- 1Hangzhou School of Clinical Medicine, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 2Department of Oncology, Hangzhou First People’s Hospital, Hangzhou, Zhejiang, China
- 3Department of Oncology, The Second Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 4The Third School of Clinical Medicine, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 5Department of Oncology, The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Chinese Medicine), Hangzhou, Zhejiang, China
Background: Neoadjuvant combination immunotherapy is changing the treatment landscape for patients with cancer. Exploring the incidence of immune-related adverse events (irAEs) in relation to this novel approach may provide valuable insights for future clinical investigations.
Methods: This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. PubMed, Embase, Cochrane Library, American Society of Clinical Oncology (ASCO), and European Society of Medical Oncology (ESMO) websites were searched for all relevant literature from their inception to November 24, 2023. We then extracted the required data from the included studies and used the R software to analyze the pooled incidence of irAEs. Subgroup analyses examined the pooled incidence of irAEs according to cancer and combination types using a random-effects model.
Results: Sixteen studies involving 501 patients were included in the meta-analysis. Considering the heterogeneity of the study design, we analyzed the randomized controlled studies (RCTs) and the single-arm studies separately. In RCTs, the incidence of any-grade irAEs was 95.0% (95% confidence interval [CI] 87.3-99.3) and that of grade ≥3 irAEs was 24.0% (95% CI 13.7-36.0). In single-arm studies, the incidence of any-grade irAEs was 89.4% (95% CI 75.0-98.0) and grade ≥3 irAEs was 20.3% (95% CI 8.7-35.2). In both RCTs and single arms, the most common any- grade irAEs were rash and fatigue, while the most common grade ≥3 irAEs was abnormal liver function and colitis. Due to irAEs, 9.4% of patients in RCTs and 6.9% of patients in single-arm studies did not complete the prescribed neoadjuvant treatment cycle.
Conclusion: This study comprehensively summarized the incidence of irAEs in neoadjuvant combination immunotherapy. The occurrence of irAEs varies depending on the cancer and combination types. Our meta-analysis provides clinicians with essential guidance for the management of patients with cancer.
Systematic review registration: https://www.crd.york.ac.uk/prospero, identifier CRD42023387969.
1 Introduction
In recent years, immune checkpoint inhibitors (ICIs), such as programmed death-1 (PD-1), programmed death ligand-1 (PD-L1), and cytotoxic T-lymphocyte antigen-4 (CTLA-4), have made significant progress in the treatment of various tumors (1, 2). Compared with conventional chemotherapy, ICIs can significantly extend the overall survival time of cancer patients and reduce the occurrence of adverse events (AEs) (3, 4). Currently, ICI neoadjuvant therapy is used for various tumors, such as lung cancer (5), melanoma (6), and esophageal cancer (7). In a meta-analysis of NSCLC, neoadjuvant immunotherapy was associated with significantly higher rates of pathological complete responses than neoadjuvant chemotherapy (8). Owing to its remarkable therapeutic effects, a large number of clinical trials have been conducted to investigate the efficacy and safety of neoadjuvant immunotherapy for malignant tumors.
The combination of the two ICIs has become an essential component of neoadjuvant immunotherapy. In a phase II clinical study, patients with resectable malignant melanoma who received neoadjuvant combination immunotherapy achieved a pathologic complete response rate of 57% (9). The NICHE-2 study showed that neoadjuvant combination immunotherapy in patients with colon cancer resulted in a high rate of pathologic complete response (10). In addition to the effectiveness of neoadjuvant immunotherapy, safety is a concern, especially for dual-agent immunotherapy. Most immune-related adverse events (irAEs) tend to be self-limiting or ameliorated using multiple strategies. However, in some cases, life-threatening fatal events can occur (11).
Considering that a new approach for combination immunotherapy may be added to neoadjuvant therapy for malignant tumors in the near future, it is necessary for clinicians to gain a comprehensive understanding of the safety of this approach. In this review, we conducted a comprehensive safety assessment by searching for clinical trials of neoadjuvant combination immunotherapy for malignancies and pooled the incidence of irAEs by meta-analysis.
2 Methods
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement (12) and was registered at PROSPERO (CRD42023387969).
2.1 Literature search and screening
Three databases PubMed, Embase, Cochrane Library, American Society of Clinical Oncology (ASCO), and European Society of Medical Oncology (ESMO) websites were searched for relevant literature from their inception until November 24, 2023. The search strategy for PubMed is presented in Supplementary Table S1. After importing documents into Endnote reference management software (Version X9), filtering was performed.
According to the population, issue of interest, comparison, outcome, and study design (PICOS) method (13), studies meeting the following criteria were included: (i) populations: the study was conducted in resectable cancer patients, (ii) interventions: neoadjuvant combination immunotherapy was used as an intervention (only dual immune checkpoint inhibitors were included), (iii) outcomes: irAEs for outcome indicators were reported, (iv) study designs: RCTs, single-arm, and non-RCT studies were all included, and (v) the full text was published in English. The exclusion criteria were as follows: (i) neoadjuvant immunotherapy combined with other treatment modalities, such as chemotherapy, targeted therapy, and radiotherapy, (ii) no mention of irAEs in the outcome indicator; and (iii) only a summary without a full-text report. The above processes were independently completed by two authors.
