- Department of Dermatology, Xijing Hospital, Fourth Military Medical University, Xi’an, China
Vitiligo is an autoimmune disease that leads to disfiguring depigmented lesions of skin and mucosa. Although effective treatments are available for vitiligo, there are still some patients with poor responses to conventional treatment. Refractory vitiligo lesions are mostly located on exposed sites such as acral sites and lips, leading to significant life stress. Understanding the causes of refractory vitiligo and developing targeted treatments are essential to enhance vitiligo outcomes. In this review, we summarized recent treatment approaches and some potential methods for refractory vitiligo. Janus kinase inhibitors have shown efficacy in refractory vitiligo. A variety of surgical interventions and fractional carbon dioxide laser have been widely applied to combination therapies. Furthermore, melanocyte regeneration and activation therapies are potentially effective strategies. Patients with refractory vitiligo should be referred to psychological monitoring and interventions to reduce the potential pathogenic effects of chronic stress. Finally, methods for depigmentation and camouflage may be beneficial in achieving uniform skin color and improved quality of life. Our ultimate focus is to provide alternative options for refractory vitiligo and to bring inspiration to future research.
1 Introduction
Vitiligo is a chronic depigmenting disease resulting from disordered immunity and the loss of functional melanocytes (1). The clinical presentation is mainly the presence of depigmented skin and mucosa. As a disfiguring disease, vitiligo affects about 0.5% to 2% of the world’s population without gender bias (2, 3). Whether or not the lesions occur on exposed parts of the skin, vitiligo patients usually have cosmetic anxiety and reduced quality of life (QoL). So far, autoimmunity theory, melanocyte adhesion theory, somatic mosaicism theory, neural theory, microvascular theory, and genetics have all been proposed to explain vitiligo pathogenesis (4). Among the numerous established theories, autoimmunity is the most popular one. It is generally recognized that the aberrantly enhanced innate immunity combined with adaptive immunity promotes the targeted killing of melanocytes by CD8+ cytotoxic T-lymphocytes (5, 6). Since the pathogenesis is not entirely clear, there is no single magic bullet in vitiligo management.
Based on the understanding of the mechanism, vitiligo has two main therapeutic goals, one is to prevent disease progression by controlling the abnormal inflammatory state; the second is to induce re-pigmentation by improving the quantity and function of melanocytes in the lesions (7, 8). Vitiligo patients exist in the condition of progressive or stable periods, and there are different treatments for different periods. Thus far, first-line therapy for patients with stable vitiligo usually consists of narrow-band UVB (NB-UVB), topical/systemic corticosteroids, and calcineurin inhibitor therapies (9). Steroid oral minipulse therapy is usually recommended for patients with rapid progression (10). As a Janus kinase (JAK) 1/JAK2 inhibitor, ruxolitinib has passed the clinical phase III trial and has already been approved by the Food and Drug Administration for the treatment of nonsegmental vitiligo (11). While many patients are adequately treated with conventional therapies, a subset of vitiligo patients fail to respond to these treatments and remain refractory.
There is no consensus definition of “refractory” vitiligo, but the term implies the persistence of the disease and resistance to medication/phototherapy (12). Refractory vitiligo has some anatomical location preferences, such as acral and joint sites, bony prominences, and lip (13, 14). There are several potential reasons for the preference of these locations. Most popular theories hold that refractory lesions have fewer pilosebaceous follicles and perilesional melanocytes (15). In addition, these low-response areas have a greater susceptibility to repeated friction and traumatic stimulation. Due to the tissue structure and repeated friction experience, lesions of refractory vitiligo have relatively thicker stratum corneum, which likely affects the penetration of topical drugs and phototherapy (16). Moreover, lesions with white hair are more difficult to get re-pigmentation, since white hair follicles have a lower percentage of intact melanocyte reservoirs than black hair follicles (17). White hair is also one of the characteristics of segmental vitiligo. Segmental vitiligo progresses rapidly and usually damages the follicular melanocyte reservoirs at the early stage (18). This may explain why segmental vitiligo is less responsive to conventional treatment. Psychological stressors are also considered as potential vitiligo triggers (19). The possible causes of refractory vitiligo are summarized in Figure 1. Firstly, the absence of pilosebaceous follicles and melanocyte stem cells/melanocytes at the lesions resembles scarce “seeds” for melanin production. Secondly, chronic psychological stress and friction stimulation create an “atrocious climate” for melanocyte survival. Thirdly, abnormal local immunity and thick stratum corneum result in an “aberrant soil” for melanocyte growth and drug effect. In aggregate, these factors may individually or collectively impede melanocyte survival and hinder melanin production, ultimately contributing to refractory vitiligo. Various medicines were used in individuals suffering from refractory vitiligo, yet they often showed poor effects. The chronic disease duration and poor therapeutic effects can result in persistent depression and low QoL in refractory vitiligo patients.
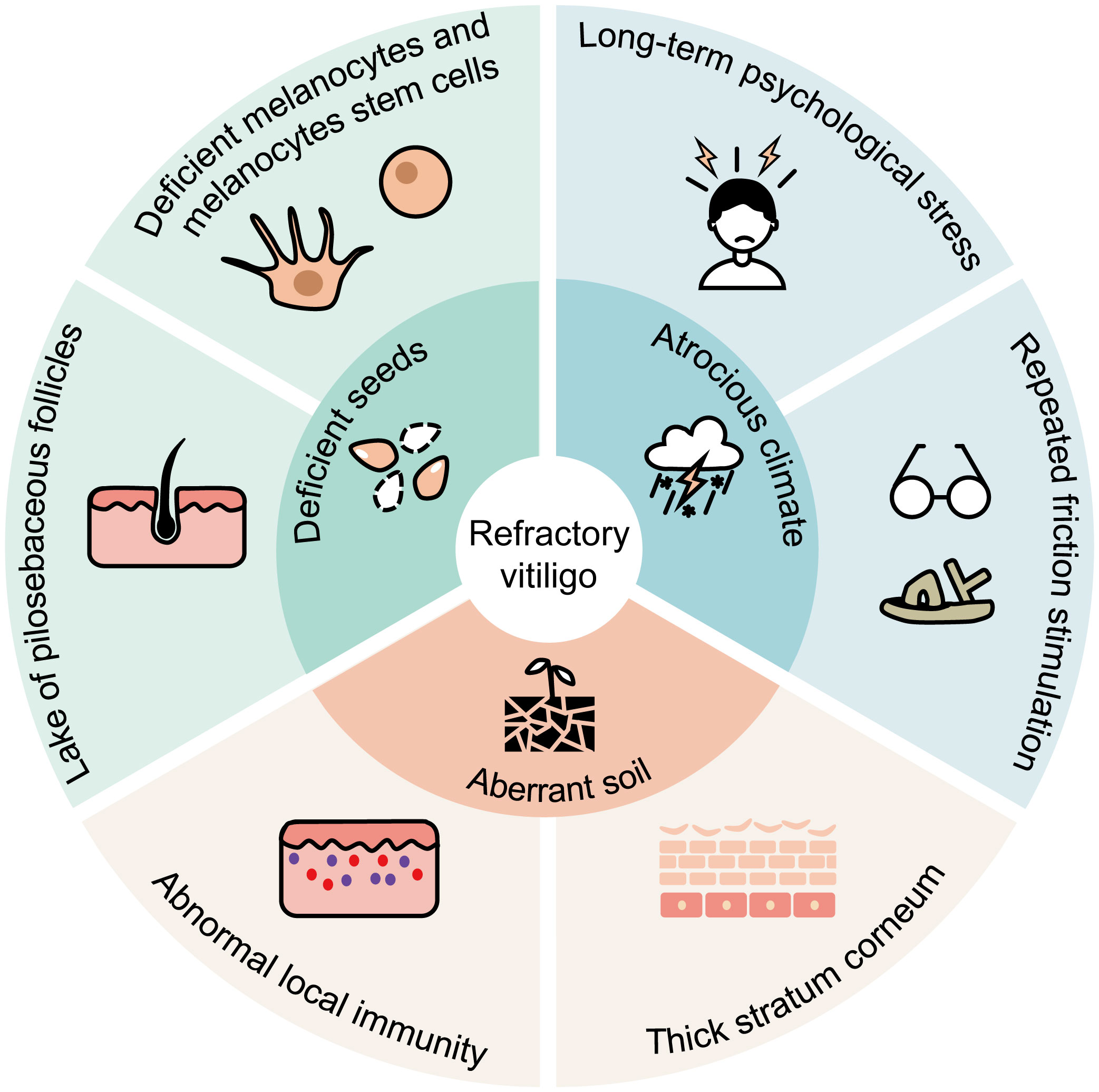
Figure 1 Clinical images depict a vitiligo patient before and after 24-weeks of continuous NB-UVB treatment, followed by recurrence at the original site after stopping treatment.
Refractory vitiligo is a puzzle in vitiligo management. Some therapeutic attempts have shown decent effects and have been widely adopted in many countries. In this review, we aim to elaborate on currently available treatment options in the treatment of refractory vitiligo and potential therapeutic strategies.
2 JAK inhibitor therapy
Previous study has confirmed that Interferon (IFN)-γ and CD8+ T cells play a central role in the pathophysiology of vitiligo (20). IFN-γ can activate the JAK and signal transducer and activator of transcription 1 (STAT1) signaling pathway by binding with its receptor, and eventually result in further recruitment of CD8+ T cells to skin lesions and melanocyte destruction (21). This process can be inhibited using JAK inhibitors such as ruxolitinib and tofacitinib (22, 23). Therefore, JAK inhibitors have recently been extensively evaluated in multiple phase II and III clinical trials, demonstrating remarkable efficacy in vitiligo management (11, 24).
Recently, several JAK inhibitors appear to be effective in patients with refractory vitiligo. As a first-generation JAK1/3 inhibitor, oral tofacitinib in combination with NB-UVB and topical calcineurin inhibitors therapy can achieve significant Vitiligo Area Severity Index (VASI) improvements on the acral regions, trunk, and extremities after 16-week treatment compared with the control group (25). Baricitinib, a newer JAK1/2 inhibitor, was reported to provide satisfactory re-pigmentation and good tolerance for patients with generalized refractory vitiligo (26). Baricitinib has also been found to promote tyrosinase activity, melanin content, and tyrosinase, tyrosinase-related protein-1 gene expression of melanocytes damaged model in vitro (27). As an oral selective JAK1 inhibitor, upadacitinib monotherapy caused an average improvement of 38.65% in VASI for 12 refractory patients and ameliorated their quality of life (28). No serious adverse events were noted in these studies. Similar to other treatments, JAK inhibitors have a better re-pigmentation effect on the facial area, which may be due to the high density of hair follicles and chronic sun exposure (29). As non-invasive treatments, JAK inhibitors can be administered to patients with progressing vitiligo. To date, small case series and clinical studies have been reported that JAK inhibitors are relatively safe and effective target therapies for refractory vitiligo. Nevertheless, the current research is still insufficient, and further study is needed to provide more evidence to prove the efficacy of JAK inhibitors.
