsequently found that exosomes have a variety of physiological functions (11). In other areas such as endocrine, cardiovascular, and ophthalmologic diseases, exosomes can serve as biomarkers for clinical diagnosis (12, 13). The function and contents within the exosomes are heterogeneous due to different origins, and even exosomes secreted by the same cell at different time can exhibit different morphologies and functions. In recent years, exosomes have been found to play an important role in tumor metastasis, immune microenvironment formation, and non-programmed tumor cell death (14).
There are various strategies for exosome isolation, including ultracentrifugation, ultrafiltration, size-exclusion chromatography, precipitation, immunoaffinity-based capture, and microfluidic separation (8, 15, 16). All these techniques allows researchers to unveil the mystery of exosomes. Currently, several researches on exosomes in lung cancer have made good progress, revealing the mechanism in lung cancer progression, drug resistance, and metastasis, and emphasizing the significance of exosomes in the early diagnosis (17). With the increasing knowledge of exosomes, researchers have used innovative techniques to generate engineered exosomes as a novel therapeutic strategy for lung cancer (18).
In this paper, we will briefly introduce the relevant mechanism of exosomes in lung cancer development in recent years. Then based on the previous understanding, we analyze the potential of exosomes as diagnostic markers as well as drug delivery carriers. Finally, we discuss the challenges and potential directions for future applications of exosomes in lung cancer research.
Drug resistance refers to the development of tolerance in tumor cells to antitumor therapy, which can greatly reduce the effectiveness of antitumor therapy. Tumor drug resistance can be achieved through a variety of mechanisms, such as altered drug kinetics and enhanced drug efflux and metabolism, affecting tumor cell cycle and promoting proliferation and inhibiting apoptosis (19). Existing studies have shown that exosomes derived from different sources of cells in the tumor microenvironment(TME) can affect tumor drug resistance by influencing tumor proliferation and immunity (20), which include immune cells, tumor cells, and tumor-associated fibroblasts.
Exosomes can act as intermediate carriers to deliver drug resistance information from drug-resistant tumor cells to tumor cells that have not acquired resistance For example, researchers found that cisplatin-resistant NSCLS cells induced by hypoxic environment could secrete PKM2 exosomes to transfer resistance capability to cisplatin-sensitive NSCLS (21). As a result, exosomes can also serve as a drug-carrying tool to inhibit tumor drug resistance in lung (22).
Tumor metastasis is a major cause of poor prognosis and death, and epithelial mesenchymal transition (EMT) is one of the important pathways for tumor progression and metastasis (23). Normally, EMT helps cells to migrate in the embryo, and tumor cells that undergo EMT show changes of weakened intercellular adhesion and enhanced cell motility, which increases the likelihood of metastasis of tumor cells (24).
Exosomes from tumor-associated fibroblasts (CAFs) enhance cell stemness and EMT in colorectal cancer, thereby promoting tumor metastasis (25). KRT6B in tumor-derived exosomes was also found to promote cancer metastasis by inducing EMT in bladder cancer (26). Similar phenomena were found in kidney cancer (27), liver cancer (28), and breast cancer (29). In lung cancer, exosomes constituting the TME can also mediate EMT in lung cancer, thus promoting metastasis. Researchers found that miRNAs contained in exosomes produced by mysenchymal stem cells under hypoxic environments could elevate the expression of markers associated with EMT in lung cancer by regulating the STAT3 signaling pathway (30). Exosomes from hypoxic LUAD cells could significantly increase the migration of normoxic lung adenocarcinoma cells via SATB2to activate MEK/ERK pathway-mediated EMT (31).
Apart from a direct effect on EMT, lung adenocarcinoma cells may indirectly regulate EMT in lung adenocarcinoma by interfering with exosome secretion from CAFs as well (32). Additionally, different states of tumor cells and other cells in the TME can exert an influence on the EMT process. Exosomes, as one of the constitutive components of the TME, thereby intervene in the progression of the tumor process greatly.
