- 1Department of Dermatology, West China Hospital, Sichuan University, Chengdu, China
- 2Laboratory of Dermatology, Clinical Institute of Inflammation and Immunology, Frontiers Science Center for Disease-related Molecular Network, West China Hospital, Sichuan University, Chengdu, China
- 3Tianfu Jincheng Laboratory & Institute of Future Medical Innovation, City of Future Medicine, Chengdu, China
The risk of infection and malignancy may be a concern for patients with psoriasis receiving interleukin (IL)-17 and IL-23 inhibitors, particularly with long-term treatments. We aimed to estimate the short-term risks and long-term incidence rates of infection and malignancy with IL-17 or IL-23 antagonists in adult patients with psoriasis and psoriatic arthritis through this comprehensive meta-analysis (PROSPERO registration number: CRD42022363127). We searched PubMed, MEDLINE, Web of Science and ClinicalTrials.gov until May 17, 2023 for randomized placebo-controlled trials and long-term (≥ 52 weeks) open-label extension studies. The estimates of short-term risk ratios (RRs) and long-term exposure-adjusted incidence rates (EAIRs) were pooled using R software 4.1.1 and STATA 16.0. This review included 45 randomized placebo-controlled studies and 27 open-label extension studies. Short-term RRs of serious infection, overall infection and malignancy were 1.45 (95% confidence intervals, 95% CI: 0.81-2.59), 1.20 (95% CI: 1.06-1.35), 0.83 (95% CI: 0.41-1.71) with IL-17 inhibitors; and 0.68 (95% CI: 0.38-1.22), 1.13 (95% CI: 1.00-1.28), 0.87 (95% CI: 0.37-2.04) with IL-23 inhibitors. Increased short-term risks of nasopharyngitis and Candida infection with IL-17 inhibitors were found. Long-term EAIRs of serious infection, overall infection, nonmelanoma skin cancer (NMSC), malignancies excluding NMSC, nasopharyngitis and upper respiratory tract infection were 1.11/100 patient-years (PYs), 57.78/100PYs, 0.47/100PYs, 0.24/100PYs, 15.07/100PYs, 8.52/100PYs, 3.41/100PYs with IL-17 inhibitors; and 1.09/100PYs, 48.50/100PYs, 0.40/100PYs, 0.43/100PYs, 10.75/100PYs, 5.84/100PYs with IL-23 inhibitors. Long-term EAIR of Candida infection was 3.41/100PYs with IL-17 inhibitors. No active or reactivated tuberculosis was ever reported in all the trials, and only a few cases of latent tuberculosis, hepatitis, and herpes zoster were reported during the long-term extension periods. No evidence of increased EAIRs of infection and malignancy with longer durations was found. Our study suggested that short-term risk and long-term incidence of infections and malignancies in psoriasis patients receiving IL-17 inhibitors and IL-23 inhibitors are generally low. However, close monitoring is required for nasopharyngitis and Candida infection with IL-17 inhibitors.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022363127.
1 Introduction
Psoriasis is a chronic, inflammatory and immune-mediated skin disorder occurring worldwide, with plaque psoriasis (PsO) being the most common phenotype (1). Psoriatic arthritis (PsA), affecting approximately 30% of psoriasis patients, is a progressive and inflammatory musculoskeletal disorder resulting in declined quality of life, disability and early mortality (2, 3). PsO and PsA are associated with an increased prevalence of comorbidities, including cardiovascular disease, metabolic syndromes, and depression, which lead to substantial burdens for individual patients and society (4).
Currently, a variety of cytokines have been found to play pivotal roles in the pathogenesis of psoriasis, including interleukin (IL)-17 and IL-23 (5). A number of therapeutic agents targeting the IL-17 or IL-23 pathway have been approved in the treatment of PsO and PsA, and they have demonstrated excellent efficacy in both clinical trials and real-world settings (6). With these biologics, complete or nearly complete clearance of psoriatic lesions is now attainable. However, in addition to being implicated in the pathogenesis of psoriasis, the IL-17 family and IL-23 perform fundamental functions in innate and adaptive immunity (7, 8). Hence, concerns about infection and malignancy associated with deficiency of IL-17 or IL-23 immunity have been raised, especially in patients receiving long-term therapies (9).
To date, IL-17 inhibitors and IL-23 inhibitors have been proved generally safe and well-tolerated (6). However, individual trials lack large sample sizes or long-term trial durations to detect the risk of rare adverse events (AEs) such as serious infections, rare infections and malignancies (10). Furthermore, long-term safety is one of the key considerations in the treatment strategy for psoriasis, while most meta-analyses focused on the randomized controlled trials (RCTs), whose durations were routinely limited to tens of weeks (11). It is unclear, however, whether the short-term safety data could be generalized to long-term treatments. There is growing evidence from open-label extension studies demonstrating the long-term efficacy and safety profiles, with the follow-up periods extending up to several years (11). Nevertheless, to the best of our knowledge, comprehensive studies that generalize and quantify the current safety data, particularly the long-term data, are still lacking (11, 12).
To evaluate the short- and long-term safety profile of IL-17 or IL-23 inhibitors, we conducted this systematic review and meta-analysis, which assessed the short-term risk and long-term incidence rate of (1) overall infection, (2) serious infection, (3) malignancy, (4) nasopharyngitis, (5) upper respiratory tract infection, (6) Candida infection, (7) tuberculosis, (8) hepatitis, and (9) herpes zoster with IL-17 inhibitors and IL-23 inhibitors in adult patients with PsO or PsA.
2 Methods
2.1 Study design and systematic literature search
This study was designed as a systematic review and meta-analysis, according to the Preferred Reporting Item for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Table S1). A predefined protocol (PROSPERO registration number: CRD42022363127) was used to conduct the literature search, study selection, quality assessment, data extraction, and statistical analyses. The literature search was performed in PubMed, MEDLINE, Web of Science and ClinicalTrials.gov until May 17, 2023 for randomized placebo-controlled trials and long-term extension studies of IL-17 or IL-23 inhibitors approved for PsO and PsA. The search terms included “psoriasis or psoriatic arthritis”, combined with “IL-17 inhibitors or IL-23 inhibitors or IL-17 antagonists or IL-23 antagonists”. Publications were limited to the English language. Detailed searching strategy and results were summarized in the Supplementary Materials. Reference lists of selected articles were hand-searched to retrieve more literature.
2.2 Selection and quality assessment
We included (1) phase 2 or 3 randomized placebo-controlled trials; (2) open-label extension (OLE) studies with maintenance duration of more than 52 weeks. The included trials were required to report at least one of the primary outcomes: serious infection, overall infection or malignancy. Studies were excluded if they were not clinical trials on adult patients, or not reporting original data. Studies on IL-17/IL-23 inhibitors combined with other drugs instead of monotherapy were also excluded. The titles and abstracts of potentially eligible articles were screened by two independent investigators (S Wu and Y Xu). Afterwards, the full texts of the remaining studies were reviewed to identify if they met the inclusion criteria. Discrepancies between the investigators regarding the inclusion of the studies were resolved by discussion and consultation of a third researcher (L Guo). The risk of bias was evaluated by two independent reviewers (S Wu and Y Xu), using the Cochrane risk of bias tool for randomized studies of interventions (13). The divergence of opinions was resolved by consultation of another researcher (L Guo). The results were summarized and presented using Review Manager 5.4.1.
2.3 Outcomes, definitions and interventions
The primary outcomes of our study were the short-term risks and long-term incidence rates of (1) serious infection, (2) overall infection, (3) malignancy. We identified serious infections as infections considered to be serious adverse events (SAEs) by researchers of the trials. Malignancies were divided into nonmelanoma skin cancer (NMSC) and malignancies excluding NMSC in open-label extension studies according to the original literature. Secondary outcomes of our study were the short-term risks and long-time incidence rates of (1) nasopharyngitis, (2) upper respiratory tract infection, (3) Candida infection, (4) tuberculosis (latent or active), (5) hepatitis, (6) herpes zoster. The original literature researching IL-23 inhibitors lacked the data of Candida infection. Nasopharyngitis, upper respiratory tract infection, Candida infection, tuberculosis, hepatitis, and herpes zoster were selected as infections of special interest. Nasopharyngitis and upper respiratory tract infection are considered the most frequent AEs with IL-17 or IL-23 inhibitors, while Candida infection, tuberculosis, and herpes zoster are opportunistic infections which may be induced by deficiency of IL-17 or IL-23. Hepatitis here refers to infections with hepatitis virus, while other types of hepatitis not caused by infection, such as autoimmune hepatitis, were not included. IL-17 antagonists approved for the treatment of PsO or PsA include brodalumab, ixekizumab, secukinumab, and bimekizumab. IL-23 antagonists approved for the treatment of PsO or PsA include guselkumab, risankizumab, and tildrakizumab. Secukinumab and ixekizumab are monoclonal antibodies selectively binding IL-17A. Brodalumab targets the IL-17 receptor A unit (IL-17RA), inhibiting IL-17A, IL-17F, IL-17C and IL-17E/IL-25. Bimekizumab selectively neutralizes both IL-17A and IL-17F. Guselkumab, risankizumab and tildrakizumab are monoclonal antibodies blocking the p19 subunit of IL-23.
2.4 Data extraction and analyses
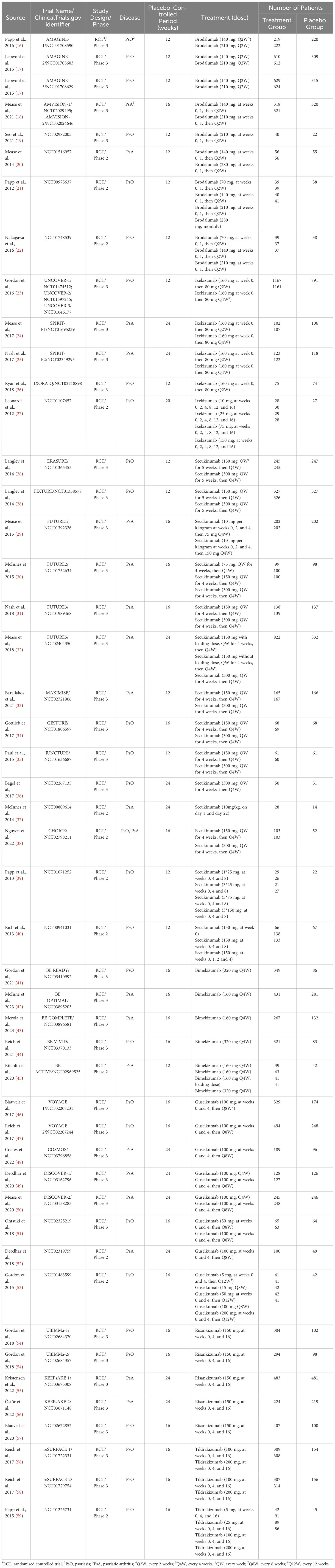
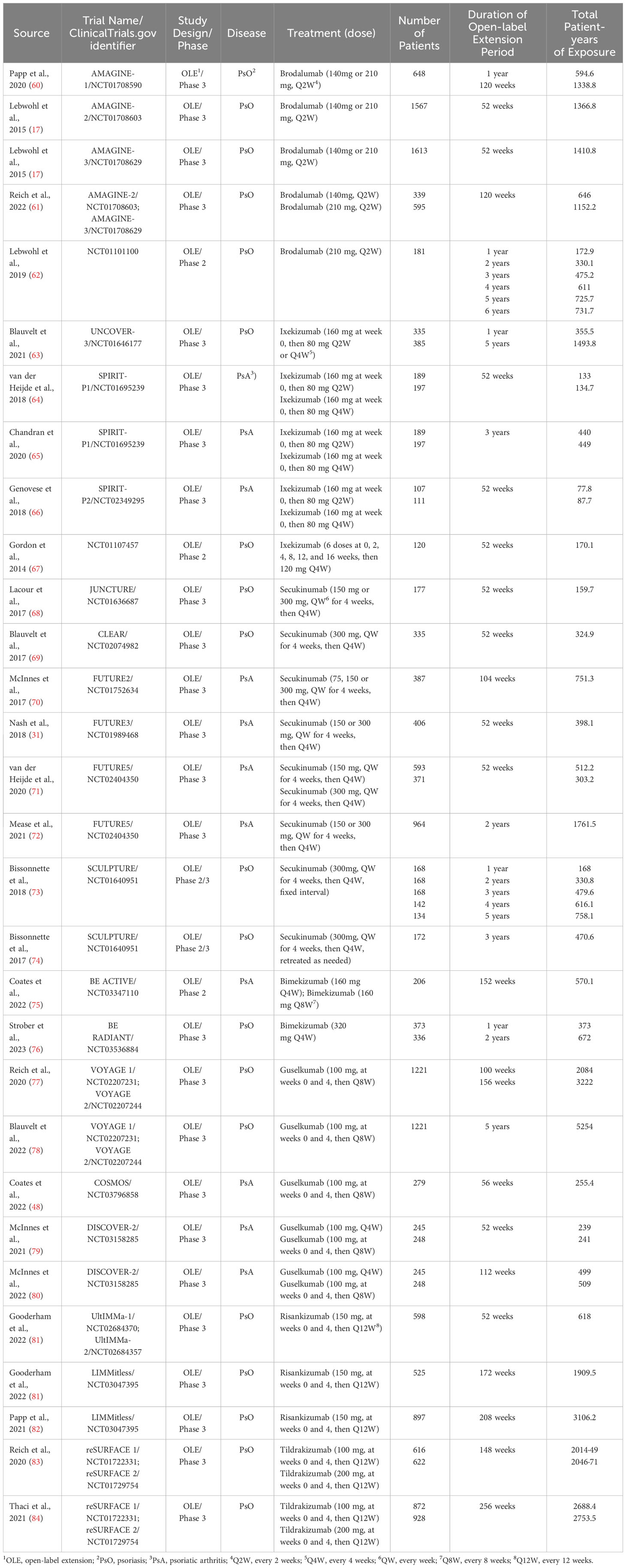
For included publications, the following information was collected: author, year of publication, trial name, ClinicalTrials.gov identifier, study design and phase, and type of disease. For RCTs, the following data were extracted: the number of patients in treatment and placebo groups, treatment dose, duration of the placebo-controlled periods, the number of serious infections, overall infections, malignancies, nasopharyngitis, upper respiratory tract infection and Candida infection, and the type of malignancies in both the treatment group and the placebo group. For OLE studies, we extracted the following data: treatment dose, number of patients, duration of the open-label extension periods, total patient-years (PYs) of exposure, exposure-adjusted incidence rate (EAIR) with 95% CIs of serious infection, overall infection, NMSC, malignancies excluding NMSC, nasopharyngitis, upper respiratory tract infection and Candida infection. Two reviewers (S Wu, L Yang) independently performed the data extraction. Disagreements were resolved by discussion and consultation of the third researcher (L Guo), and reviewers were unanimous in their final decisions. For AEs with limited data which were hard to be estimated by quantitative meta-analyses, qualitative descriptions, evaluations and summary tables were presented.