3 Surgical intervention
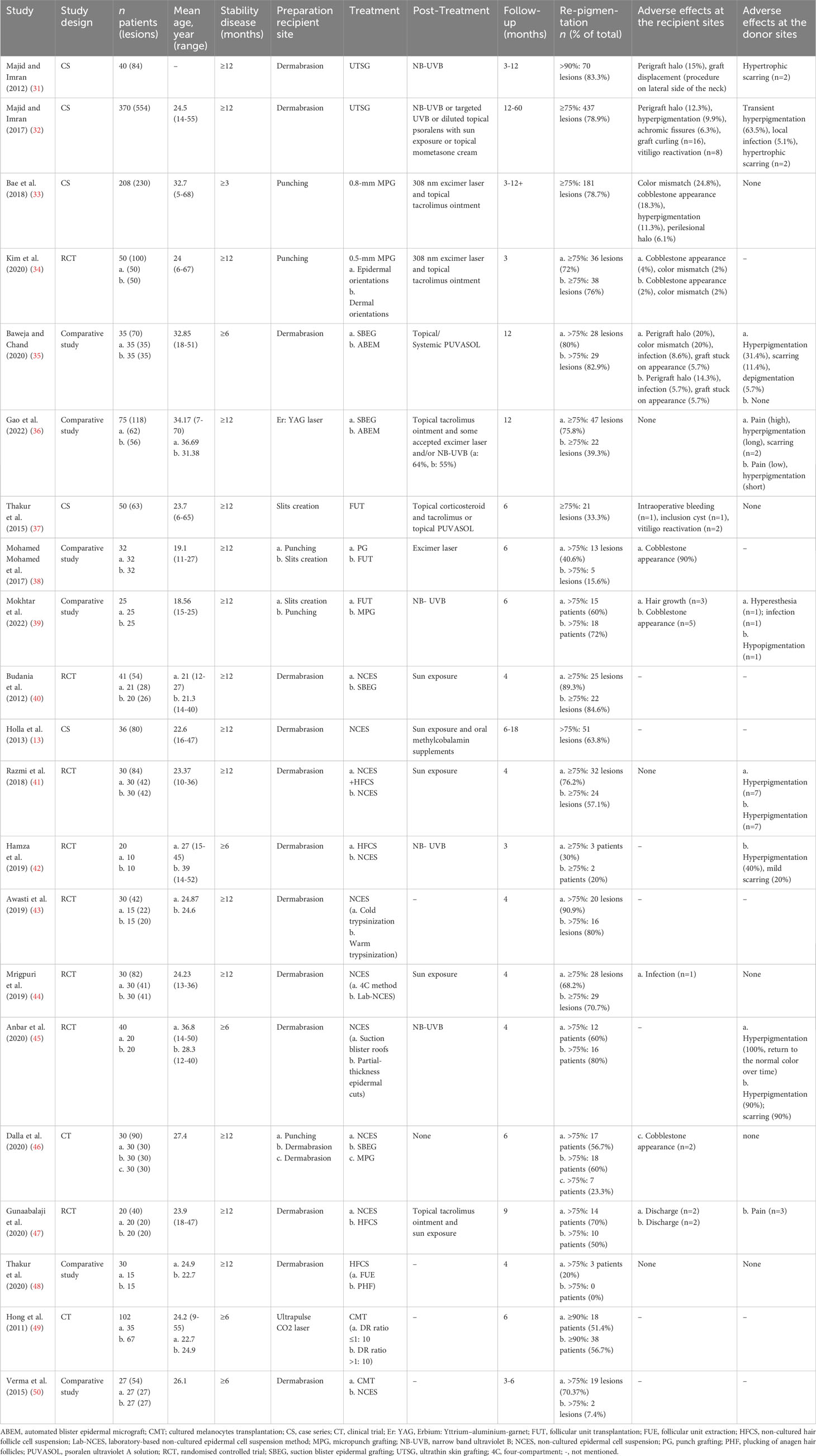
Insufficient local melanocyte reservoirs may be a primary obstacle for re-pigmentation in refractory vitiligo. Surgical intervention can be used to solve this problem. Dermatologists can replenish melanocytes or melanocyte stem cells for vitiligo lesions by various transplantation techniques. Generally, surgical intervention can be roughly classified into two categories, tissue grafting and cellular grafting (30). Tissue grafting has a long history of application with relatively simple operations. In recent years, cellular grafting has advanced significantly. There are continuous methodological and technical innovations in both surgical types. However, each surgical intervention has its pros and cons, and clinicians should apply them according to the individual conditions of vitiligo patients (Table 1).
3.1 Tissue grafting
3.1.1 Thin skin grafting
The earliest reported surgical intervention is thin skin grafting. After the administration of local anesthetics, skin grafts of a certain thickness could be harvested using manual or motorized dermatomes. Ultrathin skin grafting has been the main method in thin skin grafting with grafts composed entirely of epidermis. A retrospective analysis of 370 vitiligo patients has shown that 78.9% of lesions demonstrated excellent response after ultrathin skin grafting, and >98% pigmentation could be maintained for 4 years (32). However, thin skin grafting requires specialized surgical techniques, and the obtained grafts are irregular in shape and cannot be fully utilized. The common side effects of thin skin grafting are a “perigraft halo” of depigmentation in the recipient site and hypertrophic scarring in the donor site (31). Due to greater trauma and lower utilization, thin skin grafting has become less commonly used nowadays.
3.1.2 Punch grafting
PG is a cost-effective and easy-implementation method, which enables operation in an outpatient setting. The simplest method is to transfer 1-1.5 mm superficial grafts directly from donor to recipient sites. A randomized controlled trial has shown that superficial grafts had pigmentation effects similar to deep grafts with fewer side effects (51). Due to the small size of grafts, punch grafting is suitable for irregular lesions. In recent years, motorized 0.8-mm micropunch grafting (MPG) can produce uniform and elaborate grafts. A retrospective study has indicated that 78.7% (181/230) lesions achieved ≥75% re-pigmentation after a median of 6 months (33). Following that, one study has demonstrated that motorized 0.5-mm micropunch grafting could be used irrespective of the graft orientation (34). Micro-punch technologies are convenient with reduced appearance of cobblestone. However, the British Association of Dermatologists guidelines have indicated that there is insufficient evidence to recommend punch grafting in vitiligo (52). This is possible because punch grafting has seldom clinical trials in larger cohorts and relatively high evidence of cobblestone appearance.
3.1.3 Suction blister grafting
Suction blister grafting (SBEG) is performed using suction devices to create local negative pressure and produce blisters. Subsequently, the roots of blisters will be collected and transplanted to the recipient area after dermabrasion (53, 54). SBEG has the advantage of low cost and no scar formation with good cosmetic results. SBEG is time-consuming and painful, while automated blister epidermal micrograft (ABEM) can alleviate these shortcomings (35). Similarly, SBEG is not appropriate for large-sized lesions, as the donor-recipient size ratio (DR ratio) is 1:1. Although ABEM is expensive, it is relatively convenient and provides a larger transplanting area. In a comparative study involving 75 patients, SBEG had better re-pigmentation than ABEM, while ABEM allowed for improved treatment satisfaction with a better operative experience (36).
3.1.4 Follicular unit transplantation
Follicular unit transplantation (FUT) is characterized by the ability to transplant hair follicles unit with undifferentiated melanocyte stem cell reservoirs (37). After anesthesia, the conventional method uses a 1-mm biopsy punch or surgical removal of rectangular fragment skin to get hair follicles from the occipital region and then transplants them to the recipient areas. FUT is a labor-intensive, safe, and effective procedure with insignificant scarring. Analogous eyelash transplantation can be used to treat vitiligo-associated eyelash leucotrichia (55). Recently, minimally invasive transplantation and cosmetic technology of FUT have been developed. Using a trichiasis electrolyzer, depigmented hair follicles could be damaged and removed before transplanting to reduce depigmented hair regeneration (56). One comparative study on MPG and FUT has found that FUT had a relatively slow process of re-pigmentation, potentially because melanocytes took a long time to migrate from the hair follicle to the epidermis (39). One point to note is that FUT usually results in undesired terminal hair growth. According to a series of case reports, some methods of FUT can induce re-pigmentation while limiting additional hair growth at the same time. First, abdominal vellus hair punch grafts could be a treatment option for preventing undesired hair growth (57). Moreover, non-intact hair bulb follicle transplantation may cause less hairy and have higher cosmetic efficacy than intact hair bulbs (58).
3.2 Cellular grafting
3.2.1 Non-cultured epidermal cell suspension transplantation
In recent years, cellular grafting has advanced rapidly. Non-cultured epidermal cell suspension (NCES) transplantation is the most common technique of cellular grafting (59). The skin used for the suspension can be prepared from partial-thickness epidermal cuts or suction blister roof grafts (45). After that, cell suspensions are extracted by laboratory methods and transplanted into de-epithelialized recipient sites. With good hair re-pigmentation, the DR ratio of NCES transplantation can be as high as 1:5-1:10 (60). In a randomized controlled study involving 41 patients with stable vitiligo, NCES has been reported to be significantly superior to SBEG in terms of re-pigmentation at 16 weeks post-surgery (40). On the other hand, NCES preparation requires a complicated laboratory setup, some studies have concentrated on streamlining processes and improving treatment efficacy. The quality of cell suspension preparation is significantly related to treatment outcome. Cold trypsinization outperforms warm trypsinization in the preparation of cell suspension while providing a higher yield of viable melanocytes (43). The four-compartment (4C) method for epidermal cell suspension preparation has been shown to reduce costs and simplify conventional NCES preparation (44). What calls for special attention is that the donor-to-recipient expansion ratios should be considered in clinical practice. A recent systematic review showed that higher expansion ratios had a close relationship with lower re-pigmentation percentages (61).
3.2.2 Non-cultured hair follicle cell suspension transplantation
Hair follicle cell suspension (HFCS) collects follicular units and has comparable effective re-pigmentation to NCES, with fewer transplanted cells and donor area complications (42, 47). Hair follicles, especially the outer root sheath, contain plenty of melanocyte stem cells for excellent pigmentation. Therefore, dermatologists should pay attention to protecting these areas when harvesting hair follicles. Follicular unit extraction has better re-pigmentation than plucking of anagen hair follicles, which is likely attributed to better protection of the outer root sheath of hair follicles (48). Optimal re-pigmentation is dependent on the number of melanocytes and hair follicle stem cells as well as skin immune homeostasis, which indicates two directions to improve the treatment effect (62). Collagenase type 1 can release cells from the outer root sheath which may help to improve the therapeutic effect of HFCS (63). In addition, a randomized self-controlled study involving 30 vitiligo patients has found that the combination of NCES and HFCS attained superior re-pigmentation than NCES in range, speed, and color matching (41). One possible explanation is that keratinocytes in NCES produce integral growth factors and support melanocyte growth (64).
3.2.3 Cultured epidermal suspension transplantation
Cultured epidermal suspension (CES) isolates melanocytes and keratinocytes from the collected skin. After secondary culture, the autologous epidermal grafts will be transplanted at the recipient lesion (65). There are two types of CES, melanocyte-keratinocyte transplantation and pure melanocyte transplantation, and both methods are complicated. Melanocyte transplantation expands melanocytes in the laboratory for several weeks before transplanting (66). The distinct advantage of CES is that the maximum DR ratio of CES can be 1: 60 with a lesion size of approximately 120 cm2 (49). A comparative study has shown that lesions treated with CES had better re-pigmentation than those treated with NCES at the 12th-week follow-up, which was probably attributed to the high density of melanocytes in CES (50). A note of caution is the choice of the donor site. It has been reported that melanocytes obtained from the face as the donor site grew fastest and had the longest total propagation time, while melanocytes from the chest and back grew the lowest (67). As a result, CES is time-consuming and expensive with harsh application conditions, which limit its application (68). Furthermore, the long-term safety of CES remains controversial. The growth factors used in melanocyte culture may increase the risk of melanoma (69).
