
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 06 September 2023
Sec. Vaccines and Molecular Therapeutics
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1244373
 Nani Xu1†
Nani Xu1† Yu Xu2†
Yu Xu2† Rongrong Dai3†
Rongrong Dai3† Lin Zheng1†
Lin Zheng1† Pan Qin1
Pan Qin1 Peng Wan2
Peng Wan2 Yejing Yang1
Yejing Yang1 Jianmin Jiang4,5
Jianmin Jiang4,5 Hangjie Zhang4,5*
Hangjie Zhang4,5* Xiaowei Hu1*
Xiaowei Hu1* Huakun Lv4,5*
Huakun Lv4,5*Introduction: China experienced a record surge of coronavirus disease 2019 cases in December 2022, during the pandemic.
Methods: We conducted a randomized, parallel-controlled prospective cohort study to evaluate efficacy and antibody duration after a fourth-dose booster with Ad5-nCoV or inactivated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine.
Results: A total of 191 participants aged ≥18 years who had completed a three-dose regimen of the inactivated SARS-CoV-2 vaccine 6 months earlier were recruited to receive the intramuscular Ad5-nCoV booster or the inactivated SARS-CoV-2 vaccine. The Ad5-nCoV group had significantly higher antibody levels compared with the inactivated vaccine group at 6 months after the fourth vaccination dose. After the pandemic, the breakthrough infection rate for the Ad5-nCoV and the inactivated vaccine groups was 77.89% and 78.13%, respectively. Survival curve analysis (p = 0.872) and multivariable logistic regression analysis (p = 0.956) showed no statistically significant differences in breakthrough infection between the two groups.
Discussion: Compared with a homologous fourth dose, a heterologous fourth dose of Ad5-nCoV elicited a higher immunogenic response in healthy adults who had been immunized with three doses of inactivated vaccine. Nevertheless, the efficacy of the two vaccine types was equivalent after the pandemic.
On 30 January 2020, the World Health Organization declared that the coronavirus disease 2019 (COVID-19) outbreak constituted a public health emergency of international concern (1). To date, 768.0 million confirmed cases and 6.9 million deaths have been recorded globally (2). Despite the administration of 13.39 billion vaccine doses (2), the COVID-19 epidemic has not yet resolved. On 27 January 2023, the World Health Organization determined that the ongoing COVID-19 pandemic continued to constitute a public health emergency of international concern (3). Emerging variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that are capable of escaping an immune attack have reduced the protection conferred by vaccines (4–7). In addition, the effectiveness of the vaccine, which included a two-dose regimen and a booster (third dose) vaccine, has declined over time (8–11).
Studies in Canada, Singapore, and other countries have shown that a second booster immunization provides additional protection against COVID-19 and reduces severe illness and death (12–16). More than 71.7% of the Chinese population has been immunized with three doses of inactivated SARS-CoV-2 vaccine (17). Previous studies in China have shown that the efficacy of the first booster dose of Ad5-nCoV (heterologous booster) is superior to that of inactivated vaccine (homologous booster) (18, 19). Furthermore, a heterologous fourth dose with either aerosolized Ad5-nCoV or intramuscular Ad5-nCoV was safe and highly immunogenic in healthy adults who were previously immunized with three doses of the CoronaVac vaccine within 28 days (20). However, antibody levels following a fourth dose of Ad5-nCoV at 6 months after vaccination with three doses of inactivated SARS-CoV-2 vaccine have not yet been assessed.
On 7 December 2022, the Chinese government issued the “New ten measures” as an adjustment to its “Twenty measures” COVID-19 prevention and control policy released on 11 November 2022 (21). As a result, the COVID-19 epidemic in China changed dramatically. On 1 February 2023, the Chinese Center for Disease Control and Prevention reported an overall infection rate of 87.54% based on data reported between 9 December 2022 and 30 January 2023 (22, 23). In this context, assessing the real-world breakthrough infection rate after the fourth dose of immunization with Ad5-nCoV or inactivated SARS-CoV-2 vaccine is important.
This parallel-controlled prospective cohort study aimed to investigate antibody levels and breakthrough infection after a fourth immunization dose of Ad5-nCoV or inactivated SARS-CoV-2 vaccine.
A single-center, randomized, parallel-controlled prospective cohort study of a second booster dose (or fourth vaccine dose) with a heterologous booster (Ad5-nCoV) or a homologous booster (inactivated SARS-CoV-2 vaccine) was conducted in Xihu District, Hangzhou City, Zhejiang Province, China. Participants aged ≥18 years with stable medical conditions who had completed a three-dose regime of the inactivated SARS-CoV-2 vaccine (CoronaVac or Covilo) 6 months earlier were recruited from the community.
A screening visit before the participants were enrolled allowed exclusion of the following conditions: history of infection with SARS-CoV-2; pregnant or lactating; use of immunosuppressives; fever; history of severe anaphylaxis to vaccines; severe and/or uncontrolled respiratory disease and cardiovascular disease; hypertension (systolic pressure ≥ 180 mmHg/diastolic pressure ≥ 110 mmHg); diabetes; neurologic illness; and other underlying diseases that could interfere with the evaluation of the primary study endpoints. Previous SARS-CoV-2 infection history was confirmed with participant recall and by checking participants’ recent medical visits.
The Research Ethics Committee of the Zhejiang Provincial Center of Disease Control and Prevention reviewed and approved the study protocol. The informed consent form was signed by all participants before enrolment.
In May 2022, 200 eligible participants were randomly assigned to receive one dose of Ad5-nCoV (Convidecia, 0.5 mL of 5 × 1010) viral particles) or inactivated vaccine (CoronaVac or Covilo, 0.5 mL) via intramuscular injection. Randomization was conducted using a sealed envelope system in which the number associated with each vaccine group was displayed at a 1:1 ratio. The type of inactivated vaccine administered as the fourth dose was matched to the vaccine type each participant had already received as a first booster dose (or third dose).
Blood samples were collected from each participant at baseline, before participants received the fourth dose of vaccination and again 6 months later, in December 2022. A commercial anti–SARS-CoV-2 RBD IgG ELISA kit (Vazyme Medical Technology, Nanjing, China) was used to measure the wild-type SARS-CoV-2 Receptor Binding Domain (RBD)-specific IgG response. The Reed–Muench method was also used to assess the levels of neutralizing antibody against the Omicron BA.4/5 subvariant using a pseudovirus-neutralization test, which consisted of a vesicular stomatitis virus pseudovirus system that expresses the spike glycoprotein (50% neutralization titer).
