
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 16 November 2023
Sec. Multiple Sclerosis and Neuroimmunology
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1280020
This article is part of the Research TopicMultiple Sclerosis and Related Disorders: Challenges and Approaches to Mechanisms, Biomarkers, and Therapeutic TargetsView all 11 articles
 Xiang Zhang1
Xiang Zhang1 Hongjun Hao2
Hongjun Hao2 Tao Jin3
Tao Jin3 Wei Qiu4
Wei Qiu4 Huan Yang5
Huan Yang5 Qun Xue6
Qun Xue6 Jian Yin7
Jian Yin7 Ziyan Shi8
Ziyan Shi8 Hai Yu1
Hai Yu1 Xiaopei Ji6
Xiaopei Ji6 Xiaobo Sun4
Xiaobo Sun4 Qiuming Zeng5
Qiuming Zeng5 Xiaoni Liu1
Xiaoni Liu1 Jingguo Wang1
Jingguo Wang1 Huining Li9
Huining Li9 Xiaoyan He10
Xiaoyan He10 Jing Yang11
Jing Yang11 Yarong Li1
Yarong Li1 Shuangshuang Liu12
Shuangshuang Liu12 Alexander Y. Lau13
Alexander Y. Lau13 Feng Gao2
Feng Gao2 Shimin Hu12,14
Shimin Hu12,14 Shuguang Chu15
Shuguang Chu15 Ding Ding1
Ding Ding1 Hongyu Zhou8*
Hongyu Zhou8* Haifeng Li12*
Haifeng Li12* Xiangjun Chen1*
Xiangjun Chen1*Background: Cerebrospinal fluid oligoclonal band (CSF-OCB) is an established biomarker in diagnosing multiple sclerosis (MS), however, there are no nationwide data on CSF-OCB prevalence and its diagnostic performance in Chinese MS patients, especially in the virtue of common standard operation procedure (SOP).
Methods: With a consensus SOP and the same isoelectric focusing system, we conducted a nationwide multi-center study on OCB status in consecutively, and recruited 483 MS patients and 880 non-MS patients, including neuro-inflammatory diseases (NID, n = 595) and non-inflammatory neurological diseases (NIND, n=285). Using a standardized case report form (CRF) to collect the clinical, radiological, immunological, and CSF data, we explored the association of CSF-OCB positivity with patient characters and the diagnostic performance of CSF-OCB in Chinese MS patients. Prospective source data collection, and retrospective data acquisition and statistical data analysis were used.
Findings: 369 (76.4%) MS patients were OCB-positive, while 109 NID patients (18.3%) and 6 NIND patients (2.1%) were OCB-positive, respectively. Time from symptom onset to diagnosis was significantly shorter in OCB-positive than that in OCB-negative MS patients (13.2 vs 23.7 months, P=0.020). The prevalence of CSF-OCB in Chinese MS patients was significantly higher in high-latitude regions (41°-50°N)(P=0.016), and at high altitudes (>1000m)(P=0.025). The diagnostic performance of CSF-OCB differentiating MS from non-MS patients yielded a sensitivity of 76%, a specificity of 87%.
Interpretation: The nationwide prevalence of CSF-OCB was 76.4% in Chinese MS patients, and demonstrated a good diagnostic performance in differentiating MS from other CNS diseases. The CSF-OCB prevalence showed a correlation with high latitude and altitude in Chinese MS patients.
Multiple sclerosis (MS) is a typical chronic inflammatory demyelinating disease of the central nervous system (CNS) (1). The clinical manifestations of MS are diverse, and the core diagnostic points are neurological deficits disseminated in time and space. The diagnosis of MS is challenging, and it should be prudent to differentiate it from other diseases with similar clinical manifestations (2, 3), especially in other inflammatory demyelinating diseases, such as neuromyelitis optica spectrum disorders (NMOSD), and myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD). Therefore, MS-related biomarkers have become the focus of ongoing research. Although in recent years many diagnostic biomarkers have been reported to be related to MS, few of them have clinical applicability and reliability (4, 5). The presence of immunoglobulin G (IgG) oligoclonal band (OCB) in cerebrospinal fluid (CSF) indicates intrathecal synthesis of immunoglobulin in response to chronic inflammation in CNS (6, 7). CSF-OCB was found in MS patients in the 1960s (8). Since then, it was confirmed as an established biomarker in diagnosing MS and is widely used in the diagnosis of MS globally (9–14).
In the 2017 McDonald diagnostic criteria of MS, OCB is included and can be used as a substitution for dissemination in time (15), which promotes the early diagnosis of MS in patients with the clinically isolated syndrome (CIS) (13, 16, 17). However, the expert panel emphasized that this criteria should be used prudently in Asian patients (15), because of the higher prevalence of non-MS demyelinating diseases in Asia and some of these patients also have CSF-OCB but with short segmental spinal lesions and atypical cerebral lesions. Due to the more important role of CSF-OCB in the diagnosis of MS, Chinese scholars recognized the lack of nationwide data on CSF-OCB in Chinese MS patients might lead to under or over-diagnosis of MS. CSF-OCB was reported in over 85% of MS patients in Europe and the United States (14, 18, 19). However, the prevalence of CSF-OCB in Chinese MS patients was reported as about 30~70% by several single centers (17, 20–23), and was lower than that reported in Western countries. In China, MS was defined as a rare disease (24) with an estimated prevalence of 1~5 per 100,000 (25). Nevertheless, due to the large population in China, the total number of MS patients in China is still large. Till now there are no nationwide data on CSF-OCB positivity and its diagnostic performance in Chinese MS patients.
Using different testing methods of CSF-OCB in different regional studies was previously presumed as the difference in reported OCB positivity in Chinese MS patients and the difference from that reported in Western countries. China is a vast country, hence there might be differences in regional CSF-OCB prevalence due to differences in latitude and altitude. There are also different backgrounds in culture and conventions in different regions of China. Therefore, experts in 12 regional referring MS centers in mainland of China formed the Multiple Sclerosis Collaborative Research Group in 2019 and started the project “CNS-OCB, China National Study for Oligo-Clonal Band”in 2020. We first developed a consensus standard operation procedure (SOP) with an isoelectric focusing system and validated the inter-laboratory agreement (26). With this SOP and the same isoelectric focusing system, we conducted a nationwide multi-center study on OCB status in consecutively, and recruited 483 MS patients and 880 non-MS patients. Using a standardized case report form (CRF) to collect the clinical, radiological, immunological, and CSF data, we explored the association of CSF-OCB positivity with patient characters and the diagnostic performance of CSF-OCB in Chinese MS patients.
All patients included in this study were diagnosed with MS or non-MS diseases in the recruiting centers from May 2020 to May 2022 (ChiCTR2000040363). This study was approved by the Ethics Committee of Shanghai Huashan Hospital and all other participating centers. The study was conducted according to the principles of the Declaration of Helsinki. All patients have signed the informed consent and agreed with sample collection and data publication. Prospective source data collection, and retrospective data acquisition and statistical data analysis were used. The data was collected at all centers with a structured database issued by a cooperation project (“CNS-OCB, China National Study for Oligo-Clonal Band”) from 2019, and acquired from the database of each center according to the inclusion and exclusion criteria.
