
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 10 August 2023
Sec. Autoimmune and Autoinflammatory Disorders : Autoimmune Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1250115
This article is part of the Research TopicPemphigus and pemphigoid diseases: in memoriam Detlef ZillikensView all 38 articles
Laminin 332 is a heterotrimeric structural protein of the basal membrane zone (BMZ) of the skin and adjacent mucosal tissues. The importance of laminin 332 for the structural integrity of the BMZ is demonstrated by mutations in any of the three genes encoding for its three chains causing variants of junctional epidermolysis bullosa. Autoimmunity against laminin 332 is observed in mucous membrane pemphigoid (MMP) and in the rare patients with orf-induced pemphigoid. MMP is an autoimmune blistering disease with predominant mucosal manifestations and autoantibodies against the BMZ of the skin and orifice-close mucous membranes. The main autoantigens of MMP are type XVII collagen (BP180) and laminin 332 targeted in about 80% and 10-20% of patients, respectively. An increasing number of studies has highlighted the association of anti-laminin 332 MMP and malignancies that can be revealed in about a quarter of these patients. This data has led to the recommendation of current guidelines to assay for anti-laminin 332 reactivity in all MMP patients. The present review focuses on anti-laminin 332 MMP describing clinical features, its pathophysiology, and detection of serum anti-laminin 332 IgG. In addition, the available data about the occurrence of malignancies in anti-laminin 332 MMP, the underlying tumor entities, and its biology are detailed.
Laminin 332 is a heterotrimer and essential structural protein of the basal membrane zone (BMZ) of the skin, adjacent mucosal tissues including the mouth, pharynx, larynx, trachea, esophagus but also kidney, lung, and small intestine (1). The importance of laminin 332 for the structural integrity of the BMZ is demonstrated by mutations in any of the three genes LAMA3, LAMB3 and LAMC2, that cause a variant of junctional epidermolysis bullosa (2, 3). Autoimmunity against laminin 332 is observed in the autoimmune blistering disease mucous membrane pemphigoid (MMP) and in the very rare patients with orf-induced pemphigoid (4, 5). Furthermore, autoantibodies against laminin 332 have been described in individual patients with bullous pemphigoid, anti-p200 pemphigoid, and epidermolysis bullosa acquisita in addition to the disease-typical autoantibodies against, i.e. BP180/type XVII collagen, p200 protein, and type VII collagen, respectively (6–11). The present review focuses on anti-laminin 332 MMP summarizing clinical features, its pathophysiology, and detection of serum anti-laminin 332 IgG. In addition, the current data about the association between anti-laminin 332 MMP and malignancies are highlighted.
The current review is dedicated to the late Detlef Zillikens, director and chair of the Department of Dermatology, University of Lübeck, Germany. Detlef Zillikens has been one of the leading experts on autoimmune blistering diseases. With an enormous workload and his friendly, optimistic, supportive, and caring nature he has established in Lübeck one of the world largest research hubs for these disorders. As one of his first students in 1993, close collaborator, mentee, and friend, E.S. owes him the greatest thanks for constant support, motivation, and fruitful discussions. S.P. got to know Detlef Zillikens in 2016 when starting her PhD thesis and owes him the greatest respect and thanks for his support of a young scientist and incessantly enjoyment of research. He was able to close the gap between science and clinic due to his dedication for both disciplines and his view of the entire picture. Both authors will strive to continue Detlef’s work and guard the best memories of him.
Laminins are cross- or T-shaped heterotrimers of an α, β and γ chain with three short arms (single chains) and one long arm formed by all three chains (12). Laminins are integral proteins of the BMZ of the skin and surface-close mucosal tissues. Here, they are essential components of the anchoring filaments connecting the hemidesmosome with type VII collagen (13). Their physiological functions include adhesion of the epidermis to the dermis and epithelium to the lamina propria, respectively, cell migration and, cell signaling (12).
Laminin 332, previously termed laminin 5, epiligrin, nicein, and kalinin is composed of the α3, β3 and γ2 chains and expressed in the BMZ of e.g. oral mucosa, conjunctiva, skin, kidney, lung, and small intestine (1). In the skin, laminin 332 is synthesized by keratinocytes as a 460 kDa precursor protein that is extracellularly cleaved by proteases. As such, the α3 chain (190-200 kDa) is processed into a 165 kDa fragment, the 155 kDa γ2 chain in a 105 kDa fragment, while the 140 kDa β3 chain remains uncleaved (13). Laminin 332 interacts with BP180 (type XVII collagen), the NC-1 domain of type VII collagen (14, 15), with α3β1, α6β4, and α6β1 integrin as well as with syndecan-1 and syndecan-4 (16, 17).
MMP is a clinically and immunopathologically heterogeneous disease defined as pemphigoid disorder with prevailing involvement of orifice-close mucosal tissues (18). As a pemphigoid disorder, MMP is characterized by autoantibodies that bind to the BMZ of the skin and/or mucosa (19, 20). Clinical heterogenicity is reflected by the involvement of different mucosal sites, most frequently the mouth (in about three quarters of patients) and conjunctivae (in about 50-65% of patients) followed by nasopharynx and genitalia, and more rarely, larynx, esophagus, and trachea. In about a quarter of patients, in addition to mucosal manifestations, skin lesions are present (Figure 1) (21, 22). The high disease burden of MMP is due to frequently painful oral and genital lesions, life-threatening complications such as airway obstruction and esophageal strictures, conjunctival disease leading to vision impairment and finally, blindness, and the association with a malignancy in about a quarter of patients with anti-laminin 332 reactivity (22).
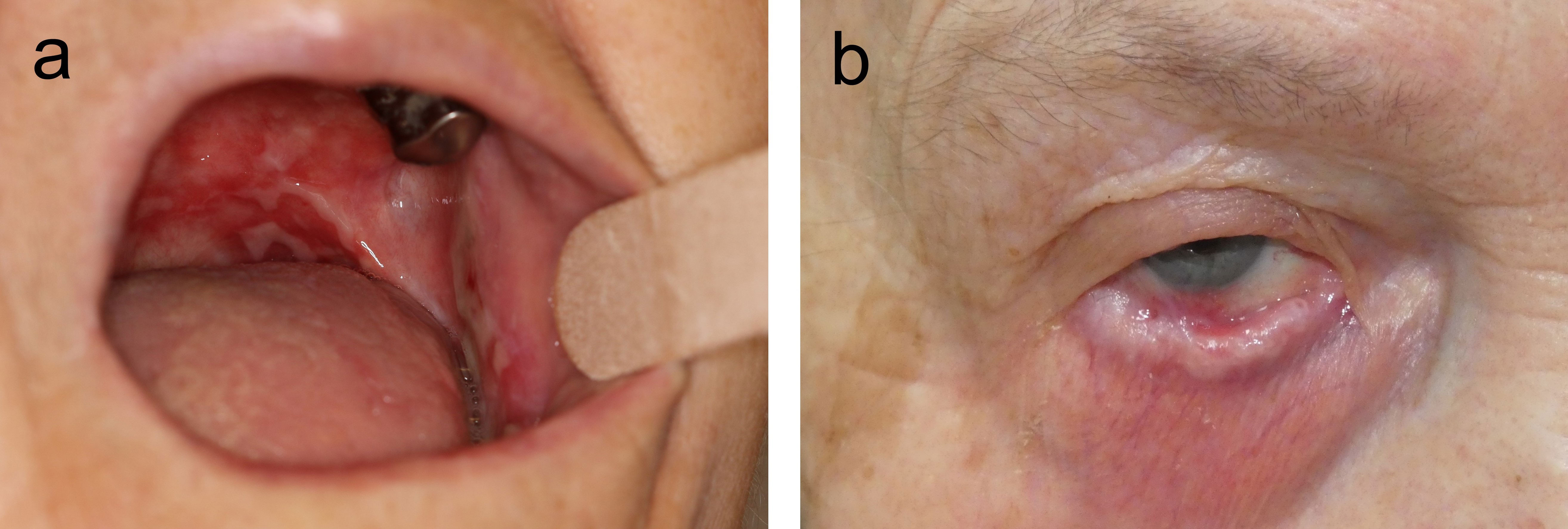
Figure 1 Clinical manifestations of mucous membrane pemphigoid. Extensive oral lesions in an 83-year-old female patient (A) and symblephara and shortening of the inferior fornix in a 72-year-old female (B).
Immunopathological heterogenicity stems from the different target antigens and the autoantibody isotype. While in most MMP patients, autoantibodies belong predominantly to the IgG isotype, the majority of patients also reveal IgA autoantibodies, and in some, the autoantibody response is restricted to IgA (22–24). BP180 (type XVII collagen) as main target antigen in MMP is recognized by about 70-80% of patients followed by laminin 332 in 10-20% of patients. In less than 5% of MMP patients, type VII collagen is recognized. Reactivity against BP230, that can be found in 10-30% of cases, is nearly always accompanied by autoantibodies against one of the three other target antigens (21, 24). In some MMP patients, autoantibodies against α6β4 integrin have been described (25–28). The relevance of these α6β4 integrin-specific antibodies in MMP is, however disputed (24, 29). Patients with mostly mucosal manifestation and predominant IgA reactivity, that previously may have been classified as linear IgA disease, and those with autoantibodies against type VII collagen previously diagnosed as epidermolysis bullosa acquisita, are now regarded within the spectrum of MMP (21).
