
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 17 November 2023
Sec. Autoimmune and Autoinflammatory Disorders : Autoimmune Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1309835
This article is part of the Research Topic Immune system disorders: from molecular mechanisms to clinical implications View all 28 articles
Background: The neutrophil-to-lymphocyte ratio (NLR) is recognized as a biomarker for systemic inflammation and immune activation. However, its connection with the mortality risk in individuals with rheumatoid arthritis (RA) is not well understood. This study aimed to investigate the association between NLR and all-cause and cardiovascular mortality risk in U.S. adults with RA.
Methods: Data were gathered from the National Health and Nutrition Examination Survey (NHANES) cycles spanning 1999 to March 2020. We included adults aged ≥20 years. The NLR was computed by dividing the neutrophil count by the lymphocyte count from complete blood counts. The maximally selected rank statistics method helped identify the optimal NLR cutoff value associated with significant survival outcomes. Multivariable logistic regression models were performed to investigate the relationship between the NLR and the all-cause and cardiovascular mortality of RA. Restricted cubic spline (RCS) analyses were utilized to detect whether there were linear or non-linear relationships between NLR and mortality.
Results: In this study, 2002 adults with RA were included, with 339 having a higher NLR (≥3.28) and 1663 having a lower NLR (<3.28). During a median follow-up of 84 months, 79 RA individuals died. Participants with higher NLR had a 2-fold increased risk of all-cause (HR = 2.02, 95% CI: 1.53-2.66) and cardiovascular mortality (HR = 2.48, 95% CI: 1.34-4.57) versus lower NLR, after adjusting for demographics, socioeconomic status, and lifestyle factors. Kaplan-Meier analysis revealed that the survival rate for the higher NLR group was significantly lower than the lower NLR group, in terms of both all-cause and cardiovascular mortality (both P<0.0001). The RCS curve demonstrated a positive linear association between the NLR and all-cause and cardiovascular mortality.
Conclusion: A higher NLR was independently predictive of elevated long-term mortality risk in U.S. adults with RA. The NLR may serve as an inexpensive, widely available prognostic marker in RA.
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by systemic inflammation that can lead to joint damage and disability (1). RA is a global public health concern, with prevalence estimates of 17.6 million cases worldwide in 2020, reflecting a 14.1% increase since 1990. Projections indicate that the RA burden will continue to rise, with forecasts predicting that the number of individuals affected globally will reach 31.7 million by 2050 (2). Patients with RA have a reduced life expectancy, with cardiovascular disease being the leading cause of premature mortality (3, 4). Understanding the factors influencing RA incidence and mortality is crucial for effective management and intervention strategies. Persistent inflammation in RA plays a crucial role in its pathogenesis and progression and is also linked to the increased cardiovascular risk and mortality observed in RA (4, 5). Therefore, markers of inflammation may have prognostic utility in this population.
A perfect prognostic scoring system should offer prognostic parameters that are easily discernible during diagnosis and be cost-effective in clinical practice, ensuring accessibility and affordability for widespread use. The neutrophil-to-lymphocyte ratio (NLR) stands as a burgeoning biomarker, offering insights into systemic inflammation and immune system activation (6). It provides a comprehensive perspective on both innate (neutrophils) and adaptive (lymphocytes) immune responses, showcasing its potential as an essential indicator in understanding the body’s inflammatory and immune states (7). Additionally, the NLR can be easily calculated from routine complete blood counts, making it highly accessible for clinical use. Its association with unfavorable outcomes has been demonstrated across a spectrum of diseases (8, 9).
In RA, previous studies have touched upon the relationship between NLR and the presence of the disease (10, 11). Recently, there has been a surge in studies exploring the potential of these hematologically derived biomarkers to differentiate between RA patients with active disease and those in remission (12–14). However, these investigations were often limited by small sample sizes. Additionally, while these studies explored the connection between NLR and RA incidence or disease activity, there is a notable gap in the literature regarding the association of NLR with the survival status of RA patients.
In light of the necessity for affordable and accessible prognostic markers in RA, our objective was to investigate the association between NLR and the risk of all-cause and cardiovascular mortality. We conducted these analyses using a vast, nationally representative sample of U.S. adults with RA obtained from the National Health and Nutrition Examination Survey (NHANES) dataset.
NHANES is an ongoing program conducted by the Centers for Disease Control and Prevention (CDC) of America to assess the health and nutritional status of US adults and children. The survey combines interviews, physical examinations, and laboratory tests. Detailed methods can be extracted from the official website (http://www.cdc.gov/nchs/nhanes.htm, accessed on 1 October 2023).
The original survey protocol underwent rigorous ethical scrutiny and received approval from the Institutional Review Board of the CDC. Prior to their participation, all individuals involved in the study willingly provided informed consent by signing consent forms. The present study, which utilized data derived from the approved survey, was reviewed by the Institutional Review Board of our center. Following careful evaluation, the Board determined that the present study fell under the category of exemption, signifying that it met the necessary ethical standards and regulations and therefore did not require additional ethical approval.
