- 1Université Paris Cité, Centre de Recherche sur l’Inflammation, Institut National de la santé et de la recherche médicale (INSERM) UMR1149, Centre national de la recherche scientifique (CNRS) EMR8252, Faculté de Médecine site Bichat, Paris, France
- 2Université Paris Cité, Laboratoire d’Excellence Inflamex, Paris, France
- 3Inflammation, Infection and Immunity Laboratory, TMDU Advanced Research Institute, Tokyo Medical and Dental University (TMDU), Tokyo, Japan
Editorial on the Research Topic
The fundamental biology of basophils in health and disease
1 Quick history of basophils research and emerging hot topics
Basophils are one of the rarest immune cell types, representing less than 1% of circulating leucocytes in humans. They were discovered more than 140 years ago by Paul Ehrlich, but basophils research has suffered from their rarity and from “shifting trends” in immunology. Indeed, from 1985 to 2009, the number of publications on basophils stalled (Figure 1A), while “newer” immune cells grabbed a steady focus (i.e., “Dendritic cells”). The year 2009 saw a renewal of basophils research, which may have arisen from several anterior breakthroughs, beginning with thorough descriptions of the regulation and dynamics of basophil degranulation (1–6), of their expression of IL-4 (7, 8), and of human basophils promoting B cell IgE production without exogenous IL-4 (9) in the 1980s-1990s. The democratization of flow cytometry in the 2000s enabled better protocols of purification and deeper characterization of the human basophil (10, 11), which fostered the development of the Basophil Activation Test (BAT) (12). Mice basophil research showed that basophils are a primary source of IL-4 in helminth infection (13), mediate delayed hypersensitivity reactions after intravenous IgE sensitization and intradermal allergen challenge (14), and promote in vivo antibody responses (15), Th2 responses (16), and IgG-driven anaphylaxis (17). This formed the announcement of a “rebirth” of basophils research in 2009, with numerous major publications characterizing how basophils are activated and promote Th2 responses (18–27).
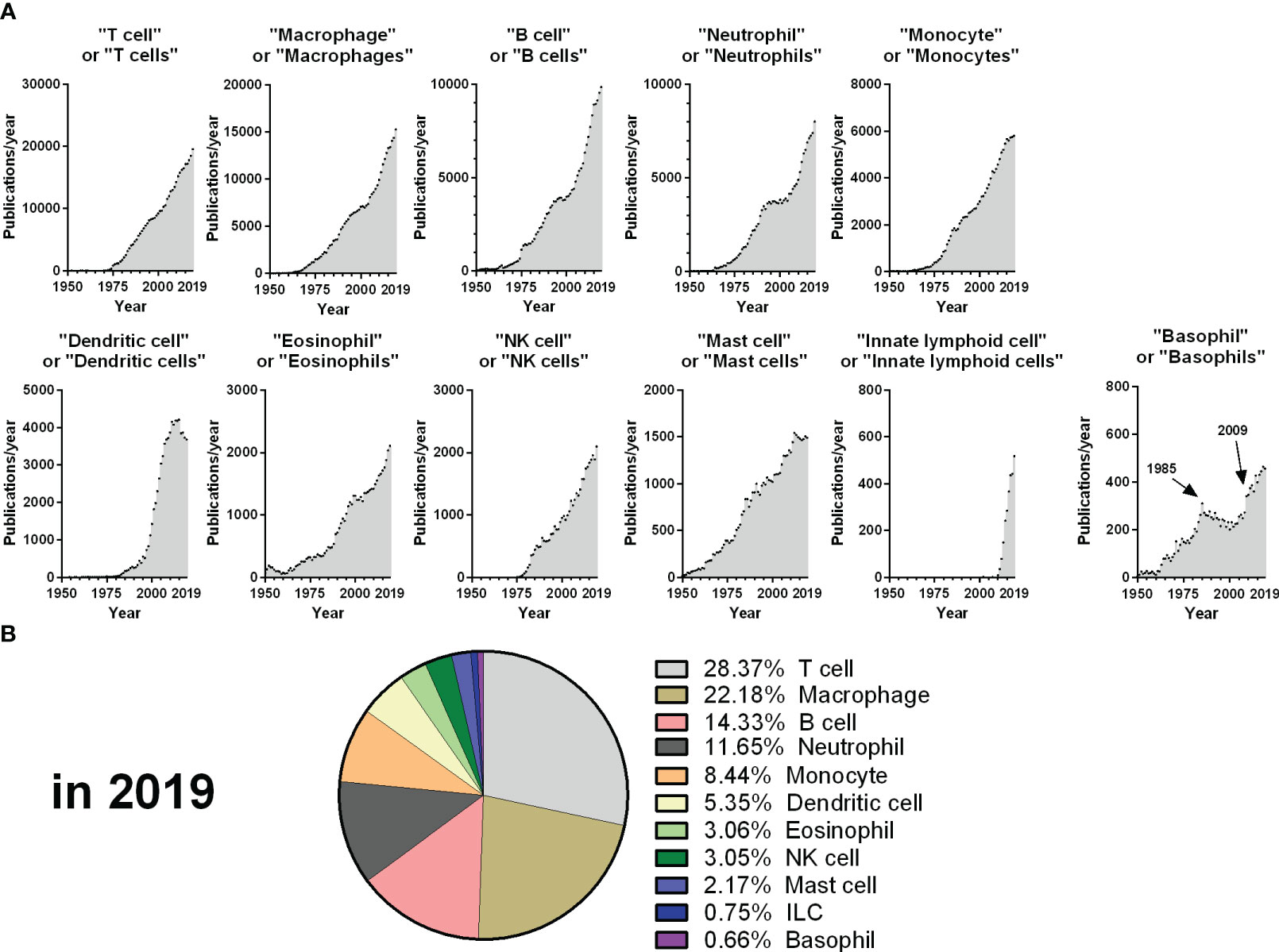
