
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 23 October 2023
Sec. Viral Immunology
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1246751
 Yiting Wang2†
Yiting Wang2† Bennan Zhao1†
Bennan Zhao1† Xinyi Zhang3†
Xinyi Zhang3† Xia Zhang1
Xia Zhang1 Fengjiao Gao1
Fengjiao Gao1 Xiaoyan Yuan1
Xiaoyan Yuan1 Xiaoxia Ren1
Xiaoxia Ren1 Maoquan Li2*
Maoquan Li2* Dafeng Liu1*‡
Dafeng Liu1*‡Background: Previous infections and vaccinations have produced preexisting immunity, which differs from primary infection in the organism immune response and may lead to different disease severities and prognoses when reinfected.
Objectives: The purpose of this retrospective cohort study was to investigate the impact of immune breakthroughs on disease progression and prognosis in patients with COVID-19.
Methods: A retrospective cohort study was conducted on 1513 COVID-19 patients in Chengdu Public Health Clinical Medical Center from January 2020 to November 2022. All patients were divided into the no immunity group (primary infection and unvaccinated, n=1102) and the immune breakthrough group (previous infection or vaccination, n=411). The immune breakthrough group was further divided into the natural immunity subgroup (n=73), the acquired immunity subgroup (n=322) and the mixed immunity subgroup (n=16). The differences in clinical and outcome data and T lymphocyte subsets and antibody levels between two groups or between three subgroups were compared by ANOVA, t test and chi-square test, and the relationship between T lymphocyte subsets and antibody levels and the disease progression and prognosis of COVID-19 patients was assessed by univariate analysis and logistic regression analysis.
Results: The total critical rate and the total mortality rate were 2.11% and 0.53%, respectively. The immune breakthrough rate was 27.16%. In the no immunity group, the critical rate and the mortality rate were all higher, and the coronavirus negative conversion time was longer than those in the immune breakthrough group. The differences in the critical rate and the coronavirus negative conversion time between the two groups were all statistically significant (3.72% vs. 0.24%, 14.17 vs. 11.90 days, all p<0.001). In addition, in the no immunity group, although lymphocyte counts and T subsets at admission were higher, all of them decreased consistently and significantly and were significantly lower than those in the immune breakthrough group at the same time from the first week to the fourth week after admission (all p<0.01). The total antibody levels and specific Immunoglobulin G (IgG) levels increased gradually and were always significantly lower than those in the immune breakthrough group at the same time from admission to the fourth week after admission (all p<0.001). Moreover, in the natural immunity subgroup, lymphocyte counts and T subsets at admission were the highest, and total antibody levels and specific IgG levels at admission were the lowest. Then, all of them decreased significantly and were the lowest among the three subgroups at the same time from admission to one month after admission (total antibody: from 546.07 to 158.89, IgG: from 6.00 to 3.95) (all p<0.001). Those in the mixed immunity subgroup were followed by those in the acquired immunity subgroup. While lymphocyte counts and T subsets in these two subgroups and total antibody levels (from 830.84 to 1008.21) and specific IgG levels (from 6.23 to 7.51) in the acquired immunity subgroup increased gradually, total antibody levels (from 1100.82 to 908.58) and specific IgG levels (from 7.14 to 6.58) in the mixed immunity subgroup decreased gradually. Furthermore, T lymphocyte subsets and antibody levels were negatively related to disease severity, prognosis and coronavirus negative conversion time. The total antibody, specific IgM and IgG levels showed good utility for predicting critical COVID-19 patients and dead COVID-19 patients.
Conclusion: Among patients with COVID-19 patients, immune breakthroughs resulting from previous infection or vaccination, could decelerate disease progression and enhance prognosis by expediting host cellular and humoral immunity to accelerate virus clearance, especially in individuals who have been vaccinated and previously infected.
Clinical trial registry: Chinese Clinical Trial Register ChiCTR2000034563.
Since mid-December 2019, the COVID-19 pandemic caused by SARS-CoV-2 has been ongoing and evolved into a major global health threat (1). By April 2023, over 762 million confirmed cases and over 6.8 million deaths have been reported globally (2). SARS-CoV-2 elicits both innate and adaptive immune responses, including the development of specific T cells and antibodies. Efficient immune responses are indispensable for the regulation and eradication of pathogen infections (3).
The adaptive immune system consists of three lymphocyte types: B cells, CD3+CD4+ T cells, and CD3+CD8+ T cells (4). T-lymphocytes and immune antibodies are necessary to control viral infections. Similar to severe influenza and other respiratory viral infections, lymphopenia is frequently observed in COVID-19 and exhibits a positive correlation with the clinical disease severity (5, 6). Adaptive responses of immune antibodies provide the first line of defense during viral infections and are important for long-term immunity and immune memory (7). Therefore, the number of T cells and antibodies can be diagnostic and predictive factors for identifying patients who will have severe disease (6, 8).
The topic of pre-existing immunity to SARS-CoV-2 infection, acquired through natural infection or vaccination, has gained significant attention currently (9). Several countries have also reported cases of breakthrough infections among individuals who were vaccinated or had a previous infection (9–11). Studies have shown that the maintenance of IgG and T-cell responses persists in most patients for at least 3–4 months following infection (12) and even more than 13 months (13). Vaccination is considered key to reducing the risk of SARS-CoV-2 infection, severe illness, and mortality risks (14). The immune breakthrough is speculated to be attributed to factors including high viral load exposure, infection with a different viral strain, and antibody-dependent enhancement (10). In the post-COVID-19 era, understanding the potential influence of immune breakthrough is crucial to improving COVID-19 prevention and control measures. Therefore, we conducted a population-based study to investigate the presence of SARS-CoV-2 T lymphocytes and antibody levels, carefully examining the fluctuations in these levels among patients with varying immune statuses.
This was a retrospective cohort study. All 1,513 patients with COVID-19 from the hospital isolation ward who presented to the Public Health Clinical Centre of Chengdu from January 16, 2020, to September 30, 2022, were retrospectively recruited (Figure 1, Table 1). The study was approved by the Public and Health Clinical Centre of Chengdu Ethics Committee (ethics approval number: PJ-K2020-26-01). Written informed consent was waived by the Ethics Commission of the designated hospital because this study was related to emerging infectious diseases.
