
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 02 October 2023
Sec. Autoimmune and Autoinflammatory Disorders : Autoimmune Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1267749
 Shuhei Yoshida1
Shuhei Yoshida1 Masayuki Miyata2
Masayuki Miyata2 Eiji Suzuki3
Eiji Suzuki3 Takashi Kanno3
Takashi Kanno3 Yuya Sumichika1
Yuya Sumichika1 Kenji Saito1
Kenji Saito1 Haruki Matsumoto1
Haruki Matsumoto1 Jumpei Temmoku1
Jumpei Temmoku1 Yuya Fujita1
Yuya Fujita1 Naoki Matsuoka1
Naoki Matsuoka1 Tomoyuki Asano1
Tomoyuki Asano1 Shuzo Sato1
Shuzo Sato1 Kiyoshi Migita1*
Kiyoshi Migita1*Background: The ORAL Surveillance trial showed a potentially higher incidence of malignancy and major adverse cardiovascular events (MACEs) associated with tofacitinib than those associated with tumor necrosis factor (TNF) inhibitors (TNFis). However, few studies have compared the safety of non-TNFis or other Janus kinase (JAK) inhibitors (JAKis). This study was aimed at comparing the incidence rates (IRs) of malignancies and MACEs in patients with rheumatoid arthritis (RA) treated using interleukin-6 (IL-6) inhibitors (IL-6is) or JAKis.
Methods: We retrospectively analyzed 427 patients with RA who were treated using an IL-6i (n = 273) or a JAKi (n = 154). We determined the IRs of malignancy and MACEs, and the standardized incidence ratio (SIR) of malignancies and investigated factors related to malignancy and MACEs. After adjusting the clinical characteristic imbalance by propensity score matching (PSM), we compared the IRs of adverse events between the JAKi and IL-6i groups.
Results: After PSM, the observational period was determined to be 605.27 patient-years (PY), and the median observational period was determined to be 2.28 years. We identified seven cases of malignancy (IR: 2.94 per 100 PY) in the JAKi-treated group and five cases (IR: 1.36 per 100 PY) in the IL-6i-treated group after PSM. The IR of MACEs was 2.56 and 0.83 (per 100 PY) in the JAKi- and IL-6i-treated groups. The IRRs of JAKi-treated patients versus IL-6i-treated patients were 2.13 (95% confidence interval (CI): 0.67–7.42) for malignancy and 3.03 (95% CI: 0.77–15.21) for MACE. There were no significant differences in IRR for malignancy and MACE between both groups after PSM. Univariate and multivariable Cox regression analyses revealed that older age and JAKi use were independent risk factors for malignancy, while older age, hypertension, and JAKi use were independent risk factors for MACEs. The overall malignancy SIR was significantly higher in the JAKi-treated group compared to the general population (2.10/100 PY, 95% CI: 1.23–2.97).
Conclusion: The IRs of malignancy and MACE in patients with RA after PSM were comparable between IL-6i-treated and JAKi-treated patients. However, the SIR of malignancy in JAKi treatment was significantly higher than in the general population; therefore, further safety studies comparing JAKi to non-TNFi biologic disease-modifying antirheumatic drugs (bDMARDs) are needed.
Rheumatoid arthritis (RA) is the most common type of autoimmune arthritis; it causes marked inflammation of the joint cartilage and bone damage and affects various other organs (1). The use of disease-modifying antirheumatic drugs (DMARDs), particularly biologic DMARDs (bDMARDs) and targeted synthetic DMARDs (tsDMARDs), such as Janus kinase (JAK) inhibitors (JAKis), theoretically enables remission to be the therapeutic goal in all patients with RA. In addition, these drugs can prevent the long-term progression of joint damage and physical dysfunction (2).
The JAK/signal transducer and activator of transcription (STAT) pathway is involved in the signal transduction of several cytokine receptors (3). JAKis inhibit the JAK-STAT pathway, leading to the inhibition of interleukin (IL)-6 and various other cytokines (4). In Japan, five JAKis have been approved for the treatment of RA: tofacitinib, baricitinib, peficitinib, upadacitinib, and filgotinib. Each JAKi has a different selectivity for JAK against each JAK isoform (5). The clinical efficacy of JAKis has been well established in large randomized controlled trials (RCTs) (6); however, there are concerns regarding the risk of adverse events (7). Recently, the ORAL Surveillance trial provided important data on the comparative safety of tofacitinib and tumor necrosis factor (TNF) inhibitors (TNFis) in the treatment of RA, raising concerns about the occurrence of malignancies and major adverse cardiovascular events (MACEs) during JAKi treatment (8). However, little is known about the comparative safety of other non-TNFi bDMARDs or JAKis other than tofacitinib. Furthermore, in the real world, JAKis tend to be introduced in patients who cannot tolerate methotrexate (MTX) because of comorbidities or in whom multiple bDMARDs have failed, which is quite different from those recruited in RCTs (9). Therefore, it would be of great interest to investigate the factors affecting MACEs and malignancy incidence of non-TNFis and JAKis in patients with RA in real-world clinical practice. We conducted the present multicenter cohort study to determine and compare the incidence rates (IRs) of MACEs and malignancies in RA patients treated with an interleukin-6 inhibitor (IL-6i) or a JAKi in clinical settings.