Neovascularization is required for lung cancer growth and metastasis, and is also a factor that affects the prognosis of lung cancer (33–35). The significance of anti-vascularization in lung cancer treatment has been noted early on, and anti-vascular endothelial growth factor (VEGF) drugs have been approved for use in the clinic (36). Studies on the mechanism of neovascularization in lung cancer are more limited, but some specific miRNAs, sirtuin1, and notch pathway have been found to play a role in neovascularization (37, 38). The Tumor Microenvironment (TME) refers to the collective assembly of cells, molecules, and physical factors surrounding a tumor, interacting with tumor cells and influencing the growth, spread, and therapeutic responses of the tumor. Hypoxic conditions are common in the TME. One study indicated that miR-23a in exosomes of lung cancer cells under hypoxia targets ZO-1 protein and prolyl hydroxylase, thereby enhancing angiogenesis and vascular permeability (39), while another study found that miR-197-3p in exosomes of lung adenocarcinoma origin targets TIMP2, TIMP3 to promote neoangiogenesis (40). MiR-3157-3p in exosomes from NSCLS was also found to down-regulate the expression of TIMP2, KLF2, ZO-1, and Occludin, and up-regulate the expression of VEGF, MMP2, and MMP9, ultimately leading to increased angiogenesis (41). Currently, anti-angiogenic drugs, such as bevacizumab, apatinib, abciximab, have been applied in the clinical treatment of lung cancer patients, with good efficacy. Since lung cancer exosomes is believed to be one of the mechanisms of neovascularization, and existing research found that the miRNAs therein seem to be the key, one may opens up the idea that if the application of the inhibitors of these miRNAs, or the inhibition of secretion of tumor exosomes, is possible to achieve good results? Of course, this requires more in-depth research to verify the safety and efficacy.
The human immune system recognizes and removes tumor cells from the body, and tumor cells can evade immune recognition and removal by inhibiting immune cell proliferation or activation. For example, tumor-derived exosomes have been found to alter mitochondrial function to inhibit the proliferation of cytotoxic T cells (42). Natural killer cells(NKC) are sentinel cells of the immune system (43) that recognize tumor cells and remove them without additional activation. During immune evasion of tumor cells, a large number of bioactive molecules contained in tumor cell-derived exosomes, such as transforming growth factor-β (TGF-β), programmed death ligand (PD-L1), ligand for natural killer cell activated receptor NKG2D (MICA/B), apoptosis-associated protein Fas-L, etc., will recognize the cognate receptor on NK cells and abolish the anti-tumor activity. Regarding macrophages, tumor-derived exosomes have also been found to inhibit polarization, achieving immunosuppression and allowing tumor progression (44, 45).
The lymphatic system is responsible for immune cell activation, and the researchers found that exosomes secreted by tumor cells containing immunosuppressive protein PD-L1 can inactivate immune cells to protect themselves from being killed (46). This observation gives clue to researchers that if one can inhibit some tumor cell-derived exosomes, it will also be possible to address the dilemma of insensitivity to immune checkpoint inhibitors in many patients. However, this also raises new challenges in developing medications that can act on tumor exosomes.
Besides the effect of tumor-derived exosomesaffect on immune cells, exosomes secreted by immune cells also exert impact on the tumor immune response. In addition to recognizing and killing tumor cells, NK cells exosomes also modulate the immune response of T cells (47) and are cytotoxic to cancer cells (48). Exosomes secreted by M1-polarized macrophages were found to promote M1 polarization of macrophages, thereby enhancing anti-tumor immunity and inhibiting tumor growth (49).
Large number of researches have validated the function of exosomes in the progression of lung cancer (50), and people are also thinking about the role of exosomes in the clinical context. Early diagnosis of lung cancer can significantly improve the survival rate of patients (51, 52). Although low-dose computed tomography (LDCT) has improved the diagnosis rate of lung nodules, LDCT has poor specificity and is prone to unnecessary surgery, so non-invasive means to diagnose benign and malignant lung nodules is a very promising research direction.
Based on the changes of miRNA profiles in plasma exosomes, researchers identified miR-500a-3p, miR-501-3p, and miR-502-3p, up-regulated within lung cancer, which revealed the possibility of early diagnosis of lung cancer by plasma exosomes (53). Subsequently, another researcher team constructed a diagnostic model to distinguish benign and malignant lung nodules based on plasma exosomal miRNAs, which was also reported encouraging outcomes for lung nodules with a diameter of less than 1 cm (54). Combined with the current deep learning algorithm Shin et al. validated the feasibility of human plasma exosomes as potential tumor-associated biomarkers (55). Subsequently, a set of miRNAs, i.e., miR-200b-3p, miR-3124-5p and miR-92b-5p, in serum exosomes could be used as diagnostic and prognostic markers for SCLC (56) (Figure 2). Another study showed that exosomes of SCLC cells with molecules such as Hippo, Rap1, and Wnt could also be used as indicators to determine prognosis (57). At present, a series of studies have verified the feasibility of exosomes in the early diagnosis of lung cancer. Combining the advantages of non-invasiveness of exosomal examination with existing tests is expected to enhance the early diagnosis and prognosis of lung cancer.