Few data about the frequency of MMP are available. With an incidence between 1.3 and 2.0/million/year in France and Germany, respectively, and a prevalence of 24.6 patients/million in Germany, MMP is certainly a rare disease (30–33). MMP arises independently of ethnicity and geographical region, mainly affects individuals in the 7th and 8th decennium, and appears to be more frequent in females (22).
Diagnosis of MMP, like in all autoimmune blistering diseases, is grounded on three pillars; clinical manifestations, direct immunofluorescence (IF) microscopy, and serology (20, 24). The clinical prerequisite is predominant mucosal involvement. Direct IF reveals linear deposits of IgG, IgA, and or C3 at the cutaneous or mucosal BMZ in a non-lesional biopsy (Figure 2). Since the initial biopsy only provides a sensitivity of 50-70% depending on the biopsy site, current guidelines recommend to repeat the biopsy for direct IF at least once after an initially negative result (24, 29).
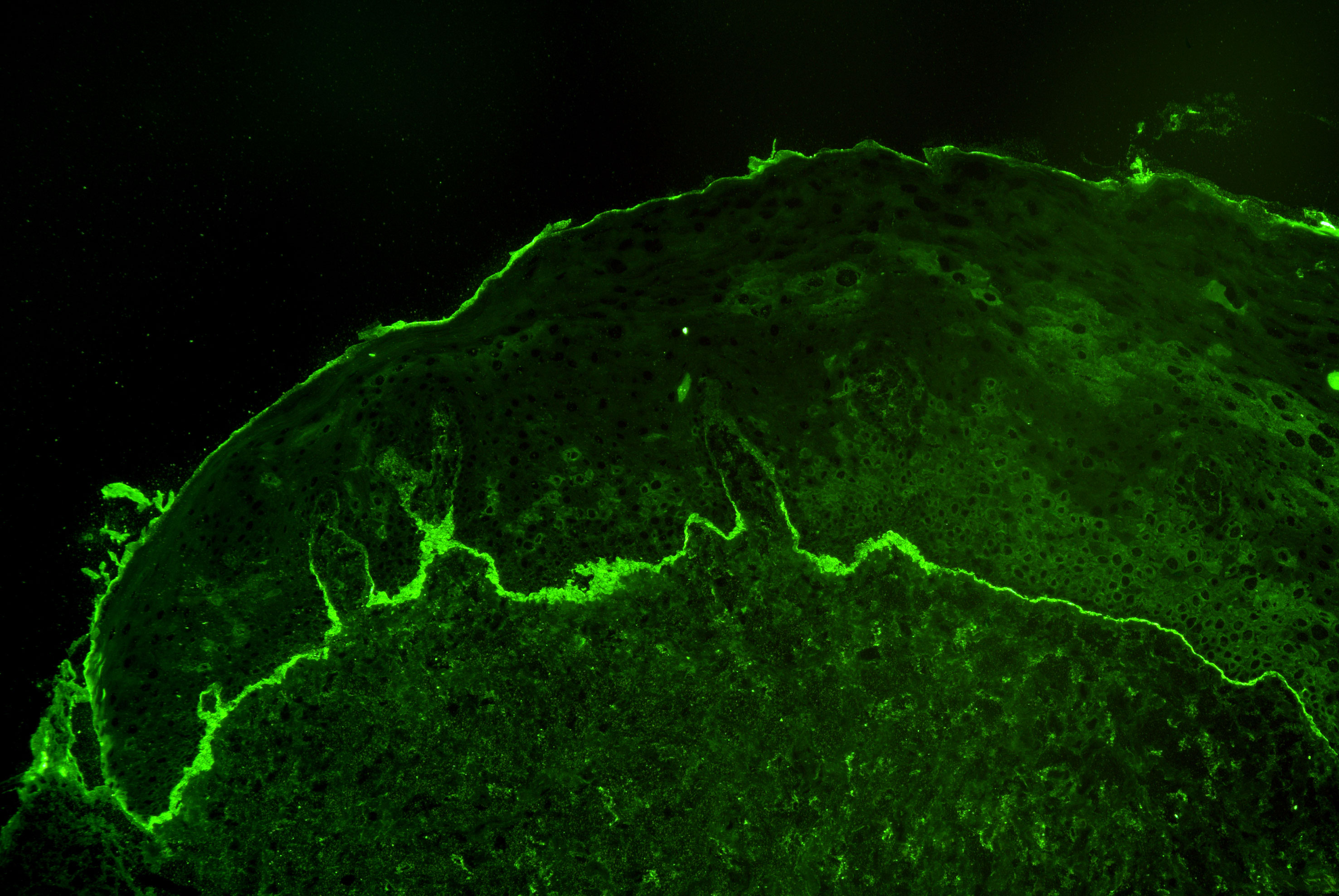
Figure 2 Linear deposits of complement C3 at the basement membrane zone by direct immunofluorescence microscopy of a perilesional biopsy in a patient with mucous membrane pemphigoid.
Detecting of circulating autoantibodies against the above-mentioned antigens is complex, mainly based on in-house assays, and reviewed elsewhere (22, 24). The detection of anti-laminin 332 IgG is detailed below.
Treatment of MMP is greatly hampered by the lack of randomized controlled studies. National and international guidelines propose treatment regimens (24, 34–37). The S3 European guidelines included a systematic literature review and recommend dapsone, methotrexate, tetracycline, and topical corticosteroids as first line treatment for mild and moderate MMP. For severe MMP, dapsone plus cyclophosphamide and/or oral corticosteroids are suggested and, if not successful, dapsone plus rituximab followed by latter two drugs combined with high-dose intravenous immunoglobulin (24). A slightly different step-ladder approach was published in the recent German S2k guideline (29).
In 1992, laminin 332 has been described as a target antigen in MMP by Kim Yancey and co-workers (4). Since then, numerous case reports and case series have reported IgG serum autoantibodies against this protein. It was only in 2019, when a highly standardized and specific assay for serum anti-laminin 332 IgG became widely available (38).
A patient with anti-laminin 332 MMP can clinically not be differentiated from a MMP patient with autoimmunity against BP180 or type VII collagen. In a systematic review of published cases and cohorts, Amber et al. reported significantly more pharyngo-laryngeal and oro-pharyngo-laryngeal involvement in MMP patients with reactivity against laminin 332 (39). In the so far largest study with 133 anti-laminin 332 MMP patients from Kurume, Japan, the oral cavity was the by far most frequently affected mucosal site (in 89% of patients) followed by conjunctivae (in 43%), pharynx (in 19%), larynx (15%), genital mucosa (in 11%), nasal mucosa (in 6%), and esophagus (in 3%) (40). Compared with MMP patients independent of the target antigen as recently reported in 154 MMP patient and as reviewed by Du et al., nasal lesions appear to occur less frequently in anti-laminin 332 MMP compared to 20-40% in all MMP patients, while oral lesions may be slightly more prominent (in 80-85% of all patients) (22, 41). These differences have, however, not been systematically evaluated and may also be related to the different ethnicity or other so far unrecognized factors.
Recently, a significant association of laminin 332-reactive MMP with male sex was reported (41). The most striking and clinically relevant feature that differentiates anti-laminin 332 MMP from MMP with other autoantibody reactivities, the association with malignancies in about a quarter of patients, is detailed below.
Several methods have been applied to detect anti-laminin 332 reactivity in skin and mucosal biopsies as well as in serum. Direct and indirect immunogold electron microscopy show deposits of immunoreactants at the lamina lucida/lamina densa interface of the BMZ in anti-laminin 332 MMP. In patients with autoantibodies against BP180 or type VII collagen, immunoreactants label the lamina lucida or the subbasal lamina-anchoring fibril zone, respectively (4, 42–45). Direct immunogold electron microscopy requires, however, fresh biopsy material that needs to be processed within hours and is only performed in few centers worldwide (46).
For the detection of serum autoantibodies against laminin 332, indirect immunogold electron microscopy is unpractical and as such, several in-house assays have been described including (i) immunoprecipitation of radiolabeled keratinocytes that was also applied in the original report of anti-laminin 332 IgG in MMP (4, 47), (ii) immunoblotting with various substrates such as (a) conditioned media of cultured SCC-25 cells (48), (b) cultured primary human keratinocytes (47, 49), (c) cultured HaCaT keratinocytes (50), (d) cultured A-431 human epidermoid carcinoma cells (50), (e) extracts of human epidermal sheets (50), (f) extracellular matrix of cultured human keratinocytes (45, 50), (g) extract of human placental amnion (51), (h) recombinant fragments of the α3 chain (52), (i) human laminin 332 purified from cultured human keratinocytes (53), (j) primary human oral mucosal keratinocytes (54), and (k) immortalized human oral mucosal keratinocytes (54), and (iii) ELISA. When immunoprecipitation was compared to immunoblotting with five different substrates, i.e. (b-f), immunoprecipitation was identified as the most sensitive method followed by Western blotting with extracellular matrix of cultured human keratinocytes (II f) (50).
For ELISA, purified laminin 332 from conditioned medium of cultured SCC-25 cells (47, 55, 56), recombinant laminin 332 (57), laminin 332 purified from supernatant of cultured primary human keratinocytes (57), or extracellular matrix of cultured HaCaT keratinocytes were used (57). In particular the ELISA employing purified laminin 332 from conditioned medium of cultured SCC-25 cells has subsequently revealed conflicting results. Bekou et al. reported anti-laminin 332 IgG in 40% of bullous pemphigoid sera, although anti-laminin 332 reactivity is not present in latter patients (38, 55, 58, 59). Bernard et al. described serum anti-laminin 332 IgG in 31 of 154 MMP patients; when 19 of the 31 laminin 332-reactive sera were retested, anti-laminin 332 reactivity was only confirmed in 4 of the 19 sera (60).
