- Department of Nephrology, The First Affiliated Hospital of Anhui University of Chinese Medicine, Hefei, China
Despite various treatment methods, the remission rate of membranous nephropathy remains limited. Refractory membranous nephropathy especially lacks effective treatment plans. Telitacicept achieves comprehensive inhibition of CD20-positive B cells, plasma cells, and T cells, thereby bringing new hope to the treatment of membranous nephropathy and refractory membranous nephropathy. Here, we report a case of a 46-year-old man with membranous nephropathy. Although the combined treatment with glucocorticoid, tacrolimus, mycophenolate mofetil, cyclophosphamide, and rituximab was not successful, the patient achieved complete remission of urinary protein after glucocorticoid combined with telitacicept. This is the first report on the application of telitacicept in the treatment of membranous nephropathy, especially refractory membranous nephropathy. The application of telitacicept in the treatment of membranous nephropathy deserves further attention.
1 Introduction
The histomorphological substrate of membranous nephropathy (MN) is the accumulation of immune deposits in the subcutaneous space above the glomerular filtration barrier. Most frequently, MN presents as proteinuria in nephrotic range, with or without other elements of nephrotic syndrome. MN occurs in all regions and races, and it has an annual incidence rate of 10%–12% in North America and 2%–17% in Europe. The average age at diagnosis is 50–60 years, and the male-to-female ratio is 2:1. Spontaneous remission of untreated MN occurs in approximately one-third of patients, with a 10-year renal survival rate of 60%–80% (1). In the past decade, the understanding of the pathogenesis of MN has substantially improved. Unlike other autoimmune kidney diseases, the pathogenic circulating autoantibodies against M-type phospholipase A2 receptor (PLA2R1) and thrombospondin type-1 domain-containing 7A (THSD7A) are considered the main factors leading to MN (2). Regarding the treatment of MN, the Kidney Disease: Improving Global Outcomes (KDIGO) 2021 guidelines emphasize that appropriate treatment plans should be selected based on the clinical risk assessment of progressive loss of kidney function (3). The use of rituximab in the treatment of MN was first reported in 2002 (4). Relevant clinical studies of MN have confirmed that the remission rate of rituximab treatment is 57%–89% (5, 6), while that of glucocorticoid combined with cyclophosphamide (CYC) and that of glucocorticoid combined with tacrolimus are about 88% and 53%, respectively (7). However, regardless of the treatment plan, the remission rate remains limited. Determining a new treatment plan is a great challenge when the abovementioned drug treatments fail. Considering the pathogenesis of MN, compared with traditional CD20-targeting biological inhibitors, telitacicept realizes the overall inhibition and regulation of lymphocyte growth process, including plasma cells and T cells, greatly reducing the risk of circulating and in situ immune complex formation, thereby achieving therapeutic effects. This is the first report of complete remission of refractory MN in 24h proteinuria following telitacicept treatment.
2 Case report
During physical and biochemical examination in October 2019, a 46-year-old man showed edema of both lower limbs and urinary protein level of 3+. In November 2019, the serum albumin (SA) level was 28.9 g/L, 24h proteinuria was 2.87 g, and blood PLA2R and THSD7A were negative.
2.1 Kidney biopsy results
The results of immunofluorescence were as follows: IgG, ++++; IgA, +; IgM, +; C3, +++; C1q, -; Kappa, +++; Lambda, +++; IgG1, ++; IgG2, -; IgG3, -; and IgG4, +++. Light microscopy showed that the number of glomerular cells was slightly increased, the basement membrane was thickened in segments, nail process was occasionally formed, mesangial cells and matrix were slightly proliferated, and subepithelial eosinophils were deposited. Congo red staining was negative. Immunofluorescence of PLA2R was positive, while immunohistochemistry of THSD7A was negative. Electron microscopic examination showed the following results. The basement membrane was irregularly thickened (the thickness of the thin part was about 240 nm–300 nm, the thickness of many parts was about 380 nm–700 nm, and the thickness of the thickest part was 1300 nm), and the foot processes of podocytes were diffusely fused (> 90%). A large amount of electron-dense matter deposition was found in the basement membrane. Pathological diagnosis was MN stages I–II (Figure 1). Chest computed tomography (CT) was performed, and tumor markers, hepatitis B virus, hepatitis C virus, and human immunodeficiency virus were screened. The results of all of these tests were negative. There was no history of nonsteroidal anti-inflammatory drug use.
