- 1Pediatric Immuno-Rheumatology Unit, Fondazione IRCSS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
- 2Center for Autoinflammatory Diseases and Immunodeficiencies, IRCCS Istituto Giannina Gaslini, Genoa, Italy
- 3DINOGMI, Università degli Studi di Genova, Genova, Italy
- 4Pediatric Nephrology, Dialysis, and Transplant Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
- 5Unit of Pediatric Dermatology, Foundation IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
Juvenile systemic lupus erythematosus (jSLE) is a complex inflammatory autoimmune disorder. In the last decades, genetic factors and activation pathways have been increasingly studied to understand their potential pathogenetic role better. Genetic and transcriptional abnormalities directly involved in the type I interferon (IFN) signaling cascade have been identified through family-based and genome-wide association studies. IFNs trigger signaling pathways that initiate gene transcription of IFN-stimulated genes through the activation of JAK1, TYK2, STAT1, and STAT2. Thus, the use of therapies that target the IFN pathway would represent a formidable advance in SLE. It is well known that JAK inhibitors have real potential for the treatment of rheumatic diseases, but their efficacy in the treatment of SLE remains to be elucidated. We report the case of a 13-year-old girl affected by jSLE, carrying a novel heterozygous missense variant on Three prime Repair EXonuclease 1 (TREX1), successfully treated with baricitinib on top of mofetil mycophenolate. The TREX1 gene plays an important role in DNA damage repair, and its mutations have been associated with an overproduction of type 1 interferon. This report underlines the role of translational research in identifying potential pathogenetic pathways in rare diseases to optimize treatment.
1 Introduction
Juvenile-onset systemic lupus erythematosus (jSLE) is a multisystemic autoimmune disease characterized by a heterogeneous presentation (1). Although jSLE pathogenesis has not been fully understood, several elements, including genetic and environmental factors, have been increasingly recognized as playing a key role. High serum levels of interferon (INF)α have been described in patients with SLE (2) for many years, and, more recently, INF-stimulated genes (ISG) were found to be overexpressed in patients with SLE, confirming the pivotal role of INFs in its pathogenesis (2, 3). Overexpression of these genes, measured as mRNA transcripts in peripheral blood cells, is called an “IFN signature” (2). The presence of a distinctive IFN signature is a strong clue to the pathway involved in the development of SLE and is frequently observed in jSLE at onset or during a disease flare (2–5).
The dysregulation of type I IFN signaling might be related to several genetic factors (5). Type I interferonopathies, rare Mendelian autoinflammatory disorders, are monogenic diseases characterized by exacerbated type I IFN signaling activity and an elevated ISG signature (6). Several mutations have been identified as pathogenetic in type I interferonopathies (7). One of the proposed mechanisms underpinning these conditions involves the accumulation of DNA and RNA pools caused by loss-of-function mutations involved in degradation of nucleic acids. In Aicardi-Goutières syndrome, pathogenetic mutations in the Three prime Repair EXonuclease 1 (TREX1) gene, which encodes a nuclear protein with 3’exonuclease activity (2), cause defective clearance of chromatin DNA, increased INF production, and an immune-mediated inflammatory response (8, 9).
The pathogenic role of type I IFN dysregulation in SLE, together with the growing evidence regarding the efficacy of JAK-inhibition in monogenic interferonopathies, advocates for the use of therapy to neutralize the activation of this particular pathway by blocking downstream JAK/STAT signaling in SLE (2). JAK- inhibitors (JAKi) are oral small molecules that block JAK–STAT signaling. Although JAKi are currently approved for the treatment of rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, and severe atopic dermatitis, their potential role in the treatment of SLE still needs to be tested (2–4).
In this report, we described the case of a jSLE girl with an extremely severe and refractory skin involvement carrying a novel heterozygous missense mutation in TREX1 who was successfully treated with the JAK 1/2 inhibitor baricitinib.