The numbers of subjects with infection or malignancy in the treatment and placebo groups from the eligible RCTs were recorded. Patients who received any dose of the biologics were included in the outcome measures. Risk ratios (RRs) based on the number of patients with infections or malignancies and the number of patients in each group were calculated. A pooled estimate of the RR with associated 95% confidence intervals (CIs) was produced for each outcome, with each result presented with forest plots. Heterogeneity testing was performed using the I2 test and Q-test. A common-effects model and a random-effects model were both calculated. A random-effects model would be adapted if I2 ≥50%. Subgroup analyses were conducted by biologic and indication. We performed funnel plots and “peters” tests (more than 10 studies) to estimate the publication bias (14). Sensitivity analyses were performed to measure the consistency and robustness of the outcomes. Statistical analyses were performed by R software 4.1.1 and the “meta” package. P-values less than 0.05 were considered significant.
The long-term incidence rates of infection and malignancy were determined by EAIRs with 95% CIs, calculated by events/100PYs. Data of 95% CIs were calculated based on Poisson distribution if they were not provided by the original literature (15). Data with durations of more than 52 weeks were included and analyzed. Patients who received any doses of the biologics were included in the final measures. We produced a pooled estimate of the EAIR of each outcome, with the results presented with forest plots. The results were stratified by years, aimed to determine EAIRs of different treatment durations. Statistical analyses were performed using STATA 16.0. P-values less than 0.05 were considered significant.
3 Results
3.1 Search results and trial characteristics
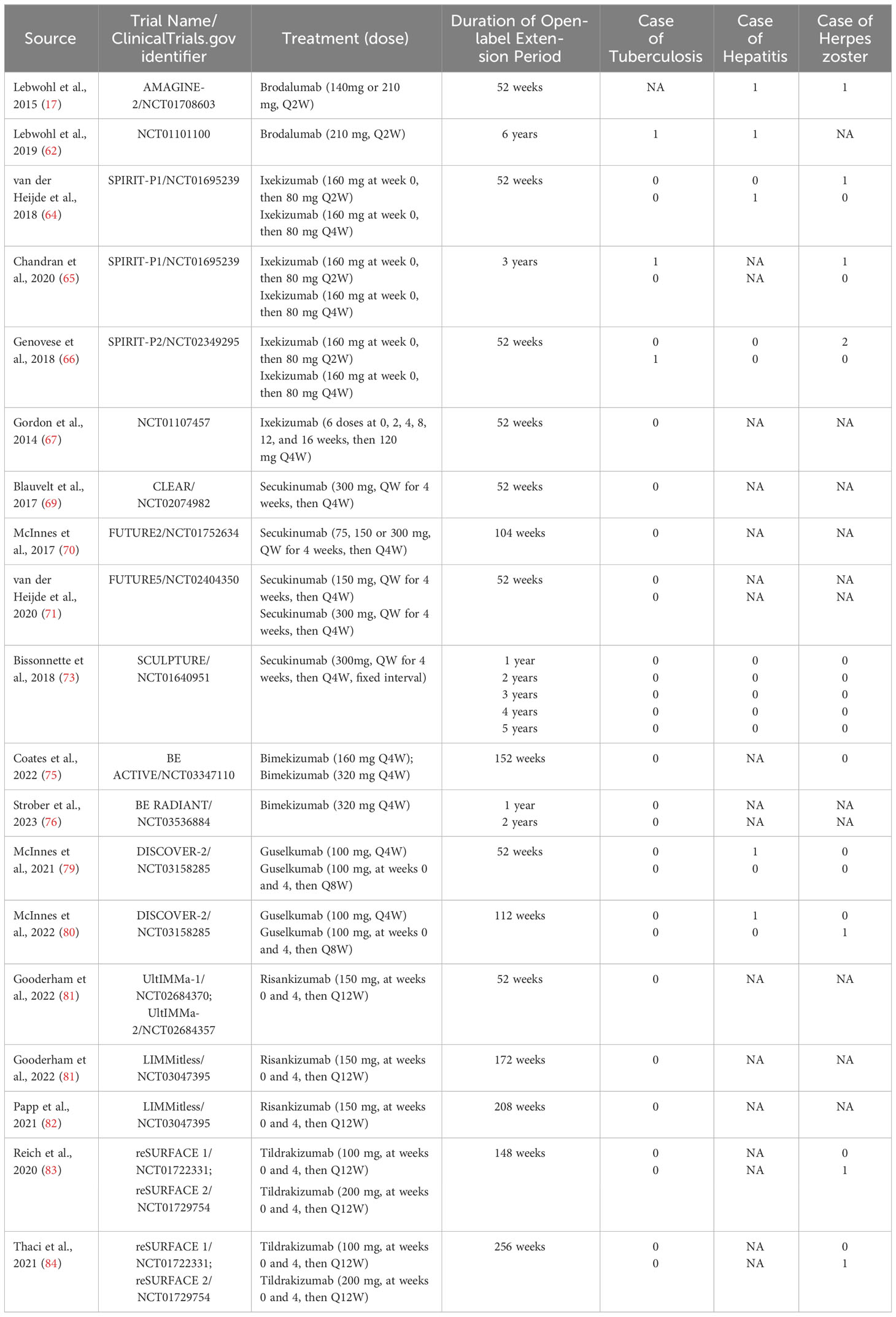
A total of 4,359 potentially relevant publications were identified through database searching, of which 4,116 were excluded after screening the title or abstract, 174 were excluded after full-text review. The researchers agreed on the major part of the study inclusion and data extraction, except for 6 publications, which were solved by consultation of the third researcher (L Guo). Then three publications were included, and three were excluded (1 study on children; 1 study on psoriasis erythrodermic; 1 study not placebo-controlled). Ultimately, 71 publications were qualified for inclusion (Figure 1) (16–84). A total of 25,496 patients were included in 44 randomized placebo-controlled studies (16–59) and 17,591 patients with 42,043.3 PYs of total exposure were included in 28 OLE studies (17, 31, 48, 60–84). The characteristics of RCTs were summarized in Table 1, and the characteristics of OLE studies were summarized in Table 2. All the included studies researched monotherapy of IL-17 or IL-23 inhibitors, with no other drug combined throughout all the trial periods. The reported cases of tuberculosis, hepatitis, and herpes zoster were summarized in Tables 3 and 4, as some of these AEs were so rare that meta-analyses were not able to be performed. Original data extracted from the included literatures were summarized in Tables S2-S4. Even when the risk of blinding was unclear in some trials, the overall risk of bias was low. The detailed risk of bias of each included study assessed by the Cochrane risk of bias tool for randomized studies of interventions was presented in Figure S1.
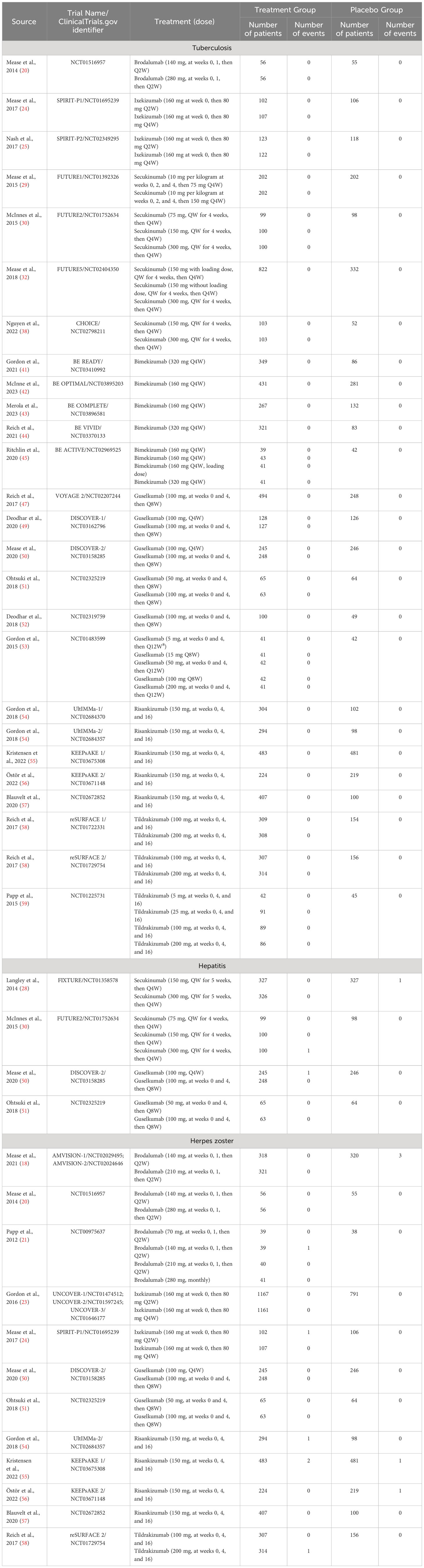
Table 3 The number of tuberculosis, hepatitis, and herpes zoster in both treatment groups and placebo groups in the included RCTs.
3.2 Short-term risk with IL-17 inhibitors
3.2.1 Serious infection
Serious infections were reported in 16 trials, including 8,267 patients in the treatment group and 3,075 patients in the placebo group. As there was no evidence of statistical heterogeneity between trials (I2 = 0.0%, P-value = 0.94), a common effects model was fit. The overall RR of serious infection was not increased with IL-17 inhibitors (RR = 1.45, 95% CI: 0.81-2.59) (Figure 2). The RR was not increased with brodalumab (RR = 0.91, 95% CI: 0.33-2.50), ixekizumab (RR = 1.93, 95% CI: 0.67-5.58), secukinumab (RR = 1.56, 95% CI: 0.44-5.48), or bimekizumab (RR = 1.70, 95% CI: 0.37-7.77). No subgroup differences were found among the four IL-17 antagonists (P-value = 0.77). There was no increase in the RR of severe infection in patients with PsO (RR = 1.08, 95% CI: 0.52-2.26) or PsA (RR = 2.20, 95% CI: 0.84-5.76) (Figure S2). No subgroup differences were found among the two indications (P-value = 0.25).
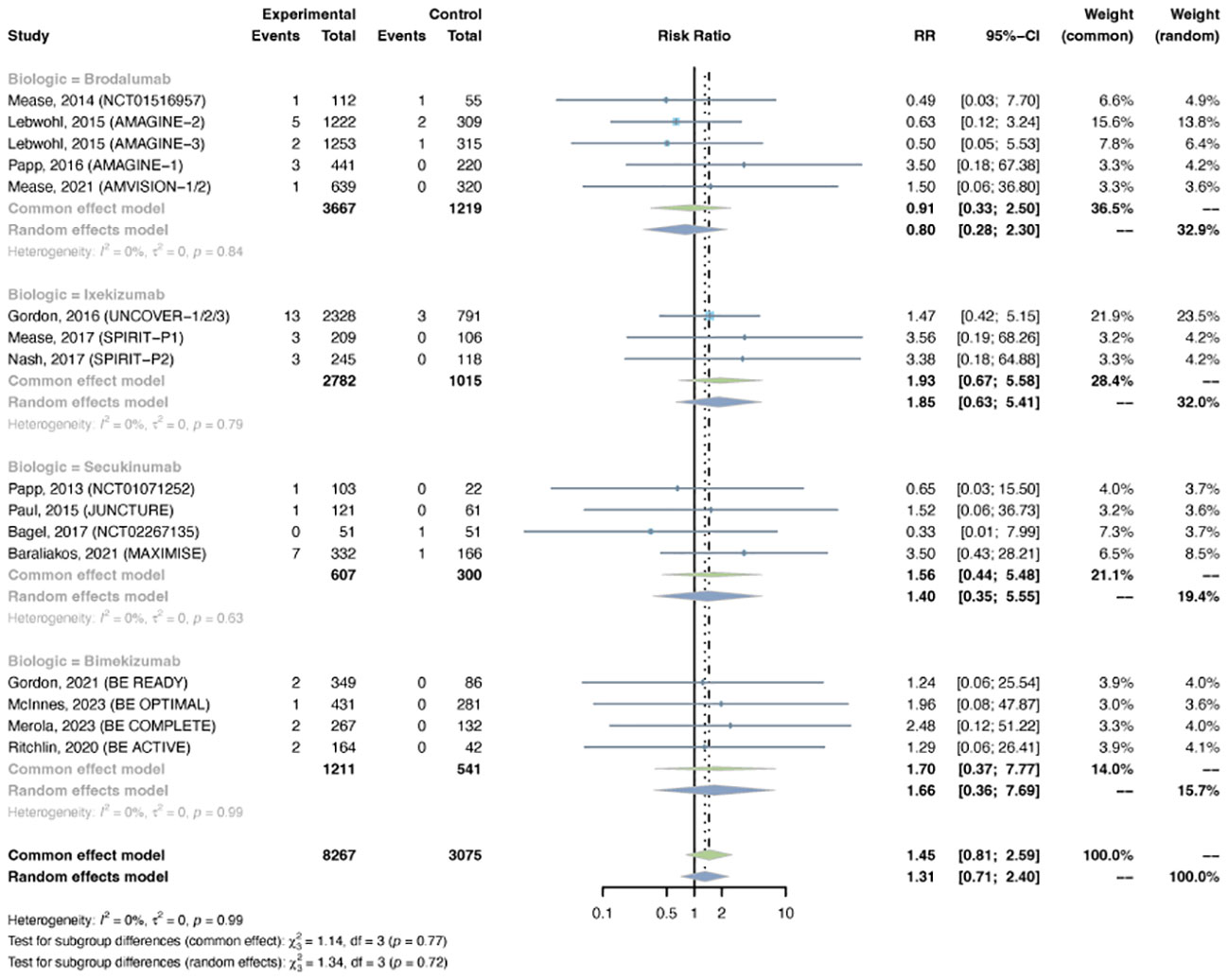
Figure 2 Pooled risk ratio (RR) of serious infection with the treatment of IL-17 inhibitors vs placebo.