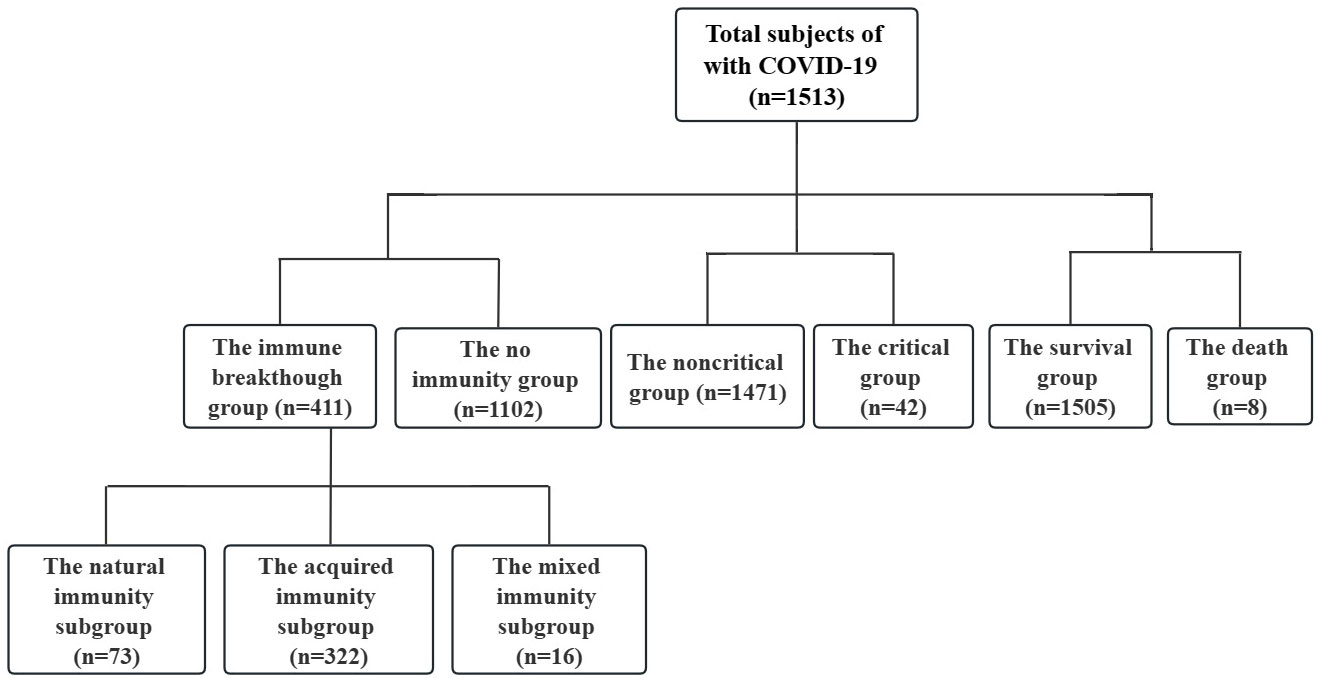
Figure 1 Patient data (n=1513). Non critical refers to the clinical type of COVID-19 that is asymptomatic, light and common. Critical refers to the clinical type of COVID-19 that is associated with severe and critical illness. No immunity refers to primary infection and no vaccination. Immune breakthrough refers to previous infection or vaccination. Natural immunity refers to previous infection. Acquired immunity refers to vaccination. Mixed immunity refers to previous infection and vaccination.
The inclusion criteria were as follows: no sex limit; age ≥18 years old; COVID-19; and inpatient isolation and treatment time >1 day.
The exclusion criteria were as follows: age<18 years old and isolation and treatment time <1 day.
The criteria for COVID-19 clinical typing, disease diagnosis and cure were in accordance with the seventh Trial Version of the Novel Coronavirus Pneumonia Diagnosis and Treatment Guidance (15).
The diagnosis criteria were cases with one of the following etiological pieces of evidence: real-time fluorescence reverse transcription-polymerase chain reaction (RT-PCR) detected the positive nucleic acid of the new coronavirus and sequencing of viral genes.
The typing criteria were as follows: (1) asymptomatic infection indicated that there were no clinical symptoms and no pneumonia manifestations on imaging; (2) the light type indicated that the clinical symptoms were mild, and there were no pneumonia manifestations on imaging; (3) the common type indicated that the clinical symptoms included fever and respiratory tract, and pneumonia could be seen on imaging; (4) the severe type indicated that the patients had any of the following criteria: respiratory distress, RR≥30 times/min; in the resting state, oxygen saturation ≤ 93%; arterial blood oxygen partial pressure (PaO2)/oxygen concentration (FiO2)≤300mmHg (1mmHg=0.133kPa), living in areas with high altitude (over 1000 meters above sea level), and PaO2/FiO2 should be corrected according to the following formula: PaO2/FiO2*[atmospheric pressure(mmHg)/760]; pulmonary imaging showed that lesions with significant progress over 50% within 24–48h were managed as heavy; (5) the critical illness type criteria included one of the following conditions: respiratory failure occurs and mechanical ventilation is needed; Shock occurs; and combining other organ failure requires intensive care units (ICU) monitoring.
The cured discharge standard was as follows: the body temperature returned to normal for more than 3 days; respiratory symptoms improved significantly; lung imaging showed a significant improvement in acute exudative lesions; and two consecutive sputum, nasopharyngeal swabs and other respiratory specimens tested negative for nucleic acid (sampling time at least 24 h apart).
The relevant serological assays were performed by Enzyme-Linked Immunosorbent Assay (ELISA) in the laboratory of the hospital.
Among the 1,513 COVID-19 cases, 1102 and 411 cases were divided into the no immunity group (primary infection and no vaccination) and the immune breakthrough group (previous infection or vaccination), respectively (Figure 1, Table 1).
Among the 411 immune breakthrough cases, 73 patients with previous infection were assigned to the natural immunity group, 322 patients who had been vaccinated (no distinction was made between vaccine type and dose) were assigned to the acquired immunity group, and 16 patients who had both previous infection and been vaccinated were assigned to the mixed immunity group (Figure 1, Table 1).
Among the 1,513 COVID-19 cases, 1,471 noncritical patients (patients with asymptomatic infection, with light and with common clinical type) were assigned to the noncritical group, and 42 critical patients (patients with severe and with critical illness clinical type) were assigned to the critical group (Figure 1, Table 1).
Among the 1,513 COVID-19 cases, 1505 surviving patients were assigned to the survival group, and 8 dead patients were assigned to the death group (Figure 1, Table 1).
The disease severity included critical illness (COVID-19 patients with severe or critical illness clinical type) and noncritical illness (COVID-19 patients with asymptomatic infection, light or common clinical type). The prognosis included death and survival within four weeks after admission. The coronavirus negative conversion time was the time from onset to the first negative nucleic acid test meeting the discharge criteria.
The data were collected from a subset of patients treated at Chengdu Public Health Clinical Medical Center from January 2020 to November 2022. All data of 1,513 cases, including clinical data, laboratory data and demographic data, were collected to establish databases. Researchers strictly controlled the accuracy, completeness and authenticity of all data.
SPSS 26.0 (SPSS, Chicago, IL, USA) and GraphPad Prism 8 (GraphPad, CA, USA) were used for statistical analyses. Measurement data with a normal distribution are presented as the mean and standard deviation, and measurement data with a nonnormal distribution are presented as the median and interquartile range (IQR). The categorical data are expressed as a percentage or proportion. Data with a normal distribution and homogeneity of variance between multiple groups were compared using one-way or two-way ANOVA, and further comparison between two groups was performed using the least significant difference (LSD) t test. Data with a normal distribution and homogeneity of variance between two groups were compared using the independent samples t test. Enumeration data are presented as percentages or proportions, and data between two or multiple groups were compared using a chi-square test. Analysis of influencing factors of disease severity and prognosis was performed using binary logistic regression analysis. Receiver operating characteristic (ROC) analysis was used to assess lymphocytes and subsets to distinguish non critical from severe COVID-19 patients. P<0.05 was considered statistically significant.
Patients and the public were not involved in the development of the research questions or in the design of the study. Patients received verbal and written information about the study; however, they were not involved in the recruitment of subjects or the conduct of the study. Additionally, the burden of the intervention was assessed by the investigators. The participants were assessed for eligibility, and data collection was performed. Dissemination of the general results (without personally identifying data) will occur on demand. The Ethics Committee of the Public Health Clinical Centre of Chengdu approved this study (ethics approval number: PJ-K2020-26-01). Written informed consent was waived by the Ethics Commission of the designated hospital because this study is related to emerging infectious diseases.
A total of 1,513 patients with COVID-19 were included in this study. Their demographic and clinical characteristics are listed in Table 1. The median age of all patients included in the study was 35 years, and males accounted for the majority (71.0%). The median coronavirus negative conversion time was 11.0 days, and the duration of hospitalization was 15.0 days.
In addition, 966 (63.40%) patients had comorbidities, 374 (25.0%) patients had one comorbidity, 233 (15.6%) patients had two comorbidities, 340 (22.8%) patients had three or more comorbidities, and 547 (36.6%) patients had no comorbidities. Among them, 42 (2.8%) patients had critical illness, 1,471 (97.2%) patients had noncritical illness, 1505 patients survived, and only 8 (0.5%) patients died.