A multicenter retrospective cohort study was conducted to evaluate the IRs of MACEs and malignancies in patients with RA treated using an IL-6i or a JAKi. The cohort consisted of patients treated at the Department of Rheumatology of Fukushima Medical University Hospital, Japanese Red Cross Fukushima Hospital, and Ohta Nishinouchi Hospital. Between April 2012 and December 2022, IL-6i or JAKi therapy was initiated in 449 patients with RA. Among these patients, 430 started receiving IL-6i or JAKi therapy in our institution, and 427 patients for whom sufficient clinical data were available were enrolled in this study. All the patients were diagnosed with RA according to the 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for RA (10). The demographic data recorded at the start of each patient’s IL-6i or JAKi treatment included age, sex, disease duration, rheumatoid factor (RF), anti-citrullinated protein antibodies (ACPAs), history of bDMARD use, coexistence of diabetes mellitus (DM) or lung disease, history of malignancy, and concomitant medication(s). The IL-6i-treated patients received tocilizumab by intravenous infusion at 8 mg/kg every 4 weeks or by subcutaneous injection of 162 mg every 2 weeks or sarilumab by subcutaneous injection of 200 mg every 2 weeks. The JAKi-treated patients received baricitinib 2 mg (in patients with renal impairment) or 4 mg once daily, tofacitinib 5 mg twice or once daily (in patients with liver impairment), upadacitinib 15 mg once daily, and filgotinib 100 mg (in patients with renal impairment) or 200 mg once daily. The study was approved by the institutional review boards of Fukushima Medical University (No. 2019-097), Japanese Red Cross Fukushima Hospital (No. 55), and Ohta Nishinouchi Hospital (No. 2022-8). An opt-out strategy was chosen for the participants, and those who declined to provide informed consent were excluded.
“Exposure” was defined as the period from the initiation of IL-6i or JAKi treatment until treatment’s discontinuation or the patient’s transfer to another hospital, death, or the end of the study period, whichever occurred first. MACEs and malignancies were focused on as adverse events. These adverse events were identified as follows. “MACEs” were defined as a composite of cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke. They were determined by the patient’s attending rheumatologist or physician treating the MACE. A malignancy was defined as a composite of cancers excluding non-melanoma skin cancers. Malignancies were confirmed by the attending rheumatologist or physician treating them. All malignancies were confirmed via histological examination. Recurrent or metastatic malignancies that occurred within 1 month of the initiation of IL-6i or JAKi treatment were excluded from the analyses. The censoring time of the above-described adverse events was defined as the time from the administration of the first dose of JAKi or IL-6i until the end of the drug treatment or the last observation point, i.e., December 31, 2022.
Data are presented as medians and interquartile ranges for continuous variables and as frequencies and percentages for qualitative variables. The Mann–Whitney U test was used to compare continuous variables, and Fisher’s exact test was used to compare qualitative variables, as appropriate. Statistical significance for all tests was defined as a two-tailed p-value of <0.05. By propensity score matching in the JAKi-treated and IL-6i-treated groups, the following were analyzed: patient age; sex; disease duration; RF and ACPA positivity; MTX, glucocorticoid (GC), and b/ts DMARD use; comorbid lung disease, hypertension, and DM; and a history of malignancy. The number of adverse events, patient-years (PY) at risk, and IR ratio (IRR) with a 95% confidence interval (CI) were determined for each outcome. The time to malignancy development and MACEs in the IL-6i-treated and JAKi-treated groups were estimated using the Kaplan–Meier analysis, and log-rank tests were used to compare the cumulative IRs between the patient groups. The standardized incidence ratio (SIR) for overall malignancy (excluding cancer in situ) was calculated using the indirect standardization method. The estimated IRs of malignancy were determined in the general Japanese population in Fukushima Prefecture in 2019, stratified by sex and age, as reported by the Center for Cancer Control and Information Service, National Cancer Center, Japan (https://ganjoho.jp/reg_stat/statistics/data/dl/index.html). Univariate and multivariate Cox regression analyses were performed to identify the factors related to the incidences of malignancy and MACEs. Variables with p-values of <0.05 in univariate Cox regression analysis were included in the multivariate Cox regression analysis. Statistical analyses were performed using SPSS Statistics software (version 25.0; IBM Corp., Armonk, NY, USA) and R ver. 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org/[accessed May 15, 2023]).