3.2.4 Jodhpur technique
Jodhpur technique (JT) is another autologous non-cultured melanocyte plus keratinocyte grafting method. JT has a simple operation and doesn’t need trypsinization. Using a dermabrader micromotor and antibiotic ointment, upper dermal cells are collected, and then the paste-like material is spread over the dermabraded recipient site (70). JT combines the donor and recipient sites by harvesting perilesional pigmented skin, avoiding two sites of superficial wounds (71). For locations lacking the above transplant technology and specialized facilities, JT is relatively simplified and cost-effective. It should be noted that JT should be strictly used for small and stable vitiligo lesions. Since large lesions mean the need for large perilesional donor sites, using JT may cause greater trauma and a higher risk of infection. Moreover, the density of transplanted melanocytes is indistinct to evaluate in JT, which makes the treatment effect uncertain.
3.3 Precautions for selecting surgical methods and factors affecting curative effect
3.3.1 Consideration of the condition of vitiligo patients
In selecting a proper treatment, the appropriate procedure is suggested to assess the basic condition and the progression of vitiligo. First, active autoimmunity is not conducive to the survival of transplanted melanocytes. Surgical methods will have a poor effect on progressive vitiligo patients and even aggravate the condition of vitiligo. Therefore, disease stability should be evaluated before considering surgical interventions based on the scoring system, such as Vitiligo Disease Activity Score (VIDA) and Vitiligo Signs of Activity Score (VSAS) (72, 73). Surgical treatments generally have the risk of the Koebner phenomenon. For patients with stable vitiligo for at least 1 year, the Koebner phenomenon can be reduced or even prevented. Second, the size of vitiligo lesions is another point to be considered when choosing appropriate methods. Generally, tissue grafting is more suitable for small skin lesions, and cellular grafting is preferred for large lesions. Moreover, dermatologists need to consider medical and patients’ conditions, such as the medical system and circumstances of the hospital, the financial situation, and the receptivity of vitiligo patients. Finally, both dermatologists and refractory vitiligo patients are supposed to have rational expectations about surgical treatments. Young ages, segmental vitiligo, and non-acral areas are reported to be associated with better treatment outcomes (30). It should be noted that surgical interventions are not once and for all, and it is difficult to get complete re-pigmentation after a single treatment. Many factors may affect the survival of transplanted melanocytes, such as individual differences, surgical technique, and postoperative treatment.
3.3.2 Comparison of different surgical methods
Surgical interventions seem to be a good treatment choice for patients with stable and refractory vitiligo. Various surgical methods bring “seed-like” melanocytes to lesions with damaged or absent melanocytes. Compared with tissue grafting, cellular grafting has become more popular due to the higher DR ratio and better therapeutic effect. Additionally, cellular grafting has fewer deficiencies such as cobblestone appearance, which is described as an important advantage (74). Thin skin grafting requires professional skill to harvest eligible grafts, and inefficient graft collection limits its therapeutic application (75). There is no significant difference between MPG and FUT in the re-pigmentation effect (12). FUT has a longer re-pigmentation time but fewer side effects, making it suitable for exposed skin (38). NCES transplantation is a more appropriate and safe method, outperforming MPG in terms of re-pigmentation rate and color matching (46). HFCS can provide a higher density of melanocytes and hair follicle stem cells, but no significant difference compared to NCES in terms of efficacy (76). The combination of HFCS and NCES has been identified to achieve better re-pigmentation (41). Meanwhile, If the final effect of cellular grafting has a little deficiency, MPG can be an adjuvant and supplement therapy (77).
3.3.3 Postoperative adjunctive therapy for improving efficacy
Surgical methods selection and individual differences should be considered when treating refractory vitiligo. Furthermore, post-surgical dressings and recipient-site preparation can have a significant impact on graft survival and re-pigmentation outcomes (78). Moreover, to prevent graft loss, the graft sites should be protected for at least two weeks after surgical treatments. Phototherapy can improve surgical outcomes in vitiligo by stimulating melanocyte proliferation, inhibiting T lymphocytes, and suppressing cytokines (79). Therefore, phototherapy represented by NB-UVB is recommended as the standard phototherapy after melanocyte transplantation (80). The NB-UVB therapy was initiated 1 week post-surgery, with a frequency of twice a week for 6 months. Patients were treated initially with a 0.2 J/cm2 dose followed by 20% increments if tolerated (12). Other adjuvant therapies such as oral cyclosporine (3 mg·kg-1·d-1 orally for the first 3 weeks followed by 1.5 mg·kg-1·d-1 for the subsequent 6 weeks), mometasone ointment (0.1% cream twice daily for 30 days), microneedle (after topical anesthesia, received 1.5- to 2-mm needle length-assisted microneedling until pinpoint bleeding was achieved. This was done after surgical treatment 1st and 2nd months), and platelet-rich plasma (PRP) (intradermal injection of 0.1 mL of PRP using insulin syringe into each point of the transplanted graft which are placed 5 mm apart within the recipient patch. This was done at the time of the surgical procedure and monthly for the next 3 months) have been reported to result in earlier re-pigmentation (81–84). Fractional carbon dioxide (CO2) laser may reduce cobblestone appearance post-tissue transplantation (38, 85). More randomized clinical trials are required to determine the effects of these adjuvant therapies.
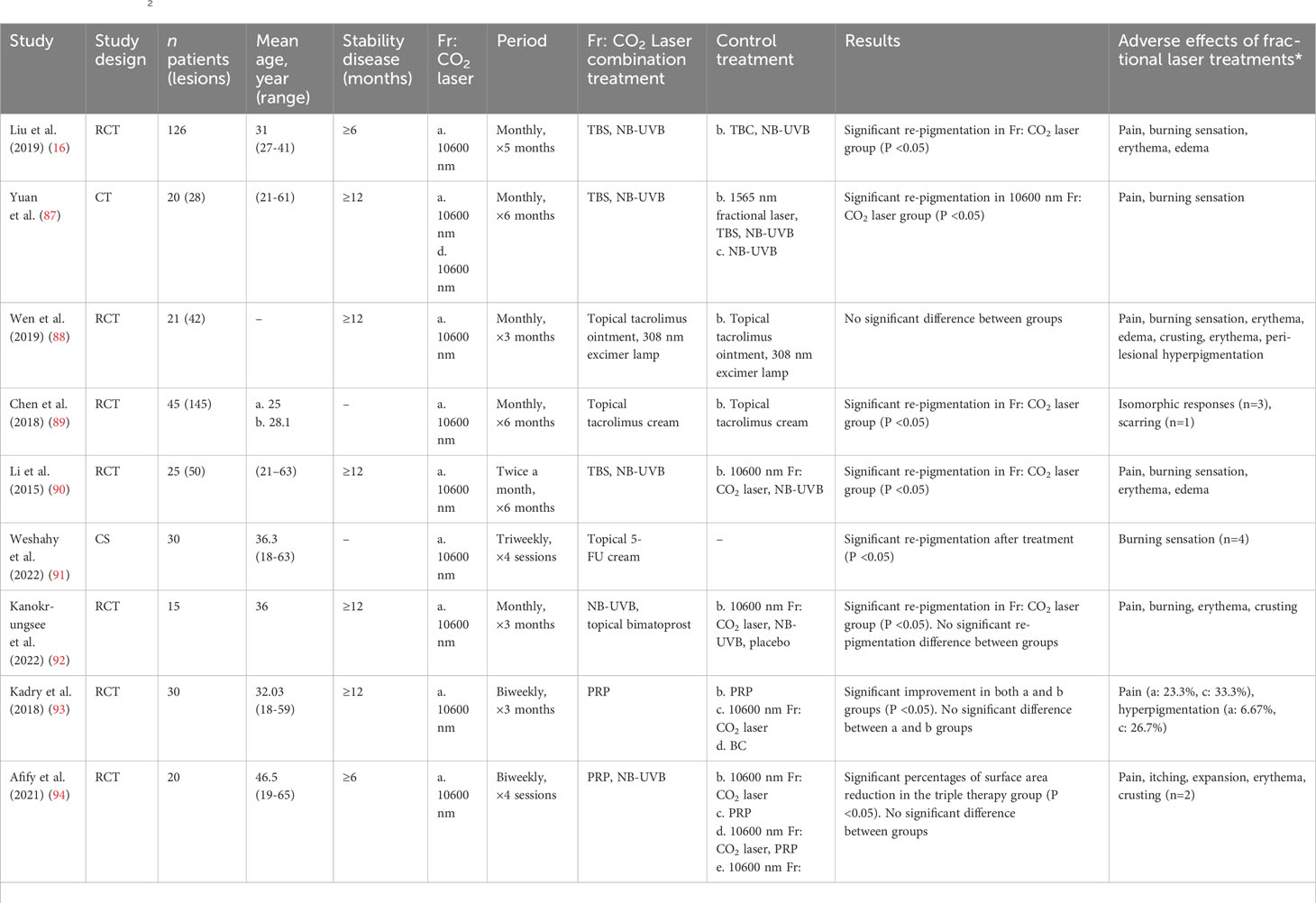
4 Transcutaneous drug delivery and re-pigmentation by fractional CO2 laser
For refractory vitiligo in the acral and bony prominent sites, the dilemma lies in the thick stratum corneum and low melanocyte density. In addition, these areas are vulnerable to chronic friction which may result in persistent abnormal immune (86). Recently, multiple clinical trials have demonstrated the effectiveness and safety of fractional CO2 laser-combined therapy. Due to the penetrating and restorative functions of fractional laser, laser-assisted drug delivery has become a feasible method for refractory vitiligo (Table 2).
4.1 Unique skin-penetrating effect of fractional CO2 laser
Although NB-UVB phototherapy is the first choice for treating vitiligo, the effects are not always satisfactory, especially in refractory vitiligo (99). Comparatively, the excimer laser can treat small and precise skin areas with high intensity, making it more effective than NB-UVB (100). Excimer laser based on the minimal blistering dose promotes a well-tolerated treatment effect for refractory vitiligo (101). Fractional CO2 laser has been introduced as a valuable “add-on” treatment modality for vitiligo (102). The most direct effect is that topical medicine can be further infiltrated through microscopic treatment zones (MTZ). MTZ are formatted by fractional photothermolysis and they can promote skin restoration (103). Various cytokines and growth factors are released during wound repair. Therefore, fractional CO2 laser may induce melanocyte migration during the inflammation and healing phases (104). Research has shown that fractional CO2 laser increases IL-4, IL-10, IL-17, and IL-23 levels, which demonstrates the restoration of the Th balance of the immune system (105). The common laser settings were as follows: pulse energy of 100 mJ, spot density ranging from 150 to 200 spots/cm2, and 2 passes over the assigned area. No anaesthesia was used during laser sessions, and the air-cooling device can provide pain relief during the procedure. The total number of treatments ranged from 1 to 10 sessions, with treatment intervals spanning from 1 week to 2 months. The duration of treatment varied between 2.5 and 5 months. The addition of fractional CO2 laser treatment has proven to be significantly advantageous for individuals with refractory vitiligo. In terms of adverse reactions, fractional CO2 laser usually induces short-lived side effects such as pain and burning sensations, but they will soon subside (106). Common adverse reactions include transient pain, erythema, oedema, and post-laser crust. Most symptoms alleviated within a day and post-laser crusting resolved within a week. Moreover, the fractional CO2 laser is not suitable for activating vitiligo due to the risk of the Koebner phenomenon.