3.2.2 Overall infection
Sixteen trials were selected, with 8,779 patients in the treatment arm and 3,347 patients in the placebo arm. There was major between-trial heterogeneity (I2 = 53%, P-value < 0.01), so a random effects model was used. Overall, the pooled RR of overall infection in patients with psoriasis receiving IL-17 inhibitors was 1.20 (95% CI: 1.06-1.35) (Figure 3). The RR was increased with ixekizumab (RR = 1.20, 95% CI: 1.06-1.35), secukinumab (RR = 1.28, 95% CI: 1.03-1.33), and bimekizumab (RR = 1.53, 95% CI: 1.16-2.01), whereas it was not increased with brodalumab (RR = 0.98, 95% CI: 0.79-1.22). The pooled RRs were 1.22 (95% CI: 1.01-1.46) in PsO, and 1.17 (95% CI: 0.99-1.39) in PsA (Figure S3).
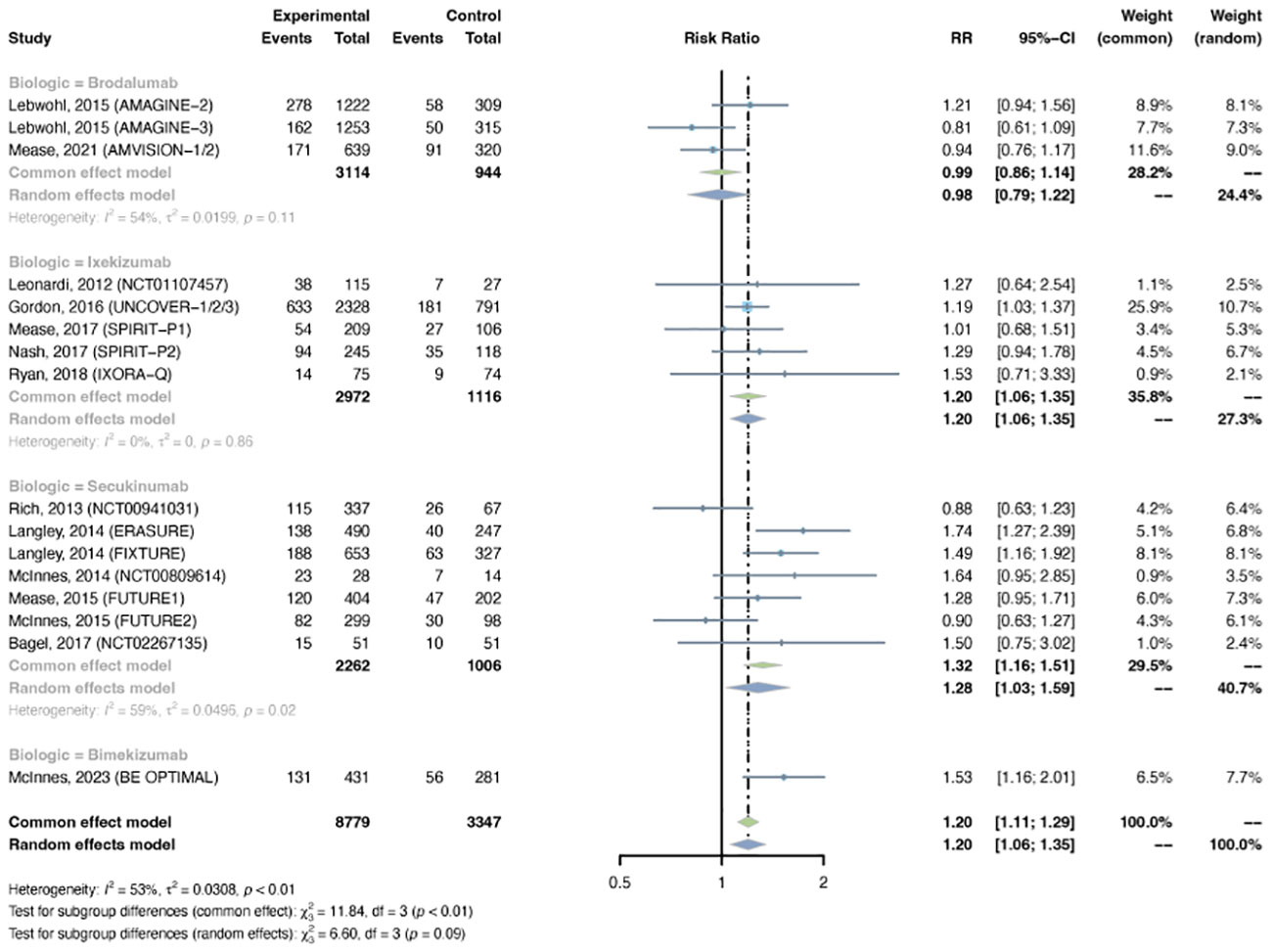
Figure 3 Pooled risk ratio (RR) of overall infection with the treatment of IL-17 inhibitors vs placebo.
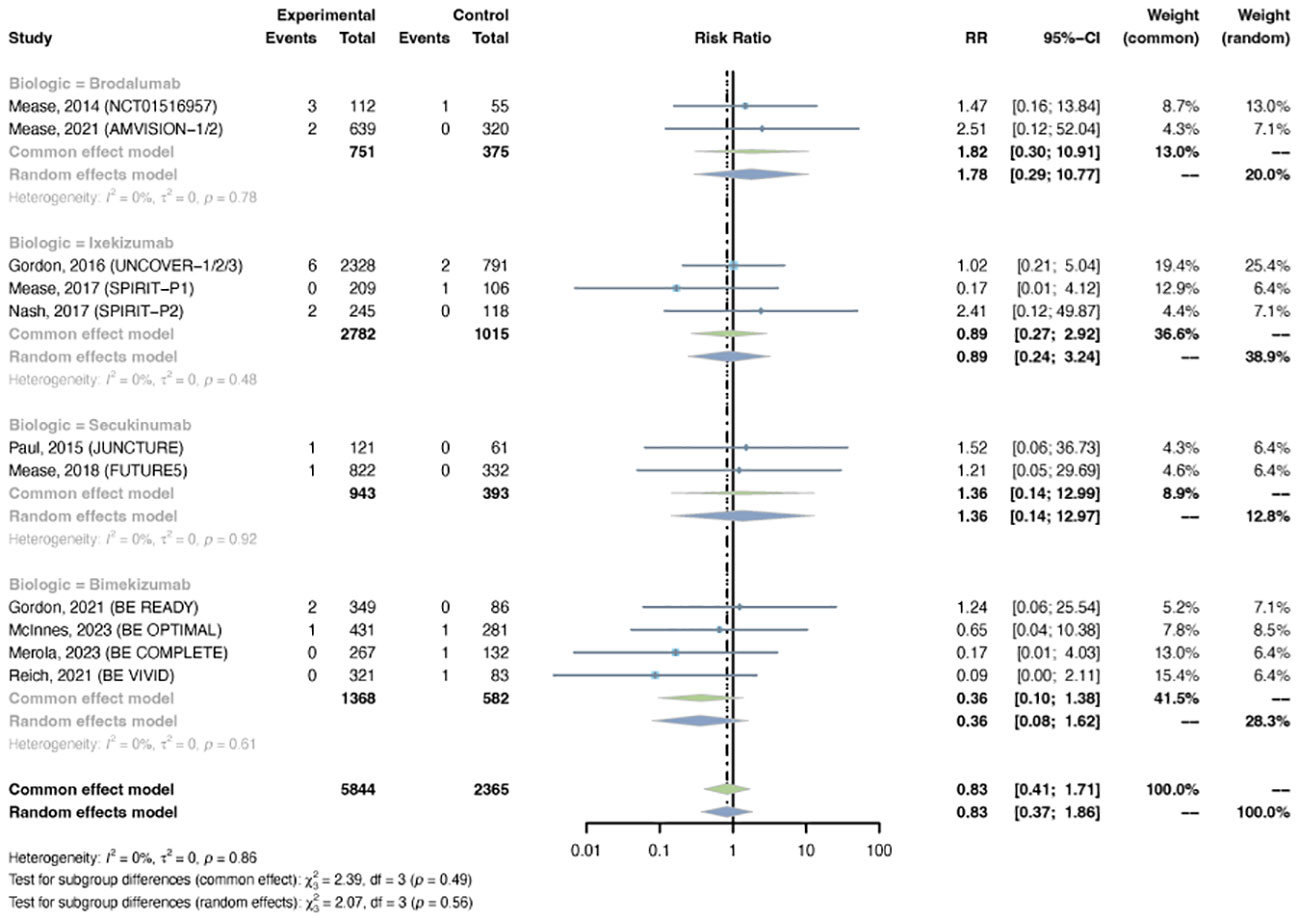
3.2.3 Malignancy
The outcomes of malignancy were reported in 11 RCTs, including 5,844 patients in the treatment group and 2,365 patients in the placebo group. A common-effects model was used based on the low heterogeneity between trials (I2= 0%, P-value = 0.86). The overall RR of malignancy was not increased in psoriasis patients using IL-17 inhibitors (RR = 0.83, 95% CI: 0.41-1.71) (Figure 4). The RR was not increased with brodalumab (RR = 1.82, 95% CI: 0.30-10.91), ixekizumab (RR = 0.89, 95% CI: 0.27-2.92), secukinumab (RR = 1.36, 95% CI: 0.14-12.99), or bimekizumab (RR = 0.36, 95% CI: 0.10-1.38). No subgroup differences were found among the different anti-IL-17 agents (P-value = 0.49). The pooled RR of malignancy was not increased in both PsO (RR = 0.77, 95% CI: 0.26-2.28) and PsA (RR = 0.88, 95% CI: 0.34-2.32) (Figure S4). No subgroup differences were found among the two conditions (P-value = 0.85).
3.2.4 Nasopharyngitis
The outcomes of nasopharyngitis were reported in 27 trials, including 8,732 patients in the treatment group and 3,551 patients in the placebo group. The overall pooled RR of nasopharyngitis in patients receiving IL-17 inhibitors was 1.17 (95% CI: 1.03-1.34) (Figure 5A). Subgroup analyses by biologic showed increased RRs of nasopharyngitis with secukinumab (RR = 1.21, 95% CI: 1.00-1.48) and bimekizumab (RR = 1.76, 95% CI: 1.16-2.67), instead of brodalumab and ixekizumab (Figure S5A). The RR of nasopharyngitis was increased in PsO, but not in PsA (Figure S5B).
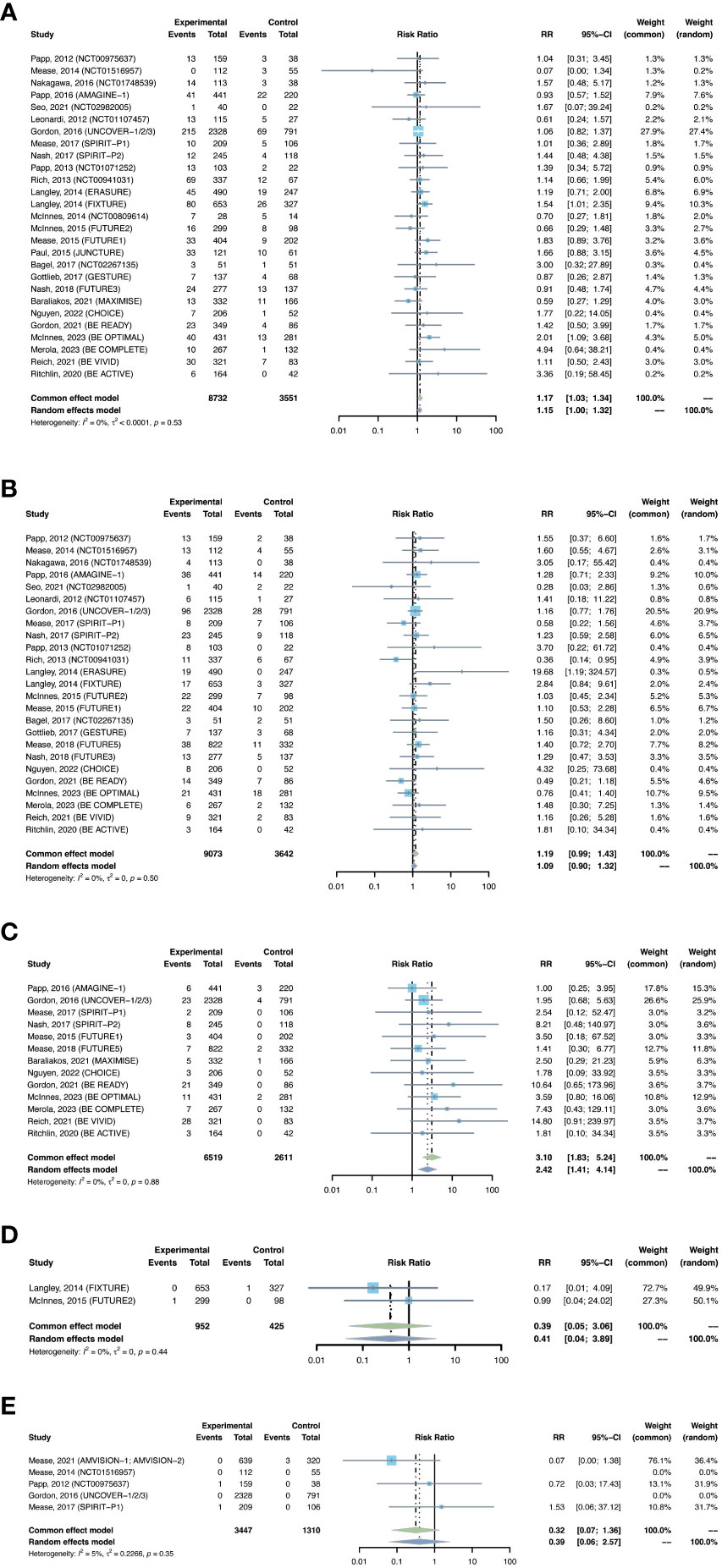
Figure 5 Pooled risk ratio (RR) of (A) nasopharyngitis; (B) upper respiratory tract infection; (C) Candida infection; (D) hepatitis; (E) herpes zoster with the treatment of IL-17 inhibitors vs placebo.