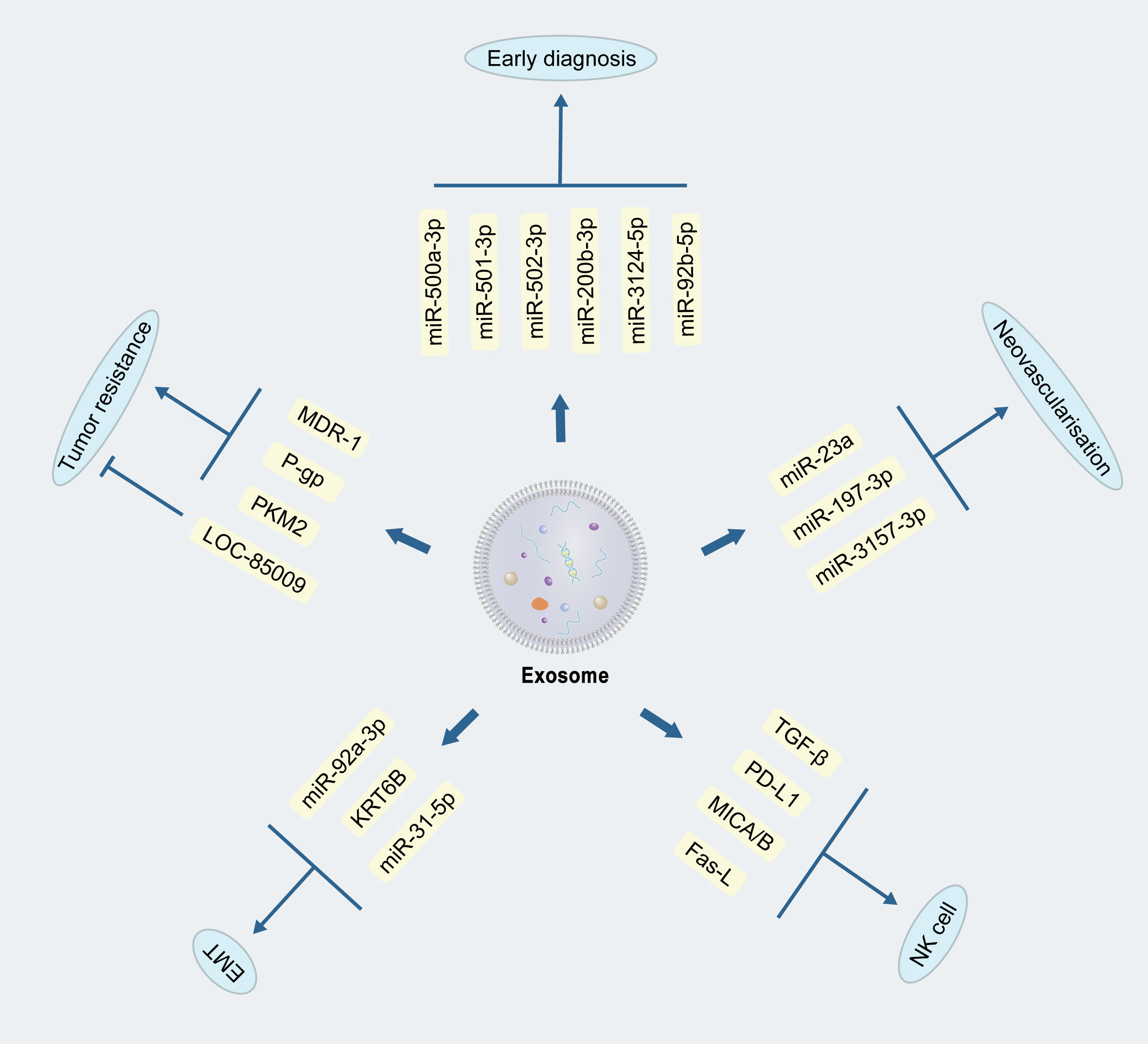
Figure 2 Mechanisms of exosomes in the development of lung cancer. Exosomes can promote or inhibit lung cancer development through different bioactive substances, as reflected in tumour drug resistance, epithelial-mesenchymal cell transformation, neovascularization, immunomodulation, and early diagnosis.
