
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 13 November 2023
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1269341
This article is part of the Research Topic New immunotherapy strategies and related therapeutic targets for gastrointestinal malignancies View all 14 articles
For colorectal cancer (CRC), surgical resection remains essential for achieving good prognoses. Unfortunately, numerous patients with locally advanced CRC and metastatic CRC failed to meet surgical indications or achieve pathological complete response after surgery. Perioperative therapy has been proven to effectively lower tumor staging and reduce recurrence and metastasis. Immune checkpoint inhibitors (ICIs) have shown unprecedented prolongation of survival time and satisfactory safety in patients with high microsatellite instability/deficient mismatch repair (MSI-H/dMMR), while the therapeutic effect obtained by patients with mismatch repair-proficient or microsatellite stable (pMMR/MSS) was considered minimal. However, recent studies found that certain CRC patients with dMMR/MSI-H presented intrinsic or acquired immune resistance, and pMMR/MSS CRC patients can also achieve better efficacy. Therefore, more predictors are required for screening patients with potential clinical benefits. Since the discovery of synergistic effects between immunotherapy, chemotherapy, and radiotherapy, different immunotherapy-based therapies have been applied to the perioperative therapy of CRC in an increasing number of research. This review comprehensively summarized the past and current progress of different combinations of immunotherapy in perioperative clinical trials for CRC, focusing on the efficacy and safety, and points out the direction for future development.
Colorectal cancer (CRC) is the third most common cancer and the leading cause of cancer death worldwide (1–4). Due to the lack of early symptoms, 36% of patients were diagnosed with locally advanced CRC (LACRC) (stage II (cT3–4, N0)/stage III (any cT, N+)), and 22% presented with distant metastasis (5). The perioperative therapy (days before and after surgery) is of great significance in promoting tumor downgrading and reducing the local recurrence and metastasis, including neoadjuvant (preoperative) therapy and adjuvant (postoperative) therapy (6–9). Given the compelling long-term durable remission in metastatic CRC (mCRC), immune checkpoint inhibitors (ICIs) have attracted great attention in the perioperative therapy of CRC. DNA mismatch repair (MMR) and Microsatellite instability (MSI) are considered important predictors of sensitivity for immunotherapy-based strategies (10) DNA mismatch repair (MMR) is an important pathway to maintain genomic stability (10, 11). Microsatellites are highly polymorphic repetitive DNA sequences in the human genome and MSI is defined as genomic instability in cancer cells due to a deficiency in MMR (dMMR) (10–12). MSI CRC accounts for 15% of all sporadic CRC, which can be divided into MSI-high (MSI-H) and MSI-low (MSI-L) according to the frequency of microsatellite marker instability (10, 11, 13). The remaining CRC is classified as microsatellite stable (MSS), with proficiency in MMR (pMMR) (10, 11). dMMR/MSI-H CRC is associated with a higher tumor mutation burden and neoantigen load and more lymphocyte infiltration than pMMR/MSS/MSI-L CRC (10, 11, 14).
dMMR/MSI-H CRC patients, whose sensitivity to ICIs is significantly higher than that of patients with pMMR/MSS/MSI-L, have derived notable pathological responses from neoadjuvant immunotherapy (14–17). However, 40% -60% of MSI-H CRC are inherently resistant to immunotherapy (14, 18) Therefore, the main challenge is to provide more benefits of immunotherapy for the majority of patients with pMMR, MSS, MSI-L, or insensitive MSI-H CRC (19) Fortunately, it is discovered that there is a synergistic effect between immunotherapy, chemotherapy, and radiotherapy (20, 21). An increasing number of clinical trials have explored the efficacy and safety of different immunotherapy-based therapies in the perioperative period (6, 17). Therefore, this article comprehensively reviewed previous achievements and the latest progress of different immunotherapy combination therapies in the perioperative period, which may provide new therapy strategies for CRC patients to achieve better efficacy and safety, as well as the mechanism of immunotherapy combination therapy and promising predictors to identify the patients with potential benefits.
In 2015, after a phase 2 clinical trial first proved that MSI CRC was a potential beneficiary, ICIs has been explored more extensively in CRC (22). Thereafter the impressive efficacy and safety of CheckMate-142 (23) and KEYNOTE-177 (16) in the treatment of dMMR/MSI-H mCRC promoted the Food and Drug Administration (FDA) ‘s approval of pembrolizumab, a programmed cell death-1 (PD-1) inhibitor, as the first-line treatment for MSI-H advanced CRC. Recently, ipilimumab combined with nivolumab, inhibitors of a cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and PD-1, has also been granted approval by the FDA for the treatment of dMMR/MSI-H mCRC (15, 24). The encouraging results motivated researchers to investigate the application of immunotherapy in the perioperative period of CRC. Recently, studies on immunotherapy combined with chemoradiotherapy and targeted drugs have been emerging. Additionally, various immunotherapy strategies have been developed to change the situation of “cold tumor” treatment, such as oncolytic virus (25, 26), cytokine therapy (27), and chimeric antigen receptor T cell therapy (28, 29).
At present, the MMR/MSI system has become the most important classification standard for CRC (30, 31). dMMR/MSI-H represents a good prognosis in early-stage CRC, while in metastatic disease it seems to confer a poor prognosis (10). Moreover, there is evidence showing that patients with dMMR/MSI-H CRC can obtain high reactivity of ICIs therapy, but for a majority of patients with pMMR or MSS, the clinical benefits from ICIs are generally minimal (12, 14, 17, 18, 32–37). However, recent studies have shown that certain CRC patients with MSI-H presented intrinsic or acquired immune resistance, while the patients with pMMR/MSS can also achieve a higher pathological complete response (pCR) rate (38, 39). Therefore, the optimal biomarkers for screening patients with potential clinical benefits are needed (40).
Currently, an increased tumor mutation burden has been observed in MSI-H CRC patients who benefit from immunotherapy, as well as MSS CRC patients, which is considered another effective biomarker (14, 19, 32). Moreover, research has identified two distinct subtypes, MSI-H1 and MSI-H2, each with different prognostic implications (41). Notably, the MSI-H1 subgroup, enriched with M2 macrophages and characterized by high PD-L2 expression, tends to indicate a lower survival rate (41). Among dMMR CRCs, beta-2-microglobulin mutations that result in complete beta-2-microglobulin loss are associated with reduced recurrence and metastasis (19).
Assessing the extent of infiltration and co-expression of CD8+ and PD-1 of T cells in tumors may be warranted to predict the overall survival rate and pCR rate of pMMR patients (38, 42). Additionally, polymerase epsilon exonuclease domain mutations (POLE EDM) (43–45), guanylate binding protein 2 expression (46), and soluble PD-L1 level may also be promising indicators to identify the pMMR patients with a favorable response to ICIs. Furthermore, CMTM6 expression in M2 macrophages (47), circulating L-arginine (48), the human gastrointestinal microbiome (49), fibroblast growth factor receptor 1-3 deficiency (50), and circulating tumor DNA (45, 51) may also play crucial roles in monitoring immunotherapy efficacy.
