- Department of General Surgery, Shengjing Hospital of China Medical University, Shenyang, China
In recent years, immune checkpoint inhibitors (ICIs) have emerged as a transformative approach in treating advanced hepatocellular carcinoma (HCC). Despite their success, challenges persist, including concerns about their effectiveness, treatment costs, frequent occurrence of treatment-related adverse events, and tumor hyperprogression. Therefore, it is imperative to identify indicators capable of predicting the efficacy of ICIs treatment, enabling optimal patient selection to maximize clinical benefits while minimizing unnecessary toxic side effects and economic losses. This review paper categorizes prognostic biomarkers of ICIs treatment into the following categories: biochemical and cytological indicators, tumor-related markers, imaging and personal features, etiology, gut microbiome, and immune-related adverse events (irAEs). By organizing these indicators systematically, we aim to guide biomarker exploration and inform clinical treatment decisions.
1 Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors, with the fifth highest incidence and third highest mortality rate of all malignancies globally (1–3). Treatment options, including hepatectomy, liver transplantation, ablative therapies, and various therapies, offer hope for early-stage patients; however, late-stage diagnoses limit surgical options. Consequently, systemic therapies, often involving conventional chemotherapy, remain the primary approach, despite their suboptimal effectiveness due to HCC’s drug-resistant nature (4). Therefore, the urgent need for novel drugs and strategies arises.
Over the last decade, multitargeted tyrosine kinase inhibitors (TKIs) like sorafenib and lenvatinib have yielded a certain degree of improvement in the survival of HCC patients, with median overall survival (OS) ranging from 11 to 14 months (5–7). In recent years, immune checkpoint inhibitors (ICIs), including anti-programmed cell death -1/programmed cell death ligand-1 (PD-1/PD-L1) and anti-cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) agents, have revolutionized the management of cancer (8–10). Although initial studies on nivolumab and pembrolizumab showed promise, later phase III clinical trials failed to meet their primary endpoints (11, 12). However, in the Phase III clinical trial IMbrave150, the combination of anti-PD-L1 and anti-vascular endothelial growth factor receptor (VEGFR) agents, atezolizumab plus bevacizumab (Atez/Bev), demonstrated significant OS improvement compared to sorafenib, becoming a recommended first-line treatment for advanced HCC (13). Additionally, recent HIMALAYA research revealed that the tremelimumab and durvalumab combination outperformed the sorafenib treatment, also gaining recognition as a first-line option for advanced HCC (14).
Despite these remarkable advancements, not all patients respond favorably to treatment. Even in patients receiving combination immunotherapy, 20% of patients are refractory to Atez/Bev, with only 20%-30% showing radiological responses. In addition, a considerable number of patients experience grade 3–4 immune-related adverse events (15, 16). Therefore, understanding the determinants of treatment response and adverse effects is crucial. As HCC systemic treatment options continue to increase, the ability to predict treatment response and survival benefits has become an exigent necessity.
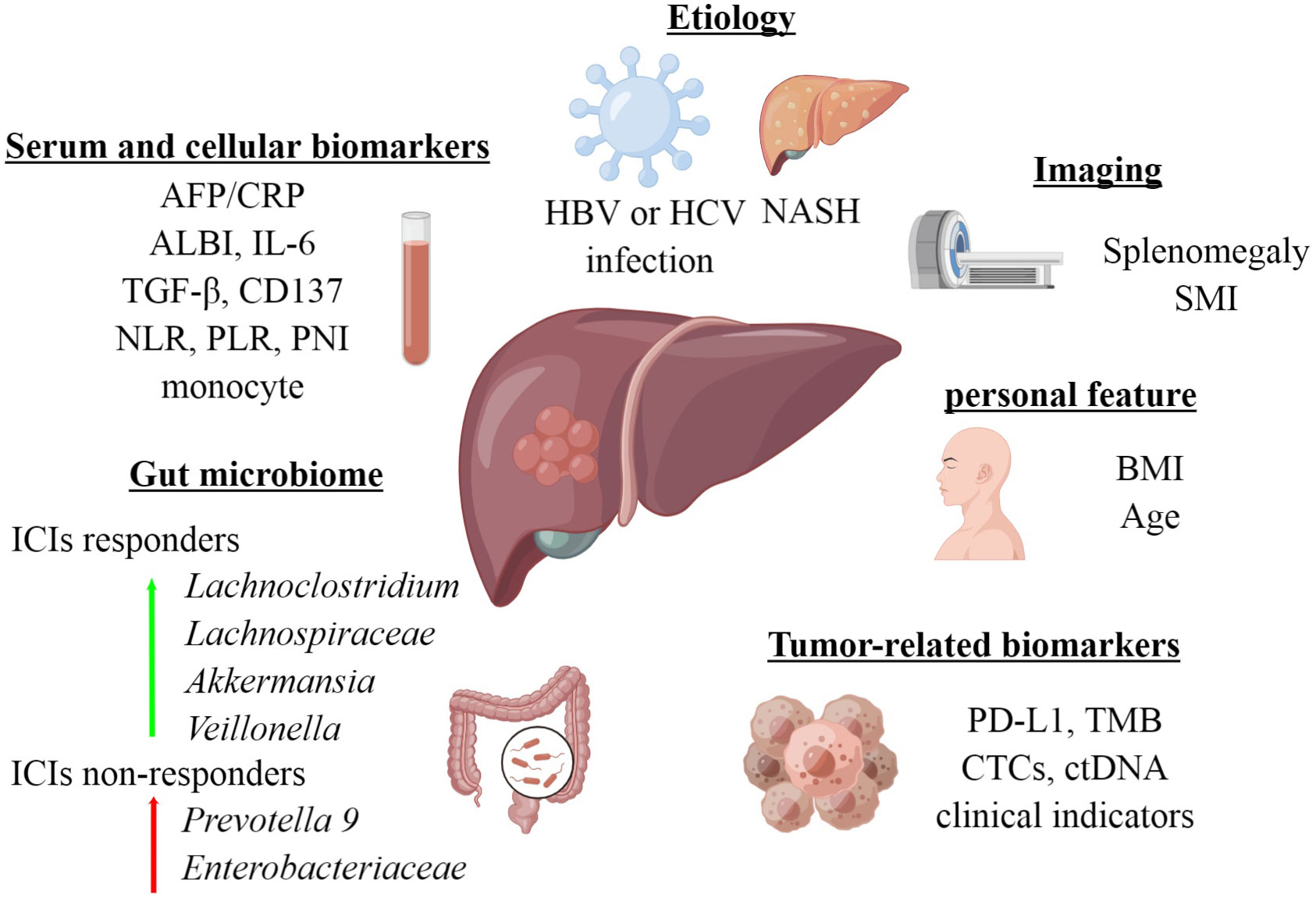
In this review, we summarize the recent clinical data on the efficacy prediction of ICIs for HCC, to provide valuable guidance for optimal treatment selection (Figure 1).
2 Serum and cellular biomarkers
2.1 Predictive biomarkers before treatment
2.1.1 AFP and CRAFITY score
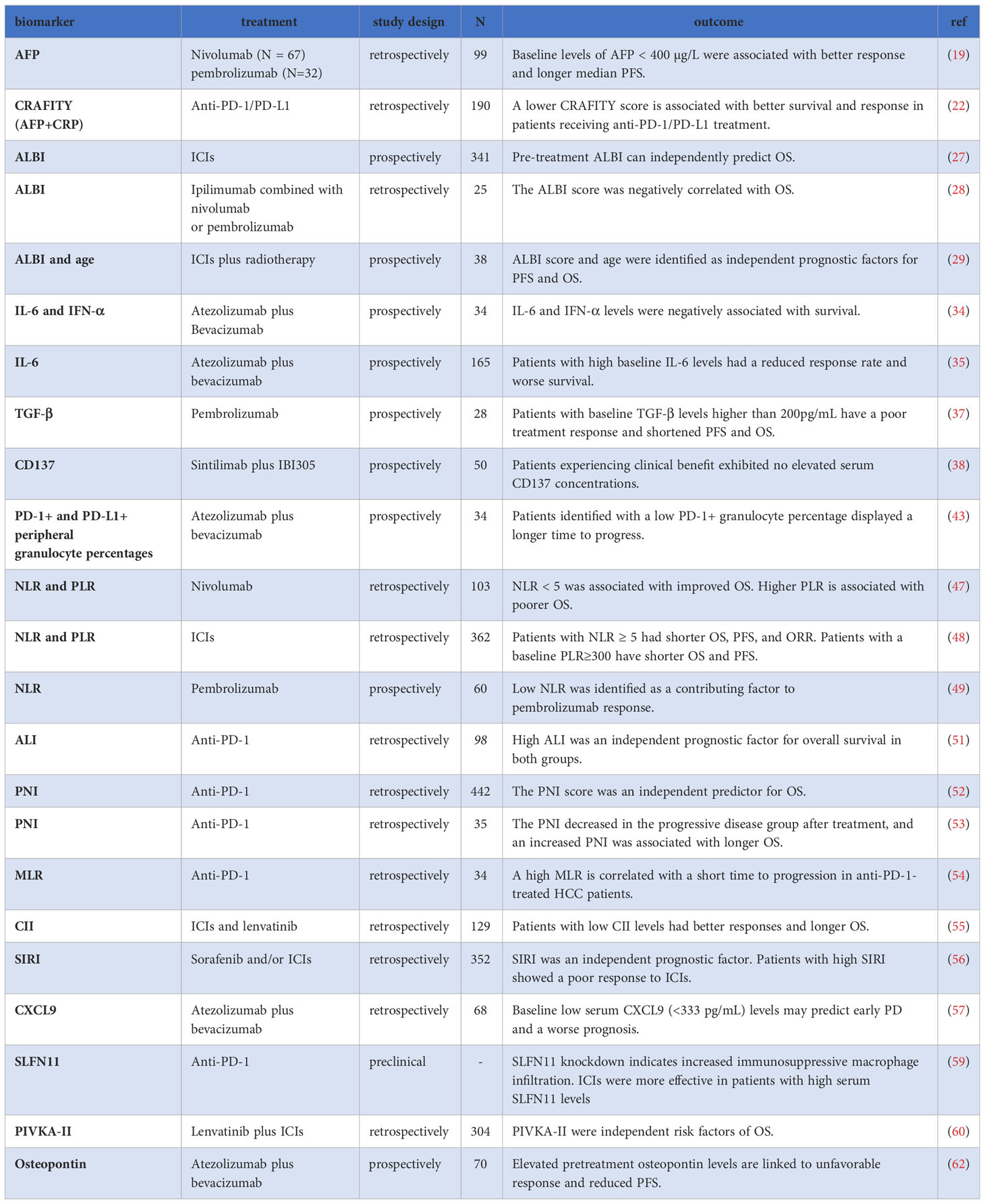
Alpha-fetoprotein (AFP) is a widely used biomarker for the diagnosis and monitoring of efficacy in HCC (17, 18). Recent studies have revealed its association with the effectiveness of ICIs in HCC. In a retrospective multicenter study involving 99 patients receiving nivolumab or pembrolizumab, those with AFP<400 µg/L before treatment demonstrated a better response and prolonged survival [median progression-free survival (PFS): 5.4 vs. 2.6 months, P < 0.05; median overall survival (OS): 21.8 vs. 8.7 months, P < 0.0001] (19). Another study corroborated these findings (20). A meta-analysis of 44 retrospective studies further highlighted that elevated AFP levels correlated with shorter OS and PFS, as well as a lower disease control rate (DCR) compared to lower AFP levels (21). Additionally, the CRAFITY score, combining baseline AFP and C-reactive protein (CRP), demonstrated efficacy in predicting ICIs response. Within this scoring system, patients with AFP≥100 ng/ml and CRP≥1 mg/dl received 2 points, those meeting one criterion scored 1 point, and patients not meeting the criteria scored 0 points. The median OS and DCR for patients with 2 points were significantly better than those with 1 point or 0 points (median OS: 27.6 months, 11.3 months, and 6.4 months, respectively; DCR: 80%, 64%, and 39%, respectively, P < 0.001) (22). The predictive capability of the CRAFITY score was further validated in multicenter studies conducted in different countries. Yang et al. suggested that the CRAFITY score could predict the survival prognosis of HCC patients treated with ICIs combined with TKIs (23). Hatanaka et al. also confirmed its predictive value in Atez/Bev treatment for DCR, OS, and immune-related adverse events (irAEs) (24). In conclusion, the CRAFITY score serves as a simple and effective predictive indicator, readily obtainable and applicable. However, it is important to consider CRP’s susceptibility to injury or infection, given its nature as an acute-phase protein. Further prospective studies are needed to verify its clinical value.
2.1.2 ALBI score
HCC mainly affects patients with advanced liver fibrosis or cirrhosis, indicating that liver function impairment significantly impacts prognosis (25). The albumin-bilirubin (ALBI) score, a simplified method for assessing liver function in HCC patients, categorizes patients into grades 1-3, with grade 1 representing the least liver damage (26). A prospective study demonstrated that the pre-treatment ALBI score can independently predict OS and serve as a stratifying biomarker for ICIs treatment (27). Another study investigated HCC patients who experienced tumor progression after prior ICIs treatment and subsequently received ipilimumab plus nivolumab or pembrolizumab, finding that all responders had a baseline ALBI grade of 1 or 2, indicating relatively preserved liver function. The study also observed a negative correlation between the ALBI score and OS (28). In a retrospective study, the baseline ALBI score and age were identified as independent prognostic factors influencing PFS and OS in HCC patients receiving ICIs combined with radiotherapy. The low-risk group (ALBI grade 1 and age≥53 years) exhibited significantly superior survival outcomes compared to the high-risk group (median OS: not reached vs. 10.1 months, P<0.005; median PFS: 15.3 months vs. 2.7 months, P<0.005) (29). A systematic review involving 4483 patients also revealed that the ALBI score could predict prognosis (30). Collectively, these studies underscore the significant importance of the ALBI score in selecting appropriate populations for ICIs treatment.
2.1.3 Cytokines
Cytokines, such as interleukin 6 (IL-6), interferon alpha (IFNα), transforming growth factor-β (TGF-β), and others, have been investigated as potential biomarkers for predicting ICIs treatment response in various cancers, including HCC (31, 32).