Among the 449 patients with RA in whom IL-6i or JAKi treatment was started at our institutions between April 2012 and December 2022, 427 were enrolled in this study (Figure 1). The background characteristics of the patients in the IL-6i-treated and JAKi-treated groups before and after the propensity score matching are summarized in Table 1. The baseline demographic and clinical characteristics of each patient treated with a JAKi (baricitinib, tofacitinib, upadacitinib, and filgotinib) are summarized in Table 2. The 273 patients in the IL-6i-treated group included 269 and 4 patients who received tocilizumab and sarilumab, respectively, and the 154 patients in the JAKi-treated group included 94, 43, 13, and 5 patients receiving baricitinib, tofacitinib, upadacitinib, and filgotinib, respectively. None of the patients had a history of using JAKi. A comparison of the two groups before propensity score weighting showed a significantly longer disease duration, higher rate of concomitant GC use, higher MTX dose, and longer observation period in the IL-6i-treated group. In contrast, the age at b/ts DMARD introduction, GC dose, and rates of hypertension and DM coexistence were significantly higher in the JAKi-treated group. The observation period for the 427 patients (135 male and 292 female patients) examined in this study was 1,264.95 PY. The median (interquartile range) length of the observation period was 2.33 (1.08–3.92) years. After propensity score matching, 220 patients with RA (70 men and 150 women) were observed for 605.27 PY. The median (interquartile range) length of the observation period was 2.28 (1.06–3.84) years. There were no significant intergroup differences after propensity score weighting, except for in the history of b/ts DMARD use and observation period. In the JAKi-treated group, malignancies and MACEs occurred only in patients treated with baricitinib and tofacitinib.
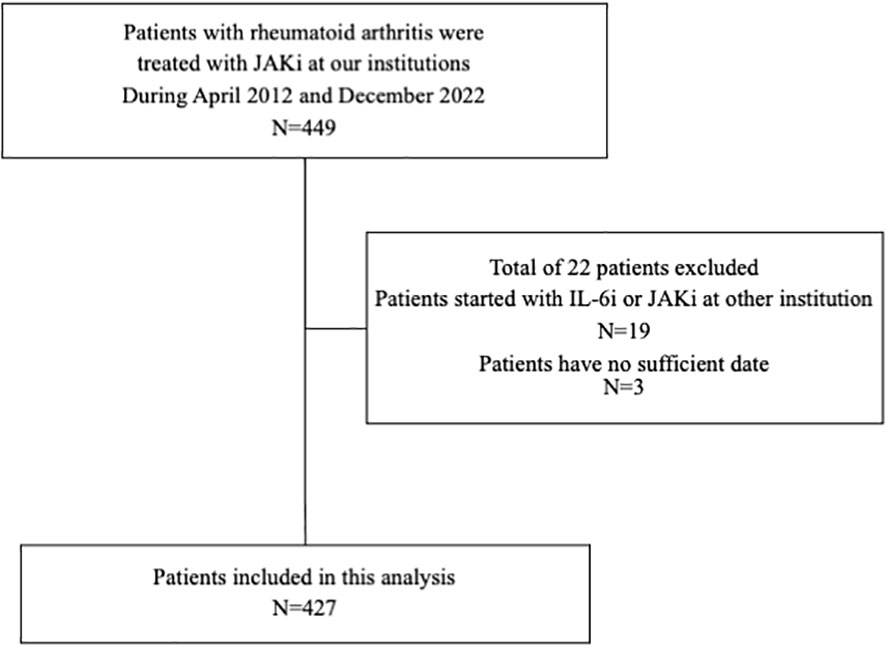
Figure 1 Flowchart showing patient selection. Among the 449 patients with RA who were initially treated with IL-6is or JAKis at our institution between April 2012 and December 2022, 427 for whom sufficient clinical data were available were enrolled in this study. RA, rheumatoid arthritis; IL-6i, interleukin-6 inhibitor; JAKi, Janus kinase inhibitor.
The IRs for malignancies are shown in Table 3. We identified 12 cases of malignancy (7.8%; IR: 3.70 per 100 PY; number needed to harm [NNH]: 27.06 PY) among 154 JAKi-treated patients. The most frequent malignancy in the JAKi-treated group was lymphoma (n = 6; 50% of all malignancies). The other six malignancies in the JAKi-treated group were lung cancer (n = 2), rectal cancer (n = 2), colon cancer (n = 1), and malignant melanoma (n = 1). Among the 273 IL-6i-treated patients, 10 had malignancies (3.7%; IR: 1.06 per 100 PY; NNH: 94.03 PY). In the IL-6i-treated group, the most frequent malignancy was lung cancer (n = 3; 30% of all malignancies). The other seven malignancies were colon cancer (n = 2), malignant lymphoma (n = 1), breast cancer (n = 1), pancreatic cancer (n = 1), prostate cancer (n = 1), and ovarian cancer (n = 1). Before propensity score weighting, the IRR for the JAKi-treated group to the IL-6i-treated group was 3.41, indicating that the JAKi-treated group was more likely to develop a malignancy than the IL-6i-treated group (95% CI: 1.48–8.30, p = 0.005). However, after propensity score weighting, there was no significant difference in the IRRs between the two groups (IRR = 2.13, 95% CI: 0.67–7.42, p = 0.20). The follow-up period of patients treated with JAKi was shorter than that of patients treated with IL-6i. Therefore, we evaluated the time-to-event outcome (malignancy) using the Kaplan–Meier curves. There was no significant difference in the cumulative incidence of malignancy between the groups (Figure 2). A comparison of the incidence of malignancy between the baricitinib and tofacitinib (JAKis) groups is shown in Table 4. The crude IR of malignancy was higher in the tofacitinib group than in the baricitinib group. There was no significant difference in the IRRs between the baricitinib and tofacitinib groups (IRR = 2.46, 95% CI: 0.77–8.45, p = 0.13). Compared with the general Japanese population, the SIR for all malignancies in JAKi treatment (2.10, 95% CI: 1.23–2.97) was significantly higher, and the SIR in IL-6i treatment (1.09, 95% CI: 0.56–1.61) was comparable (Table 5).