In this study, the 2010 McDonald criteria (27) was required to be used for MS diagnosis, and a total of 525 MS patients were consecutively recruited and met the below inclusive criteria: (1) Chinese origin; (2) 14 to 65 years old; (3) two experienced neurologists in each center confirmed the diagnosis of MS independently from the collected CRF in reference to the original medical record when needed; (4) data on demography, CSF test, MRI examination, disease duration, numbers of relapse, annualized relapse rate (ARR), Expanded Disability Status Scale (EDSS), concomitant autoantibodies of or clinical diagnosis of other autoimmune diseases were available. 42 cases were excluded for the following reasons: 9 cases due to concurrent malignant tumors, 27 cases due to meeting the 2017 McDonald criteria but not the 2010 McDonald criteria, and 6 repeated-entry cases from different regional referring neurology centers. The remaining 483 patients were analyzed (Figure 1). The concomitant connective tissue disease (CTD) or autoimmune thyroid disease (AITD) was diagnosed according to relevant diagnostic criteria by consulting rheumatologists or endocrinologists. The number of attacks during the entire disease duration and the EDSS were recorded at the time of CSF collection.
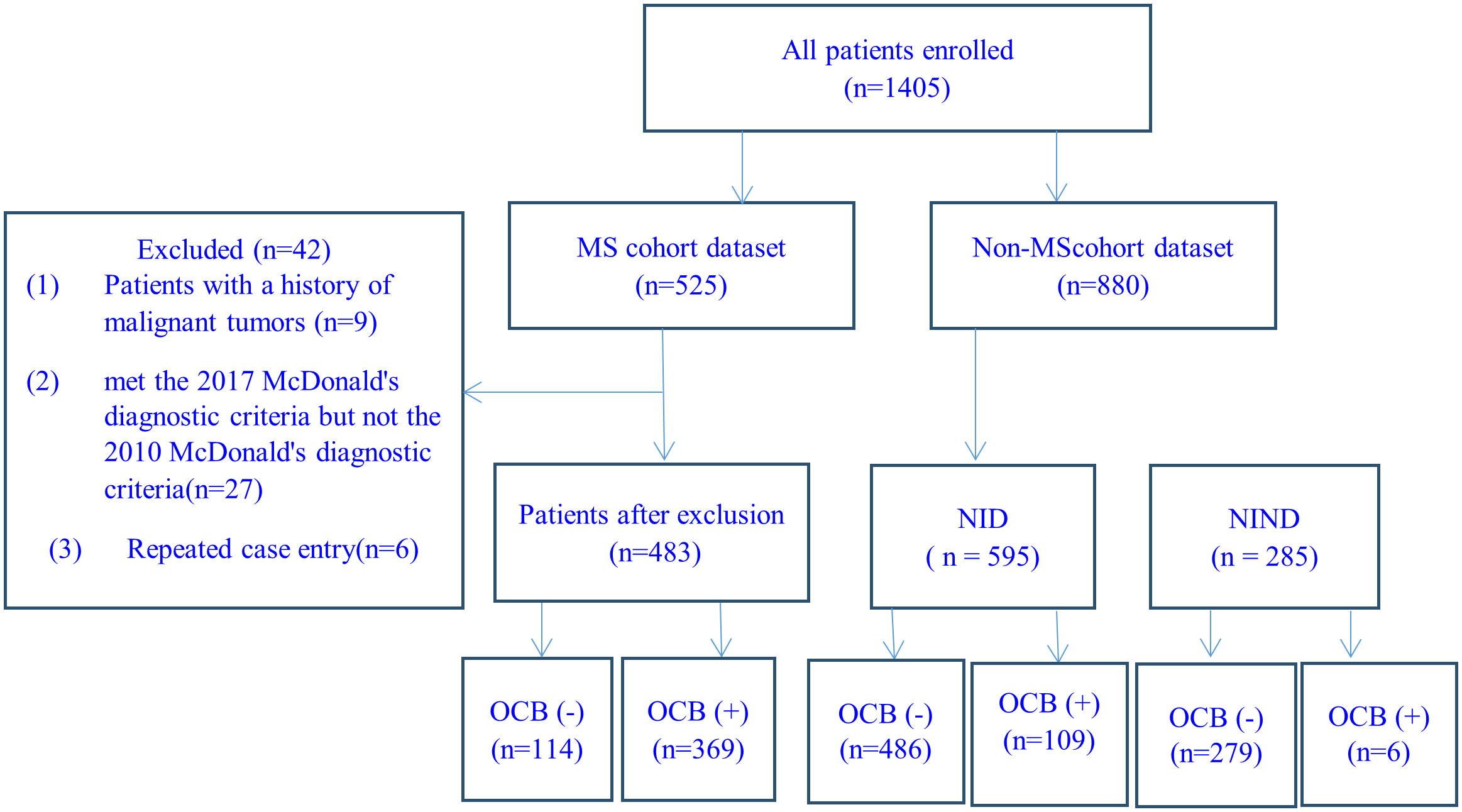
Figure 1 Case-screening flowchart. MS, multiple sclerosis; NID, neuro-inflammatory diseases; NIND: neurological non-inflammatory diseases.
CSF data of 880 consecutively recruited patients with the final diagnosis of other diseases in the 12 centers were collected during the same period, and used as the control group in the evaluation of the diagnostic performance of CSF-OCB in MS. All included patients were: (1) Chinese origin; (2) age of 14 to 65 years; (3) no MS history and two experienced neurologists excluded the diagnosis of MS when differential diagnosis presented; (4) data on demography and CSF-OCB were available. The 880 patients with non-MS diseases were divided into 2 categories: (1) neuro-inflammatory diseases (NID, n=595), including NMOSD (280 cases), MOGAD (45 cases), acute disseminated encephalomyelitis (ADEM) (15 cases), Guillain-Barré syndrome (127 cases) and autoimmune encephalitis (128 cases); (2)non-inflammatory neurological diseases (NIND, n=285), including primary headaches (56 cases), idiopathic epilepsy (20 cases), cerebrovascular diseases (79 cases), dementia (17 cases), motor neuron disease (23 cases), parkinsonism (6 cases), multiple system atrophy (6 cases), spinal vascular disease (7 cases), central nervous system involvement related to acute leukemia (10 cases), somatization disorder (7 cases), metabolic neuropathy (34 cases), hereditary neuropathy (14 cases), normal pressure hydrocephalus (4 cases) and peripheral vertigo (2 cases).
The concentrations of IgG and albumin in serum and CSF were determined by the turbidimetric scattering method. The BCB permeability was assessed using CSF/serum albumin quotient: QAlb = CSF-Alb [mg/l]/serum-Alb [g/l]. Increased BCB permeability is defined as QAlb>4+ (age/15). The IgG index, calculated as (CSF-IgG/serum-IgG)/(CSF-Alb/serum-Alb), is a measure of intrathecal IgG synthesis. An IgG index below 0.7 is considered normal. Tourtellotte IgG synthesis rate (IgG-SR), calculated as [(CSF IgG- serum IgG/369)- (CSF albumin- serum albumin/230) ×(serum IgG/serum albumin)]× 0.43 × 5, is an approach to determine intrathecal IgG synthesis with adjusting of BCB permeability. An IgG-SR below 3.3 mg/24 hours is considered normal (28, 29).