Figure 1 Evolution of publications from 1950 to 2019 for each main immune cell type. (A) The number of publications per year retrieved from the Pubmed database from 1950 to 2019 is depicted for each query, corresponding to the dot plot titles. (B) Percentage of publications retrieved from each query from the sum of all the queries represented in A for the year 2019. The year 2019 was chosen as an endpoint as the volume and dynamics of publications changed drastically from 2020 due to the COVID-19 pandemic.
From 2009 to 2019, the number of publications citing basophils rose steadily, with many discoveries deciphering the fundamental biology of basophils and their contribution to health or disease. This was supported by the generation of specific basophil-deficient mice (28, 29) and conditionally basophil-deficient mice (30, 31), which allowed unambiguous demonstrations of the various roles of basophils (32). Nowadays, we have a better understanding of several aspects of basophils biology, including their differentiation (and a pre-mature basophil state) (33–35), their heterogeneity (36–38), their responsiveness to various ligands (24, 27, 37–39), their expression of chemokine receptors (40, 41), and the mechanisms by which they can present antigens (42, 43). The controversies regarding how basophils promote the priming of T cells are underlined by Möbs et al.
A deleterious role of basophils seems evident in several allergic diseases of the skin (atopic dermatitis and chronic spontaneous urticaria), the airways (asthma and chronic rhinosinusitis), or the gut and in some anaphylactic reactions. Basophils are also detrimental in various autoimmune diseases (i.e., systemic lupus erythematosus) and chronic inflammatory or fibrotic diseases of the lungs (chronic obstructive pulmonary dysfunction), the gut (inflammatory bowel diseases), the kidneys (ischemia/reperfusion-induced fibrosis), or the heart (allograft-induced fibrosis) (32). Wiebe et al. underlined how basophils contribute to pruritus in allergic and inflammatory or autoimmune skin diseases. An updated description of the contribution of basophils to non-allergic and non-parasitic diseases, with a focus on autoimmune and chronic inflammatory disorders, has been reviewed by Poto et al. Basophils show complex capabilities to promote tumor progression or, inversely, tumor suppression. An updated description of the potential prognostic value of circulating basophils counts and a summary of their functions in various cancer or cancer models has been presented in another manuscript by Poto et al.