In sera with reactivity against the cutaneous BMZ by indirect IF microscopy on human skin, indirect IF on laminin 332-deficient skin from patients with junctional epidermolysis bullosa (being unreactive on latter substrate) as well as the fluorescence overlay antigen mapping on human salt-split skin are elegant methods to determine autoantibodies against laminin 332 (61). Another test based on indirect IF, the so-called footprint assay, demonstrated that anti-laminin 332 serum IgG can be detected in the extracellular matrix of cultured primary keratinocytes after removal of the cells from the glass coverslips. Here, the extracellular matrix of the removed individual keratinocytes appear as traces or “footprints” that can be visualized by anti-laminin 332 antibodies followed by FITC labelling (59).
A breakthrough was achieved by Goletz et al. who described an indirect IF test based on the HEK293 cells that recombinantly express the laminin 332 trimer on their cell surface (Figure 3). As negative control, HEK293 cells transfected with an empty vector are used. These cells are applied using the BIOCHIP® mosaic technology, i.e. several substrates are placed together in a single incubation field of a laboratory slide (62–65). When in an international multicenter study, 93 anti-laminin 332 MMP patient sera and 315 sera from other autoimmune blistering diseases including 153 sera from anti-laminin 332 negative MMP patients, non-inflammatory dermatoses, and heathy blood donors were probed, a sensitivity of 84% and a specificity of 99.6% were observed (38). This assay has subsequently been validated by other groups (66, 67). When the BIOCHIP® technology-based assay has recently been compared with the footprint assay using 54 anti-laminin 332 MMP sera and together 50 sera from patients with pemphigus vulgaris and healthy blood donors, both assays revealed a specificity of 100% with a slightly higher sensitivity of the footprint assay (100% versus 96.3%) (60). When 35 sera of originally laminin 332-unreactive sera were subjected to both IF tests, 3 were reactive in the BIOCHIP® assay and 7 in the footprint assay. These data show that the footprint test may be more sensitive, whereas the advantage of the BIOCHIP® assay is its high standardization and wide availability (60).
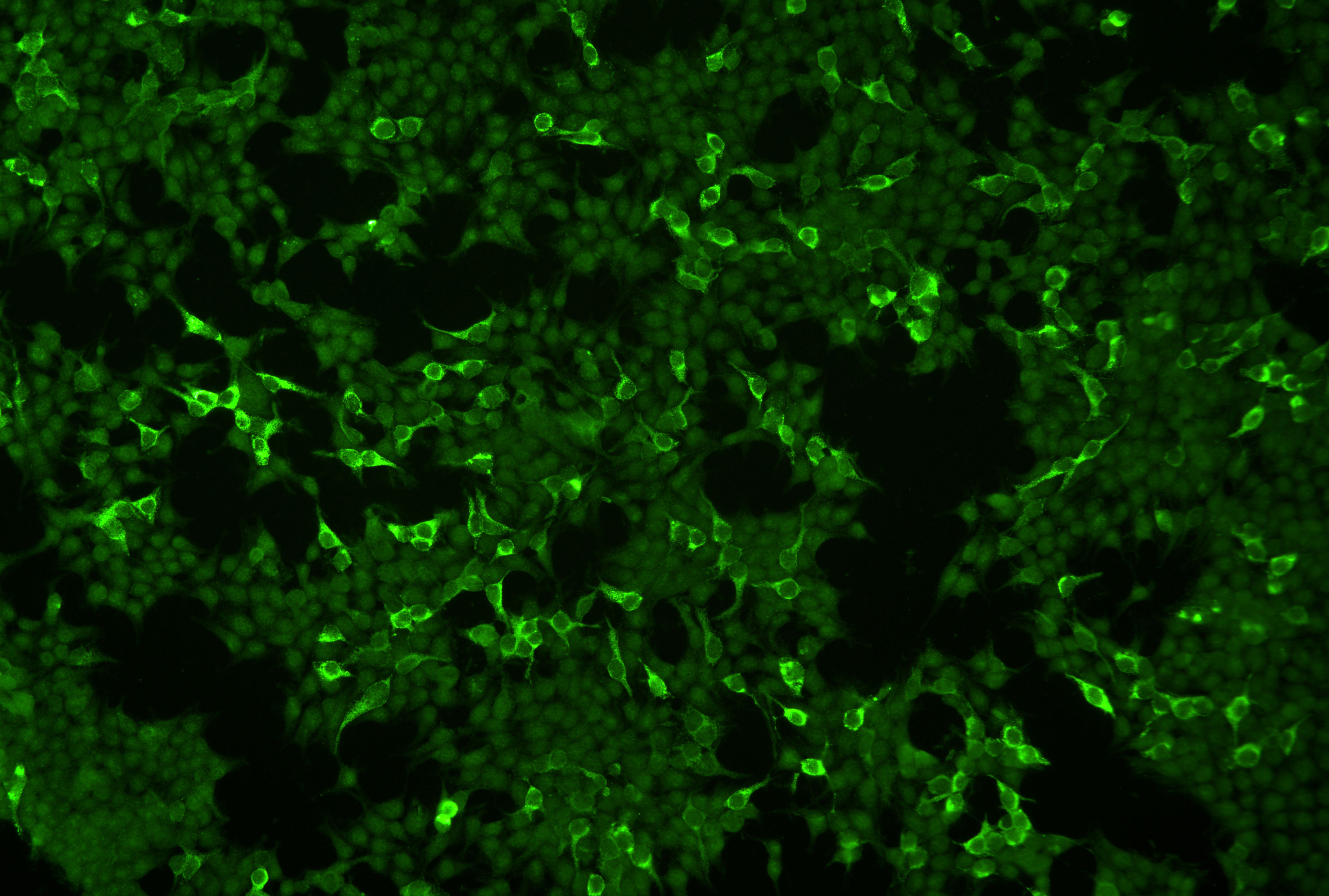
Figure 3 Indirect immunofluorescence microscopy of HEK293 cells that recombinantly express laminin 332 on the cells surface employing the Biochip™ technology. A serum of a patient with mucous membrane pemphigoid labels laminin 332-expressing cells. Non-transfected cells serve as internal negative control.
Reactivity against the different laminin chains varied considerably between studies. In 113 Japanese patients with anti-laminin 332 MMP, the γ2 chain was most frequently recognized (in 58% of patients) followed by α3 and β3 targeted in 49% and 36% of patients, respectively (40). In contrast, Goletz et al., using the BIOCHIP® technology-based IF assay in an international multicenter study with 93 sera, reported IgG4 reactivities against the α3, β3, and γ2 in 43%, 41%, and 13% of patients (38). These discrepancies maybe most likely due to the different study populations or detection methods.
In individual MMP patients, IgA and IgE antibodies against laminin 332 have also been reported (68, 69).
Since anti-laminin 332 MMP is associated with a malignancy in about a quarter of patients as detailed below, national and international guidelines recommend the detection of anti-laminin 332 serum IgG in all patients that show dermal binding by indirect IF on human salt-split skin or were unreactive in this assay (24, 29). A suggested diagnostic pathway for anti-laminin 332 MMP is depicted in Figure 4.
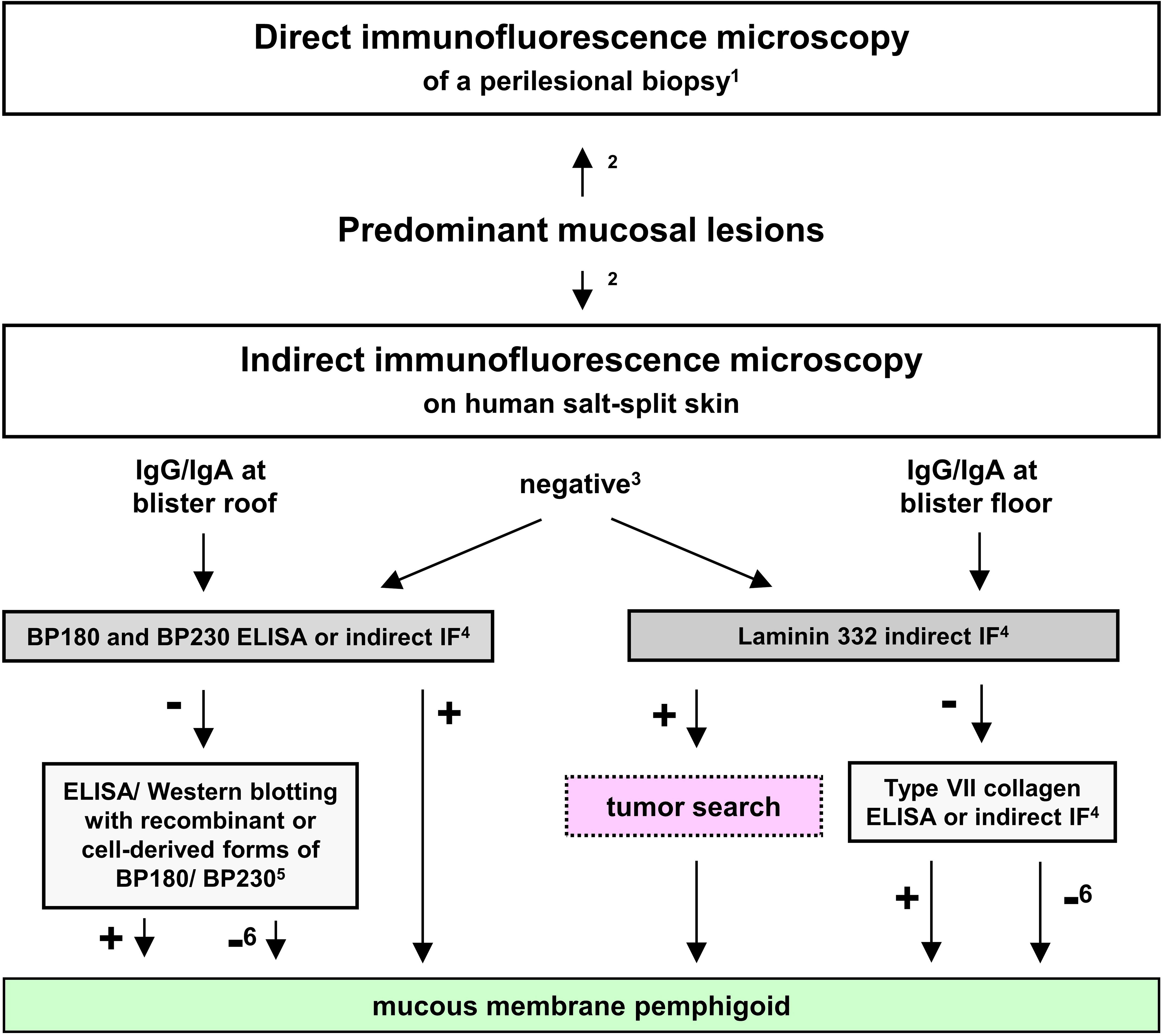
Figure 4 Proposed diagnostic algorithm for mucous membrane pemphigoid (MMP). Adopted from (22, 24, 70). 1in the oral cavity, a non-lesional biopsy is equally sensitive compared to a perilesional; 2recommended to be performed in parallel; 3in 30-50% of MMP sera; 4commercially available (for IgG); 5only available in specialized laboratories as in-house assays; 6only with positive direct and/or indirect IF microscopy.