According to the Isoelectric Focusing Electrophoresis (IEF) SOP for the detection of CSF-OCB (26, 30), the detection was performed with Sebia HYDRASYS 2 Isofocusing system PN1211 (France) according to the manufacturer’s instructions for the evaluation of IgG OCB. Briefly, CSF and serum samples were run in parallel at a concentration of 10-20 mg/L. After electrophoresis, the gel was incubated with peroxidase-labeled anti-IgG antibodies, and the bands were displayed using TTF1/2 chromogenic agents. Two inspectors independently interpreted the electrophoresis results according to the key points of interpretation, including the presence, number, and patterns of bands in the serum and CSF.
The electrophoresis results were classified into 5 main OCB types. Type I: no bands in both serum and CSF; Type II: ≥ 2 bands in CSF and no band in serum; Type III: additional bands in CSF despite of bands in serum; Type IV: identical bands in both serum and CSF; Type V: twin bands with regular and periodic spacing in both serum and CSF. CSF-OCB positivity was defined as either type II or III bands (29).
Patient characters were presented as numbers (percentages), mean ± standard deviation (SD), or median (interquartile range, IQR). Pearson χ2 test and Fisher’s exact test, as well as Student’s T test or Mann-Whitney U test, were used for comparison between OCB-positive and OCB-negative groups.
The diagnostic performance of OCB positivity was evaluated as (15):
Accuracy: [(TP+TN)/(TP+TN+FP+FN)]
Sensitivity: [TP/(TP+FN)]
Specificity: [TN/(TN+FP)]
Positive Predictive Value (PPV): [TP/(TP+FP)]
Negative Predictive Value (NPV): [TN/(TN+FN)]
The likelihood ratio for positive test result (PLR): sensitivity/(1-specificity)
The likelihood ratio for negative test result (NLR): (1-sensitivity)/specificity
True positive (TP) was defined as MS according to McDonald 2010 criteria and positive OCB, true negative (TN) was defined as non-MS and negative OCB, false positive (FP) was defined as non-MS but positive OCB, and false negative (FN) was defined as MS but negative OCB.
A two-tailed p < 0.05 was considered statistically significant. All statistical analysis was performed using SPSS 26.0 software (IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp).
The general characteristics of patients with MS were shown in Table 1. The mean age of 483 MS patients was 33.63 ± 10.58 years old. Females accounted for 63.2% of overall patients (female/male=2:1). 369 (76.4%) MS patients were OCB-positive (344 with type II, and 25 with type III), and 114 (23.6%) MS patients were OCB-negative (112 with type I, and 2 with type IV) (Table 1).
880 non-MS patients included 595 NID patients and 285 NIND patients. The mean age was 42.10 ± 16.71 years in NID patients and 45.53 ± 18.21 years in NIND patients. The female/male ratio was 1.2:1 in NID patients and 0.8:1 in NIND patients. 109 NID patients (18.3%) were OCB-positive (102 with type II, and 7 with type III), and 6 NIND patients (2.1%) were OCB-positive (5 with type II, and 1 with type III).
The patients with MS had higher prevalence of CSF-OCB than those with non-MS (76.4% vs 13.1%, P<0.001). In comparison with the NIND, NID patients had more percentage of positive CSF-OCB(18.3% vs 2.1%, P<0.001), but had less percentage of positive CSF-OCB when compared with MS (18.3% vs 76.4%, P<0.001).
The diagnostic performance of CSF-OCB differentiating MS from non-MS patients yielded an accuracy of 83%, a sensitivity of 76%, a specificity of 87%, a PPV of 76%, a NPV of 87%, a PLR of 5.85, and a NLR of 0.27. The diagnostic performance in differentiating MS from NID patients showed 79%, 76%, 82%, 77%, 81%, 4.17, and 0.29, respectively. The diagnostic performance in differentiating MS from NIND patients showed 84%, 76%, 98%, 98%, 71%, 36.29, and 0.24, respectively (Table 2).
Moreover, considering that in most clinical cases, it is mainly CNS inflammatory demyelinating diseases (CIDD) that needs to be carefully distinguished from MS, we extracted data on the OCB status of the patients with CIDD (including NMOSD, MOGAD, ADEM) and compared it with MS patients, the diagnostic performance in differentiating MS from CIDD patients showed an accuracy of 77%, a sensitivity of 76%, a specificity of 79%, a PPV of 86%, a NPV of 66%, a PLR of 3.64, and a NLR of 0.30 (Table 2).
The included MS patients were from 25 provinces and 4 cities (Beijing, Shanghai, Guangzhou, and Chongqing) (Figure 2). Based on the characteristics of the geographical condition and human geography in China, they were divided into 7 regions, including Northeast (Heilongjiang, Jilin, and Liaoning), North (Beijing, Tianjin, Shanxi, Hebei, and Inner Mongolia), East (Shanghai, Jiangsu, Zhejiang, Anhui, Jiangxi, Shandong and Fujian), Central (Henan, Hunan, and Hubei), South (Guangdong and Guangxi), Southwest (Chongqing, Sichuan, Guizhou, and Yunnan), and Northwest (Shanxi, Gansu, Qinghai, Ningxia, and Xinjiang). The prevalence of CSF-OCB was around 60% in MS patients originating in the East and South regions and more than 80% in the other regions. The provinces and cities were also classified into low latitude (20°-30°N), middle latitude (31°-40°N), and high latitude (41°-50°N) regions (Figure 2). The prevalence of CSF-OCB in MS patients in these regions was 68.1% (81/119), 77.0% (217/282), and 86.6% (71/82), significantly higher in high-latitude regions (P=0.016) (Table 3). They were also classified into low-altitude (<500m) and high-altitude (>1000m) regions (Figure 2). The prevalence of CSF-OCB in MS patients at high altitudes (82.63%, 138/167) was significantly higher than those at low altitudes (73.1%, 231/316) (P=0.025) (Table 3).