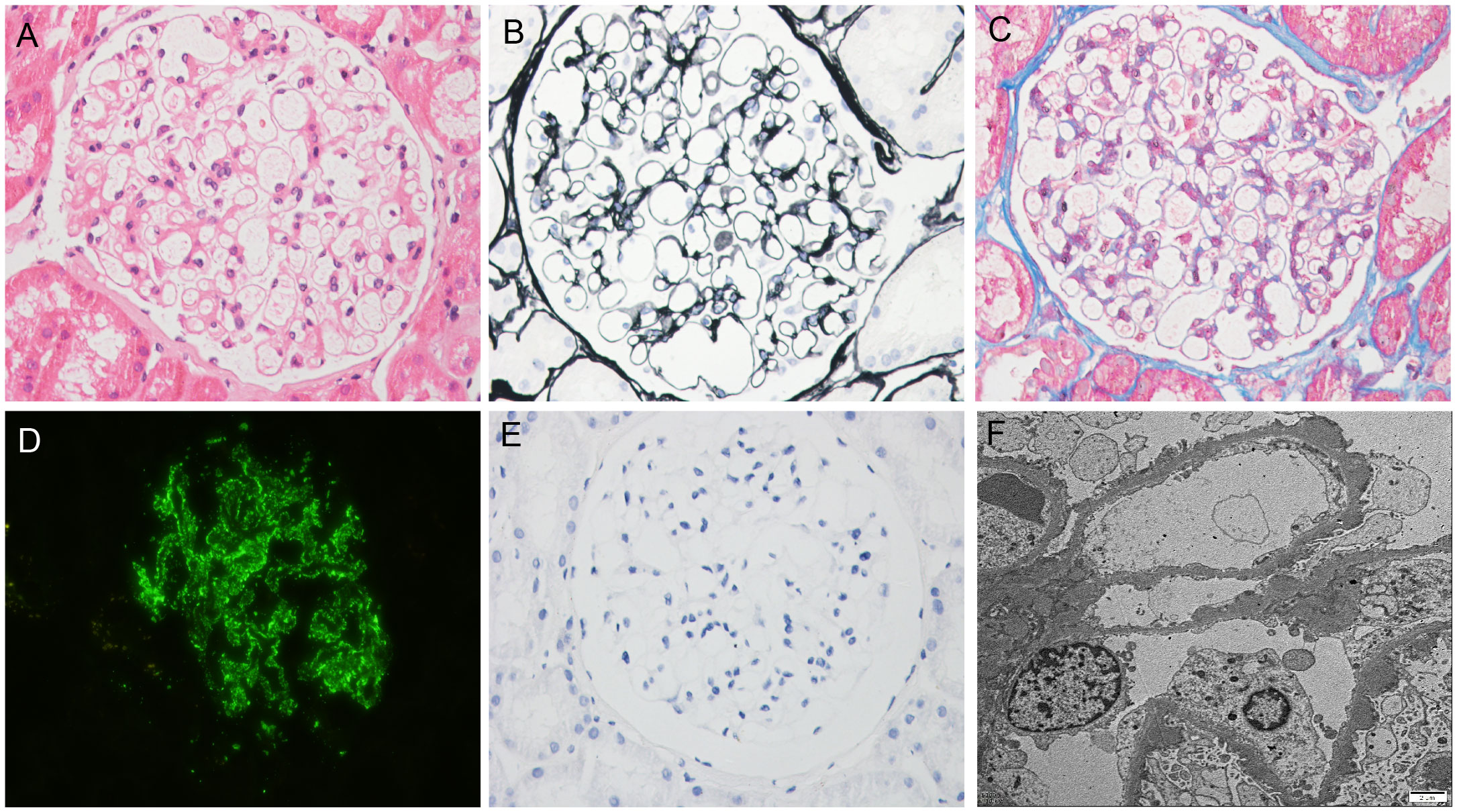
Figure 1 Biopsy findings. (A) Hematoxylin–eosin staining (×400); (B) Periodic-acid silver methenamine staining (×400); (C) Masson staining (×400); (D) Immunofluorescence staining for PLA2R (×400); (E) Immunohistochemical staining for THSD7A (×400); (F) Electron microscopy (×10000).