PD-1 and CTLA-4 are key immune checkpoints for T cells, PD-1 and programmed death-ligand 1 (PD-L1) play a role by inhibiting the proximal signaling of T cell antigen receptor, while CTLA-4 weakens costimulatory signals through the co-receptor CD28, suppressing T cell activation (52, 53). Excessive activation or expression of immune checkpoints in cancer may promote malignant proliferation and metastasis (53). Therefore, PD-1-CTLA-4 inhibitors may have a synergistic effect by simultaneously inhibiting both pathways, achieving better therapeutic effects than ICIs monotherapy. However, the effective response to PD-1 blocking requires more tumor-infiltrating lymphocytes in the tumor microenvironment, which also indicates that pMMR tumors have limited efficacy owing to the lack of tumor-infiltrating lymphocytes (54) (Figure 1).
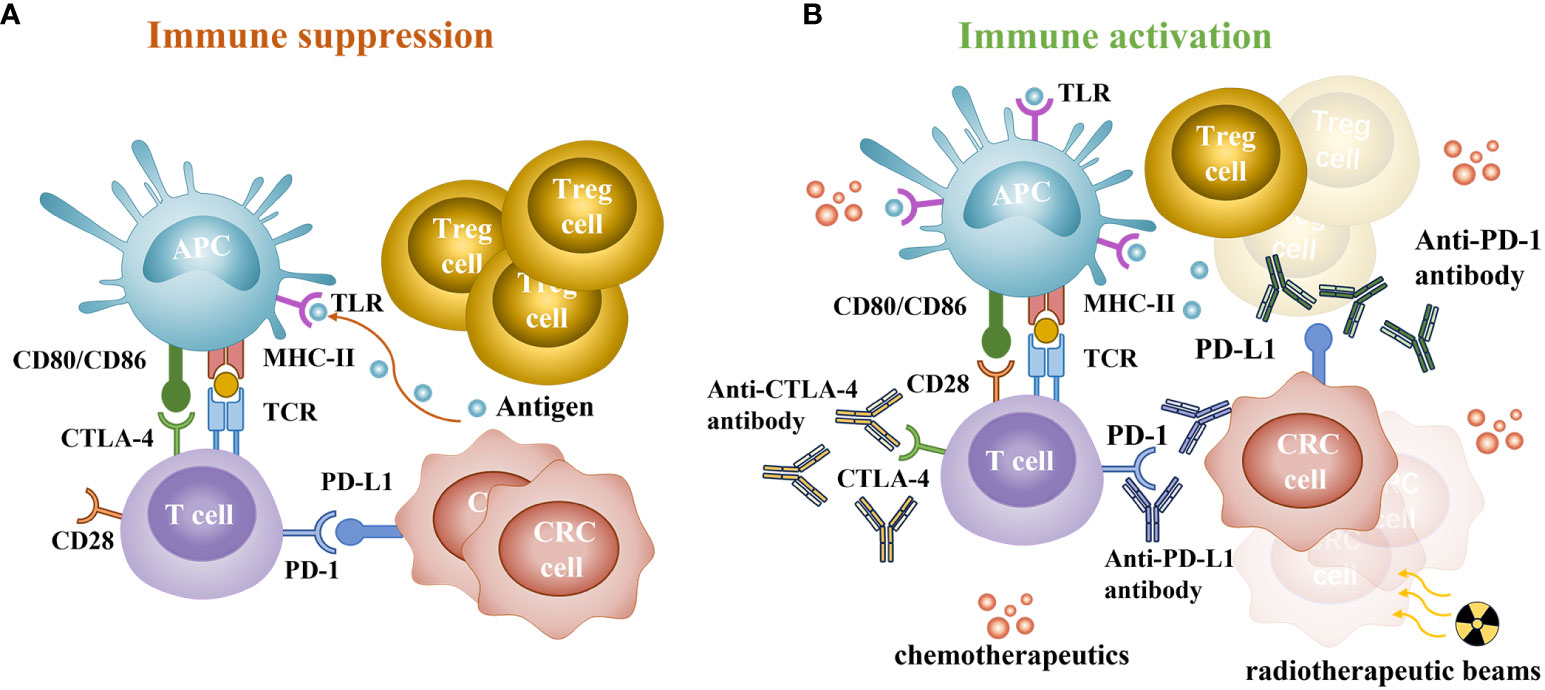
Figure 1 Immune status of patients with colorectal cancer. (A) The main way of immune suppression. CTLA-4 can competitively bind to CD80 or CD86 and inhibit activation. PD-1 is a key checkpoint for T cells, interacting with abnormally upregulated PD-L1 on cancer cells and immune cells, leading to T cell depletion and immune evasion. (B) The synergistic effect of chemoradiotherapy and immunotherapy. PD-1/PD-L1 and CTLA-4 checkpoint inhibitors can inhibit the negative feedback regulation of cancer cells and restore the anticancer function of T cells. Chemotherapeutics can induce immunogenic cell death and disrupt tumor escape strategies, increase the activity and quantity of toll-like receptors (TLR), promote DCs activation, deplete Treg cells, and reduce inhibition of T cells. Radiation induces tumor cell damage, releases a large number of damage-associated molecular patterns, and increases the formation and memory response of tumor-infiltrating lymphocytes.
The positive effects of standard chemotherapy on tumor immunity are mainly reflected in inducing immunogenic cell death and disrupting tumor escape strategies (21). Taxanes can elevate the activity of toll-like receptors and promote the activation of dendritic cells (21). Cyclophosphamide can deplete Treg cells, reducing the inhibition of tumor-infiltrating T cells (55). Therefore, the immune enhancement effect of chemotherapy may have a synergistic effect with immunotherapy. However, owing to its non-targeted effect, excessive chemotherapy can also lead to the depletion or dysfunction of immune cells (21).
Radiation induces tumor cell damage that releases a large amount of damage-associated molecular patterns, increasing the formation of tumor-infiltrating lymphocytes and memory response (56). CD8+T cells release γ-interferon that upregulates the expression of PD-L1 in tumor cells, thereby exerting a synergistic effect with ICIs (20). In the previous research, when CTLA-4 inhibitor was added to radiation, radiosensitizing anti-CTLA-4 immunotherapy was observed in breast and CRC (20).
Compared with adjuvant therapy, neoadjuvant ICIs can induce stronger and more extensive tumor-specific T cell responses, reduce the incidence of toxicity, have better compliance, and may even achieve clinical complete remission and avoid unnecessary surgery (42). Although Jiahao Zhu et al. believed that surgery can cause a decrease in tumor antigens, and damage to blood vessels and lymph nodes in the surgical area, leading to reduced survival benefits of adjuvant ICIs (33), effective adjuvant therapy may be necessary for diminishing small residual lesions and preventing recurrence and metastasis, especially for the patients that didn’t achieve pCR after surgery (57).