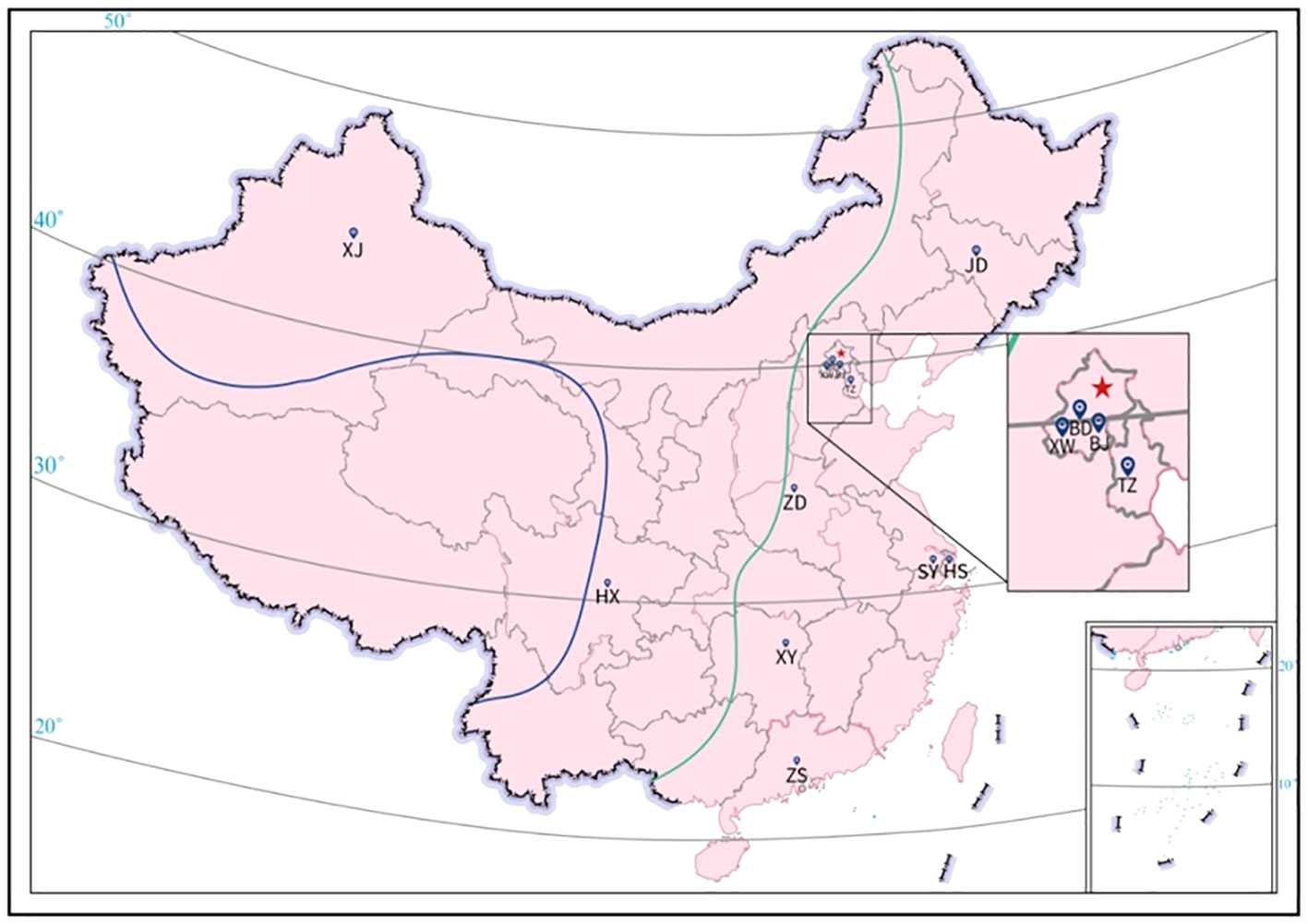
Figure 2 Geographical distribution of participating neurology centers. BD, Peking University First Hospital; BJ, Beijing Hospital; HS, Huashan Hospital affiliated to Fudan University; HX, West China Hospital of Sichuan University; JD, The First Hospital of Jilin University; SY, The First Affiliated Hospital of Soochow University; TZ; Tianjin Medical University General Hospital; XJ, People’s Hospital of Xinjiang Uygur Autonomous Region; XW, Xuanwu Hospital, Capital Medical University; XY, Xiangya Hospital of Central South University; ZD, The First Affiliated Hospital of Zhengzhou University; ZS, The Third Affiliated Hospital of Sun Yat-sen University. All patients were recruited from the 12 neurology centers in China and originated from 25 provinces and Beijing, Shanghai, Guangzhou, Chongqing (except Tibet, Hainan, Hong Kong, Macau, and Taiwan) spanning 20° and 50° North Latitude (20°-50°N). The altitude of China decreases from west to east and is commonly divided into 3 subregions: < 500m (east of green line), 1000-2000m (between green and blue line), and >4000m (southwest of blue line).
As shown in Table 1, there were no differences about age and gender between OCB positive and negative patients (P=0.129, P=0.184). Time from symptom onset to diagnosis was significantly shorter in OCB-positive patients than that in OCB-negative patients (13.2 months vs. 23.7 months, P=0.020). There were fewer patients with concomitant autoimmune diseases in the OCB-positive patients (4.3%, 16/369) than in the OCB-negative patients (13.2%, 15/114) (P=0.001). No significant differences were observed among OCB status and disease duration, the number of attacks, ARR and EDSS (P=0.499, P=0.824, P=0.907, P=0.382) (Table 1).
The attacks of MS affect various parts of the CNS, in this study T2 weighted MRI lesions in different locations of CNS were compared between OCB-positive and negative patients. More OCB-positive patients have periventricular lesions than OCB-negative patients (93.6% vs 86.5%, P= 0.017) (Table 4).
The mean level of CSF protein was lower in OCB-positive patients than that in OCB-negative patients (340.00 mg/L vs 370.00 mg/L, P<0.001). Less OCB-positive MS patients had elevated QAlb in comparison with OCB-negative MS patients (16.0%, 37/231 vs 29.3%, 22/75, P=0.011). The proportions of MS patients with increased IgG index and abnormal IgG-SR were greater in OCB-positive patients than those in OCB-negative patients (69.8%, 215/308 vs 33.7%, 32/95, P<0.001; 69.3%, 212/306 vs 38.5%, 37/96, P<0.001, respectively).
In our study, the enrolled MS patients originated from 25 provinces and 4 cities, covering most regions in mainland of China, which depicted a representative nationwide portrait of Chinese MS patients, and provided insight into the clinical and diagnostic value of CSF-OCB in Chinese MS patients. As we know, this is the first report about nationwide data on CSF-OCB positivity and its diagnostic performance in Chinese MS patients, especially in the virtue of common SOP.
We adopted the 2010 McDonald Criteria because it did not need the information of CSF-OCB, and had fairly good sensitivity for diagnosing MS in CIS patients (27). Moreover, the prevalence of CSF-OCB is easily compared with earlier researchers. Importantly, this would avoid bias from the incorporation of OCB in diagnostic studies because the 2017 McDonald criteria and other diagnostic criteria all incorporate OCB as supporting conditions (15).
OCB suggests intrathecally synthesized immunoglobulin in response to B cell activation as a result of CNS inflammation (12). It is not only detected in MS but also in other neurological disease (19). Several previous reports had shown that CSF-OCB had a high specificity for diagnosing MS. In these articles, MS was often compared to healthy individuals or patients with non-neurological inflammatory diseases (12, 30–32). However, A meta-analysis had shown that when patients with neurological inflammatory diseases were used as the control group, the specificity of CSF-OCB for diagnosing MS would reduce from 94% to 61% (32). These suggested that the differences in the control group used in research could greatly affect the evaluation of the specificity of CSF-OCB in diagnosing MS, and was one of the reasons for the significant differences about specificity reported in various studies. In clinical practice, the differential diagnosis between MS and neurological inflammatory diseases is crucial. In this study, 18.3% of patients in the NID were CSF-OCB positive, which is significantly higher than those in the NIND. Therefore, we included NID and NIND patients as the controls, and found that when NIND was used as the control, the specificity of CSF-OCB for diagnosing MS could reach 98%, while when NID was used as the control, the specificity dropped to 77%.