2.2 Data extraction and quality assessment
Two authors carefully reviewed the eligible literature and extracted relevant information, including the first author, publication year, study design, cancer type, intervention, number of patients, surgical time, follow-up time, case of irAEs (any grade and high grade), completion of neoadjuvant therapy, case of surgery delayed or not performed, and evaluation criteria of AEs.
The quality of randomized controlled trials (RCTs) was assessed using the Cochrane Collaboration’s risk of bias tool (14). Additionally, a quality assessment of non-randomized controlled studies was performed using the methodological index for non-randomized studies (MINORS) checklist (15). Two authors independently assessed the quality of the literatures and promptly consulted a third author regarding points of dispute.
2.3 Statistical analysis
The primary objective of our meta-analysis was to determine the incidence of irAEs, including any-grade irAEs as well as high-grade irAEs. The pooled incidence with 95% confidence intervals (CIs) was calculated using a random effects model in RCTs and single-arm studies, respectively. Subgroup analyses were performed to examine the pooled incidence of irAEs according to cancer and combination types. Rate consolidation was conducted using arcsine transformation. Heterogeneity among the pooled studies was assessed using the heterogeneity index (I2).
All of the above analyses were performed using R software (version 4.1.0) with the Meta package. A P value of <0.05 was considered statistically significant.
3 Results
3.1 Eligible studies and characteristics
Through a systematic literature search, 8910 relevant records were retrieved. Duplicates were removed, and titles and abstracts were browsed, leaving 68 records that required full-text reading. Among them, 3 did not have sufficient data, 17 were not the latest study results, 10 were wrong interventions, 5 were wrong outcome indicators, and 17 were not found in the full text. Sixteen studies were included; nine were non-RCTs (including eight single-arm studies) and seven were RCTs (Figure 1). The risk of bias for RCTs is detailed in Supplementary Table S2, while that for non-RCTs is detailed in Supplementary Table S3.
The main characteristics of sixteen included clinical trials are summarized in Table 1. All publications were published after 2019. In total, 501 patients received neoadjuvant combination immunotherapy. In four studies (16–19), 76 patients received durvalumab plus tremelimuma as neoadjuvant therapy. In nine studies (20–28), 326 patients received nivolumab plus ipilimumab. Thirty patients received relatlimab plus nivolumab (9), 28 patients received lirilumab plus nivolumab (29), 21 patients received Durvalumab plus Oleclumab, and 20 patients received Durvalumab plus Monalizumab (30). AEs in thirteen studies were assessed using the National Cancer Institute’s Common Terminology Criteria, whereas those in three studies were not reported.
3.2 Incidence of overall irAEs
In general, 15 trials reported the occurrence of grade ≥3 irAEs, with 11 reporting any-grade irAEs. Considering the heterogeneity of the study designs, we analyzed the RCTs and single-arm studies separately. The study published by Vos et al. was not included in the subsequent meta-analysis because it was neither an RCT study nor a single-arm study (20). In RCTs, the incidence of any-grade irAEs was 95.0% (95% CI 87.3-99.3) and that of grade ≥3 irAEs was 24.0% (95% CI 13.7-36.0).In single-arm studies, the incidence of any-grade irAEs was 89.4% (95% CI 75.0-98.0) and that of grade ≥3 irAEs was 20.3% (95% CI 8.7-35.2) (Figure 2). In addition, we measured the incidence of irAEs with immunotherapy alone. The incidence of any-grade irAEs was 87.1% (95% CI 55.9-100.0) and that of grade ≥3 irAEs was 17.3% (95% CI 10.1-25.9) (Supplementary Figure S1). There was no statistical difference in the incidence of irAEs between monotherapy and combination therapy (Supplementary Figure S2).
Considering the presence of heterogeneity, we performed a subgroup analysis according to combination and cancer types (Table 2). In RCTs, regarding the combination type, the incidence of any-grade irAEs was 97.1% (95%CI 90.9-99.9) and that of grade ≥3 irAEs was 32.6% (95%CI 17.6-49.7) for anti PD-1 plus anti CTLA-4 (nivolumab combined with ipilimumab). The incidence of any-grade irAEs was 100.0% (95% CI 76.8-100), while that of grade ≥3 irAEs was 15.3% (95% CI 1.6-39.0) for anti PD-L1 plus anti CTLA-4 (durvalumab combined with tremelimuma). Regarding the cancer type, the incidence of any-grade irAEs was 80.2% (95%CI 64.8-92.0) for non-small cell lung cancer (NSCLC), and 98.4% (95%CI 94.3-100.1) for melanoma. The incidence of grade ≥3 irAEs was 11.2% (95%CI 4.6-20.2) for NSCLC, 7.1% (95% CI 0.2-3.4) for head and neck cancers (HNCs), and 38.4% (95%CI 22.0-56.3) for melanoma.