3.2.5 Upper respiratory tract infection
The outcomes of upper respiratory tract infection were reported in 25 trials, including 9,073 patients in the treatment group and 3,642 patients in the placebo group. The overall RR of upper respiratory tract infection was found to be 1.19 (95% CI: 0.99-1.43) (Figure 5B). The risk of upper respiratory tract infection was increased with secukinumab (RR = 1.43, 95% CI: 1.05-1.96), but not with the other three drugs (Figure S6A). The RR of upper respiratory tract infection was not increased in either PsO or PsA (Figure S6B).
3.2.6 Candida infection
The outcomes of Candida infection were reported in 13 trials, including 6,519 patients in the treatment group and 2,611 patients in the placebo group. The overall RR of Candida infection was 3.10 (95% CI: 1.83-5.24) (Figure 5C). The risk of Candida infection was increased with ixekizumab (RR = 2.59, 95% CI: 1.02-6.53), and was significantly increased with bimakizumab (RR = 6.47, 95% CI: 2.29-18.33) (Figure S7A). The RR of Candida infection was increased in both PsA and in PsO (Figure S7B).
3.2.7 Tuberculosis
There were 12 studies reporting the outcomes of tuberculosis, including 3,829 patients in the treatment group and 1,587 patients in the placebo group. In all the included RCTs, there was no case of latent or active tuberculosis reported in both treatment and placebo groups (Table 3). Therefore, quantitative meta-analysis was not performed. Nevertheless, we could obviously observe a quite low short-term risk of tuberculosis with IL-17 inhibitors in population without history or evidence of tuberculosis.
3.2.8 Hepatitis
There were 2 studies reporting the outcomes of hepatitis with IL-17 inhibitors, including 952 patients in the treatment group and 425 patients in the placebo group. There were only one case of hepatitis C reported in the treatment groups (30), and one case of hepatitis B reported in the placebo groups (28) (Table 3). Generally, the incidence of hepatitis was very low in the induction periods. Meta-analysis also did not reveal an increased risk (RR = 0.39, 95% CI: 0.05-3.06) (Figure 5D). Subgroup analysis was not performed due to limited data.
3.2.9 Herpes zoster
There were 2 studies reporting the outcomes of herpes zoster, including 3,447 patients in the treatment group and 1,310 patients in the placebo group. The overall RR of herpes zoster was 0.32 (95% CI: 0.07-1.36) (Figure 5E). As shown in Table 3, the overall incidence of herpes zoster was low. Two cases were reported in the treatment group, and three cases were reported in the placebo group. Subgroup analysis was not performed due to limited data.
3.3 Long-term incidence rates with IL-17 inhibitors
3.3.1 Serious infection
The overall long-term EAIR of serious infection was 1.11/100 PYs (95% CI: 0.92-1.29) in patients with psoriatic disease using IL-17 inhibitors (Figure 6A). The EAIR of serious infection was 1.28/100 PYs for 1 year, 1.07/100 PYs for 2 years, 1.10/100 PYs for 3 years, 1.10/100 PYs for 4 years, 1.03/100 PYs for 5 years, and 1.10/100 PYs for 6 years. No evidence of an increased incidence rate of serious infection was found with a longer duration of anti-IL-17 treatment.

Figure 6 Pooled exposure-adjusted incidence rates (EAIRs) of (A) serious infection; (B) overall infection; (C) nonmelanoma skin cancer (NMSC); and (D) malignancies excluding NMSC in psoriasis patients receiving long-term anti-IL-17 treatment.
3.3.2 Overall infection
The overall long-term EAIR of overall infection was 57.78/100 PYs (95% CI: 39.89-75.68) (Figure 6B). The EAIR of overall infection was 62.01/100PYs for 1 year, 84.88/100 PYs for 2 years, 24.65/100 PYs for 3 years, and 17.60/100 PYs for 5 years, respectively. There was no evidence showing an increase in the incidence rate of overall infection with the prolonged duration of anti-IL-17 treatment.
3.3.3 NMSC
The overall long-term EAIR of NMSC was 0.47/100PYs (95% CI: 0.26-0.68) in psoriasis patients receiving IL-17 inhibitors (Figure 6C). The data were limited to 2-year outcomes.
3.3.4 Malignancies excluding NMSC
The overall long-term EAIR of malignancies excluding NMSC was 0.24/100 PYs (95% CI: 0.14-0.35) in psoriasis patients receiving IL-17 inhibitors (Figure 6D). The EAIR of malignancies excluding NMSC was 0.14/100PYs for 1 year, 0.34/100PYs for 2 years, 0.36/100PYs for 3 years, and 0.53/100PYs for 5 years. No evidence of significantly increased incidence rate of malignancies excluding NMSC was found with longer duration.
3.3.5 Nasopharyngitis
The overall long-term EAIR of nasopharyngitis was 15.07/100PYs (95% CI: 12.97-17.17) in psoriasis patients receiving IL-17 inhibitors (Figure 7A). The EAIR of nasopharyngitis was 18.02/100PYs for 1 year, 12.46/100PYs for 2 years, 12.34/100PYs for 3 years, 16.10/100PYs for 4 years, and 11.14/100PYs for 5 years. No evidence of an increased incidence rate of nasopharyngitis was found with longer duration.

Figure 7 Pooled exposure-adjusted incidence rates (EAIRs) of (A) nasopharyngitis; (B) upper respiratory tract infection; (C) Candida infection in psoriasis patients receiving long-term anti-IL-17 treatment.
3.3.6 Upper respiratory tract infection
The overall long-term EAIR of upper respiratory tract infection was 8.52/100PYs (95% CI: 6.77-10.27) (Figure 7B). The EAIR of upper respiratory tract infection was 9.40/100PYs for 1 year, 9.32/100PYs (95% CI: 6.90-15.33) for 2 years, 6.21/100PYs for 3 years, and 2.80/100PYs for 5 years. No evidence of an increased incidence rate of upper respiratory tract infection was found with the prolonged duration.
3.3.7 Candida infection
The overall long-term EAIR of Candida infection was 3.41/100PYs (95% CI: 2.70-4.12) in psoriasis patients receiving IL-17 inhibitors (Figure 7C). The EAIR of Candida infection was 4.68/100PYs for 1 year, 3.23/100PYs for 2 years, 2.26/100PYs for 3 years, 3.26/100PYs for 4 years, 2.50/100PYs for 5 years, and 5.60/100PYs for 6 years. No evidence of an increased incidence rate of Candida infection was found with the prolonged duration.
3.3.8 Tuberculosis
There were 12 studies reporting the long-term incidence of tuberculosis. One case of latent tuberculosis was reported in the six-year OLE period of brodalumab (210 mg, every 2 weeks) (62); one case was reported in the three-year OLE period of Ixekizumab (160 mg at week 0, then 80mg every 2 weeks) (65); and one case was reported in the one-year OLE period of Ixekizumab (160 mg at week 0, then 80mg every 4 weeks) (66). Other included studies all reported 0/100PYs of tuberculosis. No active or reactivated tuberculosis cases were ever reported in all the included studies. No quantitative meta-analysis was performed due to the limited data. In general, the long-term EAIRs were quite low.
3.3.9 Hepatitis
There were 5 studies reporting the long-term incidence of hepatitis. One case of hepatitis was reported in the one-year and six-year OLE periods of brodalumab (210 mg, every 2 weeks) (17); and one case of hepatitis B was reported in the one-year OLE period of ixekizumab (160 mg at week 0, then 80mg every 4 weeks) (64). No cases of hepatitis were reported by other included studies. No quantitative meta-analysis was performed due to the limited data.
3.3.10 Herpes zoster
There were 6 studies reporting the long-term incidence of herpes zoster. One case of herpes zoster was reported in the one-year OLE period of brodalumab (210 mg, every 2 weeks) (17); one case in the one-year and three-year OLE periods of ixekizumab (160 mg at week 0, then 80mg every 2 weeks) (64, 65); 2 in the one-year period of ixekizumab (160 mg at week 0, then 80mg every 2 weeks) (66). Other included studies reported 0/100PYs of EAIRs though the whole trials. No quantitative meta-analysis was performed due to the limited data. In general, the EAIRs were very low.
3.4 Short-term risk with IL-23 inhibitors
3.4.1 Serious infection
Serious infections were reported in 14 trials, including 5,024 patients in the treatment group and 2,177 patients in the placebo group. A common-effects model was adapted based on low heterogeneity (I2 = 0%, p = 0.96). The overall RR of serious infection was not increased with IL-23 inhibitors (RR = 0.68, 95% CI: 0.38-1.22) (Figure 8). The pooled RR was 0.70 (95% CI: 0.26-1.90) with guselkumab, 0.70 (95% CI: 0.31-1.56) with risankizumab, and 0.55 (95% CI: 0.11-2.82) with tidrakizumab. No subgroup differences were found among the three anti-IL-23 agents (P-value = 0.96). The pooled RR was not increased in either PsO (RR = 0.70, 95% CI: 0.28-1.76) or PsA (RR = 0.67, 95% CI: 0.31-1.42) (Figure S8). No subgroup differences were found among the two conditions (P-value = 0.94).
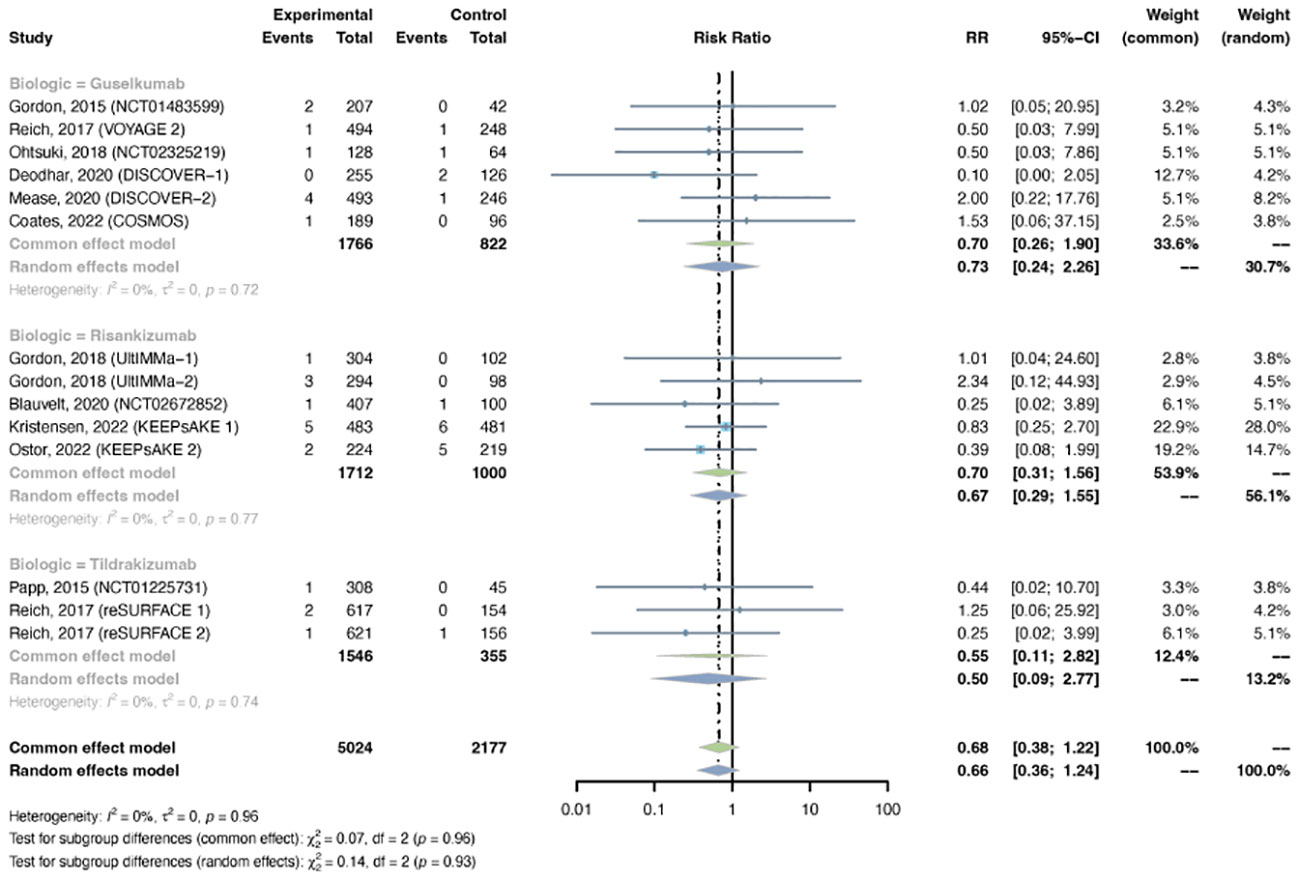
Figure 8 Pooled risk ratio (RR) of serious infection with the treatment of IL-23 inhibitors vs placebo.
3.4.2 Overall infection
The outcomes of overall infection were reported in 13 trials, including 4,438 patients in the treatment group and 1,655 patients in the placebo group. The overall RR of overall infection in psoriasis patients using IL-23 inhibitors was 1.13 (95% CI: 1.00-1.28) (Figure 9). Statistical heterogeneity was low between trials (I2 = 0%, P-value = 0.68). The pooled RR of overall infection was moderately increased with risankizumab (RR = 1.37, 95% CI: 1.02-1.85), instead of guselkumab (RR = 1.05, 95% CI: 0.91-1.22) or tidrakizumab (RR = 1.25, 95% CI: 0.86-1.83). The pooled RR was increased in PsO (RR = 1.21, 95% CI: 1.04-1.41), but not in PsA (RR = 0.98, 95% CI: 0.80-1.21) (Figure S9).