In clinical practice, it is crucial for MS to be distinguished from CIDD. The significant difference in OCB prevalence between MS and CIDD (76.4% vs 21.0%) shown in this study (diagnostic spectivity 79%) suggests that negative CSF-OCB could be a “red flag” for the diagnosis of MS. When some patients with central demyelinating disease have clinical and/or imaging manifestations that are very similar to MS, and may even be diagnosed as MS according to MS diagnostic criteria at a certain time point, their negative OCB results need to be constantly reminded to doctors during long-term follow-up to pay attention to whether changes in the patient’s clinical and imaging manifestations that were inconsistent with MS, so as to timely revise the diagnosis. Moreover, the time from the onset of disease to clinical diagnosis in our cohort was almost one-year earlier in OCB-positive patients than that in OCB-negative patients, supporting that OCB detection also facilitates early MS diagnosis for Chinese patients with MS.
In previous studies, Zheng et al. reported that the OCB positive rate was 62.5% in Zhejiang, a province of East China (17). Lu et al. have revealed that the OCB positive rate was 59.8% in Guangdong Province and Hong Kong SAR, two regions belonging to South China (20). In the survey of MS patients in north China, GU et al. and Chen et al. reported that the positive rates of OCB were 69.3% and 72.7% respectively (33, 34). According to China’s seven commonly used geographical divisions, China’s territory can be divided into North, South, Central, East, Northeast, Northwest, and Southwest. These 7 regions have obvious differences in topography, climate, vegetation types, production modes, habits, customs, and culture. In this study, the overall OCB positivity was 76.4% in Chinese patients with MS, and was about 60% in East and South China which was consistent with the results reported by Zheng et al. and Lu et al (17, 20), and was about 90% in North China, slightly higher than the results reported by GU et al. and Chen et al (33, 34). Thus, the inconsistencies of the previous reports were well explained in the analysis considering the geographical distribution difference of MS patients as the dominant factor.
Our results also showed that the geographical distribution of high OCB prevalence demonstrated a high-latitude feature. Lechner-Scott et al. have prospectively collected and analyzed CSF-OCB data from MSBase, a large international, multi-center database, and found that the frequency of OCB increased with latitude (35). In Lechner-Scott’s paper, the OCB positive rate was 85~100% in the regions located North of 40°N, which is similar to the latitudes in our study. The OCB positive rate was 50~90% in the 30°~40°N regions, and again, this is similar to the latitudes in this study. As to the regions in 20°~30°N, the OCB positive was 35% and 59%, which is lower than the same latitude in our investigation. In another report on the positive OCB and/or increased IgG index and latitude from Japan (36), CSF-OCB prevalence of MS in the northern region(42°-45°N) was higher than that in the southern region (33°-35°N), the average positive rate of the OCB and/or increased IgG index was 58.7%, and was similar to that reported in this study in the eastern coastal region of China (61.5%), which geographical characteristics are similar to those of Japan on the whole. Latitudinally the OCB prevalence in China was comparable to Europe and the United States (18, 35, 37), especially in high-latitudinal regions.
As to the altitude, only a few papers have revealed the relationship between MS morbidity and altitude (38, 39), but no publications had reported the relationship between CSF-OCB positivity and altitude. As shown in Table 3, we noticed that the OCB prevalence of patients observed in this study showed a trend related to altitude, that was, the OCB prevalence of patients in high-altitude areas was higher than that in low-altitude areas. As we know, this is the first report about the relationship between CSF-OCB prevalence and altitude.
People living in high-latitude and high-altitude areas of China, as a whole, tend to have a diet dominated by rice and pasta, with less consumption of vegetables, fruits, and fish, and have lower levels of vitamin D (40, 41). They also have specific human leukocyte antigen (HLA) enabling them to better adapt to high-altitude and high-latitude environments (42, 43).It has been confirmed that the risk of MS was associated with various geographic, environmental, ethnic, and lifestyle factors, especially genetic susceptibility(HLA), diet customs and vitamin D levels, which might promote the occurrence of MS by the influence on the status or function of immune cells, and logically the state of OCB produced by plasma cells in CNS (36, 44–48). However, the relationship between these factors and the productivity of OCB in China still lacks strong validation, and further research is needed to verify.
There were many studies on the correlation between OCB positivity and long-term MS activity and prognosis, but the results were controversial (49). Some studies reported that OCB positivity correlated with disease activity, such as ARR, and EDSS (50, 51), but in some other studies, the opposite conclusion was reported (20, 52). In this study, no significant differences were observed among OCB status and EDSS, ARR, disease course, and the number of relapses. Because this study was only a cross-sectional observation study, the correlation between OCB positivity and long-term MS activity and prognosis in Chinese people needs further study.
Both MRI and CSF-OCB are key indicators in the diagnostic criteria of MS (15). In this study, the lesion locations of MS were summarized as optical nerve, cortical/juxtacortical, periventricular, brain stem, cerebellum, cervical spinal cord, thoracic spinal cord, and lumbar spinal cord. Zhao et al. reported that in comparison with the OCB-negative MS patients, the OCB-positive group had a higher proportion of cerebellar lesions (53); Huttner et al. Suggested that MRI lesions and OCB status were independent of each other (54). However, we found that patients with positive OCB had more periventricular lesions than patients with negative OCB, while there was no significant difference in the proportion of patients with other lesions.
The BCB is an important physiological natural barrier of the CNS and peripheral environment, which can prevent the entry of various proteins and chemicals, including immunoglobulin. The presence of OCB in CSF indicates the existence of chronic persistent inflammation in CNS, suggesting the production of immunoglobulin in CNS, rather than that caused by the peripheral immunoglobulin entering CNS (55). In this study, BCB disruption was only observed in a small proportion (~20%) of MS patients in which most of the patients were OCB negative, and was negatively correlated to OCB positivity, as with previous studies (12, 56, 57).
IgG index and IgG-SR are important parameters of CSF analysis about CNS immune response. Like OCB, they are also designed to evaluate the presence of immunoglobulin synthesis and inflammatory processes in CNS and are widely used in the clinical diagnosis of MS (10, 29). Several studies have reported that an IgG index>0.7 was closely correlated with OCB positivity and was a prognostic marker of early disease activity (58, 59).In agreement with these reports, this study also showed that the proportions of MS patients with increased IgG index and abnormal IgG-SR were greater in OCB-positive patients than those in OCB-negative patients (28, 58–60).
The strength of this study is based on the following: 1. The sample size is large and the SOP adopted in CSF-OCB testing was previously validated. 2. The twelve regional referring MS centers cover the difference in latitude and altitude and represent the typical culture and convention in China. 3. Accurate diagnosis of MS and non-MS demyelinating diseases was guaranteed with the same CRF and we aimed to evaluate the diagnostic performance of CSF-OCB in MS patients, the possibility of misclassification of MS and non-MS demyelinating diseases was tiny based on the consensus from regional and nationwide experts on doubtful patients. 4. Using the 2010 Mcdonald criteria, which is based only with clinical and radiological data, avoiding the inclusion bias by using MS patients diagnosed with criteria that adopt the role of CSF-OCB.