Imported cases accounted for 85.0% of the total, while domestically transmitted cases made up the remaining 15.0%. Regarding immune status, 411 (27.2%) patients had preexisting immunity by previous infection or vaccination, and 1102 (72.8%) patients had primary infection. Among vaccinated patients, 13 (0.9%) patients accepted one dose, 235 (15.5%) patients accepted two doses, 84 (5.6%) patients accepted three doses and 6 (0.4%) patients accepted four doses.
In the immune breakthrough group, the proportion of domestically transmitted cases was significantly higher than that in the no immunity group (Table 2) (p<0.05), while the critical illness rate was significantly lower than that in the no immunity group (Table 2) (0.24% vs. 3.72%, p<0.05). The mortality rate was slightly lower than that in the no immunity group, although the difference was not statistically significant (Table 2) (p=0.083).
The age (Figure 2A) was slightly younger than that in the no immunity group (p<0.05), and there was no significant difference in BMI (Figure 2B) or duration of hospitalization (Figure 2C) between the two groups (all p>0.05). However, in the immune breakthrough group, the coronavirus negative conversion time (Figure 2D) was significantly shorter than that in the no immunity group (p<0.001).
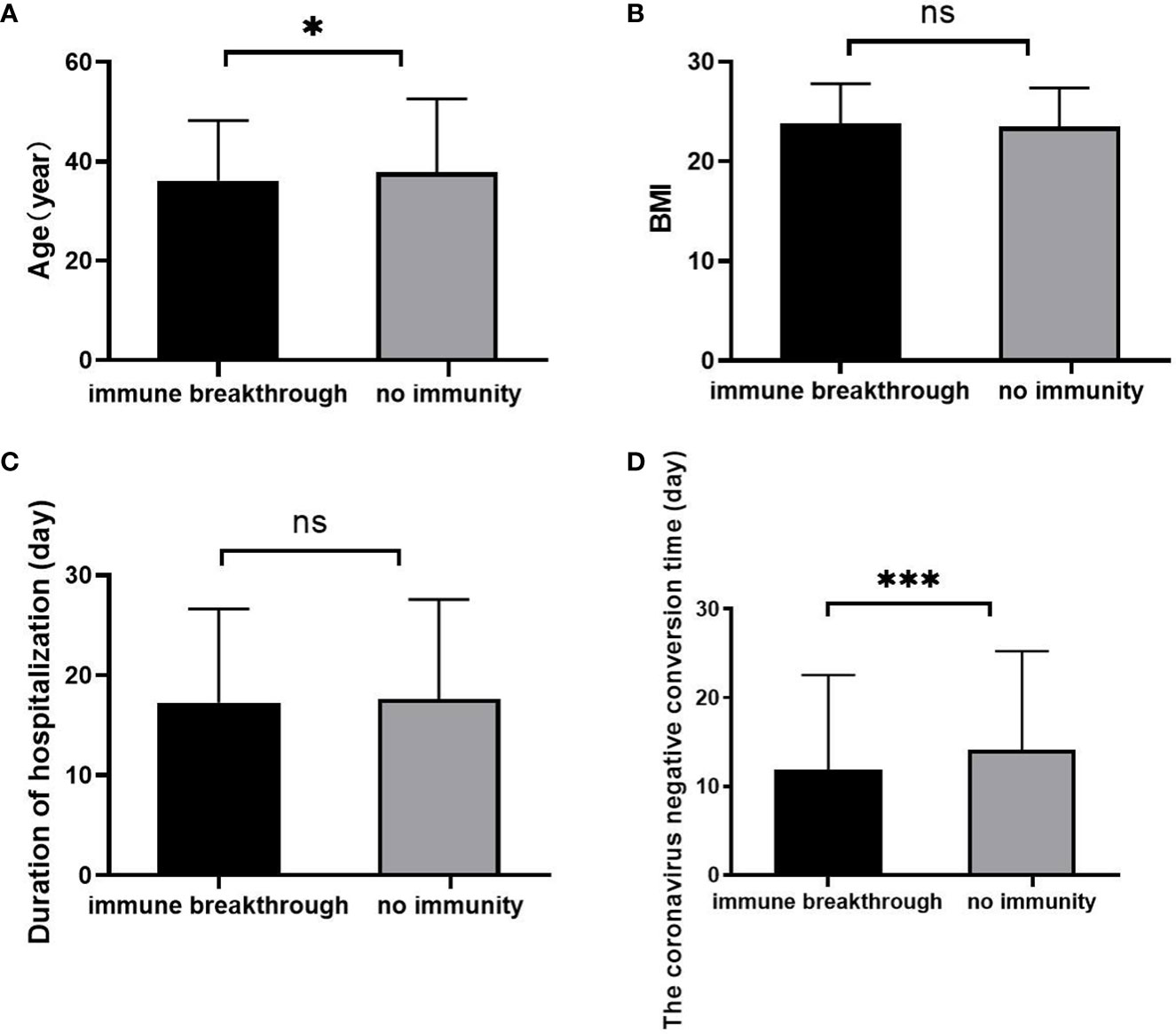
Figure 2 Comparison of age, BMI, duration of hospitalization and coronavirus negative conversion time between the immune breakthrough group and the no immunity group (n=1513; the immune breakthrough group and the no immunity group, n=411 and 1102, respectively). (A) Age. (B) BMI. (C) Duration of hospitalization (day). (D) The coronavirus negative conversion time (day). BMI, body mass index. Unpaired t tests were used for comparisons between two groups, nsp>0.05, *p<0.05, ***p<0.001.
In addition, in the no immunity group, CD3+ counts, CD3+CD4+ counts, CD3+CD8+ counts and lymphocyte counts at admission were higher than those in the immune breakthrough group (Figures 3A–C, E) (all p<0.05), but all of them then decreased to the lowest level at the first week and were always significantly lower than those in the immune breakthrough group from the first week to the fourth week (Figures 3A–C, E) (all p<0.01). The ratio of CD3+CD4+ to CD3+CD8+ cells between the two groups from onset to the fourth week after onset was always similar to each other (Figure 3D) (p>0.01). Moreover, in the no immunity group, total antibody levels and specific IgG levels from onset to the fourth week were always significantly lower than those in the immune breakthrough group (Figures 3F, H) (all p<0.001). In the no immunity group, the specific IgM levels were always lower than those in the immune breakthrough group from the first week to the fourth week, but a significant difference was found only at the first week after onset (Figure 3G).