2 Case report
A 13-year-old girl adopted from Cambodia presented with malar rash, leukopenia (WBC 2620/mmc, L 1130/mm3, N 1130/mm3), hypocomplementemia C3 75 mg/dl (90-180 n.v.), C4 8 (10-40 mg/dl n.v.), hypergammaglobulinemia, increased level of ESR (49 mm/h 0-20 n.v.), positivity of anti-dsDNA (1278 UI/ml 0-27 n.v.), anti-Sm (693.5 UA 0-20 n.v.), and anti-SSA/Ro antibodies (1374.8 UA 0-20 n.v.). The diagnosis of jSLE was made, both ACR1997 (9) and SLICC-2012 (10) criteria were satisfied, and she was started on oral prednisone (2 mg/kg/day) and hydroxychloroquine. One year later, while she was on a low dose of oral prednisone (2.5 mg/day), a disease flare occurred, with hematological and dermatological involvement, requiring an increase of the daily prednisone dose up to 25 mg/day. However, further tapering of corticosteroids led to a new flare with severe vasculitic lesions mainly involving the face which are associated with leukopenia and increased acute phase reactants. The child was therefore started on mycophenolate mofetil (MMF) at a daily dose of 1000 mg.
Despite MMF treatment, each attempt to taper systemic steroids failed, and the patient experienced disease relapses, characterized by severe skin involvement with vasculitis, alopecia, and lymphopenia and elevation of anti-dsDNA, requiring again high doses of corticosteroids (50 mg/day) and an increase in the daily MMF dose up to 1500 mg/die. However, cutaneous involvement remained uncontrolled, with severe facial erythematous infiltrative plaques and vasculitic lesions, which are associated with stable high titers of anti ds DNA and elevation of ESR, despite the addition of a topical steroid (Figure 1A). Skin involvement was refractory to multiple therapeutic approaches during the following years, which included monthly infusions of intravenous immunoglobulin (IVIG) (14 total infusions) and association with tacrolimus (1.5 mg twice a day) or azathioprine (100 mg/day) with steroid dependence and corticosteroid toxicity (hypertrichosis, weight gain, irritability) (Figure 1B).
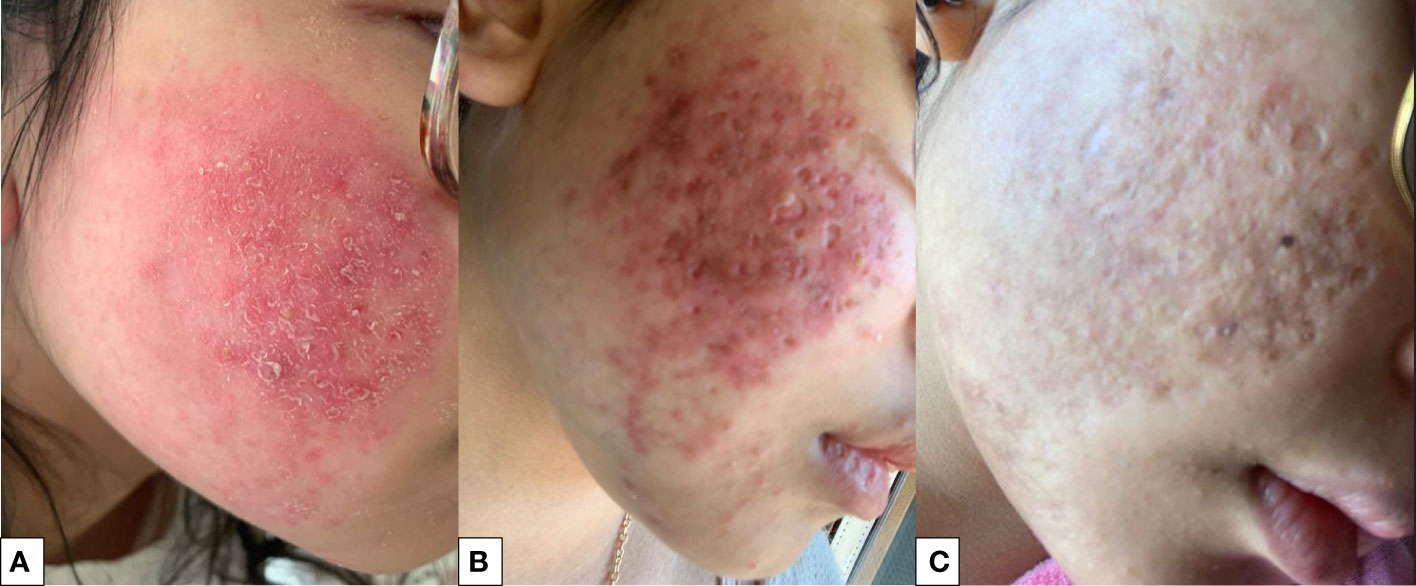
Figure 1 (A) Skin infiltrative lesions during treatment with steroids and MMF (B) skin infiltrative lesions right before treatment with baricitinib; (C) skin after 18 months of treatment with baricitinib.
