- 1Discipline of Clinical Pharmacology, College of Medicine and Public Health, Flinders University, Adelaide, SA, Australia
- 2Department of Clinical Pharmacology, Flinders Medical Centre, Southern Adelaide Local Health Network, Adelaide, SA, Australia
- 3Department of Biomedical Sciences, University of Sassari, Sassari, Italy
Introduction: Novel biomarkers of inflammation and oxidative stress might enhance the early recognition, management, and clinical outcomes of patients with rheumatic diseases (RDs). We assessed the available evidence regarding the pathophysiological role of neopterin, the oxidation product of 7,8-dihydroneopterin, a pteridine generated in macrophages activated by interferon-γ, by conducting a systematic review and meta-analysis of studies reporting its concentrations in biological fluids in RD patients and healthy controls.
Methods: We searched electronic databases for relevant articles published between inception and 31 August 2023. The risk of bias and the certainty of evidence were assessed using the Joanna Briggs Institute Critical Appraisal Checklist and the Grades of Recommendation, Assessment, Development and Evaluation Working Group system, respectively.
Results: In 37 studies, when compared to healthy controls, RD patients had significantly higher concentrations of neopterin both in plasma or serum (standard mean difference, SMD=1.31, 95% CI 1.01 to 1.61; p<0.001; moderate certainty of evidence) and in the urine (SMD=1.65, 95% CI 0.86 to 2.43, p<0.001; I2 = 94.2%, p<0.001; low certainty of evidence). The results were stable in sensitivity analysis. There were non-significant associations in meta-regression and subgroup analysis between the effect size and age, male to female ratio, year of publication, sample size, RD duration, C-reactive protein, erythrocyte sedimentation rate, specific type of RD, presence of connective tissue disease, analytical method used, or biological matrix investigated (plasma vs. serum). By contrast, the effect size was significantly associated with the geographical area in studies assessing serum or plasma and with the type of RD in studies assessing urine.
Discussion: Pending additional studies that also focus on early forms of disease, our systematic review and meta-analysis supports the proposition that neopterin, a biomarker of inflammation and oxidative stress, can be useful for the identification of RDs. (PROSPERO registration number: CRD42023450209).
Systematic review registration: PROSPERO, identifier CRD42023450209
Introduction
Rheumatic diseases (RDs) is an umbrella term that includes a wide number of chronic, disabling conditions characterized by inflammation and oxidative stress affecting the musculoskeletal system and other organ and tissues. Broadly speaking, RDs can have a predominantly autoimmune (e.g., progressive systemic sclerosis, pSS, rheumatoid arthritis, RA, systemic lupus erythematosus, SLE, Sjogren’s syndrome, SSj, systemic sclerosis, SSc, and idiopathic inflammatory myositis, IIM), mixed-autoimmune-autoinflammatory (e.g., ankylosing spondylitis, AS, axial spondylarthritis, axSpA, psoriatic arthritis, PsA, and Behcet’s disease, BD), or autoinflammatory component (e.g., familial Mediterranean fever, FMF) (1–3). The availability of a wide range of anti-inflammatory and immunomodulatory medications has revolutionised the management of clinically overt RDs over the last 20-30 years, with significant improvements in symptom control and quality of life of affected patients (4–7) (8–10). However, despite these advances, significant challenges remain with the identification of early forms of RD. This issue, in turn, prevents the implementation of strategies for the rapid control of dysregulated immune and inflammatory pathways and, potentially, the achievement of more favourable long-term clinical outcomes (11–16). Therefore, a significant body of research has been conducted to identify novel biomarkers of RDs which could better assist physicians to make an early diagnosis, in addition to clinical assessment and conventional biomarkers of inflammation, e.g., C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) (17–25).
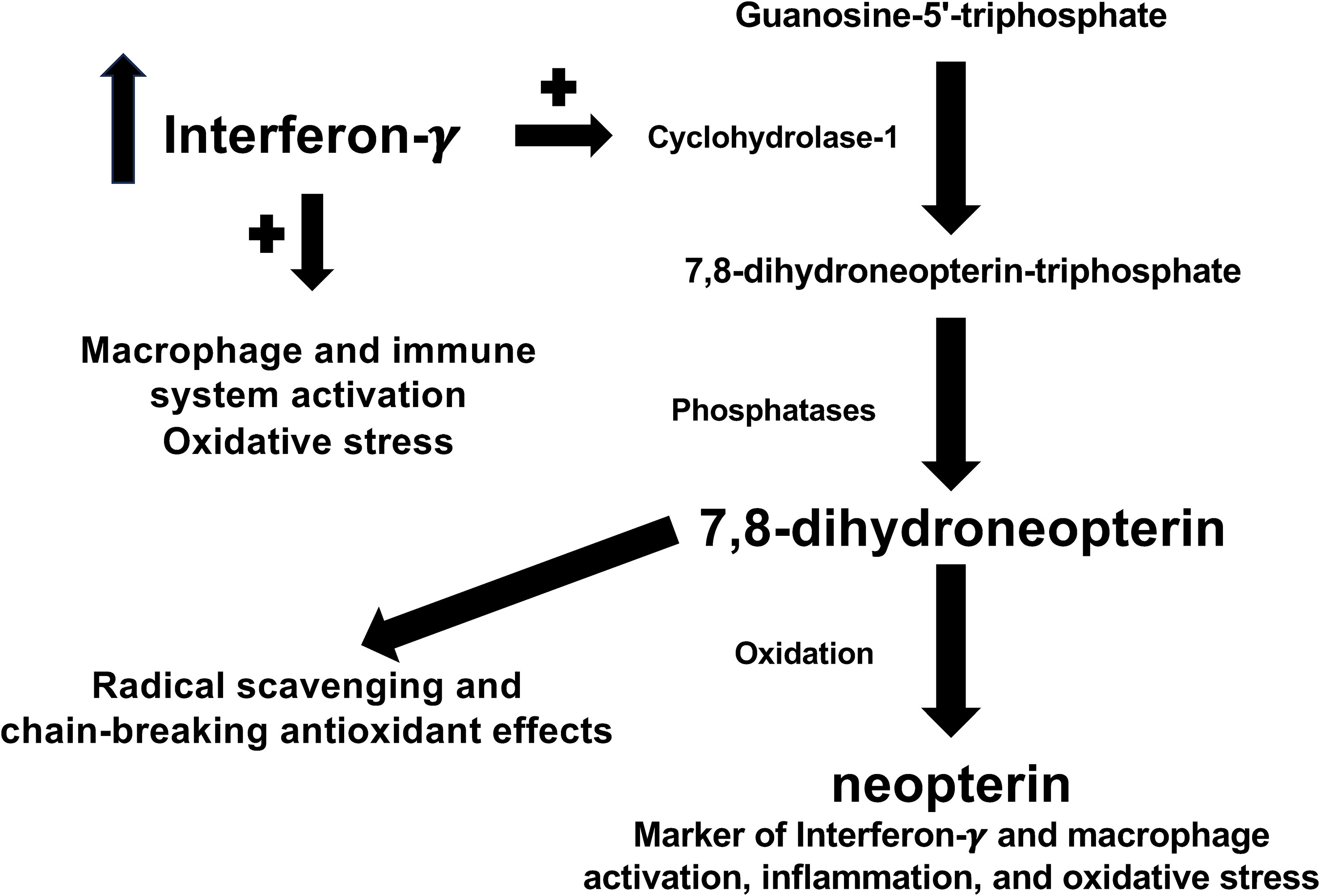
In the ongoing search for novel cellular and biochemical pathways underlying the pathophysiology of RDs, increasing attention has been given to the pleiotropic effects of the cytokine interferon-γ (26). When produced in excess, interferon-γ exerts detrimental effects on the homeostatic control of inflammatory and immune pathways in a range of experimental and clinical studies of RDs (27–30). Therefore, the identification of biomarkers that adequately reflect the activation of interferon-γ might be particularly useful for diagnosis and management. One such biomarker is neopterin, a pteridine analogue generated from the oxidation of 7,8-dihydroneopterin, a potent radical scavenging and chain-breaking antioxidant derived from the interferon-γ-mediated conversion of guanosine-5’-triphosphate (GTP) by GTP cyclohydrolase-1 in activated macrophages (Figure 1) (31–34). The potential advantages of measuring neopterin in the clinical evaluation of RDs include, in addition to its role as a marker of macrophage activation, the determination in different biological fluids and its rapid elimination by the kidney, which allows assessing fluctuations in disease activity and early effects of treatment (35–40).
Therefore, we investigated the potential clinical utility of neopterin by conducting a systematic review and meta-analysis of studies investigating the concentrations of this pteridine metabolite in different biological fluids in patients with RD and healthy controls. We also investigated associations between the effect size of the differences in neopterin concentrations and several parameters, including RD duration, type of RD (autoimmune, mixed autoimmune-autoinflammatory, or autoinflammatory disease), CRP, and ESR.
Materials and methods
Search strategy, eligibility criteria, and study selection
We systematically searched for relevant publications in the electronic databases PubMed, Web of Science, and Scopus from inception to 31 August 2023 using the following terms and their combination: “rheumatic diseases” OR “rheumatoid arthritis” OR “psoriatic arthritis” OR “ankylosing spondylitis” OR “systemic lupus erythematosus” OR “systemic sclerosis” OR “Sjogren’s syndrome” OR “connective tissue diseases” OR “vasculitis” OR “Behçet’s disease” OR “idiopathic inflammatory myositis” OR “polymyositis” OR “dermatomyositis”AND “neopterin”. Two investigators independently reviewed each abstract and, if relevant, the full-text articles and their references for additional studies. The eligibility criteria included: (i) the assessment of neopterin concentrations in biological fluids (plasma/serum, urine, synovial fluid, saliva, and cerebrospinal fluid, (ii) the comparison between patients with RDs and healthy controls conducted in case-control studies, (iii) the inclusion of patients ≥18 years of age, and (iv) the availability of the full-text of the publication in English language.
The following study and patient variables were independently extracted from selected manuscripts in an ad hoc standardized form for further analysis: first author, year of publication, study country, sample size, age, male to female ratio, CRP, ESR, RD duration, sample matrix investigated (serum or plasma), and assay method used to measure neopterin.
We assessed the risk of bias using the Joanna Briggs Institute Critical Appraisal Checklist for analytical studies. Studies addressing ≥75%, ≥50% and <75%, and <50% of checklist items were considered as having a low, moderate, and high risk, respectively (41). We also assessed the certainty of evidence using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) Working Group system. GRADE assesses the study design (randomized vs. observational), the risk of bias (JBI checklist), the presence of unexplained heterogeneity, the indirectness of the evidence, the imprecision of the results (sample size, 95% confidence interval width and threshold crossing), the effect size (small, SMD <0.5, moderate, SMD 0.5-0.8, and large, SMD >0.8) (42), and the probability of publication bias (43). The results were presented according to the guidelines provided in Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 statement (Supplementary Tables 1 and 2) (44). The review protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO registration number: CRD42023450209) (45).
Statistical analysis
We generated forest plots of standardized mean differences (SMDs) and 95% confidence intervals (CIs) to assess differences in neopterin concentrations between RD patients and healthy controls (p<0.05 for statistical significance). If necessary, the mean and standard deviation values were extrapolated from medians and interquartile ranges or medians and ranges (46, 47). The heterogeneity of the SMD across studies was tested by using the Q statistic (p<0.10 for statistical significance). Heterogeneity was considered low when the I2 value was ≤25%, moderate when the I2 value was >25% and <75%, and high when the I2 value was ≥75% (48, 49). A random-effect model based on the inverse-variance method was used in the presence of high heterogeneity. Sensitivity analysis was conducted to investigate the stability of the results by assessing the influence of individual studies on the overall risk estimate (50). Publication bias was assessed using the Begg’s adjusted rank correlation test and the Egger’s regression asymmetry test (p<0.05 for statistical significance) (51, 52). The “trim-and-fill” method was used to further test and eventually correct the occurrence of publication bias (53). Univariate meta-regression and subgroup analyses were conducted to investigate the presence of associations between the effect size (SMD) and the following parameters: year of publication, study continent, sample size, age, male to female ratio, CRP, ESR, disease duration, sample matrix investigated, and analytical method used to measure neopterin. Statistical analyses were performed using Stata 14 (Stata Corp., College Station, TX, USA).