This prospective cohort study utilized data from the continuous NHANES conducted between 1999 and March 2020. Exclusion criteria encompassed individuals younger than 20 years, those with incomplete data regarding complete blood count parameters, participants lacking information on RA or other forms of arthritis, and individuals with missing data for other essential covariates. The flowchart of participates inclusion and exclusion is depicted in Figure 1.
Participants were specifically asked “Has a doctor or other health professional ever told you that you had arthritis?” and they could respond with either “Yes” or “No.” To further differentiate between types of arthritis, individuals with a positive response were asked to specify the type of arthritis, with response options including “Rheumatoid arthritis,” “Osteoarthritis,” “Psoriatic arthritis,” “Other,” “Refused,” and “Do not know.”
The NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count from the same automated complete blood count sample. The optimal NLR cutoff point of 3.28 for mortality prediction was determined using the maximally selected rank statistics method (Figure 2).
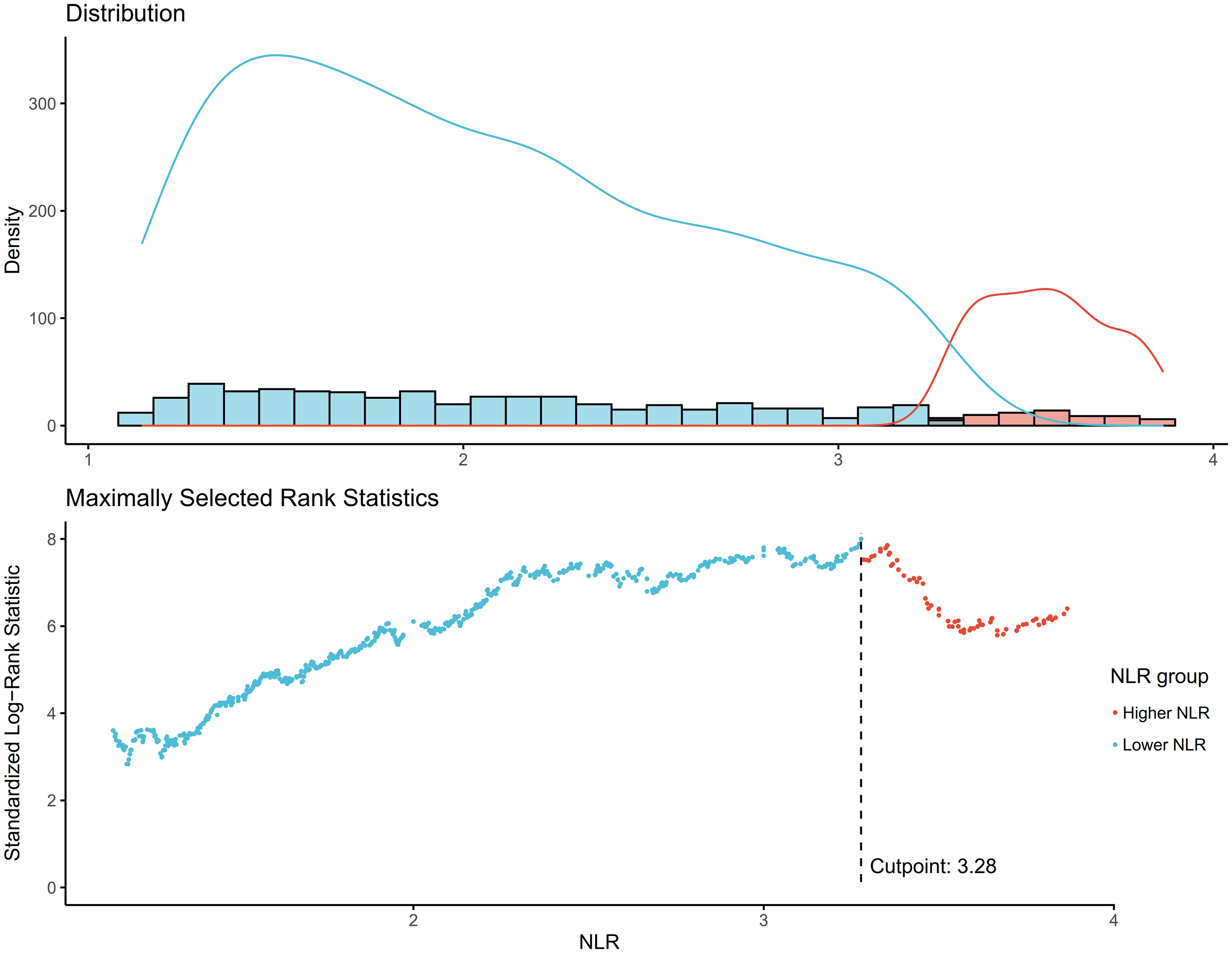
Figure 2 Determination of the NLR cutoff point using maximally selected rank statistics. Standardized Log-Rank Statistic was utilized in the calculation.
The primary outcome was all-cause mortality, ascertained using mortality-linked files (MLFs) documenting death from any cause. These mortality data were sourced from the National Death Index (NDI) database, accessible at https://www.cdc.gov/nchs/data-linkage/mortality-public.htm. Each participant’s follow-up duration was calculated from their date of participation until the date of death or until December 31, 2019, which marked the final update date of the NDI database.