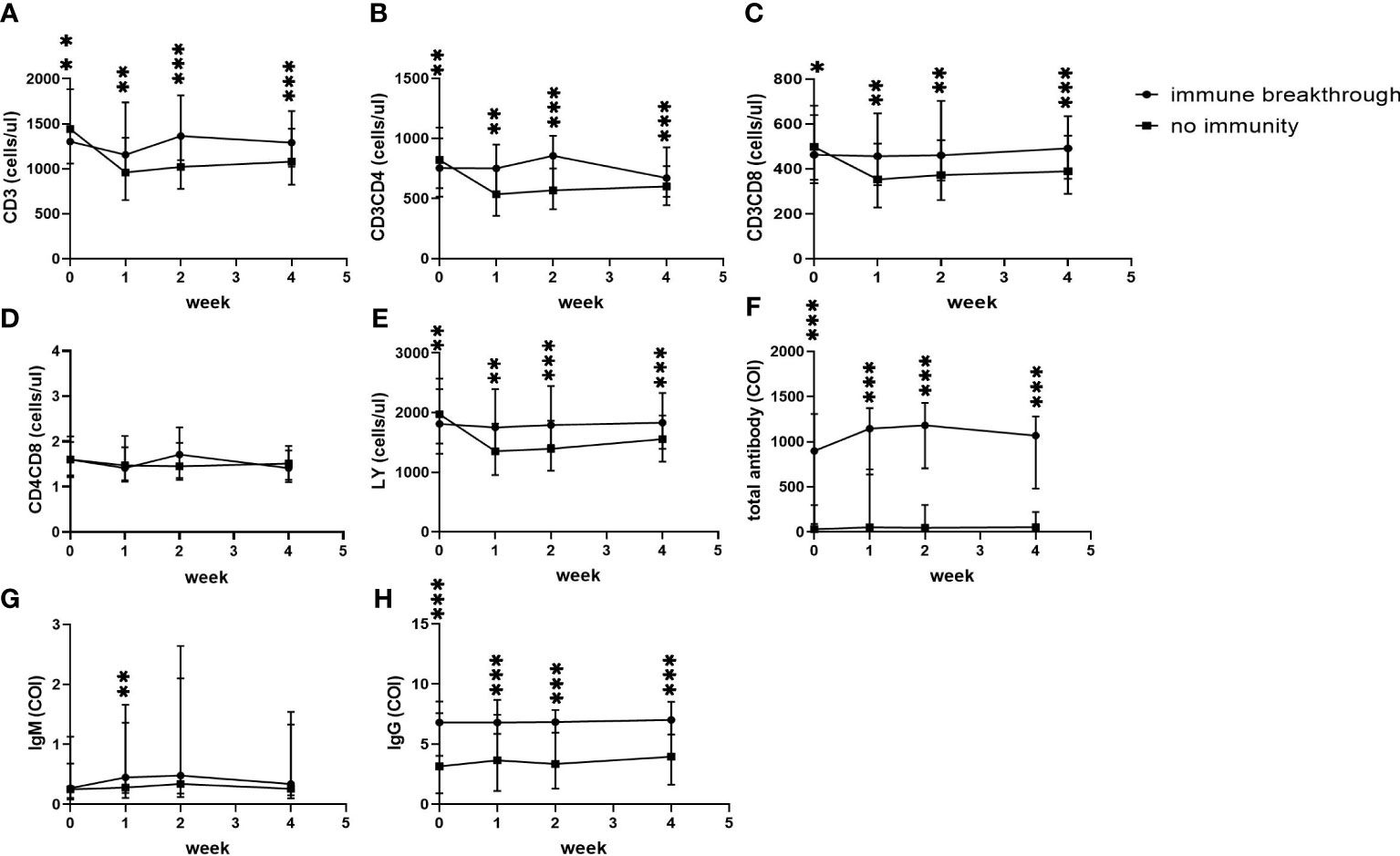
Figure 3 Comparison of lymphocyte counts, T subset counts and antibody levels between the immune breakthrough group and the no immunity group within 4 weeks (n=1513; the immune breakthrough group and the no immunity group, n=411 and 1102, respectively). LY, lymphocyte; IgM, immunoglobulin M; IgG, immunoglobulin G; (A) CD3+ counts. (B) CD3+CD4+ counts. (C) CD3+CD8+ counts. (D) CD4+CD8+ counts. (E) LY. (F) Total antibody levels. (G) IgM levels. (H) IgG levels. Two-way ANOVA was used for intergroup comparisons within 4 weeks (A–H, P all<0.01). Unpaired t tests were used for comparisons between two groups at the same time point, *p<0.05, **p<0.01, ***p<0.001.
Metabolic diseases such as fatty liver, diabetes, hypertension, hyperlipidemia, etc. were the most common in both groups, with no difference (p all <0.05) (Table 3). The incidence of cardiovascular disease was higher in the no immunity group (p<0.05) (Table 3). Besides, the incidence of hypoxemia (p<0.001) and cardiovascular disease (p<0.05) was significantly higher in the no immunity group than in the immune breakthrough group (Table 3).
Compared to the natural immunity subgroup and the acquired immunity subgroup, age was slightly younger (p<0.05), and the duration of hospitalization was obviously shorter than that in the mixed immunity subgroup (p<0.01) (Table 4). The coronavirus negative conversion time in the mixed immunity subgroup was also slightly shorter than that in the other two groups, but the difference was not statistically significant (Table 4) (p=0.057).
Moreover, in the acquired immunity subgroup, CD3+ counts, CD3+CD4+ counts, CD3+CD8+ counts and lymphocyte counts at admission were the highest and then decreased to the lowest level at the first month among the three groups. All of them in the other two groups showed an increase from admission to the first month after admission, and those in the mixed immunity group reached their highest levels (Figures 4A–D). In the mixed immunity subgroup, the total antibody levels and specific IgG levels were the highest at admission and then decreased slightly over the following month (Figures 4E, F). Those in the natural immunity subgroup were the lowest at admission, with a sharp decrease during the following month (Figures 4E, F). Only those in the mixed immunity subgroup rose from second place on admission to first place at one month (Figures 4E, F).
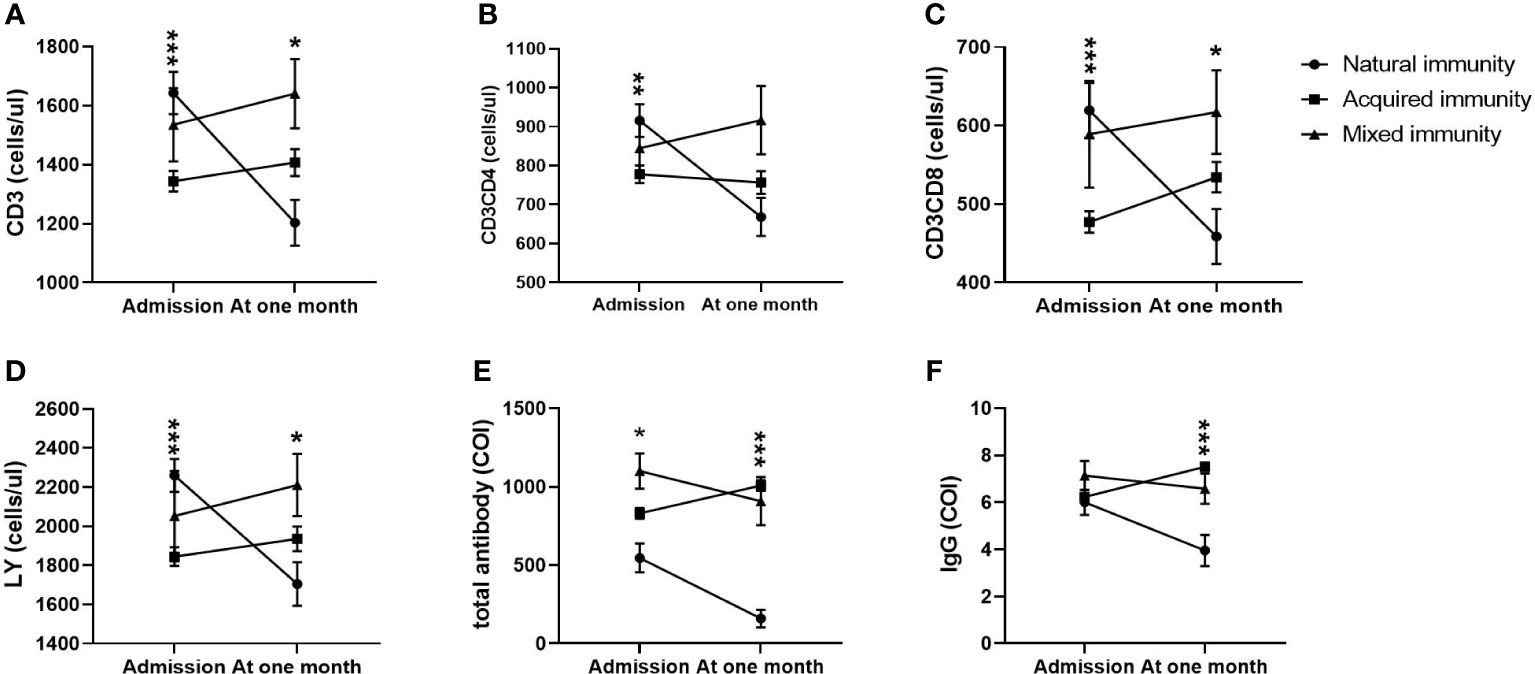
Figure 4 Comparison of lymphocyte counts, T subset counts and antibody levels among the natural immunity subgroup, the acquired immunity subgroup and the mixed immunity subgroup within 4 weeks (n=411; the natural immunity subgroup, the acquired immunity subgroup and the mixed immunity subgroup, n=73, 322 and 16, respectively). LY, lymphocyte; IgM, immunoglobulin M; IgG, immunoglobulin G. (A) CD3+ counts. (B) CD3+CD4+ counts. (C) CD3+CD8+ counts. (D) LY. (E) Total antibody levels. (F) IgG levels. Two-way ANOVA was used for intergroup comparisons within 4 weeks (A–F, P all<0.01). Unpaired t tests were used for comparisons between two groups at the same time point, *p<0.05, **p<0.01, ***p<0.001.