Exosomes act as a carrier of information passed from cell to cell, altering the responsiveness of the immune system and the microenvironment. Understanding how the immune system reads the information in exosomes and how exosomes affect immune cells is important for the development of new strategies that will allow immune cells to behave effectively. For example, exosomes secreted by lung cancer cells contain miR-21 and miR-29a, which can be taken up by surrounding tumor-associated macrophages (TAMs) to influence cytokine secretion (58), which regulate tumor growth and metastasis. Exosome secretion has been found to be pH-sensitive, with the pH of the tumor microenvironment affecting both exosome secretion and the contents (59). The acidic environment in the tumor microenvironment tends to lead to the death of immune cells. This is why some researches have proposed that neutralizing the acidic environment of the TME using proton pump inhibitors or other buffering therapies can reduce the immune escape of tumor (60), and these may be the directions in the future to produce an anti-tumor therapy by restricting the function of tumor exosomes. Exosomes may be a potential treatment for tumors as well. While drug therapy can lead to drug resistance and cell therapy can lead to the risk of so-called “cytokine storm” in the body, exosomes may be able to circumvent these side effects. In addition, exosomes have been shown to cross the blood-brain and blood-testis barriers, a vital property in the context of cancer cells, since these cells can evade elimination by immune cells through various immune escape mechanisms. Nevertheless, exosomes have the potential to bypass such mechanisms and still target and kill cancer cells effectively.
One of the problems in the development of exosome-related tools is that the content is variable and complex. On the other hand, the development of engineered exosomes allows researchers to use exosomes as carriers, in which specific content is loaded. This technology may be one of the future drug delivery strategies for targeting tumor cells, and good results have been detected in lab. Still, more research is needed.
Apart from the deepened understanding of exosomes in lung cancer in recent years, there is still much limitation in the clinical application of exosome-related knowledge and techniques. First, the difference in the delivery mechanisms of exosomes from various cancer cells as compared to normal cells are not clearly investigated. Unveiling the factors influencing category and amount of cargo within the exosomes will remarkably help us gain a better picture of cancer biology. Secondly, one should keep in mind that the distribution and concentration of exosomes is not even across different systems. For example, blood-brain barrier has a significant impact on exosome biology and induces a divergence between those in circulation and in cerebrospinal fluids (61, 62). Does alveolar-capillary barrier has similar effect should be depicted in the future, as this will provide valuable clue to the development of better laboratory methods with more accuracy. Third, a dynamic observation of exosomes from sputum, blood, and within the tumor in response to therapies is still poorly conducted in detail. A comparison and integration of these information can shed light on cancer biology and therapeutic targets.
People first discovered exosomes in the culture fluid of reticulocytes, which was then thought to be nothing more than a garbage truck for cells to remove waste. Decades later, exosomes once again attract much attention with a new face. With the deepening of research, it is found that exosomes have rich biological functions, which can regulate the growth of the cells, signaling. Then, researchers found that exosomes have specificity, and some of their special components can be used as markers for identifying exosomes. (fig4, Progress in the understanding of exosome research, three stages). Researches also found that the contents in exosomes are related to the cell state and cell origin, which also laid the foundation for exosome-based therapeutics and diagnostics. In recent years, the proposal of engineered exosomes has expanded the applications of exosomes, allowing researchers to modify the surface of exosomes to make them targeted or to evade recognition by other cells. The contents of exosomes can also be customized to carry specific drugs or miRNAs. In short, with the deepening of research, the functional development of exosomes will be more complete.
Apart from the deepened understanding of exosomes in lung cancer in recent years, there is still much limitation in the clinical application of exosome-related knowledge and techniques. First, the difference in the delivery mechanisms of exosomes from various cancer cells as compared to normal cells are not clearly investigated. Unveiling the factors influencing category and amount of cargo within the exosomes will remarkably help us gain a better picture of cancer biology. Secondly, one should keep in mind that the distribution and concentration of exosomes is not even across different systems. For example, blood-brain barrier has a significant impact on exosome biology and induces a divergence between those in circulation and in cerebrospinal fluids (61, 62). Does alveolar-capillary barrier has similar effect should be depicted in the future, as this will provide valuable clue to the development of better laboratory methods with more accuracy. Third, a dynamic observation of exosomes from sputum, blood, and within the tumor in response to therapies is still poorly conducted in detail. A comparison and integration of these information can shed light on cancer biology and therapeutic targets.