Results
Systematic search and characteristics of the included studies
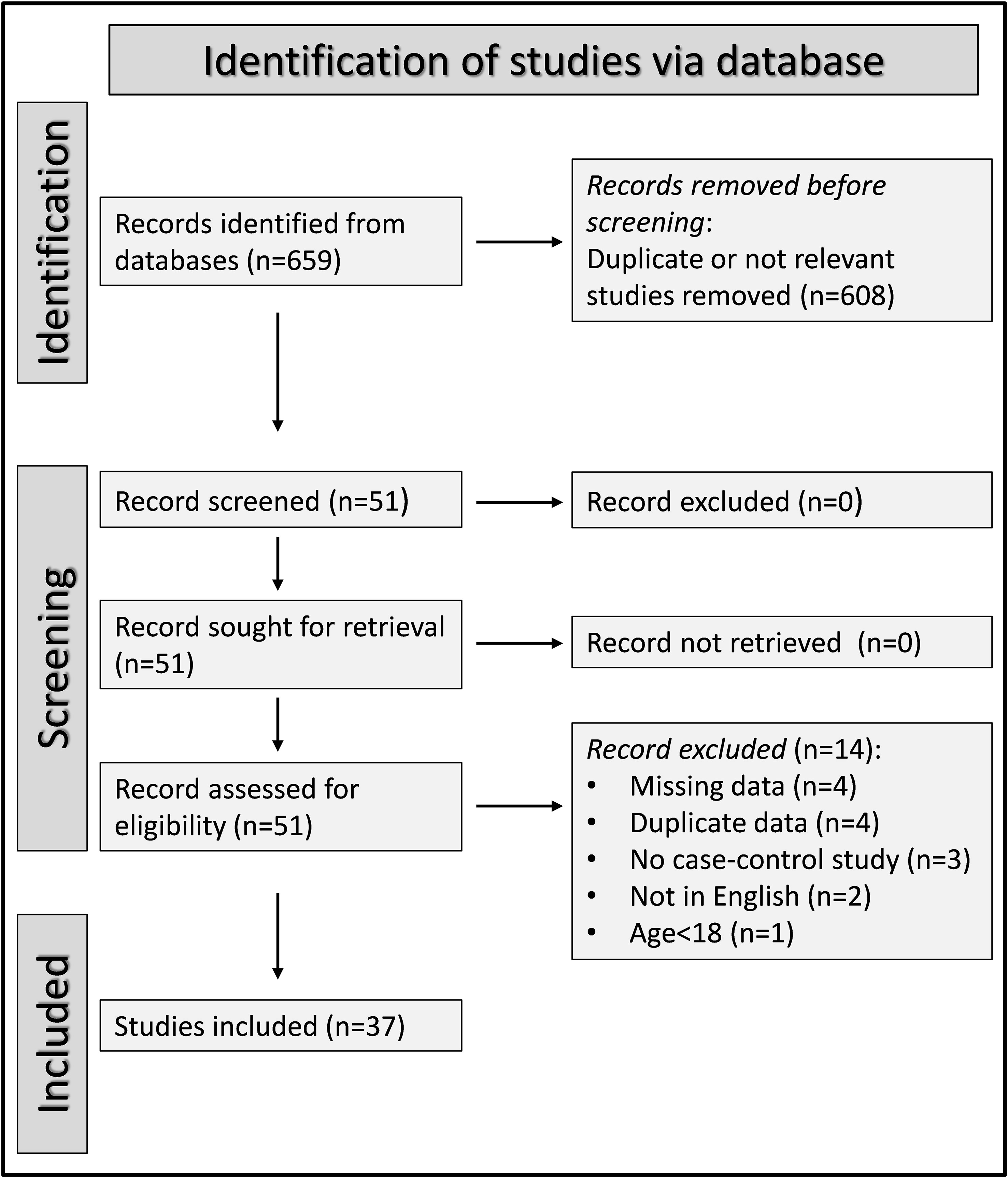
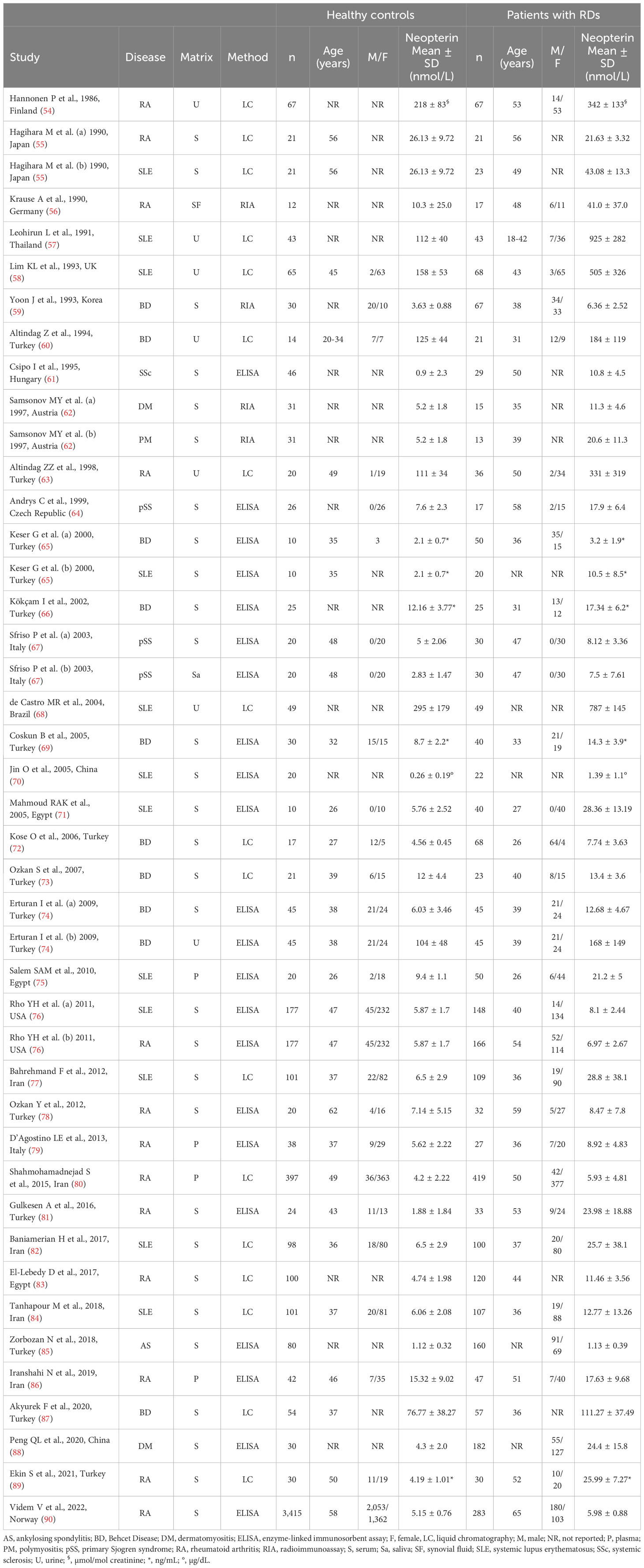
A flow chart describing the screening process is presented in Figure 2. We initially identified 659 articles. A total of 608 were excluded after the first screening because they were either duplicates or irrelevant. After a full-text review of the remaining 51 articles, a further 14 were excluded because of missing data (n=4), duplicate data (n=4), incorrect study design (n=3), non-English language used (n=2), and inclusion of children or adolescents (n=1). Therefore, 37 studies (43 study groups, 34 investigating plasma/serum, seven urine, one saliva, and one synovial fluid) were selected for analysis (Table 1) (54–90).
Serum or plasma neopterin
Study characteristics
Thirty studies (34 study groups) reported serum or plasma neopterin concentrations in a total of 2,618 RD patients (mean age 43 years, 32% males) and 5,318 healthy controls (mean age 42 years, 47% males) (55, 59, 61, 64–67, 69–87, 89, 90).
Twenty studies were conducted in Asia (55, 59, 65, 66, 69, 70, 72–74, 77, 78, 80–82, 84–89), six in Europe (61, 62, 64, 67, 79, 90), three in Africa (71, 75, 83), and the remaining one in America (76). Ten study groups included patients with RA (55, 76, 78–81, 83, 86, 89, 90), nine with SLE (55, 65, 70, 71, 75–77, 82, 84), eight with BD (59, 65, 66, 69, 72–74, 87), three with IIM (62, 88), two with pSS (64, 67), one with SSc (61), and one with AS (85). The analytical methods used for measuring neopterin included an enzyme-linked immunosorbent assay (ELISA) in 18 studies (61, 64–67, 69–71, 74–76, 78, 79, 81, 85, 86, 88, 90), liquid chromatography with fluorimetric detection in 10 (55, 72, 73, 77, 80, 82–84, 87, 89), and radioimmunoassay in two (59, 62). Serum was analysed in 26 studies (55, 59, 61, 62, 64–67, 69–74, 76–78, 81–85, 87–90), and plasma in the remaining four (75, 79, 80, 86). RD duration, reported in 11 study groups, ranged between four and 11 years (61, 67, 71, 73–75, 78, 80, 81, 83, 90).
The risk of bias was low in 14 studies (61, 69, 75–77, 79, 81, 82, 84, 85, 87–90), and moderate in the remaining 16 (55, 59, 62, 64–67, 70–74, 78, 80, 83, 86) (Supplementary Table 3). All studies had an initially low certainty of evidence given the cross-sectional design (rating 2, ⊕⊕⊝⊝) (55, 59, 61, 64–67, 69–87, 89, 90).
Results of individual studies and syntheses
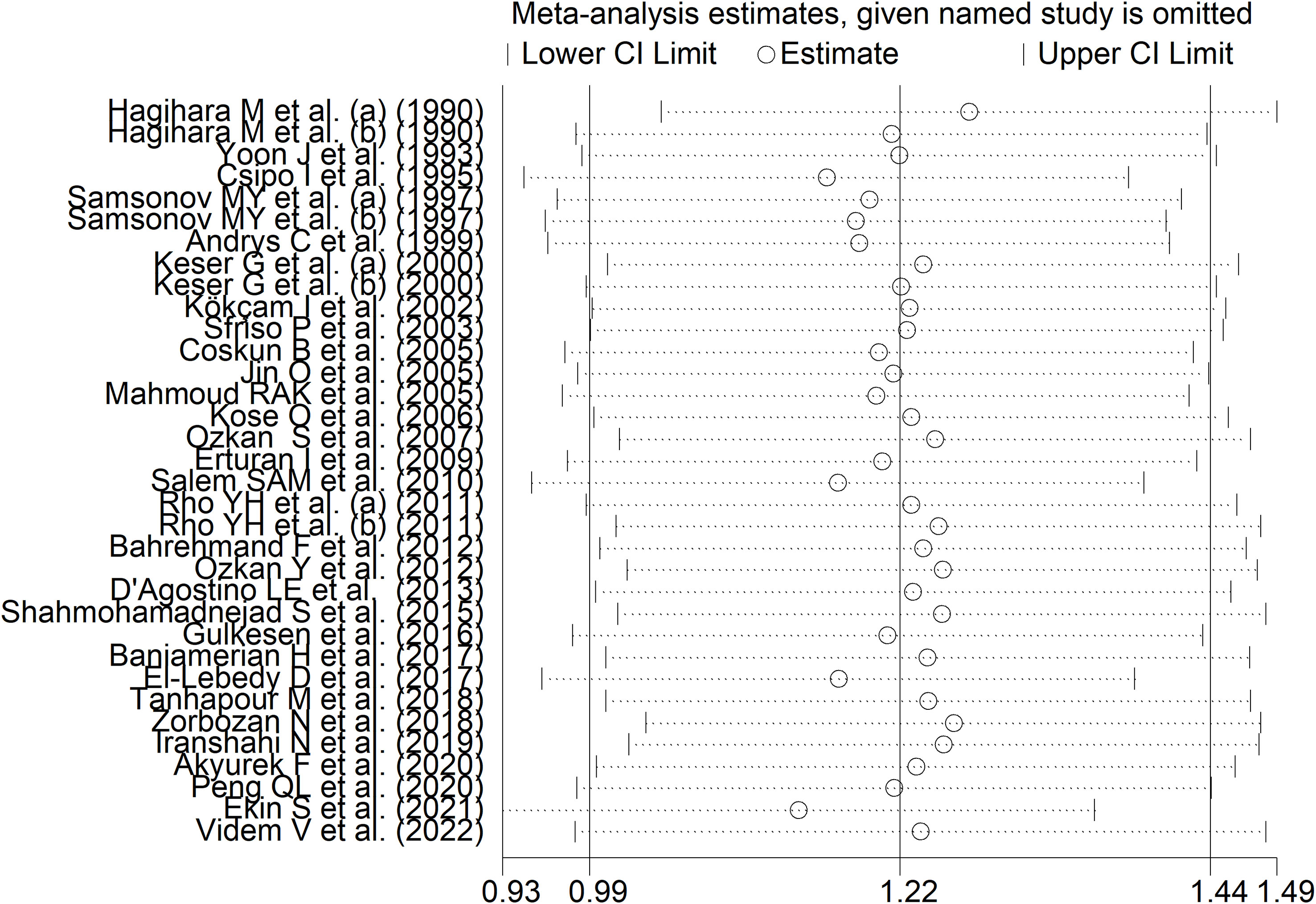
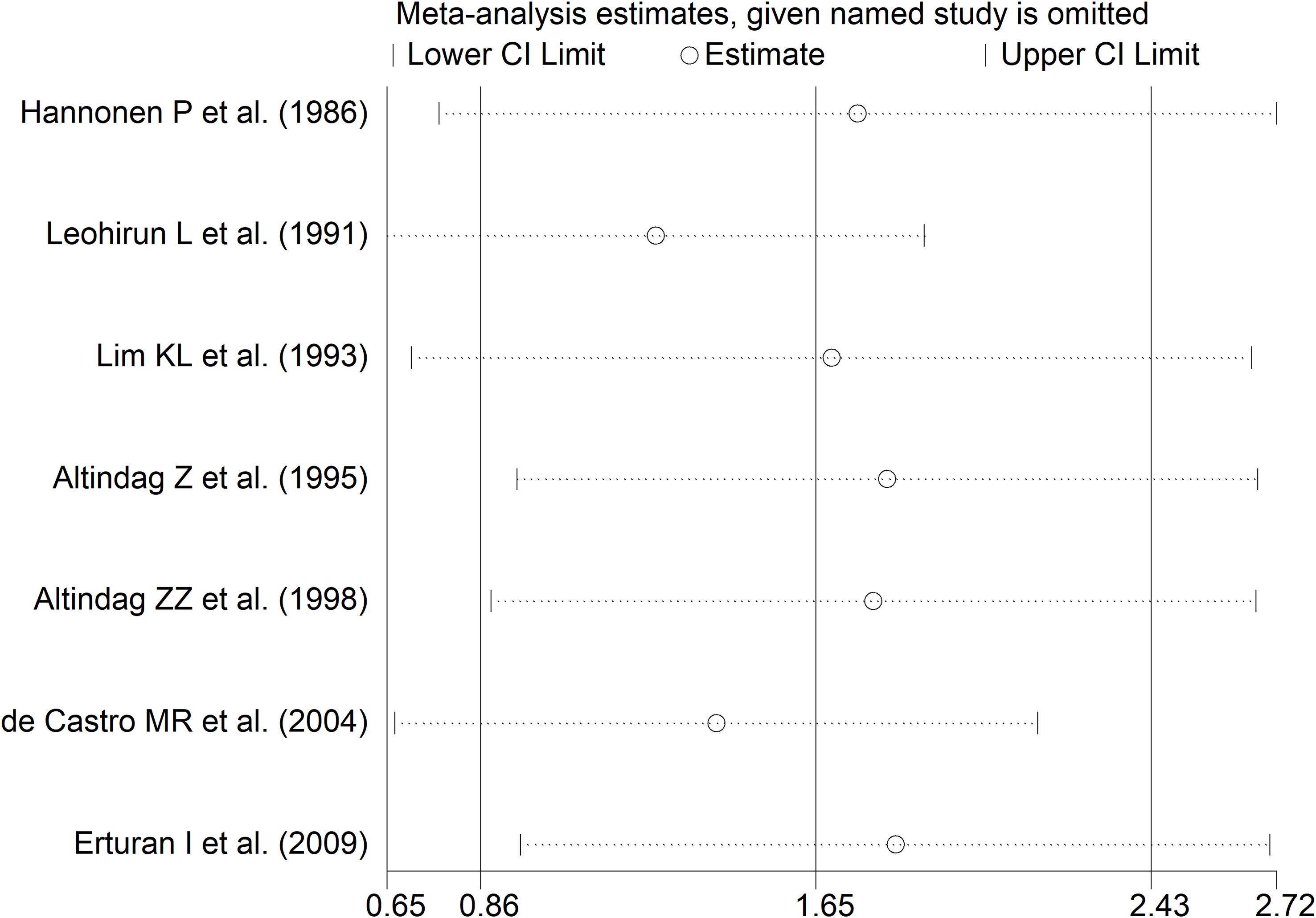
RD patients had significantly higher neopterin concentrations compared to healthy controls (SMD=1.22, 95% CI 0.99 to 1.44, p<0.001; I2 = 91.8%, p<0.001; Figure 3). In sensitivity analysis, the corresponding pooled SMD values were not influenced when individual studies were sequentially removed, with the effect size ranging between 1.14 and 1.27 (Figure 4). The effect size was also similar to the primary analysis after removing three studies accounting for 65% of the overall participant population (SMD=1.31, 95% CI 1.01 to 1.61; p<0.001; I2 = 91.2%, p<0.001) (76, 80, 90).