For critical cases, the age was significantly older, the number of comorbidities and the imported cases were higher (p all=0.000). Most T-lymphocyte subsets (except CD3+CD8+% and CD19+%) and all antibody levels (p all<0.05) were significantly higher in critical cases, whereas the level of CD56+% (p=0.024) was higher in non-critical cases (Table 5). Similarly, all the death cases were imported cases, with an average age of 77.5 years (p=0.000), a slightly higher proportion of females (p=0.034), an increased BMI index (p=0.013), and all combined with more than 3 diseases (p=0.000), without a history of previous infection. CD3+, CD3+CD4+, CD3+CD8+, LY, LY%, CD19+ and all antibody levels in non-critical cases were higher (p all <0.01) (Table 5).
The influencing factors for disease severity by multiple linear regression analysis were age, lymphocyte percentage, source of cases and CD3+CD8+ counts (Table 6). Moreover, the influencing factors for prognosis were age, sex, and source of cases (Table 6).
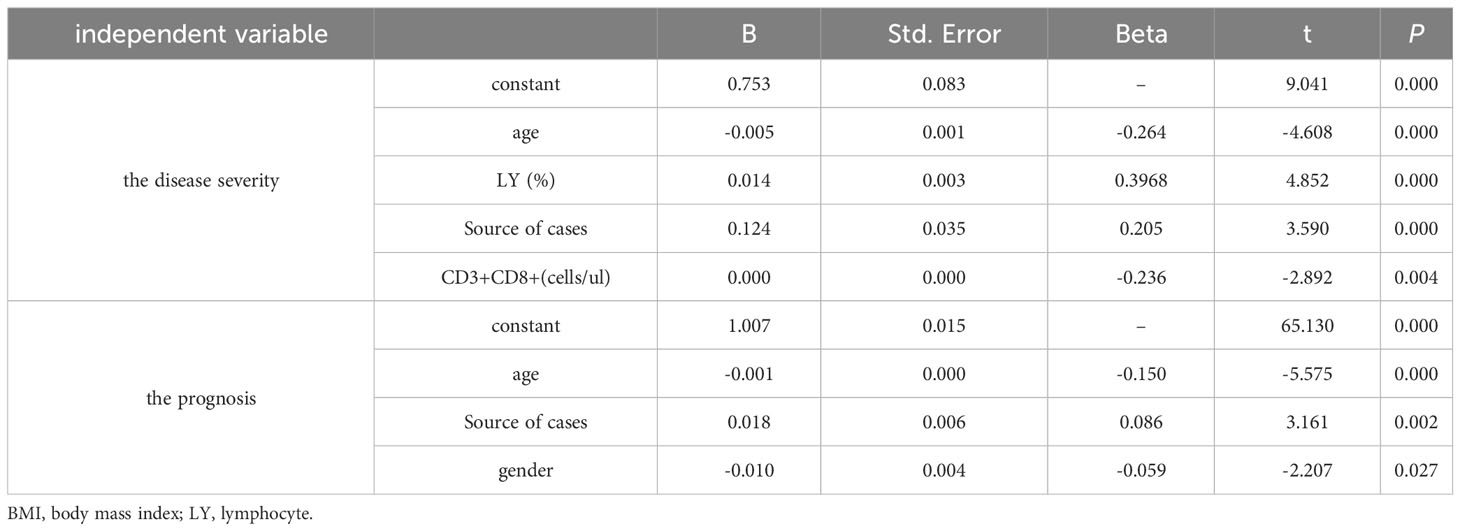
Table 6 Multiple stepwise regression analysis of influencing factors of disease severity and prognosis (n=95).
According to the ROC analysis, the total antibody, IgM and IgG levels showed good utility for predicting critical COVID-19 patients (Table 7). The areas under the curve of total antibody, IgM and IgG for disease severity were 0.854, 0.904 and 0.794, respectively (Table 7, Figure 5). The sensitivities were 98.10%, 76.40%, 96.10%, while the specificities were 75.00%, 100.00%, 75.00% (Table 7).
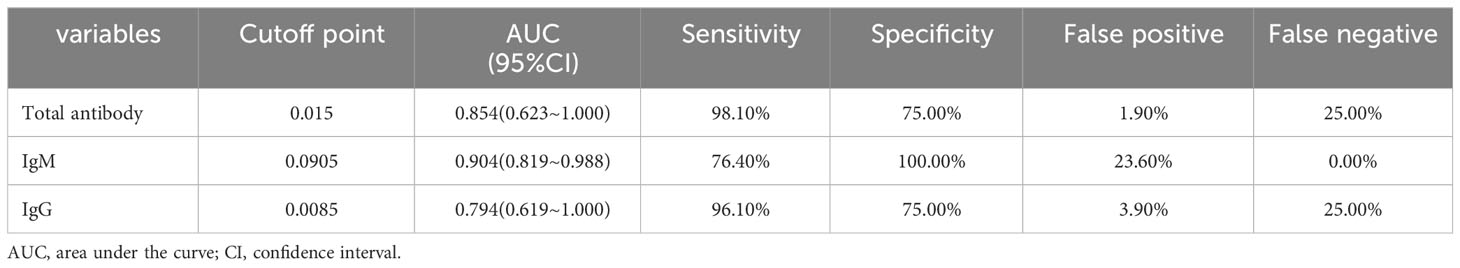
Table 7 The performance of various methods for distinguishing between critical cases and non-critical cases (n=1513).
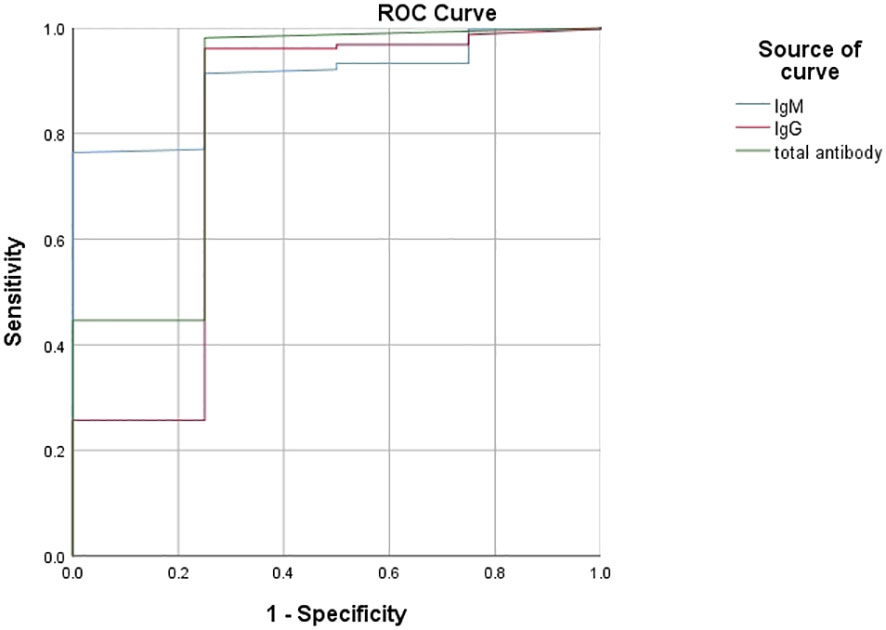
Figure 5 Using characteristics of antibody levels for discriminating the critical cases from the noncritical patients (n=1513; critical and noncritical groups, n=42 and 1471, respectively). ROC analysis showing the performance of antibody levels in distinguishing critical cases from noncritical patients. ROC, receiver operating characteristic curve.