4.2 Combination drugs of fractional CO2 laser therapy
The fractional CO2 laser can produce microscopic vertical canaliculi. Altering the skin barrier up to the depth of the dermis, the fractional laser allows topical medications and UV radiation to penetrate deeper (87). The combination of laser with medical treatment is effective in recalcitrant acral and bony prominent sites (107). Multiple medicines have been reported for laser-assisted combination therapies in recent years (84, 88–94, 98, 108). Specifically, tacrolimus and topical corticosteroids are both first-line treatments for vitiligo with definite curative effects (5, 109). The 0.1% tacrolimus ointment was applied twice daily for 6 months, along with 3 - 6 sessions of fractional CO2 laser at monthly intervals (88, 89). For topical corticosteroids, compound betamethasone dipropionate solution (2.5 mg/ml) was applied to the treated area for 2 h with gauze occlusion. The dosage was determined as 0.25 g per 1% body surface area, administered immediately after the laser treatment. The entire treatment course lasted for 5 months, with monthly intervals (16). 5-fluorouracil is a medicine for skin tumors, which may improve melanocyte migration through activation of the CXCL12/CXCR4 axis (110). Patients applied 5% 5-fluorouracil cream for 5 days consecutively following each laser treatment session. There were 4 treatment sessions, 3 weeks apart. Patients were cautioned against exposing lesions to sunlight post-session (91). Bimatoprost is a prostaglandin F2α analogue, which could improve cutaneous pigmentation by activating prostaglandin F receptor and melanosome uptake by keratinocytes (111). Bimatoprost 0.01% solution was prepared by dissolving pharmaceutical grade bimatoprost powder in an ethanol/water base. Applying one drop of each bottle over the treatment area of four-square centimeters, twice daily for 12 weeks. Conducting 3 sessions of fractional CO2 laser at monthly intervals (92). PRP is effective in healing and regeneration since it contains a variety of mitogenic/chemotactic growth factors (112, 113). Patient’s peripheral vein was aspirated for an 8 ml blood sample, which was then centrifuged at 1,500 rpm for 5 minutes. A 30-G needle was used for superficial intradermal microinjections (0.1 mL per injection, with a 1 cm spacing). Conducting 6 sessions of fractional CO2 laser with intervals of 2 weeks (93). These combinations demonstrate the broad applicability of laser-assisted treatments, which are considered relatively safe treatment options. Treatment-related adverse events, primarily attributed to fractional laser, are generally resolved without special intervention soon after they were reported. Overall, laser-assisted combination therapies promote the penetration and prolong the duration of drug action. Larger clinical trials are needed to investigate drug selection of laser-assisted treatments and improve the clinical efficacy for refractory vitiligo.
4.3 Effect of multiple laser-combination treatments
Fractional CO2 laser therapy can also enhance the efficiency of NB-UVB refractory vitiligo, and they are frequently used in conjunction with medication as triple therapies (17, 92, 94–96, 98). Similarly, the added benefit of fractional CO2 laser has been demonstrated in surgical therapies (84, 97). However, more is not always better. Triple combination therapy of fractional CO2 laser, topical tacrolimus, and 308 nm excimer lamp was not superior to double therapy (88). The failure of the treatment to achieve the expected effect may be attributed to the large molecular of tacrolimus, as well as the thin crust and tissue necrosis caused by fractional laser (114, 115). In another study, when comparing the re-pigmentation area, there was no statistically significant difference between double and triple therapies about fractional CO2 laser, PRP, and NB-UVB (94). Consequently, large-scale randomized controlled trials are required to determine the length, interval, and strategy of treatment of fractional laser when studying the combined efficacy of effective treatments. A longer period is needed to observe laser-combination treatment outcomes and more comprehensive methods to evaluate response.
4.4 Similar treatments to fractional CO2 laser
In general, MTZ and dermabrasion are the main advantages of fractional CO2 laser. The following methods may have similar beneficial effects. Fractional erbium: YAG (Er: YAG) laser is a relatively gentle laser to promote drug absorption (116). Compared with fractional CO2 laser, fractional Er: YAG laser produces a thinner beam profile and MTZ, which results in faster recovery. However, this feature also leads to a longer treatment period for re-pigmentation. Another common method for inducing drug delivery is microneedling. Microneedling is a simple and cost-effective therapy (117). The treatment experience of microneedling is less painful in comparison to fractional CO2 laser. While the standardization and precision of microneedling are relatively weaker than fractional CO2 laser. More clinical trials are needed to compare the different efficacy of these treatments.
5 Melanocyte regenerative therapies
The clinical management of refractory vitiligo is challenging. The aforementioned surgical treatment delivers melanocytes and melanocyte stem cells to lesional areas. In the lesions with thick stratum corneum, fractional laser-combination therapies improve the survival of melanocytes by ameliorating the local environment. In general, re-pigmentation in refractory vitiligo mainly depends on sufficient and available melanocytes. Being the source of melanocytes, Melanocyte stem cells are mostly located in the bulge area of hair follicles (118). The bulge area is an immune privilege site that evades the immune-killing effect. Therefore, some vitiligo patients can achieve perifollicular re-pigmentation due to the retained melanocyte stem cells. Functional melanocytes in the perilesional skin also contribute to vitiligo re-pigmentation. Moreover, studies have confirmed that stem cells that can differentiate into melanocytes are also present in the dermis of hairless vitiligo lesions (119). In general, stimulating original melanocyte stem cells and supplementing regenerative melanocyte differentiation are promising therapeutic directions for refractory vitiligo.
5.1 Promotion of melanocytes and melanocyte stem cell activation
Treatment for improving melanocyte regeneration represents a promising approach. However, most of these methods are still in the research phase. α-melanocyte-stimulating hormone (α-MSH) is a critical regulatory protein that promotes melanogenesis and melanocyte proliferation. As a potent α-MSH analog, afamelanotide has been reported to be an effective treatment option for vitiligo. In a randomized controlled trial, the administration of subcutaneous afamelanotide implants could increase the re-pigmentation rate and total area for vitiligo patients treated with NB-UVB (120). Mesenchymal stem cells can maintain the immune homeostasis of the local environment. They have been demonstrated to promote melanocyte proliferation and anti-apoptosis by targeting the phosphatase and tensin homolog/phosphatidylinositol 3 kinase/protein kinase B pathway (121). Adipose tissue extracellular fraction is detected to ameliorate the capability to counteract oxidative stress and activate the Wnt/β-catenin pathway in vitro (122). The two methods mentioned above may be helpful to improve the effect of surgical transplantation.
Moreover, research about treatment directions can also focus on the pathways of melanogenesis. Wnt signal regulation has been discovered to promote melanocyte regeneration. The Wnt/β-catenin signaling pathway can activate the transcription of melanogenesis genes such as microphthalmia-associated transcription factor (123). Microinjury caused by fractional laser has been identified to improve vitiligo pigmentation via the Wnt/β-catenin pathway (124). Furthermore, p53, transforming growth factor β, and their downstream effectors have varying roles in promoting melanogenesis (125, 126). The activation of these signal pathways appears to have positive prospects for the re-pigmentation of vitiligo.
5.2 Stem cell therapies for melanocyte regeneration
Stem cells have unlimited proliferation potential and they can differentiate into downstream lineages. Therefore, stem cell therapies hold great promise in replenishing melanocytes for refractory vitiligo. Embryonic stem cells are the first discovered stem cell line. Human embryonic stem cell-derived melanocytes have limited immunogenicity, which is promising to be used in cellular grafting for refractory vitiligo (127). However, the main restriction is the potential ethical issues due to allogeneic transplantation. It has been reported that mouse bone marrow mesenchymal stem cells could be successfully induced to melanocytes. In mouse tissue-engineering experiments, the induced melanocytes survived in transplanted tissues successfully and had similar biological functions to normal melanocytes (128). In autologous cellular therapy, adipose-derived stem cells have been reported to generate proliferative melanocyte precursors (129). Melanocyte precursor cells can be converted into melanocytes through the activation of the typical Wnt pathway and atypical Wnt signaling pathway (130). Induced pluripotent stem cells (iPSCs) have significant advantages. In addition to the conventional advantages of stem cells, iPSCs can be established from individual patients which indicates that they have fewer ethical issues than embryonic stem cells. A related study has demonstrated that human iPSC-derived melanocytes could be integrated into the mouse hair bulb and reconstitute pigmented hair follicles (131). Therefore, this method is promising to play long-term roles in vitiligo re-pigmentation.
6 Psychotherapy and adjuvant therapy
Previous research has shown that the number of vitiligo patients with depression is approximately 5 times higher than that of healthy controls (132). Various psychosocial comorbidities, such as stigmatization and sleep disturbance are also significantly increased (133). These abnormalities have a negative impact on patients’ QoL, especially in patients with refractory vitiligo. Chronic stress has been reported to induce melanocyte dysfunction by overexpressed neuropeptides, such as the substance P and neuropeptide Y (134, 135). Therefore, testing and monitoring psychological distress are recommended once vitiligo is diagnosed. Multidisciplinary treatment strategies and psychoeducation are required to address the vitiligo-associated burden of the disease (136).
To improve patients’ QoL, the following methods can be used as adjuvant therapies. Firstly, if all other treatments fail in patients with widespread vitiligo, depigmentation therapies could be an alternative option to achieve uniform skin color. Q-switched neodymium: YAG laser, bleaching creams (e.g., mono-benzyl ether of hydroquinone), trichloroacetic acid, and cryotherapy are the most common methods for depigmentation (137–141). On the other hand, cosmetic camouflage can improve the QoL of vitiligo patients, particularly in children and teenagers (142). Dihydroxyacetone, general cosmetics, and various topical camouflage agents have little impact on skin lesions (143). Furthermore, micro-pigmentation, also known as medical tattooing, could involve injecting pigment particles into lesions with needles (144). Micro-pigmentation is also an effective alternative treatment for regions with non-hair bearing and prone to friction (145). Overall, vitiligo patients need ongoing psychoeducation and professional psychotherapy when necessary. Meanwhile, the above adjuvant therapies can be appropriately considered for refractory vitiligo.
7 Future and perspective
Precisely, the primary treatment approaches for vitiligo revolve around coordinating immune homeostasis and promoting melanogenesis. Current research has recognized multiple cytokines and pathways implicated in the pathogenesis of vitiligo. To achieve ideal therapeutic efficacy, a treatment approach targeting multi-pathogenic pathways demonstrates promising potential. The recent successful example of JAK inhibitors in the treatment of vitiligo confirmed this review. JAK/STAT inhibitors not only block IFN-γ signaling, but also enhance Hedgehog and Wnt signaling in epidermal pigmentation, which are crucial for melanocyte migration, proliferation, and differentiation (146). The Wnt/β-catenin signaling pathway is also of great significance, as it potentially protects melanocytes against oxidative stress-induced damage, suppresses the differentiation of CD8+ effector T cells, and enhances the activity of regulatory T cells (147–149). For patients with active and generalized vitiligo, the administration of systemic therapies with proven curative effects and high safety profiles may be advantageous (150). In the future, targeting molecular and cellular changes associated with vitiligo will address the unmet need for treating refractory vitiligo. Before a novel single drug to cure vitiligo is developed, designing rational combination treatments based on the existing drug will become the trend.