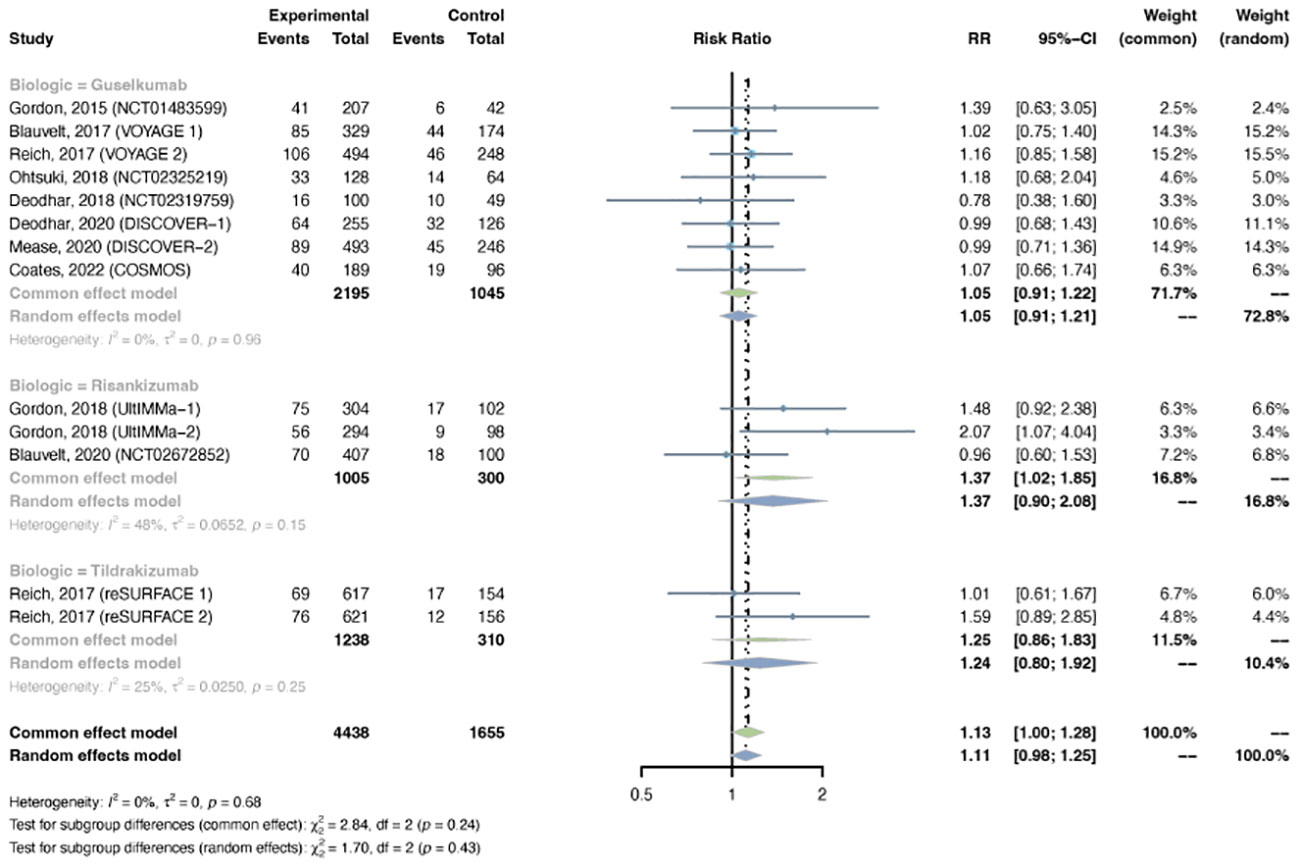
Figure 9 Pooled risk ratio (RR) of overall infection with the treatment of IL-23 inhibitors vs placebo.
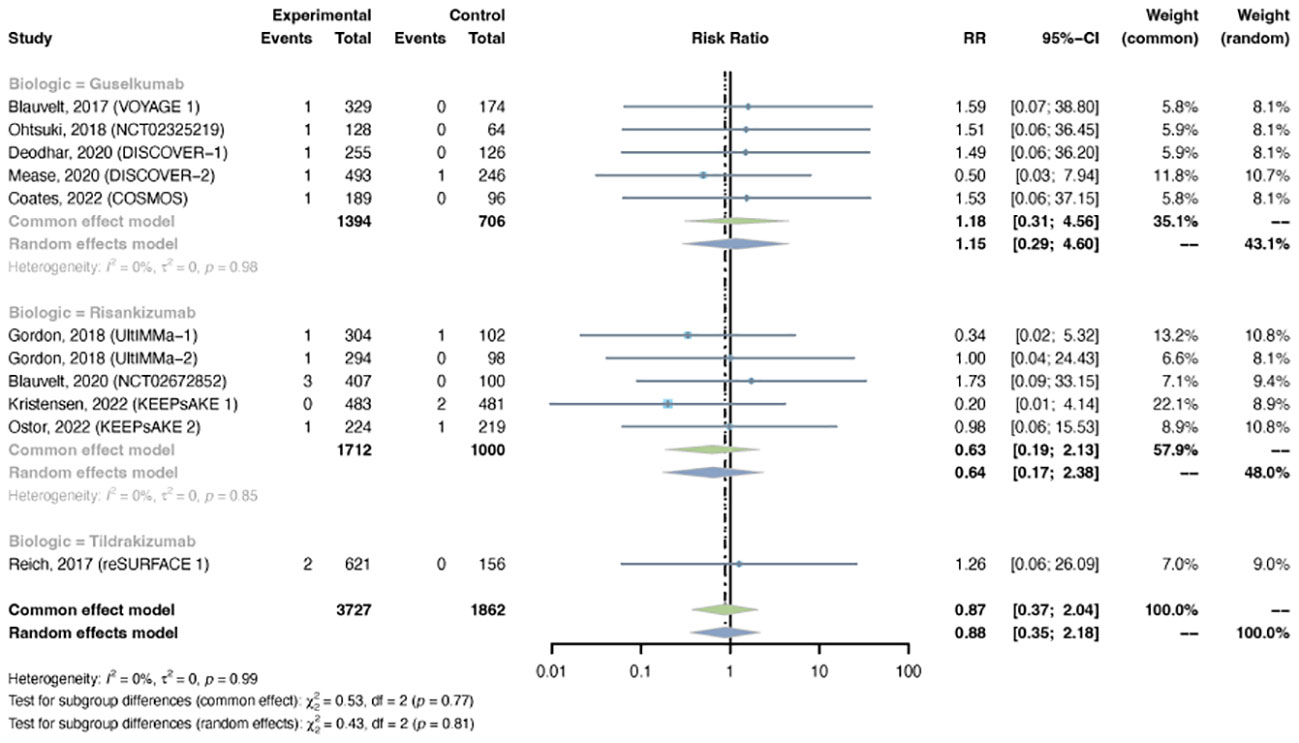
3.4.3 Malignancy
The outcomes of malignancy were reported in 11 trials, including 3,727 patients in the treatment group and 1,862 patients in the placebo group. No evidence of statistical heterogeneity between trials was found (I2 = 0.0%, P-value = 0.99), so a common-effects model was adapted. The pooled RR of malignancy in psoriasis patients with IL-23 inhibitors was not increased (RR = 0.87, 95% CI: 0.37-2.04) (Figure 10). The RR of malignancy was not increased with either guselkumab (R = 1.18, 95% CI: 0.31-4.56), risankizumab (RR = 0.63, 95% CI: 0.19-2.13), or tidrakizumab (RR = 1.26, 95% CI: 0.06-26.09). No subgroup differences were found among the three anti-IL-23 agents (P-value = 0.77). The pooled RR was not increased in PsO (RR = 1.10, 95% CI: 0.33-3.66) or PsA (RR = 0.67, 95% CI: 0.19-2.33) (Figure S10). No subgroup differences were found among the two conditions (P-value = 0.57).
3.4.4 Nasopharyngitis
The outcomes of nasopharyngitis were reported in 14 studies, including 4,855 patients in the treatment group and 2,200 in the placebo group. The overall RR of nasopharyngitis was not increased with IL-23 inhibitors (RR = 1.15, 95% CI: 0.94-1.41) (Figure 11A). Subgroup analysis by biologic showed that none of the anti-IL-23 agents could improve the risk of nasopharyngitis (Figure S11A). Subgroup analysis by indications suggested that the risk of nasopharyngitis was not increased in either PsO or PsA (Figure S11B).
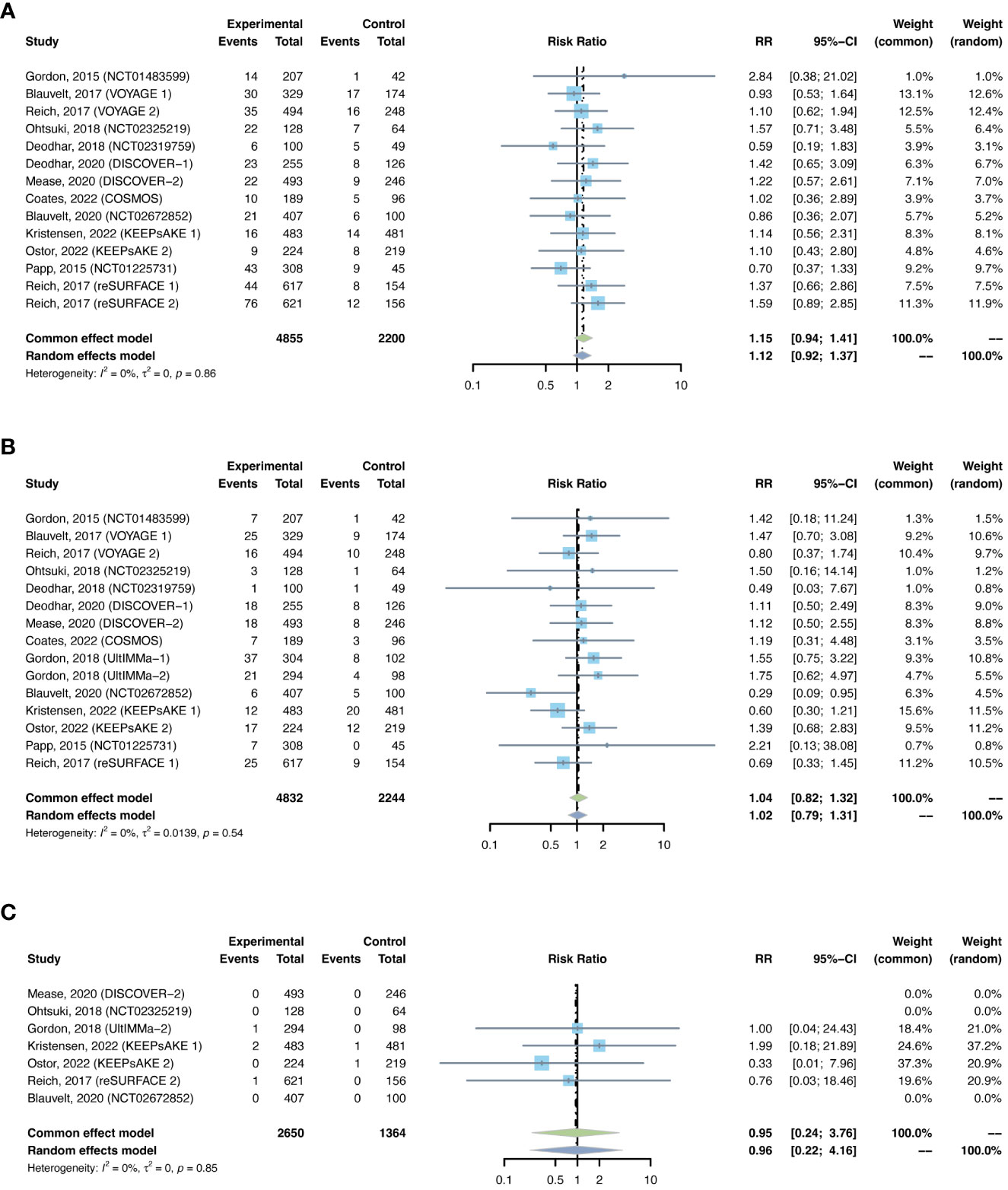
Figure 11 Pooled risk ratio (RR) of (A) nasopharyngitis; (B) upper respiratory tract infection; (C) herpes zoster with the treatment of IL-23 inhibitors vs placebo.
3.4.5 Upper respiratory tract infection
The outcomes of upper respiratory tract infection were reported in 15 studies, including 4,832 patients in the treatment group and 2,244 patients in the placebo group. The overall RR of upper respiratory tract infection was not increased with IL-23 inhibitors (RR = 1.04, 95% CI: 0.82-1.32) (Figure 11B). Subgroup analysis by biologic showed that none of the anti-IL-23 agents could improve the risk of upper respiratory tract infection (Figure S12A). Subgroup analysis by indications suggested that the risks of upper respiratory tract infection were not increased in either PsO or PsA (Figure S12B).
3.4.6 Tuberculosis
There were 14 studies reporting the outcomes of tuberculosis, including 4,935 patients in the treatment group and 2,130 patients in the placebo group. Similar to IL-17 inhibitors, there was zero case of latent or active tuberculosis reported in both treatment and placebo groups with IL-23 inhibitors (Table 3). As a result, quantitative meta-analysis was not performed. A very low short-term risk of tuberculosis with IL-23 inhibitors was reported in population without history or evidence of tuberculosis.
3.4.7 Hepatitis
There were 2 studies reporting the outcomes of hepatitis with IL-23 inhibitors, including 621 patients in the treatment group and 310 patients in the placebo group. There were only one case of hepatitis B reported in the treatment groups (50) (Table 3). Meta-analysis was not performed due to the limited data. Generally, the incidence of hepatitis was found very low in the induction periods.