In single-arm studies, regarding the combination type, the incidence of any-grade irAEs was 91.0% (95%CI 69.5-100.0) and that of grade ≥3 irAEs was 32.9% (95%CI 19.1-48.3) for anti PD-1 plus anti CTLA-4. The incidence of any-grade irAEs was 92.9% (95% CI 76.5-99.1), while that of grade ≥3 irAEs was 21.6% (95% CI 11.5-33.8) for anti PD-L1 plus anti CTLA-4. Regarding the cancer type, the incidence of any-grade was 66.7% (95% CI 29.9-92.5) for NSCLC, 75.0% (95% CI 55.1-89.3) for HNCs, and 98.0% (95% CI 92.9-99.8) for melanoma. The incidence of grade ≥3 irAEs was 33.3% (95% CI 7.5-70.1) for NSCLC, 10.7% (95% CI 2.3-28.2) for HNCs, and 8.5% (95%CI 0.0-58.2) for melanoma.
3.3 Incidence of specific irAEs
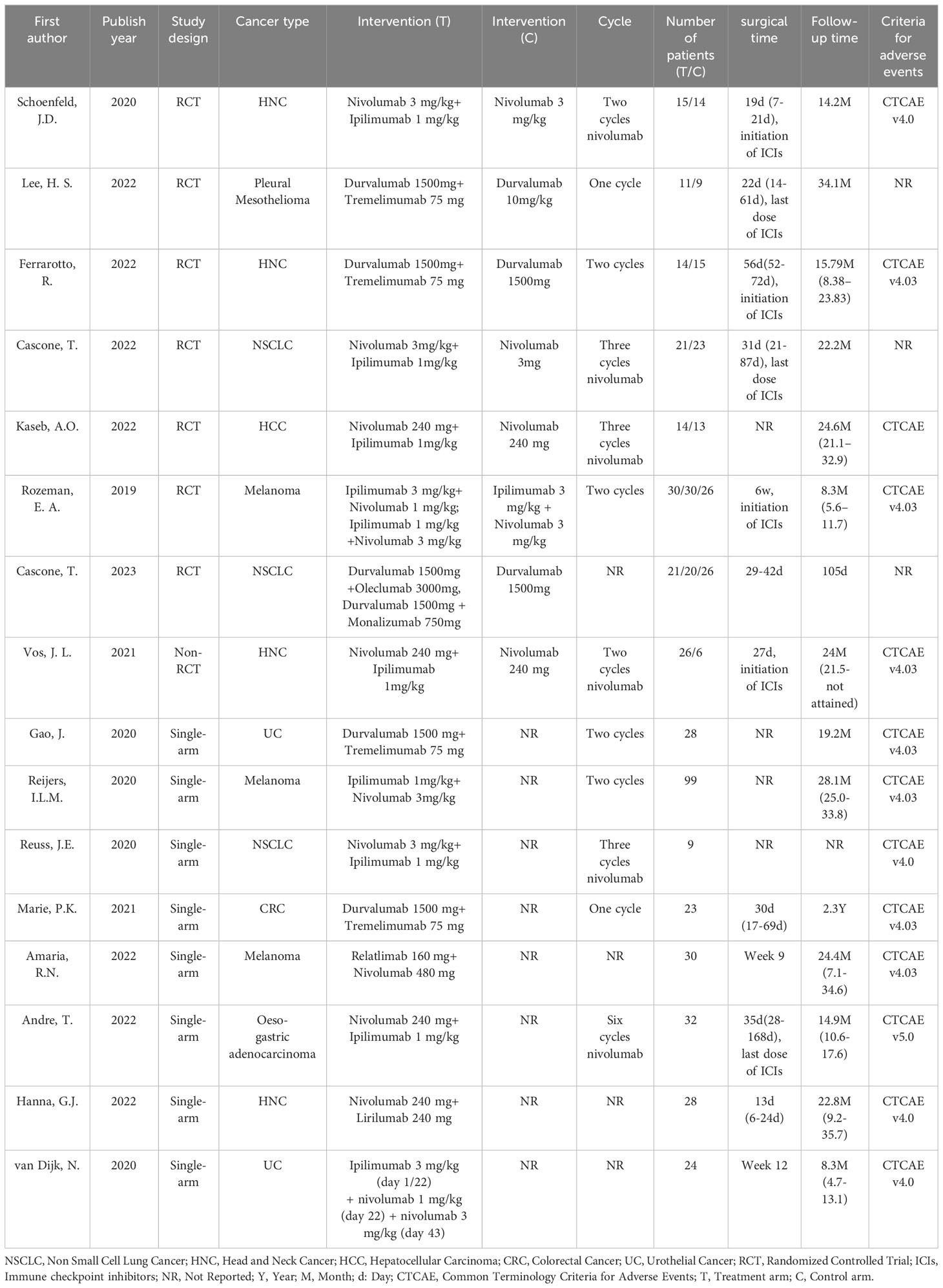
A total of 146 irAEs were reported in the 16 included trials; the 10 most common irAEs are described in Figure 3. In RCTs, the most common any-grade irAEs were rash (33.9%, 95% CI 13.0-58.8), fatigue (32.7%, 95% CI 19.4-47.5), diarrhea (18.8%, 95%CI 4.8-39.4), hyperthyroidism (17.9%, 95%CI 6.1-34.0), and pruritus (17.2%, 95% CI 8.8–27.8). The most common grade ≥3 irAEs were AST increased (10.4%, 95% CI 2.5-22.7), ALT increased (9.3%, 95% CI 2.1-20.8), colitis (5.5%, 95% CI 0.0-22.5), diarrhea (5.4%, 95% CI 2.1-10.1), and rash (4.7%, 95% CI 1.0-10.9).