IL-6, an inflammatory factor associated with HCC development and progression, has been studied in relation to ICIs treatment (33). High baseline serum IL-6 levels were found to be an independent risk factor for disease progression in patients receiving Atez/Bev, resulting in significantly shorter PFS and OS compared to those with low levels. Additionally, the study also demonstrated that patients with high baseline IFN-α levels had shorter OS compared to those with low levels (34). Another multicenter prospective research of 165 patients also showed that high baseline IL-6 levels were associated with a reduced response rate and worse PFS (HR= 2.93, P = 0.003) and OS (HR= 3.02, P = 0.021) (35). However, it is worth noting that IL-6 levels may be influenced by other inflammatory conditions, reducing its specificity as a predictive biomarker.
TGF-β, central in inflammation, fibrogenesis, and immunomodulation in the HCC microenvironment (36), has shown potential as a predictive biomarker for ICIs efficacy. In a study of 28 unresectable HCC patients treated with pembrolizumab, among several plasma biomarkers, only baseline TGF-beta cytokine levels in peripheral blood were significantly higher in non-responders compared to responders. Patients with baseline TGF-β levels higher than 200pg/mL had poor treatment response and shorter PFS and OS (37).
CD137, a cell surface marker expressed on activated T cells, has also shown promise as a biomarker for ICIs response. Patients with higher serum CD137 concentrations demonstrated significantly better clinical benefit and longer PFS (median PFS, 14.2 months vs. 4.1 months, P=0.001) (38).
While these findings highlight the potential of cytokines as biomarkers for predicting ICIs response in HCC, additional research and larger prospective studies are needed to confirm their utility, establish their clinical significance, and determine their specificity in predicting treatment outcomes.
2.1.4 Peripheral blood cell
Peripheral blood components play a crucial role in various diseases and have been utilized to assess mortality risk in patients across multiple conditions, including HCC (39–42).
In a prospective study of 34 patients treated with Atez/Bev, the baseline percentage of peripheral granulocytes and their PD-1 and PD-L1 expression emerged as predictors of patients’ response and prognosis, with lower PD-1+ granulocyte percentage associated with a better response and longer time to progression (43).
The neutrophil to lymphocytes ratio (NLR), an inflammatory marker, has gained attention in HCC (44). Elevated NLR is believed to contribute to HCC recurrence by creating a protumorigenic microenvironment through both relative neutrophilia and lymphocytopenia (45). Recent studies have found that NLR is related to the prognosis of ICIs treatment (45, 46). Notably, a study by Dharamapuri et al. revealed that in HCC patients treated with nivolumab, having a pre-treatment NLR<5 (23 months vs. 10 months, P=0.004) and a post-treatment NLR<5 (35 months vs. 9 months, P<0.001) were significantly associated with improved OS (47). Another study confirmed that patients with NLR≥5 had poorer objective response rate (ORR), OS, and PFS (48). Furthermore, NLR was identified as a facilitating factor for pembrolizumab response in a single-arm prospective phase 2 clinical trial (49). Moreover, Tada et al. found that NLR before treatment (cut-off value of 3) was an independent predictor of response in HCC patients treated with Atez/Bev and was negatively correlated with OS (50). A meta-analysis also demonstrated that patients with high NLR levels had significantly poorer OS and PFS, lower ORR and DCR, and higher hyperprogressive disease (21). Moreover, the Advanced Lung Cancer Inflammation Index (ALI), calculated as body mass index (BMI) * Albumin/NLR, has the potential as a predictive biomarker. A retrospective study involving 98 patients found that high ALI was an independent prognostic factor for OS, with a hazard ratio of 0.411 (51).
The prognostic nutritional index (PNI), incorporating peripheral blood lymphocyte count and serum albumin levels, reflects the nutritional and immune status of the body. An analysis of 442 patients receiving anti-PD-1 monoclonal antibody treatment demonstrated that baseline NLR, PLR, and PNI all exhibited significant predictive ability for OS (HRs: 1.714, 1.691, 2.153, respectively; P <0.001). However, in multivariate analysis, only PNI was an independent predictor of OS: higher baseline PNI was associated with a poorer prognosis (HR = 1.770, P <0.001) (52). Changes in PNI after treatment can also serve as a predictor of ICIs treatment efficacy. In HCC patients treated with anti-PD-1 monoclonal antibodies, the PNI decreased in the progressive disease (PD) group after treatment compared to the non-PD group(P = 0.023), and an increased PNI was associated with longer OS (P = 0.014) (53).
The platelet lymphocyte rate (PLR) also has potential predictive value for the prognosis of HCC patients receiving ICIs treatment. A study has shown that patients with a baseline PLR≥300 have shorter median OS (6.4 months vs. 16.5 months) and PFS (1.8 months vs. 3.7 months). Moreover, PLR remains an independent prognostic factor for both OS and PFS (48). A high PLR after treatment is associated with a negative impact on survival prognosis (HR=1.002, P<0.001) (47).
The baseline monocyte-to-lymphocyte ratio (MLR) has been associated with ICIs effectiveness. In patients treated with anti-PD-1 monoclonal antibodies, the low MLR group exhibited a longer tumor progression time, with a median metastasis time of 33 weeks compared to 18 weeks in the high MLR group when disease progresses (54).
The circulating immune index (CII), calculated as the ratio of white blood cell counts to lymphocyte proportion, has also shown promise in HCC prognosis. A retrospective study involving 129 patients treated with ICIs and lenvatinib demonstrated that patients with CII ≤ 43.1 reported prolonged OS compared to those with CII > 43.1 (24.7 vs. 15.1 months, P = 0.019). CII was identified as an independent prognostic factor for OS. Moreover, patients with low CII levels demonstrated improved ORR and DCR (55).
The systemic inflammation response index (SIRI), calculated based on the absolute values of neutrophils, monocytes, and lymphocytes, has revealed evidence as an independent prognostic factor for HCC patients receiving sorafenib and/or ICIs treatment in a retrospective real-world study. High SIRI levels were associated with a poor response to ICIs, and SIRI is negatively correlated with peripheral CD3+, CD4+, and CD8+ T cell counts (56), indicating its potential link to immune cell activity.
These findings indicate that peripheral blood cell indicators hold potential as biomarkers for predicting ICIs treatment response and prognosis in HCC patients. Further research is necessary to validate their utility and explore the clinical significance in HCC management.
2.1.5 Other serum proteins
In addition to AFP, which has been widely studied, several novel serum proteins have shown potential as biomarkers in HCC patients treated with ICIs.
Baseline low serum CXCL9 (<333 pg/mL) levels may predict early PD in patients with unresectable HCC treated with Atez/Bev (57). Patients with lower serum CXCL9 (<333 pg/mL) experienced early PD in 35.3% of cases(12/34) with Atez/Bev, resulting in significantly shorter PFS relative to those without early PD(median PFS, 126 days vs. 227 days; HR: 2.41, P=0.0084).
The Schlafen (SLFN) family members play an important role in oncology and immunity (58). A study by Zhou et al. observed that SLFN11 was up-regulated in ICIs-responsive HCC patients, and knockdown of SLFN11in HCC cells led to increased macrophage migration and M2-like polarization, indicating increased immunosuppressive macrophage infiltration. Furthermore, ICIs were more effective in HCC patients with high serum SLFN11 levels, suggesting SLFN11 as a potential predictive biomarker for ICI response in HCC patients (59).
In a retrospective study involving HCC patients treated with lenvatinib plus ICIs, two independent risk factors for OS were identified: Vitamin K absence or antagonist-II (PIVKA-II) and metastasis. To enhance prognostic prediction, a prognostic model called the PIMET score was developed. Patients were stratified into three groups based on this score: PIMET-low group (without metastasis and PIVKA-II<600 mAU/mL), PIMET-int group (with metastasis or PIVKA-II>600 mAU/mL), and PIMET-high group (with metastasis and PIVKA-II>600 mAU/mL). In both the training cohort and validation cohort, the PIMET-high group showed superior OS than the PIMET-low group (60).
Osteopontin (OPN), a glycoprotein involved in tumor progression (61), has also shown potential as a prognosis indicator in HCC patients treated with Atez/Bev. In a prospective multicenter study, high pretreatment OPN levels were identified as independent predictors of PD (OR=5.444, P=0.012). Furthermore, in Child–Pugh class A group, the PFS was significantly shorter in the high OPN group than in the low OPN group (62).
These novel serum protein markers provide additional insights into predicting treatment effectiveness in HCC patients receiving ICIs. However, further research is still required to expand our understanding of this area (Table 1).
2.2 Predictive indicators during treatment
2.2.1 AFP and its related indicators
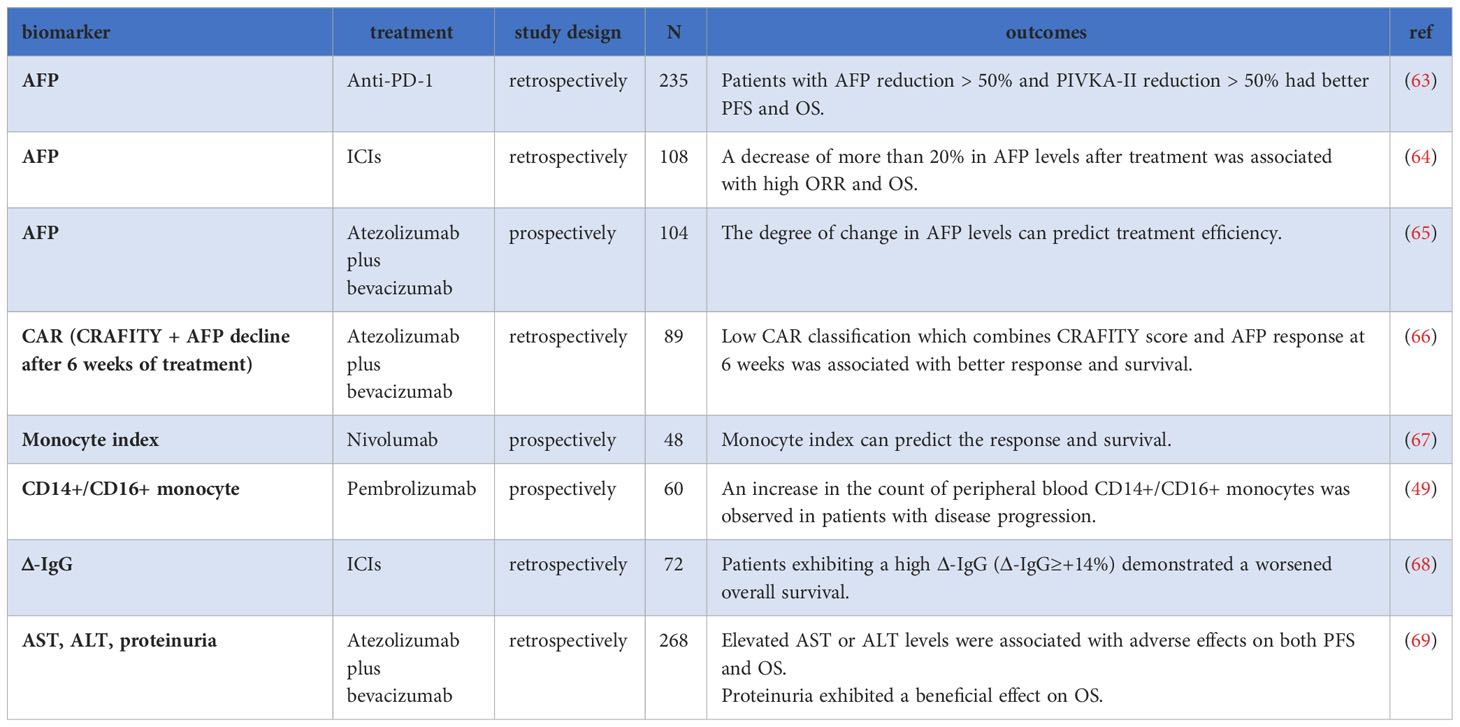
Multiple studies have highlighted the predictive value of monitoring AFP levels during treatment with ICIs. In a retrospective study involving 235 patients with HCC undergoing anti-PD-1 monoclonal antibody treatment, reductions of more than 50% in AFP or PIVKA-II levels compared to baseline were associated with improved treatment response, OS, and PFS. The AAP score, combining AFP, abnormal coagulation factor II, and ALBI, demonstrated good predictive value. Patients with an AAP score of ≥2 points had significantly longer PFS and OS (63). In another study by Kim et al., changes in AFP levels compared to baseline at 6-10 weeks and 14-18 weeks after ICIs treatment could predict treatment efficacy. An AFP response, defined as a decrease of more than 20% in AFP levels, was associated with a high ORR of 90.9% (10/11) at 6-10 weeks and 93.8% (15/16) at 14-18 weeks. Conversely, AFP progression, indicated by an increase of more than 20% in AFP levels, was associated with a lower ORR of 1.4% and 0.0% during the same periods. Additionally, the degree of change in AFP levels compared to baseline levels at 6-10 weeks and 14-18 weeks independently predicted patient OS, with HRs of 0.360 and 0.315 at 6-10 weeks, and 2.525 and 3.908 at 14-18 weeks (64). Moreover, Zhu et al. also reported that in HCC patients treated with Atez/Bev, a decrease in AFP level by ≥75% after 6 weeks of treatment effectively distinguished between treatment responders from non-responders, while an increase in AFP level by ≤10% better distinguished between disease control and disease progression (65).
Teng et al. proposed the CAR classification by combining the pre-treatment CRAFITY score with the 6-week post-treatment AFP response: Class I patients exhibited the best OS and PFS, followed by Class II and III, with statistically significant differences (OS: not reached vs.11.1 months vs. 4.3 months, P < 0.001; PFS: 7.9 months vs. 6.6 months vs. 2.6 months, P = 0.001). Class I patients also achieved a higher ORR of 35.0% compared to 18.2% and 0.0% in Class II and III, respectively (66). Additionally, a meta-analysis showed that an early AFP response was correlated with improved OS and PFS, higher ORR, and DCR compared to non-responders (21).