Given the peculiarity of the refractory skin involvement with severe vasculitis, a next-gene-sequencing (NGS) gene panel was performed, revealing a previously unreported heterozygous missense mutation on the TREX1 gene (c.374A>G; p.Asn125Ser). Furthermore, the INF signature result was strongly positive (18,81 v.n. <0,7) (Figure 2). Assuming a potential role of the TREX1-related pathway in triggering disease flares, especially skin involvement, azathioprine was switched to the JAK 1/2 inhibitor baricitinib (2 mg three times a day).
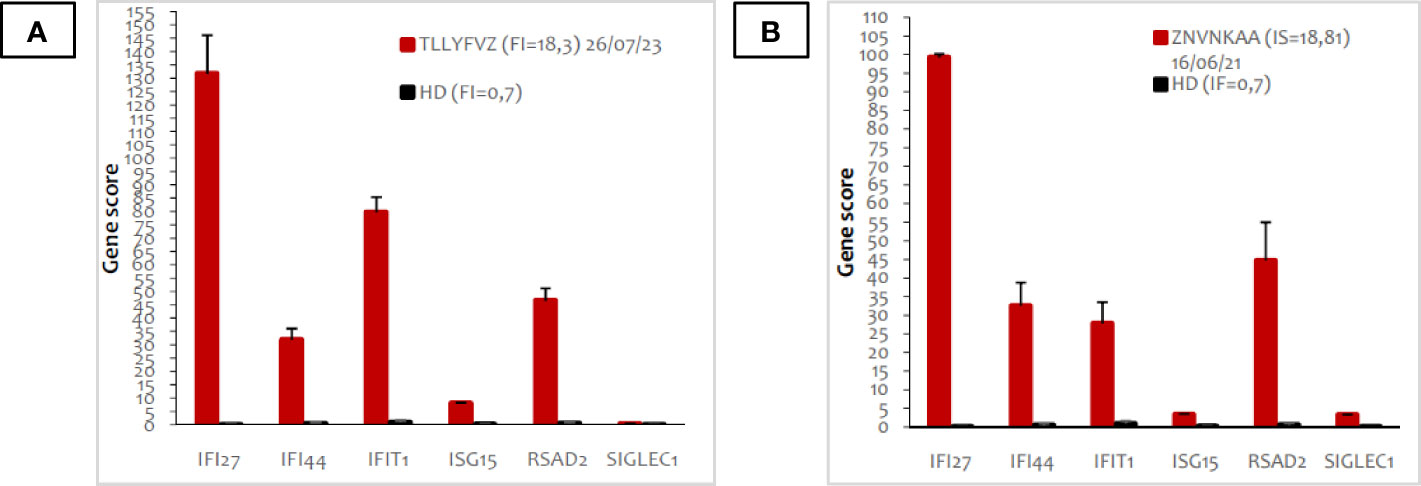
Figure 2 Peripheral blood type I interferon signature of the patient before (A) and after (B) treatment with baricitinib. The panel shows the expression of six types of I-IFN stimulated genes, indicated in the X axis. Red bars indicate expression levels in the patient; black bars represent the average of 20 healthy donors. The interferon score (FI) is indicated in the legend.
Baricitinib led to a significant improvement of skin lesions, with a prompt reduction of erythema and cutaneous swelling after a few weeks. One month after azathioprine discontinuation, a persistent proteinuria was noted (proteinuria to creatininuria ratio 0.5). Renal biopsy showed a lupic nephritis IV stage, and MMF was re-started, in association with baricitinib, achieving a good renal response.
