
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Immunol. , 12 September 2023
Sec. Vaccines and Molecular Therapeutics
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1283355
This article is a correction to:
Impact of adjuvant: Trivalent vaccine with quadrivalent-like protection against heterologous Yamagata-lineage influenza B virus
 Mallory L. Myers1
Mallory L. Myers1 John R. Gallagher1
John R. Gallagher1 De’Marcus D. Woolfork1
De’Marcus D. Woolfork1 Regan K. Stradtmann-Carvalho1
Regan K. Stradtmann-Carvalho1 Samantha Maldonado-Puga1
Samantha Maldonado-Puga1 Kevin W. Bock2
Kevin W. Bock2 Seyhan Boyoglu-Barnum3
Seyhan Boyoglu-Barnum3 Hubza Syeda3
Hubza Syeda3 Adrian Creanga3
Adrian Creanga3 Derron A. Alves2
Derron A. Alves2 Masaru Kanekiyo3
Masaru Kanekiyo3 Audray K. Harris1*
Audray K. Harris1*A Corrigendum on
Impact of adjuvant: trivalent vaccine with quadrivalent-like protection against heterologous Yamagata-lineage influenza B virus
by Myers ML, Gallagher JR, Woolfork D’MD, Stradtmann-Carvalho RK, Maldonado-Puga S, Bock KW, Boyoglu-Barnum S, Syeda H, Creanga A, Alves DA, Kanekiyo M and Harris AK (2022). Front. Immunol. 13:1002286. doi: 10.3389/fimmu.2022.1002286
In the published article, there was an error in the legend for Figure 4 as published. “quadrivalent and trivalent vaccinations” should have been “vaccinations” and “Flucelvax (Q) (green)” removed. The corrected legend appears below.
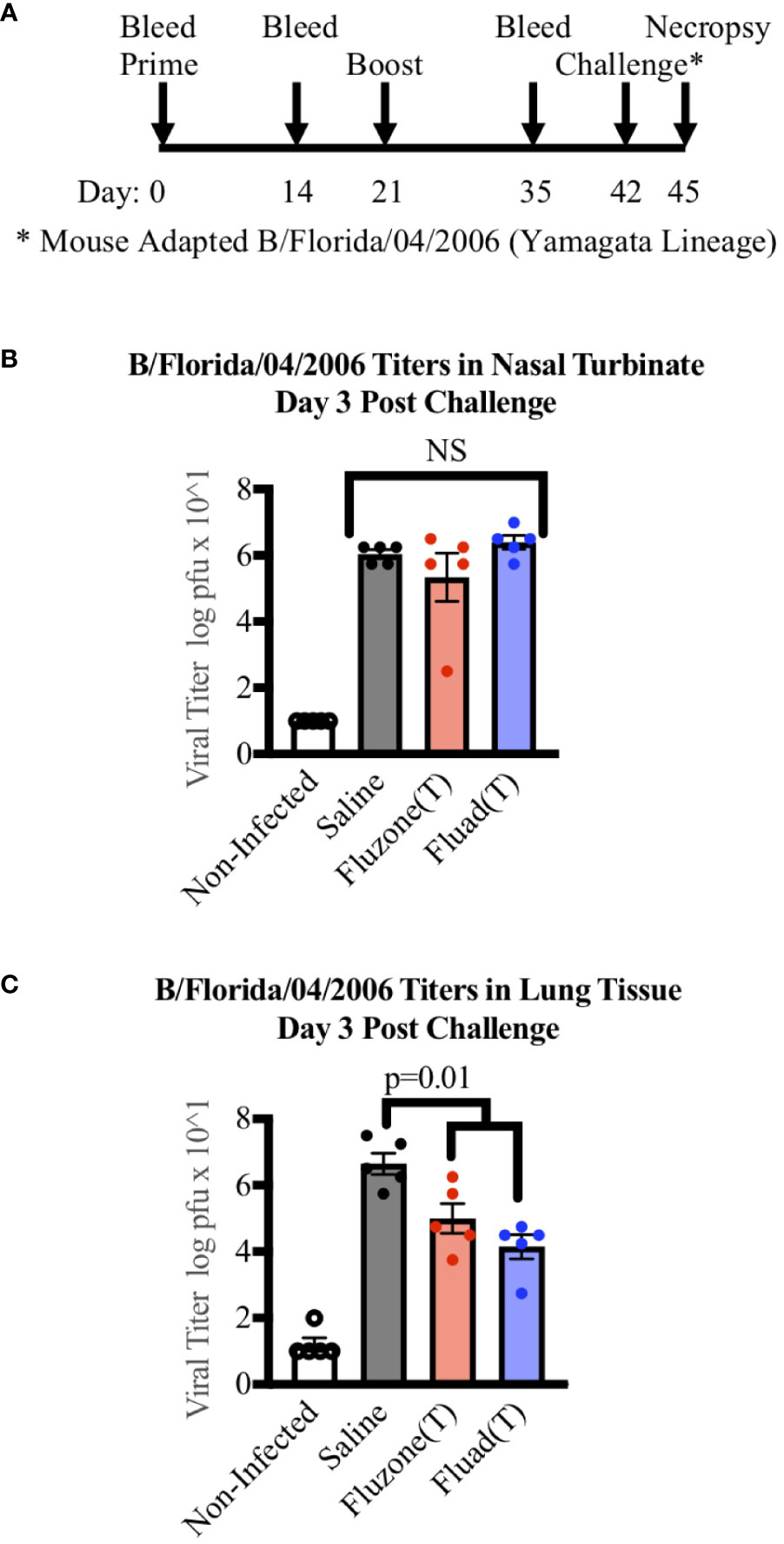
Figure 4 Measuring viral titers in the nasal turbinates and lungs of mice following vaccinations and subsequent challenge with Yamagata-lineage influenza B virus. (A) Immunization and challenge schedule with prime and boost (day 0 and 21), intranasal challenge (day 42), and bleeds (day 0, 14, and 35). On day 45 mice were euthanized for the harvesting of lungs and nasal turbinates. (B) Viral levels in the nasal turbinates of mice as measured by tissue culture infectious dose 50 (TCID50). (C) Viral levels in the left lung of mice as measured by TCID50. P-values are indicated for the significance of differences and NS denotes no statistical difference. Vaccines were Fluad (T) (blue) and Fluzone (T) (red) with saline (black) and non-infected controls (white). Yamagata-lineage challenge virus was mouse-adapted B/Florida/04/2006.