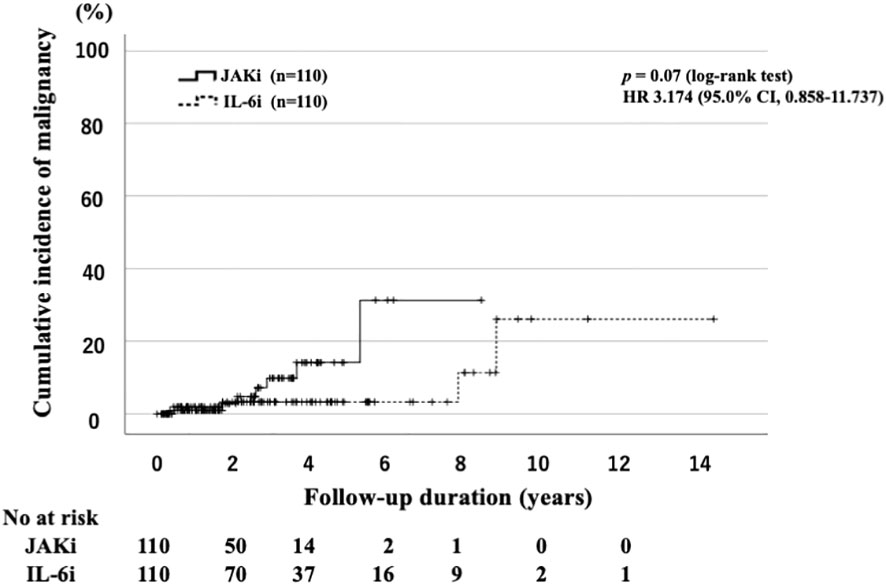
Figure 2 Cumulative incidence curves of malignancy in IL-6i-treated and JAKi-treated patients after propensity score matching. Kaplan–Meier curves showing the cumulative incidence of malignancies in patients treated with IL-6is (n = 110) and JAKis (n = 110). No significant differences were observed between IL-6i-treated and JAKi-treated groups. The starting point (0 years) was the date on which the observations began. RA, rheumatoid arthritis; IL-6i, interleukin-6 inhibitor; JAKi, Janus kinase inhibitor; No, number.
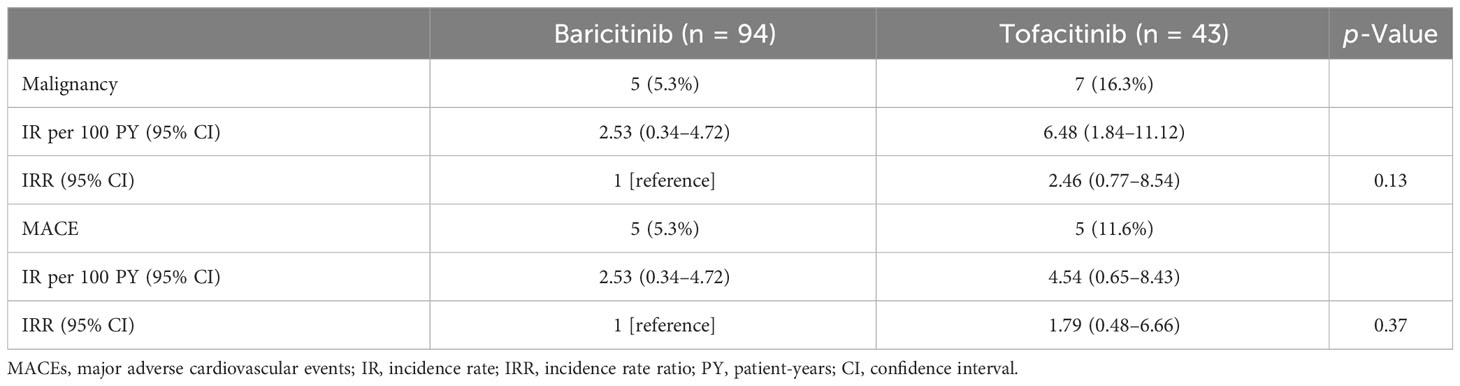
Table 4 Comparisons of the incidence rate of malignancy and MACE between patients treated with baricitinib and tofacitinib.