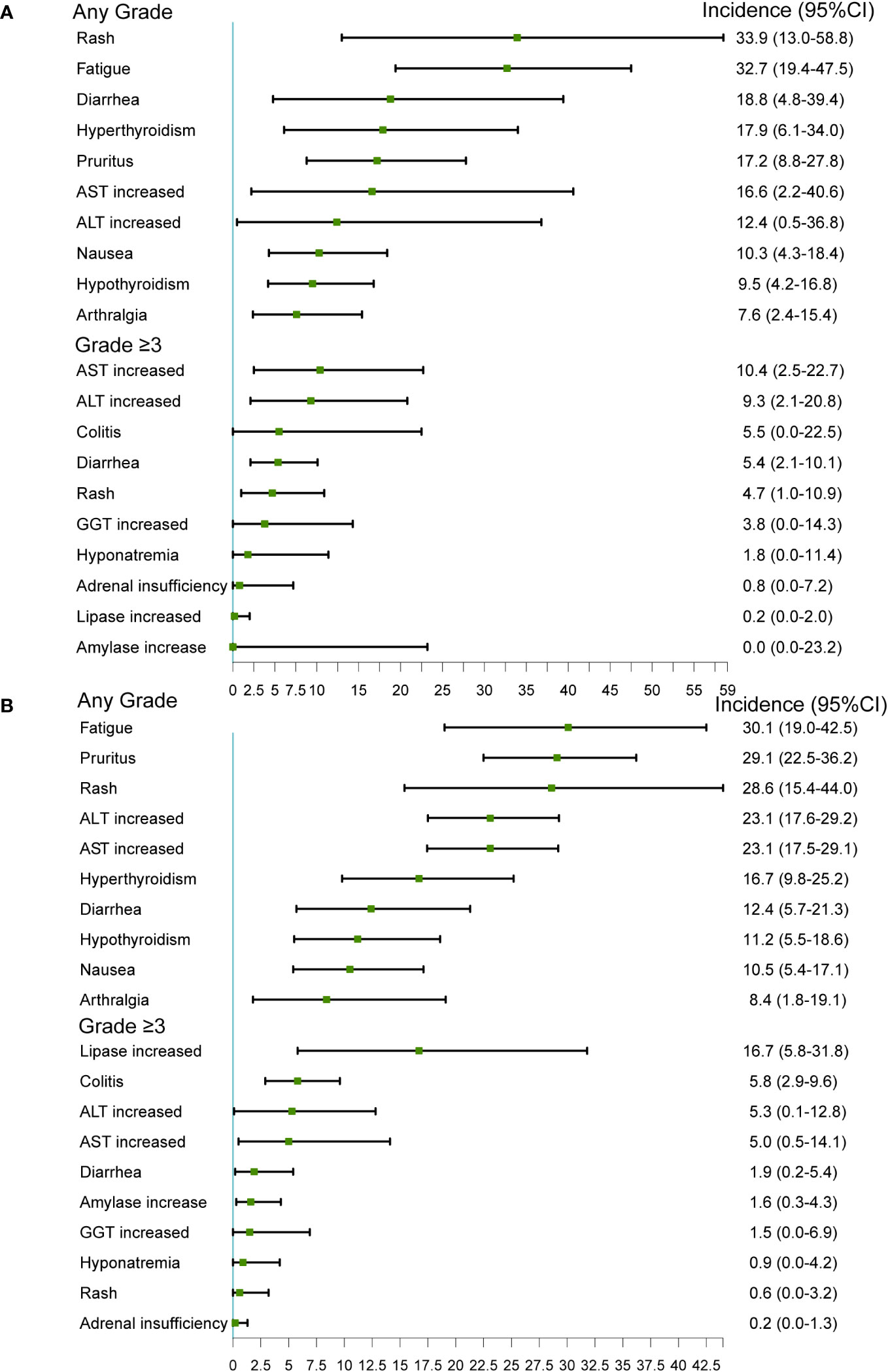
In single-arm studies, the most common any-grade irAEs were fatigue (30.1%, 95% CI 19.0-42.5), pruritus (29.1%, 95% CI 22.5-36.2), rash (28.6%, 95% CI 15.4-44.0), ALT increased (23.1%, 95% CI 17.6-29.2), and AST increased (23.1%, 95% CI 17.5-29.1). The most common grade ≥3 irAEs were lipase increased (16.7%, 95% CI 5.8-31.8), colitis (5.8%, 95%CI 2.9-9.6), ALT increased (5.3%, 95% CI 0.1-12.8), AST increased (5.0%, 95% CI 0.5-14.1), and diarrhea (1.9%, 95% CI 0.2-5.4).