Demographic covariates included age (continuous), sex (male/female), and race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, and other/mixed race). Socioeconomic status was assessed using educational attainment (less than 9th grade, 9-11th grade, high school graduate, some college, college graduate and above) and poverty-income ratio (<1.29, 1.30-3.49, ≥3.50). Lifestyle and health-related factors comprised body mass index (BMI) categorized as underweight (<18.49 kg/m2), normal (18.50-24.99 kg/m2), overweight (25.00-29.99 kg/m2), or obese (≥30.00 kg/m2); smoking status (current smoker vs nonsmoker); and health insurance status (insured vs uninsured). All multivariable models sequentially adjusted for demographic factors, socioeconomics, lifestyle factors, comorbidities, and medications where appropriate. Missing covariate data were addressed through listwise deletion.
Baseline characteristics were summarized using the mean (standard deviation) for continuous variables and the number (percentage) for categorical variables. Differences by the presence of RA or NLR groups were compared using chi-squared tests for categorical variables and t tests for continuous variables. Cox proportional hazards models were used to investigate the association between NLR and all-cause and cardiovascular mortality in individuals with RA. Three models were constructed with incremental adjustment for potential confounders. Model 1 was unadjusted. In model 2, age, sex, race/ethnicity, and educational attainment were additionally adjusted; Model 3 was additionally adjusted by age, sex, race/ethnicity, educational attainment, BMI, poverty income ratio, smoking status, and health insurance. Kaplan-Meier curves were created to illustrate the survival probabilities based on different NLR categories. Subgroup analyses were performed to assess effect modification by pertinent clinical confounders. Restricted cubic spline (RCS) model was constructed to visualize the relationship between continuous NLR and outcomes. Analyses were performed using R version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria). Two-sided P<0.05 was considered statistically significant.
Of 2002 participants with RA, 339 had higher NLR (≥3.28) while 1663 had lower NLR (<3.28) (Table 1). Participants in the higher NLR group were older than those in the lower NLR group (mean age 62.19 vs 55.48 years, P<0.001). The proportion of individuals aged 60-79 years was also higher in the higher NLR group compared to the lower NLR group (47.13% % vs 33.73%, P<0.001). The higher NLR group had a significantly greater proportion of non-Hispanic Whites compared to the lower NLR group (75.66% vs 67.57%, P<0.001). There were no significant differences between the higher and lower NLR groups in terms of educational attainment, poverty income ratio, BMI, waist circumference, smoking status, and health insurance (all P>0.05). The mean NLR was significantly higher in the higher NLR group compared to the lower NLR group (4.66 vs 1.91, P<0.001). These results indicate that NLR levels are associated with age and race/ethnicity, but not with socioeconomic factors, lifestyle factors, or other health-related variables. Participants with higher NLR were older and more likely to be non-Hispanic White.
Over a median follow-up of 84 months, 79 RA individuals died. The higher NLR group had 24 observed deaths versus only 11.7 expected deaths. In contrast, the lower NLR group had fewer observed deaths (55) than expected (67.3). In the unadjusted model (Model 1), higher NLR as a continuous variable was significantly associated with increased risk of all-cause mortality (HR per 1 unit increase 1.24, 95% CI: 1.17-1.31, P<0.001) and cardiovascular mortality (HR = 1.29, 95% CI: 1.18-1.40, P<0.001). When NLR was evaluated as a categorical variable, participants in the higher NLR group had a 2-fold higher risk of all-cause mortality (HR = 2.11, 95% CI: 1.59-2.81, P<0.001) and a 3-fold higher risk of cardiovascular mortality (HR = 3.23, 95% CI: 1.82-5.72, P<0.001) compared to the lower NLR group. Model 2 was adjusted for demographic factors including age, sex, race/ethnicity, and education. The associations remained statistically significant. Model 3 was further adjusted for BMI, poverty income ratio, smoking, and insurance in addition to demographics. The positive associations between higher NLR and elevated mortality risks persisted. As a continuous variable, each 1-unit NLR increase was linked to 20% and 22% higher all-cause and cardiovascular mortality. Categorically, the higher NLR group had 2.02 and 2.48 times the risk of all-cause and cardiovascular mortality versus lower NLR participants (Table 2). Kaplan-Meier analysis revealed that the survival rate for the higher NLR group was significantly lower than the lower NLR group, in terms of both all-cause and cardiovascular mortality (both P<0.0001) (Figure 3). These findings suggest that higher NLR, whether evaluated as a continuous or categorical variable, was consistently and independently associated with increased all-cause and cardiovascular mortality risk in US adults with RA.