At the last visit, 18 months after starting baricitinib, optimal disease control has been obtained, especially in terms of cutaneous involvement (Figure 1C) (ERS 25 mm/h 0-20n.v., anti dsDNA 412.6 UI/ml 0-27 n.v.), together with a significant reduction in glucocorticoid use (daily prednisone 5 mg/day; previously, the daily dose of prednisone was at least 10 mg/day) without any adverse event or infection reported. Figure 3 shows in details the patient’s treatment timeline, and in particular the daily steroidal dose, together with the SLEDAI-2k score over time.
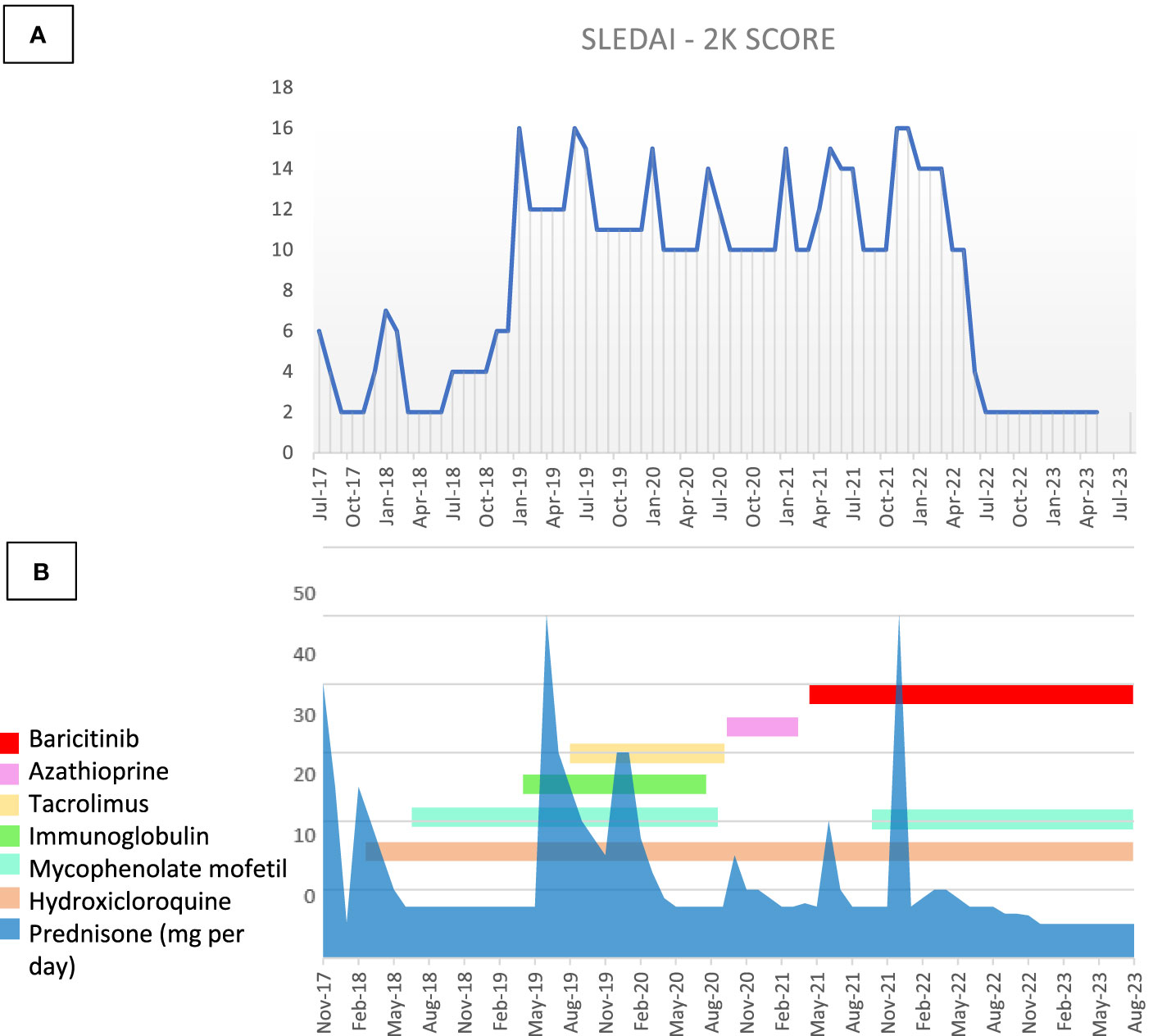
Figure 3 (A) SLEDAI-2K score and (B) therapy timeline through the disease course. Figure steroids and therapy changes over time in correlation with the modification of the SLEDAI-2K.