Exosomes of lung cancer origin can influence the metastasis and development of lung cancer through multiple mechanisms, while exosomes secreted by immune cells can also influence the progression of lung cancer. Exosomes have the potential to be a complementary means of early diagnostic tool for lung cancer, but more exploration is still needed.
JZ: Writing – original draft, Conceptualization, Methodology. XL: Writing – original draft, Writing – review & editing, Data curation. LL: Writing – original draft, Data curation. ZZ: Writing – review & editing, Methodology. CH: Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Gupta SC, Kunnumakkara AB, Aggarwal S, Aggarwal BB. Inflammation, a double-edge sword for cancer and other age-related diseases. Front Immunol (2018) 9:2160. doi: 10.3389/fimmu.2018.02160
2. Pituskin E, Joy AA, Fairchild A. Advanced cancer as a chronic disease: introduction. Semin Oncol Nurs (2021) 37:151176. doi: 10.1016/j.soncn.2021.151176
3. Nasim F, Sabath BF, Eapen GA. Lung cancer. Med Clin North Am (2019) 103:463–73. doi: 10.1016/j.mcna.2018.12.006
4. Siegel RL, Miller KD, Jemal A. Cancer statistics 2020. CA Cancer J Clin (2020) 70:7–30. doi: 10.3322/caac.21590
5. Jeon DS, Kim HC, Kim SH, Kim TJ, Kim HK, Moon MH, et al. Five-year overall survival and prognostic factors in patients with lung cancer: results from the Korean association of lung cancer registry (KALC-R) 2015. Cancer Res Treat (2023) 55:103–11. doi: 10.4143/crt.2022.264
6. Sahoo S, Adamiak M, Mathiyalagan P, Kenneweg F, Kafert-Kasting S, Thum T. Therapeutic and diagnostic translation of extracellular vesicles in cardiovascular diseases: roadmap to the clinic. Circulation (2021) 143:1426–49. doi: 10.1161/CIRCULATIONAHA.120.049254
7. Kalluri R, Lebleu VS. The biology, function, and biomedical applications of exosomes. Science (2020) 367(6478). doi: 10.1126/science.aau6977
8. Feng X, Peng Z, Yuan L, Jin M, Hu H, Peng X, et al. Research progress of exosomes in pathogenesis, diagnosis, and treatment of ocular diseases. Front Bioeng Biotechnol (2023) 11:1100310. doi: 10.3389/fbioe.2023.1100310
9. Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L, et al. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics (2020) 10:3684–707. doi: 10.7150/thno.41580
10. Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol (2019) 21:9–17. doi: 10.1038/s41556-018-0250-9
11. Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol (2007) 9:654–9. doi: 10.1038/ncb1596
12. Couch Y, Buzas EI, Di Vizio D, Gho YS, Harrison P, Hill AF, et al. A brief history of nearly EV-erything - The rise and rise of extracellular vesicles. J Extracell Vesicles (2021) 10:e12144. doi: 10.1002/jev2.12144
13. Yu W, Hurley J, Roberts D, Chakrabortty SK, Enderle D, Noerholm M, et al. Exosome-based liquid biopsies in cancer: opportunities and challenges. Ann Oncol (2021) 32:466–77. doi: 10.1016/j.annonc.2021.01.074
14. Weng Z, Zhang B, Wu C, Yu F, Han B, Li B, et al. Therapeutic roles of mesenchymal stem cell-derived extracellular vesicles in cancer. J Hematol Oncol (2021) 14:136. doi: 10.1186/s13045-021-01141-y
15. Romano E, Netti PA, Torino E. Exosomes in gliomas: biogenesis, isolation, and preliminary applications in nanomedicine. Pharm (Basel) (2020) 13(10):319. doi: 10.3390/ph13100319
16. Zhu L, Sun HT, Wang S, Huang SL, Zheng Y, Wang CQ, et al. Isolation and characterization of exosomes for cancer research. J Hematol Oncol (2020) 13:152. doi: 10.1186/s13045-020-00987-y
