- 1Department of Pathology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
- 2Department of Thoracic Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
- 3Department of Nuclear Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
- 4Hubei Key Laboratory of Molecular Imaging, Wuhan, Hubei, China
Neoadjuvant chemoimmunotherapy has demonstrated significant benefit for resectable non-small-cell lung cancer (NSCLC) excluding known EGFR/ALK genetic alterations. Recent evidence has shown that neoadjuvant chemoimmunotherapy could be clinically valuable in resectable localized driver gene-mutant NSCLC, though the data still lack robust support, especially for rare oncogenic mutations. Here, we report a patient with stage IIIA lung adenocarcinoma with a RET fusion gene and high expression of PD-L1 who underwent neoadjuvant chemoimmunotherapy and successfully attained a pathologic complete response. The patient has survived for 12 months with no recurrence or metastases after surgery. Our case suggests that this treatment strategy may be an alternative therapeutic option for resectable RET fusion-positive NSCLC patients.
Introduction
RET fusions occur in 1–2% of NSCLCs (1). A majority of patients with RET -rearranged lung cancer presented stage III–IV disease at the time of initial diagnosis (2). The selective RET inhibitors, called selpercatinib and pralsetinib, have led to improved outcomes in the field of response rates and have shown encouraging survival rates in advanced RET fusion–positive NSCLCs (3, 4), promoting the recent U. S. Food and Drug Administration approval of the RET tyrosine kinase inhibitor (TKI) for advanced or metastatic RET-fusion NSCLC patients.
The clinical manifestations of resectable NSCLC patients harboring RET fusion include an adenocarcinoma histologic subtype, younger age, never-smoker status, solid-predominant subtype, poor differentiation, smaller diameter (≤3 cm), and early lymph node metastasis (5), which may be related to early relapse. Early operable RET fusion-positive NSCLC patients receive the same standard-of-care treatments as driver gene-negative NSCLC patients. These treatments include definitive locoregional therapy and/or platinum-based chemotherapy with regular follow-up (6). Increasingly, clinical trials suggest that neoadjuvant chemoimmunotherapy has promising efficacy for resectable NSCLC (7, 8). However, most neoadjuvant chemoimmunotherapy or immunotherapy clinical trials exclude patients with EGFR/ALK mutations, resulting in a lack of convincing evidence on the efficacy in the presence of rare driver alterations (9). The performance of neoadjuvant chemoimmunotherapy as first-line therapy across resectable NSCLCs harboring RET fusions has rarely been specifically reported (9, 10). Camrelizumab, a novel programmed cell death protein 1 (PD-1) inhibitor developed in China, has shown encouraging efficacy in advanced NSCLC either as monotherapy or in combination with chemotherapy (11, 12). Several recent retrospective observational studies have indicated that camrelizumab-based treatment had satisfactory efficacy for NSCLCs in neoadjuvant setting (13–15).
Herein, we present a patient with stage IIIA lung adenocarcinoma with KIF5B-RET fusion who responded well to neoadjuvant camrelizumab plus chemotherapy and achieved pathologic complete response (pCR).
Case presentation
A 59-year-old Chinese male nonsmoker presented with a 1-month history of cough and discomfort in Nov 2021. The patient’s Eastern Cooperative Oncology Group (ECOG) performance status was 0. Computed tomography (CT) scan revealed a 3.2 cm × 2 cm × 3.2 cm soft tissue mass in the dorsal segment of the lower lobe of the right lung near the hilum (Figure 1A) and enlarged mediastinal 2R, 4R, 7th and right hilar lymph nodes (Figure 1B). There was no evidence of malignant lesions was observed in the central nervous system (Supplementary Figure 1). Endobronchial ultrasound-transbronchial needle aspiration (EBUS-TBNA) for the mediastinal lymph nodes was performed for enlarged 7th station lymph nodes (Figure 1C). Pathological examination showed low-differentiation metastatic lung adenocarcinoma with positive expression of TTF-1 and Napsin A (Figures 1D–F). Moreover, programmed cell death-ligand 1 (PD-L1) expression was found to be up to 80% in the tumor cells, as tested by the clone Dako 22C3 pharmDx antibody (Figure 1G). According to the 8th edition of American Joint Committee on Cancer (AJCC) staging system, the patient was clinically diagnosed with stage IIIA (cT2aN2M0) lung adenocarcinoma. The tumor tissue obtained by biopsy was evaluated by next-generation sequencing (NGS) of 68 solid tumor core genes. The sequencing assay showed that this patient had a RET fusion: KIF5B-RET (K15: R12, abundance: 2.14%) (Figure 1J), which was further confirmed by amplification-refractory mutation system-polymerase chain reaction technology (ADx-ARMS RT-PCR kit, Amoy Diagnostics, Xiamen, China) (Figure 1H) and in situ hybridization assay analysis with RET break-apart fluorescence (ZytoVision) (Figure 1I).