The most common irAEs varied among the different combination types and cancer types, as summarized in Supplementary Tables S4–8. In RCTs, the most common any-grade irAEs were rash (57.7%) and fatigue (42.4%) and the most common and grade ≥3 irAEs were AST increased (10.4%) and ALT increased (9.3%) in nivolumab plus ipilimumab group (Supplementary Table S4). The most common any-grade and grade ≥3 irAEs was hyperglycemia (54.6%, 18.2%) in durvalumab plus tremelimuma group (Supplementary Table S5). The most common irAEs of any-grade were acneiform rash (52.4%) in NSCLC (Supplementary Table S6), rash (28.6%) in patients with HNCs (Supplementary Table S7), and fatigue (58.2%) in melanoma group (Supplementary Table S8).
In single-arm studies, the most common any-grade irAEs were rash (32.0%) and fatigue (30.4%) and the most common grade ≥3 irAEs were lipase increased (14.9%) and ALT increased (8.2%) in nivolumab plus ipilimumab group (Supplementary Table S4). The most common any-grade irAEs was fatigue (29.6%), and the most common grade ≥3 irAEs was lipase increased (14.3%) in durvalumab plus tremelimuma group (Supplementary Table S5). The most common any grade irAEs were fatigue (42.9%, 35.9%) in the HNCs and melanoma groups and rash (44.4%) in NSCLC group (Supplementary Tables S6–8).
3.4 The impact of the irAEs in the treatment
Due to the occurrence of irAEs, a number of patients did not complete the prescribed neoadjuvant treatment cycle. In RCTs, 9.4% (95% CI 2.1–21.1) of patients did not complete the prescribed neoadjuvant treatment cycle due to irAEs (such as grade 2-4 diarrhea/colitis, grade 2-3 ALT increased, grade 2 pneumonitis/arthralgia, grade 3 rash/meningitis/radiculitis). And in single-arm studies, 6.9% (95%CI 0.4-20.1) of patients did not complete the prescribed neoadjuvant treatment cycle due to irAEs (such as colitis/ileitis/gastritis/hepatitis) (Figure 4). Treatment of severe irAEs has only been reported in a few studies. Ferrarotto, R. et al. mentioned that two patients with immune-related transaminitis and diarrhea were treated with steroids (17). One patient developed pneumonia and pneumonitis requiring steroids in the research of Cascone, T (21). In addition, there has been one case of a patient with immune-related encephalitis who was treated with steroids but died (27).
A small percentage of patients have surgery delayed due to irAEs. In RCTs, only 3 of the 188 patients reported experienced surgery delayed (Supplementary Table S9). And in single-arm studies, only 6 of the 273 patients reported experienced surgery delayed (Supplementary Table S10). Eight studies reported postoperative adjuvant treatment. Most of patients chose monotherapy immunotherapy, chemotherapy or radiotherapy during the postoperative adjuvant treatment phase. In three studies reported by Amaria, R.N., Hanna, G.J., Kaseb, A.O., the adjuvant therapy was treated in the same way as the neoadjuvant phase (Supplementary Table S11) (9, 23, 29).
4 Discussion
In this meta-analysis, we found that more than 85% of patients developed irAEs during neoadjuvant immunotherapy both in the RCTs and in the single-arm studies, and approximately 1/5 of these patients developed grade ≥ 3 irAEs. Subsequently, we conducted a subgroup analysis of different cancer and intervention types. The incidence of irAEs varied among different tumors and treatment types. In addition, we found that the most common irAEs associated with neoadjuvant combination immunotherapy were fatigue, rash, abnormal liver function (ALT/AST increased), and gastrointestinal reactions (diarrhea, colitis) in both the RCTs and single-arm studies. No more than 10% of patients stopped neoadjuvant therapy and very few patients delayed surgery due to irAEs.
The administration of ICIs during neoadjuvant therapy is believed to enhance systemic T cells, which can respond to tumor antigens and activate the immune system. Compared with adjuvant therapy after surgical excision, high-burden tumors activate more circulating tumor-specific T cells, which exert beneficial anti-tumor effects (31). Liu et al. constructed a mouse model of spontaneously metastasized breast cancer that was given immunotherapy before or after surgical resection of the primary tumor, and found that the survival rate of mice receiving neoadjuvant immunotherapy was significantly higher than that of mice receiving adjuvant therapy. Improved survival was associated with more tumor-specific CD8 T cells in the blood of the mice (32). A randomized trial of recurrent glioblastoma showed a significant improvement in overall survival in the patients received neoadjuvant pembrolizumab compared to the patients recevied PD-1 blockade alone (33). Neoadjuvant therapy showed greater amplification of tumor-resident T cell clones in peripheral blood (6). However, overreactions of the immune system are also prone to irAEs, especially with combination immunotherapy (34). The incidence of severe toxicity in the neoadjuvant population appears to exceed that in the adjuvant population (31). By analyzing blood samples from patients with melanoma before ICI treatment, Lozano et al. found that activated CD4 memory T cell abundance and T cell receptor diversity were associated with severe irAEs (35). It is critical to understand the fatal events associated with ICI treatment and promote early identification, optimal assessment, and effective management. The IMCISION trial (20) showed that in patients with HNC, the use of nivolumab plus ipilimumab prior to extensive surgery was safe, but 38% of grade≥ 3 irAEs were still observed. Two ICIs increased the risk of organ-specific irAEs compared to ICI monotherapy (36). IrAEs most commonly occurred during the first 3 months of treatment but appeared at any time after treatment or even several months after treatment was terminated (37). This highlights the need for close monitoring of the occurrence of irAEs in patients during the neoadjuvant and postoperative stages.