17. Li MY, Liu LZ, Dong M. Progress on pivotal role and application of exosome in lung cancer carcinogenesis, diagnosis, therapy and prognosis. Mol Cancer (2021) 20:22. doi: 10.1186/s12943-021-01312-y
18. Zhang J, JI C, Zhang H, Shi H, Mao F, Qian H, et al. Engineered neutrophil-derived exosome-like vesicles for targeted cancer therapy. Sci Adv (2022) 8:eabj8207. doi: 10.1126/sciadv.abj8207
19. Vasan N, Baselga J, Hyman DM. A view on drug resistance in cancer. Nature (2019) 575:299–309. doi: 10.1038/s41586-019-1730-1
20. Li I, Nabet BY. Exosomes in the tumor microenvironment as mediators of cancer therapy resistance. Mol Cancer (2019) 18:32. doi: 10.1186/s12943-019-0975-5
21. Wang D, Zhao C, Xu F, Zhang A, Jin M, Zhang K, et al. Cisplatin-resistant NSCLC cells induced by hypoxia transmit resistance to sensitive cells through exosomal PKM2. Theranostics (2021) 11:2860–75. doi: 10.7150/thno.51797
22. Yu Z, Tang H, Chen S, Xie Y, Shi L, Xia S, et al. Exosomal LOC85009 inhibits docetaxel resistance in lung adenocarcinoma through regulating ATG5-induced autophagy. Drug Resist Update (2023) 67:100915. doi: 10.1016/j.drup.2022.100915
23. Wei C, Yang C, Wang S, Shi D, Zhang C, Lin X, et al. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol Cancer (2019) 18:64. doi: 10.1186/s12943-019-0976-4
24. Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol (2019) 29:212–26. doi: 10.1016/j.tcb.2018.12.001
25. Hu JL, Wang W, Lan XL, Zeng ZC, Liang YS, Yan YR, et al. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Mol Cancer (2019) 18:91. doi: 10.1186/s12943-019-1019-x
26. Song Q, Yu H, Cheng Y, Han J, Li K, Zhuang J, et al. Bladder cancer-derived exosomal KRT6B promotes invasion and metastasis by inducing EMT and regulating the immune microenvironment. J Transl Med (2022) 20:308. doi: 10.1186/s12967-022-03508-2
27. Wang L, Yang G, Zhao D, Wang J, Bai Y, Peng Q, et al. CD103-positive CSC exosome promotes EMT of clear cell renal cell carcinoma: role of remote MiR-19b-3p. Mol Cancer (2019) 18:86. doi: 10.1186/s12943-019-0997-z
28. Lang Z, Li Y, Lin L, Li X, Tao Q, Hu Y, et al. Hepatocyte-derived exosomal miR-146a-5p inhibits hepatic stellate cell EMT process: a crosstalk between hepatocytes and hepatic stellate cells. Cell Death Discov (2023) 9:304. doi: 10.1038/s41420-023-01602-y
29. Jafari N, Kolla M, Meshulam T, Shafran JS, Qiu Y, Casey AN, et al. Adipocyte-derived exosomes may promote breast cancer progression in type 2 diabetes. Sci Signal (2021) 14:eabj2807. doi: 10.1126/scisignal.abj2807
30. Zhang X, Sai B, Wang F, Wang L, Wang Y, Zheng L, et al. Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT. Mol Cancer (2019) 18:40. doi: 10.1186/s12943-019-0959-5
31. Yu F, Liang M, Huang Y, Wu W, Zheng B, Chen C. Hypoxic tumor-derived exosomal miR-31-5p promotes lung adenocarcinoma metastasis by negatively regulating SATB2-reversed EMT and activating MEK/ERK signaling. J Exp Clin Cancer Res (2021) 40:179. doi: 10.1186/s13046-021-01979-7
32. Bai X, Shao J, Duan T, Liu X, Wang M, Li X, et al. Exo-miR-1290-induced by COX-2 overexpression promotes cancer-associated fibroblasts activation and tumor progression by CUL3-Nrf2 pathway in lung adenocarcinoma. Cell Commun Signal (2023) 21:242. doi: 10.1186/s12964-023-01268-0