There were some limitations in this study: 1. Only cross-sectional EDSS was collected, and the relapse risk was analyzed with retrospective data. 2. Long-term changes in CSF-OCB status in MS patients and non-MS demyelinating disease patients were not addressed. 3. The association between CSF-OCB status and some radiological features of MS could not be analyzed due to the cross-sectional design and only some retrospective data were acquired by reviewing the case records. Nevertheless, these are not the main focus of this study. We are collecting relevant data in a prospective cohort among all participating centers.
In conclusion, this study reported, for the first time, that the nationwide prevalence of CSF-OCB was 76.4% and conducive to early diagnosis in Chinese patients with MS, and demonstrated a good performance in differentiating MS from other CNS diseases, suggesting that CSF-OCB is a valuable clinical indicator for the diagnosis of MS in Chinese patients. In addition, the CSF-OCB prevalence showed a correlation with high latitude and altitude, reflecting the characteristics of regional distribution of OCB prevalence in Chinese patients with MS.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the Ethics Committee of Shanghai Huashan Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
XZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. HH: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. TJ: Data curation, Investigation, Methodology, Writing – review & editing. WQ: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. HuY: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. QX: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. JYi: Conceptualization, Data curation, Investigation, Writing – review & editing. ZS: Data curation, Investigation, Methodology, Writing – review & editing. HaY: Conceptualization, Data curation, Investigation, Writing – review & editing. XJ: Data curation, Investigation, Writing – review & editing. XS: Data curation, Investigation, Methodology, Writing – review & editing. QZ: Data curation, Investigation, Methodology, Writing – review & editing. XL: Data curation, Investigation, Methodology, Writing – original draft. JW: Writing – review & editing, Data curation, Investigation, Methodology. HuL: Data curation, Investigation, Software, Writing – review & editing. XH: Data curation, Investigation, Software, Writing – review & editing. JYa: Data curation, Investigation, Software, Writing – review & editing. YL: Data curation, Methodology, Writing – review & editing. SL: Data curation, Investigation, Methodology, Software, Writing – review & editing. AL: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. FG: Conceptualization, Data curation, Investigation, Writing – review & editing. SH: Data curation, Methodology, Software, Writing – review & editing. SC: Conceptualization, Investigation, Methodology, Writing – review & editing. DD: Conceptualization, Investigation, Methodology, Software, Writing – review & editing. HZ: Conceptualization, Data curation, Investigation, Supervision, Visualization, Writing – review & editing. HaL: Conceptualization, Data curation, Investigation, Supervision, Visualization, Writing – review & editing. XC: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Clinical Research Plan of SHDC (No. SHDC2020CR2027B), 2020 Medical Service and Support Capacity Improvement Project: Construction of the Cohort-Based Multidisciplinary Accurate Diagnosis and Treatment Platform for Neurological Autoimmune and Infectious Diseases, and Shanghai Municipal Science and Technology Major Project (No. 2017SHZDZX01) and State Key Laboratory of Genetic Engineering, Human Phenome Institute, Zhangjiang Fudan International Innovation Center, Fudan University.
Special appreciation for recently deceased professor Xianhao Xu who is the key advocate for our “CNS-OCB” project. Thank professors Chuanzhen Lu and Xueqiang Hu for their support for the project. In addition, appreciation is extended to the “CNS-OCB” consortium member: Yan Xu (Department of Neurology, Peking Union Medical College, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China.); Bitao Bu (Department of Neurology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.); Qi Cheng (Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai,China.); Huifang Huang (Department of Neurology, The First Affiliated Hospital of Soochow University, Suzhou, China.); Lanjun Li (Department of Neurology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, China.); Zhiguo Li (Department of Neurology, Tianjin Neurological Institute, Tianjin Medical University General Hospital, Tianjin, China.); Caiyun Liu (Department of Neurology and Neuroscience Center, The First Hospital of Jilin University, Changchun, China.); Tingting Lu (Department of Neurology, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China); Hong Wang (Department of Neurology, Beijing Hospital, Beijing, China.); Thank Zhang Yu from Biogen Medical Affairs (China) for the manuscript editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Perdaens O, van Pesch V. Molecular mechanisms of immunosenescene and inflammaging: relevance to the immunopathogenesis and treatment of multiple sclerosis. Front Neurol (2021) 12:811518. doi: 10.3389/fneur.2021.811518
2. Haralur Y, Mechtler LL. Neuroimaging of multiple sclerosis mimics. Neurol Clin (2020) 38(1):149–70. doi: 10.1016/j.ncl.2019.09.002
3. Wildner P, Stasiolek M, Matysiak M. Differential diagnosis of multiple sclerosis and other inflammatory CNS diseases. Mult Scler Relat Disord (2020) 37:101452. doi: 10.1016/j.msard.2019.101452
4. Kaisey M, Lashgari G, Fert-Bober J, Ontaneda D, Solomon AJ, Sicotte NL. An update on diagnostic laboratory biomarkers for multiple sclerosis. Curr Neurol Neurosci Rep (2022) 22(10):675–88. doi: 10.1007/s11910-022-01227-1
5. Saadeh RS, Ramos PA, Algeciras-Schimnich A, Flanagan EP, Pittock SJ, Willrich MA. An update on laboratory-based diagnostic biomarkers for multiple sclerosis and beyond. Clin Chem (2022) 68(9):1134–50. doi: 10.1093/clinchem/hvac061
6. Antel J, Bar-Or A. Roles of immunoglobulins and B cells in multiple sclerosis: from pathogenesis to treatment. J Neuroimmunol (2006) 180(1-2):3–8. doi: 10.1016/j.jneuroim.2006.06.032
7. Wootla B, Denic A, Keegan BM, Winters JL, Astapenko D, Warrington AE, et al. Evidence for the role of B cells and immunoglobulins in the pathogenesis of multiple sclerosis. Neurol Res Int (2011) 2011:780712. doi: 10.1155/2011/780712