Gibson et al., have observed the first patient with anti-laminin 332 MMP and a malignancy, a lung carcinoma, in 1997 followed by Leverkus et al., who reported solid malignancies in 2 of 5 MMP patients with serum reactivity against laminin 332 (71, 72). The association was first noted by Egan et al. who described malignancies in 10 of 35 (29%) anti-laminin 332 MMP patients (73). When all nine subsequent studies with more than three anti-laminin 332 MMP patients were evaluated, a clear association with malignancies was evident. In fact, 57 of 253 (23%) patients with anti-laminin 332 reactivity had a malignancy (Table 1). These data align well with a recent review in which Shi et al., retrieved 344 reported cases of anti-laminin 332 MMP from the literature, of whom in 75 (22%), a malignancy was described. Van Beek et al. calculated the risk for malignant neoplasms in anti-laminin 332 MMP to be 6.8-fold higher compared to the general population (41).
In the recent review by Shi et al., the most frequent tumor in 84 malignancy-associated anti-laminin 332 MMP patients retrieved from the literature, were lung carcinomas (in 23% of patients) followed by gastric (in 17%), uterine (in 13%), pancreatic (8%), colon (8%), ovary (7%), prostate (5%), and thyroid carcinoma (5%) (77). No relation between the recognized laminin chain and the tumor entity was found (77). Of the 12 malignancy-associated anti-laminin 332 MMP patients reported by Goletz et al., 3 (25%) had a lung and 2 (17%) a uterine/cervix carcinoma compatible with the data reported by Shi et al., while 2 (17%) revealed a urothel carcinoma and none has a gastric malignancy (38, 77). These data suggest that in anti-laminin 332 MMP, solid malignancies predominate with lung and uterine/cervix cancers being among the most prevalent entities, while the distribution of other solid malignancies may also depend on the population.
Interestingly, in patients with serum reactivity against α6β4 integrin, no higher rate of malignancies was found alike in MMP patients in general irrespective of the target antigen (78–80).
The exact reason for the association of ani-laminin 332 reactivity and solid cancers has not been fully elucidated yet. It is well known that laminin 332 is relevant for tumor proliferation and migration (81–83). Some solid tumors may produce excessive amounts of laminin 332 and an imbalance of extracellular matrix proteins including laminin 332 was shown to promote tumor cell migration via the Pi3-akt pathway as well as the differentiation of tumor-associated fibroblasts and tumor angiogenesis (84–86). As such, it may be hypothesized that an imbalance in laminin 332 expression during carcinogenesis induces an autoimmune response that leads to laminin 332-specific autoimmunity including anti-laminin 332 antibodies (87–89). This view is supported by the observation that MMP can regress after excision of the tumor (87, 90, 91).
Preliminary evidence for the pathogenic relevance of anti-laminin 332 IgG stems from the intraindividual correlation of anti-laminin 332 IgG serum levels with disease activity (38). Apart from in-vitro organ culture models of MMP employing normal human conjunctiva (25, 92–94), two mouse models of anti-laminin 332 MMP have been developed. One model reflects the inflammatory-poor variant of MMP and lesions develop independently of complement activation and the infiltration of inflammatory cells in the tissues, while the other model shows, oral, conjunctival, and skin lesions with inflammatory infiltrates and requires the involvement of the Fcγ-receptor and activation of C5aR1 (95–97). In latter model, dapsone has recently been shown to be effective supporting the notion that this model recapitulates important features of the human disease (98). Because most recent publications used the latter mouse model, a detailed description is depicted in Figure 5. In line with previous findings, methylprednisolone as another first-line therapy for MMP, was also able to reduce the severity of skin, although not oral lesions in this mouse model. In this study, Ghorbanalipoor et al. also showed that parsaclisib, a selective inhibitor of phosphoinositide 3-kinase delta (PI3Kδ) significantly reduced skin and oral mucosal lesions (99, 100). With regard to the characteristic symptom of scarring, typically occurring at the eyes of anti-laminin 332 MMP patients, this mouse model may also be suitable to unravel signaling pathways that contributes to this specific immunopathogenesis. Biopsies of the palpebral conjunctiva and the skin collected 28 days after the initiation of this model revealed highly condensed collagen fibrils in picro-sirius red staining and trichrome histological staining. In addition, biochemical analysis provided results on altered collagen-cross-linking signaling pathways in these tissues that are associated with fibrosis (101). Furthermore, the previously published upregulation of aldehyde dehydrogenase (ALDH1) in conjunctiva and in fibroblasts isolated from MMP patients with severe eye involvement, could be verified by transcriptome analysis of perilesional skin from this model (102). The inhibition of ALDH1 by disulfiram decreased disease severity in a mouse model for allergic eye disease (102). However, disulfiram was not effective in the anti-laminin 332 mouse model. Here, the same dosage and application of disulfiram was not able to reduce the severity of the conjunctival lesions (101).
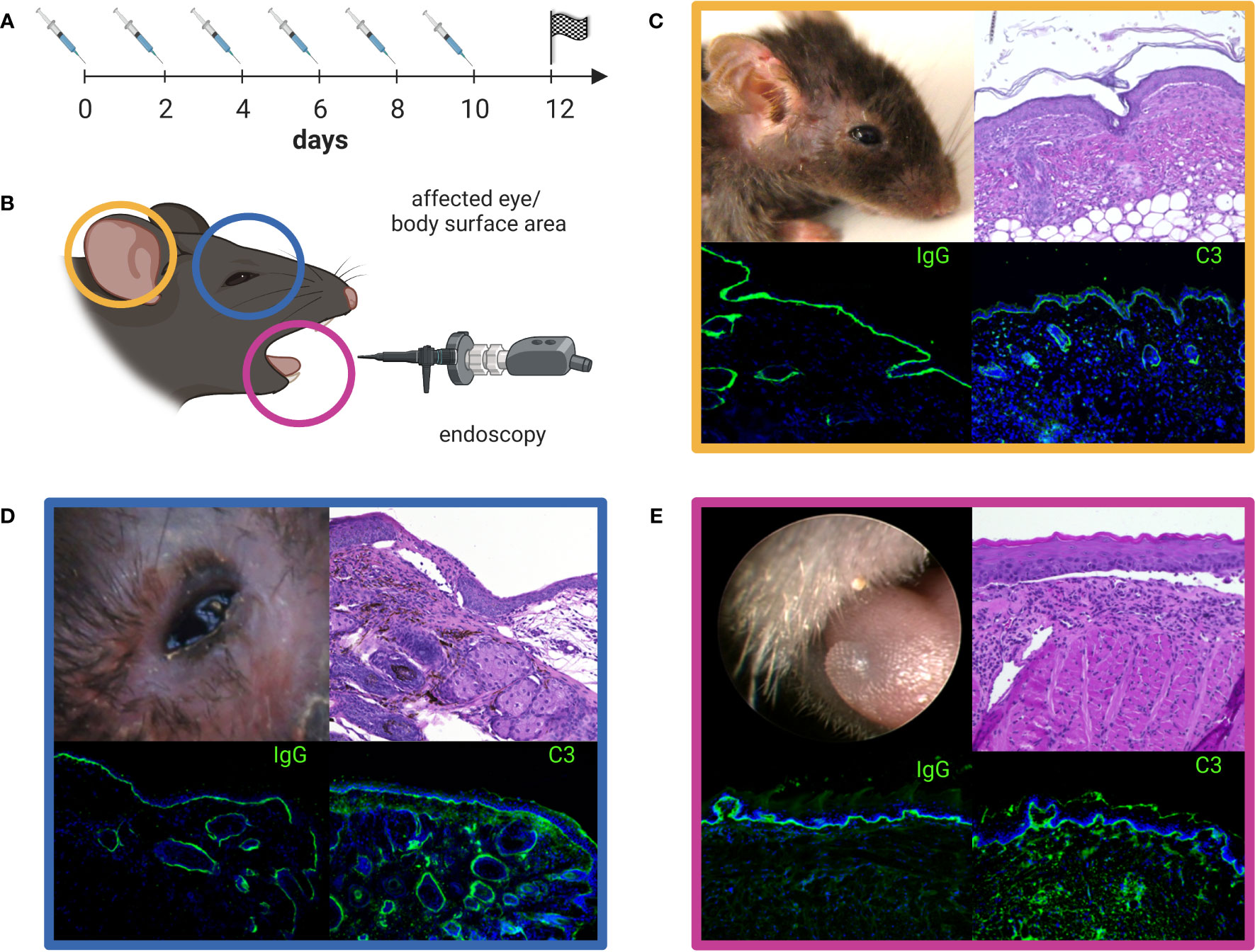
Figure 5 Anti-laminin α3 mucous membrane pemphigoid (MMP) mouse model. Rabbit anti-murine laminin α3 IgG is injected subcutaneously (s.c.) into adult C57Bl/6 mice every other day over a time period of 10 days (A). The clinical manifestation of the mouse model seen on experimental day 12 can be quantified by the use of a validated scoring system comprising the affected body surface area (yellow, C), the affected eye-area (blue, D), and the severity of oral lesions as examined by endoscopy (pink, E) (B). The color-framed boxes (C-E) show the clinical presentation (upper left panel), H&E stained lesional histopathology with an inflammatory infiltrate and split formation of the dermal-epidermal/epithelial junction (upper right panel) and, linear deposits of IgG (lower left panel) and C3 (lower right panel) along the basal membrane zone by direct immunofluorescence microscopy. Lesions, crusts and erosions of the skin are mostly restricted to the head, neck, and upper back of mice (C). The image was created with BioRender.com.
