- Department of Dermatology, Huashan Hospital, Fudan University, Shanghai, China
Pruritus is the most common symptom of dermatological disorders, and prurigo nodularis (PN) is notorious for intractable and severe itching. Conventional treatments often yield disappointing outcomes, significantly affecting patients’ quality of life and psychological well-being. The pathogenesis of PN is associated with a self-sustained “itch-scratch” vicious cycle. Recent investigations of PN-related itch have partially revealed the intricate interactions within the cutaneous neuroimmune network; however, the underlying mechanism remains undetermined. Itch mediators play a key role in pruritus amplification in PN and understanding their action mechanism will undoubtedly lead to the development of novel targeted antipruritic agents. In this review, we describe a series of pruritogens and receptors involved in mediating itching in PN, including cytokines, neuropeptides, extracellular matrix proteins, vasculogenic substances, ion channels, and intracellular signaling pathways. Moreover, we provide a prospective outlook on potential therapies based on existing findings.
1 Introduction
Prurigo nodularis (PN) is a relatively uncommon dermatosis characterized by recalcitrant chronic pruritus and keratotic nodules (1). It predominantly affects middle-aged and older individuals and Africans (2). Multiple discrete nodules are often symmetrically distributed in scratchable areas, such as the extensor surfaces of the limbs and trunk, with fewer lesions on the harder-to-reach mid-upper back, creating the classical butterfly sign (3). The pruritus associated with PN is notably intense. A previous study has shown that PN exerts a more significant impact on quality of life and carries a higher risk of psychological disorders (e.g., anxiety and depression) than other pruritic dermatoses (4). Notably, approximately half of the patients with PN exhibit coexistent atopic dermatitis (AD) or atopic predisposition (2, 5). This finding implies a potential overlap between the pathogeneses of PN and AD. Although AD and PN are both type 2 inflammatory diseases, recent transcriptomic studies have clearly revealed that PN is separated from AD. PN does not harbor the strong type 2 response pattern that is typically found in AD but is rather characterized by stromal remodeling and neurovascular dysregulation (6–11). Indeed, traditional treatments for AD, including topical steroids and antihistamines, exhibit limited efficacy against PN (1, 12). This underscores the likelihood of PN harboring distinct and yet to be elucidated pathophysiological underpinnings.
However, its exact pathogenesis remains unknown. Current understanding suggests that a persistent “itch-scratch” vicious cycle, leading to recurrent skin excoriation, crusting, and thickening, is the primary driver of nodule formation (3). Skin neuroimmune interactions and neuronal sensitization play important roles in mediating chronic itch in PN (1). Histopathologically, lesional PN skin exhibits epidermal hyperkeratosis accompanied by reduced epidermal nerve fibers (13). Fibrosis, vascular remodeling, and proliferation of afferent nerves are accompanied by mixed inflammatory cell infiltration in the dermis, including T lymphocytes, dendritic cells, mast cells, eosinophils, basophils, and macrophages (14). Notably, mast cells and eosinophils often aggregate around the peripheral sensory nerve endings, establishing close contact with them (15–17).
At the molecular level, the interactions between immune cells and sensory neurons require pruritogens and their receptors to act as intermediaries (18, 19). Pruritogens not only transmit itch by directly stimulating skin sensory neurons but also activate immune cells to release other itch mediators and indirectly stimulate sensory neurons to induce itch (20). Simultaneously, activated sensory neurons secrete pruritogens such as neuropeptides. On one hand, these neuropeptides act on the neurons themselves to increase their own sensitivity and spontaneous activity; on the other hand, they reciprocally activate immune cells and sustain and amplify inflammatory responses, thus promoting chronic itch (21). Ultimately, cutaneous C fibers project itch impulses centrally through pseudo-unipolar dorsal root ganglia (DRG) neurons to the dorsal horn of the spinal cord, which then send projection fibers to the brain (22).
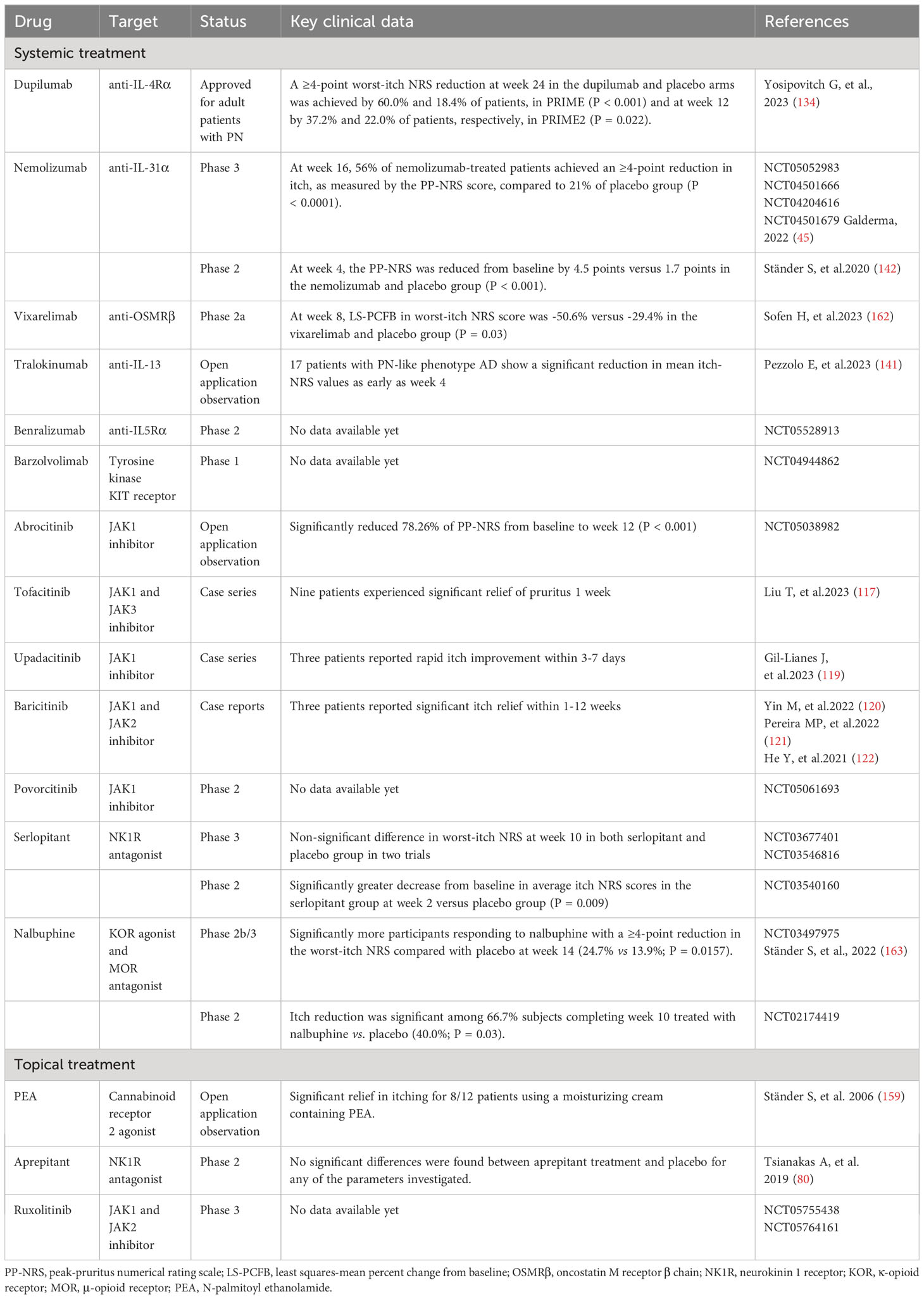
Several upregulated pruritogens have been detected within PN lesions (1), including cytokines, neuropeptides, extracellular matrix proteins, and vasculogenic mediators (Table 1). Targeting these pruritogens and their receptors is of interest for the development of emerging therapeutics for PN (Figure 1). This review aimed to provide a comprehensive summary of the roles and clinical significance of known pruritogens and receptors in the pathogenesis of pruritus in PN.
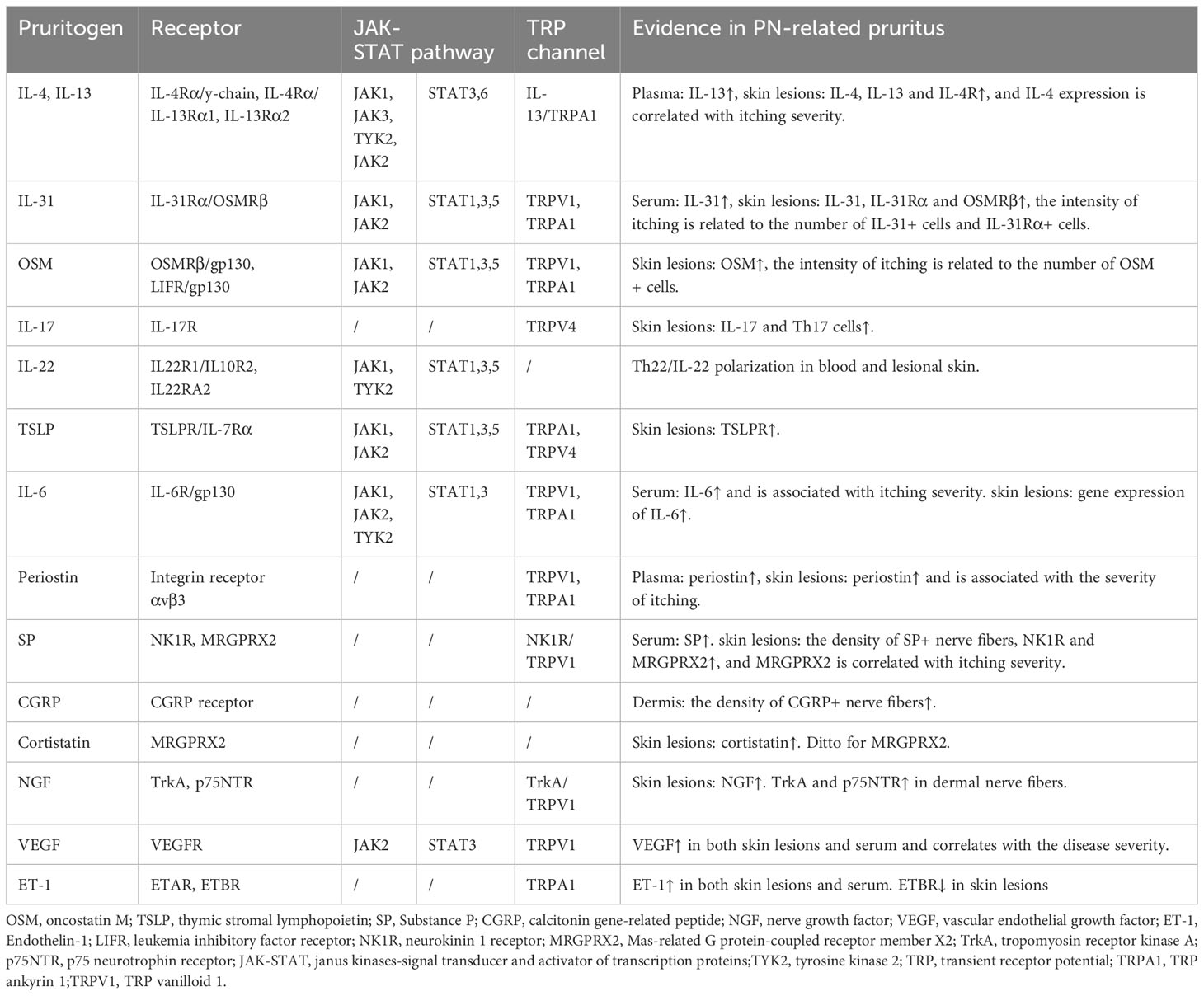
Table 1 Non-histaminergic itch mediators and their cognate receptors and channels in prurigo nodularis.