8. Holmoy T. The discovery of oligoclonal bands: a 50-year anniversary. Eur Neurol (2009) 62(5):311–5. doi: 10.1159/000235944
9. Gastaldi M, Zardini and D. Franciotta E. An update on the use of cerebrospinal fluid analysis as a diagnostic tool in multiple sclerosis. Expert Rev Mol Diagn (2017) 17(1):31–46. doi: 10.1080/14737159.2017.1262260
10. Lo Sasso B, Agnello L, Bivona G, Bellia C, Ciaccio M. Cerebrospinal fluid analysis in multiple sclerosis diagnosis: an update. Medicina (Kaunas) (2019) 55(6):245. doi: 10.3390/medicina55060245
11. Cabrera CM. Oligoclonal bands: An immunological and clinical approach. Adv Clin Chem (2022) 109:129–63. doi: 10.1016/bs.acc.2022.03.004
12. Link H, Huang YM. Oligoclonal bands in multiple sclerosis cerebrospinal fluid: an update on methodology and clinical usefulness. J Neuroimmunol (2006) 180(1-2):17–28. doi: 10.1016/j.jneuroim.2006.07.006
13. Arrambide G, Tintore M, Espejo C, Auger C, Castillo M, Río J, et al. The value of oligoclonal bands in the multiple sclerosis diagnostic criteria. Brain (2018) 141(4):1075–84. doi: 10.1093/brain/awy006
14. Alvarez-Cermeno JC, Villar LM. Multiple sclerosis: Oligoclonal bands–a useful tool to avoid MS misdiagnosis. Nat Rev Neurol (2013) 9(6):303–4. doi: 10.1038/nrneurol.2013.74
15. Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol (2018) 17(2):162–73. doi: 10.1016/S1474-4422(17)30470-2
16. van der Vuurst de Vries RM, Mescheriakova JY, Wong YYM, Runia TF, Jafari N, Samijn JP. Application of the 2017 revised McDonald criteria for multiple sclerosis to patients with a typical clinically isolated syndrome. JAMA Neurol (2018) 75(11):1392–8. doi: 10.1001/jamaneurol.2018.2160
17. Zheng Y, Shen CH, Wang S, Yang F, Cai MT, Fang W, et al. Application of the 2017 McDonald criteria in a Chinese population with clinically isolated syndrome. Ther Adv Neurol Disord (2020) 13:1756286419898083. doi: 10.1177/1756286419898083
18. Dobson R, Ramagopalan S, Davis A, Giovannoni G. Cerebrospinal fluid oligoclonal bands in multiple sclerosis and clinically isolated syndromes: a meta-analysis of prevalence, prognosis and effect of latitude. J Neurol Neurosurg Psychiatry (2013) 84(8):909–14. doi: 10.1136/jnnp-2012-304695
19. Carta S, Ferraro D, Ferrari S, Briani C, Mariotto S. Oligoclonal bands: clinical utility and interpretation cues. Crit Rev Clin Lab Sci (2022) 59(6):391–404. doi: 10.1080/10408363.2022.2039591
20. Lu T, Zhao L, Sun X, Au C, Huang Y, Yang Y. Comparison of multiple sclerosis patients with and without oligoclonal IgG bands in South China. J Clin Neurosci (2019) 66:51–5. doi: 10.1016/j.jocn.2019.05.025
21. Li B, Dong H, Zhang J, Song X, Guo L. Cerebrospinal fluid IgG profiles and oligoclonal bands in Chinese patients with multiple sclerosis. Acta Neurol Scand (2007) 115(5):319–24. doi: 10.1111/j.1600-0404.2006.00753.x
22. Ming F, Jian Q. Clinical significance of CSF-restricted OCB in neurological diseases. Chin J Clin Neurosci (2004) 12(4):365–8. doi: 10.3969/j.issn.1008-0678.2004.04.009
23. Yan W, Hongjun H, Feng G. Application of CSF oligoclonal bands in multiple sclerosis and neuromyelitis optica. Chin J Pract Nervous Dis (2013) 16(2):29–30. doi: 10.1097/WCO.0000000000000699
24. Lu Y, Gao Q, Ren X, Li J, Yang D, Zhang Z, et al. Incidence and prevalence of 121 rare diseases in China: Current status and challenges: 2022 revision. Intractable Rare Dis Res (2022) 11(3):96–104. doi: 10.5582/irdr.2022.01093
25. Jia D, Zhang Y, Yang C. The incidence and prevalence, diagnosis, and treatment of multiple sclerosis in China: a narrative review. Neurol Sci (2022) 43(8):4695–700. doi: 10.1007/s10072-022-06126-4
26. Liu XN, Chen XJ, Sun XB, Qiu W, Peng LS, Li HF, et al. [Establishment and consistency verification of the standard operation procedure for laboratory detection of immunoglobulin G oligoclonal bands in cerebrospinal fluid]. Zhonghua Yi Xue Za Zhi (2021) 101(31):2465–70. doi: 10.3760/cma.j.cn112137-20201127-03210
27. Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol (2011) 69(2):292–302. doi: 10.1002/ana.22366
28. Caroscio JT, Kochwa S, Sacks H, Cohen JA, Yahr MD. Quantitative CSF IgG measurements in multiple sclerosis and other neurologic diseases. update Arch Neurol (1983) 40(7):409–13. doi: 10.1001/archneur.1983.04050070039007
29. Gastaldi M, Zardini E, Leante R, Ruggieri M, Costa G, Cocco E, et al. Cerebrospinal fluid analysis and the determination of oligoclonal bands. Neurol Sci (2017) 38(Suppl 2):217–24. doi: 10.1007/s10072-017-3034-2
30. Freedman MS, Thompson EJ, Deisenhammer F, Giovannoni G, Grimsley G, Keir G, et al. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: a consensus statement. Arch Neurol (2005) 62(6):865–70. doi: 10.1001/archneur.62.6.865
31. Villar LM, Masjuan J, Sádaba MC, González-Porqué P, Plaza J, Bootello A, et al. Early differential diagnosis of multiple sclerosis using a new oligoclonal band test. Arch Neurol (2005) 62(4):574–7. doi: 10.1001/archneur.62.4.574
32. Petzold A. Intrathecal oligoclonal IgG synthesis in multiple sclerosis. J Neuroimmunol (2013) 262(1-2):1–10. doi: 10.1016/j.jneuroim.2013.06.014
33. Kelin C, Xue Z, Guoge L, Xixiong K, Guojun Z. Clinical significance of CSF-OCB and intrathecal IgG synthesis related indicators in multiple sclerosis. Int J Lab Med (2018) 39(23):2873–2875,2888. doi: 10.3969/j.issn.1673-4130.2018.23.007
34. Yuyu GU, Zhou J, Wang D, Liu J, Wang L, Chen K, et al. An analysis of 4514 electrophoresis samples of cerebrospinal fluid oligoclonal bands in a hospital. Labeled Immunoassays Clin Med (2021) 28(10):1681–8. doi: 10.11748/bjmy.issn.1006-1703.2021.10.012
35. Lechner-Scott J, Spencer B, de Malmanche T, Attia J, Fitzgerald M, Trojano M, et al. The frequency of CSF oligoclonal banding in multiple sclerosis increases with latitude. Mult Scler (2012) 18(7):974–82. doi: 10.1177/1352458511431729
36. Nakamura Y, Matsushita T, Sato S, Niino M, Fukazawa T, Yoshimura S, et al. Latitude and HLA-DRB1*04:05 independently influence disease severity in Japanese multiple sclerosis: a cross-sectional study. J Neuroinflamm (2016) 13(1):239. doi: 10.1186/s12974-016-0695-3