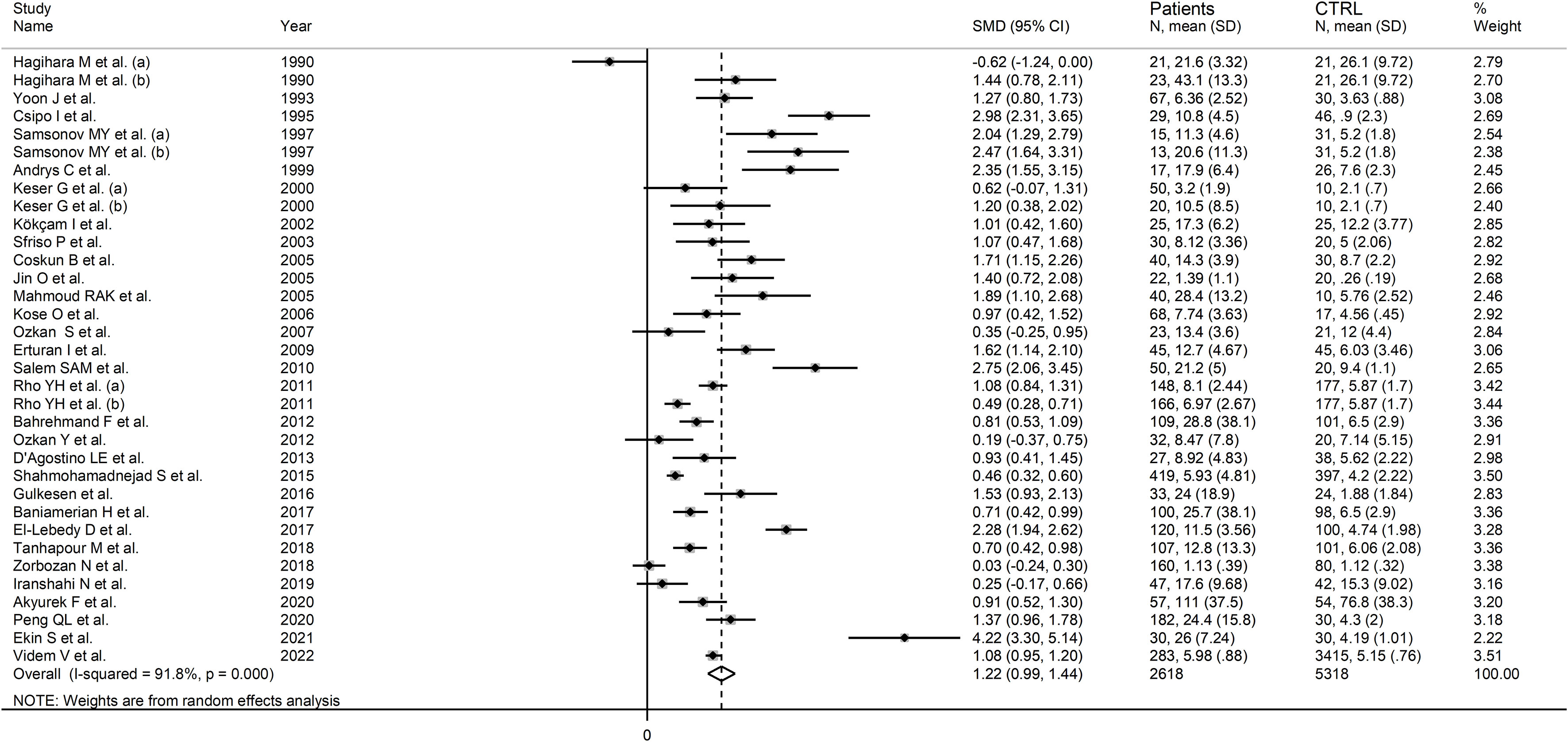
Figure 3 Forest plot of studies examining neopterin concentrations in RD patients and healthy controls in serum/plasma.
Publication bias
A significant publication bias was observed (Begg’s test, p=0.004; Egger’s test, p=0.006). The “trim-and-fill” method identified ten missing studies to be added to the left side of funnel plot to ensure symmetry (Figure 5). The resulting effect side was attenuated yet still significant (SMD=0.78, 95% CI 0.54 to 1.02, p<0.001).
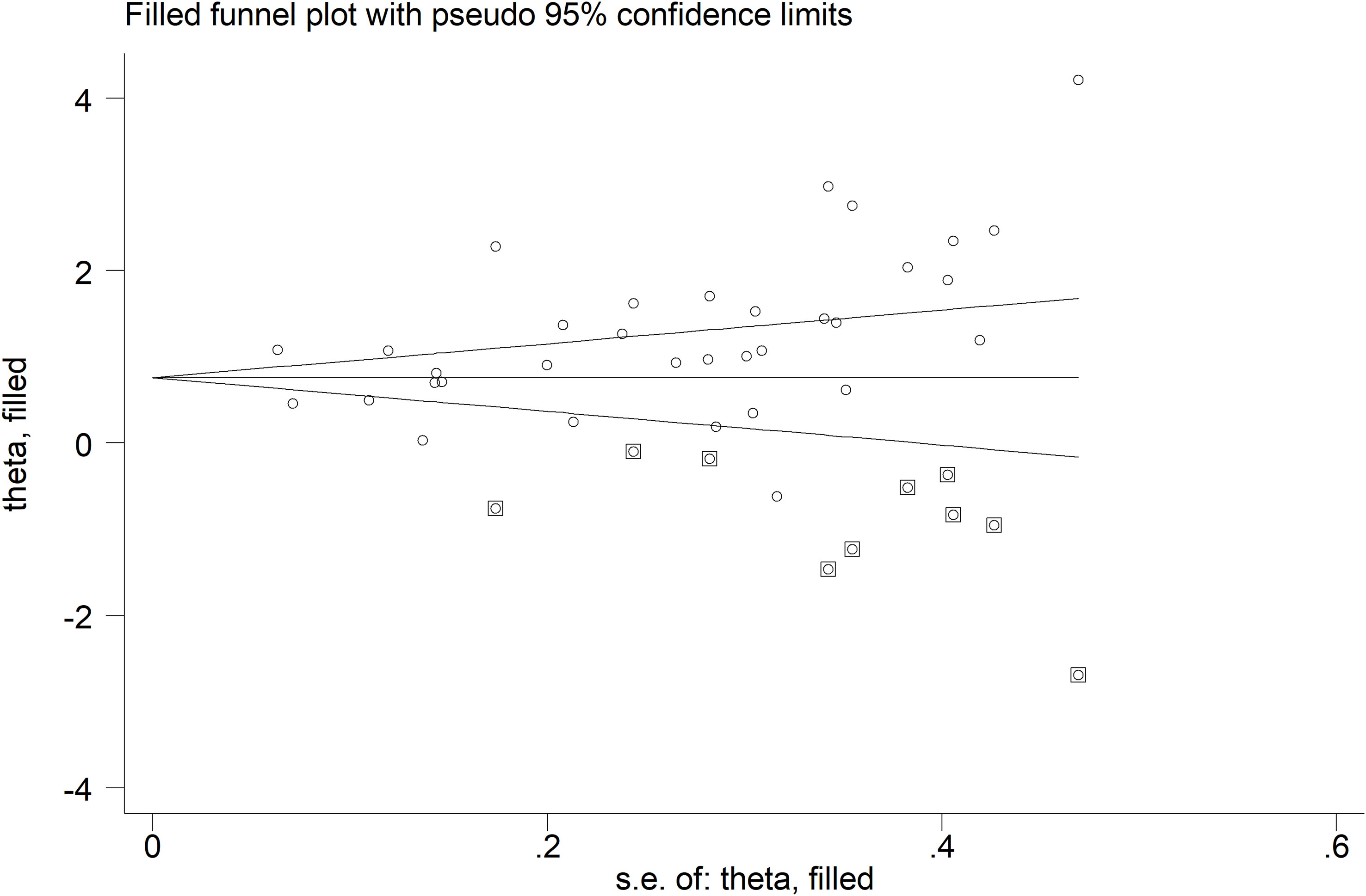
Figure 5 Funnel plot of studies investigating the association between neopterin and RDs in serum/plasma after “trimming-and-filling”. Enclosed circles and free circles indicate dummy studies and genuine studies, respectively.
Meta-regression and sub-group and analysis
There were non-significant associations between the effect size and age (t=0.13, p=0.90), male to female ratio (t=-0.34, p=0.73), year of publication (t=-0.51, p=0.61), sample size (t=-0.53, p=0.60), disease duration (t=0.83, p=0.42), CRP (t=-0.50, p=0.62), or ESR (t=0.16, p=0.87).
In subgroup analysis, there were non-significant differences (p=0.39) in SMD values between studies conducted in RA patients (SMD=1.01, 95% CI 0.57 to 1.45, p<0.001; I2 = 95.8%, p<0.001), SLE patients (SMD=1.23, 95% CI 0.90 to 1.55, p<0.001; I2 = 81.5%, p<0.001), BD patients (SMD=1.08, 95% CI 0.77 to 1.38, p<0.001; I2 = 62.6%, p=0.006), IIM patients (SMD=1.88, 95% CI 1.20 to 2.57, p<0.001; I2 = 69.6%, p=0.037), and pSS patients (SMD=1.68, 95% CI 0.43 to 2.94, p=0.008; I2 = 84.1%, p<0.001; Figure 6), with a lower heterogeneity observed in the BD and IIM subgroups. Similarly, the pooled SMD was non-significantly different (p=0.25) between studies conducted in patients with CTD (SMD=1.32, 95% CI 1.06 to 1.59, p<0.001; I2 = 92.9%, p<0.001) and without CTD (SMD=0.94, 95% CI 0.50 to 1.37, p<0.001; I2 = 92.0%, p<0.001; Figure 7). By contrast, a significant (p=0.003) increase in the effect size was observed between studies conducted in America (SMD=0.78, 95% CI 0.21 to 1.36, p=0.007; I2 = 92.3%, p<0.001), Asia (SMD=0.95, 95% CI 0.69 to 1.20, p<0.001; I2 = 88.5%, p<0.001), Europe (SMD=1.79, 95% CI 1.21 to 2.38, p<0.001; I2 = 88.8%, p<0.001) and Africa (SMD=2.32, 95% CI 1.94 to 2.69, p<0.001; I2 = 25.2%, p<0.263; Figure 8), with a lower heterogeneity observed in the African subgroup. There were non-significant differences (p=0.48) in pooled SMD between studies using liquid chromatography (SMD=1.23, 95% CI 0.95 to 1.51, p<0.001; I2 = 90.0%, p<0.001), ELISA (SMD=1.05, 95% CI 0.61 to 1.49, p<0.001; I2 = 94.3%, p<0.001), and RIA (SMD=1.86, 95% CI 1.12 to 2.61, p<0.001; I2 = 72.8%, p=0.025; Figure 9. Finally, non-significant differences (p=0.66) in pooled SMD were also observed between studies investigating serum (SMD=1.25, 95% CI 1.01 to 1.49, p<0.001; I2 = 90.9%, p<0.001) and plasma (SMD=1.04, 95% CI 0.28 to 1.79, p=0.007; I2 = 93.2%, p<0.001; Figure 10).