3.4.8 Herpes zoster
There were 7 studies reporting the outcomes of herpes zoster, including 2,650 patients in the treatment group and 1,364 patients in the placebo group. Quantitative meta-analysis suggested that the overall RR of herpes zoster was not increased with IL-23 inhibitors (RR = 0.95, 95% CI: 0.24-3.76) (Figure 11C). Subgroup analysis was not performed due to limited data.
3.5 Long-term incidence rates with IL-23 inhibitors
3.5.1 Serious infection
The overall long-term EAIR of serious infection was 1.09/100 PYs (95% CI: 0.96-1.22) in patients with psoriatic disease using IL-23 inhibitors (Figure 12A). The EAIR of serious infection was 1.33/100 PYs for 1 year, 1.18/100 PYs for 2 years, 1.13/100 PYs for 3 years, 1.20/100 PYs for 4 years, and 1.05/100 PYs for 5 years. No evidence of an increased incidence rate of serious infection was found with the prolonged duration of anti-IL-23 treatment.

Figure 12 Pooled exposure-adjusted incidence rates (EAIRs) of (A) serious infection; (B) overall infection; (C) nonmelanoma skin cancer (NMSC); and (D) malignancies excluding NMSC in psoriasis patients receiving long-term anti-IL-23 treatment.
3.5.2 Overall infection
The overall long-term EAIR of overall infection was 48.50/100 PYs (95% CI: 31.76-65.24) (Figure 12B). The EAIR of overall infection was 35.50/100PYs in psoriasis patients receiving IL-17 inhibitors for 1 year, 52.73/100 PYs for 2 years, 74.03/100 PYs for 3 years. There was also no evidence showing an increased incidence rate of overall infection with the prolonged duration of anti-IL-23 treatment.
3.5.3 NMSC
The overall long-term EAIR of NMSC was 0.40/100 PYs (95% CI: 0.32-0.48) in psoriasis patients receiving IL-23 inhibitors (Figure 12C). The EAIR of NMSC was 0.50/100PYs in psoriasis patients receiving IL-23 inhibitors for 1 year, 0.39/100PYs for 2 years, 0.42/100PYs for 3 years, 0.50/100PYs for 4 years, and 0.36/100PYs for 5 years. No evidence of an increased incidence rate of NMSC was found with the prolonged treatment duration.
3.5.4 Malignancies excluding NMSC
The overall long-term EAIR of malignancies excluding NMSC was 0.43/100 PYs (95% CI: 0.35-0.51) in psoriasis patients receiving IL-23 inhibitors (Figure 12D). The EAIR of malignancies excluding NMSC was 0.83/100PYs for 1 year, 0.29/100PYs for 2 years, 0.46/100PYs for 3 years, 0.33/100PYs for 4 years, and 0.54/100PYs for 5 years. No evidence of an increased incidence rate of malignancies excluding NMSC was found with the prolonged duration.
3.5.5 Nasopharyngitis
The overall long-term EAIR of nasopharyngitis was 10.75/100PYs (95% CI: 8.49-13.01) in psoriasis patients receiving IL-23 inhibitors (Figure 13A). The EAIR of nasopharyngitis was 7.38/100PYs for 1 year, 9.99/100PYs for 3 years, 17.30/100PYs for 4 years, and 10.60/100PYs for 5 years. No evidence of an increased incidence rate of nasopharyngitis was found with the prolonged treatment duration.
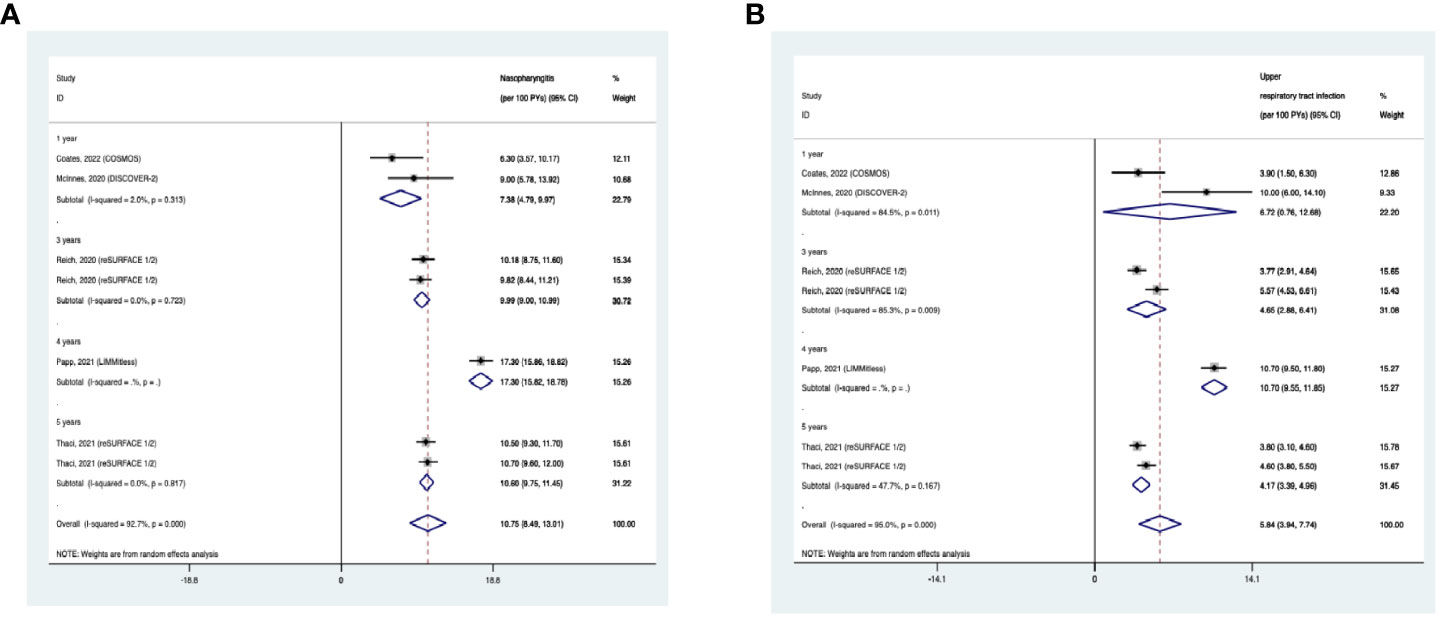
Figure 13 Pooled exposure-adjusted incidence rates (EAIRs) of (A) nasopharyngitis; (B) upper respiratory tract infection in psoriasis patients receiving long-term anti-IL-23 treatment.
3.5.6 Upper respiratory tract infection
The overall long-term EAIR of upper respiratory tract infection was 5.84/100PYs (95% CI: 3.94-7.74) (Figure 13B). The EAIR of upper respiratory tract infection was 6.72/100PYs for 1 year, 4.65/100PYs for 3 years, 10.70/100PYs for 5 years, and 4.17/100PYs. No evidence of an increased incidence rate of upper respiratory tract infection was found with the longer duration.
3.5.7 Tuberculosis
There were 6 studies reporting the long-term incidence of tuberculosis. Of all the included studies, the long-term EAIRs of tuberculosis were 0/100PYs, with no case of active, reactivated or latent tuberculosis reported throughout the whole trials. Therefore, the overall EAIR of tuberculosis with IL-23 inhibitors was estimated to be 0/100PYs. No quantitative meta-analysis was performed due to the limited data.
3.5.8 Hepatitis
There was 1 study reporting the long-term incidence of hepatitis. Only one case of hepatitis B was reported in the two-year OLE period of guselkumab (100 mg, every 4 weeks) (80). No case of hepatitis with other types of IL-23 inhibitors was reported. No quantitative meta-analysis was performed due to the limited data.
3.5.9 Herpes zoster
There were 4 studies reporting the long-term incidence of herpes zoster. One case in the two-year OLE period of guselkumab (100 mg, every 4 weeks) (80), one in the three-year and five-year OLE periods of Tildrakizumab (200 mg, at week 4 and every 12 weeks thereafter) (83, 84). Other included studies reported 0/100PYs of EAIRs though the whole trials. No quantitative meta-analysis was performed due to the limited data. In general, the EAIRs were very low.
3.6 Publication bias and sensitivity analyses
No evidence of publication bias was found with “peters” tests for serious infection (P-value = 0.66), overall infection (P-value = 0.62), malignancy (P-value = 0.82), nasopharyngitis (P-value = 0.73), upper respiratory tract infection (P-value = 0.98), or Candida infection (P-value = 0.06) with IL-17 inhibitors; serious infection (P-value = 0.63), overall infection (P-value = 0.89), malignancy (P-value = 0.22), nasopharyngitis (P-value = 0.65), or upper respiratory tract infection (P-value = 0.08) with IL-23 inhibitors. Funnel plots created for all of the above outcomes were found to be symmetrical (Figures S13, S14). Sensitivity analyses suggested that omitting any one of the studies had little effect on the final results in general (Figures S15–S18).
4 Discussion
Therapeutic agents that specifically target the IL-17 family and IL-23 have demonstrated excellent efficacy in PsO and PsA (5, 6). However, the potential for infection and malignancy has been a concern with respect to the therapies which suppress immune pathways (9, 85–87). Individual studies are not large enough in sample sizes or of long enough duration to ascertain uncommon adverse events. Hence, we performed a meta-analysis of RCTs and OLE studies to estimate the risk and incidence of infections and malignancies with treatments of IL-17 or IL-23 inhibitors. The results suggested that anti-IL-17 or anti-IL-23 treatments did not increase the short-term risk of serious infection or malignancy in adult patients with psoriatic disease. Correspondingly, the long-term incidence rates of serious infection and malignancy were quite low, in particular the incidence rate of malignancy (less than 1/100PYs). Despite the enhanced short-term risks of overall infection and some specific infections with both IL-17 inhibitors and IL-23 inhibitors, the risk ratios were moderately increased. The long-term incidence rates of overall infection were estimated to be 57.78/100PYs with IL-17 inhibitors and 48.5/100PYs with IL-23 inhibitors.
Subgroup analyses by biological agents suggested that brodalumab, ixekizumab, secukinumab, bimekizumab, guselkumab, risankizumab and tildrakizumab are safe in general, but still showed diversity in safety profile. In this study, ixekizumab, secukinumab, bimekizumab and risankizumab increased the short-term risk of overall infection, whereas other drugs did not. Specifically, ixekizumab might increase the short-term risk of Candida infection; secukinumab might increase the short-term risk of nasopharyngitis and upper respiratory tract infection; bimekizumab might increase the short-term risk of nasopharyngitis and Candida infection. None of guselkumab, risankizumab and tildrakizumab was able to increase the short-term risk of nasopharyngitis or upper respiratory tract infection. In general, the risks of infection and malignancy were comparable between patients with PsO and PsA, though there were some differences in individual AEs.
Nasopharyngitis and upper respiratory tract infection are considered the most frequent AEs with IL-17 or IL-23 antagonists (10, 11). It is interesting to note that IL-17 inhibitors did not increase the short-term risk of upper respiratory tract infection, and slightly increased the risk of nasopharyngitis. IL-23 inhibitors did not increase the short-term risk of nasopharyngitis or upper respiratory tract infection. But the long-term EAIRs of nasopharyngitis (15.07/100PYs with IL-17 inhibitors; 10.75/100PYs with IL-23 inhibitors) and upper respiratory tract infection (8.52/100PYs with IL-17 inhibitors; 5.84/100PYs with IL-23 inhibitors) were somewhat high. It is possible that nasopharyngitis and upper respiratory tract infection, albeit as two common infections in real-life settings with high incidence, are likely to have comparable incidence rates in psoriasis patients with or without using IL-17/IL-23 inhibitors. However, this finding requires further validation.
It is noteworthy that Candida infection appeared to be a prominent AE for IL-17 inhibitors. Our study suggested that the short-term risk of Candida infection was approximately 3-fold higher when administrating IL-17 antagonists compared with placebo. The long-term incidence rate was estimated to be 3.41/100PYs. It is well-acknowledged that IL-17 plays a role in host defense against bacterial and fungal infections, particularly in mucocutaneous microbial surveillance (86, 88). The deficiency of IL-17 immunity could lead to infections with Candida species (89). Hence, continued vigilance for such infection will be required during treatment with inhibitors of this pathway. In addition, It is worth noting that the risk of Candida infection was increased almost 6-fold when bimekizumab was used, which appeared to be higher than other IL-17 inhibitors. These findings were consistent with data from existing clinical trials, which demonstrated a higher incidence of Candida infection with bimekizumab than other IL-17 antagonists (41–45, 75, 76). It might be explained by the differing targets between bimekizumab and other IL-17 inhibitors (6). In comparison to ixekizumab and secukinumab which target IL-17A, bimekizumab has been shown to have dual neutralization of IL-17A and IL-17F. These findings point to the important role of IL-17F in host defense, especially at the mucocutaneous sites (86, 88). Despite higher Candida infection occurrence with IL-17 antagonists, most reported cases were mild or moderate infections, none of them were systemic, and most cases could be resolved with appropriate antifungal treatment without causing trial dropouts (41–45).
Compared with Candida infection, the incidence rates of tuberculosis, hepatitis, and herpes zoster were quite lower. For IL-17 inhibitors, zero case of tuberculosis in the induction periods was found, and only 3 cases of latent tuberculosis in long-term extension periods were reported. For IL-23 inhibitors, no case of tuberculosis in the whole induction periods and extension periods was reported. No case of active or reactivated tuberculosis was ever reported in all the included studies and throughout the whole trial periods, either with IL-17 inhibitors or IL-23 inhibitors. Short-term risks of hepatitis and herpes zoster seemed not increased according to the meta-analyses, despite limited studies and small sample sizes. Only several cases of hepatitis and herpes zoster were reported throughout the whole trial periods. The trial researchers tended not to attribute these cases to treatment-related reasons (17, 64–66, 80, 83, 84). These results supported that IL-17 inhibitors and IL-23 inhibitors may not increase the risk of tuberculosis, hepatitis, and herpes zoster in psoriasis patients without known diagnoses or previous histories of these infections. However, it did not mean that patients with history or evidence were totally safe to receive IL-17 or IL-23 inhibitors, as patients at high risk or with previous histories of tuberculosis, hepatitis or herpes zoster were already excluded in most studies. Therefore, it is noteworthy that patients with susceptibility to opportunistic infections and hepatitis always need overall examination and prudent evaluation before and during using any biologics (6, 10).