These complex roles in cancer highlight that basophils can also promote health and homeostasis in a broad array of conditions: they display unique interactions with hematopoietic and non-hematopoietic cells during lung development (38); they secrete both retinoic acid (44), IL-10 (45), and cleave extracellular ATP (46) to reduce inflammation; and they promote the resolution of infectious and sterile inflammation in the skin, liver, lungs, or heart (32, 47). Basophils have also emerged as being protective in infectious models beyond ectoparasite infections, including in a mouse model of sepsis (48) and of malarial infection (49, 50).
2 Original research, brief reports, and hypotheses
Despite these exciting discoveries, basophils remain the least studied of the main immune cells, representing less than 1% of these publications in 2019 (Figure 1B). In this context, the aim of this Research Topic was to aggregate original manuscripts exploring emerging hot topics in basophils research, which will be presented below.
IgE crosslinking induces several signaling events controlling intracellular calcium mobilization and degranulation. Hansen Selnø et al. explored the expression of the sarcoplasmic reticulum Ca2+ ATPase (SERCA2) in human basophils and its function. SERCA2 expression is strongly inversely correlated with anti-IgE-induced histamine release, and pharmaceutical inhibition or activation of SERCA proteins controls the amplitude of basophil histamine release. Thus, SERCA2 appears as a new negative regulator of basophil degranulation.
Basophil responsiveness to IgE decreases among patients suffering from chronic spontaneous urticaria (CSU), supposedly due to the presence of autoreactive antibodies against IgE or its receptor. However, Matsubara et al. showed that the response of CSU patients’ basophils to the anaphylatoxin C5a is unaltered. This suggests targeting the C5a/C5aR axis may be of critical value in patients refractory to anti-FcϵRIα treatments.
In chronic inducible urticaria, urticaria is induced by specific stimuli, such as ultraviolet light exposure. Mizuno et al. showed that the circulating basophils of these patients are more activated than those of healthy controls (as CSU patients) but without any IgE hyporesponsiveness. This highlights differences in the pathophysiology of these distinct conditions.
Peripheral basopenia in CSU patients is associated with disease activity, and basophils are found in patients’ skin lesions. Kishimoto et al. confirmed previous reports underlining a reversal of basopenia upon treatment of CSU patients with Omalizumab or anti-histamine. Then, using an oxazolone-induced contact dermatitis model, they demonstrated that the migration of circulating basophils to skin lesions provoked a transient basopenia. This supports the concept that clinical observations of basopenia reflect an active basophils extravasation.
El Hachem et al. explored the mechanisms governing basophils extravasation in the skin in FITC-induced dermatitis. They revealed that basophil migration was critically dependent on the secretion of IL-3 by T cells. They also demonstrated that IL-3-stimulated human and mice basophils relied on an autocrine retinoid acid production to drive their expression of specific integrins and mice basophil extravasation. Overall, these results strongly suggest that T cell IL-3 drives basophils autocrine secretion of retinoic acid to enable their extravasation in the inflamed skin.
The unique properties of skin-homing basophils have been described in a hypothesis and theory article by Shibuya and Kim, which suggests these basophils may have a unique identity, acquired during hematopoiesis and/or through late imprinting by the action of TSLP and epithelial-derived alarmins as mice lung basophils do under the control of IL-33 and GM-CSF (38). Skin-infiltrating basophils may externalize MRGPRX2, a receptor involved in pseudoallergic reactions and neuroimmune interactions.