In March 2023, after the pandemic and approximately 10 months after the fourth vaccination dose, the participants were followed up by telephone to collect information on breakthrough infection with SARS-CoV-2.
The level of antibodies was presented as the geometric mean titer (GMT) and geometric mean fold increase (GMFI). The 95% confidence interval (CI) was calculated on the basis of the t-distribution of the log-transformed values back-transformed to the original scale. The Student’s t-test, Chi-squared test, or Fisher’s exact test was applied to analyze categorical data. A Cox model was used to estimate the cumulative probability of breakthrough infection. A multivariable logistic regression analysis was performed to test the adjusted association between the main independent variable, which was defined as the type (inactivated vaccine or Ad5-nCoV) of the fourth-dose vaccine, and 1) the seropositivity rates of anti-RBD IgG and neutralizing antibodies against the BA.4/5 pseudovirus and 2) the risk of breakthrough infection with SARS-CoV-2. P-values of less than 0.05 were considered statistically significant. All statistical analyses were conducted using SPSS Statistics (IBM Corporation, Armonk, NY, USA) and GraphPad Prism 9 (San Diego, CA, USA).
In total, 211 volunteers aged ≥18 years who had received three doses of inactivated vaccine (CoronaVac or Covilo) ≥6 months earlier were recruited and screened for eligibility in May 2022. A total of 201 participants were sequentially enrolled and randomly assigned to two groups. One participant withdrew voluntarily after randomization. In total, 200 participants received a fourth dose of Convidecia (treatment group, heterologous booster dose, n = 100) or CoronaVac/Covilo (control group, homologous booster dose, n = 100). Finally, 95 (treatment group) and 96 (control group) participants completed both the follow-up at 6 months after vaccination and the telephone follow-up in March 2023, after the pandemic (Figure 1).
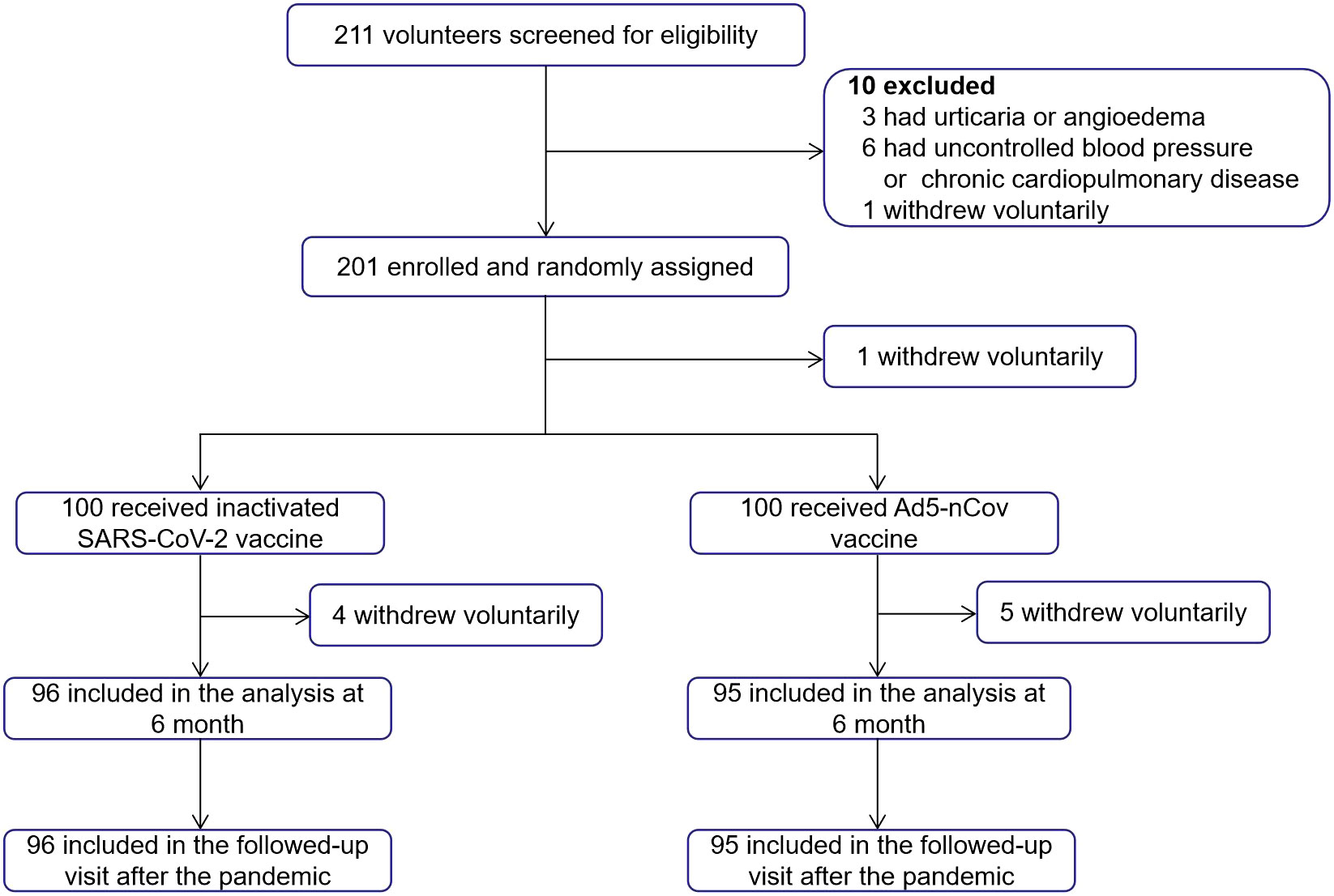
Figure 1 Trial profile. A total of 211 volunteers were recruited and screened for eligibility. Overall, 201 participants were enrolled and randomly assigned, 200 participants received the booster dose vaccination, and one participant withdrew voluntarily after randomization. Participants in each group (n = 96 and n = 95) completed both the planned visits at 6 months after vaccination and the telephone follow-up in March 2023.
Among the 191 participants who ultimately completed the follow-up, 87 (45.55%) were female and 56 (29.32%) had underlying chronic diseases. The mean patient age was 50.52 (standard deviation, 15.37) years. The mean time interval since the last booter dose of inactivated vaccine was 6.13 (standard deviation, 0.04) months. The baseline characteristics of the two vaccine groups were comparable (Table 1).
