- Department of Gastroenterology, Shengjing Hospital of China Medical University, Shenyang, China
Ulcerative colitis (UC) is a chronic idiopathic inflammatory disease mainly affecting the rectum and colon and causing diarrhoea and mucopurulent stools. UC can present with extraintestinal manifestations in various organs and systems and can be associated with various comorbidities. Autoimmune pancreatitis (AIP) is a specific type of pancreatitis associated with autoimmune abnormalities and is divided into two clinical types: type 1 (lymphoplasmacytic sclerosing pancreatitis) and type 2 (idiopathic ductocentric pancreatitis). The current study shows an association between type 2 AIP and UC, which may be related to genetic susceptibility, inflammatory factors, and immune response. The most common manifestation of AIP in patients with type 2 AIP–UC is abdominal pain with elevated pancreatic enzymes, whereas the presentation of UC in type 2 AIP–UC is more severe, with an increased risk of UC-related surgery. This review focuses on diagnosis, prevalence, pathogenesis, impact, and treatment to better understand type 2 AIP–UC, explore the molecular mechanisms of this condition, and encourage further research into the management of type 2 AIP–UC.
1 Introduction
Ulcerative colitis (UC), a major clinical subtype of inflammatory bowel disease (IBD), is a chronic idiopathic intestinal inflammatory disease (1). UC primarily involves the rectum, colonic mucosa, and submucosa (2), with abdominal pain, diarrhoea, and mucopurulent bloody stools as its main manifestations (3). Patients with UC may develop varying degrees of extraintestinal manifestations, including skin, joint, ocular, hepatic, and pulmonary disorders (4), and may have multiple comorbid conditions. The incidence and prevalence of UC are increasing with the westernisation of newly industrialised countries, and the incidence of IBD in children worldwide is rising, making UC a global disease (5, 6). The disease is currently unpredictable, recurrent and requires long-term treatment.
Autoimmune pancreatitis (AIP) was originally proposed in 1995 by Yoshida et al. to describe a group of pancreatitis cases associated with autoimmune abnormalities, resulting in hypergammaglobulinaemia or autoantibody positivity, that impair the effectiveness of glucocorticoid (GC) therapy (7). The clinical signs and symptoms of AIP vary and are nonspecific, often presenting as acute pancreatitis (AP), abdominal pain, and obstructive jaundice (8, 9). AIP presents with diffuse or focal enlargement of the pancreas and has two types of pathology: lymphoplasmacytic sclerosing pancreatitis (LPSP) and idiopathic duct-centric pancreatitis (IDCP) (10, 11). In 2010, the International Pancreatic Society classified AIP into two clinical types, type 1 AIP (corresponding to LPSP) and type 2 AIP (corresponding to IDCP), and established international consensus diagnostic criteria (ICDC) (12). These two types have similar imaging presentations but different clinical features (13). Type 1 AIP is a pancreatic manifestation of systemic immunoglobulin G4-related disease (IgG4-RD) (14, 15). Elevated serum IgG4 levels and evidence of the involvement of other organs are of high diagnostic value for type I AIP; therefore, histopathology is not necessary for the diagnosis of type 1 AIP. Type 2 AIP is currently not considered a systemic disease and mainly involves the pancreas. The lack of specific biomarkers and low positivity for serum IgG4 and other autoantibodies makes its diagnosis extremely challenging.
Although the aetiology and pathogenesis of UC and type 2 AIP are unclear, some studies have demonstrated an association between these diseases. AIP is currently considered as an autoimmune disease that can coexist with IBD; however, whether type 2 AIP is an extraintestinal manifestation of UC is controversial, as it is unclear whether its pathogenesis is related to the intestinal immune response (16, 17). The current study shows that most patients with AIP complicated by IBD have type 2 AIP, and its association with UC is stronger than that with Crohn’s disease (CD) (18). Understanding the clinical relevance and potential pathogenesis of type 2 AIP–UC may help to formulate reasonable treatment strategies. Patients with IBD presenting with abnormal pancreatic enlargement are diagnosed with probable type 2 AIP without histology if the pancreatic abnormalities resolve or improve rapidly after GC treatment, after excluding any associated malignancy. Meta-analyses have shown that patients with UC are at an increased risk of pancreatitis compared with the non-IBD population (19, 20). Pharmacological pancreatitis can be caused by drugs such as azathioprine, so in these situations, this treatment should be discontinued; however, in the case of AP manifestations due to concurrent type 2 AIP, unnecessary discontinuation should be avoided (21).
Knowledge of type 2 AIP has gradually increased in recent years, but studies on type 2 AIP–UC remain relatively scarce. Therefore, we reviewed from the diagnosis, prevalence, possible mechanisms, impact, and treatment and challenge of type 2 AIP–UC to guide the management of type 2 AIP–UC in clinical practice.
2 Diagnosis of type 2 AIP–UC
The diagnosis of UC is based on the exclusion of infections, drugs, radiotherapy, ischaemic enteritis and colorectal tumours, and requires the judgement of a specialist IBD practitioner based on a combination of gastrointestinal symptoms, colonoscopy, pathology and biochemical indices, as detailed in Figure 1A (22, 23). Faecal calreticulin (FC) is strongly correlated with the degree of endoscopic inflammation and is valuable in the assessment of initial diagnosis, recurrence and response to treatment (24).
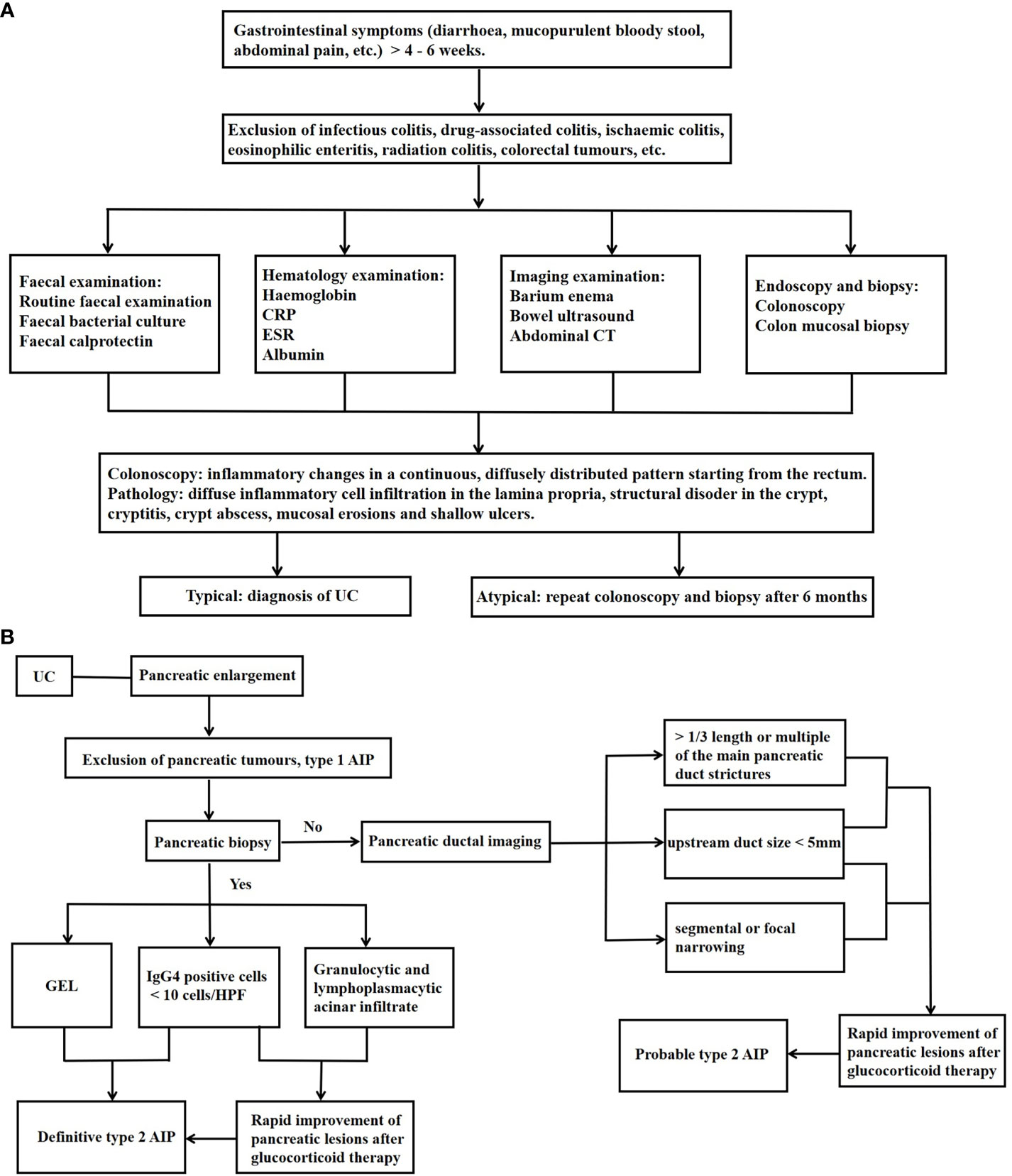
Figure 1 Diagnosis of Type 2 AIP–UC. (A) Diagnosis of UC; (B) Diagnosis of Type 2 AIP–UC. UC, ulcerative colitis; CRP, C-reactive protein; ESR,erythrocyte sedimentation rate; CT, computed tomography; GEL, granulocyte epithelial lesions; AIP, autoimmune pancreatitis; IgG, immunoglobulin G; HPF, high-power field.