In the post epidemic era, breakthrough infections of SARS-CoV-2 are being increasingly observed worldwide due to the high pervasiveness of viral spread, the emergence of novel variants (11), progressive ease of restrictive measures and waning protection against infection. In this retrospective study, we found that the critical illness rate was 2.8%, and the mortality rate was 0.5%, both of which were low, which was consistent with our previous report (16–19). We also found that the immune breakthrough rate in this study cohort was 27.2%, with 4.8% of patients having previous infections, 22.4% of patients having been vaccinated, and only 6% of patients having been fully vaccinated. This finding align with previous reports indicating that 4.0% to 5.3% of fully vaccinated individuals exhibit immune dysfunction (20, 21). A previous study reported that among fully vaccinated individuals, the incidence rate for COVID-19 breakthrough infection was 5.0 per 1000 person-months (20). Compared with partial vaccination, the incidence rate for COVID-19 breakthrough infection in full vaccination was associated with a 28% reduced risk (20). The reinfection rate among patients with COVID-19 is estimated to range from 2.3% to 21.4%, as indicated by a meta-analysis (22). The reinfection rate in our study was also in the range.
We reported the differences in disease severity and prognosis and in T lymphocyte subsets and antibody levels between different immune statuses to investigate the impact of immune breakthroughs on disease progression and prognosis in patients with COVID-19. We found that patients with preexisting immunity by previous infection or vaccination had a lower severe rate and mortality rate than those with primary infection, with all fatalities occurring exclusively among patients without previous immune protection. The results align with the findings reported in the existing literature (23–25). Since the hypoxemia secondary to infection occurred in the no immunity group, the proportion of patients with chronic obstructive pulmonary disease and cardiovascular disease was also higher in the no immunity group, suggesting that the infection combined with cardiopulmonary system diseases may be the main factor for the development of severe illness. Many studies have reported that patients with preexisting immunity have milder illnesses and lower rates of hospitalization (23, 24). Immune breakthrough patients were significantly less likely to experience severe disease or death than matched unvaccinated patients (25). With only one severe case and no deaths in preexisting immunity patients, we were unable to analyze differences in severe and mortality rates according to route of immunization. However, it also implies that pre-existing immunity diminishes the probability of progressing to severe illness following reinfection.
Similar to previous studies (3, 6), we observed higher levels of T-lymphocytes and antibodies in non-critical cases. We also found that patients with preexisting immunity by previous infection or vaccination exhibited shorter coronavirus negative conversion times and higher T lymphocyte subsets and antibody levels. SARS-CoV-2 infection is associated with lymphopenia, particularly in CD4+ T cells and CD8+ T cells (26), indicating abnormal immune function during SARS-CoV-2 infection (27). Failure to generate a timely T-cell response during natural SARS-CoV-2 infection has been linked to the development of severe COVID-19 cases (4). The immune memory generated by vaccination or natural infection serves as a reservoir of protective immunity that can rapidly expand upon re-exposure to the virus, potentially limiting viral replication during the early stages of infection (28). In addition to mitigating the severity of infection, breakthrough infections are less infectious than primary infections (29).
Preexisting immunity is associated with four major components of immunological memory: memory CD4+ T cells, memory CD8+ T cells, antibodies, and memory B cells (4). Both SARS-CoV-2-specific CD4+ and CD8+ memory T cells peaked within the initial month of infection, followed by a gradual decline over the subsequent 6 to 7 months (30). The CD4+ and CD8+ T-cell responses exhibited comparable levels between mRNA vaccination and infection, with similar T-cell production observed at both 6 months after the second dose and 6 months post-infection (31). Patricia (32) et al. found that the peak T-cell response 2 weeks after full vaccination was comparable to the peak response in mild and moderate patients. However, varying vaccine types, doses and intervals resulted in different immune memory statuses (31). In a retrospective study from Israel, in individuals who received the Pfizer mRNA vaccine (never infected), higher initial antibody levels were followed by a more rapid decline than in those with SARS-CoV-2 virus infection (33). Failure to generate sufficient IgG antibodies is linked to reduced survival (34). In contrast to our findings, some studies showed higher levels of antibodies (IgM and IgG) in severe patients than in mild-to-moderate patients (35–37). These results indicated that, in addition to antiviral efficacy, antibody responses might be associated with secondary antibody-mediated organ damage (36). Due to our subjects were mainly non critical patients, the lymphocyte and antibody levels of critical patients were not analyzed.
In addition, we further analyzed the different immune statuses of the three preexisting immune routes: natural immunity, acquired immunity and mixed immunity. The T-lymphocyte subsets exhibited the highest level one month after infection, surpassing that of the other two groups. Total antibody and IgG levels were the highest at admission and decreased slowly over the following month. Levels of anti-S1 IgG production were much higher in vaccinated individuals than in naturally infected individuals within three months of vaccination. Many studies have shown that vaccination induces a more robust and targeted immune response compared to the natural infection (32, 38). Therefore, the optimal approach to combat COVID-19 is to enhance immune function through vaccination. However, many studies have shown that mixed immunization appears to confer a more robust protective effect (39, 40). Our study also confirmed this view, with the shortest length of hospitalization and the shortest coronavirus negative conversion time results in the mixed immunization group (Table 4). A single dose of vaccine elicited higher memory T and B-cell responses in previously infected individuals (41). Due to the existence of immune memory, previously infected individuals produce more rapid and lasting cellular and humoral immunity after vaccination (42, 43). Further research found that there were significantly higher levels of antibodies in fully vaccinated individuals with natural immunity than in fully vaccinated individuals without prior infection. Vaccination after previous infection appeared to enhance and prolong immunity, even more than 1 year after the initial infection, with no sign of weakening (39). These studies suggest that in the post pandemic era, we can still fight the virus through a combination of natural immunity and vaccination.
The association between advanced age, a high number of comorbidities, and an unfavorable prognosis has been observed in previous study (16). The higher mortality rate observed in domestically transmitted cases is expected to be attributed to the limited extent of domestic transmission during the period of case collection. The large difference in the number of critical and noncritical patients (1471 vs 42) may have biased the results of the ROC analysis, but in combination with the results of the univariate analysis, it can be inferred that the antibody level is a reliable indicator of prognosis. Consistent with previous findings (5, 6, 8), critical and dead cases exhibited lower levels of T-lymphocyte subsets and antibodies, which served as reliable indicators for disease progression.
However, there are still some limitations of this study. Importantly, it was a single-center, retrospective study, and all the inherent limitations of retrospective studies are unavoidable. The incidence of critical cases, especially deaths, was minimal. Additionally, reinfected cases were extensively interviewed after a time interval, allowing the possibility of recall bias. Due to the information restrictions, the data related to the strains are in the CDC, which we cannot get. We can only infer from the time that the strains of our patients’ infection range from the original strain SARS-CoV-2 to Delta variant (B.1.617.2) and omicron (B.1.1.529). In addition, vaccine types were not available and the sequencing of vaccination and infection was not distinguished, no further analysis could be conducted.