The IRs of MACEs are listed in Table 3. We determined the IR of MACEs and identified 10 cases of MACEs (6.5%; IR: 3.08 per 100 PY; NNH: 32.13 PY) in the JAKi-treated group. The MACEs in the JAKi-treated group included acute cardiac insufficiency (n = 4), brain hemorrhage (n = 2), subarachnoid hemorrhage (n = 2), acute myocardial infarction (n = 1), and aortic dissection (n = 1). There were four cases of MACEs in the IL-6i-treated group (1.5%; IR: 0.43 per 100 PY; NNH: 233.98 PY). In the IL-6i-treated group, the four MACEs were aortic dissection (n = 2), acute myocardial infarction (n = 1), and acute cardiac insufficiency (n = 1). Before the propensity score weighting, the IRR for the JAKi-treated group to the IL-6i-treated group was 7.07, indicating that the JAKi-treated group is more likely to occur MACEs than the IL-6i-treated group (95% CI: 2.33–26.63, p < 0.001). However, after propensity score weighting, there was no significant difference in the incidence of MACEs between the JAKi-treated and IL-6i-treated groups (IRR = 3.03, 95% CI: 0.77–15.21, p = 0.11). Furthermore, there was no significant difference in the cumulative incidence of MACE between the JAKi-treated and IL-6i-treated groups (Figure 3). A comparison of the incidence of MACEs between the baricitinib and tofacitinib groups is shown in Table 4. The crude IRs of MACEs were higher in the tofacitinib group than in the baricitinib group. There was no significant difference in the IRRs between the baricitinib and tofacitinib groups (IRR = 1.79, 95% CI: 0.48–6.66, p = 0.37).
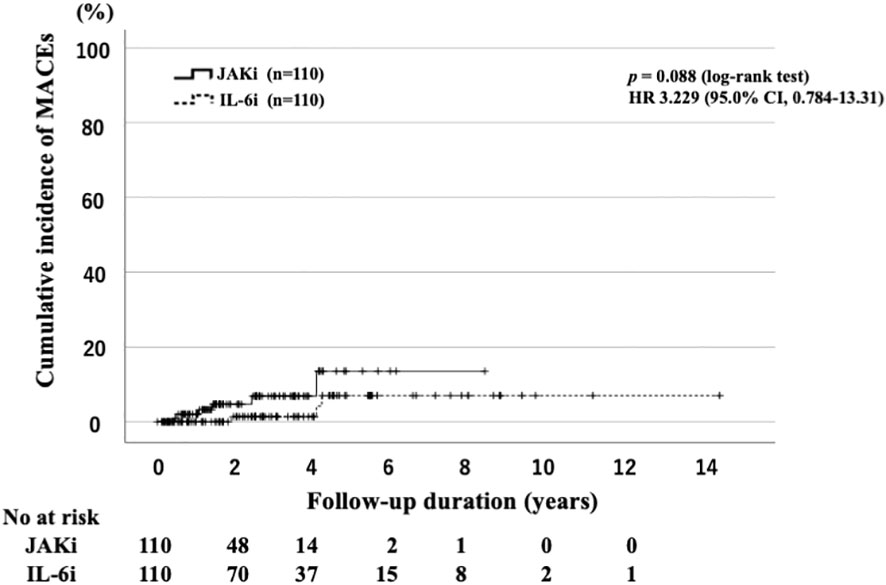
Figure 3 Cumulative incidence curves of MACEs in IL-6i-treated and JAKi-treated patients after propensity score matching. Kaplan–Meier curves showing the cumulative incidence of MACEs in patients treated with IL-6is (n = 110) and JAKis (n = 110). No significant differences were observed between IL-6i-treated and JAKi-treated groups. The starting point (0 years) was the date on which the observations began. RA, rheumatoid arthritis; IL-6i, interleukin-6 inhibitor; JAKi, Janus kinase inhibitor; MACEs, major cardiovascular events; No, number.
To identify the risk factors associated with malignancy and MACEs, we analyzed the baseline characteristics of the patients by using univariate and multivariate Cox regression analyses (Table 6). Univariate analysis indicated that age of >65 years and JAKi use were associated with the incidence of malignancy. Multivariate analysis showed similar results. Univariate analysis showed that age of >65 years, coexisting hypertension, and JAKi use were associated with MACEs.