The long-term safety analyses presented results of continuous treatments of IL-17 inhibitors and IL-23 inhibitors for up to 6 years, with > 42,000 PYs of total exposure. The cumulative long-term EAIRs of serious infection and malignancies are generally comparable to the results from the psoriasis reference population captured in the Psoriasis Longitudinal Assessment and Registry (PSOLAR) (90, 91). The long-term EAIRs of overall infection, serious infection, NMSC and malignancies excluding NMSC were also comparable between IL-17 antagonists and IL-23 antagonists. IL-23 maintains IL-17–producing Th17 cells, and is essential for their differentiation, activation, and survival (8). Therefore, inhibition of IL-23 blocks downstream production of IL-17A by Th17 cells and other cell types (87). This might explain the comparable safety profiles between IL-17 inhibitors and IL-23 inhibitors in our study. The EAIRs of infection and malignancy were stratified by years to estimate the EAIRs in different durations. While the present study was not designed to quantify the differences of EAIRs between different durations, these findings seemed to suggest that the EAIRs of infection and malignancy might be broadly consistent or even diminished with the prolonged treatment duration. These findings indicated that long-term sustained treatments with IL-17 or IL-23 inhibitors with appropriate doses could be safe with continued follow-ups of the patients.
This study has several limitations. Firstly, we did not assess the dose-related trends in the incidence rate or severity of infection and malignancy. Patients are included in our analysis once they have received at least one dose of either the anti-IL-17 or anti-IL-23 antagonists. Secondly, this study might fail to evaluate the risk in patients at high risk for infections or malignancies because of the possible exclusion of patients with previous histories of infections or malignancies in the original clinical studies. Thirdly, some of the results of the meta-analyses may still present small sample sizes due to the limited data, which may decrease the reliability. Finally, we only included RCTs and OLE studies in this review. There is a need for a meta-analysis of real-world studies evaluating the risk of infection and malignancy in patients with psoriatic disease who receive IL-17 or IL-23 antagonists.
In conclusion, IL-17 inhibitors and IL-23 inhibitors are generally safe in the treatment of psoriasis and psoriatic arthritis, with both short-term and long-term treatments. Nasopharyngitis and upper respiratory tract infection are the most frequent AEs, and Candida infection seemed to be the most common opportunistic infection. It is critical to exercise caution and maintain ongoing vigilance for nasopharyngitis and Candida infection in patients receiving IL-17 inhibitors. However, to comprehensively evaluate the potential risks of cancer and infection related to prolonged use of IL-17 inhibitors and IL-23 inhibitors in individuals with psoriasis, larger, long-term, prospective cohort studies will be required.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
SW: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing, Investigation, Software, Visualization. YX: Formal Analysis, Methodology, Software, Writing – original draft, Writing – review & editing, Data curation. LY: Formal Analysis, Methodology, Writing – original draft, Writing – review & editing, Investigation, Software. LG: Funding acquisition, Supervision, Validation, Writing – original draft, Writing – review & editing, Investigation, Methodology, Visualization. XJ: Funding acquisition, Supervision, Validation, Writing – original draft, Writing – review & editing, Conceptualization, Methodology, Resources.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work is supported by National Natural Science Foundation of China (NSFC 82273559, 82073473, and 82304047); The 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC21036); Sichuan Province Science and Technology Plan Project (2023NSFSC1546); Postdoc project of West China Hospital, Sichuan University and China Postdoctoral Science Foundation (2023HXBH10, 2023SCU12067, 2022M712246).
Acknowledgments
The authors thank the patients, the investigators and their teams who took part in the clinical trials included in this systematic review and meta-analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1294416/full#supplementary-material
References
1. M Griffiths CE, Armstrong AW, Gudjonsson JE, W N Barker JN. Psoriasis. The Lancet (2021). Available at: www.thelancet.com.
2. Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. New Engl J Med (2017) 376(10):957–70. doi: 10.1056/NEJMra1505557
3. Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. Lancet (2018) 391(10136):2273–84. doi: 10.1016/S0140-6736(18)30830-4
4. Takeshita J, Grewal S, Langan SM, Mehta NN, Ogdie A, Van Voorhees AS, et al. Psoriasis and comorbid diseases: Epidemiology. J Am Acad Dermatol (2017) 76(3):377–90. doi: 10.1016/j.jaad.2016.07.064
5. Coates LC, Soriano ER, Corp N, Bertheussen H, Callis Duffin K, Campanholo CB, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol (2022) 18(8):465–79. doi: 10.1038/s41584-022-00798-0
6. Ghoreschi K, Balato A, Enerbäck C, Sabat R. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet (2021) 397(10275):754–66. doi: 10.1016/S0140-6736(21)00184-7
7. Sbidian E, Giboin C, Bachelez H, Paul C, Beylot-Barry M, Dupuy A, et al. Factors associated with the choice of the first biologic in psoriasis: real-life analysis from the Psobioteq cohort. J Eur Acad Dermatol Venereol (2017) 31(12):2046–54. doi: 10.1111/jdv.14406
8. Whitley SK, Li M, Kashem SW, Hirai T, Igyártó BZ, Knizner K, et al. Local IL-23 is required for proliferation and retention of skin-resident memory TH17 cells. Sci Immunol (2022) 7(77):eabq3254. doi: 10.1126/sciimmunol.abq3254
9. Li X, Bechara R, Zhao J, McGeachy MJ, Gaffen SL. IL-17 receptor–based signaling and implications for disease. Nat Immunol (2019) 20(12):1594–602. doi: 10.1038/s41590-019-0514-y
10. Dávila-Seijo P, Dauden E, Descalzo MA, Carretero G, Carrascosa JM, Vanaclocha F, et al. Infections in moderate to severe psoriasis patients treated with biological drugs compared to classic systemic drugs: findings from the BIOBADADERM registry. J Invest Dermatol (2017) 137(2):313–21. doi: 10.1016/j.jid.2016.08.034
11. Rustin MHA. Long-term safety of biologics in the treatment of moderate-to-severe plaque psoriasis: review of current data. Br J Dermatol (2012) 167 Suppl 3:3–11. doi: 10.1111/j.1365-2133.2012.11208.x
12. Papp KA. The long-term efficacy and safety of new biological therapies for psoriasis. Arch Dermatol Res (2006) 298(1):7–15. doi: 10.1007/s00403-006-0660-6
13. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ (2019) 366:l4898. doi: 10.1136/bmj.l4898
14. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA (2006) 295(6):676–80. doi: 10.1001/jama.295.6.676
15. Chan KY, Wang W, Wu JJ, Liu L, Theodoratou E, Car J, et al. Epidemiology of alzheimer’s disease and other forms of dementia in China, 1990-2010: A systematic review and analysis. Lancet (2013) 381(9882):2016–23. doi: 10.1016/S0140-6736(13)60221-4
16. Papp KA, Reich K, Paul C, Blauvelt A, Baran W, Bolduc C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol (2016) 175(2):273–86. doi: 10.1111/bjd.14493
17. Lebwohl M, Strober B, Menter A, Gordon K, Weglowska J, Puig L, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. New Engl J Med (2015) 373(14):1318–28. doi: 10.1056/nejmoa1503824
18. Mease PJ, Helliwell PS, Hjuler KF, Raymond K, Mcinnes I. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheum Dis (2021) 80(2):185–93. doi: 10.1136/annrheumdis-2019-216835
19. Seo SJ, Shin BS, Lee JH, Jeong H. Efficacy and safety of brodalumab in the Korean population for the treatment of moderate to severe plaque psoriasis: A randomized, phase III, double-blind, placebo-controlled study. J Dermatol (2021) 48(6):807–17. doi: 10.1111/1346-8138.15733
20. Mease PJ, Genovese MC, Greenwald MW, Ritchlin CT, Beaulieu AD, Deodhar A, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. New Engl J Med (2014) 370(24):2295–306. doi: 10.1056/nejmoa1315231
21. Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. New Engl J Med (2012) 366(13):1181–9. doi: 10.1056/NEJMoa1109017
22. Nakagawa H, Niiro H, Ootaki K. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: Efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci (2016) 81(1):44–52. doi: 10.1016/j.jdermsci.2015.10.009
23. Gordon KB, Blauvelt A, Papp KA, Langley RG, Luger T, Ohtsuki M, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. New Engl J Med (2016) 375(4):345–56. doi: 10.1056/nejmoa1512711
24. Mease PJ, van der Heijde D, Ritchlin CT, Okada M, Cuchacovich RS, Shuler CL, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: Results from the 24-week randomised, double-blind, placebocontrolled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis (2017) 76(1):79–87. doi: 10.1136/annrheumdis-2016-209709
25. Nash P, Kirkham B, Okada M, Rahman P, Combe B, Burmester GR, et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet (2017) 389(10086):2317–27. doi: 10.1016/S0140-6736(17)31429-0
26. Ryan C, Menter A, Guenther L, Blauvelt A, Bissonnette R, Meeuwis K, et al. Efficacy and safety of ixekizumab in a randomized, double-blinded, placebo-controlled phase IIIb study of patients with moderate-to-severe genital psoriasis. Br J Dermatol (2018) 179(4):844–52. doi: 10.1111/bjd.16736
27. Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. New Engl J Med (2012) 366(13):1190–9. doi: 10.1056/NEJMoa1109997
28. Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis — Results of two phase 3 trials. New Engl J Med (2014) 371(4):326–38. doi: 10.1056/nejmoa1314258
29. Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, van der Heijde D, et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. New Engl J Med (2015) 373(14):1329–39. doi: 10.1056/nejmoa1412679
30. McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (2015) 386(9999):1137–46. doi: 10.1016/S0140-6736(15)61134-5
31. Nash P, Mease PJ, McInnes IB, Rahman P, Ritchlin CT, Blanco R, et al. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: Results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res Ther (2018) 20(1):47. doi: 10.1186/s13075-018-1551-x
32. Mease P, van der Heijde D, Landewé R, Mpofu S, Rahman P, Tahir H, et al. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: Primary results from the randomised, double-blind, phase III FUTURE 5 study. Ann Rheum Dis (2018) 77(6):890–7. doi: 10.1136/annrheumdis-2017-212687
33. Baraliakos X, Gossec L, Pournara E, Jeka S, Mera-Varela A, D'Angelo S, et al. Secukinumab in patients with psoriatic arthritis and axial manifestations: Results from the double-blind, randomised, phase 3 MAXIMISE trial. Ann Rheum Dis (2021) 80(5):582–90. doi: 10.1136/annrheumdis-2020-218808
34. Gottlieb A, Sullivan J, van Doorn M, Kubanov A, You R, Parneix A, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: Results from GESTURE, a randomized controlled trial. J Am Acad Dermatol (2017) 76(1):70–80. doi: 10.1016/j.jaad.2016.07.058
35. Paul C, Lacour JP, Tedremets L, Kreutzer K, Jazayeri S, Adams S, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: A randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol (2015) 29(6):1082–90. doi: 10.1111/jdv.12751
36. Bagel J, Duffin KC, Moore A, Ferris LK, Siu K, Steadman J, et al. The effect of secukinumab on moderate-to-severe scalp psoriasis: Results of a 24-week, randomized, double-blind, placebo-controlled phase 3b study. J Am Acad Dermatol (2017) 77(4):667–74. doi: 10.1016/j.jaad.2017.05.033
37. McInnes IB, Sieper J, Braun J, Emery P, van der Heijde D, Isaacs JD, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: A 24-week, randomised, double-blind, placebo-controlled, phase ii proof-of-concept trial. Ann Rheum Dis (2014) 73(2):349–56. doi: 10.1136/annrheumdis-2012-202646
38. Nguyen T, Churchill M, Levin R, Valenzuela G, Merola JF, Ogdie A, et al. Secukinumab in United States biologic-naïve patients with psoriatic arthritis: results from the randomized, placebo-controlled CHOICE study. J Rheumatol (2022) 49(8):894–902. doi: 10.3899/jrheum.210912
39. Papp KA, Langley RG, Sigurgeirsson B, Abe M, Baker DR, Konno P, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: A randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol (2013) 168(2):412–21. doi: 10.1111/bjd.12110
40. Rich P, Sigurgeirsson B, Thaci D, Ortonne JP, Paul C, Schopf RE, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: A randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol (2013) 168(2):402–11. doi: 10.1111/bjd.12112
41. Gordon KB, Foley P, Krueger JG, Pinter A, Reich K, Vender R, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): A multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet (2021) 397(10273):475–86. doi: 10.1016/S0140-6736(21)00126-4
42. McInnes IB, Asahina A, Coates LC, Landewé R, Merola JF, Ritchlin CT, et al. Bimekizumab in patients with psoriatic arthritis, naive to biologic treatment: a randomised, double-blind, placebo-controlled, phase 3 trial (BE OPTIMAL). Lancet (2023) 401(10370):25–37. doi: 10.1016/S0140-6736(22)02302-9