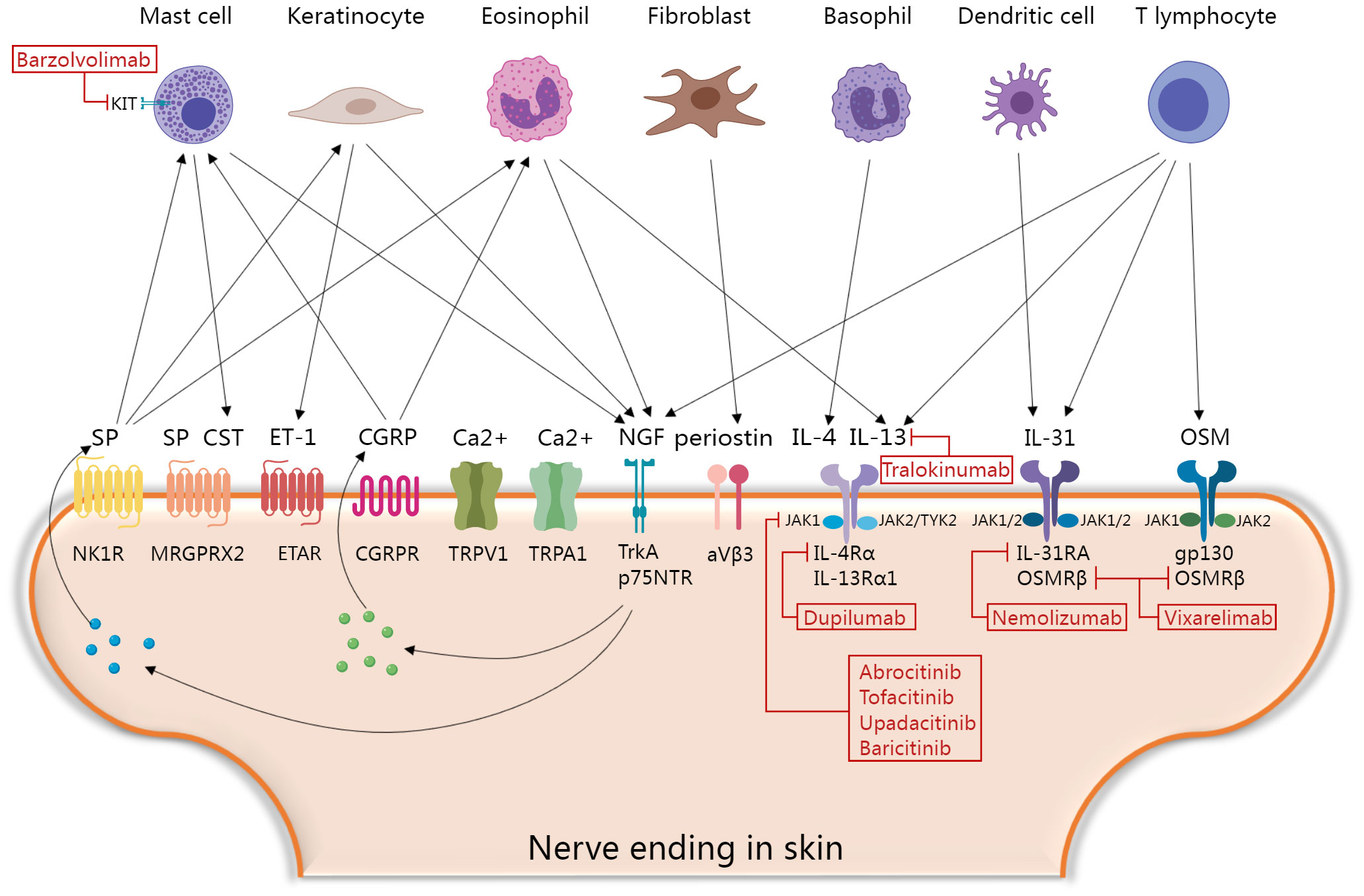
Figure 1 Molecular mechanisms of itch and current and promising therapeutic targets in prurigo nodularis. Skin immune cells, keratinocytes and fibroblasts secrete various histamine-independent itching mediators that directly or indirectly activate corresponding receptors and channels in skin nerve endings, and then promote the secretion of neuropeptides to form positive feedback loops. Finally, the skin-neuroimmune crosstalk mediating the “itch-scratch” vicious cycle of PN. Blocking this process is a key point of novel therapeutic interventions. CGRP, calcitonin gene-related peptide; CST, Cortistatin; ET-1, Endothelin-1; ETAR, the endothelin A receptors; IL, interleukin; JAK, janus kinases-signal transducer; MRGPRX2, Mas-related G protein-coupled receptor member X2; NGF, nerve growth factor; NK1R, neurokinin 1 receptor; OSM, oncostatin M; p75NTR, p75 neurotrophin receptor; SP, Substance P; TrkA, tropomyosin receptor kinase A; TYK2, tyrosine kinase 2; TRP, transient receptor potential; TRPA1, TRP ankyrin 1; TRPV1, TRP vanilloid. The figure was created with MedPeer (medpeer.cn).
2 Pruritogens
2.1 Cytokines
2.1.1 Interleukin-4 and IL-13
Although less pronounced than that in AD, T helper (Th)2 immune bias remains a significant characteristic of PN (23). IL-4 and IL-13 are the key cytokines that initiate and sustaining Th2 responses (24). Elevated levels of circulating plasma IL-13 were observed in PN (25). The expression of IL-4, IL-13, and IL-4 receptor (IL-4R) is upregulated in prurigo nodules, with IL-4 correlating with the intensity of itch (8, 11, 26, 27). IL-4 is primarily expressed in T lymphocytes and basophils, whereas IL-13 is primarily expressed in eosinophils (26). Studies have shown that IL-4 and IL-13 activate itch-sensitive neurons in both humans and mice through IL-4Rα (28). They also amplify neuronal responses to various pruritogenic stimuli, including histamine-dependent and histamine-independent pathways, thereby contributing to neuronal sensitization (29). In addition, IL-4 and IL-13 exert pruritogenic effects by recruiting eosinophils and mast cells, driving IgE synthesis, and interacting with IL-31 (30). They promote fibroblast proliferation, differentiation, and synthesis of extracellular matrix proteins such as periostin in the dermis, mediating PN-associated skin fibrosis and itch (20). Although the elicitation of itching by these cytokines has not been reported in humans, they induce pruritus in mice as acute pruritogens (31). Moreover, the combined intradermal injection of IL-4 and IL-13 in mice triggers earlier and more intense scratching behaviors than separate cytokine administration (31). These findings highlight the crucial role of IL-4 and IL-13 in promoting dysregulation of the skin neuroimmune network.
2.1.2 IL-31
IL-31 belongs to the IL-6 cytokine family, which can directly trigger pruritus in humans and is called “itch cytokine” (32). IL-31 is mainly secreted by Th2 cells, and dermal CD11c+ myeloid dendritic cells are an important source of IL-31 in PN (33, 34). In contrast to acute pruritogens, itch induced by IL-31 exhibits pronounced delayed characteristics, suggesting its potential for indirect pruritogenic actions, such as regulating the synthesis and release of central itch mediators like BNP in the DRG and skin, and induction of leukotriene B4 production in keratinocytes (35–37). IL-31 is upregulated to varying degrees in various pruritic dermatoses (38). Among them, the upregulation was most remarkable in lesional PN skin, reaching levels 50 times higher than those in healthy skin. Conversely, non-pruritic dermatoses, such as non-pruritic psoriasis, showed no elevation in IL-31 (39). Serum IL-31 levels are also increased in patients with PN (40). The receptor of IL-31 is a heterodimer composed of IL-31 receptor A (IL-31Rα) and oncostatin M receptor β chain (OSMRβ). It is expressed in various cell types including keratinocytes, immune cells, and sensory neurons (32). In PN lesions, this receptor is also notably increased, and the intensity of pruritus in PN is closely related to the number of IL-31+ cells and IL-31Rα+ cells in the dermis (33). IL-31 promotes sprouting of skin sensory nerve axons and enhances their sensitivity to pruritogens (41, 42). Transcriptomic changes in lesional skin and changes in plasma proteomics confirmed the pivotal upstream pathological role of IL-31 signaling in PN (23, 43, 44). Blocking IL-31Rα not only effectively inhibits pruritus signaling but also alleviates downstream Th2 and Th17 inflammatory responses, suppresses nerve growth factor (NGF)-mediated neuronal dysregulation, and reverses the activation of keratinocyte proliferation and pro-fibrotic reactions (23, 43, 44). These findings indicate that the IL-31/IL-31Rα axis plays an important role in the pathogenesis of PN-associated pruritus and involves intricate regulation within the epidermal-immune-neural network.
2.1.3 Oncostatin M
Oncostatin M (OSM) is a member of the IL-6 cytokine family that plays a significant role in various pathological processes in the skin, including inflammation, hyperkeratosis, and fibrosis (45, 46). OSM is upregulated in skin lesions of various pruritic dermatoses including PN (7, 47). Furthermore, the intensity of pruritus in PN is closely correlated with the number of dermal OSM+ cells but not of OSMRβ+ cells (33). Single-cell sequencing has revealed that OSM is primarily produced by dermal T cells and monocytes (42). Similar to IL-31, OSM induces delayed itching in mice and enhances histamine-induced pruritus by enhancing the excitability and sensitivity of the sensory neurons to pruritogens instead of directly activating them. OSM also indirectly induces itch by stimulating stromal cells in the skin (42). OSM has two types of receptors, the unique human type I receptors consisting of leukemia inhibitory factor receptor (LIFR) and gp130, and the type II receptors found both in humans and mice composed of gp130 and OSMRβ (45). It has been reported that OSMR is expressed by itch-selective neurotensin B (Nppb) neurons in mice. Knocking out OSMR in sensory neurons or systemic administration of gp130 inhibitors suppresses inflammatory itch in mice (42). This suggests that targeting the OSM/OSMRβ/gp130 signaling pathway holds promising potential for antipruritic therapy. Since OSMRβ is a shared receptor subunit for both OSM and IL-31, antagonizing it might be an effective approach for treating PN.