In-vitro models specific for anti-laminin 332 pemphigoid are rare. Recently Bao et al. published results about anti-laminin 332 MMP patient antibodies that were sufficient to release inflammatory mediators upon binding to keratinocytes without the presence of inflammatory cells and as such without the usage of Fc-receptors. Thus, arising the question whether blistering may be a consequence of just the binding of the anti-laminin 332 IgG and whether the complement system has a nonobligatory role in the initiation of the inflammatory response (103, 104).
Outside MMP, antibodies against laminin 332 have been detected in individual patients with bullous pemphigoid, anti-p200 pemphigoid, and epidermolysis bullosa acquisita in addition to the autoantibodies against BP180, p200 protein, and type VII collagen, respectively (6–11). The report of anti-laminin 332 reactivity in about 40% of bullous pemphigoid sera was not confirmed in subsequent studies (38, 55, 58, 59). When Holtsche et al. investigated the specificities of serum autoantibodies in anti-p200 pemphigoid, anti-laminin 332 IgG was observed in 43 (18%) of 239 patients in addition to reactivity against the p200 protein and/or laminin γ1 (10).
Autoantibody reactivity in the very rare entity orf-induced pemphigoid has puzzled investigators for many years. Recently, Yilmaz et al, showed that the major target antigen in orf-induced pemphigoid is laminin 332 (5). Of note, while a single patient with orf-induced MMP has been described, all other cases associated with orf did not show predominant mucosal involvement and, consequently may be termed orf-induced pemphigoid when antibodies against laminin 332 are detected or orf-induced epidermolysis bullosa acquisita in case of type VII collagen-specific antibodies (5, 105, 106). The reason why autoimmunity against laminin 332 is not associated with predominant mucosal manifestations when induced by an orf infection is enigmatic. It may be speculated that an underlying molecular mimicry between an orf virus protein and laminin 332 leads to autoantibodies against distinct epitopes on laminin 332 different from those targeted in anti-laminin 332 MMP. Of note, autoantibodies in orf-induced pemphigoid are predominantly of the IgG2 and IgG3 subclasses compared to IgG4 in anti-laminin 332 MMP (38, 107).
After diagnosis of MMP, testing for serum antibodies against laminin 332 and, when present, a search for the most prevalent solid tumors including chest, abdominal, and pelvic CT, gastroscopy, coloscopy, as well as urological and gynecological examinations appears to be mandatory. The anti-laminin α3 mouse model of MMP may be helpful to decipher key molecules and pathways in the pathophysiology of MMP. Only after definite preclinical data have been generated a randomized controlled treatment study will be initiated and open new therapeutic avenues for patients with this rare and frequently detrimental disorder.
Supervision: ES. Visualization: ES, SP. Writing - Review and Editing: ES, SP. All authors contributed to the article and approved the submitted version.
This work was supported by structural funding from the Schleswig-Holstein Excellence Cluster Precision Medicine in Chronic Inflammation (DFG EXC 2167/1) and the CRC 1526 Pathomechanisms of Antibody-mediated Autoimmunity: Insights from Pemphigoid Diseases (A01).
ES has research grants with Admirx, ArgenX, AstraZeneca, Biotest, Dompe, Euroimmun, CSL, Alpine Immune, and Fresenius Medical Care and in the last three years, received consulting fees and/or honoraria from Almirall, ArgenX, AstraZeneca, Janssen, Bristol-Myers Squibb, Chugai, Leo, and Sanofi. SP and ES have a patent application with Dompe.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
2. Fine JD, Bruckner-Tuderman L, Eady RA, Bauer EA, Bauer JW, Has C, et al. Inherited epidermolysis bullosa: Updated recommendations on diagnosis and classification. J Am Acad Dermatol (2014) 70:1103–26. doi: 10.1016/j.jaad.2014.01.903
3. Has C, Bauer JW, Bodemer C, Bolling MC, Bruckner-Tuderman L, Diem A, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol (2020) 183:614–27. doi: 10.1111/bjd.18921
4. Domloge-Hultsch N, Gammon WR, Briggaman RA, Gil SG, Carter WG, Yancey KB. Epiligrin, the major human keratinocyte integrin ligand, is a target in both an acquired autoimmune and an inherited subepidermal blistering skin disease. J Clin Invest (1992) 90:1628–33. doi: 10.1172/JCI116033
5. Yilmaz K, Goletz S, Pas HH, van den Bos RR, Blauvelt A, White WL, et al. Clinical and serological characterization of orf-induced immunobullous disease. JAMA Dermatol (2022) 158:670–4. doi: 10.1001/jamadermatol.2022.0290
6. Shimanovich I, Petersen EE, Weyers W, Sitaru C, Zillikens D. Subepidermal blistering disease with autoantibodies to both the P200 autoantigen and the Alpha3 chain of laminin 5. J Am Acad Dermatol (2005) 52:S90–2. doi: 10.1016/j.jaad.2004.07.036
7. Lin HY, Yanagi T, Akiyama M, Iitani MM, Moriuchi R, Natsuga K, et al. Childhood subepidermal blistering disease with autoantibodies to type vii collagen and laminin-332. Br J Dermatol (2011) 164:452–4. doi: 10.1111/j.1365-2133.2010.10065.x
8. Sakaguchi M, Bito T, Oda Y, Kikusawa A, Nishigori C, Munetsugu T, et al. Three cases of linear Iga/Igg bullous dermatosis showing iga and igg reactivity with multiple antigens, particularly laminin-332. JAMA Dermatol (2013) 149:1308–13. doi: 10.1001/jamadermatol.2013.5691
9. Nishida E, Nishio E, Murashima H, Ishii N, Hashimoto T, Morita A. Case of epidermolysis bullosa acquisita with concomitant anti-Laminin-332 antibodies. J Dermatol (2018) 45:472–4. doi: 10.1111/1346-8138.14169
10. Holtsche MM, Goletz S, von Georg A, van Beek N, Hubner F, Pigors M, et al. Serologic characterization of anti-P200 pemphigoid: Epitope spreading as a common phenomenon. J Am Acad Dermatol (2021) 84:1155–7. doi: 10.1016/j.jaad.2020.07.076
11. Kawashima H, Kageji R, Hida Y, Goto T, Ishii N, Hashimoto T. Case of pemphigoid with antibodies to Bp180 c-terminal domain and Alpha3 subunit of laminin-332 associated with chronic graft-Versus-Host disease. J Dermatol (2021) 48:e447–e8. doi: 10.1111/1346-8138.16008
12. Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, et al. A simplified laminin nomenclature. Matrix Biol (2005) 24:326–32. doi: 10.1016/j.matbio.2005.05.006
13. Goletz S, Zillikens D, Schmidt E. Structural proteins of the dermal-epidermal junction targeted by autoantibodies in pemphigoid diseases. Exp Dermatol (2017) 26:1154–62. doi: 10.1111/exd.13446
14. Chen M, Marinkovich MP, Jones JC, O'Toole EA, Li YY, Woodley DT. Nc1 domain of type vii collagen binds to the Beta3 chain of laminin 5 Via a unique subdomain within the fibronectin-like repeats. J Invest Dermatol (1999) 112:177–83. doi: 10.1046/j.1523-1747.1999.00491.x
15. Van den Bergh F, Eliason SL, Giudice GJ. Type xvii collagen (Bp180) can function as a cell-matrix adhesion molecule Via binding to laminin 332. Matrix Biol (2011) 30:100–8. doi: 10.1016/j.matbio.2010.10.005
16. Carulli S, Beck K, Dayan G, Boulesteix S, Lortat-Jacob H, Rousselle P. Cell surface proteoglycans syndecan-1 and -4 bind overlapping but distinct sites in laminin Alpha3 Lg45 protein domain. J Biol Chem (2012) 287:12204–16. doi: 10.1074/jbc.M111.300061
17. Nishiuchi R, Takagi J, Hayashi M, Ido H, Yagi Y, Sanzen N, et al. Ligand-binding specificities of laminin-binding integrins: A comprehensive survey of laminin-integrin interactions using recombinant Alpha3beta1, Alpha6beta1, Alpha7beta1 and Alpha6beta4 integrins. Matrix Biol (2006) 25:189–97. doi: 10.1016/j.matbio.2005.12.001
18. Chan LS, Ahmed AR, Anhalt GJ, Bernauer W, Cooper KD, Elder MJ, et al. The first international consensus on mucous membrane pemphigoid: Definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol (2002) 138:370–9.