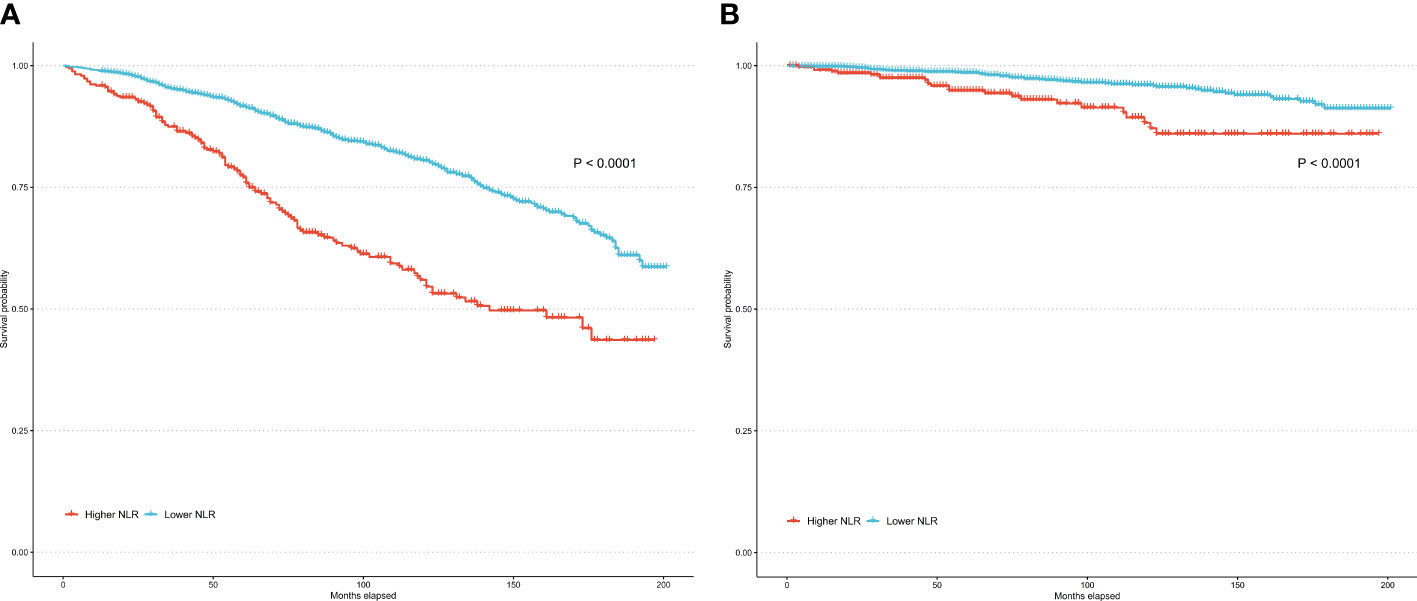
Figure 3 Kaplan–Meier curves of all-cause mortality (A) and cardiovascular mortality (B) among participates with RA.
In subgroup analyses stratified by sex, age, race/ethnicity, education, BMI, and smoking status, higher NLR was consistently associated with increased all-cause and cardiovascular mortality risk across all subgroups (Table 3). For all-cause mortality, no significant interaction was observed by sex (P=0.659), age (P=0.536), race/ethnicity (P=0.863), education (P=0.735), BMI (P=0.873), or smoking status (P=0.277). For cardiovascular mortality, a significant interaction was found by race/ethnicity (P=0.014). No other significant interactions were detected. These results indicate that the relationship between higher NLR and increased mortality risk persists across subgroups of potential confounders. The racial/ethnic difference in the association between NLR and cardiovascular mortality warrants further investigation.
Figure 4A displays the RCS curve for the association between continuous NLR and all-cause mortality risk in participates with RA. The overall association was statistically significant (P overall <0.0001). There was evidence of a nonlinear relationship, with the curve showing an initial steep increase in mortality risk at lower NLR values which plateaued at higher values (P non-linear = 0.0231). Figure 4B shows the RCS curve for the relationship between continuous NLR and cardiovascular mortality risk in participates with RA. The overall association was statistically significant (P overall = 0.0236). The curve depicts a linear relationship between increasing NLR and cardiovascular mortality without statistical evidence of nonlinearity (P non-linear = 0.5919). These findings highlight the differential relationships between this inflammatory biomarker and various mortality outcomes in individuals with RA.