2.1.4 IL-17
The Th17 cell cytokine IL-17 mediates excessive proliferation and differentiation interference of epidermal keratinocytes and plays a key pathogenic role in conditions such as psoriasis (48). Interestingly, the molecular biological features of PN are more akin to those of psoriasis than those of AD (10). Previous studies have shown an increased expression of IL-17 in lesional PN skin, coupled with an increase in the number of Th17 cells within the lesions (9, 27, 49). The origin of CD4+ Th17 cells is significantly more from PN lesions compared to from healthy skin and lesional AD skin (9). However, the relationship between IL-17 and pruritus in patients with PN remains unclear. IL-17A stimulates keratinocytes to secrete pruritogenic endothelin-1 (49). The latter is upregulated in prurigo nodules (49, 50), implying that IL-17 indirectly contributes to development of itching symptom in patients with PN.
2.1.5 IL-22
Several studies have shown that the expression of the Th22 cell cytokine IL-22 is elevated in pruritic PN lesions, which is potentially associated with impaired epidermal proliferation and differentiation and skin inflammation (27, 51). Skin RNA sequencing of patients with severe pruritus in PN revealed robust upregulation of Th22-related genes and signaling pathways, including IL-22, IL-22 receptors (IL22RA1 and IL22RA2), and IL-22-associated cytokines. Moreover, circulating IL-22 derived from both CD4+ and CD8+ T cells is significantly increased in PN, suggesting a systemic and cutaneous Th22/IL-22 polarization pattern (51). However, there is limited evidence regarding the correlation between IL-22 levels and pruritus in PN. Considering that IL-22 receptors are exclusively expressed in epithelial cells (52), IL-22 may indirectly mediate pruritus by promoting pruritogen secretion. For example, IL-22 induces keratinocytes to express substantial amounts of pruritogenic gastrin-releasing peptides (GRP) and GRP receptors (53).
2.1.6 Other cytokines
Thymic stromal lymphopoietin (TSLP) is primarily produced by epithelial cells and signals through IL-7 receptor α-chain (IL-7Rα) and TSLP receptor (TSLPR) heterodimers (54). TSLP released from keratinocytes activates TSLPR-expressing sensory neurons to trigger itch sensation in mice (55). Although levels of TSLP were not elevated in PN lesions, TSLPRs were upregulated (56). IL-6 is expressed in the dermal nerve fibers of lesional PN skin (57). Transcription level of IL-6 is increased in prurigo lesions (8). Serum IL-6 levels are significantly elevated in patients with PN and are correlated with the severity of pruritus (58). However, direct evidence linking IL-6 and TSLP to pruritus in PN is lacking.
2.2 Extracellular matrix protein
2.2.1 Periostin
Periostin is an extracellular matrix protein that plays a significant role in skeletal development, cardiovascular remodeling, and Th2 inflammation (59). Owing to its ability to induce rapid and intense scratching behavior in mice, dogs, and monkeys, it has been identified as a novel pruritogen (60). Enhanced dermal periostin expression has been observed in various pruritic skin disorders (61–63). Similarly, periostin is abundantly deposited in the dermis of PN and is significantly correlated with the severity of pruritus in PN (64). Plasma periostin was also significantly upregulated in patients with PN with severe itch (25). Single-cell sequencing has revealed that the activation of fibrotic responses is a distinguishing feature between PN and AD. The main contributor to dermal fibrosis, the COL11A1+ fibroblast subset, is likely to be the primary cellular source of periostin in PN (23). Periostin-mediated itching involves direct and indirect pathways. In the direct pathway, periostin interacts directly with itch-sensitive nerve fibers via its receptor integrin aVβ3 (60). In the indirect pathway, periostin stimulates immune cells (macrophages, eosinophils, and basophils) to release IL-31 and other pruritogenic mediators (59, 62). Periostin also stimulates keratinocytes to secrete the pruritogen TSLP, which in turn acts on fibroblasts to generate periostin, forming a “TSLP- periostin” cross-activation loop that sustains chronic itching and maintains Th2 inflammation (60). Since dermal periostin in PN shows no significant correlation with other pruritogenic mediators in the dermis (including IL-31, IL-31Rα, and OSMRβ), it might be an independent contributor to pruritus in PN (64). Further studies are required to elucidate this association. Targeting periostin or its receptor integrin aVβ3 to simultaneously treat itch and Th2 inflammation could be a novel therapeutic option for PN.
2.3 Neuropeptides
2.3.1 Substance P
Substance P (SP), secreted by sensory neurons, plays an important role in itch signaling within the peripheral and central nervous systems (65, 66). The subcutaneous injection of SP immediately induces itching in both humans and mice (67, 68). The density of SP-expressing nerve fibers was increased in the dermis of prurigo nodules but not in that of neurodermatitis, another pruritic neurogenic skin disorder (69–71). Serum SP levels and the expression of two SP receptors, neurokinin 1 receptor (NK1R) and Mas-related G protein-coupled receptor member X2 (MRGPRX2), are elevated in patients with chronic prurigo (72, 73). Additionally, the expression of MRGPRX2 closely correlates with the severity of itch (73). This evidence suggests a unique role of SP signaling in PN. At the molecular level, SP mediates mast cell activation, leading to the release of pruritogens such as histamine, leukotriene B4, and vascular endothelial growth factor (VEGF) (74–76). However, the pruritogenic effect of mast cells may not be crucial because mast cell-deficient mice still exhibit intense scratching behavior after SP stimulation (67). Keratinocytes and eosinophils activated by SP producing NGF promotes neuronal proliferation and activates afferent nerves, leading to further release of SP and sustaining the itch cycle (77). Moreover, SP directly activates sensory neurons to trigger itch (78). NK1R antagonists have shown preliminary results for the treatment of chronic pruritus in patients with PN. An 8-week phase 2 clinical trial of the NK1R antagonist serlopitant demonstrated a greater reduction in itch with oral serlopitant than with placebo (79). However, phase 3 randomized clinical trial (RCT) of the drug did not meet the primary endpoint of reducing itch (NCT03546816 and NCT03677401). Another NK1R antagonist, aprepitant, in both topical and oral formulations, did not show satisfactory antipruritic effects in placebo-controlled trials involving patients with PN (72, 80). MRGPRX2 antagonization is another potential therapeutic option for PN-associated itching. Therefore, it is necessary to investigate whether NK1R antagonists interact with MRGPRX2. Although aprepitant was incapable of antagonizing human MRGPRX2 in vitro, it was found to have an off-target effect on MrgprB2 (a homologous receptor of MRGPRX2) in animal experiments (78, 81).
2.3.2 Calcitonin gene-related peptide
Calcitonin gene-related peptide (CGRP) is the most abundant neuropeptide in human skin and is often colocalized with SP (82). Its function is similar to that of SP (83). However, intradermal injection of CGRP induces persistent erythema, but does not cause itch (84). Evidence suggests that sensory neurons expressing CGRP are necessary for both histamine-dependent and histamine-independent itch (85). CGRP antagonist blocks trypsin-induced itch in mice (86). In lesional PN skin, the density of CGRP-expressing afferent nerves in the dermis was increased, surrounded by mast cells and eosinophils (16). CGRP activates mast cells to release histamine, and eosinophils to produce NGF, another pruritogen. Histamine and NGF, in turn, promote release of CGRP by neurons, creating a bidirectional positive feedback loop between nerve fibers and immune cells; thus, amplifying and sustaining itch signaling (77). In mice, CGRP induces Th2 immune responses, promoting the production of IL-4, CCL17, and CCL22 by Langerhans cells, while suppressing Th1 responses (87). CGRP also acts as an immunoregulatory mediator that enhances IL-13 production (88). Additionally, CGRP influences endorphin levels and leads to the dysregulation of mu- and kappa-opioid receptor expression (3, 89, 90). These factors may contribute to PN-related itching; however, direct evidence of the relevance of CGRP is still lacking.
2.3.3 Cortistatin
Cortistatin (CST) is a neuropeptide that is structurally and functionally similar to somatostatin (91). It possesses various biological effects, such as regulating homeostasis in the nervous, endocrine, and cardiovascular systems, exerting anti-inflammatory effects, and promoting Th2 polarization within the immune system (92). Recently, it was identified as an endogenous pruritogen that is predominantly secreted by skin mast cells. Pricking CST on the skin of healthy individuals rapidly induces a noticeable itch sensation (73). CST and its receptor, MRGPRX2, play a pivotal role in the development of chronic itch (93). CST- and MRGPRX2-expressing cells, mostly mast cells, increased in the skin lesions of patients with chronic prurigo. Severe itch in patients with chronic prurigo is associated with the most significant upregulation of CST in the skin. Moreover, the number of cells expressing MRGPRX2 in skin lesions and serum levels of MRGPRX2 correlate with prurigo severity, itch intensity, and/or impaired quality of life (73). The pruritogenic mechanism of CST may involve binding to MRGPRX2, inducing mast cell degranulation and the release of histamine and CST, thus forming a CST autocrine feedback loop (73). MRGPRX2 is expressed in DRG sensory neurons (93, 94); however, whether CST directly activates neurons through its receptor to induce itching remains to be further elucidated.
2.4 Neurotrophin
2.4.1 Nerve growth factor
While keratinocytes are the primary source of skin-derived NGF, dermal inflammatory cells such as mast cells, eosinophils, and lymphocytes abundantly secrete NGF in lesional PN skin (95, 96). The two NGF receptors, high-affinity tropomyosin receptor kinase A (TrkA) and low-affinity p75 neurotrophin receptor (p75NTR), have a synergistic effect and are increased in the dermal nerve fibers of PN (95). Scratching can also lead to increased expression of skin-derived NGF and its receptors (97). NGF is a neurotrophic factor that primarily activates, sensitizes, and sprouts skin nerve fibers, promoting the release of neuropeptides, such as SP and CGRP (96, 98). NGF also influences the survival and function of non-neuronal cells. For example, it stimulates the proliferation and differentiation of keratinocytes; activates or enhances mast cells, eosinophils, and basophils; releases various pro-inflammatory and pruritogenic mediators; and supports the survival and differentiation of these immune cells (96). Intradermal injection of NGF in healthy individuals enhances non-histaminergic itch induced by cowhage (99). Therefore, antagonizing NGF and its receptors may be beneficial for PN treatment; however, this has not been studied in PN.
2.5 Vasculogenic substances
2.5.1 Vascular endothelial growth factor
VEGF is produced by various resident skin cells, including keratinocytes (100). Mechanical manipulation may stimulate keratinocytes to produce VEGF. It promotes endothelial cell proliferation and angiogenesis, which may be associated with the formation of prurigo nodules (101). VEGF levels in both skin lesions and serum are increased in patients with prurigo and are correlated with the severity of the condition (10, 100, 101). However, psoriasis or AD lesions do not show increased VEGF activity, suggesting a unique role of VEGF in the pathogenesis of PN (10). A case of simplex prurigo treated with bevacizumab, a monoclonal anti-VEGF antibody, showed significant improvement in itch (101). Further studies are required to determine the role of VEGF in PN-related itching.
2.5.2 Endothelin
Endothelin-1 (ET-1), initially described as a vasoconstrictor, is also a partial non-histaminergic pruritogen that independently induces a persistent itching sensation in human skin (50). In patients with PN, both skin lesions (especially in the epidermis and neurons) and serum levels of ET-1 are increased, which is possibly associated with elevated levels of IL-17 (49, 50). IL-17A induces ET-1 expression in keratinocytes via the p38 MAPK pathway (49). ET-1 signals through two receptors: the endothelin A receptors (ETAR) and endothelin B receptors (ETBR) (21). ET-1 mediates itch through ETAR in the skin nerve fibers; however, this process is negatively regulated by neutral endopeptidase 1 (ECE-1) (50). In contrast, ETBR has an antipruritic effect through peripheral κ-opioid receptors against ET-1-induced itch (102). ETBR expression is downregulated in lesional PN skin (56), further enhancing the pruritic effects of ET-1 signaling.