3 Discussion
Over the past decades, the increased knowledge of pathways involved in disease pathogenesis has increased the relevance of precision medicine (10). Monogenic disorders may provide prototypes for pathogenesis-targeted therapies (11). So far, more than 80 loci associated with susceptibility in polygenic lupus have been identified, and screening for the activation of specific patterns can aid risk stratification of patients and optimization of treatment (12–14). JAKi have been proposed to be effective in blocking inflammation mediated by interferon overproduction (15). Accordingly, an increased INF signature score may identify potential responders to JAKi (12–14). In the present study, we report a patient with jSLE with refectory cutaneous involvement and a novel heterozygous missense mutation in TREX1 who was successfully treated with baricitinib.
Despite several combinations of immunosuppressive treatments, our patient suffered from severe refractory skin involvement. The evidence of a strong positive INF signature together with the presence of a variant in the TREX1 gene were crucial to support the introduction of a JAKi. The observed heterozygous TREX-1 missense mutation (c.374A>G) replaces asparagine with serine at codon 125 of the TREX1 protein (p.Asn125Ser). The asparagine residue is highly conserved. This variant was not found in population databases nor in the dbSNP database. All algorithms developed to predict the effect of missense changes on protein structure and function (SIFT, PolyPhen-2, Align-GVGD) suggested a potential disruptive role of the variant reported. Unfortunately, the unavailability of the biological parents prevented further studies on the role of the detected variant in the family. Despite the lack of functional tests to prove our hypothesis, its rarity, in silico pathogenic prediction, clinical presentation, and impressive clinical response to JAK-inhibition highly support its pathogenetic contribution.
Indeed, TREX1 mutations have been observed with increased frequency (0.5–3%) in SLE patients compared to healthy controls (16, 17). Furthermore, deficient TREX1 activity has been related to severe cutaneous involvement both in Aicardi-Goutieres syndrome and familial chilblain lupus, suggesting skin is a target tissue of the TREX1-related pathway (17, 18).
Baricitinib is a first-generation JAKi, and it inhibits all four members of the JAK family (JAK1, JAK2, JAK3, and TYK2) with a preferential action on JAK1 and JAK2 (19, 20). After several clinical trials, JAKi have been increasingly used for adults with connective tissue disease including SLE. In the pediatric population, many case series reported the beneficial use of JAKi (20–22), especially in children with monogenic interferonopathies (17–23). Baricitinib was chosen over other JAK inhibitors due to the safety and efficacy shown in pediatric trials on JIA and SAVI syndrome (23, 24).
However, randomized controlled trials in children with jSLE to properly examine the efficacy and safety of JAK inhibitors, also in combination with disease-modifying anti rheumatic drugs (DMARDs), are lacking (25), and most of the existing data about JAKi safety are derived from adult cohorts or pre-clinical studies (26–29). Several case series supported the beneficial effect of JAKi in pediatric patients with interferonopathies caused by TREX1 mutations (12–22) and the most frequent adverse event reported was the increased risk of viral infections due to the suppression of INF signaling (15–19).
During the follow-up, we closely monitored clinical and laboratory parameters of disease activity and viral infections, in particular EBV, CMV, and polyomavirus, along with the IFN signature. This particular parameter was reassessed after 18 months of treatment, revealing stable results. In existing literature, the role of IFN-signature as a biomarker of treatment response remains ambiguous, with documented fluctuations observed throughout the day and across different follow-up timelines (17, 30). In a multicenter study that evaluates patients with monogenic interferonopathies, the IFN signature was normalized only in 5 patients with CANDLE out of the 10 who achieved clinical remission (17).