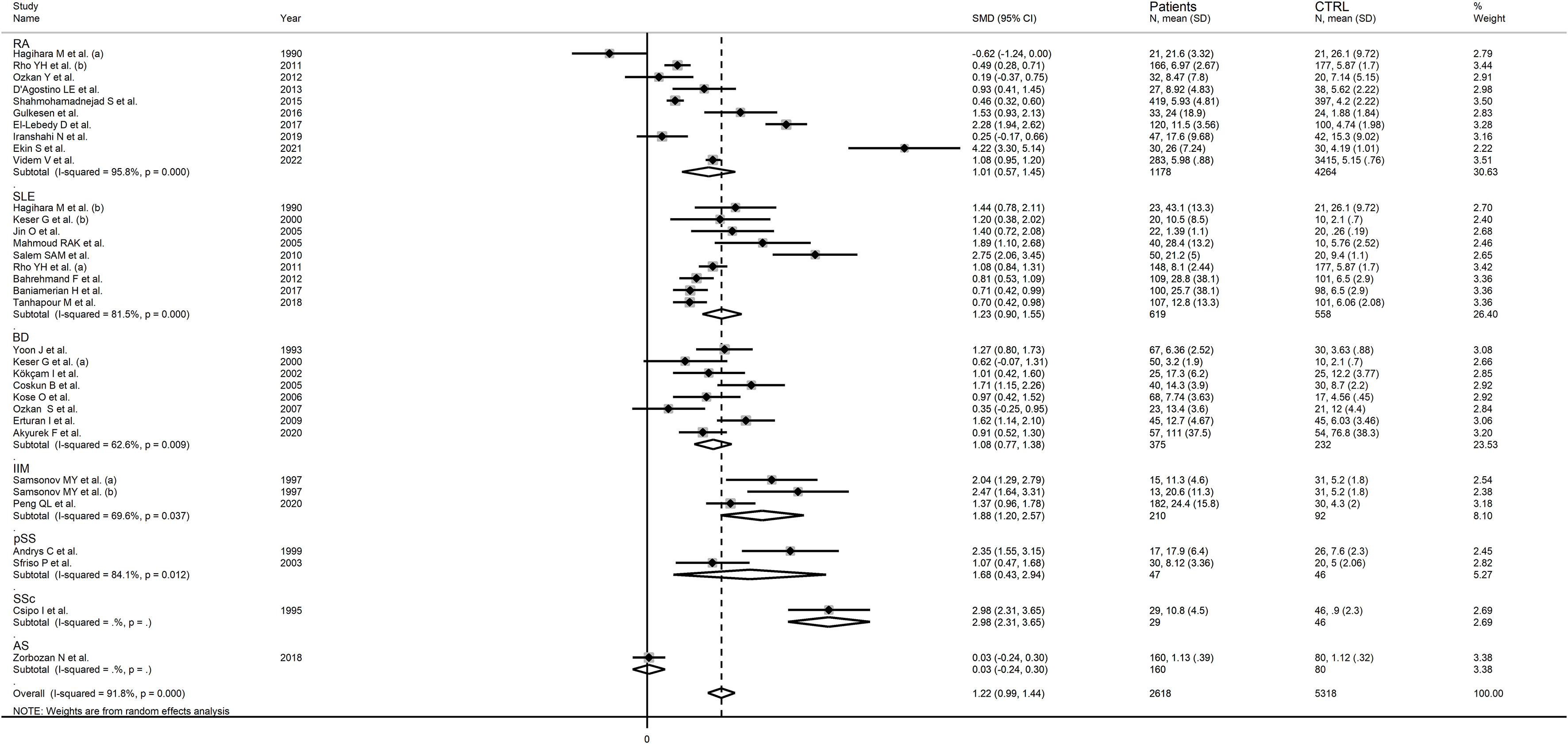
Figure 6 Forest plot of studies examining neopterin concentrations in RD patients and healthy controls in serum/plasma according to RD type.
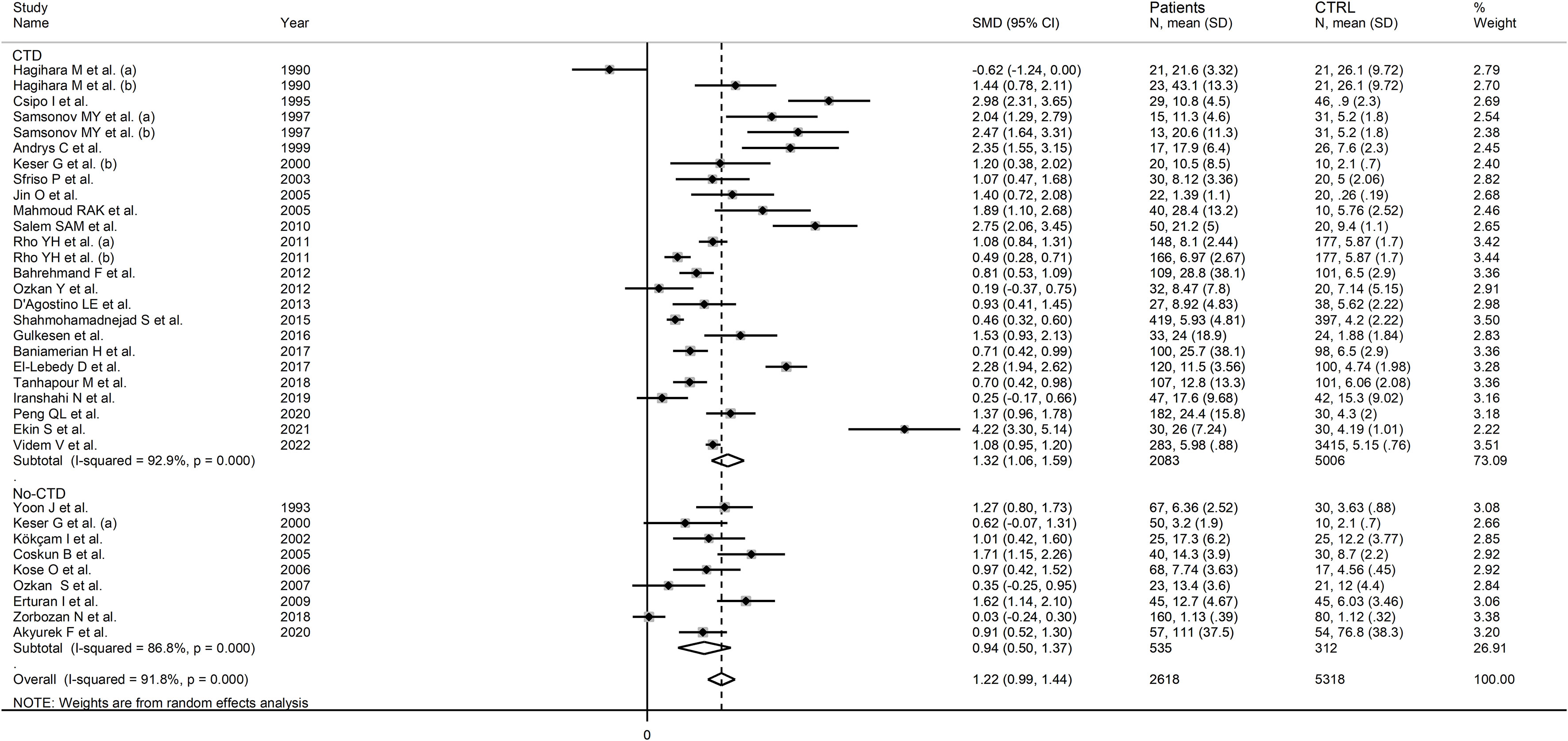
Figure 7 Forest plot of studies examining neopterin concentrations in RD patients and healthy controls in serum/plasma according to the presence of connective tissue disease.

Figure 8 Forest plot of studies examining neopterin concentrations in RD patients and healthy controls in serum/plasma according to study continent.
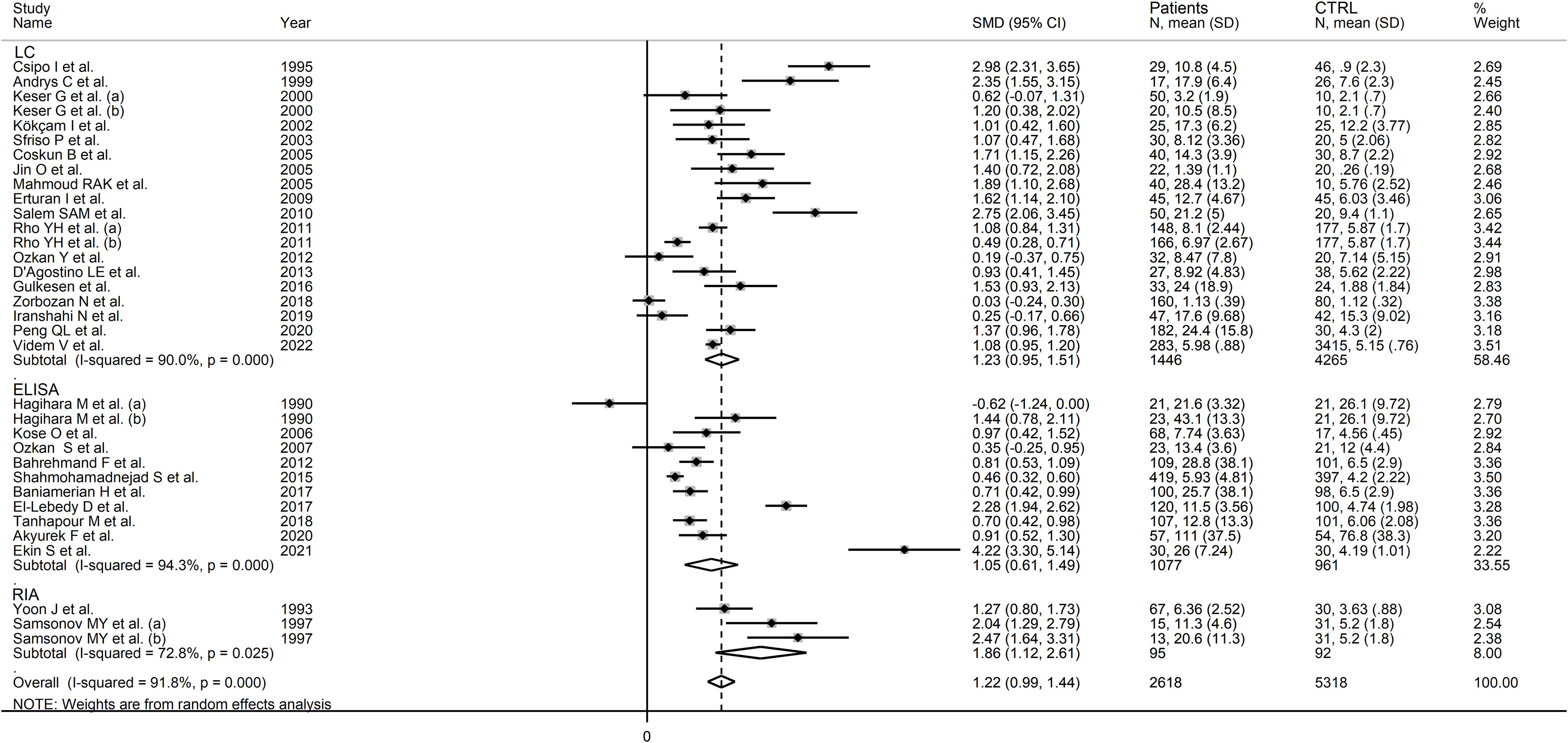
Figure 9 Forest plot of studies examining neopterin concentrations in RD patients and healthy controls in serum/plasma according to the analytical method used.
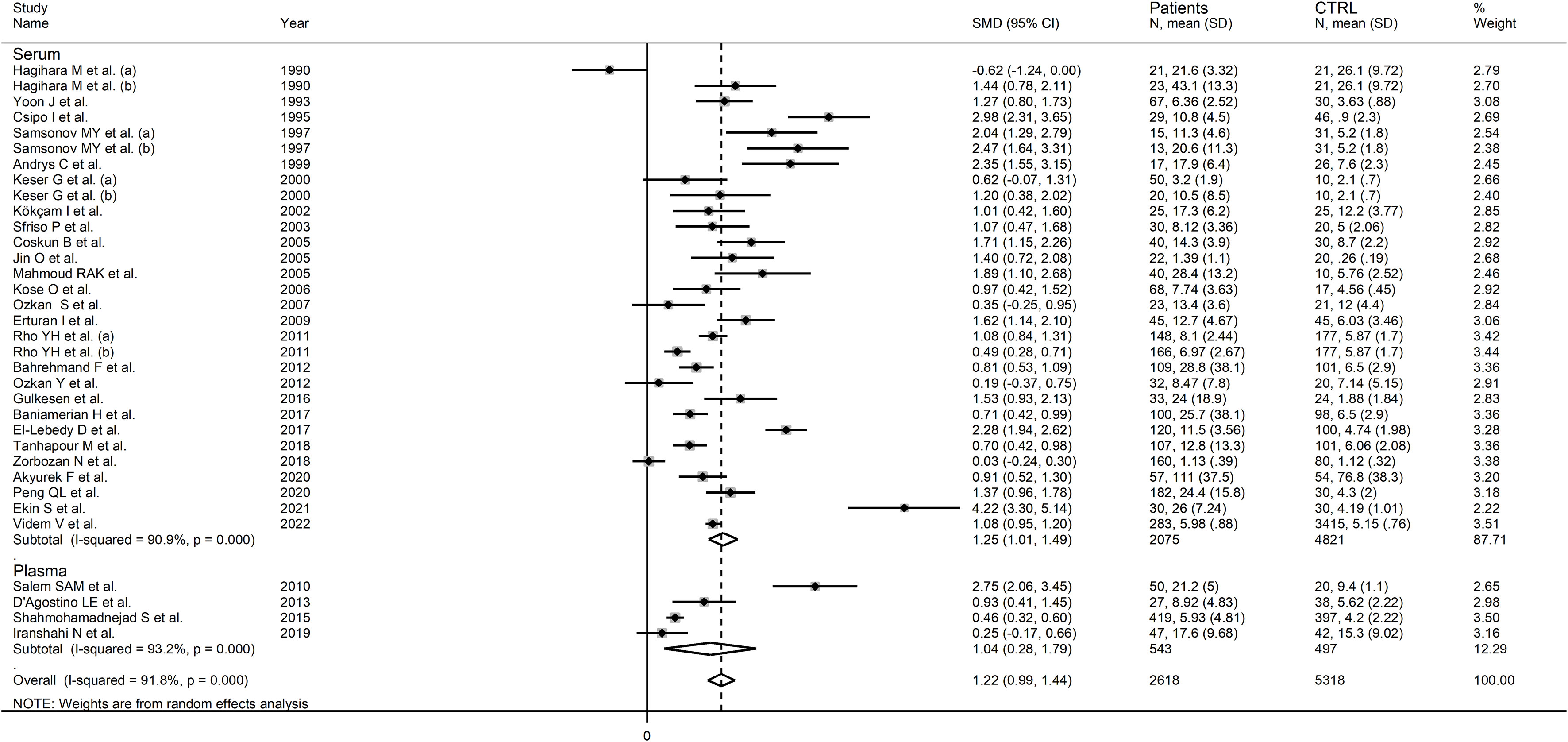
Figure 10 Forest plot of studies examining neopterin concentrations in RD patients and healthy controls in serum/plasma according to the sample matrix used for assessment (plasma or serum).