2.6 Histamine
Histamine is one of the most classical pruritogens. Its primary sources are activated mast cells and basophils (103). Immunohistochemistry showed that the number of histamine-containing mast cells in PN lesions was significantly increased, and the morphology of mast cells also changed, with enlarged cell bodies and more dendrites, but fewer intracellular granules (17, 104). Similarly, the number of activated basophils in the blood and dermis of patients with PN was also significantly higher than that in healthy controls (105). Histamine activates cutaneous sensory nerve fibers through H1 and H4 histamine receptors to elicit itch (106). However, due to the resistance of PN to antihistamine treatment, histamine is not considered a primary pruritogenic factor in PN (12).
3 Itch signaling pathways
3.1 The JAK-STAT pathway
Four members of the Janus kinase (JAK) family (JAK1, JAK2, JAK3, and TYK2) selectively bind in various combinations to different type I/II cytokine receptors and transmit their activated intracellular transcriptional signals, along with seven members of the signal transducer and activator of transcription protein (STAT) family (107). Several key cytokines, such as IL-4, IL-13, IL-31, and OSM, play roles in propagating pruritus and inflammation in PN through the JAK-STAT signaling pathway, particularly involving JAK1, STAT3, and STAT6 (28, 45, 108). Th2 cytokines are predominantly regulated by STAT6, whereas STAT3 is associated with multiple pruritogens, including IL-6, IL-22, IL-31, OSM, TSLP, and VEGF (109–111). STAT3 and STAT6 are significantly upregulated in lesional PN skin (112–114); thus, JAK inhibitors may effectively slow disease progression. Numerous case reports have shown the successful treatment of refractory PN with tofacitinib (JAK1/3 inhibitor), baricitinib (JAK1/2 inhibitor), and upadacitinib (JAK1 inhibitor) (115–124). Phase 2 clinical trials of two JAK1 inhibitors, abrocitinib (NCT05038982) and povorcitinib (NCT05061693), for the treatment of PN, as well as a phase 3 clinical trial of ruxolitinib cream (a JAK1/JAK2 inhibitor) are currently underway (NCT05755438 and NCT05764161).
3.2 Transient receptor potential channels
Transient receptor potential (TRP) channels can be activated by various physical and biochemical stimuli. Notably, most itch receptors such as G-protein-coupled receptors and cytokine receptors couple with TRP channels that act as downstream sensors (21). After activation, the channels develop to cause calcium influx and generate action potentials that propagate itch signals in the peripheral sensory neurons (19, 125). TRP channels are a class of nonselective cation channels including family members like TRP vanilloid 1 (TRPV1) and TRP ankyrin 1 (TRPA1) (126). TRPV1, also known as the capsaicin receptor, is significantly upregulated in nerve fibers and keratinocytes within lesional PN skin (127). Propagation of various histamine-independent itch signals related to the PN involves TRPV1 and/or TRPA1. For example, itching induced by IL-31 and periostin is transmitted by TRPV1+TRPA1+ neurons (60, 128). Itch, mediated by IL-13, TSLP, endothelin, and MRGPRs, is activated by TRPA1 (55, 129–131). NGF activates TRPV1 via TrkA, causing the upregulation and activation of TRPV1 in afferent nerves, subsequently releasing SP and CGRP (132). Topical capsaicin treatment alleviates itching symptoms in patients with PN, normalizes TRPV1 expression in skin lesions, and reduces SP and CGRP levels (127). The anti-itch effect of capsaicin is presumably mediated by the activation of TRPV1 on cutaneous C-fibers, leading to the depletion of neuropeptides such as SP (133). However, because of the short-acting efficacy of capsaicin and its side effects of intense burning sensations, its widespread use is limited. Further evidence is required to confirm the roles of TRPV1 and TRPA1 in PN-associated itch.
4 Promising advances in the treatment of PN
Based on two phase 3 clinical trials, dupilumab (an IL-4Rα monoclonal antibody simultaneously antagonizing IL-4 and IL-13) has become the first the U.S. Food and Drug Administration (FDA)/European Medicines Agency (EMA)/China National Medical Products Administration (NMPA)-approved treatment for adult patients with PN. Compared to placebo, significant improvements in weekly average worst itch numeric rating scale (WI-NRS) with dupilumab were observed as early as week 3 in PRIME and week 4 in PRIME2 (134). Moreover, a few cases report off-label use of dupilumab have shown good efficacy and safety in children and adolescents with PN (135–137). It took longer for dupilumab to reduce pruritus in atopic PN compared with patients with nonatopic PN (138). Two real-world studies reviewed the long-term efficacy of dupilumab. Among 19 patients with PN, 78.9% and 68.4% reported improved pruritus at weeks 16 and 52, respectively (139). The pruritus NRS score dropped to 0 at 16 weeks of treatment in 19 of 21 PN patients and was maintained for at least 104 weeks (140). A systematic review showed that 48.88% of patients with PN achieved complete relief of itching, the average time to clear itching was 19 weeks, and patients who did not achieve complete itch resolution had a longer time to first relief (138). Large real-world data collections evaluating the efficacy and safety of dupilumab in PN are still lacking.
In addition to dupilumab, a series of novel drugs targeting Th2 polarization in PN have also shown promising results. In an open-label case series, 17 patients with a PN-like phenotype of AD showed a significant reduction in mean itch-NRS values as early as week 4 after treatment with the anti-IL-13 monoclonal antibody tralokinumab (141). Recently, phase 2 and phase 3 clinical trials on nemolizumab, an anti-IL-31Rα monoclonal antibody, demonstrated the effectiveness and safety of blocking the IL-31 signaling in treating PN. Both studies reported a rapid reduction in pruritus severity and significant improvement in nodules after treatment (142, 143). The OSMRβ monoclonal antibody, vixarelimab (KPL-716), has shown promising results in a phase 2a clinical trial for PN (45). They demonstrated an average reduction of 50% in pruritus by week 8 of vixarelimab treatment, and one-third of the patients achieved lesion clearance or near clearance (45). A phase 2b trial is currently in progress (NCT03816891).
IL-5 plays a central role in the differentiation, proliferation, activation, adhesion, and survival of eosinophils, while promoting the recruitment of skin mast cells and basophils (19, 144). A phase 2 clinical trial of anti-IL5Rα antibody (benralizumab) for PN treatment is currently in preparation (NCT05528913). Blocking mast cell activation suppresses the neuro-immune axis (145, 146). Targeting mast cell tyrosine kinase KIT receptors (barzolvolimab) results in sustained and profound MC inhibition in healthy volunteers (147). A phase 1 clinical trial targeting PN with this drug has been completed and the results are awaited (NCT04944862).
Several recent omics studies have suggested that extracellular matrix remodeling, fibrosis activation, neural dysfunction, vascular system development, and keratinization may be unique pathological features of PN (6–8, 23, 43, 148). Further investigation of the relationship between these features and itching is required. Therefore, extracellular matrix proteins, such as periostin; neuroinflammatory molecules, such as neuropeptides, NGF, and MRGPRX2; and vascular substances, such as VEGF and ET, are intriguing targets for drug development. Likewise, blocking downstream cellular signaling pathways, such as with JAK inhibitors and TRP channel antagonists, could be an additional treatment option. Several JAK inhibitors have been approved for moderate-to-severe AD in many countries, and they have significantly improved patients’ pruritus and condition (149). Topical TRPV1 antagonists have demonstrated efficacy and safety in phase 2b (PAC-14028) and phase 3 (Asivatrep) clinical trials for treating atopic itch (150, 151). However, their role in the treatment of PN requires confirmation through RCT.
Cannabinoid receptors 1 and 2 (CB1 and CB2) are expressed in the central nervous system and skin nerve fibers, and are activated by various bioactive lipid mediators (152). Cannabinoid agonists alleviate pruritus in various animal models of chronic pruritus and in clinical trials of pruritic skin diseases (153–158). An open-label clinical study showed significant relief from itch in eight of 12 patients with PN using a moisturizing cream containing the CB2 agonist N-palmitoyl ethanolamide (159). However, the role of the endogenous cannabinoid system in PN requires confirmation through double-blind controlled trials. At the level of the spinal cord, an imbalance between itch-promoting μ-opioid receptor (MOR) activity and itch-inhibiting κ-opioid receptor (KOR) activity mediates non-histaminergic itch (160). A phase 2 placebo-controlled study indicated that the dual-acting KOR agonist/MOR antagonist nalbuphine extended-release (ER) tablets effectively treat PN. Furthermore, 33% of participants treated with 162 mg oral doses twice daily showed a reduction of ≥50% in itch by week 10, with a suitable safety profile (161). The phase 2b/3 PRISM clinical trial of nalbuphine ER reached its primary endpoint, with a greater proportion of participants achieving a ≥4-point reduction in WI-NRS at week 14 compared to placebo (24.7% vs 13.9%) (156). The new and emerging treatments for PN-related pruritus are summarized in Table 2.
5 Conclusion
Recent studies have provided strong evidence for unraveling the specific mechanisms underlying itching in PN. Further exploration of independent pruritogens and receptors, as well as investigation of the interactive network between skin cells and the nervous system, is crucial for a comprehensive understanding of PN. Targeting pruritus signal transmission or disrupting neuroimmune crosstalk are emerging therapeutic strategies to alleviate itching, prevent chronicity, and improve disease prognosis in PN.
Author contributions
YXS: Conceptualization, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. DW: Writing – original draft, Writing – review & editing. YZ: Writing – review & editing. ZX: Writing – review & editing. TJ: Writing – review & editing. LP: Writing – review & editing. YYS: Writing – review & editing. HT: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by the Shanghai Association for Science and Technology (grant number 21Y11905100).