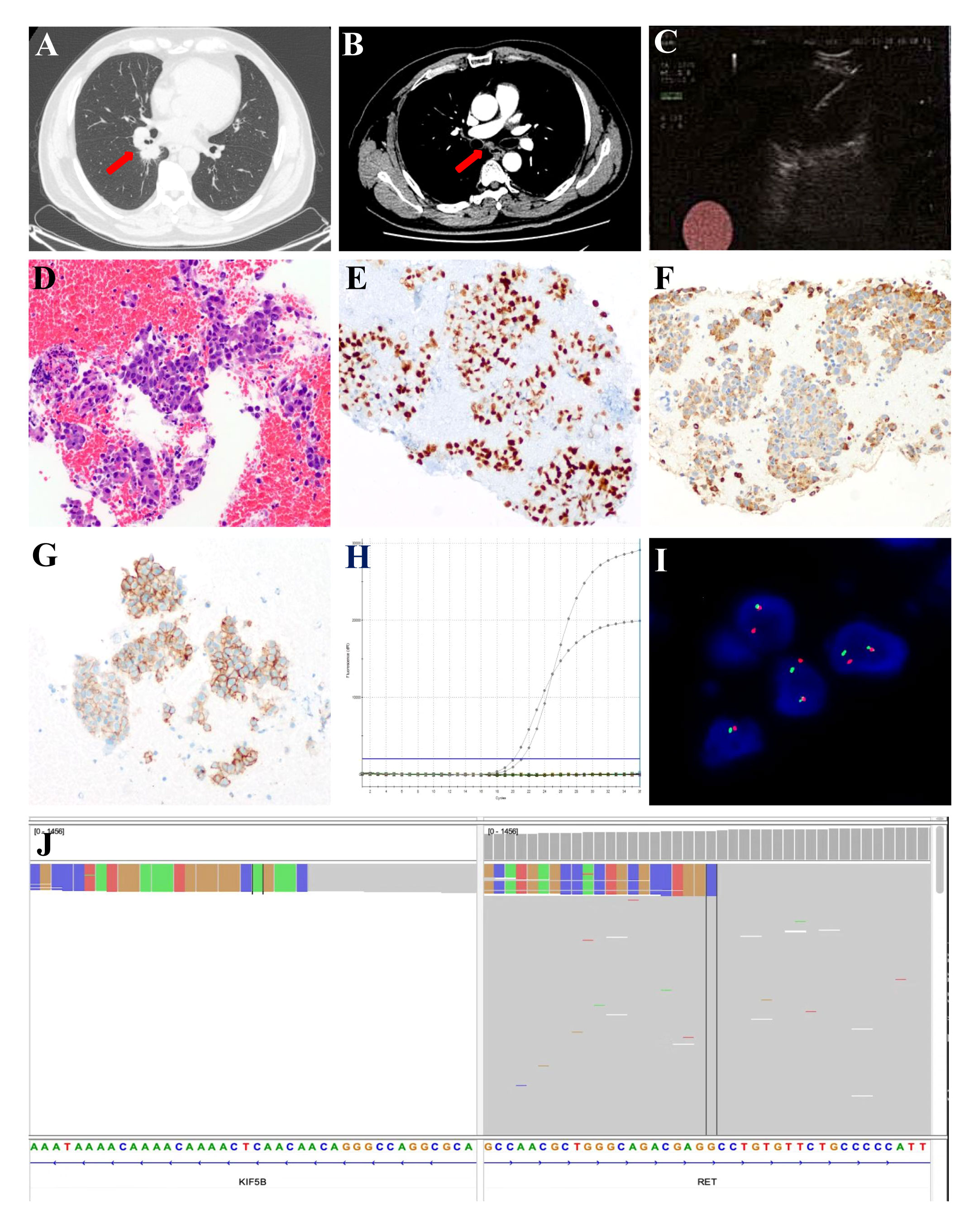
Figure 1 Chest radiological and pathological findings of the 7th station lymph nodes. (A) CT scan images of the primary tumor (red arrow) and (B) CT scan images of the enlarged 7th station lymph nodes (red arrow). (C) EBUS-TBNA for the enlarged 7th station lymph nodes. (D) HE-stained image of the 7th station lymph nodes biopsy specimens (Original magnification, ×200). Immunohistochemical features indicate positive expression of TTF-1 (E) and Napsin A (F). (G) PD-L1 assay of the 7th station lymph nodes biopsy specimens by immunohistochemical staining (Original magnification, ×200). (H) The presence of KIF5B-RET gene fusion was identified by ARMS RT-PCR. (I) RET rearrangement was detected by the break-apart fluorescence in situ hybridization (FISH) assay (Original magnification, ×1000). (J) NGS results revealed KIF5B-RET gene fusion in the Integrative Genomics Viewer genome browser. ARMS, Amplification Refractory Mutation System; CT, computed tomography; EBUS-TBNA, endobronchial ultrasound with real-time guided transbronchial needle aspiration; HE, hematoxylin and eosin; PD-L1, programmed cell death-ligand 1; RT-PCR, Reverse Transcription-Polymerase Chain Reaction.