The initial case report showed that two dMMR locally advanced rectal cancer (LARC) patients received PD-1 inhibitors (nivolumab) monotherapy to avoid adverse events (AEs) of chemoradiotherapy, which also enabled them to achieve pCR and clinical complete remission, and the latter adopted the observation and waiting (W&W) strategy without surgery (58). Moreover, the dMMR LACRC patients who are not eligible for chemotherapy, receiving anti-PD-1 inhibitors (pembrolizumab) monotherapy can also get pCR (59).
Further researches have also confirmed the value of ICIs in neoadjuvant therapy for CRC (Table 1). In a prospective phase 2 study (NCT04165772) (64), 12 patients with LARC received neoadjuvant PD-1 inhibitors (dostarlimab) monotherapy. Surprisingly, all patients achieved clinical complete remission, and no grade 3/4 AEs were reported. Moreover, Han, Kai, et al. (76) observed a high incidence rate (27.6%) of dMMR in 268 T4bM0 CRC patients. The pCR rate of the neoadjuvant ICIs monotherapy (pembrolizumab or nivolumab) group was significantly higher than that of the chemoradiotherapy group (70.0% vs. 0%). Compared with neoadjuvant chemotherapy and chemoradiotherapy, it significantly reduced the incidence of open surgery and had better disease-free survival and relatively longer overall survival. These results are also consistent with other researches (71, 72, 77, 78). Other neoadjuvant PD-1 inhibitors (toripalimab (45), sintilimab (73)) monotherapy may also have similar efficacy. A multicenter phase II study (NCT05662527) will further evaluate the efficacy and safety of neoadjuvant pembrolizumab in patients with stage I–III dMMR colon cancer (79).
Therefore, neoadjuvant monotherapy based on ICIs can significantly improve the pCR rate and avoid unnecessary surgeries, especially for those are ineligible for chemotherapy, which may translate into long-term survival benefits for dMMR LACRC, with acceptable safety and a low recurrence rate (64, 76). However, there is still a large proportion of patients who failed to achieve pCR after surgery. Effective adjuvant therapy may be necessary to reduce micro-diseases and prevent recurrence and metastasis (57). While there are limited researches on adjuvant ICIs monotherapy. Lynch syndrome is a common form of familial CRC associated with alterations in four DNA MMR genes (80). A case report shows that a Lynch syndrome patient with peritoneal metastasis received nivolumab as adjuvant therapy, achieving pCR, and no recurrence was observed during a 9-month follow-up (81). A retrospective study (75) suggests that dMMR/MSI⁃H LACRC patients who have received neoadjuvant immunotherapy can further improve their pCR rate to 75.9% by combining adjuvant anti-PD-1 treatment based on their postoperative efficacy. These researches indicated that adjuvant ICIs monotherapy can be a promising option for mCRC and LACRC. To further determine this advantage, a phase III clinical trial (NCT03803553) will evaluate the efficacy of adjuvant PD-I inhibitor (nivolumab) versus standard adjuvant chemotherapy in MSI-H CRC patients (75).
In the NICHE study (NCT03026140) (35), early-stage dMMR colon cancer patients receiving neoadjuvant CTLA-4 inhibitor (ipilimumab) and PD-1inhibitor (nivolumab) gained better pathological responses (5). The NICHE 2 study further expanded the sample size, with a pCR rate of 67% (72/112) and 5 patients experiencing 3/4 grade AEs (37). However, this combination did not indicate significant improvement in pMMR patients (35). Similarly, compared to perioperative chemotherapy (82), combining anti-CTLA-4 (tremelimumab) and anti-PD-L1 (durvalumab) did not significantly prolong median relapse-free survival (9.7 months) and overall survival (24.5 months) in pMMR CRC patients with liver metastasis (36). Numerous studies have confirmed the efficacy and safety of immunotherapy combinations, which has promoted the NCCN guidelines (v2.2022) to recommend nivolumab ± ipilimumab or pembrolizumab as neoadjuvant treatment options for resectable dMMR/MSI-H mCRC (35, 83). But current immunotherapy combinations did not improve the efficacy of pMMR patients with early-stage CRC or mCRC significantly. Nonetheless, the safety of immunotherapy combinations has been confirmed for pMMR CRC (36, 83).
Recently, two studies on immunotherapy combined with chemotherapy are underway, which will address the issue of whether the synergistic effect can also appear in perioperative therapy of LACRC. ATOMIC study (NCT02912559) (84) is exploring the efficacy of PD-L1 inhibitors (atezolizumab) combined with chemotherapy versus adjuvant chemotherapy in dMMR stage III CRC, with the primary endpoint being disease-free survival. Although the POLE EDM indicates a better response of CRC to immunotherapy plus chemotherapy, there seems to be no similar improvement in advanced CRC (43, 85). Therefore, the POLEM trial (NCT03827044) (85) aims to investigate whether adjuvant chemotherapy combined with PD-L1 inhibitor (avelumab) can improve disease-free survival in stage III dMMR/MSI-H/POLE EDM colon cancer patients.
The potential synergistic effect of immunotherapy and radiotherapy has prompted extensive research to validate its efficacy in various cancers (86–89). Not limited to dMMR-mCRC, it was reported that local radiotherapy combined with PD-1 inhibitor (sintilimab) (90) or PD-L1 inhibitor (tislelizumab) (91) can overcome the immune resistance of pMMR mCRC. Several researches are ongoing to investigate whether the synergistic effect appears in LARC. Li et al. are conducting a multicenter Ib phase study to investigate the safety and efficacy of PD-1 inhibitor (sintilimab) combined with radiotherapy for MSI-H/dMMR rectal cancer (92). Another Phase II study will evaluate whether neoadjuvant anti-PD-1therapy (pembrolizumab) and radiotherapy can improve the safety and efficacy of LARC patients (93).
Although two researches applying PD-1 inhibitors (nivolumab (74) and pembrolizumab (65), respectively) combined with chemotherapy showed no significant improvement in pCR rate, another study on 980 LARC patients suggested that the pCR rate of the SCRT with immunotherapy (PD-1 inhibitor camrelizumab) group was higher than that of the non-immunotherapy group (49.2% vs 21.6%) (66). The significant differences in this large-scale study manifested the effectiveness and safety of combined immunotherapy. In a phase II trial (NCT04231552) (50), patients with advanced rectal cancer received CAPOX combined with PD-1 inhibitor (camrelizumab) after SCRT and reached a higher pCR rate of 46.2% than that of the combination of PD-L1 inhibitor (avelumab) and mFOLFOX6 after SCRT (37.5%) (68). While it is worth noting that the mid-term results of a phase II trial (NCT04911517) (39) showed that in pMMR LARC patients, the combination of LCRT and PD-1 inhibitor (tislelizumab) also achieved a high pCR rate (50.0%). Therefore, despite the shorter radiotherapy time, SCRT may achieve similar efficacy as LCRT, combined with immunotherapy.