MRGPRX2 expression by basophils has been the subject of some controversy. In this Research Topic, Toscano et al. explored the expression and function of this receptor on basophils from patients allergic to birch pollen or hypersensitive to moxifloxacin. Circulating basophils express only very low levels of functional surface MRGPRX2, but this is very quickly externalized by specific activation (anti-IgE, fMLP) or non-specific activation (i.e., purification). Thus, the reactivity of patients’ basophils to MRGPRX2 ligands can be studied but only when using carefully controlled conditions.
Basophils are known to participate in allergic airway inflammation and allergic asthma but have mainly been studied following allergen challenge or asthma exacerbation. Here, Iype et al. analyzed the expression of activation markers on stable asthmatics basophils and reported that they express more surface CD25 but no other activation markers. As human basophils activated by IL-2 secrete type 2 cytokines, and IL-2 is associated with asthma, this pathway seems important in asthma chronicity and pathophysiology.
The development of efficient helminth vaccines is an ongoing challenge in immunology. Thuma et al. cloned a new immunogenic protein secreted by the model helminth Nippostrongylus brasiliensis (Nb) during infection, Nb-LSA1a. Immunization with Nb-LSA1a induces specific IgG1 and protective immunity against Nb infection in wild-type but not basophil-deficient mice. This strongly suggests helminth vaccination strategies should benefit from inducing basophil-dependent immunity.
Overall, the manuscripts submitted to this Research Topic underline current and emerging trends in basophils immunology: the regulation of their degranulation via FcϵRIα or MRGPRX2; their roles in chronic urticaria, pruritic diseases, asthma, or cancer; the controversies surrounding their regulation of T cell polarization and their potency in promoting anti-helminth protective immunity; the mechanisms controlling their extravasation and peripheral basopenia; and, finally, the concept of mature basophils harboring distinct specific identities.
Author contributions
CP: Writing – original draft, Writing – review & editing. HK: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dvorak AM. Ultrastructural localization of histamine in human basophils and mast cells; changes associated with anaphylactic degranulation and recovery demonstrated with a diamine oxidase-gold probe. Allergy (1997) 52:14–24. doi: 10.1111/j.1398-9995.1997.tb04807.x
2. Dvorak AM. A role for vesicles in human basophil secretion. Cell Tissue Res (1998) 293:1–22. doi: 10.1007/s004410051093
3. De Boer M, Roos D. Metabolic comparison between basophils and other leukocytes from human blood. J Immunol (1986) 136:3447–54. doi: 10.4049/jimmunol.136.9.3447
4. Macglashan jr D. Releasability of human basophils: Cellular sensitivity and maximal histamine release are independent variables. J Allergy Clin Immunol (1993) 91:605–15. doi: 10.1016/0091-6749(93)90266-I
5. Nguyen KL, Gillis S, MacGlashan DW. A comparative study of releasing and nonreleasing human basophils: Nonreleasing basophils lack an early component of the signal transduction pathway that follows IgE cross-linking. J Allergy Clin Immunol (1990) 85:1020–9. doi: 10.1016/0091-6749(90)90046-7