Most patients with type 2 AIP–UC were diagnosed with UC first or both at the same time (25–29). Diagnostic criteria for AIP include the Japanese JPS criteria (30), the Korean Kim criteria (31), the American HISORt criteria (32), and the Asian criteria (33). However, none of them differentiate between AIP types and focus on describing the characteristics of type 1 AIP. Consequently, non-IgG4 related type 2 AIP may not be well represented. Although the Italian Verona standard (34), developed in 2009, contains the characteristics of both types of AIP, it does not distinguish between them. ICDC is currently considered to have the highest accuracy and sensitivity for the diagnosis of AIP and is the most useful for the classification of type 1 and type 2 AIP (35). A recent large international cohort study of AIP–IBD has shown that ICDC is the most frequently used diagnostic criterion for AIP (29).
The ICDC-based diagnostic process for autoimmune pancreatitis is summarised in Figure 1B (12, 29). As can be seen, the presence of UC is one of the criteria for the diagnosis of probable type 2 AIP, so patients with a clear diagnosis of UC may still be diagnosed with probable type 2 AIP in the absence of a pancreatic biopsy. Bowel-related investigations in AIP patients with equivocal histopathology are also very important, and we can routinely assess the FC level at the time of diagnosis and during the follow-up period, and in the event of an FC elevation, even in the absence of intestinal symptoms, colonoscopy should be refined for early recognition of UC (26). Although histological examination is not mandatory for the diagnosis of probable type 2 AIP, pancreatic biopsy remains important for type 2 AIP. A retrospective study found that the proportion of patients with pancreatitis who underwent histological examination at the centre increased from 1% to 3% as time progressed, and that the proportion of patients with type 2 AIP to total AIP increased from 8% to 55% (36). In addition, the differentiation between type 2 AIP, which presents as a mass pattern, and pancreatic cancer is challenging (37). Studies have reported that 78% of patients with type 2 AIP who obtained a histological diagnosis of the pancreas underwent surgical resection on suspicion of pancreatic cancer (38). Enhanced magnetic resonance, PET-CT, ultrasonographic endoscopy, and serum CA199 may be helpful in this distinction (39).
Serological markers also present some value in the diagnosis of type 2 AIP–UC. Anti-neutrophil cytoplasmic antibodies (ANCA) are mainly divided into perinuclear ANCA (p-ANCA) and cytoplasmic ANCA (c-ANCA) (40). Serum p-ANCA and anti-saccharomyces cerevisiae antibodies have high specificity but low sensitivity in differentiating UC from CD (41–43). Serum Proteinase 3 ANCA (44, 45) and IgG anti-integrin αvβ6 autoantibodies (46, 47) may be potential diagnostic markers for UC. However, serological markers specific for type 2 AIP have not yet been clearly identified (48), and only a few studies that included a small number of samples have conducted preliminary explorations. Serum levels of anti-transaldolase antibodies were significantly higher in type 2 AIP than in type 1 AIP and pancreatic ductal adenocarcinoma (49). Combination of serum IgG4 and anti-amylase-α antibodies may help to diagnose and differentiate between AIP subtypes and rule out pancreatic cancer (50). In addition, neither p-ANCA nor c-ANCA were detected to be elevated in type I AIP, whereas they were elevated in 50% and 30% of type 2 AIP patients (51, 52). Anti-smooth muscle antibodies were elevated in 50% of type 2 AIP patients, compared with 17% of type 1 AIP patients (51). These findings on serological markers of type 2 AIP still need to be validated by larger studies.
3 Prevalence of type 2 AIP–UC
The association between AIP and IBD is well established, particularly between UC and type 2 AIP (52–55). The prevalence of IBD is significantly higher in patients with AIP (approximately 11%–30%) (54, 56, 57). Approximately 30%–48% of type 2 AIP cases are associated with IBD (9, 58, 59). Most patients with AIP and comorbid IBD have type 2 AIP (9). A national survey of patients with IBD–AIP in Japan showed that UC accounts for 73% of patients with concurrent type 2 AIP and IBD (type 2 AIP–IBD), which is significantly higher than the incidence of CD (60).
3.1 Prevalence of UC in patients with type 2 AIP
The prevalence of UC in type 2 AIP reported in recent studies ranges from 16%–83% (25–27, 38, 57, 59, 61–68), as shown in Table 1. Among these, the studies by Detlefsen et al. (62) and Kamisawa et al. (38) were in the context of histologically diagnosed type 2 AIP, which may have reduced the inclusion of patients with actual type 2 AIP. The studies by Notohara et al. (64), Park SH et al. (65), Schneider A et al. (57) and Czakó et al. (67) included a small number of patients with type 2 AIP, and the results may not adequately reflect the actual situation. We found that both studies from Italy used the ICDC diagnosis, but the results differed significantly for the following possible reasons: Barresi et al.’s study (62) was a national survey that included 173 centres and the diagnosis of AIP was based on the independent judgement of each centre, whereas Conti et al.’s study (26) was a single-centre study, so the heterogeneity of diagnosis was different between the two studies. In addition, about three times as many cases of type 2 AIP were histologically examined in the study of Barresi et al. (62) than that of Conti et al. (26), and the majority of type 2 AIP cases in Conti et al.’s study were probable diagnoses (26), which may have contributed to the high prevalence of type 2 AIP.
3.2 Prevalence of type 2 AIP in UC patients
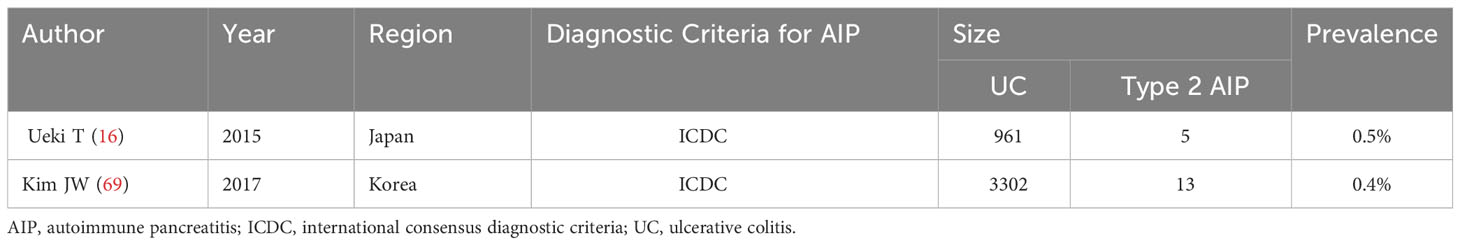
Few studies have explored the prevalence of type 2 AIP in UC, with only two large-sample studies with reliable data from Japan and Korea (16, 69), as detailed in Table 2.
4 Possible pathogenesis of type 2 AIP–UC
The pathogenesis of type 2 AIP is not fully understood, but its clinical relevance in UC suggests that there may be a common pathogenic mechanism (70, 71). From a histopathological point of view, there is a large intraepithelial neutrophil infiltration in both the granulocyte epithelial lesions (GEL) of type 2 AIP and cryptitis and crypt abscesses in UC (72, 73), which suggests a possible common pathogenesis (74). Patients with concurrent active UC and type 2 AIP have been found to experience concurrent relief after anti-tumour necrosis factor (TNF) treatment, suggesting that the two conditions may share similar pathophysiological mechanisms (75). The pathogenesis of type 2 AIP–UC may be related to genetic susceptibility, interleukin (IL)-8, T helper (Th) 17 cells and related cytokines, and programmed cell death ligand 1 (PD-L1), as shown in Figure 2.