8 Conclusion
Until today, treatment of vitiligo remains challenging. Refractory vitiligo management is representative of the vitiligo conundrum. Potentially effective therapeutic strategies aim to stimulate melanocyte regeneration and maintain an appropriate immune environment. Surgical interventions bring healthy melanocytes to depigmentation areas. Through the formation of microscopic treatment zones, fractional CO2 laser promote external medicine and phototherapy penetration. As vitiligo has serious mental health hazards, psychological testing and treatment are recommended to be included in the entire process of vitiligo. Depigmentation and camouflage treatments are meaningful for patients’ QoL improvement. Molecularly targeted therapeutics and melanocyte regeneration treatment have become the focus of interest. Although several trials have evaluated therapies for refractory vitiligo, further prospective phase III trials are sparse. Development of novel therapies is in the direction of long-acting with low side effects, to improve the durability of responses and patient compliance. Many questions regarding the progression of refractory vitiligo are still unresolved, urging us to further elucidate the pathogenesis of vitiligo and to seek additional potential treatments.
Author contributions
XW: Visualization, Writing – original draft. WW: Writing – original draft. JC: Supervision, Writing – review & editing. CL: Funding acquisition, Supervision, Writing – review & editing. SL: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by the National Natural Science Foundation of China (nos. 81930087, 82222059, and 82173416).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Picardo M, Dell'Anna ML, Ezzedine K, Hamzavi I, Harris JE, Parsad D, et al. Vitiligo. Nat Rev Dis Primers (2015) 1:15011. doi: 10.1038/nrdp.2015.11
2. Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancent (2015) 386(9988):74–84. doi: 10.1016/s0140-6736(14)60763-7
3. Zhang Y, Cai Y, Shi M, Jiang S, Cui S, Wu Y, et al. The prevalence of vitiligo: A meta-analysis. PloS One (2016) 11(9):e0163806. doi: 10.1371/journal.pone.0163806
4. Katz EL, Harris JE. Translational research in vitiligo. Front Immunol (2021) 12:624517. doi: 10.3389/fimmu.2021.624517
5. Frisoli ML, Essien K, Harris JE. Vitiligo: mechanisms of pathogenesis and treatment. Annu Rev Immunol (2020) 38:621–48. doi: 10.1146/annurev-immunol-100919-023531
6. Chen J, Li S, Li C. Mechanisms of melanocyte death in vitiligo. Med Res Rev (2021) 41(2):1138–66. doi: 10.1002/med.21754
7. Khaitan BK, Sindhuja T. Autoimmunity in vitiligo: therapeutic implications and opportunities. Autoimmun Rev (2022) 21(1):102932. doi: 10.1016/j.autrev.2021.102932
8. Yardman-Frank JM, Fisher DE. Skin pigmentation and its control: from ultraviolet radiation to stem cells. Exp Dermatol (2021) 30(4):560–71. doi: 10.1111/exd.14260
9. Boniface K, Seneschal J, Picardo M, Taieb A. Vitiligo: focus on clinical aspects, immunopathogenesis, and therapy. Clin Rev Allergy Immunol (2018) 54(1):52–67. doi: 10.1007/s12016-017-8622-7
10. Tovar-Garza A, Hinojosa JA, Hynan LS, Pandya AG. Addition of oral minipulse dexamethasone to narrowband ultraviolet B phototherapy and topical steroids helps arrest disease activity in patients with vitiligo. Br J Dermatol (2019) 180(1):193–4. doi: 10.1111/bjd.17150
11. Rosmarin D, Passeron T, Pandya AG, Grimes P, Harris JE, Desai SR, et al. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med (2022) 387(16):1445–55. doi: 10.1056/NEJMoa2118828
12. Mapar MA, Safarpour M, Mapar M, Haghighizadeh MH. A comparative study of the mini-punch grafting and hair follicle transplantation in the treatment of refractory and stable vitiligo. J Am Acad Dermatol (2014) 70(4):743–7. doi: 10.1016/j.jaad.2013.11.044
13. Holla AP, Sahni K, Kumar R, Parsad D, Kanwar A, Mehta SD. Acral vitiligo and lesions over joints treated with non-cultured epidermal cell suspension transplantation. Clin Exp Dermatol (2013) 38(4):332–7. doi: 10.1111/ced.12040
14. Parsad D, Pandhi R, Dogra S, Kumar B. Clinical study of repigmentation patterns with different treatment modalities and their correlation with speed and stability of repigmentation in 352 vitiliginous patches. J Am Acad Dermatol (2004) 50(1):63–7. doi: 10.1016/s0190-9622(03)00786-2
15. Esmat SM, El-Tawdy AM, Hafez GA, Zeid OA, Abdel Halim DM, Saleh MA, et al. Acral lesions of vitiligo: why are they resistant to photochemotherapy? J Eur Acad Dermatol Venereol (2012) 26(9):1097–104. doi: 10.1111/j.1468-3083.2011.04215.x
16. Liu L, Wu Y, Zhang J, Gu H, Luan Q, Qian L, et al. Ablative fractional co(2) laser aided delivery of long-acting glucocorticoid in the treatment of acral vitiligo: A multicenter, prospective, self-bilateral controlled study. J Dermatolog Treat (2019) 30(4):320–7. doi: 10.1080/09546634.2018.1509048
17. Seleit I, Bakry OA, Abdou AG, Dawoud NM. Immunohistochemical evaluation of vitiliginous hair follicle melanocyte reservoir: is it retained? J Eur Acad Dermatol Venereol (2015) 29(3):444–51. doi: 10.1111/jdv.12573
18. Rodrigues M, Ezzedine K, Hamzavi I, Pandya AG, Harris JE. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol (2017) 77(1):1–13. doi: 10.1016/j.jaad.2016.10.048
19. Simons RE, Zevy DL, Jafferany M. Psychodermatology of vitiligo: psychological impact and consequences. Dermatol Ther (2020) 33(3):e13418. doi: 10.1111/dth.13418
20. Harris JE, Harris TH, Weninger W, Wherry EJ, Hunter CA, Turka LA. A mouse model of vitiligo with focused epidermal depigmentation requires ifn-Γ for autoreactive cd8+ T-cell accumulation in the skin. J Invest Dermatol (2012) 132(7):1869–76. doi: 10.1038/jid.2011.463
21. Platanias LC. Mechanisms of type-I- and type-ii-interferon-mediated signalling. Nat Rev Immunol (2005) 5(5):375–86. doi: 10.1038/nri1604
22. Rosmarin D, Pandya AG, Lebwohl M, Grimes P, Hamzavi I, Gottlieb AB, et al. Ruxolitinib cream for treatment of vitiligo: A randomised, controlled, phase 2 trial. Lancet (2020) 396(10244):110–20. doi: 10.1016/s0140-6736(20)30609-7
23. Liu LY, Strassner JP, Refat MA, Harris JE, King BA. Repigmentation in vitiligo using the janus kinase inhibitor tofacitinib may require concomitant light exposure. J Am Acad Dermatol (2017) 77(4):675–82.e1. doi: 10.1016/j.jaad.2017.05.043
24. Ezzedine K, Peeva E, Yamaguchi Y, Cox LA, Banerjee A, Han G, et al. Efficacy and safety of oral ritlecitinib for the treatment of active nonsegmental vitiligo: A randomized phase 2b clinical trial. J Am Acad Dermatol (2023) 88(2):395–403. doi: 10.1016/j.jaad.2022.11.005
25. Song H, Hu Z, Zhang S, Yang L, Liu Y, Wang T. Effectiveness and safety of tofacitinib combined with narrowband ultraviolet B phototherapy for patients with refractory vitiligo in real-world clinical practice. Dermatol Ther (2022) 35(11):e15821. doi: 10.1111/dth.15821
26. Li X, Sun Y, Du J, Wang F, Ding X. Excellent repigmentation of generalized vitiligo with oral baricitinib combined with nb-uvb phototherapy. Clin Cosmet Investig Dermatol (2023) 16:635–8. doi: 10.2147/ccid.S396430
27. Dong J, Huang X, Ma LP, Qi F, Wang SN, Zhang ZQ, et al. Baricitinib is effective in treating progressing vitiligo in vivo and in vitro. Dose Response (2022) 20(2):15593258221105370. doi: 10.1177/15593258221105370
28. Su X, Luo R, Ruan S, Zhong Q, Zhuang Z, Xiao Z, et al. Efficacy and tolerability of oral upadacitinib monotherapy in patients with recalcitrant vitiligo. J Am Acad Dermatol (2023) S0190-9622(23):02410–6. doi: 10.1016/j.jaad.2023.07.1016
29. Cui J, Shen LY, Wang GC. Role of hair follicles in the repigmentation of vitiligo. J Invest Dermatol (1991) 97(3):410–6. doi: 10.1111/1523-1747.ep12480997
30. Ju HJ, Bae JM, Lee RW, Kim SH, Parsad D, Pourang A, et al. Surgical interventions for patients with vitiligo: A systematic review and meta-analysis. JAMA Dermatol (2021) 157(3):307–16. doi: 10.1001/jamadermatol.2020.5756
31. Majid I, Imran S. Ultrathin split-thickness skin grafting followed by narrowband uvb therapy for stable vitiligo: an effective and cosmetically satisfying treatment option. Indian J Dermatol Venereol Leprol (2012) 78(2):159–64. doi: 10.4103/0378-6323.93632
32. Majid I, Imran S. Ultrathin skin grafting in resistant stable vitiligo: A follow-up study of 8 years in 370 patients. Dermatol Surg (2017) 43(2):218–25. doi: 10.1097/dss.0000000000000969
33. Bae JM, Lee JH, Kwon HS, Kim J, Kim DS. Motorized 0.8-mm micropunch grafting for refractory vitiligo: A retrospective study of 230 cases. J Am Acad Dermatol (2018) 79(4):720–7 e1. doi: 10.1016/j.jaad.2018.06.016
34. Kim DS, Ju HJ, Lee HN, Choi IH, Eun SH, Kim J, et al. Skin seeding technique with 0.5-mm micropunch grafting for vitiligo irrespective of the epidermal-dermal orientation: animal and clinical studies. J Dermatol (2020) 47(7):749–54. doi: 10.1111/1346-8138.15390