6. Knol EF, Mul FP, Jansen H, Calafat J, Roos D. Monitoring human basophil activation via CD63 monoclonal antibody 435. J Allergy Clin Immunol (1991) 88:328–38. doi: 10.1016/0091-6749(91)90094-5
7. Brunner T, Heusser CH, Dahinden CA. Human peripheral blood basophils primed by interleukin 3 (IL-3) produce IL-4 in response to immunoglobulin E receptor stimulation. J Exp Med (1993) 177:605–11. doi: 10.1084/jem.177.3.605
8. Seder RA, Paul WE, Dvorak AM, Sharkis SJ, Kagey-Sobotka A, Niv Y, et al. Mouse splenic and bone marrow cell populations that express high-affinity Fc epsilon receptors and produce interleukin 4 are highly enriched in basophils. Proc Natl Acad Sci U S A. (1991) 88:2835–9. doi: 10.1073/pnas.88.7.2835
9. Gauchat JF, Henchoz S, Mazzei G, Aubry JP, Brunner T, Blasey H, et al. Induction of human IgE synthesis in B cells by mast cells and basophils. Nature. (1993) 365:340–3. doi: 10.1038/365340a0
10. Bühring HJ, Seiffert M, Giesert C, Marxer A, Kanz L, Valent P, et al. The basophil activation marker defined by antibody 97A6 is identical to the ectonucleotide pyrophosphatase/phosphodiesterase 3. Blood. (2001) 97:3303–5. doi: 10.1182/blood.V97.10.3303
11. Platz IJ, Binder M, Marxer A, Lischka G, Valent P, Bühring HJ. Hymenoptera-venom-induced upregulation of the basophil activation marker ecto-nucleotide pyrophosphatase/phosphodiesterase 3 in sensitized individuals. Int Arch Allergy Immunol (2001) 126:335–42. doi: 10.1159/000049531
12. Santos AF, Alpan O, Hoffmann H. Basophil activation test: Mechanisms and considerations for use in clinical trials and clinical practice. Allergy. (2021) 76:2420–32. doi: 10.1111/all.14747
13. Min B, Prout M, Hu-Li J, Zhu J, Jankovic D, Morgan ES, et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med (2004) 200:507–17. doi: 10.1084/jem.20040590
14. Mukai K, Matsuoka K, Taya C, Suzuki H, Yokozeki H, Nishioka K, et al. Basophils play a critical role in the development of IgE-mediated chronic allergic inflammation independently of T cells and mast cells. Immunity. (2005) 23:191–202. doi: 10.1016/j.immuni.2005.06.011
15. Denzel A, Maus UA, Gomez MR, Moll C, Niedermeier M, Winter C, et al. Basophils enhance immunological memory responses. Nat Immunol (2008) 9:733–42. doi: 10.1038/ni.1621
16. Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol (2008) 9:310–8. doi: 10.1038/ni1558
17. Tsujimura Y, Obata K, Mukai K, Shindou H, Yoshida M, Nishikado H, et al. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity. (2008) 28:581–9. doi: 10.1016/j.immuni.2008.02.008
18. Kim S, Shen T, Min B. Basophils can directly present or cross-present antigen to CD8 lymphocytes and alter CD8 T cell differentiation into IL-10-producing phenotypes. J Immunol Baltim Md (2009) 183:3033–9. doi: 10.4049/jimmunol.0900332
19. Hida S, Yamasaki S, Sakamoto Y, Takamoto M, Obata K, Takai T, et al. Fc receptor γ-chain, a constitutive component of the IL-3 receptor, is required for IL-3-induced IL-4 production in basophils. Nat Immunol (2009) 10:214–22. doi: 10.1038/ni.1686
20. Charles N, Watford WT, Ramos HL, Hellman L, Oettgen HC, Gomez G, et al. Lyn kinase controls basophil GATA-3 transcription factor expression and induction of Th2 cell differentiation. Immunity. (2009) 30:533–43. doi: 10.1016/j.immuni.2009.02.008
21. Chen K, Xu W, Wilson M, He B, Miller NW, Bengtén E, et al. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat Immunol (2009) 10:889–98. doi: 10.1038/ni.1748
22. Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y, et al. Basophils contribute to TH2-IgE responses in vivo via IL-4 production and presentation of peptide–MHC class II complexes to CD4+ T cells. Nat Immunol (2009) 10:706–12. doi: 10.1038/ni.1737
23. Torrero MN, Larson D, Hübner MP, Mitre E. CD200R surface expression as a marker of murine basophil activation. Clin Exp Allergy (2009) 39:361–9. doi: 10.1111/j.1365-2222.2008.03154.x
24. Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood. (2009) 113:1526–34. doi: 10.1182/blood-2008-05-157818
25. Ohnmacht C, Voehringer D. Basophil effector function and homeostasis during helminth infection. Blood. (2009) 113:2816–25. doi: 10.1182/blood-2008-05-154773
26. Schroeder JT, Chichester KL, Bieneman AP. Human basophils secrete IL-3: evidence of autocrine priming for phenotypic and functional responses in allergic disease. J Immunol Baltim Md (2009) 182:2432–8. doi: 10.4049/jimmunol.0801782
27. Kroeger KM, Sullivan BM, Locksley RM. IL-18 and IL-33 elicit Th2 cytokines from basophils via a MyD88- and p38alpha-dependent pathway. J Leukoc Biol (2009) 86:769–78. doi: 10.1189/jlb.0708452
28. Sullivan BM, Liang HE, Bando JK, Wu D, Cheng LE, McKerrow JK, et al. Genetic analysis of basophil function in vivo. Nat Immunol (2011) 12:527–35. doi: 10.1038/ni.2036
29. Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. (2010) 33:364–74. doi: 10.1016/j.immuni.2010.08.011
30. Sawaguchi M, Tanaka S, Nakatani Y, Harada Y, Mukai K, Matsunaga Y, et al. Role of mast cells and basophils in igE responses and in allergic airway hyperresponsiveness. J Immunol (2012) 188:1809–18. doi: 10.4049/jimmunol.1101746
31. Wada T, Ishiwata K, Koseki H, Ishikura T, Ugajin T, Ohnuma N, et al. Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J Clin Invest. (2010) 120:2867–75. doi: 10.1172/JCI42680
32. Miyake K, Ito J, Karasuyama H. Role of basophils in a broad spectrum of disorders. Front Immunol (2022) 13:902494. doi: 10.3389/fimmu.2022.902494
33. Miyake K, Ito J, Nakabayashi J, Shichino S, Ishiwata K, Karasuyama H. Single cell transcriptomics clarifies the basophil differentiation trajectory and identifies pre-basophils upstream of mature basophils. Nat Commun (2023) 14:2694. doi: 10.1038/s41467-023-38356-1
34. Matsumura T, Totani H, Gunji Y, Fukuda M, Yokomori R, Deng J, et al. A Myb enhancer-guided analysis of basophil and mast cell differentiation. Nat Commun (2022) 13:7064. doi: 10.1038/s41467-022-34906-1
35. Wanet A, Bassal MA, Patel SB, Marchi F, Mariani SA, Ahmed N, et al. E-cadherin is regulated by GATA-2 and marks the early commitment of mouse hematopoietic progenitors to the basophil and mast cell fates. Sci Immunol (2021) 6:eaba0178. doi: 10.1126/sciimmunol.aba0178
36. Vivanco Gonzalez N, Oliveria JP, Tebaykin D, Ivison GT, Mukai K, Tsai MM, et al. Mass cytometry phenotyping of human granulocytes reveals novel basophil functional heterogeneity. iScience. (2020) 23:101724. doi: 10.1016/j.isci.2020.101724
37. Pellefigues C, Mehta P, Chappell S, Yumnam B, Old S, Camberis M, et al. Diverse innate stimuli activate basophils through pathways involving Syk and IκB kinases. Proc Natl Acad Sci (2021) 118:e2019524118. doi: 10.1073/pnas.2019524118
38. Cohen M, Giladi A, Gorki AD, Solodkin DG, Zada M, Hladik A, et al. Lung single-cell signaling interaction map reveals basophil role in macrophage imprinting. Cell. (2018) 175:1031–1044.e18. doi: 10.1016/j.cell.2018.09.009