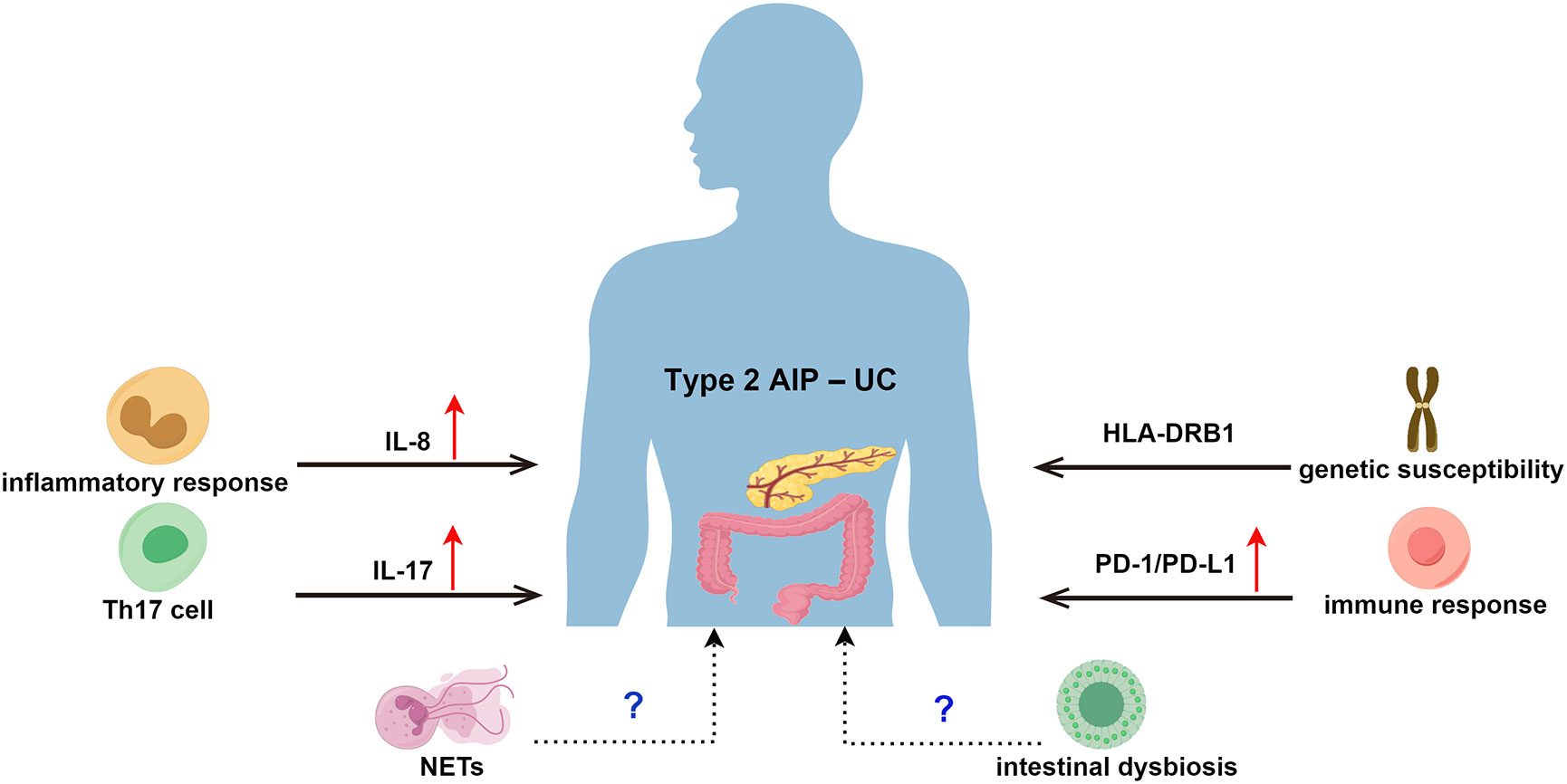
Figure 2 Possible Pathogenesis of Type 2 AIP–UC. AIP, autoimmune pancreatitis; UC, ulcerative colitis; HLA, human leukocyte antigen; IL-8, Interleukin 8; Th 17, T helper 17; IL-17, Interleukin 17; PD-1, programmed death receptor 1; PD-L1, programmed cell death ligand 1; NETs, neutrophil extracellular traps.
4.1 Genetic susceptibility
The human leukocyte antigen (HLA) is closely associated with immune tolerance. HLA molecules contain antigenic peptides that are recognised by T-cell receptors, resulting in an immune response. HLA is highly polymorphic (76), and allelic variants can alter its interaction with antigenic peptides and T cell receptors, thereby affecting the immune response (77). Specific HLA allelic variants, particularly HLA class II molecules, are strongly associated with the development of autoimmune diseases (AD), particularly HLA class II molecules (78–81). Destructive inflammatory responses to autoantigens in the context of genetic susceptibility have been suggested as potential pathogenic mechanisms of UC (82). Studies have shown that the HLA gene that is most associated with UC and CD is HLA-DRB1, with HLA-DRB1*03:01 being the predominant risk allele (83, 84). In 2002, Kawa et al. first reported that specific HLA alleles might be associated with increased susceptibility to AIP (85). Goni et al. prospectively investigated HLA alleles in 100 AIPs from Italy and Germany and reported that, despite the different histopathological characteristics of type 1 and type 2 AIPs, both subtypes were associated with a higher frequency of HLA-DRB1*16 alleles and a significant enrichment of HLA-DQB1 pure congeners (especially the HLA-DQB1*05 allele) compared to healthy controls, hypothesising that type 1 and type 2 AIPs share the same genetic susceptibility and may rely on similar immunogenic mechanisms (66). The role of HLA in the pathogenesis of type 2 AIP and UC remains unclear because of the high degree of linkage disequilibrium between HLA genotypes. However, the exact genetic risk loci and functional validation of HLA variants have not been fully elucidated, and further studies are needed to determine whether enrichment of specific HLA haplotypes leads to increased numbers of self-reactive T cells or autoantibody production (85–87).
4.2 Interleukin 8
IL-8 is a chemokine that is produced by a variety of cell types involved in inflammation, including monocytes, which can recruit a variety of immune cells, such as neutrophils and T lymphocytes, to the site of inflammation, leading to a range of responses, including inflammatory damage and tissue infiltration (88). In 2007, Ku et al. first examined the immunological profile of type 2 AIP lesions and revealed the presence of overexpressed IL-8 in ductal epithelial cells and infiltrative inflammatory cells; they found a similar pattern of IL-8 expression in crypt epithelial cells from colonic biopsy samples of patients with active UC (68). Similarly, Pearl et al. found that IL-8 levels in the diseased mucosa of patients with UC were positively correlated with the grade of inflammation and that IL-8-mediated infiltration of neutrophils may be associated with the inflammatory response in UC (89). IL8 may be the causative mechanism of type 2 AIP–UC.
4.3 Th17 cells and related cytokines
Th17 cells are a subpopulation of CD4+ Th cells that secrete cytokines such as IL-17A, IL-17F, and IL-22 (90), which have been shown to be associated with tissue inflammation and play an important role in the pathogenesis of many forms of AD (91, 92). An imbalance between regulatory and effector T cells (e.g., Th1, Th2, and Th17) is one of the key mechanisms in the immunopathology of UC, and the overactivation of Th17 cells may contribute to the development of UC (93). Patients with active UC have significantly higher numbers of Th17 cells in intestinal tissues and peripheral blood and significantly higher expression of related cytokines, such as IL-17 (94–96). Loos et al. found that Th17 cells infiltrate more significantly around the ducts in the pancreatic tissue in type 2 AIP than in type 1 AIP, accompanied by a significant increase in the number of neutrophils and a significant increase in IL-17A expression (97). Th17 cells and related cytokines are possibly involved in the mechanisms for the coexistence of UC and type 2 AIP.
4.4 Programmed cell death ligand 1
Programmed death receptor 1 (PD-1) is a co-receptor expressed on lymphocytes, monocytes, and natural killer cells and has two ligands, PD-L1 and PD-L2 (98). The PD-1/PD-L1 pathway may be involved in lymphocyte activation, T-cell development and function, immune tolerance breakdown, and AD development (99). An increasing number of studies have reported the upregulation of PD-L1 expression in the colonic epithelial cells of patients with UC, suggesting that PD-L1 may be involved in intestinal mucosal immune regulation (100–102). Gupta et al. found that 69% of samples from pancreatic ducts with type 2 AIP showed PD-L1 positive immunoreactivity, suggesting that pancreatic ductal PD-L1 may be associated with abnormal immune responses in type 2 AIP; they determined the sensitivity and specificity of PD-L1 as a marker for type 2 AIP were 70% and 99%, respectively (103). PD-L1 may also be a potential therapeutic target for the type 2 AIP–UC.