Considering that LCRT may increase toxicity and reduce tolerance of patients compared with SCRT, it remains necessary to determine the optimal combination of LARC neoadjuvant therapy. TORCH (NCT04518280) (60) explored the combination of SCRT and PD-1 inhibitor (toripalimab) for the neoadjuvant therapy of LARC. And the preliminary efficacy showed that the pCR rate and CR rate were as high as 56.2% (18/32) and 58.1% (36/62), respectively. This result suggests that SCRT combined with immunotherapy may be more advantageous. Moreover, the REGINA study (NCT04503694) (94) will investigate the efficacy of the combination of PD-1 inhibitor (Nivolumab) and chemotherapy with SCRT, while the PRIME-PR study (NCT04621370) (95) will directly compare the differences in efficacy between LCRT and SCRT in neoadjuvant immunotherapy combined with TNT.
Currently, neoadjuvant chemoradiotherapy followed by total mesorectal excision is considered the optimal treatment for LARC (74). However, recent results have shown that compared to neoadjuvant chemoradiotherapy alone (10.5%-38.0%), long-term radiation therapy (LCRT) or short-term radiation therapy (SCRT) combined with immunotherapy can significantly improve the pCR rate (37.5%-50.0%) of LARC patients without increasing the risk of AEs (96, 97). Moreover, the determination of radiotherapy strategies may further improve the safety of combination therapy.
Since cyclooxygenase-2 may mediate immune escape and inflammatory response (98), applying cyclooxygenase-2 inhibitors may elevate the responsiveness of cancer cells to ICIs. To further demonstrate its synergistic effect, a phase II trial (NCT03926338) (17) discovered that PD-1 inhibitor (toripalimab) (99) combined with cyclooxygenase-2 inhibitors (celecoxib) can achieve a higher pCR rate in dMMR/MSI-H LACRC, compared with anti-PD-1 monotherapy (15 (88%) vs 11 (65%)). Only 1 case (3%) of grade 3/4 treatment-related AEs was observed. However, in the NICHE study (NCT03026140) (35), pMMR CRC patients who received neoadjuvant CTLA-4 inhibitor (ipilimumab) combined with PD-1 inhibitor (nivolumab) and celecoxib showed no significant improvement. Therefore, the synergistic effect of targeted therapy and immunotherapy seems to be more evident in dMMR/MSI-H LACRC, while the improvement in the efficacy of pMMR CRC is limited.
Recent studies have shown that compared with chemotherapy or chemoradiotherapy, perioperative immunotherapy-based therapies can significantly improve the pCR rate of dMMR/MSI-H CRC, without increasing AEs or postoperative complications. Meanwhile, the combination strategies are also expected to further improve the efficacy of pMMR patients, especially immunotherapy combined with chemoradiotherapy or radiotherapy. However, recent studies on immunotherapy combinations and the combination of targeted treatment and ICIs seem to have failed to achieve better results in pMMR/MSS CRC. Many promising immunotherapy-based therapies still require expanding sample size and follow-up results. Furthermore, mature biomarkers for identifying CRC patients with therapeutic responses are required. Additionally, limited toxicity of immunotherapy may be related to low doses and shorter treatment duration, while the higher pCR rate may be associated with more treatment cycles and longer treatment intervals (17, 35, 42). Therefore, further research is expected to determine the optimal therapeutic combination, treatment cycle, and dosage for different populations to coordinate the relationship between efficacy and safety (100). In addition, there are many ongoing but not yet reported studies on perioperative immunotherapy for CRC, as shown in Supplementary Table 1.
J-TC: Conceptualization, Investigation, Resources, Supervision, Writing – original draft, Writing – review & editing. Y-WZ: Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Writing – review & editing. T-RH: Investigation, Resources, Writing – review & editing. J-LW: Investigation, Writing – review & editing. MQ: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Science and Technology Department of Sichuan Province Key Research and Development Project (grant no. 2022YFS0209) and 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (grant no. ZYJC21017).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1269341/full#supplementary-material
1. Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet (2019) 394(10207):1467–80. doi: 10.1016/s0140-6736(19)32319-0
2. Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol (2019) 16(12):713–32. doi: 10.1038/s41575-019-0189-8
3. Akimoto N, Ugai T, Zhong R, Hamada T, Fujiyoshi K, Giannakis M, et al. Rising incidence of early-onset colorectal cancer - a call to action. Nat Rev Clin Oncol (2021) 18(4):230–43. doi: 10.1038/s41571-020-00445-1
4. Riesco-Martinez MC, Modrego A, Espinosa-Olarte P, La Salvia A, Garcia-Carbonero R. Perioperative chemotherapy for liver metastasis of colorectal cancer: lessons learned and future perspectives. Curr Treat Opt Oncol (2022) 23(9):1320–37. doi: 10.1007/s11864-022-01008-5
5. Zhang X, Wu T, Cai X, Dong J, Xia C, Zhou Y, et al. Neoadjuvant immunotherapy for msi-H/dmmr locally advanced colorectal cancer: new strategies and unveiled opportunities. Front Immunol (2022) 13:795972. doi: 10.3389/fimmu.2022.795972
6. Matzner P, Sandbank E, Neeman E, Zmora O, Gottumukkala V, Ben-Eliyahu S. Harnessing cancer immunotherapy during the unexploited immediate perioperative period. Nat Rev Clin Oncol (2020) 17(5):313–26. doi: 10.1038/s41571-019-0319-9
7. Horowitz M, Neeman E, Sharon E, Ben-Eliyahu S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol (2015) 12(4):213–26. doi: 10.1038/nrclinonc.2014.224