Certainty of evidence
The overall level of certainty was upgraded to moderate (rating 3, ⊕⊕⊕⊝) after taking into account the low-moderate risk of bias in all studies (no rating change), the high but partly explainable heterogeneity (no rating change), the lack of indirectness (no rating change), the relatively low imprecision (confidence intervals not crossing the threshold, no rating change), the large effect size (SMD=1.22, upgrade by one level), and the presence of publication bias which was corrected using the “trim-and-fill” method (no rating change).
Urine neopterin
Study characteristics
Seven studies investigated urinary concentrations of neopterin in a total of 329 patients (mean age 46.4 years, 21.1% males) and 303 healthy controls (mean age 46.5 years, 20.5% males) (54, 57, 58, 60, 63, 68, 74). Four studies were conducted in Asia (57, 60, 63, 74), two in Europe (54, 58), and one in America (68). Three studies investigated patients with SLE (57, 58, 68), two with RA (54, 63), and two with BD (60, 74). Liquid chromatography with fluorimetric detection was used in six studies (54, 57, 58, 60, 63, 68), and ELISA in the remaining one (74).
The risk of bias was considered low in two studies (58, 63), moderate in two (57, 74), and high in the remaining three (54, 60, 68) (Supplementary Table 3). All studies had an initially low certainty of evidence given the cross-sectional design (rating 2, ⊕⊕⊝⊝) (54, 57, 58, 60, 63, 68, 74).
Results of individual studies and syntheses
The forest plot showed that RD patients had significantly higher urinary neopterin concentrations compared to healthy controls (SMD=1.65, 95% CI 0.86 to 2.43, p<0.001; I2 = 94.2%, p<0.001; Figure 11). In sensitivity analysis, the corresponding pooled SMD values were not influenced when individual studies were sequentially removed, with the effect size ranging between 1.27 and 1.83 (Figure 12).
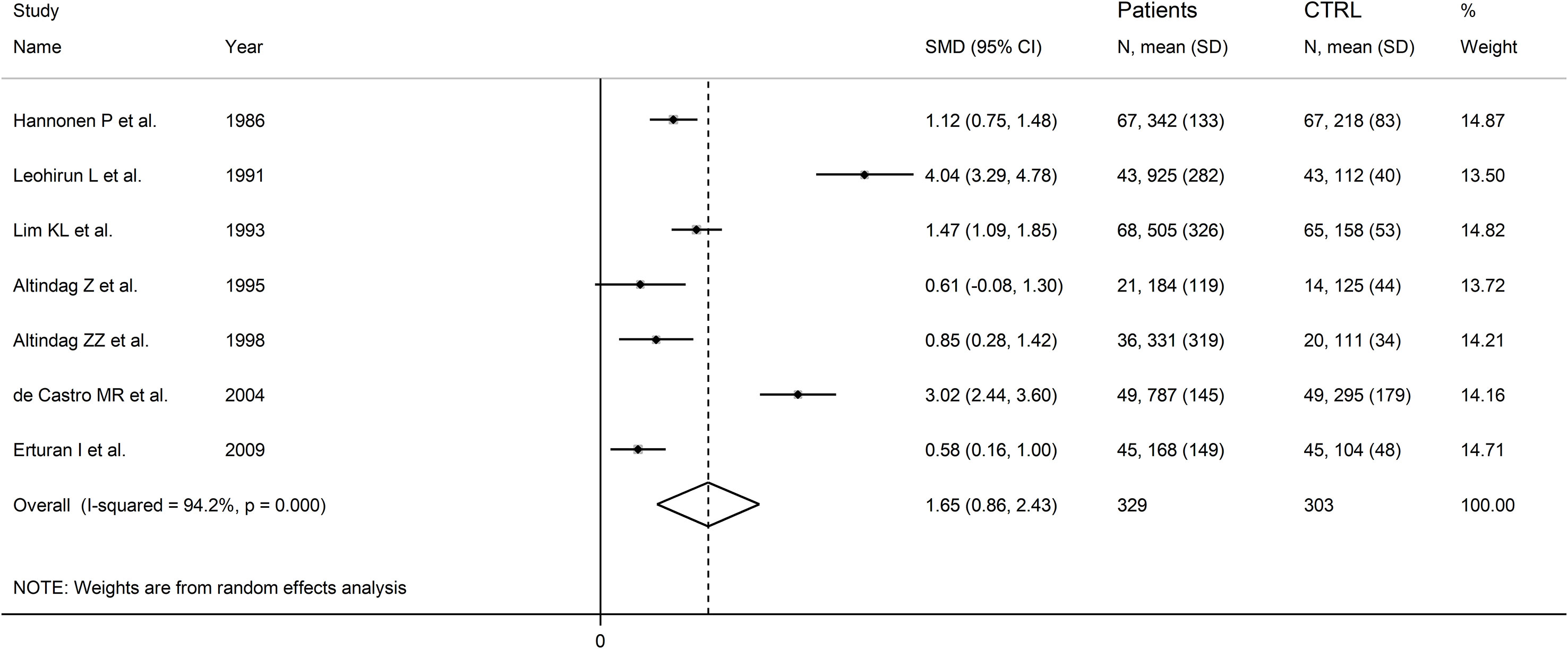
Figure 11 Forest plot of studies examining neopterin concentrations in RD patients and healthy controls in urine.
Publication bias and meta-regression analysis
Assessment of publication bias and meta-regression could not be performed because of the small number of studies.
Subgroup analysis
There were significant differences (p=0.04) in SMD values between studies conducted in SLE patients (SMD=2.82, 95% CI 1.30 to 4.33, p<0.001; I2 = 95.5%, p<0.001), RA patients (SMD=1.04, 95% CI 0.73 to 1.35, p<0.001; I2 = 0.0%, p=0.44), and BD patients (SMD=0.59, 95% CI 0.23 to 0.95, p=0.001; I2 = 0.0%, p=0.94; Figure 13), with a virtual absence of heterogeneity in the RA and BD subgroups. By contrast, there were non-significant differences (p=0.40) in SMD values between European (SMD=1.29, 95% CI 0.94 to 1.63, p<0.001; I2 = 40.9%, p<0.001), and Asian studies (SMD=1.50, 95% CI 0.10 to 2.91, p<0.001; I2 = 94.2%, p<0.001; Figure 14), with a lower heterogeneity in the European subgroup.
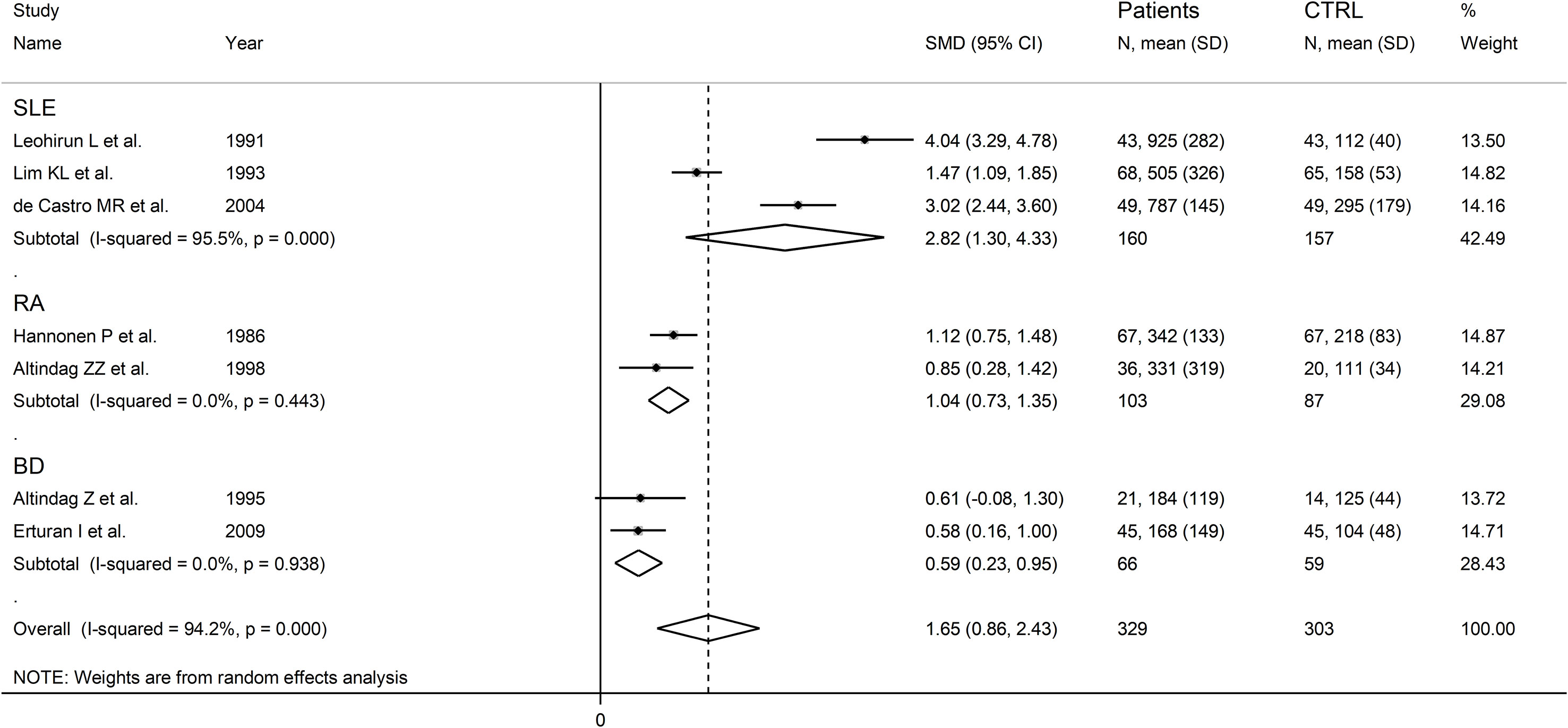
Figure 13 Forest plot of studies examining neopterin concentrations in RD patients and healthy controls in urine according to RD type.
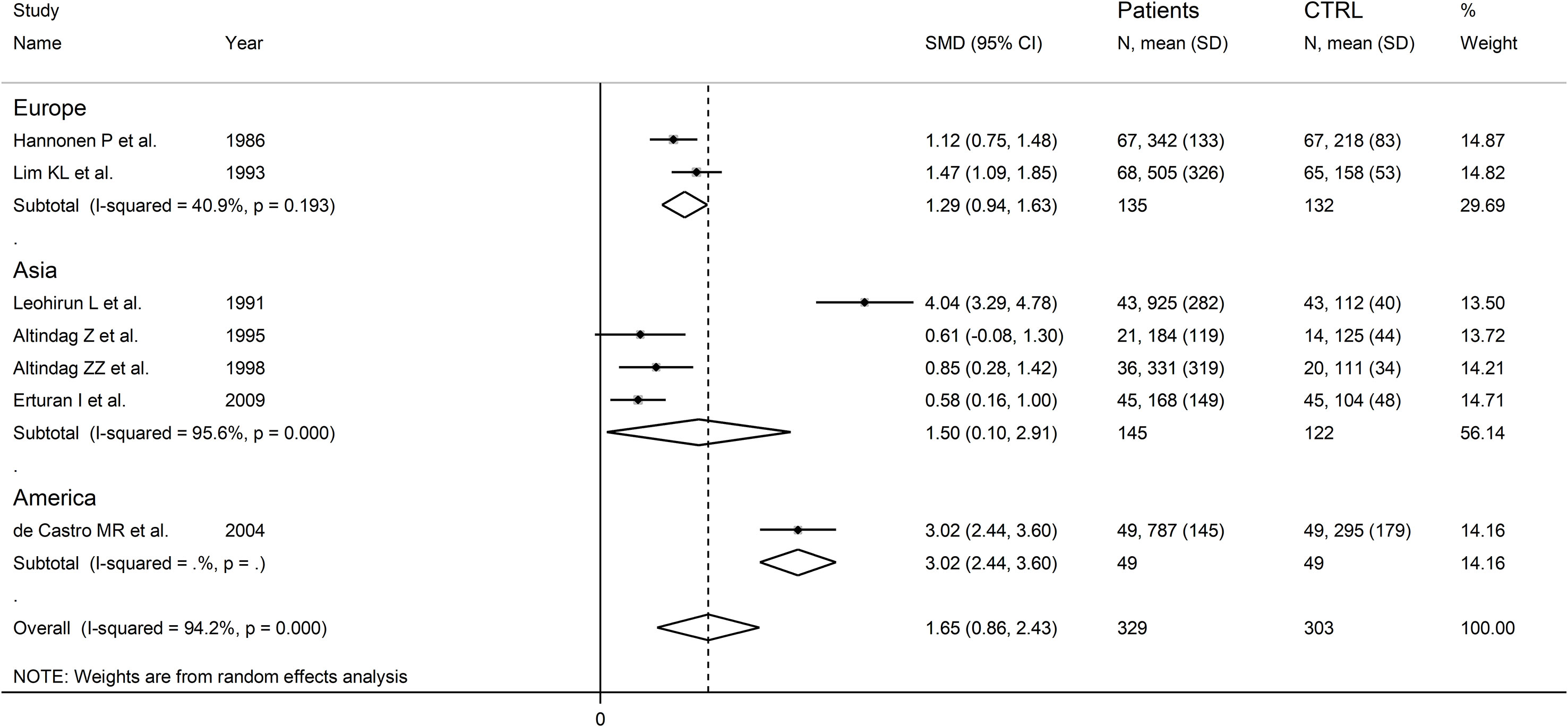
Figure 14 Forest plot of studies examining neopterin concentrations in RD patients and healthy controls in urine according to study continent.