A previous meta-analysis showed that the common AEs of adjuvant immunotherapy in patients with advanced tumors were fatigue, rash, gastrointestinal toxicity (diarrhea, nausea, and vomiting), and liver toxicity (ALT increased) (38), which was similar to our results. Once irAEs, such as immune hepatitis, gastrointestinal toxicity, occur, most can be managed by discontinue or delaying the use of ICIs or administering corticosteroids. Mild-to-moderate dermal toxicity can be effectively controlled using topical drugs; in most cases, treatment interruption is not required. Hormone replacement therapy is needed in patients with endocrine diseases, such as hypothyroidism and thyroiditis (39, 40). However, a meta-analysis has shown that corticosteroid administration is associated with poor overall survival and progression-free survival in patients with brain metastasis. Therefore, further determination of the steroid dose and duration of administration is needed to optimize survival outcomes in patients receiving ICIs (41).
Our study has several advantages. First, only AEs related to neoadjuvant immunotherapy were evaluated, which can help us understand the safety of this emerging therapy. Second, subgroup analyses based on cancer and ICI types were conducted. Third, specific AEs in neoadjuvant immunotherapy were analyzed, allowing clinicians to understand which AEs require extra attention.
However, this study had some limitations. First, the included studies were almost all single-arm, small-sample studies, and there was a large difference in sample size between individual studies. Second, the number of studies included was insufficient to analyze the safety of specific subgroups (such as the same combination of drug interventions for the same tumor type), and the presence of confounding factors may have affected the results of small-sample subgroups. Heterogeneity between studies was high, and the sources of heterogeneity could only be explored through limited subgroup analyses. Third, the occurrence, duration, and treatment of irAEs could not be analyzed. In addition, the different criteria for AEs are limitations of this study. Therefore, large-scale studies, especially RCTs, are required. We hope to provide more complete results in future studies.
5 Conclusion
Our study comprehensively analyzed the occurrence of irAEs in neoadjuvant immunotherapy. Most patients receiving neoadjuvant combination immunotherapy developed irAEs, and only about 20% of them developed grade ≥3 irAEs. The occurrence of irAEs varied slightly between different tumors and interventions, and mainly included cutaneous, gastrointestinal, and hepatic toxicity. Nearly 10% of patients needed to stop neoadjuvant immunotherapy due to irAEs, but few case had surgery delayed. It is important to correctly evaluate AEs and promptly intervene. An increasing number of immunotherapies are being evaluated; however, choosing the appropriate combination of drugs, order of administration, and dose to increase efficacy and reduce AEs remains a challenge.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
YF: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. KG: Data curation, Investigation, Methodology, Writing – original draft. HJ: Investigation, Methodology, Writing – review & editing. JJ: Investigation, Writing – review & editing. MW: Investigation, Writing – review & editing. SL: Investigation, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was financially supported by Zhejiang Lin Shengyou famous traditional Chinese medicine expert inheritance studio project (GZS202002), the medical and health research project of Zhejiang province (2024KY1328), Hangzhou medical health science and technology project (A20230054).