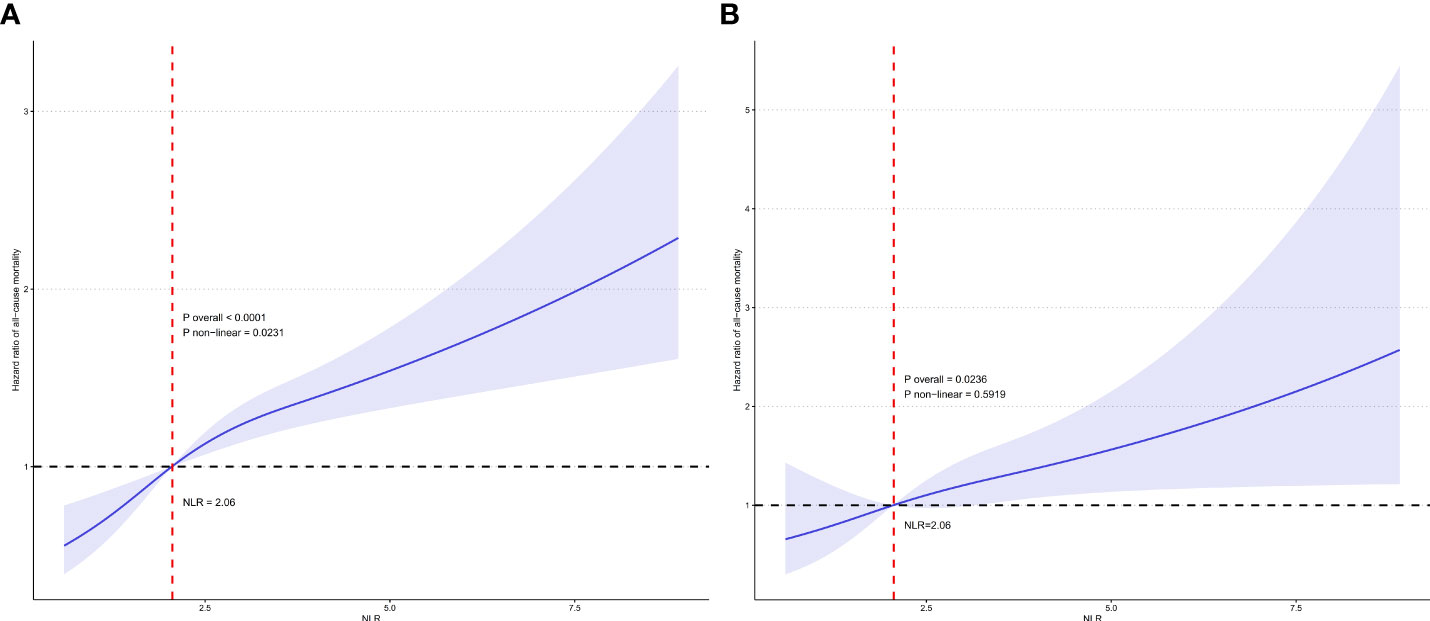
Figure 4 Restricted cubic spline (RCS) illustrating the relationship between NLR and all-cause mortality (A) and cardiovascular mortality (B) among participates with RA.
In this large nationally representative cohort of US adults, we found that a higher NLR was independently predictive of increased all-cause and cardiovascular mortality risk among adults with RA. Kaplan-Meier curves illustrated significantly lower survival probabilities for those with higher NLR. RCS analyses demonstrated linear positive associations between NLR and all-cause and cardiovascular mortality. These data highlight the potential utility of the NLR as an inexpensive and widely available marker for RA risk stratification and prognostication in clinical practice.
A number of prior studies have examined the association between NLR and RA with inconsistent findings. Several studies found significantly higher NLR values in RA patients than in healthy controls, suggesting that the NLR may be a useful biomarker for RA diagnosis (15–18). Other studies did not observe significant differences in NLR between RA and controls, implying limited diagnostic utility (18, 19). Regarding the relationship between the NLR and RA disease activity, most studies reported positive correlations, with a higher NLR associated with more active disease and an even lower response rate after TNF-α therapy or an increased risk of anti-TNF-α agent withdrawal due to a lack of efficacy (12, 14, 20–24). This indicates that the NLR could serve as an inflammatory marker reflecting RA disease activity. Compared to prior studies with small sample sizes, our study leveraged a large nationally representative cohort to provide robust evidence for the utility of the NLR as a biomarker for RA risk stratification and prognostication.
Prior studies have reported associations between higher NLR and increased mortality risk across the general population and various conditions marked by significant inflammatory components (25, 26). In glomerulonephritis, a study established a connection between NLR and mortality prediction in patients with rapidly progressive glomerulonephritis (RPGN). It was found to be associated with systemic inflammation and exhibited a negative correlation with the percentage of fibro-cellular crescents (27). In asthma, a previous study concluded that the NLR and other complete blood cell count-derived inflammatory biomarkers are associated with a higher risk of all-cause and respiratory disease mortality in adult patients (28). In colon cancer, a study found that the NLR was an independent predictor of overall mortality, with the authors concluding that the NLR may serve as a useful prognostic marker (29). Similarly, in acute coronary syndrome (ACS), a meta-analysis of over 9,000 patients demonstrated that an elevated pretreatment NLR predicted higher medium- to long-term mortality and major adverse cardiac events (MACEs) (30, 31). More recently, in COVID-19, multiple studies have shown that the NLR correlates with disease severity and mortality (32, 33). A meta-analysis concluded that evaluating the NLR could help clinicians identify severe COVID-19 cases early and initiate timely management to potentially reduce mortality (26). However, the available evidence regarding the prognostic significance of NLR for survival in RA remains limited. Our study effectively addresses this gap, providing new evidence that underscores the significance of the NLR as an independent predictor of all-cause mortality in RA.
Moreover, determining specific NLR cutoffs tailored to individual patient populations could improve the predictive accuracy of this marker in clinical settings. While a consensus on normal NLR values is lacking, there is a suggested range of 1–2 for normal values, 2–3 as a gray area indicating subclinical inflammation, and values above 3 indicating inflammation (34). Our study aligns with these recommendations, establishing an optimal NLR cutoff of 3.28, which correlated with the most significant survival outcomes. However, as noted in other conditions, the NLR alone without other inflammatory markers may have limited utility. Future research should examine combining NLR with other biomarkers to improve prognostic accuracy in RA.