35. Baweja S, Chand S. A prospective observational comparative study of novel autologous negative pressure epidermal harvesting system (Anpehs or ehs) and suction blister grafting (Sbg) in treatment of stable vitiligo. J Cutan Aesthet Surg (2020) 13(4):283–91. doi: 10.4103/jcas.Jcas_18_20
36. Gao PR, Wang CH, Lin YJ, Huang YH, Chang YC, Chung WH, et al. A comparative study of suction blister epidermal grafting and automated blister epidermal micrograft in stable vitiligo. Sci Rep (2022) 12(1):393. doi: 10.1038/s41598-021-04299-0
37. Thakur P, Sacchidanand S, Nataraj HV, Savitha AS. A study of hair follicular transplantation as a treatment option for vitiligo. J Cutan Aesthet Surg (2015) 8(4):211–7. doi: 10.4103/0974-2077.172192
38. Mohamed Mohamed EE, Younes AK, Osmand A, Mohamed R, Makki M, Younis M. Punch graft versus follicular hair transplantation in the treatment of stable vitiligo. J Cosmet Laser Ther (2017) 19(5):290–3. doi: 10.1080/14764172.2017.1303170
39. Mokhtar M, El-Ashmawy AA, Mostafa WA, Gamei MM. Clinical and dermoscopic evaluation of follicular unit transplantation vs. Mini-punch grafting in the repigmentation of resistant and stable vitiligo: A comparative study. J Cosmet Dermatol (2022) 21(11):5837–51. doi: 10.1111/jocd.15127
40. Budania A, Parsad D, Kanwar AJ, Dogra S. Comparison between autologous noncultured epidermal cell suspension and suction blister epidermal grafting in stable vitiligo: A randomized study. Br J Dermatol (2012) 167(6):1295–301. doi: 10.1111/bjd.12007
41. Razmi TM, Kumar R, Rani S, Kumaran SM, Tanwar S, Parsad D. Combination of follicular and epidermal cell suspension as a novel surgical approach in difficult-to-treat vitiligo: A randomized clinical trial. JAMA Dermatol (2018) 154(3):301–8. doi: 10.1001/jamadermatol.2017.5795
42. Hamza AM, Hussein TM, Shakshouk HAR. Noncultured extracted hair follicle outer root sheath cell suspension versus noncultured epidermal cell suspension in the treatment of stable vitiligo. J Cutan Aesthet Surg (2019) 12(2):105–11. doi: 10.4103/jcas.Jcas_136_18
43. Awasti S, Vinay K, Thakur V, Kumar R, Holla AP, Sahni K, et al. Comparison of efficacy of cold trypsinization versus warm trypsinization in preparation of autologous non-cultured epidermal cell suspension for treatment of stable vitiligo. J Eur Acad Dermatol Venereol (2019) 33(6):e237–e9. doi: 10.1111/jdv.15502
44. Mrigpuri S, Razmi TM, Sendhil Kumaran M, Vinay K, Srivastava N, Parsad D. Four compartment method as an efficacious and simplified technique for autologous non-cultured epidermal cell suspension preparation in vitiligo surgery: A randomized, active-controlled study. J Eur Acad Dermatol Venereol (2019) 33(1):185–90. doi: 10.1111/jdv.15234
45. Anbar TS, El-Ammawi TS, Mohammed SS, Abdel-Rahman AT. Noncultured epidermal suspensions obtained from partial-thickness epidermal cuts and suction blister roofs for vitiligo treatment: A prospective comparative study. J Cosmet Dermatol (2020) 19(10):2684–91. doi: 10.1111/jocd.13312
46. Dalla A, Parsad D, Vinay K, Thakur V, Sendhil Kumaran M. A prospective study to assess the efficacy of various surgical modalities in treatment of stable vitiligo patches over resistant sites. Int J Dermatol (2020) 59(7):837–42. doi: 10.1111/ijd.14924
47. Gunaabalaji DR, Pangti R, Challa A, Chauhan S, Sahni K, Arava SK, et al. Comparison of efficacy of noncultured hair follicle cell suspension and noncultured epidermal cell suspension in repigmentation of leukotrichia and skin patch in vitiligo: A randomized trial. Int J Dermatol (2020) 59(11):1393–400. doi: 10.1111/ijd.15188
48. Thakur DS, Kumar S, Kumaran MS, Srivastava N, Parsad D. Comparison of follicular unit extraction vs. Plucking of hair follicles as technique of harvesting hair follicles in non-cultured hair follicular cell suspension in vitiligo. J Eur Acad Dermatol Venereol (2020) 34(1):e34–e6. doi: 10.1111/jdv.15888
49. Hong WS, Hu DN, Qian GP, McCormick SA, Xu AE. Ratio of size of recipient and donor areas in treatment of vitiligo by autologous cultured melanocyte transplantation. Br J Dermatol (2011) 165(3):520–5. doi: 10.1111/j.1365-2133.2011.10398.x
50. Verma G, Varkhande SR, Kar HK, Rani R. Evaluation of repigmentation with cultured melanocyte transplantation (Cmt) compared with non-cultured epidermal cell transplantation in vitiligo at 12th week reveals better repigmentation with cmt. J Invest Dermatol (2015) 135(10):2533–5. doi: 10.1038/jid.2015.178
51. Komen L, Vrijman C, Prinsen CA, van der Veen JP, Luiten RM, Wolkerstorfer A. Optimising size and depth of punch grafts in autologous transplantation of vitiligo and piebaldism: A randomised controlled trial. J Dermatolog Treat (2017) 28(1):86–91. doi: 10.1080/09546634.2016.1179251
52. Eleftheriadou V, Atkar R, Batchelor J, McDonald B, Novakovic L, Patel JV, et al. British association of dermatologists guidelines for the management of people with vitiligo 2021. Br J Dermatol (2022) 186(1):18–29. doi: 10.1111/bjd.20596
53. Burm JS. Simple suction device for autologous epidermal grafting. Plast Reconstr Surg (2000) 106(5):1225–6. doi: 10.1097/00006534-200010000-00062
54. Gou D, Currimbhoy S, Pandya AG. Suction blister grafting for vitiligo: efficacy and clinical predictive factors. Dermatol Surg (2015) 41(5):633–9. doi: 10.1097/dss.0000000000000341
55. Chatterjee M, Neema S, Vasudevan B, Dabbas D. Eyelash transplantation for the treatment of vitiligo associated eyelash leucotrichia. J Cutan Aesthet Surg (2016) 9(2):97–100. doi: 10.4103/0974-2077.184042
56. Wu Y, Dai Y, Wang T, Jin H, Peng J, Xu A. The application of electrolysis of depigmented hair using a trichiasis electrolyzer combined with single hair follicle transplantation for the treatment of vitiligo-associated leukotrichia. Dermatol Ther (2022) 35(5):e15400. doi: 10.1111/dth.15400
57. Lee S, Byun J, Shin J, Choi GS. Treatment of refractory vitiligo with a vellus hair punch graft. Dermatol Surg (2019) 45(2):300–3. doi: 10.1097/dss.0000000000001526
58. Chen Y, Yan J, Chen X, Gan L, Song M, Wang J, et al. Comparative study between follicular unit transplantation with intact and non-intact hair bulb in treatment for stable vitiligo. J Dermatolog Treat (2020) 33:1–4. doi: 10.1080/09546634.2020.1782320
59. Sritanyarat T, Wongpraparut C, Jansuwan N, Yothachai P, Nuntawisuttiwong N, Silpa-archa N. Outcomes of autologous non-cultured melanocyte keratinocyte transplantation in vitiligo and nevus depigmentosus. J Dermatolog Treat (2022) 33(2):935–40. doi: 10.1080/09546634.2020.1793885
60. Gan EY, van Geel N, Goh BK. Repigmentation of leucotrichia in vitiligo with noncultured cellular grafting. Br J Dermatol (2012) 166(1):196–9. doi: 10.1111/j.1365-2133.2011.10540.x
61. Narayan VS, van den Bol LLC, van Geel N, Bekkenk MW, Luiten RM, Wolkerstorfer A. Donor to recipient ratios in the surgical treatment of vitiligo and piebaldism: A systematic review. J Eur Acad Dermatol Venereol (2021) 35(5):1077–86. doi: 10.1111/jdv.17108
62. Vinay K, Dogra S, Parsad D, Kanwar AJ, Kumar R, Minz RW, et al. Clinical and treatment characteristics determining therapeutic outcome in patients undergoing autologous non-cultured outer root sheath hair follicle cell suspension for treatment of stable vitiligo. J Eur Acad Dermatol Venereol (2015) 29(1):31–7. doi: 10.1111/jdv.12426
63. Vashisht KR, Arava SK, Tembhre MK, Parihar AS, Sharma VK, Das BK, et al. A randomized pilot study to compare hair follicle cell suspensions prepared using trypsin alone versus trypsin in combination with collagenase type I for transplantation in vitiligo. Clin Exp Dermatol (2020) 45(2):172–9. doi: 10.1111/ced.14061
64. Gauthier Y, Surleve-Bazeille JE. Autologous grafting with noncultured melanocytes: A simplified method for treatment of depigmented lesions. J Am Acad Dermatol (1992) 26(2 Pt 1):191–4. doi: 10.1016/0190-9622(92)70024-a
65. Guerra L, Capurro S, Melchi F, Primavera G, Bondanza S, Cancedda R, et al. Treatment of "Stable" Vitiligo by timedsurgery and transplantation of cultured epidermal autografts. Arch Dermatol (2000) 136(11):1380–9. doi: 10.1001/archderm.136.11.1380
66. Chen YF, Yang PY, Hung CM, Hu DN. Transplantation of autologous cultured melanocytes for treatment of large segmental vitiligo. J Am Acad Dermatol (2001) 44(3):543–5. doi: 10.1067/mjd.2001.110658
67. Yang HJ, Yang KC, Wang YF, Yang YT, Ko JL. In vitro proliferation of human epidermal melanocytes biopsied from multiple anatomical sites. J Cosmet Dermatol (2020) 19(11):3077–82. doi: 10.1111/jocd.13348
68. Löntz W, Olsson MJ, Moellmann G, Lerner AB. Pigment cell transplantation for treatment of vitiligo: A progress report. J Am Acad Dermatol (1994) 30(4):591–7. doi: 10.1016/s0190-9622(94)70067-2
69. Shih IM, Herlyn M. Role of growth factors and their receptors in the development and progression of melanoma. J Invest Dermatol (1993) 100(2 Suppl):196s–203s.