19. Amber KT, Murrell DF, Schmidt E, Joly P, Borradori L. Autoimmune subepidermal bullous diseases of the skin and mucosae: Clinical features, diagnosis, and management. Clin Rev Allergy Immunol (2018) 54:26–51. doi: 10.1007/s12016-017-8633-4
20. Beek NV, Zillikens D, Schmidt E. Bullous autoimmune dermatoses. Dtsch Arztebl Int (2021) 118:413–20. doi: 10.3238/arztebl.m2021.0136
21. Rashid H, Lamberts A, Borradori L, Alberti-Violetti S, Barry RJ, Caproni M, et al. European guidelines (S3) on diagnosis and management of mucous membrane pemphigoid, initiated by the european academy of dermatology and venereology - part i. J Eur Acad Dermatol Venereol: JEADV (2021) 35:1750–64. doi: 10.1111/jdv.17397
22. Du G, Patzelt S, van Beek N, Schmidt E. Mucous membrane pemphigoid. Autoimmun Rev (2022) 21:103036. doi: 10.1016/j.autrev.2022.103036
23. Schmidt E, Skrobek C, Kromminga A, Hashimoto T, Messer G, Brocker EB, et al. Cicatricial pemphigoid: Iga and igg autoantibodies target epitopes on both intra- and extracellular domains of bullous pemphigoid antigen 180. Br J Dermatol (2001) 145:778–83. doi: 10.1046/j.1365-2133.2001.04471.x
24. Schmidt E, Rashid H, Marzano AV, Lamberts A, Di Zenzo G, Diercks GFH, et al. European guidelines (S3) on diagnosis and management of mucous membrane pemphigoid, initiated by the european academy of dermatology and venereology - part II. J Eur Acad Dermatol Venereol: JEADV (2021) 35:1926–48. doi: 10.1111/jdv.17395
25. Chan RY, Bhol K, Tesavibul N, Letko E, Simmons RK, Foster CS, et al. The role of antibody to human Beta4 integrin in conjunctival basement membrane separation: Possible in vitro model for ocular cicatricial pemphigoid. Invest Ophthalmol Visual Sci (1999) 40:2283–90.
26. Rashid KA, Stern JN, Ahmed AR. Identification of an epitope within human integrin alpha 6 subunit for the binding of autoantibody and its role in basement membrane separation in oral pemphigoid. J Immunol (2006) 176:1968–77. doi: 10.4049/jimmunol.176.3.1968
27. Li X, Qian H, Sogame R, Hirako Y, Tsuruta D, Ishii N, et al. Integrin Beta4 is a major target antigen in pure ocular mucous membrane pemphigoid. Eur J Dermatol: EJD (2016) 26:247–53. doi: 10.1684/ejd.2016.2772
28. Maglie R, De Almeida CV, Baffa ME, Bianchi B, Caproni M, Di Zenzo G, et al. Anti-Beta4 integrin autoantibodies in patients with mucous membrane pemphigoid: A retrospective analysis from a tertiary centre in italy. J Eur Acad Dermatol Venereol: JEADV (2023) 37:e249–e51. doi: 10.1111/jdv.18617
29. Hofmann SC, Gunther C, Bockle BC, Didona D, Ehrchen J, Gaskins M, et al. S2k guideline for the diagnosis and treatment of mucous membrane pemphigoid. J Dtsch Dermatol Ges (2022) 20:1530–50. doi: 10.1111/ddg.14905
30. Bernard P, Vaillant L, Labeille B, Bedane C, Arbeille B, Denoeux JP, et al. Incidence and distribution of subepidermal autoimmune bullous skin diseases in three french regions. bullous diseases french study group. Arch Dermatol (1995) 131:48–52. doi: 10.1001/archderm.1995.01690130050009
31. Bertram F, Brocker EB, Zillikens D, Schmidt E. Prospective analysis of the incidence of autoimmune bullous disorders in lower franconia, germany. J Dtsch Dermatol Ges (2009) 7:434–40. doi: 10.1111/j.1610-0387.2008.06976.x
32. Hubner F, Recke A, Zillikens D, Linder R, Schmidt E. Prevalence and age distribution of pemphigus and pemphigoid diseases in germany. J Invest Dermatol (2016) 136:2495–8. doi: 10.1016/j.jid.2016.07.013
33. van Beek N, Weidinger A, Schneider SW, Kleinheinz A, Glaser R, Holtsche MM, et al. Incidence of pemphigoid diseases in northern germany in 2016 - first data from the schleswig-holstein registry of autoimmune bullous diseases. J Eur Acad Dermatol Venereol: JEADV (2021) 35:1197–202. doi: 10.1111/jdv.17107
34. Bedane C, Prost C, Ingen-Housz-Oro S, Joly P, Bernard P. [Mucous membrane pemphigoid. guidelines for the diagnosis and treatment. centres de reference des maladies bulleuses auto-immunes. societe francaise de dermatologie]. Ann Dermatol Venereol (2011) 138:259–63. doi: 10.1016/j.annder.2011.01.014
35. Hofmann S, Weidinger A. Epidermolysis bullosa acquisita. Hautarzt (2019) 70:265–70. doi: 10.1007/s00105-019-4387-7
36. Santi CG, Gripp AC, Roselino AM, Mello DS, Gordilho JO, Marsillac PF, et al. Consensus on the treatment of autoimmune bullous dermatoses: Bullous pemphigoid, mucous membrane pemphigoid and epidermolysis bullosa acquisita - brazilian society of dermatology. Anais Brasileiros Dermatol (2019) 94:33–47. doi: 10.1590/abd1806-4841.2019940207
37. Ujiie H, Iwata H, Yamagami J, Nakama T, Aoyama Y, Ikeda S, et al. Japanese guidelines for the management of pemphigoid (Including epidermolysis bullosa acquisita). J Dermatol (2019) 46:1102–35. doi: 10.1111/1346-8138.15111
38. Goletz S, Probst C, Komorowski L, Schlumberger W, Fechner K, van Beek N, et al. A sensitive and specific assay for the serological diagnosis of antilaminin 332 mucous membrane pemphigoid. Br J Dermatol (2019) 180:149–56. doi: 10.1111/bjd.17202
39. Amber KT, Bloom R, Hertl M. A systematic review with pooled analysis of clinical presentation and immunodiagnostic testing in mucous membrane pemphigoid: Association of anti-Laminin-332 igg with oropharyngeal involvement and the usefulness of elisa. J Eur Acad Dermatol Venereol: JEADV (2016) 30:72–7. doi: 10.1111/jdv.13397
40. Qian H, Natsuaki Y, Koga H, Kawakami T, Tateishi C, Tsuruta D, et al. The second study of clinical and immunological findings in anti-laminin 332-type mucous membrane pemphigoid examined at kurume university-diagnosis criteria suggested by summary of 133 cases. Front Immunol (2021) 12:771766. doi: 10.3389/fimmu.2021.771766
41. van Beek N, Kridin K, Buhler E, Kochan AS, Stander S, Ludwig RJ, et al. Evaluation of site- and autoantigen-specific characteristics of mucous membrane pemphigoid. JAMA Dermatol (2022) 158:84–9. doi: 10.1001/jamadermatol.2021.4773
42. Nieboer C, Boorsma DM, Woerdeman MJ, Kalsbeek GL. Epidermolysis bullosa acquisita. immunofluorescence, electron microscopic and immunoelectron microscopic studies in four patients. Br J Dermatol (1980) 102:383–92. doi: 10.1111/j.1365-2133.1980.tb06550.x
43. Yaoita H, Briggaman RA, Lawley TJ, Provost TT, Katz SI. Epidermolysis bullosa acquisita: Ultrastructural and immunological studies. J Invest Dermatol (1981) 76:288–92. doi: 10.1111/1523-1747.ep12526124
44. Prost C, Labeille B, Chaussade V, Guillaume JC, Martin N, Dubertret L. Immunoelectron microscopy in subepidermal autoimmune bullous diseases: A prospective study of igg and C3 bound in vivo in 32 patients. J Invest Dermatol (1987) 89:567–73. doi: 10.1111/1523-1747.ep12461226