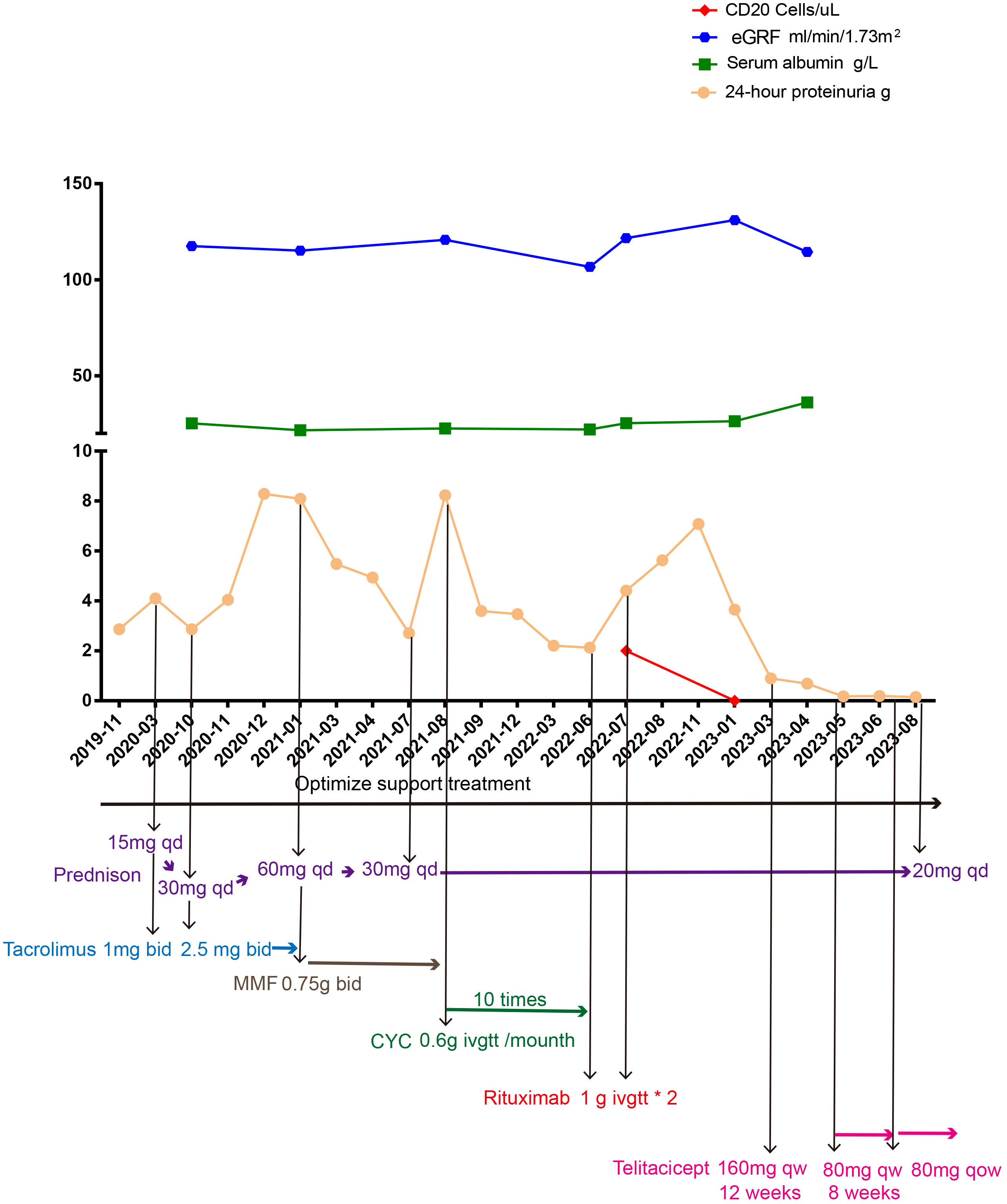
At first, clopidogrel 50 mg qd and valsartan 80 mg qd were given orally. In March 2020, 24h proteinuria (24h-P) was 4.1 g and prednisone 15 mg qd combined with tacrolimus 1 mg bid (gradually increased to 2.5 mg bid depending on the blood concentration) was added while continuing to optimize the supporting treatment scheme. In October 2020, 24h-P was 2.87 g, SA level was 25.2 g/L, serum creatinine (SC) concentration was 56.2 µmol/L, tacrolimus blood concentration was 6.5 ng/mL, and prednisone and tacrolimus were adjusted to 30 mg qd and 2.5 mg bid orally, respectively. In January 2021, the blood concentration of tacrolimus was 6.55 ng/mL, 24h-P was 8.09 g, SA level was 21.7 g/L, SC concentration was 59.3 µmol/L, and blood PLA2R and THSD7A were negative. Given that, at that time, the KDIGO 2021 clinical practice guidelines for the management of glomerular diseases had not yet been published, we recommended the patient to use rituximab or CYC. According to the KDIGO 2012 clinical practice guidelines and the evidence in the treatment of the Chinese population, mycophenolate mofetil was also introduced. Considering the economic burden and convenience of treatment, the patient insisted on using mycophenolate mofetil. We also fully reminded the patient that this prescription could pose a risk of treatment failure. The immunosuppression treatment plan was adjusted to include prednisone 60 mg qd in combination with mycophenolate mofetil 0.75 g bid orally, and prednisone was gradually reduced. In July 2021, prednisone was reduced to 30 mg qd in combination with mycophenolate mofetil 0.75 g bid. In August 2021, 24h-P was 8.23 g, SA was 22.6 g/L, SC was 52.8 µmol/L, and blood PLA2R and THSD7A were negative. This time, we recommended the patient to use rituximab or rituximab combined with tacrolimus. Due to the patient’s lack of confidence in tacrolimus and the high treatment and hospitalization costs associated with rituximab, CYC was ultimately chosen. The adjusted immunosuppressive treatment plan included prednisone 30 mg qd combined with CYC 0.6 g intravenous drip once a month. In May 2022, the patient received the 10th intravenous infusion of CYC 0.6 g. In June 2022, 24h-P was 2.13 g, SA was 22.1 g/L, SC was 60.6 µmol/L, and blood PLA2R and THSD7A were negative. We continued giving prednisone 30 mg qd orally to stop CYC, and rituximab 1.0 g intravenous drip was given. In July 2022, 24h-P was 4.41 g, SA was 25.4 g/L, SC was 60.6 µmol/L, CD20 count