Currently, reported cases do not accurately represent the infection rate due to a global reduction in testing and reporting, but the World Health Organization still reports more than 1 million new positive cases every four weeks. Hence, our findings serve as a valuable reference for predicting disease progression and treating patients with COVID-19 by monitoring changes in lymphocyte subsets and antibodies. The immune breakthrough group had lower rates of critical disease and mortality compared to the no immunity group, while the mixed immunity group showed highest levels of T-lymphocyte subsets and antibodies. Therefore, vaccination should be intensified to enhance the protective effect even in the post pandemic era, when most people have already been infected.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study was approved by the Public and Health Clinical Centre of Chengdu Ethics Committee (ethics approval number: PJ-K2020-26-31 01). Written informed consent was waived by the Ethics Commission of the designated hospital because this study was related to emerging infectious diseases.
Concept and design: YW, BZ, XinZ, XiaZ, FG, XY, XR, ML, DL; Data acquisition: YW, BZ, XinZ, XiaZ, FG, XY, XR, ML, DL; data analysis and interpretation: YW, BZ, XinZ, XiaZ, FG, XY, XR, ML, DL; Drafting manuscript: YW, BZ, XinZ, XiaZ, FG, XY, XR, ML, DL; administrative, technical, or material support: YW, BZ, XinZ, XiaZ, FG, XY, XR, ML, DL; study supervision: DL. All authors contributed to the article and approved the submitted version.
This research was supported by the Thirteenth Five-Year Project on Tackling Key Problems of National Science and Technology (2017ZX10305501008), the Nonprofit Central Research Institute Fund of the Chinese Academy of Medical Sciences (2020-PT330-005), the Sichuan Science and Technology Program (2020YFS0564), The Chengdu Municipal Science and Technology Bureau Science and Technology Huimin Major Demonstration Project (00092), the Sichuan Province Health Commission (17PJ070), the Chengdu Municipal Health Commission (2019079), and the Chengdu Science and Technology Bureau (2021-YF05-00536-SN).
We thank Dr. Xiu Li and Yaling Liu (Public Health Clinic Center of Chengdu, rehabilitation division).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Du Toit A. Outbreak of a novel coronavirus. Nat Rev Microbiol (2020) 18(3):123–. doi: 10.1038/s41579-020-0332-0
2. World Health O. Covid-19 Weekly Epidemiological Update, Edition 138, 13 April 2023. Geneva: World Health Organization (2023).
3. Zhang P, Du W, Yang T, Zhao L, Xiong R, Li Y, et al. Lymphocyte subsets as a predictor of severity and prognosis in Covid-19 patients. Int J Immunopathology Pharmacol (2021) 35:20587384211048567. doi: 10.1177/20587384211048567
4. Sette A, Crotty S. Adaptive immunity to Sars-Cov-2 and Covid-19. Cell (2021) 184(4):861–80. doi: 10.1016/j.cell.2021.01.007
5. Zhao Q, Meng M, Kumar R, Wu Y, Huang J, Deng Y, et al. Lymphopenia is associated with severe Coronavirus Disease 2019 (Covid-19) infections: A systemic review and meta-analysis. Int J Infect Dis (2020) 96:131–5. doi: 10.1016/j.ijid.2020.04.086
6. Pan P, Du X, Zhou Q, Cui Y, Deng X, Liu C, et al. Characteristics of lymphocyte subsets and cytokine profiles of patients with Covid-19. Virol J (2022) 19(1):57. doi: 10.1186/s12985-022-01786-2
7. Racine R, Winslow GM. Igm in microbial infections: taken for granted? Immunol Lett (2009) 125(2):79–85. doi: 10.1016/j.imlet.2009.06.003
8. Vengesai A, Midzi H, Kasambala M, Mutandadzi H, Mduluza-Jokonya TL, Rusakaniko S, et al. A systematic and meta-analysis review on the diagnostic accuracy of antibodies in the serological diagnosis of Covid-19. Systematic Rev (2021) 10(1):155. doi: 10.1186/s13643-021-01689-3
9. Nordström P, Ballin M, Nordström A. Risk of Sars-Cov-2 reinfection and Covid-19 hospitalisation in individuals with natural and hybrid immunity: A retrospective, total population cohort study in Sweden. Lancet Infect Dis (2022) 22(6):781–90. doi: 10.1016/S1473-3099(22)00143-8
10. Tillett RL, Sevinsky JR, Hartley PD, Kerwin H, Crawford N, Gorzalski A, et al. Genomic evidence for reinfection with Sars-Cov-2: A case study. Lancet Infect Dis (2021) 21(1):52–8. doi: 10.1016/S1473-3099(20)30764-7
11. Wang J, Kaperak C, Sato T, Sakuraba A. Covid-19 reinfection: A rapid systematic review of case reports and case series. J Invest Med (2021) 69(6):1253–5. doi: 10.1136/jim-2021-001853
12. Jiang XL, Wang GL, Zhao XN, Yan FH, Yao L, Kou ZQ, et al. Lasting antibody and T cell responses to Sars-Cov-2 in Covid-19 patients three months after infection. Nat Commun (2021) 12(1):897. doi: 10.1038/s41467-021-21155-x
13. Kucinskaite-Kodze I, Simanavicius M, Simaitis A, Zvirbliene A. Persistence of Sars-Cov-2-specific antibodies for 13 months after infection. Viruses-Basel (2021) 13(11):2123. doi: 10.3390/v13112313
14. Kent SJ, Khoury DS, Reynaldi A, Juno JA, Wheatley AK, Stadler E, et al. Disentangling the relative importance of T cell responses in Covid-19: leading actors or supporting cast? Nat Rev Immunol (2022) 22(6):387–97. doi: 10.1038/s41577-022-00716-1
15. Diagnosis and treatment protocol for novel coronavirus pneumonia (Trial Version 7). Chin Med J (Engl) (2020) 133(9):1087–95. doi: 10.1097/CM9.0000000000000819
16. Liu DF, Zheng YL, Kang J, Wang DM, Bai L, Mao Y, et al. Not only high number and specific comorbidities but also age are closely related to progression and poor prognosis in patients with Covid-19. Front Med (2022) 8:736109. doi: 10.3389/fmed.2021.736109
17. Liu DF, Wang Y, Zhao BN, Lan LJ, Liu YL, Bao L, et al. Overall reduced lymphocyte especially T and B subsets closely related to the poor prognosis and the disease severity in severe patients with Covid-19 and diabetes mellitus. Diabetol Metab Syndrome (2021) 13(1):5. doi: 10.1186/s13098-020-00622-3
18. Zhou YY, Chen Y, Zhang XY, Zhao BN, Gao FJ, Yuan XY, et al. Nutritional risk and a high Nrs2002 score are closely related to disease progression and poor prognosis in patients with Covid-19. Front Nutr (2023) 10:1089972. doi: 10.3389/fnut.2023.1089972
19. Liu DF, Lan LJ, Luo DX, Zhao BN, Wei G, He YS, et al. Lymphocyte subsets with the lowest decline at baseline and the slow lowest rise during recovery in Covid-19 critical illness patients with diabetes mellitus. Diabetes Res Clin Pract (2020) 167:108341. doi: 10.1016/j.diabres.2020.108341
20. Sun J, Zheng QL, Madhira V, Olex AL, Anzalone AJ, Vinson A, et al. Association between immune dysfunction and Covid-19 breakthrough infection after Sars-Cov-2 vaccination in the us. JAMA Internal Med (2022) 182(2):153–62. doi: 10.1001/jamainternmed.2021.7024
21. Khoury J, Najjar-Debbiny R, Hanna A, Jabbour A, Abu Ahmad Y, Saffuri A, et al. Covid-19 vaccine-long term immune decline and breakthrough infections. Vaccine (2021) 39(48):6984–9. doi: 10.1016/j.vaccine.2021.10.038
22. Piri SM, Edalatfar M, Shool S, Jalalian MN. Tavakolpour S. A systematic review on the recurrence of Sars-Cov-2 virus: frequency, risk factors, and possible explanations. Infect Dis (2021) 53(5):315–24. doi: 10.1080/23744235.2020.1871066
23. Pilz S, Theiler-Schwetz V, Trummer C, Krause R, Ioannidis JPA. Sars-Cov-2 reinfections: overview of efficacy and duration of natural and hybrid immunity. Environ Res (2022) 209:112911. doi: 10.1016/j.envres.2022.112911
24. Fabianova K, Kyncl J, Vlckova I, Jirincova H, Kostalova J, Liptakova M, et al. Covid-19 reinfections. Epidemiologie Mikrobiologie Imunologie (2021) 70(1):62–7.