To our knowledge, this is the first report of a jSLE patient with refractory cutaneous involvement carrying a mutation in the TREX1 gene who was successfully treated with a JAKi.
In 2018, a phase II trial evaluating baricitinib in adult patients with SLE proved that a 4 mg/daily dose significantly improved signs and symptoms of active disease in patients who were not adequately controlled by standard of care therapy, with a good safety profile. However, authors did not find a significance difference between baricitinib and a placebo in terms of efficacy on mucocutaneous involvement (31). Although a first phase III trial (SLE-BRAVE-I) confirmed the positive results of phase II (32), those were not replicated by a second phase III trial (SLE-BRAVE-II) (33, 34). A Phase I study found tofacitinib to be safe in SLE and to improve cardiometabolic and immunologic parameters (35), and a second phase I/II trial testing tofacitinib in adult SLE with moderate and severe skin involvement is currently ongoing (NCT03288324). Note that an ongoing clinical trial is evaluating the expression of JAK3 in blood and renal tissue during active disease in children with jSLE (NCT04293510). Results from the latter two studies may further support the use of targeted JAK-inhibition for pediatric lupus patients (20).
In conclusion, our study suggests that the p.Asn125Ser might represent a novel pathogenic TREX1 gene mutation linked to a jSLE phenotype. The impressive clinical response to JAK inhibition in our patient highlights the importance of including genetic analysis in refractory jSLE patients to foster a target treatment and optimization of care. Furthermore, our study supports the efficacy and safety of combination therapy with MMF and JAKi in a pediatric patient with jSLE with severe refractory cutaneous involvement.
Data availability statement
The datasets presented in this article are not readily available because this is a Case Report.
Ethics statement
The studies involving humans were approved by CET Lombardia 3. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
MR: Conceptualization, Writing – original draft, Writing – review & editing. EC: Writing – original draft. PB: Writing – review & editing. SV: Writing – review & editing. AM: Writing – review & editing. RC: Writing – review & editing. MG: Writing – review & editing. FM: Writing – original draft, Writing – review & editing. GF: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Publication (APC) costs were funded by Grant Ricerca Corrente, Italian Ministry of Health.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1288675/full#supplementary-material
References
1. Smith EMD, Lythgoe H, Midgley A, Beresford MW, Hedrich CM. Juvenile-onset systemic lupus erythematosus: Update on clinical presentation, pathophysiology and treatment options. Clin Immunol (2019) 209:108274. doi: 10.1016/j.clim.2019.108274
2. Gallucci S, Meka S, Gamero AM. Abnormalities of the type I interferon signaling pathway in lupus autoimmunity. Cytokine (2021) 146:155633. doi: 10.1016/j.cyto.2021.155633
3. Crow MK, Kirou KA, Wohlgemuth J. Microarray analysis of interferon-regulated genes in SLE. Autoimmunity (2003) 36(8):481–90. doi: 10.1080/08916930310001625952
4. Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med (2003) 197(6):711–23. doi: 10.1084/jem.20021553
5. Belot A, Rice GI, Omarjee SO, et al. Contribution of rare and predicted pathogenic gene variants to childhood-onset lupus: a large, genetic panel analysis of British and French cohorts. Lancet Rheumatol (2020) 2(11):E664. doi: 10.1016/S2665-9913(19)30142-0