was 2 cells/µL, and blood PLA2R and THSD7A were negative. The second intravenous drip of rituximab 1.0 g was given, and after discharge, prednisone 30 mg qd was given orally. In January 2023, 24h-P was 3.65 g, SA was 26.4 g/L, SC was 43.3 µmol/L, CD20 count was 0 cells/µL, and blood PLA2R and THSD7A were negative. Although multiple immunosuppressive treatments were in use for more than 6 months, the examination results indicated a medium to high risk. Therefore, the patient was recommended to receive additional treatment with rituximab, and observation was continued. As the patient lost confidence in rituximab, a new treatment plan was expected. Considering that resistance was treated, referring to the treatment plan for treating resistance, rituximab has to be evaluated 3 months after use. If the treatment fails, CYC (already used) should be used, or the physicians should refer to the treatment experience of individual centers. Starting from 4 February 2023, 160 mg qw (12 weeks) telitacicept was administered, and the patient continued receiving 30 mg qd prednisone orally. In March 2023, 24h-P was 0.9 g. In April 2023, 24h-P was 0.69 g, SA was 36.2 g/L, and SC was 60.2 µmol/L. Starting from 29 April 2023, 80 mg qw telitacicept was administered, and in May 2023, 24h-P was 0.18 g. We reduced the telitacicept dosage to once every other week after 8 weeks of treatment with 80 mg once a week. Urinary protein remained in a complete remission state, and prednisone was reduced to 25 mg qd on 12 August 2023 (Figure 2).
3 Discussion
This is the first report of the use of telitacicept for the treatment of refractory MN, and complete remission was achieved. The KDIGO 2021 guidelines recommend that, for refractory MN patients who did not respond to rituximab or CYC, an expert center should be consulted and therapy such as bortezomib, anti-CD38 therapy, and belimumab should be considered (3). Another challenge in the treatment of this patient was that both blood PLA2R and THSD7A were negative, and the next prescription could not be established based on the antibody titer level. Therefore, in the patient’s treatment path, we focused on adjusting the prescription based on 24h-P and SA levels. When rituximab resistance appeared and CD20 count reached 0, we considered whether to continue using rituximab or replace it with another B-cell inhibitor. For this reason, we reviewed the pathogenesis of MN and the current mechanisms of action of related drugs to establish the next step of drug prescription.