Currently, there is insufficient evidence regarding the neoadjuvant targeted therapy regimens for patients with RET-fusion resectable NSCLC. In addition, the patient could not afford highly selective RET inhibitors. After extensively discussing the standard regimen and the potential risks of pursuing a non-standard treatment option with the patient, a combination of chemotherapy and immunotherapy adopted after he gave informed consent. The patient was started on camrelizumab (200 mg) combined with carboplatin (area under the curve 5 mg/mL per min) plus pemetrexed (500 mg/m2), administered every 3 weeks for 3 cycles on 2021-12-21, 2022-01-13, and 2022-02-07. Adverse events of the drugs were well controlled and tolerable. There were no grade 4 toxicities during neoadjuvant therapy. Posttreatment radiologic evaluation by PET-CT showed that the primary lesion and mediastinal lymph nodes had decreased in size and had slowed their metabolism when compared with the disease status in Nov 2021 (Figure 2). The disease response was considered a partial response (PR) according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 after the 3rd cycles of chemoimmunotherapy. Following multidisciplinary discussion and evaluation, the patient underwent a video-assisted thoracic surgery of lower lung lobectomy plus radical mediastinal lymph node dissection (including the 2nd/4th station (3 nodes), 7th station (2 nodes), 11th station (2 nodes), 12th station (3 nodes), 13th station (1 nodes) and other paratracheal lymph nodes (6 nodes)) on 9 March 2022, which was 4 weeks after he completed neoadjuvant therapy. The postoperative pathologic examination demonstrated the absence of any remaining viable tumor cells in both the primary lesion and resected lymph nodes, thereby confirming the patient’s attainment of a pCR subsequent to neoadjuvant chemoimmunotherapy (Figures 3A, B). After surgery, the patient was treated with adjuvant immunotherapy using camrelizumab (200 mg/m2) for 13 cycles, and no recurrence or complications were observed during the 12-month follow-up (Figure 4).
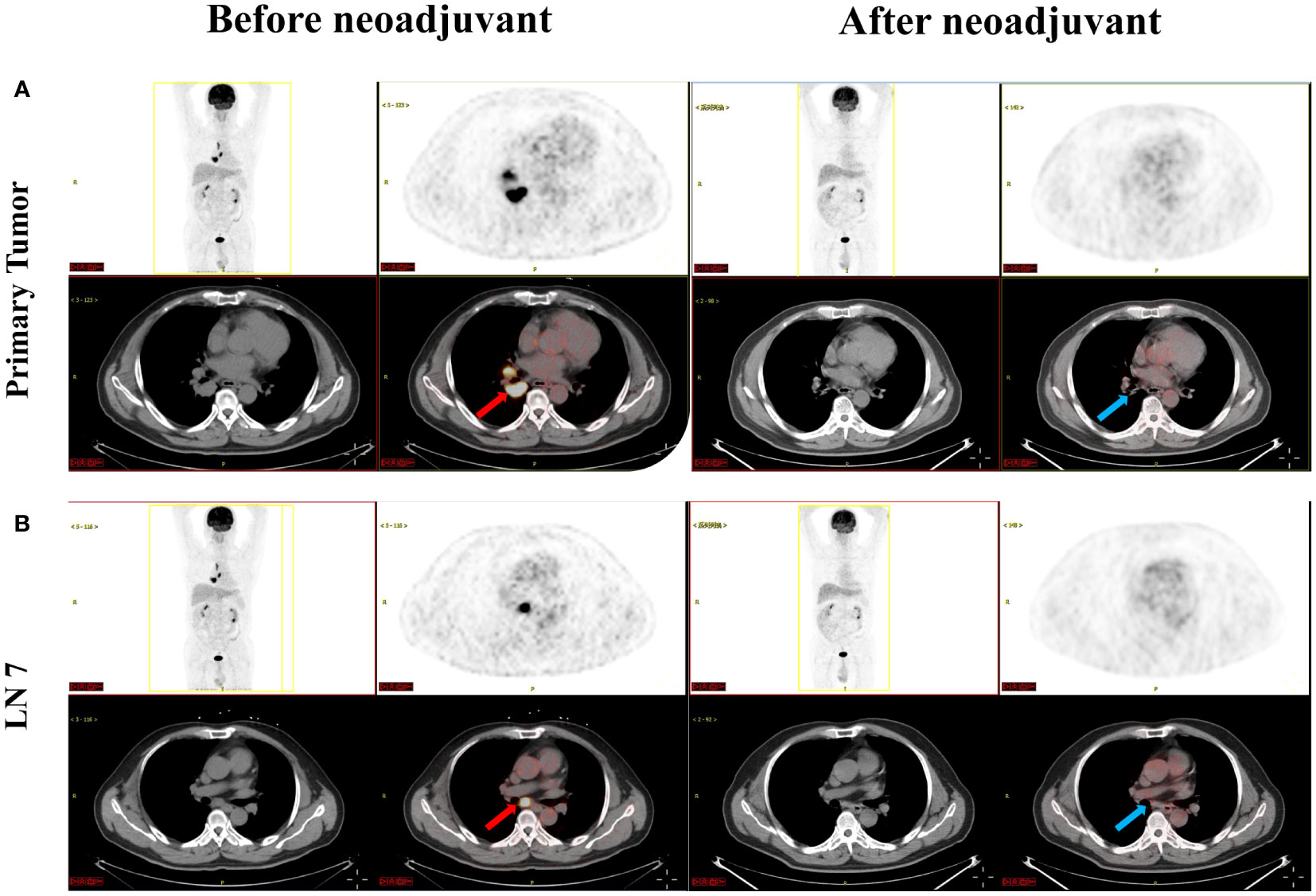
Figure 2 Images before (red arrow) and after (blue arrow) neoadjuvant camrelizumab plus chemotherapy treatment. (A) showed PET-CT of the primary tumor; (B) showed PET-CT of the 7th station lymph nodes. LN 7, 7th station lymph nodes; PET-CT, positron emission tomography-computed tomography.