In conclusion, early changes in AFP levels during treatment hold significant predictive value, and combining AFP with other biomarkers shows promise in further enhancing predictive accuracy.
2.2.2 The monocyte index
Jeon et al. conducted a prospective study on HCC patients treated with nivolumab and found that the ratio of peripheral blood circulating classical monocytes on day 7 to day 0 (cMonocyte D7/D0) was significantly higher in patients with durable clinical benefit (DCB) than in those without DCB (non-DCB). Conversely, the ratio of PD-L1+ circulating classical monocytes on day 7 to day 0 (cMonocyte-PDL1 D7/D0) was significantly higher in non-DCB patients than in DCB patients. To further evaluate this relationship, a monocyte index was constructed by dividing cMonocyte D7/D0 by cMonocyte-PDL1 D7/D0. A higher index was associated with worse survival rates, serving as an independent risk factor for PFS (HR=0.37, P=0.01) and OS (HR=0.32, P=0.03) (67). Additionally, in a single-arm phase 2 clinical trial of patients with HCC who failed sorafenib plus pembrolizumab treatment, the increased number of peripheral blood CD14+/CD16+ monocytes was associated with disease progression (P=0.002) (49). In conclusion, these results suggest that early changes in monocytes may have some significant predictive value in determining the outcomes of ICIs treatment.
2.2.3 Δ-IgG
In a retrospective study involving 72 patients, Balcar et al. investigated the role of immunoglobulin levels in ICIs treatment. Among all the immunoglobulins examined, the relative change in IgG (Δ-IgG) was found to be an independent predictor of OS in multivariable analysis. Patients could be stratified into high (Δ-IgG≥+14%) vs. low (Δ-IgG<+14%) risk groups, where the high-risk group showed a median OS decrease compared to the low-risk group (6.4 vs. 15.9 months; P = 0.001) (68). These findings suggest that Δ-IgG levels can serve as a prognostic indicator in ICIs treatment.
2.2.4 ALT, AST, and proteinuria
In a real-world study involving 268 HCC patients treated with Atez/Bev, several factors were examined for their impact on ICIs treatment. The study found that increased bilirubin levels were associated with a significantly shorter OS and PFS. The hazard ratios for OS and PFS were 2.61 (P=0.042) and 2.85 (P=0.005), respectively, in patients with increased bilirubin levels. Additionally, elevated levels of aspartate aminotransferase (AST) or alanine aminotransferase (ALT) were also associated with a significantly shorter OS and PFS. Furthermore, the study found that patients with proteinuria had a significantly longer OS, with an HR of 0.46 (95% CI: 0.23–0.92, P = 0.027). However, the impact of proteinuria on PFS was not specifically mentioned in the provided information (69). These findings suggest that increased bilirubin levels, elevated AST or ALT levels, and the presence of proteinuria can serve as prognostic indicators in HCC patients receiving Atez/Bev treatment. Monitoring and managing these factors may help in assessing patient outcomes and optimizing treatment strategies (Table 2).
3 Tumor-related biomarkers
3.1 PD-L1
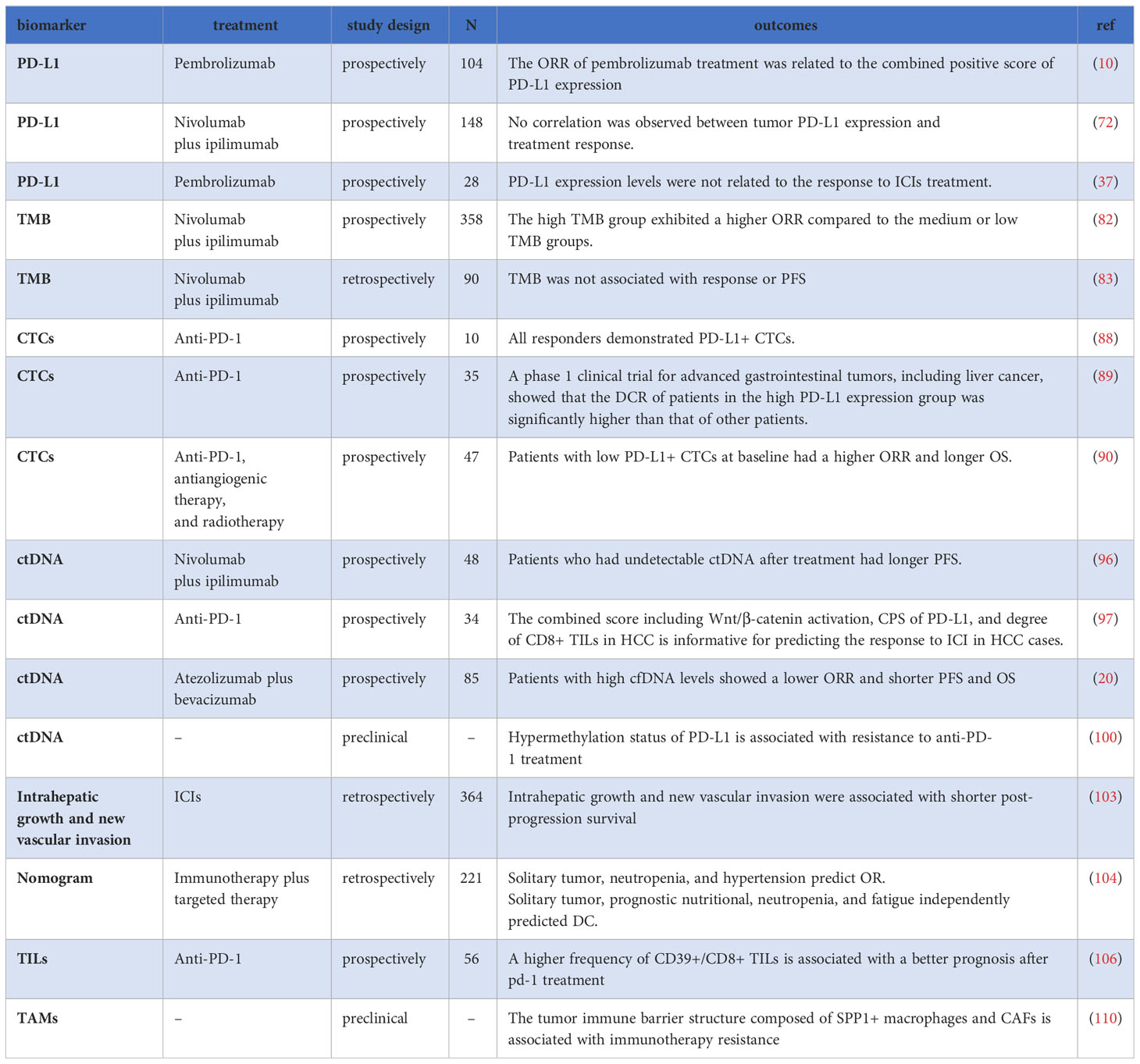
PD-L1 has been studied as a potential biomarker of response to PD-1 therapy in various types of cancer (70, 71). However, its predictive value for HCC remains controversial. In the KEYNOTE-224 study evaluating pembrolizumab in HCC, no correlation was found between ORR and PD-L1 expression on tumor cells. Instead, a relationship was observed between the response and the combined positive score (CPS) of PD-L1 expression (10). Similarly, the CheckMate040 study did not find a clear association between tumor PD-L1 expression levels and treatment response (72). However, a different study reported a link between tumor PD-L1 and plasma PD-L1/PD-1 levels and plasma interferon-gamma (IFN-γ) or interleukin-10 (IL-10) (37).
A recent meta-analysis suggested that higher PD-L1 expression levels on tumor cells and tumor proportion score were associated with a higher ORR in HCC patients treated with ICIs (73). Another meta-analysis confirmed improved ORR in PD-L1-positive patients compared to PD-L1-negative patients (26% vs. 18%, OR=1.86). However, for DCR, there was no significant difference between PD-L1-positive and PD-L1-negative patients (74).
Moreover, Mocan et al. found that high plasma PD-L1 levels were associated with shorter OS, indicating a poor prognostic biomarker for HCC. However, this study did not explore the efficacy of ICIs treatment (75). Additionally, some studies observed that HCC patients treated with sorafenib had higher PD-L1 expression levels compared with those who did not, suggesting that sorafenib treatment may influence PD-L1 expression levels (76).
Overall, due to the complex immune microenvironment of HCC, the predictive value of PD-L1 in ICIs treatment is limited. While some studies have indicated an association between PD-L1 expression and treatment response, others have found conflicting results. Further research is warranted to better understand the role of PD-L1 in estimating the response to ICIs therapy in HCC and its potential clinical implications.
3.2 Tumor mutational burden
Tumor mutational burden (TMB) refers to the total number of errors in somatic genetic coding, base substitutions, gene insertions, or deletions per million bases (77). TMB is believed to reflect the tumor’s ability to produce neoantigens and is related to the efficacy of ICIs treatment (78) in certain cancers such as melanoma and lung cancer (79–81). However, in the case of HCC, studies have shown inconsistent results. One study analyzed 358 HCC patients enrolled in the GO30140 phase 1b or IMbrave150 phase 3 trial and found that although the high TMB group exhibited a higher ORR compared with the medium or low TMB groups (56% vs. 35% or 17%), the difference in PFS did not reach statistical significance (82). Other studies have reported that the TMB level of liver cancer is generally low and is not associated with treatment response or PFS (83, 84). Furthermore, differences in tissue acquisition methods and regional variations inevitably limit the clinical applicability of TMB as a predictive biomarker (85), which requires further exploration of how TMB can be used to evaluate the therapeutic effect of ICIs in HCC.
3.3 Circulating tumor cells
Circulating tumor cells (CTCs) are cancer cells that have detached from primary tumors or metastatic sites and entered the bloodstream (86). They hold significant potential in predicting the efficacy of ICIs when measured and analyzed through liquid biopsies (87). Several studies have investigated the association between PD-L1 expression in CTCs and treatment outcomes in HCC patients.
In a prospective study of HCC patients receiving anti-PD-1 treatment, all responders demonstrated PD-L1+ CTCs at baseline, whereas only one non-responder showed PD-L1+ CTCs (88). In another phase 1 clinical trial involving advanced gastrointestinal tumors, including HCC, patients treated with anti-PD-1 antibodies were categorized into four groups based on the baseline PD-L1 expression level in CTCs: negative, low, medium, and high. Patients with high PD-L1 expression in CTCs had a significantly higher DCR than others, and DCR in patients with expression levels≥20%, the DCR is even further elevated (89). However, discordant results were observed in another study of HCC patients receiving a PD-1 inhibitor in combination with antiangiogenic therapy and radiotherapy. In this study, patients with low PD-L1+CTCs at baseline had a higher ORR (56.5% vs. 16.7%, P=0.007) and longer OS (not reached vs. 10.8 months, P=0.001) in comparison to those with high PD-L1+CTCs. Furthermore, individuals with a dynamic decrease in PD-L1+ CTC count 1 month after treatment were more likely to achieve an objective response(OR) (90).
In summary, CTCs hold promise as an underlying biomarker for highlighting the efficacy of PD-1 in the treatment of HCC. However, further research and larger-scale prospective studies are required to establish their clinical utility and consistency.
3.4 Circulating tumor DNA
Circulating tumor DNA (ctDNA) is defined as tumor-derived DNA fragments released into the bloodstream from apoptotic or necrotic tumor cells. It serves as a biomarker for ICIs treatment across various cancers, including HCC (91–95). In a study involving HCC patients treated with Atez/Bev, higher baseline ctDNA levels correlated with high TMB, and patients who had undetectable ctDNA after treatment had longer PFS (96). Another study showed that responders to ICIs exhibited a decrease in the mutation allele frequency of ctDNA during treatment (84).
Contrastingly, a prospective study of 85 HCC patients treated with Atez/Bev revealed that individuals with high levels of cell-free DNA (cfDNA) experienced significantly lower ORR, PFS, and OS compared to those with low cfDNA levels. Additionally, the presence of a TERT mutation independently predicted poor OS in multivariate analysis (20).
Analyzing specific characteristics of ctDNA fragments from HCC patients may provide prognostic insights. Notably, the activation of the Wnt/β-catenin pathway was associated with poorer disease control and PFS in HCC patients treated with anti-PD-1 antibodies (97). Extensive research on DNA methylation in HCC initiation and progression revealed hypermethylated DNA at specific genes (98). Shen et al. systematically screened 14 DNA methylation-driven survival-related genes, developing a risk model involving five methylation-driven genes. Specifically, DNA methylation levels of CYBYR, CYP2C9, and LAMB1 correlated significantly with overall survival in HCC patients (99). Recent studies identified PD-L1 K162 methylation as a regulator of PD1/PD-L1 interaction, influencing T cell activity suppression. Hypermethylation of PD-L1 was associated with resistance to anti-PD-1 treatment, highlighting its potential as a predictive biomarker for assessing anti-PD-1/PD-L1 therapy response (100).
Despite ctDNA’s easy obtainability and minimal invasiveness, current evidence regarding its efficacy in predicting ICIs treatment response in HCC remains insufficient. Further large-scale studies are crucial to validate its clinical utility. As technology advances and standard operating procedures are established, ctDNA is poised to become a valuable biomarker for evaluating ICIs treatment efficacy in the future (91).
3.5 Clinical indicators of tumor
Clinical indicators can provide valuable information about the tumor and are generally associated with the tumor’s malignant features and prognosis (101).
The presence of metastasis, which indicates that the tumor has spread to other parts of the body, has been identified as an independent risk factor for inferior OS in patients treated with lenvatinib monotherapy or lenvatinib plus ICI (60). Additionally, the presence of extrahepatic spread (metastasis outside the liver) has been associated with an inferior ORR in ICIs-treated HCC patients (102). Macrovascular invasion, which refers to tumor thrombosis in the portal vein or hepatic vein, has also been associated with poorer PFS and OS (102). In a retrospective study involving 604 HCC patients treated with ICIs after progression, intrahepatic growth, and new vascular invasion were associated with a poorer prognosis (103).