The intricate pathological processes underlying autoimmune diseases have been meticulously explored, elucidating the interplay between innate and adaptive immunity. The NLR, comprising neutrophil and lymphocyte counts, provides valuable insights into the body’s inflammatory status and immune response. A higher neutrophil count suggests an ongoing nonspecific inflammatory process, while a lower lymphocyte count indicates a relatively compromised immune system (35). Several lines of evidence suggest that dysregulated innate immunity and inflammation play key roles in RA pathogenesis (36, 37). Neutrophils are among the first responders during inflammation and immune activation in RA (37). Additionally, neutrophils may function as antigen-presenting cells (APCs) in presenting antigens and activating T cells to perpetuate chronic inflammation and autoimmunity (38). They also produce proteases such as elastase that contribute to cartilage and bone destruction in RA (39). Therefore, an elevated NLR likely implies higher activation of neutrophils and innate inflammatory pathways contributing to RA development and progression. The increased systemic inflammation marked by a high NLR could also accelerate atherosclerosis, conferring greater cardiovascular risk and mortality (40). Conversely, lymphocytes, such as regulatory T cells, normally act to suppress inflammatory responses and restore immune homeostasis (41). A lower lymphocyte count signified by a higher NLR may indicate impaired anti-inflammatory immunity. In summary, an elevated NLR represents enhanced proinflammatory innate responses along with potential deficiencies in anti-inflammatory lymphocyte activity. This imbalance could promote the inflammatory and immunopathogenic mechanisms underlying RA onset and severity. The NLR provides a simple readout of the skewing between pathological innate immunity and protective adaptive responses in RA.
Several limitations should be noted. First, the observational design of this study limit causal inferential ability. We identified associations between NLR and outcomes but cannot confirm a definitive causal relationship. Second, residual confounding is possible despite adjustments for an extensive set of potential confounders. Unmeasured factors such as comorbidities, smoking intensity, medications, and RA characteristics could influence the observed relationships. Third, the NLR was measured at a single timepoint and may not reflect variability over time or with interventions. Serial NLR measurements could provide a more accurate representation of inflammatory status. Finally, the study population comprised U.S. adults, which may restrict generalizability to other populations. Further studies in diverse cohorts are warranted to validate our findings. However, the strengths of a large nationally representative sample, long follow-up, and robust adjustment for confounders enhance the reliability of the results.
In summary, the NLR provides a simple readout of the balance between pathological innate inflammation and protective adaptive immunity. Our study of a large nationally representative cohort demonstrates that a higher NLR is independently predictive of increased risks of both all-cause and cardiovascular mortality among adults with RA. These findings highlight the potential clinical utility of the NLR as an inexpensive and widely available biomarker that can be integrated into routine care to improve risk stratification and prognostication in RA patients.
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
The original survey protocol underwent rigorous ethical scrutiny and received approval from the Institutional Review Board of CDC. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
JW: Conceptualization, Writing – review & editing. EZ: Data curation, Writing – original draft. XZ: Methodology, Writing – review & editing. YY: Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Babaahmadi M, Tayebi B, Gholipour NM, Kamardi MT, Heidari S, Baharvand H, et al. Rheumatoid arthritis: the old issue, the new therapeutic approach. Stem Cell Res Ther (2023) 14(1):268. doi: 10.1186/s13287-023-03473-7
2. Global, Regional, and National Burden of Rheumatoid Arthritis. 1990-2020, and projections to 2050: A systematic analysis of the global burden of disease study 2021. Lancet Rheumatol (2023) 5(10):e594–610. doi: 10.1016/s2665-9913(23)00211-4
3. Cai W, Tang X, Pang M. Prevalence of metabolic syndrome in patients with rheumatoid arthritis: an updated systematic review and meta-analysis. Front Med (Lausanne) (2022) 9:855141. doi: 10.3389/fmed.2022.855141
4. Schattner A. The cardiovascular burden of rheumatoid arthritis - implications for treatment. Am J Med (2023) 22:S0002-9343(23)00576-4. doi: 10.1016/j.amjmed.2023.09.004