33. Altorki NK, Markowitz GJ, Gao D, Port JL, Saxena A, Stiles B, et al. The lung microenvironment: an important regulator of tumour growth and metastasis. Nat Rev Cancer (2019) 19:9–31. doi: 10.1038/s41568-018-0081-9
34. Tan HW, Xu YM, Qin SH, Chen GF, Lau ATY. Epigenetic regulation of angiogenesis in lung cancer. J Cell Physiol (2021) 236:3194–206. doi: 10.1002/jcp.30104
35. Fang H, Sun Q, Zhou J, Zhang H, Song Q, Zhang H, et al. m(6)A methylation reader IGF2BP2 activates endothelial cells to promote angiogenesis and metastasis of lung adenocarcinoma. Mol Cancer (2023) 22:99. doi: 10.1186/s12943-023-01791-1
36. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature (2018) 553:446–54. doi: 10.1038/nature25183
37. Xie M, Liu M, He CS. SIRT1 regulates endothelial Notch signaling in lung cancer. PloS One (2012) 7:e45331. doi: 10.1371/journal.pone.0045331
38. Dimitrova N, Gocheva V, Bhutkar A, Resnick R, Jong RM, Miller KM, et al. Stromal expression of miR-143/145 promotes neoangiogenesis in lung cancer development. Cancer Discov (2016) 6:188–201. doi: 10.1158/2159-8290.CD-15-0854
39. Hsu YL, Hung JY, Chang WA, Lin YS, Pan YC, Tsai PH, et al. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene (2017) 36:4929–42. doi: 10.1038/onc.2017.105
40. Chang RM, Fu Y, Zeng J, Zhu XY, Gao Y. Cancer-derived exosomal miR-197-3p confers angiogenesis via targeting TIMP2/3 in lung adenocarcinoma metastasis. Cell Death Dis (2022) 13:1032. doi: 10.1038/s41419-022-05420-5
41. Ma Z, Wei K, Yang F, Guo Z, Pan C, He Y, et al. Tumor-derived exosomal miR-3157-3p promotes angiogenesis, vascular permeability and metastasis by targeting TIMP/KLF2 in non-small cell lung cancer. Cell Death Dis (2021) 12:840. doi: 10.1038/s41419-021-04037-4
42. Bland CL, Byrne-Hoffman CN, Fernandez A, Rellick SL, Deng W, Klinke D. J., 2ND. Exosomes derived from B16F0 melanoma cells alter the transcriptome of cytotoxic T cells that impacts mitochondrial respiration. FEBS J (2018) 285:1033–50. doi: 10.1111/febs.14396
43. Terren I, Orrantia A, Vitalle J, Zenarruzabeitia O, Borrego F. NK cell metabolism and tumor microenvironment. Front Immunol (2019) 10:2278. doi: 10.3389/fimmu.2019.02278
44. Liu J, Fan L, Yu H, Zhang J, He Y, Feng D, et al. Endoplasmic reticulum stress causes liver cancer cells to release exosomal miR-23a-3p and up-regulate programmed death ligand 1 expression in macrophages. Hepatology (2019) 70:241–58. doi: 10.1002/hep.30607
45. Luo C, Xin H, Zhou Z, Hu Z, Sun R, Yao N, et al. Tumor-derived exosomes induce immunosuppressive macrophages to foster intrahepatic cholangiocarcinoma progression. Hepatology (2022) 76:982–99. doi: 10.1002/hep.32387
46. Poggio M, Hu T, Pai CC, Chu B, Belair CD, Chang A, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell (2019) 177:414–427.e13. doi: 10.1016/j.cell.2019.02.016
47. Dosil SG, Lopez-Cobo S, Rodriguez-Galan A, Fernandez-Delgado I, ramirez-huesca M, Milan-Rois P, et al. Natural killer (NK) cell-derived extracellular-vesicle shuttled microRNAs control T cell responses. Elife (2022) 11:e76319. doi: 10.7554/eLife.76319.sa2
48. Lugini L, Cecchetti S, Huber V, Luciani F, Macchia G, Spadaro F, et al. Immune surveillance properties of human NK cell-derived exosomes. J Immunol (2012) 189:2833–42. doi: 10.4049/jimmunol.1101988
49. Gunassekaran GR, Poongkavithai Vadevoo SM, Baek MC, Lee B. M1 macrophage exosomes engineered to foster M1 polarization and target the IL-4 receptor inhibit tumor growth by reprogramming tumor-associated macrophages into M1-like macrophages. Biomaterials (2021) 278:121137. doi: 10.1016/j.biomaterials.2021.121137