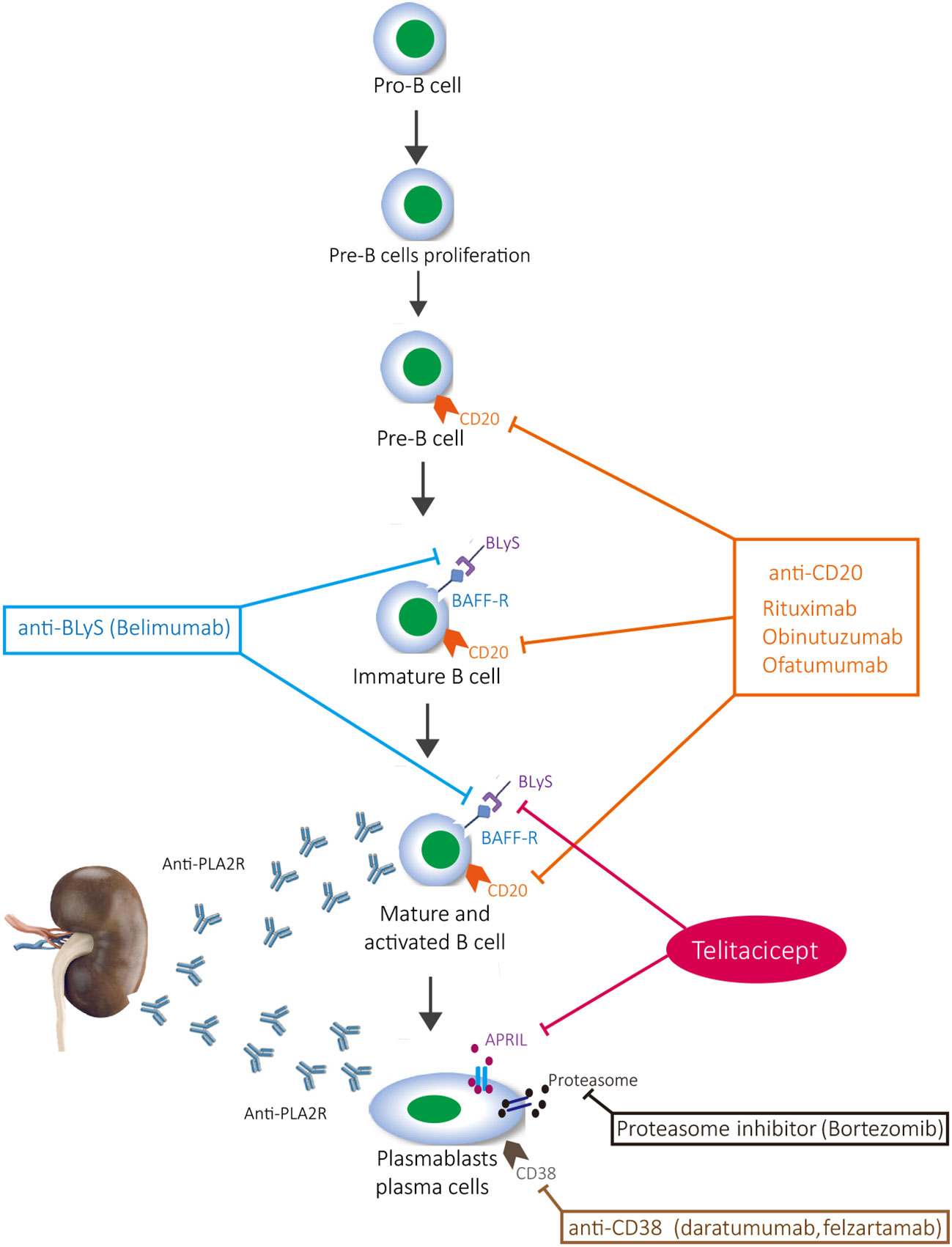
The immune mechanism of MN involves the formation and deposition of immune complexes that contain immunoglobulins and complement, which induce podocyte damage and change the glomerular basement membrane, thereby leading to the development of proteinuria. Inflammation further develops into nephrotic syndrome and, if it continues to escalate, renal failure develops (8). Activated B cells and plasma cells are the main source of antibody secretion; when plasma cells are stimulated by antigens, they can produce a large number of antibodies (9). Rituximab is a classic drug for MN, and there is evidence that rituximab is superior to cyclosporine in maintaining proteinuria remission up to 24 months (10). Obinutuzumab, ofatumumab, and belimumab also target CD20-positive B cells, but they do not target plasma cells (11). Although proteasome inhibitors (bortezomib) and anti-CD38 (daratumab and felzartamab) inhibit plasma cells, they cannot inhibit CD20-positive B cells, which has certain limitations on the activation of B cells and the inhibition of immune response (12). Furthermore, it cannot be ignored that T cells are also involved in the activation of B cells (13). Although CYC and calcineurin inhibitors (tacrolimus and cyclosporin) inhibit T cells and their secreted cytokines, they lack effective inhibition of B cells, thus making it difficult to control the immune response from the source (14). The current clinical research results also confirm the limitations of the abovementioned drugs, that is, rituximab, CYC, and calcineurin inhibitors combined with glucocorticoid, to induce remission of proteinuria (15, 16) (Figure 3). The new type of B-cell-targeted therapy is not yet supported by evidence-based medicine and is only suggested in case reports (17–21).