In a retrospective study aimed at constructing the nomogram for tumor response prediction, several factors were identified as independent predictors of treatment response: solitary tumor, neutropenia, and hypertension independently predicted ORR; tumor sizes less than 5 cm, a solitary tumor, prognostic nutritional indices greater than or equal to 54.3, neutropenia and fatigue were found to independently predict disease control (104).
Overall, considering clinical indicators, such as metastasis, macrovascular invasion, and other specific tumor characteristics, can provide valuable insights into predicting the outcomes of ICIs treatment in HCC patients. However, further research and validation studies are needed to establish their clinical significance and utility in guiding treatment decisions.
3.6 Tumor infiltrating lymphocytes and tumor-associated macrophages
Tumor infiltrating lymphocytes (TILs), lymphocytes present within and around tumor cells, have been associated with improved outcomes in HCC immunotherapy (8, 105). In a study by Liu et al., patients in the higher frequency of CD39+/CD8+ TILs group demonstrated a favorable prognosis following anti-PD-1 therapy (106). The Checkmate 040 trial revealed a non-significant trend toward prolonged survival (P=0.08) in Nivolumab-treated HCC patients with elevated CD3+/CD8+ TILs (107). Conversely, tumor-associated macrophages (TAMs), macrophages present within tumor tissues or clustering in the solid tumor microenvironment (TME), have been implicated in inducing immune suppression in the HCC TME, with their frequency correlating with poor prognosis (108). Recently, Qu et al. constructed an HCC prognostic model utilizing public databases, identifying eight M2-like TAM-related genes (PDLIM3, PAM, PDLIM7, FSCN1, DPYSL2, ARID5B, LGALS3, and KLF2) (109). In an anti-PD-1 treatment study involving eight HCC patients, a tumor–immune barrier structure comprising SPP1+ macrophages and cancer-associated fibroblasts was identified in non-responsive patients (110).
Despite their crucial roles in the TME, detecting and accessing TILs and TAMs pose significant challenges in clinical applications. The emergence of sophisticated detection technologies, such as single-cell RNA sequencing, holds promise for overcoming these challenges and establishing TILs and TAMs as predictive biomarkers for ICIs in HCC (111, 112) (Table 3).
4 Imaging and personal features
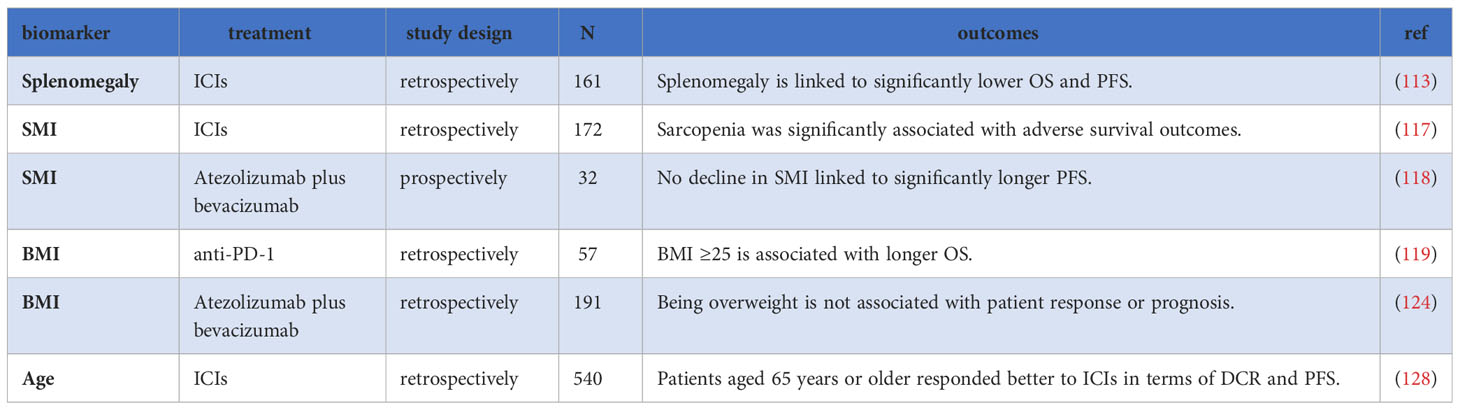
4.1 Splenomegaly
Splenomegaly refers to the enlargement of the spleen and is often confirmed using radiological imaging, although the “gold-standard” definition is based on splenic weight. In a retrospective analysis involving 161 liver cancer patients treated with ICIs, Xiao et al. observed that patients with splenomegaly had significantly lower OS and PFS compared to those without splenomegaly (113). The precise mechanism by which splenomegaly affects the efficacy of immunotherapy is not yet clear. Hypotheses include its potential influence on the number or function of splenic lymphocytes or its effects through the compression of adjacent abdominal organs (113). Further research is needed to establish and verify its predictive value.
4.2 Skeletal muscle index
The skeletal muscle index (SMI) is the ratio of the cross-sectional area of the muscles at the level of the third lumbar vertebra (L3) to the square of the height (114). It serves as an indicator of the body’s nutritional status and is commonly used to evaluate sarcopenia, which is defined as a male SMI < 43 cm2/m2 (when BMI < 25) or < 53 cm2/m2 (when BMI ≥ 26), or female SMI < 41 cm2/m2 (115, 116). A retrospective study demonstrated that sarcopenia was significantly associated with adverse survival outcomes with anti-PD-1/PD-L1 treatment (HR=5.39, P=0.004). Interestingly, the study did not find a similar relationship between BMI and survival outcomes (117). Furthermore, another study conducted by Matsumoto et al. found that in patients treated with Atez/Bev, those who did not experience a decline in SMI had significantly longer PFS compared to those with a decline in SMI (8.5 months vs. 5.8 months), while baseline sarcopenia did not show a significant association with survival outcomes in this study (118). It is worth noting that a previous retrospective study observed a trend toward worse OS in patients with sarcopenia, although statistical significance was not reached (119).
In summary, changes in muscle mass, indicated by a decline in SMI, have the potential to predict the efficacy of ICIs treatment. Further research is necessary to better understand the relationship between sarcopenia and survival outcomes in HCC. Monitoring muscle changes using SMI can provide valuable information regarding the prognosis of HCC patients.
4.3 BMI
Obesity is generally recognized as having adverse effects on health and clinical outcomes (120). However, recent studies have suggested that obesity may be associated with an improved response to ICIs in various cancer types, such as melanoma and non-small cell lung cancer (NSCLC), and it may also impact the dosing strategy of ICIs (121–123). Nevertheless, the specific effect of obesity on immunotherapy outcomes in HCC is still unclear. One retrospective study focused on HCC patients treated with anti-PD-1 antibodies found that median OS was 5 months in patients with a BMI of less than 25, while it was 17.5 months in patients with a BMI of 25 or greater (Log-rank P=0.034) (119). On the other hand, another study involving 191 HCC patients treated with Atez/Bev found that overweight (BMI ≥25) patients had similar OS compared to non-overweight patients. Additionally, BMI did not significantly influence median PFS, ORR, and DCR in this study (124).
In summary, some studies suggest a potential association between obesity and an improved response to ICIs, while others did not find significant correlations. Further research is needed to better understand the relationship between obesity and ICIs treatment outcomes in HCC.
4.4 Age
Several studies have reported comparable efficacy of ICIs in younger and older patients across various cancer types (125–127). However, in HCC, interestingly, age has been found to be a prognostic factor in patients treated with ICIs. The Keynote-240 phase III trial, which investigated pembrolizumab in HCC, conducted a subgroup analysis of patients aged 65 years or older and revealed that immunotherapy with pembrolizumab improved PFS compared to placebo in this age group (11).
Furthermore, in HCC patients receiving ICIs combined with radiotherapy treatment, older patients aged 65 years or older showed better responses to ICIs in terms of both PFS (HR=0.955, P=0.037) and OS (HR=0.931, P<0.001) (29).In another multicenter retrospective study involving 540 patients treated with ICIs, patients aged 65 years or older responded better to ICIs in terms of DCR and PFS, while maintaining similar ORR and OS (128). The analysis of public datasets revealed that the elderly group had lower expression of oncogenic pathways such as PI3K-Akt, Wnt, and IL-17, and higher tumor mutation burden compared to younger patients (128).
The gene characteristics may explain the results to some extent; however, whether age is an independent prognostic factor remains controversial, as older age may also influence liver function (129). Further research is necessary to fully understand the underlying mechanisms and validate the predictive role of age in HCC patients treated with ICIs (Table 4).
5 Etiology
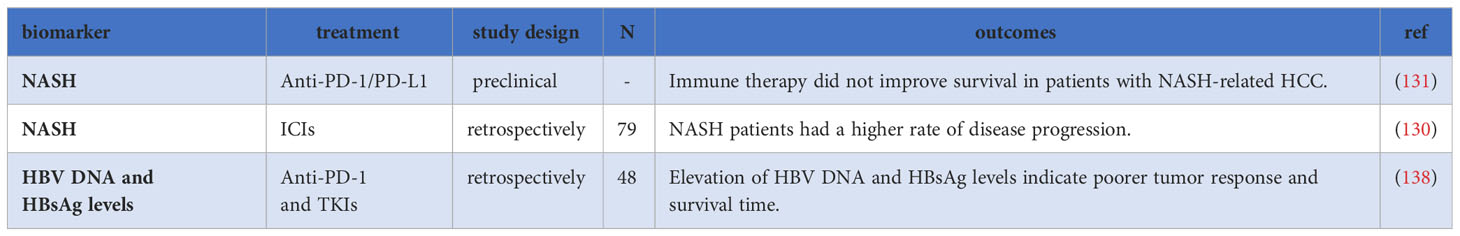
5.1 Non-alcoholic steatohepatitis
Studies have indicated that patients with HCC associated with non-alcoholic steatohepatitis (NASH) did not experience improved survival after treatment with ICIs. A retrospective study involving 75 HCC patients treated with ICIs demonstrated significantly higher rates of disease progression in patients with NASH cirrhosis compared to those without NASH cirrhosis (130). Additionally, Pfister et al. reported decreased survival in NASH-induced HCC after ICIs treatment compared to HCC caused by other factors (131). The underlying mechanisms include a loss of antitumor CD4+ T cells, and an accumulation of exhausted, unconventionally activated CD8+PD-1+ T cells, which impede tumor immune surveillance and the efficacy of immunotherapy (131, 132). Moreover, NASH-cirrhosis patients exhibit microbiome disorders and changes in the intestinal microbiome of NASH-associated HCC patients may contribute to peripheral immunosuppression, thereby impairing the efficacy of ICIs (133).
5.2 HBV and HCV
Hepatitis B virus (HBV) and Hepatitis C virus (HCV) infections significant risk factors for HCC. Phase III studies suggest that ICIs treatment may be more effective in virus-related HCC compared to non-viral-related HCC. In the IMbrave150 trial investigating Atez/Bev in HCC, the median OS of patients treated with this combination therapy was shorter in nonviral-related HCC compared to HBV- and HCV-related HCC (16). The HIMALAYA study also showed improved OS in patients with HBV who received durvalumab plus tremelimumab compared to sorafenib (HR=0.64), while this improvement was not observed in HCV-related HCC (134).
Recent meta-analyses have further supported the notion that viral infections are associated with a better prognosis after ICIs therapy in HCC. One meta-analysis of eight trials involving 3739 patients showed that ICI therapy was significantly more effective in patients with viral hepatitis compared to non-viral-related HCC (135). Another meta-analysis found that patients with viral infections achieved a better prognosis than those without infections (P = 0.018), particularly in HBV-associated HCC (P = 0.016), but not in HCV-associated HCC (P = 0.081) (136). However, there have been conflicting results, as another meta-analysis did not find a significant effect of viral etiology on ICIs treatment in HCC (137).
A study revealed that among HBV-related liver cancer patients receiving ICIs combined with TKIs treatment, patients with disease control (DC) showed a more significant decrease in HBV DNA and HBsAg levels compared to patients with PD. Higher levels of HBV DNA (HR=4.816, P=0.011) and HBsAg (HR=4.161, P=0.022) were identified as independent risk factors for survival, suggesting that higher levels of HBV DNA and HBsAg indicate poorer tumor response and outcomes (138). However, the study also found that regardless of baseline HBV DNA levels, the tumor response of HBV-related HCC patients was similar to that of other HCC patients. This suggests that a high viral load should not be a contraindication for ICIs treatment in HBV-related liver cancer patients and antiviral treatment may benefit these patients by reducing the risk of hepatic decompensation (139–141).
Overall, virus-related HCC, especially HBV-related HCC, may serve as a prognostic factor in ICIs treatment. However, the relationship between viral etiology and the response to ICIs in HCC is complex and requires further investigation (Table 5).