At enrollment and before receiving the second vaccine booster, the average GMT of anti-RBD IgG in the 191 participants was 61.78, and the GMT of neutralizing antibodies against the BA.4/5 pseudovirus was 31.68. No statistical difference was observed in the baseline antibody levels between the inactivated vaccine group and the Ad5-nCoV group (Figure 2).
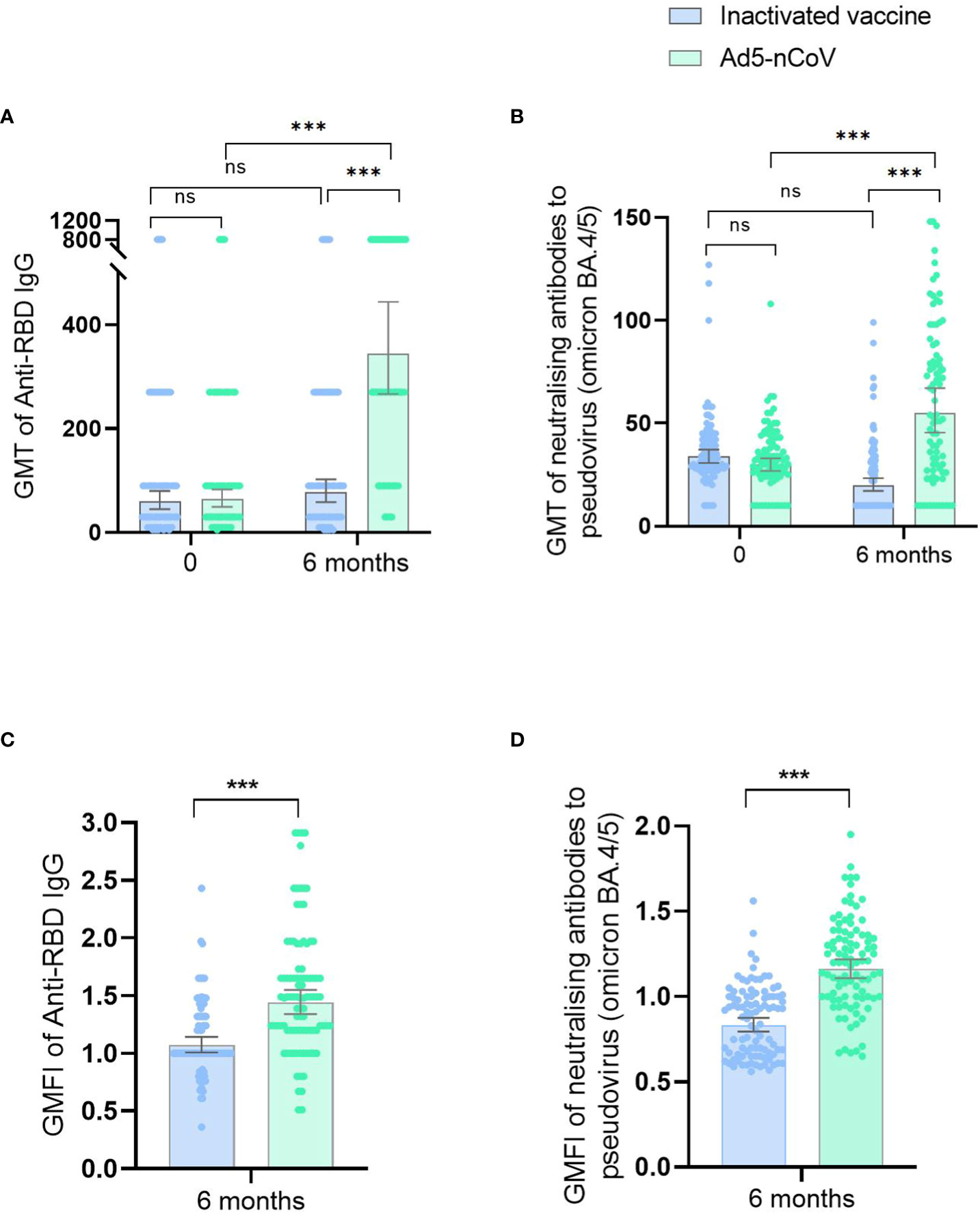
Figure 2 Antibody responses before and after booster vaccination. ID50 of SARS-CoV-2 RBD-specific IgG antibodies (A) and ID50 of pseudovirus-neutralizing antibodies against Omicron BA.4/5 (B) on day 0 (before booster vaccination) and at 6 months after booster vaccination in the inactivated SARS-CoV-2 vaccine group and the Ad5-nCoV group. ID50 of SARS-CoV-2 RBD-specific IgG antibodies (C) and ID50 of pseudovirus-neutralizing antibodies against Omicron BA.4/5 (D) at 6 months after booster vaccination in the inactivated SARS-CoV-2 vaccine group and the Ad5-nCoV group with different age subgroups. GMFIs of SARS-CoV-2 RBD-specific IgG antibodies (E) and GMFIs of pseudovirus-neutralizing antibodies against Omicron BA.4/5 (F) at 6 months after booster vaccination in the inactivated SARS-CoV-2 vaccine group and the Ad5-nCoV group. ns, no significance; *P < 0.05; **P < 0.01; ***P < 0.001.
Six months after the fourth vaccination dose, anti-RBD IgG GMTs were significantly higher in the Ad5-nCoV group (344.22; 95% CI, 266.65–444.35) than in the inactivated vaccine group (77.10; 95% CI, 58.24–102.05; p < 0.001). Similarly, the GMFI was higher for the Ad5-nCoV group (1.53) than that for the inactivated vaccine group (1.13; p < 0.001). GMTs for the neutralizing antibodies against the BA.4/5 pseudovirus were 55.26 (95% CI, 45.52–67.08) and 20.03(95% CI, 17.23–23.28) for the treatment and control groups, respectively (p < 0.001), and the GMFIs were 1.19 and 0.86 for the treatment and control groups, respectively (p < 0.001; Supplementary Table 1; Figure 2).
Compared with the baseline antibody levels before the fourth vaccination dose, no significant difference in the GMT of anti-RBD IgG was observed at 6 months after the fourth-dose vaccination in the inactivated vaccine group (p = 0.212); however, the GMT of neutralizing antibodies against the BA.4/5 pseudovirus was lower versus baseline (p < 0.001). In contrast, significantly higher GMTs of anti-RBD IgG (p < 0.001) and neutralizing antibodies against the BA.4/5 pseudovirus (p < 0.001) were observed compared with pre-immunization levels in the Ad5-nCoV group (Figure 2).