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1269067/full#supplementary-material
References
1. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science (2018) 359(6382):1350–5. doi: 10.1126/science.aar4060
2. de Miguel M, Calvo E. Clinical challenges of immune checkpoint inhibitors. Cancer Cell (2020) 38(3):326–33. doi: 10.1016/j.ccell.2020.07.004
3. Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol (2020) 38(35):4138–48. doi: 10.1200/JCO.20.01888
4. Paz-Ares LG, Ramalingam SS, Ciuleanu TE, Lee JS, Urban L, Caro RB, et al. First-line nivolumab plus ipilimumab in advanced NSCLC: 4-year outcomes from the randomized, open-label, phase 3 checkMate 227 part 1 trial. J Thorac Oncol (2022) 17(2):289–308. doi: 10.1016/j.jtho.2021.09.010
5. Provencio M, Nadal E, Insa A, García-Campelo MR, Casal-Rubio J, Dómine M, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol (2020) 21(11):1413–22. doi: 10.1016/S1470-2045(20)30453-8
6. Blank CU, Rozeman EA, Fanchi LF, Sikorska K, van de Wiel B, Kvistborg P, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med (2018) 24(11):1655–61. doi: 10.1038/s41591-018-0198-0
7. van den Ende T, de Clercq NC, van Berge Henegouwen MI, Gisbertz SS, Geijsen ED, Verhoeven RHA, et al. Neoadjuvant chemoradiotherapy combined with atezolizumab for resectable esophageal adenocarcinoma: A single-arm phase II feasibility trial (PERFECT). Clin Cancer Res (2021) 27(12):3351–9. doi: 10.1158/1078-0432.CCR-20-4443
8. Jia XH, Xu H, Geng LY, Jiao M, Wang WJ, Jiang LL, et al. Efficacy and safety of neoadjuvant immunotherapy in resectable nonsmall cell lung cancer: A meta-analysis. Lung Cancer (2020) 147:143–53. doi: 10.1016/j.lungcan.2020.07.001
9. Amaria RN, Postow M, Burton EM, Tezlaff MT, Ross MI, Torres-Cabala C, et al. Neoadjuvant relatlimab and nivolumab in resectable melanoma. Nature (2022) 611(7934):155–60. doi: 10.1038/s41586-022-05368-8
10. Chalabi M, Verschoor YL, van den Berg J, Sikorska K, Beets G, Lent AV, et al. LBA7 Neoadjuvant immune checkpoint inhibition in locally advanced MMR-deficient colon cancer: The NICHE-2 study. Ann Oncol (2022) 33:S1389. doi: 10.1016/j.annonc.2022.08.016
11. Blidner AG, Choi J, Cooksley T, Dougan M, Glezerman I, Ginex P, et al. Cancer immunotherapy-related adverse events: causes and challenges. Support Care Cancer (2020) 28(12):6111–7. doi: 10.1007/s00520-020-05705-5
12. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj (2021) 372:n71. doi: 10.1136/bmj.n71
13. Amir-Behghadami M, Janati A. Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg Med J (2020) 37(6):387. doi: 10.1136/emermed-2020-209567
14. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj (2011) 343:d5928. doi: 10.1136/bmj.d5928
15. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg (2003) 73(9):712–6. doi: 10.1046/j.1445-2197.2003.02748.x
16. Lee HS, Jang HJ, Ramineni M, Wang DY, Ramos D, Choi JM, et al. A Phase II Window of Opportunity Study of Neoadjuvant PD-L1 versus PD-L1 plus CTLA-4 Blockade for Patients with Malignant Pleural Mesothelioma. Clin Cancer Res (2022). doi: 10.1158/1078-0432.c.6533147
17. Ferrarotto R, Bell D, Rubin ML, Hutcheson KA, Johnson JM, Goepfert RP, et al. Impact of neoadjuvant durvalumab with or without tremelimumab on CD8(+) tumor lymphocyte density, safety, and efficacy in patients with oropharynx cancer: CIAO trial results. Clin Cancer Res (2020) 26(13):3211–9. doi: 10.1158/1078-0432.CCR-19-3977
18. Gao J, Navai N, Alhalabi O, Siefker-Radtke A, Campbell MT, Tidwell RS, et al. Neoadjuvant PD-L1 plus CTLA-4 blockade in patients with cisplatin-ineligible operable high-risk urothelial carcinoma. Nat Med (2020) 26(12):1845–51. doi: 10.1038/s41591-020-1086-y
19. Kanikarla Marie P, Haymaker C, Parra ER, Kim YU, Lazcano R, Gite S, et al. Pilot clinical trial of perioperative durvalumab and tremelimumab in the treatment of resectable colorectal cancer liver metastases. Clin Cancer Res (2021) 27(11):3039–49. doi: 10.1158/1078-0432.CCR-21-0163
20. Vos JL, Elbers JBW, Krijgsman O, Traets JJH, Qiao X, van der Leun AM, et al. Neoadjuvant immunotherapy with nivolumab and ipilimumab induces major pathological responses in patients with head and neck squamous cell carcinoma. Nat Commun (2021) 12(1):1–13. doi: 10.1038/s41467-021-26472-9
21. Cascone T, William WN, Weissferdt A, Leung CH, Lin HY, Pataer A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med (2021) 27(3):504–14. doi: 10.1038/s41591-020-01224-2
22. Schoenfeld JD, Hanna GJ, Jo VY, Rawal B, Chen YH, Catalano PS, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in untreated oral cavity squamous cell carcinoma: a phase 2 open-Label randomized clinical trial. JAMA Oncol (2020) 6(10):1563–70. doi: 10.1001/jamaoncol.2020.2955