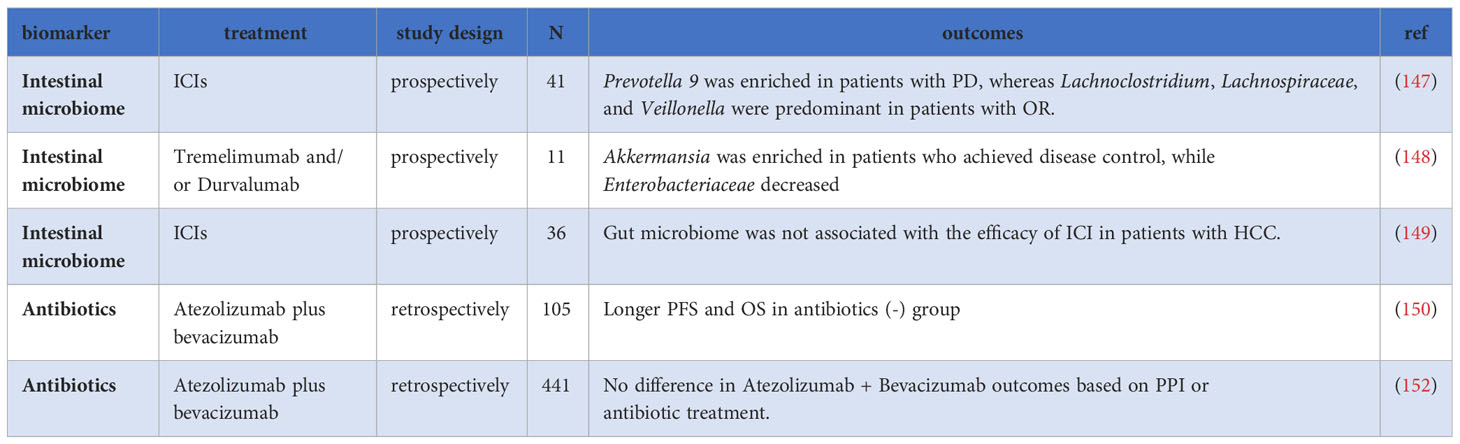
6 Gut microbiome
The gut microbiota engages in profound interactions with the host’s immune system, and its polymorphic nature is considered one of the hallmarks of cancer (19, 142). Research has demonstrated the influence of gut microbiota on the effectiveness of ICIs in various cancers (143–146). As ICIs therapy continues to evolve, the emerging importance of the gut microbiome in HCC is becoming evident. In a prospective study by Lee et al., the relationship between pre-treatment fecal samples and ICIs treatment in HCC was investigated. The study found that the composition of fecal bacteria before treatment significantly correlated with the therapeutic response (147). Prevotella 9 was enriched in PD patients, while Lachnoclostridium, Lachnospiraceae, and Veillonella were more dominant in OR patients. Patients with a more favorable microbiota composition, characterized by the absence of Prevotella 9 and enrichment of Lachnoclostridium, had better PFS and OS. Additionally, specific metabolites, such as ursodeoxycholic acid and ursocholic acid, were enriched in the feces of patients who responded well to treatment, suggesting a potential relationship between pre-treatment fecal microbiota, bile acids, and the outcome of HCC immunotherapy (147).
Moreover, changes in gut microbiota during treatment may also predict treatment outcomes. In a prospective study involving patients receiving tremelimumab and/or durvalumab treatment, Akkermansia was enriched in patients who achieved disease control, while Enterobacteriaceae decreased (148). However, another study by Shen et al. did not find significant changes in gut microbiota between pre-ICIs treatment and 8 weeks post-treatment (149).
Antibiotic use can significantly impact gut microbiota in clinical settings. The alteration of gut microbial composition and function due to antibiotic use may reduce microbial diversity and adversely affect the immune response. A retrospective study with 105 HCC patients showed that those who did not receive antibiotics had longer median PFS (9.1 months vs. 3.0 months; P=0.049) and OS (not reached vs. 11.4 months; P=0.015) compared to the group that received antibiotics (150). Furthermore, a meta-analysis of 1056 patients showed that the use of antibiotics can modify the treatment effect of ICIs in HCC patients, suggesting that early exposure or use of antibiotics during treatment may affect gut microbiota and, consequently, treatment efficacy (151).
Nevertheless, findings from a retrospective study with 441 patients treated with Atez/Bev indicated that after adjusting for imbalances in baseline patient characteristics, the differences in PFS and OS between patients with and without antibiotic treatment were not statistically significant (median PFS, 3.8 vs. 6.7 months, P=0.2; 1-year survival rate, 61.8% vs. 71.0%, P=0.6) (152). Additionally, the study observed no statistically significant difference in PFS and OS between patients with and without proton pump inhibitors (PPI)(median PFS, 7.0 vs. 6.5 months, P=0.07, 1-year survival rate 66.3% and 73.8%, P=0.9) (152).
These conflicting results underscore the necessity for further research and large-scale studies to better understand the role of gut microbiota in predicting treatment response and to validate its potential as a therapeutic target. Future investigations may focus on elucidating the underlying mechanisms of gut microbiota in HCC and exploring strategies to modulate the microbiota to enhance treatment outcomes (153) (Table 6).
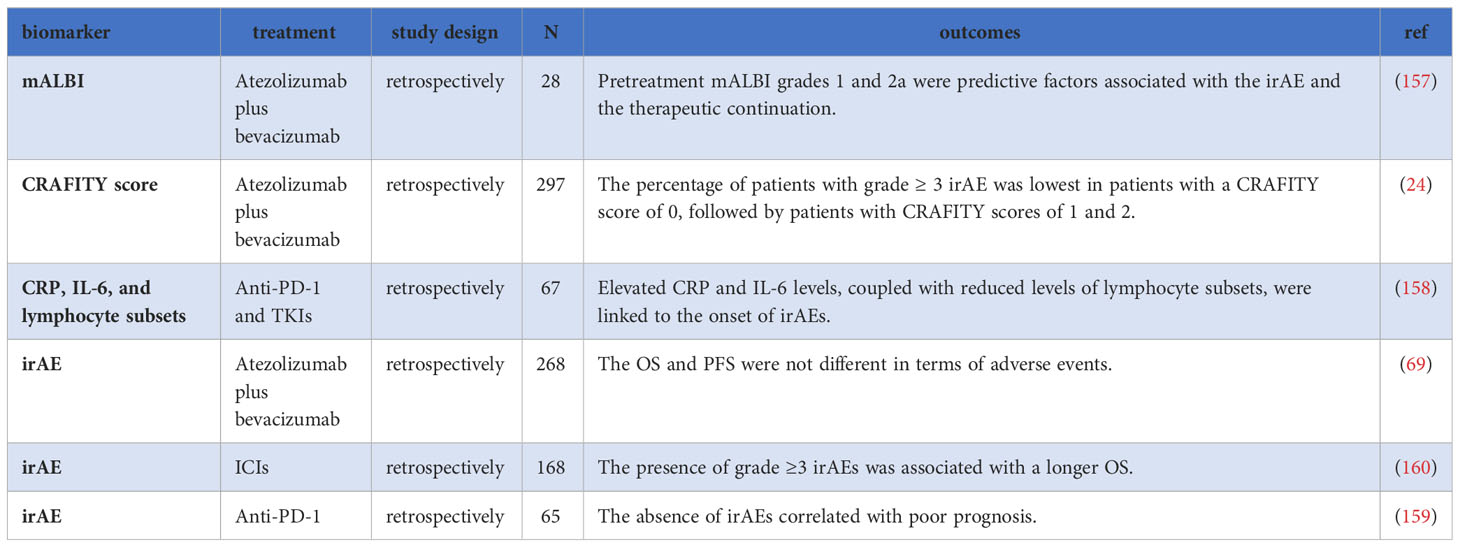
7 Immune-related adverse events
The safety profile of ICIs is a critical consideration in their clinical use. While ICIs can improve patient prognosis, they can also lead to immune-related adverse events (irAEs), causing damage to normal tissues and organs, and potentially resulting in treatment discontinuation or abandonment (154, 155). The reasons for irAEs occurring in certain patients are not yet clear, and emerging evidence suggests that different immunopathogenic mechanisms can lead to distinct histopathological phenotypes in affected organs (156).
Identifying risk factors for irAEs in HCC patients is crucial to promptly identify those who may not tolerate treatment-related toxicities, avoid unnecessary pain, and reduce healthcare costs (69). Several markers have been investigated as potential predictors of irAEs. The modified ALBI (mALBI) score, which further divides grade 2 of the ALBI score into grades 2a and 2b, has gained attention. In a retrospective study involving patients undergoing Atez/Bev treatment, a significant difference in baseline mALBI scores (P=0.02) was observed between the group that discontinued medication due to irAEs and the group that continued treatment (157). However, a systematic review did not find a significant increase in adverse events in patients with impaired liver function (30). Furthermore, Hatanaka et al. revealed that patients with a CRAFITY score of 0 exhibited a low incidence of grade≥3 irAEs, suggesting its potential as a predictive marker for irAEs (24). Additionally, in a retrospective analysis of patients treated with ICIs combined with TKIs, Yu et al. demonstrated that compared with patients who did not develop irAEs, those who developed irAEs had higher levels of CRP and IL-6 and lower levels of lymphocyte subsets (excluding natural killer cell counts), all of which may serve as potential biomarkers for irAEs (158).
The pathophysiological mechanisms underlying irAEs are believed to be related to the role of immune checkpoints in maintaining immunological homeostasis (155). Interestingly, some retrospective studies have indicated that patients who experienced irAEs exhibited improved outcomes compared to those who did not (159, 160). In a study involving 65 HCC patients who received anti-PD-1 treatment, the median PFS in the irAEs group was superior to that in the non-irAEs group. Multivariate analysis demonstrated that the absence of irAEs (HR = 6.410, P=0.017) independently correlated with poor prognosis (159). Another study involving 198 patients undergoing ICIs treatment found that the occurrence of grade ≥3 irAEs was associated with better survival compared to grades 1 and 2 (PFS: 8.5 months vs. 3.6 months vs. 1.3 months; OS: 26.9 months vs. 14.0 months vs. 4.6 months) (160). However, some concerns have been raised by other researchers regarding the grouping and statistical analysis methods employed in this study (161). Furthermore, in a multicenter study involving 268 patients treated with Atez/Bev, no significant differences were found in OS and PFS concerning irAEs (69). These conflicting results show that further research is needed to fully understand the relationship between irAEs and treatment outcomes in HCC patients. Larger-scale studies are necessary to clarify the predictive value of irAEs in the context of ICIs treatment for HCC (Table 7).
8 Discussion
Despite significant advancements in HCC treatment in recent years, the overall five-year survival of patients remains unsatisfactory due to challenges in early diagnosis, treatment response prediction, and treatment response. Therefore, there is a pressing need to explore robust biomarkers that can improve the efficacy of ICIs in HCC treatment. Currently, biomarkers for predicting ICIs response in HCC are still in the exploration stage and lack compelling evidence. The existing studies on the predictive efficacy of ICIs are limited, often comprising small sample sizes and retrospective designs. To establish stronger evidence, larger prospective studies are warranted.
Although certain potential biomarkers, such as PD-L1 and TMB, have been identified, their predictive value in HCC is limited. On the other hand, easily accessible serum biomarkers like NLR, ALBI, and the CRAFITY score offer promising methods for predicting the efficacy of ICIs, and combining multiple factors may enhance the accuracy of these predictions. Novel biomarkers such as CTCs and ctDNA have the potential to precisely reflect the preexisting immunity within the tumor tissue. However, their practical application is currently hindered by the lack of standardized procedures and reliable measurement technologies (162). The gut microbiome has emerged as another promising biomarker for HCC. With the development of fecal microbiota transplantation, it may even be utilized as a combination treatment to enhance the efficacy of ICIs in the future. Nevertheless, further research is necessary to unravel the underlying mechanisms.
Artificial intelligence (AI) and machine learning (ML) represent a transformative era in HCC immunotherapy, offering superior prognostic biomarkers compared to conventional techniques. The capacity of AI and ML to analyze vast datasets, encompassing radiology, pathology, genomics, and proteomics presents an unprecedented opportunity for precision medicine and personalized treatment (163, 164). Yet, practical implementation confronts nonnegligible challenges such as data privacy, model interpretability, and rigorous clinical validation (165, 166). Striking a balance between harnessing the power of AI, safeguarding patient privacy, enhancing model transparency, and ensuring real-world applicability through thorough validation are crucial steps in realizing the full potential of AI and ML in HCC prognosis and treatment (167).
In conclusion, the response and prognosis of ICIs treatment in HCC are influenced by various factors, including intrinsic characteristics of tumor tissue, the TME, and host immunity. Future efforts should focus on exploring novel biomarkers, and the management of ICIs treatment in HCC patients should involve dynamic monitoring and personalized evaluation, taking into account multiple indicators to maximize the role of ICIs and maximize the benefits for patients.
Author contributions
RQ: Data curation, Writing – original draft. TJ: Conceptualization, Funding acquisition, Writing – review & editing. FX: Conceptualization, Funding acquisition, Writing – review & editing, Investigation, Supervision.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported in part by the grants from the Natural Science Fund of Liaoning Province (20180551193 and 2020-MS-181 to FX) and the 345 Talent Project of Shengjing Hospital (40B to FX and 30C to TJ).