“Measuring viral titers in the nasal turbinates and lungs of mice following vaccinations and subsequent challenge with Yamagata-lineage influenza B virus. (A) Immunization and challenge schedule with prime and boost (day 0 and 21), intranasal challenge (day 42), and bleeds (day 0, 14, and 35). On day 45 mice were euthanized for the harvesting of lungs and nasal turbinates. (B) Viral levels in the nasal turbinates of mice as measured by tissue culture infectious dose 50 (TCID50). (C) Viral levels in the left lung of mice as measured by TCID50. P-values are indicated for the significance of differences and NS denotes no statistical difference. Vaccines were Fluad (T) (blue) and Fluzone (T) (red) with saline (black) and non-infected controls (white). Yamagata-lineage challenge virus was mouse-adapted B/Florida/04/2006.”
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: influenza B, MF59 adjuvant, commercial vaccine, challenge, Yamagata lineage
Citation: Myers ML, Gallagher JR, Woolfork D’MD, Stradtmann-Carvalho RK, Maldonado-Puga S, Bock KW, Boyoglu-Barnum S, Syeda H, Creanga A, Alves DA, Kanekiyo M and Harris AK (2023) Corrigendum: Impact of adjuvant: trivalent vaccine with quadrivalent-like protection against heterologous Yamagata-lineage influenza B virus. Front. Immunol. 14:1283355. doi: 10.3389/fimmu.2023.1283355
Received: 25 August 2023; Accepted: 30 August 2023;
Published: 12 September 2023.
Approved by:
Frontiers Editorial Office, Frontiers Media SA, SwitzerlandCopyright © 2023 Myers, Gallagher, Woolfork, Stradtmann-Carvalho, Maldonado-Puga, Bock, Boyoglu-Barnum, Syeda, Creanga, Alves, Kanekiyo and Harris. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Audray K. Harris, aGFycnNpYXVAbWFpbC5uaWguZ292
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.