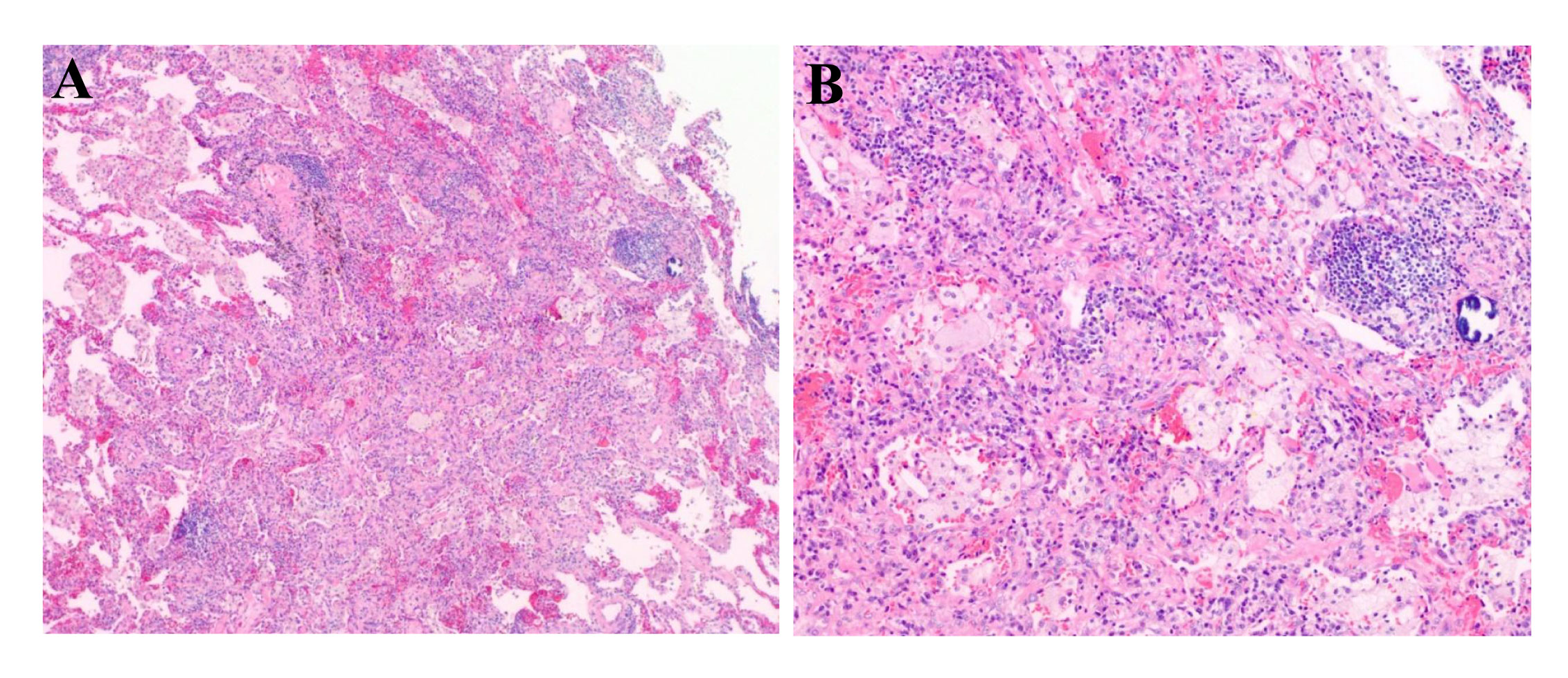
Figure 3 Picture of the postoperative pathological HE-staining of the primary lesion of the lower lobe of the right lung. (Original magnification, (A): ×40; (B): ×100). HE, hematoxylin and eosin.

Figure 4 Timeline of the patient’s treatment history. LUAD, lung adenocarcinoma; PD-L1, programmed death-ligand 1; NGS, next-generation sequencing; pCR, pathological complete response; AUC, area under the curve; DFS, disease-free survival.
All procedures performed in the human participant were in accordance with the ethical standards of the institutional and national research committee(s) and with the Helsinki Declaration (as revised in 2013). All treatments were performed with the consent of the patient. Written informed consent was obtained from the patient for publication of this study and any accompanying images.
Discussion
Patients with RET-rearranged lung adenocarcinomas make up a rare and heterogeneous NSCLC subgroup (16). Selective RET inhibitors have demonstrated remarkable and durable success against advanced RET fusion-positive NSCLC, but there is less evidence in the neoadjuvant setting. More recently, studies revealed that neoadjuvant immunotherapy plus chemotherapy has had a significant benefit in driver gene-negative patients but has also indicated its potential clinical feasibility for treating resectable localized oncogene-mutant NSCLC (9). However, the data is still limited for patients possessing RET fusion genes, and more available data are needed to support it use. Here, we describe the beneficial effect of neoadjuvant chemoimmunotherapy in a stage IIIA NSCLC patient who harbored a RET fusion gene with high PD-L1 expression.