Acknowledgments
The authors acknowledge the contribution of all investigators at the participating study sites.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet (2022) 400(10360):1345–62. doi: 10.1016/S0140-6736(22)01200-4
3. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers (2021) 7(1):6. doi: 10.1038/s41572-020-00240-3
4. Kong FH, Ye QF, Miao XY, Liu X, Huang SQ, Xiong L, et al. Current status of sorafenib nanoparticle delivery systems in the treatment of hepatocellular carcinoma. Theranostics (2021) 11(11):5464–90. doi: 10.7150/thno.54822
5. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med (2008) 359(4):378–90. doi: 10.1056/NEJMoa0708857
6. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet (2018) 391(10126):1163–73. doi: 10.1016/S0140-6736(18)30207-1
7. Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin S, Zhu AX, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol (2022) 23(8):995–1008. doi: 10.1016/S1470-2045(22)00326-6
8. Llovet JM, Castet F, Heikenwalder M, Maini MK, Mazzaferro V, Pinato DJ, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol (2022) 19(3):151–72. doi: 10.1038/s41571-021-00573-2
9. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet (2017) 389(10088):2492–502. doi: 10.1016/S0140-6736(17)31046-2
10. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol (2018) 19(7):940–52. doi: 10.1016/S1470-2045(18)30351-6
11. Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase III trial. J Clin Oncol (2020) 38(3):193–202. doi: 10.1200/JCO.19.01307
12. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol (2022) 23(1):77–90. doi: 10.1016/S1470-2045(21)00604-5
13. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. New Engl J Med (2020) 382(20):1894–905. doi: 10.1056/NEJMoa1915745
14. Abou-Alfa GK, Chan SL, Kudo M, Lau G, Kelley RK, Furuse J, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol (2022) 40(4_suppl):379–. doi: 10.1200/JCO.2022.40.4_suppl.379
15. Kudo M. Durvalumab plus tremelimumab in unresectable hepatocellular carcinoma. Hepatobiliary Surg Nutr (2022) 11(4):592–6. doi: 10.21037/hbsn-22-143
16. Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol (2022) 76(4):862–73. doi: 10.1016/j.jhep.2021.11.030
17. Mehta N, Dodge JL, Roberts JP, Hirose R, Yao FY. Alpha-fetoprotein decrease from > 1,000 to < 500 ng/mL in patients with hepatocellular carcinoma leads to improved posttransplant outcomes. Hepatology (2019) 69(3):1193–205. doi: 10.1002/hep.30413
18. Hu X, Chen R, Wei Q, Xu X. The landscape of alpha fetoprotein in hepatocellular carcinoma: where are we? Int J Biol Sci (2022) 18(2):536–51. doi: 10.7150/ijbs.64537
19. Spahn S, Roessler D, Pompilia R, Gabernet G, Gladstone BP, Horger M, et al. Clinical and genetic tumor characteristics of responding and non-responding patients to PD-1 inhibition in hepatocellular carcinoma. Cancers (Basel) (2020) 12(12):3830. doi: 10.3390/cancers12123830
20. Matsumae T, Kodama T, Myojin Y, Maesaka K, Sakamori R, Takuwa A, et al. Circulating cell-free DNA profiling predicts the therapeutic outcome in advanced hepatocellular carcinoma patients treated with combination immunotherapy. Cancers (Basel) (2022) 14(14):3367. doi: 10.3390/cancers14143367
21. Zhang L, Feng J, Kuang T, Chai D, Qiu Z, Deng W, et al. Blood biomarkers predict outcomes in patients with hepatocellular carcinoma treated with immune checkpoint Inhibitors: A pooled analysis of 44 retrospective sudies. Int Immunopharmacol (2023) 118:110019. doi: 10.1016/j.intimp.2023.110019
22. Scheiner B, Pomej K, Kirstein MM, Hucke F, Finkelmeier F, Waidmann O, et al. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy - development and validation of the CRAFITY score. J Hepatol (2022) 76(2):353–63. doi: 10.1016/j.jhep.2021.09.035
23. Yang Y, Ouyang J, Zhou Y, Zhou J, Zhao H. The CRAFITY score: A promising prognostic predictor for patients with hepatocellular carcinoma treated with tyrosine kinase inhibitor and immunotherapy combinations. J Hepatol (2022) 77(2):574–6. doi: 10.1016/j.jhep.2022.03.018
24. Hatanaka T, Kakizaki S, Hiraoka A, Tada T, Hirooka M, Kariyama K, et al. Prognostic impact of C-reactive protein and alpha-fetoprotein in immunotherapy score in hepatocellular carcinoma patients treated with atezolizumab plus bevacizumab: a multicenter retrospective study. Hepatol Int (2022) 16(5):1150–60. doi: 10.1007/s12072-022-10358-z
25. Cabibbo G, Edeline J. The prognostic role of tumour progression and liver function at progression under immunotherapy for hepatocellular carcinoma. Liver Int (2023) 43(3):528–30. doi: 10.1111/liv.15513
26. Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol (2015) 33(6):550–8. doi: 10.1200/JCO.2014.57.9151
27. Pinato DJ, Kaneko T, Saeed A, Pressiani T, Kaseb A, Wang Y, et al. Immunotherapy in hepatocellular cancer patients with mild to severe liver dysfunction: adjunctive role of the ALBI grade. Cancers (Basel) (2020) 12(7):1862. doi: 10.3390/cancers12071862
28. Wong JSL, Kwok GGW, Tang V, Li BCW, Leung R, Chiu J, et al. Ipilimumab and nivolumab/pembrolizumab in advanced hepatocellular carcinoma refractory to prior immune checkpoint inhibitors. J Immunother Cancer (2021) 9(2):e001945. doi: 10.1136/jitc-2020-001945
29. Dong D, Zhu X, Wang H, Li L, Wan M, Li S, et al. Prognostic significance of albumin-bilirubin score in patients with unresectable hepatocellular carcinoma undergoing combined immunotherapy and radiotherapy. J Med Imaging Radiat Oncol (2022) 66(5):662–70. doi: 10.1111/1754-9485.13398
30. Tian BW, Yan LJ, Ding ZN, Liu H, Han CL, Meng GX, et al. Evaluating liver function and the impact of immune checkpoint inhibitors in the prognosis of hepatocellular carcinoma patients: A systemic review and meta-analysis. Int Immunopharmacol (2023) 114:109519. doi: 10.1016/j.intimp.2022.109519
31. Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol (2013) 14(6):e218–28. doi: 10.1016/S1470-2045(12)70582-X
32. Rizzo A, Brandi G. Biochemical predictors of response to immune checkpoint inhibitors in unresectable hepatocellular carcinoma. Cancer Treat Res Commun (2021) 27:100328. doi: 10.1016/j.ctarc.2021.100328
33. Xu J, Lin H, Wu G, Zhu M, Li M. IL-6/STAT3 is a promising therapeutic target for hepatocellular carcinoma. Front Oncol (2021) 11:760971. doi: 10.3389/fonc.2021.760971
34. Myojin Y, Kodama T, Sakamori R, Maesaka K, Matsumae T, Sawai Y, et al. Interleukin-6 is a circulating prognostic biomarker for hepatocellular carcinoma patients treated with combined immunotherapy. Cancers (Basel) (2022) 14(4):883. doi: 10.3390/cancers14040883
35. Yang H, Kang B, Ha Y, Lee SH, Kim I, Kim H, et al. High serum IL-6 correlates with reduced clinical benefit of atezolizumab and bevacizumab in unresectable hepatocellular carcinoma. JHEP Rep (2023) 5(4):100672. doi: 10.1016/j.jhepr.2023.100672
36. Chen J, Gingold JA, Su X. Immunomodulatory TGF-β Signaling in hepatocellular carcinoma. Trends Mol Med (2019) 25(11):1010–23. doi: 10.1016/j.molmed.2019.06.007
37. Feun LG, Li YY, Wu C, Wangpaichitr M, Jones PD, Richman SP, et al. Phase 2 study of pembrolizumab and circulating biomarkers to predict anticancer response in advanced, unresectable hepatocellular carcinoma. Cancer (2019) 125(20):3603–14. doi: 10.1002/cncr.32339
38. Zhang W, Gong C, Peng X, Bi X, Sun Y, Zhou J, et al. Serum concentration of CD137 and tumor infiltration by M1 macrophages predict the response to sintilimab plus bevacizumab biosimilar in advanced hepatocellular carcinoma patients. Clin Cancer Res (2022) 28(16):3499–508. doi: 10.1158/1078-0432.CCR-21-3972
39. Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer (2020) 20(9):485–503. doi: 10.1038/s41568-020-0281-y
40. Xiong S, Dong L, Cheng L. Neutrophils in cancer carcinogenesis and metastasis. J Hematol Oncol (2021) 14(1):173. doi: 10.1186/s13045-021-01187-y
41. Gkolfinopoulos S, Jones RL, Constantinidou A. The emerging role of platelets in the formation of the micrometastatic niche: current evidence and future perspectives. Front Oncol (2020) 10:374. doi: 10.3389/fonc.2020.00374
42. Durán-Saenz NZ, Serrano-Puente A, Gallegos-Flores PI, Mendoza-Almanza BD, Esparza-Ibarra EL, Godina-González S, et al. Platelet membrane: an outstanding factor in cancer metastasis. Membranes (Basel) (2022) 12(2):182. doi: 10.3390/membranes12020182
43. Giovannini C, Suzzi F, Tovoli F, Bruccoleri M, Marseglia M, Alimenti E, et al. Low-baseline PD1+ Granulocytes predict responses to atezolizumab-bevacizumab in hepatocellular carcinoma. Cancers (Basel) (2023) 15(6):1661. doi: 10.3390/cancers15061661
44. Mouchli M, Reddy S, Gerrard M, Boardman L, Rubio M. Usefulness of neutrophil-to-lymphocyte ratio (NLR) as a prognostic predictor after treatment of hepatocellular carcinoma." Review article. Ann Hepatol (2021) 22:100249. doi: 10.1016/j.aohep.2020.08.067
45. Najjar M, Agrawal S, Emond JC, Halazun KJ. Pretreatment neutrophil-lymphocyte ratio: useful prognostic biomarker in hepatocellular carcinoma. J Hepatocell Carcinoma (2018) 5:17–28. doi: 10.2147/JHC.S86792
46. Nakano M, Kuromatsu R, Niizeki T, Okamura S, Iwamoto H, Shimose S, et al. Immunological inflammatory biomarkers as prognostic predictors for advanced hepatocellular carcinoma. ESMO Open (2021) 6(1):100020. doi: 10.1016/j.esmoop.2020.100020
47. Dharmapuri S, Özbek U, Lin JY, Sung M, Schwartz M, Branch AD, et al. Predictive value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in advanced hepatocellular carcinoma patients treated with anti-PD-1 therapy. Cancer Med (2020) 9(14):4962–70. doi: 10.1002/cam4.3135
48. Muhammed A, Fulgenzi CAM, Dharmapuri S, Pinter M, Balcar L, Scheiner B, et al. The systemic inflammatory response identifies patients with adverse clinical outcome from immunotherapy in hepatocellular carcinoma. Cancers (Basel) (2021) 14(1):168. doi: 10.3390/cancers14010186
49. Hong JY, Cho HJ, Sa JK, Liu X, Ha SY, Lee T, et al. Hepatocellular carcinoma patients with high circulating cytotoxic T cells and intra-tumoral immune signature benefit from pembrolizumab: results from a single-arm phase 2 trial. Genome Med (2022) 14(1):1. doi: 10.1186/s13073-021-00995-8
50. Tada T, Kumada T, Hiraoka A, Hirooka M, Kariyama K, Tani J, et al. Neutrophil-lymphocyte ratio predicts early outcomes in patients with unresectable hepatocellular carcinoma treated with atezolizumab plus bevacizumab: a multicenter analysis. Eur J Gastroenterol Hepatol (2022) 34(6):698–706. doi: 10.1097/MEG.0000000000002356
51. Li Q, Ma F, Wang JF. Advanced lung cancer inflammation index predicts survival outcomes of hepatocellular carcinoma patients receiving immunotherapy. Front Oncol (2023) 13:997314. doi: 10.3389/fonc.2023.997314
52. Mei J, Sun XQ, Lin WP, Li SH, Lu LH, Zou JW, et al. Comparison of the prognostic value of inflammation-based scores in patients with hepatocellular carcinoma after anti-PD-1 therapy. J Inflammation Res (2021) 14:3879–90. doi: 10.2147/JIR.S325600
53. Jiang Y, Tu X, Zhang X, Liao H, Han S, Jiang W, et al. Nutrition and metabolism status alteration in advanced hepatocellular carcinoma patients treated with anti-PD-1 immunotherapy. Support Care Cancer (2020) 28(11):5569–79. doi: 10.1007/s00520-020-05478-x
54. Zhu Z-F, Zhuang L-P, Zhang C-Y, Ning Z-Y, Wang D, Sheng J, et al. Predictive role of the monocyte-to-lymphocyte ratio in advanced hepatocellular carcinoma patients receiving anti-PD-1 therapy. Transl Cancer Res (2022) 11(1):160–70. doi: 10.21037/tcr-21-1760
55. Guo DZ, Zhang SY, Dong SY, Yan JY, Wang YP, Cao Y, et al. Circulating immune index predicting the prognosis of patients with hepatocellular carcinoma treated with lenvatinib and immunotherapy. Front Oncol (2023) 13:1109742. doi: 10.3389/fonc.2023.1109742
56. Zhao M, Duan X, Mi L, Shi J, Li N, Yin X, et al. Prognosis of hepatocellular carcinoma and its association with immune cells using systemic inflammatory response index. Future Oncol (2022) 18(18):2269–88. doi: 10.2217/fon-2021-1087
57. Hosoda S, Suda G, Sho T, Ogawa K, Kimura M, Yang Z, et al. Low baseline CXCL9 predicts early progressive disease in unresectable HCC with atezolizumab plus bevacizumab treatment. Liver Cancer (2022) 12(2):156–70. doi: 10.1159/000527759
58. Liu F, Zhou P, Wang Q, Zhang M, Li D. The Schlafen family: complex roles in different cell types and virus replication. Cell Biol Int (2018) 42(1):2–8. doi: 10.1002/cbin.10778
59. Zhou C, Weng J, Liu C, Liu S, Hu Z, Xie X, et al. Disruption of SLFN11 deficiency-induced CCL2 signaling and macrophage M2 polarization potentiates anti-PD-1 therapy efficacy in hepatocellular carcinoma. Gastroenterology (2023) 164(7):1261–78. doi: 10.1053/j.gastro.2023.02.005
60. Guo DZ, Zhang SY, Dong SY, Yan JY, Wang YP, Cao Y, et al. Prognostic model for predicting outcome and guiding treatment decision for unresectable hepatocellular carcinoma treated with lenvatinib monotherapy or lenvatinib plus immunotherapy. Front Immunol (2023) 14:1141199. doi: 10.3389/fimmu.2023.1141199