25. Butt AA, Nafady-Hego H, Chemaitelly H, Abou-Samra AB, Al Khal A, Coyle PV, et al. Outcomes among Patients with Breakthrough Sars-Cov-2 Infection after Vaccination. Int J Infect Dis (2021) 110:353–8. doi: 10.1016/j.ijid.2021.08.008
26. Vafaeinezhad A, Atashzar MR, Baharlou R. The immune responses against coronavirus infections: friend or foe? Int Arch Allergy Immunol (2021) 182(9):863–76. doi: 10.1159/000516038
27. Bobcakova A, Petriskova J, Vysehradsky R, Kocan I, Kapustova L, Barnova M, et al. Immune profile in patients with Covid-19: lymphocytes exhaustion markers in relationship to clinical outcome. Front Cell Infection Microbiol (2021) 11:646688. doi: 10.3389/fcimb.2021.646688
28. Jordan SC. Innate and adaptive immune responses to Sars-Cov-2 in humans: relevance to acquired immunity and vaccine responses. Clin Exp Immunol (2021) 204(3):310–20. doi: 10.1111/cei.13582
29. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, Tang P, Coyle P, Hasan MR, et al. Relative infectiousness of Sars-Cov-2 vaccine breakthrough infections, reinfections, and primary infections. Nat Commun (2022) 13(1):532. doi: 10.1038/s41467-022-28199-7
30. Cohen KW, Linderman SL, Moodie Z, Czartoski J, Lai LL, Mantus G, et al. Longitudinal analysis shows durable and broad immune memory after Sars-Cov-2 Infection with persisting antibody responses and memory B and T cells. Cell Rep Med (2021) 2(7):100354. doi: 10.1016/j.xcrm.2021.100354
31. S ette A, Crotty S. Immunological memory to Sars-Cov-2 infection and Covid-19 vaccines. Immunol Rev (2022) 310(1):27–46. doi: 10.1111/imr.13089
32. Almendro-Vazquez P, Laguna-Goya R, Ruiz-Ruigomez M, Utrero-Rico A, Lalueza A, de la Calle GM, et al. Longitudinal dynamics of Sars-Cov-2-specific cellular and humoral immunity after natural infection or Bnt162b2 vaccination. PloS Pathog (2021) 17(12):e1010211. doi: 10.1371/journal.ppat.1010211
33. Israel A, Shenhar Y, Green I, Merzon E, Golan-Cohen A, Schaeffer AA, et al. Large-scale study of antibody titer decay following Bnt162b2 mrna vaccine or Sars-Cov-2 infection. Vaccines (2022) 10(1):64. doi: 10.3390/vaccines10010064
34. Nairz M, Sahanic S, Pizzini A, Bohm A, Tymoszuk P, Mitterstiller AM, et al. Quantity of igg response to Sars-Cov-2 spike glycoprotein predicts pulmonary recovery from Covid-19. Sci Rep (2022) 12(1):3677. doi: 10.1038/s41598-022-07489-6
35. Liu XT, Zheng X, Liu B, Wu MX, Zhang ZL, Zhang GC, et al. Serum Igm against Sars-Cov-2 Correlates with in-Hospital Mortality in Severe/Critical Patients with Covid-19 in Wuhan, China. Aging-Us (2020) 12(13):12432–40. doi: 10.18632/aging.103417
36. Zhang BC, Zhou XY, Zhu CL, Song YX, Feng F, Qiu YR, et al. Immune phenotyping based on the neutrophil-to-lymphocyte ratio and igg level predicts disease severity and outcome for patients with Covid-19. Front Mol Biosci (2020) 7:157. doi: 10.3389/fmolb.2020.00157
37. Korobova ZR, Zueva EV, Arsentieva NA, Batsunov OK, Liubimova NE, Khamitova IV, et al. Changes in anti-Sars-Cov-2 igg subclasses over time and in association with disease severity. Viruses-Basel (2022) 14(5):941. doi: 10.3390/v14050941
38. Samanovic MI, Oom AL, Cornelius AR, Gray-Gaillard SL, Karmacharya T, Tuen M, et al. Vaccine-acquired Sars-Cov-2 immunity versus infection-acquired immunity: A comparison of three Covid-19 vaccines. Vaccines (2022) 10(12):2152. doi: 10.3390/vaccines10122152
39. Hall V, Foulkes S, Insalata F, Kirwan P, Saei A, Atti A, et al. Protection against Sars-Cov-2 after Covid-19 Vaccination and Previous Infection. New Engl J Med (2022) 386(13):1207–20. doi: 10.1056/NEJMoa2118691
40. Vicenti I, Basso M, Gatti F, Scaggiante R, Boccuto A, Zago D, et al. Faster decay of neutralizing antibodies in never infected than previously infected healthcare workers three months after the second Bnt162b2 mrna Covid-19 vaccine dose. Int J Infect Dis (2021) 112:40–4. doi: 10.1016/j.ijid.2021.08.052
41. Sasikala M, Shashidhar J, Deepika G, Ravikanth V, Krishna VV, Sadhana Y, et al. Immunological memory and neutralizing activity to a single dose of Covid-19 vaccine in previously infected individuals. Int J Infect Dis (2021) 108:183–6. doi: 10.1016/j.ijid.2021.05.034
42. Gobbi F, Buonfrate D, Moro L, Rodari P, Piubelli C, Caldrer S, et al. Antibody response to the Bnt162b2 mrna Covid-19 vaccine in subjects with prior Sars-Cov-2 infection. Viruses-Basel (2021) 13(3):422. doi: 10.3390/v13030422
Keywords: coronavirus disease 2019 (COVID-19), immune breakthroughs, vaccination, previous infection, disease progression, prognosis
Citation: Wang Y, Zhao B, Zhang X, Zhang X, Gao F, Yuan X, Ren X, Li M and Liu D (2023) How immune breakthroughs could slow disease progression and improve prognosis in COVID-19 patients: a retrospective study. Front. Immunol. 14:1246751. doi: 10.3389/fimmu.2023.1246751
Received: 26 June 2023; Accepted: 04 October 2023;
Published: 23 October 2023.
Edited by:
Massimo Pieri, University of Rome Tor Vergata, ItalyReviewed by:
Eleonora Nicolai, University of Rome Tor Vergata, ItalyCopyright © 2023 Wang, Zhao, Zhang, Zhang, Gao, Yuan, Ren, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dafeng Liu, bGRmMzEyQDEyNi5jb20=; Maoquan Li, OTE3NjA0NDEyQHFxLmNvbQ==
†These authors have contributed equally to this work
‡ORCID: Dafeng Liu, orcid.org/0000-0002-6792-641X
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.