The ORAL Surveillance trial provided important data concerning the safety of JAKis, showing an increased risk of MACEs and malignancy in patients with RA treated using tofacitinib compared with those treated using adalimumab among patients with cardiovascular risk factors (8). Studies have identified several risk factors for MACEs and cancer, including older age (>65 years), smoking, and a history of venous thromboembolism, MACEs, or cancer (11). The Food and Drug Administration issued warnings regarding the increased risk of MACEs and malignancy in patients with RA treated using JAKis (12). Following the ORAL Surveillance trial, several studies have been conducted to evaluate the safety of JAKis compared with TNFis in real-world patients with RA (13, 14). However, most of these studies found no evidence of an increased risk of cancer other than non-melanoma skin cancer in patients treated with JAKis compared with those with TNFis (13, 14). A recent meta-analysis also showed that JAKi treatment in real-world patients with RA was not associated with a significantly increased risk of the first primary cancer compared with those who received bDMARDs (15). However, few studies have comparatively assessed the safety of other non-TNFi bDMARDs or JAKi bDMARDs other than tofacitinib. We aimed to compare the incidence of cancer and MACEs in patients with RA treated with tocilizumab or JAKis in a real-world setting. Despite the different baseline characteristics, our data demonstrated that patients with RA treated using JAKis had a higher risk of developing MACEs and cancer than those treated with IL-6is. However, in the propensity score matching analysis, we did not observe any increase in the overall occurrence of cancer with JAKis compared to that with IL-6is. The follow-up period of patients with RA treated using JAKis was shorter than that of those treated with IL-6is. We evaluated the time-to-event outcomes (cancer and MACEs) using the Kaplan–Meier curves to minimize the influence of differences in follow-up time; however, there was no significant difference between these two groups, and the occurrence of malignancies in either the IL-6i-treated or TNFi-treated patients was generally infrequent in commercial databases with an IR of less than 10/1,000 PY (16). In our study, the IR for malignancy was 1.06/100 PY in patients with RA treated using IL-6is. Our results are consistent with those of previous studies (16).
In the ORAL Surveillance trial, the IR for malignancy was 1.13 (95% CI: 0.86–1.14) for those treated with tofacitinib 10 mg twice daily, compared with 0.77 among those treated using TNFis (95% CI: 0.55–1.04), indicating the significant risk associated with JAKis (hazard ratio (HR): 1.48; 95% CI: 1.04–2.09) (8). However, in RCTs or long-term extension studies, the overall rate of malignancy for JAKis has been reported to be similar to that associated with bDMARDs (17, 18). A recent multi-database cohort study also found no difference in the risk of malignancies, excluding non-melanoma skin cancer (NMSC), in patients with RA treated using tocilizumab compared with those treated with TNFis (19, 20). In our propensity score matching analysis, cancer IRs were similar between patients with RA treated using JAKis and IL-6is. Although the difference was not significant, the IR of malignancy in patients with RA treated using JAKis was 2.94 (95% CI: 0.80–5.08), which was higher than that in the previous studies and suggests the need for further safety assessments (14). Concerning risk factors, elderly age and the use of JAKis were identified as independent risk factors for malignancies in our study. This finding is consistent with the findings of earlier reports (11). Patients with RA are considered to have an increased risk of cancer, including lymphoma, compared with the general population (21, 22). The incidence of lymphoma was higher (6/12) in patients treated with JAKis than in those treated with tocilizumab (1/10). The mechanism by which JAKis are associated with some types of cancer is unknown; however, it can be speculated that JAKis may affect the functions of natural killer cells, which could potentially diminish the immunosurveillance of the host for cancer. Nevertheless, we could not exclude residual or unmeasured confounding factors in our propensity score matching comparisons between JAKis and tocilizumab. Further monitoring of the safety of JAKis and other bDMARDs is warranted because there are ongoing safety concerns about MACEs in patients with RA treated using JAKis.
In the ORAL Surveillance trial, which included patients with active RA aged >50 years and with a least one cardiovascular risk factor, the results indicated that the incidence of MACEs associated with tofacitinib was higher than that associated with TNFis (HR: 1.33; 95% CI: 0.91–1.94) (8). A post-hoc analysis showed a higher MACE risk with tofacitinib than with TNFis in patients with RA and a history of atherosclerotic cardiovascular disease (23). By contrast, no clear difference was observed in the risk of MACEs among patients without a history of atherosclerotic cardiovascular disease (23). In the propensity score matching analysis, the IRs of MACEs were similar between patients with RA treated using JAKis and those treated using IL-6is in our study. Although the difference was not significant, in patients treated with JAKis, the IR of MACEs was relatively high, which requires further safety assessment. Factors such as older patient age and the presence of DM were considered risk factors for MACEs in patients with RA in our study. The patients in the ORAL Surveillance trial were aged >50 years and had at least one additional cardiovascular risk factor, whereas the elderly patients in the present study had varying backgrounds, which might explain why MACEs occurred frequently in our cohort. Several studies have indicated the possibility of an increased incidence of MACEs in patients treated with JAKis (23, 24). Concerning the risk for MACEs, factors such as an advanced age (>65 years) and concomitant GC treatment were described in earlier reports (23, 24). More elderly patients with RA at risk for MACEs and cancer were enrolled in this study than in previous studies (8); this might explain why more MACEs occurred in our study.