Telitacicept is a protein that fuses a specific extracellular soluble part of transmembrane activator and calmodulin cyclin ligand interaction factor (TACI) with human IgG1 Fc segment through recombinant DNA technology. TACI receptor has strong affinity for B-lymphocyte stimulator (BLyS) and promotion-inducing ligand (APRIL). It blocks the interaction between BLyS and APRIL and their cell membrane receptors. By blocking BLyS, it inhibits the further development and maturation of immature B cells, helping to control the future development of disease. By blocking APRIL, it inhibits the differentiation of mature B cells into plasma cells, affects the secretion of autoantibodies by autoreactive plasma cells, better controls disease activity, and achieves multistage inhibition of B-cell maturation and differentiation. Meanwhile, due to the presence of TACI receptors on the surface of T cells, telitacicept also inhibits T-cell activation (22). At present, telitacicept is mainly used for the treatment of rheumatoid arthritis, systemic lupus erythematosus (SLE), multiple sclerosis, and IgA nephropathy (23). In children aged 5–18 years with active SLE, telitacicept combined with standard treatment significantly improves the response rate of refractory active SLE, reduces the dosage of glucocorticoid, and has a curative effect on lupus nephritis (24). The same effect has been demonstrated in the treatment of adult SLE (25). In a single-center, single-arm, and open-label study, eight patients with recurrent optic myelitis spectrum disorders were recruited in China. All of the patients underwent plasma exchange three times and then received telitacicept 240 mg per week for a total of 46 times. Two patients (25%) relapsed, and five patients (63%) still had no recurrence after 48 weeks of treatment (26). According to clinical empirical research on adult primary Sjogren’s syndrome (pSS), telitacicept has good clinical efficacy, tolerance, and safety in the treatment of pSS (27). In the treatment of primary glomerular disease, the current report mainly focused on a phase II clinical study of IgA nephropathy, which demonstrated that telitacicept could effectively reduce the level of urinary protein in patients (28). Recently, there have been case reports of the use of telitacicept for the treatment of refractory proliferative lupus nephritis (29) and minimal change disease (30). Based on the pathogenesis of autoimmune nephropathy and the mechanism of action of telitacicept, it is reasonable to believe that telitacicept has broad prospects in the treatment of autoimmune nephropathy (31). However, this case report has certain limitations. Due to the lack of evidence for the treatment of MN with telitacicept, the treatment regimen that we adopted needs further research and observation. It is not yet clear whether the patient’s condition will recur in the future and whether there will be adverse reactions. Further evaluation is needed to determine whether a multitarget inhibitor for B cells would be superior to single-target drugs. The therapeutic effect of telitacicept in MN deserves further attention.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Author contributions
LZ: Writing – original draft, Writing – review & editing. HJ: Methodology, Writing – review & editing. DW: Data curation, Writing – review & editing. YW: Writing – original draft, Writing – review & editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by grants from the National Natural Science Foundation of China (82004165) and Collaborative Public Relations Project Plan of Chinese and Western Medicine for Major and Difficult Diseases in Anhui Province (Approval number: 2021-70).