6. Melki I, Frémond ML. Type I interferonopathies: from a novel concept to targeted therapeutics. Curr Rheumatol Rep (2020) 22(7):32. doi: 10.1007/s11926-020-00909-4
7. Volpi S, Picco P, Caorsi R, Candotti F, Gattorno M. Type I interferonopathies in pediatric rheumatology. Pediatr Rheumatol Online J (2016) 14(1):35. doi: 10.1186/s12969-016-0094-4
8. Endo Y, Koga T, Otaki H, Furukawa K, Kawakami A. Systemic lupus erythematosus overlapping dermatomyositis owing to a heterozygous TREX1 Asp130Asn missense mutation. Clin Immunol (2021) 227:108732. doi: 10.1016/j.clim.2021.108732
9. Fredi M, Bianchi M, Andreoli L, Greco G, Olivieri I, Orcesi S, et al. Typing TREX1 gene in patients with systemic lupus erythematosus. Reumatismo (2015) 67(1):1–7. doi: 10.4081/reumatismo.2015.782
10. Hochberg MC. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum (1997) 40(9):1725. doi: 10.1002/art.1780400928
11. Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheumatol (2012) 64(8):2677–86. doi: 10.1002/art.34473
12. Yeo JG, Ng CT, Albani S. Precision medicine in pediatric rheumatology. Curr Opin Rheumatol (2017) 29(5):500–5. doi: 10.1097/BOR.0000000000000419
13. Pin A, Tesser A, Pastore S, Moressa V, Valencic E, Arbo A, et al. Biological and clinical changes in a pediatric series treated with off-label JAK inhibitors. Int J Mol Sci (2020) 21(20):7767. doi: 10.3390/ijms21207767
14. Alperin JM, Ortiz-Fernández L, Sawalha AH. Monogenic lupus: A developing paradigm of disease. Front Immunol (2018) 9:2496. doi: 10.3389/fimmu.2018.02496
15. Trindade VC, Carneiro-Sampaio M, Bonfa E, Silva CA. An update on the management of childhood-onset systemic lupus erythematosus. Paediatr Drugs (2021) 23(4):331–47. doi: 10.1007/s40272-021-00457-z
16. Richter P, Cardoneanu A, Burlui AM, Macovei LA, Bratoiu I, Buliga-Finis ON, et al. Why do we need JAK inhibitors in systemic lupus erythematosus? Int J Mol Sci (2022) 23(19):11788. doi: 10.3390/ijms231911788
17. Sanchez GAM, Reinhardt A, Ramsey S, Wittkowski H, Hashkes PJ, Berkun Y, et al. JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies. J Clin Invest. (2018) 128(7):3041–52. doi: 10.1172/JCI98814
18. Carneiro-Sampaio M, Coutinho A. Early-onset autoimmune disease as a manifestation of primary immunodeficiency. Front Immunol (2015) 6:185. doi: 10.3389/fimmu.2015.00185
19. Lee-Kirsch MA, Gong M, Chowdhury D, Senenko L, Engel K, Lee YA, et al. Mutations in the gene encoding the 3'-5' DNA exonuclease TREX1 are associated with systemic lupus erythematosus. Nat Genet (2007) 39(9):1065–7. doi: 10.1038/ng2091
20. Fiehn. C. Familial chilblain lupus - what can we learn from type I interferonopathies? Curr Rheumatol Rep (2017) 19:61. doi: 10.1007/s11926-017-0689-x
21. Chuprin J, McCormack L, Richmond JM, Rashighi M. Evaluating the use of JAK inhibitors in inflammatory connective tissue diseases in pediatric patients: an update. Expert Rev Clin Immunol (2022) 18(3):263–72. doi: 10.1080/1744666X.2022.2047022
22. Welzel T, Winskill C, Zhang N, Woerner A, Woerner A, Pfister M. Biologic disease modifying antirheumatic drugs and Janus kinase inhibitors in paediatric rheumatology - what we know and what we do not know from randomized controlled trials. Pediatr Rheumatol Online J (2021) 19(1):46. doi: 10.1186/s12969-021-00514-4
23. Kim H, Brooks KM, Tang CC. Pharmacokinetics, pharmacodynamics, and proposed dosing of the oral JAK1 and JAK2 inhibitor baricitinib in pediatric and young adult CANDLE and SAVI patients. Clin Pharmacol Ther (2018) 104(2):364–73. doi: 10.1002/cpt.936