Certainty of evidence
The overall level of certainty remained low (rating 2, ⊕⊕⊝⊝) after taking into account the low-moderate risk of bias in the majority of studies (no rating change), the high but partly explainable heterogeneity (no rating change), the lack of indirectness (no rating change), the relatively low imprecision (confidence intervals not crossing the threshold, no rating change), the large effect size (SMD=1.65, upgrade by one level), and lack of assessment of publication bias (downgrade one level).
Neopterin concentration in other biological fluids
One study reported significantly higher salivary concentrations of neopterin in pSS patients when compared with healthy subjects (9.5 ± 7.61 vs. 2.83 ± 1.47 nmol/L, p<0.005) (67), whereas another study reported that RA patients have increased concentrations of neopterin in synovial fluid when compared with healthy controls (41 ± 37 vs. 10.3 ± 25 nmol/L, p<0.001) (56) (Table 1).
Discussion
The results of our systematic review and meta-analysis have shown that the plasma/serum and urinary concentrations of neopterin, a biomarker of interferon-γ activation, are significantly higher in patients with RDs compared to healthy controls. In meta-regression analysis, the effect size of the between-group differences in plasma/serum neopterin concentrations (SMD) was not associated with a range of study and patient characteristics, including age, male to female ratio, year of publication, study sample size, RD duration, CRP, and ESR. Similarly, in subgroup analysis the SMD was not associated with the type of RD (i.e., RA, SLE, BD, and pSS), the presence of CTD, the analytical method used to determine neopterin, or the matrix used for assessment (plasma vs. serum). By contrast, there was a significant association between the SMD (plasma or urine) and the study geographical location, with progressively higher SMD values in studies conducted in America, Asia, Europe, and Africa, and between the SMD (urine) and the type of RD investigated.
Taken together, these results suggest that neopterin can significantly discriminate between physiological states and different types of RD, including an autoimmune and/or an autoinflammatory component, using a range of analytical methods that can be applied both in plasma/serum and in urine. High-performance liquid chromatography with fluorimetric detection, ELISA, and RIA were the analytical methods most used to measure neopterin in biological fluids. High-performance liquid chromatography with fluorimetric detection offers a particularly high sensitivity, enabling the simultaneous detection of low neopterin concentrations. Its specificity is also high due to compound separation in the sample, which minimize the interference from other molecules. Quantitative accuracy is achievable, particularly when coupled with sensitive fluorimetric detection. However, it demands specialized equipment and expertise for operation and maintenance, and the process is time-consuming and potentially costly (91). ELISA is particularly suitable for the assessment of a large volume of samples due to its capacity to process multiple samples simultaneously. Its execution is relatively straightforward, with many commercially available kits. The broad dynamic range of quantitative values is an advantage, covering both low and high neopterin concentrations. However, specificity relies on the quality of antibodies used, and cross-reactivity with related compounds might limit accuracy. Additionally, sensitivity might be an issue with very low concentrations (38). RIA is known for its high sensitivity, enabling the detection of very low neopterin concentrations. Quantitative accuracy is attainable with proper optimization. Specificity depends on appropriately selected antibodies, which can be highly specific. However, there are also safety concerns due to the use of radioisotopes, requiring proper handling and disposal (92). RIA can involve complex steps due to the separation of bound and free fractions. Overall, the choice among these methods should be based on the required sensitivity, available resources, and safety considerations. High-performance liquid chromatography with fluorimetric detection offers high specificity and sensitivity but requires complex and costly equipment. ELISA is simple, high-capacity, and has a broad dynamic range, but specificity might be limited. RIA provides high sensitivity and precision but carries safety issues and has limitations in reagent availability.
Another interesting observation was the absence of significant correlations between the SMD of neopterin and CRP and ESR, biomarkers that are routinely used to assess inflammation and disease activity in RDs, also suggests that the information provided by neopterin can potentially complement existing knowledge to enhance diagnostic capacity. The presence of significant geographic-related and RD type-related differences in the SMD of neopterin also suggests the potential influence of ethnicity and specific RDs in mediating the associations between interferon-γ, macrophage activation, and inflammatory and immune pathways.
Although interferon-γ is mainly produced by T helper 1 and natural killer cells, macrophages can also contribute to its formation (93, 94). In this context, there is robust evidence that interferon-γ activates macrophages to the creation of a pro-inflammatory phenotype and, at the same time, stimulates the expression of pro-inflammatory cytokines and downregulates anti-inflammatory cytokines (Figure 1) (95, 96). Furthermore, interferon-γ regulates the initial steps of the adaptative immune response by influencing dendritic cells, T-cells, and B-cells (97–99). However, the excessive production of interferon-γ is responsible for the dysregulation of inflammatory and immune pathways, a phenomenon that has been observed in several hyperinflammatory disease states, cytokine release syndromes, and autoimmune conditions (28, 100–103). Notably, in these studies neopterin was measured as a biomarker of interferon-γ activity (28, 100–103). This pteridine analogue is not directly synthesized in macrophages, rather it is the oxidized form of another pteridine analogue synthesized in these cells, 7,8-dihydroneopterin. In activated macrophages, interferon-γ is responsible for the upregulation of GTP cyclohydrolase 1, the enzyme responsible for the bioconversion of GTP into 7,8-dihydroneopterin-triphosphate, which is then transformed to 7,8-dihydroneopterin by the action of phosphatase enzymes (Figure 1) (104–106). 7,8-dihydroneopterin is a known antioxidant and free radical scavenger with protective effects on low-density lipoprotein, other proteins, and lipids (107–109). The scavenging effects of 7,8-dihydroneopterin on free radicals lead to the synthesis of several oxidation products, including neopterin (Figure 1) (110, 111). Although 7,8-dihydroneopterin might theoretically serve as a robust biomarker of immune activation and redox state its physicochemical characteristics, particularly the low fluorescence, present analytical challenges when measured in isolation and in combination with neopterin (total neopterin) (40, 112, 113). Pending further analytical studies to optimize the measurement of 7,8-dihydroneopterin in blood and other biological samples, our systematic review and meta-analysis also warrants further studies to confirm the potential utility of neopterin specifically in the early detection of RDs. In this context, the absence of significant associations between the SMD of neopterin concentrations and RD duration observed in meta-regression analysis suggests that this biomarker can effectively discriminate between physiological states and presence of RDs also in patients with relatively short disease duration.
Another interesting observation was the presence of significant differences in the SMD of neopterin according to specific geographical locations. Epidemiological studies have shown that in healthy individuals neopterin concentrations can be influenced by age, body mass index, body composition and ethnicity (114, 115). In a study of 426 healthy subjects, black participants, particularly males, had significantly higher concentrations of neopterin than white participants (114). However, opposite results, with higher neopterin concentrations in white compared to black subjects, or no ethnic-related differences were reported in other studies (116, 117). A systematic review and meta-analysis has also investigated the association between a functional polymorphism of the interferon-γ gene, +874 T/A, associated with excess production of interferon-γ (118), and the risk of autoimmune disease. In this study, there were significant differences in the frequencies of the T allele across Asian (34.1%), Middle Eastern (47.8%), Latin American (51.5%), and Caucasian subjects (74.2%). Furthermore, the T allele was significantly associated with the risk of autoimmune disease in Latin Americans, but not in Middle Eastern, Asian, or Caucasian populations (119). Clearly, additional research is warranted to investigate the influence of ethnicity on interferon-γ production, macrophage activation, neopterin concentrations, and RDs. The additional observation that the SMD of urine neopterin was significantly associated with specific types of RD also requires further studies to investigate the capacity of urine neopterin to discriminate between different types of RD. At the same time, however, the significantly higher SMD of urine neopterin observed in studies of patients with SLE vs. other types of RD opens new opportunities to investigate the utility of this biomarker to diagnose and/or assess the severity of renal involvement, specifically nephritis, often observed in this group (120).
Our study has several strengths, including the assessment of neopterin in different biological fluids in a wide range of RD types, the study of associations between the effect size and several study and patient characteristics, and a rigorous evaluation of the risk of bias and the certainty of evidence. Significant limitations include the paucity of studies investigating specific types of RD (i.e., AS, SSc, FMF, and PsA), and the high heterogeneity observed. However, we identified potential sources of heterogeneity in subgroup analyses (type of RD and study continent for both plasma/serum and urine neopterin). Furthermore, sensitivity analysis ruled out the effect of individual studies on the overall effect size.
In conclusion, this systematic review and meta-analysis has shown the presence of significant alterations in the plasma/serum and urinary concentrations of neopterin, a biomarker of interferon-γ production, macrophage activation, inflammation, and oxidative stress, in patients with RD. Further research is warranted to determine the capacity of neopterin to identify early vs. overt RD manifestations and justify its introduction in clinical practice.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
AM: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. AZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1271383/full#supplementary-material
References
1. Calle E, Gómez-Puerta JA. The spectrum of rheumatic diseases. Surgery in rheumatic and musculoskeletal disease. Handb Systemic Autoimmune Dis (2018) 15: 1–13. doi: 10.1016/B978-0-444-63887-8.00001-3
2. McGonagle D, McDermott MF. A proposed classification of the immunological diseases. PLoS Med (2006) 3(8):e297. doi: 10.1371/journal.pmed.0030297
3. Moutsopoulos HM. Autoimmune rheumatic diseases: One or many diseases? J Transl Autoimmun (2021) 4:100129. doi: 10.1016/j.jtauto.2021.100129
4. Ben Mrid R, Bouchmaa N, Ainani H, El Fatimy R, Malka G, Mazini L. Anti-rheumatoid drugs advancements: New insights into the molecular treatment of rheumatoid arthritis. BioMed Pharmacother (2022) 151:113126. doi: 10.1016/j.biopha.2022.113126
5. Jung SM, Kim WU. Targeted immunotherapy for autoimmune disease. Immune Netw (2022) 22(1):e9. doi: 10.4110/in.2022.22.e9
6. Reynolds JA, Putterman C. Progress and unmet needs in understanding fundamental mechanisms of autoimmunity. J Autoimmun (2023) 137:102999. doi: 10.1016/j.jaut.2023.102999
7. Fugger L, Jensen LT, Rossjohn J. Challenges, progress, and prospects of developing therapies to treat autoimmune diseases. Cell (2020) 181(1):63–80. doi: 10.1016/j.cell.2020.03.007
8. Havnaer A, Han G. Autoinflammatory disorders: A review and update on pathogenesis and treatment. Am J Clin Dermatol (2019) 20(4):539–64. doi: 10.1007/s40257-019-00440-y
9. Efthimiou P, Petryna O, Nakasato P, Kontzias A. New insights on multigenic autoinflammatory diseases. Ther Adv Musculoskelet Dis (2022) 14:1759720X221117880. doi: 10.1177/1759720X221117880
10. Kawakami A, Endo Y, Koga T, Yoshiura KI, Migita K. Autoinflammatory disease: clinical perspectives and therapeutic strategies. Inflammation Regen (2022) 42(1):37. doi: 10.1186/s41232-022-00217-7
11. Ten Klooster PM, Oude Voshaar MAH, Fakhouri W, de la Torre I, Nicolay C, van de Laar M. Long-term clinical, functional, and cost outcomes for early rheumatoid arthritis patients who did or did not achieve early remission in a real-world treat-to-target strategy. Clin Rheumatol (2019) 38(10):2727–36. doi: 10.1007/s10067-019-04600-7
12. Bosello S, Fedele AL, Peluso G, Gremese E, Tolusso B, Ferraccioli G. Very early rheumatoid arthritis is the major predictor of major outcomes: clinical ACR remission and radiographic non-progression. Ann Rheum Dis (2011) 70(7):1292–5. doi: 10.1136/ard.2010.142729
13. Taylor PC. Update on the diagnosis and management of early rheumatoid arthritis. Clin Med (Lond) (2020) 20(6):561–4. doi: 10.7861/clinmed.2020-0727
14. Brown M, Bradbury LA. New approaches in ankylosing spondylitis. Med J Aust (2017) 206(5):192–4. doi: 10.5694/mja16.01111
15. Sakkas LI, Simopoulou T, Katsiari C, Bogdanos D, Chikanza IC. Early systemic sclerosis-opportunities for treatment. Clin Rheumatol (2015) 34(8):1327–31. doi: 10.1007/s10067-015-2902-5
16. Md Yusof MY, Vital EM. Early intervention in systemic lupus erythematosus: time for action to improve outcomes and health-care utilization. Rheumatol Adv Pract (2022) 6(1):rkab106. doi: 10.1093/rap/rkab106
17. Mallen CD, Helliwell T, Scott IC. How can primary care physicians enhance the early diagnosis of rheumatic diseases? Expert Rev Clin Immunol (2018) 14(3):171–3. doi: 10.1080/1744666X.2018.1429919
18. Mohan C, Assassi S. Biomarkers in rheumatic diseases: how can they facilitate diagnosis and assessment of disease activity? BMJ (2015) 351:h5079. doi: 10.1136/bmj.h5079
19. Guma M, Tiziani S, Firestein GS. Metabolomics in rheumatic diseases: desperately seeking biomarkers. Nat Rev Rheumatol (2016) 12(5):269–81. doi: 10.1038/nrrheum.2016.1
20. Harrison M. Erythrocyte sedimentation rate and C-reactive protein. Aust Prescr (2015) 38(3):93–4. doi: 10.18773/austprescr.2015.034
21. Firooz N, Albert DA, Wallace DJ, Ishimori M, Berel D, Weisman MH. High-sensitivity C-reactive protein and erythrocyte sedimentation rate in systemic lupus erythematosus. Lupus (2011) 20(6):588–97. doi: 10.1177/0961203310393378
22. Ruof J, Stucki G. Validity aspects of erythrocyte sedimentation rate and C-reactive protein in ankylosing spondylitis: a literature review. J Rheumatol (1999) 26(4):966–70.