70. Kachhawa D, Kalla G. Keratinocyte-melanocyte graft technique followed by puva therapy for stable vitiligo. Indian J Dermatol Venereol Leprol (2008) 74(6):622–4. doi: 10.4103/0378-6323.45106
71. Kachhawa D, Kachhawa N, Gupta S. Mechanical harvesting of perilesional normally pigmented tissue to merge donor and recipient sites for transplantation in vitiligo. J Am Acad Dermatol (2022) 87(2):e69–70. doi: 10.1016/j.jaad.2020.08.069
72. van Geel N, Passeron T, Wolkerstorfer A, Speeckaert R, Ezzedine K. Reliability and validity of the vitiligo signs of activity score (Vsas). Br J Dermatol (2020) 183(5):883–90. doi: 10.1111/bjd.18950
73. Njoo MD, Das PK, Bos JD, Westerhof W. Association of the köbner phenomenon with disease activity and therapeutic responsiveness in vitiligo vulgaris. Arch Dermatol (1999) 135(4):407–13. doi: 10.1001/archderm.135.4.407
74. Verma R, Grewal RS, Chatterjee M, Pragasam V, Vasudevan B, Mitra D. A comparative study of efficacy of cultured versus non cultured melanocyte transfer in the management of stable vitiligo. Med J Armed Forces India (2014) 70(1):26–31. doi: 10.1016/j.mjafi.2013.09.004
75. Janowska A, Dini V, Panduri S, Macchia M, Oranges T, Romanelli M. Epidermal skin grafting in vitiligo: A pilot study. Int Wound J (2016) 13 Suppl 3(Suppl 3):47–51. doi: 10.1111/iwj.12632
76. Ghasemi M, Bajouri A, Shafiiyan S, Aghdami N. Hair follicle as a source of pigment-producing cells for treatment of vitiligo: an alternative to epidermis? Tissue Eng Regener Med (2020) 17(6):815–27. doi: 10.1007/s13770-020-00284-2
77. Bae JM, Ju HJ, Lee RW, Lee HN, Kim NB, Kim YH, et al. Micropunch grafting as an adjuvant for noncultured melanocyte-keratinocyte transplantation for refractory vitiligo. J Am Acad Dermatol (2020) 82(6):1548–50. doi: 10.1016/j.jaad.2020.03.003
78. Al-Hadidi N, Griffith JL, Al-Jamal MS, Hamzavi I. Role of recipient-site preparation techniques and post-operative wound dressing in the surgical management of vitiligo. J Cutan Aesthet Surg (2015) 8(2):79–87. doi: 10.4103/0974-2077.158439
79. Esmat S, Hegazy RA, Shalaby S, Hu SC, Lan CE. Phototherapy and combination therapies for vitiligo. Dermatol Clin (2017) 35(2):171–92. doi: 10.1016/j.det.2016.11.008
80. Lommerts J, Uitentuis S, Bekkenk M, de Rie M, Wolkerstorfer A. The role of phototherapy in the surgical treatment of vitiligo: A systematic review. J Eur Acad Dermatol Venereol (2018) 32(9):1427–35. doi: 10.1111/jdv.14950
81. Mutalik S, Shah S, Sidwadkar V, Khoja M. Efficacy of cyclosporine after autologous noncultured melanocyte transplantation in localized stable vitiligo-a pilot, open label, comparative study. Dermatol Surg (2017) 43(11):1339–47. doi: 10.1097/dss.0000000000001190
82. Saldanha KD, MaChado Filho CD, Paschoal FM. Action of topical mometasone on the pigmented halos of micrografting in patients with vitiligo. Bras Dermatol (2012) 87(5):685–90. doi: 10.1590/s0365-05962012000500002
83. Salem SAM, Fezeaa TA, El Khazragy N, Soltan MY. Effect of platelet-rich plasma on the outcome of mini-punch grafting procedure in localized stable vitiligo: clinical evaluation and relation to lesional basic fibroblast growth factor. Dermatol Ther (2021) 34(2):e14738. doi: 10.1111/dth.14738
84. Feily A, Firoozifard A, Sokhandani T, Elosegui-Rodriguez P, Perez-Rivera E, Lange CS, et al. Follicular transplantation, microneedling, and adjuvant narrow-band ultraviolet-B irradiation as cost-effective regimens for palmar-plantar vitiligo: A pilot study. Cureus (2020) 12(4):e7878. doi: 10.7759/cureus.7878
85. Bhingradia YM, Patel NK. Pinhole technique for cobblestoning in patients post mini-punch grafting for stable vitiligo. Indian J Dermatol Venereol Leprol (2021) 87(6):861–3. doi: 10.25259/ijdvl_397_20
86. Kovacs D, Bastonini E, Briganti S, Ottaviani M, D'Arino A, Truglio M, et al. Altered epidermal proliferation, differentiation, and lipid composition: novel key elements in the vitiligo puzzle. Sci Adv (2022) 8(35):eabn9299. doi: 10.1126/sciadv.abn9299
87. Yuan J, Chen H, Yan R, Cui S, Li YH, Wu Y, et al. Fractional co(2) lasers contribute to the treatment of stable non-segmental vitiligo. Eur J Dermatol (2016) 26(6):592–8. doi: 10.1684/ejd.2016.2875
88. Wen X, Hamblin MR, Xian Y, Li Y. A preliminary study of fractional co(2) laser added to topical tacrolimus combined with 308 nm excimer lamp for refractory vitiligo. Dermatol Ther (2019) 32(1):e12747. doi: 10.1111/dth.12747
89. Chen W, Zhou Y, Huang FR, Luo D, Wang DG. Preliminary study on the treatment of vitiligo with carbon dioxide fractional laser together with tacrolimus. Lasers Surg Med (2018) 50(8):829–36. doi: 10.1002/lsm.22821
90. Li L, Wu Y, Li L, Sun Y, Qiu L, Gao XH, et al. Triple combination treatment with fractional co2 laser plus topical betamethasone solution and narrowband ultraviolet B for refractory vitiligo: A prospective, randomized half-body, comparative study. Dermatol Ther (2015) 28(3):131–4. doi: 10.1111/dth.12202
91. Weshahy R, Abdelhamid MF, Sayed KS, El Desouky ED, Ramez SA. Efficacy and safety of combined fractional ablative co(2) laser and 5 fluorouracil in the treatment of acral vitiligo: an open, uncontrolled study. J Cosmet Dermatol (2022) 21(11):5636–41. doi: 10.1111/jocd.15116
92. Kanokrungsee S, Khunkhet S, Rojhirunsakool S, Thadvibun K, Sahaspot T. Triple combination therapy of narrowband ultraviolet B, fractional carbon dioxide laser and topical bimatoprost 0.01% for non-segmental vitiligo on non-facial areas: A randomized half-body, double-blind, placebo-controlled, comparative study. Dermatol Ther (2022) 35(1):e15198. doi: 10.1111/dth.15198
93. Kadry M, Tawfik A, Abdallah N, Badawi A, Shokeir H. Platelet-rich plasma versus combined fractional carbon dioxide laser with platelet-rich plasma in the treatment of vitiligo: A comparative study. Clin Cosmet Investig Dermatol (2018) 11:551–9. doi: 10.2147/ccid.S178817
94. Afify AA, Zuelfakkar NM, Eshafi MA. Fractional co2 laser, platelet rich plasma and narrow band ultraviolet B in the treatment of vitiligo (a randomized clinical trial). Lasers Med Sci (2021) 36(7):1479–86. doi: 10.1007/s10103-020-03195-9
95. Shin J, Lee JS, Hann SK, Oh SH. Combination treatment by 10 600 nm ablative fractional carbon dioxide laser and narrowband ultraviolet B in refractory nonsegmental vitiligo: A prospective, randomized half-body comparative study. Br J Dermatol (2012) 166(3):658–61. doi: 10.1111/j.1365-2133.2011.10723.x
96. Cunha PR, Scabine Pessotti N, Bonati Mattos C, Salai AF. New approach in the treatment of refractory vitiligo: co2 laser combined with betamethasone and salicylic acid solution. Dermatol Ther (2017) 30(1):e12410–e12413. doi: 10.1111/dth.12410
97. Feily A, Seifi V, Ramirez-Fort MK. Fractional co2 laser pretreatment to autologous hair transplantation and phototherapy improves perifollicular repigmentation in refractory vitiligo: A randomized, prospective, half-lesion, comparative study. Dermatol Surg (2016) 42(9):1082–8. doi: 10.1097/DSS.0000000000000844
98. Vachiramon V, Chaiyabutr C, Rattanaumpawan P, Kanokrungsee S. Effects of a preceding fractional carbon dioxide laser on the outcome of combined local narrowband ultraviolet B and topical steroids in patients with vitiligo in difficult-to-treat areas. Lasers Surg Med (2016) 48(2):197–202. doi: 10.1002/lsm.22389
99. Stinco G, Trevisan G, Buligan C, Gregoraci G, De Marchi S, di Meo N, et al. Narrow band-ultraviolet B versus clobetasol propionate foam in the treatment of vitiligo: A retrospective study. Dermatol Ther (Heidelb) (2013) 3(1):95–105. doi: 10.1007/s13555-013-0028-8
100. Cho S, Zheng Z, Park YK, Roh MR. The 308-nm excimer laser: A promising device for the treatment of childhood vitiligo. Photodermatol Photoimmunol Photomed (2011) 27(1):24–9. doi: 10.1111/j.1600-0781.2010.00558.x
101. Noborio R, Nomura Y, Nakamura M, Nishida E, Kiyohara T, Tanizaki H, et al. Efficacy of 308-nm excimer laser treatment for refractory vitiligo: A case series of treatment based on the minimal blistering dose. J Eur Acad Dermatol Venereol (2021) 35(4):e287–e9. doi: 10.1111/jdv.17047
102. Kim HJ, Hong ES, Cho SH, Lee JD, Kim HS. Fractional carbon dioxide laser as an "Add-on" Treatment for vitiligo: A meta-analysis with systematic review. Acta Derm Venereol (2018) 98(2):180–4. doi: 10.2340/00015555-2836
103. Manstein D, Herron GS, Sink RK, Tanner H, Anderson RR. Fractional photothermolysis: A new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med (2004) 34(5):426–38. doi: 10.1002/lsm.20048
104. Kumar R, Parsad D, Kanwar AJ, Kaul D. Altered levels of ets-1 transcription factor and matrix metalloproteinases in melanocytes from patients with vitiligo. Br J Dermatol (2011) 165(2):285–91. doi: 10.1111/j.1365-2133.2011.10324.x
105. Hu Y, Qi X, Hu Y, Lu Y, Liu K, Han X, et al. Effects of co2 fractional laser therapy on peripheral blood cytokines in patients with vitiligo. Dermatol Ther (2019) 32(4):e12992. doi: 10.1111/dth.12992
106. Chiu YJ, Perng CK, Ma H. Fractional co(2) laser contributes to the treatment of non-segmental vitiligo as an adjunct therapy: A systemic review and meta-analysis. Lasers Med Sci (2018) 33(7):1549–56. doi: 10.1007/s10103-018-2516-7
107. Mohammadi S, Amiri R, Khalili M, Iranmanesh B, Aflatoonian M. Treatment protocols and efficacy of combined laser with medical treatment modalities in vitiligo. J Cosmet Dermatol (2021) 21:3272–91. doi: 10.1111/jocd.14602
108. Yan R, Yuan J, Chen H, Li YH, Wu Y, Gao XH, et al. Fractional er:Yag laser assisting topical betamethasone solution in combination with nb-uvb for resistant non-segmental vitiligo. Lasers Med Sci (2017) 32(7):1571–7. doi: 10.1007/s10103-017-2282-y
109. Frisoli ML, Harris JE. Vitiligo: mechanistic insights lead to novel treatments. J Allergy Clin Immunol (2017) 140(3):654–62. doi: 10.1016/j.jaci.2017.07.011
110. Liao ZK, Hu SH, Han BY, Qiu X, Jiang S, Lei TC. Pro-pigmentary action of 5-fluorouracil through the stimulated secretion of cxcl12 by dermal fibroblasts. Chin Med J (Engl) (2021) 134(20):2475–82. doi: 10.1097/cm9.0000000000001689