24. Ramanan AV, Guly CM, Keller SY, Schlichting DE, de Bono S, Liao R, et al. Clinical effectiveness and safety of baricitinib for the treatment of juvenile idiopathic arthritis-associated uveitis or chronic anterior antinuclear antibody-positive uveitis: study protocol for an open-label, adalimumab active-controlled phase 3 clinical trial (JUVE-BRIGHT). Trials (2021) 22(1):689. doi: 10.1186/s13063-021-05651-5
25. Zhang S, Song J, Yang Y, Miao H, Yang L, Liu Y, et al. Type I interferonopathies with novel compound heterozygous TREX1 mutations in two siblings with different symptoms responded to tofacitinib. Pediatr Rheumatol Online J (2021) 19(1):1. doi: 10.1186/s12969-020-00490-1
26. Fleischmann R, Schiff M, et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol (2017) 69(3):506–17. doi: 10.1002/art.39953
27. Klaeschen AS, Wolf D, Brossart P, Bieber T, Wenzel J. JAK inhibitor ruxolitinib inhibits the expression of cytokines characteristic of cutaneous lupus erythematosus. Exp Dermatol (2017) 26(8):728–30. doi: 10.1111/exd.13253
28. Ikeda K, Hayakawa K, Fujishiro M, Kawasaki M, Hirai T, Tsushima H, et al. JAK inhibitor has the amelioration effect in lupus-prone mice: the involvement of IFN signature gene downregulation. BMC Immunol (2017) 18(1):41. doi: 10.1186/s12865-017-0225-9
29. Zimmermann N, Wolf C, Schwenke R, Lüth A, Schmidt F, Engel K, et al. Assessment of clinical response to janus kinase inhibition in patients with familial chilblain lupus and TREX1 mutation. JAMA Dermatol (2019) 155(3):342–6. doi: 10.1001/jamadermatol.2018.5077
30. Vanderver A, Adang L, Gavazzi F, McDonald K, Helman G, Frank DB, et al. Janus kinase inhibition in the aicardi-goutières syndrome. N Engl J Med (2020) 383(10):986–9. doi: 10.1056/NEJMc2001362
31. Alunno A, Padjen I, Fanouriakis A, Boumpas DT. Pathogenic and therapeutic relevance of JAK/STAT signaling in systemic lupus erythematosus: integration of distinct inflammatory pathways and the prospect of their inhibition with an oral agent. Cells (2019) 8(8):898. doi: 10.3390/cells8080898
32. Wallace DJ, Furie RA, Tanaka Y. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet (2018) 392(10143):222–31. doi: 10.1016/S0140-6736(18)31363-1
33. Morand EF, Vital EM, Petri M, van Vollenhoven R, Wallace DJ, Mosca M, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 3 trial (SLE-BRAVE-I). Lancet (2023), S0140–6736(22)02607-1. doi: 10.1016/S0140-6736(22)02607-1
34. Petri M, Bruce IN, Dörner T, Tanaka Y, Morand EF, Kalunian KC, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 3 trial (SLE-BRAVE-II). Lancet, S0140–6736(22)02546-6. doi: 10.1016/S0140-6736(22)02546-6
Keywords: systemic lupus erythematosus, Trex1, JAK-inhibitor, baricitinib, pediatrics, case report
Citation: Rossano M, Conti EA, Bocca P, Volpi S, Mastrangelo A, Cavalli R, Gattorno M, Minoia F and Filocamo G (2023) Novel heterozygous TREX1 mutation in a juvenile systemic lupus erythematosus patient with severe cutaneous involvement treated successfully with Jak-inhibitors: a case report. Front. Immunol. 14:1288675. doi: 10.3389/fimmu.2023.1288675
Received: 04 September 2023; Accepted: 08 November 2023;
Published: 06 December 2023.
Edited by:
Klaus Tenbrock, RWTH Aachen University, GermanyReviewed by:
Mikhail Kostik, Saint Petersburg State Pediatric Medical University, RussiaSezgin Sahin, Istanbul University-Cerrahpasa, Türkiye
Copyright © 2023 Rossano, Conti, Bocca, Volpi, Mastrangelo, Cavalli, Gattorno, Minoia and Filocamo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martina Rossano, bWFydGluYS5yb3NzYW5vQHBvbGljbGluaWNvLm1pLml0
 Martina Rossano
Martina Rossano Emilio Amleto Conti1
Emilio Amleto Conti1 Stefano Volpi
Stefano Volpi Antonio Mastrangelo
Antonio Mastrangelo Marco Gattorno
Marco Gattorno Francesca Minoia
Francesca Minoia Giovanni Filocamo
Giovanni Filocamo