8. van der Bij GJ, Oosterling SJ, Beelen RH, Meijer S, Coffey JC, van Egmond M. The perioperative period is an underutilized window of therapeutic opportunity in patients with colorectal cancer. Ann Surg (2009) 249(5):727–34. doi: 10.1097/SLA.0b013e3181a3ddbd
9. Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (Tme) versus preoperative chemoradiotherapy, tme, and optional adjuvant chemotherapy in locally advanced rectal cancer (Rapido): a randomised, open-label, phase 3 trial. Lancet Oncol (2021) 22(1):29–42. doi: 10.1016/s1470-2045(20)30555-6
10. Zhao P, Li L, Jiang X, Li Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-pd-1/pd-L1 immunotherapy efficacy. J Hematol Oncol (2019) 12(1):54. doi: 10.1186/s13045-019-0738-1
11. Du F, Liu Y. Predictive molecular markers for the treatment with immune checkpoint inhibitors in colorectal cancer. J Clin Lab Anal (2022) 36(1):e24141. doi: 10.1002/jcla.24141
12. Yu I, Dakwar A, Takabe K. Immunotherapy: recent advances and its future as a neoadjuvant, adjuvant, and primary treatment in colorectal cancer. Cells (2023) 12(2):258. doi: 10.3390/cells12020258
13. Pawlik TM, Raut CP, Rodriguez-Bigas MA. Colorectal carcinogenesis: msi-H versus msi-L. Dis Markers (2004) 20(4-5):199–206. doi: 10.1155/2004/368680
14. Lin A, Zhang J, Luo P. Crosstalk between the msi status and tumor microenvironment in colorectal cancer. Front Immunol (2020) 11:2039. doi: 10.3389/fimmu.2020.02039
15. Lenz HJ, Van Cutsem E, Luisa Limon M, Wong KYM, Hendlisz A, Aglietta M, et al. First-line nivolumab plus low-dose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: the phase ii checkmate 142 study. J Clin Oncol (2022) 40(2):161–70. doi: 10.1200/jco.21.01015
16. Andre T, Amonkar M, Norquist JM, Shiu KK, Kim TW, Jensen BV, et al. Health related quality of life in patients with microsatellite instability-high or mismatch repair deficient metastatic colorectal cancer treated with first-line pembrolizumab versus chemotherapy (Keynote-177): an open-label, randomised, phase 3 trial. Lancet Oncol (2021) 22(5):665–77. doi: 10.1016/s1470-2045(21)00064-4
17. Hu H, Kang L, Zhang J, Wu Z, Wang H, Huang M, et al. Neoadjuvant pd-1 blockade with toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high, locally advanced, colorectal cancer (Picc): A single-centre, parallel-group, non-comparative, randomised, phase 2 trial. Lancet Gastroenterol Hepatol (2022) 7(1):38–48. doi: 10.1016/s2468-1253(21)00348-4
18. Kwon M, An M, Klempner SJ, Lee H, Kim KM, Sa JK, et al. Determinants of response and intrinsic resistance to pd-1 blockade in microsatellite instability-high gastric cancer. Cancer Discovery (2021) 11(9):2168–85. doi: 10.1158/2159-8290.Cd-21-0219
19. Fan A, Wang B, Wang X, Nie Y, Fan D, Zhao X, et al. Immunotherapy in colorectal cancer: current achievements and future perspective. Int J Biol Sci (2021) 17(14):3837–49. doi: 10.7150/ijbs.64077
20. Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol (2015) 16(13):e498–509. doi: 10.1016/s1470-2045(15)00007-8
21. Yu WD, Sun G, Li J, Xu J, Wang X. Mechanisms and therapeutic potentials of cancer immunotherapy in combination with radiotherapy and/or chemotherapy. Cancer Lett (2019) 452:66–70. doi: 10.1016/j.canlet.2019.02.048
22. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. Pd-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372(26):2509–20. doi: 10.1056/NEJMoa1500596
23. Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (Checkmate 142): an open-label, multicentre, phase 2 study. Lancet Oncol (2017) 18(9):1182–91. doi: 10.1016/s1470-2045(17)30422-9
24. Sahin IH, Akce M, Alese O, Shaib W, Lesinski GB, El-Rayes B, et al. Immune checkpoint inhibitors for the treatment of Msi-H/Mmr-D colorectal cancer and a perspective on resistance mechanisms. Br J Cancer (2019) 121(10):809–18. doi: 10.1038/s41416-019-0599-y
25. Ren Y, Miao JM, Wang YY, Fan Z, Kong XB, Yang L, et al. Oncolytic viruses combined with immune checkpoint therapy for colorectal cancer is a promising treatment option. Front Immunol (2022) 13:961796. doi: 10.3389/fimmu.2022.961796
26. Crupi MJF, Taha Z, Janssen TJA, Petryk J, Boulton S, Alluqmani N, et al. Oncolytic virus driven T-cell-based combination immunotherapy platform for colorectal cancer. Front Immunol (2022) 13:1029269. doi: 10.3389/fimmu.2022.1029269
27. Liu C, Liu R, Wang B, Lian J, Yao Y, Sun H, et al. Blocking Il-17a enhances tumor response to anti-pd-1 immunotherapy in microsatellite stable colorectal cancer. J Immunother Cancer (2021) 9(1). doi: 10.1136/jitc-2020-001895
28. Yuan J, Li J, Gao C, Jiang C, Xiang Z, Wu J. Immunotherapies catering to the unmet medical need of cold colorectal cancer. Front Immunol (2022) 13:1022190. doi: 10.3389/fimmu.2022.1022190
29. Aparicio C, Belver M, Enríquez L, Espeso F, Núñez L, Sánchez A, et al. Cell therapy for colorectal cancer: the promise of chimeric antigen receptor (Car)-T cells. Int J Mol Sci (2021) 22(21):11781. doi: 10.3390/ijms222111781
30. Evrard C, Tachon G, Randrian V, Karayan-Tapon L, Tougeron D. Microsatellite instability: diagnosis, heterogeneity, discordance, and clinical impact in colorectal cancer. Cancers (2019) 11(10):1567. doi: 10.3390/cancers11101567
31. Jin Z, Sinicrope FA. Prognostic and predictive values of mismatch repair deficiency in non-metastatic colorectal cancer. Cancers (2021) 13(2):300. doi: 10.3390/cancers13020300
32. Kanani A, Veen T, Søreide K. Neoadjuvant immunotherapy in primary and metastatic colorectal cancer. Br J Surg (2021) 108(12):1417–25. doi: 10.1093/bjs/znab342
33. Zhu J, Lian J, Xu B, Pang X, Ji S, Zhao Y, et al. Neoadjuvant immunotherapy for colorectal cancer: right regimens, right patients, right directions? Front Immunol (2023) 14:1120684. doi: 10.3389/fimmu.2023.1120684
34. Wu Z, Hu H, Wang C, Zhang J, Cai Y, Xie X, et al. The prognostic and predictive value of mismatch repair status in patients with locally advanced rectal cancer following neoadjuvant therapy. Ann Trans Med (2022) 10(8):491. doi: 10.21037/atm-22-124