39. Suurmond J, Stoop JN, Rivellese F, Bakker AM, Huizinga TWJ, Toes REM. Activation of human basophils by combined toll-like receptor- and FcϵRI-triggering can promote Th2 skewing of naive T helper cells. Eur J Immunol (2014) 44:386–96. doi: 10.1002/eji.201343617
40. Blom LH, Bartko EA, Møller TKR, Poulsen LK, Jensen BM. FcϵRI-activated basophils express CCR4, CCR8, CCR9, CCX-CKR and XCR1. Allergy (2022) 78(2):539–43. doi: 10.1111/all.15488
41. Iikura M, Miyamasu M, Yamaguchi M, Kawasaki H, Matsushima K, Kitaura M, et al. Chemokine receptors in human basophils: inducible expression of functional CXCR4. J Leukoc Biol (2001) 70:113–20. doi: 10.1189/jlb.70.1.113
42. Otsuka A, Nakajima S, Kubo M, Egawa G, Honda T, Kitoh A, et al. Basophils are required for the induction of Th2 immunity to haptens and peptide antigens. Nat Commun (2013) 4:1738. doi: 10.1038/ncomms2740
43. Miyake K, Shiozawa N, Nagao T, Yoshikawa S, Yamanishi Y, Karasuyama H. Trogocytosis of peptide–MHC class II complexes from dendritic cells confers antigen-presentinzg ability on basophils. Proc Natl Acad Sci (2017) 114(5):1111–6. doi: 10.1073/pnas.1615973114
44. Spiegl N, Didichenko S, McCaffery P, Langen H, Dahinden CA. Human basophils activated by mast cell-derived IL-3 express retinaldehyde dehydrogenase-II and produce the immunoregulatory mediator retinoic acid. Blood. (2008) 112:3762–71. doi: 10.1182/blood-2008-01-135251
45. Kleiner S, Rüdrich U, Gehring M, Loser K, Eiz-Vesper B, Noubissi Nzeteu GA, et al. Human basophils release the anti-inflammatory cytokine IL-10 following stimulation with α-melanocyte–stimulating hormone. J Allergy Clin Immunol (2021) 147(4):1521–3.E3. S0091674921000099. doi: 10.1016/j.jaci.2020.12.645
46. Tsai SH, Kinoshita M, Kusu T, Kayama H, Okumura R, Ikeda K, et al. The ectoenzyme E-NPP3 negatively regulates ATP-dependent chronic allergic responses by basophils and mast cells. Immunity (2015) 42:279–93. doi: 10.1016/j.immuni.2015.01.015
47. Sicklinger F, Meyer IS, Li X, Radtke D, Dicks S, Kornadt MP, et al. Basophils balance healing after myocardial infarction via IL-4/IL-13. J Clin Invest (2021) 131:e136778. doi: 10.1172/JCI136778
48. Piliponsky AM, Shubin NJ, Lahiri AK, Truong P, Clauson M, Niino K, et al. Basophil-derived tumor necrosis factor can enhance survival in a sepsis model in mice. Nat Immunol (2019) 20:129–40. doi: 10.1038/s41590-018-0288-7
49. Donnelly EL, Céspedes N, Hansten G, Wagers D, Briggs AM, Lowder C, et al. The basophil IL-18 receptor precisely regulates the host immune response and malaria-induced intestinal permeability and alters parasite transmission to mosquitoes without effect on gametocytemia. ImmunoHorizons (2022) 6:630–41. doi: 10.4049/immunohorizons.2200057
Keywords: basophil, health, disease, IgE, allergy, MRGPRX2, urticaria, dermatitis
Citation: Pellefigues C and Karasuyama H (2023) Editorial: The fundamental biology of basophils in health and disease. Front. Immunol. 14:1292279. doi: 10.3389/fimmu.2023.1292279
Received: 11 September 2023; Accepted: 06 October 2023;
Published: 18 October 2023.
Edited and Reviewed by:
Francesca Granucci, University of Milano-Bicocca, ItalyCopyright © 2023 Pellefigues and Karasuyama. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christophe Pellefigues, Q2hyaXN0b3BoZS5wZWxsZWZpZ3Vlc0BpbnNlcm0uZnI=
 Christophe Pellefigues
Christophe Pellefigues Hajime Karasuyama
Hajime Karasuyama