45. Domloge-Hultsch N, Anhalt GJ, Gammon WR, Lazarova Z, Briggaman R, Welch M, et al. Antiepiligrin cicatricial pemphigoid. a subepithelial bullous disorder. Arch Dermatol (1994) 130:1521–9. doi: 10.1001/archderm.1994.01690120057008
46. Prost-Squarcioni C, Caux F, Schmidt E, Jonkman MF, Vassileva S, Kim SC, et al. International bullous diseases group: Consensus on diagnostic criteria for epidermolysis bullosa acquisita. Br J Dermatol (2018) 179:30–41. doi: 10.1111/bjd.16138
47. Terra JB, Pas HH, Hertl M, Dikkers FG, Kamminga N, Jonkman MF. Immunofluorescence serration pattern analysis as a diagnostic criterion in antilaminin-332 mucous membrane pemphigoid: Immunopathological findings and clinical experience in 10 dutch patients. Br J Dermatol (2011) 165:815–22. doi: 10.1111/j.1365-2133.2011.10474.x
48. Kirtschig G, Marinkovich MP, Burgeson RE, Yancey KB. Anti-basement membrane autoantibodies in patients with anti-epiligrin cicatricial pemphigoid bind the alpha subunit of laminin 5. J Invest Dermatol (1995) 105:543–8. doi: 10.1111/1523-1747.ep12323431
49. Lazarova Z, Hsu R, Yee C, Yancey KB. Antiepiligrin cicatricial pemphigoid represents an autoimmune response to subunits present in laminin 5 (Alpha3beta3gamma2). Br J Dermatol (1998) 139:791–7. doi: 10.1046/j.1365-2133.1998.02502.x
50. Lazarova Z, Sitaru C, Zillikens D, Yancey KB. Comparative analysis of methods for detection of anti-laminin 5 autoantibodies in patients with anti-epiligrin cicatricial pemphigoid. J Am Acad Dermatol (2004) 51:886–92. doi: 10.1016/j.jaad.2004.06.004
51. Oyama N, Bhogal BS, Carrington P, Gratian MJ, Black MM. Human placental amnion is a novel substrate for detecting autoantibodies in autoimmune bullous diseases by immunoblotting. Br J Dermatol (2003) 148:939–44. doi: 10.1046/j.1365-2133.2003.05316.x
52. Lazarova Z, Yee C, Lazar J, Yancey KB. Igg autoantibodies in patients with anti-epiligrin cicatricial pemphigoid recognize the g domain of the laminin 5 alpha-subunit. Clin Immunol (2001) 101:100–5. doi: 10.1006/clim.2001.5091
53. Hisamatsu Y, Nishiyama T, Amano S, Matsui C, Ghohestani R, Hashimoto T. Usefulness of immunoblotting using purified laminin 5 in the diagnosis of anti-laminin 5 cicatricial pemphigoid. J Dermatol Sci (2003) 33:113–9. doi: 10.1016/s0923-1811(03)00158-0
54. Kamaguchi M, Iwata H, Miyauchi T, Ujiie H, Ujiie I, Nomura T, et al. The identification of autoantigens in mucous membrane pemphigoid using immortalized oral mucosal keratinocytes. J Oral Pathol Med (2019) 48:60–7. doi: 10.1111/jop.12780
55. Bekou V, Thoma-Uszynski S, Wendler O, Uter W, Schwietzke S, Hunziker T, et al. Detection of laminin 5-specific auto-antibodies in mucous membrane and bullous pemphigoid sera by elisa. J Invest Dermatol (2005) 124:732–40. doi: 10.1111/j.0022-202X.2005.23646.x
56. Bernard P, Antonicelli F, Bedane C, Joly P, Le Roux-Villet C, Duvert-Lehembre S, et al. Prevalence and clinical significance of anti-laminin 332 autoantibodies detected by a novel enzyme-linked immunosorbent assay in mucous membrane pemphigoid. JAMA Dermatol (2013) 149:533–40. doi: 10.1001/jamadermatol.2013.1434
57. Chiorean R, Danescu S, Virtic O, Mustafa MB, Baican A, Lischka A, et al. Molecular diagnosis of anti-laminin 332 (Epiligrin) mucous membrane pemphigoid. Orphanet J Rare Dis (2018) 13:111. doi: 10.1186/s13023-018-0855-x
58. Lazarova Z, Salato VK, Lanschuetzer CM, Janson M, Fairley JA, Yancey KB. Igg anti-Laminin-332 autoantibodies are present in a subset of patients with mucous membrane, but not bullous, pemphigoid. J Am Acad Dermatol (2008) 58:951–8. doi: 10.1016/j.jaad.2008.02.035
59. Giurdanella F, Nijenhuis AM, Diercks GFH, Jonkman MF, Pas HH. Keratinocyte footprint assay discriminates antilaminin-332 pemphigoid from all other forms of pemphigoid diseases. Br J Dermatol (2020) 182:373–81. doi: 10.1111/bjd.18129
60. Goletz S, Giurdanella F, Holtsche MM, Nijenhuis M, Horvath B, Diercks GFH, et al. Comparison of two diagnostic assays for anti-laminin 332 mucous membrane pemphigoid. Front Immunol (2021) 12:773720. doi: 10.3389/fimmu.2021.773720
61. Vodegel RM, de Jong MC, Pas HH, Yancey KB, Jonkman MF. Anti-epiligrin cicatricial pemphigoid and epidermolysis bullosa acquisita: Differentiation by use of indirect immunofluorescence microscopy. J Am Acad Dermatol (2003) 48:542–7. doi: 10.1067/mjd.2003.99
62. van Beek N, Knuth-Rehr D, Altmeyer P, Assaf C, Babilas P, Bayerl C, et al. Diagnostics of autoimmune bullous diseases in german dermatology departments. J Dtsch Dermatol Ges (2012) 10:492–9. doi: 10.1111/j.1610-0387.2011.07840.x
63. Komorowski L, Muller R, Vorobyev A, Probst C, Recke A, Jonkman MF, et al. Sensitive and specific assays for routine serological diagnosis of epidermolysis bullosa acquisita. J Am Acad Dermatol (2013) 68:e89–95. doi: 10.1016/j.jaad.2011.12.032
64. van Beek N, Kruger S, Fuhrmann T, Lemcke S, Goletz S, Probst C, et al. Multicenter prospective study on multivariant diagnostics of autoimmune bullous dermatoses using the biochip technology. J Am Acad Dermatol (2020) 83:1315–22. doi: 10.1016/j.jaad.2020.01.049
65. Yang A, Xuan R, Melbourne W, Tran K, Murrell DF. Validation of the biochip test for the diagnosis of bullous pemphigoid, pemphigus vulgaris and pemphigus foliaceus. J Eur Acad Dermatol Venereol: JEADV (2020) 34:153–60. doi: 10.1111/jdv.15770
66. Gasparini G, Cozzani E, Di Zenzo G, Salemme A, Dematte E, Vassallo C, et al. Anti-laminin 332 antibody detection using biochip immunofluorescence microscopy in a real-life cohort of italian patients with mucous membrane pemphigoid. Eur J Dermatol: EJD (2022) 32:756–61. doi: 10.1684/ejd.2021.4104
67. Gornowicz-Porowska J, Jalowska M, Seraszek-Jaros A, Bowszyc-Dmochowska M, Kaczmarek E, Dmochowski M. A probing of the issue of detecting igg, Igg4 and iga antibodies to laminin 332 epitopes in mucous membrane pemphigoid: A clinical-laboratory experience of a single central european university dermatology department. Clin Cosmetic Investigational Dermatol (2022) 15:783–90. doi: 10.2147/CCID.S359589
68. Natsuga K, Nishie W, Shinkuma S, Moriuchi R, Shibata M, Nishimura M, et al. Circulating iga and ige autoantibodies in antilaminin-332 mucous membrane pemphigoid. Br J Dermatol (2010) 162:513–7. doi: 10.1111/j.1365-2133.2009.09508.x
69. Hayashi I, Shinkuma S, Shimizu S, Natsuga K, Ujiie H, Yasui C, et al. Mucous membrane pemphigoid with generalized blisters: Iga and igg autoantibodies target both laminin-332 and type xvii collagen. Br J Dermatol (2012) 166:1116–20. doi: 10.1111/j.1365-2133.2011.10776.x
70. Schmidt E, Groves R. Immunobullous disorders. In: Griffith C, Barker J, Chalmers, Bleiker T, Creamer D, editors. Rook’s Textbook of Dermatology, part 3, chapter 50, 10th edition. Chichester: Wiley-Blackwell (in press).