4.5 Other possible mechanisms to be investigated
Neutrophil extracellular traps (NETs) are released from activated neutrophils, and their structure consists of a meshwork of desmosomal chromatin fibres decorated with antimicrobial granule proteins, such as myeloperoxidase (MPO), neutrophil elastase, histone, and prothrombin G (104). NETs can act as traps that immobilise and kill microorganisms, thus limiting their spread (105). However, if NETs are overproduced and persistent, they can lead to local tissue destruction, a vicious cycle of inflammatory response, and overactivation of immune cells (106). NETs are associated with a variety of immune disorders. Dinallo et al. showed that excessive formation of NETs was present in UC, predominantly in mucosal areas with active inflammation, and that inhibition of NETs formation improved dextran sulphate sodium-induced colitis (107). Several studies have noted that pancreatic tissue from IgG4-related AIP, which is type 1 AIP, contains NETs (14, 108, 109), and NETs-related proteins are overexpressed in the inflamed colon of UC patients (107). Although there are no reports of NETs associated with type 2 AIP, the histological feature of type 2 AIP is granulocytic epitheliopathy (110), so it is reasonable to speculate that NETs might play a role in type 2 AIP–UC. In addition, alterations in the composition and function of the intestinal microbiome (also known as intestinal dysbiosis) have been associated with digestive disorders (111, 112), and the role of intestinal dysbiosis in the pathogenesis of ulcerative colitis has been widely recognised (113, 114). Recent studies have found that intestinal dysbiosis is also closely related to pancreatic diseases, and that intestinal dysbiosis may exacerbate chronic inflammation in type 1 AIP through activation of plasmacytoid dendritic cells producing IFN-α and IL-33, and TLR7-expressing M2 macrophages (115). Although there are no reports of intestinal dysbiosis associated with type 2 AIP, it is hypothesised that excessive innate immune responses of the intestinal dysbiosis may be associated with type 2 AIP–UC.
5 Clinical features of type 2 AIP–UC
5.1 Clinical manifestations of type 2 AIP–UC
Type 2 AIP mostly occurs after or in conjunction with the diagnosis of UC (25, 26, 28). A national survey in Japan found that abdominal pain was more common in patients with type 2 AIP–IBD than type 1 AIP, whereas the incidence of lower bile duct stricture and obstructive jaundice was significantly lower than in type 1 AIP (60). European studies have shown that the most common presentation of AIP in patients with type 2 AIP–UC is AP (56%–83%), i.e., abdominal pain with elevated pancreatic enzymes, but symptoms are generally mild, without local or systemic complications, and the response to GC is rapid, with persistent pain generally subsiding rapidly after the initiation of GC (26, 28). The Montreal typing of UC in patients with type 2 AIP–UC is predominantly left hemicolonic (E2) and extensively colonic (E3), with the rectal type (E1) being less common (9, 16, 25, 26, 28). Approximately 50%–70% of patients have UC in the active phase of the disease at the time of diagnosis of type 2 AIP (16, 26). Park et al. found that patients with AIP–UC had a lower body mass and higher CRP and Mayo scores than patients with UC without AIP, suggesting an increased severity of UC in patients with AIP–UC (65). Patients with type 2 AIP–UC have nearly twice the rate of severe UC compared to those with UC not complicated by type 2 AIP (16).
5.2 Recurrence rate of type 2 AIP
The recurrence of type 2 AIP usually appears on abnormal pancreatic imaging, with or without associated clinical manifestations such as abdominal pain and acute pancreatitis (52, 58). Recurrence rate of type 2 AIP is approximately 5%–34% (9, 25, 26, 38, 59, 116). Ueki et al. continued to follow up patients with UC for approximately 4 years after the diagnosis of type 2 AIP and found that 20% of patients developed type 2 AIP recurrence (16). In an Italian study, a recurrence rate of 11.1% was observed in patients with type 2 AIP–UC at a median follow-up of approximately 4–5 years, and the risk of recurrence was not higher in patients with type 2 AIP–UC compared with those without concurrent UC (26).
5.3 Surgical risks of UC
A multicentre retrospective study found that the proportion of patients with type 2 AIP–UC who underwent colectomy was approximately 20%, which was significantly higher than that of patients with UC without concurrent type 2 AIP (5%), and surgery was mostly performed after a diagnosis of type 2 AIP; the majority of UC patients requiring surgery were type E3, and the reason for surgery was overwhelmingly due to the development of acute severe colitis (9). According to the Mayo Clinic, 43% of patients with type 2 AIP–UC undergo colectomy for refractory UC (27). However, different views exist regarding the rate of surgery for UC in patients with type 2 AIP–UC. Bellocchi et al. followed patients for a mean of 55 months and found a colon resection rate of 4%–5% in patients with type 2 AIP–UC, with no increase in colon resection rates compared with patients with UC without concurrent type 2 AIP (26).
5.4 Long-term complications
A multicentre retrospective study in Italy reported that 31% of patients with type 2 AIP had pancreatic atrophy, 8% had diabetes mellitus (DM), and 10% had pancreatic calcification after 2–3 years of follow-up (62). After 4–5 years of follow-up of patients with type 2 AIP–IBD, it was found that approximately 19%–28% of patients had pancreatic exocrine insufficiency, and 12%–17% of patients had DM (9, 59). The current study showed that none of the patients with type 2 AIP-UC developed pancreatic or colorectal cancers during the follow-up period (9, 27, 59).
6 Treatment and challenges of Type 2 AIP–UC
The preferred treatment option for most type 2 AIP–UC patients is GC. The role of GC in the treatment of UC has been well documented over decades of clinical experience (117), and type 2 AIP also responds well to GC therapy (39). GC inhibit Th-1 and Th-17 differentiation, promote Th2 differentiation and Treg production, induce immunosuppression, and attenuate inflammatory responses (118). TNF inhibitors may play an important therapeutic role in patients with GC ineffectiveness or relapse. Lorenzo D et al. reported remission in recurrent type 2 AIP–UC patient after treatment with anti-TNF (adalimumab) (75), which may be related to the fact that anti-TNF therapy can target and block TNF-α, reducing the development of inflammatory processes and activation of immune system cells (119). Recently Lauri G reported the use of ustekinumab for induction and maintenance therapy in type 2 AIP–UC patients with good results (120). Ustekinumab is a monoclonal antibody targeting the p40 subunit of IL 12 and IL 23 (IL12/23p40), IL-23 promotes differentiation of Th cells into Th17 and secretes inflammatory cytokines such as IL-17 and IL-22, whereas IL-12 induces Th1 and produces other cytokines such as interferon-gamma and TNF (121), thus ustekinumab could play an important role in the treatment of type 2 AIP-UC. Neutrophil infiltration is a typical feature of type 2 AIP and UC, and Chiabrando F reported a case report on the use of colchicine, which targets neutrophils, in the treatment of type 2 AIP (122), but colchicine needs to be further explored in the treatment of UC. The neutrophilic capacity of IL-8 makes it a possible link to the pathogenesis of type 2 AIP–UC (68), but anti-inflammatory drugs targeting IL-8 are still being developed (123), which may be an important research direction for the future treatment of type 2 AIP–UC.
At present, there are still some challenges in the diagnosis and treatment of type 2 AIP–UC. On one hand, the diagnosis of type 2 AIP–UC, especially the definitive diagnosis of type 2 AIP, is still difficult (124, 125), and it is necessary to improve the understanding and differentiation of type 2 AIP. On the other hand, the presence of type 2 AIP in UC may indicate a more severe UC phenotype, which may lead to higher rates of colectomy or surgery (9, 126), and this is a challenge for the treatment of type 2 AIP–UC.
7 Conclusion
In summary, although type 2 AIP and UC have related features, their comorbidity can affect the clinical presentation of patients and the course of the disease. However, clinical studies on large samples of type 2 AIP–UC are still lacking, as well as basic research on the mechanisms involved. There is still a need to further elucidate the relationship between these two diseases and determine the exact molecular mechanisms. A better understanding of its pathogenesis could help improve disease management and identify potential biomarkers or therapeutic targets.