Acknowledgments
Thanks to my husband Jiada Huang for help with manuscript editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Boozalis E, Tang O, Patel S, Semenov YR, Pereira MP, Stander S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol (2018) 79(4):714–9.e3. doi: 10.1016/j.jaad.2018.04.047
3. Williams KA, Huang AH, Belzberg M, Kwatra SG. Prurigo nodularis: pathogenesis and management. J Am Acad Dermatol (2020) 83(6):1567–75. doi: 10.1016/j.jaad.2020.04.182
4. Brenaut E, Halvorsen JA, Dalgard FJ, Lien L, Balieva F, Sampogna F, et al. The self-assessed psychological comorbidities of prurigo in European patients: A multicentre study in 13 countries. J Eur Acad Dermatol Venereol JEADV (2019) 33(1):157–62. doi: 10.1111/jdv.15145
5. Morgan CL, Thomas M, Ständer S, Jabbar-Lopez ZK, Piketty C, Gabriel S, et al. Epidemiology of prurigo nodularis in England: A retrospective database analysis. Br J Dermatol (2022) 187(2):188–95. doi: 10.1111/bjd.21032
6. Alkon N, Assen FP, Arnoldner T, Bauer WM, Medjimorec MA, Shaw LE, et al. Single-cell rna sequencing defines disease-specific differences between chronic nodular prurigo and atopic dermatitis. J Allergy Clin Immunol (2023) 152(2):420–35. doi: 10.1016/j.jaci.2023.04.019
7. Deng J, Parthasarathy V, Marani M, Bordeaux Z, Lee K, Trinh C, et al. Extracellular matrix and dermal nerve growth factor dysregulation in prurigo nodularis compared to atopic dermatitis. Front Med (2022) 9:1022889. doi: 10.3389/fmed.2022.1022889
8. Shao Y, Zhu Y, Xiao Z, Shen Y, Dai B, Tang H, et al. Rna sequencing reveals the transcriptome profile of the atopic prurigo nodularis with severe itching. Exp Dermatol (2023) 32(1):30–40. doi: 10.1111/exd.14678
9. Calugareanu A, Specque F, Demouche S, Grolleau C, Dobos G, Merandet M, et al. Transcriptomic landscape of prurigo nodularis lesional skin cd3+ T cells using single-cell rna sequencing. J Invest Dermatol (2023) S0022-202X(23)02134-6. doi: 10.1016/j.jid.2023.05.011
10. Sutaria N, Alphonse MP, Roh YS, Choi J, Parthasarathy V, Deng J, et al. Cutaneous transcriptomics identifies fibroproliferative and neurovascular gene dysregulation in prurigo nodularis compared with psoriasis and atopic dermatitis. J Invest Dermatol (2022) 142(9):2537–40. doi: 10.1016/j.jid.2022.02.010
11. Agelopoulos K, Renkhold L, Wiegmann H, Dugas M, Süer A, Zeidler C, et al. Transcriptomic, epigenomic, and neuroanatomic signatures differ in chronic prurigo, atopic dermatitis, and brachioradial pruritus. J Invest Dermatol (2023) 143(2):264–72.e3. doi: 10.1016/j.jid.2022.08.042
12. Elmariah S, Kim B, Berger T, Chisolm S, Kwatra SG, Mollanazar N, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol (2021) 84(3):747–60. doi: 10.1016/j.jaad.2020.07.025
13. Schuhknecht B, Marziniak M, Wissel A, Phan NQ, Pappai D, Dangelmaier J, et al. Reduced intraepidermal nerve fibre density in lesional and nonlesional prurigo nodularis skin as a potential sign of subclinical cutaneous neuropathy. Br J Dermatol (2011) 165(1):85–91. doi: 10.1111/j.1365-2133.2011.10306.x
14. Weigelt N, Metze D, Ständer S. Prurigo nodularis: systematic analysis of 58 histological criteria in 136 patients. J cutaneous Pathol (2010) 37(5):578–86. doi: 10.1111/j.1600-0560.2009.01484.x
15. Johansson O, Liang Y, Marcusson JA, Reimert CM. Eosinophil cationic protein- and eosinophil-derived neurotoxin/eosinophil protein X-immunoreactive eosinophils in prurigo nodularis. Arch Dermatol Res (2000) 292(8):371–8. doi: 10.1007/s004030000142
16. Liang Y, Jacobi HH, Reimert CM, Haak-Frendscho M, Marcusson JA, Johansson O. Cgrp-immunoreactive nerves in prurigo nodularis–an exploration of neurogenic inflammation. J cutaneous Pathol (2000) 27(7):359–66. doi: 10.1034/j.1600-0560.2000.027007359.x
17. Liang Y, Marcusson JA, Jacobi HH, Haak-Frendscho M, Johansson O. Histamine-containing mast cells and their relationship to ngfr-immunoreactive nerves in prurigo nodularis: A reappraisal. J cutaneous Pathol (1998) 25(4):189–98. doi: 10.1111/j.1600-0560.1998.tb01718.x
18. Kim B, Rothenberg ME, Sun X, Bachert C, Artis D, Zaheer R, et al. Neuroimmune interplay during type 2 inflammation: symptoms, mechanisms and therapeutic targets in atopic diseases. J Allergy Clin Immunol (2023) S0091-6749(23)01070-9. doi: 10.1016/j.jaci.2023.08.017
19. Vander Does A, Ju T, Mohsin N, Chopra D, Yosipovitch G. How to get rid of itching. Pharmacol Ther (2023) 243:108355. doi: 10.1016/j.pharmthera.2023.108355
20. Kolkhir P, Akdis CA, Akdis M, Bachert C, Bieber T, Canonica GW, et al. Type 2 chronic inflammatory diseases: targets, therapies and unmet needs. Nat Rev Drug Discovery (2023) 22(9):743–67. doi: 10.1038/s41573-023-00750-1
21. Misery L, Pierre O, Le Gall-Ianotto C, Lebonvallet N, Chernyshov PV, Le Garrec R, et al. Basic mechanisms of itch. J Allergy Clin Immunol (2023) 152(1):11–23. doi: 10.1016/j.jaci.2023.05.004
22. Wang F, Kim BS. Itch: A paradigm of neuroimmune crosstalk. Immunity (2020) 52(5):753–66. doi: 10.1016/j.immuni.2020.04.008
23. Ma F, Gharaee-Kermani M, Tsoi LC, Plazyo O, Chaskar P, Harms P, et al. Single-cell profiling of prurigo nodularis demonstrates immune-stromal crosstalk driving profibrotic responses and reversal with nemolizumab. J Allergy Clin Immunol (2023) S0091-6749(23)00925-9. doi: 10.1016/j.jaci.2023.07.005
24. Lloyd CM, Snelgrove RJ. Type 2 immunity: expanding our view. Sci Immunol (2018) 3(25):eaat1604. doi: 10.1126/sciimmunol.aat1604
25. Parthasarathy V, Cravero K, Deng J, Sun Z, Engle SM, Auxier AN, et al. Circulating plasma il-13 and periostin are dysregulated type 2 inflammatory biomarkers in prurigo nodularis: A cluster analysis. Front Med (2022) 9:1011142. doi: 10.3389/fmed.2022.1011142
26. Hashimoto T, Okuno S, Okuzawa M, Satoh T. Increased sensitivity to touch-evoked itch (Punctate hyperknesis) in prurigo nodularis and type 2 inflammation: A cross-sectional pilot study. J Eur Acad Dermatol Venereol JEADV (2023) 37(6):e789–e91. doi: 10.1111/jdv.18942
27. Park K, Mori T, Nakamura M, Tokura Y. Increased expression of mrnas for il-4, il-17, il-22 and il-31 in skin lesions of subacute and chronic forms of prurigo. Eur J Dermatol EJD (2011) 21(1):135–6. doi: 10.1684/ejd.2010.1196
28. Oetjen LK, Mack MR, Feng J, Whelan TM, Niu H, Guo CJ, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell (2017) 171(1):217–28.e13. doi: 10.1016/j.cell.2017.08.006
29. Miron Y, Miller PE, Hughes C, Indersmitten T, Lerner EA, Cevikbas F. Mechanistic insights into the antipruritic effects of lebrikizumab, an anti-il-13 mab. J Allergy Clin Immunol (2022) 150(3):690–700. doi: 10.1016/j.jaci.2022.01.028
30. Dubin C, Del Duca E, Guttman-Yassky E. The il-4, il-13 and il-31 pathways in atopic dermatitis. Expert Rev Clin Immunol (2021) 17(8):835–52. doi: 10.1080/1744666x.2021.1940962
31. Campion M, Smith L, Gatault S, Métais C, Buddenkotte J, Steinhoff M. Interleukin-4 and interleukin-13 evoke scratching behaviour in mice. Exp Dermatol (2019) 28(12):1501–4. doi: 10.1111/exd.14034
32. Datsi A, Steinhoff M, Ahmad F, Alam M, Buddenkotte J. Interleukin-31: the "Itchy" Cytokine in inflammation and therapy. Allergy (2021) 76(10):2982–97. doi: 10.1111/all.14791
33. Hashimoto T, Nattkemper LA, Kim HS, Kursewicz CD, Fowler E, Shah SM, et al. Itch intensity in prurigo nodularis is closely related to dermal interleukin-31, oncostatin M, il-31 receptor alpha and oncostatin M receptor beta. Exp Dermatol (2021) 30(6):804–10. doi: 10.1111/exd.14279
34. Liu T, Chu Y, Li S, Wang Y, Zhong X, Fang H, et al. Myeloid dendritic cells are increased in the lesional skin and associated with pruritus in patients with prurigo nodularis. MedComm (2023) 4(1):e204. doi: 10.1002/mco2.204
35. Hawro T, Saluja R, Weller K, Altrichter S, Metz M, Maurer M. Interleukin-31 does not induce immediate itch in atopic dermatitis patients and healthy controls after skin challenge. Allergy (2014) 69(1):113–7. doi: 10.1111/all.12316
36. Meng J, Moriyama M, Feld M, Buddenkotte J, Buhl T, Szöllösi A, et al. New mechanism underlying il-31-induced atopic dermatitis. J Allergy Clin Immunol (2018) 141(5):1677–89.e8. doi: 10.1016/j.jaci.2017.12.1002
37. Andoh T, Harada A, Kuraishi Y. Involvement of leukotriene B4 released from keratinocytes in itch-associated response to intradermal interleukin-31 in mice. Acta dermato-venereol (2017) 97(8):922–7. doi: 10.2340/00015555-2697
38. Borgia F, Custurone P, Li Pomi F, Cordiano R, Alessandrello C, Gangemi S. Il-31: state of the art for an inflammation-oriented interleukin. Int J Mol Sci (2022) 23(12):6507. doi: 10.3390/ijms23126507
39. Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, et al. Il-31: A new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol (2006) 117(2):411–7. doi: 10.1016/j.jaci.2005.10.033
40. Chaowattanapanit S, Wongjirattikarn R, Chaisuriya N, Ungarreevittaya P, Poosekeaw P, Winaikosol K, et al. Increased il-31 expression in serum and tissue protein in prurigo nodularis. Ther Adv chronic Dis (2022) 13:20406223221112561. doi: 10.1177/20406223221112561
41. Feld M, Garcia R, Buddenkotte J, Katayama S, Lewis K, Muirhead G, et al. The pruritus- and th2-associated cytokine il-31 promotes growth of sensory nerves. J Allergy Clin Immunol (2016) 138(2):500–8.e24. doi: 10.1016/j.jaci.2016.02.020
42. Tseng PY, Hoon MA. Oncostatin M can sensitize sensory neurons in inflammatory pruritus. Sci Trans Med (2021) 13(619):eabe3037. doi: 10.1126/scitranslmed.abe3037