Our study had several limitations. The number of patients (n = 427) and the duration of the follow-up period (April 2012 through December 2022) were not sufficient to detect all adverse events. Furthermore, the median follow-up period for the patients after propensity score matching was only 2.3 years, despite the 10-year recruitment period. The follow-up period was shorter in the JAKi group than in the IL-6i group, and that may affect the fewer adverse events in the JAKi group. Therefore, this study may not be adequately powered to determine the risk of malignancies or MACEs associated with the long-term use of JAKis or bDMARDs. Malignancy and MACE are rare outcomes, and not all factors that were significantly different in univariate Cox regression analysis could be included in the multivariate analysis. The choice of treatment was made at the discretion of each rheumatologist with no standardized protocol. In addition, the JAKi used in this study was not limited to the same agent. We also did not have data on family history of cancer, body mass index, smoking, and alcohol intake. Some patients with RA receive more than one b/ts DMARD, and previous b/ts DMARD exposure may influence the risk of cancer or MACEs. Adverse events, such as MACEs, may be affected by disease activity (25); however, the precise disease activity was not evaluated in this study.
In conclusion, there was no significant difference in the incidences of cancer and MACEs between patients with RA treated using JAKis and IL-6is in our propensity-matched analysis with a retrospective cohort design. The SIR of malignancy in JAKi treatment was significantly higher than in the general population. Although the difference was not significant, the IRs of MACEs and cancer seemed higher in patients with RA treated using JAKis than in those treated using IL-6is, suggesting the need for more safety studies comparing JAKis and non-TNFi bDMARDs.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the institutional review boards of Fukushima Medical University (No. 2019-097), Japanese Red Cross Fukushima Hospital (No. 55), and Ohta Nishinouchi Hospital (No. 2022–8). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because an opt-out strategy was chosen for the participants, and those who declined to provide informed consent were excluded.
SY: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. MM: Data curation, Supervision, Validation, Writing – review & editing. ES: Data curation, Supervision, Writing – review & editing, Validation. TK: Data curation, Supervision, Validation, Writing – review & editing. YS: Data curation, Validation, Writing – review & editing. KS: Data curation, Validation, Writing – review & editing. HM: Data curation, Validation, Writing – review & editing. JT: Data curation, Validation, Writing – review & editing. YF: Data curation, Validation, Writing – review & editing. NM: Data curation, Validation, Writing – review & editing. TA: Data curation, Validation, Writing – review & editing. SS: Data curation, Validation, Writing – review & editing. KM: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
The authors declare financial support was received for the research, authorship, and/or publication of this article. The study was supported by the Japan Grant-in-Aid for Scientific Research (20K08777).
We would like to thank Editage (www.editage.jp) for the English language review.
KM has received research grants from Chugai Pharmaceutical Co., Ltd. and Novartis Pharma K.K., which were unrelated to this study.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet (2016) 388(10055):2023–38. doi: 10.1016/s0140-6736(16)30173-8
2. Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, et al. Rheumatoid arthritis. Nat Rev Dis Primers (2018) 4:18001. doi: 10.1038/nrdp.2018.1
3. Villarino AV, Kanno Y, O'Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol (2017) 18(4):374–84. doi: 10.1038/ni.3691
4. Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol (2017) 13(4):234–43. doi: 10.1038/nrrheum.2017.23
5. O'Shea JJ, Gadina M. Selective Janus kinase inhibitors come of age. Nat Rev Rheumatol (2019) 15(2):74–5. doi: 10.1038/s41584-018-0155-9
6. Choy EH. Clinical significance of Janus Kinase inhibitor selectivity. Rheumatol (Oxford) (2019) 58(6):953–62. doi: 10.1093/rheumatology/key339
7. Winthrop KL, Cohen SB. Oral surveillance and JAK inhibitor safety: the theory of relativity. Nat Rev Rheumatol (2022) 18(5):301–4. doi: 10.1038/s41584-022-00767-7
8. Ytterberg SR, Bhatt DL, Mikuls TR, Koch GG, Fleischmann R, Rivas JL, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med (2022) 386(4):316–26. doi: 10.1056/NEJMoa2109927
9. Liu L, Yan YD, Shi FH, Lin HW, Gu ZC, Li J. Comparative efficacy and safety of JAK inhibitors as monotherapy and in combination with methotrexate in patients with active rheumatoid arthritis: A systematic review and meta-analysis. Front Immunol (2022) 13:977265. doi: 10.3389/fimmu.2022.977265
10. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum (2010) 62(9):2569–81. doi: 10.1002/art.27584
11. Curtis JR, Yamaoka K, Chen Y-H, Bhatt DL, Gunay LM, Sugiyama N, et al. Malignancy risk with tofacitinib versus TNF inhibitors in rheumatoid arthritis: results from the open-label, randomised controlled ORAL Surveillance trial. Ann Rheumatic Dis (2023) 82(3):331–43. doi: 10.1136/ard-2022-222543
12. Kragstrup TW, Glintborg B, Svensson AL, McMaster C, Robinson PC, Deleuran B, et al. Waiting for JAK inhibitor safety data. RMD Open (2022) 8(1). doi: 10.1136/rmdopen-2022-002236
13. Khosrow-Khavar F, Desai RJ, Lee H, Lee SB, Kim SC. Tofacitinib and risk of Malignancy: results from the safety of tofacitinib in routine care patients with rheumatoid arthritis (STAR-RA) study. Arthritis Rheumatol (2022) 74(10):1648–59. doi: 10.1002/art.42250
14. Uchida T, Iwamoto N, Fukui S, Morimoto S, Aramaki T, Shomura F, et al. Comparison of risks of cancer, infection, and MACEs associated with JAK inhibitor and TNF inhibitor treatment: a multicenter cohort study. Rheumatol (Oxford) (2023). doi: 10.1093/rheumatology/kead229
15. Bezzio C, Vernero M, Ribaldone DG, Alimenti E, Manes G, Saibeni S. Cancer risk in patients treated with the JAK inhibitor tofacitinib: systematic review and meta-analysis. Cancers (Basel) (2023) 15(8). doi: 10.3390/cancers15082197
16. Lopez-Olivo MA, Tayar JH, Martinez-Lopez JA, Pollono EN, Cueto JP, Gonzales-Crespo MR, et al. Risk of Malignancies in patients with rheumatoid arthritis treated with biologic therapy: a meta-analysis. Jama (2012) 308(9):898–908. doi: 10.1001/2012.jama.10857
17. Kremer JM, Bingham CO 3rd, Cappelli LC, Greenberg JD, Madsen AM, Geier J, et al. Postapproval comparative safety study of tofacitinib and biological disease-modifying antirheumatic drugs: 5-year results from a United States-based rheumatoid arthritis registry. ACR Open Rheumatol (2021) 3(3):173–84. doi: 10.1002/acr2.11232
18. Cohen SB, Tanaka Y, Mariette X, Curtis JR, Lee EB, Nash P, et al. Long-term safety of tofacitinib up to 9.5 years: a comprehensive integrated analysis of the rheumatoid arthritis clinical development programme. RMD Open (2020) 6(3). doi: 10.1136/rmdopen-2020-001395
19. Kim SC, Pawar A, Desai RJ, Gale S, Klearman M, Sarsour K, et al. OP0002 No difference in the risk of Malignancy in tocilizumab versus tnf inhibitor initiators in patients with rheumatoid arthritis: a multi-database cohort study. Ann Rheumatic Dis (2018) 77(Suppl 2):50–. doi: 10.1136/annrheumdis-2018-eular.3352
20. Kim SC, Pawar A, Desai RJ, Solomon DH, Gale S, Bao M, et al. Risk of Malignancy associated with use of tocilizumab versus other biologics in patients with rheumatoid arthritis: A multi-database cohort study. Semin Arthritis Rheum (2019) 49(2):222–8. doi: 10.1016/j.semarthrit.2019.03.002
21. Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of Malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther (2015) 17(1):212. doi: 10.1186/s13075-015-0728-9
22. Hashimoto A, Chiba N, Tsuno H, Komiya A, Furukawa H, Matsui T, et al. Incidence of Malignancy and the risk of lymphoma in Japanese patients with rheumatoid arthritis compared to the general population. J Rheumatol (2015) 42(4):564–71. doi: 10.3899/jrheum.140533
23. Charles-Schoeman C, Buch MH, Dougados M, Bhatt DL, Giles JT, Ytterberg SR, et al. Risk of major adverse cardiovascular events with tofacitinib versus tumour necrosis factor inhibitors in patients with rheumatoid arthritis with or without a history of atherosclerotic cardiovascular disease: a post hoc analysis from ORAL Surveillance. Ann Rheum Dis (2023) 82(1):119–29. doi: 10.1136/ard-2022-222259
24. Khosrow-Khavar F, Kim SC, Lee H, Lee SB, Desai RJ. Tofacitinib and risk of cardiovascular outcomes: results from the Safety of TofAcitinib in Routine care patients with Rheumatoid Arthritis (STAR-RA) study. Ann Rheum Dis (2022) 81(6):798–804. doi: 10.1136/annrheumdis-2021-221915
Keywords: rheumatoid arthritis, tofacitinib, baricitinib, JAK inhibitor, IL-6 inhibitor, malignancy, major adverse cardiovascular events
Citation: Yoshida S, Miyata M, Suzuki E, Kanno T, Sumichika Y, Saito K, Matsumoto H, Temmoku J, Fujita Y, Matsuoka N, Asano T, Sato S and Migita K (2023) Safety of JAK and IL-6 inhibitors in patients with rheumatoid arthritis: a multicenter cohort study. Front. Immunol. 14:1267749. doi: 10.3389/fimmu.2023.1267749
Received: 27 July 2023; Accepted: 13 September 2023;
Published: 02 October 2023.
Edited by:
Michele Maria Luchetti Gentiloni, Marche Polytechnic University, ItalyReviewed by:
Raimon Sanmarti, Hospital Clinic of Barcelona, SpainCopyright © 2023 Yoshida, Miyata, Suzuki, Kanno, Sumichika, Saito, Matsumoto, Temmoku, Fujita, Matsuoka, Asano, Sato and Migita. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kiyoshi Migita, bWlnaXRhQGZtdS5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.