Compared with their younger peers, participants ≥60 years had significantly lower GMTs of anti-RBD IgG (p = 0.001) and neutralizing antibodies against the BA.4/5 pseudovirus (p = 0.015) in the inactivated vaccine group and lower GMTs of neutralizing antibodies against the BA.4/5 pseudovirus (p = 0.018) in the Ad5-nCoV group (Table 2; Figure 2). In the inactivated vaccine group, persons with underlying chronic diseases had lower GMTs of anti-RBD IgG (p = 0.033) compared with those without diseases (Table 2).
Between 13 December 2022 and 16 January 2023, 66.49% (127/191) of participants developed symptoms and had positive nucleic acid or antigen test results, 9.95% (19/191) of participants developed symptoms and had suspected infection without nucleic acid or antigen test results, 1.57% (3/191) of participants were asymptomatic and had positive nucleic acid or antigen test results, and 21.99% (42/191) were asymptomatic with or without nucleic acid or antigen test results. The breakthrough infection peak occurred between 20 December 2022 and 26 December 2022 counted 47.12% (90/191) during this time (Figure 3). The breakthrough infection rates for the Ad5-nCoV and the inactivated vaccine groups were 77.89% and 78.13%, respectively. Survival curve analysis adjusted for sex, age, chronic diseases, and influenza vaccine history showed no statistically significant difference in breakthrough infection between the two groups (p = 0.872; Figure 4).
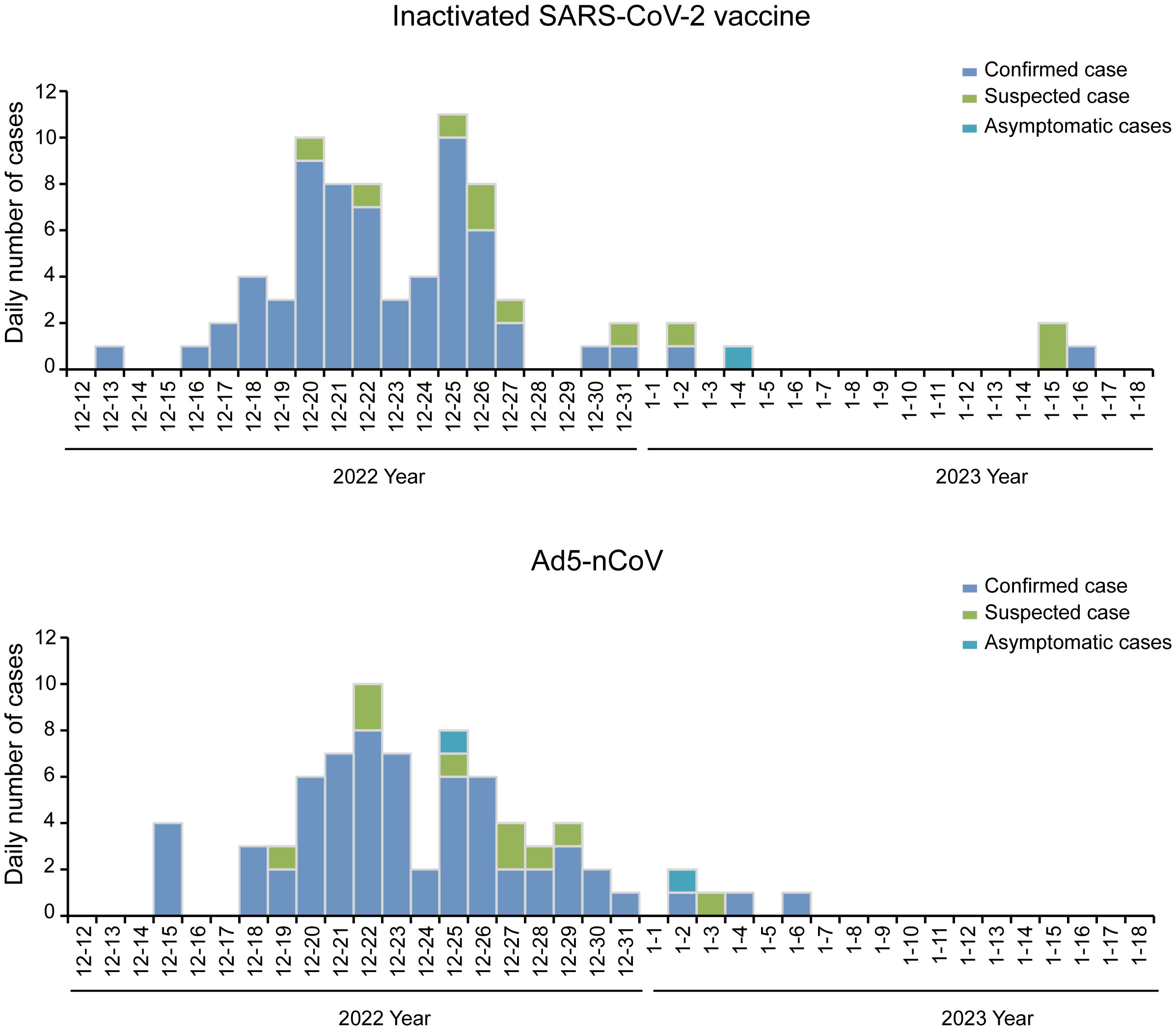
Figure 3 Epidemiologic curve of breakthrough cases. Daily numbers of confirmed cases, suspected cases, and asymptomatic cases. In March 2023, the participants were followed up by telephone to collect information on breakthrough infection. The breakthrough infection occurred from 13 December 2022 to 16 January 2023. Peak occurred between 20 December 2022 and 26 December 2022, counted 47.12% (90/191) during this time.