Author contributions
NN: Writing – original draft. DW: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Valmiki S, Ahuja V, Puri N, Paul J. miR-125b and miR-223 contribute to inflammation by targeting the key molecules of NFκB pathway. Front Med (Lausanne) (2020) 6:31. doi: 10.3389/fmed.2019.0031
2. Ouyang W, Wu M, Wu A, Xiao H. Circular RNA_0001187 participates in the regulation of ulcerative colitis development via upregulating myeloid differentiation factor 88. Bioengineered (2022) 13:12863–75. doi: 10.1080/21655979.2022.207757
3. Yu Y, Zhang G, Han T, Huang H. Analysis of the pharmacological mechanism of Banxia Xiexin decoction in treating depression and ulcerative colitis based on a biological network module. BMC Complement Med Ther (2020) 20:199. doi: 10.1186/s12906-020-02988-
4. Chen YL, Zhang YL, Dai YC, Tang ZP. Systems pharmacology approach reveals the antiinflammatory effects of Ampelopsis grossedentata on dextran sodium sulfate-induced colitis. World J Gastroenterol (2018) 24:1398–409. doi: 10.3748/wjg.v24.i13.139
5. Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet (2017) 390:2769–78. doi: 10.1016/S0140-6736(17)32448-
6. Sýkora J, Pomahačová R, Kreslová M, Cvalínová D, Štych P, Schwarz J. Current global trends in the incidence of pediatric-onset inflammatory bowel disease. World J Gastroenterol (2018) 24:2741–63. doi: 10.3748/wjg.v24.i25.274
7. Yoshida K, Toki Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, et al. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci (1995) 40:1561–8. doi: 10.1007/BF0228520
8. de Pretis N, Frulloni L. Autoimmune pancreatitis type 2. Curr Opin Gastroenterol (2020) 36:417–20. doi: 10.1097/MOG.000000000000065
9. Lorenzo D, Maire F, Stefanescu C, Gornet JM, Seksik P, Serrero M, et al. Features of autoimmune pancreatitis associated with inflammatory bowel diseases. Clin Gastroenterol Hepatol (2018) 16:59–67. doi: 10.1016/j.cgh.2017.07.03
10. Chari ST, Kloeppel G, Zhang L, Notohara K, Lerch MM, Shimosegawa T, et al. Histopathologic and clinical subtypes of autoimmune pancreatitis: the Honolulu consensus document. Pancreas (2010) 39:549–54. doi: 10.1097/MPA.0b013e3181e4d9e
11. Notohara K, Burgart LJ, Yadav D, Chari S, Smyrk TC. Idiopathic chronic pancreatitis with periductal lymphoplasmacytic infiltration: clinicopathologic features of 35 cases. Am J Surg Pathol (2003) 27:1119–27. doi: 10.1097/00000478-200308000-0000
12. Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas (2011) 40:352–8. doi: 10.1097/MPA.0b013e3182142fd
13. Song TJ, Kim JH, Kim MH, Jang JW, Park DH, Lee SS, et al. Comparison of clinical findings between histologically confirmed type 1 and type 2 autoimmune pancreatitis. J Gastroenterol Hepatol (2012) 27:700–8. doi: 10.1111/j.1440-1746.2011.06934.x
14. Watanabe T, Minaga K, Kamata K, Kudo M, Strober W. Mechanistic insights into autoimmune pancreatitis and igG4-related disease. Trends Immunol (2018) 39:874–89. doi: 10.1016/j.it.2018.09.00
15. Okazaki K, Uchida K. Current concept of autoimmune pancreatitis and igG4-related disease. Am J Gastroenterol (2018) 113:1412–6. doi: 10.1038/s41395-018-0184-
16. Ueki T, Kawamoto K, Otsuka Y, Minoda R, Maruo T, Matsumura K, et al. Prevalence and clinicopathological features of autoimmune pancreatitis in Japanese patients with inflammatory bowel disease. Pancreas (2015) 44:434–40. doi: 10.1097/MPA.000000000000026
17. Hedin CRH, Vavricka SR, Stagg AJ, Schoepfer A, Raine T, Puig L, et al. Pathogenesis of extraintestinal manifestations: implications for IBD research, diagnosis, and therapy. J Crohns Colitis (2019) 13:541–54. doi: 10.1093/ecco-jcc/jjy19
18. Roque Ramos L, DiMaio CJ, Sachar DB, Atreja A, Colombel JF, Torres J. Autoimmune pancreatitis and inflammatory bowel disease: Case series and review of the literature. Dig Liver Dis (2016) 48:893–8. doi: 10.1016/j.dld.2016.05.00
19. Pedersen JE, Ängquist LH, Jensen CB, Kjærgaard JS, Jess T, Allin KH. Risk of pancreatitis in patients with inflammatory bowel disease - a meta-analysis. Dan Med J (2020) 67:A08190427.
20. Tél B, Stubnya B, Gede N, Varjú P, Kiss Z, Márta K, et al. Inflammatory bowel diseases elevate the risk of developing acute pancreatitis: A meta-analysis. Pancreas (2020) 49:1174–81. doi: 10.1097/MPA.000000000000165
21. Massironi S, Fanetti I, Viganò C, Pirola L, Fichera M, Cristoferi L, et al. Systematic review-pancreatic involvement in inflammatory bowel disease. Aliment Pharmacol Ther (2022) 55:1478–91. doi: 10.1111/apt.1694
22. Gros B, Kaplan GG. Ulcerative colitis in adults: A review. JAMA (2023) 330:951–65. doi: 10.1001/jama.2023.15389
23. D’Incà R, Sturniolo G. Biomarkears in IBD: what to utilize for the diagnosis? Diagnostics (Basel) (2023) 13:2931. doi: 10.3390/diagnostics1318293
24. Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis (2019) 13:144–64. doi: 10.1093/ecco-jcc/jjy11
25. Oh D, Song TJ, Moon SH, Kim JH, Lee NJ, Hong SM, et al. Type 2 autoimmune pancreatitis (Idiopathic duct-centric pancreatitis) highlighting patients presenting as clinical acute pancreatitis: A single-center experience. Gut Liver (2019) 13:461–70. doi: 10.5009/gnl1842
26. Conti Bellocchi MC, Marconato E, Lamonaca L, Cattani Mottes M, Ciccocioppo R, Carrara S, et al. The features and clinical outcomes of inflammatory bowel disease associated with autoimmune pancreatitis: A greater awareness is needed. Med (Baltimore) (2022) 101:e28602. doi: 10.1097/MD.000000000002860
27. Hart PA, Levy MJ, Smyrk TC, Takahashi N, Abu Dayyeh BK, Clain JE, et al. Clinical profiles and outcomes in idiopathic duct-centric chronic pancreatitis (type 2 autoimmune pancreatitis): the Mayo Clinic experience. Gut (2016) 65:1702–9. doi: 10.1136/gutjnl-2015-30927
28. Almeida P, Almeida C, Gompertz M, Berger Z. Association between autoimmune pancreatitis and ulcerative colitis: a report of 12 patients. Rev Esp Enferm Dig (2020) 112:682–7. doi: 10.17235/reed.2020.6677/201
29. Eder P, Verstock B, Culver E, Dragoni G, Kredel LI, Wypych J, et al. Autoimmune pancreatitis in patients with inflammatory bowel disease - a real-world multicentre collaborative ECCO CONFER study. J Crohns Colitis (2023) 17:1791-9. doi: 10.1093/ecco-jcc/jjad09
30. Shimosegawa T. Working Group Members of the Japan Pancreas Society; Research Committee for Intractable Pancreatic Disease by the Ministry of Labor, Health and Welfare of Japan. The amendment of the Clinical Diagnostic Criteria in Japan (JPS2011) in response to the proposal of the International Consensus of Diagnostic Criteria (ICDC) for autoimmune pancreatitis. Pancreas (2012) 41:1341–2. doi: 10.1097/MPA.0b013e3182706ed
31. Park SW, Chung JB, Otsuki M, Kim MH, Lim JH, Kawa S, et al. Conference report: Korea-Japan symposium on autoimmune pancreatitis. Gut Liver. (2008) 2:81–7. doi: 10.5009/gnl.2008.2.2.8
32. Chari ST, Takahashi N, Levy MJ, Smyrk TC, Clain JE, Pearson RK, et al. A diagnostic strategy to distinguish autoimmune pancreatitis from pancreatic cancer. Clin Gastroenterol Hepatol (2009) 7:1097–103. doi: 10.1016/j.cgh.2009.04.02
33. Otsuki M, Chung JB, Okazaki K, Kim MH, Kamisawa T, Kawa S, et al. Asian diagnostic criteria for autoimmune pancreatitis: consensus of the Japan-Korea Symposium on Autoimmune Pancreatitis. J Gastroenterol (2008) 43:403–8. doi: 10.1007/s00535-008-2205-
34. Frulloni L, Scattolini C, Falconi M, Zamboni G, Capelli P, Manfredi R, et al. Autoimmune pancreatitis: differences between the focal and diffuse forms in 87 patients. Am J Gastroenterol (2009) 104:2288–94. doi: 10.1038/ajg.2009.32
35. Sumimoto K, Uchida K, Mitsuyama T, Fukui Y, Kusuda T, Miyoshi H, et al. A proposal of a diagnostic algorithm with validation of International Consensus Diagnostic Criteria for autoimmune pancreatitis in a Japanese cohort. Pancreatology (2013) 13:230–7. doi: 10.1016/j.pan.2013.02.01
36. Schneider A, Michaely H, Weiss C, Hirth M, Rückert F, Wilhelm TJ, et al. Prevalence and incidence of autoimmune pancreatitis in the population living in the southwest of Germany. Digestion (2017) 96:187–98. doi: 10.1159/00047931
37. Fritz S, Bergmann F, Grenacher L, Sgroi M, Hinz U, Hackert T, et al. Diagnosis and treatment of autoimmune pancreatitis types 1 and 2. Br J Surg (2014) 101:1257–65. doi: 10.1002/bjs.957
38. Kamisawa T, Chari ST, Giday SA, Kim MH, Chung JB, Lee KT, et al. Clinical profile of autoimmune pancreatitis and its histological subtypes: an international multicenter survey. Pancreas (2011) 40:809–14. doi: 10.1097/MPA.0b013e3182258a1
39. Li Y, Song H, Meng X, Li R, Leung PSC, Gershwin ME, et al. Autoimmune pancreatitis type 2 (idiopathic duct-centric pancreatitis): A comprehensive review. J Autoimmun (2023) 140:103121. doi: 10.1016/j.jaut.2023.10312
40. Sinico RA, Radice A. Antineutrophil cytoplasmic antibodies (ANCA) testing: detection methods and clinical application. Clin Exp Rheumatol (2014) 32:S112–7.