35. Verschoor YL, Jvd B, Beets G, Sikorska K, Aalbers A, Av L, et al. Neoadjuvant nivolumab, ipilimumab, and celecoxib in mmr-proficient and Mmr-deficient colon cancers: final clinical analysis of the niche study. JCO (2022) 40(16_suppl):3511. doi: 10.1200/JCO.2022.40.16_suppl.3511
36. Kanikarla Marie P, Haymaker C, Parra ER, Kim YU, Lazcano R, Gite S, et al. Pilot clinical trial of perioperative durvalumab and tremelimumab in the treatment of resectable colorectal cancer liver metastases. Clin Cancer Res (2021) 27(11):3039–49. doi: 10.1158/1078-0432.Ccr-21-0163
37. Chalabi M, Verschoor YL, van den Berg J, Sikorska K, Beets G, Lent AV, et al. Neoadjuvant immune checkpoint inhibition in locally advanced mmr-deficient colon cancer: the niche-2 study. Ann Oncol (2022) 33(7):S1389–S. doi: 10.1016/j.annonc.2022.08.016
38. Hou W, Yi C, Zhu H. Predictive biomarkers of colon cancer immunotherapy: present and future. Front Immunol (2022) 13:1032314. doi: 10.3389/fimmu.2022.1032314
39. Gao J, Zhang X, Yang Z, Zhang J, Bai Z, Deng W, et al. Interim result of phase ii, prospective, single-arm trial of long-course chemoradiotherapy combined with concurrent tislelizumab in locally advanced rectal cancer. Front Oncol (2023) 13:1057947. doi: 10.3389/fonc.2023.1057947
40. Wu M, Huang Q, Xie Y, Wu X, Ma H, Zhang Y, et al. Improvement of the anticancer efficacy of Pd-1/Pd-L1 blockade via combination therapy and pd-L1 regulation. J Hematol Oncol (2022) 15(1):24. doi: 10.1186/s13045-022-01242-2
41. Hu W, Yang Y, Qi L, Chen J, Ge W, Zheng S. Subtyping of microsatellite instability-high colorectal cancer. Cell Commun Signal CCS (2019) 17(1):79. doi: 10.1186/s12964-019-0397-4
42. Chalabi M, Fanchi LF, Dijkstra KK, Van den Berg JG, Aalbers AG, Sikorska K, et al. Neoadjuvant immunotherapy leads to pathological responses in mmr-proficient and mmr-deficient early-stage colon cancers. Nat Med (2020) 26(4):566–76. doi: 10.1038/s41591-020-0805-8
43. Wen L, Chen Z, Ji X, Fong WP, Shao Q, Ren C, et al. Pathological complete response to immune checkpoint inhibitor in patients with colorectal cancer liver metastases harboring pole exonuclease domain mutation. J Immunother Cancer (2022) 10(7):e004487. doi: 10.1136/jitc-2022-004487
44. Ma X, Riaz N, Samstein RM, Lee M, Makarov V, Valero C, et al. Functional landscapes of pole and pold1 mutations in checkpoint blockade-dependent antitumor immunity. Nat Genet (2022) 54(7):996–1012. doi: 10.1038/s41588-022-01108-w
45. Qin Q, Yang K, Ma T, Wang H, Yu P, Yuan M, et al. Serial circulating tumor DNA in monitoring the effect of neoadjuvant and adjuvant immunotherapy in patients with colon cancer: case series and review of the literature. J Immunother (2022) 45(8):358–62. doi: 10.1097/cji.0000000000000436
46. Wang H, Zhou Y, Zhang Y, Fang S, Zhang M, Li H, et al. Subtyping of microsatellite stability colorectal cancer reveals guanylate binding protein 2 (Gbp2) as a potential immunotherapeutic target. J Immunother Cancer (2022) 10(4)e004302. doi: 10.1136/jitc-2021-004302
47. Wu X, Lan X, Hu W, Zhang W, Lai X, Xu S, et al. Cmtm6 expression in M2 macrophages is a potential predictor of Pd-1/Pd-L1 inhibitor response in colorectal cancer. Cancer Immunol Immunother (2021) 70(11):3235–48. doi: 10.1007/s00262-021-02931-6
48. Peyraud F, Guegan JP, Bodet D, Nafia I, Fontan L, Auzanneau C, et al. Circulating L-arginine predicts the survival of cancer patients treated with immune checkpoint inhibitors. Ann Oncol (2022) 33(10):1041–51. doi: 10.1016/j.annonc.2022.07.001
49. Xu S, Yin W, Zhang Y, Lv Q, Yang Y, He J. Foes or friends? Bacteria enriched in the tumor microenvironment of colorectal cancer. Cancers (2020) 12(2):372. doi: 10.3390/cancers12020372
50. Lin Z, Cai M, Zhang P, Li G, Liu T, Li X, et al. Phase ii, single-arm trial of preoperative short-course radiotherapy followed by chemotherapy and camrelizumab in locally advanced rectal cancer. J Immunother Cancer (2021) 9(11):e003554. doi: 10.1136/jitc-2021-003554
51. Wang C, Chevalier D, Saluja J, Sandhu J, Lau C, Fakih M. Regorafenib and nivolumab or pembrolizumab combination and circulating tumor dna response assessment in refractory microsatellite stable colorectal cancer. Oncologist (2020) 25(8):e1188–94. doi: 10.1634/theoncologist.2020-0161
52. Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers (2020) 6(1):38. doi: 10.1038/s41572-020-0160-6
53. Johdi NA, Sukor NF. Colorectal cancer immunotherapy: options and strategies. Front Immunol (2020) 11:1624. doi: 10.3389/fimmu.2020.01624
54. Coukos G. Neoadjuvant immune-checkpoint blockade in resectable colon cancer. Nat Med (2020) 26(4):473–4. doi: 10.1038/s41591-020-0826-3
55. Scurr M, Pembroke T, Bloom A, Roberts D, Thomson A, Smart K, et al. Low-dose cyclophosphamide induces antitumor T-cell responses, which associate with survival in metastatic colorectal cancer. Clin Cancer Res (2017) 23(22):6771–80. doi: 10.1158/1078-0432.Ccr-17-0895
56. Herrera FG, Ronet C, Ochoa de Olza M, Barras D, Crespo I, Andreatta M, et al. Low-dose radiotherapy reverses tumor immune desertification and resistance to immunotherapy. Cancer Discovery (2022) 12(1):108–33. doi: 10.1158/2159-8290.Cd-21-0003
57. Kuan FC, Lai CH, Ku HY, Wu CF, Hsieh MC, Liu TW, et al. The survival impact of delayed surgery and adjuvant chemotherapy on stage ii/iii rectal cancer with pathological complete response after neoadjuvant chemoradiation. Int J Cancer (2017) 140(7):1662–9. doi: 10.1002/ijc.30562
58. Zhang J, Cai J, Deng Y, Wang H. Complete response in patients with locally advanced rectal cancer after neoadjuvant treatment with nivolumab. Oncoimmunology (2019) 8(12):e1663108. doi: 10.1080/2162402x.2019.1663108