61. Amilca-Seba K, Sabbah M, Larsen AK, Denis JA. Osteopontin as a regulator of colorectal cancer progression and its clinical applications. Cancers (Basel) (2021) 13(15):3793. doi: 10.3390/cancers13153793
62. Yamauchi R, Ito T, Yoshio S, Yamamoto T, Mizuno K, Ishigami M, et al. Serum osteopontin predicts the response to atezolizumab plus bevacizumab in patients with hepatocellular carcinoma. J Gastroenterol (2023) 58(6):565–74. doi: 10.1007/s00535-023-01985-w
63. Sun X, Mei J, Lin W, Yang Z, Peng W, Chen J, et al. Reductions in AFP and PIVKA-II can predict the efficiency of anti-PD-1 immunotherapy in HCC patients. BMC Cancer (2021) 21(1):775. doi: 10.1186/s12885-021-08428-w
64. Kim HI, Lim J, Shim JH. Role of the alpha-fetoprotein response in immune checkpoint inhibitor-based treatment of patients with hepatocellular carcinoma. J Cancer Res Clin Oncol (2022) 148(8):2069–77. doi: 10.1007/s00432-021-03727-y
65. Zhu AX, Dayyani F, Yen CJ, Ren Z, Bai Y, Meng Z, et al. Alpha-fetoprotein as a potential surrogate biomarker for atezolizumab + Bevacizumab treatment of hepatocellular carcinoma. Clin Cancer Res (2022) 28(16):3537–45. doi: 10.1158/1078-0432.CCR-21-3275
66. Teng W, Lin C-C, Su C-W, Lin P-T, Hsieh Y-C, Chen W-T, et al. Combination of CRAFITY score with Alpha-fetoprotein response predicts a favorable outcome of atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma. Am J Cancer Res (2022) 12(4):1899–911. doi: 10.1016/S0168-8278(22)01098-4
67. Jeon SH, Lee YJ, Kim H-D, Nam H, Ryoo B-Y, Park S-H, et al. Dynamic changes in peripheral blood monocytes early after anti-PD-1 therapy predict clinical outcomes in hepatocellular carcinoma. Cancer Immunol Immunother CII (2022) 72(2):371–84. doi: 10.1007/s00262-022-03258-6
68. Balcar L, Bauer D, Pomej K, Meischl T, Mandorfer M, Reiberger T, et al. Early changes in immunoglobulin G levels during immune checkpoint inhibitor treatment are associated with survival in hepatocellular carcinoma patients. PloS One (2023) 18(4):e0282680. doi: 10.1371/journal.pone.0282680
69. Takaki S, Kurosaki M, Mori N, Tsuji K, Ochi H, Marusawa H, et al. Effects on survival of the adverse event of atezolizumab plus bevacizumab for hepatocellular carcinoma: a multicenter study by the Japan Red Cross Liver Study Group. Invest New Drugs (2023) 41(2):340–9. doi: 10.1007/s10637-023-01349-4
70. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet (2019) 393(10183):1819–30. doi: 10.1016/S0140-6736(18)32409-7
71. Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet (2019) 393(10189):2404–15. doi: 10.1016/S0140-6736(19)30723-8
72. Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the checkMate 040 randomized clinical trial. JAMA Oncol (2020) 6(11):e204564. doi: 10.1001/jamaoncol.2020.4564
73. Zhou X, Cao J, Topatana W, Xie T, Chen T, Hu J, et al. Evaluation of PD-L1 as a biomarker for immunotherapy for hepatocellular carcinoma: systematic review and meta-analysis. Immunotherapy (2023) 15(5):353–65. doi: 10.2217/imt-2022-0168
74. Yang Y, Chen D, Zhao B, Ren L, Huang R, Feng B, et al. The predictive value of PD-L1 expression in patients with advanced hepatocellular carcinoma treated with PD-1/PD-L1 inhibitors: a systematic review and meta-analysis. Cancer Med (2023) 12(8):9282–92. doi: 10.1002/cam4.5676
75. Mocan T, Ilies M, Nenu I, Craciun R, Horhat A, Susa R, et al. Serum levels of soluble programmed death-ligand 1 (sPD-L1): A possible biomarker in predicting post-treatment outcomes in patients with early hepatocellular carcinoma. Int Immunopharmacol (2021) 94:107467. doi: 10.1016/j.intimp.2021.107467
76. Hou M-M, Rau K-M, Kang Y-K, Lee J-S, Pan H, Yuan Y, et al. 77 Association between programmed death-ligand 1 (PD-L1) expression and gene signatures of response or resistance to tislelizumab monotherapy in hepatocellular carcinoma (HCC). J ImmunoTher Cancer (2020) 8(Suppl 3):A47–A8. doi: 10.1136/jitc-2020-SITC2020.0077
77. Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature (2013) 500(7463):415–21. doi: 10.1038/nature12477
78. Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther (2017) 16(11):2598–608. doi: 10.1158/1535-7163.MCT-17-0386
79. Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (2015) 348(6230):124–8. doi: 10.1126/science.aaa1348
80. Yarchoan M, Albacker LA, Hopkins AC, Montesion M, Murugesan K, Vithayathil TT, et al. PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI Insight (2019) 4(6):e126908. doi: 10.1172/jci.insight.126908
81. Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med (2014) 371(23):2189–99. doi: 10.1056/NEJMoa1406498
82. Zhu AX, Abbas AR, de Galarreta MR, Guan Y, Lu S, Koeppen H, et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat Med (2022) 28(8):1599–611. doi: 10.1038/s41591-022-01868-2
83. Zhu AX, Guan Y, Abbas AR, Koeppen H, Lu S, Hsu C-H, et al. Abstract CT044: Genomic correlates of clinical benefits from atezolizumab combined with bevacizumab vs. atezolizumab alone in patients with advanced hepatocellular carcinoma (HCC). Cancer Res (2020) 80(16_Supplement):CT044–CT. doi: 10.1158/1538-7445.AM2020-CT044
84. Araujo DV, Wang A, Torti D, Leon A, Marsh K, McCarthy A, et al. Applications of circulating tumor DNA in a cohort of phase I solid tumor patients treated with immunotherapy. JNCI Cancer Spectr (2021) 5(3):pkaa122. doi: 10.1093/jncics/pkaa122
85. Muhammed A, D'Alessio A, Enica A, Talbot T, Fulgenzi CAM, Nteliopoulos G, et al. Predictive biomarkers of response to immune checkpoint inhibitors in hepatocellular carcinoma. Expert Rev Mol Diagn (2022) 22(3):253–64. doi: 10.1080/14737159.2022.2049244
86. Maheswaran S, Haber DA. Circulating tumor cells: a window into cancer biology and metastasis. Curr Opin Genet Dev (2010) 20(1):96–9. doi: 10.1016/j.gde.2009.12.002
87. Ahn JC, Teng P-C, Chen P-J, Posadas E, Tseng H-R, Lu SC, et al. Detection of circulating tumor cells and their implications as a biomarker for diagnosis, prognostication, and therapeutic monitoring in hepatocellular carcinoma. Hepatol (Baltimore Md) (2021) 73(1):422–36. doi: 10.1002/hep.31165
88. Winograd P, Hou S, Court CM, Lee YT, Chen PJ, Zhu Y, et al. Hepatocellular carcinoma-circulating tumor cells expressing PD-L1 are prognostic and potentially associated with response to checkpoint inhibitors. Hepatol Commun (2020) 4(10):1527–40. doi: 10.1002/hep4.1577
89. Yue C, Jiang Y, Li P, Wang Y, Xue J, Li N, et al. Dynamic change of PD-L1 expression on circulating tumor cells in advanced solid tumor patients undergoing PD-1 blockade therapy. Oncoimmunology (2018) 7(7):e1438111. doi: 10.1080/2162402X.2018.1438111
90. Su K, Guo L, He K, Rao M, Zhang J, Yang X, et al. PD-L1 expression on circulating tumor cells can be a predictive biomarker to PD-1 inhibitors combined with radiotherapy and antiangiogenic therapy in advanced hepatocellular carcinoma. Front Oncol (2022) 12:873830. doi: 10.3389/fonc.2022.873830
91. Li Y, Zheng Y, Wu L, Li J, Ji J, Yu Q, et al. Current status of ctDNA in precision oncology for hepatocellular carcinoma. J Exp Clin Cancer Res (2021) 40(1):140. doi: 10.1186/s13046-021-01940-8
92. von Felden J, Craig AJ, Garcia-Lezana T, Labgaa I, Haber PK, D'Avola D, et al. Mutations in circulating tumor DNA predict primary resistance to systemic therapies in advanced hepatocellular carcinoma. Oncogene (2021) 40(1):140–51. doi: 10.1038/s41388-020-01519-1
93. Fu Y, Yang Z, Hu Z, Yang Z, Pan Y, Chen J, et al. Preoperative serum ctDNA predicts early hepatocellular carcinoma recurrence and response to systemic therapies. Hepatol Int (2022) 16(4):868–78. doi: 10.1007/s12072-022-10348-1
94. Howell J, Atkinson SR, Pinato DJ, Knapp S, Ward C, Minisini R, et al. Identification of mutations in circulating cell-free tumour DNA as a biomarker in hepatocellular carcinoma. Eur J Cancer (2019) 116:56–66. doi: 10.1016/j.ejca.2019.04.014
95. Zhu GQ, Liu WR, Tang Z, Qu WF, Fang Y, Jiang XF, et al. Serial circulating tumor DNA to predict early recurrence in patients with hepatocellular carcinoma: a prospective study. Mol Oncol (2022) 16(2):549–61. doi: 10.1002/1878-0261.13105
96. Hsu C-H, Lu S, Abbas A, Guan Y, Zhu AX, Aleshin A, et al. Longitudinal and personalized detection of circulating tumor DNA (ctDNA) for monitoring efficacy of atezolizumab plus bevacizumab in patients with unresectable hepatocellular carcinoma (HCC). J Clin Oncol (2020) 38(15_suppl):3531–. doi: 10.1200/JCO.2020.38.15_suppl.3531
97. Morita M, Nishida N, Sakai K, Aoki T, Chishina H, Takita M, et al. Immunological microenvironment predicts the survival of the patients with hepatocellular carcinoma treated with anti-PD-1 antibody. Liver Cancer (2021) 10(4):380–93. doi: 10.1159/000516899
98. Hernandez-Meza G, von Felden J, Gonzalez-Kozlova EE, Garcia-Lezana T, Peix J, Portela A, et al. DNA methylation profiling of human hepatocarcinogenesis. Hepatology (2021) 74(1):183–99. doi: 10.1002/hep.31659
99. Shen B, Wen Z, Lv G, Wang J, Han R, Jiang J. Identification and analysis of DNA methylation-driven signatures for prognostic and immune microenvironments evaluation in hepatocellular carcinoma. Front Genet (2022) 13:1022078. doi: 10.3389/fgene.2022.1022078
100. Huang C, Ren S, Chen Y, Liu A, Wu Q, Jiang T, et al. PD-L1 methylation restricts PD-L1/PD-1 interactions to control cancer immune surveillance. Sci Adv (2023) 9(21):eade4186. doi: 10.1126/sciadv.ade4186
101. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol (2022) 76(3):681–93. doi: 10.1016/j.jhep.2021.11.018
102. Han CL, Tian BW, Yan LJ, Ding ZN, Liu H, Mao XC, et al. Efficacy and safety of immune checkpoint inhibitors for hepatocellular carcinoma patients with macrovascular invasion or extrahepatic spread: a systematic review and meta-analysis of 54 studies with 6187 hepatocellular carcinoma patients. Cancer Immunol Immunother (2023) 72(7):1957–69. doi: 10.1007/s00262-023-03390-x
103. Talbot T, D'Alessio A, Pinter M, Balcar L, Scheiner B, Marron TU, et al. Progression patterns and therapeutic sequencing following immune checkpoint inhibition for hepatocellular carcinoma: An international observational study. Liver Int (2023) 43(3):695–707. doi: 10.1111/liv.15502
104. Chen Q, Deng Y, Zhao C, Huang Z, Zhang W, Yang Y, et al. Nomogram for tumour response based on prospective cohorts of hepatocellular carcinoma patients receiving immunotherapy combined with targeted therapy: development and validation. Ann Transl Med (2023) 11(5):199. doi: 10.21037/atm-22-3045
105. Brummel K, Eerkens AL, de Bruyn M, Nijman HW. Tumour-infiltrating lymphocytes: from prognosis to treatment selection. Br J Cancer (2023) 128(3):451–8. doi: 10.1038/s41416-022-02119-4
106. Liu T, Tan J, Wu M, Fan W, Wei J, Zhu B, et al. High-affinity neoantigens correlate with better prognosis and trigger potent antihepatocellular carcinoma (HCC) activity by activating CD39(+)CD8(+) T cells. Gut (2021) 70(10):1965–77. doi: 10.1136/gutjnl-2020-322196
107. Sangro B, Melero I, Wadhawan S, Finn RS, Abou-Alfa GK, Cheng AL, et al. Association of inflammatory biomarkers with clinical outcomes in nivolumab-treated patients with advanced hepatocellular carcinoma. J Hepatol (2020) 73(6):1460–9. doi: 10.1016/j.jhep.2020.07.026
108. Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol (2020) 15:123–47. doi: 10.1146/annurev-pathmechdis-012418-012718
109. Qu X, Zhao X, Lin K, Wang N, Li X, Li S, et al. M2-like tumor-associated macrophage-related biomarkers to construct a novel prognostic signature, reveal the immune landscape, and screen drugs in hepatocellular carcinoma. Front Immunol (2022) 13:994019. doi: 10.3389/fimmu.2022.994019
110. Liu Y, Xun Z, Ma K, Liang S, Li X, Zhou S, et al. Identification of a tumour immune barrier in the HCC microenvironment that determines the efficacy of immunotherapy. J Hepatol (2023) 78(4):770–82. doi: 10.1016/j.jhep.2023.01.011
111. Greten TF, Villanueva A, Korangy F, Ruf B, Yarchoan M, Ma L, et al. Biomarkers for immunotherapy of hepatocellular carcinoma. Nat Rev Clin Oncol (2023) 20(11):780–98. doi: 10.1038/s41571-023-00816-4
112. Li XY, Shen Y, Zhang L, Guo X, Wu J. Understanding initiation and progression of hepatocellular carcinoma through single cell sequencing. Biochim Biophys Acta Rev Cancer (2022) 1877(3):188720. doi: 10.1016/j.bbcan.2022.188720
113. Xiao L-S, Hu C-Y, Cui H, Li R-N, Hong C, Li Q-M, et al. Splenomegaly in predicting the survival of patients with advanced primary liver cancer treated with immune checkpoint inhibitors. Cancer Med (2022) 11(24):4880–8. doi: 10.1002/cam4.4818
114. Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol (2013) 31(12):1539–47. doi: 10.1200/JCO.2012.45.2722