111. Scott G, Leopardi S, Printup S, Malhi N, Seiberg M, Lapoint R. Proteinase-activated receptor-2 stimulates prostaglandin production in keratinocytes: analysis of prostaglandin receptors on human melanocytes and effects of pge2 and pgf2alpha on melanocyte dendricity. J Invest Dermatol (2004) 122(5):1214–24. doi: 10.1111/j.0022-202X.2004.22516.x
112. Chen J, Wan Y, Lin Y, Jiang H. Current art of combination therapy with autologous platelet-rich plasma for stable vitiligo: A meta-analysis. Int Wound J (2021) 18(3):251–60. doi: 10.1111/iwj.13524
113. Xiong S, Qiu L, Zhao J, Zheng H, Cui D, Su Y, et al. The role of platelet concentrates in facial fat grafting. Ann Plast Surg (2018) 81(6S Suppl 1):S117–s23. doi: 10.1097/sap.0000000000001498
114. Banzhaf CA, Ortner VK, Philipsen PA, Haedersdal M. The ablative fractional coagulation zone influences skin fluorescence intensities of topically applied test molecules-an in vitro study with fluorescence microscopy and fluorescence confocal microscopy. Lasers Surg Med (2019) 51(1):68–78. doi: 10.1002/lsm.23034
115. Pople PV, Singh KK. Development and evaluation of colloidal modified nanolipid carrier: application to topical delivery of tacrolimus, part ii–in vivo assessment, drug targeting, efficacy, and safety in treatment for atopic dermatitis. Eur J Pharm Biopharm (2013) 84(1):72–83. doi: 10.1016/j.ejpb.2012.11.026
116. Huang C, Li P, Wang B, Deng Y, Li J, Mao M, et al. Multi-factors associated with efficacy and adverse events of fractional erbium:Yag laser-assisted delivery of topical betamethasone for stable vitiligo: A retrospective analysis. Lasers Surg Med (2020) 52(7):590–6. doi: 10.1002/lsm.23198
117. Jha AK, Sonthalia S. 5-fluorouracil as an adjuvant therapy along with microneedling in vitiligo. J Am Acad Dermatol (2019) 80(4):e75–e6. doi: 10.1016/j.jaad.2018.12.008
118. Meyer KC, Klatte JE, Dinh HV, Harries MJ, Reithmayer K, Meyer W, et al. Evidence that the bulge region is a site of relative immune privilege in human hair follicles. Br J Dermatol (2008) 159(5):1077–85. doi: 10.1111/j.1365-2133.2008.08818.x
119. Kumar R, Parsad D, Rani S, Bhardwaj S, Srivastav N. Glabrous lesional stem cells differentiated into functional melanocytes: new hope for repigmentation. J Eur Acad Dermatol Venereol (2016) 30(9):1555–60. doi: 10.1111/jdv.13686
120. Toh JJH, Chuah SY, Jhingan A, Chong WS, Thng STG. Afamelanotide implants and narrow-band ultraviolet B phototherapy for the treatment of nonsegmental vitiligo in asians. J Am Acad Dermatol (2020) 82(6):1517–9. doi: 10.1016/j.jaad.2020.01.035
121. Zhu L, Lin X, Zhi L, Fang Y, Lin K, Li K, et al. Mesenchymal stem cells promote human melanocytes proliferation and resistance to apoptosis through pten pathway in vitiligo. Stem Cell Res Ther (2020) 11(1):26. doi: 10.1186/s13287-019-1543-z
122. Bellei B, Papaccio F, Filoni A, Caputo S, Lopez G, Migliano E, et al. Extracellular fraction of adipose tissue as an innovative regenerative approach for vitiligo treatment. Exp Dermatol (2019) 28(6):695–703. doi: 10.1111/exd.13954
123. Regazzetti C, Joly F, Marty C, Rivier M, Mehul B, Reiniche P, et al. Transcriptional analysis of vitiligo skin reveals the alteration of wnt pathway: A promising target for repigmenting vitiligo patients. J Invest Dermatol (2015) 135(12):3105–14. doi: 10.1038/jid.2015.335
124. Han X, Chang L, Qiu Z, Lin M, Wang Y, Liu D, et al. Micro-injury induces hair regeneration and vitiligo repigmentation through wnt/Β-catenin pathway. Stem Cells Dev (2022) 31(5-6):111–8. doi: 10.1089/scd.2021.0276
125. Yang G, Li Y, Nishimura EK, Xin H, Zhou A, Guo Y, et al. Inhibition of pax3 by tgf-beta modulates melanocyte viability. Mol Cell (2008) 32(4):554–63. doi: 10.1016/j.molcel.2008.11.002
126. Cui R, Widlund HR, Feige E, Lin JY, Wilensky DL, Igras VE, et al. Central role of P53 in the suntan response and pathologic hyperpigmentation. Cell (2007) 128(5):853–64. doi: 10.1016/j.cell.2006.12.045
127. Wang J, Zeng X, Liu Y, Lian W, Lv H, Wei K, et al. Human embryonic stem cell-derived melanocytes exhibit limited immunogenicity. Biochem Biophys Res Commun (2021) 573:151–7. doi: 10.1016/j.bbrc.2021.07.103
128. Xie Y, Xu Z, Shi W, Mei X. Biological function and application of melanocytes induced and transformed by mouse bone marrow mesenchymal stem cells. Regener Ther (2022) 21:148–56. doi: 10.1016/j.reth.2022.06.007
129. Zavala G, Sandoval C, Meza D, Contreras R, Gubelin W, Khoury M. Differentiation of adipose-derived stem cells to functional cd105(Neg) cd73(Low) melanocyte precursors guided by defined culture condition. Stem Cell Res Ther (2019) 10(1):249. doi: 10.1186/s13287-019-1364-0
130. Hosaka C, Kunisada M, Koyanagi-Aoi M, Masaki T, Takemori C, Taniguchi-Ikeda M, et al. Induced pluripotent stem cell-derived melanocyte precursor cells undergoing differentiation into melanocytes. Pigment Cell Melanoma Res (2019) 32(5):623–33. doi: 10.1111/pcmr.12779
131. Liu LP, Li YM, Guo NN, Li S, Ma X, Zhang YX, et al. Therapeutic potential of patient ipsc-derived imelanocytes in autologous transplantation. Cell Rep (2019) 27(2):455–66.e5. doi: 10.1016/j.celrep.2019.03.046
132. Wang G, Qiu D, Yang H, Liu W. The prevalence and odds of depression in patients with vitiligo: A meta-analysis. J Eur Acad Dermatol Venereol (2018) 32(8):1343–51. doi: 10.1111/jdv.14739
133. Ezzedine K, Eleftheriadou V, Jones H, Bibeau K, Kuo FI, Sturm D, et al. Psychosocial effects of vitiligo: A systematic literature review. Am J Clin Dermatol (2021) 22(6):757–74. doi: 10.1007/s40257-021-00631-6
134. Chen M, Cai J, Zhang X, Liao Z, Zhong M, Shang J, et al. Keratinocytes take part in the regulation of substance P in melanogenesis through the hpa axis. J Dermatol Sci (2022) 106(3):141–9. doi: 10.1016/j.jdermsci.2022.04.011
135. Tu C, Zhao D, Lin X. Levels of neuropeptide-Y in the plasma and skin tissue fluids of patients with vitiligo. J Dermatol Sci (2001) 27(3):178–82. doi: 10.1016/s0923-1811(01)00134-7
136. Lai YC, Yew YW, Kennedy C, Schwartz RA. Vitiligo and depression: A systematic review and meta-analysis of observational studies. Br J Dermatol (2017) 177(3):708–18. doi: 10.1111/bjd.15199
137. Komen L, Zwertbroek L, Burger SJ, van der Veen JP, de Rie MA, Wolkerstorfer A. Q-switched laser depigmentation in vitiligo, most effective in active disease. Br J Dermatol (2013) 169(6):1246–51. doi: 10.1111/bjd.12571
138. Tan ES, Sarkany R. Topical monobenzyl ether of hydroquinone is an effective and safe treatment for depigmentation of extensive vitiligo in the medium term: A retrospective cohort study of 53 cases. Br J Dermatol (2015) 172(6):1662–4. doi: 10.1111/bjd.13642
139. Nofal A, Fawzy MM, Alakad R. The use of trichloroacetic acid as a depigmenting therapy in universal vitiligo. J Dtsch Dermatol Ges (2021) 19(2):241–6. doi: 10.1111/ddg.14316
140. van Geel N, Depaepe L, Speeckaert R. Laser (755 nm) and cryotherapy as depigmentation treatments for vitiligo: A comparative study. J Eur Acad Dermatol Venereol (2015) 29(6):1121–7. doi: 10.1111/jdv.12762
141. El-Mofty M, Mostafa WZ, Esmat S, Zayed A, Mashaly H, Hussien MF, et al. Site-oriented depigmentation in vitiligo patients using Q-switched nd:Yag laser (1,064/532 nm), cryotherapy and chemical peels: A comparative study. Dermatol Ther (2019) 32(5):e13052. doi: 10.1111/dth.13052
142. Ramien ML, Ondrejchak S, Gendron R, Hatami A, McCuaig CC, Powell J, et al. Quality of Life in Pediatric Patients before and after Cosmetic Camouflage of Visible Skin Conditions. J Am Acad Dermatol (2014) 71(5):935–40. doi: 10.1016/j.jaad.2014.07.029
143. Li M, Wang F, Ding X, Xu Q, Du J. Evaluation of the potential interference of camouflage on the treatment of vitiligo: an observer-blinded self-controlled study. Dermatol Ther (2021) 34(1):e14545. doi: 10.1111/dth.14545
144. Ju HJ, Eun SH, Lee HN, Lee JH, Kim GM, Bae JM. Micropigmentation for vitiligo on light to moderately colored skin: updated evidence from a clinical and animal study. J Dermatol (2020) 47(5):464–9. doi: 10.1111/1346-8138.15282
145. Sharma A, Agrawal S, Dhurat R, Mhatre M, Surve R, Kerure A. Micropigmentation-a revived therapeutic tool for recalcitrant, difficult-to-treat periungual vitiligo. Dermatol Ther (2020) 33(4):e13568. doi: 10.1111/dth.13568
146. Birlea SA, Costin GE, Roop DR, Norris DA. Trends in regenerative medicine: repigmentation in vitiligo through melanocyte stem cell mobilization. Med Res Rev (2017) 37(4):907–35. doi: 10.1002/med.21426
147. Graham JA, Fray M, de Haseth S, Lee KM, Lian MM, Chase CM, et al. Suppressive regulatory T cell activity is potentiated by glycogen synthase kinase 3{Beta} Inhibition. J Biol Chem (2010) 285(43):32852–9. doi: 10.1074/jbc.M110.150904
148. Tang L, Fang W, Lin J, Li J, Wu W, Xu J. Vitamin D protects human melanocytes against oxidative damage by activation of wnt/Β-catenin signaling. Lab Invest (2018) 98(12):1527–37. doi: 10.1038/s41374-018-0126-4
149. Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, et al. Wnt signaling arrests effector T cell differentiation and generates cd8+ Memory stem cells. Nat Med (2009) 15(7):808–13. doi: 10.1038/nm.1982
Keywords: refractory vitiligo, treatment, surgery, fractional laser, regenerative medicine, psychotherapy
Citation: Wang X, Wu W, Chen J, Li C and Li S (2024) Management of the refractory vitiligo patient: current therapeutic strategies and future options. Front. Immunol. 14:1294919. doi: 10.3389/fimmu.2023.1294919
Received: 15 September 2023; Accepted: 30 November 2023;
Published: 04 January 2024.
Edited by:
Shahnawaz Jadeja, University College Dublin, IrelandReviewed by:
Jayvadan Jayantilal Vaishnav, Maharaja Sayajirao University of Baroda, IndiaRosalba Buquicchio, Antonio Perrino Hospital, Italy
Copyright © 2024 Wang, Wu, Chen, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuli Li, bGlzaGxpQGZtbXUuZWR1LmNu; Chunying Li, bGljaHlpbmdAZm1tdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Xinju Wang†
Xinju Wang† Wei Wu
Wei Wu Jianru Chen
Jianru Chen Chunying Li
Chunying Li Shuli Li
Shuli Li