5. Liu B, Wang J, Li YY, Li KP, Zhang Q. The association between systemic immune-inflammation index and rheumatoid arthritis: evidence from Nhanes 1999-2018. Arthritis Res Ther (2023) 25(1):34. doi: 10.1186/s13075-023-03018-6
6. Shahrabi S, Saki N, Safa M, Pezeshki SMS. Complete blood count test in rheumatology: not just a screening test. Clin Lab (2023) 69(6). doi: 10.7754/Clin.Lab.2022.221012
7. García-Escobar A, Vera-Vera S, Tébar-Márquez D, Rivero-Santana B, Jurado-Román A, Jiménez-Valero S, et al. Neutrophil-to-lymphocyte ratio an inflammatory biomarker, and prognostic marker in heart failure, cardiovascular disease and chronic inflammatory diseases: new insights for a potential predictor of anti-cytokine therapy responsiveness. Microvasc Res (2023) 150:104598. doi: 10.1016/j.mvr.2023.104598
8. Li J, Zhang K, Zhang Y, Gu Z, Huang C. Neutrophils in Covid-19: recent insights and advances. Virol J (2023) 20(1):169. doi: 10.1186/s12985-023-02116-w
9. Uribe-Querol E, Rosales C. Neutrophils actively contribute to obesity-associated inflammation and pathological complications. Cells (2022) 11(12). doi: 10.3390/cells11121883
10. Song BW, Kim AR, Moon DH, Kim YK, Kim GT, Ahn EY, et al. Associations of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and monocyte-to-lymphocyte ratio with osteoporosis and incident vertebral fracture in postmenopausal women with rheumatoid arthritis: A single-center retrospective cohort study. Medicina (Kaunas) (2022) 58(7). doi: 10.3390/medicina58070852
11. Song BW, Kim AR, Kim YK, Kim GT, Ahn EY, So MW, et al. Diagnostic value of neutrophil-to-lymphocyte, platelet-to-lymphocyte, and monocyte-to-lymphocyte ratios for the assessment of rheumatoid arthritis in patients with undifferentiated inflammatory arthritis. Diagnost (Basel) (2022) 12(7). doi: 10.3390/diagnostics12071702
12. Liu X, Li J, Sun L, Wang T, Liang W. The association between neutrophil-to-lymphocyte ratio and disease activity in rheumatoid arthritis. Inflammopharmacology (2023) 31(5):2237–44. doi: 10.1007/s10787-023-01273-2
13. Choe JY, Lee CU, Kim SK. Association between novel hematological indices and measures of disease activity in patients with rheumatoid arthritis. Medicina (Kaunas) (2023) 59(1). doi: 10.3390/medicina59010117
14. Zinellu A, Mangoni AA. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio and disease activity in rheumatoid arthritis: A systematic review and meta-analysis. Eur J Clin Invest (2023) 53(2):e13877. doi: 10.1111/eci.13877
15. Erre GL, Paliogiannis P, Castagna F, Mangoni AA, Carru C, Passiu G, et al. Meta-analysis of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio in rheumatoid arthritis. Eur J Clin Invest (2019) 49(1):e13037. doi: 10.1111/eci.13037
16. Jin Z, Cai G, Zhang P, Li X, Yao S, Zhuang L, et al. The value of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as complementary diagnostic tools in the diagnosis of rheumatoid arthritis: A multicenter retrospective study. J Clin Lab Anal (2021) 35(1):e23569. doi: 10.1002/jcla.23569
17. Zhang Y, Yin Y, Kuai S, Shan Z, Pei H, Wang J. Combination of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as diagnostic biomarker for rheumatoid arthritis. Int J Clin Exp Med (2016) 9(11):22076–81.
18. Peng Y-F, Cao L, Zeng Y-H, Zhang Z-X, Chen D, Zhang Q, et al. Platelet to lymphocyte ratio and neutrophil to lymphocyte ratio in patients with rheumatoid arthritis. Open Med (2015) 10(1). doi: 10.1515/med-2015-0037
19. Chen Q, Chen DY, Xu XZ, Liu YY, Yin TT, Li D. Platelet/lymphocyte, lymphocyte/monocyte, and neutrophil/lymphocyte ratios as biomarkers in patients with rheumatoid arthritis and rheumatoid arthritis-associated interstitial lung disease. Med Sci Monit (2019) 25:6474–81. doi: 10.12659/msm.916583
20. Wang Z, Kong L, Zhang H, Sun F, Guo Z, Zhang R, et al. Tumor necrosis factor alpha -308g/a gene polymorphisms combined with neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio predicts the efficacy and safety of anti-Tnf-A Therapy in patients with ankylosing spondylitis, rheumatoid arthritis, and psoriasis arthritis. Front Pharmacol (2021) 12:811719. doi: 10.3389/fphar.2021.811719
21. Targońska-Stępniak B, Grzechnik K, Kolarz K, Gągoł D, Majdan M. Systemic inflammatory parameters in patients with elderly-onset rheumatoid arthritis (Eora) and young-onset rheumatoid arthritis (Yora)-an observational study. J Clin Med (2021) 10(6). doi: 10.3390/jcm10061204
22. Targońska-Stępniak B, Zwolak R, Piotrowski M, Grzechnik K, Majdan M. The relationship between hematological markers of systemic inflammation (Neutrophil-to-lymphocyte, platelet-to-lymphocyte, lymphocyte-to-monocyte ratios) and ultrasound disease activity parameters in patients with rheumatoid arthritis. J Clin Med (2020) 9(9). doi: 10.3390/jcm9092760
23. Lee HN, Kim YK, Kim GT, Ahn E, So MW, Sohn DH, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio as predictors of 12-week treatment response and drug persistence of anti-tumor necrosis factor-A Agents in patients with rheumatoid arthritis: A retrospective chart review analysis. Rheumatol Int (2019) 39(5):859–68. doi: 10.1007/s00296-019-04276-x