23. Kaseb AO, Hasanov E, Cao HST, Xiao L, Vauthey JN, Lee SS, et al. Perioperative nivolumab monotherapy versus nivolumab plus ipilimumab in resectable hepatocellular carcinoma: a randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol (2022) 7(3):208–18. doi: 10.1016/S2468-1253(21)00427-1
24. Reijers ILM, Menzies AM, van Akkooi ACJ, Versluis JM, van den Heuvel NMJ, Saw RPM, et al. Personalized response-directed surgery and adjuvant therapy after neoadjuvant ipilimumab and nivolumab in high-risk stage III melanoma: the PRADO trial. Nat Med (2022) 28(6):1178–88. doi: 10.1038/s41591-022-01851-x
25. Reuss JE, Anagnostou V, Cottrell TR, Smith KN, Verde F, Zahurak M, et al. Neoadjuvant nivolumab plus ipilimumab in resectable non-small cell lung cancer. J Immunother Cancer (2020) 8(2):1–8. doi: 10.1136/jitc-2020-001282
26. André T, Tougeron D, Piessen G, de la Fouchardière C, Louvet C, Adenis A, et al. Neoadjuvant nivolumab plus ipilimumab and adjuvant nivolumab in localized deficient mismatch repair/Microsatellite instability-High gastric or esophagogastric junction adenocarcinoma: the GERCOR NEONIPIGA phase II study. J Clin Oncol (2023) 2022:JCO2200686. doi: 10.1200/JCO.2022.40.4_suppl.244
27. Rozeman EA, Menzies AM, van Akkooi ACJ, Adhikari C, Bierman C, van de Wiel BA, et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial. Lancet Oncol (2019) 20(7):948–60. doi: 10.1016/S1470-2045(19)30151-2
28. van Dijk N, Gil-Jimenez A, Silina K, Hendricksen K, Smit LA, de Feijter JM, et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial. Nat Med (2020) 26(12):1839–44. doi: 10.1038/s41591-020-1085-z
29. Hanna GJ, O’Neill A, Shin KY, Wong K, Jo VY, Quinn CT, et al. Neoadjuvant and adjuvant nivolumab and lirilumab in patients with recurrent, resectable squamous cell carcinoma of the head and neck. Clin Cancer Res (2022) 28(3):468–78. doi: 10.1158/1078-0432.CCR-21-2635
30. Cascone T, Kar G, Spicer JD, García-Campelo R, Weder W, Daniel DB, et al. Neoadjuvant durvalumab alone or combined with novel immuno-oncology agents in resectable lung cancer: the phase II neoCOAST platform trial. Cancer Discov (2023) 13(11):2394–411. doi: 10.1158/2159-8290.CD-23-0436
31. Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science (2020) 367(6477):1–9. doi: 10.1126/science.aax0182
32. Liu J, Blake SJ, Yong MC, Harjunpää H, Ngiow SF, Takeda K, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov (2016) 6(12):1382–99. doi: 10.1158/2159-8290.CD-16-0577
33. Cloughesy TF, Mochizuki AY, Orpilla JR, Hugo W, Lee AH, Davidson TB, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med (2019) 25(3):477–86. doi: 10.1038/s41591-018-0337-7
34. Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin (2020) 70(2):86–104. doi: 10.3322/caac.21596
35. Lozano AX, Chaudhuri AA, Nene A, Bacchiocchi A, Earland N, Vesely MD, et al. T cell characteristics associated with toxicity to immune checkpoint blockade in patients with melanoma. Nat Med (2022) 28(2):353–62. doi: 10.1038/s41591-021-01623-z
36. Li M, Hou X, Chen J, Yu J, Chen M, Wang N, et al. Comparing organ-specific immune-related adverse events for immune checkpoint inhibitors: A Bayesian network meta-analysis. Clin Transl Med (2021) 11(2):e291. doi: 10.1002/ctm2.291
37. Weber JS, Hodi FS, Wolchok JD, Topalian SL, SChadendorf D, Larkin J, et al. Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J Clin Oncol (2017) 35(7):785–92. doi: 10.1200/JCO.2015.66.1389
38. Zhang L, Sun L, Zhou Y, Yu J, Lin Y, Wasan HS, et al. Association of survival and immune-related adverse events with anti-PD-1/PD-L1 and anti-CTLA-4 inhibitors, alone or their combination for the treatment of cancer: A systematic review and meta-analysis of 13 clinical trials. Front Oncol (2021) 11:575457. doi: 10.3389/fonc.2021.575457
39. Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer (2017) 5(1):95. doi: 10.1186/s40425-017-0300-z
40. Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2017) 28(suppl_4):iv119–42. doi: 10.1093/annonc/mdx225
Keywords: neoadjuvant immunotherapy, immune checkpoint inhibitors, adverse events, safety, resectable cancer
Citation: Feng Y, Guo K, Jin H, Jiang J, Wang M and Lin S (2024) Adverse events of neoadjuvant combination immunotherapy for resectable cancer patients: a systematic review and meta-analysis. Front. Immunol. 14:1269067. doi: 10.3389/fimmu.2023.1269067
Received: 29 July 2023; Accepted: 18 December 2023;
Published: 05 January 2024.
Edited by:
Yutian Zou, Sun Yat-sen University Cancer Center (SYSUCC), ChinaReviewed by:
Brigida Anna Maiorano, IRCCS Casa Sollievo della Sofferenza Hospital, ItalySofiya Latifyan, Centre Hospitalier Universitaire Vaudois (CHUV), Switzerland
Copyright © 2024 Feng, Guo, Jin, Jiang, Wang and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shengyou Lin, c2hlbmd5b3VsaW5AemNtdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Yuqian Feng
Yuqian Feng Kaibo Guo
Kaibo Guo Huimin Jin
Huimin Jin Jing Jiang
Jing Jiang Menglei Wang4
Menglei Wang4 Shengyou Lin
Shengyou Lin