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ronco P, Beck L, Debiec H, Fervenza FC, Hou FF, Jha V, et al. Membranous nephropathy. Nat Rev Dis Primers (2021) 7(1):69. doi: 10.1038/s41572-021-00303-z
2. Hoxha E, Reinhard L, Stahl RAK. Membranous nephropathy: new pathogenic mechanisms and their clinical implications. Nat Rev NephrolB (2022) 18(7):466–78. doi: 10.1038/s41581-022-00564-1
3. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int (2021) 100(4S):S1–S276. doi: 10.1016/j.kint.2021.05.021
4. Remuzzi G, Chiurchiu C, Abbate M, Brusegan V, Bontempelli M, Ruggenenti P. Rituximab for idiopathic membranous nephropathy. Lancet (2002) 360(9337):923–4. doi: 10.1016/S0140-6736(02)11042-7
5. Fervenza FC, Abraham RS, Erickson SB, Irazabal MV, Eirin A, Specks U, et al. Rituximab therapy in idiopathic membranous nephropathy: A 2-year study. Clin J Am Soc Nephrol (2010) 5(12):2188–98. doi: 10.2215/CJN.0508061
6. Ruggenenti P, Cravedi P, Chianca A, Perna A, Ruggiero B, Gaspari F, et al. Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol (2012) 23(8):1416–25. doi: 10.1681/ASN.2012020181
7. Lin W, Li HY, Lin S, Zhou T. Efficacy and safety of tacrolimus vs cyclophosphamide in the therapy of patients with idiopathic membranous nephropathy: a meta-analysis. Drug Des Devel Ther (2019) 13:2179–86. doi: 10.2147/DDDT.S209211
8. Chung EYM, Wang YM, Keung K, Hu M, McCarthy H, Wong G, et al. Membranous nephropathy: Clearer pathology and mechanisms identify potential strategies for treatment. Front Immunol (2022) 13:1036249. doi: 10.3389/fimmu.2022.1036249
9. Salhi S, Ribes D, Fortenfant F, Faguer S. Plasma cell-directed therapy for rituximab-refractory PLA2R+ membranous nephropathy. Nephrol Dial Transplant (2023) 135(6). doi: 10.1093/ndt/gfad135
10. Fervenza FC, Appel GB, Barbour SJ. Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med (2019) 381(1):36–46. doi: 10.1056/NEJMoa1814427
11. Krustev E, Clarke AE, Barber MRW. B cell depletion and inhibition in systemic lupus erythematosus. Expert Rev Clin Immunol (2023) 19(1):55–70. doi: 10.1080/1744666X.2023.2145281
12. Rojas-Rivera JE, Ortiz A, Fervenza FC. Novel treatments paradigms: membranous nephropathy. Kidney Int Rep (2023) 8(3):419–31. doi: 10.1016/j.ekir.2022.12.011
13. Zhao Q, Dai H, Liu X, Jiang H, Liu W, Feng Z, et al. Helper T cells in idiopathic membranous nephropathy. Front Immunol (2021) 12:665629. doi: 10.3389/fimmu.2021.665629
14. Rojas-Rivera JE, Carriazo S, Ortiz A. Treatment of idiopathic membranous nephropathy in adults: KDIGO 2012, cyclophosphamide and cyclosporine A are out, rituximab is the new normal. Clin Kidney J (2019) 12(5):629–38. doi: 10.1093/ckj/sfz127
15. Bose B, Chung EYM, Hong R, Strippoli GFM, Johnson DW, Yang WL, et al. Immunosuppression therapy for idiopathic membranous nephropathy: systematic review with network meta-analysis. J Nephrol (2022) 35(4):1159–70. doi: 10.1007/s40620-022-01268-2
16. Chen Y, Schieppati A, Cai G, Chen X, Zamora J, Giuliano GA, et al. Immunosuppression for membranous nephropathy: a systematic review and meta-analysis of 36 clinical trials. Clin J Am Soc Nephrol (2013) 8(5):787–96. doi: 10.2215/CJN.07570712
17. Boyer-Suavet S, Andreani M, Lateb M, Savenkoff B, Brglez V, Benzaken S, et al. Neutralizing anti-rituximab antibodies and relapse in membranous nephropathy treated with rituximab. Front Immunol (2020) 10:3069. doi: 10.3389/fimmu.2019.03069