41. Prideaux L, De Cruz P, Ng SC, Kamm MA. Serological antibodies in inflammatory bowel disease: a systematic review. Inflammation Bowel Dis (2012) 18:1340–55. doi: 10.1002/ibd.2190
42. Kaul A, Hutfless S, Liu L, Bayless TM, Marohn MR, Li X. Serum anti-glycan antibody biomarkers for inflammatory bowel disease diagnosis and progression: a systematic review and meta-analysis. Inflammation Bowel Dis (2012) 18(10):1872–84. doi: 10.1002/ibd.2286
43. Takedatsu H, Mitsuyama K, Fukunaga S, Yoshioka S, Yamauchi R, Mori A, et al. Diagnostic and clinical role of serum proteinase 3 antineutrophil cytoplasmic antibodies in inflammatory bowel disease. J Gastroenterol Hepatol (2018) 33:1603-7. doi: 10.1111/jgh.1414
44. Horn MP, Peter AM, Righini Grunder F, Leichtle AB, Spalinger J, Schibli S, et al. PR3-ANCA and panel diagnostics in pediatric inflammatory bowel disease to distinguish ulcerative colitis from Crohn’s disease. PloS One (2018) 13:e0208974. doi: 10.1371/journal.pone.020897
45. Mizuochi T, Arai K, Kudo T, Nambu R, Tajiri H, Aomatsu T, et al. Diagnostic accuracy of serum proteinase 3 antineutrophil cytoplasmic antibodies in children with ulcerative colitis. J Gastroenterol Hepatol (2021) 36:1538–44. doi: 10.1111/jgh.1529
46. Livanos AE, Dunn A, Fischer J, Ungaro RC, Turpin W, Lee SH, et al. Anti-integrin αvβ6 autoantibodies are a novel biomarker that antedate ulcerative colitis. Gastroenterology (2023) 164(4):619–29. doi: 10.1053/j.gastro.2022.12.04
47. Rydell N, Ekoff H, Hellström PM, Movérare R. Measurement of serum igG anti-integrin αvβ6 autoantibodies is a promising tool in the diagnosis of ulcerative colitis. J Clin Med (2022) 11:1881. doi: 10.3390/jcm1107188
48. de Pretis N, Amodio A, De Marchi G, Marconato E, Ciccocioppo R, Frulloni L. The role of serological biomarkers in the diagnosis and management of autoimmune pancreatitis. Expert Rev Clin Immunol (2022) 18:1119–24. doi: 10.1080/1744666X.2022.212537
49. Felix K, Hauck O, Schnölzer M, Kempf T, Warnken U, Schneider K, et al. Identification of novel serum autoantibodies for differential diagnosis of autoimmune pancreatitis and pancreatic ductal adenocarcinoma. Pancreas (2016) 45:1309–19. doi: 10.1097/MPA.000000000000064
50. Sánchez Castañón M, Zuliani V, Amodio A, Campagnola P, Granato A, Gabbrielli A, et al. Role of amylase-α2A autoantibodies in the diagnosis of autoimmune pancreatitis. Pancreas (2015) 44:1078–82. doi: 10.1097/MPA.000000000000041
51. Detlefsen S, Mortensen MB, Pless TK, Cribe AS, de Muckadell OB. Laparoscopic and percutaneous core needle biopsy plays a central role for the diagnosis of autoimmune pancreatitis in a single-center study from Denmark. Pancreas (2015) 44:845–58. doi: 10.1097/MPA.000000000000031
52. Maire F, Le Baleur Y, Rebours V, Vullierme MP, Couvelard A, Voitot H, et al. Outcome of patients with type 1 or 2 autoimmune pancreatitis. Am J Gastroenterol (2011) 106:151–6. doi: 10.1038/ajg.2010.31
53. Ramos LR, Sachar DB, DiMaio CJ, Colombel JF, Torres J. Inflammatory bowel disease and pancreatitis: A review. J Crohns Colitis (2016) 10:95–104. doi: 10.1093/ecco-jcc/jjv15
54. Fukuda S, Akiyama S, Tarakji A, Hamdeh S, Suzuki H, Tsuchiya K. Prevalence and clinical features of patients with autoimmune pancreatitis and inflammatory bowel disease: A systematic review and meta-analysis. J Gastroenterol Hepatol (2022) 37:1474–84. doi: 10.1111/jgh.1589
55. Zen Y. Type 2 autoimmune pancreatitis: consensus and controversies. Gut Liver (2022) 16:357–65. doi: 10.5009/gnl21024
56. Bezzio C, Della Corte C, Vernero M, Di Luna I, Manes G, Saibeni S. Inflammatory bowel disease and immune-mediated inflammatory diseases: looking at the less frequent associations. Therap Adv Gastroenterol (2022) 15:17562848221115312. doi: 10.1177/17562848221115312
57. Schneider A, Hirth M, Weiss C, Weidner P, Antoni C, Thomann A, et al. Prevalence of inflammatory bowel disease in alcoholic, non-alcoholic and autoimmune pancreatitis. Z Gastroenterol (2018) 56:469–78. doi: 10.1055/s-0043-12388
58. Hart PA, Kamisawa T, Brugge WR, Chung JB, Culver EL, Czakó L, et al. Long-term outcomes of autoimmune pancreatitis: a multicentre, international analysis. Gut (2013) 62:1771–6. doi: 10.1136/gutjnl-2012-30361
59. Nikolic S, Lanzillotta M, Panic N, Brismar TB, Moro CF, Capurso G, et al. Unraveling the relationship between autoimmune pancreatitis type 2 and inflammatory bowel disease: Results from two centers and systematic review of the literature. United Eur Gastroenterol J (2022) 10:496–506. doi: 10.1002/ueg2.1223
60. Kawa S, Okazaki K, Notohara K, Watanabe M, Shimosegawa T, The Research Committee for Intractable Pancreatic Disease, et al. Autoimmune pancreatitis complicated with inflammatory bowel disease and comparative study of type 1 and type 2 autoimmune pancreatitis. J Gastroenterol (2015) 50:805–15. doi: 10.1007/s00535-014-1012-
61. Buechter M, Manka P, Heinemann FM, Lindemann M, Juntermanns B, Canbay A, et al. Outcome and genetic factors in igG4-associated autoimmune pancreatitis and cholangitis: A single center experience. Gastroenterol Res Pract (2017) 2017:6126707. doi: 10.1155/2017/612670
62. Barresi L, Tacelli M, Crinò SF, Attili F, Petrone MC, De Nucci G, et al. Multicentric Italian survey on daily practice for autoimmune pancreatitis: Clinical data, diagnosis, treatment, and evolution toward pancreatic insufficiency. United Eur Gastroenterol J (2020) 8:705–15. doi: 10.1177/205064062092430
63. Detlefsen S, Zamboni G, Frulloni L, Feyerabend B, Braun F, Gerke O, et al. Clinical features and relapse rates after surgery in type 1 autoimmune pancreatitis differ from type 2: a study of 114 surgically treated European patients. Pancreatology (2012) 12:276–83. doi: 10.1016/j.pan.2012.03.05
64. Notohara K, Nishimori I, Mizuno N, Okazaki K, Ito T, Kawa S, et al. Clinicopathological features of type 2 autoimmune pancreatitis in Japan: results of a multicenter survey. Pancreas (2015) 44:1072–7. doi: 10.1097/MPA.000000000000043
65. Park SH, Kim D, Ye BD, Yang SK, Kim JH, Yang DH, et al. The characteristics of ulcerative colitis associated with autoimmune pancreatitis. J Clin Gastroenterol (2013) 47:520–5. doi: 10.1097/MCG.0b013e31827fd4a
66. Goni E, Regel I, Mahajan UM, Amodio A, De Marchi G, Beyer G, et al. HLA-DRB1∗16 and -DQB1∗05 alleles are strongly associated with autoimmune pancreatitis in a cohort of hundred patients. Pancreatology (2022) 22:466–71. doi: 10.1016/j.pan.2022.03.01