115. Shiroyama T, Nagatomo I, Koyama S, Hirata H, Nishida S, Miyake K, et al. Impact of sarcopenia in patients with advanced non-small cell lung cancer treated with PD-1 inhibitors: A preliminary retrospective study. Sci Rep (2019) 9(1):2447. doi: 10.1038/s41598-019-39120-6
116. Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol (2015) 63(1):131–40. doi: 10.1016/j.jhep.2015.02.031
117. Xiao L-S, Li R-N, Cui H, Hong C, Huang C-Y, Li Q-M, et al. Use of computed tomography-derived body composition to determine the prognosis of patients with primary liver cancer treated with immune checkpoint inhibitors: a retrospective cohort study. BMC Cancer (2022) 22(1):737. doi: 10.1186/s12885-022-09823-7
118. Matsumoto H, Tsuchiya K, Nakanishi H, Hayakawa Y, Yasui Y, Uchihara N, et al. Clinical usefulness of monitoring muscle volume during atezolizumab plus bevacizumab therapy in patients with unresectable hepatocellular carcinoma. Cancers (2022) 14(14):3551. doi: 10.3390/cancers14143551
119. Akce M, Liu Y, Zakka K, Martini DJ, Draper A, Alese OB, et al. Impact of sarcopenia, BMI, and inflammatory biomarkers on survival in advanced hepatocellular carcinoma treated with anti-PD-1 antibody. Am J Clin Oncol (2021) 44(2):74–81. doi: 10.1097/COC.0000000000000787
120. Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism (2019) 92:121–35. doi: 10.1016/j.metabol.2018.11.001
121. Ahmed M, von Itzstein MS, Sheffield T, Khan S, Fattah F, Park JY, et al. Association between body mass index, dosing strategy, and efficacy of immune checkpoint inhibitors. J Immunother Cancer (2021) 9(6):e002349. doi: 10.1136/jitc-2021-002349
122. Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, Sorich MJ. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non-small cell lung cancer. JAMA Oncol (2020) 6(4):512–8. doi: 10.1001/jamaoncol.2019.5241
123. Xu H, Cao D, He A, Ge W. The prognostic role of obesity is independent of sex in cancer patients treated with immune checkpoint inhibitors: A pooled analysis of 4090 cancer patients. Int Immunopharmacol (2019) 74:105745. doi: 10.1016/j.intimp.2019.105745
124. Vithayathil M, D'Alessio A, Fulgenzi CAM, Nishida N, Schönlein M, von Felden J, et al. Impact of body mass index in patients receiving atezolizumab plus bevacizumab for hepatocellular carcinoma. Hepatol Int (2023) 17(4):904–14. doi: 10.1007/s12072-023-10491-3
125. Elias R, Giobbie-Hurder A, McCleary NJ, Ott P, Hodi FS, Rahma O. Efficacy of PD-1 & PD-L1 inhibitors in older adults: a meta-analysis. J Immunother Cancer (2018) 6(1):26. doi: 10.1186/s40425-018-0336-8
126. Wong SK, Nebhan CA, Johnson DB. Impact of patient age on clinical efficacy and toxicity of checkpoint inhibitor therapy. Front Immunol (2021) 12:786046. doi: 10.3389/fimmu.2021.786046
127. Poropatich K, Fontanarosa J, Samant S, Sosman JA, Zhang B. Cancer immunotherapies: are they as effective in the elderly? Drugs Aging (2017) 34(8):567–81. doi: 10.1007/s40266-017-0479-1
128. Xiao L, Liao Y, Wang J, Li Q, Zhu H, Hong C, et al. Efficacy and safety of immune checkpoint inhibitors in elderly patients with primary liver cancer: a retrospective, multicenter, real-world cohort study. Cancer Immunol Immunother (2023) 72(7):2299–308. doi: 10.1007/s00262-023-03417-3
129. Chang PE, Ong WC, Lui HF, Tan CK. Is the prognosis of young patients with hepatocellular carcinoma poorer than the prognosis of older patients? A comparative analysis of clinical characteristics, prognostic features, and survival outcome. J Gastroenterol (2008) 43(11):881–8. doi: 10.1007/s00535-008-2238-x
130. Chang J, Ryan JS, Ajmera V, Ting S, Tamayo P, Burgoyne A. Responses to immunotherapy in hepatocellular carcinoma patients with nonalcoholic steatohepatitis cirrhosis. J Clin Oncol (2022) 40(4_suppl):389–. doi: 10.1200/JCO.2022.40.4_suppl.389
131. Pfister D, Núñez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature (2021) 592(7854):450–6. doi: 10.1038/s41586-021-03362-0
132. Ma C, Kesarwala AH, Eggert T, Medina-Echeverz J, Kleiner DE, Jin P, et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature (2016) 531(7593):253–7. doi: 10.1038/nature16969
133. Behary J, Amorim N, Jiang XT, Raposo A, Gong L, McGovern E, et al. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat Commun (2021) 12(1):187. doi: 10.1038/s41467-020-20422-7
134. Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evidence (2022) 1(8):EVIDoa2100070. doi: 10.1056/EVIDoa2100070
135. Haber PK, Puigvehí M, Castet F, Lourdusamy V, Montal R, Tabrizian P, et al. Evidence-based management of hepatocellular carcinoma: systematic review and meta-analysis of randomized controlled trials (2002-2020). Gastroenterology (2021) 161(3):879–98. doi: 10.1053/j.gastro.2021.06.008
136. Liu T, Li Q, Lin Z, Wang P, Chen Y, Fu Y, et al. Viral infections and the efficacy of PD-(L)1 inhibitors in virus-related cancers: Head and neck squamous cell carcinoma and hepatocellular carcinoma. Int Immunopharmacol (2021) 100:108128. doi: 10.1016/j.intimp.2021.108128
137. Ho WJ, Danilova L, Lim SJ, Verma R, Xavier S, Leatherman JM, et al. Viral status, immune microenvironment and immunological response to checkpoint inhibitors in hepatocellular carcinoma. J Immunother Cancer (2020) 8(1):e000394. doi: 10.1136/jitc-2019-000394
138. Pan S, Yu Y, Wang S, Tu B, Shen Y, Qiu Q, et al. Correlation of HBV DNA and hepatitis B surface antigen levels with tumor response, liver function and immunological indicators in liver cancer patients with HBV infection undergoing PD-1 inhibition combinational therapy. Front Immunol (2022) 13:892618. doi: 10.3389/fimmu.2022.892618
139. Cabibbo G, Celsa C, Calvaruso V, Petta S, Cacciola I, Cannavò MR, et al. Direct-acting antivirals after successful treatment of early hepatocellular carcinoma improve survival in HCV-cirrhotic patients. J Hepatol (2019) 71(2):265–73. doi: 10.1016/j.jhep.2019.03.027
140. Sapena V, Enea M, Torres F, Celsa C, Rios J, Rizzo GEM, et al. Hepatocellular carcinoma recurrence after direct-acting antiviral therapy: an individual patient data meta-analysis. Gut (2022) 71(3):593–604. doi: 10.1136/gutjnl-2020-323663
141. Choi WM, Yip TC, Wong GL, Kim WR, Yee LJ, Brooks-Rooney C, et al. Hepatocellular carcinoma risk in patients with chronic hepatitis B receiving tenofovir- vs. entecavir-based regimens: Individual patient data meta-analysis. J Hepatol (2023) 78(3):534–42. doi: 10.1016/j.jhep.2022.12.007
142. Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discovery (2022) 12(1):31–46. doi: 10.1158/2159-8290.CD-21-1059
143. Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science (2015) 350(6264):1084–9. doi: 10.1126/science.aac4255
144. Zheng Y, Wang T, Tu X, Huang Y, Zhang H, Tan D, et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J Immunother Cancer (2019) 7(1):193. doi: 10.1186/s40425-019-0650-9
145. Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science (2015) 350(6264):1079–84. doi: 10.1126/science.aad1329
146. Peng Z, Cheng S, Kou Y, Wang Z, Jin R, Hu H, et al. The gut microbiome is associated with clinical response to anti-PD-1/PD-L1 immunotherapy in gastrointestinal cancer. Cancer Immunol Res (2020) 8(10):1251–61. doi: 10.1158/2326-6066.CIR-19-1014
147. Lee P-C, Wu C-J, Hung Y-W, Lee CJ, Chi C-T, Lee IC, et al. Gut microbiota and metabolites associate with outcomes of immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. J For Immunother Cancer (2022) 10(6):e004779. doi: 10.1136/jitc-2022-004779
148. Ponziani FR, De Luca A, Picca A, Marzetti E, Petito V, Del Chierico F, et al. Gut dysbiosis and fecal calprotectin predict response to immune checkpoint inhibitors in patients with hepatocellular carcinoma. Hepatol Commun (2022) 6(6):1492–501. doi: 10.1002/hep4.1905
149. Shen YC, Lee PC, Kuo YL, Wu WK, Chen CC, Lei CH, et al. An exploratory study for the association of gut microbiome with efficacy of immune checkpoint inhibitor in patients with hepatocellular carcinoma. J Hepatocell Carcinoma (2021) 8:809–22. doi: 10.2147/JHC.S315696
150. Maesaka K, Sakamori R, Yamada R, Doi A, Tahata Y, Ohkawa K, et al. Pretreatment with antibiotics is associated with reduced therapeutic response to atezolizumab plus bevacizumab in patients with hepatocellular carcinoma. PloS One (2023) 18(2):e0281459. doi: 10.1371/journal.pone.0281459
151. Zhang L, Chen C, Chai D, Li C, Guan Y, Liu L, et al. The association between antibiotic use and outcomes of HCC patients treated with immune checkpoint inhibitors. Front Immunol (2022) 13:956533. doi: 10.3389/fimmu.2022.956533
152. Hatanaka T, Kakizaki S, Hiraoka A, Tada T, Hirooka M, Kariyama K, et al. Association of PPI and antibiotics with the clinical outcomes of HCC patients receiving atezolizumab and bevacizumab: A multicenter analysis. Hepatol Res (2023) 53(8):737–48. doi: 10.1111/hepr.13905
153. Huo R, Chen Y, Li J, Xu Q, Guo J, Xu H, et al. Altered gut microbiota composition and its potential association in patients with advanced hepatocellular carcinoma. Curr Oncol (2023) 30(2):1818–30. doi: 10.3390/curroncol30020141
154. Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer (2016) 54:139–48. doi: 10.1016/j.ejca.2015.11.016
155. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med (2018) 378(2):158–68. doi: 10.1056/NEJMra1703481
156. Esfahani K, Elkrief A, Calabrese C, Lapointe R, Hudson M, Routy B, et al. Moving towards personalized treatments of immune-related adverse events. Nat Rev Clin Oncol (2020) 17(8):504–15. doi: 10.1038/s41571-020-0352-8
157. Tanaka T, Takata K, Yokoyama K, Fukuda H, Yamauchi R, Fukunaga A, et al. Pretreatment modified albumin-bilirubin grade is an important predictive factor associated with the therapeutic response and the continuation of atezolizumab plus bevacizumab combination therapy for patients with unresectable hepatocellular carcinoma. Curr Oncol (2022) 29(7):4799–810. doi: 10.3390/curroncol29070381
158. Yu Y, Wang S, Su N, Pan S, Tu B, Zhao J, et al. Increased circulating levels of CRP and IL-6 and decreased frequencies of T and B lymphocyte subsets are associated with immune-related adverse events during combination therapy with PD-1 inhibitors for liver cancer. Front In Oncol (2022) 12:906824. doi: 10.3389/fonc.2022.906824
159. Xu S, Lai R, Zhao Q, Zhao P, Zhao R, Guo Z. Correlation between immune-related adverse events and prognosis in hepatocellular carcinoma patients treated with immune checkpoint inhibitors. Front Immunol (2021) 12:794099. doi: 10.3389/fimmu.2021.794099
160. Ng KYY, Tan SH, Tan JJE, Tay DSH, Lee AWX, Ang AJS, et al. Impact of immune-related adverse events on efficacy of immune checkpoint inhibitors in patients with advanced hepatocellular carcinoma. Liver Cancer (2022) 11(1):9–21. doi: 10.1159/000518619
161. Wang Y, Huang Y, Yang H, Mao Y. Letter regarding "Impact of immune-related adverse events on efficacy of immune checkpoint inhibitors in patients with advanced hepatocellular carcinoma". Liver Cancer (2023) 12(1):85–6. doi: 10.1159/000526804
162. Pallozzi M, Di Tommaso N, Maccauro V, Santopaolo F, Gasbarrini A, Ponziani FR, et al. Non-invasive biomarkers for immunotherapy in patients with hepatocellular carcinoma: current knowledge and future perspectives. Cancers (2022) 14(19):4631. doi: 10.3390/cancers14194631
163. Gao Q, Yang L, Lu M, Jin R, Ye H, Ma T. The artificial intelligence and machine learning in lung cancer immunotherapy. J Hematol Oncol (2023) 16(1):55. doi: 10.1186/s13045-023-01456-y
164. Hectors SJ, Lewis S, Besa C, King MJ, Said D, Putra J, et al. MRI radiomics features predict immuno-oncological characteristics of hepatocellular carcinoma. Eur Radiol (2020) 30(7):3759–69. doi: 10.1007/s00330-020-06675-2
165. Khalid N, Qayyum A, Bilal M, Al-Fuqaha A, Qadir J. Privacy-preserving artificial intelligence in healthcare: Techniques and applications. Comput Biol Med (2023) 158:106848. doi: 10.1016/j.compbiomed.2023.106848
166. Khan B, Fatima H, Qureshi A, Kumar S, Hanan A, Hussain J, et al. Drawbacks of artificial intelligence and their potential solutions in the healthcare sector. BioMed Mater Devices (2023) 1–8. doi: 10.1007/s44174-023-00063-2
Keywords: immunotherapy, hepatocellular carcinoma, biomarker, PD-L1, HCC, immune checkpoint inhibitor
Citation: Qin R, Jin T and Xu F (2023) Biomarkers predicting the efficacy of immune checkpoint inhibitors in hepatocellular carcinoma. Front. Immunol. 14:1326097. doi: 10.3389/fimmu.2023.1326097
Received: 22 October 2023; Accepted: 11 December 2023;
Published: 22 December 2023.
Edited by:
Yunfei Xu, Shandong University, ChinaReviewed by:
Xin Ye, Shandong Provincial Qianfoshan Hospital, ChinaZheng Zhong, Lyell Immunopharma, Inc., United States
Copyright © 2023 Qin, Jin and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianqiang Jin, anRxMTk5NTA4MjdAMTYzLmNvbQ==; Feng Xu, Znh1QGNtdS5lZHUuY24=
 Ran Qin
Ran Qin Tianqiang Jin
Tianqiang Jin Feng Xu
Feng Xu