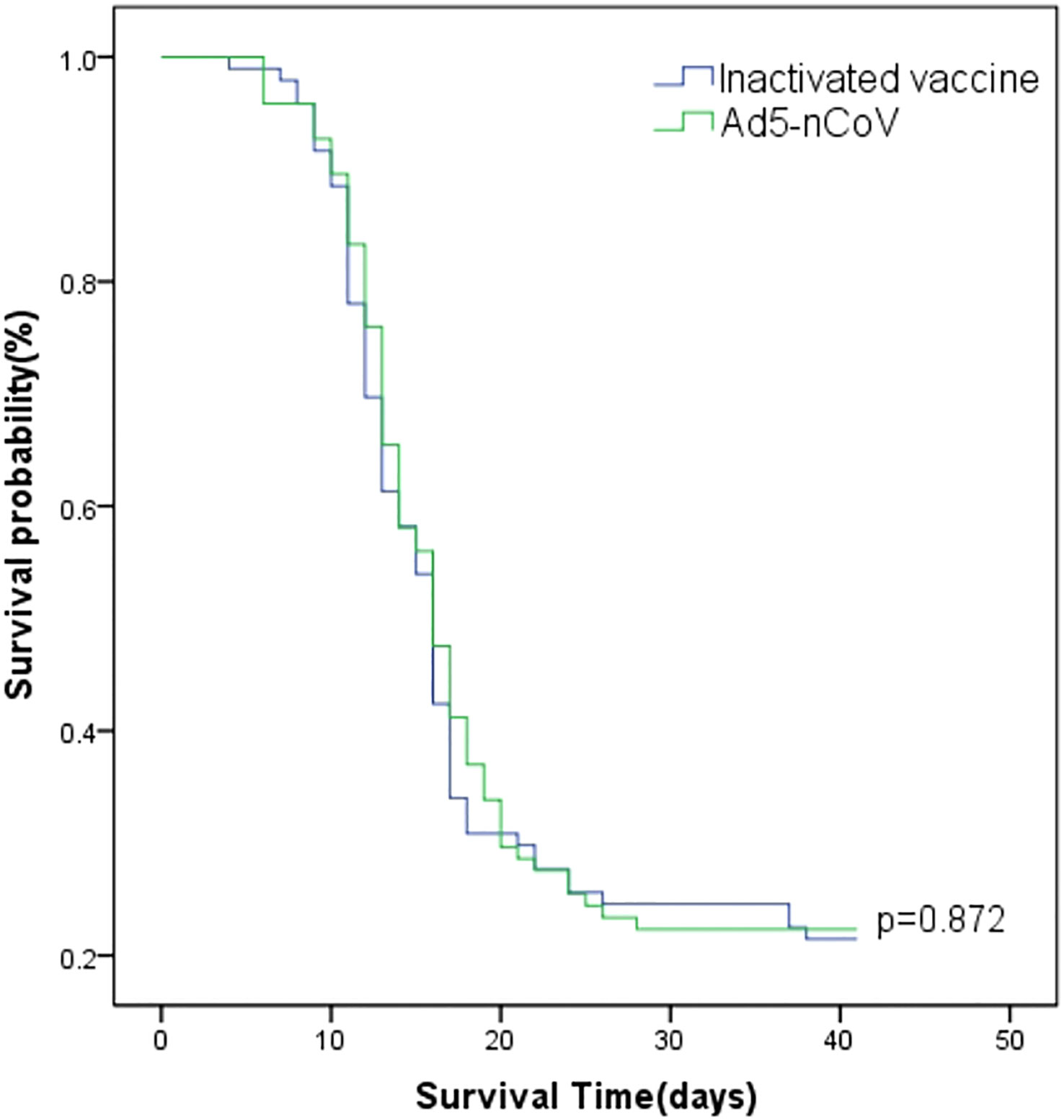
Figure 4 Survival curves of breakthrough infection. The Cox model estimate shows the cumulative probability of breakthrough infection by vaccination type adjusted for sex, age, chronic diseases, and influenza vaccine history, starting on 12 December 2022.
In total, 14.14% (27/191) of participants visited outpatient clinics for treatment and 50.26% (96/191) of participants significantly recovered from symptoms within 3 days. However, 1.05% (2/191) of participants (female, inactivated vaccine booster) had symptoms from which they had not significantly recovered more than 2 months after infection. No statistical differences were observed in the incidence of COVID-19 (p = 0.952), medical treatment situation (p = 0.601), or symptom recovery time (p = 0.784) between the two vaccine groups (Table 3). The most common symptom among all the participants was fever (63.35%, 121/191), followed by cough (50.79%, 97/191; Figure 5). Among all participants, 56.54% (108/191) had at least one of the general symptoms of tiredness/fatigue, headache/dizziness, overall aches, or chills; 60.73% (116/191) had at least one of the general symptoms of pharyngodynia, rhinobyon, nasal mucus discharge, cough, or expectoration; and 17.28% (33/191) had at least one of the digestive tract symptoms of change or decrease in taste or smell, diarrhea, nausea, emesis, or stomachache. No statistical difference in symptoms other than fever was observed between the two vaccine groups. The incidence of fever in the Ad5-nCoV group (54.74%) was lower than that in the inactivated vaccine group (71.88%, p = 0.014); the incidence of high fever especially was lower in the treatment (7.37%) versus the control 27.08%) group (p < 0.001; Table 3).

Figure 5 Breakthrough symptoms of the two vaccine groups. The incidence of fever in the Ad5-nCoV group (54.74%) was lower than that in the inactivated vaccine group (71.88%, p = 0.014); the incidence of high fever especially was lower in the Ad5-nCoV group (7.37%) than that in the inactivated vaccine (27.08%, p < 0.001). *p < 0.05; ***p < 0.001.
Participants aged 18–59 years in the inactivated vaccine group were more likely to have digestive tract symptoms compared with who aged ≥60 years (p = 0.011; Table 4). Those who exercised ≥3 h per week in the Ad5-nCoV group and participants with a history of influenza vaccine uptake in the inactivated vaccine group recovered significantly faster than who exercised <3 h per week (p = 0.035 and p = 0.038; Table 5).
A multivariable logistic regression analysis was performed to test the adjusted association between the main independent variable, defined as the type (inactivated vaccine or Ad5-nCoV) of the fourth vaccine dose vaccine and 1) the seropositivity rate of anti-RBD IgG and neutralizing antibodies against the BA.4/5 pseudovirus and 2) the risk of breakthrough infection with SARS-CoV-2. The association was tested after adjustment for sex, age, body mass index, exercise, sleep, underlying chronic disease, and influenza vaccination history.