67. Czakó L, Gyökeres T, Topa L, Sahin P, Takács T, Vincze A, et al. Autoimmune pancreatitis in Hungary: a multicenter nationwide study. Pancreatology (2011) 11:261–7. doi: 10.1159/00032709
68. Ku Y, Hong SM, Fujikura K, Kim SJ, Akita M, Abe-Suzuki S, et al. IL-8 expression in granulocytic epithelial lesions of idiopathic duct-centric pancreatitis (Type 2 autoimmune pancreatitis). Am J Surg Pathol (2017) 41:1129–38. doi: 10.1097/PAS.000000000000089
69. Kim JW, Hwang SW, Park SH, Song TJ, Kim MH, Lee HS, et al. Clinical course of ulcerative colitis patients who develop acute pancreatitis. World J Gastroenterol (2017) 23:3505–12. doi: 10.3748/wjg.v23.i19.350
70. Hart PA, Zen Y, Chari ST. Recent advances in autoimmune pancreatitis. Gastroenterology (2015) 149:39–51. doi: 10.1053/j.gastro.2015.03.01
71. Zen Y, Deshpande V. Tumefactive inflammatory diseases of the pancreas. Am J Pathol (2019) 189:82–93. doi: 10.1016/j.ajpath.2018.05.02
72. Zhang L, Chari S, Smyrk TC, Deshpande V, Klöppel G, Kojima M, et al. Autoimmune pancreatitis (AIP) type 1 and type 2: an international consensus study on histopathologic diagnostic criteria. Pancreas (2011) 40:1172–9. doi: 10.1097/MPA.0b013e318233bec
73. Magro F, Langner C, Driessen A, Ensari A, Geboes K, Mantzaris GJ, et al. European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis (2013) 7:827–51. doi: 10.1016/j.crohns.2013.06.00
74. Tsen A, Alishahi Y, Rosenkranz L. Autoimmune pancreatitis and inflammatory bowel disease: an updated review. J Clin Gastroenterol (2017) 51:208–14. doi: 10.1097/MCG.000000000000073
75. Lorenzo D, Vullierme MP, Rebours V. Antitumor necrosis factor therapy is effective for autoimmune pancreatitis type 2. Am J Gastroenterol (2020) 115:1133–4. doi: 10.14309/ajg.000000000000066
76. The MHC sequencing consortium. Complete sequence and gene map of a human major histocompatibility complex. The MHC sequencing consortium. Nature (1999) 401(6756):921–3. doi: 10.1038/4485
77. Naito T, Okada Y. HLA imputation and its application to genetic and molecular fine-mapping of the MHC region in autoimmune diseases. Semin Immunopathol (2022) 44:15–28. doi: 10.1007/s00281-021-00901-
78. Dendrou CA, Petersen J, Rossjohn J, Fugger L. HLA variation and disease. Nat Rev Immunol (2018) 18:325–39. doi: 10.1038/nri.2017.14
79. Bodis G, Toth V, Schwarting A. Role of human leukocyte antigens (HLA) in autoimmune diseases. Rheumatol Ther (2018) 5:5–20. doi: 10.1007/s40744-018-0100-z
80. Rajaei E, Jalali MT, Shahrabi S, Asnafi AA, Pezeshki SMS. HLAs in autoimmune diseases: dependable diagnostic biomarkers? Curr Rheumatol Rev (2019) 15:269–76. doi: 10.2174/157339711566619011514322
81. Matzaraki V, Kumar V, Wijmenga C, Zhernakova A. The MHC locus and genetic susceptibility to autoimmune and infectious diseases. Genome Biol (2017) 18:76. doi: 10.1186/s13059-017-1207-
82. Pitchumoni CS, Chari S. Ulcerative colitis and autoimmune pancreatitis. J Clin Gastroenterol (2013) 47:469. doi: 10.1097/MCG.0b013e31828a709
83. Ashton JJ, Latham K, Beattie RM, Ennis S. Review article: the genetics of the human leucocyte antigen region in inflammatory bowel disease. Aliment Pharmacol Ther (2019) 50:885–900. doi: 10.1111/apt.1548
84. Goyette P, Boucher G, Mallon D, Ellinghaus E, Jostins L, Huang H, et al. High-density mapping of the MHC identifies a shared role for HLA-DRB1*01:03 in inflammatory bowel diseases and heterozygous advantage in ulcerative colitis. Nat Genet (2015) 47:172–9. doi: 10.1038/ng.317
85. Kawa S, Ota M, Yoshizawa K, Horiuchi A, Hamano H, Ochi Y, et al. HLA DRB10405-DQB10401 haplotype is associated with autoimmune pancreatitis in the Japanese population. Gastroenterology (2002) 122:1264–9. doi: 10.1053/gast.2002.3302
86. Ota M, Katsuyama Y, Hamano H, Umemura T, Kimura A, Yoshizawa K, et al. Two critical genes (HLA-DRB1 and ABCF1)in the HLA region are associated with the susceptibility to autoimmune pancreatitis. Immunogenetics (2007) 59:45–52. doi: 10.1007/s00251-006-0178-
87. Park DH, Kim MH, Oh HB, Kwon OJ, Choi YJ, Lee SS, et al. Substitution of aspartic acid at position 57 of the DQbeta1 affects relapse of autoimmune pancreatitis. Gastroenterology (2008) 134:440–6. doi: 10.1053/j.gastro.2007.11.02
88. Nourshargh S, Perkins JA, Showell HJ, Matsushima K, Williams TJ, Collins PD. A comparative study of the neutrophil stimulatory activity in vitro and pro-inflammatory properties in vivo of 72 amino acid and 77 amino acid IL-8. J Immunol (1992) 148:106–11. doi: 10.4049/jimmunol.148.1.106
89. Pearl DS, Shah K, Whittaker MA, Nitch-Smith H, Brown JF, Shute JK, et al. Cytokine mucosal expression in ulcerative colitis, the relationship between cytokine release and disease activity. J Crohns Colitis (2013) 7:481–9. doi: 10.1016/j.crohns.2012.07.02
90. Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell (2010) 140:845–58. doi: 10.1016/j.cell.2010.02.02
91. Yasuda K, Takeuchi Y, Hirota K. The pathogenicity of Th17 cells in autoimmune diseases. Semin Immunopathol (2019) 41:283–97. doi: 10.1007/s00281-019-00733-
92. Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood (2010) 115:335–43. doi: 10.1182/blood-2009-04-21608
93. Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet (2012) 380:1606–19. doi: 10.1016/S0140-6736(12)60150-
94. Jiang W, Su J, Zhang X, Cheng X, Zhou J, Shi R, et al. Elevated levels of Th17 cells and Th17-related cytokines are associated with disease activity in patients with inflammatory bowel disease. Inflammation Res (2014) 63:943–50. doi: 10.1007/s00011-014-0768-
95. Gomez-Bris R, Saez A, Herrero-Fernandez B, Rius C, Sanchez-Martinez H, Gonzalez-Granado JM. CD4 T-cell subsets and the pathophysiology of inflammatory bowel disease. Int J Mol Sci (2023) 24:2696. doi: 10.3390/ijms2403269
96. Gui X, Li J, Ueno A, Iacucci M, Qian J, Ghosh S. Histopathological features of inflammatory bowel disease are associated with different CD4+ T cell subsets in colonic mucosal lamina propria. J Crohns Colitis (2018) 12:1448–58. doi: 10.1093/ecco-jcc/jjy11
97. Loos M, Lauffer F, Schlitter AM, Kleeff J, Friess H, Klöppel G, et al. Potential role of Th17 cells in the pathogenesis of type 2 autoimmune pancreatitis. Virchows Arch (2015) 467:641–8. doi: 10.1007/s00428-015-1850-