50. Khan FH, Reza MJ, Shao YF, Perwez A, Zahra H, Dowlati A, et al. Role of exosomes in lung cancer: A comprehensive insight from immunomodulation to theragnostic applications. Biochim Biophys Acta Rev Cancer (2022) 1877:188776. doi: 10.1016/j.bbcan.2022.188776
51. Liu J, Ren L, Li S, Li W, Zheng X, Yang Y, et al. The biology, function, and applications of exosomes in cancer. Acta Pharm Sin B (2021) 11:2783–97. doi: 10.1016/j.apsb.2021.01.001
52. Chang L, Li J, Zhang R. Liquid biopsy for early diagnosis of non-small cell lung carcinoma: recent research and detection technologies. Biochim Biophys Acta Rev Cancer (2022) 1877:188729. doi: 10.1016/j.bbcan.2022.188729
53. Zhang JT, Qin H, Man Cheung FK, Su J, Zhang DD, Liu SY, et al. Plasma extracellular vesicle microRNAs for pulmonary ground-glass nodules. J Extracell Vesicles (2019) 8:1663666. doi: 10.1080/20013078.2019.1663666
54. Zheng D, Zhu Y, Zhang J, Zhang W, Wang H, Chen H, et al. Identification and evaluation of circulating small extracellular vesicle microRNAs as diagnostic biomarkers for patients with indeterminate pulmonary nodules. J Nanobiotechnol (2022) 20:172. doi: 10.1186/s12951-022-01366-0
55. Shin H, Oh S, Hong S, Kang M, Kang D, Ji YG, et al. Early-stage lung cancer diagnosis by deep learning-based spectroscopic analysis of circulating exosomes. ACS Nano (2020) 14:5435–44. doi: 10.1021/acsnano.9b09119
56. Kim DH, Park H, Choi YJ, Im K, Lee CW, Kim DS, et al. Identification of exosomal microRNA panel as diagnostic and prognostic biomarker for small cell lung cancer. biomark Res (2023) 11:80. doi: 10.1186/s40364-023-00517-1
57. Cao B, Wang P, Gu L, Liu J. Use of four genes in exosomes as biomarkers for the identification of lung adenocarcinoma and lung squamous cell carcinoma. Oncol Lett (2021) 21:249. doi: 10.3892/ol.2021.12510
58. Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci USA (2012) 109:E2110–6. doi: 10.1073/pnas.1209414109
59. Logozzi M, Mizzoni D, Angelini DF, Di Raimo R, Falchi M, Battistini L, et al. Microenvironmental pH and exosome levels interplay in human cancer cell lines of different histotypes. Cancers (Basel) (2018) 10(10):370. doi: 10.3390/cancers10100370
60. Gillies RJ, Pilot C, Marunaka Y, Fais S. Targeting acidity in cancer and diabetes. Biochim Biophys Acta Rev Cancer (2019) 1871:273–80. doi: 10.1016/j.bbcan.2019.01.003
61. Ramos-Zaldivar HM, Polakovicova I, Salas-Huenuleo E, Corvalan AH, Kogan MJ, Yefi CP, et al. Extracellular vesicles through the blood-brain barrier: a review. Fluids Barriers CNS (2022) 19:60. doi: 10.1186/s12987-022-00359-3
Keywords: exosome, lung cancer, immunotherapy, miRNA, EMT, neovascularization
Citation: Zhao J, Li X, Liu L, Zhu Z and He C (2023) Exosomes in lung cancer metastasis, diagnosis, and immunologically relevant advances. Front. Immunol. 14:1326667. doi: 10.3389/fimmu.2023.1326667
Received: 23 October 2023; Accepted: 04 December 2023;
Published: 14 December 2023.
Edited by:
Jinhong Zhu, Harbin Medical University Cancer Hospital, ChinaReviewed by:
Yang Du, Peking Union Medical College Hospital (CAMS), ChinaCopyright © 2023 Zhao, Li, Liu, Zhu and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunyan He, Y2h1bnlhbmhlNTMwQDE2My5jb20=; Zhen Zhu, enplc2t5QGhvdG1haWwuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.