43. Tsoi LC, Hacini-Rachinel F, Fogel P, Rousseau F, Xing X, Patrick MT, et al. Transcriptomic characterization of prurigo nodularis and the therapeutic response to nemolizumab. J Allergy Clin Immunol (2022) 149(4):1329–39. doi: 10.1016/j.jaci.2021.10.004
44. Deng J, Liao V, Parthasarathy V, Cornman HL, Kambala A, Kwatra MM, et al. Modulation of neuroimmune and epithelial dysregulation in patients with moderate to severe prurigo nodularis treated with nemolizumab. JAMA Dermatol (2023) 159(9):977–85. doi: 10.1001/jamadermatol.2023.2609
45. Hermanns HM. Oncostatin M and interleukin-31: cytokines, receptors, signal transduction and physiology. Cytokine Growth factor Rev (2015) 26(5):545–58. doi: 10.1016/j.cytogfr.2015.07.006
46. Richards CD, Gandhi R, Botelho F, Ho L, Paolini JF. Oncostatin M induction of monocyte chemoattractant protein 1 is inhibited by anti-oncostatin M receptor beta monoclonal antibody kpl-716. Acta dermato-venereol (2020) 100(14):adv00197. doi: 10.2340/00015555-3505
47. Boniface K, Diveu C, Morel F, Pedretti N, Froger J, Ravon E, et al. Oncostatin M secreted by skin infiltrating T lymphocytes is a potent keratinocyte activator involved in skin inflammation. J Immunol (Baltimore Md 1950) (2007) 178(7):4615–22. doi: 10.4049/jimmunol.178.7.4615
48. Blauvelt A, Chiricozzi A. The immunologic role of il-17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol (2018) 55(3):379–90. doi: 10.1007/s12016-018-8702-3
49. Wong LS, Yen YT, Lin SH, Lee CH. Il-17a Induces Endothelin-1 Expression through P38 pathway in Prurigo Nodularis. J Invest Dermatol (2020) 140(3):702–6.e2. doi: 10.1016/j.jid.2019.08.438
50. Kido-Nakahara M, Buddenkotte J, Kempkes C, Ikoma A, Cevikbas F, Akiyama T, et al. Neural peptidase endothelin-converting enzyme 1 regulates endothelin 1-induced pruritus. J Clin Invest (2014) 124(6):2683–95. doi: 10.1172/jci67323
51. Belzberg M, Alphonse MP, Brown I, Williams KA, Khanna R, Ho B, et al. Prurigo nodularis is characterized by systemic and cutaneous T helper 22 immune polarization. J Invest Dermatol (2021) 141(9):2208–18.e14. doi: 10.1016/j.jid.2021.02.749
52. Dudakov JA, Hanash AM, van den Brink MR. Interleukin-22: immunobiology and pathology. Annu Rev Immunol (2015) 33:747–85. doi: 10.1146/annurev-immunol-032414-112123
53. Lou H, Lu J, Choi EB, Oh MH, Jeong M, Barmettler S, et al. Expression of il-22 in the skin causes th2-biased immunity, epidermal barrier dysfunction, and pruritus via stimulating epithelial th2 cytokines and the grp pathway. J Immunol (Baltimore Md 1950) (2017) 198(7):2543–55. doi: 10.4049/jimmunol.1600126
54. Ebina-Shibuya R, Leonard WJ. Role of thymic stromal lymphopoietin in allergy and beyond. Nat Rev Immunol (2023) 23(1):24–37. doi: 10.1038/s41577-022-00735-y
55. Wilson SR, Thé L, Batia LM, Beattie K, Katibah GE, McClain SP, et al. The epithelial cell-derived atopic dermatitis cytokine tslp activates neurons to induce itch. Cell (2013) 155(2):285–95. doi: 10.1016/j.cell.2013.08.057
56. Zhong W, Wu X, Zhang W, Zhang J, Chen X, Chen S, et al. Aberrant expression of histamine-independent pruritogenic mediators in keratinocytes may be involved in the pathogenesis of prurigo nodularis. Acta dermato-venereol (2019) 99(6):579–86. doi: 10.2340/00015555-3150
57. Nordlind K, Chin LB, Ahmed AA, Brakenhoff J, Theodorsson E, Lidén S. Immunohistochemical localization of interleukin-6-like immunoreactivity to peripheral nerve-like structures in normal and inflamed human skin. Arch Dermatol Res (1996) 288(8):431–5. doi: 10.1007/bf02505230
58. Konda D, Chandrashekar L, Rajappa M, Kattimani S, Thappa DM, Ananthanarayanan PH. Serotonin and interleukin-6: association with pruritus severity, sleep quality and depression severity in prurigo nodularis. Asian J Psychiatry (2015) 17:24–8. doi: 10.1016/j.ajp.2015.07.010
59. Hashimoto T, Mishra SK, Olivry T, Yosipovitch G. Periostin, an emerging player in itch sensation. J Invest Dermatol (2021) 141(10):2338–43. doi: 10.1016/j.jid.2021.03.009
60. Mishra SK, Wheeler JJ, Pitake S, Ding H, Jiang C, Fukuyama T, et al. Periostin activation of integrin receptors on sensory neurons induces allergic itch. Cell Rep (2020) 31(1):107472. doi: 10.1016/j.celrep.2020.03.036
61. Hashimoto T, Kursewicz CD, Fayne RA, Nanda S, Shah SM, Nattkemper L, et al. Pathophysiologic mechanisms of itch in bullous pemphigoid. J Am Acad Dermatol (2020) 83(1):53–62. doi: 10.1016/j.jaad.2019.07.060
62. Hashimoto T, Satoh T, Yokozeki H. Pruritus in ordinary scabies: il-31 from macrophages induced by overexpression of thymic stromal lymphopoietin and periostin. Allergy (2019) 74(9):1727–37. doi: 10.1111/all.13870
63. Murota H, Lingli Y, Katayama I. Periostin in the pathogenesis of skin diseases. Cell Mol Life Sci CMLS (2017) 74(23):4321–8. doi: 10.1007/s00018-017-2647-1
64. Hashimoto T, Nattkemper LA, Kim HS, Kursewicz CD, Fowler E, Shah SM, et al. Dermal periostin: A new player in itch of prurigo nodularis. Acta dermato-venereol (2021) 101(1):adv00375. doi: 10.2340/00015555-3702
65. Ständer S, Spellman MC, Kwon P, Yosipovitch G. The nk1 receptor antagonist serlopitant for treatment of chronic pruritus. Expert Opin investigational Drugs (2019) 28(8):659–66. doi: 10.1080/13543784.2019.1638910
66. Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev (2014) 94(1):265–301. doi: 10.1152/physrev.00031.2013
67. Andoh T, Nagasawa T, Satoh M, Kuraishi Y. Substance P induction of itch-associated response mediated by cutaneous nk1 tachykinin receptors in mice. J Pharmacol Exp Ther (1998) 286(3):1140–5.
68. Amatya B, Nordlind K, Wahlgren CF. Responses to intradermal injections of substance P in psoriasis patients with pruritus. Skin Pharmacol Physiol (2010) 23(3):133–8. doi: 10.1159/000270385
69. Haas S, Capellino S, Phan NQ, Böhm M, Luger TA, Straub RH, et al. Low density of sympathetic nerve fibers relative to substance P-positive nerve fibers in lesional skin of chronic pruritus and prurigo nodularis. J Dermatol Sci (2010) 58(3):193–7. doi: 10.1016/j.jdermsci.2010.03.020
70. Abadía Molina F, Burrows NP, Jones RR, Terenghi G, Polak JM. Increased sensory neuropeptides in nodular prurigo: A quantitative immunohistochemical analysis. Br J Dermatol (1992) 127(4):344–51. doi: 10.1111/j.1365-2133.1992.tb00452.x
71. Vaalasti A, Suomalainen H, Rechardt L. Calcitonin gene-related peptide immunoreactivity in prurigo nodularis: A comparative study with neurodermatitis circumscripta. Br J Dermatol (1989) 120(5):619–23. doi: 10.1111/j.1365-2133.1989.tb01346.x
72. Ohanyan T, Schoepke N, Eirefelt S, Hoey G, Koopmann W, Hawro T, et al. Role of substance P and its receptor neurokinin 1 in chronic prurigo: A randomized, proof-of-concept, controlled trial with topical aprepitant. Acta dermato-venereol (2018) 98(1):26–31. doi: 10.2340/00015555-2780
73. Kolkhir P, Pyatilova P, Ashry T, Jiao Q, Abad-Perez AT, Altrichter S, et al. Mast cells, cortistatin, and its receptor, mrgprx2, are linked to the pathogenesis of chronic prurigo. J Allergy Clin Immunol (2022) 149(6):1998–2009.e5. doi: 10.1016/j.jaci.2022.02.021
74. Ebertz JM, Hirshman CA, Kettelkamp NS, Uno H, Hanifin JM. Substance P-induced histamine release in human cutaneous mast cells. J Invest Dermatol (1987) 88(6):682–5. doi: 10.1111/1523-1747.ep12470339
75. Andoh T, Katsube N, Maruyama M, Kuraishi Y. Involvement of leukotriene B(4) in substance P-induced itch-associated response in mice. J Invest Dermatol (2001) 117(6):1621–6. doi: 10.1046/j.0022-202x.2001.01585.x
76. Kohara H, Tajima S, Yamamoto M, Tabata Y. Angiogenesis induced by controlled release of neuropeptide substance P. Biomaterials (2010) 31(33):8617–25. doi: 10.1016/j.biomaterials.2010.07.079
77. Mollanazar NK, Smith PK, Yosipovitch G. Mediators of chronic pruritus in atopic dermatitis: getting the itch out? Clin Rev Allergy Immunol (2016) 51(3):263–92. doi: 10.1007/s12016-015-8488-5
78. Azimi E, Reddy VB, Pereira PJS, Talbot S, Woolf CJ, Lerner EA. Substance P activates mas-related G protein-coupled receptors to induce itch. J Allergy Clin Immunol (2017) 140(2):447–53.e3. doi: 10.1016/j.jaci.2016.12.980
79. Ständer S, Kwon P, Hirman J, Perlman AJ, Weisshaar E, Metz M, et al. Serlopitant reduced pruritus in patients with prurigo nodularis in a phase 2, randomized, placebo-controlled trial. J Am Acad Dermatol (2019) 80(5):1395–402. doi: 10.1016/j.jaad.2019.01.052
80. Tsianakas A, Zeidler C, Riepe C, Borowski M, Forner C, Gerss J, et al. Aprepitant in anti-histamine-refractory chronic nodular prurigo: A multicentre, randomized, double-blind, placebo-controlled, cross-over, phase-ii trial (Aprepru). Acta dermato-venereol (2019) 99(4):379–85. doi: 10.2340/00015555-3120
81. Azimi E, Reddy VB, Shade KC, Anthony RM, Talbot S, Pereira PJS, et al. Dual action of neurokinin-1 antagonists on mas-related gpcrs. JCI Insight (2016) 1(16):e89362. doi: 10.1172/jci.insight.89362
82. Wallengren J. Neurokinin-1 and cytokine receptors as targets for therapy of chronic prurigo. J Eur Acad Dermatol Venereol JEADV (2019) 33(12):2221–2. doi: 10.1111/jdv.16031
83. Choi JE, Di Nardo A. Skin neurogenic inflammation. Semin immunopathol (2018) 40(3):249–59. doi: 10.1007/s00281-018-0675-z
84. Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature (1985) 313(5997):54–6. doi: 10.1038/313054a0