Compared with the participants who received the inactivated vaccine as the fourth dose, the participants who received Ad5-nCoV experienced an increase in the seropositivity rate of anti-RBD IgG [relative risk (RR) = 8.58; 95% CI, 3.53–20.86] and neutralizing antibodies against the BA.4/5 pseudovirus (RR = 24.52; 95% CI, 6.62–90.87) at 6 months after the last vaccination and were at a lower risk of fever caused by SARS-CoV-2 (RR = 0.41; 95% CI, 0.22–0.78). Compared with their male counterparts, female participants were at a higher risk of fever (RR = 2.44; 95% CI, 1.23–4.83) and general symptoms (RR = 2.44; 95% CI, 1.06–3.87) caused by SARS-CoV-2 and at a higher risk of symptom recovery time ≥3 days (RR = 2.45; 95% CI, 1.27–4.70). Compared with participants aged 18–59 years, participants aged ≥60 years had a lower seropositivity rate of neutralizing antibodies against BA.4/5 pseudovirus (RR = 0.18; 95% CI, 0.05–0.64) and were at a lower risk of digestive tract symptoms (RR = 0.29; 95% CI, 0.10–0.85). Compared with participants with a body mass index <24.0 kg/m2, participants with a body mass index ≥24.0 kg/m2 had a higher risk of digestive tract symptoms (RR = 2.82; 95% CI, 1.12–7.14; Table 6).
Neutralizing antibodies are reported to act as a correlate of protection against COVID-19; therefore, a boost in these antibodies suggests an induced response associated with vaccine efficacy (24). Previous studies have shown that in individuals previously vaccinated with three doses of inactivated SARS-CoV-2 vaccine, heterologous regimens with intramuscular Ad5-nCoV induced significantly higher titers of neutralizing antibodies against both wild-type SARS-CoV-2 and the BA.4/5 pseudovirus at days 14 and 28 after vaccination than the homologous booster schedule with inactivated virus (20). The findings of our 10-month randomized, parallel-controlled prospective cohort study further indicated that the second heterologous booster with intramuscular Ad5-nCoV induced higher titers and seropositivity rates of anti-RBD IgG and neutralizing antibodies against the BA.4/5 pseudovirus than the second homologous booster with inactivated vaccine at 6 months after vaccination. Similarly, the third dose (first booster) of the heterologous vaccine with Ad5-nCoV induced a higher antibody response than the homologous vaccines (18, 19, 25, 26), indicating that the heterologous booster regimens containing Ad5-nCoV were superior to the homologous schedule with regard to the first and second boosters (third and fourth doses). Similar results have been reported that heterologous boosting resulted in more robust immune responses than homologous boosting with other COVID-19 vaccines such as mRNA-1273 (Moderna), BNT162b2 (Pfizer–BioNTech), AZD1222 (Astra Zeneca), (Ad26.COV2-S, Janssen), and ChAdOx1 nCoV-19 vaccine (AZD1222, AstraZeneca) (27–31).
The overall infection rate during the nationwide COVID-19 pandemic that followed the Chinese government’s “New ten measures” policy issued on 7 December 2022 (21) was estimated at 87.54% (22). By following participants who received a fourth-dose vaccination with Ad5-nCoV or the inactivated SARS-CoV-2 vaccine, we observed that 78.01% (149/191) of participants were infected or suspected to be infected with SARS-CoV-2. The breakthrough infection peak occurred between 20 December 2022 and 26 December 2022, consistent with the nationwide peak on 22 December 2022 (23). Furthermore, no differences were observed between the Ad5-nCoV booster group and the inactivated SARS-CoV-2 vaccine booster group in breakthrough infection rate, medical treatment situation, or symptom recovery time. However, to the best of our knowledge, ours was the first study to report that the fourth dose of Ad5-nCoV is associated with a lower incidence SARS-CoV-2–related fever than the inactivated vaccine booster, indicating that the heterologous booster regimen may moderately alleviate some symptoms of infection.
In addition, our study found that compared with male participants, female participants were at an increased risk of fever and general symptoms caused by SARS-CoV-2 and at a greater risk of symptom recovery time ≥3 days, supporting previous findings that the female sex is a risk factor for long COVID-19 (32). Vaccine responses are widely reported to be weaker in older adults, who experience immunosenescence and a more rapid waning of antibodies than younger people (33–35). We further observed that participants aged ≥60 years had a lower seropositivity rate of neutralizing antibodies against the BA.4/5 pseudovirus versus participants aged 18–59 years. However, no statistical difference in breakthrough infection was observed between the age groups (18–59 vs. ≥60 years). Higher age and body mass index were associated with an increased risk of digestive tract symptoms in our study. Digestive system involvement may protect patients with mild and moderate symptoms from lymphocyte depletion caused by SARS-CoV-2 (36); however, the relevant mechanism of action remains unclear.
Our study has several limitations. First, only adults with stable medical conditions over 18 years of age were recruited; the inclusion of people who require additional protection against COVID-19 such as immunocompromised individuals may have produced more marked results than those observed. Therefore, the recruitment criteria prevented our results from being representative of the general population. Second, because blank controls (i.e., participants who do not receive a fourth vaccination dose) were not recruited into the study, we could not assess the efficacy of a four-dose immunization regimen against breakthrough infection with SARS-CoV-2. Therefore, the protective effect of the second heterologous booster vaccination regimen remains uncertain. Third, the sample size was too small to investigate the number of potentially severe and fatal cases caused by SARS-CoV-2. A larger sample size would have increased statistical power to allow the identification of influencing factors among subpopulations. Fourth, information on breakthrough infection in the follow-up was based only on participant recall, introducing potential recall bias. Finally, live virus neutralization antibodies against wild-type SARS-CoV-2, Omicron BA.4/5, or other current Omicron subvariants such as BF.7, BQ.1, and XBB that could increase the generalizability of immunogenicity results were not used in our study and must be further explored.