23. Houttekiet C, de Vlam K, Neerinckx B, Lories R. Systematic review of the use of CRP in clinical trials for psoriatic arthritis: a concern for clinical practice? RMD Open (2022) 8(1):e001756. doi: 10.1136/rmdopen-2021-001756
24. Muftuoglu AU, Yazici H, Yurdakul S, Tuzun Y, Pazarli H, Gungen G, et al. Behcet's disease. Relation of serum C-reactive protein and erythrocyte sedimentation rates to disease activity. Int J Dermatol (1986) 25(4):235–9. doi: 10.1111/j.1365-4362.1986.tb02232.x
25. Korkmaz C, Ozdogan H, Kasapcopur O, Yazici H. Acute phase response in familial Mediterranean fever. Ann Rheum Dis (2002) 61(1):79–81. doi: 10.1136/ard.61.1.79
26. Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol (2004) 75(2):163–89. doi: 10.1189/jlb.0603252
27. Zhang J. Yin and yang interplay of IFN-gamma in inflammation and autoimmune disease. J Clin Invest (2007) 117(4):871–3. doi: 10.1172/JCI31860
28. De Benedetti F, Prencipe G, Bracaglia C, Marasco E, Grom AA. Targeting interferon-gamma in hyperinflammation: opportunities and challenges. Nat Rev Rheumatol (2021) 17(11):678–91. doi: 10.1038/s41584-021-00694-z
29. Kelchtermans H, Billiau A, Matthys P. How interferon-gamma keeps autoimmune diseases in check. Trends Immunol (2008) 29(10):479–86. doi: 10.1016/j.it.2008.07.002
30. Ding H, Wang G, Yu Z, Sun H, Wang L. Role of interferon-gamma (IFN-gamma) and IFN-gamma receptor 1/2 (IFNgammaR1/2) in regulation of immunity, infection, and cancer development: IFN-gamma-dependent or independent pathway. BioMed Pharmacother (2022) 155:113683. doi: 10.1016/j.biopha.2022.113683
31. Baxter-Parker G, Prebble HM, Cross S, Steyn N, Shchepetkina A, Hock BD, et al. Neopterin formation through radical scavenging of superoxide by the macrophage synthesised antioxidant 7,8-dihydroneopterin. Free Radic Biol Med (2020) 152:142–51. doi: 10.1016/j.freeradbiomed.2020.03.002
32. Prebble H, Cross S, Marks E, Healy J, Searle E, Aamir R, et al. Induced macrophage activation in live excised atherosclerotic plaque. Immunobiology (2018) 223(8-9):526–35. doi: 10.1016/j.imbio.2018.03.002
33. Oettl K, Greilberger J, Dikalov S, Reibnegger G. Interference of 7,8-dihydroneopterin with peroxynitrite-mediated reactions. Biochem Biophys Res Commun (2004) 321(2):379–85. doi: 10.1016/j.bbrc.2004.06.157
34. Gieseg SP, Maghzal G, Glubb D. Protection of erythrocytes by the macrophage synthesized antioxidant 7,8 dihydroneopterin. Free Radic Res (2001) 34(2):123–36. doi: 10.1080/10715760100300121
35. Huber C, Batchelor JR, Fuchs D, Hausen A, Lang A, Niederwieser D, et al. Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon-gamma. J Exp Med (1984) 160(1):310–6. doi: 10.1084/jem.160.1.310
36. Fukushima T, Nixon JC. Analysis of reduced forms of biopterin in biological tissues and fluids. Anal Biochem (1980) 102(1):176–88. doi: 10.1016/0003-2697(80)90336-X
37. Flavall EA, Crone EM, Moore GA, Gieseg SP. Dissociation of neopterin and 7,8-dihydroneopterin from plasma components before HPLC analysis. J Chromatogr B Analyt Technol BioMed Life Sci (2008) 863(1):167–71. doi: 10.1016/j.jchromb.2007.12.019
38. Westermann J, Thiemann F, Gerstner L, Tatzber F, Kozak I, Bertsch T, et al. Evaluation of a new simple and rapid enzyme-linked immunosorbent assay kit for neopterin determination. Clin Chem Lab Med (2000) 38(4):345–53. doi: 10.1515/CCLM.2000.050
39. Estelberger W, Weiss G, Petek W, Paletta B, Wachter H, Reibnegger G. Determination of renal clearance of neopterin by a pharmacokinetic approach. FEBS Lett (1993) 329(1-2):13–6. doi: 10.1016/0014-5793(93)80182-T
40. Gieseg SP, Baxter-Parker G, Lindsay A. Neopterin, inflammation, and oxidative stress: what could we be missing? Antioxidants (Basel) (2018) 7(7):80. doi: 10.3390/antiox7070080
41. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, editors. Joanna Briggs Institute Reviewer’s Manual. Adelaide, Australia: Johanna Briggs Institute (2017).
42. Cohen J. Statistical power analysis. Curr Dir Psychol Sci (1992) 1(3):98–101. doi: 10.1111/1467-8721.ep10768783
43. Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol (2011) 64(4):401–6. doi: 10.1016/j.jclinepi.2010.07.015
44. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
45. Booth A, Clarke M, Ghersi D, Moher D, Petticrew M, Stewart L. An international registry of systematic-review protocols. Lancet (2011) 377(9760):108–9. doi: 10.1016/S0140-6736(10)60903-8
46. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol (2014) 14:135. doi: 10.1186/1471-2288-14-135
47. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol (2005) 5:13. doi: 10.1186/1471-2288-5-13
48. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21(11):1539–58. doi: 10.1002/sim.1186
49. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
50. Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech Bulletin (1999) 47:15–7.
51. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics (1994) 50(4):1088–101. doi: 10.2307/2533446
52. Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol (2001) 54(10):1046–55. doi: 10.1016/S0895-4356(01)00377-8
53. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics (2000) 56(2):455–63. doi: 10.1111/j.0006-341X.2000.00455.x
54. Hannonen P, Tikanoja S, Hakola M, Mottonen T, Viinikka L, Oka M. Urinary neopterin index as a measure of rheumatoid activity. Scand J Rheumatol (1986) 15(2):148–52. doi: 10.3109/03009748609102081
55. Hagihara M, Nagatsu T, Ohhashi M, Miura T. Concentrations of neopterin and biopterin in serum from patients with rheumatoid arthritis or systemic lupus erythematosus and in synovial fluid from patients with rheumatoid or osteoarthritis. Clin Chem (1990) 36(4):705–6. doi: 10.1093/clinchem/36.4.705
56. Krause A, Protz H, Goebel KM. Correlation between synovial neopterin and inflammatory activity in rheumatoid arthritis. Ann Rheum Dis (1989) 48(8):636–40. doi: 10.1136/ard.48.8.636
57. Leohirun L, Thuvasethakul P, Sumethkul V, Pholcharoen T, Boonpucknavig V. Urinary neopterin in patients with systemic lupus erythematosus. Clin Chem (1991) 37(1):47–50. doi: 10.1093/clinchem/37.1.47
58. Lim KL, Jones AC, Brown NS, Powell RJ. Urine neopterin as a parameter of disease activity in patients with systemic lupus erythematosus: comparisons with serum sIL-2R and antibodies to dsDNA, erythrocyte sedimentation rate, and plasma C3, C4, and C3 degradation products. Ann Rheum Dis (1993) 52(6):429–35. doi: 10.1136/ard.52.6.429
59. Yoon J, Lee SH, Bang D, Lee S, Kim JC, Chung TH. Elevated serum levels of neopterin in patients with Behçet's disease. Ann Dermatol (1993) 5(2):74–8. doi: 10.5021/ad.1993.5.2.74
60. Altindağ Z, Şahin G, Akpek G, Koç Y, Işimer A, Kansu E, et al. Urinary neopterin levels as an indicator of disease activation in Behçet’s disease. Pteridines (1995) 6:79–83. doi: 10.1515/pteridines.1995.6.2.79
61. Csipo I, Czirjak L, Szanto S, Szerafin L, Sipka S, Szegedi G. Decreased serum tryptophan and elevated neopterin levels in systemic sclerosis. Clin Exp Rheumatol (1995) 13(2):269–70.
62. Samsonov MY, Nassonov EL, Tilz GP, Geht BM, Demel U, Gurkina GT, et al. Elevated serum levels of neopterin in adult patients with polymyositis/dermatomyositis. Br J Rheumatol (1997) 36(6):656–60. doi: 10.1093/rheumatology/36.6.656
63. Altindag ZZ, Sahin G, Inanici F, Hascelik Z. Urinary neopterin excretion and dihydropteridine reductase activity in rheumatoid arthritis. Rheumatol Int (1998) 18(3):107–11. doi: 10.1007/s002960050067
64. Andrys C, Krejsek J, Slezak R, Drahosova M, Kopecky O. Serum soluble adhesion molecules (sICAM-1, sVCAM-1, sE-selectin) and neopterin in patients with Sjogren's syndrome. Acta Med (Hradec Kralove) (1999) 42(3):97–101. doi: 10.14712/18059694.2019.151
65. Keser G, Oksel F, Aksu K, Kabasakal Y, Gumusdis G, Doganavs argil E, et al. Serum neopterin levels in Behcet's syndrome. Clin Rheumatol (2000) 19(4):328–9.