71. Gibson GE, Daoud MS, Pittelkow MR. Anti-epiligrin (Laminin 5) cicatricial pemphigoid and lung carcinoma: Coincidence or association? Br J Dermatol (1997) 137:780–2. doi: 10.1111/j.1365-2133.1997.tb01118.x
72. Leverkus M, Schmidt E, Lazarova Z, Brocker EB, Yancey KB, Zillikens D. Antiepiligrin cicatricial pemphigoid: An underdiagnosed entity within the spectrum of scarring autoimmune subepidermal bullous diseases? Arch Dermatol (1999) 135:1091–8. doi: 10.1001/archderm.135.9.1091
73. Egan CA, Lazarova Z, Darling TN, Yee C, Cote T, Yancey KB. Anti-epiligrin cicatricial pemphigoid and relative risk for cancer. Lancet (2001) 357:1850–1. doi: 10.1016/S0140-6736(00)04971-0
74. Matsushima S, Horiguchi Y, Honda T, Fujii S, Okano T, Tanabe M, et al. A case of anti-epiligrin cicatricial pemphigoid associated with lung carcinoma and severe laryngeal stenosis: Review of japanese cases and evaluation of risk for internal malignancy. J Dermatol (2004) 31:10–5. doi: 10.1111/j.1346-8138.2004.tb00497.x
75. Hayakawa T, Furumura M, Fukano H, Li X, Ishii N, Hamada T, et al. Diagnosis of oral mucous membrane pemphigoid by means of combined serologic testing. Oral Surg Oral Med Oral Pathol Oral Radiol (2014) 117:483–96. doi: 10.1016/j.oooo.2013.12.402
76. Li X, Qian H, Natsuaki Y, Koga H, Kawakami T, Tateishi C, et al. Clinical and immunological findings in 55 patients with anti-laminin 332-type mucous membrane pemphigoid. Br J Dermatol (2021) 185:449–51. doi: 10.1111/bjd.20099
77. Shi L, Li X, Qian H. Anti-laminin 332-type mucous membrane pemphigoid. Biomolecules (2022) 12:1461. doi: 10.3390/biom12101461
78. Nayar M, Wojnarowska F. No association between cicatricial pemphigoid and malignant disease. Br J Dermatol (1991) 125:193–4. doi: 10.1111/j.1365-2133.1991.tb06077.x
79. Letko E, Gurcan HM, Papaliodis GN, Christen W, Foster CS, Ahmed AR. Relative risk for cancer in mucous membrane pemphigoid associated with antibodies to the Beta4 integrin subunit. Clin Exp Dermatol (2007) 32:637–41. doi: 10.1111/j.1365-2230.2007.02463.x
80. Malik M, Gurcan HM, Christen W, Ahmed AR. Relationship between cancer and oral pemphigoid patients with antibodies to Alpha6-integrin. J Oral Pathol Med (2007) 36:1–5. doi: 10.1111/j.1600-0714.2006.00483.x
81. Giannelli G, Antonaci S. Biological and clinical relevance of laminin-5 in cancer. Clin Exp Metastasis (2000) 18:439–43. doi: 10.1023/a:1011879900554
82. Marinkovich MP. Tumour microenvironment: Laminin 332 in squamous-cell carcinoma. Nat Rev Cancer (2007) 7:370–80. doi: 10.1038/nrc2089
83. Guess CM, Quaranta V. Defining the role of laminin-332 in carcinoma. Matrix Biol (2009) 28:445–55. doi: 10.1016/j.matbio.2009.07.008
84. Waterman EA, Sakai N, Nguyen NT, Horst BA, Veitch DP, Dey CN, et al. A laminin-collagen complex drives human epidermal carcinogenesis through phosphoinositol-3-Kinase activation. Cancer Res (2007) 67:4264–70. doi: 10.1158/0008-5472.CAN-06-4141
85. Cavaco ACM, Rezaei M, Caliandro MF, Lima AM, Stehling M, Dhayat SA, et al. The interaction between laminin-332 and Alpha3beta1 integrin determines differentiation and maintenance of cafs, and supports invasion of pancreatic duct adenocarcinoma cells. Cancers (2018) 11:14. doi: 10.3390/cancers11010014
86. Rousselle P, Scoazec JY. Laminin 332 in cancer: When the extracellular matrix turns signals from cell anchorage to cell movement. Semin Cancer Biol (2020) 62:149–65. doi: 10.1016/j.semcancer.2019.09.026
87. Taniuchi K, Takata M, Matsui C, Fushida Y, Uchiyama K, Mori T, et al. Antiepiligrin (Laminin 5) cicatricial pemphigoid associated with an underlying gastric carcinoma producing laminin 5. Br J Dermatol (1999) 140:696–700. doi: 10.1046/j.1365-2133.1999.02773.x
88. Yasuda H, Nakagawa M, Kiyokawa H, Yoshida E, Yoshimura T, Koshikawa N, et al. Unique biological activity and potential role of monomeric laminin-Gamma2 as a novel biomarker for hepatocellular carcinoma: A review. Int J Mol Sci (2019) 20:226. doi: 10.3390/ijms20010226
89. Koga K, Anan T, Fukumoto T, Fujimoto M, Nabeshima K. Ln-gamma 2 chain of laminin-332 is a useful marker in differentiating between benign and malignant sclerosing adnexal neoplasms. Histopathology (2020) 76:318–24. doi: 10.1111/his.13974
90. Uchiyama K, Yamamoto Y, Taniuchi K, Matsui C, Fushida Y, Shirao Y. Remission of antiepiligrin (Laminin-5) cicatricial pemphigoid after excision of gastric carcinoma. Cornea (2000) 19:564–6. doi: 10.1097/00003226-200007000-00033
91. Ding DC, Chu TY, Hsu YH. Remission of anti-epiligrin cicatricial pemphigoid after excision of cervical adenocarcinoma. J Cutaneous Pathol (2014) 41:692–3. doi: 10.1111/cup.12348
92. Colon JE, Bhol KC, Razzaque MS, Ahmed AR. In vitro organ culture model for mucous membrane pemphigoid. Clin Immunol (2001) 98:229–34. doi: 10.1006/clim.2000.4972
93. Branisteanu DC, Stoleriu G, Branisteanu DE, Boda D, Branisteanu CI, Maranduca MA, et al. Ocular cicatricial pemphigoid (Review). Exp Ther Med (2020) 20:3379–82. doi: 10.3892/etm.2020.8972
94. Diebold Y, Garcia-Posadas L. Is the conjunctiva a potential target for advanced therapy medicinal products? Pharmaceutics (2021) 13:1140. doi: 10.3390/pharmaceutics13081140
95. Lazarova Z, Yee C, Darling T, Briggaman RA, Yancey KB. Passive transfer of anti-laminin 5 antibodies induces subepidermal blisters in neonatal mice. J Clin Invest (1996) 98:1509–18. doi: 10.1172/JCI118942
96. Lazarova Z, Hsu R, Briggaman RA, Yancey KB. Fab fragments directed against laminin 5 induce subepidermal blisters in neonatal mice. Clin Immunol (2000) 95:26–32. doi: 10.1006/clim.2000.4845
97. Heppe EN, Tofern S, Schulze FS, Ishiko A, Shimizu A, Sina C, et al. Experimental laminin 332 mucous membrane pemphigoid critically involves C5ar1 and reflects clinical and immunopathological characteristics of the human disease. J Invest Dermatol (2017) 137:1709–18. doi: 10.1016/j.jid.2017.03.037
98. Murthy S, Schilf P, Patzelt S, Thieme M, Becker M, Kroger L, et al. Dapsone suppresses disease in preclinical murine models of pemphigoid diseases. J Invest Dermatol (2021) 141:2587–95 e2. doi: 10.1016/j.jid.2021.04.009
99. Ghorbanalipoor S, Emtenani S, Parker M, Kamaguchi M, Osterloh C, Pigors M, et al. Cutaneous kinase activity correlates with treatment outcomes following Pi3k delta inhibition in mice with experimental pemphigoid diseases. Front Immunol (2022) 13:865241. doi: 10.3389/fimmu.2022.865241
100. Ghorbanalipoor S, Emtenani S, Parker M, Kamaguchi M, Osterloh C, Pigors M, et al. Corrigendum: Cutaneous kinase activity correlates with treatment outcomes following Pi3k delta inhibition in mice with experimental pemphigoid diseases. Front Immunol (2022) 13:1099535. doi: 10.3389/fimmu.2022.1099535
101. Patzelt S, Pigors M, Steenbock H, Diel L, Boch K, Chakievska L, et al. Increased fibrosis in a mouse model of anti-laminin 332 mucous membrane pemphigoid remains unaltered by inhibition of aldehyde dehydrogenase. Front Immunol (2021) 12:812627. doi: 10.3389/fimmu.2021.812627
102. Ahadome SD, Abraham DJ, Rayapureddi S, Saw VP, Saban DR, Calder VL, et al. Aldehyde dehydrogenase inhibition blocks mucosal fibrosis in human and mouse ocular scarring. JCI Insight (2016) 1:e87001. doi: 10.1172/jci.insight.87001
103. Bao L, Li J, Solimani F, Didona D, Patel PM, Li X, et al. Subunit-specific reactivity of autoantibodies against laminin-332 reveals direct inflammatory mechanisms on keratinocytes. Front Immunol (2021) 12:775412. doi: 10.3389/fimmu.2021.775412
104. Bao L, Perez White BE, Li J, Patel PM, Amber KT. Gene expression profiling of laminin Alpha3-blocked keratinocytes reveals an immune-independent mechanism of blistering. Exp Dermatol (2022) 31:615–21. doi: 10.1111/exd.14501
105. Zuelgaray E, Salle de Chou C, Gottlieb J, Battistella M, Vignon-Pennamen MD, Bagot M, et al. Human orf complicated by epidermolysis bullosa acquisita. Br J Dermatol (2018) 178:547–50. doi: 10.1111/bjd.15496
106. Daneshpazhooh M, Mahmoudi H, Toosi R, Tavakolpour S, Schmidt E, Zillikens D. Post-orf epidermolysis bullosa acquisita. J Eur Acad Dermatol Venereol: JEADV (2019) 33:e118–e9. doi: 10.1111/jdv.15299
Keywords: autoimmunity, diagnosis, malignancy, immunofluorescence, guidelines, BP180, type VII collagen, laminin 332
Citation: Patzelt S and Schmidt E (2023) Autoimmunity against laminin 332. Front. Immunol. 14:1250115. doi: 10.3389/fimmu.2023.1250115
Received: 29 June 2023; Accepted: 24 July 2023;
Published: 10 August 2023.
Edited by:
Angelo Valerio Marzano, University of Milan, ItalyReviewed by:
Alessandra Marconi, University of Modena and Reggio Emilia, ItalyCopyright © 2023 Patzelt and Schmidt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Enno Schmidt, ZW5uby5zY2htaWR0QHVrc2guZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.