In conclusion, a heterologous fourth dose with Ad5-nCoV caused higher antibody levels than a homologous fourth dose with the inactivated SARS-CoV-2 vaccine at 6 months after the last vaccination and decreased the risk of fever caused by SARS-CoV-2 in healthy adults who had been immunized with three doses of the inactivated vaccine. However, the two vaccine types showed equivalent efficacy after the pandemic. Our findings support the heterologous administration of Ad5-nCoV over the homologous administration of the inactivated SARS-CoV-2 vaccine. Furthermore, next-generation vaccines may be needed to provide better protection against COVID-19 by addressing the immune escape of SARS-CoV-2 variants.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by The Research Ethics Committee of the Zhejiang Provincial Center of Disease Control and Prevention (ethics code number: 2022-021-01). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
XH, HL, JJ, LZ, and HZ were the principal investigators who designed the research and coordinated the study; NX, PQ, YY, and RD led and participated in the site work, including the recruitment, follow-up, and data collection; PW and YX supervised the study; HZ was responsible for laboratory analyses; NX and HZ did the statistical analysis and wrote the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Key Research and Development Program of Zhejiang Province (2021C03200), the Key Program of Health Commission of Zhejiang Province/Science Foundation of National Health Commission (WKJ-ZJ-2221), the Major Program of Zhejiang Municipal Natural Science Foundation (LD22H190001), the Explorer Program of Zhejiang Municipal Natural Science Foundation (LQ23H100001), the General Plan for Agricultural and Social Development Research Projects of Hangzhou(20201203B27), and the General Project of Hangzhou Health Science and Technology Plan (B20230116).
We would like to thank the local designated hospitals for their help with the field survey.
YX and PW are employees of CanSino Biologics and contributed to the conceptualization of the study clinical protocol and electronic case report form design but did not participate in the analysis or interpretation of the data presented in the manuscript.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1244373/full#supplementary-material
1. Statement on the second meeting of the international health regulations (2005) emergency committee regarding the outbreak of novel coronavirus (2019-nCoV). (2020). Available at: https://www.who.int/news/item/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov).
2. Organization WH. (2023). Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
3. Organization WH. Statement on the fourteenth meeting of the international health regulations (2005) emergency committee regarding the coronavirus disease (COVID-19) pandemic. (2023). Available at: https://www.who.int/news/item/30-01-2023-statement-on-the-fourteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic.
4. Arashiro T, Arima Y, Muraoka H, Sato A, Oba K, Uehara Y, et al. Covid-19 vaccine effectiveness against symptomatic sars-Cov-2 infection during delta-dominant and omicron-dominant periods in japan: A multi-center prospective case-control study (Fascinate study). Clin Infect Dis (2022) 76(3):e108–15. doi: 10.1093/cid/ciac635
5. Gram MA, Emborg HD, Schelde AB, Friis NU, Nielsen KF, Moustsen-Helms IR, et al. Vaccine effectiveness against sars-Cov-2 infection or covid-19 hospitalization with the alpha, delta, or omicron sars-Cov-2 variant: A nationwide danish cohort study. PloS Med (2022) 19(9):e1003992. doi: 10.1371/journal.pmed.1003992
6. Tseng HF, Ackerson BK, Luo Y, Sy LS, Talarico CA, Tian Y, et al. Effectiveness of mrna-1273 against sars-Cov-2 omicron and delta variants. Nat Med (2022) 28(5):1063–71. doi: 10.1038/s41591-022-01753-y
7. Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med (2022) 386(16):1532–46. doi: 10.1056/NEJMoa2119451
8. Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg Y, et al. Duration of effectiveness of vaccines against sars-Cov-2 infection and covid-19 disease: Results of a systematic review and meta-regression. Lancet (2022) 399(10328):924–44. doi: 10.1016/S0140-6736(22)00152-0
9. Risk M, Hayek SS, Schiopu E, Yuan L, Shen C, Shi X, et al. Covid-19 vaccine effectiveness against omicron (B.1.1.529) variant infection and hospitalisation in patients taking immunosuppressive medications: A retrospective cohort study. Lancet Rheumatol (2022) 4(11):e775–e84.
10. Ioannou GN, Bohnert ASB, O'Hare AM, Boyko EJ, Maciejewski ML, Smith VA, et al. Effectiveness of mrna covid-19 vaccine boosters against infection, hospitalization, and death: A target trial emulation in the omicron (B.1.1.529) variant era. Ann Intern Med (2022) 175(12):1693–706. doi: 10.7326/M22-1856
11. Kawasuji H, Morinaga Y, Tani H, Saga Y, Kaneda M, Murai Y, et al. Effectiveness of the third dose of Bnt162b2 vaccine on neutralizing omicron variant in the japanese population. J Infect Chemother (2022) 28(9):1273–8. doi: 10.1016/j.jiac.2022.05.009
12. Grewal R, Kitchen SA, Nguyen L, Buchan SA, Wilson SE, Costa AP, et al. Effectiveness of a fourth dose of covid-19 mrna vaccine against the omicron variant among long term care residents in ontario, canada: Test negative design study. BMJ (2022) 378:e071502. doi: 10.1136/bmj-2022-071502
13. Tan CY, Chiew CJ, Lee VJ, Ong B, Lye DC, Tan KB. Effectiveness of a fourth dose of covid-19 mrna vaccine against omicron variant among elderly people in singapore. Ann Intern Med (2022) 175(11):1622–3. doi: 10.7326/M22-2042
14. Intawong K, Chariyalertsak S, Chalom K, Wonghirundecha T, Kowatcharakul W, Ayood P, et al. Heterologous third and fourth dose vaccines reduce severity and mortality in covid-19 patients during the periods of delta and omicron predominance in thailand. Int J Infect Dis (2022) 126:31–8. doi: 10.1016/j.ijid.2022.11.006
15. Kim J, Seo H, Kim HW, Kim D, Kwon HJ, Kim YK. Effect of previous covid-19 vaccination on humoral immunity 3 months after sars-Cov-2 omicron infection and booster effect of a fourth covid-19 vaccination 2 months after sars-Cov-2 omicron infection. Viruses (2022) 14(11). doi: 10.3390/v14112458
16. Kislaya I, Machado A, Magalhaes S, Rodrigues AP, Franco R, Leite PP, et al. Covid-19 mrna vaccine effectiveness (Second and first booster dose) against hospitalisation and death during omicron Ba.5 circulation: Cohort study based on electronic health records, portugal, may to july 2022. Euro Surveill (2022) 27(37):2200697.
17. New China News Agency. The immunisation coverage with the full primary series of covid-19 vaccines in china is 89.7%. (2022).