59. Demisse R, Damle N, Kim E, Gong J, Fakih M, Eng C, et al. Neoadjuvant immunotherapy-based systemic treatment in mmr-deficient or msi-high rectal cancer: case series. J Natl Compr Cancer Netw JNCCN (2020) 18(7):798–804. doi: 10.6004/jnccn.2020.7558
60. Wang Y, Shen L, Wan J, Zhang H, Wu R, Wang J, et al. Short-course radiotherapy combined with capox and toripalimab for the total neoadjuvant therapy of locally advanced rectal cancer: A randomized, prospective, multicentre, double-arm, phase ii trial (Torch). BMC Cancer (2022) 22(1):274. doi: 10.1186/s12885-022-09348-z
61. Zheng R, Wang BS, Li Z, Chi P, Xu B. Combining Chemotherapy and Tislelizumab with Preoperative Split-Course Hypofraction Radiotherapy for Locally Advanced Rectal Cancer: Study Protocol of a Prospective, Single-Arm, Phase Ii Trial. BMJ Open (2023) 13(3):e066976. doi: 10.1136/bmjopen-2022-066976
62. Salvatore L, Bens M, Corallo S, Bergamo F, Pellegrini I, Rasola C, et al. Phase Ii Study of Preoperative (Preop) Chemoradiotherapy (Ctrt) Plus Avelumab (Ave) in Patients (Pts) with Locally Advanced Rectal Cancer (Larc): the Avana Study. JCO (2021) 39(15_suppl):3511. doi: 10.1200/JCO.2021.39.15_suppl.3511
63. Fan Y, Zhu X, Xu C, Ding C, Hu J, Hong Q, et al. Neoadjuvant Arterial Embolization Chemotherapy Combined Pd-1 Inhibitor for Locally Advanced Rectal Cancer (Neci Study): a Protocol for a Phase II Study. BMJ Open (2023) 13(3):e069401. doi: 10.1136/bmjopen-2022-069401
64. Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, et al. Pd-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med (2022) 386(25):2363–76. doi: 10.1056/NEJMoa2201445
65. Rahma OE, Yothers G, Hong TS, Russell MM, You YN, Parker W, et al. Use of total neoadjuvant therapy for locally advanced rectal cancer: initial results from the pembrolizumab arm of a phase 2 randomized clinical trial. JAMA Oncol (2021) 7(8):1225–30. doi: 10.1001/jamaoncol.2021.1683
66. Zhai ML, Zhang FY, Yang JR, Zhang S, Zhao L, Lin ZY, et al. Current status of neoadjuvant therapy for locally advanced rectal cancer in Wuhan Union hospital cancer center. Radiat Oncol (London England) (2022) 17(1):109. doi: 10.1186/s13014-022-02081-8
67. Grassi E, Zingaretti C, Petracci E, Corbelli J, Papiani G, Banchelli I, et al. Phase II study of capecitabine-based concomitant chemoradiation followed by durvalumab as a neoadjuvant strategy in locally advanced rectal cancer: the pandora trial. ESMO open (2023) 8(5):101824. doi: 10.1016/j.esmoop.2023.101824
68. Shamseddine A, Zeidan Y, Bouferraa Y, Turfa R, Kattan J, Mukherji D, et al. So-30 efficacy and safety of neoadjuvant short-course radiation follow ed by mfolfox-6 plus avelumab for locally-advanced rectal adenocarcino ma: averectal study. Ann Oncol 32:S215. doi: 10.1016/j.annonc.2021.05.054
69. Wu AW, Li YJ, Ji DB, Zhang L, Zhang XY, Cai Y, et al. Pkuch 04 trial: total neoadjuvant chemoradiation combined with neoadjuvant pd-1 blockade for pmmr/mss locally advanced middle to low rectal cancer. JCO (2022) 40(16_suppl):3609.
70. Liu XZ, Xiong Z, Xiao BY, Yu GY, Li YJ, Yao YF, et al. Multicenter Real-World Study on Safety and Efficacy of Neoadjuvant Therapy in Combination with Immunotherapy for Colorectal Cancer. Zhonghua wei chang wai ke za zhi = Chinese J Gastrointest Surg (2022) 25(3):219–27. doi: 10.3760/cma.j.cn441530-20220228-00070
71. Xiao BY, Zhang X, Cao TY, Li DD, Jiang W, Kong LH, et al. Neoadjuvant immunotherapy leads to major response and low recurrence in localized mismatch repair-deficient colorectal cancer. J Natl Compr Cancer Network JNCCN (2023) 21(1):60–6.e5. doi: 10.6004/jnccn.2022.7060
72. Pei F, Wu J, Zhao Y, He W, Yao Q, Huang M, et al. Single-agent neoadjuvant immunotherapy with a pd-1 antibody in locally advanced mismatch repair-deficient or microsatellite instability-high colorectal cancer. Clin Colorect Cancer (2023) 22(1):85–91. doi: 10.1016/j.clcc.2022.11.004
73. Chen G, Jin Y, Guan WL, Zhang RX, Xiao WW, Cai PQ, et al. Neoadjuvant pd-1 blockade with sintilimab in mismatch-repair deficient, locally advanced rectal cancer: an open-label, single-centre phase 2 study. Lancet Gastroenterol Hepatol (2023) 8(5):422–31. doi: 10.1016/s2468-1253(22)00439-3
74. Bando H, Tsukada Y, Inamori K, Togashi Y, Koyama S, Kotani D, et al. Preoperative chemoradiotherapy plus nivolumab before surgery in patients with microsatellite stable and microsatellite instability-high locally advanced rectal cancer. Clin Cancer Res (2022) 28(6):1136–46. doi: 10.1158/1078-0432.Ccr-21-3213
75. Zhang X, Yang R, Wu T, Cai X, Li G, Yu K, et al. Efficacy and safety of neoadjuvant monoimmunotherapy with pd-1 inhibitor for dmmr/msi⁃H locally advanced colorectal cancer: A single-center real-world study. Front Immunol (2022) 13:913483. doi: 10.3389/fimmu.2022.913483
76. Han K, Tang J-H, Liao L-E, Jiang W, Sui Q-Q, Xiao B-Y, et al. Neoadjuvant immune checkpoint inhibition improves organ preservation in T4bm0 colorectal cancer with mismatch repair deficiency: A retrospective observational study. Dis Colon Rectum (2022) 66(10):e996–e1005. doi: 10.1097/dcr.0000000000002466
77. Ludford K, Raghav K, Murphy MAB, Fleming ND, Nelson D, Lee MS, et al. Neoadjuvant pembrolizumab in localized/locally advanced solid tumors with mismatch repair deficiency. Ann Oncol (2021) 32:S1210–S. doi: 10.1016/j.annonc.2021.08.1703
78. Eefsen RL, Larsen JS, Klarskov LL, Altaf R, Høgdall E, Ingeholm P, et al. Therapy with pembrolizumab in treatment-naïve patients with nonmetastatic, mismatch repair deficient colorectal cancer. Int J Cancer (2023) 152(10):2145–52. doi: 10.1002/ijc.34420
79. Justesen TF, Gögenur I, Tarpgaard LS, Pfeiffer P, Qvortrup C. Evaluating the efficacy and safety of neoadjuvant pembrolizumab in patients with stage I-iii mmr-deficient colon cancer: A national, multicentre, prospective, single-arm, phase ii study protocol. BMJ Open (2023) 13(6):e073372. doi: 10.1136/bmjopen-2023-073372