18. Schmidt T, Schulze M, Harendza S, Hoxha E. Successful treatment of PLA2R1-antibody positive membranous nephropathy with ocrelizumab. J Nephrol (2021) 34(2):603–6. doi: 10.1007/s40620-020-00874-2
19. Sethi S, Kumar S, Lim K, Jordan SC. Obinutuzumab is effective for the treatment of refractory membranous nephropathy. Kidney Int Rep (2020) 5(9):1515–8. doi: 10.1016/j.ekir.2020.06.030
20. Barrett C, Willcocks LC, Jones RB, Tarzi RM, Henderson RB, Cai G, et al. Effect of belimumab on proteinuria and anti-phospholipase A2 receptor autoantibody in primary membranous nephropathy. Nephrol Dial Transplant (2020) 35(4):599–606. doi: 10.1093/ndt/gfz086
21. Salhi S, Ribes D, Colombat M, Fortenfant F, Faguer S. Bortezomib plus dexamethasone for rituximab-resistant PLA2R+ membranous nephropathy. Kidney Int (2021) 100(3):708–9. doi: 10.1016/j.kint.2021.04.011
22. Shi F, Xue R, Zhou X, Shen P, Wang S, Yang Y. Telitacicept as a BLyS/APRIL dual inhibitor for autoimmune disease. Immunopharmacol Immunotoxicol (2021) 43(6):666–73. doi: 10.1080/08923973.2021.1973493
23. Dhillon S. Telitacicept: first approval. Drugs (2021) 81(14):1671–5. doi: 10.1007/s40265-021-01591-1
24. Sun L, Shen Q, Gong Y, Li Y, Lv Q, Liu H, et al. Safety and efficacy of telitacicept in refractory childhood-onset systemic lupus erythematosus: A self-controlled before-after trial. Lupus (2022) 31(8):998–1006. doi: 10.1177/09612033221097812
25. Chen R, Fu R, Lin Z, Huang C, Huang W. The efficacy and safety of telitacicept for the treatment of systemic lupus erythematosus: a real life observational study. Lupus (2023) 32(1):94–100. doi: 10.1177/09612033221141253
26. Ding J, Jiang X, Cai Y, Pan S, Deng Y, Gao M, et al. Telitacicept following plasma exchange in the treatment of subjects with recurrent neuromyelitis optica spectrum disorders: A single-center, single-arm, open-label study. CNS Neurosci Ther (2022) 28(10):1613–23. doi: 10.1111/cns.13904
27. Xu D, Fang J, Zhang S, Huang C, Huang C, Qin L, et al. Efficacy and safety of telitacicept in primary Sjögren’s syndrome: a randomized, double-blind, placebo-controlled, phase 2 trial. Rheumatol (Oxford) (2023), kead265. doi: 10.1093/rheumatology/kead265
28. Lv J, Liu L, Hao C, Li G, Fu P, Xing G, et al. Randomized phase 2 trial of telitacicept in patients with IgA nephropathy with persistent proteinuria. Kidney Int Rep (2022) 8(3):499–506. doi: 10.1016/j.ekir.2022.12.014
29. Chen JW, Zhan JY, Liang SP, Wang XD, Lin CS, Xu Q. A patient with refractory proliferative lupus nephritis treated with telitacicept: A case report. Int J Rheum Dis Dis (2023) 26(7):1417–21. doi: 10.1111/1756-185X.14752
30. Li S, Ding L, Yang YJ, Yang XD. Telitacicept for minimal change disease. Kaohsiung J Med Sci (2023) 39(7):748–9. doi: 10.1002/kjm2.12719
Keywords: case report, successful, treatment, refractory membranous nephropathy, telitacicept
Citation: Zhang L, Jin H, Wang D and Wang Y (2023) Case report: Successful treatment of refractory membranous nephropathy with telitacicept. Front. Immunol. 14:1268929. doi: 10.3389/fimmu.2023.1268929
Received: 28 July 2023; Accepted: 28 September 2023;
Published: 17 October 2023.
Edited by:
Kathrin Eller, Medical University of Graz, AustriaReviewed by:
Balazs Odler, Medical University of Graz, AustriaMohamed Tharwat Hegazy, Cairo University, Egypt
Copyright © 2023 Zhang, Jin, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yiping Wang, d3lwd3lwNTRAYWxpeXVuLmNvbQ==
 Lei Zhang
Lei Zhang Hua Jin
Hua Jin Dong Wang
Dong Wang Yiping Wang
Yiping Wang