Encouragingly, the selective RET inhibitors, selpercatinib and pralsetinib, have shown potent and durable clinical activity, with a well-tolerated safety profile in patients with advanced RET-altered NSCLC (3, 4). However, resectable RET fusion-positive NSCLC patients treated with selective TKI adjuvant and neoadjuvant therapy have only been found in scattered case reports to date. Zhou, N. et al. reported on the efficacy of pralsetinib as neoadjuvant treatment in a patient diagnosed with stage IIIA lung adenocarcinoma harboring KIF5B-RET rearrangement, transforming the unresectable tumor into a resectable tumor. In their study, the postoperative pathologic results revealed tumor regression rate of 74% and no residual viable tumor cells were observed in the resected lymph nodes (17). An, L. et al. reported a KIF5B-RET fusion patient with stage IB lung adenocarcinoma who achieved stable disease(SD) for more than 9 months with second-line adjuvant pralsetinib treatment (18). Because high-grade evidence of RET selective TKIs as neoadjuvant targeted therapy from large studies is scarce and our patient could not afford the cost of treatment, we could not perform targeted therapy.
The activity of adjuvant immunotherapy in advanced NSCLC patients carrying rare driver oncogene alterations, including RET fusion, has not been well characterized. A few studies have suggested that immune checkpoint inhibitors (ICIs) as adjuvant therapy show modest activity in RET-rearranged NSCLCs. A retrospective multicenter study compared the activity of therapeutics that included multikinase inhibitors (MKIs), chemotherapy, and single-agent immunotherapy in 45 patients with locally advanced or metastatic RET-altered NSCLC. The findings indicated that there was no significant difference among the groups with regard to progression-free survival (PFS). Notably, the disease control rate (DCR) by ICIs was 60% (2). In addition, Guisier et al. have shown the effectiveness of ICIs as second-line treatment in nine patients with RET translocation. For these patients, the overall response rate (ORR) was 38% and the median progression-free survival (mPFS) was 7.6 months (95% CI, 2.3–NR) (19). The RET-MAP study, the largest cohort study on this topic reported to date, reported 52 patients with RET-fusion positive NSCLC treated with ICI monotherapy with a high ORR of 23%. Two patients had complete responses (CR) to single-agent immunotherapy, and the median duration of response (mDOR) was 9.4 months (20). Nevertheless, in other retrospective studies, the efficacy of immunotherapy as monotherapy in patients with advanced RET fusion-positive NSCLC has not been satisfactory, with ORR ranging from 0% to 7.7% and mPFS ranging from 2.1 to 3.4 months, respectively (21–23). Considering the small sample size of the above-mentioned studies, the ICI activity in RET-positive NSCLC remains uncertain. In a recent study on the use of ICIs in combination with chemotherapy for patients with advanced RET fusion-positive NSCLC, the 12 patients who received chemoimmunotherapy as first-line treatment had an ORR of 70% (7/10) (24). The results suggested that immunotherapy combined with chemotherapy may have promising efficacy in RET fusion-positive patients, prompting the question of whether resectable NSCLC patients with RET fusions could also benefit from chemoimmunotherapy.
Growing evidence has demonstrated significant efficacy and favorable safety profile of neoadjuvant chemoimmunotherapy in the treatment of resectable NSCLC (25–29). Recently released data from Checkmate-816 showed that the combination of nivolumab and chemotherapy as neoadjuvant therapy resulted in significantly longer event-free survival (EFS) and a higher pCR proportion than chemotherapy alone in resectable NSCLC patients without known EGFR/ALK mutations. The addition of nivolumab to neoadjuvant chemotherapy did not result in an increase in adverse events or hinder surgery (26). Based on these clinical trials, the FDA granted approval for the use of nivolumab in combination with platinum doublet chemotherapy as neoadjuvant treatment for patients with resectable NSCLC. At present, there is a scarcity of data regarding the treatment of oncogene-driven NSCLC using novel neoadjuvant modalities. Zhang, C. et al. conducted a study using a large consecutive multicenter cohort of 40 patients with oncogene-mutant NSCLC (including EGFR, KRAS, RET fusion, ROS1 fusion, ALK fusion, HER2 and BRAF insertion) treated with neoadjuvant immunotherapy. In their cohort, the ORR was 62.5%, the major pathological response (MPR) rate was 37.5%, and the pCR rate was 12.5%. Among the three patients with RET fusion-positive NSCLC, one individual underwent neoadjuvant immunotherapy and achieved a PR, while the remaining two patients received a combination of neoadjuvant immunotherapy and chemotherapy resulting in SD and MPR, respectively (9). These findings initially indicated that neoadjuvant immunotherapy may no longer be considered a contraindication for surgically resectable localized NSCLC with oncogene mutations.