85. McCoy ES, Taylor-Blake B, Street SE, Pribisko AL, Zheng J, Zylka MJ. Peptidergic cgrpα Primary sensory neurons encode heat and itch and tonically suppress sensitivity to cold. Neuron (2013) 78(1):138–51. doi: 10.1016/j.neuron.2013.01.030
86. Costa R, Marotta DM, Manjavachi MN, Fernandes ES, Lima-Garcia JF, Paszcuk AF, et al. Evidence for the role of neurogenic inflammation components in trypsin-elicited scratching behaviour in mice. Br J Pharmacol (2008) 154(5):1094–103. doi: 10.1038/bjp.2008.172
87. Ding W, Stohl LL, Wagner JA, Granstein RD. Calcitonin gene-related peptide biases langerhans cells toward th2-type immunity. J Immunol (Baltimore Md 1950) (2008) 181(9):6020–6. doi: 10.4049/jimmunol.181.9.6020
88. Antúnez C, Torres MJ, López S, Rodriguez-Pena R, Blanca M, Mayorga C, et al. Calcitonin gene-related peptide modulates interleukin-13 in circulating cutaneous lymphocyte-associated antigen-positive T cells in patients with atopic dermatitis. Br J Dermatol (2009) 161(3):547–53. doi: 10.1111/j.1365-2133.2009.09318.x
89. Li L, Wang X, Yu LC. Involvement of opioid receptors in the cgrp-induced antinociception in the nucleus accumbens of rats. Brain Res (2010) 1353:53–9. doi: 10.1016/j.brainres.2010.07.042
90. Neugebauer V, Mazzitelli M, Cragg B, Ji G, Navratilova E, Porreca F. Amygdala, neuropeptides, and chronic pain-related affective behaviors. Neuropharmacology (2020) 170:108052. doi: 10.1016/j.neuropharm.2020.108052
91. Dalm VA, Van Hagen PM, de Krijger RR, Kros JM, Van Koetsveld PM, van der Lely AJ, et al. Distribution pattern of somatostatin and cortistatin mrna in human central and peripheral tissues. Clin Endocrinol (2004) 60(5):625–9. doi: 10.1111/j.1365-2265.2004.02024.x
92. van Hagen PM, Dalm VA, Staal F, Hofland LJ. The role of cortistatin in the human immune system. Mol Cell Endocrinol (2008) 286(1-2):141–7. doi: 10.1016/j.mce.2008.03.007
93. Robas N, Mead E, Fidock M. Mrgx2 is a high potency cortistatin receptor expressed in dorsal root ganglion. J Biol Chem (2003) 278(45):44400–4. doi: 10.1074/jbc.M302456200
94. Al Hamwi G, Riedel YK, Clemens S, Namasivayam V, Thimm D, Müller CE. Mas-related G protein-coupled receptors X (Mrgprx): orphan gpcrs with potential as targets for future drugs. Pharmacol Ther (2022) 238:108259. doi: 10.1016/j.pharmthera.2022.108259
95. Johansson O, Liang Y, Emtestam L. Increased nerve growth factor- and tyrosine kinase a-like immunoreactivities in prurigo nodularis skin – an exploration of the cause of neurohyperplasia. Arch Dermatol Res (2002) 293(12):614–9. doi: 10.1007/s00403-001-0285-8
96. Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev (2006) 86(4):1309–79. doi: 10.1152/physrev.00026.2005
97. Yamaoka J, Di ZH, Sun W, Kawana S. Changes in cutaneous sensory nerve fibers induced by skin-scratching in mice. J Dermatol Sci (2007) 46(1):41–51. doi: 10.1016/j.jdermsci.2006.12.007
98. Verge VM, Richardson PM, Wiesenfeld-Hallin Z, Hökfelt T. Differential influence of nerve growth factor on neuropeptide expression in vivo: A novel role in peptide suppression in adult sensory neurons. J Neurosci Off J Soc Neurosci (1995) 15(3 Pt 1):2081–96. doi: 10.1523/jneurosci.15-03-02081.1995
99. Rukwied RR, Main M, Weinkauf B, Schmelz M. Ngf sensitizes nociceptors for cowhage- but not histamine-induced itch in human skin. J Invest Dermatol (2013) 133(1):268–70. doi: 10.1038/jid.2012.242
100. Krull C, Schoepke N, Ohanyan T, Brachaczek M, Maurer M, Lange-Asschenfeldt B, et al. Increased angiogenesis and vegf expression correlates with disease severity in prurigo patients. J Eur Acad Dermatol Venereol JEADV (2016) 30(8):1357–61. doi: 10.1111/jdv.13406
101. Krause K, Krull C, Kessler B, Lange-Asschenfeldt B, Maurer M, Metz M. Effective control of recalcitrant pruritus by bevacizumab: A possible role for vascular endothelial growth factor in chronic itch? Acta dermato-venereol (2013) 93(2):175–9. doi: 10.2340/00015555-1445
102. Ji W, Liang J, Zhang Z. Endothelin B receptors exert antipruritic effects via peripheral K-opioid receptors. Exp Ther Med (2012) 4(3):503–6. doi: 10.3892/etm.2012.624
103. Nakashima C, Ishida Y, Kitoh A, Otsuka A, Kabashima K. Interaction of peripheral nerves and mast cells, eosinophils, and basophils in the development of pruritus. Exp Dermatol (2019) 28(12):1405–11. doi: 10.1111/exd.14014
104. Liang Y, Jacobi HH, Marcusson JA, Haak-Frendscho M, Johansson O. Dendritic mast cells in prurigo nodularis skin. Eur J Dermatol EJD (1999) 9(4):297–9.
105. Ito Y, Satoh T, Takayama K, Miyagishi C, Walls AF, Yokozeki H. Basophil recruitment and activation in inflammatory skin diseases. Allergy (2011) 66(8):1107–13. doi: 10.1111/j.1398-9995.2011.02570.x
106. Ohsawa Y, Hirasawa N. The role of histamine H1 and H4 receptors in atopic dermatitis: from basic research to clinical study. Allergol Int Off J Japanese Soc Allergol (2014) 63(4):533–42. doi: 10.2332/allergolint.13-RA-0675
107. Chovatiya R, Paller AS. Jak inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol (2021) 148(4):927–40. doi: 10.1016/j.jaci.2021.08.009
108. Jiang H, Harris MB, Rothman P. Il-4/il-13 signaling beyond jak/stat. J Allergy Clin Immunol (2000) 105(6 Pt 1):1063–70. doi: 10.1067/mai.2000.107604
109. Philips RL, Wang Y, Cheon H, Kanno Y, Gadina M, Sartorelli V, et al. The jak-stat pathway at 30: much learned, much more to do. Cell (2022) 185(21):3857–76. doi: 10.1016/j.cell.2022.09.023
110. Huang IH, Chung WH, Wu PC, Chen CB. Jak-stat signaling pathway in the pathogenesis of atopic dermatitis: an updated review. Front Immunol (2022) 13:1068260. doi: 10.3389/fimmu.2022.1068260
111. Wang H, Byfield G, Jiang Y, Smith GW, McCloskey M, Hartnett ME. Vegf-mediated stat3 activation inhibits retinal vascularization by down-regulating local erythropoietin expression. Am J Pathol (2012) 180(3):1243–53. doi: 10.1016/j.ajpath.2011.11.031
112. Agrawal D, Sardana K, Mathachan SR, Bhardwaj M, Ahuja A, Jain S. A prospective study examining the expression of stat 1, 3, 6 in prurigo nodularis lesions with its immunopathogenic and therapeutic implications. J cosmetic Dermatol (2022) 21(9):4009–15. doi: 10.1111/jocd.14709
113. Agrawal D, Sardana K, Mathachan SR, Bhardwaj M, Ahuja A, Jain S. A prospective study examining the effect of selected topical and systemic drugs on pruritus grading system score and stat 6 expression in patients of prurigo nodularis. Indian J Dermatol (2021) 66(6):638–44. doi: 10.4103/ijd.ijd_341_21
114. Fukushi S, Yamasaki K, Aiba S. Nuclear localization of activated stat6 and stat3 in epidermis of prurigo nodularis. Br J Dermatol (2011) 165(5):990–6. doi: 10.1111/j.1365-2133.2011.10498.x
115. Yew YW, Yeo PM. Comparison between dupilumab and oral janus kinase inhibitors in the treatment of prurigo nodularis with or without atopic dermatitis in a tertiary care center in Singapore. JAAD Int (2023) 13:13–4. doi: 10.1016/j.jdin.2023.06.005
116. Peng C, Li C, Zhou Y, Wang Q, Xie P, Li T, et al. Tofacitinib for prurigo nodularis: A case report. Clinical cosmetic investigational Dermatol (2022) 15:503–6. doi: 10.2147/ccid.S354025
117. Liu T, Chu Y, Wang Y, Zhong X, Yang C, Bai J, et al. Successful treatment of prurigo nodularis with tofacitinib: the experience from a single center. Int J Dermatol (2023) 62(5):e293–e5. doi: 10.1111/ijd.16568
118. Molloy OE, Kearney N, Byrne N, Kirby B. Successful treatment of recalcitrant nodular prurigo with tofacitinib. Clin Exp Dermatol (2020) 45(7):918–20. doi: 10.1111/ced.14320
119. Gil-Lianes J, Morgado-Carrasco D, Riquelme-Mc Loughlin C. Treatment of chronic prurigo with upadacitinib: A case series. J Eur Acad Dermatol Venereol JEADV (2023). doi: 10.1111/jdv.19462. [published online ahead of print, 2023 Aug 23]
120. Yin M, Wu R, Chen J, Dou X. Successful treatment of refractory prurigo nodularis with baricitinib. Dermatol Ther (2022) 35(8):e15642. doi: 10.1111/dth.15642
121. Pereira MP, Zeidler C, Ständer S. Improvement of chronic nodular prurigo with baricitinib. J Eur Acad Dermatol Venereol JEADV (2022) 36(6):e486–e8. doi: 10.1111/jdv.17991
122. He Y, Ji S, Yu Q. Effectiveness of baricitinib in prurigo-type atopic dermatitis: A case report. Dermatol Ther (2021) 34(2):e14878. doi: 10.1111/dth.14878
123. Agrawal D, Sardana K, Mathachan SR, Ahuja A. A case of recalcitrant prurigo nodularis with heightened expression of stat 3 and stat 6 and its dramatic response to tofacitinib. Indian Dermatol Online J (2023) 14(4):564–6. doi: 10.4103/idoj.idoj_508_22
124. Fakhraie S, Chovatiya R. Janus kinase inhibition in the treatment of prurigo nodularis. Dermatitis contact atopic occupational Drug (2023). doi: 10.1089/derm.2023.0036. [published online ahead of print, 2023 Aug 24]
125. Sutaria N, Adawi W, Goldberg R, Roh YS, Choi J, Kwatra SG. Itch: pathogenesis and treatment. J Am Acad Dermatol (2022) 86(1):17–34. doi: 10.1016/j.jaad.2021.07.078
126. Moore C, Gupta R, Jordt SE, Chen Y, Liedtke WB. Regulation of pain and itch by trp channels. Neurosci Bull (2018) 34(1):120–42. doi: 10.1007/s12264-017-0200-8
127. Ständer S, Moormann C, Schumacher M, Buddenkotte J, Artuc M, Shpacovitch V, et al. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp Dermatol (2004) 13(3):129–39. doi: 10.1111/j.0906-6705.2004.0178.x
128. Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, et al. A sensory neuron-expressed il-31 receptor mediates T helper cell-dependent itch: involvement of trpv1 and trpa1. J Allergy Clin Immunol (2014) 133(2):448–60. doi: 10.1016/j.jaci.2013.10.048
129. Oh MH, Oh SY, Lu J, Lou H, Myers AC, Zhu Z, et al. Trpa1-dependent pruritus in il-13-induced chronic atopic dermatitis. J Immunol (Baltimore Md 1950) (2013) 191(11):5371–82. doi: 10.4049/jimmunol.1300300
130. Liang J, Ji Q, Ji W. Role of transient receptor potential ankyrin subfamily member 1 in pruritus induced by endothelin-1. Neurosci Lett (2011) 492(3):175–8. doi: 10.1016/j.neulet.2011.02.009
131. Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, et al. Trpa1 is required for histamine-independent, mas-related G protein-coupled receptor-mediated itch. Nat Neurosci (2011) 14(5):595–602. doi: 10.1038/nn.2789
132. Zhang X, Huang J, McNaughton PA. Ngf rapidly increases membrane expression of trpv1 heat-gated ion channels. EMBO J (2005) 24(24):4211–23. doi: 10.1038/sj.emboj.7600893
133. Patowary P, Pathak MP, Zaman K, Raju PS, Chattopadhyay P. Research progress of capsaicin responses to various pharmacological challenges. Biomed pharmacother = Biomed pharmacother (2017) 96:1501–12. doi: 10.1016/j.biopha.2017.11.124
134. Yosipovitch G, Mollanazar N, Ständer S, Kwatra SG, Kim BS, Laws E, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med (2023) 29(5):1180–90. doi: 10.1038/s41591-023-02320-9
135. Fachler T, Maria Faitataziadou S, Molho-Pessach V. Dupilumab for pediatric prurigo nodularis: A case report. Pediatr Dermatol (2021) 38(1):334–5. doi: 10.1111/pde.14464
136. Fang HY, Lian CH. The effectiveness and safety of dupilumab in the management of refractory prurigo nodularis in 45 chinese patients: A real-life observational study. J Dermatol (2023) 50(8):1084–7. doi: 10.1111/1346-8138.16803
137. Giovannini M, Mori F, Oranges T, Ricci S, Barni S, Canessa C, et al. Dupilumab treatment of prurigo nodularis in an adolescent. Eur J Dermatol EJD (2021) 31(1):104–6. doi: 10.1684/ejd.2020.3947
138. Husein-ElAhmed H, Steinhoff M. Dupilumab in prurigo nodularis: A systematic review of current evidence and analysis of predictive factors to response. J Dermatol Treat (2022) 33(3):1547–53. doi: 10.1080/09546634.2020.1853024
139. Georgakopoulos JR, Croitoru D, Felfeli T, Alhusayen R, Lansang P, Shear NH, et al. Long-term dupilumab treatment for chronic refractory generalized prurigo nodularis: A retrospective cohort study. J Am Acad Dermatol (2021) 85(4):1049–51. doi: 10.1016/j.jaad.2021.02.038
140. Selvaraj KJ, Stewart T, Frew JW. Maintenance of response to dupilumab in prurigo nodularis: A retrospective cohort study. JAAD Int (2023) 11:143–4. doi: 10.1016/j.jdin.2023.02.006
141. Pezzolo E, Gambardella A, Guanti M, Bianchelli T, Bertoldi A, Giacchetti A, et al. Tralokinumab shows clinical improvement in patients with prurigo nodularis-like phenotype atopic dermatitis: A multicenter, prospective, open-label case series study. J Am Acad Dermatol (2023) 89(2):430–2. doi: 10.1016/j.jaad.2023.04.056
142. Ständer S, Yosipovitch G, Legat FJ, Lacour JP, Paul C, Narbutt J, et al. Trial of nemolizumab in moderate-to-severe prurigo nodularis. New Engl J Med (2020) 382(8):706–16. doi: 10.1056/NEJMoa1908316
143. Galderma. Galderma Announces Positive Data from Phase Iii Trial, Demonstrating Efficacy and Safety of Nemolizumab in Patients with Prurigo Nodularis (2022). Available at: https://www.galderma.com/news/galderma-announces-positive-data-phase-iii-trial-demonstrating-efficacy-and-safety-nemolizumab (Accessed 25, 2023).
144. Hassani M, Koenderman L. Immunological and hematological effects of il-5(Rα)-targeted therapy: an overview. Allergy (2018) 73(10):1979–88. doi: 10.1111/all.13451
145. Zhang S, Sumpter TL, Kaplan DH. Neuron-Mast cell cross-talk in the skin. J Invest Dermatol (2022) 142(3 Pt B):841–8. doi: 10.1016/j.jid.2021.10.006
146. Gupta K, Harvima IT. Mast cell-neural interactions contribute to pain and itch. Immunol Rev (2018) 282(1):168–87. doi: 10.1111/imr.12622
147. Alvarado D, Maurer M, Gedrich R, Seibel SB, Murphy MB, Crew L, et al. Anti-kit monoclonal antibody cdx-0159 induces profound and durable mast cell suppression in a healthy volunteer study. Allergy (2022) 77(8):2393–403. doi: 10.1111/all.15262
148. Patel JR, Joel MZ, Lee KK, Kambala A, Cornman H, Oladipo O, et al. Single-cell rna sequencing reveals dysregulated fibroblast subclusters in prurigo nodularis. bioRxiv preprint server Biol (2023) 2023.01.29.526050. doi: 10.1101/2023.01.29.526050
149. Trier AM, Kim BS. Insights into atopic dermatitis pathogenesis lead to newly approved systemic therapies. Br J Dermatol (2023) 188(6):698–708. doi: 10.1093/bjd/ljac016
150. Lee YW, Won CH, Jung K, Nam HJ, Choi G, Park YH, et al. Efficacy and safety of pac-14028 cream - a novel, topical, nonsteroidal, selective trpv1 antagonist in patients with mild-to-moderate atopic dermatitis: A phase iib randomized trial. Br J Dermatol (2019) 180(5):1030–8. doi: 10.1111/bjd.17455
151. Park CW, Kim BJ, Lee YW, Won C, Park CO, Chung BY, et al. Asivatrep, a trpv1 antagonist, for the topical treatment of atopic dermatitis: phase 3, randomized, vehicle-controlled study (Captain-ad). J Allergy Clin Immunol (2022) 149(4):1340–7.e4. doi: 10.1016/j.jaci.2021.09.024
152. Avila C, Massick S, Kaffenberger BH, Kwatra SG, Bechtel M. Cannabinoids for the treatment of chronic pruritus: A review. J Am Acad Dermatol (2020) 82(5):1205–12. doi: 10.1016/j.jaad.2020.01.036
153. Massimini M, Dalle Vedove E, Bachetti B, Di Pierro F, Ribecco C, D'Addario C, et al. Polyphenols and cannabidiol modulate transcriptional regulation of th1/th2 inflammatory genes related to canine atopic dermatitis. Front vet Sci (2021) 8:606197. doi: 10.3389/fvets.2021.606197
154. Todurga Seven ZG, Çakır Gündoğdu A, Ozyurt R, Özyazgan S. The effects of cannabinoid agonist, heat shock protein 90 and nitric oxide synthase inhibitors on increasing il-13 and il-31 levels in chronic pruritus. Immunol investigations (2022) 51(7):1938–49. doi: 10.1080/08820139.2022.2083973
155. Haruna T, Soga M, Morioka Y, Hikita I, Imura K, Furue Y, et al. S-777469, a novel cannabinoid type 2 receptor agonist, suppresses itch-associated scratching behavior in rodents through inhibition of itch signal transmission. Pharmacology (2015) 95(1-2):95–103. doi: 10.1159/000371890
156. Visse K, Blome C, Phan NQ, Augustin M, Ständer S. Efficacy of body lotion containing N-palmitoylethanolamine in subjects with chronic pruritus due to dry skin: A dermatocosmetic study. Acta dermato-venereol (2017) 97(5):639–41. doi: 10.2340/00015555-2593
157. Pulvirenti N, Nasca MR, Micali G. Topical adelmidrol 2% Emulsion, a novel aliamide, in the treatment of mild atopic dermatitis in pediatric subjects: A pilot study. Acta dermatovenerol Croatica ADC (2007) 15(2):80–3.
158. Yuan C, Wang XM, Guichard A, Tan YM, Qian CY, Yang LJ, et al. N-palmitoylethanolamine and N-acetylethanolamine are effective in asteatotic eczema: results of a randomized, double-blind, controlled study in 60 patients. Clin Interventions Aging (2014) 9:1163–9. doi: 10.2147/cia.S65448
159. Ständer S, Reinhardt HW, Luger TA. Topical cannabinoid agonists. An effective new possibility for treating chronic pruritus. Der Hautarzt; Z fur Dermatol Venerol und verwandte Gebiete (2006) 57(9):801–7. doi: 10.1007/s00105-006-1180-1
160. Kim BS, Inan S, Ständer S, Sciascia T, Szepietowski JC, Yosipovitch G. Role of kappa-opioid and mu-opioid receptors in pruritus: peripheral and central itch circuits. Exp Dermatol (2022) 31(12):1900–7. doi: 10.1111/exd.14669
161. Weisshaar E, Szepietowski JC, Bernhard JD, Hait H, Legat FJ, Nattkemper L, et al. Efficacy and safety of oral nalbuphine extended release in prurigo nodularis: results of a phase 2 randomized controlled trial with an open-label extension phase. J Eur Acad Dermatol Venereol JEADV (2022) 36(3):453–61. doi: 10.1111/jdv.17816
162. Sofen H, Bissonnette R, Yosipovitch G, Silverberg JI, Tyring S, Loo WJ, et al. Efficacy and safety of vixarelimab, a human monoclonal oncostatin M receptor B Antibody, in moderate-to-severe prurigo nodularis: A randomised, double-blind, placebo-controlled, phase 2a study. EClinicalMedicine (2023) 57:101826. doi: 10.1016/j.eclinm.2023.101826
163. Ständer S. Nalbuphine: aspiring to become another treatment for prurigo nodularis? (2022). Available at: https://conferences.medicom-publishers.com/specialisation/dermatology/eadv-2022/nalbuphine-aspiring-to-become-another-treatment-for-prurigo-nodularis (Accessed 25, 2023).
Keywords: prurigo nodularis, pruritus, pruritogen, pathogenetic mechanism, therapeutic target
Citation: Shao Y, Wang D, Zhu Y, Xiao Z, Jin T, Peng L, Shen Y and Tang H (2023) Molecular mechanisms of pruritus in prurigo nodularis. Front. Immunol. 14:1301817. doi: 10.3389/fimmu.2023.1301817
Received: 25 September 2023; Accepted: 13 November 2023;
Published: 23 November 2023.
Edited by:
Jian Gao, Shanghai Children’s Medical Center, ChinaReviewed by:
Yan Zhou, The First Affiliated Hospital of Xi’an Jiaotong University, ChinaJianjun Qiao, Zhejiang University, China
Jie Li, Central South University, China
Copyright © 2023 Shao, Wang, Zhu, Xiao, Jin, Peng, Shen and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Tang, dGFuZ2h1aWh1YXNoYW5AMTYzLmNvbQ==
†These authors have contributed equally to this work
 Yixin Shao
Yixin Shao Duoqin Wang†
Duoqin Wang†