18. Li JX, Wu SP, Guo XL, Tang R, Huang BY, Chen XQ, et al. Safety and immunogenicity of heterologous boost immunisation with an orally administered aerosolised Ad5-ncov after two-dose priming with an inactivated sars-Cov-2 vaccine in chinese adults: A randomised, open-label, single-centre trial. Lancet Respir Med (2022) 10(8):739–48. doi: 10.1016/S2213-2600(22)00087-X
19. Zhang Z, Wu S, Liu Y, Li K, Fan P, Song X, et al. Aerosolized Ad5-ncov booster vaccination elicited potent immune response against the sars-Cov-2 omicron variant after inactivated covid-19 vaccine priming. medRxiv (2022), 22271816. doi: 10.1101/2022.03.08.22271816
20. Tang R, Zheng H, Wang BS, Gou JB, Guo XL, Chen XQ, et al. Safety and immunogenicity of aerosolised Ad5-ncov, intramuscular Ad5-ncov, or inactivated covid-19 vaccine coronavac given as the second booster following three doses of coronavac: A multicentre, open-label, phase 4, randomised trial. Lancet Respir Med (2023) 11(8):698–708. doi: 10.1016/S2213-2600(23)00049-8
21. CHINA SCIO. COVID-19 response further optimized with 10 new measures. National Heath Commission of the People’s Republic of China (2022).
22. Bai Y, Peng Z, Wei F, Jin Z, Wang J, Xu X, et al. Study on the Covid-19 epidemic in mainland china between november 2022 and january 2023, with prediction of its tendency. J Biosaf Biosecur (2023) 5(1):39–44. doi: 10.1016/j.jobb.2023.03.001
23. China CDC. The situation of the novel coronavirus infection in China. (2023). Available at: https://www.chinacdc.cn/jkzt/crb/zl/szkb_11803/jszl_13141/202304/t20230408_264979.
24. Gilbert PB, Donis RO, Koup RA, Fong Y, Plotkin SA, Follmann D. A covid-19 milestone attained - a correlate of protection for vaccines. N Engl J Med (2022) 387(24):2203–6. doi: 10.1056/NEJMp2211314
25. Jin L, Tang R, Wu S, Guo X, Huang H, Hou L, et al. Antibody persistence and safety after heterologous boosting with orally aerosolised Ad5-ncov in individuals primed with two-dose coronavac previously: 12-month analyses of a randomized controlled trial. Emerg Microbes Infect (2023) 12(1):2155251. doi: 10.1080/22221751.2022.2155251
26. Li J, Hou L, Guo X, Jin P, Wu S, Zhu J, et al. Heterologous Ad5-ncov plus coronavac versus homologous coronavac vaccination: A randomized phase 4 trial. Nat Med (2022) 28(2):401–9. doi: 10.1038/s41591-021-01677-z
27. Cohen G, Jungsomsri P, Sangwongwanich J, Tawinprai K, Siripongboonsitti T, Porntharukchareon T, et al. Immunogenicity and reactogenicity after heterologous prime-boost vaccination with coronavac and Chadox1 ncov-19 (Azd1222) vaccines. Hum Vaccines Immunotherapeutics (2022) 5(18):000–. doi: 10.1080/21645515.2022.2052525
28. Baek YJ, Kim W-J, Ko J-H, Lee Y-J, Ahn JY, Kim JH, et al. A heterologous Azd1222 priming and Bnt162b2 boosting regimen more efficiently elicits neutralizing antibodies, but not memory t cells, than the homologous Bnt162b2 regimen. Vaccine (2023) 10(41):1694–702. doi: 10.1016/j.vaccine.2023.01.063
29. Assawakosri S, Kanokudom S, Chansaenroj J, Suntronwong N, Auphimai C, Nilyanimit P, et al. Persistence of immunity against omicron Ba.1 and Ba.2 variants following homologous and heterologous covid-19 booster vaccines in healthy adults after a two-dose Azd1222 vaccination. Int J Infect Dis (2022) 000(122):793–801. doi: 10.1016/j.ijid.2022.07.038
30. Costa Clemens SA, Weckx L, Clemens R, Almeida Mendes AV, Ramos Souza A, Silveira MBV, et al. Heterologous versus homologous covid-19 booster vaccination in previous recipients of two doses of coronavac covid-19 vaccine in brazil (Rhh-001): A phase 4, non-inferiority, single blind, randomised study. Lancet (2022) 10324(399):521–9. doi: 10.1016/S0140-6736(22)00094-0
31. Atmar RL, Lyke KE, Deming ME, Jackson LA, Branche AR, El Sahly HM, et al. Homologous and heterologous covid-19 booster vaccinations. New Engl J Med (2022) 11(386):1046–57. doi: 10.1056/NEJMoa2116414
32. Jagadeesh N, Deva V, Kapadi S, Shaw D. Risk factors of 120-day mortality among hip fractures with concomitant covid-19 infection. Cureus (2022) 14(12):e32637. doi: 10.7759/cureus.32637
33. Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev (2019) 32(2). doi: 10.1128/CMR.00084-18
34. Oliveira-Silva J, Reis T, Lopes C, Batista-Silva R, Ribeiro R, Marques G, et al. Long-term serological sars-Cov-2 igg kinetics following mrna covid-19 vaccine: Real-world data from a large cohort of healthcare workers. Int J Infect Dis (2022) 122:1–7. doi: 10.1016/j.ijid.2022.05.026
35. Kang YM, Minn D, Lim J, Lee KD, Jo DH, Choe KW, et al. Comparison of antibody response elicited by Chadox1 and Bnt162b2 covid-19 vaccine. J Korean Med Sci (2021) 36(46):e311. doi: 10.3346/jkms.2021.36.e311
Keywords: SARS-CoV-2, COVID-19, fourth dose, Ad5-nCoV, breakthrough infection
Citation: Xu N, Xu Y, Dai R, Zheng L, Qin P, Wan P, Yang Y, Jiang J, Zhang H, Hu X and Lv H (2023) Study of efficacy and antibody duration to fourth-dose booster of Ad5-nCoV or inactivated SARS-CoV-2 vaccine in Chinese adults: a prospective cohort study. Front. Immunol. 14:1244373. doi: 10.3389/fimmu.2023.1244373
Received: 22 June 2023; Accepted: 16 August 2023;
Published: 06 September 2023.
Edited by:
Mrinmoy Sanyal, Stanford University, United StatesReviewed by:
Larry Ellingsworth, Novavax, Inc., United StatesCopyright © 2023 Xu, Xu, Dai, Zheng, Qin, Wan, Yang, Jiang, Zhang, Hu and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hangjie Zhang, aGp6aGFuZ0BjZGMuemouY24=; Xiaowei Hu, eGhqa2h4d0AxMjYuY29t; Huakun Lv, aGtsdkBjZGMuemouY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.