80. Boland PM, Yurgelun MB, Boland CR. Recent progress in lynch syndrome and other familial colorectal cancer syndromes. CA: Cancer J Clin (2018) 68(3):217–31. doi: 10.3322/caac.21448
81. Tonello M, Nappo F, Vassallo L, Di Gaetano R, Davoli C, Pizzolato E, et al. Complete pathological response of colorectal peritoneal metastases in lynch syndrome after immunotherapy case report: is a paradigm shift in cytoreductive surgery needed? BMC Gastroenterol (2022) 22(1):17. doi: 10.1186/s12876-021-02084-x
82. Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative folfox4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (Eortc 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol (2013) 14(12):1208–15. doi: 10.1016/s1470-2045(13)70447-9
83. Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, et al. Colon cancer, version 2.2021, nccn clinical practice guidelines in oncology. J Natl Compr Cancer Network JNCCN (2021) 19(3):329–59. doi: 10.6004/jnccn.2021.0012
84. Sinicrope FA, Ou FS, Zemla T, Nixon AB, Mody K, Levasseur A, et al. Randomized Trial of Standard Chemotherapy Alone or Combined with Atezolizumab as Adjuvant Therapy for Patients with Stage Iii Colon Cancer and Deficient Mismatch Repair (Atomic, Alliance A021502). JCO (2019) 37(15):e15169. doi: 10.1200/JCO.2019.37.15_suppl.e15169
85. Lau D, Kalaitzaki E, Church DN, Pandha H, Tomlinson I, Annels N, et al. Rationale and design of the polem trial: avelumab plus fluoropyrimidine-based chemotherapy as adjuvant treatment for stage iii mismatch repair deficient or pole exonuclease domain mutant colon cancer: a phase iii randomised study. ESMO Open (2020) 5(1):e000638. doi: 10.1136/esmoopen-2019-000638
86. Segal NH, Cercek A, Ku G, Wu AJ, Rimner A, Khalil DN, et al. Phase ii single-arm study of durvalumab and tremelimumab with concurrent radiotherapy in patients with mismatch repair-proficient metastatic colorectal cancer. Clin Cancer Res (2021) 27(8):2200–8. doi: 10.1158/1078-0432.Ccr-20-2474
87. McLaughlin M, Patin EC, Pedersen M, Wilkins A, Dillon MT, Melcher AA, et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer (2020) 20(4):203–17. doi: 10.1038/s41568-020-0246-1
88. Qian JM, Schoenfeld JD. Radiotherapy and immunotherapy for head and neck cancer: current evidence and challenges. Front Oncol (2020) 10:608772. doi: 10.3389/fonc.2020.608772
89. Filippi AR, Fava P, Badellino S, Astrua C, Ricardi U, Quaglino P. Radiotherapy and immune checkpoints inhibitors for advanced melanoma. Radiother Oncol (2016) 120(1):1–12. doi: 10.1016/j.radonc.2016.06.003
90. Zhou P, Wang Y, Qin S, Han Y, Yang Y, Zhao L, et al. Abscopal effect triggered by radiation sequential mono-immunotherapy resulted in a complete remission of pmmr sigmoid colon cancer. Front Immunol (2023) 14:1139527. doi: 10.3389/fimmu.2023.1139527
91. Liu S, Zhang Y, Lin Y, Wang P, Pan Y. Case report: the msi-L/P-mmr metastatic rectal cancer patient who failed systemic therapy responds to anti-pd-1 immunotherapy after stereotactic body radiation-therapy. Front Immunol (2022) 13:981527. doi: 10.3389/fimmu.2022.981527
92. Li X, Fang C, Wang X, Yu Y, Wang Z, Qiu M. Neoadjuvant treatment of sintilimab plus hypofractionated radiotherapy for msi-H/dmmr rectal cancer: a prospective, multicenter, phase ib study. Cancer Med (2022) 11(23):4405–10. doi: 10.1002/cam4.4720
93. Corrò C, Buchs NC, Tihy M, Durham-Faivre A, Bichard P, Frossard JL, et al. Study protocol of a phase ii study to evaluate safety and efficacy of neo-adjuvant pembrolizumab and radiotherapy in localized rectal cancer. BMC Cancer (2022) 22(1):772. doi: 10.1186/s12885-022-09820-w
94. Bregni G, Senti C, Reina EA, Gkolfakis P, Moretti L, Veron A, et al. 505tip regina: A phase ii trial of neoadjuvant regorafenib (Rego) in c ombination with nivolumab (Nivo) and short-course radiotherapy (Scrt) in intermediate-risk, stage ii-iii rectal cancer (Rc). Ann Oncol 32:S579. doi: 10.1016/j.annonc.2021.08.1024
95. Hanna CR, O'Cathail SM, Graham JS, Saunders M, Samuel L, Harrison M, et al. Durvalumab (Medi 4736) in combination with extended neoadjuvant regimens in rectal cancer: A study protocol of a randomised phase ii trial (Prime-rt). Radiat Oncol (London England) (2021) 16(1):163. doi: 10.1186/s13014-021-01888-1
96. Chen L, Yang X, Zhang Y, Liu J, Jiang Q, Ji F, et al. Survival outcomes analysis according to mismatch repair status in locally advanced rectal cancer patients treated with neoadjuvant chemoradiotherapy. Front Oncol (2022) 12:920916. doi: 10.3389/fonc.2022.920916
97. Cercek A, Roxburgh CSD, Strombom P, Smith JJ, Temple LKF, Nash GM, et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol (2018) 4(6):e180071. doi: 10.1001/jamaoncol.2018.0071
98. Hashemi Goradel N, Najafi M, Salehi E, Farhood B, Mortezaee K. Cyclooxygenase-2 in cancer: a review. J Cell Physiol (2019) 234(5):5683–99. doi: 10.1002/jcp.27411
99. Keam SJ. Toripalimab: first global approval. Drugs (2019) 79(5):573–8. doi: 10.1007/s40265-019-01076-2
Keywords: colorectal cancer, perioperative therapy, immune checkpoint inhibition, microsatellite instability-high, mismatch repair deficiency, mismatch repair proficiency, microsatellite stable
Citation: Chen J-T, Zhou Y-W, Han T-R, Wei J-L and Qiu M (2023) Perioperative immune checkpoint inhibition for colorectal cancer: recent advances and future directions. Front. Immunol. 14:1269341. doi: 10.3389/fimmu.2023.1269341
Received: 29 July 2023; Accepted: 25 October 2023;
Published: 13 November 2023.
Edited by:
Ganesan Ramamoorthi, Moffitt Cancer Center, United StatesReviewed by:
Jianwei Zhang, The Sixth Affiliated Hospital of Sun Yat-sen University, ChinaCopyright © 2023 Chen, Zhou, Han, Wei and Qiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meng Qiu, cWl1bWVuZ0Bob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.