98. Lote H, Cafferkey C, Chau I. PD-1 and PD-L1 blockade in gastrointestinal Malignancies. Cancer Treat Rev (2015) 41:893–903. doi: 10.1016/j.ctrv.2015.09.00
99. Gianchecchi E, Delfino DV, Fierabracci A. Recent insights into the role of the PD-1/PD-L1 pathway in immunological tolerance and autoimmunity. Autoimmun Rev (2013) 12:1091–100. doi: 10.1016/j.autrev.2013.05.00
100. Rajabian Z, Kalani F, Taghiloo S, Tehrani M, Rafiei A, Hosseini-Khah Z, et al. Over-expression of immunosuppressive molecules, PD-L1 and PD-L2, in ulcerative colitis patients. Iran J Immunol (2019) 16:62–70. doi: 10.22034/IJI.2019.3940
101. Nakazawa A, Dotan I, Brimnes J, Allez M, Shao L, Tsushima F, et al. The expression and function of costimulatory molecules B7H and B7-H1 on colonic epithelial cells. Gastroenterology (2004) 126:1347–57. doi: 10.1053/j.gastro.2004.02a.00
102. Nguyen J, Finkelman BS, Escobar D, Xue Y, Wolniak K, Pezhouh M. Overexpression of programmed death ligand 1 in refractory inflammatory bowel disease. Hum Pathol (2022) 126:19–27. doi: 10.1016/j.humpath.2022.04.01
103. Gupta R, Neyaz A, Chougule A, Akita M, Zen Y, Forcione D, et al. Autoimmune pancreatitis type 2: diagnostic utility of PD-L1 immunohistochemistry. Am J Surg Pathol (2019) 43:898–906. doi: 10.1097/PAS.000000000000128
104. Islam MM, Takeyama N. Role of neutrophil extracellular traps in health and disease pathophysiology: recent insights and advances. Int J Mol Sci (2023) 24:15805. doi: 10.3390/ijms24211580
105. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science (2004) 303:1532–5. doi: 10.1126/science.109238
106. Demkow U. Molecular mechanisms of neutrophil extracellular trap (NETs) degradation. Int J Mol Sci (2023) 24:4896. doi: 10.3390/ijms2405489
107. Dinallo V, Marafini I, Di Fusco D, Laudisi F, Franzè E, Di Grazia A, et al. Neutrophil extracellular traps sustain inflammatory signals in ulcerative colitis. J Crohns Colitis (2019) 13:772–84. doi: 10.1093/ecco-jcc/jjy21
108. Arai Y, Yamashita K, Kuriyama K, Shiokawa M, Kodama Y, Sakurai T, et al. Plasmacytoid dendritic cell activation and IFN-α Production are prominent features of murine autoimmune pancreatitis and human igG4-related autoimmune pancreatitis. J Immunol (2015) 195:3033–44. doi: 10.4049/jimmunol.150097
109. Minaga K, Watanabe T, Arai Y, Shiokawa M, Hara A, Yoshikawa T, et al. Activation of interferon regulatory factor 7 in plasmacytoid dendritic cells promotes experimental autoimmune pancreatitis. J Gastroenterol (2020) 55:565–76. doi: 10.1007/s00535-020-01662-
110. Hayashi H, Miura S, Fujishima F, Kuniyoshi S, Kume K, Kikuta K, et al. Utility of endoscopic ultrasound-guided fine-needle aspiration and biopsy for histological diagnosis of type 2 autoimmune pancreatitis. Diagnostics (Basel) (2022) 12:2464. doi: 10.3390/diagnostics1210246
111. Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol (2017) 17:219–32. doi: 10.1038/nri.2017
112. Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med (2016) 375:2369–79. doi: 10.1056/NEJMra160026
113. Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol (2012) 9:599–608. doi: 10.1038/nrgastro.2012.15
114. Chen L, Wilson JE, Koenigsknecht MJ, Chou WC, Montgomery SA, Truax AD, et al. NLRP12 attenuates colon inflammation by maintaining colonic microbial diversity and promoting protective commensal bacterial growth. Nat Immunol (2017) 18:541–51. doi: 10.1038/ni.369
115. Yoshikawa T, Watanabe T, Kamata K, Hara A, Minaga K, Kudo M. Intestinal dysbiosis and autoimmune pancreatitis. Front Immunol (2021) 12:62153. doi: 10.3389/fimmu.2021.62153
116. Tacelli M, Celsa C, Magro B, Barresi L, Guastella S, Capurso G, et al. Risk factors for rate of relapse and effects of steroid maintenance therapy in patients with autoimmune pancreatitis: systematic review and meta-analysis. Clin Gastroenterol Hepatol (2019) 17:1061–1072.e8. doi: 10.1016/j.cgh.2018.09.05
117. Bruscoli S, Febo M, Riccardi C, Migliorati G. Glucocorticoid therapy in inflammatory bowel disease: mechanisms and clinical practice. Front Immunol (2021) 12:69148. doi: 10.3389/fimmu.2021.69148
118. Cannarile L, Delfino DV, Adorisio S, Riccardi C, Ayroldi E. Implicating the role of GILZ in glucocorticoid modulation of T-cell activation. Front Immunol (2019) 10:182. doi: 10.3389/fimmu.2019.0182
119. Souza RF, Caetano MAF, Magalhães HIR, Castelucci P. Study of tumor necrosis factor receptor in the inflammatory bowel disease. World J Gastroenterol (2023) 29:2733–46. doi: 10.3748/wjg.v29.i18.273
120. Lauri G, D’Amico F, Allocca M, Palumbo D, Della-Torre E, De Cobelli F, et al. Ustekinumab as induction and maintenance therapy in patients with inflammatory bowel disease and type II autoimmune pancreatitis: report of two cases. J Crohns Colitis (2023) 17(9):1552–4. doi: 10.1093/ecco-jcc/jjad07
121. Gara SK, Guntipalli P, Marzban S, Taqi M, Aryal V, Khan QUA, et al. Clinical outcomes of ustekinumab in inflammatory bowel disease. Cureus (2023) 15:e46833. doi: 10.7759/cureus.4683
122. Chiabrando F, Lanzillotta M, Palumbo D, Pedica F, Caruso M, Capurso G, et al. Treating type 2 autoimmune pancreatitis with colchicine: A case series. Ann Intern Med (2021) 174:1775–6. doi: 10.7326/L21-028
123. Matsushima K, Yang D, Oppenheim JJ. Interleukin-8: An evolving chemokine. Cytokine (2022) 153:155828. doi: 10.1016/j.cyto.2022.15582
124. Moon SH. A multidisciplinary approach is essential for differentiating autoimmune pancreatitis from pancreatic adenocarcinoma. Clin Endosc (2023) 56:457–9. doi: 10.5946/ce.2023.11
125. Cho MK, Moon SH, Song TJ, Kim RE, Oh DW, Park DH, et al. Contrast-enhanced endoscopic ultrasound for differentially diagnosing autoimmune pancreatitis and pancreatic cancer. Gut Liver (2018) 12:591–6. doi: 10.5009/gnl1739
Keywords: ulcerative colitis, autoimmune pancreatitis, inflammatory bowel disease, prevalence, pathogenesis, treatment
Citation: Nan N and Wang D (2023) Type 2 autoimmune pancreatitis associated with ulcerative colitis. Front. Immunol. 14:1288390. doi: 10.3389/fimmu.2023.1288390
Received: 04 September 2023; Accepted: 23 November 2023;
Published: 06 December 2023.
Edited by:
Li-Tung Huang, Kaohsiung Chang Gung Memorial Hospital, TaiwanCopyright © 2023 Nan and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongxu Wang, ZG9uZ3h1d2FuZ2R4QDEyNi5jb20=
 Nan Nan
Nan Nan Dongxu Wang
Dongxu Wang