37. Peña-Sánchez M, Lestayo Lestayo O Z, Farril L, Valido Luna L, Betancourt Loza M, González-García S, Hernández-Díaz ZM, et al. CSF oligoclonal band frequency in a Cuban cohort of patients with multiple sclerosis. comparison with Latin American countries and association with latitude. Mult Scler Relat Disord (2020) 45:102412. doi: 10.1016/j.msard.2020.102412
38. Tian DC, Zhang C, Yuan M, Yang X, Gu H, Li Z, et al. Incidence of multiple sclerosis in China: A nationwide hospital-based study. Lancet Reg Health West Pac (2020) 1:100010. doi: 10.1016/j.lanwpc.2020.100010
39. Lauer K. Environmental associations with the risk of multiple sclerosis: the contribution of ecological studies. Acta Neurol Scand Suppl (1995) 161(Suppl 161):77–88. doi: 10.1111/j.1600-0404.1995.tb05861.x
40. Li L, Li K, Li J, Luo Y, Cheng Y, Jian M, et al. Ethnic, geographic, and seasonal differences of vitamin D status among adults in south-west China. J Clin Lab Anal (2020) 34(12):e23532. doi: 10.1002/jcla.23532
41. Mata-Greenwood E, Westenburg HCA, Zamudio S, Illsley NP, Zhang L. Decreased vitamin D levels and altered placental vitamin D gene expression at high altitude: role of genetic ancestry. Int J Mol Sci (2023) 24(4). doi: 10.3390/ijms24043389
42. Wang B, Zhang YB, Zhang F, Lin H, Wang X, Wan N, et al. On the origin of Tibetans and their genetic basis in adapting high-altitude environments. PloS One (2011) 6(2):e17002. doi: 10.1371/journal.pone.0017002
43. Zhang YB, Li X, Zhang F, Wang DM, Yu J. A preliminary study of copy number variation in Tibetans. PloS One (2012) 7(7):e41768. doi: 10.1371/journal.pone.0041768
44. Guglielmetti M, Al-Qahtani WH, Ferraris C, Grosso G, Fiorini S, Tavazzi E, et al. Adherence to mediterranean diet is associated with multiple sclerosis severity. Nutrients (2023) 15(18). doi: 10.3390/nu15184009
45. Lehman PC, Ghimire S, Price JD, Ramer-Tait AE, Mangalam AK. Diet-microbiome-immune interplay in multiple sclerosis: Understanding the impact of phytoestrogen metabolizing gut bacteria. Eur J Immunol (2023) 53(11):e2250236. doi: 10.1002/eji.202250236
46. Jacobs BM, Tank P, Bestwick JP, Noyce AJ, Marshall CR, Mathur R, et al. Modifiable risk factors for multiple sclerosis have consistent directions of effect across diverse ethnic backgrounds: a nested case-control study in an English population-based cohort. J Neurol (2023). doi: 10.1007/s00415-023-11971-0
47. Amato MP, Derfuss T, Hemmer B, Liblau R, Montalban X, Soelberg Sørensen P, et al. Environmental modifiable risk factors for multiple sclerosis: Report from the 2016 ECTRIMS focused workshop. Mult Scler (2018) 24(5):590–603. doi: 10.1177/1352458516686847
48. Olsson T, Barcellos LF, Alfredsson L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat Rev Neurol (2017) 13(1):25–36. doi: 10.1038/nrneurol.2016.187
49. Magliozzi R, Cross AH. Can CSF biomarkers predict future MS disease activity and severity? Mult Scler (2020) 26(5):582–90. doi: 10.1177/1352458519871818
50. Ben Noon G, Vigiser I, Shiner T, Kolb H, Karni A, Regev K. Reinforcing the evidence of oligoclonal bands as a prognostic factor in patients with Multiple sclerosis. Mult Scler Relat Disord (2021) 56:103220. doi: 10.1016/j.msard.2021.103220
51. Rojas JI, Tizio S, Patrucco L, Cristiano E. Oligoclonal bands in multiple sclerosis patients: worse prognosis? Neurol Res (2012) 34(9):889–92. doi: 10.1179/1743132812Y.0000000088
52. Frau J, Villar LM, Sardu C, Secci MA, Schirru L, Ferraro D, et al. Intrathecal oligoclonal bands synthesis in multiple sclerosis: is it always a prognostic factor? J Neurol (2018) 265(2):424–30. doi: 10.1007/s00415-017-8716-4
53. Zhao L, Abrigo J, Chen Q, Au C, Ng A, Fan P, et al. Advanced MRI features in relapsing multiple sclerosis patients with and without CSF oligoclonal IgG bands. Sci Rep (2020) 10(1):13703. doi: 10.1038/s41598-020-70693-9
54. Huttner HB, Schellinger PD, Struffert T, Richter G, Engelhorn T, Bassemir T, et al. MRI criteria in MS patients with negative and positive oligoclonal bands: equal fulfillment of Barkhof's criteria but different lesion patterns. J Neurol (2009) 256(7):1121–5. doi: 10.1007/s00415-009-5081-y
55. Meinl E, Krumbholz M, Derfuss T, Junker A, Hohlfeld R. Compartmentalization of inflammation in the CNS: a major mechanism driving progressive multiple sclerosis. J Neurol Sci (2008) 274(1-2):42–4. doi: 10.1016/j.jns.2008.06.032
56. Akaishi T, Takahashi T, Nakashima I. Oligoclonal bands and periventricular lesions in multiple sclerosis will not increase blood-brain barrier permeability. J Neurol Sci (2018) 387:129–33. doi: 10.1016/j.jns.2018.02.020
57. Deisenhammer F, Zetterberg H, Fitzner B, Zettl UK. The cerebrospinal fluid in multiple sclerosis. Front Immunol (2019) 10:726. doi: 10.3389/fimmu.2019.00726
58. Zheng Y, Cai MT, Yang F, Zhou JP, Fang W, Shen CH, et al. IgG index revisited: diagnostic utility and prognostic value in multiple sclerosis. Front Immunol (2020) 11:1799. doi: 10.3389/fimmu.2020.01799
59. Simonsen CS, Flemmen HØ, Lauritzen T, Berg-Hansen P, Moen SM, Celius EG. The diagnostic value of IgG index versus oligoclonal bands in cerebrospinal fluid of patients with multiple sclerosis. Mult Scler J Exp Transl Clin (2020) 6(1):2055217319901291. doi: 10.1177/2055217319901291
60. Belimezi M, Kalliaropoulos A, Mentis AA, Chrousos GP. Diagnostic significance of IgG and albumin indices versus oligoclonal band types in demyelinating disorders. J Clin Pathol (2021) 76(3):166–71. doi: 10.1136/jclinpath-2021-207766
Keywords: oligoclonal bands, cerebrospinal fluid, multiple sclerosis, prevalence, diagnostic performance, China
Citation: Zhang X, Hao H, Jin T, Qiu W, Yang H, Xue Q, Yin J, Shi Z, Yu H, Ji X, Sun X, Zeng Q, Liu X, Wang J, Li H, He X, Yang J, Li Y, Liu S, Lau AY, Gao F, Hu S, Chu S, Ding D, Zhou H, Li H and Chen X (2023) Cerebrospinal fluid oligoclonal bands in Chinese patients with multiple sclerosis: the prevalence and its association with clinical features. Front. Immunol. 14:1280020. doi: 10.3389/fimmu.2023.1280020
Received: 19 August 2023; Accepted: 27 October 2023;
Published: 16 November 2023.
Edited by:
Shougang Guo, Shandong Provincial Hospital, ChinaReviewed by:
Faiez Al Nimer, Karolinska Institutet (KI), SwedenCopyright © 2023 Zhang, Hao, Jin, Qiu, Yang, Xue, Yin, Shi, Yu, Ji, Sun, Zeng, Liu, Wang, Li, He, Yang, Li, Liu, Lau, Gao, Hu, Chu, Ding, Zhou, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangjun Chen, eGlhbmdqY2hlbkBmdWRhbi5lZHUuY24=; Haifeng Li, ZHJsaGZAMTYzLmNvbQ==; Hongyu Zhou, OTI0MzM5ODM2QHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.