24. Ghang B, Kwon O, Hong S, Lee CK, Yoo B, Kim YG. Neutrophil-to-lymphocyte ratio is a reliable marker of treatment response in rheumatoid arthritis patients during tocilizumab therapy. Mod Rheumatol (2017) 27(3):405–10. doi: 10.1080/14397595.2016.1214340
25. Song M, Graubard BI, Rabkin CS, Engels EA. Neutrophil-to-lymphocyte ratio and mortality in the United States general population. Sci Rep (2021) 11(1):464. doi: 10.1038/s41598-020-79431-7
26. Li X, Liu C, Mao Z, Xiao M, Wang L, Qi S, et al. Predictive values of neutrophil-to-lymphocyte ratio on disease severity and mortality in Covid-19 patients: A systematic review and meta-analysis. Crit Care (2020) 24(1):647. doi: 10.1186/s13054-020-03374-8
27. Toraman A, Neşe N, Özyurt BC, Kürşat S. Association between neutrophil-lymphocyte & Platelet lymphocyte ratios with prognosis & Mortality in rapidly progressive glomerulonephritis. Indian J Med Res (2019) 150(4):399–406. doi: 10.4103/ijmr.IJMR_1234_17
28. Ke J, Qiu F, Fan W, Wei S. Associations of complete blood cell count-derived inflammatory biomarkers with asthma and mortality in adults: A population-based study. Front Immunol (2023) 14:1205687. doi: 10.3389/fimmu.2023.1205687
29. Demirkol S, Balta S, Celik T, Arslan Z, Unlu M, Cakar M, et al. Assessment of the relationship between red cell distribution width and cardiac syndrome X. Kardiol Pol (2013) 71(5):480–4. doi: 10.5603/kp.2013.0094
30. Dong CH, Wang ZM, Chen SY. Neutrophil to lymphocyte ratio predict mortality and major adverse cardiac events in acute coronary syndrome: A systematic review and meta-analysis. Clin Biochem (2018) 52:131–6. doi: 10.1016/j.clinbiochem.2017.11.008
31. Tudurachi BS, Anghel L, Tudurachi A, Sascău RA, Stătescu C. Assessment of inflammatory hematological ratios (Nlr, Plr, Mlr, Lmr and monocyte/Hdl-cholesterol ratio) in acute myocardial infarction and particularities in young patients. Int J Mol Sci (2023) 24(18). doi: 10.3390/ijms241814378
32. Ben Jemaa A, Salhi N, Ben Othmen M, Ben Ali H, Guissouma J, Ghadhoune H, et al. Evaluation of individual and combined Nlr, Lmr and Clr ratio for prognosis disease severity and outcomes in patients with Covid-19. Int Immunopharmacol (2022) 109:108781. doi: 10.1016/j.intimp.2022.108781
33. Wang S, Fu L, Huang K, Han J, Zhang R, Fu Z. Neutrophil-to-lymphocyte ratio on admission is an independent risk factor for the severity and mortality in patients with coronavirus disease 2019. J Infect (2021) 82(2):e16–e8. doi: 10.1016/j.jinf.2020.09.022
34. Kourilovitch M, Galarza–Maldonado C. Could a simple biomarker as neutrophil-to-lymphocyte ratio reflect complex processes orchestrated by neutrophils? J Trans Autoimmun (2023) 6:100159. doi: 10.1016/j.jtauto.2022.100159
35. Zahorec R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl Lek Listy (2021) 122(7):474–88. doi: 10.4149/BLL_2021_078
36. Edilova MI, Akram A, Abdul-Sater AA. Innate immunity drives pathogenesis of rheumatoid arthritis. BioMed J (2021) 44(2):172–82. doi: 10.1016/j.bj.2020.06.010
37. Zhang L, Yuan Y, Xu Q, Jiang Z, Chu CQ. Contribution of neutrophils in the pathogenesis of rheumatoid arthritis. J BioMed Res (2019) 34(2):86–93. doi: 10.7555/jbr.33.20190075
38. Li Y, Wang W, Yang F, Xu Y, Feng C, Zhao Y. The regulatory roles of neutrophils in adaptive immunity. Cell Commun Signaling (2019) 17(1):147. doi: 10.1186/s12964-019-0471-y
39. Carmona-Rivera C, Carlucci PM, Goel RR, James E, Brooks SR, Rims C, et al. Neutrophil extracellular traps mediate articular cartilage damage and enhance cartilage component immunogenicity in rheumatoid arthritis. JCI Insight (2020) 5(13). doi: 10.1172/jci.insight.139388
40. González-Sierra M, Quevedo-Rodríguez A, Romo-Cordero A, González-Chretien G, Quevedo-Abeledo JC, de Vera-González A, et al. Relationship of blood inflammatory composite markers with cardiovascular risk factors and subclinical atherosclerosis in patients with rheumatoid arthritis. Life (Basel) (2023) 13(7). doi: 10.3390/life13071469
Keywords: neutrophil, lymphocyte, inflammation, mortality, rheumatoid arthritis
Citation: Zhou E, Wu J, Zhou X and Yin Y (2023) The neutrophil-lymphocyte ratio predicts all-cause and cardiovascular mortality among U.S. adults with rheumatoid arthritis: results from NHANES 1999-2020. Front. Immunol. 14:1309835. doi: 10.3389/fimmu.2023.1309835
Received: 08 October 2023; Accepted: 07 November 2023;
Published: 17 November 2023.
Edited by:
Ruben Dario Motrich, National Scientific and Technical Research Council (CONICET), ArgentinaReviewed by:
Takemichi Fukasawa, The University of Tokyo Graduate School of Medicine, JapanCopyright © 2023 Zhou, Wu, Zhou and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yufeng Yin, eWlueXVmZW5nQDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.