The investigation of predictive biomarkers for chemoimmunotherapy is still ongoing. PD-L1 status, which is widely recognized as a predictive biomarker for immunotherapy in advanced NSCLC, tends to be expressed at low-to-intermediate levels in RET-rearranged lung cancers. Previous reports have reported that the tumor proportion score of PD-L1≥1% ranged from 42% to 77.8% and high PD-L1 expression (≥50%) was observed in 19% to 37% of tumor cells in these patients (20, 22, 30, 31). The predictive role of PD-L1 in immunotherapy for patients with advanced RET-rearranged lung cancer remains a subject of controversy. Offin et al. conducted a study involving 13 advanced RET-rearranged NSCLC patients to demonstrate the effectiveness of ICIs. The mPFS in all patients was 3.4 months (95% CI, 2.1 to 5.6 months). However, patients with higher levels of PD-L1 expression (50% and 30%) experienced shorter PFS durations of 1.3 months and 2.5 months, respectively (22). In a separate case series, Rodriguez et al. observed that patients with RET-arranged lung cancers and high PD-L1 expression exhibited positive responses to immunotherapy (32). Furthermore, Baby, S. et al. documented a case of a patient with metastatic RET-rearranged NSCLC and 100% positive PD-L1 expression who obtained a CR lasting for 29 months and ongoing, following frontline pembrolizumab treatment (33). Similarly, Nakasuka, T. et al. reported the positive impact of pembrolizumab in a patient with 90% positive PD-L1 expression advanced NSCLC harboring the CCDC6–RET fusion gene and NF1/TP53 mutations (34). In the present case, PD-L1 expression positivity reached 80%, which likely accounts for part of the favorable outcome of this treatment strategy. Taken together, more effective predictive biomarkers for identifying patients with oncogene-addicted NSCLC who would benefit from chemoimmunotherapy require further exploration and validation in future clinical trials.
In the present case, ICI-based regimen was applied in neoadjuvant therapy and postoperative adjuvant therapy setting, and immune-mediated toxicity was tolerable during treatment period. No RET-TKIs was used in this reported case. Additionally, the toxicity risk associated with concurrent or sequential treatment with ICIs and RET-TKIs has not been adequately explored so far and only sporadic reports involved. Gu et al. reported a case of the pralsetinib efficacy and safety in a stage IIIA NSCLC with 3 concurrent RET fusions (CCDC6-RET, LINCO1264-RET and SEMA5A-RET), who was first treated with multiple lines of chemotherapy and switched to ICI (toripalimab) for 2 cycles but failed to respond (35). Despite with a renal insufficient background, adverse events of pralsetinib therapy included grade 2 creatinine elevation and grade 1 proteinuria, hypertension and rash after dose reduction to 100 mg. The above case suggested that sequential RET-TKIs therapy after ICIs failure may still be effective and toxicity-manageable. Actually, ICIs-based regimens were commonly used as later lines therapy in advanced RET fusion-positive patients according to the limited evidence from real-world studies. In a retrospective, multicenter study, ICIs were used as ≥2nd-line treatment in 9 advanced RET-fusion NSCLC patients and researchers reported 10% of enrolled patients were observed grade 3 to grade 5 immune-mediated AEs (most frequent was colitis) (19). Furthermore, another RET−fusion positive case with brain metastases and disease progression after previous RET-TKIs therapy, the toxicity of subsequent ICIs-based treatment was manageable and no ICIs discontinuance for severe adverse events occurred (36). At present, related research concerning the toxicity risk associated with concurrent or sequential adiministration with ICIs and RET-TKIs is very limited and further study will be explored in the future.
Conclusion
In summary, we provide the first direct evidence that resectable NSCLC with RET fusion and strongly positive PD-L1 staining (tumor proportion score = 80%) had a significant response to neoadjuvant chemoimmunotherapy, indicating that neoadjuvant chemoimmunotherapy may be a promising candidate for treating resectable RET fusion-positive NSCLC patients with high expression of PD-L1. Future investigations will help to clarify the optimal timing to use chemotherapy plus immunotherapy in these patients and the potential clinical feasibility in neoadjuvant setting.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the ethics committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MD: Data curation, Investigation, Writing – original draft. NW: Data curation, Investigation, Writing – original draft. QX: Data curation, Investigation, Writing – original draft. YL: Writing – review & editing. WC: Writing – review & editing. JF: Writing – review & editing. DZ: Writing – review & editing, Conceptualization. SW: Conceptualization, Writing – review & editing. XN: Writing – review & editing, Funding acquisition, Project administration.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (grant number 82072333); and the Natural Science Foundation of Hubei Province (grant number 2023AFB986).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1258762/full#supplementary-material
Supplementary Figure 1 | The images of PET-CT in central nervous system. (A) and (B) showed no sign of malignant lesions was observed in the brain.