43. Merola JF, Landewé R, McInnes IB, Mease PJ, Ritchlin CT, Tanaka Y, et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-α inhibitors: a randomised, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE). Lancet (2023) 401(10370):38–48. doi: 10.1016/S0140-6736(22)02303-0
44. Reich K, Papp KA, Blauvelt A, Langley RG, Armstrong A, Warren RB, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet 397(10273):487–498. doi: 10.1016/S0140-6736(21)00125-2
45. Ritchlin CT, Kavanaugh A, Merola JF, Schett G, Scher JU, Warren RB, et al. Bimekizumab in patients with active psoriatic arthritis: results from a 48-week, randomised, double-blind, placebo-controlled, dose-ranging phase 2b trial. Lancet (2020) 395(10222):427–40. doi: 10.1016/S0140-6736(19)33161-7
46. Blauvelt A, Papp KA, Griffiths CEM, Randazzo B, Wasfi Y, Shen YK, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: Results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol (2017) 76(3):405–17. doi: 10.1016/j.jaad.2016.11.041
47. Reich K, Armstrong AW, Foley P, Song M, Wasfi Y, Randazzo B, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: Results from the phase III, double-blind, placebo- and active comparator–controlled VOYAGE 2 trial. J Am Acad Dermatol (2017) 76(3):418–31. doi: 10.1016/j.jaad.2016.11.042
48. Coates LC, Gossec L, Theander E, Bergmans P, Neuhold M, Karyekar CS, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis who are inadequate responders to tumour necrosis factor inhibitors: results through one year of a phase IIIb, randomised, controlled study (COSMOS). Ann Rheum Dis (2022) 81(3):359–69. doi: 10.1136/annrheumdis-2021-220991
49. Deodhar A, Helliwell PS, Boehncke WH, Kollmeier AP, Hsia EC, Subramanian RA, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet (2020) 395(10230):1115–25. doi: 10.1016/S0140-6736(20)30265-8
50. Mease PJ, Rahman P, Gottlieb AB, Kollmeier AP, Hsia EC, Xu XL, et al. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet (2020) 395(10230):1126–36. doi: 10.1016/S0140-6736(20)30263-4
51. Ohtsuki M, Kubo H, Morishima H, Goto R, Zheng R, Nakagawa H. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: Efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol (2018) 45(9):1053–62. doi: 10.1111/1346-8138.14504
52. Deodhar A, Gottlieb AB, Boehncke WH, Dong B, Wang Y, Zhuang Y, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet (2018) 391(10136):2213–24. doi: 10.1016/S0140-6736(18)30952-8
53. Gordon KB, Duffin KC, Bissonnette R, Prinz JC, Wasfi Y, Li S, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. New Engl J Med (2015) 373(2):136–44. doi: 10.1056/nejmoa1501646
54. Gordon KB, Strober B, Lebwohl M, Augustin M, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet (2018) 392(10148):650–61. doi: 10.1016/S0140-6736(18)31713-6
55. Kristensen LE, Keiserman M, Papp K, McCasland L, White D, Lu W, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 1 trial. Ann Rheum Dis (2022) 81(2):225–31. doi: 10.1136/annrheumdis-2021-221019
56. Östör A, Van den Bosch F, Papp K, Asnal C, Blanco R, Aelion J, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 2 trial. Ann Rheum Dis (2022) 81(3):351–8. doi: 10.1136/annrheumdis-2021-221048
57. Blauvelt A, Leonardi CL, Gooderham M, Papp KA, Philipp S, Wu JJ, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: A phase 3 randomized clinical trial. JAMA Dermatol (2020) 156(6):649–58. doi: 10.1001/jamadermatol.2020.0723
58. Reich K, Papp KA, Blauvelt A, Tyring SK, Sinclair R, Thaçi D, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet (2017) 390(10091):276–88. doi: 10.1016/S0140-6736(17)31279-5
59. Papp K, Thaçi D, Reich K, Riedl E, Langley RG, Krueger JG, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol (2015) 173(4):930–9. doi: 10.1111/bjd.13932
60. Papp K, Menter A, Leonardi C, Soung J, Weiss S, Pillai R, et al. Long-term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: subgroup analysis of a randomized phase III trial (AMAGINE-1)*. Br J Dermatol (2020) 183(6):1037–48. doi: 10.1111/bjd.19132
61. Reich K, Iversen L, Puig L, Lambert J, Mrowietz U, Kaplan Saday K, et al. Long-term efficacy and safety of brodalumab in moderate-to-severe plaque psoriasis: a post hoc pooled analysis of AMAGINE-2 and -3. J Eur Acad Dermatol Venereol (2022) 36(8):1275–83. doi: 10.1111/jdv.18068
62. Lebwohl MG, Blauvelt A, Menter A, Papp KA, Guenthner S, Pillai R, et al. Efficacy, safety, and patient-reported outcomes in patients with moderate-to-severe plaque psoriasis treated with brodalumab for 5 years in a long-term, open-label, phase II study. Am J Clin Dermatol (2019) 20(6):863–71. doi: 10.1007/s40257-019-00466-2
63. Blauvelt A, Lebwohl MG, Mabuchi T, Leung A, Garrelts A, Crane H, et al. Long-term efficacy and safety of ixekizumab: A 5-year analysis of the UNCOVER-3 randomized controlled trial. J Am Acad Dermatol (2021) 85(2):360–8. doi: 10.1016/j.jaad.2020.11.022
64. van der Heijde D, Gladman DD, Kishimoto M, Okada M, Rathmann SS, Moriarty SR, et al. Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: 52-week results from a phase III study (SPIRIT-P1). J Rheumatol (2018) 45(3):367–77. doi: 10.3899/jrheum.170429
65. Chandran V, van der Heijde D, Fleischmann RM, Lespessailles E, Helliwell PS, Kameda H, et al. Ixekizumab treatment of biologic-naïve patients with active psoriatic arthritis: 3-year results from a phase III clinical trial (SPIRIT-P1). Rheumatol (United Kingdom) (2020) 59(10):2774–84. doi: 10.1093/rheumatology/kez684
66. Genovese MC, Combe B, Kremer JM, Tsai TF, Behrens F, Adams DH, et al. Safety and efficacy of ixekizumab in patients with PsA and previous inadequate response to TNF inhibitors: Week 52 results from SPIRIT-P2. Rheumatol (United Kingdom) (2018) 57(11):2001–11. doi: 10.1093/rheumatology/key182
67. Gordon KB, Leonardi CL, Lebwohl M, Blauvelt A, Cameron GS, Braun D, et al. A 52-week, open-label study of the efficacy and safety of ixekizumab, an anti-interleukin-17A monoclonal antibody, in patients with chronic plaque psoriasis. J Am Acad Dermatol (2014) 71(6):1176–82. doi: 10.1016/j.jaad.2014.07.048
68. Lacour JP, Paul C, Jazayeri S, Papanastasiou P, Xu C, Nyirady J, et al. Secukinumab administration by autoinjector maintains reduction of plaque psoriasis severity over 52 weeks: results of the randomized controlled JUNCTURE trial. J Eur Acad Dermatol Venereol (2017) 31(5):847–56. doi: 10.1111/jdv.14073
69. Blauvelt A, Reich K, Tsai TF, Tyring S, Vanaclocha F, Kingo K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: Results from the CLEAR study. J Am Acad Dermatol (2017) 76(1):60–69.e9. doi: 10.1016/j.jaad.2016.08.008
70. McInnes IB, Mease PJ, Ritchlin CT, Rahman P, Gottlieb AB, Kirkham B, et al. Secukinumab sustains improvement in signs and symptoms of psoriatic arthritis: 2 year results from the phase 3 FUTURE 2 study. Rheumatol (United Kingdom) (2017) 56(11):1993–2003. doi: 10.1093/rheumatology/kex301
71. van der Heijde D, Mease PJ, Landewé RBM, Rahman P, Tahir H, Singhal A, et al. Secukinumab provides sustained low rates of radiographic progression in psoriatic arthritis: 52-week results from a phase 3 study, FUTURE 5. Rheumatol (United Kingdom) (2020) 59(6):1325–34. doi: 10.1093/rheumatology/kez420
72. Mease PJ, Landewé R, Rahman P, Tahir H, Singhal A, Boettcher E, et al. Secukinumab provides sustained improvement in signs and symptoms and low radiographic progression in patients with psoriatic arthritis: 2-year (end-of-study) results from the FUTURE 5 study. RMD Open (2021) 7(2):e001600. doi: 10.1136/rmdopen-2021-001600
73. Bissonnette R, Luger T, Thaçi D, Toth D, Lacombe A, Xia S, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol (2018) 32(9):1507–14. doi: 10.1111/jdv.14878
74. Bissonnette R, Luger T, Thaçi D, Toth D, Messina I, You R, et al. Secukinumab sustains good efficacy and favourable safety in moderate-to-severe psoriasis after up to 3 years of treatment: results from a double-blind extension study. Br J Dermatol (2017) 177(4):1033–42. doi: 10.1111/bjd.15706
75. Coates LC, McInnes IB, Merola JF, Warren RB, Kavanaugh A, Gottlieb AB, et al. Safety and efficacy of bimekizumab in patients with active psoriatic arthritis: three-year results from a phase IIb randomized controlled trial and its open-label extension study. Arthritis Rheumatol (2022) 74(12):1959–70. doi: 10.1002/art.42280
76. Strober B, Paul C, Blauvelt A, Thaçi D, Puig L, Lebwohl M, et al. Bimekizumab efficacy and safety in patients with moderate to severe plaque psoriasis: two-year interim results from the open-label extension of the randomized BE RADIANT phase 3b trial. J Am Acad Dermatol (2023) 89(3):486–95. doi: 10.1016/j.jaad.2023.04.063
77. Reich K, Griffiths CEM, Gordon KB, Papp KA, Song M, Randazzo B, et al. Maintenance of clinical response and consistent safety profile with up to 3 years of continuous treatment with guselkumab: Results from the VOYAGE 1 and VOYAGE 2 trials. J Am Acad Dermatol (2020) 82(4):936–45. doi: 10.1016/j.jaad.2019.11.040
78. Blauvelt A, Tsai TF, Langley RG, Miller M, Shen YK, You Y, et al. Consistent safety profile with up to 5 years of continuous treatment with guselkumab: Pooled analyses from the phase 3 VOYAGE 1 and VOYAGE 2 trials of patients with moderate-to-severe psoriasis. J Am Acad Dermatol (2022) 86(4):827–34. doi: 10.1016/j.jaad.2021.11.004
79. McInnes IB, Rahman P, Gottlieb AB, Hsia EC, Kollmeier AP, Chakravarty SD, et al. Efficacy and safety of guselkumab, an interleukin-23p 19–specific monoclonal antibody, through one year in biologic-naive patients with psoriatic arthritis. Arthritis Rheumatol (2021) 73(4):604–16. doi: 10.1002/art.41553
80. McInnes IB, Rahman P, Gottlieb AB, Hsia EC, Kollmeier AP, Xu XL, et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naive patients with active psoriatic arthritis. Arthritis Rheumatol (2022) 74(3):475–85. doi: 10.1002/art.42010
81. Gooderham M, Pinter A, Ferris LK, Warren RB, Zhan T, Zeng J, et al. Long-term, durable, absolute Psoriasis Area and Severity Index and health-related quality of life improvements with risankizumab treatment: a post hoc integrated analysis of patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol (2022) 36(6):855–65. doi: 10.1111/jdv.18010
82. Papp KA, Lebwohl MG, Puig L, Ohtsuki M, Beissert S, Zeng J, et al. Long-term efficacy and safety of risankizumab for the treatment of moderate-to-severe plaque psoriasis: interim analysis of the LIMMitless open-label extension trial beyond 3 years of follow-up. Br J Dermatol (2021) 185(6):1135–45. doi: 10.1111/bjd.20595
83. Reich K, Warren RB, Iversen L, Puig L, Pau-Charles I, Igarashi A, et al. Long-term efficacy and safety of tildrakizumab for moderate-to-severe psoriasis: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2) through 148 weeks. Br J Dermatol (2020) 182(3):605–17. doi: 10.1111/bjd.18232
84. Thaci D, Piaserico S, Warren RB, Gupta AK, Cantrell W, Draelos Z, et al. Five-year efficacy and safety of tildrakizumab in patients with moderate-to-severe psoriasis who respond at week 28: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2)*. Br J Dermatol (2021) 185(2):323–34. doi: 10.1111/bjd.19866
85. Chang SH. T helper 17 (Th17) cells and interleukin-17 (IL-17) in cancer. Arch Pharm Res (2019) 42(7):549–59. doi: 10.1007/s12272-019-01146-9
86. Tangye SG, Puel A. The th17/IL-17 axis and host defense against fungal infections. J Allergy Clin Immunol Pract (2023) 11(6):1624–34. doi: 10.1016/j.jaip.2023.04.015
87. Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: From mechanisms to therapeutic testing. Nat Rev Immunol (2014) 14(9):585–600. doi: 10.1038/nri3707
88. Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity (2009) 30(1):108–19. doi: 10.1016/j.immuni.2008.11.009
89. Yamanaka-Takaichi M, Ghanian S, Katzka DA, Torgerson RR, Alavi A. Candida infection associated with anti-IL-17 medication: A systematic analysis and review of the literature. Am J Clin Dermatol (2022) 23(4):469–80. doi: 10.1007/s40257-022-00686-z
90. Kalb RE, Fiorentino DF, Lebwohl MG, Toole J, Poulin Y, Cohen AD, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: Results from the psoriasis longitudinal assessment and registry (PSOLAR). JAMA Dermatol (2015) 151(9):961–9. doi: 10.1001/jamadermatol.2015.0718
Keywords: psoriasis, psoriatic arthritis, malignancy, infection, biologics, safety
Citation: Wu S, Xu Y, Yang L, Guo L and Jiang X (2023) Short-term risk and long-term incidence rate of infection and malignancy with IL-17 and IL-23 inhibitors in adult patients with psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Front. Immunol. 14:1294416. doi: 10.3389/fimmu.2023.1294416
Received: 14 September 2023; Accepted: 10 November 2023;
Published: 29 November 2023.
Edited by:
Irma Convertino, University of Pisa, ItalyReviewed by:
George E. Fragoulis, Laiko General Hospital of Athens, GreeceFrancisco Capani, Universidad Abierta Interamericana, Argentina
Copyright © 2023 Wu, Xu, Yang, Guo and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linghong Guo, bGluaG9tLmd1b0Bmb3htYWlsLmNvbQ==; Xian Jiang, amlhbmd4aWFuQHNjdS5lZHUuY24=
†ORCID: Xian Jiang, orcid.org/0000-0001-5109-6055
 Shuwei Wu
Shuwei Wu Yuanyuan Xu
Yuanyuan Xu Lihua Yang
Lihua Yang Linghong Guo
Linghong Guo Xian Jiang1,2*†
Xian Jiang1,2*†