66. Kokcam I, Naziroglu M. Effects of vitamin E supplementation on blood antioxidants levels in patients with Behcet's disease. Clin Biochem (2002) 35(8):633–9. doi: 10.1016/S0009-9120(02)00400-9
67. Sfriso P, Ostuni P, Botsios C, Andretta M, Oliviero F, Punzi L, et al. Serum and salivary neopterin and interferon-gamma in primary Sjogren's syndrome. Correlation with clinical, laboratory and histopathologic features. Scand J Rheumatol (2003) 32(2):74–8. doi: 10.1080/03009740310000067
68. de Castro MR, Di Marco GS, Arita DY, Teixeira LC, Pereira AB, Casarini DE. Urinary neopterin quantification by reverse-phase high-performance liquid chromatography with ultraviolet detection. J Biochem Biophys Methods (2004) 59(3):275–83. doi: 10.1016/j.jbbm.2004.03.004
69. Coskun B, Saral Y, Godekmerdan A, Erden I, Coskun N. Activation markers in Behcet's disease. Skinmed (2005) 4(5):282–6. doi: 10.1111/j.1540-9740.2005.03865
70. Jin O, Sun LY, Zhou KX, Zhang XS, Feng XB, Mok MY, et al. Lymphocyte apoptosis and macrophage function: correlation with disease activity in systemic lupus erythematosus. Clin Rheumatol (2005) 24(2):107–10. doi: 10.1007/s10067-004-0972-x
71. Mahmoud RA, El-Gendi HI, Ahmed HH. Serum neopterin, tumor necrosis factor-alpha and soluble tumor necrosis factor receptor II (p75) levels and disease activity in Egyptian female patients with systemic lupus erythematosus. Clin Biochem (2005) 38(2):134–41. doi: 10.1016/j.clinbiochem.2004.11.002
72. Kose O, Arca E, Akgul O, Erbil K. The levels of serum neopterin in Behcet's disease–objective marker of disease activity. J Dermatol Sci (2006) 42(2):128–30. doi: 10.1016/j.jdermsci.2006.02.001
73. Ozkan Y, Yardim-Akaydin S, Sepici A, Engin B, Sepici V, Simsek B. Assessment of homocysteine, neopterin and nitric oxide levels in Behcet's disease. Clin Chem Lab Med (2007) 45(1):73–7. doi: 10.1515/CCLM.2007.018
74. Erturan I, Basak PY, Ozturk O, Ceyhan AM, Akkaya VB. Is there any relationship between serum and urine neopterin and serum interferon-gamma levels in the activity of Behcet's disease? J Eur Acad Dermatol Venereol (2009) 23(12):1414–8. doi: 10.1111/j.1468-3083.2009.03334.x
75. Salem SA, Farouk HM, Mostafa AA, Hassan IM, Osman WM, Al-Shamy HA, et al. Keratinocyte and lymphocyte apoptosis: relation to disease outcome in systemic lupus erythematosus patients with and without cutaneous manifestations. Eur J Dermatol (2010) 20(1):35–41. doi: 10.1684/ejd.2010.0812
76. Rho YH, Solus J, Raggi P, Oeser A, Gebretsadik T, Shintani A, et al. Macrophage activation and coronary atherosclerosis in systemic lupus erythematosus and rheumatoid arthritis. Arthritis Care Res (Hoboken) (2011) 63(4):535–41. doi: 10.1002/acr.20365
77. Bahrehmand F, Vaisi-Raygani A, Kiani A, Rahimi Z, Tavilani H, Navabi SJ, et al. Matrix metalloproteinase-2 functional promoter polymorphism G1575A is associated with elevated circulatory MMP-2 levels and increased risk of cardiovascular disease in systemic lupus erythematosus patients. Lupus (2012) 21(6):616–24. doi: 10.1177/0961203312436857
78. Ozkan Y, Mete G, Sepici-Dincel A, Sepici V, Simsek B. Tryptophan degradation and neopterin levels in treated rheumatoid arthritis patients. Clin Rheumatol (2012) 31(1):29–34. doi: 10.1007/s10067-011-1767-5
79. D'Agostino LE, Ventimiglia F, Verna JA, Colina Ade L, Aguirre Y, Arturi A, et al. Correlation between DAS-28 and neopterin as a biochemical marker of immune system activation in early rheumatoid arthritis. Autoimmunity (2013) 46(1):44–9. doi: 10.3109/08916934.2012.722143
80. Shahmohamadnejad S, Vaisi-Raygani A, Shakiba Y, Kiani A, Rahimi Z, Bahrehmand F, et al. Association between butyrylcholinesterase activity and phenotypes, paraoxonase192 rs662 gene polymorphism and their enzymatic activity with severity of rheumatoid arthritis: correlation with systemic inflammatory markers and oxidative stress, preliminary report. Clin Biochem (2015) 48(1-2):63–9. doi: 10.1016/j.clinbiochem.2014.08.016
81. Gulkesen A, Akgol G, Tuncer T, Kal GA, Telo S, Poyraz AK, et al. Relationship between leptin and neopterin levels and disease activation parameters in patients with rheumatoid arthritis. Arch Rheumatol (2016) 31(4):333–9. doi: 10.5606/ArchRheumatol.2016.5893
82. Baniamerian H, Bahrehmand F, Vaisi-Raygani A, Rahimi Z, Pourmotabbed T. Angiotensin type 1 receptor A1166C polymorphism and systemic lupus erythematosus: correlation with cellular immunity and oxidative stress markers. Lupus (2017) 26(14):1534–9. doi: 10.1177/0961203317711008
83. El-Lebedy D, Hussein J, Ashmawy I, Mohammed AM. Serum level of neopterin is not a marker of disease activity in treated rheumatoid arthritis patients. Clin Rheumatol (2017) 36(9):1975–9. doi: 10.1007/s10067-016-3433-4
84. Tanhapour M, Miri A, Vaisi-Raygani A, Bahrehmand F, Kiani A, Rahimi Z, et al. Synergism between apolipoprotein E E4 allele and paraoxonase (PON1) 55-M allele is associated with risk of systemic lupus erythematosus. Clin Rheumatol (2018) 37(4):971–7. doi: 10.1007/s10067-017-3859-3
85. Zorbozan N, Demir S, Çobankara V. Evaluation of the relationship between TNFα, sTNFR1, sTNFR2, sIL2R, IL6, neopterin with disease activity in ankylosing spondylitis. Turkish J Biochem (2018) 43(5):487–94. doi: 10.1515/tjb-2017-0350
86. Iranshahi N, Assar S, Amiri SM, Zafari P, Fekri A, Taghadosi M. Decreased gene expression of epstein-barr virus-induced gene 3 (EBI-3) may contribute to the pathogenesis of rheumatoid arthritis. Immunol Invest (2019) 48(4):367–77. doi: 10.1080/08820139.2018.1549066
87. Akyurek F, Tuncez Akyurek F. Investigation of pregnancy associated plasma protein-A and neopterin levels in Behcet's patients. Dermatol Ther (2020) 33(4):e13443. doi: 10.1111/dth.13443
88. Peng QL, Zhang YM, Liang L, Liu X, Ye LF, Yang HB, et al. A high level of serum neopterin is associated with rapidly progressive interstitial lung disease and reduced survival in dermatomyositis. Clin Exp Immunol (2020) 199(3):314–25. doi: 10.1111/cei.13404
89. Ekin S, Sivrikaya A, Akdag T, Yilmaz S, Gulcemal S. Elevated levels of neopterin and pentraxin 3 in patients with rheumatoid arthritis. Horm Mol Biol Clin Investig (2021) 42(4):419–23. doi: 10.1515/hmbci-2021-0012
90. Videm V, Houge IS, Liff MH, Hoff M. Inflammation mediates approximately one quarter of excess relative all-cause mortality in persons with rheumatoid arthritis: the Trondelag Health Study. Sci Rep (2022) 12(1):18599. doi: 10.1038/s41598-022-21977-9
91. Carru C, Zinellu A, Sotgia S, Serra R, Usai MF, Pintus GF, et al. A new HPLC method for serum neopterin measurement and relationships with plasma thiols levels in healthy subjects. BioMed Chromatogr (2004) 18(6):360–6. doi: 10.1002/bmc.325
92. Mayersbach P, Augustin R, Schennach H, Schonitzer D, Werner ER, Wachter H, et al. Commercial enzyme-linked immunosorbent assay for neopterin detection in blood donations compared with RIA and HPLC. Clin Chem (1994) 40(2):265–6. doi: 10.1093/clinchem/40.2.265
93. Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol (2007) 96:41–101. doi: 10.1016/S0065-2776(07)96002-2
94. Darwich L, Coma G, Pena R, Bellido R, Blanco EJ, Este JA, et al. Secretion of interferon-gamma by human macrophages demonstrated at the single-cell level after costimulation with interleukin (IL)-12 plus IL-18. Immunology (2009) 126(3):386–93. doi: 10.1111/j.1365-2567.2008.02905.x
95. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep (2014) 6:13. doi: 10.12703/P6-13
96. Chistiakov DA, Myasoedova VA, Revin VV, Orekhov AN, Bobryshev YV. The impact of interferon-regulatory factors to macrophage differentiation and polarization into M1 and M2. Immunobiology (2018) 223(1):101–11. doi: 10.1016/j.imbio.2017.10.005
97. Pearl JE, Saunders B, Ehlers S, Orme IM, Cooper AM. Inflammation and lymphocyte activation during mycobacterial infection in the interferon-gamma-deficient mouse. Cell Immunol (2001) 211(1):43–50. doi: 10.1006/cimm.2001.1819
98. Swindle EJ, Brown JM, Radinger M, DeLeo FR, Metcalfe DD. Interferon-gamma enhances both the anti-bacterial and the pro-inflammatory response of human mast cells to Staphylococcus aureus. Immunology (2015) 146(3):470–85. doi: 10.1111/imm.12524
99. Peng SL, Szabo SJ, Glimcher LH. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc Natl Acad Sci U S A (2002) 99(8):5545–50. doi: 10.1073/pnas.082114899
100. Yang SL, Xu XJ, Tang YM, Song H, Xu WQ, Zhao FY, et al. Associations between inflammatory cytokines and organ damage in pediatric patients with hemophagocytic lymphohistiocytosis. Cytokine (2016) 85:14–7. doi: 10.1016/j.cyto.2016.05.022
101. Bracaglia C, de Graaf K, Pires Marafon D, Guilhot F, Ferlin W, Prencipe G, et al. Elevated circulating levels of interferon-gamma and interferon-gamma-induced chemokines characterise patients with macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Ann Rheum Dis (2017) 76(1):166–72. doi: 10.1136/annrheumdis-2015-209020
102. Teachey DT, Lacey SF, Shaw PA, Melenhorst JJ, Maude SL, Frey N, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discovery (2016) 6(6):664–79. doi: 10.1158/2159-8290.CD-16-0040
103. Kokic V, Martinovic Kaliterna D, Radic M, Perkovic D, Cvek M, Capkun V. Relationship between vitamin D, IFN-gamma, and E2 levels in systemic lupus erythematosus. Lupus (2016) 25(3):282–8. doi: 10.1177/0961203315605367
104. Troppmair J, Nachbaur K, Herold M, Aulitzky W, Tilg H, Gastl G, et al. In-vitro and in-vivo studies on the induction of neopterin biosynthesis by cytokines, alloantigens and lipopolysaccharide (LPS). Clin Exp Immunol (1988) 74(3):392–7.
105. Muller MM, Curtius HC, Herold M, Huber CH. Neopterin in clinical practice. Clin Chim Acta (1991) 201(1-2):1–16. doi: 10.1016/0009-8981(91)90019-9
106. Schoedon G, Troppmair J, Fontana A, Huber C, Curtius HC, Niederwieser A. Biosynthesis and metabolism of pterins in peripheral blood mononuclear cells and leukemia lines of man and mouse. Eur J Biochem (1987) 166(2):303–10. doi: 10.1111/j.1432-1033.1987.tb13515.x
107. Gieseg SP, Reibnegger G, Wachter H, Esterbauer H. 7,8 Dihydroneopterin inhibits low density lipoprotein oxidation in vitro. Evidence that this macrophage secreted pteridine is an anti-oxidant. Free Radic Res (1995) 23(2):123–36. doi: 10.3109/10715769509064027
108. Duggan S, Rait C, Platt A, Gieseg S. Protein and thiol oxidation in cells exposed to peroxyl radicals is inhibited by the macrophage synthesised pterin 7,8-dihydroneopterin. Biochim Biophys Acta (2002) 1591(1-3):139–45. doi: 10.1016/S0167-4889(02)00272-0
109. Gieseg SP, Cato S. Inhibition of THP-1 cell-mediated low-density lipoprotein oxidation by the macrophage-synthesised pterin, 7,8-dihydroneopterin. Redox Rep (2003) 8(2):113–5. doi: 10.1179/135100003125001396
110. Widner B, Mayr C, Wirleitner B, Fuchs D. Oxidation of 7,8-dihydroneopterin by hypochlorous acid yields neopterin. Biochem Biophys Res Commun (2000) 275(2):307–11. doi: 10.1006/bbrc.2000.3323
111. Shchepetkina AA, Hock BD, Miller A, Kennedy MA, Gieseg SP. Effect of 7,8-dihydroneopterin mediated CD36 down regulation and oxidant scavenging on oxidised low-density lipoprotein induced cell death in human macrophages. Int J Biochem Cell Biol (2017) 87:27–33. doi: 10.1016/j.biocel.2017.03.017
112. Dántola ML, Vignoni M, Capparelli AL, Lorente C, Thomas AH. Stability of 7,8-dihydropterins in air-equilibrated aqueous solutions. Helv Chimica Acta (2008) 91(3):411–25. doi: 10.1002/hlca.200890046
113. Lindsay A, Janmale T, Draper N, Gieseg SP. Measurement of changes in urinary neopterin and total neopterin in body builders using SCX HPLC. Pteridines (2014) 25(2):53–63. doi: 10.1515/pteridines-2014-0003
114. Spencer ME, Jain A, Matteini A, Beamer BA, Wang NY, Leng SX, et al. Serum levels of the immune activation marker neopterin change with age and gender and are modified by race, BMI, and percentage of body fat. J Gerontol A Biol Sci Med Sci (2010) 65(8):858–65. doi: 10.1093/gerona/glq066
115. Sotgia S, Zinellu A, Mangoni AA, Serra R, Pintus G, Caruso C, et al. Cellular immune activation in Sardinian middle-aged, older adults and centenarians. Exp Gerontol (2017) 99:133–7. doi: 10.1016/j.exger.2017.10.005
116. Diamondstone LS, Tollerud DJ, Fuchs D, Wachter H, Brown LM, Maloney E, et al. Factors influencing serum neopterin and beta 2-microglobulin levels in a healthy diverse population. J Clin Immunol (1994) 14(6):368–74. doi: 10.1007/BF01546321
117. Currie MS, Rao MK, Blazer DG, Cohen HJ. Age and functional correlations of markers of coagulation and inflammation in the elderly: functional implications of elevated crosslinked fibrin degradation products (D-dimers). J Am Geriatr Soc (1994) 42(7):738–42. doi: 10.1111/j.1532-5415.1994.tb06534.x
118. Pravica V, Perrey C, Stevens A, Lee JH, Hutchinson IV. A single nucleotide polymorphism in the first intron of the human IFN-gamma gene: absolute correlation with a polymorphic CA microsatellite marker of high IFN-gamma production. Hum Immunol (2000) 61(9):863–6. doi: 10.1016/S0198-8859(00)00167-1
119. Lee YH, Bae SC. Association between interferon-gamma +874 T/A polymorphism and susceptibility to autoimmune diseases: a meta-analysis. Lupus (2016) 25(7):710–8. doi: 10.1177/0961203315624557
Keywords: neopterin, rheumatic diseases, inflammation, oxidative stress, biomarkers, metabolism
Citation: Mangoni AA and Zinellu A (2023) A systematic review and meta-analysis of neopterin in rheumatic diseases. Front. Immunol. 14:1271383. doi: 10.3389/fimmu.2023.1271383
Received: 02 August 2023; Accepted: 04 September 2023;
Published: 18 September 2023.
Edited by:
Philippe Saas, Etablissement Français du Sang AuRA, FranceReviewed by:
Natsumi Inoue, Cincinnati Children’s Hospital Medical Center, United StatesDietmar Fuchs, Innsbruck Medical University, Austria
Copyright © 2023 Mangoni and Zinellu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arduino A. Mangoni, YXJkdWluby5tYW5nb25pQGZsaW5kZXJzLmVkdS5hdQ==
 Arduino A. Mangoni
Arduino A. Mangoni Angelo Zinellu
Angelo Zinellu