References
1. Qiu Z, Ye B, Wang K, Zhou P, Zhao S, Li W, et al. Unique genetic characteristics and clinical prognosis of female patients with lung cancer harboring ret fusion gene. Sci Rep (2020) 10(1):10387. doi: 10.1038/s41598-020-66883-0
2. Lu C, Dong XR, Zhao J, Zhang XC, Chen HJ, Zhou Q, et al. Association of genetic and immuno-characteristics with clinical outcomes in patients with ret-rearranged non-small cell lung cancer: A retrospective multicenter study. J Hematol Oncol (2020) 13(1):37. doi: 10.1186/s13045-020-00866-6
3. Gainor JF, Curigliano G, Kim DW, Lee DH, Besse B, Baik CS, et al. Pralsetinib for ret fusion-positive non-small-cell lung cancer (Arrow): A multi-cohort, open-label, phase 1/2 study. Lancet Oncol (2021) 22(7):959–69. doi: 10.1016/S1470-2045(21)00247-3
4. Drilon A, Oxnard GR, Tan DSW, Loong HHF, Johnson M, Gainor J, et al. Efficacy of selpercatinib in ret fusion-positive non-small-cell lung cancer. New Engl J Med (2020) 383(9):813–24. doi: 10.1056/NEJMoa2005653
5. Wang R, Hu H, Pan Y, Li Y, Ye T, Li C, et al. Ret fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J Clin Oncol (2012) 30(35):4352–9. doi: 10.1200/JCO.2012.44.1477
6. NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelinesa®). Non-small cell lung cancer (2023). Available at: www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
7. Saw SPL, Ong BH, Chua KLM, Takano A, Tan DSW. Revisiting neoadjuvant therapy in non-small-cell lung cancer. Lancet Oncol (2021) 22(11):e501–e16. doi: 10.1016/S1470-2045(21)00383-1
8. Uprety D, Mandrekar SJ, Wigle D, Roden AC, Adjei AA. Neoadjuvant immunotherapy for nsclc: current concepts and future approaches. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer (2020) 15(8):1281–97. doi: 10.1016/j.jtho.2020.05.020
9. Zhang C, Chen HF, Yan S, Wu L, Yan LX, Yan XL, et al. Induction immune-checkpoint inhibitors for resectable oncogene-mutant nsclc: A multicenter pooled analysis. NPJ Precis Oncol (2022) 6(1):66. doi: 10.1038/s41698-022-00301-8
10. Zhao ZR, Lin ZC, Shen JF, Xie ZH, Jiang L. Neoadjuvant immunotherapy in oncogene-positive non-small cell lung cancer: A multicenter study. Ann Thorac Surg (2023) 116(4):703–10. doi: 10.1016/j.athoracsur.2022.11.035
11. Reguart N, Reyes R. Spotlight on camrelizumab in advanced squamous lung cancer: another feather in the cap of Chinese checkpoint inhibitors. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer (2022) 17(4):477–80. doi: 10.1016/j.jtho.2022.01.001
12. Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (Camel): A randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med (2021) 9(3):305–14. doi: 10.1016/S2213-2600(20)30365-9
13. Liu SY, Chen Q, Zhou C, Zhang H, Li W, Chen J, et al. Neoadjuvant camrelizumab for non-small cell lung cancer: A retrospective multicenter, real-world study (Ctong2004). Cancer Immunol Immunother (2023) 72(7):2257–65. doi: 10.1007/s00262-023-03412-8
14. Hou X, Shi X, Luo J. Efficacy and safety of camrelizumab (a pd-1 inhibitor) combined with chemotherapy as a neoadjuvant regimen in patients with locally advanced non-small cell lung cancer. Oncol Lett (2022) 24(1):215. doi: 10.3892/ol.2022.13336
15. Zhou H, Lin L, Qin T, Ren W, Tan Y, Yang Q, et al. Neoadjuvant camrelizumab, nab-paclitaxel, and carboplatin in patients with stage ib-iiia non-small cell lung cancer (Nane-lc): A study protocol of prospective, single-arm, multicenter, phase ii study. J Thorac Dis (2021) 13(11):6468–75. doi: 10.21037/jtd-21-1022
16. Michels S, Scheel AH, Scheffler M, Schultheis AM, Gautschi O, Aebersold F, et al. Clinicopathological characteristics of ret rearranged lung cancer in European patients. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer (2016) 11(1):122–7. doi: 10.1016/j.jtho.2015.09.016
17. Zhou N, Li T, Liang M, Ren F, Ni H, Liu W, et al. Use of pralsetinib as neoadjuvant therapy for non-small cell lung cancer patient with ret rearrangement. Front Oncol (2022) 12:848779. doi: 10.3389/fonc.2022.848779
18. An L, Chen P, Wang J, Qin X, Liu T, Gao Y, et al. Case report: recurrent lung infections following treatment with pralsetinib for an elderly patient with ret-fusion positive nsclc. Front Oncol (2022) 12:1024365. doi: 10.3389/fonc.2022.1024365
19. Guisier F, Dubos-Arvis C, Viñas F, Doubre H, Ricordel C, Ropert S, et al. Efficacy and safety of anti-pd-1 immunotherapy in patients with advanced nsclc with braf, her2, or met mutations or ret translocation: gfpc 01-2018. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer (2020) 15(4):628–36. doi: 10.1016/j.jtho.2019.12.129
20. Aldea M, Marinello A, Duruisseaux M, Zrafi W, Conci N, Massa G, et al. Ret-map: an international multicenter study on clinicobiologic features and treatment response in patients with lung cancer harboring a ret fusion. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer (2023) 18(5):576–86. doi: 10.1016/j.jtho.2022.12.018
21. Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the immunotarget registry. Ann Oncol (2019) 30(8):1321–8. doi: 10.1093/annonc/mdz167
22. Offin M, Guo R, Wu SL, Sabari J, Land JD, Ni A, et al. Immunophenotype and response to immunotherapy of ret-rearranged lung cancers. JCO Precis Oncol (2019) 3. doi: 10.1200/po.18.00386
23. Lee J, Ku BM, Shim JH, La Choi Y, Sun JM, Lee SH, et al. Characteristics and outcomes of ret-rearranged Korean non-small cell lung cancer patients in real-world practice. Jpn J Clin Oncol (2020) 50(5):594–601. doi: 10.1093/jjco/hyaa019
24. Bhandari NR, Hess LM, Han Y, Zhu YE, Sireci AN. Efficacy of immune checkpoint inhibitor therapy in patients with ret fusion-positive non-small-cell lung cancer. Immunotherapy (2021) 13(11):893–904. doi: 10.2217/imt-2021-0035
25. Provencio M, Serna-Blasco R, Nadal E, Insa A, García-Campelo MR, Casal Rubio J, et al. Overall survival and biomarker analysis of neoadjuvant nivolumab plus chemotherapy in operable stage iiia non-small-cell lung cancer (Nadim phase ii trial). J Clin Oncol (2022) 40(25):2924–33. doi: 10.1200/jco.21.02660
26. Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. New Engl J Med (2022) 386(21):1973–85. doi: 10.1056/NEJMoa2202170
27. Provencio M, Nadal E, Insa A, Garcia-Campelo MR, Casal-Rubio J, Domine M, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (Nadim): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol (2020) 21(11):1413–22. doi: 10.1016/S1470-2045(20)30453-8
28. Zhao ZR, Yang CP, Chen S, Yu H, Lin YB, Lin YB, et al. Phase 2 trial of neoadjuvant toripalimab with chemotherapy for resectable stage iii non-small-cell lung cancer. Oncoimmunology (2021) 10(1):1996000. doi: 10.1080/2162402X.2021.1996000
29. Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Gao S, et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. New Engl J Med (2023) 389(6):491–503. doi: 10.1056/NEJMoa2302983
30. Negrao MV, Skoulidis F, Montesion M, Schulze K, Bara I, Shen V, et al. Oncogene-specific differences in tumor mutational burden, pd-L1 expression, and outcomes from immunotherapy in non-small cell lung cancer. J Immunother Cancer (2021) 9(8):e002891. doi: 10.1136/jitc-2021-002891
31. Tan Q, Ji Y, Wang XL, Wang ZW, Qi XW, Liu YK. [Clinicopathological features of patients with ret fusion-positive non-small cell lung cancer]. Zhonghua Bing Li Xue Za Zhi (2023) 52(2):124–8. doi: 10.3760/cma.j.cn112151-20220717-00614
32. Knetki-Wróblewska M, Wojas-Krawczyk K, Kowalski DM, Krzakowski M. Non-small-cell lung cancer patients with coexistence of high pd-L1 expression and ret fusion-which path should we follow? Case reports and literature review. J Clin Med (2022) 11(6):1630. doi: 10.3390/jcm11061630
33. Baby S, Khalil F, Tanvetyanon T. Frontline pembrolizumab for the treatment of ret-rearranged non-small cell lung cancer: A case report. Cancer Treat Res Commun (2021) 28:100423. doi: 10.1016/j.ctarc.2021.100423
34. Nakasuka T, Ohashi K, Watanabe H, Kubo T, Matsumoto S, Goto K, et al. A case of dramatic reduction in cancer-associated thrombus following initiation of pembrolizumab in patient with a poor performance status and pd-L1(+) lung adenocarcinoma harboring ccdc6-ret fusion gene and nf1/tp53 mutations. Lung Cancer (2021) 156:1–4. doi: 10.1016/j.lungcan.2021.03.022
35. Gu L, Ji W, Xu Y, Han Y, Jian H. Durable response to low-dose pralsetinib in a renal insufficient patient with nsclc harboring concurrent ccdc6-ret, linco1264-ret, and sema5a-ret fusions: A case report. Med (Baltimore) (2022) 101(47):e31480. doi: 10.1097/MD.0000000000031480
Keywords: lung adenocarcinoma, stage IIIA, RET fusion, neoadjuvant chemoimmunotherapy, pathological complete response
Citation: Dai M, Wang N, Xia Q, Liao Y, Cao W, Fan J, Zhou D, Wang S and Nie X (2024) Neoadjuvant chemoimmunotherapy achieved a pathologic complete response in stage IIIA lung adenocarcinoma harboring RET fusion: a case report. Front. Immunol. 14:1258762. doi: 10.3389/fimmu.2023.1258762
Received: 14 July 2023; Accepted: 12 December 2023;
Published: 03 January 2024.
Edited by:
Fan Yang, Peking University People’s Hospital, ChinaReviewed by:
Hao Zhang, The Affiliated Hospital of Xuzhou Medical University, ChinaRachel E. Sanborn, Earle A. Chiles Research Institute, United States
Copyright © 2024 Dai, Wang, Xia, Liao, Cao, Fan, Zhou, Wang and Nie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiu Nie, bmlleGl1eWlzaGlAMTI2LmNvbQ==; Sihua Wang, c2lodWFfd2FuZ0AxMjYuY29t; Diwei Zhou, emR3YmxrQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Minqian Dai1†
Minqian Dai1† Yongde Liao
Yongde Liao Jun Fan
Jun Fan Sihua Wang
Sihua Wang Xiu Nie
Xiu Nie